- 1Environmental Science Laboratory, Department of Sustainable Natural Resources, School of Earth Sciences and Engineering, Botswana International University of Science and Technology (BIUST), Palapye, Botswana
- 2Plant Biotechnology Laboratory, Department of Biological Sciences and Biotechnology, School of Life Sciences, Botswana International University of Science and Technology (BIUST), Palapye, Botswana
Introduction: Savanna ecosystems, an important contributor to Botswana’s economy and occupy up to 86 percent of the land mass of Botswana serving as habitat for wildlife and livestock that are being affected by frequent wildfire which are attributed in part to climate change. While the impact of wildfires on the environment has been extensively studied, there are some uncertainties as to their short-term effects on vegetation dynamics as well as the ability for vegetation to recover from fires. In addition, the impact on soil biogeochemical properties, soil microbial community dynamics, and their interaction within the savanna ecosystem in Botswana, needs to be understood to effectively manage the increasing occurrence of wildfires.
Methodology: A comparative study was conducted on a burned area and unburned adjacent area within a period after 6 months after wildfire occurrence.
Results and discussion: Our findings reveal that the ecological impacts of fire on vegetation, soil chemical properties, and microbial community dynamics were not uniform but were strongly mediated by site-specific conditions and the soil type of the associated plant species. Wildfire consistently reduced vegetation cover, plant biomass, and net primary productivity. In contrast, wildfire increased plant species diversity and evenness by disrupting competitive dominance. The impact of wildfire significant increases in soil pH and exchangeable cations (P, K, and Mg) only occurred at the nutrient-rich Mmashoro site and were further localized to the soils under Combretum apiculatum. Conversely, total organic matter increased post-fire at both sites, likely due to ash deposition. The effects of the wildfire on soil microbial community was also site-specific. In Palapye, fire significantly suppressed metabolic activity and functional evenness, leading to a less balanced community but was poorer in key mineral nutrients (K and Mg). Conversely, in Mmashoro, the microbial community associated with the fire-adapted tree C. apiculatum exhibited significant functional resilience, which retained high metabolic activity post-fire. We conclude that certain keystone plant species can mitigate the impacts of fire on belowground processes, creating resilient patches within the landscape. These findings underscore that effective fire management and conservation strategies in savanna ecosystems must be context-specific, accounting for the unique vegetation and soil characteristics of the area.
1 Introduction
About 86 percent of the land mass of Botswana is covered by the savanna ecosystem which supports the subsistence livelihoods of 80 percent of the population (Woods and Sekhwela, 2003). Between 1820 and 1935, travelers to Botswana described the landscape as grass dominated savanna (Woods and Sekhwela, 2003). This ecosystem contributes substantially to Botswana’s gross domestic product (GDP) through agriculture and tourism, and it also provides forage for domestic and wild animals (de Torres Curth et al., 2012). Savanna ecosystem is highly impacted by various factors, with wildfires standing out as the most influential. Wildfire is a biogeochemical agent that has been an integral component of savanna ecosystems worldwide for over 300 million years, playing a vital role in their creation and maintenance (Bond and Keeley, 2005; Bowman et al., 2009; Ryan and Williams, 2011). Wildfires contribute significantly to soil degradation and the reduction of essential elements through soil erosion and vaporization (Gómez-Rey et al., 2013; Agbeshie et al., 2022). They can disrupt soil physicochemical properties (including texture, pH, electrical conductivity (EC), total organic matter, and nutrients) and biological properties (Jhariya and Raj, 2014; Ghazoul et al., 2015; Silvério et al., 2019; Agbeshie et al., 2022) while also increasing water repellency (Novara et al., 2013). Although wildfire reduces the total organic matter of the soil, some instances were identified in Sicilian grasslands where this has remained constant (Novara et al., 2013). Furthermore, burning organic matter produces a large amount of readily accessible nutrients that can be utilized for plant recuperation (Kauffman et al., 1992; Chungu et al., 2020; Arunrat et al., 2024; Johnson et al., 2024; Hu and Wan, 2024; Ji et al., 2025). Mineral ash produced by burning biomass can also affect soil pH, as well as microbial activities associated with the decomposition of organic matter and recycling of the nutrients (Kauffman et al., 1992; Chungu et al., 2020; Arunrat et al., 2024; Johnson et al., 2024; Hu and Wan, 2024; Ji et al., 2025). Soil microbial communities serve an important function in the breakdown of organic matter within the bulk soil and the rhizosphere through their metabolic processes. The microbes secrete degrading enzymes within the rhizosphere that regulate the decomposition and accumulation of organic matter (Nannipieri et al., 2003). The rhizosphere serves as the epicenter for microbial metabolism and activity which contribute a significant role in the maintenance of carbon and nutrient recycling within the terrestrial ecosystem (Turpault et al., 2007; Kuzyakov and Blagodatskaya, 2015; Zhao et al., 2022). The rhizosphere differs from bulk soil due to various biological, chemical, and physical processes driven by root growth, nutrients, water uptake, respiration, rhizo-deposition, and increased microbial growth (Yadav and Islam, 2023). Soil microorganisms form complex communities that drive 80–90% of soil processes and these includes decomposition, mineralization, and immobilization that are imperative for recycling nutrients and degrading pollutant in the environment (Nannipieri et al., 2003). Microorganisms respond rapidly to environmental changes, and shifts in their biomass, metabolic processes, or community composition can serve as early indicators of positive or negative changes in the entire ecosystem (Barreiro et al., 2015). Wildfires significantly alter the carbon source consumption profile of microbial communities within the rhizosphere, reducing the diversity and efficiency of carbon metabolism (Mubyana-John et al., 2007; Aponte et al., 2022).
Wildfire have the ability to alter the vegetation structure and composition through biomass destruction (Bond and Keeley, 2005; Jhariya and Raj, 2014), and replacement of grass species with shrubs, i.e., encroachment (Van Auken, 2000; Franzese et al., 2009; Ghermandi et al., 2010; Oddi et al., 2010), thus affecting the wildlife due to the damage of habitats and food sources (Ryan and Williams, 2011; Dube, 2015). Conversely, the absence of wildfires can turn shrub lands into forests (Ryan and Williams, 2011). Therefore, fire plays a significant role in controlling the balance of woody vegetation and grasses (Bond et al., 2005; Bond and Keeley, 2005; Franzese et al., 2009; Oddi et al., 2010; Ghermandi et al., 2010; de Torres Curth et al., 2012; Dudinszky and Ghermandi, 2013). Biomass combustion also emits substantial amounts of climate warming gases such as carbon dioxide (CO2) and methane (CH4), which contributes to global warming (Levine et al., 1995; Carter and Hulme, 1999; Kikstra et al., 2022; Muñoz et al., 2024; Hassan, 2024; Filonchyk et al., 2024; Reisinger, 2024; Riishojgaard and Tarasova, 2024; Su et al., 2024). In contrast, wildfires also have important functions in the ecosystem such as managing and controlling vegetation density structure (Nyamadzawo et al., 2013) and enhancing the seed germination in various plant species (Keeley and Fotheringham, 2000). They promote heat-induced germination, which is commonly observed in hard-seeded species characterized by a physical seed coat that acts as a barrier to water absorption (Bond and Keeley, 2005). The heat shock from the wildfires, together with elevated soil temperature on open sites, combine with smoke from charred wood to trigger germination by cracking the seed and inducing sprouting for tree species within the savanna ecosystem (Keeley and Fotheringham, 2000; Bond and Keeley, 2005). In this ecosystem, heat triggered germination is commonly associated with plant species from the Fabaceae and Sterculaceae families (Bond and Keeley, 2005).
In Botswana, wildfires frequently occur between April and June, but the most intense fires occur in the dry season, starting from August to October (Maabong and Mphale, 2021). The high intensity of the wildfires is due to the high fuel load that easily ignites during the dry season (Dube, 2013; Maabong and Mphale, 2021), and strong winds which can increase their spread (Dube, 2013; Maabong and Mphale, 2021). Between 2006 and 2017, about 2,582 fire incidences were reported in Botswana, burning a total area of approximately 70,944,239 ha (Maabong and Mphale, 2021). Recent studies related to wildfires were conducted in the Chobe enclave of northern Botswana by Cassidy et al. (2022), which focused on fire frequency and seasonality. Another study conducted in the Okavango Delta, looked at how fire influences the microbial community structure by using phospholipid ester-linked fatty acids (PLFA) (Mubyana-John et al., 2007). However, in Botswana, there are limited studies that assess the impacts of wildfire on vegetation recovery, soil biochemical properties, and community level physiological profiling in the savanna ecosystem.
Therefore, this study provides novel information on the short-term impacts of wildfire on microbial community level physiological profiling using Biolog EcoPlate™ in a savanna ecosystem. The information generated from this study will assist in monitoring the microbial community dynamics in the bulk and rhizosphere soils within the savanna ecosystem which will aid in the maintenance of the soil vitality, productivity, and these will inform policies aimed at climate change mitigation. We hypothesized that wildfire would function as a major disturbance whose effects are strongly influenced by site-specific conditions, inducing a divergent response between vegetation and soil microbial dynamics activity. Specifically, the short-term impacts of wildfires would result in the reduction of vegetation cover, plant biomass, net primary productivity (NPP), and carbon substrate utilization pattern and an increment in soil pH, and EC, in both sites. Moreover, we predict a rapid increase in total organic matter content, species evenness and species diversity, available P and exchangeable Ca whereas exchangeable cations (K, Mg, and Na) will decrease depending on the plant species in Palapye. However, we predict a slight increase in total organic matter content, available P, and exchangeable cations (K, Mg, Ca, and Na) in Mmashoro. Given the massive wildfire incidences in Botswana and their essential negative and positive roles on ecosystem functions, this study aims to assess: (i) the immediate short-term impacts of wildfire on vegetation recovery, and (ii) the subsequent impact of wildfire and vegetation on soil biochemical properties (including pH, EC, total organic matter content, available P, and exchangeable cations (K, Mg, Ca, and Na)), as well as community level physiological profiling of the microbial community in the bulk and rhizosphere soil of dominant vegetation in the savanna ecosystem of Botswana.
2 Materials and methods
2.1 Study site description
This study was conducted in 12 100 m2 quadrats. Three quadrats were demarcated in burned (Experimental) sites and three quadrats were marked in unburned (Control) sites both in Palapye and Mmashoro, Central Botswana (Figure 1). The coordinates of the study sites in Palapye and Mmashoro were marked with a Handheld GPS device (Garmin 62 s) and the captured points were loaded into the ArcGIS database to mark the sampling sites on the map. The wildfire incidents within Palapye and Mmashoro occurred September 4–6, 2022 and September 17–20, 2022 respectively, and sampling in both sites was done 6 months after the fires occurred. Palapye has a semi –arid tropical climate with an annual mean precipitation of 396 mm, varying between 129 mm and 988 mm (Akinyemi, 2017), while Mmashoro is characterized by a warm semi-arid climate with annual mean precipitation of 420 mm, varying between 250 mm and 650 mm (Batanani, 2020). The vegetation composition in Palapye is characterized by shrub and tree savanna dominated by Colophospermum mopane (Benth.) J. Léonard, Senegalia nigrescens (Oliv.) P. J. H. Hurter, Terminalia sericea Burch. ex DC, Combretum apiculatum Sond, and Vachellia tortilis (Forssk.) Gallaso & Banfi (Bekker and De Wit, 1991; Gajaje et al., 2024). The vegetation structure in Mmashoro is composed of shrub and tree savanna dominated by C. apiculatum, Combretum hereroense Schinz, T. sericea, Bauhinia petersiana Bolle subsp. macrantha (Oliv.) Brummitt & J. H. Ross, and Tylosema esculentum (Burch.) A. Schreib (Bekker and De Wit, 1991; Van Wyk, 2013). The soil types in the study area in Palapye are predominantly Ferralic/Luvic Arenosols, Ferric/Haplic Lixisols, and Lithic Leptosols (Akinyemi et al., 2021). The soil types in the Mmashoro study area is mainly comprised of Ferralic arenosols and Petric Calcisols (De Wit and Nachtergaele, 1990). Based on the findings from the Moderate Resolution Imaging Spectroradiometer (MODIS) and Visible Infrared Imaging Radiometer Suite (VIIRS), the Palapye and Mmashoro wildfires had fire power that ranged from 1 to 10.5 Mw (Megawatts) with a lower heat intensity (based on color temperature) that ranged from 306 to 368 K. The spatial resolution of the fire was 250 m, as provided by MODIS.
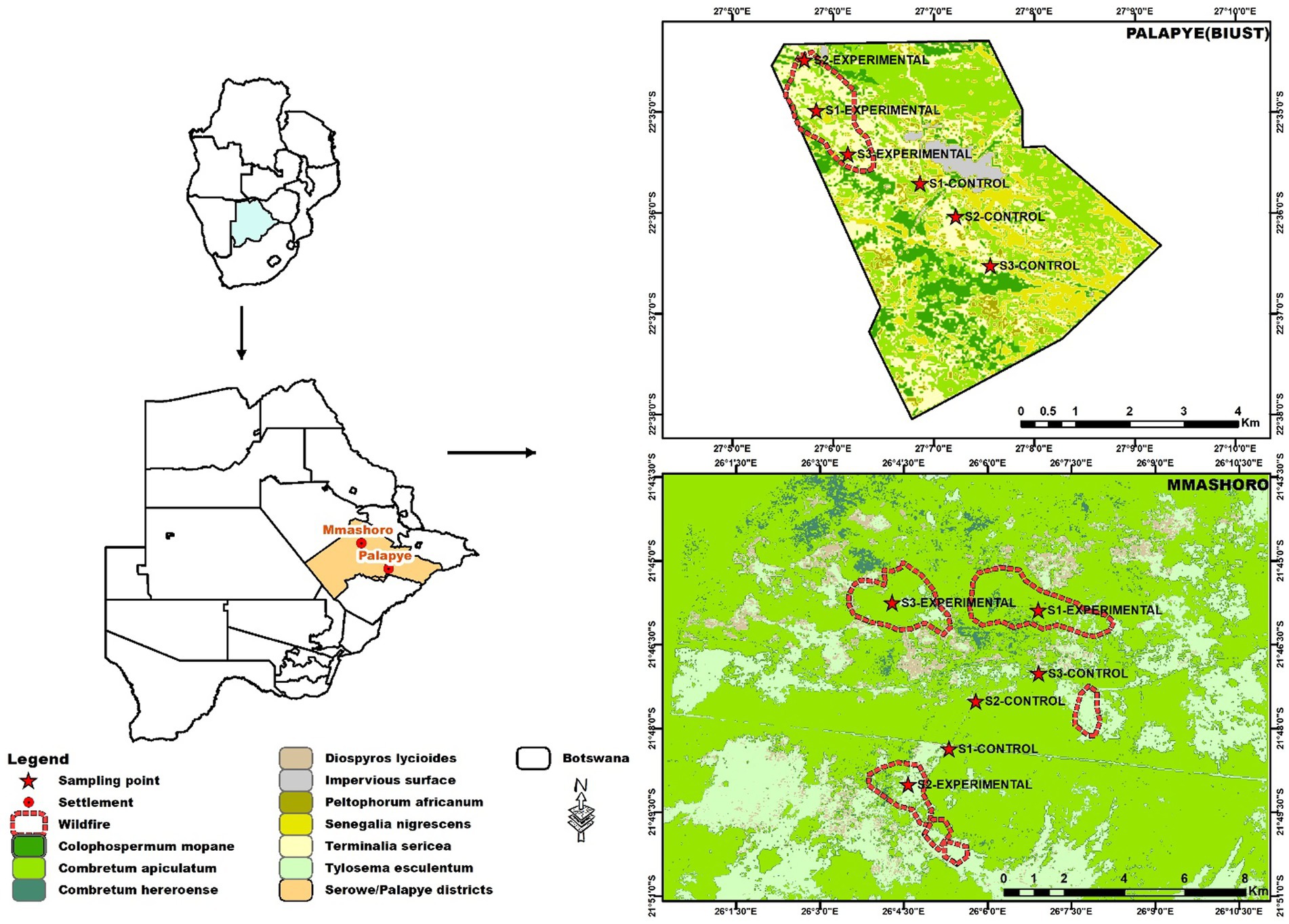
Figure 1. Location of the study area, sampling points, fire scar [European Space Agency (ESA) Climate change Initiative (CCI) Progaramme], and vegetation types (Bekker and De Wit, 1991) within Palapye and Mmashoro (Map by: Morati Mpalo).
