- 1Graduate Program of Environmental Science, Forest Governance Research Group, Institute of Energy and Environment, University of São Paulo, São Paulo, Brazil
- 2Amure Ikpeng School, Xingu Indigenous Park, Brazil
- 3Samauma Village, Xingu Indigenous Territory, Querência, Brazil
- 4The Conservation and Management of Common Pool Resources Group (CGCommons) – Center for Environmental Studies and Research at the University of Campinas (NEPAM/Unicamp), Forest Governance Research Group, Institute of Energy and Environment, University of São Paulo, São Paulo, Brazil
- 5Xingu Program, Instituto Socioambiental (ISA), São Paulo, Brazil
The indigenous systems of agricultural and forest management in the Amazon are characterized by a deep knowledge of ecological processes, biodiversity, and the use and management of fire. The influence of these systems on the distribution of biodiversity includes semi-domesticated and domesticated species and landscapes, which have led to extensive anthropogenic or cultural forests. However, in many places, the livelihoods of indigenous peoples are being transformed by the intensification of agriculture and social, ecological, and economic changes, putting at risk the sustainability of production systems and food security and sovereignty of these peoples. In the last years, in the Xingu Indigenous Territory (XIT), the food production systems and the form of occupation of territories have changed, affecting the recovery of secondary forests, which now demand a too long period. The increase in the number and frequency of fires has aggravated this situation, due to a drier climate that has become predominant in the region. Changes in climate are attributed to deforestation in the neighboring municipalities, especially in the headwaters of the Xingu river basin. This study was conducted among the Kawaiwete (Tupi-Guarani) and the Ikpeng (Carib-Arara) peoples in the XIT, in the state of Mato Grosso, Brazil. The main objective was to develop alternative techniques of forest management based on indigenous and scientific knowledge more adapted to the new livelihood contexts, aiming to favor forest regeneration in areas dominated by shifting cultivation. We sought to answer the following questions: (I) How do forests regenerate during the fallow period? (II) How can local management improve forest regeneration? (III) Are there indicator species for secondary succession, soil recovery, and vulnerability to fires? (IV) Is the increase in the number of fires affecting the sustainability of the shifting cultivation systems? Our results show that some local practices based on indigenous knowledge have the potential to facilitate natural regeneration, such as choosing forest areas that have been recovered for agricultural use, limiting the number of cultivation cycles, protecting and selecting of individual trees during cultivation period, and attracting seed dispersers. Assisted natural regeneration strategies grounded on indigenous knowledge are promising ways to restore degraded lands of the XIT.
Introduction
In the region of the Xingu Indigenous Territory (XIT), in the state of Mato Grosso (Brazil), several factors are affecting indigenous shifting cultivation systems, with consequences to the natural regeneration of forests. Changes in the type of occupation of the territory by indigenous peoples and in local rules that guide the use of agricultural environments may be the main causes of it. However, external factors, such as the intense and rapid change in land use around the XIT, which occurred in the last 50 years, have affected environmental conditions within the territory (Sanches and Villas-Bôas, 2008; Durigan et al., 2013; Sanches and Futemma, 2019). As a result of the increase in value of land for planting grains, there has been an increase in regional deforestation rates, which in turn has led to changes in the regional microclimate (Silvério et al., 2015), rainfall regimes, and levels of soil moisture (Nobre et al., 1991; Li et al., 2006; Morton et al., 2013), contributing to an increase in unintended fires (Nepstad et al., 2004; Brando et al., 2014; Alencar et al., 2015). With a drier climate, several accidents have occurred during the burning of areas to open traditional swiddens, leading to losses of forest areas. Thus, indigenous peoples in the XIT began to face challenges in finding strategies to ensure the sustainability of agricultural systems and adapting them to the new environmental context.
In many tropical regions, there is an increasing pressure to intensify indigenous productive systems (Denevan et al., 1984; Charlton, 1987; Denevan, 2001) as a result of delimitation of territories and population growth (Johnson, 1983; Freire, 2003, 2007; Toledo and Salick, 2006). In a political-economic context of accelerated cultural and social changes, the current challenge is how to reconcile or adapt traditional management practices while maintaining a sustainable use of these indigenous territories, which are increasingly circumscribed by the pressures of the surroundings (Le Tourneau, 2015; Jusys, 2018). Many indigenous productive systems in tropical forests are based on shifting cultivation, which depends on a fallow period for the regeneration of secondary forests and soil fertility (Ewel, 1986; Mertz et al., 2009; Ribeiro Filho et al., 2013, 2018). When subjected to agricultural intensification processes, the fallow time is shortened and may be insufficient to ensure sustainability, leading to forest degradation (Marquardt et al., 2013; van Vliet et al., 2013).
In the Amazon region, although the origin of anthropogenic forests is still under debate, evidence shows that there is an important contribution of indigenous food production systems and local communities to the generation and maintenance of biodiversity (Clement, 1999; Miller and Nair, 2006; Balée, 2010; Shepard and Ramirez, 2011; Sutherland et al., 2013; Tengö et al., 2014, 2017). Forest and agricultural management often leads to changes in species composition and in soils (Smith, 1980; Balée, 1993; Heckenberger and Neves, 2009; Arroyo-Kalin, 2010; Junqueira et al., 2011; Woods et al., 2013; Schmidt et al., 2014; Levis et al., 2018, 2020), and are based on a deep knowledge of ecological dynamics and on social and cultural rules (Berkes and Berkes, 2009).
The shifting cultivation practiced by indigenous Amazonian peoples depends on the multifunctional management of the landscape during the cultivation phase (van Vliet et al., 2012; Mukul, 2016), which includes the simultaneous cultivation of several cultivated/domesticated and semi-domesticated species (Hett et al., 2012). The combination of social and ecological dynamics leads to different successional trajectories (Wangpakapattanawong et al., 2010; Chazdon, 2014b; Chazdon and Uriarte, 2016; Uriarte and Chazdon, 2016). One of the most important aspects of indigenous adaptive management is the use of plants according to their potential to promote the natural regeneration of the forest and the recovery of biodiversity during forest succession in the post-cultivation fallow period (Wangpakapattanawong et al. op. cit; Chazdon, 2013), safeguarding fundamental species, including those used in material and immaterial culture.
However, despite the importance of the forestry and agricultural management of indigenous peoples for the diversification of Amazonian landscapes (Neves et al., 2003; Clement et al., 2015), less attention has been paid to forest recovery processes after agricultural cultivation (Moran, 1996; Jakovac et al., 2015; Hartman et al., 2016), including the development or regrowth of young plants (Tschakert et al., 2007; Jakovac et al., 2015; Uriarte and Chazdon, 2016). Likewise, little is known about the effects of the increase in population pressure, climate change, and the loss of environments and ecological knowledge in some local systems (Magnuszewski et al., 2015; Mukul et al., 2016a, b).
In this paper, we investigate agricultural management by indigenous people in two areas within the XIT that are undergoing processes of agricultural intensification and environmental changes (2013–2016). The areas belong to the Ikpeng and the Kawaiwete peoples. We seek to answer the following questions: (I) How do forests regenerate during the fallow period? (II) How can local management improve forest regeneration? (III) Are there indicator species for secondary succession, soil recovery, and vulnerability to fires? (IV) Is the increase in the number of fires affecting the sustainability of the shifting cultivation systems?
Materials and Methods
The project Fogo do Índio—Management alternatives adapted to climate change for the conservation of forests in the Xingu Indigenous Park was conducted with a technical partnership formalized in 2016 between the non-governmental organization Instituto Socioambiental (ISA) and the Associação Terra Indígena Xingu (ATIX), which represents the interests of the indigenous peoples of the Xingu. The Cooperation Agreement contemplates strategic projects and activities in areas considered a priority to the indigenous peoples of the XIT, such as supporting initiatives aiming to raise awareness of and protect the Xingu peoples from the impacts of climate change. The construction of the project started in the Moygu Ikpeng village in response to internal demands resulting from a gradual increase in the number of fires in the XIT region, which caused several impacts on local livelihoods. All activities were conducted with the consent of the leaders and the people from the indigenous communities involved in the Moygu/Arayo Ikpeng1 and Samaúma Kawaiwete villages, counting with a fundamental role of their elders. The main local interlocutors who acted in mobilizing communities and who had an active involvement in research activities are co-authors of this article.
The Xingu Indigenous Territory (XIT) and the Villages Analyzed in This Study
The XIT has an area of 2.8 million hectares and occupies a region of great biological and cultural diversity in the northeast of the state of Mato Grosso, Brazil, in the Parecis Plateau (Sanches et al., 2012). This region has distinct physiognomies that follow a gradual climatic and edaphic variability, and the species are characteristic of an ecological transition area (Radam Brasil, 1985). In this region, Evergreen Seasonal Forest predominates (Ivanauskas et al., 2008). However, there are also different savanna formations, among other wetlands and lowland swamps (Sanches et al., 2012). The predominant climate in the southern Amazon border is tropical rainy savanna (Aw), according to the Köppen classification (1948). Temperatures are above 18°C in two well-defined seasons: a rainy season (more than 1,200 mm of rainfall) and a dry season (less than 100 mm of rainfall). Seasons may vary between 4 and 7 months per year (Ivanauskas et al., op. cit.).
In addition to these natural characteristics, the XIT holds a significant part of the Brazilian sociodiversity. There are 16 indigenous peoples in this location2. Most of these peoples did not originally inhabit this region but were transferred there when the Brazilian government created the territory in 1961 (BRASIL, 1961). The limitation of land for establishing indigenous territories resulted in the loss of mobility for villages and farming areas of various peoples, leading to a scarcity of recovered agricultural land in the vicinity of villages.
In this study, two villages inhabited by different peoples were included: Samauma (Kawaiwete, with a population of 40 individuals) and Moygu/Arayo (Ikpeng, with a total of 300 individuals), both in the middle Xingu River region (Figure 1). Kawaiwete and Ikpeng livelihoods are similar regarding the way they perform subsistence activities such as hunting and fishing (Rodgers, 2002; Txicão and Leão, 2019). There are also similarities in the collection of forest products used for domestic and ritual purposes, in addition to forest-based shifting cultivation practices that complement their systems (Menget, 1981; Schmidt, 2001; Rodgers, 2013; Silva, 2016; Athayde and Lugo, 2018). However, these peoples have peculiar and distinct characteristics regarding their knowledge systems, the languages they speak, and their different origins, in addition to other characteristics of an ethnic and cultural nature (Dole, 2001).
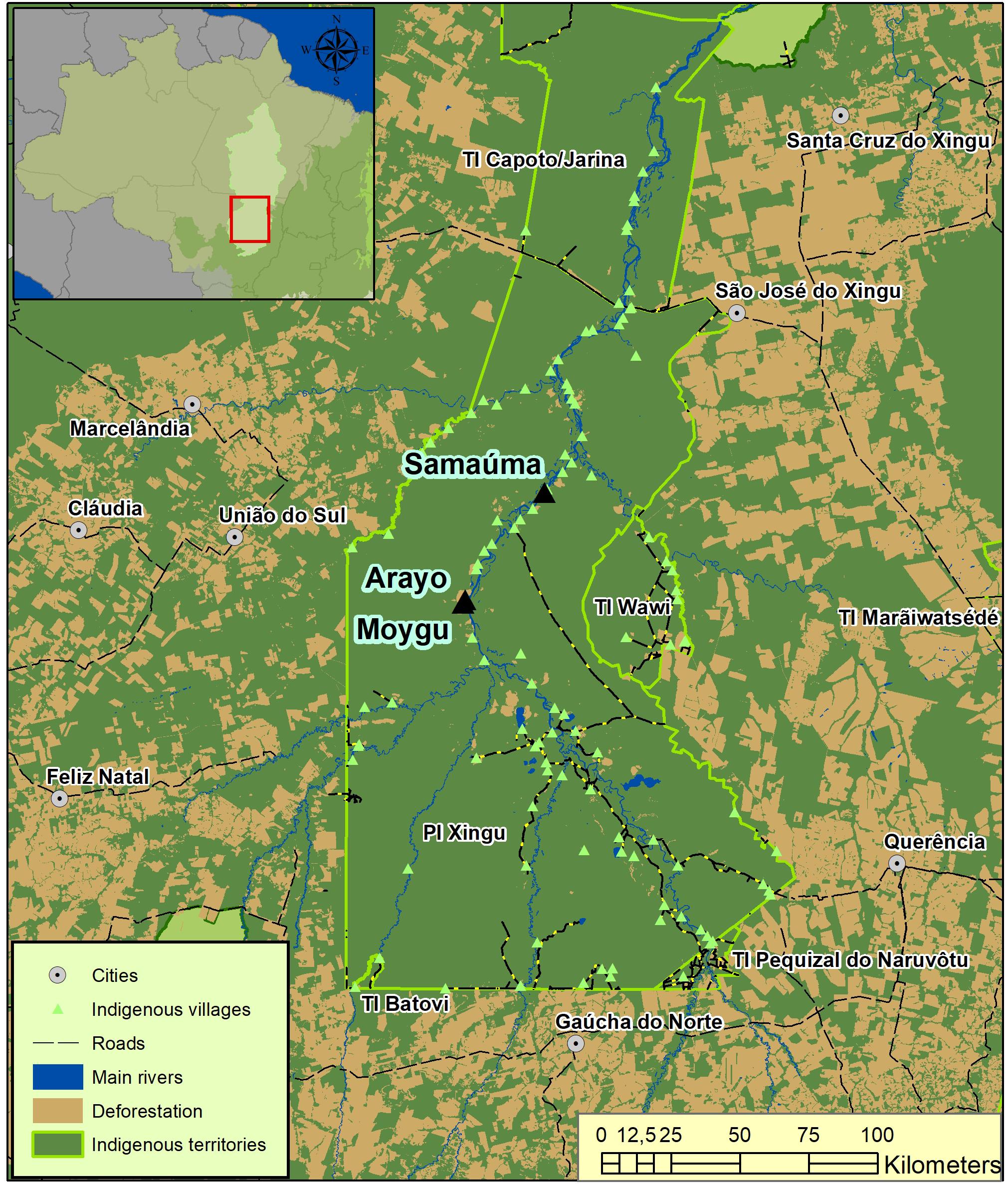
Figure 1. Location of the Xingu River basin, and the villages of Samaúma Kawaiwete, Moygu Ikpeng, and Arayo Ikpeng within the boundaries of the TIX and surrounded by adjacent municipalities that have lost more than 66% of the forest cover in the last 40 years (map by Ricardo Abad, courtesy Instituto Socioambiental, Xingu Program).
