- 1Engineering School for Sustainable Infrastructure and Environment, University of Florida, Gainesville, FL, United States
- 2Marine Laboratory, Sanibel Captiva Conservation Foundation, Sanibel, FL, United States
- 3Nicholas School of the Environment, Duke University, Durham, NC, United States
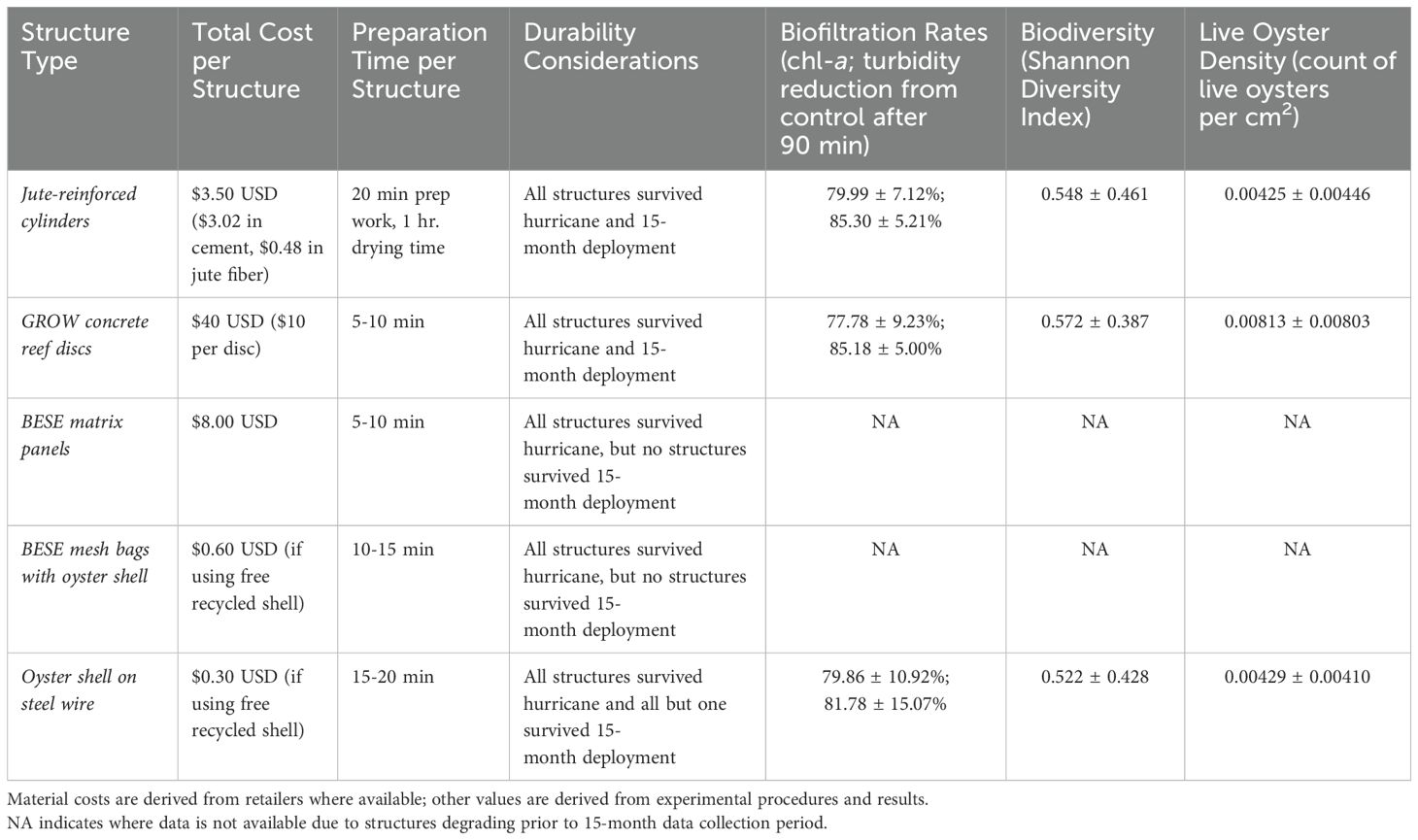
Oyster gardening, in which hanging oyster recruitment substrates are suspended from docks, has become an increasingly common and accessible technique for coastal communities to support local oyster populations for biodiversity enhancement, habitat restoration, and ecological functions including water filtration. However, little research has been done to evaluate materials and methods for oyster garden structures regarding cost, ease of assembly, durability, and ecosystem benefits, making it difficult to scale up efforts and maximize project success and sustainability. We conducted a field experiment in a residential canal system on Sanibel Island, Florida where we deployed a variety of oyster garden structure types to evaluate their performance in oyster recruitment, durability, water filtration rate, and biodiversity. Additionally, the occurrence of Hurricane Ian during the deployment provided an opportunity to evaluate how these structures resisted severe storm events. We tested a total of five structures: (1) a conventional design made of drilled oyster shell on steel wire (shell structures); and four alternatives, (2) GROW concrete discs (disc structures); (3) jute fiber coated with calcium sulfoaluminate cement (jute structures); (4) BESE biodegradable plastic matrix panels (BESE matrix panel structures); and (5) BESE biodegradable plastic mesh bags filled with oyster cultch (bag structures). All structures survived Hurricane Ian; however, both BESE structure types ultimately disintegrated without recruiting oysters. Disc, jute, and shell wire structures demonstrated similarly high levels of durability, oyster recruitment and growth, and biofiltration. Thus, we conclude that structure type selection may be based on material and labor availability and whether cost and biodegradability are prioritized. We show that oyster gardening can provide ecosystem services, including biofiltration, in residential canal sites that have lost “natural” shoreline morphology. Investments in oyster gardening are low risk in the face of natural hazards, supporting the use of the practice in storm-prone areas. However, residential canals are prone to adverse water quality, including low dissolved oxygen, which we show may undermine oyster survival and growth in certain cases; location and season thus need to be considered for garden deployment. Our results reveal material options for providing sustainability, durability, oyster recruitment, and biodiversity for oyster gardening projects while minimizing adverse environmental impacts.
1 Introduction
Coastal communities are increasingly under threat from a suite of environmental hazards, including habitat and water quality degradation, necessitating interventions that promote habitat resilience and bolster ecosystem functioning (Morris et al., 2018; Smith et al., 2020). Nature-based solutions (NBS) are one such intervention type that weaves natural systems and processes into the built environment to promote ecosystem services and resilience for the benefit of both humans and nature (Bridges et al., 2021). Oyster reef restoration is a form of coastal NBS which addresses the urgent issue of oyster reef loss. Oyster reefs provide valuable ecosystem services including habitat provisioning, sediment stabilization, and water quality improvement, but over 85% of oyster reefs have been lost globally in the last 200 years due largely to anthropogenic pressures, amplifying the need for more creative, large-scale alternative approaches to restoration interventions (Jamil et al., 2024; Xu et al., 2024).
While coastal NBS have expanded substantially in the last ten years, at this early stage, there are currently no widely agreed upon guidelines for project design and implementation to meet many aspects of project needs (Smith et al., 2020). Coastal NBS often involve the deployment of physical structures designed to enhance the recruitment and growth of foundation species, which physically modify their habitat, to address hazards and enhance ecosystem functioning. These structures often employ materials characteristic of traditional terrestrial construction and restoration techniques, but their application in marine environments with unique environmental conditions and engineering goals, as well as the application of novel materials deployed without adequate evaluation of their durability, may undermine project performance and sustainability goals (Barry, 2021; Palinkas et al., 2022). Novel materials, including reduced-impact artificial alternatives to conventional materials such as concrete and plastic, are currently being developed for use in NBS, including in oyster restoration (Goelz et al., 2020; Sakr and Altieri, 2025). Rigorous, systematic testing of these materials is necessary to improve sustainable practices and project performance (i.e., ecosystem service enhancement) while minimizing adverse effects such as material degradation impacts (Sakr and Altieri, 2025).