2.2 Assessment of the impacts of wildfire on vegetation diversity
2.2.1 Vegetation diversity
A vegetation diversity assessment of all plant species was conducted within three quadrats of the burned sites and three quadrats of the unburned sites following the protocol by Heard and Channon (1997), and Gajaje et al. (2024). Woody species (trees and shrubs) were studied using 100 m2 quadrats, while grasses and herbaceous species were studied using three 1 m2 mini quadrats randomly placed in the main quadrat (100 m2) to establish their density and population (Gajaje et al., 2024). Crown diameter and height were determined through visual observation and by using a tape measure. Crown cover area (Equation 1) and vegetation cover (%) (Equation 2) were determined following a method described by Elzinga et al. (2001), Bonham (2013), and Gülci (2019). Morphological identification of all plant species within the burned and unburned quadrats was conducted by examining plant anatomical parts (leaves, bark, flowers, and fruits) with the assistance of a botanist and verified with several field guidebooks (Oudtshoorn, 2002; Heath and Heath, 2009; Van Wyk, 2013; Roodt, 2015; Van Rooyen and Van Rooyen, 2019). The frequencies of all plants were recorded to determine species richness (Equation 3), diversity (Equation 4), and evenness (Equation 5) using Menhinick’s richness index, Shannon-Wiener index, and Shannon index evenness (Davari et al., 2011; Kanagaraj et al., 2017). The dominant vegetation (grasses, herbs, shrubs, and trees) were determined in three quadrats of burned and three quadrats of unburned sites using the Simpson dominance index (Equation 6) (Thukral et al., 2019; Ulfah et al., 2019; Firmaningrum et al., 2021).
Crown cover area represents the overall area of land occupied by the vegetation, where r is the radius of the plant canopy, radius was derived from dividing the diameter by 2.
Vegetation cover is expressed as a percentage, and it represents the portion of the ground area occupied by the flora (vegetation).
Where d is Menhinick richness index, s is an overall quantity of plant species in the quadrat, and N is an overall number of individual species in the quadrat. The Shannon-Wiener diversity Index (H) given by Equation 2 represents the proportion of species to their importance within a community:
Where i is the overall quantity of individuals of single species, Pi (a decimal fraction) is the quantity of individuals in one species divided by population, and ln is the natural logarithm. The species evenness was determined based on the following equation (Kanagaraj et al., 2017):
Where EH represents evenness, H depicts Shannon-Wiener diversity Index, and s denotes an entire number of species.
Simpson’s dominance index is a statistical metric applied in the field of ecology to quantitatively assess the level of biodiversity within a given ecosystem (Thukral et al., 2019; Ulfah et al., 2019; Firmaningrum et al., 2021). It denotes the degree to which a species dominates within an ecological community (Thukral et al., 2019; Ulfah et al., 2019; Firmaningrum et al., 2021). The calculation of the index is derived from the assessed relative abundance of various species present within the community (Thukral et al., 2019; Ulfah et al., 2019; Firmaningrum et al., 2021).
Where ni is the overall number of individual species and N represents the grand total of all species in an area.
2.2.2 Net primary productivity (NPP) and plant biomass of dominant vegetation
The net primary productivity of dominant vegetation (grasses, herbs, shrubs, and trees) from three quadrats (100 m2) in burned and unburned sites were measured in triplicate using a Handheld CI-340 Photosynthesis system (CID Bioscience, Inc). Dominant plant species were targeted because they are abundant and significantly impact community structure and ecological communities through several processes (Emery and Gross, 2006; Chen et al., 2023). The impact of the dominant plant species on important ecological functions includes litter biomass, pH, soil water content, net primary productivity within and between trophic levels (Emery and Gross, 2006; Chen et al., 2023). The photosynthesis meter’s operating principle is based on the notion of gas exchange on the leaf and typically monitors the concentrations of carbon dioxide (CO2) and water vapor (H2O) via the stomata. The time interval between the measurements was set to 30 s. Leaf area varied as per plant leaf size. The flow rate was set at +0.30 Ipm, and readings were determined using the open system mode. Plant biomass of the dominant vegetation was determined using Equation 7 (Field et al., 1998; Gwynn-Jones et al., 2002; Chapin et al., 2011);
2.3 Assessment of chemical properties of soil impacted by wildfire
2.3.1 Bulk and rhizosphere soil sampling and processing
Soil samples from the bulk and rhizosphere soil from dominant vegetation (grasses, herbs, shrubs, and trees) were gathered from the 0–20 cm soil layer from three quadrats of the burned and three quadrats of unburned sites in Palapye and Mmashoro following the protocol by Singh and Dubey (2012). Soil adhering to the root surface was diligently brushed off with sterile brush and gathered as rhizosphere. Soil not adhering to the roots was collected as bulk soil. The analysis of the bulk and rhizosphere soil was done in triplicate to determine their biochemical properties, and microbial community functional structure by Biolog EcoPlate™. Before chemical analysis, soil samples were air dried at ambient temperature for 3 days and filtered using a 2 mm stainless steel sieve, while soil samples for Biolog EcoPlate™ were kept at 4°C in a refrigerator and analyzed within 7 days from the date of sampling.
2.3.2 Soil pH, electrical conductivity (EC), total organic matter, and available fractions of calcium (ca), potassium (K), magnesium (Mg), sodium (Na), and phosphorus (P) analysis
The analysis of soil chemical properties was done following the same protocol described in UltraJr (2020), Ultra (2020). In brief, a portable pH/EC meter was used to measure soil pH and EC in a water suspension (1:2, soil/water) after 1 h of shaking. The total organic matter of the bulk and rhizosphere soil of the dominant plant species was evaluated through loss of ignition (LOI) method (Ball, 1964) using a blast furnace at 550°C for 16 h. It was calculated based on Equation 7:
Available Phosphorus (P) content of the bulk and rhizosphere soil samples was quantified using Bray P-2 method following the protocol described in Bray and Kurtz (1945). The exchangeable cations (Ca, K, Mg, and Na) were extracted with 0.01 M Calcium chloride solution (1:20 Soil; CaCl2) and then shaken for an hour and filtered with a Whatman filter paper No 42 based on the protocol described by Taupedi and Ultra (2022). The concentration of the exchangeable cations (Ca, K, Mg, and Na) was determined using ICP-OES (Thermo Scientific™ iCAP™ 7,000 Plus Series).
2.4 Community level physiological profiling (CLPP) of the bulk and rhizosphere soil (soil microbial community functional diversity) by biolog EcoPlate™ method
The microbial functional diversity of the bulk and rhizosphere soil samples was estimated using the Biolog EcoPlate™ (Biolog, Int). The experiment was conducted following the protocol by Ultra et al. (2013) and 31 carbon sources were used in this study. The Biolog EcoPlate™ method assesses functional diversity of microbial communities in soil, water, and sediments (Garland and Mills, 1991). This method offers insight into the metabolic capabilities of microbial communities by assessing their ability to use various carbon sources (Garland and Mills, 1991). Each carbon source is linked to a distinct metabolic pathway, allowing for the analysis of the microbial community function (Garland and Mills, 1991). Ten grams (g) of soil were weighed and put in a glass beaker containing 0.1 M NaH2PO4 (pH 6) solution, and the mixture was thoroughly shaken to obtain a 10−2 dilution. The soil slurry was further diluted to 10−3 with a 0.15 M NaCl solution. The diluted soil mixture was transferred in triplicate into the wells of Biolog EcoPlate™ microplates (31 carbon substrates) using a multichannel pipette. The microplates were incubated in darkness at room temperature (25°C) for 8 days. The rate of carbon substrate utilization absorbance or Optical Density (OD) was quantified at 595 nm using a Multiskan FC Microplate Photometer (ThermoFisher Scientific) every 24 h for 8 days. Furthermore, absorbance values of the microplate wells containing the substrate were subtracted from the control wells (water).
Average Well Color Development (AWCD) (Equation 8) was derived from the mean of the blanked values of 31 wells in a single reading time and it denoted the speed and magnitude of the development of microbial communities (microbial metabolic activity) through the usage of 31 carbon substrates (Garland and Mills, 1991). The Shannon index (Equation 9) was used to assess functional richness of the microbial community; and the McIntosh index (Equation 10) represented functional evenness of the microbial community (Zak et al., 1994; Buyer and Kaufman, 1997; Manjunath et al., 2018; Németh et al., 2021). The formulas used to calculate the AWCD, Shannon index (H′), Simpson index (D), and McIntosh index (U) were given by:
Where n is the overall number of carbon sources in the category (Biolog ecoplates, n = 31), and C represents OD reading of the substrates in each well at 595 nm, and R is the optical density of the blank (water) (Németh et al., 2021). The Pi value represents the OD measurement of each well as a proportion to the total OD readings of all wells on the plate after 6 days of incubation (Németh et al., 2021). Pi = (C-R)/∑(C-R). Where Ni is the OD reading of a well and Ni = (C-R).
2.5 Data analysis
The data of the vegetation diversity and soil chemical properties of the bulk and rhizosphere soil of the dominant plant species from burned and unburned area from Palapye (C. virgata, D. cineria, and C. apiculatum) and Mmashoro (D. eriantha, T. esculentum, B. petersiana, and C. apiculatum) were analyzed using two–way analysis of variance (ANOVA) using Tukey multiple comparison to detect significant difference (p < 0.05). The data of the community level physiological profiling (AWCD), and soil microbial community functional diversity indices (Shannon-Weiner index, Simpson index, and McIntosh Index) of the bulk and rhizosphere soil of C. virgata, D. cineria, and C. apiculatum from Palapye burned and unburned area and D. eriantha, T. esculentum, B. petersiana, and C. apiculatum from Mmashoro burned and unburned area were evaluated using three-way analysis of variance (ANOVA) with Tukey multiple comparison to detect any significant difference (p < 0.05) using SPSS 12. The data was presented as Mean ± standard deviation. The normality of the variances of the data was tested using Shapiro–Wilk test and the p > 0.05 indicating that the data were normally distributed. The carbon substrate utilization pattern was analyzed by Principal Component (PC) and bi-plots of the PCs generated were prepared to elucidate segregation and similarities between samples.
3 Results
3.1 Short-term impacts of wildfire on vegetation diversity in savanna in Botswana
3.1.1 Plant species composition
The vegetation diversity assessment conducted at burned and unburned area in Palapye, and Mmashoro shows that the vegetation type belongs to shrub and tree savanna (Figure 2). Based on the results, about 39 plant species were recorded in Palapye, and 59 plant species in Mmashoro (Supplementary Table 1). A total of 7 plant species were classified as grass (Poaceae family); 12 plant species from 8 families were classified as herbs; and 6 plant species from 4 families were classified as shrubs within Palapye (Supplementary Table 1). Additionally, 14 plant species from 8 families were classified as trees. According to the results in Mmashoro, 20 plant species were classified as grass; 3 plant species as sedges; and 19 plant species from 10 families were categorized as herbs (Supplementary Table 1). Furthermore, 7 plant species from 5 families were classified as shrubs, and 7 plant species from 4 families were categorized as trees (Supplementary Table 1). The dominant grass species identified in Palapye’s burned and unburned area (Table 1) include Chloris virgata Sw, Urochloa mosambicensis (Hack.), and Melinis repens (Wild.) Zizka. Dominant herbaceous species (Supplementary Table 1) were Crotalaria podocarpa DC, Chamaecrista biensis (Steyaert) Lock, Heliotropium ciliatum Kaplan, Gisekia africana (Lour.) Kuntze, and Orthanthera jasminiflora (Decne.) N. E. Br. ex Schinz. Dominant shrub species (Supplementary Table 1) include Dichrostachys cinerea (L.) Wight & Arn, Grewia retinervis Burret, Mundulea sericea (Willd.) A. Chev, Senegalia nigrescens (Oliv.) P. J. H. Hurter, and Strychnos spinosa Lam. The dominant tree species (Supplementary Table 1) are Colophospermum mopane (Benth.) J. Léonard, Combretum apiculatum Sond, Sclerocarya birrea (Oliv.) P. J. H. Hurter, and Strychnos madagascariensis Poir. The dominant plant species in Mmashoro’s burned and unburned area were D. eriantha, T. esculentum, B. petersiana, and C. apiculatum. The new plants species that emerged after burning in Palapye and Mmashoro are U. mocambicensis, M. repens, Tragia okanyua Pax, Verbena officinalis L, Dipcadi glaucum (Ker Gawl.) Baker, Euphorbia prostrata Aiton, G. africana, and H. ciliatum. The following plant species emerged in Mmashoro: Pomaria burchellii (DC.) B. B. Simpson & G. P. Lewis, Dactylotenium giganteum B. S. Fisher & Schweick., Dichanthium annulatum (Forssk.) Stapf, Centropodia glauca (Nees) Cope, and Brachiaria nigropedata (Ficalho & Hiern) Stapf.

Figure 2. Palapye burned (a) and unburned (b) sites, and Mmashoro burned (c), and unburned (d) sites, and the picture was photographed on the 27 April 2023 (7 months after burning).
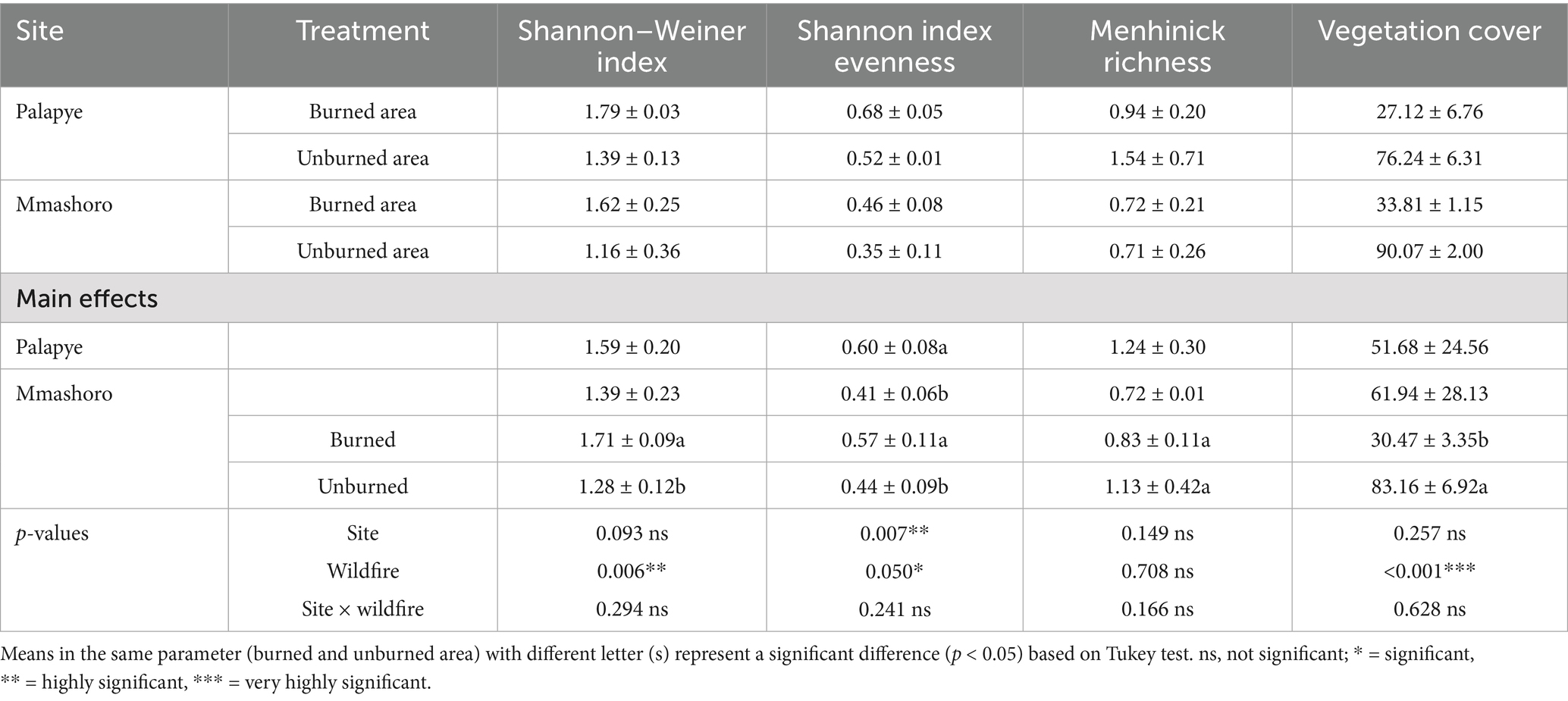
Table 1. Plant species diversity, evenness, richness, and vegetation cover in burned and unburned area in Palapye, and Mmashoro in a savanna ecosystem of Botswana.