The Kawaiwete people are the most populous in the XIT. They are widely distributed throughout the territory in more than 30 villages in the middle and lower Xingu River regions. They speak Tupi-Guarani and are originally from the Tapajós and Arinos river basins. Part of its population was transferred to the Xingu region in the late 1950s (Grümberg, 2004), while another part remained in its old traditional territory in the northwest of Mato Grosso (Athayde and Lugo, op. cit.). The Kawaiwete agriculture is one of the richest compared to those of other peoples in the Xingu (Villas-Bôas and Villas-Bôas, 1986) mainly because their food system presents a greater diversity of crops, among which peanuts (Arachis sp.), corn (Zea sp.), cassava (Manihot sp.), beans (Phaseolus spp. and Vigna spp.), broad beans (Phaseolus sp.), sweet potato (Ipomea sp.), yam (Dioscorea spp.), arrowleaf elephant ear (Xanthosoma spp.), pepper (Capsicum spp.), and tobacco (Nicotiniana sp.) (Grümberg, 2004) stand out.
The Ikpeng people, contacted in 1964 in the tributary region of the middle Ronuro River, were only transferred from their former territory to XIT in 1967 due to the risks of invasion by gold miners (Simões, 1963; Menget, 1981, 2001). The ethnic and cultural origins of the Ikpeng are related to indigenous groups in the northern Amazon and the Guianas, whose language is part of the Arara complex, a subgroup of the Caribbean family (Menget, 2001; Rodgers, 2002). Some of the rare records of Ikpeng agriculture show the cultivation of varieties of crops of beans, peanuts, and tubers (Menget, 1981). Corn (Zea sp.) is relevant in this system. It is used in the preparation of a type of baked bread (pako) and a fermented drink (wonkinom-egr⋅t—beverage of the spirits), still prepared today for the end of the Pomeri feast (Rodgers, 2002), but that was not known by the peoples of the upper Xingu (Menget, 1981). Some of the agroforestry management techniques used by the Ikpeng were incorporated from the systems that already existed among peoples of the upper Xingu, such as cultivation of fruit trees in cassava swiddens, mainly pequi (Caryocar sp.) and mangaba (Hancornia sp.) (Carneiro, 1983 p. 68; Schmidt, 2006), as well as the cleaning of plots, as we present below (Item 3.2). Currently, the Ikpeng are distributed across four villages in the XIT, including the Moygu and Arayo villages, which are part of this study (Figure 1).
Inside the XIT, forests that grow on anthropogenic soils, called “Amazonian Dark Earth” (ADE), assume a strategic importance for the Xingu peoples (Clement et al., 2003; Schmidt, 2013; Schmidt et al., 2014). Its location is restricted to regions of stream headwaters or near ponds (Kern et al., 2003), coinciding with archeological remains (Smith, 1980; Neves et al., 2003). Although both peoples use the ADE, especially for crops more demanding in nutrients, mainly corn (Zea sp.), this soil is more important for the Kawaiwete people, as these lands are used for planting varieties of peanuts (Arachis sp.) and broad beans (Phaseolus spp.) (Silva, 2016).
Forests in soils of non-anthropogenic origin (nADE), also referred to locally as “red earth,” are classified as “Perennial Forest Season—in true red-yellow Podzolic Soils” (Ivanauskas et al., 2008; IBGE, 2012). They represent the predominant landscape matrix in the XIT and, in addition to presenting different types of uses for these peoples, they are also prioritized for the cultivation of cassava varieties (Manihot sp.), which are the basis of local food. Among the Kawaiwete, some varieties are used in the preparation of the puba flour (Grümberg, 2004), while the Ikpeng cultivate varieties indicated for starch extraction to make beiju—tariwe—and others that are used to make a type of sweet porridge—the perereba.
The areas of the municipalities adjacent to the XIT have lost more than 66% of forest cover in the last 40 years (Nascimento et al., 2018; Figure 1). This is the main factor responsible for the greater vulnerability of forests in the region to fires, which have become more frequent (Nepstad et al., 2004; Brando et al., 2014; Alencar et al., 2015), affecting many plants species and strategic environments. The period of occupation of the villages in both locations coincides with the beginning of changes in the regions adjacent to the XIT.
Survey and Participation in the Project
The project activities were conducted between 2013 and 2016, in three stages: (I) co-construction of the project and adaptation of the intercultural research method; (II) data gathering on the successional process, agricultural and forest management techniques, and forest degradation and recovery indicators; and (III) in-field survey of the vegetation and indicators of successional stages, degraded areas, and vulnerability to fires (Table 1).
The project involved 67 people from both villages (100%) at its different stages. In Stage II, the interviews were undertaken with nine Ikpeng men (13% of participants), aged between 30 and 70 years old, and three women aged between 50 and 70 years old (4%). As the Samauma Kawaiwete village comprises a single family, we interviewed only three men, aged 39–70 years old (4%), and only one elderly woman, aged approximately 70 years old (1%). The other participants (61%) were directly involved in Stages I and III, including students from indigenous schools, girls and boys aged 15–18 years old, in addition to young people who aided in the interviews and translated the indigenous languages into Portuguese (Table 1). Here the indigenous names for species, types of management, and phases of forest succession were kept in the original language and written in italics.
(I) Co-construction of the project and adaptation of the intercultural research method:
The project was constructed in a participatory manner with the villages involved and adopted an intercultural research perspective. Participatory projects can provide information on the way natural assets are managed by a given community in a consensual way among all forest users (AFN, 2002) in a process of co-production of knowledge (Bergold and Thomas, 2012). Intercultural research was developed in partnership with the communities in order to access knowledge about local history, characteristics of natural environments and management techniques, and uses of plants, soils, and animals (Cabalzar, 2017). This approach allows relations among the different knowledge systems (scientific and local) to be more balanced and complementary, and based on local conceptions (Candau, 2012, p. 245). Intercultural studies have been used to access the local knowledge of other Amazonian indigenous peoples on the recovery of the agricultural fallows (Schmidt et al., 2010a) and participatory forest management (Schmidt, 2001; Schmidt and Ikpeng, 2006; Schmidt et al., 2010b).
Surveys on shifting cultivation systems were conducted through intercultural courses and workshops in the Moygu, Arayo (Ikpeng), and Samauma (Kawaiwete) villages (Table 1). The surveys sought to identify the main factors contributing to changes in the local systems in order to understand the regeneration processes of secondary forests according to local knowledge. To this end, the communities chose informants who had a renowned knowledge about the territory, as well as about changes in landscapes, to monitor and aid the field research.
(II) Data gathering on the successional process, agricultural and forest management techniques, and forest degradation and recovery indicators;
We conducted semi-structured interviews (Bernard, 2006) with 16 key informants chosen by the communities on issues related to the changes observed in the availability of land for cultivation, forest regeneration, and species used in material culture based on the following questions: How swiddens were cultivated in the past and what has changed today? How does the forest grow again? What are the plants that emerge first? Which ones emerge after a few years? Which ones are essential to the recovery of the forest? These information were essential for identifying the types of plants that are part of the initial, intermediate, final, or mature secondary forest formations, as well as for providing information on impact indicators based on the abundance or scarcity of certain plant species and other types of resources.
Most interviews were recorded in indigenous languages, later translated into Portuguese, and transcribed in full with the aid of some local young people3. During the interviews, we had the collaboration of indigenous teachers, especially from the Amure Ikpeng School, and of some students who participated in the surveys and discussions on environmental changes and possible forms of adaptation.
(III) In-field survey of vegetation and indicators of successional stages, degraded areas, and vulnerability to fires
The field survey sought to identify the predominant environments around both villages and to get to know the local landscape using “monitored trails” with the participation of experienced farmers chosen by the communities (Brondizio and Neves, 1996; Brondízio, 2006; Munari, 2009) (Table 1). The current and older agricultural areas were visited, allowing a qualitative characterization of secondary succession. The areas were characterized and classified according to regeneration phase (initial, intermediate, and final), establishing the trajectories of successional stages since the end of the cultivation period, according to the Kawaiwete and Ikpeng knowledge systems.
In the Samauma village, areas of anthropogenic ADE were visited between the initial and intermediate stages. Three swiddens were in production and three were in fallow. The more advanced stages, on the other hand, could not be located in the areas close to the village, and an oral description by the farmers on them was obtained. In the Moygu/Arayo Ikpeng villages, five swiddens in production on non-anthropogenic soils (nADE, red earth) were visited between the initial and intermediate stages, in addition to seven fallow areas. Likewise, visits to secondary forests were not made at more advanced stages, as they are far from the village and were described only orally. The oral description of forests at a more advanced stage aimed to characterize the reference environments.
The agricultural fallow areas were described according to the history of use—present fallow age and age at the last time it was cut—and the history of recurrence of fires (one, two, three or more times). However, it was not possible to locate and consider agricultural fallows that had not been impacted by fires, so that secondary “control” areas at more advanced stages could not be visited. For each of these areas, the characteristics of structure, composition, type of soil, and other aspects were collected and scientific illustrations were made. The plants from each successional stage were identified, as well as their uses in material/immaterial culture, to better understand how the losses caused by the changes could affect the cultural systems of the indigenous peoples under study.
The plants were identified in indigenous and scientific language, the latter with the support of Dr. Natalia Ivanauskas, a botanist specialist, who conducted the identification of several species from photographic images captured during field surveys. For this purpose, the APG IV botanical classification system (Iv et al., 2016) was used. As there was no collection of botanical material in the areas and the identification was conducted in loco, some of them are still preliminary. Unidentified species are here identified as (ni). The uses of plants were grouped into pre-established categories adapted from Cook (1995): (1) medicinal—including for humans and animals; (2) food—including drinks; (3) construction—including wood, fibers, and plants useful for the manufacture of canoes; (4) material—including handicrafts, hunting gear, paints, hygienic substances, toys, etc.; (5) fuel—firewood, torches, lighters; (6) social use—rituals, magic, smoking, hallucinogenic, drugs; (7) environmental use—including restorative plants, time markers, ornamentation; and (8) poison—including fish poison.
The areas under cultivation were visited to characterize the agricultural system and its influence on the regeneration of forests in the XIT. Through participant observation (Bernard, 2006), we identified together with experienced farmers, elders and leaders, techniques and practices used during the cultivation cycle and monitored some cleaning and product collection activities by recording practices, regenerating plants, and changes in the system. This survey considered local impact indicators, mainly regarding management and forest regeneration during the initial phase of fallow, when the forest is subjected to different historical and land use intensities (Defoer and Budelman, 2000; quoted in Marquardt et al., 2013), in order to identify species that “catalyze” forest regeneration (Chazdon, 2013).
To describe regeneration in areas used for cultivation, we defined a gradient of intensity of agricultural management and recovery of fallow areas based on descriptors of intensification of land use, such as (1) the number of fallow cycles, (2) the method for weeding during the cultivation period, (3) the age of the previous fallow when the forest was cut down, (4) the size of the area used, and (5) the dominant plants (Lawrence, 2004; Mertz et al., 2009; van Vliet et al., 2012; Jakovac et al., 2015, 2016a). These descriptors were recorded and discussed with some participants. Based on this information, lists of species were made according to different levels of land use intensity and the potential for regeneration of agricultural environments that remain in fallow, in addition to their vulnerability to fires. The information obtained in the Samauma Kawaiwete village (Supplementary Table 1) and in the Moygu/Arayo Ikpeng village (Supplementary Table 2) is presented below.
Results
Regeneration of Forests During the Shifting Cultivation Cycle
The Kawaiwete recognize different landscape units during the shifting cultivation cycle according to the successional phases of secondary forests. The production phase includes the cultivation phases (ko), when selective cleaning is conducted; the harvest phase, lasting 2–3 years; and the final phase, when young plants of the forest begin to regenerate (kofet jeapat). The secondary forest then begins to grow, passing to the stage of new fallow (capoeira, in Portuguese) (3–15 years—ojemowyt), medium fallow “growing” (15–30 years—ojemowyrete), until reaching the stage of fallow “turning into a forest” (30 and over 40 years old—ojewyt koferete ramu) (Figure 2).
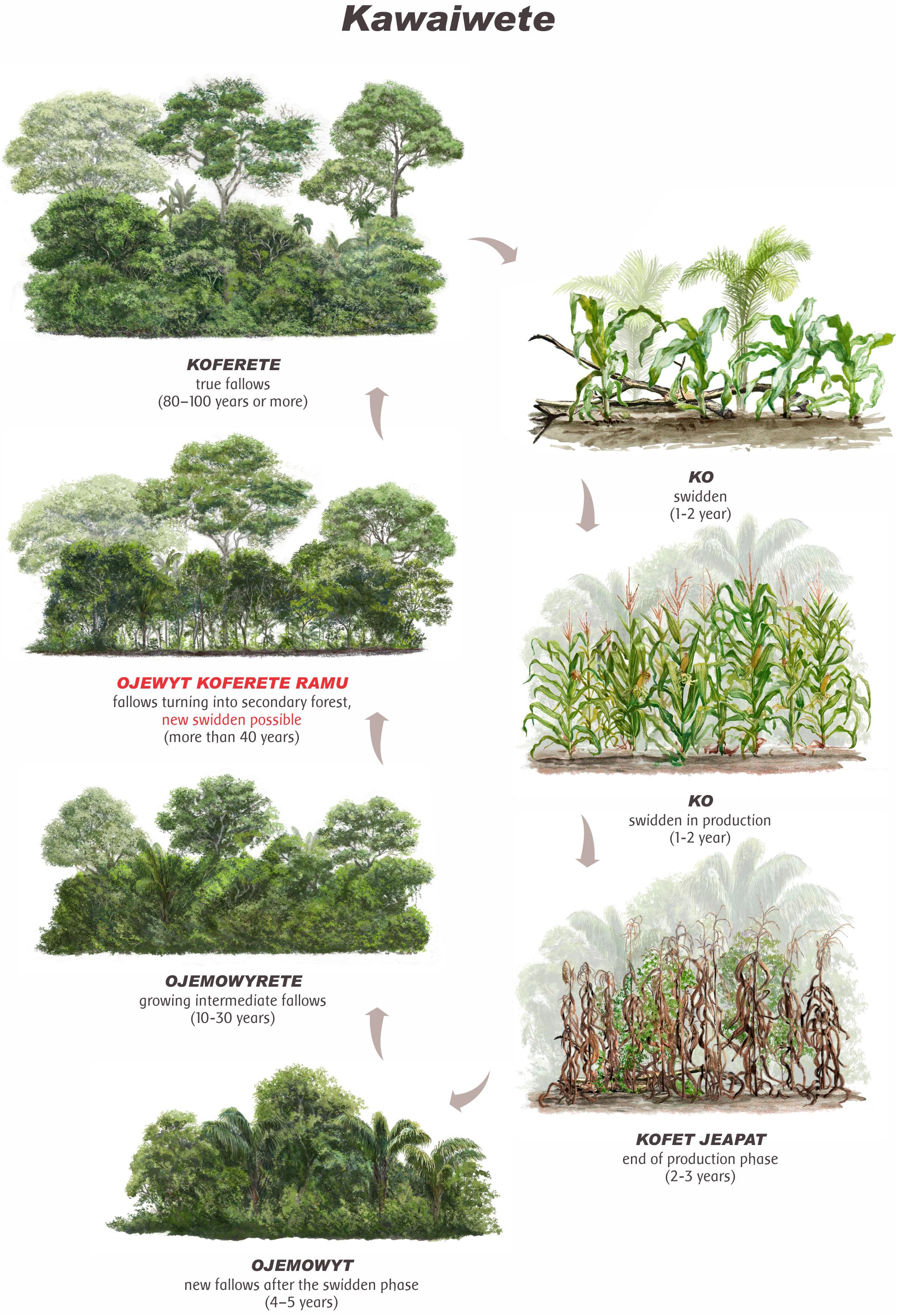
Figure 2. Illustration of Kawaiwete maize (Zea mays L.) shifting cultivation cycle in anthropogenic soils (ADE). When the fallow reaches the OJEWYT KOFERETE RAMU phase (in red), soils and vegetation are considered as recovered and a new cycle may start (Ilustração de Gustavo Marigo).
