- 1Hebei Key Laboratory of Close-to-Nature Restoration Technology of Wetlands, School of Eco-Environment, Hebei University, Baoding, China
- 2Technical Centre for Soil, Agriculture and Rural Ecology and Environment, Ministry of Ecology and Environment, Beijing, China
- 3Huarui Agricultural Company, Zhangye, China
- 4Engineering Research Center of Ecological Safety and Conservation in Beijing-Tianjin-Hebei (Xiong’an New Area) of MOE, Baoding, China
Cow dung reduces the bioavailability of heavy metals (HMs) in wheat soils. However, interactive influence of soil chemical properties, microbial communities and functional genes in HM immobilization need to be further investigated. Therefore, the effects of cow dung on heavy metals content in soil and wheat, soil microbial community structure, and heavy metal resistance genes (MRGs) were tested with pot experiment. Cow dung reduced the bioavailable forms of Cd by 8% to 21%, thereby reducing the Cd content of wheat grains by 30.5% to 46.1%. Bioavailable forms of Cd in soil were significantly and negatively correlated with soil chemical properties, relative abundance of microbial genera (such as Bradyrhizobium spp. and Mycolicibacterium spp.), and relative abundance of MRGs (such as actA and fpvA). Concurrently, the bioavailable forms of Cu and Zn exhibited significant correlations with Shannon index and MRGs (such as copC). These results indicated that changes in soil environmental factors (soil chemical properties, microbial communities and MRGs) are conducive to reducing the bioavailability of Cd. However, the high levels of Cu and Zn in cow dung may also disrupt soil ecology, resulting in a decline in microbial diversity and impacting the abundance of MRGs. Therefore, the rates of application of cow dung should be carefully selected and should not exceed 2.5% (w/w). These results may provide scientific evidence for the safe use of cow dung and remediation of heavy metals in wheat soils.
1 Introduction
In recent decades, HMs pollution of agricultural soils has become a social concern (Wang N. et al., 2021). Cadmium (Cd) is considered an environmental nuisance compared to other HMs (Zhou and Li, 2022). The accumulation of Cd in soil degrades soil resources (Sun et al., 2019), impedes crop growth (Rahi et al., 2022), and threatens human health through the food chain (Wang et al., 2010). With the rapid development of industry and the acceleration of urbanization, the activities of enterprises emitting heavy metals have intensified the pollution pressure on the surrounding environment. Studies have shown that the concentrations of Cd, copper (Cu), and zinc (Zn) in wheat grains increases with decreasing distance from smelters (Li et al., 2020). Therefore, effective methods to mitigate the adverse effects of Cd contamination on soils and crops are urgent.
Because most HMs persist in soil owing to their immobile nature (Jamali et al., 2009), the key to ameliorating soil heavy metal contamination is to reduce the bioavailability of HMs. Livestock manure is a readily available renewable resource with great potential for reducing the bioavailability of HMs. The application of chicken and pig dung significantly reduces the accumulation of Cd in rice kernels, mainly by increasing the soil pH and reducing the active-state Cd in the soil (Wang et al., 2024). Azhar et al. (2019) found that the use of livestock manure improves soil properties and plant growth by reducing Cd bioavailability in the soil and limiting Cd uptake by plants. Meanwhile, treatment with livestock manure has been reported to reduce Cd bioavailability and increase nutrient levels in the soil, thereby increasing crop yields (Wang Y. et al., 2021). Therefore, application of livestock manure may be an effective way to reduce the bioavailability of HMs, improve soil fertilization, and promote crop growth.
Livestock dung also exerts a passivating effect on HMs by altering soil microbial activity. According to Haider et al. (2022), microbes such as Trichoderma harzianum and Bacillus subtilis can reduce Cd bioavailability through biotransformation and biomineralization, significantly decreasing the toxicity of Cd in the root zone of plants, promoting root growth, and increasing crop yield. In addition, microorganisms can exhibit resistance to specific HMs through the expression of MRGs, which promotes redox and complexation reactions and passivates HMs (Yin et al., 2019). Moreover, soil microorganisms can work together with soil passivators to reduce the bioavailability of HMs; for instance, phosphogypsum-based passivate (Ca-mPG) promotes the enrichment of arsenic-resistant bacteria along with good reduction in heavy metal bioavailability (Ma et al., 2023). Overall, livestock manure reduces heavy metal bioavailability while affecting soil ecosystems.
Cow dung is the most produced livestock manure in China (Liu et al., 2020) and possesses less Cd than chicken and pig dung (Mu et al., 2020). Therefore, rational application of cow dung ensures a manageable risk of Cd accumulation in soil (Zhou et al., 2015). Many studies have discussed the effect of cow dung on the morphology and bioavailability of HMs in soil (Clemente et al., 2007; Kibria et al., 2023; Aina et al., 2024; Zhang et al., 2025). However, the interaction of soil HMs with environmental factors still needs to be studied in depth. In this study, wheat was used as the planting crop, and different mass fractions of cow dung were applied in a potting experiment. We investigated (1) the effect of the application of cow dung on the bioavailability of HMs and the uptake of Cd by wheat, (2) the interaction and coexistence of heavy metal bioavailability with environmental factors after the application of cow dung, and (3) differences in the effects of different applied rates of cow dung on the soil environment. The results of the study provide a theoretical basis and data support for the safe use of livestock manure and promote sustainable agricultural development.
2 Materials and methods
2.1 Experimental materials
Soil was obtained from an area of cadmium-contaminated farmland in Henan Province, China. The basic chemical properties of the soil were as follows: pH, 6.80; electrical conductivity (EC), 430us cm−1; soil organic matter (SOM), 26.54 g kg−1; total nitrogen (TN), 1.56 g kg−1; total phosphorus (TP), 0.42 g kg−1; available nitrogen (AN), 80.89 mg kg−1; and available phosphorus (AP), 44.50 mg kg−1. The soil heavy metal contents were Cd at 1.22 mg kg−1, Cu at 29.54 mg kg−1, and Zn at 192.54 mg kg−1. Cow dung was obtained from Gansu Huarui Agricultural Co. and used in the study after drying, fermentation, and compaction. The basic chemical properties of the cow dung were as follows: pH, 7.66; TN, 10.90 g kg−1; TP, 19.97 g kg−1; Cd, 0.15 mg kg−1; Cu, 47.01 mg kg−1; and Zn, 193.51 mg kg−1. Wheat seeds were selected from Yongliang 15. The experimental pots were cylindrical plastic containers with a diameter of 30 cm and a depth of 20 cm.
2.2 Wheat potting experiment
The experimental site was located in Huarui Ranch, Zhangye City, Gansu Province (100°625′E, 38°375′N). The Soil was naturally air dried, removed from large gravel and plant debris, and sieved through a 10 mm diameter sieve. 4 kg of soil was placed in each pot and cow dung was added to the pots according to the mass fraction of the soil and mixed well with the soil at nine application rates of 0% (w/w) (CK, the control), 2.5% (CD025), 5% (CD050), 7.5% (CD075), 10% (CD100), 12.5% (CD125), 15% (CD150), 17.5% (CD175), and 20% (CD200). Each cow dung application rate was set in 3 parallels for a total of 27 potted plants. Nitrogen, phosphorus, and potassium fertilizers (0.27 g/kg of urea, 0.25 g/kg of monoammonium phosphate, and 0.25 g/kg of potassium chloride, respectively) were applied to ensure normal growth of wheat. In July 2023, 50 wheat grains were sown in each pot, and the grains were buried at a depth of 2–3 cm. After 1 week of wheat germination, 15 plants with similar growth were kept; the sowing distance between each plant was kept even. Water was applied daily to keep the soil moist. Wheat matured in November of the same year, with simultaneous collection of wheat and soil. The whole experimental process was done outdoors in a temperate continental climate, with an altitude of 1673 m above sea level, an average annual temperature of 5.6 °C, a rainfall of 256.4 mm, and a sunshine duration of 2768.1 h. Wheat rhizosphere soil was collected using sterile spoons and divided into two parts: one part was naturally air-dried and used for chemical analyses, and the other part was stored at −80 °C until the completion of the macrogenomic analyses. After removing the topsoil, roots, stems, leaves and grains of wheat were collected separately, dehydrated at 105 °C, then dried to constant weight at 60 °C and crushed for determination of chemical properties. During the wheat harvest, 15 ears of wheat with uniform growth were selected from each pot, and the number of grains per ear was counted. The grains were threshed to measure the yield, and the thousand-grain weight and yield of each pot of wheat were obtained.