3.1.2 Plant diversity, richness, evenness, and vegetation cover
Analysis of plant community metrics revealed that wildfire had a significant impact on vegetation diversity and structure across the two savanna sites (Table 1). The Shannon–Weiner diversity index was significantly higher (p = 0.006), in burned area (1.71) compared to unburned area (1.28) indicating that wildfire can enhance species diversity by suppressing the prevalence of dominant species and aiding the colonization of less dominant species. Similarly, species evenness was significantly greater (p = 0.050) in burned area (0.57) than in unburned ones (0.44), indicating a greater evenness in species abundance after a fire. Although species richness (as determined by the Menhinick index) was not significantly affected by fire (p = 0.708), burned area tended to have slightly lower richness values than unburned area. The findings suggest that fire alters community structure by reallocating species abundances, thereby increasing diversity without necessarily adding new species. In contrast, vegetation cover was significantly reduced in burned areas (p < 0.001), with mean cover decreasing from 83.16% in unburned area to 30.47% in burned area. This reduction is attributable to the immediate loss of aboveground biomass due to combustion and the early stages of post-fire recovery. Site comparisons revealed that Palapye (0.60) exhibited significantly higher (p = 0.007) species evenness than Mmashoro (0.41), potentially attributable to local environmental variables, including soil characteristics, the existing plant community before the fire, and herbivory levels. However, no significant site differences were detected for the Shannon index (p = 0.093), species richness (p = 0.149), or vegetation cover (p = 0.257). The results revealed that there was no significant site × wildfire interaction for any of the variables, indicating that the effects of wildfire on plant diversity and cover were relatively uniform across both study sites.
3.1.3 Net primary productivity and plant biomass of dominant vegetation from burned and unburned areas
The significant three-way interaction (site, plant species, and wildfire; p = 0.016 for NPP and p = 0.046 for biomass) highlights the complex interplay between local environmental conditions, vegetation type, and disturbance (fire) regime in shaping ecosystem productivity (Table 2). This suggest that plant species respond differently to wildfire depending on growth form and inherent physiological traits. The results shows that C. apiculatum from unburned area exhibited the highest NPP and biomass at both sites. Among the two, C. apiculatum from unburned area in Mmashoro site had the highest NPP (832.59 kg C ha−1 yr.−1), and biomass (2081.48 kg ha−1). In contrast, D. cinerea and C. virgata recorded the lowest values in burned area in Palapye, suggesting limited post-fire productivity.
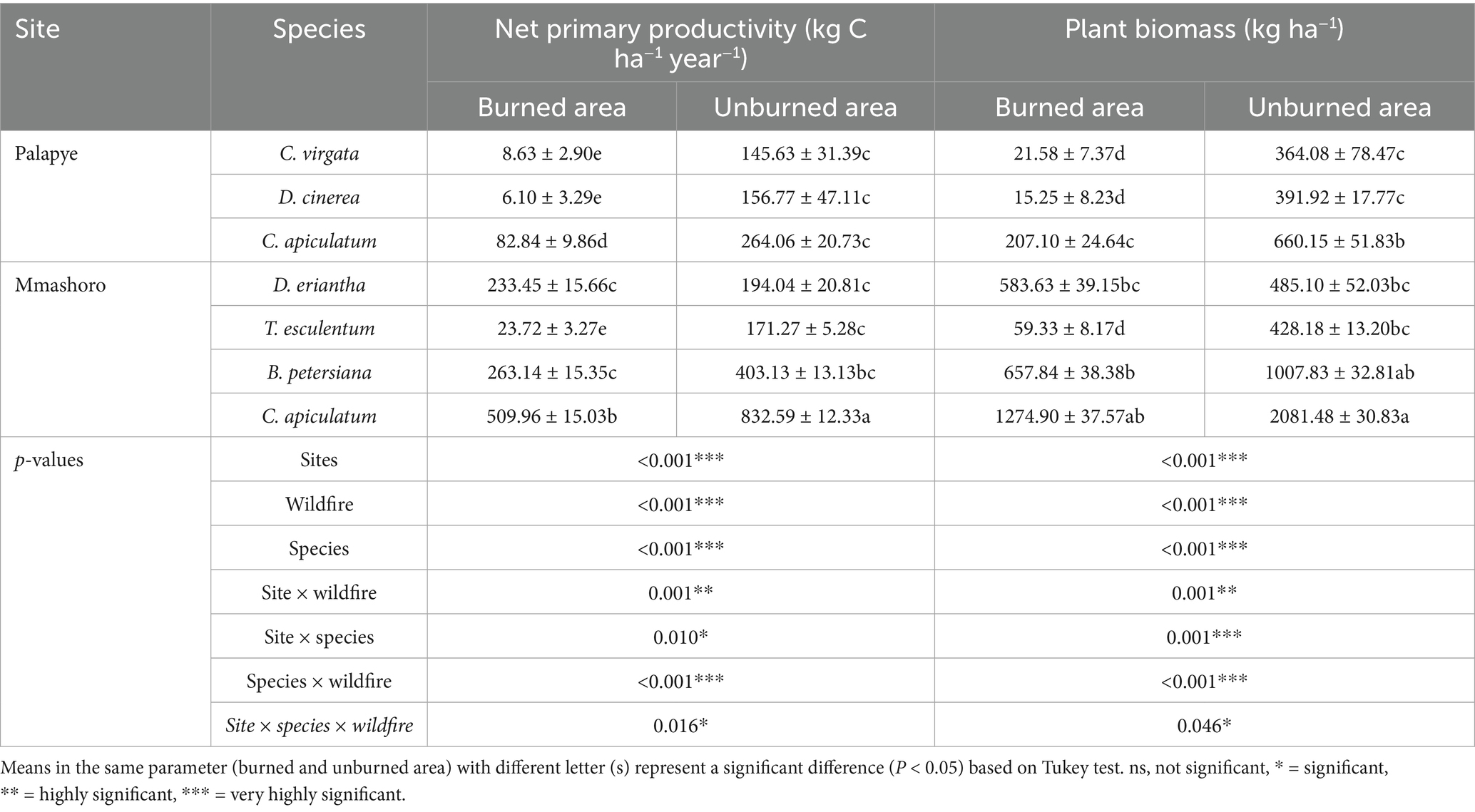
Table 2. Net primary productivity, and plant biomass of dominant vegetation in burned and unburned area in Palapye and Mmashoro in a savanna ecosystem.
3.2 Impacts of wildfire on soil chemical properties of the bulk soil and rhizosphere in savanna of Botswana
The three-way interactions (site, wildfire, and soil type) were significant (p = 0.031) and these shows that the effects of wildfire on soil pH is mainly dependent on the site, and soil type. The results shows general increase in pH values on the bulk and rhizosphere soil of the various plant species from burned areas, particularly in Mmashoro site (Table 3). Moreover, Mmashoro site (7–7.67) has a significantly higher pH than Palapye site (5–5.82). These may indicate that the two sites might have distinct soil pH profiles. Wildfire may directly influence soil pH through ash deposition that induces the rhizosphere effect in both Palapye and Mmashoro sites. The three-way interactions (site, wildfire, and soil type) were significant (p = 0.017) and these shows that their impacts of wildfire on soil EC depend on site and soil type. The results revealed that Mmashoro site have a highly significant soil EC than Palapye site. Furthermore, the results shows that the rhizosphere soil of B. petersiana and C. apiculatum have higher EC than other plant species. Based on the results, three-way interactions (site, wildfire, and soil type) were significant (p = 0.023) and these shows that the impacts of wildfire on OM levels are mainly dependent on site and soil type and these shows that their impacts are consistent across combinations. The TOM levels in Palapye were significantly higher as compared than Mmashoro. Moreover, the TOM levels of the burned areas were significantly higher than unburned areas. The results shows that C. apiculatum and C. virgata from burned area in Palapye had highest TOM levels (2.94%) than other plant species. Based on the results, the three-way interactions (site, wildfire, and soil type) were highly significant (p = 0.022) on available P, and these show that the impacts of the wildfire were not consistent and may differ based on site and soil type (plant species). Therefore, the levels of available P increased by wildfire, can also vary per site due to distinct available P profiles, and soil type can differ because of inherent characteristics of the plant species. The available P levels in Palapye was significantly greater than Mmashoro. Moreover, the available P levels of the burned areas were significantly higher than unburned areas. The results shows that C. virgata from burned area in Palapye had highest available P (217.10 mg kg−1) than other plant species. The results shows that the three-way interactions (site, wildfire, and soil type) were significant (p = 0.047), and this indicate that their impact of wildfire on exchangeable Ca (Exch Ca) is mainly dependent on site and soil type (Table 4). Therefore, the wildfire occurrence might increase Exch Ca levels, this can vary per site due to unique Exch Ca profiles. The Exch Ca levels in Palapye was significantly larger than Mmashoro. Moreover, the Exch Ca of the burned areas were significantly higher than unburned areas. The results shows that C. apiculatum and C. virgata from burned area in Palapye had highest Exch Ca levels (10408.5 and 11,412 mg kg−1). The results indicate that the three-way interactions (site, wildfire, and soil type) were significant (p = 0.031) and these shows that the impacts of wildfire on Exch K levels are mainly dependent on site and soil type. The Exch K levels Mmashoro was significantly higher than Palapye. Additionally, the Exch K levels of the burned areas were significantly higher than unburned areas. The results shows that C. apiculatum from burned area in Mmashoro had highest Exch K levels (136.56 mg kg−1). Based on the results, three-way interactions (site, wildfire, and soil type) were significant (p = 0.011) on exchangeable Mg (Exch Mg) and these shows that the impacts of wildfire on Exch Mg levels are mainly dependent on site and soil type. The Exch Mg levels in Mmashoro was significantly higher as compared than Palapye. Additionally, the Exch Mg levels of the burned areas were significantly higher than unburned areas. The results indicate that C. apiculatum from burned area in Mmashoro had highest Exch Mg levels (158.34 mg kg−1). The significant three-way interactions among site, wildfire, and soil type (p = 0.021) shows that the impacts of wildfire on exchangeable Na (Exch Na) levels are not uniform on the site and soil type. The Exch Na levels in Mmashoro was marginally greater than Palapye. Moreover, the Exch Na levels of the burned areas were slightly higher than unburned areas. The results indicate that C. apiculatum and B. petersiana from burned area in Mmashoro had highest Exch Na levels (8.07 and 8.64 mg kg−1). Therefore, the wildfire does not exert the same effect on the soil chemistry and the impact is mediated by the vegetation and inherent properties of the site.
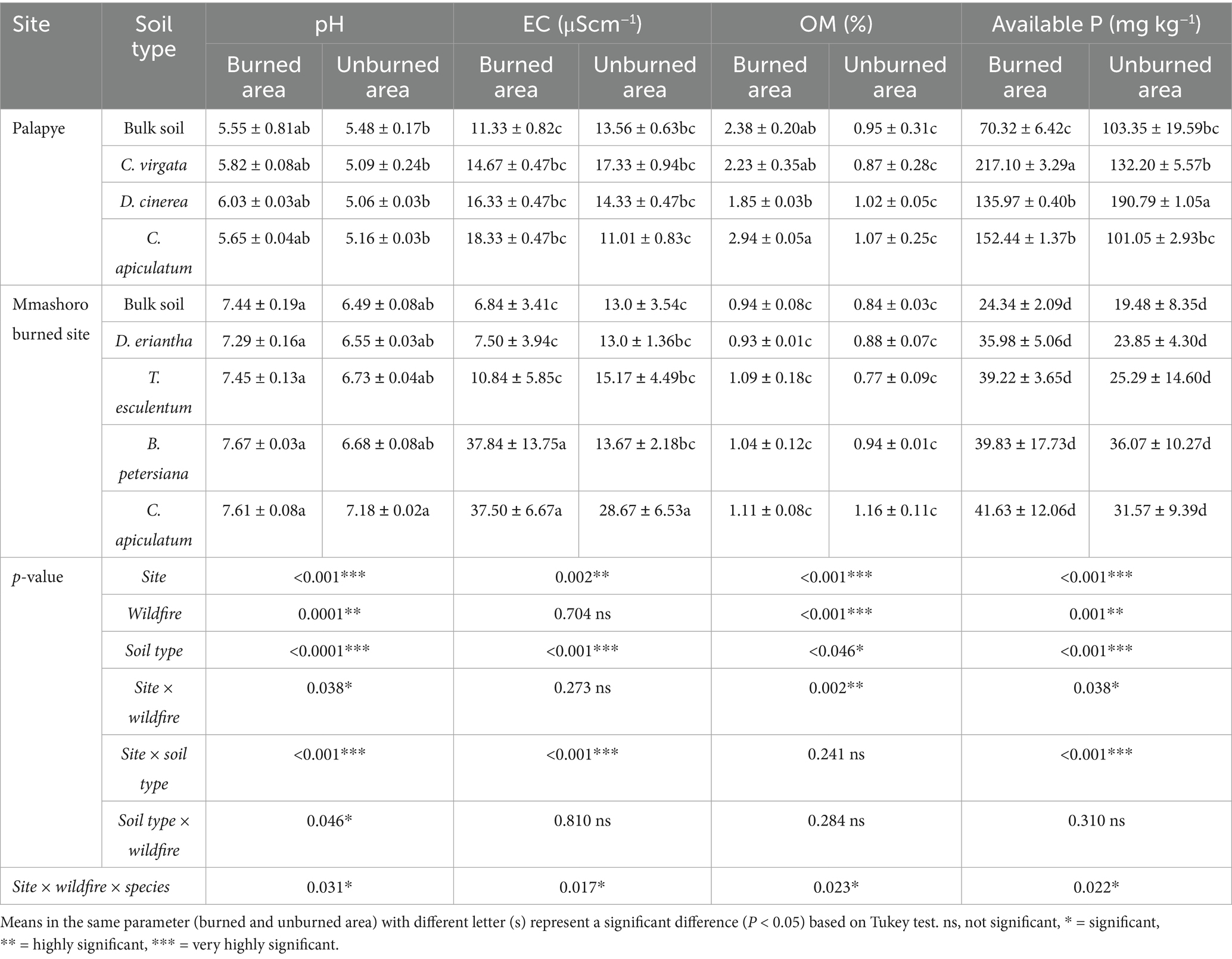
Table 3. Soil biochemical properties (pH, EC, TOM and available P) of the bulk and rhizosphere soil of dominant plant species in burned and unburned area in Palapye, and Mmashoro in a savanna ecosystem of Botswana.
