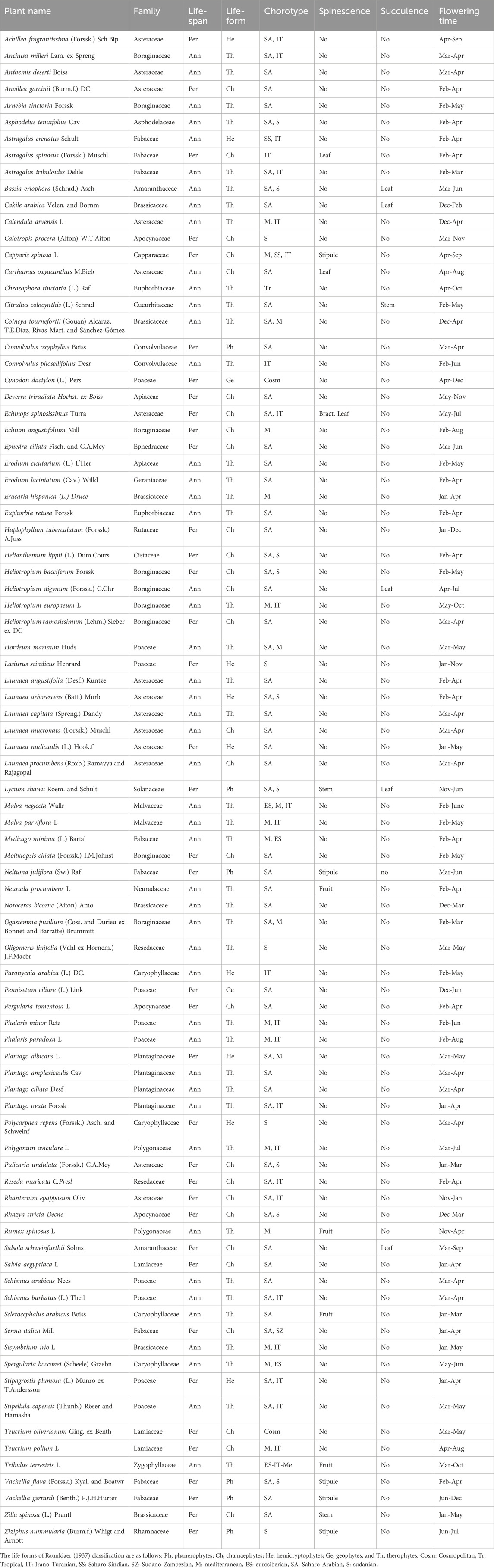
- 1Chair of Climate Change, Environmental Development and Vegetation Cover, Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 2Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia
- 3King Abdulaziz Royal Reserve Development Authority, Riyadh, Saudi Arabia
The soil seed bank (SSB) composition is crucial for ecosystem vitality and restoration. The present study aimed to evaluate the SSB and vegetation composition in Raudhat Altinhat, Saudi Arabia, an arid ecosystem. A total of 14 plots were determined and studied for their aboveground vegetation during winter-spring and summer-fall seasons. Soil samples were collected for chemical and physical analyses to determine the edaphic factors affecting vegetation composition. Soil sample of 4000 cm3 was collected and analyzed for SSB composition via direct emergent method. Eighty-six plant species (55.8% annuals and 44.2% perennials) were determined in aboveground vegetation. Asteraceae, Poaceae, Boraginaceae, and Fabaceae were major families. During winter-spring season, communities of Ziziphus nummularia, Zilla spinosa-Rhazya stricta, and R. stricta were identified, where most of soil variables did not show significant variation among communities, except for salinity, Ca, Mg, SO4, and NO3. Also, during summer-fall season, communities of R. stricta, Z. nummularia, and Cynodon dactylon were identified, and most soil variables did not show significant variation among communities, except for Mg and SO4 contents. SSB collected during winter-spring season comprised 23 species, mainly annuals (87.0%). Trigonella stellata was the predominant plant in SSB (average of 465 seeds/m2), followed by Phalaris minor (167 seeds/m2). During summer-fall season SSB had 22 plants, mainly annual (86.4%), while C. dactylon, Launaea nudicaulis, and V. gerrardi were identified as perennials. Trigonella stellata is the most predominant plant (159 seeds/m2). A negative correlation was observed between aboveground vegetation and SSB composition during both seasons. The species diversity indexes showed that aboveground vegetation during winter-spring season attained higher species richness and evenness, compared to SSB. However, during summer-fall season, species richness of aboveground vegetation and SSB was consistent, while species evenness was higher for SSB compared to aboveground vegetation. Our results showed that Raudhat Altinhat site needed rehabilitation and strict protection against all unmanaged activities, such as overgrazing and logging. Z. nummularia community showed high degradation and very low regeneration, and no seed germination in SSB. Therefore, further study is needed to determine the causes of the degradation and the best way to rehabilitate and recruit this very important shrub.
1 Introduction
In arid regions, which encompass a substantial proportion of the world’s biodiversity (Zhang and Chen, 2022), plant diversity is essential for the preservation of terrestrial biodiversity and the stability of ecosystems (Zhang et al., 2023). These ecosystems, which are primarily constituted of grasses and shrubs, are essential for the maintenance of social and environmental balance (Santini et al., 2022). Nevertheless, arid regions are experiencing an increase in desertification as a result of stressors induced by climate change, including extreme temperatures, limited water and nutrient availability, and high soil salinity (Khattab et al., 2024). Moreover, the depletion of soil seed banks (SSBs) and the alteration of aboveground vegetation are all crucial contributors to biodiversity loss, as a result of anthropogenic activities such as grazing and intensive land use (Cao et al., 2022). This degradation poses a threat to ecosystem services, such as soil fertility, carbon sequestration, and water regulation, which are essential for the conservation of biodiversity and the wellbeing of humans (Guo et al., 2023). Therefore, conservation strategies, such as the utilisation of native SSBs, provide a viable solution for the restoration of ecological resilience and plant diversity in arid ecosystems, taking into account the slow natural recovery of these ecosystems (Luo et al., 2023; Islam and Jacob, 2024).
Soil seed banks, which serve as reservoirs of plant propagules (Hopfensperger, 2007), facilitate ecosystem resilience and regeneration by preserving viable seeds within the soil, including those on the surface, that are capable of replacing mature plants (Gallagher, 2014). These seed banks are vital for the stabilisation of ecosystems, particularly in arid regions where the recovery of vegetation is contingent upon the persistence of seeds (Aragón-Gastélum et al., 2018). In spite of the majority of seeds are derived from local progenitor vegetation, the seed pool is also enriched by external sources, which contributes to the diversity of species (Solomon, 2011). According to Thompson et al. (1997), seed banks are categorized based on the longevity of their seeds as transient or persistent. Transient seed banks germinate within a short period, while persistent seed banks remain dormant but viable for extended periods through bet-hedging strategies, guaranteeing survival during unfavourable conditions. This dormancy mechanism enables seed assemblages to function as genetic reservoirs, thereby facilitating the regeneration of future vegetation (Guo et al., 2023). In this sense, Ooi (2015) and Mohammed and Denboba (2020) noted that species-specific dormancy and germination traits significantly influence the persistence of SSBs, as they determine their capacity to endure environmental fluctuations and disturbances.
The interaction between SSBs and aboveground vegetation is essential for the resilience of ecosystems, particularly in arid regions and degraded landscapes (Shaukat and Siddiqui, 2004). According to Guo et al. (2023), the composition of seed banks is substantially influenced by environmental factors, vegetation type, and disturbance history. On one hand, it is highly likely that the substantial similarity between the species composition of SSBs and the aboveground vegetation cover in arid ecosystems is primarily attributable to localised seed distribution mechanisms and limited seed dispersal (Shaukat and Siddiqui, 2004). On the other hand, the discrepancies observed between the species in SSBs and those in the vegetation above may be partially explained by differences in their reproductive strategies (Amiaud and Touzard, 2004). Research conducted in Saudi Arabia on this topic showed that SSBs in arid environments often differ significantly from aboveground vegetation due to the dominance of annual plants and the tendency of seeds to persist as a survival strategy in harsh climatic conditions. This variation is the result of the fact that a substantial proportion of seeds remain dormant in the soil, awaiting favourable conditions for germination, rather than immediately contributing to the visible plant community (Al-Huqail et al., 2025). Assaeed and Al-Doss (2002) have found that anthropogenic activities and grazing pressure have an impact on both species richness and seed bank densities in arid rangelands, which in turn affects long-term vegetation recovery. Globally, some ecosystems exhibit significant correlations between SSBs and standing vegetation, while others, particularly forests and wetlands, exhibit low similarity. This is frequently due to dispersal mechanisms and differential seed dormancy (Hopfensperger, 2007). Nonetheless, it is imperative to comprehend these dynamics in order to develop effective restoration strategies that ensure the regeneration of diverse and functional plant communities by utilising SSBs (Yang et al., 2021).
Raudhats, lowland channels for floodwaters in Saudi Arabia, have a surface composed of sand and clay that improves their fertility, making them ecologically vital refugia for mesophytic plants that flourish during the rainy season (Al-Huqail et al., 2025). The raudhat ecosystems are essential for the preservation of plant biodiversity, as the region’s arid to hyper-arid climate restricts vegetation diversity (Assaeed and Al-Doss, 2002). However, they are facing escalating threats from anthropogenic and climatic factors. In this sense, Al-Qarawi (2011) argued that the resilience of both aboveground vegetation and the SSB has been diminished as a result of human activities such as overgrazing, off-road driving, camping, logging, and medicinal plant harvesting, which have contributed to habitat degradation. Furthermore, the loss of biodiversity is further exacerbated by abiotic stressors, such as high temperatures and low rainfall patterns, which are associated with climate change (Alghanem et al., 2023). These factors present substantial obstacles to conservation and restoration strategies.
Even though the literature had many studies investigating the relationship between SSB and aboveground vegetation from different aspects, very few studies (Assaeed and Al-Doss, 2002; Al-Huqail et al., 2025) were found to cover this topic under the context of Saudi Arabia and within Raudhat ecosystems. Moreover, no studies have addressed this topic in the study area (Raudhat Altinhat). This study hypothesizes that Raudhat Altinhat contains a substantial reservoir of viable seeds, which, under favourable environmental conditions, can contribute to the regeneration of degraded plant communities by acting as a natural source of vegetation cover. Furthermore, the study suggests that the composition and diversity of the SSB and aboveground vegetation vary across different vegetation types, primarily as a result of variations in soil properties. These variations subsequently affect the potential for vegetation recovery and ecological restoration. Therefore, this study aims to achieve the following objectives: (i) analyze the aboveground vegetation in the Raudhat Altinhat ecosystem, (ii) identify the SSB composition, and (iii) examine the relationship between the SSB, aboveground vegetation, and soil properties.