2.3 Physical and chemical analysis
Soil pH was measured with a pH meter (PHS-3C, Ray Magnetics) after mixing soil with deionized water 1:2.5 ratio (Lu, 1999). Using the principle that potassium dichromate oxidizes organic carbon in soil under acidic conditions, SOM content is calculated by titrating the remaining potassium dichromate (Lu, 1999). Soil TN was measured by Kjeldahl method (Lu, 1999). Soil samples were melted with sodium hydroxide to convert all phosphorus-containing minerals and organophosphorus compounds in the soil to soluble phosphates, which were then used in a molybdate colorimetric method to determine soil TP (Bao, 2000). Soil AN and AP were determined using the alkali diffusion method and soil molybdate colorimetric method, respectively (Lu, 1999). Due to the high content of Cu and Zn in livestock manure, which may have some impact on soil ecology, in addition to Cd, Cu and Zn in soil were also determined in this study (Yuan et al., 2020). The morphology of soil Cd, Cu, and Zn was analyzed by modified continuous extraction method (BCR) (You et al., 2023), where HMs were defined in order of decreasing bioavailability as acid extractable (AcidExt), reducible (Red), oxidizable (Oxi), and residual (Res) fractions (Guo et al., 2022). The heavy metal content of the liquid to be measured was determined by ICP-MS (7900, Agilent, United States). To represent heavy metal bioavailability more directly, bioavailability factor (BF) was studied together with heavy metal fractions. Guo et al. had defined BF as: BF = (AcidExt content + Red content)/Total content (Guo et al., 2022). Wheat samples were digested using HNO3 and HClO4 (4:1) (Lu, 1999), and the HMs content of the wheat fractions was then determined by ICP-MS (7900, Agilent, United States). The detailed determination method can be seen in Supplementary Table S1.
2.4 Macrogenomic analysis
Soil DNA was extracted by CTAB method (Hultman et al., 2015) a and DNA concentration were measured using the Agilent 5400 system (Agilent, United States). DNA libraries were constructed using the NEBNext® UltraTM DNA Library Prep Kit for Illumina (NEB, United States, Catalog#: E7370 L). Library preparation was completed by end repair, fragment screening, PCR amplification and purification. Library quality was assessed by Agilent 5400 system (Agilent, United States), and finally library concentration was quantified by QPCR (1.5 nM). According to the library concentration and target data volume, the libraries were up-sequenced on the Illumina NovaSeq 6000 platform using the PE150 strategy to receive raw macrogenomic data of microorganisms in soil samples. Macrogenomic sequencing was performed using the Illumina NovaSeq platform to receive raw data on the microorganisms in each soil sample. The annotation of microbial species was accomplished by using Kraken2 and a self-constructed microbial nucleic acid database (Sequences belonging to bacteria, fungi, archaea and viruses were screened from NT nucleic acid database and RefSeq whole genome database of NCBI) to calculate the number of sequences of microorganisms in the plotted samples and to predict the relative abundance of microorganisms using Bracken (Lu et al., 2017). Functional genes of microorganisms were annotated using HUMAnN2 software. Sequences after QC and de-hosting were aligned with the protein database (UniRef90). Based on the comparison results, annotation information and relative abundance tables can be obtained for several functional databases such as BacMet.
2.5 Statistical analysis
One-way analysis of variance (ANOVA) and Kruskal–Wallis test were performed using IBM SPSS Statistics 27 with a significance level of p < 0.05. Soil microbial alpha analyses were carried out on an online platform (https://www.bioincloud.tech/), where the Shannon index and chao1 index represent microbial diversity and richness, respectively, and which was also used to calculate phylum and genus proportions of microorganisms in different soil samples. Principal Coordinate Analysis (PCoA) and Non-metric multidimensional scaling (NMDS) analysis of soil microbial diversity based on the Bray-Curtis distance was used to analyze changes in the overall composition of soil microbes between treatments using the “vegan” package in RStudio, and the “ggcor” and “dplyr” packages in RStudio were used to calculate the Mantel correlations between environmental factors and Cd content in soil and wheat. Spearman correlation analyses of soil heavy metal morphology, BF and environmental factors were performed using the “Hmisc” package in RStudio, retaining correlation coefficients (r) > 0.4 and significant P-values (P < 0.05). Finally, the data from the correlation analysis were imported into the Gephi software (version 0.10) using the “igraph” package and visualized by adjusting the position and size of each point.
3 Results
3.1 Effect of cow dung on soil chemical properties and heavy metal forms
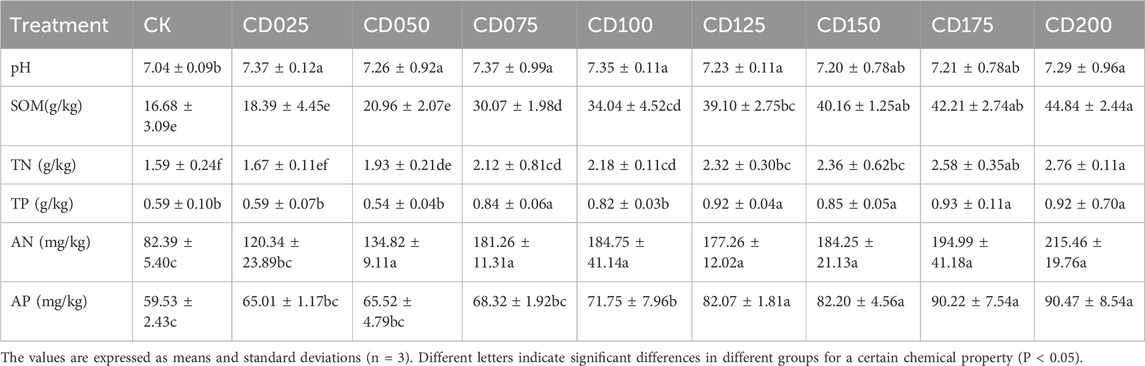
The application of cow dung significantly altered a wide range of soil chemical properties (Table 1). Significant differences were observed in pH, SOM, TN, TP, AP and AN at different cow dung application rates as compared to CK, and soil nutrients were elevated with increasing cow dung application rates. The proportion of bioavailable forms (AcidExt + Red) of Cd decreased to different degrees in all treatment groups after the addition of cow dung (Figure 1). Cow dung had a relatively significant passivating effect on the AcidExt and Red of Cd, with a maximum reduction of 8% for the Red of Cd and 13% for the AcidExt of Cd. At the 15% dosage, the bioavailable form of Cd was reduced by up to 21% of the total heavy metal. However, the application of cow dung increased the bioavailability of Cu and Zn to some extent (Figure 1). At 20% rate, cow dung increased the bioavailable forms of Cu and Zn by up to 26% and 18%, respectively.
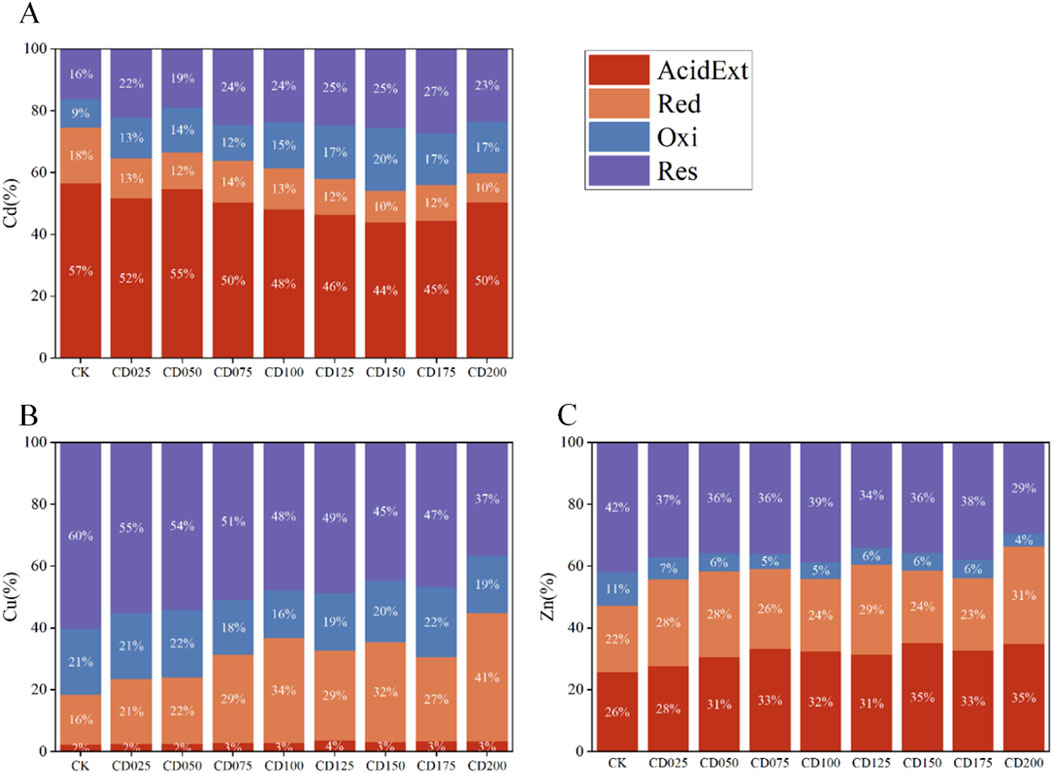
Figure 1. Changes in the morphology of (A) Cd, (B) Cu, and (C) Zn after treatment of cow dung at different rates.
