- Department of Biology, Indiana University Bloomington, Bloomington, IN, United States
While it has long been hypothesized that belowground carbon (C) allocation in plants is tightly coupled to nutrient uptake, empirical tests of this are rare, especially for woody plants. We grew tree saplings of nine species in soils enriched in isotopically-labeled nitrogen (N) and after several months, pulse-labeled trees with 13CO2. This approach allowed us to track how 13C allocation from foliage to absorptive root tissue related to 15N movement from soil to plant tissues as a measure of each species' N return on C investment. We hypothesized that tree species known to associate with ectomycorrhizal (ECM) fungi would have greater belowground C fluxes than those that associate with arbuscular mycorrhizal (AM) fungi, and that species with greater belowground C allocation would acquire the most soil N. Overall, we found large interspecific differences in both the amount of recently-fixed C allocated belowground and plant N uptake, yet no differences in either flux between AM and ECM trees (P > 0.05). Moreover, we found no differences between mycorrhizal groups in terms of their N return on C investment. However, mycorrhizal type influenced the relationship between belowground C allocation and N uptake, which was positive among AM species (r = 0.42; P = 0.001) and negative among ECM species (r = −0.44; P = 0.003), suggesting that the relationship between C allocation and plant nutrition is more complex than theory predicts. Collectively, our results suggest that tree species' nutrient return on C investment can differ greatly among species, and that efforts to model these dynamics should consider the traits and tradeoffs that underlie these dynamics.
Introduction
Plant productivity is frequently limited by nutrients (Elser et al., 2007; LaBauer and Treseder, 2008) and consequently a large portion of primary production is allocated belowground to acquire nutrients from soil (Gill and Finzi, 2016). While numerous studies have highlighted the importance of C allocation and fine root production in forests (Raich and Nadelhoffer, 1989; Litton et al., 2007; McCormack et al., 2015), our understanding of the factors that control belowground C fluxes remains limited (Grayston et al., 1997; Epron et al., 2012). This results from the fact that dynamic C allocation belowground (as opposed to static measurements of C pools such as fine root biomass and root: shoot relationships) is challenging to study in situ and is likely to be highly variable within and among plant species (Grayston et al., 1997). Given that variation in belowground C allocation can affect soil C storage, nutrient retention and plant uptake (Pendall et al., 2004; Cheng et al., 2014), an improved understanding of species-specific effects of belowground C dynamics is needed to accurately predict how future species shifts will affect ecosystem functioning (Nguyen, 2003; Heimann and Reichstein, 2008; Cheng et al., 2014).
Trees can allocate anywhere from 5 to 25% of recently fixed C belowground (Grayston et al., 1997; Phillips and Fahey, 2005; Drake et al., 2011), yet the impacts of belowground C fluxes on nutrient availability in forests are not well-established. Theory predicts trees will preferentially allocate energy (i.e., C) to acquire the most limiting resource (Bloom et al., 1985; Chapin et al., 1990; Franklin et al., 2012). In most temperate forests, nitrogen (N) limits net primary production (LaBauer and Treseder, 2008), suggesting that belowground C allocation and N acquisition should be coupled processes. Using a meta-analytical and modeling approach, Finzi et al. (2015) found support for such C-for-N “return on investment” in forests. They estimated that up to one-third of total soil N mineralization may be fueled by C allocated belowground and delivered to soil via rhizodeposition. In a separate meta-analysis, Gill and Finzi (2016) reported that forests with the largest belowground C fluxes have the fastest rates of N mineralization—consistent with the idea that more C allocated belowground should result in more plant N uptake, at least in N-limited forests. However, greater belowground C allocation under low soil N availability could have the opposite effect on plant uptake. If belowground C inputs to soil increase plant-microbe competition for N, increases in N immobilization could reduce plant N uptake (Kuzyakov and Xu, 2013). Such a scenario would be likely in plants that produce C that is of low chemical quality as a microbial substrate.
Theory also predicts that the C cost of N uptake is a function of not only soil N availability but also the type of mycorrhizal fungi a plant associates with (Brzostek et al., 2015). Recent work shows plant mycorrhizal association can be considered an integrative plant functional trait, with plants from distinct mycorrhizal groups displaying differences in other plant nutrient traits such as leaf and root N (Averill et al., 2019), leaf and root litter decay rates (Keller and Phillips, 2019; See et al., 2019) and foliar resorption (Zhang et al., 2018). Moreover, trees associated with ectomycorrhizal (ECM) fungi are believed to allocate more C to their symbionts, which in turn have the capacity to mine N from SOM. In contrast, trees associated with arbuscular mycorrhizal (AM) fungi, typically lacking such capabilities, are thought to allocate less C to their symbionts as they commonly grow in more N-rich soils (Phillips et al., 2013; Lin et al., 2016). Several studies have shown that ECM trees may release more C to soil (Phillips and Fahey, 2005; Yin et al., 2014) and that such inputs may lead to root-accelerated N cycling in ECM-dominated stands (Brzostek et al., 2012; Yin et al., 2014; but see Wurzburger and Brookshire, 2017). However, the return on investment of AM and ECM trees may differ depending on soil N availability. In a modeling analysis, Brzostek et al. (2015) reported that while ECM trees have a greater N return on C investment in low N soils, AM trees may have a greater N return on C investment in high N soils. This may explain, in part, why AM trees are becoming increasingly abundant (relative to ECM trees) in areas that receive the highest levels of N deposition (Jo et al., 2019).
Improved quantification of interspecific differences in belowground C allocation, N uptake, and N return on C investment may facilitate the inclusion of belowground traits into other plant resource use theory (e.g., the plant economic spectrum). Numerous studies have reported weak links between species' aboveground and belowground traits (Mommer and Weemstra, 2012; Weemstra et al., 2016), a pattern that has been attributed to the multidimensionality of roots (Mccormack and Iversen, 2019) and the lack of appropriate belowground trait data. If belowground C allocation and N uptake can be predicted by other more easily measured belowground traits, improved parameterizations can be used to model these critical plant-soil fluxes at large spatial scales in the wake of global changes (McCormack et al., 2017).