2 Materials and methods
2.1 Study area
The study was conducted in Raudat Altinhat (26.0807° N, 46.5072° E) in the northeast of Riyadh region, central Saudi Arabia (Figure 1). The area is belonging to the King Abdulaziz Royal Reserve. The site is a low-lying plain covering approximately 32 km2, bordered by the sand dunes of Al-Dahna to the east. Due to numerous wadi/valley canals, including Wadi Al-Shwaki, Wadi Al-Tayri, Wadi Al-Atiq, and Wadi Al-Wadi, the Raudat has superior soil and vegetation compared to neighboring areas. The vegetation is primarily composed of Vachellia and Ziziphus trees, along with various seasonal wildflowers, which flourish particularly during springtime (Saudipedia, 2024). The average mean temperature and average annual rainfall are 25.22°C and 119.82 mm, respectively, for the years 1991–2020 (World Bank, 2024). The soil in the selected site is primarily composed of loamy sand and sandy soil (Saudipedia, 2024). The selection of sampling sites within the study area was conducted to ensure a comprehensive representation of vegetation characteristics, including diversity and intensity. Additionally, the topography features of the entire Raudhat ecosystem were considered to facilitate a systematic assessment of the ecosystem’s spatial heterogeneity. These features encompass water streams, water accumulation areas, land aspects, depressions, and sandy plains.
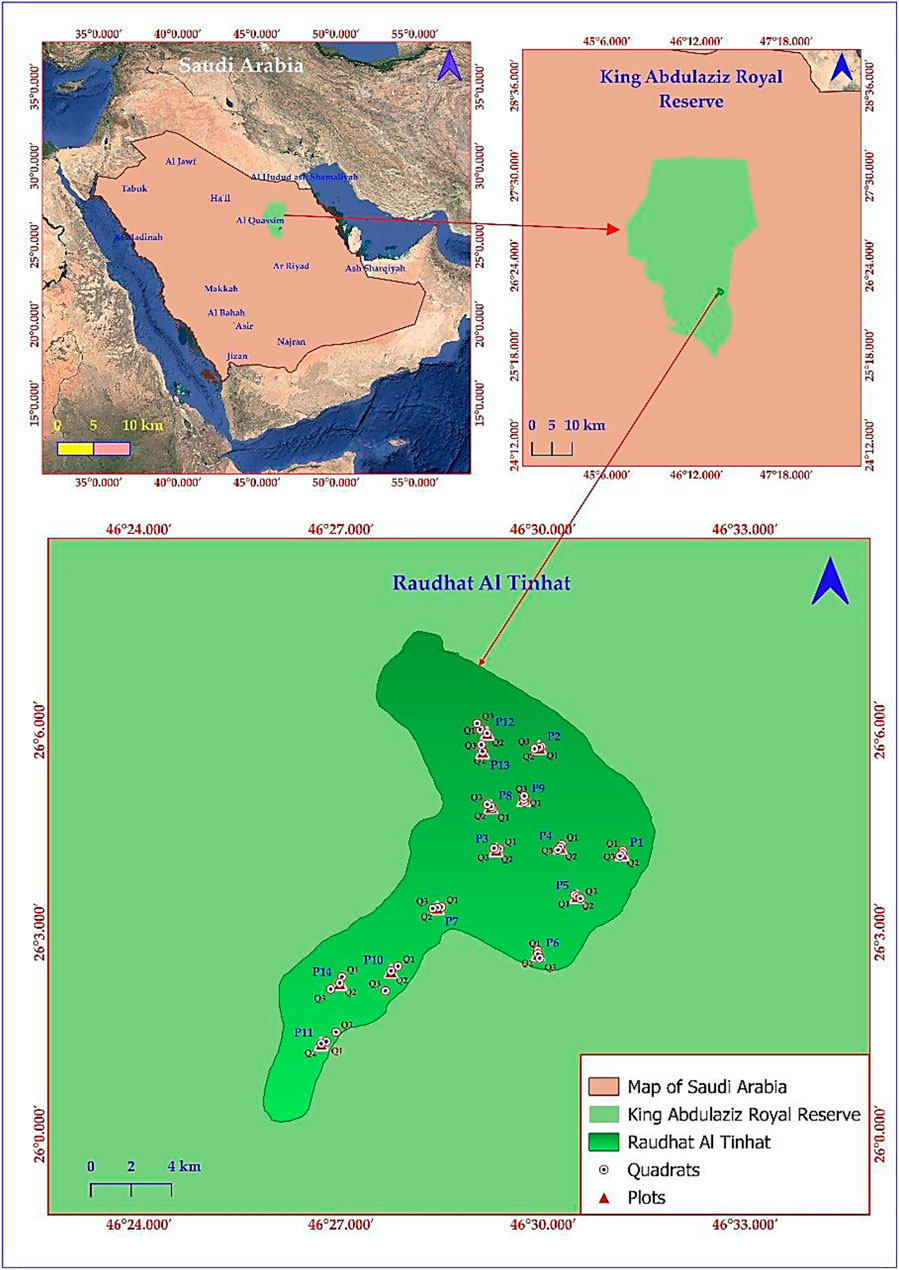
Figure 1. A map of Saudi Arabia showing the location of Raudhat Altinhat and the sampled plots (P1-P14).
2.2 Aboveground vegetation analysis
Fourteen plots were selected to cover the whole area of the Raudhat Altinhat. Within each plot, three quadrats were selected for the vegetation analysis. The minimal area for each quadrat was determined to be 10 m × 10 m in areas for herbaceous vegetation, and 20 m × 20 m in areas with shrub vegetation. In total, 42 quadrats were performed. The species in each quadrat were identified and named in accordance with the protocols of Collenette (1999), Chaudhary (1999), Chaudhary (2000), and Chaudhary (2001), and were subsequently reconfirmed by the Royal Botanical Garden, Kew (POWO, 2023). These species were observed at a variety of sites during the summer-fall and winter-spring seasons of 2023 and 2024. Plant density was calculated in accordance with Bonham (2013), and plant cover was determined using the Braun-Blanquet scale (Mueller-Dombois and Ellenberg, 1974). Before calculating the species importance value index (IVI) to ascertain the species dominance, the density, cover, and frequency data were converted to their respective relative values using the following equations:
Various published materials and online resources, including https://flora.org.il/en/plants/, http://www.theplantlist.org/1/, and https://powo.science.kew.org/, were used to examine the chorotype of each species, and the species life forms were identified according to Raunkiaer (1937). The life forms and chorotypes of all identified plants were examined in accordance with Raunkiaer (1937). The species richness (Simpson index) and species evenness (Shannon evenness) of all sites were ascertained using the subsequent equations:
where Pi = ni/N = proportional abundance of species, “i” in a habitat made up of “S” is the number of species, “ni” is the number of sites contai006Eing species i, and N = S ni. The species richness and species evenness were calculated according to the following equations:
2.3 Soil seed bank sampling
Plastic bags were used to collect a central soil sample with a volume of 4,000 cm3 (20 cm width × 20 cm length × 10 cm depth) in each quadrat where the vegetation survey was conducted. A total of 42 soil seed bank samples were collected, labeled, and transferred to the Range Science Lab, College of Food Science and Agriculture at King Saud University in Riyadh, Saudi Arabia, for further preparation and analysis. The seedling emergence techniques are employed to evaluate the seed density of the seed bank (Baker, 1989). Approximately 60% of the previous studies have employed direct greenhouse germination to evaluate the densities and species composition of soil seed banks (Padonou et al., 2022). This method is a common method for studying the soil seed bank in the desert ecosystem (Jiang et al., 2013; Loydi and Collins, 2021; Luo et al., 2021; Gomaa et al., 2023; Al-Huqail et al., 2025). The soil samples were first passed through a 4 mm mesh sieve to remove any detritus, including plant fragments, roots, and coarse stones, before being stored at 2 °C for a month to break seed dormancy (Niu et al., 2022). This was done prior to the initiation of the germination trial. A manual examination was conducted on the excluded material to detect the presence of seeds. The rectangular perforated plastic trays (45 × 45 × 7 cm) were lined with sterilised sand substrate that was less than 2 cm thick at the bottom. The sieved soil samples were spread to a thickness of approximately 2 cm. The trays were positioned on benches in a greenhouse that was adjusted to maintain a temperature of 25 °C ± 2 during the trial period. Water was introduced daily to ensure that the soil remained moist. All germinated seedling species were identified and their numbers were recorded. Subsequently, they were removed at weekly intervals. The germination trials were terminated when no new seedlings emerged. The plants were identified in accordance with the methods of Collenette (1999), Chaudhary (1999), and Chaudhary (2001). Additionally, the relative density of each species was determined using the following equation, and the number of species in each tray was counted:
2.4 Soil analysis
Soil samples were collected from each quadrat under investigation at a depth of 5–25 cm in polythene bags for soil chemical and physical analyses. The soil samples that were collected were labeled and transported to the laboratory for additional processing and preparation. The soil samples were immediately distributed over paper sheets to air-dry at room temperature (25°C ± 3°C) upon arrival in the laboratory. Subsequently, they were sieved through a 2 mm sieve to remove any extraneous materials, including gravel and plant elements. Mualem’s approach was employed to evaluate soil porosity (Mualem, 1976), while Bouyoucos’s method was employed to determine soil texture (Bouyoucos, 1962). The method of Keen and Raczkowski (1921) was performed to ascertain the water holding capacity (WHC). The graduated cylinder method (Blake and Hartge, 1986) was used to ascertain the particle density of the soil. The pH and electrical conductivity (EC) were estimated using soil-water extracts (1:5) (Rowell, 1994). The calcimeter method, as outlined by Page (1982), was employed to ascertain the calcium carbonate content. The total nitrogen (N) was determined using the Kjeldahl method, as outlined by Bremner and Mulvaney (1982), and the total phosphorus (P) was determined spectrophotometrically in accordance with Nelson and Sommers (Nelson and Sommers, 1982). The soil organic matter content was determined by the loss on ignition method at 550°C according to (Jones, 2001). The exchangeable sodium (Na) and potassium (K) were determined using the flame photometer method (PHF 80 B Biologie Spectrophotometer), as described by Rhoades (1982). The atomic absorption spectrometer (A Perkin-Elmer, Model 2,380, United States) was employed to estimate the calcium (Ca) and magnesium (Mg) as described by Allen et al. (1974). The sulphuric acid method was used to measure the carbonate (CO3) and bicarbonate (HCO3) in the soil samples through titration (Page, 1982). Finally, AgNO3 was used to determine the chloride (Cl) content through a titration technique (Jackson, 1962).