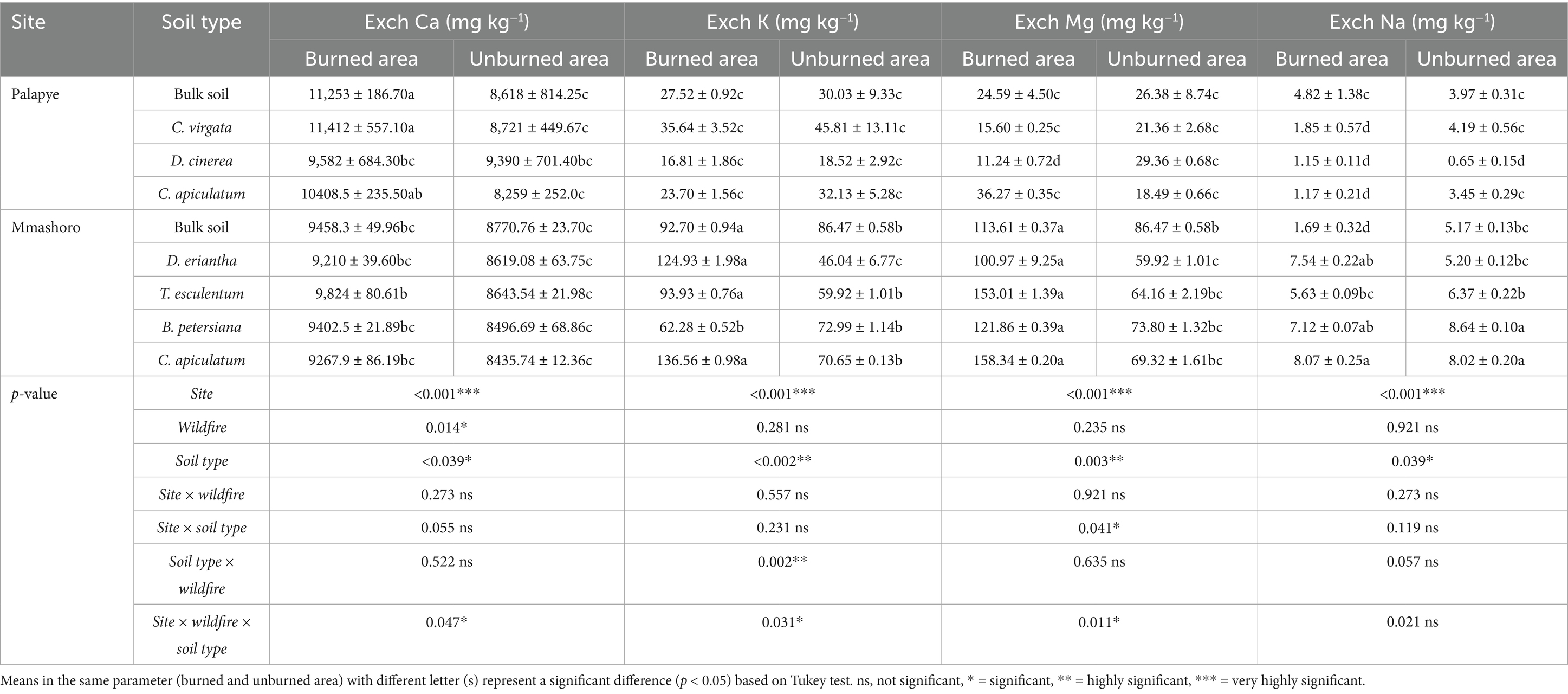
Table 4. Soil biochemical properties (Exchangeable Ca, K, Mg, and Na levels) of the bulk and rhizosphere soil of dominant plant species in burned and unburned area in Palapye, and Mmashoro in a savanna ecosystem of Botswana.
3.3 Impacts of wildfire on community level physiological profiling (CLPP) (soil microbial community functional structures) in savanna of Botswana
3.3.1 Metabolic activity of microbial community in the bulk and rhizosphere soil as affected by wildfires based on the average well color development (AWCD)
The results show that the optical density measured at 595 nm (OD595) increased for all wells analyzed after 6 days of incubation. This indicates that the carbon substrates were oxidized by microbial communities in soils from both burned and unburned areas (Table 2) in Palapye and Mmashoro. Based on the results, the significant three-way interaction among site, wildfire, and soil type (p = 0.046) and suggests that the effects of wildfire varies depending on site and soil type. Metabolic activity of microbial communities from Palapye was significantly higher (p = 0.006) than Mmashoro. Moreover, the metabolic activity from unburned area were significantly higher (p < 0.001) than burned area in both sites.
3.3.2 Microbial functional diversity indices of the bulk soil and rhizosphere of dominant vegetation affected by wildfires
The results shows that the three-way interaction between site, wildfire, and soil type on microbial communities richness was very significant (p = 0.038) and these indicate that the impacts of wildfire are mainly driven by site and soil type (Table 5). The microbial community richness of Palapye was slightly higher than Mmashoro. Burned areas have a higher microbial community richness than unburned areas in both sites. Based on the results on McIntosh index, the three-way interaction between site, wildfire, and soil type on microbial communities evenness was very significant (p = 0.017) and these indicate that the impacts of wildfire are mainly influenced by site and soil type. The microbial community richness of Palapye was marginally higher than Mmashoro. Unburned areas have a higher microbial community evenness than burned areas in both sites.
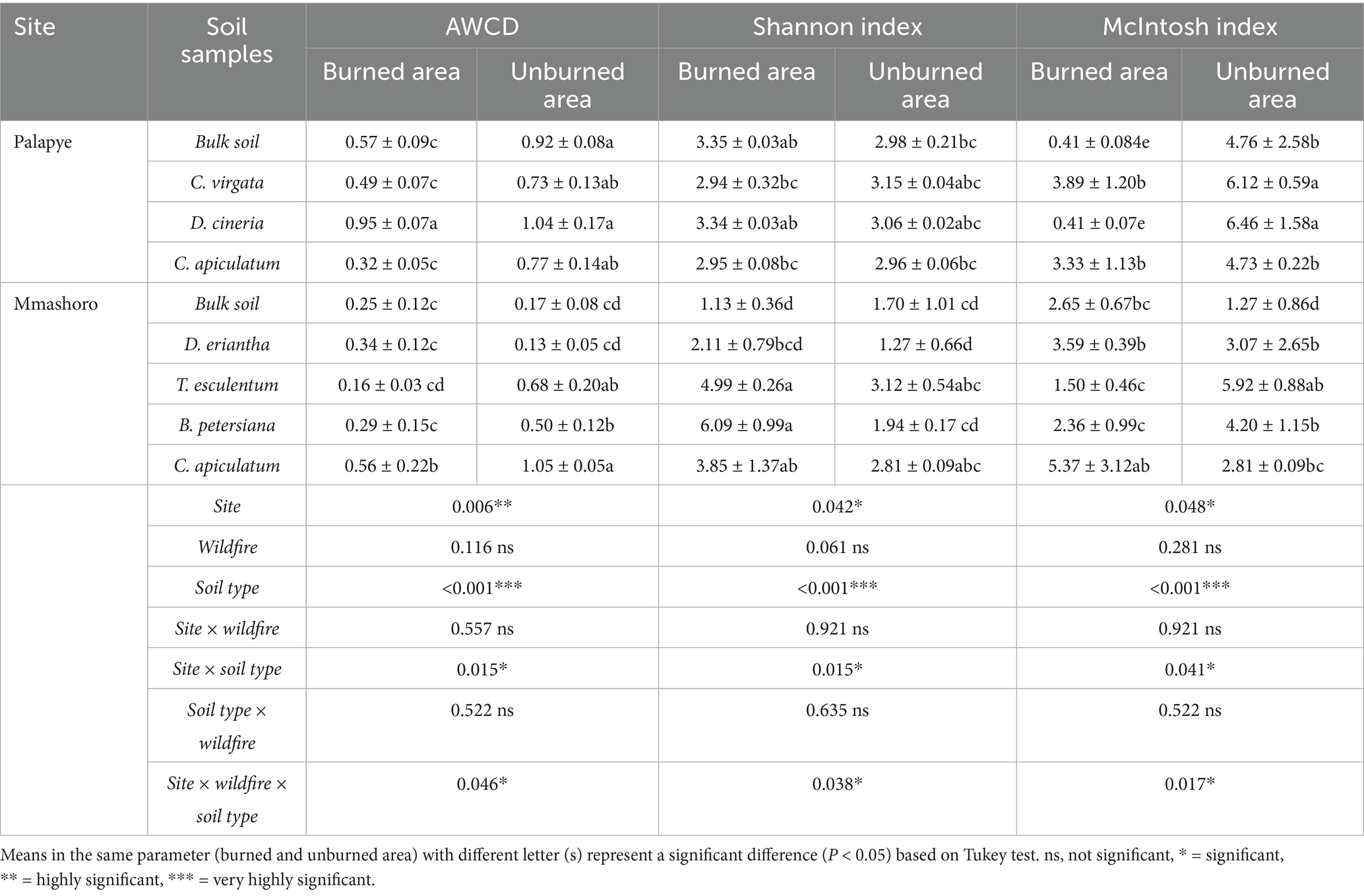
Table 5. Microbial metabolic activity and diversity indices of soil microbial communities in Palapye, and Mmashoro burned and unburned area based on the average well color development (AWCD) after 6 days of incubation.
3.3.3 Community level physiological profiling of the microbial community of bulk and rhizosphere soil of dominant plants species as affected by wildfires based on carbon substrate utilization pattern using principal component analysis
Principal Component Analysis (PCA) of carbon substrate utilization patterns revealed distinct functional capability among microbial communities from two different sites (Palapye and Mmashoro) were associated with different plant species, and between burned and unburned area. PC1 separated the samples based on the most powerful factor being wildfire and PC2 reveals the second most important factor namely; species-specific differences. The first two principal components, PC1 and PC2, accounted for a combined 51.80% of the total variance in carbon substrate utilization (PC1: 37.68%, PC2: 14.12%), indicating moderate discrimination power of the PCA, which is common in complex ecological datasets. Our results concurred with various studies by McCune and Grace (2002), and Gotelli and Ellison (2013) who reported that moderate degree of discrimination is the most common and meaningful result in complex community datasets. The striking pattern in bi-plot is the clear segregation of the bulk and rhizosphere soil samples (C. virgata, T. esculentum, B. petersiana, and C. apiculatum) from unburned area (C) from both sites (Palapye and Mmashoro) on the right side of PC1 axis (37.68% of the total variance). This indicates that these microbial communities, regardless of their specific location or associated plant species, share a similar functional profile and also possess more diverse substrate utilization profiles. These samples from unburned area were strongly associated with a wide array of carbon sources, particularly carbohydrates, amino acids, and complex polymers, as shown by the vectors pointing in their direction (e.g., L-glutamic acid, α-ketobutyric acid, methyl esters, and Tween compounds). This suggests high metabolic versatility within microbial communities in the unburned area. Therefore, these microbes utilize a wide-array of distinct carbon sources, which symbolizes a healthy and functionally diverse ecosystem. In contrast, microbial communities associated with the bulk and rhizosphere soil samples of the same plant species under burned area (E) clustered distinctly on the negative side of PC1, indicating reduced or altered substrate utilization. Based on the results, very few vectors point (D-Galacturonic acid) to the left, and these prove that samples from burned area have weak associations with most carbon substrates, especially those strongly correlated with PC1, implying a potential functional shift or suppression in microbial activity following fire disturbance. For instance, D. cinerea and bulk soil (E) samples from burned area were separated farthest from the origin, highlighting their divergence in microbial function relative to unburned counterparts. The distinct separation suggests that wildfire reduced microbial functional diversity and shifted substrate preferences. The results shows that PC2 accounted for 14.12% of the overall variance and these provide secondary level of distinction based on specific plant species and site conditions, which revealed a pronounced difference between samples from burned and unburned area. Moreover, C. apiculatum from burned area and T. esculentum from unburned area in Mmashoro site are higher on PC2, associated with carbon substrates such as Itanconic acid whereas D. cinerea and C. virgata from unburned area from Palapye were low on PC2 and they were associated with distinct carbon substrates such as L-phenylalanine that belong to amino acid group. The results also demonstrated that C. apiculatum and C. virgata from burned area in Mmashoro and Palapye clustered on the positive side of PC2 and PC1 with samples from unburned area of both sites and this suggests that C. apiculatum and C. virgata are resilient or adapted to fire-prone ecosystem. However, samples from burned area are more clustered tightly but still some little separation and this indicates that regardless of homogenization effect of fire, there are some minor species–specific variation that lingers in the microbial function.
3.4 Relationship between soil microbial functional structures and soil chemical properties
Based on results, the correlation analysis showed strong connection between soil chemical properties and microbial functional traits (Table 6). Average Well Color Development (AWCD), that serves an indicator for overall microbial metabolic activity, was positively correlated with total organic matter (r = 0.546), available phosphorus (r = 0.611), and electrical conductivity (r = 0.369) and this indicate that microbial activity in the soil of the savanna ecosystem is mainly influenced by the availability of labile carbon sources and nutrients. Notably, AWCD exhibited a significantly inverse correlation relationship with soil pH (r = −0.506), demonstrating that mildly acidic conditions stimulates microbial communities and enhances their metabolism possibly due to improved nutrient solubility or favorable conditions for acidophilic microbial taxa. Similarly, negative correlations were observed with exchangeable cations such as potassium, magnesium, and sodium (all r < −0.5), which could reflect osmotic stress or ion toxicity effects under higher cation concentrations. The McIntosh index, a representative metric for functional evenness within microbial communities, revealed the strongest and only statistically significant positive correlation with available phosphorus (r = 0.729, p < 0.05). This indicates that phosphorus enrichment facilitates equitable carbon substrate utilization across microbial taxa. Additionally, McIntosh index had positive associations with TOM (r = 0.484) and calcium (r = 0.544) support the contribution of organic inputs and nutrient availability in sustaining microbial communities. Conversely, McIntosh values were inversely correlated with pH and cations such as K, Mg, and Na, and this imply that alkaline or cation-rich environments may suppress microbial functional balance. The Shannon diversity index showed only weak correlations, the strongest being with EC (r = 0.622).
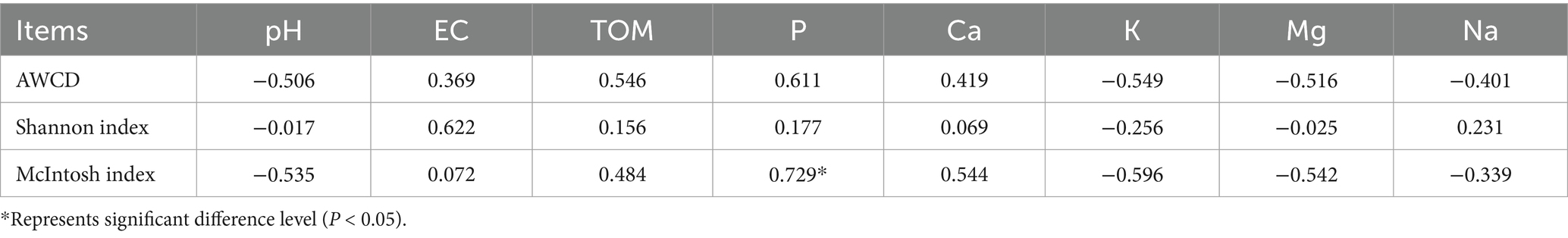
Table 6. The correlation analysis of microbial metabolic activity (AWCD), diversity indices of soil microbial communities, soil chemical properties (pH, EC, and TOM), and concentration of available P and exchangeable essential micronutrients (P, Ca, K, Mg, and Na) of burned area.