3.2 Effect of cow dung on Cd content in wheat
After examining the HMs in the wheat tissues of all groups, we found that different rates of cow dung reduced Cd content in wheat roots, stems, leaves, and grains as compared to those in the CK control (Figure 2). The addition of cow dung reduced the Cd content in wheat roots by 14.2%–35.9%; significant reductions (P < 0.05) were observed at 2.5%, 5%, 10%, 12.5%, 15%, 17.5%, and 20% applications of cow dung, with the highest reduction at 12.5% application. The Cd content in wheat stems was reduced by 4.4%–42.3%; significant reductions (P < 0.05) were observed at 5%, 10%, and 12.5%, with the highest reduction at 7.5% application. The Cd content in wheat leaves was reduced by 23.6%–40.2%; all application rates displayed a significant reduction (P < 0.05), with the highest reduction at 12.5% application. The Cd content in wheat grains was reduced by 30.5%–46.1%; all application rates revealed significant reduction (P < 0.05), with the highest reduction at 2.5% application. The wheat yield of CK was 11.07 g/pot, and the wheat yield after the application of cow dung ranged from 10.28 g/pot to 14.22 g/pot, which did not change significantly compared with CK (Supplementary Table S2). Therefore, after the application of cow dung, the Cd content of wheat grains in each pot decreased compared with CK (Supplementary Table S2).
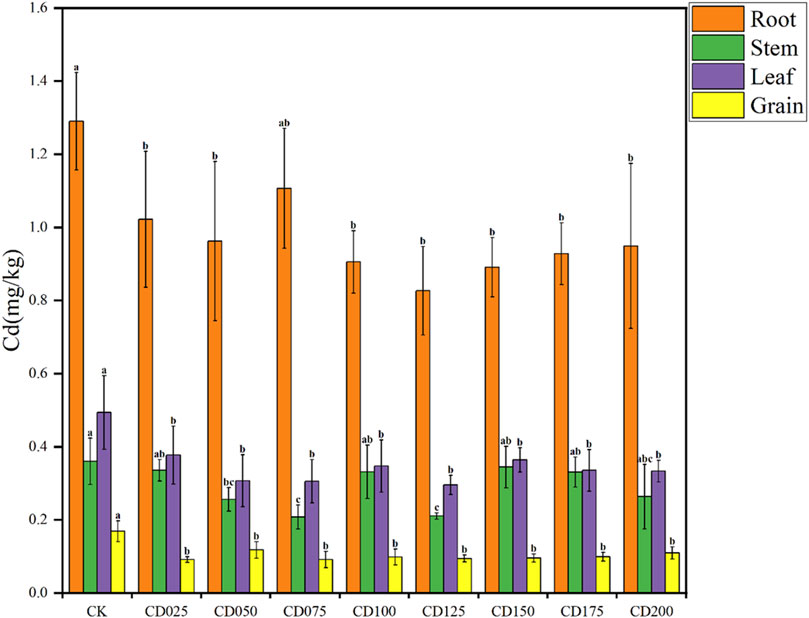
Figure 2. Cd content in roots, stems, leaves and grains of wheat under the influence of different rates of cow dung. Different letters indicate significant differences in cadmium content in different groups (P < 0.05).
Without the addition of cow dung, the cadmium content in wheat kernels reached 0.17 ± 0.03 mg/kg, which exceeded the Chinese regulation of 0.1 mg/kg for wheat grains and their products (NY 816-2004) and the addition of cow dung resulted in six groups of wheat grains meeting the Chinese standard for Cd content.The data showed that cow dung significantly reduced the accumulation of Cd in wheat, especially in leaves and grains, at application rates ranging from 2.5% to 20%; however, there was no linear relationship between the Cd content in various parts of wheat and the amount of cow dung applied.
3.3 Effect of cow dung on soil microorganisms
3.3.1 Alpha diversity
Application of cow dung significantly altered soil microbial alpha diversity. Two alpha diversity indexes were calculated, including the Chao1 index and Shannon index. The Shannon index and Chao1 index characterize microbial abundance and diversity, respectively (Xiang et al., 2023). The Shannon index of soil microorganisms was significantly (p < 0.05) reduced when 5% and above rate cow dung was applied (Figure 3A), and the Shannon index decreased the most when cow dung was dosed at 15%. In contrast, the Chao1 index was significantly reduced in comparison to CK when cow dung was applied at up to 15%, but there was no significant difference in the samples with other application rates (Figure 3B). These results indicate that the application of cow dung reduced the diversity of wheat rhizosphere soil microorganisms but had little effect on the Chao1 index, and soil microbial abundance changed less.
Figure 3. Soil microbial alpha diversity under the influence of different rates of cow dung. (A) Chao1 index. (B) Shannon index. Different letters indicate significant differences in alpha diversity among different groups (P < 0.05).
3.3.2 Relative abundance of microbial phyla and genera
Differences in soil microbial phyla and genera were observed after the application of cow dung. The relative abundance of microorganisms under different cow dung application rates is shown in Figures 4A,B. Among the top 15 microbial phyla with high relative abundance, the dominant phyla (RA > 10%) included Actinomycetota and Pseudomonadota. Meanwhile, the relative abundance of Pseudomonadota and Planctomycetota increased, but not significantly, with a corresponding decrease in the abundance of microorganisms in other phyla after cow dung application. At the genus level, Mycolicibacterium (3.5%–27.6%), Streptomyces (7.7%–15.3%), and Bradyrhizobium (1.6%–8.9%) had the highest relative abundance. The relative abundance of the six microbial genera differed significantly (P < 0.05) after the application of cow dung (Supplementary Table S3). The relative abundance of Mycolicibacterium, Bradyrhizobium, Shinella, and Sphingopyxis increased significantly (P < 0.05), with a corresponding decrease in the relative abundance of Nocardioides and Mycobacterium.
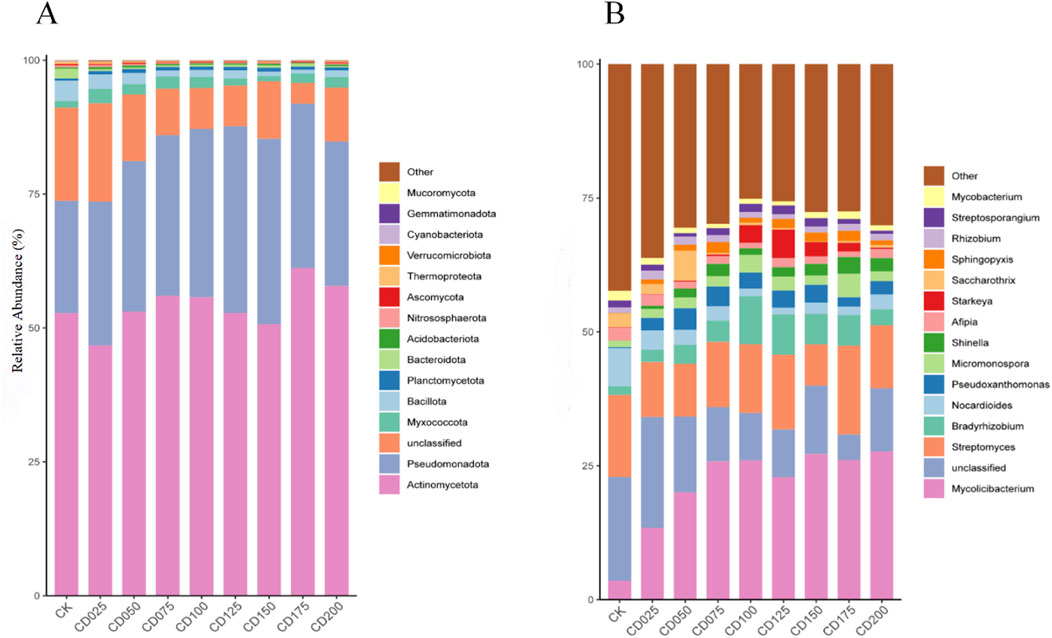
Figure 4. Relative abundance of soil microorganisms under the influence of different rates of cow dung. (A) Phylum level (B) Genus level.
3.3.3 Beta diversity
The overall structure of the soil microbial community was further investigated using PCoA and NMDS analysis. The PCoA analysis (Figure 5A) showed that the contributions of PCoA1 (first principal coordinate) and PCoA2 (second principal coordinate) were 40.23% and 14.96%, respectively, and the cumulative contribution of the two was 55.19%, which indicates a large separation of the microbial communities of CD and CK. NMDS analyses (Figure 5B) had a Stress <0.2, also indicating a large separation of microbial communities in CD and CK. These results indicated significant changes in the microbial community structure of wheat rhizosphere soil by the application of cow dung compared to CK.