The goals of this study were to quantify interspecific differences in belowground C allocation and N uptake, as a means of estimating interspecific variation in N return on C investment. To this end, we conducted a dual 13C and 15N pulse labeling greenhouse experiment with nine tree species (5 AM, 4 ECM species) where we simultaneously traced the flux of recently photosynthesized 13C belowground and the uptake of 15N into tree saplings. We hypothesized that ECM trees would allocate more C belowground (relative to AM trees) resulting in greater N uptake from soil. Accordingly, we predicted that relative to AM trees, ECM trees would allocate a greater percentage of fixed 13C to their absorptive roots, resulting in greater soil-derived 15N recovered in plant tissues. Moreover, across all species we expected to find a positive relationship between the amount of 13C allocated belowground and plant 15N.
Materials and Methods
Experimental Set-Up
We conducted a dual (13C and 15N) isotopic labeling study of nine tree species, comprising two types of mycorrhizal associations (AM and ECM) under greenhouse conditions. We selected five AM-associated tree species: Acer saccharum (sugar maple), Juglans nigra (black walnut), Liriodendron tulipifera (tulip poplar), Prunus serotina (black cherry), Sassafras albidum (sassafras), and four ECM-associated tree species: Betula nigra (river birch), Quercus alba (white oak) Quercus rubra (red oak), and Tilia americana (American basswood). At the start of the study, saplings were 2 years old (obtained from Vallonia State Nursery, Indiana, USA) except for S. albidum and T. americana (obtained from Cold Stream Farm Nursery, Michigan, USA) which were 1 year old. All trees were “bare root” (i.e., obtained without soil) and consequently likely not colonized by mycorrhizal fungi.
Ten experimental saplings of each species were grown in a forest soil-sand mix (due to some sapling death, the final number of replicates varied by species slightly, Table 1; additional ‘unlabeled control' saplings of each species were also grown in the same conditions). We collected all soil from Lilly-Dickie Woods in Brown County, Indiana, USA (39°14 N, 86°12 W; altitude 290 m). For detailed description of the soil and microbial community properties of these soils see Cheeke et al. (2017) and Craig et al. (2018). Within this forest, we selected from two stands with either >90% basal area of AM-associated or ECM-associated tree species. At each stand, we removed the standing litter layer and collected soil from the top 10 cm (including Oe and Oa, where present, and A horizons). We removed large clumps of roots and large rocks and then mixed each soil type with equal parts sand that had been steamed at 80°C for 4 h. Saplings were planted in individual pots (19.7 cm width × 45.7 cm height, 9.63 liters volume) in an AM or ECM soil: sand matrix in a fully reciprocal species × soil type design. However, using two-way analysis of variance (ANOVA) we found no soil type or species × soil type effect for any variable measured (P > 0.10 in all cases) and subsequently grouped all replicates of each species together for data analysis. We allowed all saplings to grow in pots for ~2 months to develop their rooting systems.
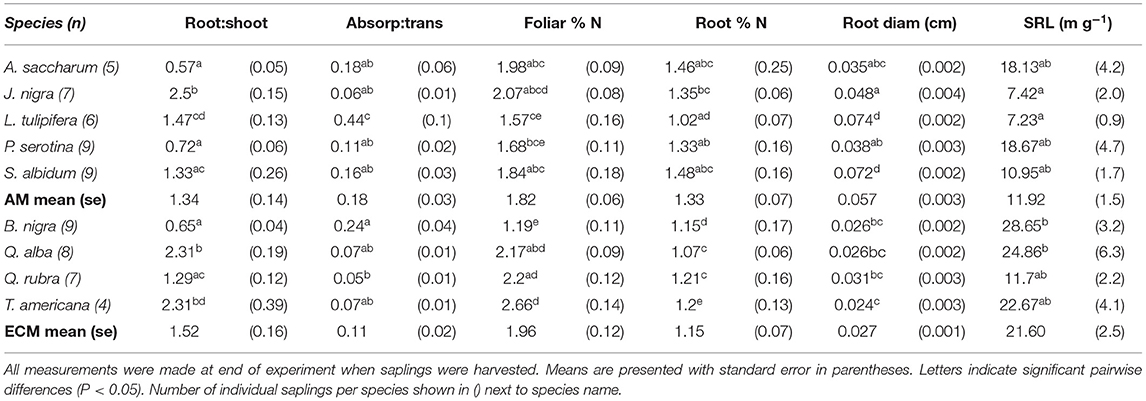
Table 1. Species-specific differences in root mass: shoot mass, absorptive root mass: transport root mass, foliar and root % N, absorptive root diameter, and specific root length (SRL).
After 2 months, we added 15N-labeled plant leaf material to each pot (hereafter referred to as particulate organic N, PO15N). The addition of an organic source of 15N (as opposed to just adding inorganic 15N) allowed us to investigate the degree to which trees can shift their belowground C allocation to mine N from organic matter. To do this, we removed saplings from their pots, mixed in 0.75 g/1 L soil of PON15 to the bulk soil, and repotted the saplings. The same procedure was performed for saplings that did not receive PO15N, to serve as unlabeled controls (one unlabeled sapling per soil type × species combination). Six soil-only controls were also included. PO15N was produced by growing a different set of saplings (same tree species, obtained from the same respective nurseries) in a 50:50 sand: Turface® fritted clay mixture amended with 15N-labeled Hoagland's solutions wherein 30% of the N was 98% 15N (15NO3 and K15NO3). After several months, we collected foliage from all nine species, dried it at 60°C for 48 h and ground it into a coarse powder using a mortar and pestle. We combined equal masses of foliage from each species to make a homogeneous composite source of PO15N.
All saplings were grown for an additional 2 months before pulse labeling with 13C. This allowed time for the PO15N to decompose and plants to take it up. Sapling height was measured twice over the course of the experiment, once immediately after repotting and once immediately prior the start of the 13C pulse labeling period.
13C Pulse Labeling of Saplings
To track the flux of recently photosynthesized C belowground, we used a custom-made gas-tight chamber, following the protocol of Kannenberg and Phillips (2017). In brief, we pulse-labeled four saplings at a given time (groupings were selected using a randomized block design with species as the blocking factor), with the stem and foliage of each sapling housed within the gas-tight, transparent chamber while the pot (and therefore all soil and belowground tissues) remained outside the chamber. Thus, all 13C recovered in the roots or soils resulted from belowground C allocation dynamics within each plant. Labeling occurred on sunny or partly sunny days under full greenhouse lighting and lasted for 4 h during mid-day. After flushing the chamber with pure N2 gas, we injected 50 mL of 99% 13CO2 into the chamber at the beginning of each labeling period. We injected a second 50 mL of 13CO2 after 2 h to prevent significant drawdown of CO2 inside the chamber over the course of the labeling period. The control saplings, as described above, were placed in the chamber and exposed to the same conditions as the labeled trees with the exception of using standard CO2 gas rather than 99% 13CO2.