2.5 Data analysis
Hierarchical cluster analysis was conducted using Community Analysis Package, version 1.2 (Pisces Conservation Ltd. IRC House, Pennington, Lymington, UK), where the importance value index (IVI) of all identified plant species dataset was subjected to an agglomerate hierarchical clustering via Ward’s linkage to identify the various plant communities within each season. The datasets of the IVI and the soil variables of all examined sites were prepared and subjected to ordination analysis using canonical correspondence analysis (CCA) in order to evaluate the relationships between the soil variables and vegetation composition. The MVSP software program, version 3.22 (Kovach Computing Services, United Kingdom), was employed to generate the CCA. A one-way ANOVA was conducted using CoStat software (v. 6.311, CoHort Software, CA, United States) on the raw data of all soil parameters of the plant communities of the winter-spring and summer-fall seasons to compare variances across the identified plant communities and their soil characteristics. Duncan’s multiple range test was then performed. Correlation analysis was conducted on a dataset that comprises the density percentage of all identified plant species in the soil seed bank composition and the aboveground vegetation via the JMP® Pro 16.0.0 software program. In addition, the JMP® Pro was used for hierarchical cluster analysis, the constellation plot, and the heatmap between the aboveground vegetation and soil seed bank composition were generated to demonstrate a distinct and representative correlation among the investigated sites.
3 Results and discussion
3.1 Floristic composition
The vegetation survey of the different sites of Raudhat Altinhat showed the presence of 86 plant species (Table 1). These plant species were 38 (44.2%) perennial plants and 48 (55.8%) annuals. These plant species belong to 27 families, where the major families are Asteraceae, Poaceae, Boraginaceae, and Fabaceae, which attained 47.7% of the total identified species (Supplementary Figure S1A). The plant species of these families are usually reported in such arid regions of Saudi Arabia (Assaeed and Al-Doss, 2002; Abd-ElGawad et al., 2021; Al-Huqail et al., 2025), and this could be attributed to the adaptability of the species of these plants belonging to these families for such arid environmental conditions (Waheed and Arshad, 2024). Among the life forms of the identified plant species, therophytes/annuals were the most represented (47.7%), followed by chamaephytes (32.6%), while the geophyte life form was the lowest represented (Supplementary Figure S1B). The predominance of annual species can be ascribed to stressful environments, where the seeds of these species germinate during the favorable/rainy season and complete their life cycle before the start of the arid season (Gutterman, 2000). In addition, these annual plant species developed various survival strategies to tolerate the harsh conditions, such as phenotypic plasticity and powerful flowering and seed dispersal tactics (Venable et al., 2008; Stotz et al., 2021).
The chorological analysis of the identified plant species showed that 55.8% are mono-regional (found in one biogeographical region), while 40.7% of the recorded species are categorized as bi-regional and 3.5% as pluri-regional species. The Saharo-Arabian element was the most representative, where it was determined with 37.2% as mono-regional and 29.1% as bi-regional. In this context, the Irano-Turanian chorotype was also found in a high percentage (30.2%), which is 3.5% mono-regional, 23.3% bi-regional, and 3.5 pluri-regional (Supplementary Figure S1C). This structure of the chorotype in the present study is in agreement with other studies in similar environmental conditions in Saudi Arabia (Abd-ElGawad et al., 2021; Elkordy et al., 2022; Al-Huqail et al., 2025). Among the studied species, 17.4% of species have been reported as spiny plant species that showed adaptability to desert or drought conditions (Batanouny, 2000). Also, six plant species were reported as leaf or stem succulent plants.
3.2 Current vegetation analysis
3.2.1 Winter-spring season communities
The vegetation analysis of the Raudhat Altinhat was performed during the winter-spring and summer-autumn seasons. Regarding the vegetation composition during the winter-spring season, the cluster analysis revealed three plant communities (Figure 2A). The first community consisted of 38 plant species and was dominated by the shrubs of Ziziphus nummularia, which attained an IVI of 144.3, while the other important associated species in this community were Cynodon dactylon, Zilla spinosa, and Hordeum marinum, where they attained an IVI of 107.8, 104.1, and 95.2, respectively. The second identified plant community in the Raudhat Altinhat during the winter-spring season is the community of Z. spinosa- Rhazya stricta, and this community consists of 41 species. In this community, other important associated species were determined, such as Vachellia gerrardi, Convolvulus pilosellifolius, Malva parviflora, and Z. nummularia (Table 2).
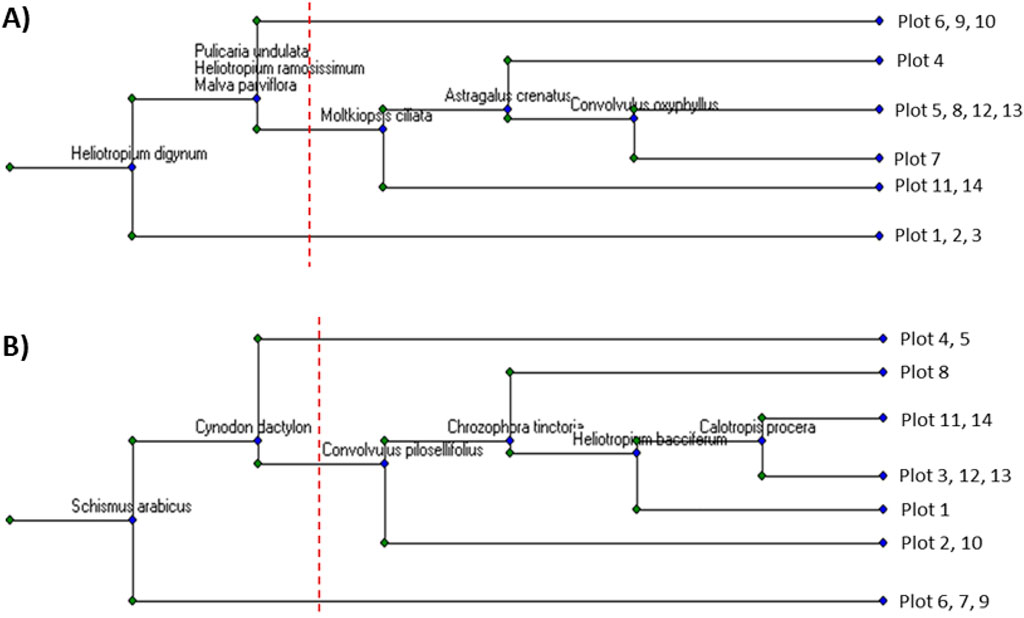
Figure 2. The cluster analysis of the plant communities within Raudhat Altinhat during (A) the winter-spring season and (B) the summer-fall season.
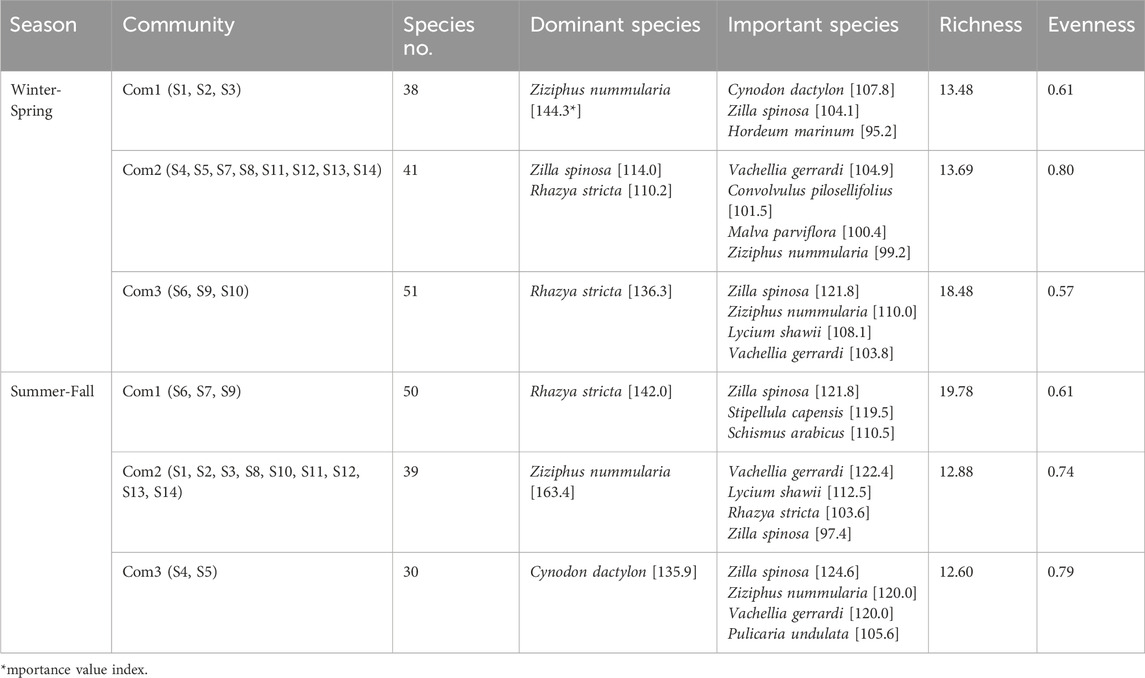
Table 2. Plant communities and diversity indexes of Raudhat Altinhat, Saudi Arabia, during the winter-spring and summer-fall seasons.
The last community in Altinhat site is the most diverse (containing 51 plant species) and dominated by the R. stricta which showed an IVI of 136.3, while the other associated important species in this community were Z. spinosa, Z. nummularia, Lycium shawii, and V. gerrardi which showed an IVI of 121.8, 110.0, 108.1, and 103.8, respectively. The detailed importance values of all identified species within each community were presented in Supplementary Table S1. Regarding species richness and evenness, the third community (R. stricta community) attained the highest species richness (18.48), while the first community showed the lowest species richness (13.48). On the other side, the second community attained the highest species evenness (0.80), while the third community revealed the lowest species evenness among the identified plant communities (Table 2).
