- 1Department of Chemistry, Life Sciences and Environmental Sustainability, University of Parma, Parma, Italy
- 2Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy
- 3Institute for Electromagnetic Sensing of the Environment, National Research Council of Italy, Milano, Italy
- 4Department of Design, Technology Architecture, Land and Environment, University of Rome La Sapienza, Rome, Italy
- 5School of Life Sciences, University of Essex, Colchester, United Kingdom
Despite the global ecological and societal importance of deep lakes and their associated biota and ecosystem services, the relationships between water and sediment features and the spatial patterns of macrophyte assemblages remain poorly understood in these ecosystems, especially below 4–5 m depth. We aimed to fill this gap by providing new evidence of macrophyte community assembly rules over a wide range of colonized depths (up to 20 m). The macrophyte communities of five deep volcanic lakes in Central Italy, covering a wide range of dimensions (from 1.7 to 114.5 km2), maximum depths (from 33 to 165 m), and trophic status [12.4–41.3 μg of total phosphorus (TP) L-1], were explored. We applied linear mixed effect models and multivariate Multiscale Codependence Analysis (mMCA) to investigate macrophyte depth patterns and environmental drivers at nested spatial scales ranging from micro (at the scale of single vegetation belt) to large (whole lake study site) scales. A weak or absent macrophyte spatial structure was reported for the most impacted lakes (Vico and Nemi lakes), as well as for the most pristine lakes (Bracciano and Bolsena lakes). A well-defined structure was observed exclusively in Martignano Lake, an intermediate site both in terms of trophic status (17.1 μg TP L-1) and area (2.02 km2). Overall, distinctive macrophyte patterns were found at the largest lake scale, reflecting a clear distinction between shallow (up to 3 m) and deep vegetated bands (>3 m), dominated by vascular plants and large charophytes, respectively. Conversely, no strong spatial structure was detected at the microscale (i.e., with metric resolution, comparing the different study plots with each other). The low species diversity and the constant presence of only one dominant species per vegetated band can explain this result. Beyond light availability, sediment features (TP and organic matter content) emerged as significant in determining the arrangement of macrophytes in relation to depth, offering a more informed view of macrophyte spatial processes and their functional implications in deep lakes.
1 Introduction
Despite occupying a relatively small portion of the Earth’s surface, deep freshwater lakes have a disproportionally high biodiversity compared to terrestrial ecosystems, and provide key services like fisheries, drinking water and recreational activities (Hayford et al., 2015; Salmaso et al., 2018; Heino et al., 2021). Unfortunately, a worldwide decline in lake quality has been observed, in terms of pollution, water temperature and reduction of biodiversity (Zhang et al., 2017; Jenny et al., 2020). This decline is due to direct anthropogenic overexploitation, as well as to the close relationship between lakes and watershed conditions (Heino et al., 2021), which makes them susceptible to environmental factors, threats and changes across their watersheds.
Deep lakes present steep environmental gradients along the depth profile in terms of light availability, temperature, nutrients, oxygen and wind/wave disturbance (Bornette and Puijalon, 2011; Lewerentz et al., 2021). These gradients influence the distribution of submerged plants and vegetation composition, in turn affecting the functions and services provided by these ecosystems (Spence, 1982; Thomaz, 2021). Depth changes of a few meters are sufficient for the environmental conditions and availability of resources to undergo significant variations (e.g., light attenuation follows a logarithmic function; see Wetzel, 2001).
Notwithstanding the general recognition of the key role played by light intensity in regulating the presence and distribution of macrophytes in deep waters (Spence, 1982), we lack systematic studies on the relationships between water column and sediment conditions and the spatial structure of macrophyte communities within deep lentic systems. Macrophytes inhabit a challenging environment (O’Hare, 2015) and their spatial structure is determined by a variety of factors acting at different spatial scales. Broad descriptors include latitude, altitude and temperature (Rooney and Kalff, 2000; Lacoul and Freedman, 2006). At the lake scale, multiple factors such as topography, turbidity, water chemistry and sediment characteristics are important spatial drivers of macrophyte community structure (Bornette and Puijalon, 2011), but competition, herbivory and disease can also play a major role (Lacoul and Freedman, 2006; Van Onsem and Triest, 2018). Nevertheless, there is a paucity of literature dedicated to intra-lake macrophyte community structure and ecological processes. Indeed, the importance of environmental variables in structuring communities varies depending on the spatial scale considered (Alahuhta et al., 2016), but lakes are often thought to be homogeneous ecosystems, and little is known about intra-lake processes (but see Lewerentz et al., 2021). In fact, available studies tend to investigate large-scale spatial processes, without accounting for differences in macrophyte communities within lakes (Alahuhta et al., 2013; Alahuhta et al., 2015; Alahuhta et al., 2018; Alahuhta et al., 2025), or they do not address macrophyte communities in deeper water. For example, Wang et al. (2020), while determining the environmental and spatial drivers of local communities, limited the deep-water belt to 3–6 m, as well as Lewerentz and colleagues (2021) who explored the macrophyte depth diversity gradient (DDG) down to a depth of −5 m. Similarly, Tian et al. (2023) did not include assessment of spatial patterns beyond shallow water depths in their study of intra-lake variations in macrophyte communities in relation to trophic status.
The aforementioned abiotic factors have a differential effect on various macrophyte growth forms at the local scale (Trindade et al., 2018), with emergent species less affected by environmental changes than submerged species under stable water level conditions (Alahuhta et al., 2016). Light availability is one of the most limiting factors for submerged macrophyte growth (Spence, 1982; Wen et al., 2022). It decreases along the depth gradient thereby determining zonation of hydrophytes along the littoral area of lakes (Lehmann et al., 1997; Azzella et al., 2014). Deeper areas are often colonized by charophytes in clear lakes, because of their low light tolerance, while vascular species tend to occupy the shallower areas (Bolpagni et al., 2016; Murphy et al., 2018).
Spatial processes (e.g., dispersal) can confound interpretation of the effect of environmental variables on species distributions because they may influence the community structure regardless of local environmental conditions (Clappe et al., 2018; Török et al., 2020). Accounting for spatial components of macrophyte structure may allow us to integrate interactions between abiotic factors and dispersal processes (Alahuhta et al., 2021; Lobatode Magalhães et al., 2022). Therefore, we should include spatial information in lake macrophyte community studies (Lewerentz et al., 2021). Focusing on the appropriate scale can improve the quality of lake macrophyte research because communities respond differently to environmental conditions according to the spatial scale considered (Alahuhta et al., 2016). Capers et al. (2010) suggest that environmental variation and spatial processes (e.g., dispersal) contribute similarly to macrophyte structure, both at local and regional scales, although a great amount of stochasticity is still involved.
Starting from the evidence collected by Azzella et al. (2017), who explored the co-occurrence patterns of macrophytes in five deep Mediterranean lakes up to 20 m depth, the aim of this study is to further deepen the understanding of the spatial relationships between submerged vegetation community structure and major environmental drivers (i.e., water and sediment features), including the relative contribution of both vascular and charophyte components, using the same data set. Using Canonical Correspondence Analysis, Azzella et al. (2017) observed the existence of recurrent macrophyte distribution patterns, which are closely dependent on the trophic status of the lakes: as trophic loads increased, species tended to distribute themselves more and more randomly. This suggests that a more thorough approach including spatial components may capture the processes underlying community structure and its environmental drivers. To overcome the limitations of the previous study, here we apply a spatial-based approach known as multivariate Multiscale Codependence Analysis (mMCA) (Guénard and Legendre, 2018) with the aim of highlighting complex spatial patterns in environmental processes such as the re-assembly of macrophyte communities in response to trophic changes. Indeed, mMCA is specifically designed to incorporate spatiotemporal information into species distribution modelling in a multivariate context. We hypothesize that more pristine lakes show stronger spatial structure driven by community-environmental relationships at a wider range of spatial scales than more impacted lakes: i.e., we expect to find that environmental drivers would act on macrophyte communities at smaller and larger spatial scales simultaneously in pristine lakes. Increasingly impacted lakes suffer from the progressive loss of potential colonization areas due to a reduced light availability in deeper areas, which can trigger a spatial rearrangement of macrophyte species (i.e., a progressive migration towards shallower depths) with a consequent increase in competition among species.