Harvesting and Laboratory Analyses
We sampled foliage from each pot immediately following pulse labeling and soil from each pot 1, 3, and 5 days after pulse labeling. We also sampled foliage immediately prior to labeling to provide a baseline from which to calculate total 13CO2 fixation by each sapling during the 4 h labeling period. To sample foliage, we cut or punched holes in two leaves from each sapling and composited these subsamples into one sample per sapling per time point. We then oven-dried foliage samples at 60°C for 48 h, ground the tissue to a fine powder, and weighed 3 mg of each sample into tin capsules for 13C and 15N analysis (see below).
We sampled soil from each pot by taking two soil cores (2.5 cm diameter) to the depth of the pot and compositing the cores into one soil sample per pot per time point. The soil was immediately transferred to the lab, where we sieved and picked out all roots. For soil samples with sufficient root biomass, we processed the root tissue as described above for foliage tissue and analyzed root 13C and 15N (see below). Due to insufficient root biomass in some cores, the sample size for absorptive root measurements varied slightly among species.
Five days post labeling, we harvested each entire pot. We separated plant biomass into foliage, stem, and transport and absorptive root pools, and collected a representative subsample from the homogenized soil. We also collected two absorptive root (1st-3rd) samples from each sapling. Root samples were thoroughly cleaned of soil with water and immediately scanned on an Epson GT 20000 scanner. Mass-specific root length (SRL) and average root diameter measurements were obtained from the scanned images using WinRhizo software (Regent Instruments, Quebec City, Quebec, Canada). Plant tissues, including scanned root samples, were oven-dried at 60°C for 48 h and weighed. Foliage and root tissues (not including scanned roots) were processed as described above. Transport and absorptive roots were separated into distinct pools from the intact oven-dried root systems of each sapling following Kong and Ma (2014).
Isotopic Analyses
We determined δ13C and δ15N, as well as elemental C and N, for plant pools using a Costech ECS 4010 Elemental Analyzer (Costech Analytical Technologies, Valencia, CA) coupled to a ThermoFinnigan DELTA plus XP isotope ratio mass spectrometer (Thermo Fischer Scientific, San Jose, CA). Carbon isotopic ratios were converted to the Vienna Pee Dee Belemite (VPDB) international scale by calibration with Acetanilide 1 and EDTA-1 and N isotopic ratios were compared to atmospheric N2 (Schimmelmann et al., 2009). Analyses were conducted at the Stable Isotope Research Facility (Dept. of Earth and Atmospheric Sciences, Indiana University) and the Purdue Stable Isotope Facility (Earth, Atmospheric and Planetary Sciences Department, Purdue University). A subset of samples were analyzed at both facilities and results were highly correlated between facilities (R2 > 0.95, P < 0.001).
We quantified the incorporation of newly photosynthesized 13C and 15N into distinct plant tissue pools (foliar, stem, transport and absorptive roots) by first calculating atom % for C and N separately as:
where STND represents the standard isotopic value for VPDB (0.0111802) and atmospheric N2 (0.0036764) for 13C and 15N, respectively. Excess δ13C and δ15N for each pool were then calculated as:
where atom %L is atom % of labeled material and atom %UL is atom % unlabeled material, DW is the dry weight of the plant (mg), and % C or % N represents the elemental composition of the tissue. Isotopic enrichment of foliage, stem and absorptive root tissue was measured for each individual sapling. Isotopic enrichment of transport roots was measured on two randomly selected experimental saplings per species and one control sapling per species. Whole plant 15N excess (ng N) was calculated by adding foliar, stem, transport and absorptive root 15N excess pools together. We then calculated the N return on C investment in two ways: (1) plant 15N (ng N) / absorptive root 13C (ng C/ ng foliar 13C) and (2) whole plant N content (g N)/absorptive root biomass (g C).
Statistical Analysis
We first tested if foliar atom % 13C was significantly different in labeled compared to unlabeled samples using a Student's t-test. We used a one-way analysis of variance (ANOVA) and Tukey's HSD post-hoc test to assess species differences in sapling growth (% change in height), SRL, absorptive root diameter, and tissue 13C and 15N content. We then used step-wise multiple linear regression to assess if there were significant relationships between 13C and 15N in plant tissues and if such relationships varied by mycorrhizal group. We subsequently explored how belowground C allocation and N uptake relate to other plant traits in a multidimensional trait space using PCA. All analyses were conducted in R 3.3.2 (R Development Core Team).
Results
C Labeling of Plants
We found a significant increase in foliar atom % 13C in labeled compared to unlabeled plants after the 4 h 13CO2 pulse labeling treatment (t = 13.598, P < 0.001), indicating successful labeling of foliar tissue in our experimental saplings (foliar atom % 13C labeled mean = 1.25, standard error = 0.13; unlabeled mean = 1.08, standard error = 0.00046). While there was significant variation in leaf mass [F(8, 54) = 7.581, P < 0.001] and tree height [F(8, 54) = 98.8, P < 0.001] {and change in height over time [F(8, 54) = 10.7, P < 0.001]} among species, there was no difference in these metrics between mycorrhizal groups (mean tree height across species at end of experiment: 83.11 cm ± 5.13; mean increase in height across species: 28.22 cm ± 3.74). Species-specific plant traits are presented in Table 1.
On average, ~10% of fixed 13CO2 was transferred from the foliage to the roots within 5 days post-labeling (estimated as the ratio of foliar 13C enrichment immediately after labeling to absorptive root 13C 5 days post-labeling), indicating significant movement of newly photosynthesized C from aboveground to belowground tissues. There was no time point × species interaction for root 13C enrichment, and therefore peak 13C enrichment across time points for each sapling was used in subsequent analyses. Root 13C enrichment (as calculated in Equation 2) varied significantly among species [F(8, 54) = 2.3, P < 0.001, Figure 1A; F(8, 54) = 3.575, P < 0.001, Figure 1B] but not between mycorrhizal types. There was a 16-fold difference in root 13C enrichment (ng C/ ng foliar 13C) among species; Acer saccharum (sugar maple; AM-associated) had significantly higher root 13C enrichment compared to T. americana (American basswood; ECM-associated).