In the anthropogenic dark earth (ADE), the true fallow (koferete) is considered the most important forest formation for agriculture as it is strategic to the most demanding crops. The forest at this later stage, with at least 40 years old, present a greater diversity of species, a closed canopy and stratified structure, a clean understory, and richer soils, which can exceed 25 m in depth, with a predominance of large trees (>40 cm DAP) and the presence of certain indicator plants. Among the species mentioned are jatyta’yp [Maclura tinctoria (L.) Engl], jatua’yp [Guarea cf. guidonia (L.) Sleumer], pino [Attalea maripa (Aublet) Drude], tukumã (Astrocaryum aculeatum G. Mey.), and yky’ryp (Thyrsodium spruceanum) (Supplementary Table 1). The following illustration represents the successional trajectory in the management of the shifting cultivation cycle in ADE according to the farmers interviewed in the Samauma village (Figure 2). Traditionally, for the Kawaiwete, the fallow should be reused for a new shifting cultivation cycle only after reaching the stage between 30 and 40 years (ojewyt koferete ramu).
Similarly, the Ikpeng also classify the secondary forest development phases into succession stages during the shifting cultivation cycle. In this case, we investigated the shifting cultivation cycle in non-anthropologenic earth (nADE), or in “red earth” (akyun), used mainly for the cultivation of cassava (Manihot sp.) and some fruit trees, although the Ikpeng also use dark earth environments (ADE, iruktowowon).
The old forests (irwa) are sought after for planting new gardens (tukto) and can be used for up to three production cycles (ine). They are managed by selective cleaning, harvesting, and replanting techniques up to the end of the cultivation stage and the beginning of the new fallow stage (irïnpïn). The initial fallow stage (3–5 years, awmtu) is followed by the fallow stage (up to approximately 15 years, tukto rïnpïn), advancing to fallow at an advanced stage of growth (15–30 years, iprororetpïn), until reaching the secondary forest stage “with a clean understory” (30 years or older, oremyegetpïn) (Figure 3).
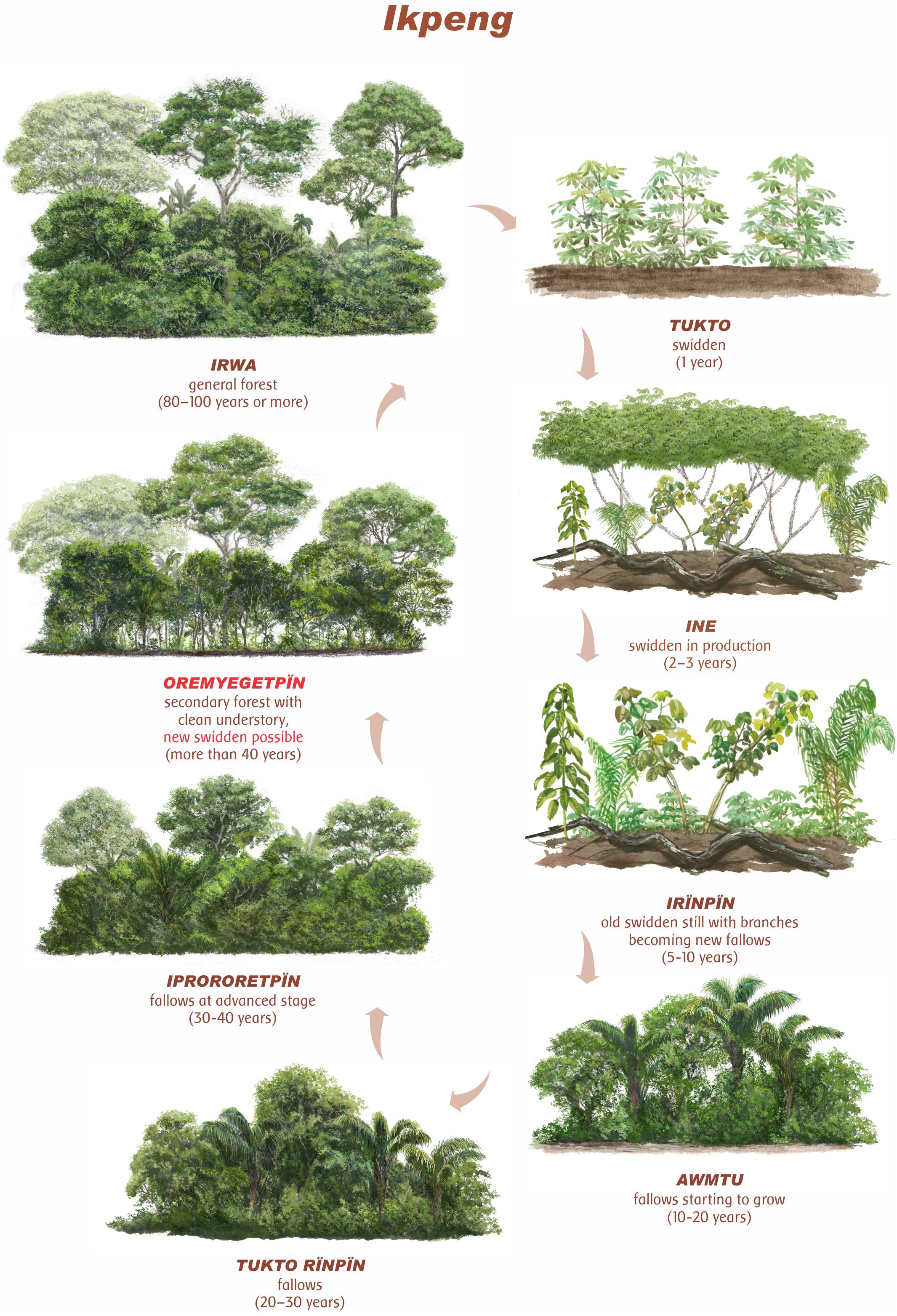
Figure 3. Illustration of Ikpeng cassava (Manihot esculenta Crantz) shifting cultivation cycle in non-anthropogenic soils (nADE). When the fallow reaches the OREMYEGETPÏN phase (in red), soils and vegetation are considered as recovered and a new cycle may start (Ilustração de Gustavo Marigo).
According to Ikpeng farmers, the gardens should preferably be opened at this last stage, which characterizes the most advanced stage of secondary forest growth, with trees already developed (>45 cm DBH) and a canopy height higher than 25 m. At this stage, some tree species are recognized as indicator species, such as kumpang [Emmotum Emmotum nitens (Benth.) Miers], magra (Sacoglotis guianensis Benth.), motoe [Helicostylis tomentosa (Poepp. and Endl.) J. F. Macbr.], oyot [Dypterix odorata (Aubl.) Wild.], yego (Vochysia ferruginea Mart.), yemkat (Apeiba cf. tibourbou Aubl.), katepo (Hymenaea courbaril L.), raegï (Xylopia amazonica R. E. Fries), roromi [Dialium guianense (Aublet) Sandw.], muret (Byrsonima intermedia A. Juss.), ototo (Astrocaryum aculeatum G. Mey), among others (Kampot Ikpeng, personal account, 2016). The fruiting stage of some species, such as megriut (Inga marginata Willd.), katamaut (Inga thibaudiana DC.), potkïngis (Vitex montevidensis Cham.), tomela (Byrsonima crispa A. Juss.), which attract birds and other animals, is also considered as an indicator of the maturity of the forest formation (oremyegetpïn) and of the appropriate time for the opening of a new swidden.
In this type of more developed formation, the soils are more structured (“fluffy,” according to local speech; oromãkura) as a result of the accumulation of leaf remains, tree branches, and fruit peels on the soil surface, forming a darker layer (yongonwulun). However, finding fallow areas with these ideal characteristics of development stage for reuse is increasingly difficult in the current regional context. Close to villages, areas of fallows at early stages (awmtu) or slightly more developed (tukto rïpïn) predominate. Figure 3 shows the succession trajectories of the Ikpeng shifting cultivation cycle according to farmers interviewed in Moygu and Arayo Ikpeng villages.
Techniques and Local Rules That Facilitate Forest Regeneration
The Kawaiwete traditionally manage swiddens to favor forest regeneration, for instance, by choosing the right places to open new plots, keeping some specific trees, cleaning the understory, and above all, limiting the number of years for cultivation at the same location contribute to forest recovery. As explained during the interviews, the most intensive cleaning should be conducted only at the initial stage of the swidden, when most plants are eliminated so as not to hinder the development of crops, and species with specific uses are selected and kept, thus helping forest regeneration (Supplementary Table 1).
However, nowadays many farmers prefer to leave the swiddens very clean throughout the cultivation phase, so as not to hinder the growth of cassava roots or the peanut production, but they end up weakening the soil:
“[.] No, no, they are all cut, that’s why we clean the cassava, it’s very clean, we remove everything that grows. This is also done with peanuts; it has to be kept very clean, we do not leave any young trees behind” (Kape Kaiabi, personal account, 2016).
In cases where excessive cleaning has already occurred in previous cycles, the impacts can be even more drastic for regenerating areas. Although changes in management techniques are visible, it is not known exactly why they occurred. However, some farmers have shown resistance to changing the traditional way of cleaning, as they believe that it can hinder the development of cassava and compromise the feeding of their families. Some farmers also recognized that it is worth trying a more balanced management of swiddens and perform selective cleanings only around plants, decreasing clearing intensity gradually as crops develop, to favor forest regeneration during the fallow.
In the Moygu Ikpeng village, the method of opening swiddens has also changed. In the past, there were fewer swiddens and the people always followed the guidelines of the village leaders to manage areas under recovery. As a result, it was possible to plan ahead the opening of new cultivation areas for the families. However, nowadays, the swiddens have become more individualized and often managed by young people with little agricultural experience, leading to the opening of secondary forest areas still at their early stages and without criteria. However, farmers also recognized that the method used for clearing swiddens can help the secondary forest to recover faster during the fallow and maintain important useful resources.
In the past, plots were cultivated for 1 year only, natural clearings were used, and cleaning was scarcely conducted during regeneration. Thus, such care helped the forest to recover faster, allowing it to be reused in a few years. According to the current Ikpeng rules, cassava swiddens can be used for up to three cycles of planting/harvesting. Nowadays there is no consensus on how to plant cassava and at the same time help the forest to regenerate. This has made it difficult to plan the use of agricultural fallows (Kampot Ikpeng, personal account, 2015).
These shifts in ancient Ikpeng agricultural management are attributed to the peoples of the upper Xingu, with whom they lived for several years after the time of contact (Menget, 2001), and may be causing changes in the local landscape: “[.] We are currently cleaning the swiddens because we learned it from Kalapalo, Kamayura. Our old swiddens recovered quickly, a lot of raegï (Xylopia sp.) and yego (Vochysia sp.) grew naturally instead of cassava, do you understand? (Amputxa Ikpeng, elder and farmer, personal account, 2015). However, the origin of this form of cleaning is not known, as well as the reason it occurs among the Kawaiwete.
Kawiago Ikpeng, an experienced farmer from the Moygu village, explained that the farmers themselves end up harming the forest regeneration by over-cleaning the plots. He also recognizes that the low productivity of cassava is due to the land itself, which became weak because fallows were reused at their initial stages of succession or because they were accidentally set on fire in previous periods:
“[.] The ancients did not live fixed in a same place. When they opened swiddens, they harvested, and soon moved to another village. Nowadays we live in the same place, we don’t have woods anymore, it is too far away, that makes us use the swidden several times.” (Kawiago Ikpeng, farmer, personal account, 2016).
Even so, some farmers manage their plots and fallows in ways that favor forest regeneration. Purat Ikpeng, a spiritual leader of the Moygu village, showed us a swidden plot that was at the final stage of cultivation (ïrïnpïn), still with some remaining cassava branches (Figure 3), where he performed only selective cleaning (tukto tawengkeremtowo). During the cultivation phase, he protected and let many types of trees to grow. The “real trees” (yay were keni) that were protected helped the regeneration of the fallows.
This selective cleaning system is based on two cultivation cycles, which may last between 2 and 3 years (30 months). The most intense cleanings take place in the first year, between 3 and 4 months of cassava growth. While weeding, farmers select the species that will remain in the system after selective cleaning. From the second year, the first-cycle cassava begins to be harvested and its branches are replanted taking advantage of the same location of the collected roots. At this stage, the regenerating trees that were left in the first year are already grown and can be pruned so that they do not hinder the second-cultivation cycle cassava. From the second year onward, regeneration trees are left to grow, the last cassava harvest takes place, and the regeneration phase of fallow begins with several species of trees already established.
After the third year, the swidden already has several trees important for local use, including fruit trees such as pequi-do-xingu (Caryocar brasiliensis Cambess.) and cashew (Anacardium sp.). Other species are arakto (ni)—for constructions; ykwalapi (ni)—medicinal use; raegï (Xilopya amazonica R. E. Fries) and mopya [Attalea maripa (Aublet) Drude]—wood and straw used in the construction of houses; yepkuy (Himatanthus sp.)—various uses, including paint fixing; motoe [Helicostylis tomentosa (Poepp. and Endl.) J. F. Macbr.] and tomkorowo (Talisia sp.)—highly prized foods, bait for hunting; and ponmu (Cochlospermum sp.)—medical use and fiber for the tucum oil press—ototo (Astrocaryum aculeatum G. Mey). Figure 4, adapted from Bahuchet and Betsch (2012) for the selective cleaning techniques used by the Wayãpi people of the Guianas, which are very similar, shows the technique used in the Moygu Ikpeng village.
Indicator Species for Secondary Succession, Soil Recovery, and Resistance to Fires
Certain trees or plants have cultural uses among the Ikpeng and Kawaiwete, but some also have functional characteristics that may favor the regeneration of secondary forests and the recovery of soils. Others are characteristic of certain sites (types of soil or successional stages), resistant to fires, or subject to certain levels of protection, among other characteristics that may favor a fast establishment in the cultivated areas.