Oyster gardening is an oyster restoration technique that allows waterfront communities to enhance water quality and habitat availability along developed shorelines. Historically, oyster gardening was defined as the practice of placing hard substrate on the benthos to encourage oyster settlement and recruitment. This form of oyster gardening has been promoted along the Atlantic coast of the United States for decades; however, oyster gardening can take many other forms (Bersoza Hernández et al., 2018; Brumbaugh and Coen, 2009). One increasingly popular approach involves hanging substrates from docks and other over-water structures into the water column to support oyster recruitment (Brumbaugh and Coen, 2009; Hamilton et al., 2005). This technique often relies on a distributed management strategy whereby oyster gardens are maintained by local volunteers or civic groups, often at multiple locations including private docks and marinas (Brumbaugh and Coen, 2009). These oyster gardens can employ either substrate pre-seeded with oyster spat or bare substrate onto which oyster spat recruit naturally in the water column from nearby reefs (Brumbaugh and Coen, 2009; Griffin, 2016). Community-driven oyster gardening projects have been conducted throughout the United States, including in Virginia, Alabama, Florida, Maryland, New York, and Delaware, as well as in other countries such as Australia (Boström-Einarsson et al., 2022; Brumbaugh and Coen, 2009; Krasny et al., 2014; Oesterling and Petrone, 2012). Oyster garden substrates enhance the development of oyster communities which remove sediments and algae via biofiltration, reducing nutrient pollution and improving local water quality (Griffin, 2016; Marenghi and Ozbay, 2010). Oyster gardens have also been shown to locally enhance the biodiversity of fish and invertebrates and to provide critical nursery habitat (Marenghi and Ozbay, 2010). These services are particularly important in developed canal systems, where hardened seawalls and steep, deep cross-sections prevent the establishment of shallow-water habitats and limit ecosystem functionality. Once oysters have recruited to oyster gardens, they can be translocated to support larger-scale benthic reef restoration; additionally, oyster gardening provides opportunities for community engagement and education as well as oyster consumption by humans where water quality allows (Brumbaugh and Coen, 2009; Griffin, 2016; Oesterling and Petrone, 2012).
Hanging oyster gardens are constructed using a diverse range of structures and materials, as there is currently no standard design protocol for selecting among options (Hamilton et al., 2005). Common existing structures employ plastics, such as polyvinyl chloride (PVC) tubes or polyethylene aquaculture mesh bags; metal, such as gabion cages or recycled crab traps; or locally available materials such as recycled hockey sticks (Anderson et al., 2019; Hamilton et al., 2005; Marenghi and Ozbay, 2010; Rink2Reef®, 2024). Alternative oyster reef substrate materials have been increasingly used in coastal restoration applications, including in the state of Florida where biodegradable plastics, native stone, fiber mesh, and jute-cement structures have all seen successful use as part of reef enhancement efforts (Nitsch et al., 2021; Sakr and Altieri, 2025; Walters et al., 2022). The success of these materials supports the evaluation of their potential for expanded usage and functionality in oyster gardening applications. With oyster gardening still in the early stages of development and proliferation, it is particularly important that design aspects such as material selection be evaluated to ensure that future efforts are both as broadly sustainable and as functional as possible.
It is also important to understand the functionality of oyster garden structures across areas that vary in water quality to inform the placement and design of future efforts. Abiotic water quality factors such as salinity and dissolved oxygen (DO) can have large effects on the long-term success of restored oyster communities, while seasonality can further affect success based on both substrate installation time and the magnitude of water quality impacts during warm or rainy seasons (Anderson et al., 2019). Sufficient spat availability is also necessary for natural oyster establishment, though even in areas where adult oysters are able to survive and reproduce, water quality impairment may impede the survival of juvenile recruits (Brumbaugh and Coen, 2009; Morris et al., 2019).
Here, we tested the functional performance of five different oyster garden types to evaluate established and alternative structural designs: (1) drilled oyster shell on steel wire; (2) GROW concrete discs; (3) jute fiber coated with calcium sulfoaluminate cement; (4) BESE biodegradable plastic matrix panels; and (5) BESE biodegradable plastic mesh bags filled with oyster cultch (Figure 1). We selected this suite of structures to represent commonly available yet reduced-impact alternatives to conventionally used oyster substrate materials such as traditional concretes and plastics. We evaluated structure performance via durability, oyster recruitment and growth, biodiversity, and biofiltration. We carried out our experiment from docks in a residential canal system on Sanibel Island, FL, which is a typical setting for oyster garden projects. Hurricane Ian (Category 4) struck our study area during the deployment window, allowing us to evaluate the response of each structure type to intense physical stress and sustained water quality impairment. Our study allowed us to systematically evaluate the performance of a variety of structures and revealed differences in suitability among structure types for oyster gardening applications.
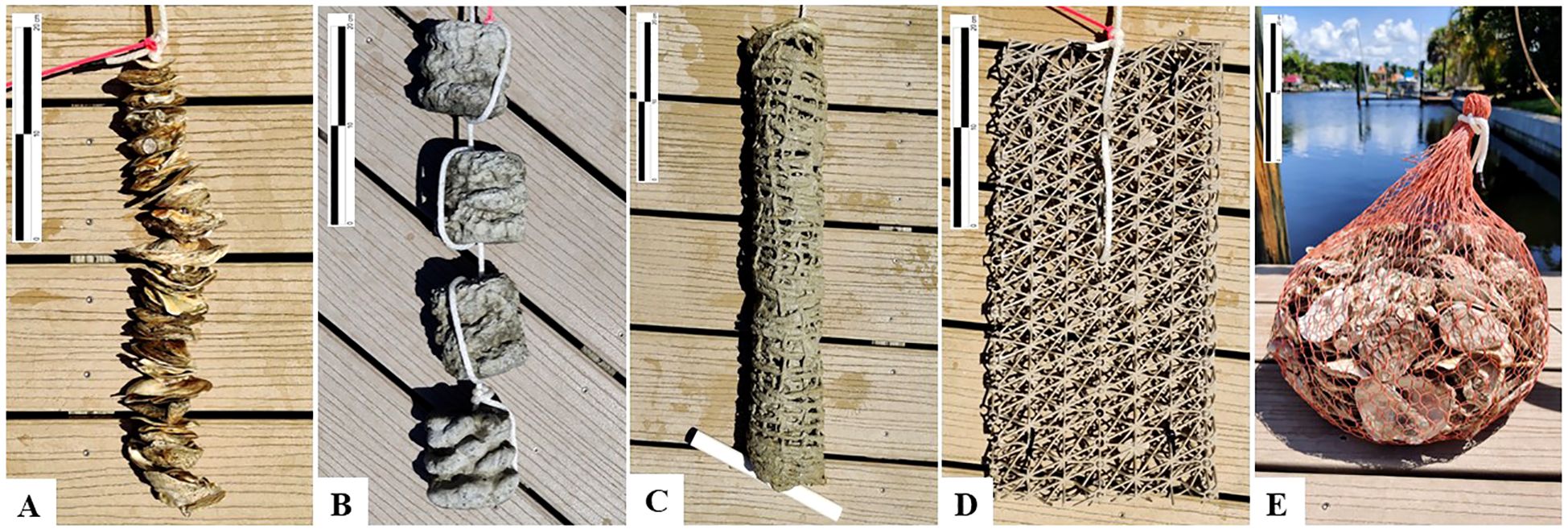
Figure 1. Photographs of tested structures prior to deployment: (A) oyster shell drilled onto 12-gauge steel wire (“wire” structure); (B) concrete disc structure (“disc” structure); (C) jute-reinforced calcium sulfoaluminate structure (“jute” structure); (D) BESE biodegradable plastic matrix panel structure (“matrix panel” structure); (E) BESE biodegradable plastic mesh bag filled with oyster cultch (“mesh bag” structure). Scale bars represent 20 cm.
2 Methods
2.1 Study system
This experiment was conducted in a constructed canal system on Sanibel Island, a barrier island in the southwest area of Florida, USA on the Gulf of Mexico coast (26°26’46.7”N 82°02’14.0”W). This residential canal system has a single outlet to San Carlos Bay in the north (Figure 2). Approximately half of island’s watershed (5 km2) empties through a control weir situated at the western end of the canal system (Flores and Albright, 2017) (Figure 2). Nutrient-rich runoff which enters the canal system through the control weir is unable to easily flush out of the system to the bay due to restricted flow. Thus, whenever discharges occur, the canal experiences a salinity and nutrient impairment gradient with lower water quality close to the weir and higher quality close to the outlet. Following intense rain events (>150 mm precipitation), chlorophyll-a levels at higher water quality areas during the summer wet season were observed at 5-15 µg/L, with DO levels at 3-5 mg/L and salinity levels at 22-27 ppt; at lower water quality areas during the same measurement window, chlorophyll-a was found to be as high as 50-60 µg/L, with DO levels < 1 mg/L and salinity levels <10 ppt (Thompson and Milbrandt, 2021). During dry season conditions, salinity and DO in the canal system were found to more closely reflect typical unimpaired levels for estuarine canals (Thompson and Milbrandt, 2021). Crassostrea virginica, the eastern oyster, which is the primary oyster species observed in this region, generally prefers salinity levels between 14-28 ppt, though it is able to tolerate brief exceedances of this range, as well as DO levels above 4 mg/L (Kennedy et al., 1996; Shepard et al., 2018). The concrete seawalls lining the canal system showed recruitment by various intertidal species, including live adult oysters and barnacles, from the mean high water line to the bottom of the canal (approximately 1.3 m depth) in both low and high water quality areas, suggesting viable levels of natural spat availability for oyster gardening.