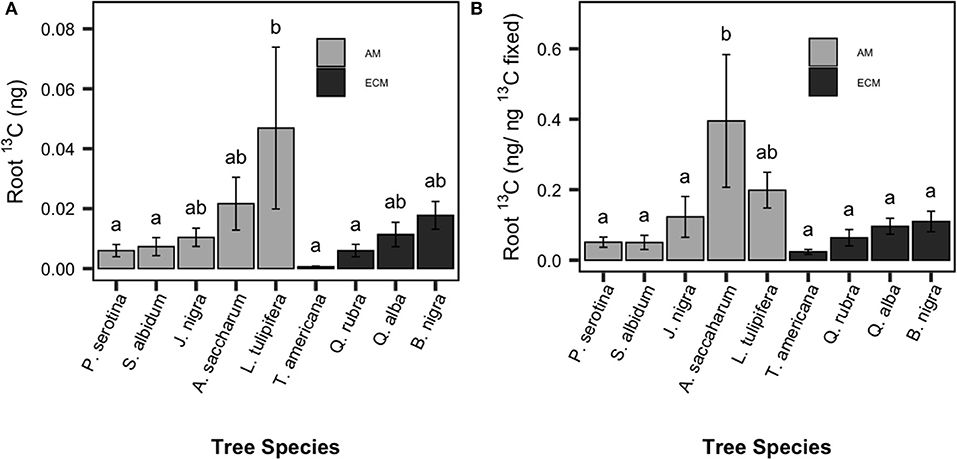
Figure 1. Species patterns in allocation of recently-fixed C allocated to absorptive roots. Total 13C absorptive root enrichment (A) and relative 13C absorptive root enrichment per unit 13C fixed (B) shown. Species means are show with standard error bars. Light bars indicate AM-associated tree species, dark bars indicated ECM-associated species. Letters indicate significant pairwise differences (P < 0.05).
15N Labeling of Plants
The addition of PO15N to each pot resulted in significantly enriched 15N-soil in the experimental vs. control pots (P < 0.001). As evidenced by 15N-enriched plant tissue in our experimental saplings, a detectable proportion of this soil-derived 15N pool was subsequently decomposed and taken up by plants. Similar to belowground C allocation, plant 15N uptake varied among species but not between mycorrhizal types [F(8, 54) = 2.58, P = 0.015, Figure 2]. Among species, there was a 4-fold difference in plant 15N enrichment, with A. saccharum (sugar maple; AM-associated) having the greatest PO15N uptake among the nine species.
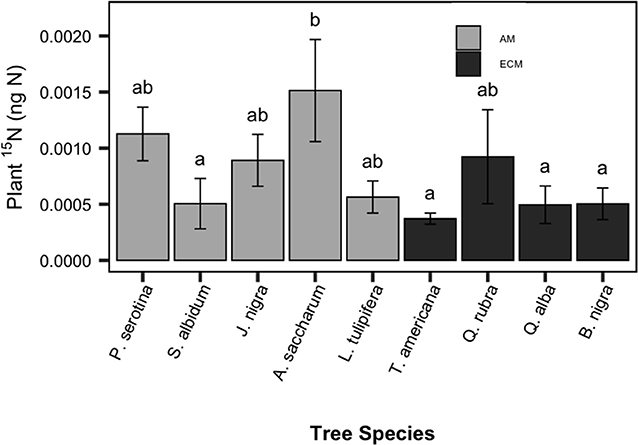
Figure 2. Species patterns in sapling plant tissue 15N enrichment, compared to saplings grown without 15N-labeled particulate organic matter. Species means are shown with standard error bars. Light bars indicate AM-associated tree species, dark bars indicated ECM-associated species. Letters indicate significant pairwise differences (P < 0.10).
Plant N Return on C Investment
By comparing root 13C tissue enrichment to plant 15N tissue enrichment (quantifying new plant N uptake), we can assess the relationship between plant N demand and dynamic belowground C allocation. Among species, there was a more than 10-fold difference in mean plant 15N enrichment: absorptive root 13C enrichment (P = 0.012, SI Figure 1). We found a significant positive relationship between plant 15N enrichment and absorptive root 13C for AM species (r2 = 0.18, P = 0.001) but a significant negative relationship for ECM species (r2 = 0.19, P = 0.003; Figure 3). When accounting for variation in plant N content among species, we found similar but weaker relationships with a negative relationship for ECM species (r2 = 0.15, P = 0.017) but no significant relationship among AM species (SI Figure 2). However, there were no mycorrhizal type differences in N return on belowground C investment when calculating this ratio either by 13C and 15N tissue enrichment or total C and N tissue concentrations (as described in methods; SI Figure 1). Using PCA, we found variation in increased root 13C aligned with increased allocation to absorptive compared to transport roots. Meanwhile, mass-specific root length (SRL) and root diameter were orthogonal to root 13C enrichment. The first two orthogonal PCA axes explained 31.5 and 20.9%, respectively, of the variation in our dataset (Figure 4).
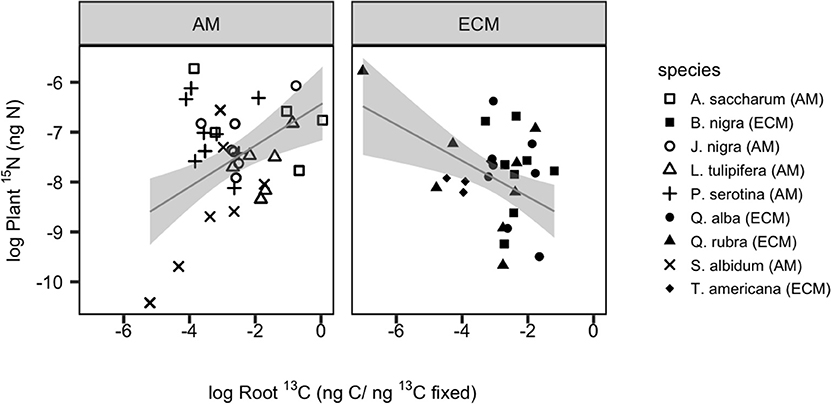
Figure 3. Variation in belowground allocation of recently-fixed C (as measured by absorptive root 13C enrichment) in relation to plant N uptake (as measured by whole plant 15N enrichment). Individual species are shown with unique symbols, with filled shaped representing ECM species. Linear regression lines of best fit for each mycorrhizal type are shown with 95% confidence interval individually. For AM species, r2 = 0.18, P = 0.001; for ECM species, r2 = 0.19, P = 0.003.