2 Materials and methods
2.1 Study sites
The present study used data collected by Azzella et al. (2017) from five deep volcanic lakes of Central Italy (Bolsena, Bracciano, Martignano, Nemi and Vico lakes; Figure 1), varying in terms of dimension (from 1.7 to 114.5 km2), maximum depth (from 33 to 165 m), and trophic status [from 12.4 to 41.3 μg L-1 of total phosphorus (TP), with 10 and 30 μg L-1 being the thresholds between oligotrophic, mesotrophic and eutrophic conditions (Azzella et al., 2017)]. These well-studied lakes had comparable plant communities in the past (Azzella et al., 2013; Pinzani et al., 2025), with a similar distribution along the depth gradient within each lake (Azzella, 2012). In pristine condition they are Chara-lakes according to the Italian lake typology (Tartari et al., 2006). They were selected to represent a trophic gradient resulting from anthropogenic impact. Physical and chemical conditions of the target lakes are presented in Table 1, Supplementary Table SI1 and Azzella et al. (2017). Bracciano and Bolsena lakes represent near-pristine conditions (with TP values close to oligotrophic conditions), Nemi and Vico lakes are the most impacted sites (with TP in the range of 37–41 μg L-1), while Martignano Lake has intermediate conditions (17.1 μg TP L-1). These lakes greatly differ also in dissolved oxygen (DO) patterns along the depth gradient (Supplementary Figure SI1). During daytime, in Martignano and Nemi lakes the DO peaked at 12 m depth with values of 155.7% (±5.6) and 130.2% (±4.2), and then it rapidly decreased below the maximum depth of macrophytes growth (Zcmax). At 20 m depth, DO showed values respectively of 81.0% (±17.5), 46.0% (±12.5) and 0% in Martignano, Vico and Nemi lakes, respectively. Similarly, trophic parameters showed appreciable differences among lakes, highlighting an increase in trophic status in Martignano and more prominently in Nemi and Vico. Water total nitrogen (TN) reached the highest concentrations in Nemi (515 μg L-1) and Vico (473 μg L-1) at 20 m depth and the lowest in Bracciano (50 μg l L-1). Concerning sediment features, an increasing trend was evident in sediment organic matter content from more pristine to more impacted lakes. For further insights on the methodological procedures of water and sediment sampling and analysis and physical and chemical conditions of the target lakes, see Table 1, Supplementary Table SI1 and Azzella et al. (2017).
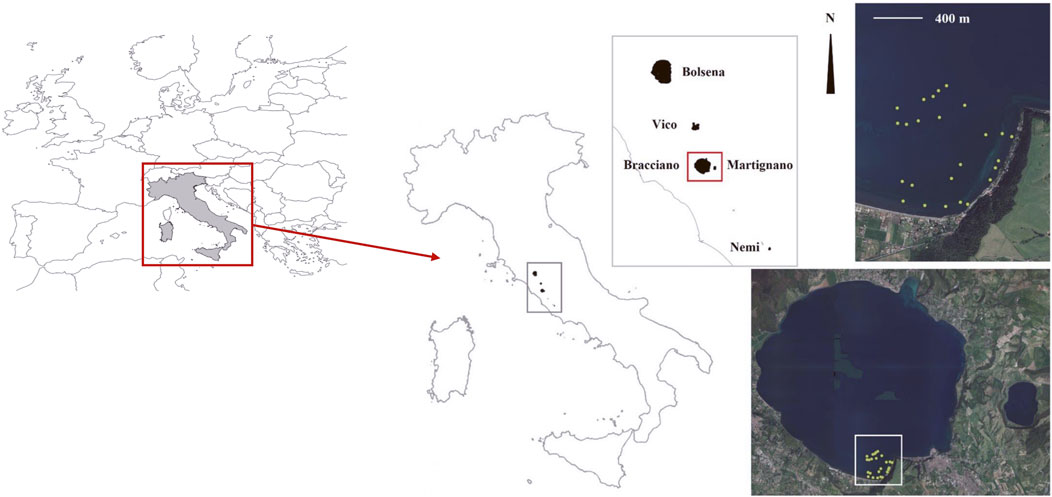
Figure 1. Map of the study area with a zoom on Bracciano Lake as an example of sampling design. Yellow dots represent the surveyed plots.
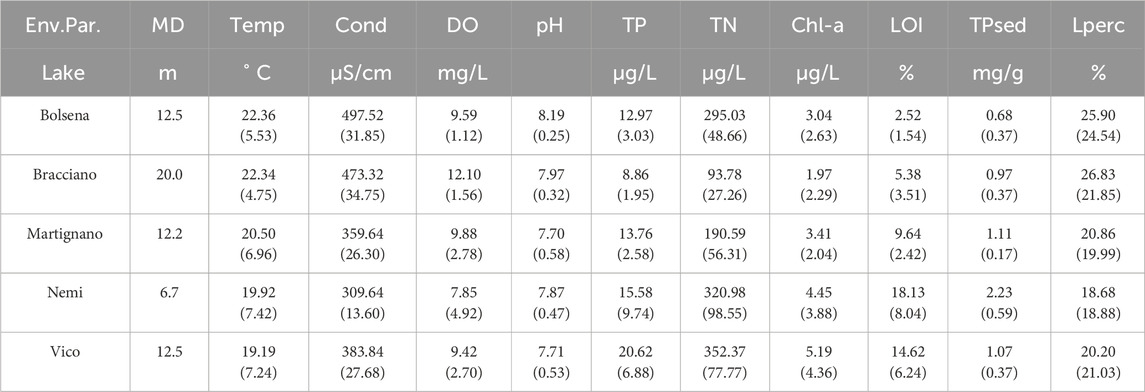
Table 1. Maximum vegetation colonization depth (MD = Max Depth, data from sampling), mean and standard deviation (in brackets) of main environmental parameters (Env.Par.) for the five investigated lakes. MD = Maximum depth, Temp = Temperature, Cond = Conductivity, DO = Dissolved oxygen, TP = Water Total Phosphorus, TN = Water Total Nitrogen, Chl-a = Water Chlorophyll-a, LOI = Sediment Organic Matter Content, TPsed = Sediment Total Phosphorus, Lperc = light availability (data from Azzella et al., 2017).
2.2 Sampling design and data collection
The data used in this study were collected during the previous study that aimed at determining co-occurrence patterns of macrophytes in the five lakes (Azzella et al., 2017), while here we use their data to investigate the spatial structure of the communities and their relationships with water and sediment features in more detail, implementing a mMCA strategy. This is a significant enhancement of the earlier study through which we intend to address critical issues highlighted by Azzella et al. (2017), primarily associated with the importance of whole-lake trophic status and dynamics in explaining the role and importance of environmental determinants as macrophyte filters.
In each lake, a homogeneous littoral sector of about 1 km (linear distance) parallel to the shore was selected (Figure 1). These areas did not show evident artificial alteration of littorals or point-like sources capable of altering the submerged vegetation (Azzella et al., 2013). At the same time, the selected sectors were not significantly affected by fetch, allowing the vegetation to be representative of the whole lake macrophyte community. These sectors were identified based on preliminary surveys covering the entire surface of the lakes (Azzella, 2012), allowing the identification of those areas characterized by the highest development (in terms of maximum growth depths) of macrophytes, not affected by unfavorable local conditions, such as steep and/or rocky bottoms. Within each target sector in each lake, in the depth range from 0 to 20 m, 25 plots of 4 m2 were selected and surveyed, for a total of 125 plots. The sampling was carried out in 2013, during the peak of the growing season (July-August; Azzella et al., 2017). The sampling design was arranged to have five plots randomly selected at five pre-defined water depths (centered at 1.5, 3, 6, 12 and 20 ± 0.5 m of depth) which correspond to the core areas occupied by the vegetation bands characterizing Bracciano, the reference site among those explored. In the absence of significant chemical and physical perturbations, each of the five target lakes (Bracciano, Bolsena, Martignano, Nemi and Vico) should be characterized by all five vegetation belts (currently present only in Bracciano) (for further insights see Azzella et al., 2017).