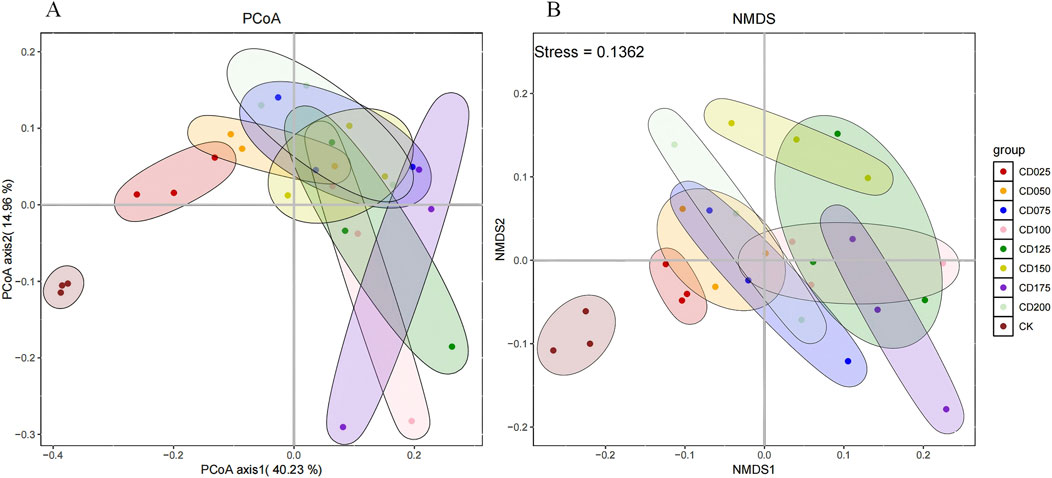
Figure 5. (A) PCoA and (B) NMDS analysis of Beta diversity of the microbial community under the influence of different applications of cow dung.
3.4 Effect of application cow dung on MRGs
A total of 149 gene names associated with Cd, Cu, and Zn were screened using reads-based macrogenomic analysis. Among them, 45 were associated with Cd, 75 with Cu, 69 with Zn (Supplementary Figure S1). Cow dung application significantly altered the relative abundance of some MRGs. The Kruskal–Wallis test showed that of the 149 genes, 29 showed significant changes in relative abundance after application with cow dung (P < 0.05) (Supplementary Table S4). Among them, 8 MRGs are associated with Cd, such as actR and actS; 15 MRGs are associated with Cu, such as copA and copB; and 15 MRGs are associated with Zn, such as acrD and actA. Based on these data, we investigated the interactions between the environmental factors and HMs by subjecting the morphology and BF of Cd, Cu, and Zn to Spearman analysis with soil chemical properties, MRGs, and microbial community structures (including relative abundance of microorganisms of the top five phyla and genera and the Chao1 and Shannon indes), respectively. Correlation grid plots were then constructed (Figure 6). These observations found that 13, 14, and 12 MRGs were moderately and above correlated (r > 0.4) with Cd, Cu, and Zn heavy metal morphology and BF, respectively.
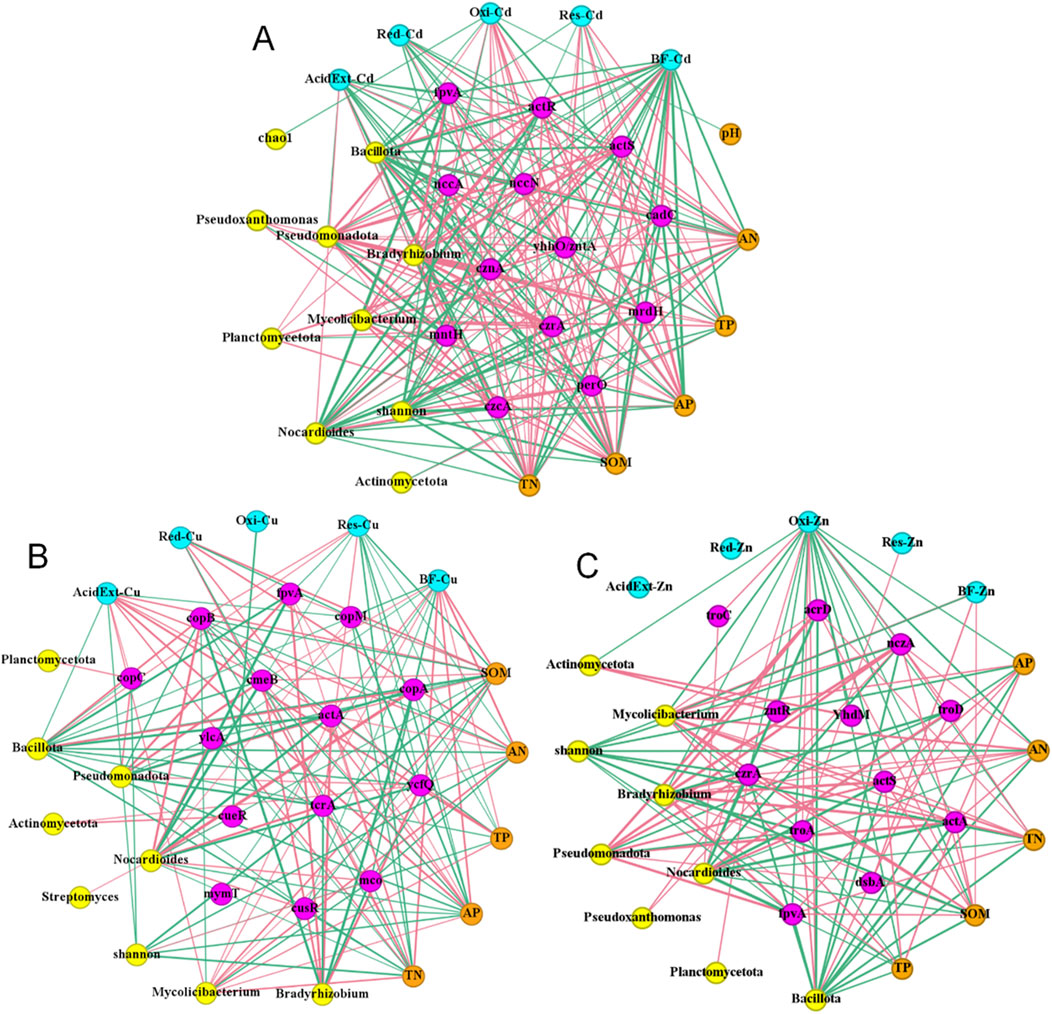
Figure 6. Spearman correlation analysis morphology and bioavailability factor of (A) Cd, (B) Cu, and (C) Zn in relation to environmental factors.
3.5 Correlation between environmental factors and bioavailability of HMs
Spearman correlation analysis was conducted among environmental factors and soil HMs (Figure 6). Node colour represents the classification of nodes, blue represents the morphology and bioavailability factor of HMs, purple represents functional genes, yellow represents soil microbial community, orange represents soil chemical properties, the color of the connecting line between two nodes represents the relevance of the node, red is positive correlation and green is negative correlation, and the wider lines. The thickness of the line between two nodes represents the strength of the correlation between the nodes, the thicker the line, the stronger the correlation. At the phylum level, Pseudomonadota was significantly negatively correlated with BF-Cd. Meanwhile, Bacillota was significantly positively correlated with BF-Cd and Oxi-Zn and negatively correlated with BF-Cu. At the genus level, Mycolicibaterium was significantly negatively correlated with BF-Cd and positively correlated with BF-Cu and BF-Zn. Bradyrhizobium was significantly negatively correlated with AcidExt-Cd, BF-Cd, and Oxi-Zn. Nocardioides was positively correlated with BF-Cd and Oxi-Zn. In addition, the Shannon index was significantly positively correlated with BF-Cd and significantly negatively correlated with AcidExt-Cu and BF-Zn. MRGs were also closely related to soil HMs. Here, actR, actS, and AcidExt-Cd were significantly negatively correlated; actA, copC, and AcidExt-Cu were significantly positively correlated; and troC, troD, and Oxi-Zn were significantly positively correlated.
The morphology and BF of HMs were significantly correlated with soil chemical properties; SOM, TN, AN, TP, and AP were more highly correlated with HMs, and pH was less correlated with HMs. Soil microbial communities and MRGs were closely related to soil chemical properties. Genes, such as cadC and perO, decreased in relative abundance in the soil environment and were negatively correlated with soil nutrients. In contrast, increased relative abundance of genes such as actR and fpvA was positively correlated with soil nutrients. The relative abundance of Mycolicibaterium and Bradyrhizobium increased and was positively correlated with soil physical and chemical properties, while the relative abundance of Bacillota and Nocardioides decreased and was positively correlated with soil physical and chemical properties. Meanwhile, soil microbial communities and MRGs were also significantly correlated.