In the Samauma Kawaiwete village, the forest species surveyed are organized into local categories: (I) adapted to the successional stages—initial, intermediate or advanced; (II) indicators of specific environments such as koferete (ADE) or “trees that own fallows” such as kaarete (nADE); (III) indicator of burnt forest (ywokaiwet); (IV) those that “animate the regeneration” and “help the earth to become dark” (yja mama kaap) or protected trees for being the “owner of the swidden” (Supplementary Table 1). For the recovery of koferete, plants called yja mama kaap are required, which care for and help to recover the soil because [.] “they produce many leaves that fall quickly and the soil acquires a darker appearance, with good and more fluffy earth” (Kape Kawaiwete, personal account, 2016).
In the past, during the opening phase of a Kawaiwete agricultural site, some adult trees were kept at the edges of swiddens to provide more shade and moisture to the lands, also serving as a source of propagules. These trees were called “trees owners of the swidden” and were not felled because, in addition to helping with regeneration, they had several uses in material culture. Among them, are the jatua’yp [Guarea cf. guidonia (L.) Sleumer], jatuywa (Hymenaea courbaril L.), jatytayp (Maclura tinctoria L. Engl.), yangyp (Dinizia excelsa Ducke), and ykyr’yp (Thyrsodium spruceanum Benth.) (Supplementary Table 1). According to Kape Kawaiwete, an elder farmer (personal account, 2016):
“[.] To me, they are left the jatua’yp; so when it bears fruit, the seeds are born in the middle of the swiddens. Jatua’yp is born in the middle of the swiddens; its seeds are red. These are those that the ancestors did not fell; so species returned, and fallows soon grew.”
However, nowadays the practice is to cut all the trees down, even if such practices are recognized as enhancing recovery of fallows. This type of management has not been used in most Kawaiwete villages anymore and possibly in most villages in the XIT. As a result, secondary forests have become poorer in composition because certain types of plants are no longer found in the regenerating forest, such as jatua’yp [Guarea cf. guidonia (L.) Sleumer], jatetayp (Maclura tinctoria L. Engl.), y’ga (Inga spp.), and ykyr’yp (Thyrsodium spruceanum Benth.). Other useful trees used in medicine—asiraryp (Zanthoxylum rhoifolium Lam.), for collecting fruit—ynga (Inga spp.), api (Pseudolmedia macrophylla Trécul), and for wood—yperoyp (ni), ykyr’yp (Thyrsodium spruceanum Benth.), yogyp (Dinizia cf excelsa), ajuyp [Ocotea leucoxylon (Sw.) Laness], ajuwyuun (Ocotea guianensis Aubl.), ywyiyp (ni), tukumãm (Astrocaryum aculeatum G. Mey)—no longer exist (Supplementary Table 1). These species, with the exception of Astocaryum sp., do not tolerate fires and no longer regenerate spontaneously in changed or intensely burned areas. For Kape, a Kaiabi elder of the Samauma Kawaiwete village (personal account, 2016):
“[.] The land is finished; the land and the trees that existed no longer do; it is not the same as before. Jatyta’yp is a sign of where there is dark earth; there is no more dark earth; there is no jatyta’yp, there is no jatua’yp; the trees that existed before do not exist anymore.”
For the Ikpeng of the Moygu and Arayo villages, the most conserved forests at advanced stages of succession (irwa otepnïnpo) contain trees considered as vulnerable, which cannot withstand impacts (such as frequent fires), and/or have relevant uses for material culture. Among them are poret (Annonaceae) and raegï (Xylopia amazonica R. E. Fries), which are highly sought after and used for building traditional houses. They are increasingly rare in areas close to the villages. Currently, there are also no more species that indicate the recovery of forests, such as muret (Byrsonima intermedia A. Juss.), katepo (Hymenaea courbaril L.), tïrampo [Guarea cf. guidonia (L.) Sleumer], kumpang [Emmotum nitens (Benth.) Miers], and yego (Vochysia ferruginea Mart.) (Supplementary Table 2). The yego is considered a sacred tree for the Ikpeng people. It is used as a bench for the initiation of children during the festivity panango atpotpot (piercing children’s ears, one of the stages of the festive event Pomeri; Rodgers, 2002, 2013), as well as for building traditional houses.
Like the Kawaiwete, Ikpeng farmers also used to protect some trees during the opening of swiddens in the past and recognized their value, as explained by Oporike (Tome) Ikpeng, a leader of the Moygu Ikpeng village: “[.] These trees were kept in the swiddens because they had a strong spirit; it wasn’t just anyone who could cut them down” (Oporike Tomé Ikpeng, personal account, 2016).
During the cultivation phase, the “real trees” (yay were keni) were tolerated in swiddens by means of selective cleaning. They indicated that fallows were following their successional recovery, so that the forest could be reused for agriculture, yielding a productive yield. Among them were katepo (Hymenaea courbaril L.), kuryum (Enterolobium schomburgkii Benth.), oyot (Dipteryx odorata (Aubl.) Wild.), pitpirak (Qualea cf. paraensis Ducke), raegï (Xylopia amazonica R. E. Fries), yeron (Ocotea leucoxylon (Sw.) Laness.), and txiworo (Trattinickia cf, rhoifolia Wild.) (Supplementary Table 2). Other tall trees, which form a wide canopy and favor a quick shading of the area, protecting regenerating plants, are called “mother trees” (okewï) and “grandmother trees” (iramrungmo). Other species are important because they “call the other plants” (awmtu), such as kumpang (Emmotum nitens (Benth.) Miers), kuryum (Enterolobium schomburgkii Benth.), and katepo (Hymenaea courbaril L.) (Pura Ikpeng, Makawa Ikpeng, personal account, 2015). Finally, some plants “are [also] born by the spirit oyng,” a non-human entity who plants many trees for the forest to be reborn (Totopiat Ikpeng, personal account, 2015).
Totopiat Ikpeng, the main spiritual leader of the Moygu village, when questioned about how forests regenerate, explained very slowly: [.] “everything has its owner. the owner of the river—orongo—the owner of the land—momtïng. and the owner of the bush in general—oyoreta.” In his understanding, [.] “the forest needs oyng, the spirit that freshens the earth and helps the forest to grow back—orong ewyangtenopni. When he cries, the tears make the earth moist4. Plants are born together, kerekere-umi, ogolak, which is next to the kayakpo, tïrampo, pïrigu, mawa. These are plants that oyng gives birth to it to first refresh the earth” (Totopya Ikpeng, personal account, 2015).
As noted, in addition to presenting some priority uses, many of these plants have functional characteristics in the recovery of secondary forests. However, the Ikpeng have observed that many species that re-sprouted in disturbed secondary areas (engrotet) are no longer sprouting due to the increase in the number of fires and the excessive cleaning in agricultural areas during the cassava cultivation cycle.
Impacts on the Sustainability of Systems With the Increase in the Number of Fires
The availability of recovered areas for opening new plots is increasingly critical. In choosing the area for cultivation, in addition to other indicators, the main criterion is the stage of development of fallows measured by the forest structure and tree sizes. The lack of good lands in areas close to Kawaiwete and Ikpeng villages implies the clearing of secondary forests that are still very young, with less than 5–10 years in fallow age, indicating an intensification of the traditional system.
However, even areas that remain for longer periods in fallow, between 10 and 15 years, have their recovery affected by the history of fires or previous uses. As a result, many species characteristic of secondary forests—and that are important for forest regeneration and soil recovery—no longer grow spontaneously nowadays. Examples such as jatua’yp (Guarea cf. guidonia (L.) Sleumer), jatetayp (Maclura tinctoria L. Engl.), y”ga (Inga spp.) for the Kawaiwete and katepo (Hymenaea courbaril L.), kuryum (Enterolobium schomburgkii Benth.), oyot [Dipteryx odorata (Aubl.) Wild.], pitpirak (Qualea cf. paraensis Ducke), raegï (Xylopia amazonica R. E. Fries), yeron [Ocotea leucoxylon (Sw.) Laness.] and txiworo (Trattinickia cf, rhoifolia Wild.) for the Ikpeng.
For this reason, important resources characteristic of late stages of regeneration become less accessible, rare, or even extinct in areas close to human occupations, like (Pseudolmedia macrophylla Trécul), [Guarea cf. guidonia (L.) Sleumer], (Xylopia amazonica R. E. Fries), (Inga spp.), (Talisia sp.), (Byrsonima crispa A. Juss), and (Vochysia ferruginea Mart.).
Discussion
The indigenous peoples of the Amazon exert an important influence on the structure and composition of its forests through the succession management conducted during the cleaning and gardening of plots (Irvine, 1989; Pinedo-Vasquez et al., 2012). Practices conducted during cultivation or fallow management can favor forest recovery. In addition to selective clearing, the frequency and duration of other processes, such as the protection of useful species, seed dispersal and attraction of fauna, selection of desirable phenotypes and cultivation of propagules, may favor a greater concentration of useful plants and to improve soils (Levis et al., 2018).
The agroforestry management includes food attractive to animals for hunt and, therefore, the fauna plays an important role by contributing to the dispersion of seeds that are brought from other forested areas close to swiddens (Denevan et al., 1984; Zent and Zent, 2012; Bahuchet et Betsch, op. cit.; Thomas, 2014). Areas in fallow create wetter and more fertile microhabitats than areas that are open and thus drier (Uhl et al., 1981; Uhl, 1983). Seeds dispersed by animals are more likely to establish themselves and accelerate the initial recovery of the area (Unruh, 1988). Several of these species also serve as food for humans. They are multiplied in backyards, gardens, or even dispersed in displacements during collection activities. Fire management, at low intensities and frequency, when combined with these other practices, may also lead to an improved soil fertility, a process that indirectly favors forest recovery (Junqueira et al., 2011; Levis et al., 2017).
However, in several regions of the Amazon agricultural intensification processes in indigenous lands are taking place, either by changing habits to more sedentary livelihoods, circumscription of their original territories by legal delimitation (Vickers, 1983; Denevan, 2001; Freire, 2003, 2007) or illegal advancement of miners and loggers, a need for access to schools, health centers or urban areas, as well as the advancement of the agricultural frontier and the impacts of large construction works (Jusys, 2018; Fa et al., 2020).
In the two cases studied here, the reality is no different. In the past, both the Kawaiwete and the Ikpeng presented patterns in the use of the territory characterized by the frequent mobility of villages, which were moved when the local natural resources were depleted. This lack of mobility, in the long run, results in an increase in pressure on secondary formations, with impacts to local livelihoods. Inside the XIT, it has become increasingly difficult to observe older villages changing place, as this decision depends on the availability of specific environments that are rich in dark earth and that are close to the main river channel, which must be negotiated with other indigenous people.
The lack of mobility is directly related to the time and space necessary for the continuity of the shifting cultivation and territory management system, making difficult a full recovery of secondary forests and leading to the intensification of agricultural use (Uriarte and Chazdon, 2016; Chazdon, 2017), in addition to surpassing the limits of the system (van Vliet et al., 2012). The history of intensification of land use with the increase in cutting and burning practices leads to a reduction in soil fertility, which ends up causing a decrease in species that depend on seeds to regenerate to the detriment of those that have the ability to regrowth and are more adapted to this new context. This leads to a decrease in species diversity in managed landscapes, as Jakovac et al. (2016a) observed in other regions of the Amazon. With the increase in the number of cultivation cycles, the frequency of cleaning also increases, and, in this way, the system may reach a state of interrupted succession, where regeneration starts to rely more on re-sprouting to compensate for the loss of species that reproduce by seeds (Jakovac et al., 2015). The factors that contribute to these changes are often difficult to understand, since the systems are dynamic and correspond to different sociocultural contexts (Wiersum, 2004; Mansourian et al., 2019).
Local Transformations and Forest Regeneration
From the circumstances presented, in the studied Kawaiwete and Ikpeng villages there is a conjunction of local factors of change that are being aggravated by regional transformations. In the past, the processes of clearing and cultivating Kawaiwete and Ikpeng swiddens followed specific rules that, although still present in local knowledge, had their application hampered by changes in the form of occupation of the territory. Currently, the period during which plots are left in fallow varies from 5–10 years. The history of previous uses of the same plots and accidents with fires aggravates this situation, which impairs the processes of vegetation regeneration, soil recovery, and recovery of key species in this process.
With less mobility in villages, in addition to occasional forest fires, agricultural areas already show signs of soil degradation even before they are cultivated, affecting the regeneration of many forest species during the fallow phase. The decrease in the size of plots close to the Kawaiwete and Ikpeng villages leads to the cutting down of secondary forests even at initial stages of regeneration (up to 10 years). These often present little biomass, individuals with a baseline DBH area below 5 cm, and heights up to 3 m, where fast-growing trees and low longevity predominate. In other words, the criteria used in the choice of new swidden plots does no longer take into account aspects of forest structure or other local indicators as in the past. As a result, many species characteristic to these areas—which are important for forest regeneration and soil recovery—no longer appear spontaneously. For this reason, the resources characteristic of late stages of regeneration become less accessible, rare, or even extinct in areas close to human occupations.
The decision of using initial stages seems to be related to the ease of access to areas or to their practicality, since the branches and thin shrubs do not need much effort to be cut down, unlike swiddens cleared in a more developed fallow or the older and more distant forests (Figures 2, 3). For the same reasons, agricultural areas may end up being reused for cultivation cycles of less demanding species, such as manioc, beyond the limits dictated by traditional local rules (two to three cycles). Limitations in the available workforce may be one of the factors that lead to this decision, making it easier to work in a nearby area and in a fallow of thin trees instead of having to look for more distant areas with thick trees where the human-hour work would be more demanding. However, the effect of using young fallows may be the opposite, as there seems to be a relationship between low productivity in swiddens and an increased need for work. When poorer soils start to predominate, the need for weeding and for cultivating other sites in order to compensate for the low yields of the swiddens increases, as Jakovac et al. (2016b, 2017) also reported.
On the other hand, some indigenous families began to seek other areas of old forests for the most demanding crops that need recovered soils, mainly maize (Zea sp.), peanuts (Arachis sp.), banana (Musa sp.), broad beans (Phaseolus spp.), sweet potato (Ipomea sp.), and yam (Taro sp.). The dependence on more distant places demands more physical effort and inputs, including those from outside the village, because people need vehicles and fuel to transport production either by tractor or motorboats. In other cases, the loss of resilience in red earth systems made farmers use dark earth (ADE) plots for less demanding crops, as observed in the Samauma village. These soils, which were previously indicated only for demanding crops and grown only once, are now reused for planting cassava, without enough fallow time after the cultivation of corn and peanuts, for example. As Junqueira et al. (2010) reported, although the ADE is more fertile and allows for a more intensive use when compared to the nADE, a prolonged and intensive cultivation period without respecting the fallow period may compromise regeneration and cause soil degradation (Junqueira et al., 2016).