All the species present in the target plots were identified, and their relative cover-abundance recorded using percentage classes from 0% to 100% at 5% intervals. Each plot was also characterized in terms of water quality (conductivity, pH, DO, nitrate and ammonia ions, soluble reactive phosphorous, chlorophyll-a, TN, TP and light attenuation expressed as the proportion of incoming radiation reaching the plot depth = Lperc) and sediment characteristics (total phosphorus = TPsed, organic matter = OM expressed as LOI = dry weight Loss on Ignition, density and porosity). Standard approaches and methods were followed to collect physical and chemical data; details are reported by Azzella et al. (2017).
2.3 Vegetation features of study lakes
In all lakes, a total of 24 macrophyte species was recorded, of which 13 were vascular, including one bryophyte, and 10 were Characeae species (Supplementary Table SI2). The most common species in terms of number of colonized plots was Ceratophyllum demersum (present in 27 plots out of 125), followed by Chara polyacantha (24, syn. C. aculeolata) and Myriophyllum spicatum (24). Vegetation mainly covered the shallowest littoral areas of lakes (in the range 1.5–12.0 m of depth), whereas it was normally absent at the depth of 20 m (except in Bracciano Lake) and below 12 m in Nemi and Vico (Azzella et al., 2017). Vascular species were poorly represented in Bolsena and Bracciano Lakes (Supplementary Figure SI2), while they occupied the shallowest littoral vegetation belt in Martignano and Nemi Lakes. In Vico, instead, vascular species occupied only the 6 m belt. Charophytes were dominant in Bracciano and Bolsena (Supplementary Figure SI2), occupying part of the littoral and the deepest plots in Martignano, but were completely absent from Nemi. In Vico, charophytes were present in the shallowest plots, in contrast with vascular species’ distributions (for a full account of these patterns, see Azzella et al., 2017).
2.4 Statistical analyses
To test our hypothesis, an mMCA was performed (Guénard and Legendre, 2018) using the software R (R Core Team, 2021). This analysis uses three types of information: the response variables are the species abundances per site (plots); the explanatory variables are the environmental variables recorded at each site; and lastly, spatial information about the sites, which can be one-dimensional or bi-dimensional, using plot coordinates. In this way, we intended to improve on the approach used by Azzella et al. (2017), i.e., null model analysis and CCA, analyzing the same dataset, offering a holistic perspective on the drivers of the spatial structure of macrophyte communities.
Before running the mMCA, we used linear mixed effect models to test the effect of vegetation on pH and DO, which could be influenced by the presence of submerged macrophytes, showing wide daily variations. This step was necessary to understand whether these variables reflected lake conditions and could therefore be considered explanatory variables of macrophyte structure, rather than mirroring the influence of macrophytes. We tested the combined effect of water depth and presence of plants on the chosen variables. We used data from vegetated and non-vegetated plots surveyed during the 2013 study, together with data measured in the center of the lakes (in a non-vegetated location) at the corresponding water depth, derived from Azzella et al. (2017). We included random slopes described by the interaction between lakes and vegetation presence. The significance of predictor variables was explored by calculating the confidence intervals with the function ‘confint’, and the best model was selected using ANOVA and comparing the AIC. The R package lme4 was used to perform the analyses (Kuznetsova et al., 2017).
In the first step of the mMCA analysis, the space (study area within each lake) is organized into a number of spatial Eigenvectors, called MEMs (Moran’s eigenvector maps; Dray et al., 2006), that describe the given space from the largest (lower order of MEM) to the smallest scale (higher order of MEM) (Grimaldo et al., 2016). The eigenmap function in the R package codep was used to obtain eigenvector maps (Guénard and Legendre, 2018). Then, a PCA is run with the species abundance data, reducing community structure to the first two PCA axes which are used in the analysis. Finally, spatial structure is defined based on the covariation of community composition and environment at each successive spatial scale investigated, in this case ranging from the micro scale (comparison between the different vegetation bands) to the larger scale (lake study area).
For each lake two analyses were carried out: one mMCA using all species’ abundances to investigate the whole community structure, and one mMCA using the relative proportion of vascular species cover compared to charophyte cover, to assess the contribution of these two taxonomic components. In both cases, collinearity in the environmental variables was checked and redundant variables (linear correlation coefficient r > 0.7) were omitted. Plots with 0% vegetation cover were also omitted from the analysis, and species abundances were Hellinger transformed to mitigate the broad differences between total abundances within plots and to cope with the high proportion of zeros. Finally, a permutation test was performed to test for significance of the mMCA model output for each lake. The R packages ggplot2 (Wickham, 2016) and ggrepel (Slowikowski, 2021) were implemented to graphically represent the community structure PCA as well as the lake environmental descriptors.
3 Results
The linear mixed effects models revealed no significant correlation between vegetation and DO or pH. Indeed, ANOVA revealed no significant difference between the model with and without plants (p > 0.1); AIC values of both models were similar. Therefore, we kept DO and pH as explanatory variables in the mMCA analyses. Due to collinearity, the environmental variables included in the mMCA analyses were: DO (mg L-1), pH, Lperc (%), water TP (µg L-1), TN (µg L-1), sediment OM (%) and TPsed (mg g-1).
3.1 Micro-to macro-spatial macrophyte structure
Our results indicate that environmental drivers exert significant effects on underwater macrophyte distribution almost exclusively at larger scales (Table 2). This is supported by the findings from all the studied lakes except for Nemi. The latter lake is the most impacted system in this study, and no spatial structure in the macrophyte community was found. In this lake the vegetation included only vascular species and no charophytes were observed.
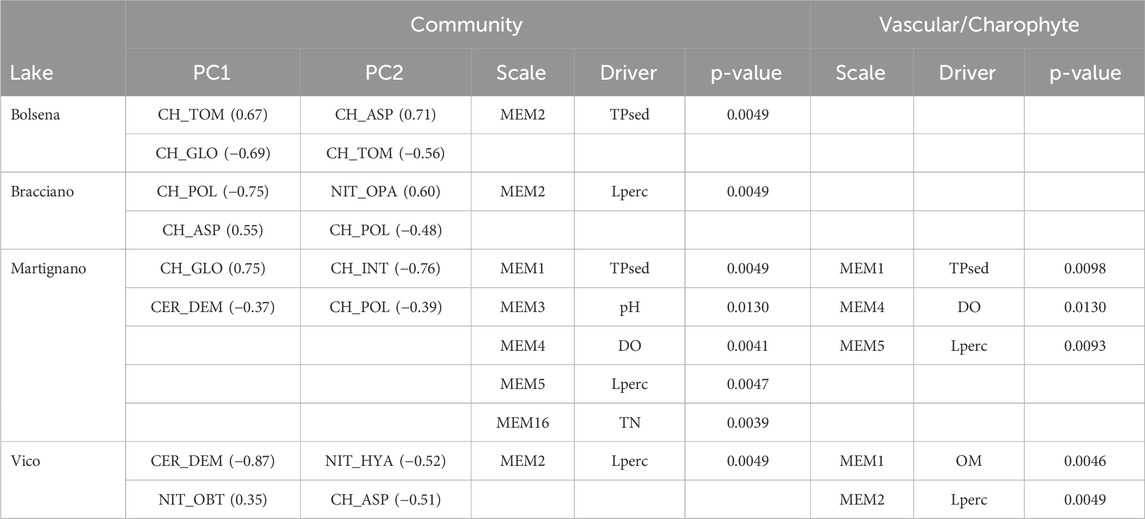
Table 2. Summary of the mMCA results. “Community” refers to the analyses on the community including all species, “Vascular/Charophyte” refers to the comparison of vascular and charophyte community composition. In PC1 and PC2 columns the two species with higher loadings for each PC axis are indicated, with loading values in brackets (for complete name reference see Supplementary Table SI2). Significant environmental drivers are reported, together with the relative p-value and the scale at which they affect the community (lower MEM order means broader scale); no information relating to Lake Nemi is reported in the table as no significant driver was found. Refer to text for driver abbreviations.