Mantel correlation analysis was conducted among environmental factors and soil HMs (Figure 7). Nodes represent the classification of environmental fators, and the lines connecting the nodes to the matrix represent the mantel correlation between the environmental features and Cd, the wider the line represents the stronger the connection, and the colour of the line represents the significance of the connection, with blue representing a very high significance, pink representing a high significance, and grey representing a non-significant correlation. The matrix represents the Spearman correlation between Cd morphology, bioavailability factor and plant heavy metal content, with red representing a positive correlation and blue a negative correlation. The Mantel correlation analysis of environmental factors with Cd bioavailability revealed that soil chemical properties, microbial communities, and MRGs significantly influenced soil Cd bioavailability in the form of Cd and BF, and were strongly correlated with Cd content in wheat roots, stems, leaves and grains. In addition, Cd content in wheat roots was significantly correlated with soil Cd morphology and BF, but Cd content in the aboveground parts of wheat (stems, leaves, and grains) was weakly correlated with soil Cd morphology and BF.
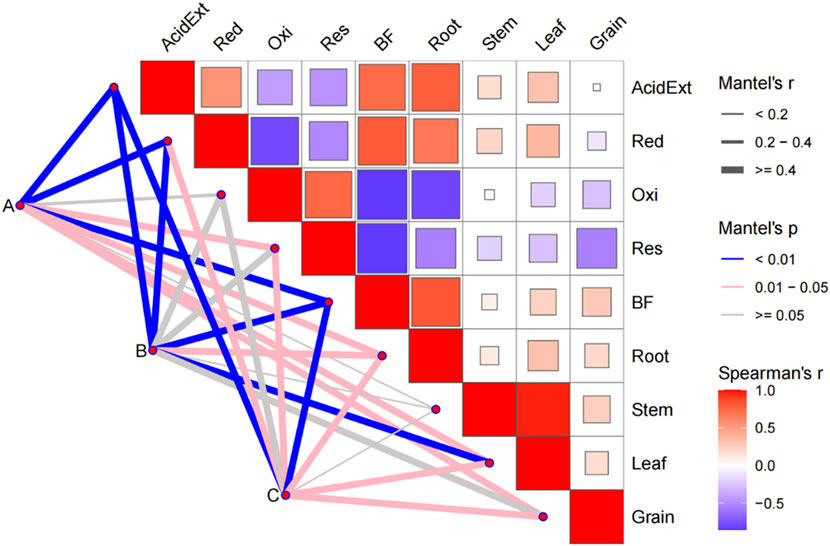
Figure 7. Mantel correlation study of Cd morphology, bioavailability factor and plant Cd content with environmental factors, (A) soil chemical properties, (B) microbial community structure, (C) heavy metal resistance genes.
4 Discussion
4.1 Application of cow dung contributes to safe wheat production on contaminated arable land
This study indicates that cow dung significantly reduces the Cd content in various parts of wheat, especially in the grain, by 30.5%–46.1%, thereby improving the quality of wheat. Meanwhile, wheat production has not changed significantly, and cadmium content in wheat grains in each pot decreased, ruling out the dilution effect. Studies have shown that The AcidExt and Red forms of HMs are readily available for plant uptake and utilization (Nemati et al., 2011). In this study, application of cow dung reduced the AcidExt and Red forms of Cd by 8%–21%, thus effectively reducing the uptake of HMs by wheat. In addition, changes in soil chemical properties were evident after application with different concentrations of cow dung, with increases in pH, SOM, TP, and AP content in the soils of all treated groups; all these changes may alter the morphology of HMs and reduce the bioavailability of HMs. First, the application of cow dung increased the soil pH. A higher pH increases the adsorption of heavy metal ions on soil particles, thus reducing heavy metal bioavailability (Zhong et al., 2020), and higher soil pH also leads to precipitation of HMs thereby making them unavailable (Liao et al., 2005). Second, cow dung application increased the SOM content to varying degrees. It has been shown that increased SOM content increases the number of adsorption sites in soil particles and effectively reduces the bioavailability of metals in soil (Hong et al., 2022). Third, the addition of cow dung significantly increased the content of TP and AP in the soil. Previous studies have shown that insoluble phosphorus in cow dung directly adsorbs heavy metal ions from soil (Valipour et al., 2016), whereas an increase in AP promotes its reaction and precipitation with HMs such as Cd (Chen et al., 2006). However, AP reacts with Cu and Zn to precipitate less; the fact that cow dung originally contains more Cu and Zn may be the main reason for the increase in the bioactive state of Cu and Zn after application cow dung (Cao et al., 2004). Further studies have shown that Cd and Zn have the same valence state and approximately the same ionic radius, and that they may share the same transporter in the cytoplasm for transport in plants, and that competition for the transporter binding site by Zn can lead to a decrease in the mobility of Cd, thereby inhibiting the uptake of Cd by wheat. (Wang et al., 2022). Thus, The higher Zn content of cow dung may also antagonise Cd and reduce Cd uptake by wheat.
4.2 Environmental factors affecting the bioavailability of Cd
4.2.1 Soil chemical properties
As discussed in Section 4.1, the addition of cow dung alters the soil chemical properties, thereby reducing the bioavailability of Cd. In this study, the soil chemical properties, including SOM, TN, AN, TP, and AP, were all significantly positively correlated with Cd-BF, indicating the positive effect of cow dung addition on Cd toxicity. Meanwhile, soil chemical properties and microbial community structure were significantly correlated with MRGs. These observations were consistent with previous findings that Cd bioavailability is not only affected by one factor and that soil chemical properties can affect heavy metal bioavailability directly or indirectly by altering microbial communities and functions (Fu et al., 2023). In addition, no significantly correlation was found among soil pH and the available form of Cd, indicating that the change in soil pH after application of cow dung had a small effect on the bioavailability of Cd. This may be due to the fact that both the soil and cow dung were neutral, and although the application of cow dung increased the soil pH to prevent the conversion of HMs to biologically effective forms, the change in the adsorption capacity of soil heavy metals was relatively small (Hussain et al., 2019). Studies have found that smaller changes in pH may have little effect on metal immobilization (Sauvé et al., 2000; Xu et al., 2018), which is consistent with the results of the present study.
Here, soil chemical properties were also significantly correlated with the amount of Cd in wheat, suggesting that soil chemical properties could influence Cd uptake in wheat. Higher SOM and AP levels can reduce soil heavy metal bioavailability, thereby reducing heavy metal uptake in wheat, which is consistent with previous research (Rizwan et al., 2016). However, the small correlation between Cd content in aboveground parts of wheat and soil Cd morphology suggests that cow dung application mainly reduces Cd uptake by wheat roots by decreasing soil Cd bioavailability. In contrast, Cd translocation to the aboveground parts of wheat is affected by a complex set of factors that are less affected by changes in soil Cd morphology (Nocito et al., 2011).
4.2.2 Microbial community structure
In this study, cow dung application had a significant effect on soil microorganisms, likely altering the relative abundance of soil microbial phyla and genera and reducing the bioavailability of Cd. First, the application of cow dung can directly increase the relative abundance of microorganisms, such as Pseudomonadota, and also indirectly elevate the relative abundance of microorganisms, such as Mycolicibacterium, Bradyrhizobium and other microorganisms, by enhancing the growth and multiplication of microorganisms through the addition of soil nutrients (Tagele et al., 2023). The significant negative correlation of Pseudomonadota with AcdiExt-Cd and BF-Cd in this study indicates that the increase in Pseudomonadota abundance after cow dung application is beneficial to the decrease in Cd bioavailability. This phenomenon may be due to the significant OM degrading ability of Pseudomonadota to degrade lignin and cellulose to negatively charged fatty acids, which can combine with heavy metal cations to form stable compounds (Cui et al., 2020; Klimek et al., 2024). Second, Bradyrhizobium was positively correlated with various soil nutrients and negatively correlated with AcadiExt-Cd and BF-Cd, suggesting that an increase in Bradyrhizobium abundance benefited the decrease in Cd bioavailability. This result is likely because microorganisms, such as Bradyrhizobium, are inherently Cd-resistant (Duan et al., 2020). Numerous studies have reported that microorganisms with heavy metal resistance can reduce heavy metal bioefficacy through biosorption, redox, and other means (Silver and Phung, 1996; Khudur et al., 2019; Insuk et al., 2020; Sujkowska-Rybkowska et al., 2020). Notably, the present study also found a significant negative correlation between Mycolicibacterium and BF-Cd, which might be due to the fact that cow dung increased the relative abundance of Mycolicibacterium, which belongs to the group of Gram-positive bacteria with phosphomuconic acid on their cell wall surfaces. These bacteria are negatively charged and can reduce cationic metal activity through electrostatic adsorption, thereby reducing the bioavailability of Cd (Jacob et al., 2018). Overall, soil microorganisms can reduce soil heavy metal bioavailability through metabolism, expression of resistance genes or direct adsorption. In addition, the relative abundance of the soil microorganisms was found to be significantly correlated with Cd in wheat, suggesting that changes in soil microbial abundance reduced Cd uptake by wheat by reducing the bioavailability of Cd.