Impact of Fires on the Sustainability of the Kawaiwete and Ikpeng Systems
Fires started to occur more intensely in the Kawaiwete and Ikpeng villages mainly due to environmental changes that occurred in the last decades in the XIT region (Nepstad et al., 2004; Morton et al., 2013; Brando et al., 2014). As already explained, the loss of forest cover in the Xingu river basin changed the microregional rainfall regime and seasonal events that were recognized by the indigenous peoples; it also led to an increase in the flammability of forests inside the XIT. Fires alter the structure and composition of forests and more vulnerable tree species do not resist, becoming rare or disappearing from these areas, such as observed by Chazdon et al. (2009) and Rozendaal et al. (2019). The degree of vulnerability of species to fire defines their survival in affected areas. Depending on the intensity and recurrence of fires, tree species can (1) extinguish locally, (2) remain stable, (3) be stimulated by regeneration, or (4) re-sprout, starting to dominate the forest formation in an opportunistic way. Participants recognized the degree of vulnerability of plants to fires during the interviews, as their importance to the forest regeneration processes (Supplementary Tables 1, 2).
As a result, fallows or secondary forests around the villages have become poorer in composition (less biological and cultural richness) due to frequent fires, leading to the loss of raw materials and conditions that were previously more favorable for the establishment of species that are considered by indigenous people as key regeneration species, such as the “regeneration-animating” plants (ywakamaap for the Kawaiwete) and “real trees”—yay were kenin—used in the material and immaterial culture of these peoples.
Traditional Kawaiwete and Ikpeng Forest Management Practices
The changes reported by the Kawaiwete and the Ikpeng in the traditional management of swidden plots corroborate research conducted in other tropical regions, that points to shorter fallow periods due to limitations in access to traditional territories and increase in pressures for demographic or for socioeconomic reasons (Johnson, 1983, p. 52; Thrupp et al., 1997; Marquardt et al., 2013). For the Ikpeng people, as already mentioned, agricultural areas were traditionally cultivated only once. This is no longer possible due to the permanence of villages in a same place, leading nearby areas to be frequently reused, as Jakovac et al. (2017) also observed for farmers in the Amazon.
Regarding the impacts on the regeneration of fallows, the Kawaiwete and Ikpeng local knowledge seems to value preventive care during cultivation phases aiming to prevent the forest from being degraded, instead of resorting to concepts or ways of restoring exhausted environments. In this sense, traditional local knowledge seems to indicate rules or “care” in the way environments should be managed based on resilience limits, as observed for other places (Lugo, 1995; Camacho et al., 2012; Chazdon, 2014a; Magnuszewski et al., 2015; Chazdon and Guariguata, 2016; Brancalion et al., 2016; Mukul, 2016; Vásquez-Grandón et al., 2018).
The experiences reported here indicate that the Kawaiwete and the Ikpeng manage to favor forest regeneration while the field is being cultivated (Marquardt et al., 2013). According to the observed agricultural rules and practices, some tree species were not cut during the opening of plots as a way to protect seed-bearing species during the fallow time. According to research conducted in tropical regions, this practice contributes to ensure fertile agricultural areas, as it favors forest regeneration (Abdoellah et al., 1993; cited in Balée, 2010; Chazdon, 2014a). Karen farmers in Thailand, for example, left between 20 and 40 trees in swiddens at an average of 244/ha (Schmidt-Vogt, 2001). As a result, the structure and composition of the forest changes following a process that is similar as that of domesticating landscapes (Denevan et al., 1984; Wiersum, 2004; Clement et al., 2015).
The careful cleaning of areas surrounding crops, with little removal of regenerating plants, can also favor the recovery of the forest (Rodrigues, 1993; Silva, 2016, p. 146). As the Kawaiwete and the Ikpeng, the vast majority of indigenous and traditional people that practice forest-based agriculture use “selective cleaning.” Some plants are protected and favored during soil preparation, resulting in the spontaneous development of useful species (Denevan et al., 1984, Descola, 1988; Denevan, 2001; Bahuchet and Betsch, 2012; Figures 4, 5). However, this traditional technique is not followed by all the Kawaiwete and Ikpeng farmers nowadays, despite apparently presenting good results. Likewise, some regenerating species could also be pruned during the crop growth phase, such as manioc, instead of being uprooted (Johnson, 1983; Wiersum, 2004; Uprety et al., 2012; Pye-Smith, 2013; Peltier et al., 2014; Chazdon, 2017; FAO, 2018). The use of nitrogen-fixing species, as is the case observed in the Samauma village, is a strategy widely used by tropical forest farmers (Unruh, 1988; Chazdon and Guariguata, 2016, p. 717), and, in a way, is related to the yja mamakaap (”trees that grew together”) of the Kawaiwete5.
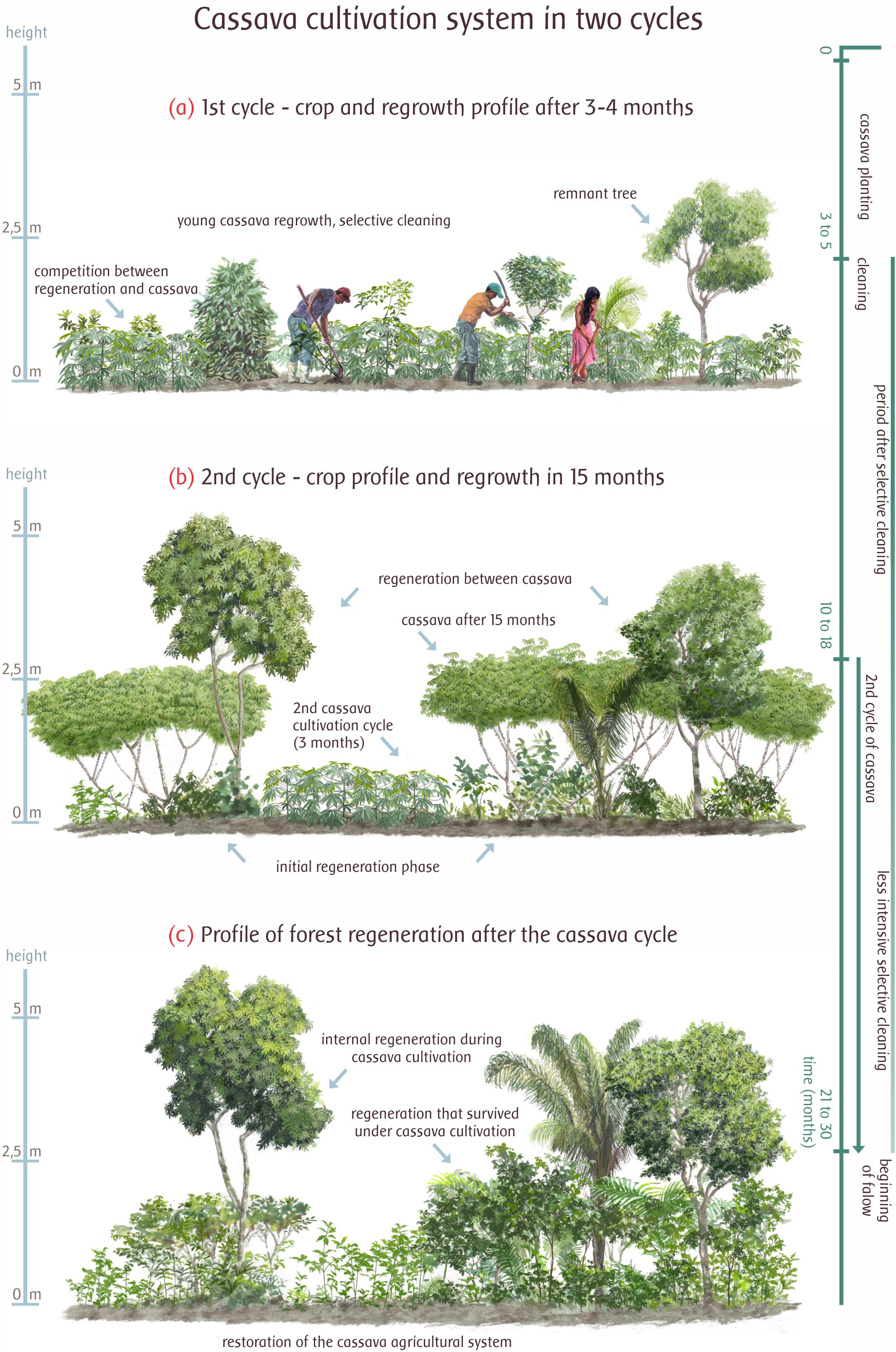
Figure 4. Selective cleaning management (tukto tawengkeremtowo) and forest regrowth in the Ikpeng cassava (Manihot esculenta Crantz) shifting cultivation cycle. (a) When the plot is opened, certain tree species are tolerated and left in the plot. As the crop grows, selective cleaning is repeated after 3–5 months (b). After the first harvest, cassava is planted again and left to grow among the young regenerating trees (2–5 m) that may be pruned to avoid competition (10–18 months). A less intensive selective cleaning is performed. (c) At the end of the cultivation phase (21–30 months) the protected trees help other regenerating secondary growth species to thrive (Illustration by Gustavo Marigo—adapted according to Bahuchet and Betsch, 2012).

Figure 5. Three-year-old cultivation plot (ïrïnpïn) submitted to selective cleaning management (tukto tawengkeremtowo) in Moygu village. See young trees that were left to grow for different purposes: medicinal— (arakto (ni), ykwalapi (ni); construction—raegï (Xylopya amazonica R. E. Fr.), mopya (Attalea maripa (Aubl.) Drude); ritual—yepkuy (Himatanthus sucuuba (Spruce ex Müll. Arg.) Woodson); food and food for hunting—motoe (Helicostylis tomentosa (Poepp. and Endl.) J. F. Macbr.), food, food for hunting and ritual—inot (Caryocar cf. Brasiliensis Cambess); among others (Photo by Marcus V. C Schmidt, Moygu Village 2008).
Finally, according to the perception of many indigenous peoples in the Amazon, the regeneration of forests also seems to assume a mythical and spiritual dimension. Some concepts refer to domains that are claimed by human and non-human beings (Rodgers, 2002; Fausto, 2008; Oliveira, 2016). In this case, forests are understood as a large plantation of creative spirits (Descola, 1996), as the peoples of the XIT. Thus, the plants over which the spirit Oyng cries to refresh the earth are plants that should be considered in forest recovery projects (Supplementary Table 2).
Assisted Natural Regeneration (ANR) as a Way of Managing Indigenous Shifting Cultivation Systems in the XIT
In the Kawaiwete and Ikpeng villages, traditional agroforestry management is being replaced by techniques that take into account the practicality and ease of access to areas, with food production as a priority, rather than a good regeneration of fallows. As the more mature forests are far away from villages, there is no alternative but to reuse the same area several times, even if this means degrading the secondary forest. Therefore, as it seems, it is not just a question of disregarding techniques that favor regeneration, but of the lack of local conditions to put these techniques into practice. Therefore, the mere recovery of some rules that previously governed the access to resources may not respond to the challenges for the maintenance of production systems more adapted to the current reality (Denslow, 1980; Wiersum, 2004; Uprety et al., 2012; Chazdon, 2013, 2017; Tengö et al., 2014).
The Ikpeng believe that the best way to recover areas affected by the increasing episodes of recurrent fires in recent years would be to leave the forests to recover on their own. According to preliminary assessments considering the species identified here, the restoration of burnt forests can take place with few interventions, since many areas impacted by the fires are close to the remnants of more enclosed forests, which favor the flow of seeds6. To this end, the forest should be protected from fires and there should be investments in broad firebreaks and in local agreements, in addition to monitoring the growth of species characteristic of more advanced stages in fallow areas. However, there are cases where it becomes necessary to create favorable conditions for the maintenance of the productivity and the resilience of these systems (Hames and Vickers, 1983, p. 25).
The methods used by the peoples in the XIT are methods of assisted natural regeneration (ANR), as Wiersum (2004); Walpole (2010), Marquardt et al. (2013); Chazdon (2014a), and Thomas (2014) described. The main methods are: (1) tolerate adult trees at the borders of swiddens in such a way that they begin to produce seeds that regenerate the cultivated site, (2) limit the crop cycles so as not to affect the potential for the establishment of regenerating plants, (3) select individuals during cleaning at the cultivation phase, which favors the regeneration of useful and functional species, (4) use fire protection techniques, and (5) know functional plants that favor regeneration processes. In this regard, the ANR should be considered a tool to promote livelihoods within the context of traditional forest management (Butic and Ngidlo, 2003; Wiersum, 2004; Walpole, 2010; Marquardt et al., 2013; Thomas, 2014).
One of the main reasons for forest degradation is the depletion of soils (Chazdon, 2003), when the resilience limits are exceeded and prevent forest succession (Jakovac et al., 2016a, b). Therefore, cultivation systems with accumulated ash and coal from controlled burning (Schmidt et al., 2014) may improve soil structure and fertility and accelerate the recovery of fallows (Junqueira et al., 2010). On a local scale, ANR processes must also consider the previous legacies of agricultural management because they influence the structural, compositional, functional, and dynamic attributes of regenerating forests (Uriarte and Chazdon, 2016).
ANR strategies also include the sociocultural values of the communities involved and may include approaches to recover traditional indigenous ecological management practices, in which their knowledge can support management strategies and silvicultural practices (Coomes et al., 2000; Sajise, 2003; Uprety et al., 2012). In this regard, it is essential to maintain the social structures that govern these mechanisms of agricultural production and landscape conservation aiming to achieve a long-term production stability (Bahuchet and Betsch, 2012).
Transforming degraded lands into improved fallow lands may represent a large investment in labor and harvests could be very low during the first seasons, as Marquardt et al. (2013) argued. However, in situations as the XIT, where there is a limitation in the availability of fertile lands and difficulty in moving the villages, this form of “adaptive management” may improve the conditions of weakened or degraded soils, which limit the natural regeneration of forests (Wiersum, 2004; Joslin et al., 2011; Pinedo-Vasquez et al., 2012). Farmers remembered that in the past, the fields were smaller, circular, and separated by a forest matrix (Eden and Andrade, 1987; Chazdon, 2003, 2014b, 2017; Chazdon et al., 2009; Ferguson et al., 2003). These aspects almost no longer exist in the oldest and most populous villages in the XIT. Thus, other factors should also be considered in future ANR strategies, such as the size of the cultivated area, the presencxe of remaining trees in the surroundings, the number of years of use, and the control of fires in areas under recovery (Uriarte and Chazdon, 2016).