3.2.2 Summer-fall season communities
On the other hand, during the summer-fall season, fourteen plots were performed in Raudhat Altinhat, and the data of the importance value index (IVI) were subjected to cluster analysis, which showed three plant communities (Figure 2B). The first community is dominated by R. stricta (IVI = 142.0) with an association of 49 plant species, among these, Z. spinosa, and Stipellula capensis, and Schismus arabicus those that attained an IVI of 121.8, 119.5, and 110.5, respectively, were determined as most important that attained higher importance value index (Table 2). The second community that was identified in Rawadat Altinhat was the community of Z. nummularia, and this community comprises 39 plant species. This community is also associated with other important species such as V. gerrardi (IVI = 122.4), L. shawii (IVI = 112.5), R. stricta (IV = 103.6), and Z. spinosa (IVI = 97.4). The last community in Rawadat Altinhat is dominated by C. dactylon, which attained an IVI of 135.9, respectively, while other important species in this community were Z. spinosa, Z. nummularia, V. gerrardi, and Pulicaria undulata (Table 1). The detailed importance values of all identified species in each community were presented in Supplementary Table S2. For the plant species richness and evenness analyses, the community of R. stricta (first community) showed the highest species richness (19.78), followed by the second community (Z. nummularia community). On the other side, the third community (C. dactylon community) attained the highest species evenness compared to the other identified plant communities.
3.3 Vegetation-soil relationship
3.3.1 Winter-spring season
Based on the identified plant communities during the winter-spring season, most of the assessed soil variables did not show significant variation among the plant communities, except for salinity, Ca, Mg, SO4, and NO3 (Table 3). The soil of the community of Z. spinosa-R. stricta showed the highest content of the salts (0.51 dS/m), while the soil of R. stricta community (com 3) attained the lowest (0.30 dS/m). This could be ascribed to the colonization of the woody plant communities along the border of the water streams, while the Rhazya community usually colonizes the plain areas (Abd-ElGawad et al., 2021). The same observation was reported for calcium and magnesium, where the soil of Z. spinosa-R. stricta community showed the highest content. The canonical correspondence analysis (CCA) showed that Z. nummularia community is separated on the right part of the biplot, and showed a correlation to the bulk density, sand, NH4, NO3, SO4, and HCO3 (Figure 3A). The dominant species of this community (Z. nummularia) showed a close positive correlation to the bulk density, sand, NH4, and NO3.
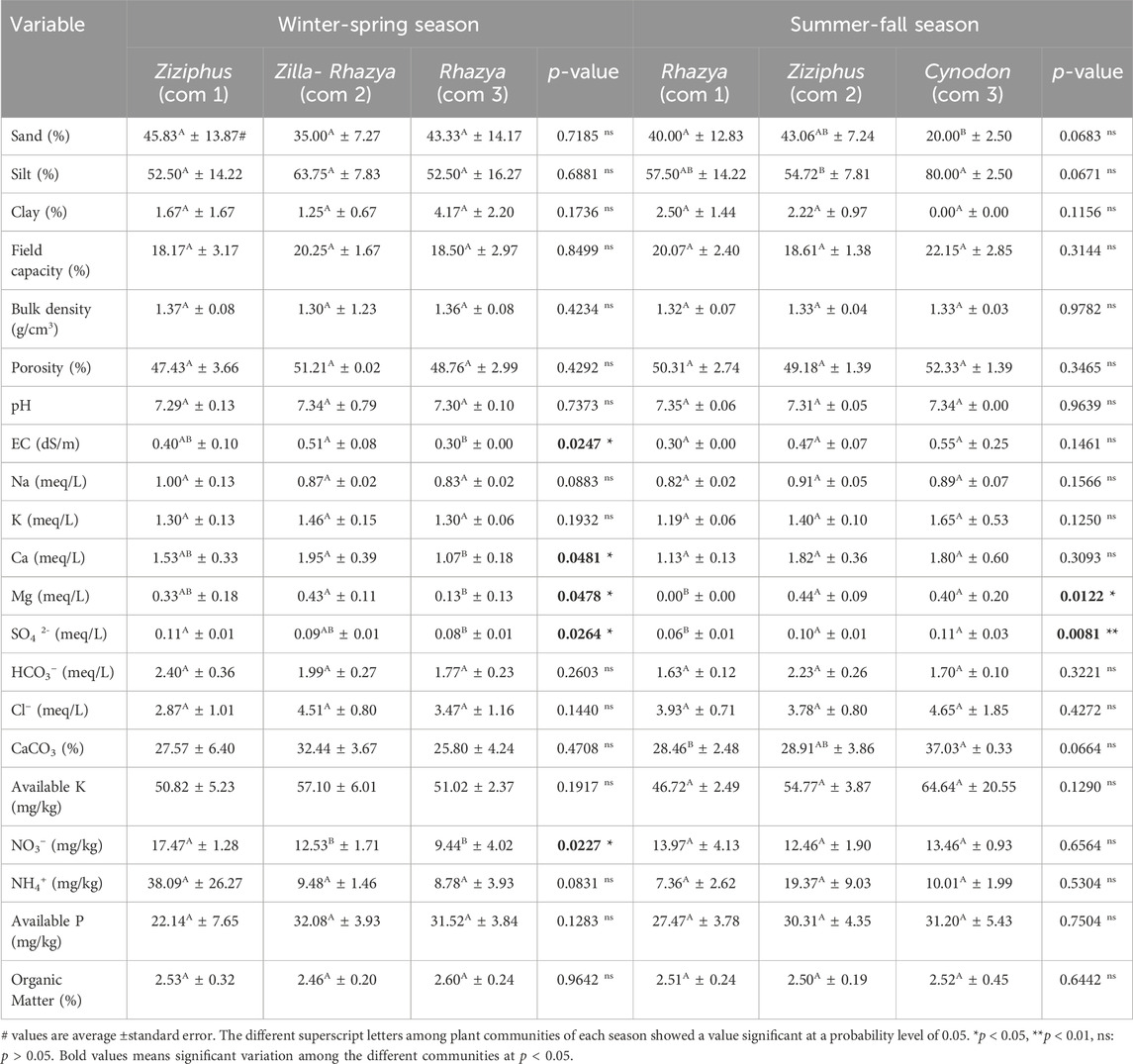
Table 3. The soil variables of the identified plant communities in Rawadat Altinhat during winter-spring and summer-fall seasons.
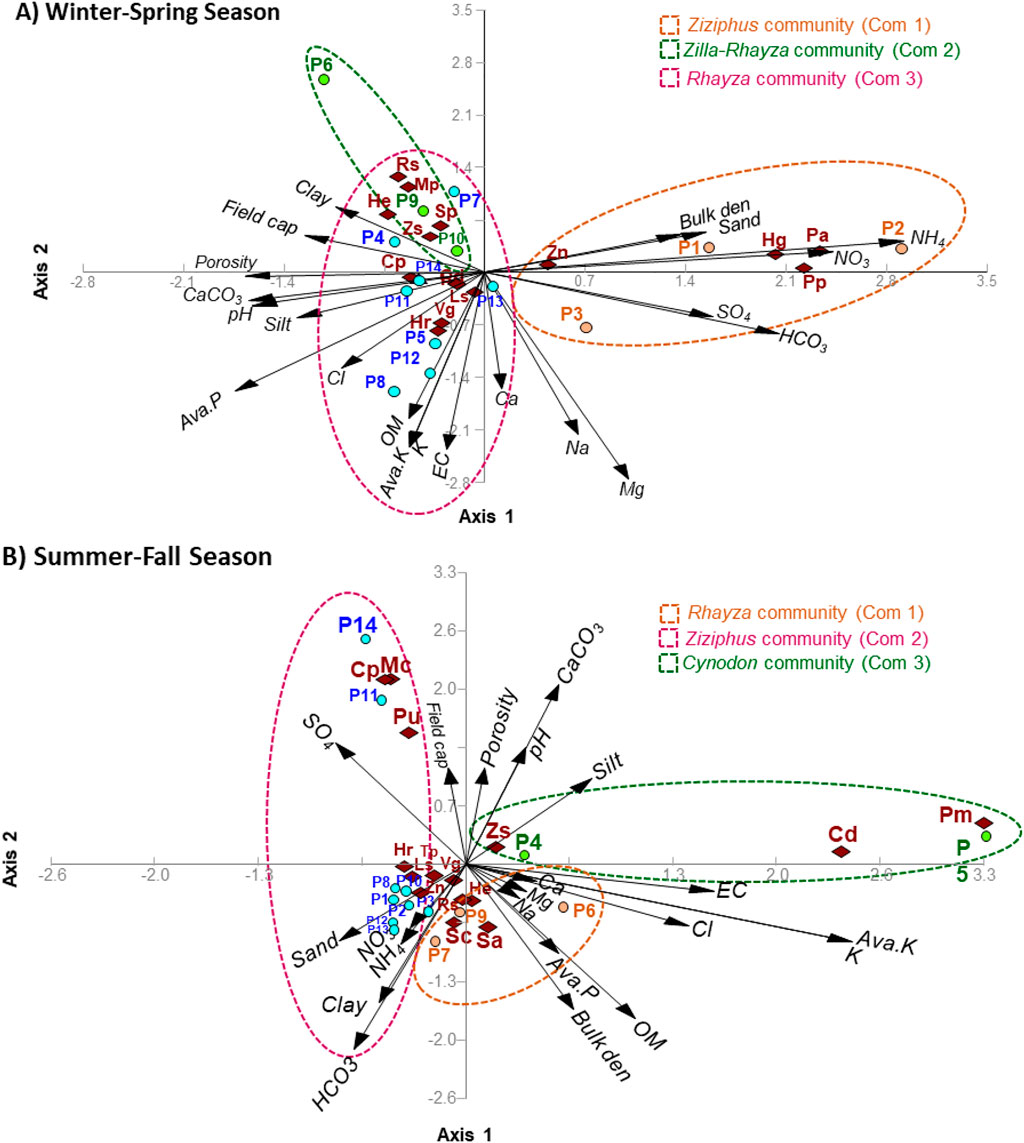
Figure 3. Canonical correspondence analysis (CCA) showed the relationships between the dominant and important plants of the identified communities and the different soil variables within (A) the winter-spring season and (B) the summer-fall season. Fc: field capacity, EC: electrical conductivity, Cp: Calotropis procera, Cd: Cynodon dactylon, Cp: Calotropis procera, He: Heliotropium europaeum, Hg: Hordeum glaucum, Hr: Heliotropium ramosissimum, Ls: Lycium shawii, Mc: Moltkiopsis ciliata, Mp: Malva parviflora, Pa: Plantago amplexicaulis, Pm: Phalaris minor, Pp: Phalaris paradoxa, Pu: Pulicaria undulata, Rs: Rhazya stricta, Sa: Schismus arabicus, Sc: Stipellula capensis, Sp: Stipagrostis plumose, Tp: Teucrium polium, Vg: Vachellia gerrardi, Zn: Ziziphus nummularia, Zs: Zilla spinosa.