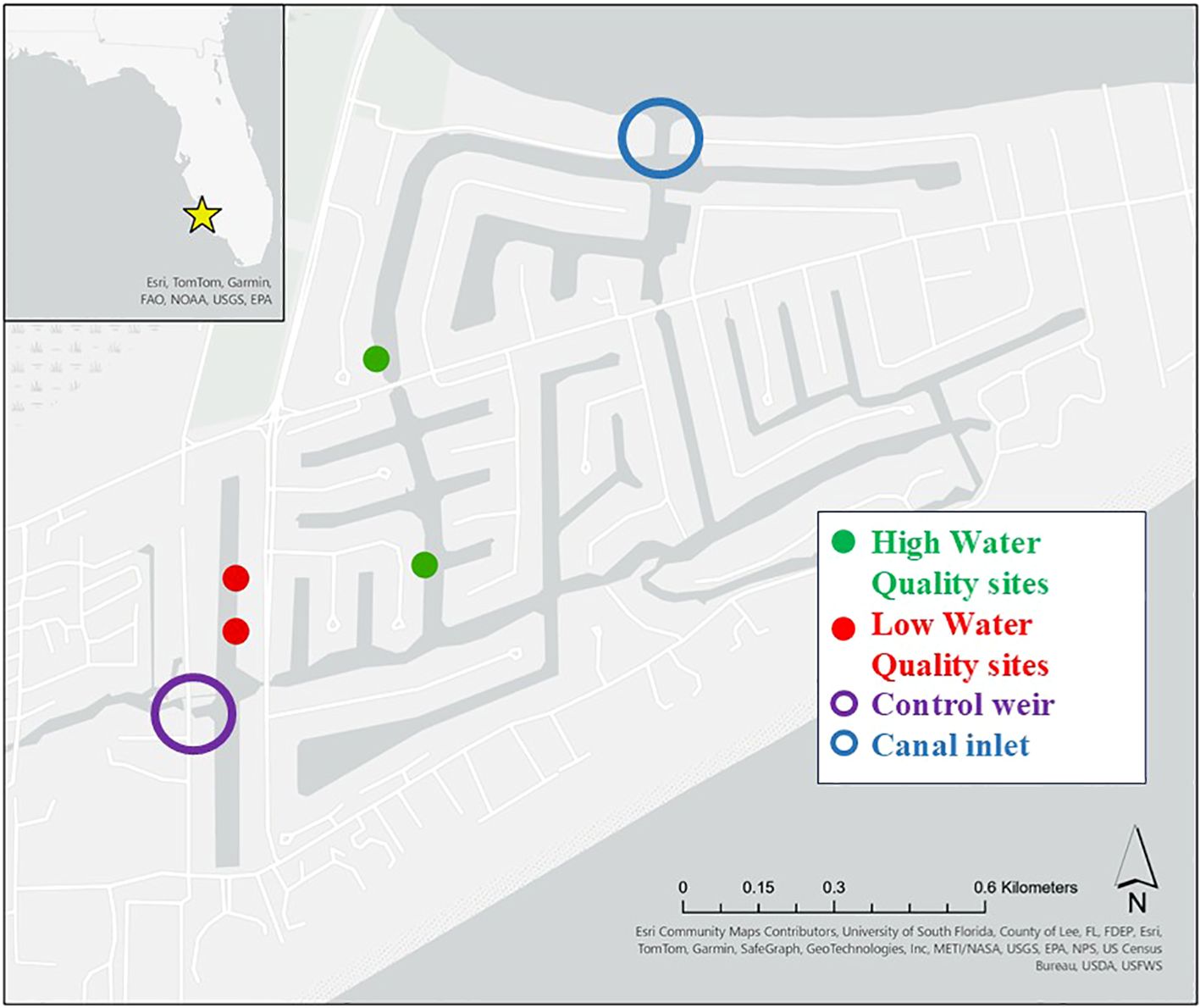
Figure 2. Map of the canal study system on the east end of Sanibel Island (26°26’46.7”N 82°02’14.0”W) showing the location of experimental structure deployment sites, the control weir, and the canal inlet.
2.2 Oyster garden structure types
The materials selected for this experiment were intended to represent a range of novel materials which are currently commonly available but are yet to be deployed at full scale in oyster gardening applications, as well as a conventional option in the wire shell structures (Figure 1). The materials tested also have reduced environmental impacts relative to conventional materials such as traditional concretes and plastics. BESE material was selected for its potential functional suitability and its precedented usage in other coastal restoration applications (Comba et al., 2022; Marin-Diaz et al., 2021; Nitsch et al., 2021). Jute-reinforced calcium sulfoaluminate cement and drilled oyster shell both employ materials commonly available in coastal areas which have also been used in other coastal restoration settings (Barry, 2020; Sanibel-Captiva Conservation Foundation, 2022). The concrete reef discs used represent a novel type and application of concrete formulation currently available for public purchase.
The wire shell garden structure (hereafter: wire structures) consists of oyster shell recycled from restaurants and aged (out of water for >90 days; Bushek et al., 2004). The shells have holes drilled in their centers and are strung onto to 12-gauge steel wire tied off at one end to form a structure 40 cm long and approximately 10 cm in diameter with a surface area of approximately 2,000 cm2 based on its exterior geometry. This structure type has been used in other oyster gardening projects in Florida and was included as a reference compared to the other more novel, less widely used materials (Sanibel-Captiva Conservation Foundation, 2022).
The concrete discs (hereafter: disc structures) are designed by the company GROW Oyster Reefs, LLC to enhance oyster recruitment and growth for oyster garden applications without using artificial aggregates or admixtures. Each disc is 9 cm high and 10 cm in diameter and weighs 2.3 kg. Each garden structure was made by tying four discs onto a polypropylene rope with 3 cm spacing to expose horizontal surfaces on the top and bottom of each disc, such that the total length was 46 cm with a total surface area of approximately 2,400 cm2 for four discs.
The jute-reinforced calcium sulfoaluminate cement tubes (hereafter: jute structures) were made by coating sheets of jute mesh with commercially available pre-mixed calcium sulfoaluminate cement and casting them on cylindrical forms to solidify. These tubes, which were 60 cm long and 10 cm in diameter with an exterior surface area of approximately 2,200 cm2 based on their geometry, had high porosity and rugosity intended to enhance oyster recruitment. The structures were secured vertically onto ropes using a short PVC pipe attached to the end of each rope to prevent them from sliding off; alternative materials such as wood could potentially have been used for this purpose.
The biodegradable plastic panels (hereafter: matrix panel structures) were manufactured by the Dutch company BESE using material sourced from post-processed potato starch waste (BESE-Elements, 2018). These matrix panel structures were made by cutting existing panels in half widthwise and affixing two halves front-to-back using built-in snapping features in order to increase rigidity, resulting in a structure measuring 45 cm long by 25 cm wide and 8 cm deep. Each panel was designed with a total surface area of 80 m2 including internal rugosity space (BESE-Elements, 2018). These panels are not explicitly designed for use as hanging oyster recruitment structures, and their inclusion in this experiment was intended to test the expansion of their application.
The biodegradable plastic oyster mesh bags (hereafter: bag structures) were made using a mesh biopolymer also manufactured by BESE using source material from post-processed potato starch waste (BESE Ecosystem Restoration Products, n.d.). Each bag consisted of two layers of 1 cm mesh BESE biopolymer filled with 6.8 kg of aged recycled oyster shell and secured with a plastic zip tie, ultimately forming a roughly spherical garden structure 40 cm in diameter with high internal rugosity.
2.3 Experimental design
We installed replicate structures at four sites (i.e., docks) in the canal system, with two sites in low water quality areas and two in high water quality areas (Figure 2). Two replicates of each of the five structure types were initially placed at each site, for ten structures in total per site and eight total replicates of each type across sites. Structures were suspended from docks from a polypropylene rope such that the top of each structure was 0.30 m below the mean high-water line, as determined using the upper limit of proximal barnacle recruitment (Kaye, 1964), and remained fully submerged during low tide. Average water depth in the canal system is approximately 1.3 m, and the minimum distance from the bottom of each suspended structure to the benthic sediment was approximately 0.75 m. Structures were randomly ordered at each study site in a line along the outer edge of each dock with approximately 1.5 meter spacing.