4.2.3 MRGs
The increase in the abundance of some MRGs after cow dung application may also explain the decrease in Cd bioavailability. MRGs were significantly correlated with soil chemical properties dominated by soil nutrients and relative microbial abundance; the selective pressures and coexisting microbial interactions in heavy metal-contaminated environments may contribute to gene transfer and changes in the abundance of MRGs (He et al., 2023). In this study, the abundance of nine MRGs, including actR and actS, was found to be significantly negatively correlated with Cd bioavailability, suggesting that the enrichment of multiple MRGs was conducive to the reduction of heavy metal bioavailability, achieved through a series of complex biological reactions, including biosorption, complexation, and redox (Xavier et al., 2019). Here, actR and actS have acid-resistant capabilities that mitigate small-scale pH decreases in soils by maintaining hydrogen ion balance, thereby reducing the conversion of HMs to bioavailable forms (Liu et al., 2015). fpvA is located in the extracellular membrane and can form complexes with other proteins and bind heavy metal ions, such as Cd2+, Zn2+, and Cu2+, thereby reducing heavy metal bioavailability (Braud et al., 2009). Notably, fpvA was also significantly negatively correlated with AcidExt-Cd, Res-Cu, and Res-Zn, suggesting that the increase in the bioavailability of Cu and Zn might have contributed to the enrichment of fpvA, which in turn reduced the bioavailability of Cd (Braud et al., 2009). In addition, MRGs were significantly correlated with wheat Cd content, again suggesting that changes in the abundance of MRGs were beneficial for reducing soil Cd bioavailability.
4.3 Excessive application of cow dung May cause damage to the microbiological system
4.3.1 Reduction of microbial diversity by excessive application of cow dung
In this study, the application rates of cow dung should not be excessive. First, rarer microorganisms, such as Mycetohabitans, Tannerella, and Rhodopirellula, disappeared from the groups with cow dung application rates of 15% and above (Supplementary Table S5). This result was likely due to the introduction of microorganisms from cow dung into the soil exacerbated microbial competition, causing rare microbial genera to be filtered out of competition and thus reducing microbial diversity and disrupting the microbial ecosystem (Chen et al., 2021). Second, cow dung increased the bioavailability of copper and zinc (AcidExt + Red). Microbial diversity is often used as an indicator of metal contamination (Xiang et al., 2024; El-Sharkawy et al., 2025; Wang et al., 2025), and increased bioavailability of Cu and Zn may exacerbate soil pollution, lead to changes in soil microbial communities, and reduce microbial diversity [58]. In this study, Shannon index was significantly negatively correlated with AcidExt-Cu and BF-Zn, suggesting that higher levels of Cu and Zn in cow dung may reduce soil microbial diversity and thus cause damage to the microbial system.
4.3.2 Excessive application of cow dung affects MRG expression
Here, we found that BF-Cu and BF-Zn was significantly correlated with various MRGs. Increased copper and zinc bioavailability may affect the expression of MRGs, which indirectly affects the colonisation of microorganisms with resistance genes (He et al., 2023). Abnormal growth and reproduction of microorganisms in a defined environment affects the space for other microorganisms to grow, leading to a reduction in microbial diversity and disruption of microbial ecosystems (Ghoul and Mitri, 2016). In this study, AcidExt-Cu was significantly and positively correlated with copC and actA. The increased effectiveness of Cu may lead to considerable production of metal-binding proteins by copC and substantial expression of actA to prevent localized low pH-induced Cu toxicity (Cha and Cooksey, 1991; Tiwari et al., 1996). These studies suggest that the increase in the bioavailability of Cu and Zn may lead to an overenrichment of MRGs. Overexpression of MRGs may cause an imbalance in the expression of antibiotic resistance genes and mobile genetic elements, resulting in a potential risk of soil environmental contamination. resulting in damage to the microbial system (Engin et al., 2023). In addition, microorganisms, such as Bacillota and Nocardioides, are significantly correlated with various MRGs, such as copA and czcD, suggesting that an increase in the bioavailability of Cu and Zn can also indirectly alter the abundance of heavy metal resistance genes by affecting the relative abundance of soil microbes (Guo et al., 2022). Meanwhile, Microbial diversity is also a key factor in the expression of MRGs (Zeng et al., 2023). In this study, we observed that the Shannon index was significantly positively correlated with MRGs such as yebM and copZ, and negatively correlated with AcidExt-Cu and BF-Zn. This finding suggests that an increase in the bioavailability of Cu and Zn may have reduced the microbial diversity, lowering the expression of some MRGs.
4.4 Limitations
A pot experiment was used in this study with the following limitations: first, potting experiments greatly simplify the complexity of actual agroecosystems, where unnatural inter-root environments and controlled irrigation may result in soil microorganisms that differ significantly from field soils (Johnson et al., 2017). Secondl field experiments usually have a longer period of time, and the stability of the experimental results is higher, and it is difficult to replicate pot experiments (Wan et al., 2020; Zhao et al., 2022; Wu et al., 2023). Third, pot experiments are spatially limited and do not reflect spatial differences in restoration effects in the field (Mulyani et al., 2022). With clear limitations, the data and results provided in this study are still of important reference value, which efficiently compare the remediation effects of different cow dung application rates on Cd-contaminated soils and the interaction effects of heavy metal bioavailability and environmental factors, and can and provide a pre-basis and scientific support for the design of subsequent field experiments.
5 Conclusion
In this study, we found that the application of cow dung was effective in reducing the bioavailability of Cd, thereby reducing Cd uptake by wheat. Application of cow dung significantly reduced Cd content in all parts of wheat, especially leaves and grains. Correlation analysis showed that changes in soil chemical properties (such as AP and SOM), microbial community abundance (such as Bradyrhizobium spp. and Mycolicibacterium spp.) and the abundance of MRGs (such as actA and fpvA) contributed to the reduction of the bioavailable forms of Cd. Based on antagonism, increased Zn bioavailability also decreased Cd uptake in wheat. However, microorganisms and higher levels of Cu and Zn introduced in cow dung could also be potentially harmful to the soil environment. Microorganisms introduced with cow dung increase microbial competition in the soil and may result in a decrease in microbial diversity. Increased bioavailability of Cu and Zn can also disrupt the pre-existing microbial ecosystems, leading to reduced microbial diversity and affecting the expression of functional microbial genes. In conclusion, HMs bioavailability interacts with environmental factors, and cow dung applied at different rates will have different impacts on the soil environment. Therefore, application rates of cow dung should be carefully selected to avoid excessive application. Considering the Cd content of wheat grains and soil microbial diversity, the application of cow dung should not exceed 2.5% (w/w). This study provides a scientific basis for the safe use of cow dung and the remediation of heavy metals in wheat fields.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
GL: Data curation, Investigation, Visualization, Writing – original draft. ZH: Supervision, Writing – original draft. JS: Data curation, Project administration, Writing – original draft. CL: Data curation, Funding acquisition, Writing – original draft. HS: Investigation, Methodology, Writing – original draft. YF: Funding acquisition, Writing – original draft. JL: Data curation, Writing – original draft. YM: Resources, Writing – original draft. HW: Writing – review and editing. RZ: Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Key Research and Development Program of China (2023YFD1701805) and the Youth Science and Technology Innovation Fund Program of Technical Centre for Soil, Agriculture and Rural Ecology and Environment, Ministry of Ecology and Environment (QKC2024011).
Acknowledgments
The authors thank the financial support from Chenfeng Liu and YF. We also thank the Huarui Agricultural Company for providing experimental sites.
Conflict of interest
Author YM was employed by Huarui Agricultural Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1675922/full#supplementary-material
References
Aina, O. E., Mugivhisa, L. L., Olowoyo, J. O., and Obi, C. L. (2024). Heavy metals and potential health risk assessment of Lactuca sativa and Daucus carrota from soil treated with organic manures and chemical fertilizer. Environ. Monit. Assess. 196, 538–15. doi:10.1007/s10661-024-12687-y
Azhar, M., Zia Ur Rehman, M., Ali, S., Qayyum, M. F., Naeem, A., Ayub, M. A., et al. (2019). Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 227, 72–81. doi:10.1016/j.chemosphere.2019.04.041
Bao, S. D. (2000). Soil and agricultural chemistry analysis. 3rd Edn. Beijing: China Agriculture Press. Available online at: http://www.researchgate.net/publication/301822463_Soil_and_agricultural_chemistry_analysis (Accessed March 5, 2024).