On the other side, the community of R. stricta showed a low correlation to the clay content and field capacity. The community of Z. spinosa-R. stricta has been segregated on the central-left position of the CCA biplot, where it showed no substantial correlation to the soil variables; however, in specifically, the dominant tree V. gerrardi showed a positive correlation to the organic matter, available potassium, potassium content, and salinity (Figure 3A). In this community, plot six was segregated alone, reflecting specific characteristics where it is located in the southeastern part of the Raudhat Altinhat along a separate valley that has specific microhabitat conditions with about 574 m. a.s.l. elevation, which is relatively higher than other plots. In general, communities two and three are overlapped and mainly affected by the field capacity, clay, porosity, available phosphorus, and available potassium, while they showed a negative correlation to the sand fraction content, NH4, and NO3.
3.3.2 Summer-fall season
For the plant communities determined in the summer-fall season, most of the assessed soil variables did not show significant variation among the plant communities, except for Mg and SO4 contents (Table 3). Based on the CCA, the C. dactylon community showed a positive correlation to the silt content, pH, and salinity (Figure 3B). On the other side, the community of R. stricta is segregated on the lower part of the CCA biplot, which showed a positive correlation to the bulk density, organic matter, potassium, available potassium, calcium, magnesium, and sodium. The last community (Z. nummularia community) is separated on the left side of the CCA biplot. Some sites (11 and 14) of this community showed a correlation to the sulfate content. In contrast, the rest sites, which are segregated in the lower part of the CCA, showed a positive correlation to sand content, clay content, NO3, NH4, and HCO3 (Figure 3B).
3.4 Soil seed bank composition
3.4.1 Winter-spring season
The analysis of the soil seed bank collected during the winter-spring season revealed the presence of 23 plant species in the soil seed bank, mainly annual species (87.0%). The most representative families were Asteraceae (21.7%) and Poaceae (16.4%). The herb Trigonella stellata is the most predominant plant in the soil seed bank (average of 465 seeds/m2 among all plots), where plot two and plot one showed the highest content of its seeds (1,389 and 1,367 seeds/m2, respectively). The second dominant seeds in the soil were the seeds of Phalaris minor which attained an average seed bank of 167 seeds/m2 (Figure 4). About 29.2% of the determined plants attained an average soil seed bank density in the range of 10–38 seeds/m2, while 62.5% of the species attained a range of one to eight seeds/m2 (Figure 4). In this context, plots one to three showed the highest richness of the soil seed bank, where they had 41.0% of all identified individuals from all soil seed bank samples. The annual plants usually produce massive amounts of seeds to cope with the harsh conditions in the desert ecosystem (Gomaa et al., 2023). The annuals in the desert ecosystem flourish after the rains, grow fast, and produce seeds that enrich the soil’s seed bank as a strategy to escape from the dry season (Lan and Zhang, 2008; Welles and Funk, 2021).
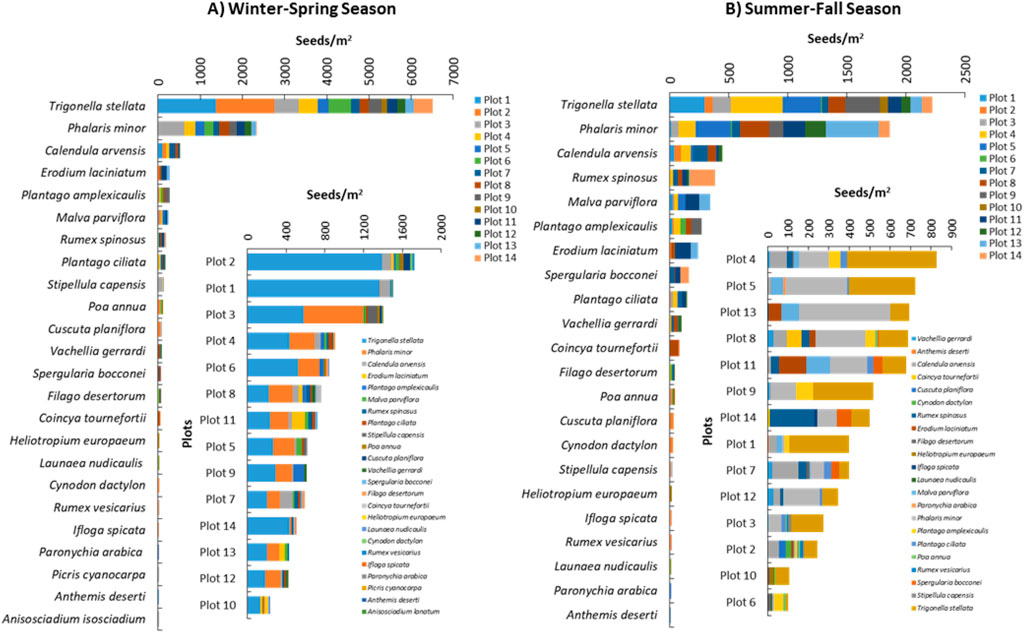
Figure 4. Soil seed bank composition of the studied plots of Raudhat Altinhat, Saudi Arabia during (A) the winter-spring season and (B) the summer-fall season.
During the winter-spring season, three perennial species were identified in the soil seed bank; namely, V. gerrardi, Launaea nudicaulis, and C. dactylon. The abundance analysis of these three species showed that they had average soil seed bank densities among the studied sites of 6, 2, and two seeds/m2, respectively (Figure 4). The limited seed bank of grasslands and woodlands due to their dispersal capacity to accumulate seeds in the soil and most of these plant species do not establish persistent seed banks (Dölle and Schmidt, 2009). Regarding the Vachellia seeds, the size of the seeds plays a very important role in its soil seed bank establishment, as the big seeds usually fall on the surface of the soil and can be easily detected by insects and other animals that eat seeds fastly, thereby, affecting the soil seed bank of the plant with bigger seeds in size (Sternberg et al., 2003).
3.4.2 Summer-fall season
For the soil seed bank during the summer-fall season, the analysis revealed the presence of 22 plant species identified by the direct germination of the soil seed bank (Figure 4). These species are mainly annual (86.4%), while only three perennial species were identified, namely, C. dactylon, L. nudicaulis, and V. gerrardi. The most representative plant families were Asteraceae and Poaceae, which attained 36.4% of all determined families. The herb T. stellata is the most predominant plant in the soil seed bank (average of 159 seeds/m2 among all plots), where plot 4, plot 5, plot 1, and plot nine showed the highest content of its seeds (439, 322, 294, and 294 seeds/m2, respectively). The second dominant seeds in the soil were the seeds of P. minor which attained an average seed bank of 133 seeds/m2 (Figure 4). The observed variation between the winter-spring and summer-fall seasons regarding the soil seed bank composition is known for many ecosystems (Assaeed and Al-Doss, 2002; Céspedes et al., 2012; Chu et al., 2019), particularly in arid or semiarid areas (Chu et al., 2019). Rainfall has been reported as the most crucial factor affecting the soil seed bank density and its stocking rate (Pol et al., 2014; Chu et al., 2019). In the present study, the species richness of the soil seed bank is varied between the winter-spring and summer-fall seasons, which could be ascribed to the rainfall, however, the two main plant species (T. stellata and P. minor) with high seed density are still the dominant among the plants in the soil seed bank (Figure 4).
3.5 Correlation between aboveground vegetation and soil seed bank
A dataset of the relative densities of all identified plant species of the aboveground vegetation as well as that determined in the soil seed banks is prepared and subjected to the Pearson correlation coefficient (r) to show the correlation between the aboveground (current) vegetation and the soil seed bank of various studied plots (Figure 5). As an average of all studied plots, a low negative correlation was observed between the aboveground vegetation and soil seed bank composition either during the winter-spring season (r = −0.060) or the summer-fall season (r = −0.036).
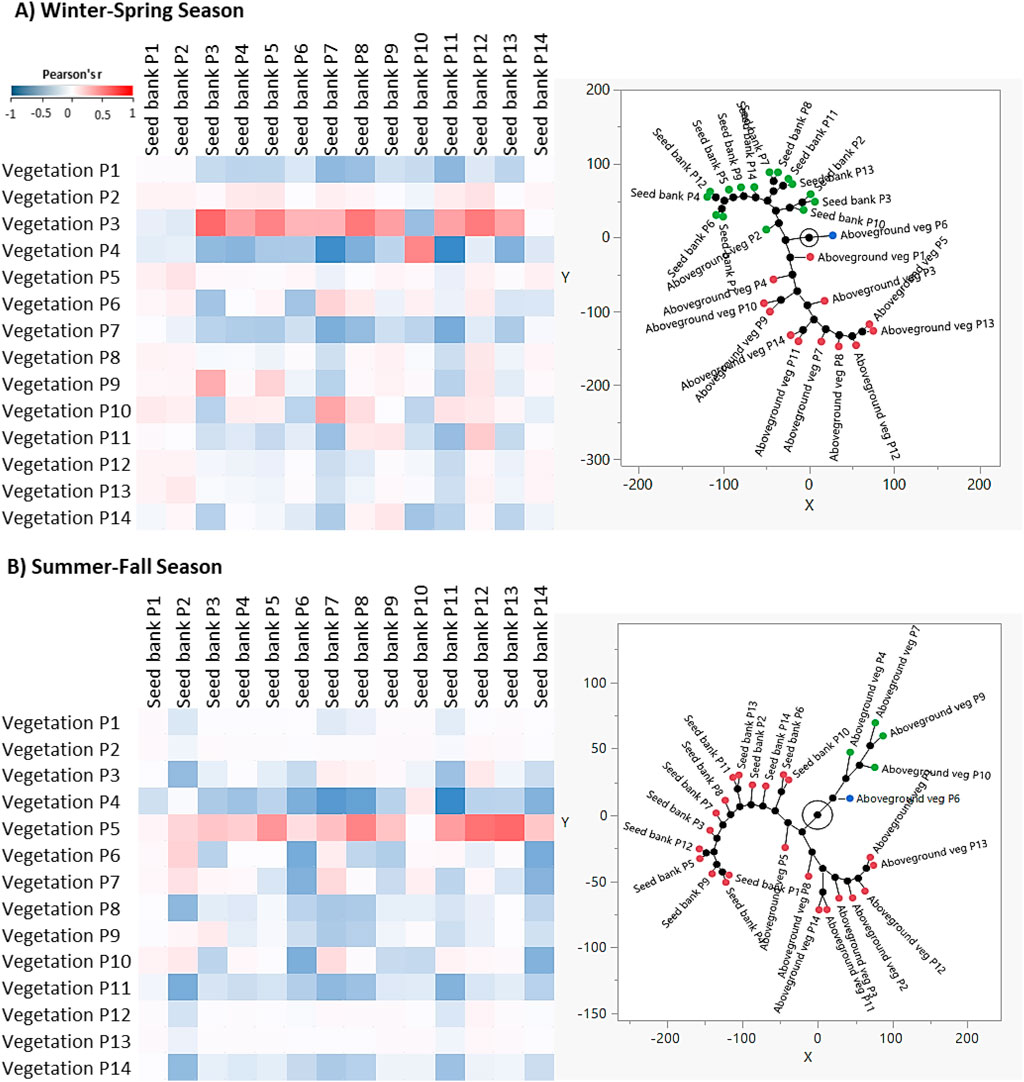
Figure 5. The correlation between the aboveground vegetation and soil seed bank composition during (A) the winter-spring season and (B) the summer-fall season. The left figure shows the heatmap analysis and the right figure shows the constellation plot.