To overcome these challenges and move to more sustainable cultivation systems there is a need to bridge local knowledge systems with scientific knowledge systems and co-produce knowledge adapted to uncertainty and environmental change (Armitage et al., 2011; Tengö et al., 2017). Ikpeng and Kawaiwete younger generations are open to innovations and some initiatives are being implemented with external organizations to try and overcome the knowledge gap, such as the agroecology high school program in the Ikpeng village7, the muvuca seed-planting system by ISA8 and the Prevfogo fire-fighting training program by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA)9. Knowledge co-production and adaptive management are processes mediated by institutions that need to create the conditions for “social learning, defined here as the iterative action, reflection, and deliberation of individuals and groups engaged in sharing experiences and ideas to resolve complex challenges collaboratively (Diduck et al., 2005; Keen et al., 2005)” (Armitage et al., 2011, p. 995). However, at this stage, the agroecology program and the muvuca system have not yet incorporated the local knowledge of older generations on shifting cultivation, forest regeneration and fallow indicators discussed here, and are based on external forest restoration models that need to be adapted. The Prevfogo has been more successful in training local fire fighters and cultivators on fire management techniques adapted to the new environmental conditions, but needs to be upscaled to include all the villages in XIT.
Conclusion
Maintaining agricultural productivity in indigenous lands in the Amazon is a priority, especially considering the increase in deforestation rates and in the number of fires in the Xingu Indigenous Land (XIT). In areas where processes of agricultural intensification have led to the degradation of lands for cultivation, it is necessary to involve indigenous farmers in forestry landscape recovery initiatives considering their ancient knowledge in order to understand the causes and consequences of the permanence of these shifting cultivation systems.
Our results show how traditional practices can favor the regeneration of forests after disturbances according to the knowledge of the Kawaiwete and the Ikpeng peoples. Traditional knowledge can provide important information about the functional and ecological roles of pioneer species during natural regeneration (Reyes-García et al., 2018). However, the potential for natural regeneration is influenced by the intensity of land use. The more conserved the forests, the greater the power of recovery of forest remains in the landscape (Uriarte and Chazdon, 2016). We suggest that the restoration of degraded areas in villages in the XIT should use ANR techniques grounded on indigenous knowledge. This implies the adaptive co-construction of knowledge on cultivation systems that value and strength traditional practices of these indigenous peoples, as well as assimilate new practices comprising a more “agro-forestry-restorer” profile, and fundamentally a greater control of fires.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical approval was not provided for this study on human participants because this article represents part of the results of a project developed by a non-governmental organization—Instituto Socioambiental (ISA) that works in partnership with indigenous peoples in this area of study. ISA develops activities to protect and defend the rights of these peoples and carries out collaborative and intercultural research projects on the impacts of fires, in order to identify strategies to favor the conservation of forests in Indigenous Territory. This project was carried out through a formal partnership with these peoples, represented by local associations and indigenous representatives participated in the project and are co-authors of the text that is being submitted for this Frontiers special. The indigenous authors participated in the conception of the research and in the elaboration of the text. They are very pleased to be recognized and represented in this scientific article and the results value their knowledge of their people. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MS author of the project and research, conducted interviews in the communities and surveys in the forests of Xingu, and specialized in intercultural research and knowledge co-construction processes. YI professor and researcher, was responsible for the mobilization and planning of research between Ikpeng villages, helped in the interviews and translation of local knowledge about environmental changes, indigenous management, and forest regeneration. TK researcher from the village Samauma Kawaiwete, was responsible for mobilizing the people who participated in the project, in field surveys and in interviews about the knowledge of the forest, environmental changes, and the agricultural management of his people. RS technical reviewer of the scientific text and played a fundamental role in structuring the text. KO technical and administrative support to carry out the project in the Indigenous Territory. CA supervisor of MS, has reviewed and reorganized the manuscript after Frontiers first review, has contributed with theoretical inputs to the interpretation of data, as well as revised the manuscript’s translation to English. All authors contributed to the article and approved the submitted version.
Funding
This project was carried out through a non-governmental organization (Instituto Socioambiental), the project was carried out with funds from the National Fund for Climate Change, of the Ministry of Environment, linked to the federal government. Although MS now linked to the University of São Paulo, as a doctoral student, this project was not linked to any university. The research was funded by the Climate Change National Fund of the Brazilian Ministry of Environment. The grant (Agreement 813130/2014) was awarded to Instituto Socioambiental (ISA).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors CA.
Acknowledgments
This work could not be conducted without the consent, trust, and teachings of the indigenous peoples of the Xingu. We thank Manuela Carneiro da Cunha for the great encouragement, Daniel Vieira and Raissa Ribeiro for their support in data analysis, Natalia Ivanauskas for preliminary botanical identifications, Gustavo Marigo (@vibremnatureza) for the illustrations, and Arminda Jardim for the spelling check. This project was conducted with logistical and technical support from the Xingu Program of the Instituto Socioambiental (ISA).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.605925/full#supplementary-material
Footnotes
- ^ The Moygu and Arayo villages are in a same region in the middle Xingu River and thus, although there is a political separation, here we consider them as areas of common use.
- ^ The inhabitants of the XIT are the Aweti, Kalapalo, Kamaiurá, Kuikuro, Matipu, Mehinako, Nahukuá, Naruvotu, Trumai, Wauja (Waurá), Yawalapiti, Ikpeng, Kawaiwete, Kĩsêdjê, Tapayuna, and Yudja (https://pib.socioambiental.org/en/xingu).
- ^ The interviews were transcribed with the help of Rosana Gasparini (UNIFESP), Oreme Ikpeng and Kamatxi Ikpeng, and Tari Kaiabi and Myayup Kaiabi.
- ^ Relating to morning dew.
- ^ This concept was also identified in the Capivara Kawaiwete village, where a group of similar plants were called “trees owner of fallows,” necessary for the recovery of the dark earth (koferete).
- ^ The analyses of results were conducted with the support of Dr. Daniel M. Vieira of EMBRAPA-CENARGEN 8.
- ^ Integrated Technical High School in Agroecology, linked to the State Secretariat of Education of Mato Grosso (Seduc/MT).
- ^ https://www.sementesdoxingu.org.br/site/muvuca-que-vira-floresta/
- ^ https://www.ibama.gov.br/ultimas-2/2043-forca-tarefa-combate-14-incendios-no-parque-indigena-do-xingu
References
Abdoellah, O., Lahjie, A. B., Wangsadidjaja, S. S., Hadikusumah, H., Iskandar, J., and Sukmananto, B. (1993). “Communities and forest management in East Kalimantan: pathway to environmental stability,” in Southeast Asia Sustainable Forest Management Network. Mark Poffenberger and Betsy McGean Research Network Report n°3, (Berkeley, CA: Center for Southeast Asia Studies, International and Area Studies, University of California), 65.
AFN (2002). Asia Forest Network - Participatory Rural Appraisal for Community Forest Management. Fort George G. Meade: AFN.
Alencar, A. A., Brando, P. M., Asner, G. P., and Putz, F. E. (2015). Landscape fragmentation, severe drought, and the new Amazon forest fire regime. Ecol. Appl. 25, 1493–1505. doi: 10.1890/14-1528.1
Armitage, D., Berkes, F., Dale, A., Kocho-Schellenberg, E., and Patton, E. (2011). Co-management and the co-production of knowledge: Learning to adapt in Canada’s Arctic. Global Environ. Change 21, 995–1004. doi: 10.1016/j.gloenvcha.2011.04.006
Arroyo-Kalin, M. (2010). The amazonian formative: crop domestication and anthropogenic soils. Diversity 2, 473–504. doi: 10.3390/d2040473
Athayde, S., and Lugo, J. S. (2018). Adaptive strategies to displacement and environmental change among the kaiabi indigenous people of the Brazilian Amazon. Soc. Nat. Resources 31, 666–682. doi: 10.1080/08941920.2018.1426801
Bahuchet, S., and Betsch, J. M. (2012). L’agriculture itinérante sur brûlis, une menace sur la forêt tropicale humide? Revue d’ethnoécologie 1, 1–32. doi: 10.4000/ethnoecologie.768
Balée, W. (1993). Indigenous transformation of Amazonian forests: an example from Maranhão. Brazil. L’Homme 33, 231–254. doi: 10.3406/hom.1993.369639
Balée, W. (2010). Contingent diversity on anthropic landscapes. Diversity 2, 163–181. doi: 10.3390/d2020163
Bergold, J., and Thomas, S. (2012). Participatory Research Methods: A Methodological Approach in Motion. Historical Social Research, 191–222.
Berkes, F., and Berkes, M. K. (2009). Ecological complexity, fuzzy logic, and holism in indigenous knowledge. Futures 41, 6–12. doi: 10.1016/j.futures.2008.07.003
Bernard, H. R. (2006). Research Methods in Anthropology. Qualitative and Quantitative Approaches, 4th Edn. Lanham, MD: AltaMira Press, 803.
Brancalion, P. H. S., Nave, A. G., Schweizer, D., Farah, F., Lamonato, F., Mangueira, J. R., et al. (2016). Balancing economic costs and ecological outcomes of passive and active restoration in agricultural landscapes: The case of Brazil. Biotropica 48, 856–867. doi: 10.1111/btp.12383
Brando, P. M., Balch, J. K., Nepstad, D. C., Morton, D. C., Putz, F. E., Coe, M. T., et al. (2014). Abrupt increases in Amazonian tree mortality due to drought–fire interactions. Proc. Natl. Acad. Sci. U S A. 111, 6347–6352. doi: 10.1073/pnas.1305499111
Brondízio, E. S. (2006). “Intensificação agrícola, identidade econômica e invisibilidade de pequenos produtores amazônicos: caboclos e colonos em uma perspectiva comparada,” in Sociedades Caboclas Amazônicas: modernidade e Invisibilidade, eds C. Adams, R. S. S. Murrieta, and W. A. Neves (São Paulo: AnaBlume), 135–236.
Brondizio, E. S., and Neves, W. A. (1996). “Populações caboclas do Estuário do Amazonas: a percepção do ambiente natural,” in Uma Estratégia Latino-Americana Para a Amazônia, ed. C. Pavan (São Paulo: Mmarhal/Memorial/Unesp), 167–182.
Butic, M., and Ngidlo, R. (2003). “Muyong forest of Ifugao: assisted natural regeneration in traditional forest management,” in Advancing Assisted Natural Regeneration (ANR) in Asia and the Pacific, eds P. C. Dugan, P. B. Durst, D. J. Ganz, and P. J. McKenzie (Rockville, MA: RAP Publication), 53.
Cabalzar, A. (2017). Pesquisas Interculturais, do Local ao Global. Povos Indígenas do Brasil 2011-2015. São Paulo: Instituto Socioambiental, 240–242.
Camacho, L. D., Combalicer, M. S., Yeo-Chang, Y., Combalicer, E. A., Carandang, A. P., Camacho, S. C., et al. (2012). Traditional forest conservation knowledge/technologies in the Cordillera. Northern Philippines. Forest Pol. Econ. 22, 3–8. doi: 10.1016/j.forpol.2010.06.001
Candau, V. M. F. (2012). Diferenças culturais, interculturalidade e educação em direitos humanos. Educação e Sociedade. 33, 235–250.
Carneiro, R. L. (1983). “The cultivation of manioc among the kuikuru of the upper xingu,” in Adaptative Responses of Native Amazonians, eds H. Raymond, B. E. William, and T. Wickers (Cambridge, MA: Academic Press), 64–111. doi: 10.1016/b978-0-12-321250-4.50007-5
Charlton, C. A. (1987). Problems and prospects for sustainable agricultural systems in the humid tropics. Appl. Geography 7, 153–174. doi: 10.1016/0143-6228(87)90047-6
Chazdon, R. L. (2003). Tropical forest recovery: legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Systemat. 6, 51–71. doi: 10.1078/1433-8319-00042
Chazdon, R. L. (2014a). Second Growth: the Promise of Tropical Forest Regeneration in an Age of Deforestation. Chicago, IL: Univ. Chicago Press, 448. doi: 10.7208/chicago/9780226118109.001.0001
Chazdon, R. L. (2014b). The role of natural regeneration in large-scale. forest and landscape restoration: challenge and opportunity. Build. Foundat. Global Nat. Regenerat. Partnership 55.
Chazdon, R. L. (2017). Landscape restoration, natural regeneration, and the forests of the future. Ann. Missouri. Bot. Gard. 102, 251–257. doi: 10.3417/2016035
Chazdon, R. L., and Guariguata, M. R. (2016). Natural regeneration as a tool for large-scale forest restoration in the tropics: prospects and challenges. Biotropica 48, 844–855. doi: 10.1111/btp.12381
Chazdon, R. L., Peres, C. A., Dent, F. D., Sheil, D., Lugo, A. E., Lamb, D., et al. (2009). The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417. doi: 10.1111/j.1523-1739.2009.01338.x
Chazdon, R. L., and Uriarte, M. (2016). Natural regeneration in the context of large-scale forest and landscape restoration in the tropics. Biotropica 48, 709–715. doi: 10.1111/btp.12409
Chazdon, R. L. (2013). Making tropical succession and landscape reforestation successful. J. Sustainable Forestry 32, 649–658. doi: 10.1080/10549811.2013.817340
Clement, C. R. (1999). 1492 and the loss of Amazonian crop genetic resources. I. The relation between domestication and human population decline. Econ. Bot. 53, 188–202. doi: 10.1007/BF02866498
Clement, C. R., Denevan, W. M., Heckenberger, M. J., Junqueira, A. B., Neves, E. G., Teixeira, W. G., et al. (2015). The domestication of Amazonia before European conquest. Proc. R. Soc. B. 282:20150813. doi: 10.1098/rspb.2015.0813
Clement, C. R., McCann, J. M., and Smith, N. J. (2003). “Agrobiodiversity in amazonia and its relationship with dark earths,” in Amazonian Dark Earths: Origin, Properties, Management, eds J. Lehmann, D. C. Kern, B. Glaser, and W. Woods (Dordrecht: Kluwer Academic Publishers), 159–178. doi: 10.1007/1-4020-2597-1_9
Cook, F. E. M. (1995). Economic Botany Data Collection Standard. Royal Botanic Gardens, Kew: TDWG, 146.
Coomes, O. T., Grimard, F., and Burt, G. J. (2000). Tropical forests and shifting cultivation: Secondary forest fallow dynamics among traditional farmers of the Peruvian Amazon. Ecol. Econ. 32, 109–124. doi: 10.1016/s0921-8009(99)00066-x
Defoer, T., and Budelman, A. (2000). Managing Soil. (Fertility)in the Tropics. Amsterdam: Resource Guide, FAO and KIT Press, The Netherlands’s Royal Tropical Institute.
Denevan, W. M. (2001). Cultivated Landscapes of Native Amazonia and the Andes. Oxford: Oxford University Press, 396. doi: 10.1017/s0022216x02506544
Denevan, W. M., Treacy, J. M., Alcorn, J. B., Padoch, C., Denslow, J., and Paitan, S. F. (1984). Indigenous agroforestry in the Peruvian Amazon: bora indian management of swidden fallows. Interciencia 9, 346–357.