Lperc (proportion of incoming radiation reaching the plot depth) emerged as the key large-scale driver of macrophyte community structure in Bracciano and Vico, for the whole macrophyte community, and only in Vico for the vascular versus charophyte models (MEM2, p < 0.01 in all cases). OM (sediment organic matter content) was also a significant driver at the lake scale (MEM1, p < 0.05). Similarly, at a large scale, TPsed (sediment total phosphorus) structured the community of Bolsena (MEM2, p < 0.01). For Bracciano and Bolsena none of the environmental variables included in the analysis were significant in distinguishing between belts dominated by charophytes and vascular species. Overall, the broad-scale descriptors captured the distinction between shallow and deep areas (>3 m).
Martignano presented a highly structured macrophyte community–showing that species distribution was influenced by different drivers at different scales–both at large and at small spatial scales. The environmental variables involved in significant (all p < 0.01) spatial-ecological relationships were TPsed at MEM1, pH at MEM3, DO (dissolved oxygen concentration) at MEM4, Lperc at MEM5 and TN (total nitrogen concentration) at MEM16. In this lake, we found that TPsed (p < 0.05), DO (p < 0.01) and Lperc (p < 0.01) were also significant in the models of vascular versus charophyte community structure at larger scales (MEM1, MEM4 and MEM5, respectively).
3.2 Site-specific macrophyte spatial patterns
For Bracciano, the mMCA analysis identified 19 significant (p < 0.05) spatial Eigenvectors. The first two PCA axes together explained 83% of the variation in community structure. Characeae were the dominant species driving the variation: C. aspera, C. polyacantha, Nitella opaca and C. globularis were the species with the highest loadings (Figure 2A). Therefore, C. aspera was found in shallow plots where Lperc was higher, whereas C. polyacantha, C. globularis and N. opaca were found in deeper plots with lower Lperc (Figure 2B).
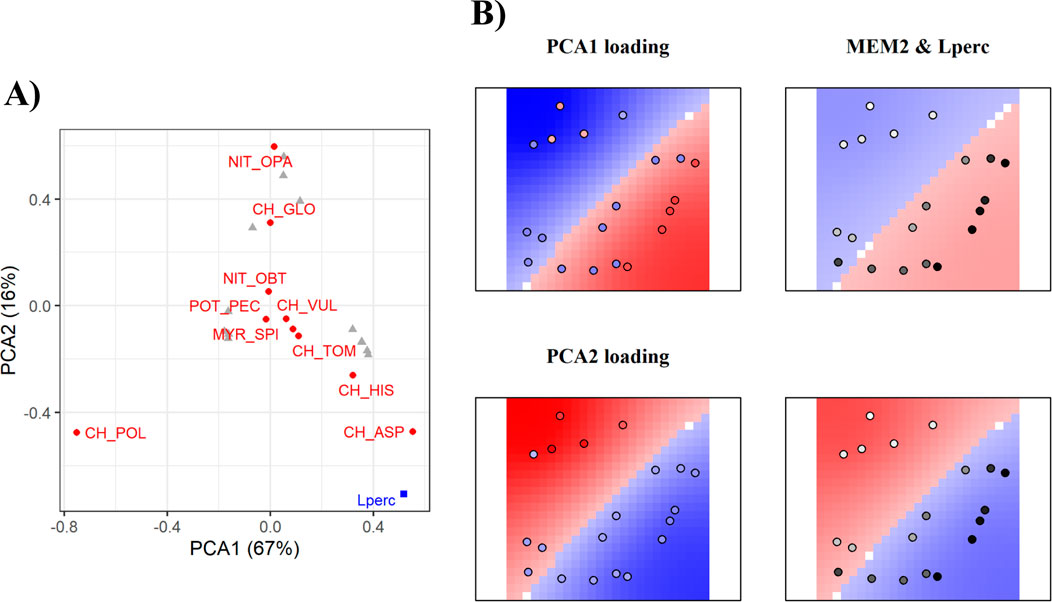
Figure 2. Output of the mMCA analyses for Bracciano Lake. On the left the first two principal components of the community structure (A): species are in red (for complete name reference see Supplementary Table SI2), significant environmental variables in the mMCA are in blue, plots are indicated with grey triangles. On the right, the representation of significant spatial Eigenvectors (B). Red corresponds to positive values, blue to negative values. The first column of maps represents plots and their loadings in the corresponding PCA. The remaining maps on the right indicate the spatial pattern (MEM) and the significant environmental variables acting at the given scale. The background represents the loadings of the spatial Eigenvector (MEM). White dots or areas in the background correspond to loadings around zero. The intensity of grey in the dots indicates the value of the environmental variable at a given plot (darker dots indicate higher values).
In Bolsena, 16 significant (p < 0.05) spatial Eigenvectors were identified. The first two PCA axes explained 79% of the species variation, and again charophytes were the most important group in the community, represented by C. tomentosa, C. globularis, and C. aspera (Table 1). Species like C. tomentosa and C. aspera grew in shallow plots with low TPsed, while C. globularis was present in deeper plots with high TPsed. Vascular species were found nearest to the shore.
In Martignano we obtained 19 significant (p < 0.05) spatial Eigenvectors. The first two PCA axes described 62% of the variation, and we can observe a higher importance of vascular species in the community structure. The species with highest loadings were C. globularis, C. intermedia and C. demersum (Figure 3A). Positive loadings on PC1 were related to low TPsed, pH and Lperc, and high DO. Positive loadings on PC2 were more related to high TPsed, DO, and low pH and Lperc (Figure 3B). Water TN, though significant, was not very descriptive (Figure 3B). In this lake, C. globularis was found in deeper plots with high DO and low pH and Lperc whereas C. intermedia was found in approximately opposite conditions, including plots with low TPsed. C. demersum grew mainly in shallow plots where TPsed was higher. Vascular species were present near the shore where plots presented high TPsed and Lperc or low DO.
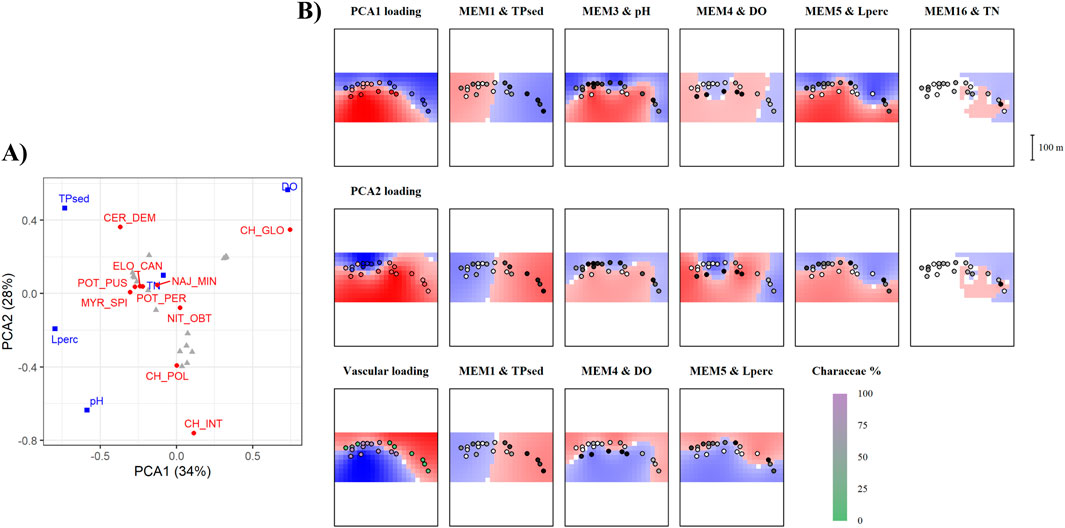
Figure 3. Output of the mMCA analyses for Martignano Lake. Refer to Figure 2 caption for interpretation of the figure. (A) Output of the PCA analysis. (B) Output of the mMCA analyses showing environmental drivers acting at different spatial scales. Here, the last row of maps represents the mMCA on vascular vs. charophytes. Shading green in the bottom left map indicates the proportion of charophyte species.