4 Discussion
4.1 Short-term impacts of wildfire on vegetation diversity in savanna in Botswana
The findings indicate that wildfire was a major driver of change in the plant community structure at both savanna sites, characterized by an enhancement of species diversity and evenness alongside a significant decrease in vegetation cover. The elevated Shannon-Weiner diversity index and evenness in burned area demonstrated that fire disturbance fosters a plant community with greater functional balance and diversity compared to unburned areas. These result are harmonious with previous studies in other savanna ecosystems, which suggests that fire can reduce the prevalence of dominant competitors, thus facilitating the colonization of early-successional or pioneer species (Higgins et al., 2000; Bond and Keeley, 2005). Pioneer species such as U. mosambicensis, C. virgata, and M. repens were identified in both sites and these species are normally found in disturbed soils and roadsides (Kgosikoma et al., 2012; Roodt, 2015). Increased species evenness observed in burned areas provide support to the theory that fire promotes co-existence by destabilizing competitive dominance and expanding available ecological niches (Abades et al., 2014). Our results are harmonious with the recent understanding that wildfire performs a critical role for the succession of grasses globally (Simpson et al., 2016), and has been directly connected with increased herbaceous population (Wienk et al., 2004). Increased growth of grasses and herbs in burned areas was induced by fire that broke down their dormant seeds which enhanced germination, and permitted more light for understory vegetation (Jhariya and Raj, 2014). The species evenness in Palapye was marginally higher than Mmashoro and this might be attributable to unique soil type and elevated total organic matter and nutrient availability (Silva et al., 2013; Sebata, 2017). This may also be explained by the high recovery rate of the shrubs and trees following the wildfires, as evidenced by resprouting (Souchie et al., 2017; Machida et al., 2021) of species such as M. sericea, D. cinerea, and S. madagascariensis. Dichrostachys cinerea is widely recognized as a strong encroacher species, particularly in savanna and semi-arid ecosystems, where it aggressively expands into grasslands and alters ecosystem structure and function (Moleele et al., 2002; Smit, 2004). The fire’s impact was limited to increasing diversity and evenness; species richness, by contrast, was statistically unaffected. This implies that the fire’s main effect was not the colonization of new species, but rather a shift in the population dynamics of the pre-existing community. Our findings are consistent with previous research in fire-prone ecosystems, which indicates that species richness often stays constant post-disturbance, particularly when the local flora is already fire-adapted (Govender et al., 2006). Nevertheless, a significant reshuffling of dominant species can still occur, especially in the initial stages of post-fire recovery. The significant main effect of wildfire resulted in a reduction of vegetation cover was observed in burned area, a direct consequence of the combustion of plant biomass. Conversely, the main effect of site and two-way interaction between site and wildfire did not have a significant impact on vegetation cover. The outcomes of this study are comparable to studies by Sheuyange et al. (2005) and Memoli et al. (2022), who reported that wildfires temporarily decreased the coverage of shrubs, trees and encouraged the cover of herbaceous species especially in savanna ecosystems with limited rainfall. Fire may change vegetation composition, structure, and variety (Jhariya and Oraon, 2012; Jhariya et al., 2013; Kittur et al., 2014), but vegetation cover is a critical determinant in altering soil nutrient levels following a fire event (Caon et al., 2014). This phenomenon represents a classic short-term impact in the savanna ecosystems (prone to fire), in which surface vegetation is combusted and the rate of recovery is determined by factors including rainfall, species-specific life cycles, and vegetative regrowth capabilities (Trollope et al., 2002; Sankaran et al., 2008). A cross-site comparison indicated that species evenness was significantly greater in Palapye than Mmashoro. However, the sites did not differ significantly in terms of overall diversity, species richness, or vegetation cover. The greater species evenness observed at Palapye might be explained by local environmental variables, including soil characteristics (edaphic conditions), the existing plant community, or historical land-use patterns, all of which are known to mediate a community’s response to fire (Moleele et al., 2002). The fire-induced changes in diversity and cover were consistent across both sites, as evidenced by the non-significant site and fire interaction, which suggests a broadly uniform reaction of these savanna ecosystems to fire.
4.2 Short-term impacts of wildfire on net primary productivity and plant biomass
The findings reveal that NPP and plant biomass are significantly influenced by wildfire, type of plant species, and site-specific conditions (Table 2). Across all species and site, both NPP and aboveground biomass were substantially lower in burned area compared to their unburned counterparts, and these highlight the profound effect of fire on plant growth and carbon sequestration. This reduction in NPP and biomass is attributable to immediate loss of vegetation, the destruction of photosynthetic tissues, and post-fire environmental stressors, including diminished soil moisture and nutrient depletion (Bond and van Wilgen, 1996; Sankaran et al., 2004; Moore et al., 2021). The impact of the fire was most significant at the Palapye site, where burned conditions led to substantial decreases in both NPP and biomass for all species. C. virgata provides a clear example of this trend, showing a dramatic 16-fold drop in NPP (from 145.63 to 8.63 kg C ha−1 yr.−1) and a 17-fold fall in biomass (from 364.08 to 21.58 kg ha−1) after the fire. This study demonstrated that D. cinerea, a known encroacher species, performed poorly post-fire in Palapye, and both NPP and biomass remained below 16 kg C ha−1 yr.−1 and 20 kg ha−1, respectively. These results suggest that although D. cinerea is a successful encroacher under undisturbed conditions, it may be temporarily suppressed by fire, especially in more arid or nutrient-poor sites. In contrasts, Mmashoro site demonstrated a more robust post-fire recovery, a trend that was most significant among the D. eriantha and C. apiculatum. Specifically, D. eriantha and C. apiculatum exhibited a robust level of NPP (233.45 and 509.96 kg C ha−1 yr.−1, respectively) and biomass (583.63 and 1274.90 kg ha−1, respectively) even in the burned area. C. apiculatum exhibited the greatest productivity under both burned and unburned area, which suggests a high degree of fire tolerance and a capacity for rapid post-disturbance regeneration. These observations could be explained by a combination of more favorable local conditions, like soil quality and rainfall, and inherent species characteristics that promote resilience, such as the ability to resprout from the base and store carbon underground (Trollope, 1984; Scholes and Archer, 1997).
4.3 Short-term impacts of wildfire on soil chemical properties of the bulk soil and rhizosphere in a savanna ecosystem of Botswana
The significant three-way interaction (site, wildfire, and soil type) suggest that species-specific root-soil interactions modulate pH shifts, with fire intensifying or mitigating these effects depending on the site’s baseline conditions (Table 3). Plant species (C. apiculatum and B. petersiana) from burned area in Mmashoro site recorded pH values exceeding 7.6, while the corresponding unburned area had values around 6.5–7.2. This increase is consistent with the known liming effect of fire, where the combustion of organic matter results in the accumulation of basic cations (e.g., Ca2+, Mg2+, K+) in ash, which temporarily raises pH (Certini, 2005). In contrast, Palapye soils remained acidic, even under burned conditions (pH 5.5–6.0), suggesting buffering by underlying soil properties such as higher iron and aluminum oxides, or variation in soil texture and mineralogy. The slight increase in soil pH can be attributed to the liming effect of fire, which results in burned and charred biomass that lead to the accumulations of basic cations such as Ca2+, Mg2+, and K+ in ash (DeBano and Conrad, 1978; Certini, 2005; Molina et al., 2007; Boerner et al., 2009; Schafer and Mack, 2010; Tüfekçioğlu et al., 2010; Aref et al., 2011; Verma and Jayakumar, 2012; Badía et al., 2014; Bodí et al., 2014; Francos et al., 2019; Fernández-García et al., 2019; Agbeshie et al., 2022). The results demonstrate that the main drivers of soil electrical conductivity (EC) in the studied savanna ecosystems are the intrinsic properties of the location (site) and the vegetation (soil type), rather than the direct, uniform effect of fire (Table 3). The lack of a significant main effect for wildfire is a critical finding that indicate the influence of wildfire on soil chemical properties is not direct, but rather exhibits considerable variation depending on contextual factors. This aligns with other studies that showing that the ecological effects of fire are mediated by local environmental and biotic factors (Neary et al., 1999). The significant impact of soil type on EC highlights the function of vegetation as “ecosystem engineers,” which actively alter the chemical properties of their local soil environment (Jones et al., 1994). Various plant species influence soil properties in unique ways through mechanisms like nutrient uptake patterns, litter breakdown processes, and the cultivation of specific microbial communities within the rhizosphere. The most significant observation was the substantial and highly specific increase in EC, which occurred only in the burned soils associated with B. petersiana and C. apiculatum at the Mmashoro location. This specific and significant interaction explains why a generalized fire effect was not detected; the impact of fire was intense but restricted to a particular combination of site and vegetation. This phenomenon is likely attributable to the combustion of plant biomass and the subsequent leaching of soluble inorganic salts from the deposited ash layer after wildfire occurrence (Certini, 2005; Bodí et al., 2014). The exceptionally high EC values suggest that these two plant species may accumulate higher concentrations of base cations (K+, Ca2+, Mg2+) in their rhizosphere than other species at the site. Upon combustion, these cations are converted into soluble oxides and carbonates, which, when leached by rainfall, cause a sharp, localized increase in soil solution conductivity (Raison, 1979). These ashes which contain various minerals that exist as oxides and carbonates, also stimulate plant growth (Certini, 2005; Bodí et al., 2014). This leads to vegetation recovery of plant species such as grasses and herbs within the ecosystem. The rainfall received in December and January might have dissolved these minerals through the process of leaching, which allows basic cations (calcium and magnesium) to infiltrate the soil and rhizosphere (Romero-Matos et al., 2023).
The results reveal that Total Organic Matter (TOM) in the soil is mainly regulated by a complex hierarchy of factors, and with site and immediate impact of wildfire emerging as the most significant (Table 3). Although soil type (as defined by vegetation) is a contributing factor, its impact is overshadowed by the dominant, interactive influence of site and wildfire. Contrary to the common assumption that fire diminishes organic matter, the study revealed that burned areas contained significantly greater TOM levels than their unburned counterparts. The increment of total organic matter levels within burned sites can be attributed to ash and char deposition that are rich in organic and inorganic compounds, and rapid vegetation recovery (Campos et al., 2016; Liu et al., 2018; Alcañiz et al., 2018; Alexakis et al., 2021). Wildfire can temporarily improve soil porosity and infiltration, which enables microbial access to substrates and these enhance organic matter accumulation. Moreover, early-successional plants (grass, herbs, and shrubs) rapidly colonize burned area and these contribute to elevated organic matter through litter input and increased root turnover. The findings of this study shows that plant species differ in their contributions to soil organic matter, possibly due to variation in litter input and root dynamics. However, the lack of significant interactions with soil type indicates that this influence represents a background process rather than a key factor driving post-fire changes. Organic matter plays an important function in ecosystem dynamics by facilitating ion exchange, interacting with minerals from clay, forming soil aggregates, and aiding in the absorption and dispersion of nutrients and water from plants (Caon et al., 2014). The results indicate that the effects of wildfire on available Phosphorus levels significantly varied per site and plant species. Wildfire significantly increased available phosphorus levels on the rhizosphere soil of C. virgata (Table 3). Our findings were consistent with Guidi et al. (1988), and Cade-Menun et al. (2000), who reported that combustion convert organic P from plant residues and microbial biomass into orthophosphate, which is accessible to plants. Wildfires can create a flush of nutrients when preceded by the rainy season that might temporarily boost microbial metabolic activity, aiding in the breaking down of the organic matter, nutrient cycling and nutrient uptake, which supports plant growth, and boosts the recovery of vegetation after the rainy season (Xu et al., 2012; Pellegrini et al., 2015). This can stimulate early stages of vegetation recovery by promoting the growth of pioneer species such as C. virgata, M. repens, U. mosambicensis, and H. ciliatum (Kgosikoma et al., 2012; Gajaje et al., 2024). The variation of available nutrients including P within the rhizosphere of the various dominant plant species can be attributed to unique individual plant traits and physiology. Different plant species have unique root structures that affect their ability to access and absorb nutrients from the soil. Some plant species such as Strychnos species (S. madagascariensis and S. spinosa), form a symbiotic relationship with mycorrhizal fungus, which stimulates the uptake of P through the increased surface area for absorption (Mwamba, 2006).
The results shows that the effects of wildfire Exchangeable K (Exch K) levels was mainly driven by site and soil type (plant species) and their impacts were not consistent (Table 4). The results shows that Palapye and Mmashoro have unique Exch K levels and these shows the two sites have different Exch K sources (soil parent material). The variation in geology between the sites can result in unique inherent potassium levels. Differences in geology between the sites would lead to different inherent K concentrations. This phenomenon is supported by our results, showing that Mmashoro soils (136.56 mg kg−1) were generally higher in K levels and these suggest a more K rich environment compared to Palapye soils (<46 mg kg−1). The results revealed that the impact of wildfire on Exch K levels was mainly dependent on the affected plant species and this reinforces the concept that vegetation is a key driver of nutrient cycling and concentration processes. This trend is well reflected in the Mmashoro dataset from burned area; C. apiculatum, D. eriantha, and T. esculentum showed significantly higher K levels, while other species did not. Some plant species are efficient in accumulating and storing potassium in their biomass. During combustion, organic K is rapidly converted into simple, water-soluble inorganic forms (like K₂O and K₂CO₃) and deposited in the ash (Certini, 2005; Caon et al., 2014). Subsequent rainfall leaches these compounds into the soil, causing a sharp, immediate increase in exchangeable K, which is the form available to plants. Different plant species exhibit distinct efficiencies in soil potassium uptake and possess varying levels of potassium in their biomass, which affects litter composition. Plant species exhibit distinct efficiencies in soil potassium uptake and potassium content of their tissues, and these reflect some variation in litter quality. The results shows that C. apiculatum from burned area in Mmashoro site have higher Exch K levels than other plant species. Our findings are harmonious with studies by Ludwig et al. (2004), and Prieto et al. (2012), who reported that deep-rooted species like Combretum enrich surface soils through the creation of localized hotspots of fertility. This process occurs through two ways; (1) deposition of nutrient-rich litter into the soil (Ludwig et al., 2004), and (2) deep roots transport water to shallow soil layers through a process called hydraulic lift (Ludwig et al., 2004; Cardon and Whitbeck, 2011; Prieto et al., 2012). This moisture sustains microbial activity and nutrient mineralization, making nutrients available for uptake by shallow roots, thus functioning as an efficient nutrient cycling system (Ludwig et al., 2004; Prieto et al., 2012).
Based on the results, the impact of fire on exchangeable magnesium (Exch Mg) was highly influenced by site and soil type of the specific plant species it burns (Table 4). The study shows that Mg was significantly higher in Mmashoro (158 mg kg−1) as compared to lower levels at Palapye (<36 mg kg−1) might be attributed to differences in soil parent material. Magnesium is a mineral element derived from the weathering of rocks. The significant site variation indicates that Mmashoro is underpinned by magnesium rich geological formations (e.g., dolomite, biotite, serpentine) as compared to Palapye (Brady and Weil, 2016). The study shows that different plant species have varying capacities for Mg uptake, storage in their tissues, and release through litter fall. The study demonstrated that T. esculentum and C. apiculatum from burned area had higher concentrations of Exch Mg levels. Plant species that grows in an environment rich in Mg accumulates higher Mg concentration in their tissues. During wildfire occurrence, their biomass are consumed by fire, which mineralizes the organically-bound Mg, and convert it into soluble forms that are deposited in the ash (Kauffman et al., 1992; Caon et al., 2014; de Sousa Ferreira et al., 2023). Consequently, fire generates a localized surge in magnesium availability within the soil. The results revealed that the impacts of wildfire on exchangeable Na (Exch Na) was not consistent and significantly different per site and soil type This study shows that some plant species in Mmashoro (C. apiculatum and B. petersiana) had higher Exch Na levels (8.64 mg kg−1) regardless of fire treatment. In contrast, Palapye soils had lower Exch Na (5 mg kg−1) concentration. This spatial (site) variation in Exch Na levels may stem from differences in parent material composition and evapotranspiration levels. In semi-arid environments, elevated evapotranspiration can concentrate salts levels in the upper soil horizon through a process known as salinization (Munns and Tester, 2008; Sposito, 2008). The study revealed higher Na levels under species like C. apiculatum and B. petersiana at the Na-richer Mmashoro site and these suggest these above mentioned species might be adapted to Na than others.