After initial installation of all structures in June 2022 to capture summer and early fall oyster recruitment peaks, subsequent monitoring occurred in Sept. 2022, Dec. 2022, July 2023, and Oct. 2023. Hurricane Ian (Category 4) occurred in late September 2022 between the September and December monitoring events. To quantify the survivorship of each structure type, the percentage remaining of each structure was determined at each time point; we defined a structure as surviving if at least 33% of the original mass was visibly remaining. Oyster density was calculated by dividing the total number of live oysters visibly observed on the exterior of each structure by the geometric surface area of each structure type. We used electronic calipers to measure oyster shell height from umbo to furthest margin edge (Martin and Hall, 2006; Shepard et al., 2018) for 50 randomly selected live oysters on each replicate structure; if fewer than 50 live oysters were present, all live oysters were measured. To quantify epifaunal biodiversity, we photographed the entire external surface of each structure and derived data using the image analysis program CoralNet to determine the Shannon diversity index (SDI) for each structure. No destructive or disruptive sampling techniques were used which impacted future monitoring events.
After the original structures had been deployed for 1 year, we determined that the most successful structures based on recruitment and structural survivorship were the two concrete types: the jute and disc structures. We installed additional replicates (n = 3) of each of these structure types in June 2023 at each of the four sites, with final data collection for these structures occurring at the same time (Oct. 2023, 3 months after installation) as final data collection for the initially installed structures. This second deployment allowed us to better evaluate potential variations in performance between the two installations and determine whether the final values observed in Oct. 2023 were a function of the hurricane and cumulative community development. The absence of major hurricanes during this second deployment window allowed us to better understand the structures’ performance without a major water quality impairment event.
To measure potential differences in biofiltration services provided by each of the surviving structure types, we subjected the structures to a field biofiltration assay in Oct. 2023. Individual structures were placed in an opaque plastic container on the docks above the gardens with 56 L of canal water, as well as 5 mL of PhytoFeast™ aquarium algal feed to increase initial chlorophyll-a concentrations and ensure a measurable magnitude of biofiltration responses (Hauser, 2015). All containers were covered with a non-airtight lid to provide shade and fitted with a battery-powered aquarium bubbler for aeration and water circulation. One container at each site filled with the same amount of canal water and algal feed but with no garden structure served as a control to account for other potential sources of change in chlorophyll-a and turbidity, such as ambient algal growth or settlement of suspended material. We recorded the initial chlorophyll-a concentration and turbidity of each container using a handheld YSI ProDSS probe immediately prior to adding a structure and then every 15 minutes for a total of 90 minutes. Biofiltration levels were analyzed using the percent change in chlorophyll-a and turbidity levels between each treatment group and the average of all control containers. Biofiltration data was only collected for structures at the two high water quality sites, as structures at low water quality sites had no visible recruitment by filter-feeding organisms.
2.4 Statistical analysis
We used two-way analysis of variance (ANOVA) to test the effects of structure type and water quality designation, as well as interactions between the two, on variables including density of live oysters, heights of live oyster shells, diversity, and, for structures at high water quality sites, chlorophyll-a and turbidity changes in the biofiltration assay; Tukey HSD tests were used for post-hoc analysis. We conducted all statistical analyses using the software R (Version 4.4.1) (R Core Team, 2021). Normality and homogeneity of variance assumptions of ANOVA were assessed from residuals plots. Oyster density data from Sept. 2022 were cube-root transformed prior to analysis to meet the normality and homogeneity of variance assumptions of ANOVA. All other data met test assumptions.
3 Results
Data were analyzed for each time point separately to account for the design of the experiment and events during the study period. The results from Sept. 2022 show the performance of all structures after the initial 3 months of deployment (Supplementary Table S1). The following time point, from Dec. 2022, shows the response of the structures in the aftermath of Hurricane Ian, which had resulted in the loss of most previously observed oyster establishment and biodiversity (Supplementary Table S2). The first set of results from Oct. 2023 shows the performance of the initially deployed structures which survived after 15 months, providing insight into the structures’ recovery post-hurricane, as well as biofiltration data for structures at high water quality sites which were collected only at this final time point (Supplementary Table S3). The other set of results from Oct. 2023 shows the performance of the second deployment of structures from June 2023 to Oct. 2023, providing additional replication to support our findings from the original deployment series (Supplementary Table S4).
3.1 Performance of all structures in September 2022
In Sept. 2022, we found that all structures had survived the initial three months of deployment. Mesh bag and matrix panels had no observed live oysters, while disc, jute and wire structures all had similar densities and heights of live oysters (Figure 3, Supplementary Table S5). Oyster density was not affected by water quality (ANOVA, F(1, 29) = 3.877, P = 0.0608), but oyster shell height in high water quality areas was 32% greater on average than those in low water quality areas (ANOVA, F(1, 18) = 16.659, P < 0.001) (Figure 3). Shannon diversity index (SDI) was not affected by water quality (ANOVA, F(1, 34) = 1.987, P = 0.169), but mesh bag and matrix panel structures had significantly lower SDIs than disc, jute, and wire structures (ANOVA, F(4, 34) = 6.408, P < 0.001) (Figure 3).
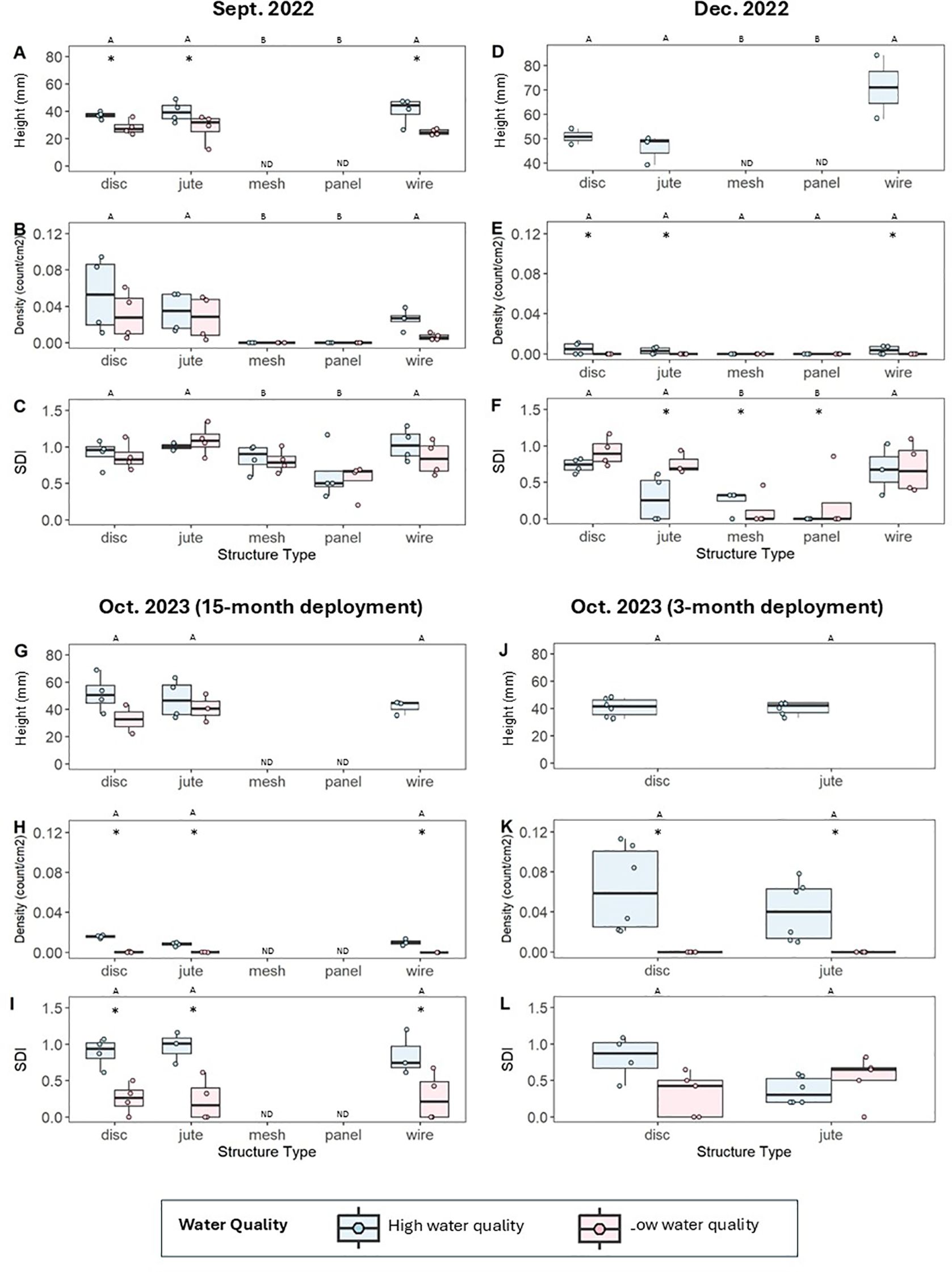
Figure 3. Shell height of live oysters (mm; measured from umbo to furthest tip of margin), density of live oysters (count/cm2), and Shannon Diversity Index (SDI) for structures in Sept. 2022 (A-C), Dec. 2022 (D-F), and Oct. 2023 [including those which were deployed for 15 months (G-I) and those which were deployed for 3 months (J-L)]. Structures for each metric are separated by those at high water quality (blue) and low water quality (red) sites. Only jute and disc structures were redeployed for the 3-month deployment. There was no data (ND) for oyster heights on mesh and panel structures in Sept. 2022 and Dec. 2022 due to an absence of live oysters, and in Oct. 2023 due to the structures having disintegrated. Different letters indicate significant pairwise differences in response variables by structure type as determined by Tukey HSD tests, while asterisks indicate significant differences within each structure type based on water quality as determined by two-way ANOVA tests.