In Vico, one of the two most impacted lakes, we obtained 16 significant (p < 0.05) spatial Eigenvectors. PC1 and PC2 accounted for 74% of the variation altogether, and we could observe a clear distinction between charophytes and vascular species. The most representative species here were N. obtusa, C. globularis C. demersum, N. hyalina and C. aspera (Table 1; Figure 4A). Compared to other lakes, here we found vascular species (e.g., C. demersum) in the deepest plots, where Lperc was lowest, and charophytes in the littoral area, with higher Lperc (Figure 4B).
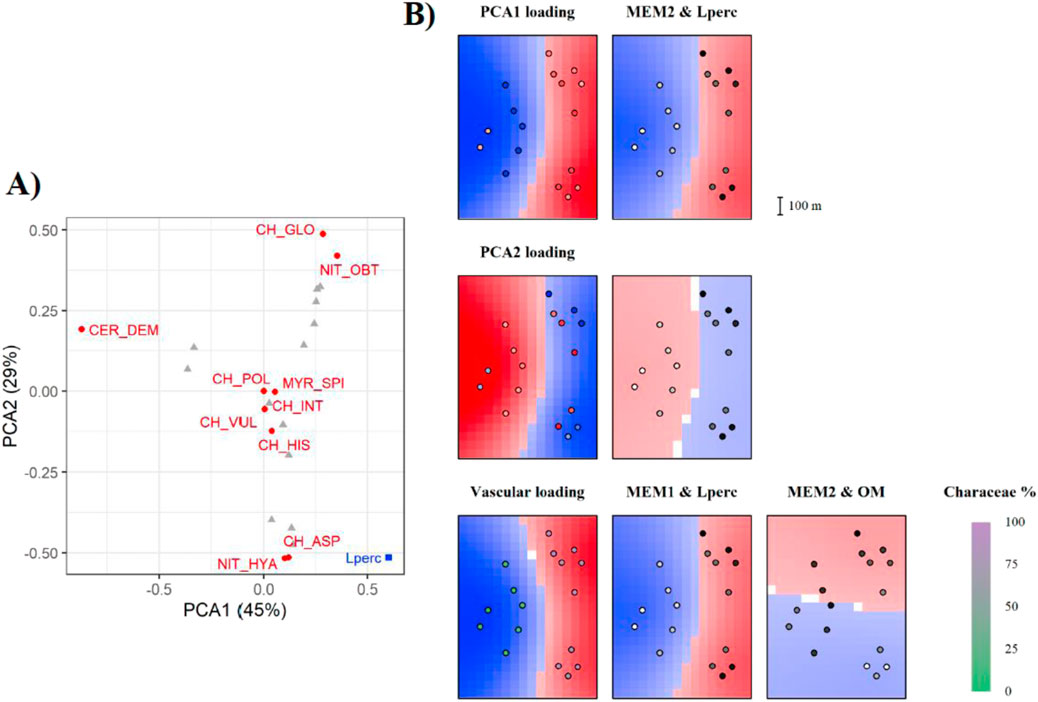
Figure 4. Output of the mMCA analyses for Vico Lake. Refer to Figure 2 caption for interpretation of the figure. (A) Output of the PCA analysis. (B) Output of the mMCA analyses showing environmental drivers acting at different spatial scales. Here, the last row of maps represents the mMCA on vascular vs. charophytes. Shading green in the bottom left map indicates the proportion of charophyte species.
4 Discussion
Findings from the present study highlight a clear partitioning between shallow (up to 3 m) and deep vegetated bands (>3 m) colonized by vascular macrophytes and large charophytes (e.g., C. polyacantha, C. tomentosa), respectively. On the contrary, no robust spatial structure was detected at the microscale (therefore across the different depth-related vegetation bands), both in pristine and impacted deep lakes, only partially supporting the hypothesis that more pristine lakes are characterized by a clearly differentiated macrophytes zonation.
Indeed, the vascular component was poorly represented in near-pristine lakes, while charophytes were absent from one of the impacted sites, reducing the macrophyte species diversity of these lakes and therefore the potential of the analysis to detect clear patterns of community structure.
4.1 Ecological drivers of macrophyte vegetation in deep lakes
Light availability (expressed as percentage) was the most common driver among our sites. This is not surprising, as light is widely considered the most limiting factor for submerged vegetation growth (Bini et al., 1999; Bornette and Puijalon, 2011; Chen et al., 2022; Cui et al., 2024). Interestingly, however, light was the only significant driver both in near pristine and more impacted lakes. Indeed, light is the most important environmental filter in lentic environments, where it can determine very rapid and substantial changes in conditions both in space and time, hence the establishment of light-demanding rather than low light-tolerant submerged species (Rodrigues and Thomaz, 2010; Luhtala et al., 2016; Zhang et al., 2020). A higher reduction in light availability along the depth gradient induces a limitation in the area suitable for colonization by submerged macrophytes, resulting in a lower number of vegetated plots in the eutrophic lakes investigated in this study.
Sediment total phosphorus (TPsed) was the second most common driver in our study, confirming the importance of sediment features for macrophyte assemblages (Bolpagni and Pino, 2017; Dainez-Filho et al., 2019; Marzocchi et al., 2019). The observed pattern reflects local sediment characteristics as well as a higher influence of sediment metabolism in the water column in shallower areas compared to deeper ones (Dalla Vecchia and Bolpagni, 2022).
As for dissolved oxygen (DO), its availability is indicative of the metabolism of a lake, including the consumption by autotrophic organisms and decomposition processes, especially in deep water (Misra, 2010). Therefore, the spatial patterns determined by DO at medium scale may reflect plant interactions with other species. On the other hand, pH is related to a plant’s carbon acquisition strategy (Rørslett, 1991), with higher pH favoring the presence of species adapted to use bicarbonate for photosynthesis (Iversen et al., 2019). Indeed, in Martignano we see how MEM3, related to pH, clearly differentiates the charophyte band from the vascular species band of vegetation, which may reflect differences in bicarbonate use between the two groups, although charophytes can be efficient bicarbonate users (Sand-Jensen et al., 2018). Further, it may also reflect the water depth gradient (Supplementary Figure SI1). The low predictive power of nitrogen in this lake is probably related to an unbalanced stoichiometry of nitrogen and phosphorus in the water; although nitrogen is abundant, the concentration of phosphorus remains relatively low, dampening its effect (de Baar, 1994; Xia et al., 2014).
4.2 Spatial relationships of macrophyte vegetation in deep lakes
Only relatively few drivers are involved in regulating the depth distribution of macrophytes in deep lakes: above all light availability (Sculthorpe, 1971; Spence, 1982). Based on our data, this means that pristine (or near-pristine deep lakes, with TP < 15 μg L-1) and impacted lakes (>35 μg L-1) have the same main (almost exclusive) driver at the largest spatial scales. In both these two extreme cases there are no other limiting factors as important as light. In near-pristine lakes, the contextual low availability of nutrients allows a progressive depth arrangement of macrophyte species according to their different adaptation to submergence (Stross et al., 1988; Schwarz et al., 2002). Conversely, in impacted lakes the reduction of light availability is so critical that the effects of other drivers (mainly nutrients) are probably masked. Therefore, we demonstrate that the spatial structure of macrophytes in near-pristine conditions is not driven by environmental constraints other than light availability. The absence of a complex environmental filter could lead to a higher relative importance of species interactions in structuring the community, highlighting the importance of species-specific traits and resource-use efficiency (Fu et al., 2023). This is in line with recent updates on context dependency in freshwater metacommunity studies (Alahuhta et al., 2025 and references therein).