4.4 Impacts of wildfire on microbial metabolic activity, soil microbial community functional diversity, and carbon substrate utilization pattern in savanna of Botswana
The results revealed that the impacts of wildfire on microbial metabolic community was not uniform and mainly driven by site and soil type associated by the plant species (Table 5). This trend can be explained by the microbial community under C. apiculatum at Palapye demonstrated a sharp decline in metabolic activity after a fire, whereas in Mmashoro, the microbial community under the same species indicated a slight reduction. This slight decline could be attributable to the microbial community adaptation to harsher conditions and lower-activity conditions in Mmashoro. Our findings are congruent with Allison and Martiny (2008) and Fierer (2017) who reported that microbial communities can adapt to environmental stress through functional resilience and stress tolerance, and this often results in lower metabolic activity. Soil microbial communities are necessary for maintaining the fertility of the soil and plant yield by decomposing the organic matter to release plant accessible elements (Chen et al., 2024). The findings show that unburned area of Palapye have higher AWCD values than Mmashoro, and these suggests that the two sites exhibit inherently distinct baseline microbial activity levels. This outcome is anticipated, since microbial communities are mainly influenced by the characteristics of their habitat. The higher AWCD observed at Palapye could be attributed to increased organic matter content (key energy source), improved moisture retention capacity, or a lightly acidic (5–6.03) but near-neutral pH, all of which are known to foster robust microbial growth and metabolic activity (Fierer, 2017). The variation in AWCD values under various plant species suggest that their associated microbial communities possess unique metabolic capabilities (Grayston et al., 1998). The most direct impact of the fire disturbance was the reduction of AWCD in burned area that was severe in the Palapye site (e.g., reduction from 0.92 to 0.57 in bulk soil). Our findings are also congruent with previous studies by de Miera et al. (2020) who revealed a decrease in total soil microbial metabolic activity and microbial functional diversity after wildfire occurrence which can be attributed to heat induced microbial mortality (Dooley and Treseder, 2012; Xu et al., 2012), and toxic compounds like polycyclic aromatic hydrocarbons (PAHs) that are produced by burning of plant debris (Haritash and Kaushik, 2009; de Miera et al., 2020). Hazardous compounds can remain in the soil even after the fire has been extinguished, adversely reducing microbial biomass, hence lowering AWCD levels (de Miera et al., 2020). The Shannon index diversity analysis proved that the effect of wildfire on the balance and richness of microbial community is not consistent and mainly dependent of site and soil type that is associated with unique plant species (Table 2). The results indicate that Palapye have a higher Shannon diversity than Mmashoro, which suggests that the two locations support microbial communities with inherently different functional diversity. This demonstrates that different plant species possess functionally distinct microbial communities in their distinctive habitat. This is consistent with the understanding that plants release a unique cocktail of root exudates and produce chemically distinct litter, which selectively promotes certain microbial groups with specific metabolic pathways (Grayston et al., 1998). Increased microbial metabolic activity within the rhizosphere can be attributed to the exudation of organic carbon by the roots of the plants (Hartmann et al., 2009). There is a more conducive environment at Palapye that possess higher biodiversity, organic carbon availability, and soil moisture stability, could support a greater diversity of microbial functions through expanded niche availability (Fierer, 2017). A diverse and rich soil microbial community have direct effects on nutrient availability by improving carbon storage and nutrient cycling, chelation, climate regulation, mineralization, and solubilization, and eventually enhancing the physical condition, expansion, and maturation of the plant species (Delgado-Baquerizo et al., 2017; Peng et al., 2022a; Peng et al., 2022b; Dai et al., 2023; Chen et al., 2024; Jayaramaiah et al., 2025). Soil microbial community impacts plant growth and yield through a wide range of processes, such as increasing nutrient absorption, stimulating root development, regulating plant hormones, and boosting the capability of plant to combat environmental stresses, and diseases (Chen et al., 2024). Based on the McIntosh index results, the effect of wildfire is not consistent and mainly influenced by site and soil type (Table 6). The finding revealed that rhizosphere soil of plant species such as D. cinerea (6.46) and C. virgata (6.12) from unburned area in Palapye had higher microbial evenness and these suggest the presence of a balanced microbial communities in unburned (undisturbed) area. However, rhizosphere soil of D. cinerea (McIntosh; 0.41) and C. virgata (McIntosh; 3.89) from burned area had a great reduction in evenness, and these indicate dominance by a few functional groups post-fire. Fire-induced disturbances create a filtering effect, which only allows microorganisms that can adapt in a new environment (Fierer, 2017). The heat from the fire denatures the specialized and less resilient microbes that resulted to the high evenness of the original community. Wildfires can disrupt the number, structure, and function of microbial communities by denaturing microbial cells by heat or altering soil conditions (Agbeshie et al., 2022). The combustion of the fresh litter and biomass results in a pyrogenic carbon (charcoal), which is difficult to be degraded by microbes (Campos et al., 2016; Liu et al., 2018; Alcañiz et al., 2018; Alexakis et al., 2021). The post-fire new environment favors a small number of organisms; fast-growing opportunists (r-strategists) fire-adapted microbes that can rapidly utilize simple and available substrates and soluble nutrients released in the ash, and highly specialized microbes that are specialized to degrade the intricate aromatic molecular structure of charcoal (Deacon et al., 2006).
The Principal Component Analysis (PCA) of the carbon substrate utilization patterns (Figure 3) offers a robust visualization of the functional dynamics within the soil microbial communities, reinforcing and expanding the insights gained from the univariate diversity indices (Table 5). The PCA was highly effective in the discrimination of the microbial communities from burned and unburned area from both sites (Palapye and Mmashoro) and these demonstrates that wildfire serves as a dominant contributor of functional divergence, substantially modifying the metabolic capabilities of the soil microbiome, while the microbial communities from unburned area exhibit comparable functional traits and a high level of adaptability. The microbial communities from unburned area are strongly correlated with a wide range of carbon sources such as carbohydrates (D, L-a-Glycerol phosphate), carboxylic acids (α-ketobutyric acid), amino acids (L-glutamic acid), and complex polymers (Tween compounds), and these indicate high metabolic versatility (Garland and Mills, 1991). The undisturbed and unburned ecosystem possess a steady influx of different organic materials such as litter fall, root exudates, and microbial byproducts that sustain a diverse microbial community characterized by functional redundancy and complexity (Bardgett and Van Der Putten, 2014). This community possesses a diverse enzymatic activity capable of decomposing grading various types of carbon substrates, which is a key indicator of a healthy and resilient soil ecosystem (Zak et al., 1994). The tight-clustering indicates that this functional trait is consistent across undisturbed savanna soils, irrespective of vegetation type.
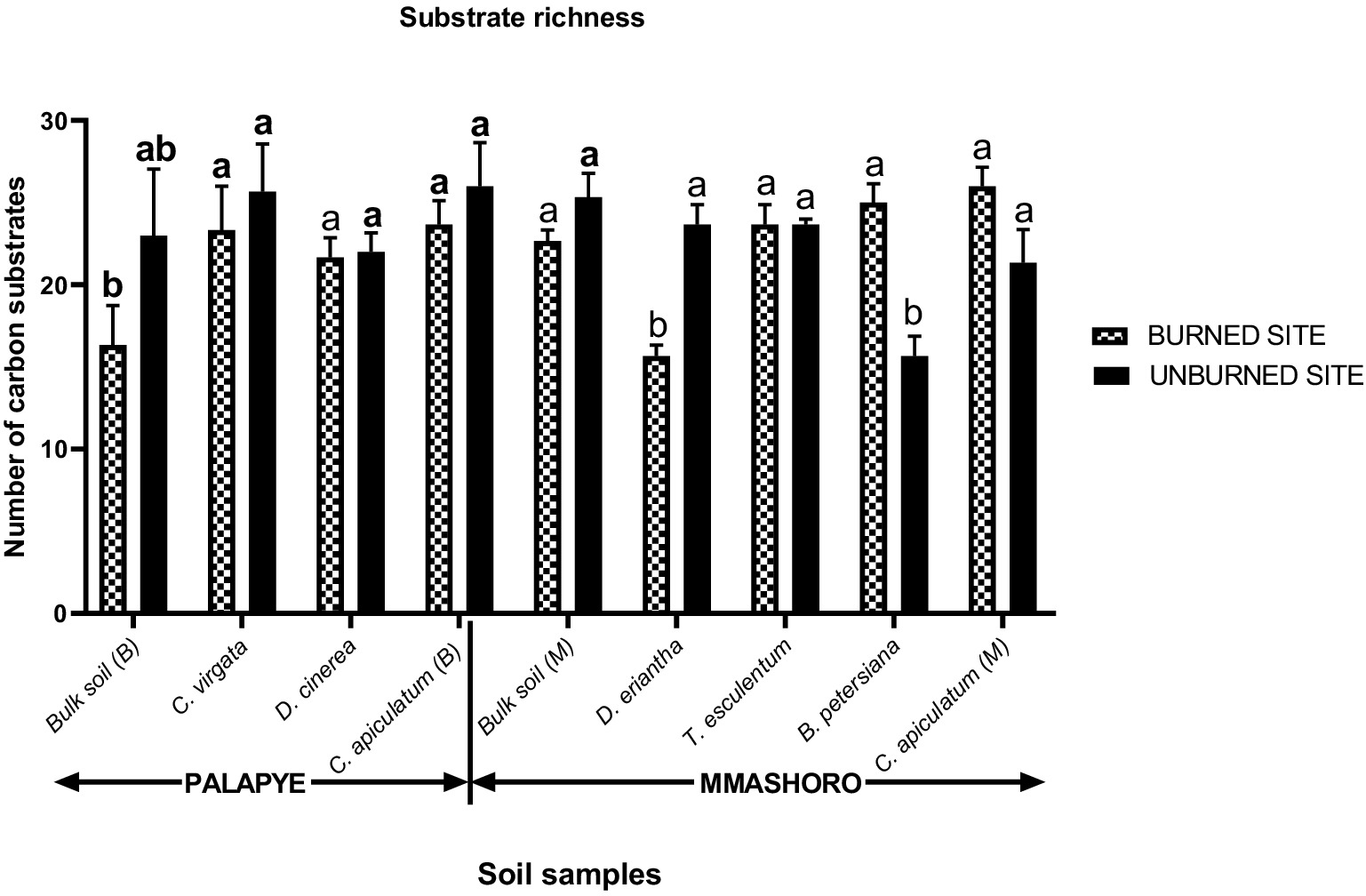
Figure 3. Bi-plot of the PC1 and PC2 extracted using Principal component analysis of the carbon substrate utilization of soil microbial communities of the bulk and rhizosphere soil of plant species (C. virgata, D. cinerea, and C. apiculatum) from Palapye site (P) and Mmashoro (D. eriantha, T. esculentum, B. petersiana, and C. apiculatum) in burned (E) and unburned (C) area in in the savanna biome in Botswana. C-Control, E-Experimental, M- Mmashoro, and P-Palapye.
In contrast, the most striking outcome of the microbial communities from burned area in both sites (Palapye and Mmashoro) reveal that post-fire communities are functionally impaired and this can be attributed to the wildfire that denature the microbes and deplete the labile carbon sources (fresh litter) that the adapted microbial community relies on (Certini, 2005; Dooley and Treseder, 2012). Although fire is the main driver of the functional shift, microbial communities’ exhibit greater heterogeneity and evidence of resilience are linked to specific plant species (vegetation) and site. The unique position of samples (D. cinerea and D eriantha) from unburned area demonstrate that each plant species possess unique functional microbial community in its rhizosphere. This is congruent with the principle that plant species-specific releases root exudates and produce litter with unique chemical composition that leads to the selection of different microbial communities (Grayston et al., 1998; Garbeva et al., 2008; Nannipieri et al., 2008; Prescott and Grayston, 2013) (Figure 4).
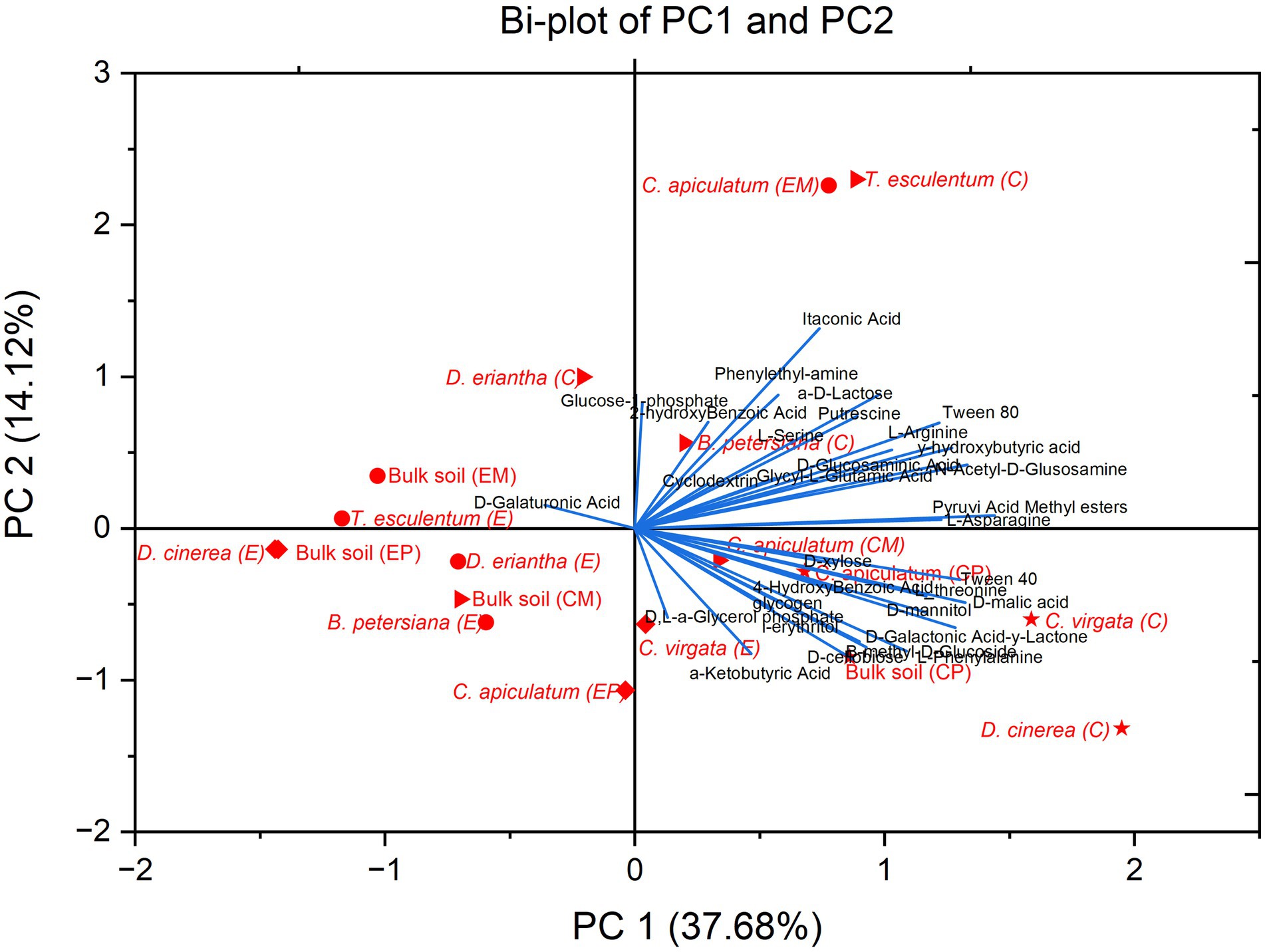
Figure 4. Bi-plot of the PC1 and PC2 extracted using Principal component analysis of the carbon utilization of soil microbial communities of the bulk and rhizosphere soil of C. virgata, D. cineria, and C. apiculatum from Palapye site, and D. eriantha, T. esculentum, B. petersiana, and C. apiculatum from Mmashoro site under burned and unburned area in the savanna biome in Botswana.