3.2 Performance of structures following Hurricane Ian
In early Dec. 2022, we found that all structures had survived Hurricane Ian, which occurred in late Sept. 2022; however, on average only 7% of live oysters remained on the structures following the hurricane (ANOVA, F(4, 30) = 5.550, P < 0.001) (Figure 3, Supplementary Table S6). At this timepoint, no live oysters were observed at low water quality sites, and both live oyster shell height and density were similar across structure types at the high-water quality sites (ANOVA, F(3, 29) ≥ 1.608, P ≤ 0.112) (Figure 3). SDI was lower overall on mesh bag and matrix panel structures (ANOVA, F(4, 29) = 9.643, P < 0.001) and was also affected by water quality (ANOVA, F(1, 29) = 4.760, P = 0.0374) (Figure 3).
3.3 Performance of structures in October 2023
All jute and disc structures survived throughout the 15-month deployment from June 2022 to Oct. 2023. One wire structure replicate out of eight was observed to break in Sept. 2022 after our monitoring; all others survived. By Oct. 2023, all 8 BESE bag structures and all 8 BESE matrix panel structures had severely degraded. The following analyses for Oct. 2023 data are for the surviving jute, disc, and wire structures which were deployed for 15 months.
No live oysters were observed on any structure at low water quality sites but were observed on all surviving structures at high water quality sites. Dead oysters were observed on all surviving structures at low water quality sites. Structure type did not affect live oyster shell heights at high water quality sites (ANOVA, F(2, 12) = 0.112, P = 0.115) (Figure 3, Supplementary Table S7). SDI did not vary among structure types (ANOVA, F(2,19) = 0.156, P = 0.856), but was lower for structures in low water quality sites (ANOVA, F(1, 19) = 17.009, P < 0.001) (Figure 3). Filtration by all structure types led to reductions in chlorophyll-a and turbidity relative to controls, with no differences in filtration rate detected among structure types. The reduction values observed represent 75-85% lower chlorophyll-a and 80-90% lower turbidity relative to controls (Figure 4).
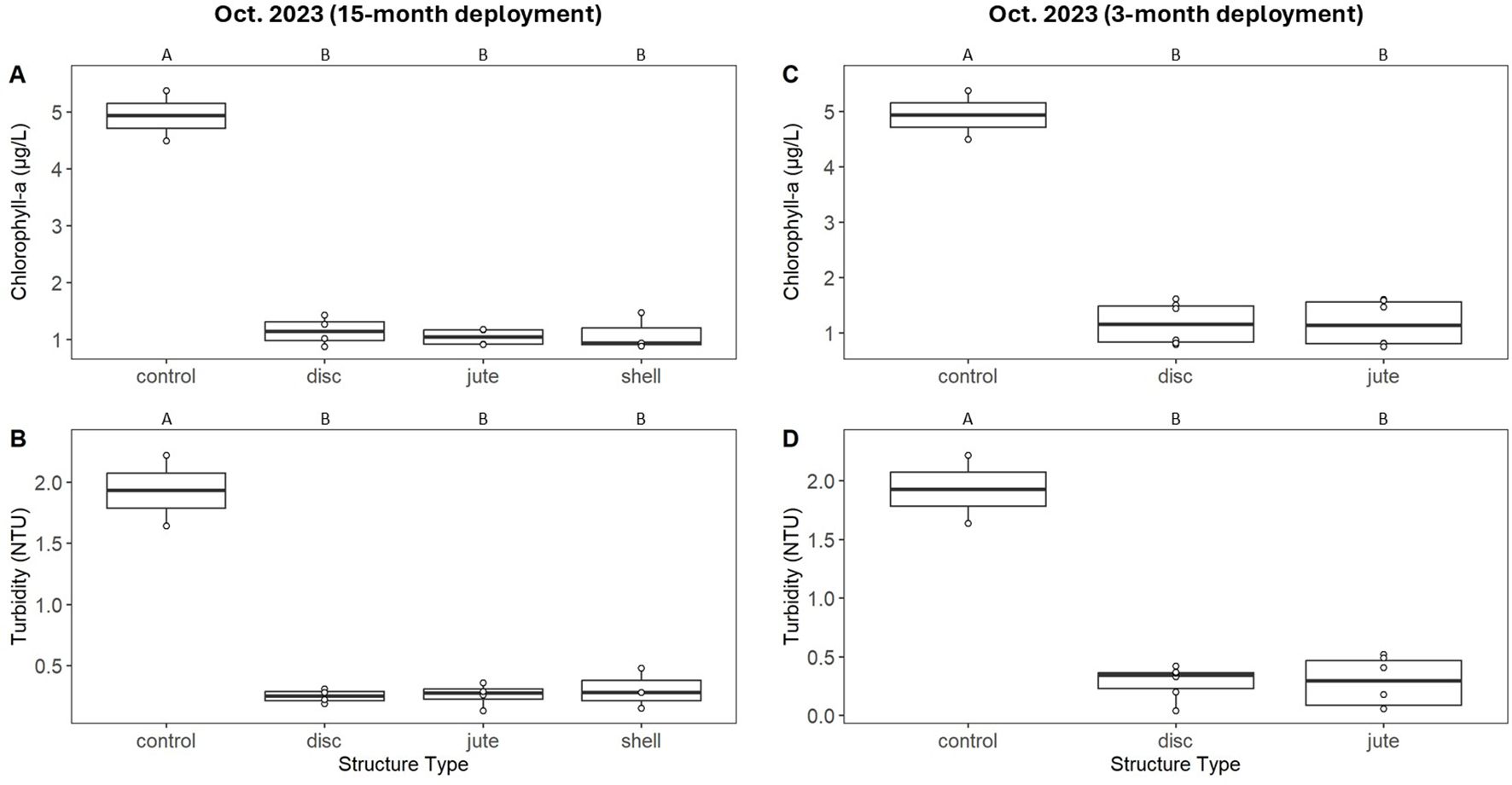
Figure 4. Biofiltration for structures deployed for 15 months (A, B) and 3 months (C, D) alongside control treatments. Chlorophyll-a and turbidity values represent final measurements after 90 minutes of immersion in 56 L of chlorophyll-spiked canal water.
3.4 Performance of second deployment jute and disc structures
For jute and disc structures that were deployed for 3 months during the second deployment from June 2023 to Oct. 2023, live oysters were only observed on structures at high water quality sites; thus, the following analyses only include those structures. No dead oysters were observed on structures at low water quality sites. There was no significant effect of structure type on heights of live oysters (ANOVA, F(1, 10) = 0.056, P = 0.818), oyster density (ANOVA, F(1, 20) = 1.123, P = 0.302), or SDI (ANOVA, F(1, 20) = 0.000, P = 0.988) (Figure 3, Supplementary Table S8). All structure types demonstrated reductions in chlorophyll-a and turbidity relative to controls, with no variation in filtration rates among structure types. These reduction values represent 70-80% lower chlorophyll-a and 80-90% lower turbidity relative to controls (Figure 4).