Moreover, the absence of clear macrophyte spatial drivers in oligo-mesotrophic conditions is probably an effect of the low species diversity of submerged assemblages. They are typically dominated by only one taxon (i.e., a dominant vascular plant or macroalga) or often just a few species, in turn capable of transgressing their optimal growth depth (Tanner et al., 1985). This could represent a confounding behaviour for evaluating the spatial structure of species-poor macrophyte assemblages. In fact, evidence is accumulating on the intrinsic high spatial dynamism of submerged macrophytes, which is much greater than previously expected (Bresciani et al., 2012; Bolpagni et al., 2016; Ghirardi et al., 2019).
Only Martignano Lake exhibited strong spatial structuring of macrophyte assemblages at multiple scales with many environmental drivers acting simultaneously. This lake is characterized by a meso-eutrophic status, defining intermediate conditions that support higher biodiversity and prevent the dominance of few species that would become very competitive in more pronounced eutrophic or oligotrophic conditions (Bakker et al., 2013). The several drivers identified by mMCA analysis for this lake may define unique combinations of conditions and niches that can be occupied by various representatives of both Characeae and vascular species, as well as by herbivore communities–which may be fundamental in influencing the diversity, patterns and abundance of freshwater macrophytes (Sheldon, 1987; Bakker et al., 2016). The spatial arrangement of vascular plants and charophytes in this meso - to eutrophic lake is therefore well-defined and could be detected by the analysis, because the community is composed of a balanced abundance of a good number (6) of representative species (Ceratophyllum demersum, Chara polyacantha, C. globularis, Myriophyllum spicatum, Potamogeton perfoliatus, Stuckenia pectinata). Our study included only one lake with mesotrophic conditions; therefore, it is difficult to generalize our results. Nonetheless, this takes us one step further in supporting our expectations that we would see a difference in community drivers at different levels of lake trophic status. Indeed, previous studies have highlighted the importance of the nutrient content of water and sediments for the structuring of macrophyte communities (e.g., Bini et al., 1999), and, more generally, the importance of the abiotic environment as a leading driver in selecting macrophytes with similar traits within communities (Alahuhta et al., 2013; Alahuhta et al.,2025).
4.3 Future insights
This study highlights the need to further explore the complex mechanisms underlying macrophyte depth arrangements, in addition to reaffirming the true spatial and ecological significance of the depth gradient. In just a few meters of lake depth, substantial ecosystem variations emerge which are comparable to those that characterize the succession of vegetation belts along entire mountain ranges. When it comes to extreme environments for plant growth (e.g., cold environments, high salinity habitats and deserts; Bechtold, 2018), lake depths are rarely mentioned. We must urgently change our perception of the ecological requirements and environmental relationships of macrophytes and understand their ecological-evolutionary mechanisms to offer effective actions to recover lake macrophyte meadows and thereby maintain adequate levels of ecosystem service provision from large, deep lakes. This could be achieved by integrating studies investigating other biotic components of aquatic ecosystems, to account for interactions between macrophytes and other organisms such as herbivorous species (e.g., fish, water birds), grazers like Lymnaea stagnalis and other aquatic snails (capable of regulating the abundance and impacts of epiphytes) and bacteria.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ADV: Formal Analysis, Writing – original draft, Visualization, Data curation, Writing – review and editing, Investigation. RB: Investigation, Writing – review and editing, Conceptualization, Methodology, Supervision, Funding acquisition, Resources, Writing – original draft, Data curation. AL: Writing – review and editing, Formal Analysis, Investigation. DN: Investigation, Resources, Writing – review and editing, Data curation. MB: Data curation, Investigation, Writing – review and editing, Resources. MA: Data curation, Conceptualization, Methodology, Writing – review and editing, Validation, Supervision, Investigation. MW: Validation, Writing – review and editing, Data curation, Visualization, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is based on data collected in the frame of the project MIVOLS (Macrophytes of the Italian VOlcanic Lake System) supported by Parma and La Sapienza universities (Italy). This work has also benefited from the funds, equipment and framework of the COMP-R Initiative, funded by the ‘Departments of Excellence’ program of the Italian Ministry for Education, University and Research (MIUR, 2023–2027). RB is partially funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4, funded by the European Union–NextGenerationEU; Award Number: Project code CN_00000033, CUP B63C22000650007, Project title “National Biodiversity Future Center - NBFC”, Cascading grant call by Spoke 3 “Assessing and monitoring terrestrial and freshwater biodiversity and its evolution: from taxonomy to genomics and citizen science”, Project title “development of the Italian MAcrophytes Database (iMAD)”. ADV is funded by the MSCA-Global-2023 fellowship DIVE IN “Predicting DIVErsity of INvasive aquatic plants” (GA No. 101147317).
Acknowledgments
This work is based on data collected in the frame of the project MIVOLS (Macrophytes of the Italian VOlcanic Lake System) supported by Parma and La Sapienza universities (Italy). We thank Kevin Murphy (University of Glasgow, Scotland), Sidinei M. Thomaz (State University of Maringá, Brazil) for kindly commenting on the prior to submission, and the Specialty Chief Editor of the freshwater section of Frontiers in Environmental Science Angela H. Arthington for valuable suggestions and critical review of the Special thanks also go to the Regional Park of Bracciano and Martignano for logistic support and supply of instrumentation during the 2013 survey of lakes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1614281/full#supplementary-material
References
Alahuhta, J., Baastrup-Spohr, L., de Winton, M., Fernández-Aláez, C., Lukács, B. A., Sass, L., et al. (2025). The signature of biotic interactions on lake macrophytes differs among seven metacommunities of three continents. Hydrobiologia. doi:10.1007/s10750-025-05866-6
Alahuhta, J., Kanninen, A., Hellsten, S., Vuori, K. M., Kuoppala, M., and Hämäläinen, H. (2013). Environmental and spatial correlates of community composition, richness and status of boreal lake macrophytes. Ecol. Indic. 32, 172–181. doi:10.1016/J.ECOLIND.2013.03.031
Alahuhta, J., Lindholm, M., Baastrup-Spohr, L., García-Girón, J., Toivanen, M., Heino, J., et al. (2021). Macroecology of macrophytes in the freshwater realm: patterns, mechanisms and implications. Aquat. Bot. 168, 103325. doi:10.1016/J.AQUABOT.2020.103325
Alahuhta, J., Lindholm, M., Bove, C. P., Chappuis, E., Clayton, J., de Winton, M., et al. (2018). Global patterns in the metacommunity structuring of lake macrophytes: regional variations and driving factors. Oecologia 188, 1167–1182. doi:10.1007/S00442-018-4294-0
Alahuhta, J., Luukinoja, J., Tukiainen, H., and Hjort, J. (2016). Importance of spatial scale in structuring emergent lake vegetation across environmental gradients and scales: GIS-based approach. Ecol. Indic. 60, 1164–1172. doi:10.1016/j.ecolind.2015.08.045
Alahuhta, J., Rääpysjärvi, J., Hellsten, S., Kuoppala, M., and Aroviita, J. (2015). Species sorting drives variation of boreal lake and river macrophyte communities. Community Ecol. 16, 76–85. doi:10.1556/168.2015.16.1.9
Azzella, M. M. (2012). Flora, vegetazione e indicatori macrofitici dei laghi vulcanici d'Italia. Available online at: https://iris.uniroma1.it/handle/11573/918808.