The most striking outcome is that C. apiculatum (E) from burned area clustered on the positive side of PC1, alongside the samples of [D. eriantha (C), T. esculentum (C), B. petersiana (C), and C. apiculatum (C)] from unburned area. Additionally, C. apiculatum from burned area in Palapye is positioned closer to the origin and this suggest that it might have retained slightly more functional diversity. This suggest that microbial community linked to C. apiculatum is not only resilient but also functionally adapted to post-fire conditions, and also retains higher level of metabolic capabilities. This could be attributed to several factors; woody species like C. apiculatum may change fire regime on their base by creating localized refugia that modify the soil temperature during combustion and this allow the survival of the larger microbial population (Neary et al., 1999; Keeley and Zedler, 2009); the charcoal and ash derived from the combustion of C. apiculatum may exhibit unique chemical properties richer in labile compounds or possess a structure more conducive to microbial colonization and activity than the pyrogenic residues of other species (Lehmann et al., 2011); the plant species like C. apiculatum may be adapted to fire-prone ecosystem with fast post-fire root recovery and exudation that promptly restores and support a functional microbial community (Higgins et al., 2000) (Figure 5).
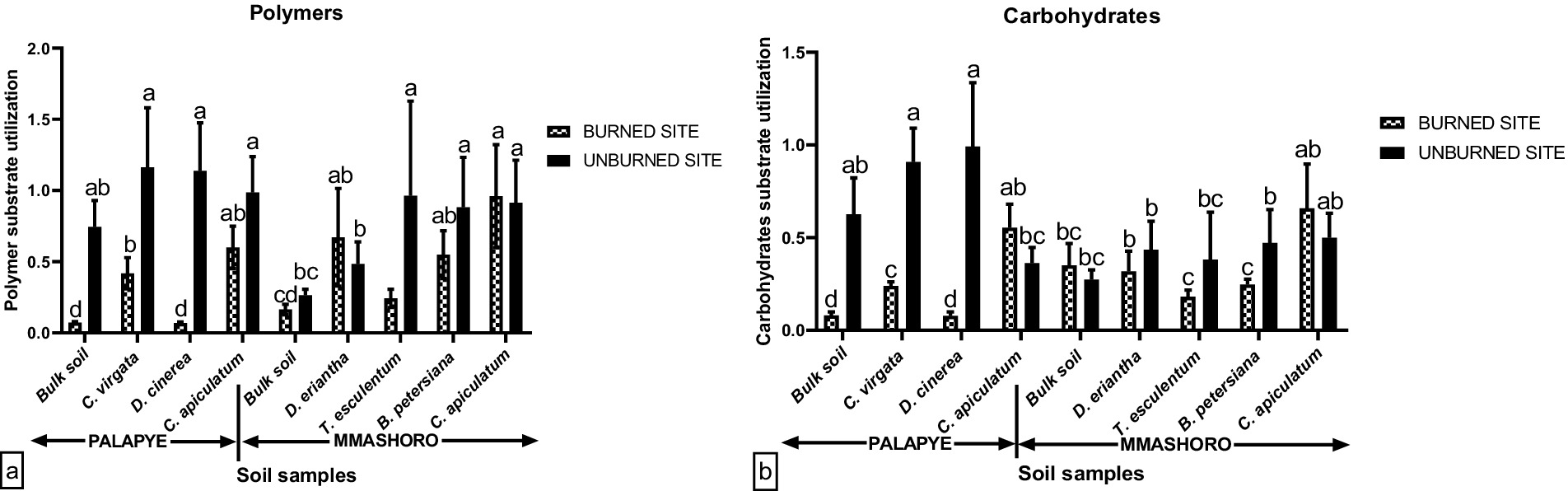
Figure 5. Average polymer (a) and Carbohydrates (b) carbon substrate group’s utilization by soil microbial communities in the bulk and rhizosphere soil of C. virgata, D. cineria, and C. apiculatum from Palapye site, and D. eriantha, T. esculentum, B. petersiana, and C. apiculatum from Mmashoro site under burned and unburned area in the savanna biome in Botswana. Means with similar letters are not significantly different at (P < 0.05).
4.5 Short-term impacts of wildfire on the relationship between soil microbial functional structures and soil chemical properties
The correlation analysis showed vital associations between soil chemical properties and microbial functional traits (Table 6). Average Well Color Development (AWCD), was directly proportional to the total organic matter, available phosphorus, and electrical conductivity and this indicate that microbial metabolic activity in the soil of the savanna ecosystem is mainly influenced by the availability of labile carbon sources and nutrients. Our finding concurred with Delgado-Baquerizo et al. (2013), and Weil et al. (2017) who reported that microbial metabolic activity is strongly impacted by the presence of labile carbon substrates and nutrients in semi-arid environments such as the savanna ecosystem. The observed negative correlation between AWCD and pH show the stimulatory effect of mildly acidic environments on microbial function, and this is might be linked to better nutrient accessibility and the ecological preferences of acid-adapted microbes. This is consistent with proven principle that specify pH as one of the main variable in controlling microbial structures (Fierer and Jackson, 2006). Similarly, ACWD was negatively correlated with exchangeable cations such as potassium, magnesium, and sodium, and these suggests that higher concentrations of cations cause osmotic stress or ion toxicity effects (Munns and Tester, 2008; Kumar et al., 2020; Gupta et al., 2024). The McIntosh index revealed the most robust positive correlation with available phosphorus. This demonstrate that an increase in phosphorus availability promotes a more even distribution of metabolic functions among microbial taxa, which supports the hypothesis that phosphorus is a primary limiting nutrient in these savanna ecosystems (Lambers et al., 2008) (Figures 6, 7). Additionally, McIntosh index had positive correlation with TOM and calcium, and these findings highlights the significance of organic inputs and nutrient availability for the sustenance of microbial communities (Delgado-Baquerizo et al., 2013; Weil et al., 2017). The negative correlation between McIntosh values and soil pH, as well as cations like K, Mg, and Na, indicates that environments with high alkalinity or cation concentrations may reduce microbial functional equilibrium (Munns and Tester, 2008; Kumar et al., 2020; Gupta et al., 2024). The Shannon diversity index was not strongly correlated with most variables, but its strongest link was with electrical conductivity (EC). These findings align with broader ecological theory that enhanced resource supply can mitigate competition and promote functional redundancy in the ecosystem (Tilman, 1999; Banning and Murphy, 2008) (Table 7).
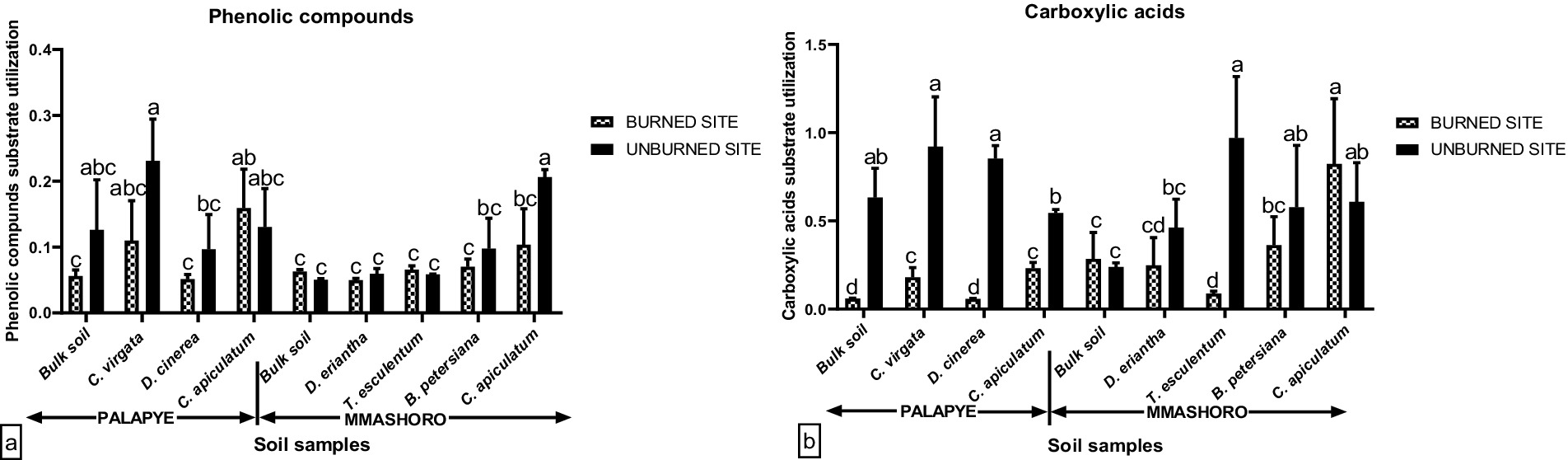
Figure 6. Average Phenolic compounds (a), and Carboxylic acids (b) carbon substrate group’s utilization by soil microbial communities in the bulk and rhizosphere soil of C. virgata, D. cineria, and C. apiculatum from Palapye site, and D. eriantha, T. esculentum, B. petersiana, and C. apiculatum from Mmashoro site under burned and unburned area in the savanna biome in Botswana. Means with similar letters are not significantly different at (P < 0.05).
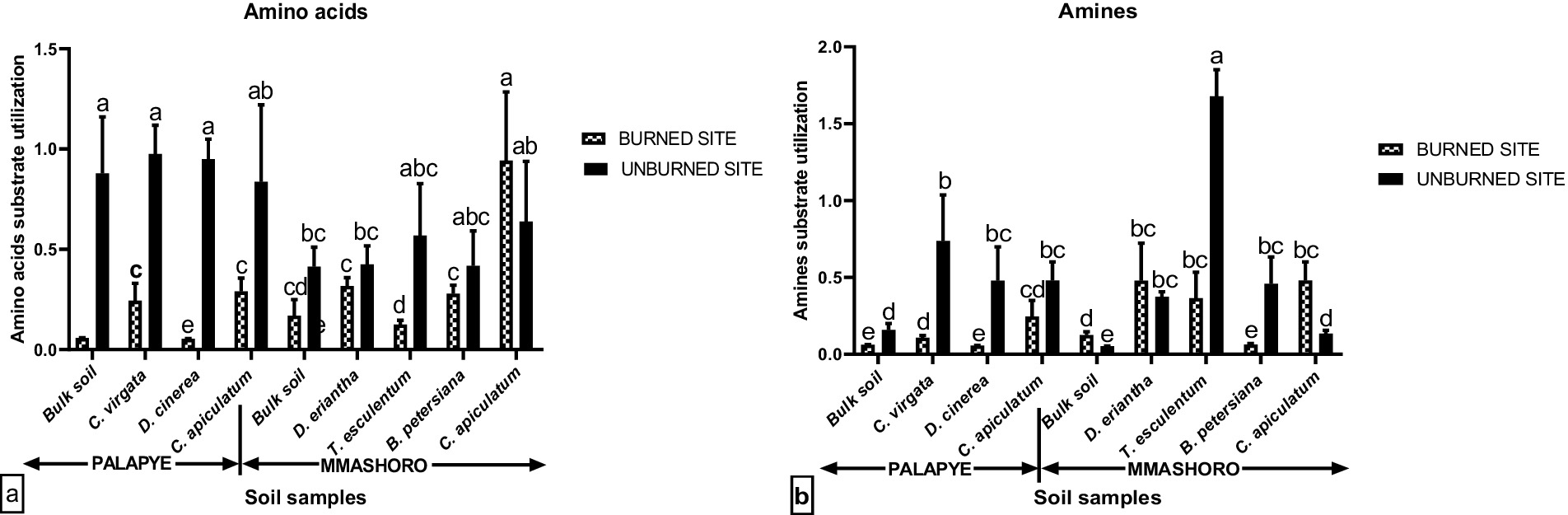
Figure 7. Average amino acids (a), and amines (b) carbon substrate group’s utilization by soil microbial communities in the bulk and rhizosphere soil of C. virgata, D. cineria, and C. apiculatum from Palapye site, and D. eriantha, T. esculentum, B. petersiana, and C. apiculatum from Mmashoro site under burned and unburned area in the savanna biome in Botswana. Means with similar letters are not significantly different at (P < 0.05).
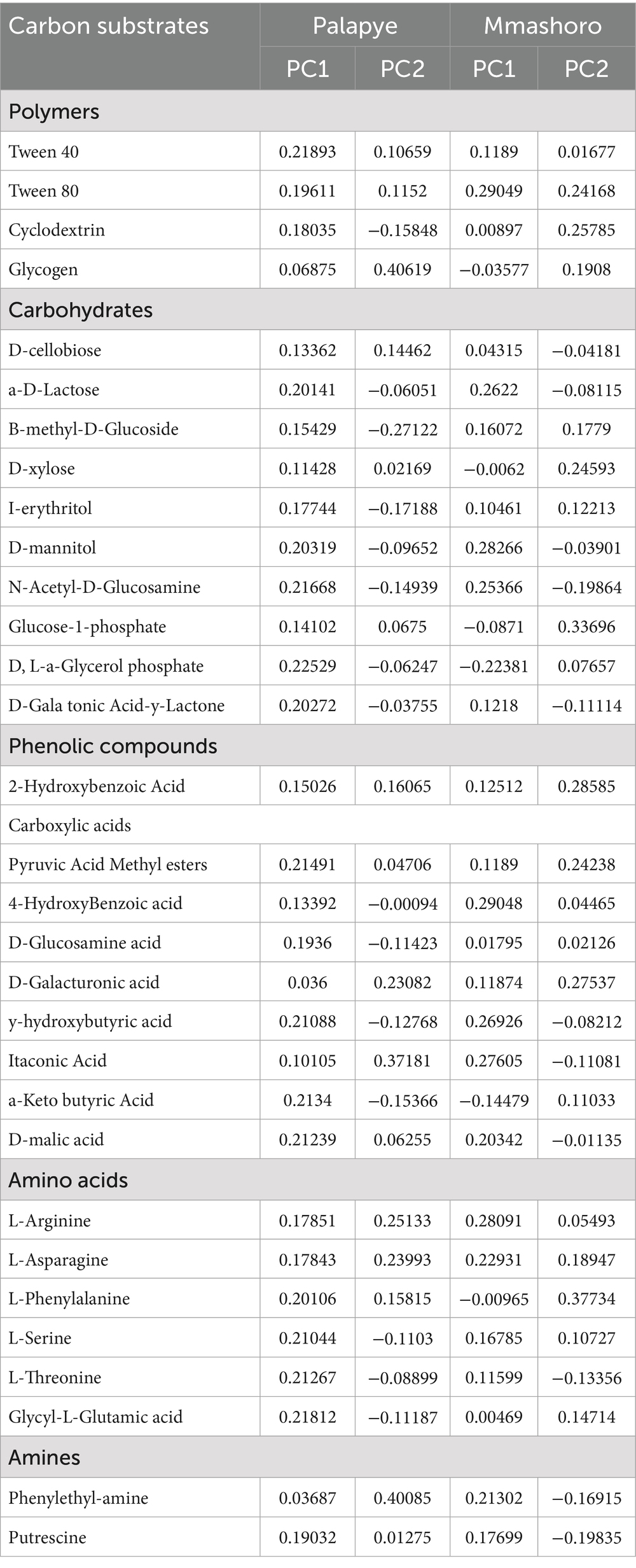
Table 7. Principal carbon substrates utilization pattern of the microbial communities from burned and unburned area with high loading plots for PC1 and PC2 (loading values fall in the range between −0.500 and 0.500).