Braud, A., Hoegy, F., Jezequel, K., Lebeau, T., and Schalk, I. J. (2009). New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ. Microbiol. 11, 1079–1091. doi:10.1111/j.1462-2920.2008.01838.x
Cao, X., Ma, L. Q., Rhue, D. R., and Appel, C. S. (2004). Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 131, 435–444. doi:10.1016/j.envpol.2004.03.003
Cha, J. S., and Cooksey, D. A. (1991). Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. U. S. A. 88, 8915–8919. doi:10.1073/pnas.88.20.8915
Chen, S. B., Zhu, Y. G., and Ma, Y. B. (2006). The effect of grain size of rock phosphate amendment on metal immobilization in contaminated soils. J. Hazard. Mater. 134, 74–79. doi:10.1016/j.jhazmat.2005.10.027
Chen, Q., Hu, H., Yan, Z., Li, C., Nguyen, B. T., Zheng, Y., et al. (2021). Termite mounds reduce soil microbial diversity by filtering rare microbial taxa. Environ. Microbiol. 23, 2659–2668. doi:10.1111/1462-2920.15507
Clemente, R., Paredes, C., and Bernal, M. (2007). A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in a contaminated calcareous soil from Murcia (Spain). Agric. Ecosyst. and Environ. 118, 319–326. doi:10.1016/j.agee.2006.06.002
Cui, H., Ou, Y., Wang, L., Yan, B., Li, Y., and Ding, D. (2020). The passivation effect of heavy metals during biochar-amended composting: emphasize on bacterial communities. Waste Manag. 118, 360–368. doi:10.1016/j.wasman.2020.08.043
Duan, C., Liu, Y., Zhang, H., Chen, G., and Song, J. (2020). Cadmium pollution impact on the bacterial community of haplic cambisols in northeast China and inference of resistant genera. J. Soil Sci. Plant Nutr. 20, 1156–1170. doi:10.1007/s42729-020-00201-5
El-Sharkawy, G., Alotaibi, M. O., Zuhair, R., Mahmoud, E., El Baroudy, A., Omara, A. E.-D., et al. (2025). Ecological assessment of polluted soils: linking ecological risks, soil quality, and biota diversity in contaminated soils. Sustainability 17, 1524. doi:10.3390/su17041524
Engin, A. B., Engin, E. D., and Engin, A. (2023). Effects of co-selection of antibiotic-resistance and metal-resistance genes on antibiotic-resistance potency of environmental bacteria and related ecological risk factors. Environ. Toxicol. Pharmacol. 98, 104081. doi:10.1016/j.etap.2023.104081
Fu, T., Zhang, B., Gao, X., Cui, S., Guan, C.-Y., Zhang, Y., et al. (2023). Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci. Total Environ. 856, 158810. doi:10.1016/j.scitotenv.2022.158810
Ghoul, M., and Mitri, S. (2016). The ecology and evolution of microbial competition. Trends Microbiol. 24, 833–845. doi:10.1016/j.tim.2016.06.011
Guo, H., Liu, H., and Wu, S. (2022). Immobilization pathways of heavy metals in composting: interactions of microbial community and functional gene under varying C/N ratios and bulking agents. J. Hazard. Mater. 426, 128103. doi:10.1016/j.jhazmat.2021.128103
Haider, F. U., Farooq, M., Naveed, M., Cheema, S. A., Ain, N. U., Salim, M. A., et al. (2022). Influence of biochar and microorganism co-application on stabilization of cadmium (Cd) and improved maize growth in Cd-contaminated soil. Front. Plant Sci. 13, 983830. doi:10.3389/fpls.2022.983830
He, Z., Shen, J., Li, Q., Yang, Y., Zhang, D., and Pan, X. (2023). Bacterial metal(loid) resistance genes (MRGs) and their variation and application in environment: a review. Sci. Total Environ. 871, 162148. doi:10.1016/j.scitotenv.2023.162148
Hong, Y., Li, D., Xie, C., Zheng, X., Yin, J., Li, Z., et al. (2022). Combined apatite, biochar, and organic fertilizer application for heavy metal co-contaminated soil remediation reduces heavy metal transport and alters soil microbial community structure. Sci. Total Environ. 851, 158033. doi:10.1016/j.scitotenv.2022.158033
Hultman, J., Waldrop, M. P., Mackelprang, R., David, M. M., McFarland, J., Blazewicz, S. J., et al. (2015). Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 521, 208–212. doi:10.1038/nature14238
Hussain, A., Ali, S., Rizwan, M., Rehman, M. Z. U., Qayyum, M. F., Wang, H., et al. (2019). Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 173, 156–164. doi:10.1016/j.ecoenv.2019.01.118
Insuk, C., Kuncharoen, N., Cheeptham, N., Tanasupawat, S., and Pathom-aree, W. (2020). Bryophytes harbor cultivable actinobacteria with plant growth promoting potential. Front. Microbiol. 11, 563047. doi:10.3389/fmicb.2020.563047
Jacob, J. M., Karthik, C., Saratale, R. G., Kumar, S. S., Prabakar, D., Kadirvelu, K., et al. (2018). Biological approaches to tackle heavy metal pollution: a survey of literature. J. Environ. Manag. 217, 56–70. doi:10.1016/j.jenvman.2018.03.077
Jamali, M. K., Kazi, T. G., Arain, M. B., Afridi, H. I., Jalbani, N., Kandhro, G. A., et al. (2009). Heavy metal accumulation in different varieties of wheat (Triticum aestivum L.) grown in soil amended with domestic sewage sludge. J. Hazard. Mater. 164, 1386–1391. doi:10.1016/j.jhazmat.2008.09.056
Johnson, S. P., Miller, Z. J., Lehnhoff, E. A., Miller, P. R., and Menalled, F. D. (2017). Cropping systems modify soil biota effects on wheat (Triticum aestivum) growth and competitive ability. Weed Res. 57, 6–15. doi:10.1111/wre.12231
Khudur, L. S., Shahsavari, E., Webster, G. T., Nugegoda, D., and Ball, A. S. (2019). The impact of lead co-contamination on ecotoxicity and the bacterial community during the bioremediation of total petroleum hydrocarbon-contaminated soils. Environ. Pollut. 253, 939–948. doi:10.1016/j.envpol.2019.07.107
Kibria, K. Q., Islam, M. A., Hoque, S., Hossain, M. Z., and Islam, M. A. (2023). Effect of organic amendments on cadmium bioavailability in soil and its accumulation in rice grain. Bull. Environ. Contam. Toxicol. 110, 74–79. doi:10.1007/s00128-023-03717-5
Klimek, D., Herold, M., and Calusinska, M. (2024). Comparative genomic analysis of Planctomycetota potential for polysaccharide degradation identifies biotechnologically relevant microbes. BMC Genomics 25, 523. doi:10.1186/s12864-024-10413-z
Li, L., Zhang, Y., Ippolito, J. A., Xing, W., Qiu, K., and Yang, H. (2020). Lead smelting effects heavy metal concentrations in soils, wheat, and potentially humans. Environ. Pollut. 257, 113641. doi:10.1016/j.envpol.2019.113641
Liao, B., Guo, Z., Probst, A., and Probst, J.-L. (2005). Soil heavy metal contamination and acid deposition: experimental approach on two forest soils in Hunan, Southern China. Geoderma 127, 91–103. doi:10.1016/j.geoderma.2004.11.019
Liu, T., Tian, C. F., and Chen, W. X. (2015). Site-specific ser/thr/tyr phosphoproteome of sinorhizobium meliloti at stationary phase. PLoS ONE 10, e0139143. doi:10.1371/journal.pone.0139143
Liu, W.-R., Zeng, D., She, L., Su, W.-X., He, D.-C., Wu, G.-Y., et al. (2020). Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 734, 139023. doi:10.1016/j.scitotenv.2020.139023
Lu, R. K. (1999). Soil and agro-chemistry analytical methods. China Agric. Sci. Technol. Press. Available online at: http://www.researchgate.net/publication/284071334_Soil_and_agro-chemistry_analytical_methods_M (Accessed May 11, 2024).