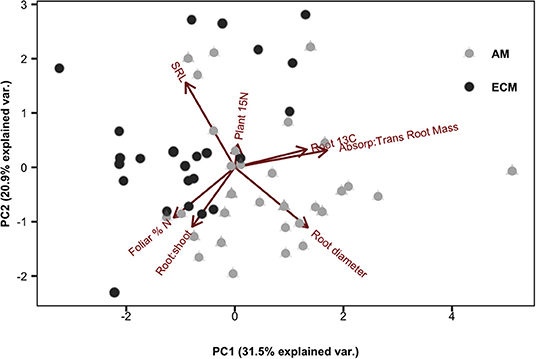
Figure 4. Principal component analysis of individual plant traits including: specific root length (SRL), absorptive root diameter, plant 15N, absorptive: transport root mass, root 13C (per unit 13C fixed), root: shoot mass, and foliar % N.
Discussion
Trees allocate a significant amount of C belowground to acquire limiting resources, yet we lack a predictive understanding of what controls belowground C flux and how this relates to plant nutrient uptake. Using a dual 13C and 15N isotopic labeling approach under controlled greenhouse conditions, we examined how transfer of recently fixed C belowground varied across nine different tree species that are known to associate with either AM or ECM fungi. We then assessed how variation in belowground C allocation related to plant N uptake. We found that fixed 13CO2 was rapidly transferred from sapling foliage to absorptive roots, and this rate varied across species but not between mycorrhizal groups (Figure 1). Notably, we found that the percentage of 13C allocated to absorptive roots was related to plant N uptake, but this relationship depended on plant mycorrhizal type: AM trees showed a positive relationship between root 13C and plant 15N, while this relationship was negative for ECM trees (Figure 3). This suggests that the N return on C invested belowground may be greater for AM tree species relative to ECM tree species and that plants' return on belowground investment may be mediated by distinct plant functional group traits.
Belowground C Dynamics
Our results highlight the importance of species identity in driving belowground C allocation patterns. Most evidence of species differences to date comes from studies comparing only a few species (e.g., Phillips and Fahey, 2005; Yin et al., 2018), mainly non-woody plants (e.g., Cheng, 2009) and often from broadly distinct plant functional groups (e.g., Dijkstra et al., 2009). Here, we expand on this work and report evidence of species-specific patterns across nine temperate deciduous tree species.
Notably, principal component analysis revealed close alignment between the vectors describing the variation in absorptive: transport root biomass and those for root 13C enrichment (Figure 4). Why were species with the most absorptive roots the strongest sinks for recently-fixed C? One hypothesis is that absorptive roots contain the greatest density of rhizosphere microbes, which increase C sink strength by assimilating C released from roots as rhizodeposits. Other pulse-labeling studies have found a correlation between 13C recovered in roots and microbially processed 13C recovered in the soil or as CO2 efflux (SI Figure 3; Dilkes et al., 2004; Phillips and Fahey, 2005; Pausch et al., 2012; Karst et al., 2016), indicating that much of the recently-fixed C allocated to roots ultimately ends up in soil (Karst et al., 2016). Alternatively, interspecific differences in belowground C allocation may relate to differences in C surplus and transport. Under nutrient limiting conditions, plants export C from leaves to other tissues to avoid biochemical inhibition of photosynthesis (Sharkey et al., 1986). In a study using three of the nine species used in this study (sugar maple, tulip poplar and white oak), export of recently-fixed C out of leaves was greater for sugar maple and tulip poplar relative to white oak (Kannenberg and Phillips, 2017), which aligns with our findings (Figure 1).
We did not find any evidence to support our hypothesis that ECM trees allocate more C belowground than AM trees. This contrasts with both theory and field studies of mature trees that have reported that ECM-associated tree species allocate more C to absorptive roots, mycorrhizal hyphae and root exudates compared to AM-associated species (Brzostek et al., 2012, 2014; Yin et al., 2014, but see Liese et al., 2017). One possible explanation for our finding of no mycorrhizal group differences is that our roots were likely only weakly colonized by mycorrhizal fungi. While we did not measure mycorrhizal colonization of root tissues in this study, a previous investigation using a subset of species (maple, tulip and white oak) and similar growing conditions found that few of the roots contained mycorrhizal structures (Kannenberg and Phillips, 2017). Thus, the lack of a mycorrhizal type effect on rhizosphere C flux in our study may point to the importance of mycorrhizal fungal partners as key drivers of root C allocation and movement of C from plant to soil pools (Jones et al., 2004; Högberg et al., 2008). To the extent that plants are able to actively regulate C transfer to mycorrhizal partners, and given some evidence that ECM fungi typically have a higher plant C cost compared to AM fungi (Field et al., 2017), AM and ECM plant functional type differences in rhizosphere C fluxes may be more pronounced when mycorrhizal colonization rates are high. This warrants future field studies quantifying AM and ECM differences in root C fluxes into the soil and assessing the degree to which % mycorrhizal colonization drives these patterns.
Plant N Uptake and N Return on C Investment
Our results also reveal significant species variation in plant 15N uptake, with evidence that more acquisitive root traits (e.g., specific root length) enable greater N uptake (Figure 4). While we initially hypothesized greater plant 15N uptake would be positively related to root 13C across all species, only AM species showed a significant positive relationship. In contrast, root 13C and plant 15N were negatively related across ECM species (Figure 3). These distinct patterns of C-N coupling between mycorrhizal types may be due, in part, to differences in rhizodeposition stoichiometry. Stoichiometric variation in root-derived compounds could mediate opposite patterns in microbially-mediated soil N cycling and plant N uptake (Drake et al., 2013). For example, release of high-quality substrates into the rhizosphere should stimulate microbial activity, induce a positive priming effect and increase plant-available inorganic N. In contrast, low quality rhizodeposits may induce greater microbial community N limitation, enhanced microbial N immobilization and lower plant-available N (Cheng and Kuzyakov, 2005; Kuzyakov and Xu, 2013). Many ECM plants contain higher concentrations of condensed tannins and phenolics than AM plants (Midgley et al., 2015; Fox et al., manuscript in preparation), and these compounds can slow the decay of root-derived inputs (Kraus et al., 2003; Sun et al., 2018). To the extent that root chemistry reflects rhizodeposit chemical quality, rhizodeposition stoichiometry could play a key role in driving distinct patterns of soil C-N coupling between mycorrhizal types.