Denslow, J. (1980). Gap partitioning among tropical rainforest trees. Biotropica 12, 47–55. doi: 10.2307/2388156
Descola, P. (1988). La Selva Culta: Simbolismo y Praxis en la Ecología de los Andes. Lima: Lima e Quito, Ediciones Abya-Yala e Instituto Francés de Estudios Andinos. 468. doi: 10.4000/books.ifea.1600
Diduck, A., Bankes, N., Clark, D., and Armitage, D. (2005). “Unpacking social learning in social-ecological systems: case studies of polar bears and narwhal management in northern Canada,” in Breaking Ice: Renewable Resource and Ocean Management, ed. F. Berkes (Calgary: University of Calgary Press), 269–291.
Dole, G. (2001). “Retrospectiva da história comparativa das culturas do alto Xingu: um esboço das origens culturais alto-xinguanas,” in Os Povos do Alto Xingu: História e Cultura, eds B. Franchetto and M. Heckenberger (Rio de Janeiro: Editora da UFRJ), 63–76.
BRASIL (1961). Decreto n° 50455. Cria o Parque Nacional do Xingu. Diário Oficial da União: Seção 1, Vol. 4. (Brasília, DF: Poder Executivo, ano 139), 3492. Available online at: https://www2.camara.leg.br/legin/fed/decret/1960-1969/decreto-50455-14-abril-1961-390087-norma-pe.html (accessed March 31, 2020).
Durigan, G., Guerin, N., and da Costa, J. M. N. (2013). Ecological restoration of Xingu Basin headwaters: motivations, engagement, challenges and perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20120165. doi: 10.1098/rstb.2012.0165
Eden, M. J., and Andrade, A. (1987). Ecological aspects of swidden cultivation among the Andoke and Witoto. Hum. Ecol. 15, 339–359. doi: 10.1007/bf00888030
Ewel, J. J. (1986). Designing agricultural ecosystems for the humid tropics. Ann. Rev. Ecol. Syst. 17, 245–271. doi: 10.1146/annurev.es.17.110186.001333
Fa, J. E., Watson, J. E., Leiper, I., Potapov, P., Evans, T. D., Burgess, N. D., et al. (2020). Importance of indigenous peoples’ lands for the conservation of intact forest landscapes. Front. Ecol. Environ. 18:135–140. doi: 10.1002/fee.2148
FAO (2018). Caracterización de los Sistemas Agroforestales Kuxur Rum y Quesungual en el Corredor Seco de Guatemala y Honduras. Rome: FAO.
Fausto, C. (2008). Donos demais: maestria e domínio na Amazônia. Mana 14, 329–366. doi: 10.1590/s0104-93132008000200003
Ferguson, B. G., Vandermeer, J., Morales, H., and Griffith, D. M. (2003). Post-Agricultural succession in El petén, guatemala. Conserv. Biol. 17, 818–828. doi: 10.1046/j.1523-1739.2003.01265.x
Freire, G. (2003). Tradition, change and land rights - Land use and territorial strategies among the Piaroa. Critique Anthropol. 23, 349–372. doi: 10.1177/0308275x03234006
Freire, G. N. (2007). Indigenous shifting cultivation and the new amazonia: a piaroa example of economic articulation. Hum. Ecol. 35, 681–696. doi: 10.1007/s10745-007-9120-y
Grümberg, G. (2004). Os Kaiabi do Brasil Central : História e Etnografia; [tradução Eugênio G. Wenzel; tradução dos mitos João Dornstauder]. São Paulo: Instituto Socioambiental.
Hames, R. B., and Vickers, W. T. (1983). “Introduction,” in Adaptive Responses of Native Amazonians. Studies in Anthropology, eds R. B. Hames and W. T. Vickers (New York, NY: Academic Press), 516. doi: 10.1016/b978-0-12-321250-4.50005-1
Hartman, B. D., Cleveland, D. A., and Chadwick, O. A. (2016). Linking changes in knowledge and attitudes with successful land restoration in indigenous communities. Restorat. Ecol. 24, 749–760. doi: 10.1111/rec.12347
Heckenberger, M., and Neves, E. G. (2009). Amazonian archaeology. Annu. Rev. Anthropol. 38, 251–266. doi: 10.1146/annurev-anthro-091908-164310
Hett, C., Castella, J. C., Heinimann, A., Messerli, P., and Pfund, J. L. (2012). A landscape mosaic approach for characterizing swidden systems from a REDD+ perspective. Appl. Geography 32, 608–618. doi: 10.1016/j.apgeog.2011.07.011
IBGE (2012). Manual Técnico da Vegetação Brasileira. Série Manuais Técnicos em Geociências 1, 2a edição revista e ampliada. Rio de Janeiro: IBGE.
Irvine, D. (1989). Succession management and resource distribution in an Amazonian rain forest. Adv. Econ. Bot. 7, 223–237.
Iv, A. P. G., Angiosperm Phylogeny, and Group. (2016). An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Linnean Soc. Lond. Botan. J. Linnean Soc. 181, 1–20.
Ivanauskas, N. M., Monteiro, R., and Rodrigues, R. R. (2008). Classificação fitogeográfica das florestas do Alto Rio Xingu. Acta Amazonica 38, 387–402. doi: 10.1590/s0044-59672008000300003
Jakovac, C. C., Dutrieux, L. P., Siti, L., Peña-Claros, M., and Bongers, F. (2017). Spatial and temporal dynamics of shifting cultivation in the middle-Amazonas river: expansion and intensification. PLoS One 12:e0181092. doi: 10.1371/journal.pone.0181092
Jakovac, C. C., Peña-Claros, M., Kuyper, T. W., and Bongers, F. (2015). Loss of secondary-forest resilience by land-use intensification in the Amazon. J. Ecol. 103, 67–77. doi: 10.1111/1365-2745.12298
Jakovac, C. C., Bongers, F., Kuyper, T. W., Mesquita, R. C., and Peña-Claros, M. (2016a). Land use as a filter for species composition in Amazonian secondary forests. J. Veg. Sci. 27, 1104–1116. doi: 10.1111/jvs.12457
Jakovac, C. C., Peña-Claros, M., Mesquita, R. C., Bongers, F., and Kuyper, T. W. (2016b). Swiddens under transition: consequences of agricultural intensification in the Amazon. Agric EcosystEnviron. 218, 116–125. doi: 10.1016/j.agee.2015.11.013
Johnson, A. (1983). “Machiguenga gardens,” in Adaptative Responses of Native Amazonians, 518, eds R. B. Hames and W. T. Wickers (Cambridge, MA: Academic Press), 27–63. doi: 10.1016/b978-0-12-321250-4.50006-3
Joslin, A. H., Markewitz, D., Morris, L. A., Oliveira, F., de, A., Figueiredo, R. O., et al. (2011). Five native tree species and manioc under slash-and-mulch agroforestry in the eastern Amazon of Brazil: plant growth and soil responses. Agroforest Syst. 81, 1–14. doi: 10.1007/s10457-010-9356-1
Junqueira, A. B., Shepard, G. H. Jr., and Clement, C. R. (2010). Secondary forests on anthropogenic soils in Brazilian Amazonia conserve agrobiodiversity. Biodivers. Conserv. 19, 1933–1961. doi: 10.1007/s10531-010-9813-1
Junqueira, A. B., Shepard, G. H., and Clement, C. R. (2011). Secondary forests on anthropogenic soils of the middle Madeira river: valuation, local knowledge, and landscape domestication in Brazilian Amazonia. Econ. Bot. 65, 85–99. doi: 10.1007/s12231-010-9138-8
Junqueira, A. B., Stomph, T. J., Clement, C. R., and Struik, P. C. (2016). Variation in soil fertility influences cycle dynamics and crop diversity in shifting cultivation systems. Agricul. Ecosystems Environ. 215, 122–132. doi: 10.1016/j.agee.2015.09.01
Jusys, T. (2018). Changing patterns in deforestation avoidance by different protection types in the Brazilian Amazon. PLoS One 13:e0195900. doi: 10.1371/journal.pone.0195900
Keen, M., Brown, V. A., and Dyball, R. (eds) (2005). Social Learning in Environmental Management. London: Earthscan.
Kern, D. C., D’Aquino, G., Rodrigues, T. E., Frazão, F. J. L., Sombroek, W., Myers, T. P., et al. (2003). “Distribution of amazonian dark earths in the Brazilian Amazon,” in Amazonian Dark Earths: Origin, Properties, Management, eds J. Lehmann, D. Kern, B. Glaser, and W. Woods (Alphen aan den Rijn: Kluwer Academic Publishers), 51–76. doi: 10.1007/1-4020-2597-1_4
Lawrence, D. (2004). Erosion of tree diversity during 200 years of shifting cultivation in Bornean rain forest. Ecol. Appl. 14, 1855–1869. doi: 10.1890/03-5321
Le Tourneau, F. M. (2015). The sustainability challenges of indigenous territories in Brazil’s Amazonia. Curr. Opin. Environ. Sust. 14, 213–220. doi: 10.1016/j.cosust.2015.07.017
Levis, C., Costa, F. R. C., Bongers, F., Peña-Claros, M., Clement, C. R., Junqueira, A. B., et al. (2017). Response to Comment on “Persistent effects of pre-Columbian plant domestication on Amazonian forest composition”. Science 355, 925–931. doi: 10.1126/science.aan8837
Levis, C., Flores, B. M., Moreira, P. A., Luize, B. G., Alves, R. P., Franco-Moraes, J., et al. (2018). How people domesticated amazonian forests. Front. Ecol. Evol. 5:171. doi: 10.3389/fevo.2017.00171
Levis, C., Peña-Claros, M., Clement, C. R., et al. (2020). Pre-Columbian soil fertilization and current management maintain food resource availability in old-growth Amazonian forests. Plant Soil 450, 29–48.
Li, W., Fu, R., and Dickinson, E. (2006). Amazon in the 21st century as assessed by the coupled models for the IPCC AR4. J. Geophys. Res. 111:D02111. doi: 10.1029/2005jd006355
Magnuszewski, P., Ostasiewicz, K., Chazdon, R., Salk, C., Pajak, M., Sendzimir, J., et al. (2015). Resilience and alternative stable states of tropical forest landscapes under shifting cultivation regimes. PLoS One 10:e0137497. doi: 10.1371/journal.pone.0137497
Mansourian, S., Parrotta, J., Balaji, P., Belwood-Howard, I., Bhasme, S., Bixler, R. P., et al. (2019). Putting the pieces together: integration for forest landscape restoration implementation. Land Degrad Dev. 31, 419–429. doi: 10.1002/ldr.3448
Marquardt, K., Milestad, R., and Salomonsson, L. (2013). Improved fallows: a case study of an adaptive response in Amazonian swidden farming systems. Agric Hum Values 30, 417–428. doi: 10.1007/s10460-012-9415-5
Menget, P. (1981). “From forest to mouth: reflections on the Txicao theory of substance,” in Food Taboos in Lowland South America. Working Papers on South American Indians, eds K. M. Kensinger and W. H. Kracke (Bennington, VL: Bennington College), 2–17.
Menget, P. (2001). Em Nome dos Outros. Classificação das Relações Sociais entre os Txicáo do Alto Xingu. Lisboa: Museu Nacional de Etnologia Assírio & Alvim, 334.
Mertz, O., Padoch, C., Fox, J., Cramb, R. A., Leisz, S. J., Lam, N. T., et al. (2009). Swidden change in Southeast Asia: understanding causes and consequences. Hum. Ecol. 37, 259–264. doi: 10.1007/s10745-009-9245-2
Miller, R. P., and Nair, P. K. R. (2006). Indigenous agroforestry systems in amazonia: from prehistory to today. Agroforestry Systems 66, 151–164. doi: 10.1007/s10457-005-6074-6071
Moran, E. F. (1996). “Nurturing the Forest: Strategies of Native Amazonians,” Reprinted from Redefining Nature, eds R. Ellen and K. Fukui (London: ACT Publication), 531–555.
Morton, D. C., Le Page, Y., DeFries, R., Collatz, G. J., and Hurtt, G. C. (2013). Understorey fire frequency and the fate ofburned forests in southern Amazonia. Philos. Trans. R. Soc. Biol. Sci. 368:20120163. doi: 10.1098/rstb.2012.0163
Mukul, S. A. (2016). Shifting Cultivation in the Upland Secondary Forests of the Philippines: Biodiversity and Carbon Stock Assessment, and Ecosystemservices Trade-Offs In Land-Use Decisions. PhD thesis, The University of Queensland: Australia. doi: 10.14264/uql.2016.222.
Mukul, S. A., Herbohn, J., and Firn, J. (2016a). “High resilience of tropical forest diversity and structure after shifting cultivation in the Philippines uplands. applied vegetation science,” in Shifting Cultivation in the Upland Secondary Forests of the Philippines: Biodiversity and Carbon Stock Assessment, and Ecosystemservices Trade-offs in Land-use Decisions, PhD thesis, ed. S. A. Mukul (Australia: The University of Queensland).
Mukul, S. A., Herbohn, J., and Firn, J. (2016b). Tropical secondary forests regenerating after shifting cultivation in the Philippines uplands are important carbon sinks. Sci. Rep. 6:22483. doi: 10.1038/srep22483
Munari, L. C. (2009). Memória Social e ecologia histórica: a agricultura de coivara das populações quilombolas do Vale do Ribeira e sua relação com a formação da Mata Atlântica local. São Paulo: IB-USP São Paulo.
Nascimento, A. A., Horta, A., and Ono, K. Y. (2018). Com Quantas Fogueiras se Faz Uma Floresta? Projeto Fogo do Índio – Alternativas de Manejo Para a Conservação das Florestas no Parque Indígena do Xingu. São Paulo: Instituto Socioambiental, 18. (Unpublished).
Nepstad, D., Lefebvre, P., Lopes, da Silva, U., Tomasella, J., Schlesinger, P., et al. (2004). Amazon drought and its implications for forest flammability and tree growth: a basin-wide analysis. Global Change Biol. 10, 704–717. doi: 10.1111/j.1529-8817.2003.00772.x
Neves, E. G., Petersen, J. B., Bartone, R. N., and Silva, C. D. (2003). “Historical and socio-cultural origins of Amazonian Dark Earths,” in Amazonian Dark Earths: Origin, Properties, Management, eds J. Lehmann, D. C. Kern, B. Glaser, and W. I. Woods (Berlin: Springer Science and Business Media), 29–50. doi: 10.1007/1-4020-2597-1_3
Nobre, C. A., Sellers, P. J., and Shukla, J. (1991). Amazonian deforestation and regional climate change. J. Climate 4, 957–988.