Azzella, M. M., Bolpagni, R., and Oggioni, A. (2014). A preliminary evaluation of lake morphometric traits influence on the maximum growing depth of macrophytes. J. Limnol. 73, 1–7. doi:10.4081/jlimnol.2014.932
Azzella, M. M., Bresciani, M., Nizzoli, D., and Bolpagni, R. (2017). Aquatic vegetation in deep lakes: Macrophyte co-occurrence patterns and environmental determinants. J. Limnol. 76, 97–108. doi:10.4081/jlimnol.2017.1687
Azzella, M. M., Iberite, M., Fascetti, S., and Rosati, L. (2013). Loss detection of aquatic habitats in Italian volcanic lakes using historical data. Plant Biosystems-An Int. J. Deal. all Aspects Plant Biol. 147, 521–524. doi:10.1080/11263504.2013.772080
Bakker, E. S., Sarneel, J. M., Gulati, R. D., Liu, Z., and van Donk, E. (2013). Restoring macrophyte diversity in shallow temperate lakes: biotic versus abiotic constraints. Hydrobiologia 710, 23–37. doi:10.1007/s10750-012-1142-9
Bakker, E. S., Wood, K. A., Pagès, J. F., Veen, G. F., Christianen, M. J. A., Santamaría, L., et al. (2016). Herbivory on freshwater and marine macrophytes: a review and perspective. Aquat. Bot. 135, 18–36. doi:10.1016/j.aquabot.2016.04.008
Bechtold, U. (2018). Plant life in extreme environments: how do you improve drought tolerance? Front. Plant Sci. 9, 543. doi:10.3389/fpls.2018.00543
Bini, L. M., Thomaz, S. M., Murphy, K. J., and Camargo, A. F. M. (1999). Aquatic macrophyte distribution in relation to water and sediment conditions in the Itaipu reservoir, Brazil. Hydrobiologia 415, 147–154. doi:10.1023/A:1003856629837
Bolpagni, R., Laini, A., and Azzella, M. M. (2016). Short-term dynamics of submerged aquatic vegetation diversity and abundance in deep lakes. Applied Vegetation Science 19 (4), 711–723. doi:10.1111/avsc.12245
Bolpagni, R., and Pino, F. (2017). Sediment nutrient drivers of the growth dynamics of the rare fern Marsilea quadrifolia. Hydrobiologia 792, 303–314. doi:10.1007/S10750-016-3064-4
Bornette, G., and Puijalon, S. (2011). Response of aquatic plants to abiotic factors: a review. Aquat. Sci. 73, 1–14. doi:10.1007/s00027-010-0162-7
Bresciani, M., Bolpagni, R., Braga, F., Oggioni, A., and Giardino, C. (2012). Retrospective assessment of macrophytic communities in southern Lake Garda (Italy) from in situ and MIVIS (multispectral infrared and visible imaging spectrometer) data. J. Limnol. 71, 19. doi:10.4081/jlimnol.2012.e19
Capers, R. S., Selsky, R., and Bugbee, G. J. (2010). The relative importance of local conditions and regional processes in structuring aquatic plant communities. Freshw. Biol. 55, 952–966. doi:10.1111/j.1365-2427.2009.02328.x
Chen, J., Chou, Q., Ren, W., Su, H., Zhang, M., Cao, T., et al. (2022). Growth, morphology and C/N metabolism responses of a model submersed macrophyte, Vallisneria natans, to various light regimes. Ecol. Indic. 136, 108652. doi:10.1016/J.ECOLIND.2022.108652
Clappe, S., Dray, S., and Peres-Neto, P. R. (2018). Beyond neutrality: disentangling the effects of species sorting and spurious correlations in community analysis. Ecology 99, 1737–1747. doi:10.1002/ECY.2376
Cui, Z., Huang, Q., Sun, J., Wan, B., Zhang, S., Shen, J., et al. (2024). The secchi disk depth to water depth ratio affects morphological traits of submerged macrophytes: development patterns and ecological implications. Sci. Total Environ. 907, 167882. doi:10.1016/J.SCITOTENV.2023.167882
Dainez-Filho, M. S., Michelan, T. S., Louback-Franco, N., Souza, D. C., Cafofo, E. G., and Thomaz, S. M. (2019). Role of sediment structuring by detritus on colonization and interspecific competition of one native and one invasive submerged macrophyte. Hydrobiologia 834, 63–74. doi:10.1007/S10750-019-3909-8
Dalla Vecchia, A., and Bolpagni, R. (2022). The importance of being petioled: leaf traits and resource-use strategies in Nuphar lutea. Hydrobiologia 849, 3801–3812. doi:10.1007/s10750-022-04803-1
Dray, S., Legendre, P., and Peres-Neto, P. (2006). Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). doi:10.1016/j.ecolmodel.2006.02.015
de Baar, H. (1994). von Liebig’s Law of the Minimum and Plankton Ecology. Prog. Oceanogr. 33, 347–386. doi:10.1016/0079-6611(94)90022-1
Fu, H., Guo, J., He, X., Chen, Y., Wu, Z., Ge, Y., and Cai, G. (2023). Individual traits modify environmental effects on interaction, connectivity, and productivity of macrophyte community. Hydrobiologia 851 (21), 5059–5070. doi:10.1007/S10750-023-05185-8/
Ghirardi, N., Bolpagni, R., Bresciani, M., Valerio, G., Pilotti, M., and Giardino, C. (2019). Spatiotemporal dynamics of submerged aquatic vegetation in a deep lake from Sentinel-2 data. Water 11, 563. doi:10.3390/W11030563
Grimaldo, J. T., Bini, L. M., Landeiro, V. L., O’Hare, M. T., Caffrey, J., Spink, A., et al. (2016). Spatial and environmental drivers of macrophyte diversity and community composition in temperate and tropical calcareous rivers. Aquat. Bot. 132, 49–61. doi:10.1016/J.AQUABOT.2016.04.006
Guénard, G., and Legendre, P. (2018). Bringing multivariate support to multiscale codependence analysis: assessing the drivers of community structure across spatial scales. Methods Ecol. Evol. 9, 292–304. doi:10.1111/2041-210X.12864
Hayford, B. L., Caires, A. M., Chandra, S., and Girdner, S. F. (2015). Patterns in benthic biodiversity link lake trophic status to structure and potential function of three large, deep lakes. PLOS ONE 10, e0117024. doi:10.1371/JOURNAL.PONE.0117024
Heino, J., Alahuhta, J., Bini, L. M., Cai, Y., Heiskanen, A. S., Hellsten, S., et al. (2021). Lakes in the era of global change: moving beyond single-lake thinking in maintaining biodiversity and ecosystem services. Biol. Rev. 96, 89–106. doi:10.1111/BRV.12647
Iversen, L. L., Winkel, A., Baastrup-Spohr, L., Hinke, A. B., Alahuhta, J., Baattrup-Pedersen, A., et al. (2019). Catchment properties and the photosynthetic trait composition of freshwater plant communities. Science 366, 878–881. doi:10.1126/science.aay5945
Jenny, J. P., Anneville, O., Arnaud, F., Baulaz, Y., Bouffard, D., Domaizon, I., et al. (2020). Scientists’ warning to humanity: rapid degradation of the world’s large lakes. J. Gt. Lakes. Res. 46, 686–702. doi:10.1016/J.JGLR.2020.05.006
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi:10.18637/jss.v082.i13
Lacoul, P., and Freedman, B. (2006). Environmental influences on aquatic plants in freshwater ecosystems. Environ. Rev. 14, 89–136. doi:10.1139/A06-001
Lehmann, A., Castella, E., and Lachavanne, J. B. (1997). Morphological traits and spatial heterogeneity of aquatic plants along sediment and depth gradients, Lake Geneva, Switzerland. Aquat. Bot. 55, 281–299. doi:10.1016/S0304-3770(96)01078-9
Lewerentz, A., Hoffmann, M., and Sarmento Cabral, J. (2021). Depth diversity gradients of macrophytes: shape, drivers, and recent shifts. Ecol. Evol. 11, 13830–13845. doi:10.1002/ECE3.8089
Lobato-de Magalhães, T., Murphy, K., Efremov, A., Davidson, T. A., Molina-Navarro, E., Wood, K. A., et al. (2022). How on Earth did that get there? Natural and human vectors of aquatic macrophyte global distribution. Hydrobiologia 850, 1515–1542. doi:10.1007/S10750-022-05107-0
Luhtala, H., Kulha, N., Tolvanen, H., and Kalliola, R. (2016). The effect of underwater light availability dynamics on benthic macrophyte communities in a Baltic sea archipelago coast. Hydrobiologia 776, 277–291. doi:10.1007/S10750-016-2759-X
Marzocchi, U., Benelli, S., Larsen, M., Bartoli, M., and Glud, R. N. (2019). Spatial heterogeneity and short-term oxygen dynamics in the rhizosphere of vallisneria spiralis: implications for nutrient cycling. Freshw. Biol. 64, 532–543. doi:10.1111/FWB.13240
Misra, A. K. (2010). Modeling the depletion of dissolved oxygen in a lake due to submerged macrophytes. Nonlinear Analysis Model. Control 15, 185–198. doi:10.15388/NA.2010.15.2.14353
Murphy, F., Schmieder, K., Baastrup-Spohr, L., Pedersen, O., and Sand-Jensen, K. (2018). Five decades of dramatic changes in submerged vegetation in Lake Constance. Aquat. Bot. 144, 31–37. doi:10.1016/J.AQUABOT.2017.10.006
O’Hare, M. T. (2015). Aquatic vegetation - a primer for hydrodynamic specialists. J. Hydraulic Res. 53, 687–698. doi:10.1080/00221686.2015.1090493
Pinzani, L., Pelella, E., Azzella, M. M., and Ceschin, S. (2025). A bibliographic review on vascular flora of Italian volcanic lakes. Inland Waters, 1–10. doi:10.1080/20442041.2025.2475684
R Core Team (2021). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/.