Overall, the positive relationship between belowground C allocation and plant N uptake observed among AM species aligns with theory suggesting plants allocate more energy belowground to acquire limiting resources and subsequently benefit from this preferential allocation. Why then did ECM trees show the opposite pattern (i.e., a negative relationship between belowground C allocation and N uptake)? Plant N uptake capacity also mediates the nature of this “C for N” exchange. That is, plants with a poor ability to take up N (per unit C) may need to allocate substantially more C to absorptive roots to compensate for the low N uptake capacity, while plants with high N uptake capacity per unit C would be expected to allocate less C to absorptive roots. Such a pattern would result in a negative relationship between absorptive root 13C and plant 15N, as observed among ECM species.
Importantly, allocation of newly fixed C to absorptive roots is only one of a suite of possible nutrient uptake strategies and our results highlight the degree to which complex suites of morphological and physiochemical traits control plant-soil C and N dynamics. As such, a simple “C for N” economic investment framework may not fully capture how plant C allocation relates to resource uptake across plant functional types. For example, two morphological root traits (i.e., SRL and absorptive root diameter) were orthogonal to root 13C, while absorptive: transport root mass aligned closely with root 13C, suggesting important but complex links between plant morphological traits and their effects on element cycling. This builds on previous work that has shown such traits to be an important factor driving species-specific patterns of rhizosphere priming effects, given that root morphology influences the rhizosphere surface area: root biomass ratio (Cheng et al., 2014). Additionally, root maintenance incurs a C cost that is not captured by commonly measured root traits. Differences between mycorrhizal groups in this maintenance cost could also help explain distinct C-N coupling patterns between AM and ECM plant species. Finally, roots are tasked with acquiring diverse resources belowground, and different suites of traits may be advantageous for different resource environments. For example, plants in water-limited environments may prioritize deeper, woody roots while nutrient-limited plants may allocate more energy toward shallow, absorptive roots. Taken together, our work suggests that evidence of a well-coordinated root economic spectrum may remain elusive (Mommer and Weemstra, 2012; Mccormack and Iversen, 2019) due to the multi-functional nature of roots.
Our greenhouse results motivate future field studies of species effects on rhizosphere C-N coupling. Additional studies are needed to understand how effects of saplings may differ from those of mature trees. While saplings may have higher rhizodeposition (Fu and Cheng, 2002), mature trees may exert stronger rhizosphere effects given increased total root mass (Kuzyakov, 2002). Additionally, although biotic and abiotic soil properties are recognized as important factors driving rhizosphere dynamics (Jones et al., 2004), how these factors interact with plant species effects under field conditions to influence C-N coupling in forests remains poorly quantified. For example, while N-limitation is common in temperate forests, other nutrients (e.g., Ca, P) as well as water can also control plant productivity and C allocation. Additionally, while N stocks tend to be greater in AM than ECM temperate forest soils (Craig et al., 2018), more of this N may be bound up in protected forms in AM soils (Craig et al., 2019) and therefore not as susceptible to microbial priming. Future research should consider how such soil physiochemical variables may mediate belowground C dynamics and plant nutrition.
Overall, we found species-specific differences in belowground C allocation across nine tree species, indicating the importance of species identity in driving rhizosphere C dynamics. Additionally, we found the relationship between belowground C allocation and plant N uptake depended on plant mycorrhizal type. While roots and root-microbe interactions are increasingly being recognized as key components of ecosystem and earth system models (Ostle et al., 2009; McCormack et al., 2017), the sensitivity of these models to species identity remains poorly understood. Moreover, species effects on rhizosphere dynamics appear to be sensitive to global change (Cheng et al., 2012; Sulman et al., 2017). Thus, future data-fusion efforts combining greenhouse and field studies with modeling experiments are called for to develop accurate and predictive carbon-climate products.
Data Availability Statement
Data are publicly available in the figshare repository, accessible here: https://figshare.com/articles/13C_and_15N_pulse_labeling_data_from_greenhouse_experiment/11323337.
Author Contributions
AK and RP conceived and designed the experiment, revised, and wrote the final manuscript. AK carried out the experiment, performed the data analysis, and wrote the first draft.
Funding
We acknowledge the support of the U.S. Department of Energy Office of Biological and Environmental Research, Terrestrial Ecosystem Science Program (Award# DESC0016188) and the US National Science Foundation Ecosystem Studies Program (1153401).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to two reviewers for their comments and feedback which greatly improved our manuscript. We thank Daniel Boyes, Dan Du, and Megan Du for assistance in the lab, and Sally Keller for her unparalleled tree planting skills. Special thanks to Steve Kannenberg for construction of the growth chamber. Peter Sauer was helpful in experimental design and analysis of the isotope samples. We thank Phillips Lab members and Saskia Klink for comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2019.00081/full#supplementary-material
References
Averill, C., Bhatnagar, J. M., Dietze, M. C., Pearse, W. D., and Kivlin, S. N. (2019). Global imprint of mycorrhizal fungi on whole-plant nutrient economics. PNAS 116, 23163–23168. doi: 10.1073/pnas.1906655116
Bloom, A. J., Chapin, F. S., Mooney, H. A., Bloom, A. J., and Mooney, H. A. (1985). Resource limitation in plants - an economic analogy. Ann. Rev. Ecol. Syst. 16, 363–392. doi: 10.1146/annurev.es.16.110185.002051
Brzostek, E. R., Dragoni, D., Brown, Z. A., and Phillips, R. P. (2015). Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol. 206, 1274–1282. doi: 10.1111/nph.13303
Brzostek, E. R., Fisher, J. B., and Phillips, R. P. (2014). Modeling the carbon cost of plant nitrogen acquisition: mycorrhizal trade-offs and multi-path resistance uptake improve predictions of retranslocation. J. Geophys. Res Biogeogr. 119. doi: 10.1002/2014JG002660
Brzostek, E. R., Greco, A., Drake, J. E., and Finzi, A. C. (2012). Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115, 65–76. doi: 10.1007/s10533-012-9818-9
Chapin, F. S. III., Schulze, E.-D., and Mooney, H. A. (1990). The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 21, 423–447. doi: 10.1146/annurev.es.21.110190.002231
Cheeke, T. E., Phillips, R. P., Brzostek, E. R., Rosling, A., Bever, J. D., and Fransson, P. (2017). Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol. 214, 432–442. doi: 10.1111/nph.14343
Cheng, L., Booker, F. L., Tu, C., Burkey, K. O., Zhou, L., Shew, H. D., et al. (2012). Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337, 1084–1087. doi: 10.1126/science.1224304
Cheng, W. (2009). Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C–N budgets. Soil Biol. Biochem. 41, 1795–1801. doi: 10.1016/j.soilbio.2008.04.018
Cheng, W., and Kuzyakov, Y. (2005). Root Effects on Soil Organic Matter Decomposition. Page Roots and Soil Management: Interactions Between Roots and the Soil. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America.