Oliveira, J. C. (2016). Mundos de roças e florestas. Boletim do Museu Paraense Emílio Goeldi Ciências Humanas 11, 115–131. doi: 10.1590/1981.81222016000100007
Peltier, R., Dubiez, E., Diowo, S., Gigaud, M., Marien, J. N., Marquant, B., et al. (2014). Assisted natural regeneration in slash-and-burn agriculture: results in the democratic republic of the congo. Bois et Forêts des Tropiques. 68, 67–79. doi: 10.19182/bft2014.321.a31220
Pinedo-Vasquez, M., Hecht, S., and Padoch, C. (2012). “Amazonia,” in Traditional Forest-Related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, World Forests, Chap. 4, eds J. A. Parrotta and R. L. Trosper (Berlin: Springer), doi: 10.1007/978-94-007-2144-9_4
Pye-Smith, C. (2013). The Quiet Revolution: How Niger’s Farmers are Re-Greening the Parklands of the Sahel. ICRAF Trees for Change no.12. Nairobi: World Agroforestry Centre
Radam Brasil (1985). Fitogeografia brasileira. Boletim Técnico do Projeto Radam Brasil. Rio de Janeiro: Departamento Nacional de Produção Mineral, 206.
Reyes-García, V., Fernández-Llamazares, Á, McElwee, P., Molnár, Z., Öllerer, K., Wilson, S. J., et al. (2018). The contributions of Indigenous peoples and local communities to ecological restoration. Restor. Ecol. 27, 3–8. doi: 10.1111/rec.12894
Ribeiro Filho, A. A., Adams, C., Manfredini, S., Munari, L. C., da Silva, Junior, J. A., et al. (2018). Dynamics of the soil fertility in quilombola shifting cultivation communities of the Atlantic Rainforest. Brazil. Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém 13, 79–106.
Ribeiro Filho, A. A., Adams, C., and Murrieta, R. S. S. (2013). The impacts of shifting cultivation on tropical forest soil: a review. Bol. Mus. Para. Emílio Goeldi. Cienc. Hum., Belém. 8, 693–727.
Rodgers, D. (2002). A soma anômala: a questão do suplemento no xamanismo e menstruação Ikpeng. Mana 8, 91–125. doi: 10.1590/s0104-93132002000200004
Rodgers, D. (2013). The filter trap. Swarms, anomalies, and the quasi-topology of Ikpeng shamanism. HAU: J. Ethnographic Theory 3, 77–105. doi: 10.14318/hau3.3.005
Rodrigues, A. J. (1993). The ecology of the Kayabi Indians of Xingu, Brazil: Soil And Agroforestry Management. PHD Thesis, Darwin College, Cambridge University: Cambridge.
Rozendaal, D. M. A., Bongers, F., Aide, T. M., Alvarez-Dávila, E., Ascarrunz, N., and Balvanera, P. (2019). Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 5:eaau3114.
Sajise, P. (2003). “Working with nature: technical and social dimensions of assisted natural regeneration,” in Advancing Assisted Natural Regeneration (ANR) in Asia and the Paci?c, eds P. C. Dugan, P. B. Durst, D. J. Ganz, and P. J. McKenzie (Rome: FAO), 5–16.
Sanches, R. A., and Futemma, C. R. T. (2019). Seeds network and collective action for the restoration and conservation of Xingu River’s springs (Mato Grosso, Brazil). Desenvolv. Meio Ambiente. 50, 127–150. doi: 10.5380/dma.v50i0.59435
Sanches, R. A., Rossete, A. N., Rezende, A. C. P., Aves, H. Q., and Villas-Bôas, A. (2012). Subsídios para a proteção das áreas úmidas da bacia do rio Xingu (Mato Grosso, Brasil). Revista Árvore 36, 489–498. doi: 10.1590/s0100-67622012000300011
Sanches, R. A., and Villas-Bôas, A. (2008). “Os formadores do rio Xingu: desafios para a preservação e gestão ambiental,” in Seria Melhor Mandar Ladrilhar? Biodiversidade Como, Para Que, Por Quê, ed. N. Bensusan (Brasília: Instituto Socioambiental, Editora Universidade de Brasília), 303–316.
Schmidt, M. J. (2013). Amazonian dark earths: pathways to sustainable development in tropical rainforests? Bol. Mus. Para. Emílio Goeldi. Cienc. Hum. 8, 11–38. doi: 10.1590/s1981-81222013000100002
Schmidt, M. J., Daniel, A. R. P., Moraes, C. P., Valle, R. B. M., Caromano, C. F., Texeira, W. G., et al. (2014). Dark earths and the human built landscape in Amazonia: a widespread pattern of anthrosol formation. J. Archaeol. Sci. 42, 152–165.
Schmidt, M. V. C. (2001). Etnosilvicultura Kaiabi no Parque Indígena do Xingu: Subsídios ao manejo de recursos florestais. [Dissertação de Mestrado] [São Carlos]. Brasil: Escola de Engenharia, Universidade de São Paulo, 185. doi: 10.11606/d.18.2017.tde-01022017-125404
Schmidt, M. V. C., and Ikpeng, K. (2006). “Ikpeng - mobilização para resgatar recursos naturais tradicionais,” in Povos Indígenas no Brasil - 2001/2005, (São Paulo: Instituto Socioambiental - ISA), 671–674.
Schmidt, M. V. C., Tuyuka, J. B. R., Cabalzar, A., and Van der Veld, P. J. (2010a). “Capoeiras Tuyka. Processos de Restauração de Recuperação de Terras Degradadas,” in Conhecimentos e Práticas dos Povos Indígenas do Rio Negro (noroeste amazônico), I. Edn, 1, ed. O. Manejo do Mundo (São Paulo: Instituto Socioambiental - ISA), 157–166.
Schmidt, M. V. C., Van der Veld, P. J., Cabalzar, A., Tuyuka, T. A. B., Tuyuka, R. B. R., Tukano, V. A., et al. (2010b). “Manejo florestal participativo da sorva. produção sustentável do banco tukano – kumurô,” in Conhecimentos e Práticas dos Povos Indígenas do Rio Negro (noroeste amazônico), I. Edn, 1, ed. O. Manejo do Mundo (São Paulo: Instituto Socioambiental - ISA), 169–180.
Schmidt, M. V. C. (2006). Pequi é Fruta Cultural Indígena. Parque Indígena do Xingu. Povos Indígenas no Brasil 2001/2005. São Paulo: Instituto Socioambiental, 675–678.
Schmidt-Vogt, D. (2001). Secondary forest in swidden agriculture in the highlands of thailand. J. Trop. Forest Sci. 13, 748–767.
Shepard, G. H., and Ramirez, H. (2011). “Made in Brazil”: human dispersal of the Brazil Nut (Bertholletia excelsia, Lecythidaceae) in Ancient Amazonia. Econ. Botany 65, 44–65. doi: 10.1007/s12231-011-9151-6
Silva, G. M. S. (2016). Peanut Diversity Management by the Kaiabi (Tupi Guarani) Indigenous People, Brazilian Amazon. PhD Thesis, Gainesville, FL: University of Florida, 507.
Silvério, D. V., Brando, P. M., Macedo, M. N., Beck, P. S. A., Bustamante, M., and Coe, M. T. (2015). Agricultural expansion dominates climate changes in southeastern Amazonia: the overlooked non-GHG forcing. Environ. Res. Lett. 10, 104015. doi: 10.1088/1748-9326/10/10/104015
Simões, M. E. (1963). Os “Txikão” e outras tribos marginais do Alto Xingu. Revista do Museu Paulista. Nova Série 14, 76–105.
Smith, N. J. H. (1980). Anthrosols and human carrying capacity in Amazonia. Ann. Assoc. Am. Geogr. 70, 553–566. doi: 10.1111/j.1467-8306.1980.tb01332.x
Sutherland, W. J., Gardner, T., Haider, L. J., and Dicks, L. V. (2013). How can local and traditional knowledge be effectively incorporated into international assessments? Oryx 48, 1–2. doi: 10.1017/s0030605313001543
Tengö, M., Brondizio, E. S., Elmqvist, T., Malmer, P., and Spierenbur, M. (2014). Connecting diverse knowledge systems for enhanced ecosystem governance: the multiple evidence base approachg. Ambio 43, 579–591. doi: 10.1007/s13280-014-0501-3
Tengö, M., Hill, R., Malmer, P., Raymond, C. M., Spierenburg, M., Danielsen, F., et al. (2017). Weaving knowledge systems in IPBES, CBD and beyond—lessons learned for sustainability. Curr. Opin. Environ. Sustainabil. 26-27, 17–25. doi: 10.1016/j.cosust.2016.12.005
Thomas, E. (2014). “Assisted natural regeneration,” in Genetic Considerations in Ecosystem Restoration Using Native Tree Species. The State of the World’s Forest Genetic Resources. Publisher: Thematic Study. PART 3, eds M. Bozzano, R. Jalonen, E. Thomas, D. Boshier, L. Gallo, S. Cavers, et al. (Rome: FAO), 148–149. doi: 10.1016/j.foreco.2014.07.015
Thrupp, L. A., Hecht, S., Browder, J., Lynch, O. J., Megateli, N., and O’Brien, W. (1997). The Diversity and Dynamics of Shifting Cultivation: Myths, Realities, and Policy. Washington, D.C: World Resources Institute, 53.
Toledo, M., and Salick, J. (2006). Secondary succession and indigenous management. Biotropica 38, 161–170. doi: 10.1111/j.1744-7429.2006.00120.x
Tschakert, P., Coomes, O. T., and Potvin, C. (2007). Indigenous livelihoods, slash-and-burn agriculture, and carbon stocks in Eastern Panama. Ecol. Econ. 60, 807–820. doi: 10.1016/j.ecolecon.2006.02.001
Txicão, K., and Leão, M. F. (2019). A pesca dos Ikpeng com cipó timbó-açu: elementos da cultura e da natureza que podem ser utilizados no ensino de ciências. Experiências em Ensino de Ciências 14, 481–494. doi: 10.14295/ambeduc.v24i1.8300
Uhl, C. (1983). You Can Keep a Good Forest Down. Natural History 4:71–79. Agroforestry Systems 11: 175-197, 1990. Printed in the Netherlands: Kluwer Academic Publishers, 175.
Uhl, C., Clark, K., Clark, H., and Murphy, P. (1981). Early plant succession after cutting and burning in the upper rib negro region of the Amazon basin. J. Ecol. 69, 631–649. doi: 10.2307/2259689
Unruh, J. D. (1988). Ecological aspects of site recovery under swidden-fallow management in the Peruvian Amazon. Agroforestry Systems 7, 161–184. doi: 10.1007/bf00046850
Uprety, Y., Asselin, H., Bergeron, Y., Doyon, F., and Boucher, J.-F. (2012). Review article: contribution of traditional knowledge to ecological restoration: practices and application 1. Ecoscience 19, 225–237. doi: 10.2980/19-3-3530
Uriarte, M., and Chazdon, R. L. (2016). Incorporating natural regeneration in forest landscape restoration in tropical regions: synthesis and key research gaps. Biotropica 48, 915–924. doi: 10.1111/btp.12411
van Vliet, N., Adams, C., Vieira, I. C. G., and Mertz, O. (2013). Slash and burn” and “shifting” cultivation systems in forest agriculture frontiers from the Brazilian Amazon. Soc. Nat. Resources: Int. J. 26, 1454–1467. doi: 10.1080/08941920.2013.820813
van Vliet, N., Mertz, O., Heinimann, A., Langanke, T., Pascual, U., Schmook, B., et al. (2012). Trends, drivers and impacts of changes in swidden cultivation in tropical forest-agriculture frontiers: a global assessment. Global Environ. Change: Hum. Policy Dimens. 22, 418–429. doi: 10.1016/j.gloenvcha.2011.10.009
Vásquez-Grandón, A., Donoso, P. J., and Gerding, V. (2018). Forest degradation: when is a forest degraded? Forests 9:726. doi: 10.3390/f9110726
Vickers, W. T. (1983). “The territorial dimensions of siona-secoya and encahellado adaptation,” in Adaptative Responses of Native Amazonians, eds R. B. Hames and W. T. Wickers (Cambridge, MA: Academic Press), doi: 10.1016/b978-0-12-321250-4.50019-1
Villas-Bôas, C., and Villas-Bôas, O. (1986). Xingu: os Kayabi do rio São Manoel. Porto Alegre: Kuarup, 116.
Walpole, P. (2010). Bringing back the forests through assisted natural regeneration. Institute of environmental Science for Social Change. Available online at: https://essc.org.ph/content/view/277/153/ (accessed May 10, 2020).
Wangpakapattanawong, P., Kavinchan, N., Vaidhayakarn, C., Schmidt-Vogt, D., and Elliott, S. (2010). Fallow to forest: applying indigenous and scientific knowledge of swidden cultivation to tropical forest restoration. Forest Ecol. Manag. 260, 1399–1406. doi: 10.1016/j.foreco.2010.07.042
Wiersum, K. F. (2004). Forest gardens as an ‘intermediate’ land-use system in the nature–culture continuum: characteristics and future potential. Agroforestry Systems 61, 123–134. doi: 10.1023/b:agfo.0000028994.54710.44
Woods, W. I., Denevan, W. M., and Rebellato, L. (2013). “How many years do you get for counterfeiting a paradise?,” in Soils, Climate and Society: Archaeological Investigations in Ancient America, eds J. D. Wingard and S. E. Hayes (Boulder: University Press of Colorado), 1–20.
Keywords: Xingu Indigenous Territory, Kawaiwete people, Ikpeng people, local ecological knowledge, shifting cultivation, swidden fallows, assisted natural regeneration, indigenous forest management
Citation: Schmidt MVC, Ikpeng YU, Kayabi T, Sanches RA, Ono KY and Adams C (2021) Indigenous Knowledge and Forest Succession Management in the Brazilian Amazon: Contributions to Reforestation of Degraded Areas. Front. For. Glob. Change 4:605925. doi: 10.3389/ffgc.2021.605925
Received: 13 September 2020; Accepted: 22 March 2021;
Published: 26 April 2021.
Edited by:
Catarina C. Jakovac, Wageningen University and Research, NetherlandsReviewed by:
Carolina Levis, Federal University of Santa Catarina, BrazilNivaldo Peroni, Federal University of Santa Catarina, Brazil
Copyright © 2021 Schmidt, Ikpeng, Kayabi, Sanches, Ono and Adams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcus Vinícius C. Schmidt, bXNtYXJjdXN0b3BlMkBnbWFpbC5jb20=
 Marcus Vinícius C. Schmidt
Marcus Vinícius C. Schmidt Yakuna Ullillo Ikpeng
Yakuna Ullillo Ikpeng Tariaiup Kayabi
Tariaiup Kayabi Rosely Alvim Sanches
Rosely Alvim Sanches Katia Yukari Ono5
Katia Yukari Ono5 Cristina Adams
Cristina Adams