4 Discussion
This study demonstrates the role of material selection and site conditions in optimizing oyster gardening practices for improving structure durability, oyster recruitment, biofiltration, and biodiversity. By systematically evaluating the performance of a diverse set of materials under varying environmental conditions, including exposure to extreme weather events, our findings provide actionable insights for designing sustainable, resilient, and effective oyster gardening solutions. All structures tested in our field experiment were durable enough to survive a major hurricane three months after their installation, demonstrating their suitability in residential canal systems at risk for intense storm events and reflecting similar observations of structural survivorship through hurricane events in other restoration applications (Barry, 2020; MacDonnell et al., 2022) (Table 1). Both types of biodegradable plastic structures fully degraded within 15 months without recruiting significant amounts of oysters, indicating that these are not effective options for oyster gardening. Jute, disc, and wire structures performed relatively well and similarly to one another in terms of oyster recruitment, biodiversity provisioning, and biofiltration (Table 1). Oyster recruitment was lower at sites in areas of low water quality near the canal weir, and there was no survivorship of oysters at those sites over the timescale of our study. Physical conditions and site selection are important components of oyster garden programs and NBS more broadly, which, alongside material type and project design, can strongly affect project success.
While all structures survived Hurricane Ian, the deterioration of all mesh bag and matrix panel structures by 15 months of deployment shows that both are poorly suited for hanging oyster garden applications (Table 1). The matrix panels are not primarily designed for oyster recruitment, and neither the panels nor bags are designed for applications where they would be hanging in the water column as in this study. These materials are designed for relatively rapid degradation, which may be accommodated or even desirable for structures in areas where oyster recruitment is high and oysters can establish structural integrity before material degradation occurs (Howie and Bishop, 2021; Sakr and Altieri, 2025). However, such levels of oyster recruitment were not observed on these structures in our experiment. Our findings reflect past research showing that BESE biopolymer mesh bags filled with oyster cultch can degrade before substantial oyster recruitment can occur (Comba et al., 2022). While this mesh is designed to last for a year or longer in the water, the truncated lifespan observed in our experiment may have been due to higher environmental temperature, biofouling, or salinity (or other water chemistry variables) than were present at the sites where this material was tested (BESE Ecosystem Restoration Products, n.d.; Comba et al., 2022). Similarly, while past studies have shown that BESE matrix panel structures may last 4-6 years and support oyster recruitment in Florida waters, variations in environmental conditions, structural orientation, or forces associated with attachment method may explain their reduced durability and recruitment in our experiment (Nitsch et al., 2021; Temmink et al., 2021). The expected lifespan of such materials should therefore consider site-specific physical conditions (i.e., wave energy, salinity, and temperature) as well as deployment technique.
The results of the second deployment of jute and disc structures aligned with the results of the initially deployed structures, providing additional support for their recruitment performance and demonstrating their performance without the occurrence of a major water quality impairment event (as experienced by structures in the first deployment). In the first deployment, structures were in the water for a total of 15 months, with all jute and disc structures surviving and demonstrating meaningful performance during their initial 3-month deployment period and again at high water quality sites by the end of the end of their 15-month deployment. This end time coincided with the end of the 3-month deployment of the second set of jute and disc structures which had similar performance to the first deployment structures. While there is not currently information on the long-term durability of the type of concrete discs used here, our findings reflect other studies where similarly constructed jute-cement reef structures have remained intact for over 3 years (Walters et al., 2022). The similar performance of the first and second deployments, despite the first having experienced a major hurricane, indicates the resilient function of these structures. These findings provide additional replication to substantiate our assessment of these structure types and support their consistent value through time for full-scale applications.
Jute, disc, and wire structures had similar oyster recruitment to one another, while BESE mesh bag and matrix panel structures had lower recruitment of oysters and other invertebrate species (Table 1). The lack of recruitment onto mesh bags was unexpected considering that they were filled with the same oyster shell which provided successful recruitment when used in the drilled-shell wire structures. Bagged oyster shell using conventional plastic has traditionally served as suitable substrate for oyster recruitment (Anderson et al., 2019; Bersoza Hernández et al., 2018; Walters et al., 2022). While our experiment was not designed to isolate specific causes for the lack of oyster recruitment on either BESE biodegradable structure type, preemption of space by fouling organisms is a likely mechanism. Mesh bag and matrix panel structures were heavily fouled (87.5 ± 12.5% cover) by sessile organisms such as hydroids, anemones, bryozoans, and algae, even when the other structure types at the same sites and timepoints demonstrated successful oyster recruitment. These organisms can potentially limit juvenile oyster recruitment by settling onto usable settlement space, increasing biodiversity but precluding oysters’ habitat-forming and filtration benefits (Barnes et al., 2010). A number of other factors may have contributed to low oyster recruitment on mesh bags, including the small mesh size of the bag (1 cm, compared to 2 cm mesh typically seen in polyethylene aquaculture bags), the physical barrier of two layers of mesh bag, or increased refugia for oyster predators such as mud crabs and oyster drills which were observed at our site (Comba et al., 2022). Oyster settlement is also chemically mediated by substrate characteristics, with oysters having been shown to preferentially recruit to oyster shell over plastic substrates (Bae et al., 2022; Barnes et al., 2010; Tamburri et al., 2008). While live oyster density following Hurricane Ian was observed to decrease substantially on all structures which previously had oyster recruitment, this may have occurred in conjunction with other end-of-year seasonal factors including decreases in water temperature and oyster recruitment. However, we believe that the extreme nature of the hurricane disturbance and the totality of oyster loss observed provide reasonable support for this loss occurring largely as a result of the storm. Previous research has shown that warm temperatures and sustained oyster recruitment can occur in southwest Florida through December (Dye et al., 2022; Parker et al., 2013), while the presence of live adult oysters on the canal walls throughout the experiment indicate that complete winter oyster mortality is not typical. Standardization of oyster density measurements for novel structure types is an increasingly important issue as more complex structures become common; while simplified geometric surface areas were used in this study, more advanced methods for measuring surface area may need to be incorporated into design and manufacturing specifications.
The biodiversity results underscore the multifaceted ecological benefits of oyster gardening structures, demonstrating their importance for enhancing local habitat complexity and supporting diverse epifaunal communities. Jute, disc, and wire structures all demonstrated higher biodiversity compared to mesh bag and matrix panel structures, emphasizing the importance of substrate type in supporting diverse assemblages of marine organisms. This shows that oyster gardens can serve as important refuge and nursery habitats in developed waterways such as canals that otherwise lack these natural spaces. Notably, the variability in biodiversity across water quality conditions underscores the context dependency of ecological outcomes, with high water quality areas supporting greater diversity and recruitment success. The taxa observed on our experimental structures included various species of anemones, barnacles, mussels, hydroids, sponges, tunicates, crabs, worms, snails, and various types of algae. The biodiversity supported by oyster reefs augments complex food webs by enhancing secondary productivity while also supporting additional filter feeding species with value for water quality (Searles et al., 2022). By enhancing local biodiversity, oyster gardening can contribute to broader conservation goals including bolstering ecosystem resilience and mitigating habitat loss, reinforcing its value as a tool for habitat restoration and as a model for integrating ecological co-benefits into NBS objectives.
Water quality conditions were strongly associated with both the successes and limitations of our experiment. Our biofiltration results highlight the potential of oyster gardening structures to enhance water quality in coastal environments, strengthening the case for integrating oyster gardening into broader coastal restoration and resilience strategies. All jute, disc, and wire structures reached similarly high levels of chlorophyll-a and turbidity reduction after 90 minutes, suggesting that oyster gardens can serve as effective local biofilters for coastal communities facing challenges from algal blooms and eutrophication. The comparable biofiltration rates measured for these structures suggest that material choice can be guided by factors such as cost, durability, and environmental impact without compromising performance in water quality enhancement. However, our findings also underscore the role of existing water quality conditions for site selection. Biofiltration benefits were only measurable at sites with sufficiently high water quality for oysters to establish, indicating a threshold in conditions where oyster gardens can effectively perform. While adult oysters were observed on adjacent seawalls prior to and during the experiment, water quality impairment during the experiment may have prevented the survivorship of larvae or growth of new spat. Regardless of structure type, gardens should be deployed in areas conducive to oyster recruitment and growth. Anderson et al. (2019) showed that the success of oyster gardens pre-seeded with spat varied in association with algal blooms and temperature at different sites. As foundation species growth is a key component of much of coastal NBS functionality, thorough site characterization or pilot testing can improve deployment success (Anderson et al., 2019; Dunlop et al., 2023). However, with sufficient numbers, oyster gardens may engineer improvements to water quality such that they extend the spatial extent of suitable sites via positive feedbacks.