Rodrigues, R. B., and Thomaz, S. M. (2010). Photosynthetic and growth responses of Egeria densa to photosynthetic active radiation. Aquatic Botany, 92 (4), 281–284. doi:10.1016/J.AQUABOT.2010.01.009
Rooney, N., and Kalff, J. (2000). Inter-annual variation in submerged macrophyte community biomass and distribution: the influence of temperature and lake morphometry. Aquat. Bot. 68, 321–335. doi:10.1016/S0304-3770(00)00126-1
Rørslett, B. (1991). Principal determinants of aquatic macrophyte richness in northern European lakes. Aquat. Bot. 39, 173–193. doi:10.1016/0304-3770(91)90031-Y
Salmaso, N., Anneville, O., Straile, D., and Viaroli, P. (2018). European large perialpine lakes under anthropogenic pressures and climate change: present status, research gaps and future challenges. Hydrobiologia 824, 1–32. doi:10.1007/S10750-018-3758-X
Sand-Jensen, K., Jensen, R. S., Gomes, M., Kristensen, E., Martinsen, K. T., Kragh, T., et al. (2018). Photosynthesis and calcification of charophytes. Aquat. Bot. 149, 46–51. doi:10.1016/J.AQUABOT.2018.05.005
Schwarz, A. M., de Winton, M., and Hawes, I. (2002). Species-specific depth zonation in New Zealand charophytes as a function of light availability. Aquat. Bot. 72, 209–217. doi:10.1016/S0304-3770(01)00201-7
Sheldon, S. P. (1987). The effects of herbivorous snails on submerged macrophyte communities in Minnesota lakes. Ecology 68, 1920–1931. doi:10.2307/1939883
Slowikowski, K. (2021). Ggrepel: automatically position non-overlapping text labels with ggplot2. R package version 0.9.1. Available online at: https://CRAN.R-project.org/package=ggrepel.
Spence, D. H. N. (1982). The zonation of plants in freshwater lakes. Advances in Ecological Research, 12 (C), 37–125. doi:10.1016/S0065-2504(08)60077-X
Stross, R. G., Huvane, J., and Sokol, R. C. (1988). Internal structure of deep-dwelling nitella meadows. Aquat. Bot. 29, 329–345. doi:10.1016/0304-3770(88)90077-0
Tanner, C. C., Clayton, J. S., and Coffey, D. T. (1985). Notes on the submerged vegetation of Lakes heron, Clearwater, and camp Canterbury, South Island, New Zealand. N. Z. J. Bot. 23, 213–218. doi:10.1080/0028825X.1985.10425327
Tartari, G., Buraschi, E., Copetti, D., Salerno, F., Monguzzi, C., Pagnotta, R., et al. (2006). Characterization of Italian lake types. Int. Ver. für theoretische Angew. Limnol. Verhandlungen 29, 1811–1816. doi:10.1080/03680770.2006.11903001
Thomaz, S. M. (2021). Ecosystem services provided by freshwater macrophytes. Hydrobiologia 2021, 2757–2777. doi:10.1007/S10750-021-04739-Y
Tian, Y., Lv, C., Huang, L., Shan, H., Wang, H., Wen, Z., et al. (2023). Seasonal variation and nutrient jointly drive the community structure of macrophytes in lakes with different trophic states. Front. Mar. Sci. 10, 1182823. doi:10.3389/FMARS.2023.1182823
Török, P., Bullock, J. M., Jiménez-Alfaro, B., and Sonkoly, J. (2020). The importance of dispersal and species establishment in vegetation dynamics and resilience. J. Veg. Sci. 31, 935–942. doi:10.1111/jvs.12958
Trindade, C. R. T., Landeiro, V. L., and Schneck, F. (2018). Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 823, 217–230. doi:10.1007/S10750-018-3709-6
van Onsem, S., and Triest, L. (2018). Turbidity, waterfowl herbivory, and propagule banks shape submerged aquatic vegetation in ponds. Front. Plant Sci. 871, 1514. doi:10.3389/fpls.2018.01514
Wang, H., Wen, Z., Zhang, Z., Zhang, X., Fu, H., Cao, Y., et al. (2020). Environmental vs. spatial drivers of submerged macrophyte community assembly in different seasons and water depths in a mesotrophic bay of Erhai Lake, China. Ecol. Indic. 117, 106696. doi:10.1016/J.ECOLIND.2020.106696
Wen, Z., Wang, H., Zhang, Z., Cao, Y., Yao, Y., Gao, X., et al. (2022). Depth distribution of three submerged macrophytes under water level fluctuations in a large plateau lake. Aquat. Bot. 176, 103451. doi:10.1016/J.AQUABOT.2021.103451
Wetzel, R. G. (2001). “Light in inland waters,” in Limnology. Editor R. G. Wetzel third ed. (San Diego: Academic Press), 49–69.
Xia, C., Yu, D., Wang, Z., and Xie, D. (2014). Stoichiometry patterns of leaf carbon, nitrogen and phosphorous in aquatic macrophytes in eastern China. Ecol. Eng. 70, 406–413. doi:10.1016/J.ECOLENG.2014.06.018
Zhang, Y., Qin, B., Shi, K., Zhang, Y., Deng, J., Wild, M., et al. (2020). Radiation dimming and decreasing water clarity fuel underwater darkening in lakes. Sci. Bull. 65, 1675–1684. doi:10.1016/J.SCIB.2020.06.016
Keywords: macrophyte spatial models, charophytes, freshwater biodiversity, mMCA, environmental drivers, water depth gradients
Citation: Dalla Vecchia A, Bolpagni R, Laini A, Nizzoli D, Bresciani M, Azzella MM and Wilkes M (2025) Spatial relationships between macrophyte assemblages, water and sediment features in deep lakes. Front. Environ. Sci. 13:1614281. doi: 10.3389/fenvs.2025.1614281
Received: 18 April 2025; Accepted: 04 July 2025;
Published: 13 August 2025.
Edited by:
Mateja Germ, University of Ljubljana, SloveniaReviewed by:
Aneta Spyra, University of Silesia in Katowice, PolandChaochao Lv, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Dalla Vecchia, Bolpagni, Laini, Nizzoli, Bresciani, Azzella and Wilkes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossano Bolpagni, cm9zc2Fuby5ib2xwYWduaUB1bmlwci5pdA==
†These authors share last authorship
 Alice Dalla Vecchia
Alice Dalla Vecchia Rossano Bolpagni
Rossano Bolpagni Alex Laini2
Alex Laini2 Daniele Nizzoli
Daniele Nizzoli Mariano Bresciani
Mariano Bresciani Mattia Martin Azzella
Mattia Martin Azzella