Lu, J., Breitwieser, F. P., Thielen, P., and Salzberg, S. L. (2017). Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 3, e104. doi:10.7717/peerj-cs.104
Ma, M., Xu, X., Ha, Z., Su, Q., Lv, C., Li, J., et al. (2023). Deep insight on mechanism and contribution of arsenic removal and heavy metals remediation by mechanical activation phosphogypsum. Environ. Pollut. 336, 122258. doi:10.1016/j.envpol.2023.122258
Mu, H.-Y., Zhuang, Z., Li, Y.-M., Qiao, Y.-H., Chen, Q., Xiong, J., et al. (2020). Heavy metal contents in animal manure in China and the related soil accumulation risks. Environ. Sci. 41, 986–996. doi:10.13227/j.hjkx.201903078
Mulyani, O., Joy, B., and Kurnia, D. (2022). The various forms of cow manure waste as adsorbents of heavy metals. Appl. Sci. 12, 5763. doi:10.3390/app12115763
Nemati, K., Bakar, N. K. A., Abas, M. R., and Sobhanzadeh, E. (2011). Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 192, 402–410. doi:10.1016/j.jhazmat.2011.05.039
Nocito, F. F., Lancilli, C., Dendena, B., Lucchini, G., and Sacchi, G. A. (2011). Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell and Environ. 34, 994–1008. doi:10.1111/j.1365-3040.2011.02299.x
Rahi, A. A., Younis, U., Ahmed, N., Ali, M. A., Fahad, S., Sultan, H., et al. (2022). Toxicity of Cadmium and nickel in the context of applied activated carbon biochar for improvement in soil fertility. Saudi J. Biol. Sci. 29, 743–750. doi:10.1016/j.sjbs.2021.09.035
Rizwan, M., Ali, S., Abbas, T., Zia-ur-Rehman, M., Hannan, F., Keller, C., et al. (2016). Cadmium minimization in wheat: a critical review. Ecotoxicol. Environ. Saf. 130, 43–53. doi:10.1016/j.ecoenv.2016.04.001
Sauvé, S., Hendershot, W., and Allen, H. E. (2000). Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 34, 1125–1131. doi:10.1021/es9907764
Silver, S., and Phung, L. T. (1996). Bacterial heavy metal resistance: new Surprises. Annu. Rev. Microbiol. 50, 753–789. doi:10.1146/annurev.micro.50.1.753
Sujkowska-Rybkowska, M., Banasiewicz, J., Rekosz-Burlaga, H., and Stępkowski, T. (2020). Anthyllis vulneraria and Lotus corniculatus on calamine heaps form nodules with Bradyrhizobium liaoningense-related strains harboring novel in Europe symbiotic nifD haplotypes. Appl. Soil Ecol. 151, 103539. doi:10.1016/j.apsoil.2020.103539
Sun, Y., Li, H., Guo, G., Semple, K. T., and Jones, K. C. (2019). Soil contamination in China: current priorities, defining background levels and standards for heavy metals. J. Environ. Manag. 251, 109512. doi:10.1016/j.jenvman.2019.109512
Tagele, S. B., Kim, R.-H., Jeong, M., Lim, K., Jung, D.-R., Lee, D., et al. (2023). Soil amendment with cow dung modifies the soil nutrition and microbiota to reduce the ginseng replanting problem. Front. Plant Sci. 14, 1072216. doi:10.3389/fpls.2023.1072216
Tiwari, R. P., Reeve, W. G., Dilworthan, M. J., and Glenn, A. R. (1996). An essential role for actA in acid tolerance of rhizobium melilotix. Microbiol. Read. 142 (Pt 3), 601–610. doi:10.1099/13500872-142-3-601
Valipour, M., Shahbazi, K., and Khanmirzaei, A. (2016). Chemical immobilization of lead, cadmium, copper, and nickel in contaminated soils by phosphate amendments. Clean. Soil Air Water 44, 572–578. doi:10.1002/clen.201300827
Wan, Y., Huang, Q., Wang, Q., Yu, Y., Su, D., Qiao, Y., et al. (2020). Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 384, 121293. doi:10.1016/j.jhazmat.2019.121293
Wang, Z., Chai, L., Yang, Z., Wang, Y., and Wang, H. (2010). Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, changsha, China. J. Environ. Qual. 39, 1616–1623. doi:10.2134/jeq2010.0007
Wang, N., Wu, X., Liao, P., Zhang, J., Liu, N., Zhou, Z., et al. (2021). Morphological transformation of heavy metals and their distribution in soil aggregates during biotransformation of livestock manure. Biocatal. Agric. Biotechnol. 32, 101963. doi:10.1016/j.bcab.2021.101963
Wang, Y., Zhang, Y., Li, J., Lin, J.-G., Zhang, N., and Cao, W. (2021). Biogas energy generated from livestock manure in China: current situation and future trends. J. Environ. Manag. 297, 113324. doi:10.1016/j.jenvman.2021.113324
Wang, Y., Xing, W., Liang, X., Xu, Y., Wang, Y., Huang, Q., et al. (2022). Effects of exogenous additives on wheat Cd accumulation, soil Cd availability and physicochemical properties in Cd-contaminated agricultural soils: a meta-analysis. Sci. Total Environ. 808, 152090. doi:10.1016/j.scitotenv.2021.152090
Wang, H., Liu, H., Li, J., Chen, S., Uz Zaman, Q., Sultan, K., et al. (2024). Combined passivators regulate physiological, antioxidant potential and metals accumulation in potato grown in metals contaminated soil. Sci. Total Environ. 912, 168956. doi:10.1016/j.scitotenv.2023.168956
Wang, Z., Deng, G., Hu, C., Hou, X., Zhang, X., Fan, Z., et al. (2025). Microbial diversity and community assembly in heavy metal-contaminated soils: insights from selenium-impacted mining areas. Front. Microbiol. 16, 1561678. doi:10.3389/fmicb.2025.1561678
Wu, P., Guo, Z., Hua, K., and Wang, D. (2023). Long-term application of organic amendments changes heavy metals accumulation in wheat grains by affecting soil chemical properties and wheat yields. J. Soils Sediments 23, 2136–2147. doi:10.1007/s11368-023-03473-3
Xavier, J. C., Costa, P. E. S., Hissa, D. C., Melo, V. M. M., Falcão, R. M., Balbino, V. Q., et al. (2019). Evaluation of the microbial diversity and heavy metal resistance genes of a microbial community on contaminated environment. Appl. Geochem. 105, 1–6. doi:10.1016/j.apgeochem.2019.04.012
Xiang, Y., Liu, Y., Niazi, N. K., Bolan, N., Zhao, L., Zhang, S., et al. (2023). Biochar addition increased soil bacterial diversity and richness: large-scale evidence of field experiments. Sci. Total Environ. 893, 164961. doi:10.1016/j.scitotenv.2023.164961
Xiang, Y., Lan, J., Dong, Y., Zhou, M., Hou, H., and Huang, B.-T. (2024). Pollution control performance of solidified nickel-cobalt tailings on site: bioavailability of heavy metals and microbial response. J. Hazard. Mater. 471, 134295. doi:10.1016/j.jhazmat.2024.134295
Xu, Y., Seshadri, B., Sarkar, B., Wang, H., Rumpel, C., Sparks, D., et al. (2018). Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 621, 148–159. doi:10.1016/j.scitotenv.2017.11.214
Yin, K., Wang, Q., Lv, M., and Chen, L. (2019). Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 360, 1553–1563. doi:10.1016/j.cej.2018.10.226
You, M., Hu, Y., and Meng, Y. (2023). Chemical speciation and bioavailability of potentially toxic elements in surface sediment from the Huaihe River, Anhui Province, China. Mar. Pollut. Bull. 188, 114616. doi:10.1016/j.marpolbul.2023.114616
Yuan, K., Xiong, S., Liang, J., Li, Y., Qiao, Y., Li, H., et al. (2020). Status and risk analysis of copper and zinc pollution in livestock manure. J. Agro-Environment Sci. 39, 1837–1842. doi:10.11654/jaes.2020-0142
Zeng, J., Pan, Y., Hu, R., Liu, F., Gu, H., Ding, J., et al. (2023). The vertically-stratified resistomes in mangrove sediments was driven by the bacterial diversity. J. Hazard. Mater. 458, 131974. doi:10.1016/j.jhazmat.2023.131974
Zhang, X., Yu, Q., Gao, B., Hu, M., Chen, H., Liang, Y., et al. (2025). Organic amendments enhance the remediation potential of economically important crops in weakly alkaline heavy metal-contaminated bauxite residues. Agriculture 15, 15. doi:10.3390/agriculture15010015
Zhao, W., Deng, J., Chi, S., Wang, W., Xu, L., Huang, Q., et al. (2022). Sustainability assessment of topsoil ecology in Chongqing, China based on the application of livestock and poultry manure. J. Clean. Prod. 358, 131969. doi:10.1016/j.jclepro.2022.131969
Zhong, X., Chen, Z., Li, Y., Ding, K., Liu, W., Liu, Y., et al. (2020). Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 400, 123289. doi:10.1016/j.jhazmat.2020.123289
Zhou, M., and Li, Z. (2022). Recent advances in minimizing cadmium accumulation in wheat. Toxics 10, 187. doi:10.3390/toxics10040187
Keywords: cow dung, heavy metals, soil chemical properties, soil microorganisms, heavy metal resistance genes
Citation: Liu G, Han Z, Sun J, Liu C, Shi H, Fei Y, Liang J, Mu Y, Wang H and Zhang R (2025) Remediation of cadmium-contaminated wheat soil with cow dung: interactions between soil chemical properties, microbial communities, functional genes, and heavy metal bioavailability. Front. Environ. Sci. 13:1675922. doi: 10.3389/fenvs.2025.1675922
Received: 29 July 2025; Accepted: 20 August 2025;
Published: 04 September 2025.
Edited by:
Khandaker Rayhan Mahbub, Primary Industries and Resources South Australia, AustraliaReviewed by:
Qin Yao, Heilongjiang Bayi Agricultural University, ChinaHaichao Fu, Henan Agricultural University, China
Copyright © 2025 Liu, Han, Sun, Liu, Shi, Fei, Liang, Mu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Zhang, emhhbmdyb25nQHRjYXJlLW1lZS5jbg==; Hongjie Wang, d2FuZ2hqQGhidS5lZHUuY24=
 Getong Liu
Getong Liu Ziyu Han2
Ziyu Han2