Our study reveals how material properties, ease of acquisition, and performance should all be considered in oyster garden design (Table 1). Jute structures offer several benefits in addition to those measured in this study: the constituent materials are inexpensive and commercially available, the structures can be easily built using volunteer labor with minimal training or experience, there is high customizability in the size and shape of gardens made, and the structures are biodegradable (Table 1). This material has been used in full-scale living shorelines in Florida to create reef structures and breakwaters, but research on its use for oyster gardening is limited (Barry, 2020; Soucy, 2020; Walters et al., 2022). The concrete disc structures used in this experiment were manufactured by a proprietary vendor; they are fully comprised of cement and are not biodegradable, and they offer limited design customizability but maximum durability (Table 1). Their delivery in ready-made form allows for rapid deployment with minimal labor, though their price is also the highest out of the structures tested (Table 1). These concrete discs are available for full-scale application but are still in the early stages of broader adoption. Wire structures made with oyster shell on steel wire are inexpensive but similarly limited in design customizability and employ a non-biodegradable steel wire (Table 1). The size and durability of these structures could be improved by using multiple wire strands per garden or thicker gauge steel, which may have prevented the single observed instance of shell garden loss. BESE mesh bags and matrix panels are both comprised of commercially available biodegradable materials. The panels offer the benefits of ready-made structures, and the mesh bags allow for use of natural shell material without the need for drilling holes for a metal wire. While they have been used successfully for oyster recruitment and other coastal ecosystem enhancement and restoration applications, we found they were not well suited in this test for use in oyster gardening, in part because biodegradation preceded sufficient oyster recruitment (Comba et al., 2022; Marin-Diaz et al., 2021; Nitsch et al., 2021). Given material cost, ease of use, and degradation considerations in addition to experimentally measured performance, it is the ultimate recommendation of the authors that jute reinforced with calcium sulfoaluminate cement, drilled oyster shell on steel wire, full-scale oyster gardening projects, with optimal material dependent on project-specific constraints and objectives.
Our study highlights the importance of systematically testing material options to ensure structural and ecological suitability and helps establish the value of alternative materials which accomplish the ecosystem enhancement goals of NBS with reduced adverse environmental impacts. We suggest that many other NBS project types would benefit from a similar approach of systematic materials testing, particularly relating to the use of natural and reduced-impact materials, that also considers the environmental context of performance. Beyond material performance, future systematic design evaluation should be expanded to incorporate homeowner engagement and participation in NBS activities. As the scale of future NBS activities continues to increase, the impacts and benefits of the materials used are amplified, increasing the urgency of critically evaluating options to ensure more sustainable future development.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
AS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. EM: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JM: Data curation, Formal analysis, Supervision, Validation, Visualization, Writing – review & editing. AA: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Formal analysis, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Science Foundation Graduate Research Fellowship Program (NSF award no. 1650114) to support material costs, travel costs, and expenses for labor and writing. This work was also supported by the U.S. Army Corps of Engineers Engineering With Nature® Initiative through a Cooperative Ecosystem Studies Unit Agreement to support material costs, travel costs, and expenses for labor and writing.
Acknowledgments
Thank you to Christine Angelini and Tom Ankersen for inspiring and supporting this experiment, and to everyone at the Sanibel-Captiva Conservation Foundation’s Marine Lab and Sea School, especially A.J. Martignette and Kealy Pfau, who provided material and logistical support. We sincerely thank Ed Meyer, Susan and Mars Harlan, Ran Niehoff, and Bill and Barbara Millar for allowing us to hang oyster gardens from their docks, as well as Mark Clark, Todd van Natta, Charli Pezoldt, Farah Aryan, and Alyssa Guariniello for field and material support. GROW Oyster Reefs, LLC provided the concrete discs used in our experiment; bioplastic materials were purchased from BESE. Dr. Jeff King was national lead of the U.S. Army Corps of Engineers Engineering with Nature® Initiative at the time of this manuscript's publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1576407/full#supplementary-material
References
Anderson L., Sacks P., Donnelly M., Barker V., Anderson S., and Walters L. (2019). Oyster reef enhancement utilizing gardened oysters in a subtropical estuary. Restor. Ecol. 27, 966–973. doi: 10.1111/rec.12975
Bae S., Ubagan M. D., Shin S., and Kim D. G. (2022). Comparison of recruitment patterns of sessile marine invertebrates according to substrate characteristics. Int. J. Environ. Res. Public Health 19, 1083. doi: 10.3390/ijerph19031083
Barnes B. B., Luckenbach M. W., and Kingsley-Smith P. R. (2010). Oyster reef community interactions: The effect of resident fauna on oyster (Crassostrea spp.) larval recruitment. J. Exp. Marine Biol. Ecol. 391, 169–177. doi: 10.1016/j.jembe.2010.06.026
Barry S. (2020). If you build it, oysters will come (Gainesville, Florida: UF/IFAS Nature Coast Biological Station). Available at: https://blogs.ifas.ufl.edu/ncbs/2020/08/18/if-you-build-it-oysters-will-come/ (Accessed September 30, 2022).
Barry S. (2021). A Partnership for PROS: Plastic Free Restoration of Oyster Shorelines (Gainesville, Florida: UF/IFAS Nature Coast Biological Station). Available at: https://blogs.ifas.ufl.edu/ncbs/2021/09/03/a-partnership-for-pros-plastic-free-restoration-of-oyster-shorelines/ (Accessed January 21, 2024).
Bersoza Hernández A., Brumbaugh R. D., Frederick P., Grizzle R., Luckenbach M. W., Peterson C. H., et al. (2018). Restoring the eastern oyster: How much progress has been made in 53 years? Front. Ecol. Environ. 16, 463–471. doi: 10.1002/fee.1935
BESE Ecosystem Restoration Products. BESE-mesh biopolymer (Culemborg, The Netherlands: Bese Products). Available at: https://www.bese-products.com/biodegradable-products/bese-mesh-biopolymer/ (Accessed April 4, 2024).
BESE-Elements (2018). (Culemborg, The Netherlands). Available online at: https://www.bese-products.com/biodegradable-products/bese-elements/ (Accessed April 4, 2024).
Boström-Einarsson L., Martínez-Baena F., Diggles B., Firby L., and McLeod I. M. (2022). An ecological assessment of Australia’s first community oyster gardens. Ecol. Manage. Restor. 23, 244–251. doi: 10.1111/emr.12565
Bridges T., King J., Simm J. D., Beck M., Collins G., Lodder Q., et al. (2021). International Guidelines on Natural and Nature-Based Features for Flood Risk Management. Eds. Bridges T., King J., Simm J. D., Beck M., Collins G., Lodder Q., and Mohan R. (Vicksburg, MS: USACE). doi: 10.21079/11681/41946
Brumbaugh R. D. and Coen L. D. (2009). Contemporary approaches for small-scale oyster reef restoration to address substrate versus recruitment limitation: A review and comments relevant for the olympia oyster, ostrea lurida carpenter 1864. J. Shellf. Res. 28, 147–161. doi: 10.2983/035.028.0105
Bushek D., Richardson D., Bobo M. Y., and Coen L. D. (2004). Quarantine of oyster shell cultch reduces the abundance of Perkinsus marinus. J. Shellf. Res. 23, 369–374. Available at: https://www.researchgate.net/publication/228486632.
Comba D., Palmer T. A., Breaux N. J., and Pollack J. B. (2022). Evaluating biodegradable alternatives to plastic mesh for small-scale oyster reef restoration. Restor. Ecol. doi: 10.1111/rec.13762
Dunlop T., Glamore W., and Felder S. (2023). Restoring estuarine ecosystems using nature-based solutions: Towards an integrated eco-engineering design guideline. Sci. Total Environ. 873, 162362. doi: 10.1016/j.scitotenv.2023.162362
Dye B., Jose F., Richard J., Mortensen J. B., and Milbrandt E. C. (2022). An agent-based model accurately predicts larval dispersal and identifies restoration and monitoring priorities for eastern oyster () in a Southwest Florida estuary. Restor. Ecol. 30, e13487. doi: 10.1111/rec.13487
Flores P. and Albright J. (2017). Final TMDL Report Nutrient TMDLs for Sanibel Slough (WBIDs 2092F1 and 2092F2) (Tallahassee, FL: Florida Department of Environmental Protection). Available at: https://floridadep.gov/sites/default/files/FINAL_nut_sanibel_08142017.pdf (Accessed June 20, 2024).
Goelz T., Vogt B., and Hartley T. (2020). Alternative substrates used for oyster reef restoration: A review. J. Shellf. Res. 39, 1–12. doi: 10.2983/035.039.0101
Griffin M. (2016). Fifteen Years of Rhode Island Oyster Restoration: A Performance Evaluation and Cost-Benefit Analysis (Kingston, RI: The University of Rhode Island). doi: 10.23860/thesis-griffin-matthew-2016
Hamilton K. A., Swann D. L., and Rikard F. S. (2005). Evaluation of two off-bottom oyster, crassostrea virginica, culture methods for use in oyster gardening in Alabama. J. Appl. Aquacult. 16, 1–16. doi: 10.1300/J028v16n03_01
Hauser L. W. (2015). Predicting episodic ammonium excretion by freshwater mussels via gape response and heart rate (Iowa City, IA: The University of Iowa). Available at: https://www.proquest.com/docview/1708652816/abstract/7E82E953D0FC41B9PQ/1 (Accessed May 6, 2024).