The correlation analysis between the aboveground vegetation and soil seed bank during the winter-spring season showed a low negative correlation for all studied plots, except for plot 3, where it showed a low positive correlation (r = 0.141). However, during the summer-fall season, all plots revealed a negative correlation between the aboveground vegetation and the soil seed banks, except for plot 5 and plot 7, where they showed a positive correlation (Figure 5; Supplementary Table S3). The negative correlation between the aboveground vegetation and soil seed bank could be ascribed to the degradation of the sites due to overgrazing, camping, and logging. The Raudhat Altinhat site was not protected before, but after the establishment of the King Abdulaziz Royal Reserve in 2018, the area become protected and it is expected that the vegetation of the site will be recovered over time. The difference between the aboveground vegetation and the composition of the soil seed bank increased with decreasing disturbance intensity (Dölle and Schmidt, 2009).
In a similar study, Savadogo et al. (2016) reported a low correlation between the aboveground vegetation and soil seed bank species in the degraded areas of the National Park transboundary biosphere reserve, West Africa. In this context, the study of the soil seed bank of Raudhat Alkhafs, northeast of Riyadh City, Saudi Arabia, also showed low correlation in the northern part between the vegetation and soil seed bank that was attributed to the overgrazing and anthropogenic activities (Al-Huqail et al., 2025). The ecosystem of Raudhats in Saudi Arabia was accessible to various human activities like overgrazing, camping, cutting of wood for firing, and harvesting medicinal plants (Al-Qarawi, 2011). These anthropogenic activities, coupled with the environmental stresses and harsh environmental conditions, disrupt the relationships between the aboveground vegetation and the soil seed bank in this ecosystem (Al-Qarawi, 2011; Al-Huqail et al., 2025). Various studies have reported that anthropogenic activity leading to disruption of the soil seed bank as well as the vegetation, and in consequence, negatively impacts their relationships and harms the vitality of the natural ecosystems (Amiaud and Touzard, 2004; Amrein et al., 2005; dos Santos et al., 2018; Shinoda and Akasaka, 2019; Luo et al., 2023). Among the various anthropogenic activities, the overgrazing in Saudi Arabia is the most common (Mirreh and Al Diran, 2014; Al-Rowaily et al., 2015), and this overgrazing can alter the soil seed bank by decreasing the production of seeds and affecting the seed bank composition due to the selection of certain and more preferable plants by animals (Tessema et al., 2012; van Langevelde et al., 2016; Gonzalez and Ghermandi, 2021).
The species diversity indexes showed that aboveground vegetation during the winter-spring season attained higher species richness (25.1) and evenness (1.9), compared to the soil seed bank, which showed species richness of 5.7 and species evenness of 1.1 (Figure 6). This observed variation between the aboveground vegetation and SSB during the winter season could be attributed to the rainfall, where most annual species seeds germinated and flourished after the rainy season, affecting the SSB pool (Gomaa, 2014; Shi et al., 2022; Al-Huqail et al., 2025).
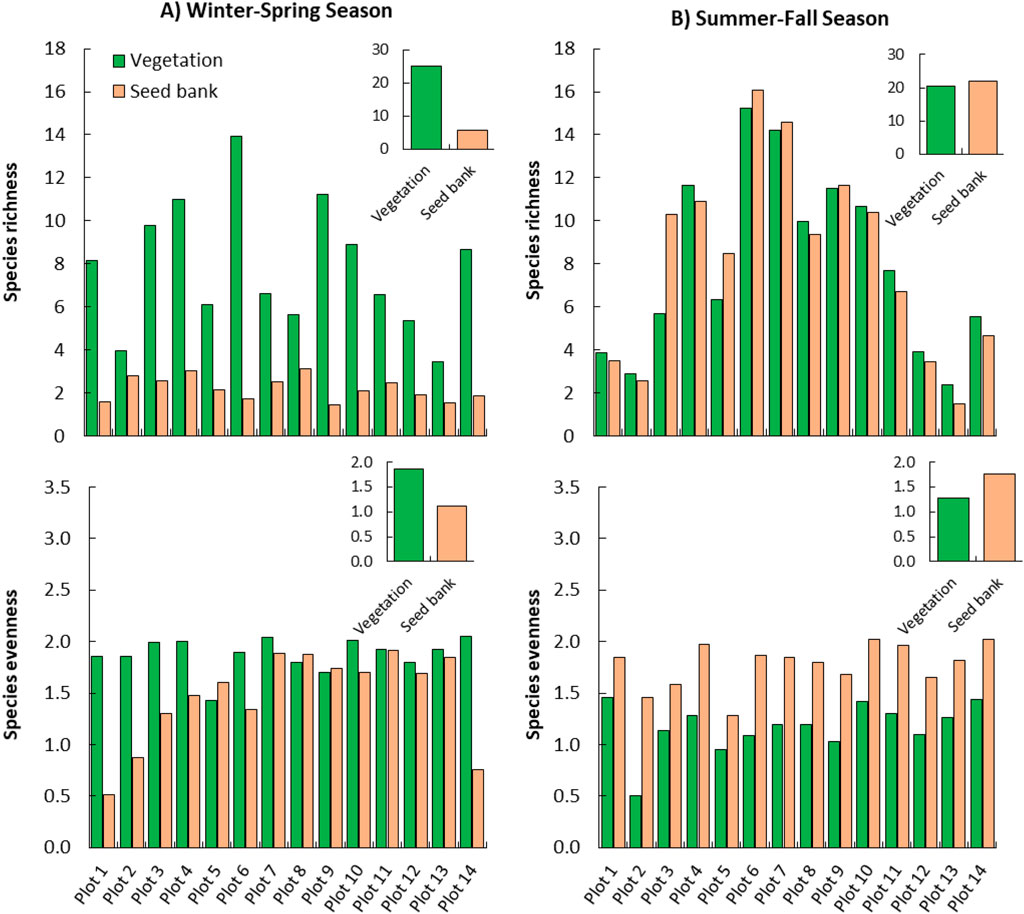
Figure 6. The species richness and evenness of the aboveground vegetation and soil seed bank among the studied plots during (A) the winter-spring season and (B) the summer-fall season. The small figure within each figure shows the overall species richness or evenness.
In this context, the vegetation in the desert habitat usually produces more dormant seeds for most taxa compared to less stressful habitats (Shi et al., 2022), which could explain the low species richness and evenness of the soil seed bank in the present study. This dormancy is considered a good strategy by the desert plant to sustain a long-term seed bank within unpredictable environments, like deserts (Kleemann and Gill, 2013). On the other hand, during the summer-fall season, the species richness of the aboveground vegetation and soil seed bank was consistent, while the species evenness was higher for the soil seed bank (1.8) compared to the aboveground vegetation (1.3). This clear decline in the species richness and evenness during the summer-fall season is attributed to the harsh and dry conditions, where perennial species some few summer annuals that adapted to the drought conditions, can survive (Abd-ElGawad et al., 2021). Also, the considerable species richness and evenness of the SSB during summer could be ascribed to the accumulation of seeds in the soil after the rainy season (Gomaa et al., 2023). Our results showed that the Raudhat Altinhat site needed rehabilitation and strict protection against all unmanaged activities, such as overgrazing and logging. Based on our survey, the Z. nummularia community showed a high degradation level as well as very low regeneration, and no seed germination in the soil seed bank. Therefore, this issue needs further studies to determine specifically the causes for the degradation of Z. nummularia community and the best way to rehabilitate and recruit these very important shrubs on the site. Also, it is worth mentioning that in our study, the seedling emergence method was used, which provides an underestimate for the total number of seeds in the soil due to seed dormancy. Therefore, a further study to compare using seedling emergence method with othermethods such as seed extraction or using chemical treatments for estimating the soil seed bank in desert ecosystem with comparision to the aboveground vegetation.