Cheng, W., Parton, W. J., Gonzalez-Meler, M. A., Phillips, R., Asao, S., McNickle, G. G., et al. (2014). Synthesis and modeling perspectives of rhizosphere priming. New Phytol. 201, 31–44. doi: 10.1111/nph.12440
Craig, M. E., Lovko, N., Flory, S. L., Wright, J. P., and Phillips, R. P. (2019). Impacts of an invasive grass on soil organic matter pools vary across a tree-mycorrhizal gradient. Biogeochemistry 144, 149–164. doi: 10.1007/s10533-019-00577-2
Craig, M. E., Turner, B. L., Liang, C., Clay, K., Johnson, D., and Phillips, R. P. (2018). Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil carbon and nitrogen. Glob. Chang. Biol. 24, 3317–3330. doi: 10.1111/gcb.14132
Dijkstra, F. A., Bader, N. E., Johnson, D. W., and Cheng, W. (2009). Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability? Soil Biol. Biochem. 41, 1080–1087. doi: 10.1016/j.soilbio.2009.02.013
Dilkes, N. B., Jones, D. L., and Farrar, J. (2004). Temporal dynamics of carbon partitioning and rhizodeposition in wheat. Plant Physiol. 134, 706–715. doi: 10.1104/pp.103.032045
Drake, J. E., Darby, B. A., Giasson, M. A., Kramer, M. A., Phillips, R. P., and Finzi, A. C. (2013). Stoichiometry constrains microbial response to root exudation-insights from a model and a field experiment in a temperate forest. Biogeosciences 10, 821–838. doi: 10.5194/bg-10-821-2013
Drake, J. E., Gallet-Budynek, A., Hofmockel, K. S., Bernhardt, E. S., Billings, S. A., Jackson, R. B., et al. (2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357. doi: 10.1111/j.1461-0248.2011.01593.x
Elser, J. J., Bracken, M. E., Cleland, E. E., Gruner, D., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Epron, D., Bahn, M., Derrien, D., Lattanzi, F. A., Pumpanen, J., Gessler, A., et al. (2012). Pulse-labelling trees to study carbon allocation dynamics : a review of methods, current knowledge and future prospects. Tree Physiol. 32, 776–798. doi: 10.1093/treephys/tps057
Field, K. J., Davidson, S. J., Alghamdi, S. A., and Cameron, D. (2017). “Magnitude, dynamics and control of the carbon flow to mycorrhizas,” in Mycorrhizal Mediation of Soil, eds N. Johnson, J. Jansa, and K. Gehring (Chichester: John Wiley & Sons), 375–393.
Finzi, A. C., Abramoff, R. Z., Spiller, K. S., Brzostek, E. R., Darby, B. A., and Kramer, M. A. (2015). Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 21, 2082–2094. doi: 10.1111/gcb.12816
Franklin, O., Johansson, J., Dewar, R. C., Dieckmann, U., Mcmurtrie, R. E., Brännström, A., et al. (2012). Modeling carbon allocation in trees : a search for principles. Tree Physiol. 32, 648–666. doi: 10.1093/treephys/tpr138
Fu, S., and Cheng, W. (2002). Rhizosphere priming effects on the decomposition of soil organic matter in C4 and C3 grassland soils. Plant Soil 238, 289–294. doi: 10.1023/A:1014488128054
Gill, A. L., and Finzi, A. C. (2016). Belowground carbon flux links biogeochemical cycles and resource-use efficiency at the global scale. Ecol. Lett. 12, 1419–1428. doi: 10.1111/ele.12690
Grayston, S. J., Vaughan, D., and Jones, D. (1997). Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5, 29–56. doi: 10.1016/S0929-1393(96)00126-6
Heimann, M., and Reichstein, M. (2008). Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292. doi: 10.1038/nature06591
Högberg, P., Högberg, M. N., Göttlicher, S. G., Betson, N., Keel, S. G., Metcalfe, D. B., et al. (2008). High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 177, 220–228. doi: 10.1111/j.1469-8137.2007.02238.x
Jo, I., Fei, S., Oswalt, C. M., Domke, G. M., and Phillips, R. P. (2019). Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci. Adv. 5:eaav6358. doi: 10.1126/sciadv.aav6358
Jones, D. L., Hodge, A., and Kuzyakov, Y. (2004). Plant and mycorrhizal regulation rhizodeposition. New Phytol. 163, 459–480. doi: 10.1111/j.1469-8137.2004.01130.x
Kannenberg, S. A., and Phillips, R. P. (2017). Soil microbial communities buffer physiological responses to drought stress in three hardwood species. Oecologia 183, 631–641. doi: 10.1007/s00442-016-3783-2
Karst, J., Gaster, J., Wiley, E., and Landhäusser, S. M. (2016). Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol. 37, 154–164. doi: 10.1093/treephys/tpw090
Keller, A. B., and Phillips, R. P. (2019). Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytol. 222, 556–564. doi: 10.1111/nph.15524
Kong, D., and Ma, C. (2014). Acquisition of ephemeral module in roots: a new view and test. Sci. Rep. 4, 4–7. doi: 10.1038/srep05078
Kraus, T. E. C., Dahlgren, R. A., and Zasoski, R. J. (2003). Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil. 256, 41–66. doi: 10.1023/A:1026206511084
Kuzyakov, Y. (2002). Review: factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 165, 382–396. doi: 10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-%23
Kuzyakov, Y., and Xu, X. (2013). Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 198, 656–669. doi: 10.1111/nph.12235
LaBauer, D. S., and Treseder, K. K. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystem is globally distributed. Ecology 89, 371–379. doi: 10.1890/06-2057.1
Liese, R., Lübbe, T., Albers, N. W., and Meier, I. C. (2017). The mycorrhizal type governs root exudation and nitrogen uptake of temperate tree species. Tree Physiol. 38, 83–95. doi: 10.1093/treephys/tpx131