Howie A. H. and Bishop M. J. (2021). Contemporary oyster reef restoration: responding to a changing world. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.689915
Jamil A., Ahmad A., Zhao Y., Zhao Y., Yang C., Li Y., et al. (2024). Advances in global oyster reef restoration: innovations and sustainable ecological approaches. Sustainability 16. doi: 10.3390/su16229795
Kaye C. A. (1964). The Upper Limit of Barnacles as an Index of Sea-Level Change on the New England Coast during the past 100 Years. J. Geol. 72, 580–600. doi: 10.1086/627015
Kennedy V. S., Newell R. I. E., and Eble A. F. (1996). The Eastern Oyster: Crassostrea Virginicaz. Available online at: https://repository.library.noaa.gov/view/noaa/45763 (Accessed March 28, 2025).
Krasny M. E., Crestol S. R., Tidball K. G., and Stedman R. C. (2014). New York City’s oyster gardeners: Memories and meanings as motivations for volunteer environmental stewardship. Landscape Urban Plann. 132, 16–25. doi: 10.1016/j.landurbplan.2014.08.003
MacDonnell C., Tiling K., Encomio V., van der Heide T., Teunis M., Lengkeek W., et al. (2022). Evaluating a novel biodegradable lattice structure for subtropical seagrass restoration. Aquat. Bot. 176, 103463. doi: 10.1016/j.aquabot.2021.103463
Marenghi F. and Ozbay G. (2010). Preliminary habitat assessment of floating oyster (Crassostrea virginica) gardens (Delaware). Ecol. Restor. 28, 254–257. doi: 10.3368/er.28.3.254
Marin-Diaz B., Fivash G. S., Nauta J., Temmink R. J. M., Hijner N., Reijers V. C., et al. (2021). On the use of large-scale biodegradable artificial reefs for intertidal foreshore stabilization. Ecol. Eng. 170, 106354. doi: 10.1016/j.ecoleng.2021.106354
Martin D. E. and Hall S. G. (2006). Oyster shucking technologies: Past and present. Int. J. Food Sci. Technol. 41, 223–232. doi: 10.1111/j.1365-2621.2005.01052.x
Morris R. L., Bilkovic D. M., Boswell M. K., Bushek D., Cebrian J., Goff J., et al. (2019). The application of oyster reefs in shoreline protection: Are we over-engineering for an ecosystem engineer? J. Appl. Ecol. 56, 1703–1711. doi: 10.1111/1365-2664.13390
Morris R. L., Konlechner T. M., Ghisalberti M., and Swearer S. E. (2018). From grey to green: Efficacy of eco-engineering solutions for nature-based coastal defence. Global Change Biol. 24, 1827–1842. doi: 10.1111/gcb.14063
Nitsch C. K., Walters L. J., Sacks J. S., Sacks P. E., and Chambers L. G. (2021). Biodegradable material for oyster reef restoration: first-year performance and biogeochemical considerations in a coastal lagoon. Sustainability 13. doi: 10.3390/su13137415
Oesterling M. and Petrone C. (2012). Non-Commercial Oyster Culture, or Oyster Gardening (Gainesville, FL: Southern Regional Aquaculture Center). Available at: https://shellfish.ifas.ufl.edu/wp-content/uploads/Non-Commercial-Oyster-Culture_SRAC-4307.pdf (Accessed August 14, 2024).
Palinkas C. M., Orton P., Hummel M. A., Nardin W., Sutton-Grier A. E., Harris L., et al. (2022). Innovations in coastline management with natural and nature-based features (NNBF): lessons learned from three case studies. Front. Built Environ. 8. doi: 10.3389/fbuil.2022.814180
Parker M. L., Arnold W. S., Geiger S. P., Gorman P., and Leone E. H. (2013). Impacts of freshwater management activities on eastern oyster (Crassostrea virginica) density and recruitment: recovery and long-term stability in seven Florida estuaries. J. Shellf. Res. 32, 695–708. doi: 10.2983/035.032.0311
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed May 6, 2024).
Rink2Reef® (2024). Available online at: https://rink2reef.org/ (Accessed May 6, 2024).
Sakr A. and Altieri A. H. (2025). Living in a material world: Support for the use of natural and alternative materials in coastal restoration and living shorelines. Ecol. Eng. 211, 107462. doi: 10.1016/j.ecoleng.2024.107462
Sanibel-Captiva Conservation Foundation (2022). Coastal watch volunteers Assist with Vertical Oyster Garden Pilot Program (Sanibel, FL: SCCF). Available at: https://sccf.org/blog/2022/07/19/coastal-watch-volunteers-assist-with-vertical-oyster-garden-pilot-program/ (Accessed March 17, 2025).
Searles A. R., Gipson E. E., Walters L. J., and Cook G. S. (2022). Oyster reef restoration facilitates the recovery of macroinvertebrate abundance, diversity, and composition in estuarine communities. Sci. Rep. 12, 8163. doi: 10.1038/s41598-022-11688-6
Shepard C., Brenner J., Goodin K. L., and Ames K. W. (2018). “Chapter 5. Ecological Resilience Indicators for Oyster Reefs,” in Ecological Resilience Indicators for Five Northern Gulf of Mexico Ecosystems (Arlington, VA: NatureServe). Available at: https://www.natureserve.org/sites/default/files/chapter_5_-_oyster_indicators.pdf (Accessed March 28, 2025).
Smith C. S., Rudd M. E., Gittman R. K., Melvin E. C., Patterson V. S., Renzi J. J., et al. (2020). Coming to terms with living shorelines: A scoping review of novel restoration strategies for shoreline protection. Front. Marine Sci. 7. doi: 10.3389/fmars.2020.00434
Soucy B. (2020). Alternative Material Selection for Oyster Restoration with an Emphasis on Living Docks (Melbourne, FL: Florida Institute of Technology). Available at: https://repository.lib.fit.edu/handle/11141/3177 (Accessed January 21, 2022).
Tamburri M. N., Luckenbach M. W., Breitburg D. L., and Bonniwell S. M. (2008). Settlement of crassostrea ariakensis larvae: effects of substrate, biofilms, sediment and adult chemical cues. J. Shellf. Res. 27, 601–608. doi: 10.2983/0730-8000(2008)27[601:SOCALE]2.0.CO;2
Temmink R. J. M., Angelini C., Fivash G. S., Swart L., Nouta R., Teunis M., et al. (2021). Life cycle informed restoration: Engineering settlement substrate material characteristics and structural complexity for reef formation. J. Appl. Ecol. 58, 2158–2170. doi: 10.1111/1365-2664.13968
Thompson M. and Milbrandt E. (2021). City of Sanibel Clean Canals Program, Year One Monitoring Report (Sanibel, FL: Sanibel Captiva Conservation Foundation). Available at: https://www.mysanibel.com/content/download/27945/file/Sanibel%20Clean%20Canals%20Program%20Year%20One%20Monitoring%20Report%202020.pdf (Accessed February 7, 2025).
Walters L. J., Roddenberry A., Crandall C., Wayles J., Donnelly M., Barry S. C., et al. (2022). The use of non-plastic materials for oyster reef and shoreline restoration: understanding what is needed and where the field is headed. Sustainability 14. doi: 10.3390/su14138055
Keywords: alternative materials, biodegradable plastic, coastal restoration, community science, filtration, oyster gardening, biodiversity, hurricane
Citation: Sakr A, Mazor LD, Milbrandt EC, Morton JP and Altieri AH (2025) A changing of the garden: evaluating the performance and ecosystem functionality of alternative oyster garden structures in residential waterways. Front. Ecol. Evol. 13:1576407. doi: 10.3389/fevo.2025.1576407
Received: 13 February 2025; Accepted: 24 April 2025;
Published: 23 May 2025.
Edited by:
Bianca Charbonneau, Engineer Research and Development Center (ERDC), United StatesReviewed by:
Asad Jamil, Tianjin University, ChinaBecca Hatchell, Florida Fish and Wildlife Research Institute, United States
Copyright © 2025 Sakr, Mazor, Milbrandt, Morton and Altieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adrian Sakr, YWRyaWFuc2FrckB1ZmwuZWR1
 Adrian Sakr
Adrian Sakr Logan D. Mazor
Logan D. Mazor Eric C. Milbrandt
Eric C. Milbrandt Joseph P. Morton
Joseph P. Morton Andrew H. Altieri
Andrew H. Altieri