4 Conclusion
The present study showed that the Rawadat Altinhat, an arid ecosystem, is colonized mainly by woodland communities such as Z. nummularia and V. gerrardi communities, as well as some other communities such as R. stricta, Z. spinosa, and C. dactylon communities. The most affecting soil variables shaping these communities were salinity, Ca, Mg, SO4, and NO3. The vegetation survey of the different sites of Raudhat Altinhat showed the presence of 86 plant species (55.8% annuals and 44.2% perennials). Among 27 identified families, Asteraceae, Poaceae, Boraginaceae, and Fabaceae were the major families (47.7% of the total identified species). The predominance of annual species can be ascribed to stressful environments, where the seeds of these species germinate during the favorable/rainy season and complete their life cycle before the start of the arid season. A total of 23 plant species were identified in the SSB of samples collected during winter-spring season, while during summer-fall season the SSB had 22 plants. The SSB analysis revealed the dominance of annual species (>87%) in both seasons. The herb T. stellata is the most predominant plant in the soil seed bank during the winter-spring season (average of 465 seeds/m2 among all plots) as well as in the summer-fall season (159 seeds/m2). The second dominant seeds in the soil were the seeds of P. minor. A negative correlation was observed between the aboveground vegetation and soil seed bank composition either during the winter-spring season (r = −0.060) or the summer-fall season (r = −0.036). The species diversity indexes showed that aboveground vegetation during the winter-spring season attained higher species richness and evenness, compared to the soil seed bank, while during the summer-fall season, the species richness of the aboveground vegetation and soil seed bank was consistent, while the species evenness was higher for the soil seed bank compared to the aboveground vegetation. Our results showed that the Raudhat Altinhat site needed rehabilitation and strict protection against all unmanaged activities such as overgrazing and logging. In addition, a high degree of degradation was observed for the Z. nummularia shrubs as well as limited regeneration, and no seed germination in the SSB. Therefore, further study is recommended to assess the causes of this degradation and to assess the best way to rehabilitate and recruit these very important shrubs on the site. Based on our data, the perennial species is very limited in the SSB, and it is worth mentioning that in the degraded desert ecosystem, it is important to consider supporting the ecosystem with shrub and perennial herb seeds that could crucially limit their restoration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
AA-E: Investigation, Formal Analysis, Writing – original draft, Writing – review and editing, Visualization, Conceptualization. AA-H: Writing – review and editing, Conceptualization, Investigation, Project administration, Formal Analysis. AAI: Investigation, Writing – review and editing, Resources. TA: Investigation, Writing – review and editing, Resources. AAS: Writing – review and editing, Investigation. OA: Writing – review and editing, Formal Analysis. BD: Investigation, Formal Analysis, Writing – review and editing. HK: Writing – review and editing, Formal Analysis, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by King Abdulaziz Royal Reserve Development Authority, and supported by the Deanship of Scientific Research, King Saud University, through Vice Deanship of Scientific Research Chairs: Chair of Climate Change, Environmental Development and Vegetation Cover.
Acknowledgments
The authors are grateful to King Abdulaziz Royal Reserve Development Authority for funding. Also, the authors are grateful to the Deanship of Scientific Research, King Saud University for funding through the Vice Deanship of Scientific Research Chairs: Chair of Climate Change, Environmental Development and Vegetation Cover.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1650193/full#supplementary-material
References
Abd-Elgawad, A. M., Assaeed, A. M., Al-Rowaily, S. L., Dar, B. M., and Malik, J. A. (2021). Moisture and salinity drive the vegetation composition of wadi Hargan, Riyadh, Saudi Arabia. Diversity 13 (11), 587. doi:10.3390/d13110587
Al-Huqail, A. A., Al-Harbi, H. F., Alowaifeer, A. M., El-Sheikh, M. A., Assaeed, A. M., Alsaleem, T. S., et al. (2025). Correlation between aboveground vegetation composition and soil seed bank of Raudhat desert habitat: a case study of Raudhat Alkhafs, Saudi Arabia. BMC Plant Biol. 25 (1), 136. doi:10.1186/s12870-025-06162-0
Al-Qarawi, A. A. (2011). Vegetation analysis in the Rawdhat Om Al-Khefas, Central Saudi Arabia. Aust. J. Basic Appl. Sci. 5 (12), 3264–3269.
Al-Rowaily, S. L., El-Bana, M. I., Al-Bakre, D. A., Assaeed, A. M., Hegazy, A. K., and Ali, M. B. (2015). Effects of open grazing and livestock exclusion on floristic composition and diversity in natural ecosystem of Western Saudi Arabia. Saudi J. Biol. Sci. 22 (4), 430–437. doi:10.1016/j.sjbs.2015.04.012
Alghanem, S., and Alhaithloul, H. (2023). Species diversity and floristic composition of Rawdhat Abalworood vegetation in Al-Asyah, Al-Qassim Region, Saudi Arabia. Appl. Ecol. and Environ. Res. 21 (5), 4703–4719. doi:10.15666/aeer/2105_47034719
Allen, S. E., Grimshaw, H., Parkinson, J. A., and Quarmby, C. (1974). Chemical analysis of ecological materials. Blackwell Scientific Publications.
Amiaud, B., and Touzard, B. (2004). The relationships between soil seed bank, aboveground vegetation and disturbances in old embanked marshlands of Western France. Flora 199 (1), 25–35. doi:10.1078/0367-2530-00129
Amrein, D., Rusterholz, H.-P., and Baur, B. (2005). Disturbance of suburban Fagus forests by recreational activities: effects on soil characteristics, above-ground vegetation and seed bank. Appl. Veg. Sci. 8 (2), 175–182. doi:10.1658/1402-2001(2005)008[0175:dosffb]2.0.co;2
Aragón-Gastélum, J. L., Flores, J., Jurado, E., Ramírez-Tobías, H. M., Robles-Díaz, E., Rodas-Ortiz, J. P., et al. (2018). Potential impact of global warming on seed bank, dormancy and germination of three succulent species from the Chihuahuan Desert. Seed Sci. Res. 28 (4), 312–318. doi:10.1017/s0960258518000302
Assaeed, A. M., and Al-Doss, A. A. (2002). Soil seed bank of a desert range site infested with Rhazya stricta in Raudhat al-Khafs, Saudi Arabia. Arid Land Res. Manag. 16 (1), 83–95. doi:10.1080/153249802753365340
Baker, H. G. (1989). in Ecology of soil seed banks. Editors M. A. Leck, V. T. Parker, and R. L. Simpson (San Diego, CA, US: Academic Press), 9–21.
Batanouny, K. H. (2000). Plants in the deserts of the Middle East. Springer Science and Business Media.
Blake, G. R., and Hartge, K. H. (1986). Methods of soil analysis. American Society of Agronomy, Inc. Soil Science Society of America, Inc., 377–382.
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analyses of soils. Agron. J. 54 (5), 464–465. doi:10.2134/agronj1962.00021962005400050028x
Bremner, J., and Mulvaney, C. J. a.S. a.M. (1982). “Total nitrogen,”. Methods of soil analysis. Editors A. L. Page, R. H. Miller, and D. R. Keeney, 2, 595–624.
Cao, J., Li, B., Qi, R., Liu, T., Chen, X., Gao, B., et al. (2022). Negative impacts of human disturbances on the seed bank of subalpine forests are offset by climatic factors. Sci. Total Environ. 851, 158249. doi:10.1016/j.scitotenv.2022.158249
Céspedes, B., Torres, I., Urbieta, I. R., and Moreno, J. M. (2012). Effects of changes in the timing and duration of the wet season on the germination of the soil seed bank of a seeder-dominated Mediterranean shrubland. Plant Ecol. 213, 919–931. doi:10.1007/s11258-012-0053-1
Chaudhary, S. A. (1999). Flora of the Kingdom of Saudi Arabia, 1. Riyadh, Saudi Arabia: Ministry of Agriculture and Water.
Chaudhary, S. A. (2000). Flora of the Kingdom of Saudi Arabia, 2. Riyadh, Saudi Arabia: Ministry of Agriculture and Water.
Chaudhary, S. A. (2001). Flora of the Kingdom of Saudi Arabia, 3. Riyadh, Saudi Arabia: Ministry of Agriculture and Water.
Chu, H., Zhang, C., Dong, Q., Shang, Z., Degen, A. A., Yang, X., et al. (2019). The effect of grazing intensity and season on the soil seed bank and its relation with above-ground vegetation on the alpine steppe. Agric. Ecosyst. and Environ. 285, 106622. doi:10.1016/j.agee.2019.106622
Collenette, S. (1999). Wildflowers of Saudi Arabia. National Commission for Wildlife Conservation and Development. Riyadh, Saudi Arabia: (NCWCD).
Dölle, M., and Schmidt, W. (2009). The relationship between soil seed bank, above-ground vegetation and disturbance intensity on old-field successional permanent plots. Appl. Veg. Sci. 12 (4), 415–428. doi:10.1111/j.1654-109x.2009.01036.x
Dos Santos, D. M., Da Silva, K. A., Dos Santos, J. M. F. F., and De Lima Araújo, E. (2018). Soil seed bank and its importance in the natural regeneration of degraded areas. Ethnobiol. Conservation 7, 1–7. doi:10.15451/ec2018-03-07.05-1-7
Elkordy, A., Nour, I. H., Ellmouni, F. Y., Al Shaye, N. A., Al-Bakre, D. A., and El-Banhawy, A. (2022). Floristic diversity of Jabal Al-Ward, Southwest Tabuk region, Kingdom of Saudi Arabia. Agronomy 12 (11), 2626. doi:10.3390/agronomy12112626
Gallagher, R. S. (2014). Seeds: the ecology of regeneration in plant communities. Wallingford, United Kingdom: CABI Publishing.
Gomaa, N. H. (2014). Microhabitat variations and seed bank-vegetation relationships in a desert wadi ecosystem. Flora 209 (12), 725–732. doi:10.1016/j.flora.2014.09.004
Gomaa, N. H., Hegazy, A. K., and Alhaithloul, H. a.S. (2023). Facilitation by Haloxylon persicum shrubs enhances density and richness of soil seed bank of annual plants in a hyper-arid ecosystem. Plants 12 (6), 1276. doi:10.3390/plants12061276
Gonzalez, S. L., and Ghermandi, L. (2021). Overgrazing causes a reduction in the vegetation cover and seed bank of Patagonian grasslands. Plant Soil 464 (1), 75–87. doi:10.1007/s11104-021-04931-y
Guo, N., Sang, C., Huang, M., Zhang, R., Degen, A. A., Ma, L., et al. (2023). Long-term active restoration of degraded grasslands enhances vegetation resilience by altering the soil seed bank. Agron. Sustain. Dev. 43 (1), 6. doi:10.1007/s13593-022-00862-9
Gutterman, Y. (2000). Environmental factors and survival strategies of annual plant species in the Negev Desert, Israel. Plant Species Biol. 15 (2), 113–125. doi:10.1046/j.1442-1984.2000.00032.x
Hopfensperger, K. N. (2007). A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 116 (9), 1438–1448. doi:10.1111/j.0030-1299.2007.15818.x
Islam, M. A., and Jacob, S. (2024). Terrestrial environment and ecosystems of Kuwait: assessment and restoration. Springer, 329–340.
Jiang, D., Wang, Y., Oshida, T., Luo, Y., Wang, H., and Zhou, Q. (2013). Review of research on soil seed banks in desert regions. Disaster Adv. 6 (3), 315–322.