Lin, G., Mccormack, M. L., Ma, C., and Guo, D. (2016). Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 213, 1440–1451. doi: 10.1111/nph.14206
Litton, C. M., Raich, J. W., and Ryan, M. G. (2007). Carbon allocation in forest ecosystems. Glob. Chang. Biol. 13, 2089–2109. doi: 10.1111/j.1365-2486.2007.01420.x
McCormack, M. L., Dickie, I. A., Eissenstat, D. M., Fahey, T., Fernandez, C. W., Guo, D., et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 207, 505–518. doi: 10.1111/nph.13363
McCormack, M. L., Guo, D., Iversen, C. M., Chen, W., Eissenstat, D., Fernandez, C. W., et al. (2017). Building a better foundation: improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 215, 27–37. doi: 10.1111/nph.14459
Mccormack, M. L., and Iversen, C. M. (2019). Physical and functional constraints on viable belowground acquisition strategies. Front. Plant Sci. 10, 1–12. doi: 10.3389/fpls.2019.01215
Midgley, M. G., Brzostek, E., and Phillips, R. P. (2015). Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. J. Ecol. 103, 1454–1463. doi: 10.1111/1365-2745.12467
Mommer, L., and Weemstra, M. (2012). The role of roots in the resource economics spectrum. New Phytol. 195, 725–727. doi: 10.1111/j.1469-8137.2012.04247.x
Nguyen, C. (2003). Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23, 375–396. doi: 10.1051/agro:2003011
Ostle, N. J., Smith, P., Fisher, R., Woodward, F. I., Fisher, J., Smith, J. U., et al. (2009). Integrating plant – soil interactions into global carbon cycle models. J. Ecol. 97, 851–863. doi: 10.1111/j.1365-2745.2009.01547.x
Pausch, J., Tian, J., and Riederer, M. (2012). Estimation of rhizodeposition at field scale:upscaling of a 14 C labeling study. Plant Soil 364, 273–285. doi: 10.1007/s11104-012-1363-8
Pendall, E., Pendall, E., Bridgham, S., Hanson, P. J., Hungate, B., Kicklighter, D. W., et al. (2004). Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phytol. 162, 311–322. doi: 10.1111/j.1469-8137.2004.01053.x
Phillips, R. P., Brzostek, E., and Midgley, M. G. (2013). The mycorrhizal-associated nutrient economy : a new framework for predicting carbon – nutrient couplings in temperate forests. New Phytol. 199, 41–51. doi: 10.1111/nph.12221
Phillips, R. P., and Fahey, T. J. (2005). Patterns of rhizosphere carbon flux in sugar maple (Acer saccharum) and yellow birch (Betula allegheniensis) saplings. Glob. Chang. Biol. 11, 983–995. doi: 10.1111/j.1365-2486.2005.00959.x
Raich, J. W., and Nadelhoffer, K. J. (1989). Belowground carbon allocation in forest ecosystems: global trends. Ecology 70, 1346–1354. doi: 10.2307/1938194
Schimmelmann, A., Albertino, A., Sauer, P. E., Qi, H., Molinie, R., and Mesnard, F. (2009). Nicotine, acetanilide and urea multi-level 2H-, 13C- and 15N-abundance reference materials for continuous-flow isotoe ratio mass spectrometry. Rapid Commun. Mass Sp. 23, 3513–3521. doi: 10.1002/rcm.4277
See, C. R., Luke Mccormack, M., Hobbie, S. E., Flores-Moreno, H., Silver, W. L., and Kennedy, P. G. (2019). Global patterns in fine root decomposition : climate, chemistry, mycorrhizal association and woodiness. Ecol. Lett. 22, 946–953. doi: 10.1111/ele.13248
Sharkey, T. D., Stitt, M., Heineke, D., Gerhardt, R., Raschke, K., Heldt, H., et al. (1986). Limitation of photosynthesis by carbon metabolism II: O2-insensitive CO2 uptake results from limitation of triose phosphate utilization. Plant Physiol. 81, 1123–1129. doi: 10.1104/pp.81.4.1123
Sulman, B. N., Brzostek, E. R., Medici, C., Shevliakova, E., Menge, D. N., and Phillips, R. P. (2017). Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol. Lett. 20, 1043–1053. doi: 10.1111/ele.12802
Sun, T., Hobbie, S. E., Berg, B., Zhang, H., Wang, Q., Wang, Z., et al. (2018). Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. U.S.A. 115, 10392–10397. doi: 10.1073/pnas.1716595115
Weemstra, M., Mommer, L., Visser, E. J. W., Van Ruijven, J., Kuyper, T., Mohren, G. M. J., et al. (2016). Towards a multidimensional root trait framework: a tree root review New Phytol. 211, 1159–1169. doi: 10.1111/nph.14003
Wurzburger, N., and Brookshire, E. N. J. (2017). Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology 98, 1491–1497. doi: 10.1002/ecy.1827
Yin, H., Wheeler, E., and Phillips, R. P. (2014). Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol. Biochem. 78, 1–9. doi: 10.1016/j.soilbio.2014.07.022
Yin, L., Dijkstra, F. A., Wang, P., Zhu, B., and Cheng, W. (2018). Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol. 218, 1036–1048. doi: 10.1111/nph.15074
Keywords: carbon cycling, isotopic approaches, nitrogen uptake, resource allocation, rhizodeposition, species patterns
Citation: Keller AB and Phillips RP (2019) Relationship Between Belowground Carbon Allocation and Nitrogen Uptake in Saplings Varies by Plant Mycorrhizal Type. Front. For. Glob. Change 2:81. doi: 10.3389/ffgc.2019.00081
Received: 19 September 2019; Accepted: 20 November 2019;
Published: 13 December 2019.
Edited by:
Stephanie N. Kivlin, The University of Tennessee, Knoxville, United StatesReviewed by:
Laura Bogar, University of California, Santa Barbara, United StatesChao Wang, Institute of Applied Ecology (CAS), China
Copyright © 2019 Keller and Phillips. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adrienne B. Keller, a2VsbGVyYWJAaW5kaWFuYS5lZHU=
 Adrienne B. Keller
Adrienne B. Keller Richard P. Phillips
Richard P. Phillips