Jones, J. B. (2001). Laboratory guide for conducting soil tests and plant analysis. Boca Raton, FL, United States: CRC Press.
Keen, B. A., and Raczkowski, H. (1921). The relation between the clay content and certain physical properties of a soil. J. Agric. Sci. 11 (4), 441–449. doi:10.1017/s0021859600004469
Khattab, H. I., Sadak, M. S., Dawood, M. G., Elkady, F. M. A., and Helal, N. M. (2024). Foliar application of esculin and digitoxin improve the yield quality of salt-stressed flax by improving the antioxidant defense system. BMC Plant Biol. 24 (1), 963. doi:10.1186/s12870-024-05626-z
Kleemann, S. G. L., and Gill, G. S. (2013). Seed dormancy and seedling emergence in ripgut brome (Bromus diandrus) populations in Southern Australia. Weed Sci. 61 (2), 222–229. doi:10.1614/ws-d-12-00083.1
Lan, H., and Zhang, F. (2008). Reviews on special mechanisms of adaptability of early-spring ephemeral plants to desert habitats in Xinjiang. Acta Bot. Boreali-Occidentalia Sin. 28 (7), 1478–1485.
Loydi, A., and Collins, S. L. (2021). Extreme drought has limited effects on soil seed bank composition in desert grasslands. J. Veg. Sci. 32 (5), e13089. doi:10.1111/jvs.13089
Luo, C., Guo, X. P., Feng, C. D., Ye, J. P., Li, P. F., and Li, Z. T. (2021). Spatial patterns of soil seed banks and their relationships with above-ground vegetation in an arid desert. Appl. Veg. Sci. 24 (4), e12616. doi:10.1111/avsc.12616
Luo, C., Guo, X., Feng, C., and Xiao, C. (2023). Soil seed bank responses to anthropogenic disturbances and its vegetation restoration potential in the arid mining area. Ecol. Indic. 154, 110549. doi:10.1016/j.ecolind.2023.110549
Mirreh, M. M., and Al Diran, M. S. (2014). in Range management in arid zones. Editors S. A. S. Omar, M. A. Razzaque, and F. Alsdirawi (London, UK: Routledge), 189–194.
Mohammed, S. A., and Denboba, M. A. (2020). Study of soil seed banks in ex-closures for restoration of degraded lands in the central Rift Valley of Ethiopia. Sci. Rep. 10 (1), 956. doi:10.1038/s41598-020-57651-1
Mualem, Y. (1976). A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 12 (3), 513–522. doi:10.1029/wr012i003p00513
Mueller-Dombois, D., and Ellenberg, H. (1974). Aims and methods of vegetation ecology. Wiley and Sons.
Nelson, D., and Sommers, L. (1982). in Methods for soil analysis. Editor A. L. Page (Madison, USA: ASA Monograph), 539–579.
Niu, F., Huo, L., Wang, Z., Liu, J., Gao, Z., Li, M., et al. (2022). Effects of nitrogen addition and watering on soil seed bank germination in a semiarid grassland on the Loess Plateau of China. Land Degrad. and Dev. 34 (1), 142–155. doi:10.1002/ldr.4449
Ooi, M. K. (2015). Seed bank dynamics and climate change in semi-arid ecosystems: a focus on physically dormant species. Rev. Bras. Geogr. Física 8, 651–659. doi:10.5935/1984-2295.20150021
Padonou, E. A., Akakpo, B. A., Tchigossou, B., and Djossa, B. (2022). Methods of soil seed bank estimation: a literature review proposing further work in Africa. iForest-Biogeosciences For. 15 (2), 121–127. doi:10.3832/ifor3850-015
Page, A. L. (1982). “Methods of Soil Analysis. Part 2,” in Chemical and microbiological properties (American Society of Agronomy, Soil Science Society of America).
Pol, R. G., Sagario, M. C., and Marone, L. (2014). Grazing impact on desert plants and soil seed banks: implications for seed-eating animals. Acta Oecol. 55, 58–65. doi:10.1016/j.actao.2013.11.009
POWO (2023). Plants of the world online. Kew: Facilitated by the Royal Botanic Gardens. Available online at: http://www.plantsoftheworldonline.org/.
Rhoades, J. (1982). in Methods of soil analysis: part 2: chemical and microbiological properties. Editors A. L. Page, R. H. Miller, and D. R. Keeney (Madison, USA: American Society of Agronomy).
Rowell, D. (1994). Soil science: method and applications. Essex, England: Pearson Education Limited.
Santini, N. S., Chamizo, S., Lucas-Borja, M. E., and Muñoz-Rojas, M. (2022). Editorial: restoration of degraded terrestrial ecosystems. Front. Ecol. Evol. 10, 863845. doi:10.3389/fevo.2022.863845
Saudipedia (2024). Nature reserves in Saudi Arabia. Available online at: https://saudipedia.com/en/article/2903/geography/reserves/nature-reserves-in-saudi-arabia#h-24.
Savadogo, P., Sanou, L., Dayamba, S. D., Bognounou, F., and Thiombiano, A. (2016). Relationships between soil seed banks and above-ground vegetation along a disturbance gradient in the W National Park trans-boundary biosphere reserve, West Africa. J. Plant Ecol. 10 (2), 349–363. doi:10.1093/jpe/rtw025
Shaukat, S. S., and Siddiqui, I. A. (2004). Spatial pattern analysis of seeds of an arable soil seed bank and its relationship with above-ground vegetation in an arid region. J. Arid Environ. 57 (3), 311–327. doi:10.1016/s0140-1963(03)00112-5
Shi, Y.-F., Wang, Z.-R., Xu, B.-X., Huo, J.-Q., Hu, R., Zhao, Y., et al. (2022). Rainfall amount determines annual herb controls over soil seed bank and its similarity with vegetation in the Tengger Desert. Ecol. Process. 11 (1), 5. doi:10.1186/s13717-021-00346-w
Shinoda, Y., and Akasaka, M. (2019). Species turnover differentiates diversity–disturbance relationships between aboveground vegetation and soil seedbank. Plant Ecol. 220 (6), 595–603. doi:10.1007/s11258-019-00938-9
Solomon, T. B. (2011). Soil seed bank dynamics in relation to land management and soil types in the semi-arid savannas of Swaziland. Afr. J. Agric. Res. 6, 2494–2505. doi:10.5897/AJAR10.1054
Sternberg, M., Gutman, M., Perevolotsky, A., and Kigel, J. (2003). Effects of grazing on soil seed bank dynamics: an approach with functional groups. J. Veg. Sci. 14 (3), 375–386. doi:10.1658/1100-9233(2003)014[0375:eogoss]2.0.co;2
Stotz, G. C., Salgado-Luarte, C., Escobedo, V. M., Valladares, F., and Gianoli, E. (2021). Global trends in phenotypic plasticity of plants. Ecol. Lett. 24 (10), 2267–2281. doi:10.1111/ele.13827
Tessema, Z. K., De Boer, W. F., Baars, R. M. T., and Prins, H. H. T. (2012). Influence of grazing on soil seed banks determines the restoration potential of aboveground vegetation in a semi-arid savanna of Ethiopia. Biotropica 44 (2), 211–219. doi:10.1111/j.1744-7429.2011.00780.x
Thompson, K., Bakker, J. P., and Bekker, R. M. (1997). The soil seed banks of North West Europe: methodology, density and longevity. Cambridge University Press.
Van Langevelde, F., Tessema, Z. K., De Boer, W. F., and Prins, H. H. (2016). Soil seed bank dynamics under the influence of grazing as alternative explanation for herbaceous vegetation transitions in semi-arid rangelands. Ecol. Model. 337, 253–261. doi:10.1016/j.ecolmodel.2016.07.013
Venable, D. L., Flores-Martinez, A., Muller-Landau, H. C., Barron-Gafford, G., and Becerra, J. X. (2008). Seed dispersal of desert annuals. Ecology 89 (8), 2218–2227. doi:10.1890/07-0386.1
Waheed, M., and Arshad, F. (2024). Adaptive convergence and divergence underpin the diversity of Asteraceae in a semi-arid lowland region. Flora 317, 152554. doi:10.1016/j.flora.2024.152554
Welles, S. R., and Funk, J. L. (2021). Patterns of intraspecific trait variation along an aridity gradient suggest both drought escape and drought tolerance strategies in an invasive herb. Ann. Bot. 127 (4), 461–471. doi:10.1093/aob/mcaa173
World Bank (2024). Climate change knowledge portal. Available online at: https://climateknowledgeportal.worldbank.org/.
Yang, X., Baskin, C. C., Baskin, J. M., Pakeman, R. J., Huang, Z., Gao, R., et al. (2021). Global patterns of potential future plant diversity hidden in soil seed banks. Nat. Commun. 12 (1), 7023. doi:10.1038/s41467-021-27379-1
Zhang, T., and Chen, Y. (2022). The effects of landscape change on habitat quality in arid desert areas based on future scenarios: tarim River Basin as a case study. Front. Plant Sci. 13, 1031859. doi:10.3389/fpls.2022.1031859
Keywords: habitat degradation, soil repository, raudhat ecosystems, plant regeneration, rehabilitation, species richness
Citation: Abd-ElGawad AM, Al-Huqail AA, Alowaifeer AM, Alsaleem TS, Assaeed AM, Azab OM, Dar BA and Kassem HS (2025) Soil seed bank dynamics and vegetation composition in raudhat Altinhat, Saudi Arabia: implications for arid ecosystem restoration. Front. Environ. Sci. 13:1650193. doi: 10.3389/fenvs.2025.1650193
Received: 19 June 2025; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Z. Y. Yuan, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Hameed Ullah, Quaid-i-Azam University, PakistanSina Attarroshan, Islamic Azad University Central Tehran Branch, Iran
Copyright © 2025 Abd-ElGawad, Al-Huqail, Alowaifeer, Alsaleem, Assaeed, Azab, Dar and Kassem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed M. Abd-ElGawad, YWlicmFoaW0yQGtzdS5lZHUuc2E=; Asma A. Al-Huqail, YWFsaHVxYWlsQGtzdS5lZHUuc2E=
 Ahmed M. Abd-ElGawad
Ahmed M. Abd-ElGawad Asma A. Al-Huqail1*
Asma A. Al-Huqail1* Abdulaziz M. Assaeed
Abdulaziz M. Assaeed Basharat A. Dar
Basharat A. Dar Hazem S. Kassem
Hazem S. Kassem