5 Conclusion
This study offers a comprehensive analysis of the short-term impacts of wildfire on the savanna ecosystems and revealed that fire effects are not uniform but highly dependent on site-specific factors like soil type and plant species. The study proves that it is crucial to understand the role of pre-fire nutrient reserves in the vegetation for post-fire nutrient pulses that have the potential to contribute to stable carbon pools. The findings of this study reveal that wildfire increase total organic matter levels, and these offers a critical insight into carbon sequestration and soil health. Even though, wildfire reduces the vegetation cover, net primary productivity and plant biomass, carbon substrate utilization pattern, microbial functional diversity, and metabolic activity, the natural recovery of the vegetation is induced by fire induced soil changes. Crucially, this study shows that certain keystone species are resilient and can mitigate the impact of fire-disturbance on microbial communities, highlighting the ecological significance of biodiversity. Thus, it is important to continuously monitor the persisting impacts of wildfires on the soil ecosystem and health in the savanna ecosystem. The broader implication is that holistic approach to fire management strategies is essential and must be tailored to the specific environmental context and vegetation composition of the landscape. The continuous monitoring of the post-fire impacts is vital to refine strategies on the sustainable management of savanna ecosystem in a changing climate.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WM: Data curation, Investigation, Validation, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis. MM: Data curation, Conceptualization, Investigation, Writing – original draft, Formal analysis, Visualization. VU: Resources, Writing – original draft, Visualization, Validation, Methodology, Supervision, Conceptualization, Data curation. GR: Writing – original draft, Resources, Visualization, Methodology, Validation, Supervision. BN: Writing – original draft, Validation, Visualization, Conceptualization, Methodology. GMT: Funding acquisition, Project administration, Validation, Resources, Supervision, Conceptualization, Writing – original draft, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work has received funding from the O. R. Tambo Africa Research Chairs Initiative as supported by the Botswana International University of Science and Technology, the Ministry of Tertiary Education, Science and Technology, the National Research Foundation of South Africa (NRF), the Department of Science and Innovation of South Africa (DSI), the International Development Research Center of Canada (IDRC), and the Oliver and Adelaide Tambo Foundation (OATF), with grant number: (UID) 136696.
Acknowledgments
We thank Trust Manyiwa and Serwalo Mokgosi for assisting with vegetation assessment and soil sample collection. We would also express our gratitude to Phenyo Tlale and Morati Mpalo for assistance with the sampling map.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1605456/full#supplementary-material
References
Abades, S. R., Gaxiola, A., and Marquet, P. A. (2014). Fire, percolation thresholds and the savanna forest transition: a neutral model approach. J. Ecol. 102, 1386–1393. doi: 10.1111/1365-2745.12321
Agbeshie, A. A., Abugre, S., Atta-Darkwa, T., and Awuah, R. (2022). A review of the effects of forest fire on soil properties. J. For. Res. 33, 1419–1441. doi: 10.1007/s11676-022-01475-4
Akinyemi, F. (2017). Climate change and variability in semi-arid Palapye, eastern Botswana: an assessment from smallholder farmers’ perspective. Wea. Clim. Soc. 9, 349–365. doi: 10.1175/WCAS-D-16-0040.1
Akinyemi, F. O., Tlhalerwa, L. T., and Eze, P. N. (2021). Land degradation assessment in an African dryland context based on the composite land degradation index and mapping method. Geocarto Int. 36, 1838–1854. doi: 10.1080/10106049.2019.1678673
Alcañiz, M., Outeiro, L., Francos, M., and Úbeda, X. (2018). Effects of prescribed fires on soil properties: a review. Sci. Total Environ. 613, 944–957. doi: 10.1016/j.scitotenv.2017.09.144
Alexakis, D., Kokmotos, I., Gamvroula, D., and Varelidis, G. (2021). Wildfire effects on soil quality: application on a suburban area of West Attica (Greece). Geosci. J. 25, 243–253. doi: 10.1007/s12303-020-0011-1
Allison, S. D., and Martiny, J. B. (2008). Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 105, 11512–11519. doi: 10.1073/pnas.0801925105
Aponte, H., Galindo-Castañeda, T., Yáñez, C., Hartmann, M., and Rojas, C. (2022). Microbial community-level physiological profiles and genetic prokaryotic structure of burned soils under Mediterranean sclerophyll forests in Central Chile. Front. Microbiol. 13:824813. doi: 10.3389/fmicb.2022.824813
Aref, I. M., El Atta, H. A., and Mohamed Al Ghamde, A. R. (2011). Effect of forest fires on tree diversity and some soil properties. Int. J. Agric. Biol. 13, 659–664.
Arunrat, N., Kongsurakan, P., Solomon, L. W., and Sereenonchai, S. (2024). Fire impacts on soil properties and implications for sustainability in rotational shifting cultivation: a review. Agriculture 14:1660. doi: 10.3390/agriculture14091660
Badía, D., Martí, C., Aguirre, A. J., Aznar, J. M., González-Pérez, J., De La Rosa, J., et al. (2014). Wildfire effects on nutrients and organic carbon of a Rendzic phaeozem in ne Spain: changes at cm-scale topsoil. Catena 113, 267–275.
Ball, D. (1964). Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 15, 84–92. doi: 10.1111/j.1365-2389.1964.tb00247.x
Banning, N. C., and Murphy, D. V. (2008). Effect of heat-induced disturbance on microbial biomass and activity in forest soil and the relationship between disturbance effects and microbial community structure. Appl. Soil Ecol. 40, 109–119. doi: 10.1016/j.apsoil.2008.03.011
Bardgett, R. D., and Van Der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Barreiro, A., Fontúrbel, M., Lombao, A., Martín, A., Vega, J., Fernández, C., et al. (2015). Using phospholipid fatty acid and community level physiological profiling techniques to characterize soil microbial communities following an experimental fire and different stabilization treatments. Catena 135, 419–429. doi: 10.1016/j.catena.2014.07.011
Batanani, S. (2020). Seed germination of morama bean (tylosema esculentum (burch) a. schreib) from different collection sites in Botswana. Gaborone: Botswana University of Agriculture and Natural Resources.
Bekker, R., and De Wit, P. (1991). Vegetation map of Republic of Botswana. Soil mapping and advisory services project. Ag: Dp. Bot/85/011. Gaborone: Food and agricultural organisation and government of Botswana.
Bodí, M. B., Martin, D. A., Balfour, V. N., Santín, C., Doerr, S. H., Pereira, P., et al. (2014). Wildland fire ash: production, composition and eco-hydro-geomorphic effects. Earth Sci. Rev. 130, 103–127. doi: 10.1016/j.catena.2013.08.002
Boerner, R. E., Huang, J., and Hart, S. C. (2009). Impacts of fire and fire surrogate treatments on forest soil properties: a meta-analytical approach. Ecol. Appl. 19, 338–358. doi: 10.1890/07-1767.1
Bond, W. J., and Keeley, J. E. (2005). Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394. doi: 10.1016/j.tree.2005.04.025
Bond, W. J., Woodward, F. I., and Midgley, G. F. (2005). The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–537
Bowman, D. M., Balch, J. K., Artaxo, P., Bond, W. J., Carlson, J. M., Cochrane, M. A., et al. (2009). Fire in the earth system. ScienceAsia 324, 481–484. doi: 10.1126/science.1163886
Brady, N., and Weil, R. (2016). The nature and properties of soils. Columbus: Eua Pearson Education.
Bray, R. H., and Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46. doi: 10.1097/00010694-194501000-00006
Buyer, J. S., and Kaufman, D. D. (1997). Microbial diversity in the rhizosphere of corn grown under conventional and low-input systems. Appl. Soil Ecol. 5, 21–27. doi: 10.1016/S0929-1393(96)00127-8
Cade-Menun, B. J., Berch, S. M., Preston, C. M., and Lavkulich, L. (2000). Phosphorus forms and related soil chemistry of podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Can. J. For. Res. 30, 1726–1741.
Campos, I., Abrantes, N., Keizer, J. J., Vale, C., and Pereira, P. (2016). Major and trace elements in soils and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Sci. Total Environ. 572, 1363–1376. doi: 10.1016/j.scitotenv.2016.01.190
Caon, L., Vallejo, V. R., Ritsema, C. J., and Geissen, V. (2014). Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth-Sci. Rev. 139, 47–58. doi: 10.1016/j.earscirev.2014.09.001
Cardon, Z. G., and Whitbeck, J. L. (2011). The rhizosphere: An ecological perspective. Amsterdam: Elsevier.
Carter, T., and Hulme, M. (1999). Interim characterizations of regional climate and related changes up to 2100 associated with the provisional Sres emissions scenarios: Guidance for lead authors of the Ipcc working group ii third assessment report. Washington, DC: Ipcc Working Group Ii Technical Support Unit.
Cassidy, L., Perkins, J., and Bradley, J. (2022). Too much, too late: fires and reactive wildfire management in northern Botswana’s forests and woodland savannas. Afr. J. Range Forage Sci. 39, 160–174. doi: 10.2989/10220119.2022.2033833
Certini, G. (2005). Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10. doi: 10.1007/s00442-004-1788-8
Chapin, F. S., Matson, P. A., Vitousek, P. M., Chapin, F. S., Matson, P. A., and Vitousek, P. M. (2011). Temporal dynamics. Principles Terrestr. Ecosyst. Ecol., 339–367. doi: 10.1007/978-1-4419-9504-9_12
Chen, J., Liu, Z., Cui, H., Song, H., Wang, J., Gao, H., et al. (2023). Direct and indirect effects of dominant plants on ecosystem multifunctionality. Front. Plant Sci. 14:1117903. doi: 10.3389/fpls.2023.1117903
Chen, Q., Song, Y., An, Y., Lu, Y., and Zhong, G. (2024). Soil microorganisms: their role in enhancing crop nutrition and health. Diversity 16:734. doi: 10.3390/d16120734
Chungu, D., Ng’andwe, P., Mubanga, H., and Chileshe, F. (2020). Fire alters the availability of soil nutrients and accelerates growth of Eucalyptus grandis in Zambia. J. For. Res. 31, 1637–1645. doi: 10.1007/s11676-019-00977-y
Dai, Z., Guo, X., Lin, J., Wang, X., He, D., Zeng, R., et al. (2023). Metallic micronutrients are associated with the structure and function of the soil microbiome. Nat. Commun. 14:8456. doi: 10.1038/s41467-023-44182-2
Davari, N., Jouri, M. H., and Ariapour, A. (2011). Comparison of measurement indices of diversity, richness, dominance, and evenness in rangeland ecosystem (case study: Jvaherdeh-Ramesar). J. Rangeland Sci. 2, 389–398.
De Miera, L. E. S., Pinto, R., Gutierrez-Gonzalez, J. J., Calvo, L., and Ansola, G. (2020). Wildfire effects on diversity and composition in soil bacterial communities. Sci. Total Environ. 726:138636. doi: 10.1016/j.scitotenv.2020.138636
De Sousa Ferreira, L., De Souza Oliveira, V., Paula Marchiori, J. J., Ferreira, T. C., Bernabé, A. C. B., Boone, G. T. F., et al. (2023). The nutrient magnesium in soil and plant: a review. Int. J. Plant Soil Sci. 35, 136–144. doi: 10.9734/ijpss/2023/v35i82890
De Torres Curth, M. I., Ghermandi, L., and Biscayart, C. (2012). Are Fabiana imbricata shrublands advancing over northwestern Patagonian grasslands? A population dynamics study involving fire and precipitation. J. Arid Environ. 83, 78–85. doi: 10.1016/j.jaridenv.2012.03.011
De Wit, P., and Nachtergaele, F. (1990). Soil map of the Republic of Botswana. Soil mapping and advisory services project Fao/bot/85/011. Map 1 of 2. Rome: FAO.
Deacon, L. J., Pryce-Miller, E. J., Frankland, J. C., Bainbridge, B. W., Moore, P. D., and Robinson, C. H. (2006). Diversity and function of decomposer fungi from a grassland soil. Soil Biol. Biochem. 38, 7–20. doi: 10.1016/j.soilbio.2005.04.013
Debano, L. F., and Conrad, C. E. (1978). The effect of fire on nutrients in a chaparral ecosystem. Ecology 59, 489–497. doi: 10.2307/1936579
Delgado-Baquerizo, M., Morillas, L., Maestre, F. T., and Gallardo, A. (2013). Biocrusts control the nitrogen dynamics and microbial functional diversity of semi-arid soils in response to nutrient additions. Plant Soil 372, 643–654. doi: 10.1007/s11104-013-1779-9
Delgado-Baquerizo, M., Trivedi, P., Trivedi, C., Eldridge, D. J., Reich, P. B., Jeffries, T. C., et al. (2017). Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 31, 2330–2343. doi: 10.1111/1365-2435.12924
Dooley, S. R., and Treseder, K. K. (2012). The effect of fire on microbial biomass: a meta-analysis of field studies. Biogeochemistry 109, 49–61. doi: 10.1007/s10533-011-9633-8
Dube, O. P. (2013). Challenges of wildland fire management in Botswana: towards a community inclusive fire management approach. Weather Climate Extr. 1, 26–41. doi: 10.1016/j.wace.2013.08.001
Dube, E. (2015). Environmental challenges posed by veld fires in fragile regions: the case of the Bulilima and Mangwe districts in southern Zimbabwe. Jamba J. Disaster. Risk Stud. 7, 1–8. doi: 10.4102/jamba.v7i1.224
Dudinszky, N., and Ghermandi, L. (2013). Fire as a stimulant of shrub recruitment in northwestern Patagonian (Argentina) grasslands. Ecol. Res. 28, 981–990. doi: 10.1007/s11284-013-1080-7
Elzinga, C. L., Salzer, D. W., Willoughby, J. W., and Gibbs, J. P. (2001). Monitoring plant and animal populations: A handbook for field biologists. New York, NY: John Wiley & Sons.
Emery, M., and Gross, L. (2006). Dominant species identity regulates invasibility of old-field plant communities. Oikos 115, 549–558. doi: 10.1111/j.2006.0030-1299.15172.x
Fernández-García, V., Miesel, J., Baeza, M. J., Marcos, E., and Calvo, L. (2019). Wildfire effects on soil properties in fire-prone pine ecosystems: indicators of burn severity legacy over the medium term after fire. Appl. Soil Ecol. 135, 147–156. doi: 10.1016/j.apsoil.2018.12.002
Field, C. B., Behrenfeld, M. J., Randerson, J. T., and Falkowski, P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. doi: 10.1126/science.281.5374.237
Fierer, N. (2017). Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590. doi: 10.1038/nrmicro.2017.87
Fierer, N., and Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. 103, 626–631. doi: 10.1073/pnas.0507535103
Filonchyk, M., Peterson, M. P., Zhang, L., Hurynovich, V., and He, Y. (2024). Greenhouse gases emissions and global climate change: examining the influence of Co2, Ch4, and N2O. Sci. Total Environ. 935:173359.
Firmaningrum, N., Sari, L., Pursetyo, K., Zein, A., Idris, M., and Cahyoko, Y. (2021). Biodiversity studies of echinoderms in Sedati waters, Sidoarjo district, East Java province. Iop Conf. Series Earth Environ. Sci. 718:012089. doi: 10.1016/j.scitotenv.2024.173359
Francos, M., Úbeda, X., and Pereira, P. (2019). Impact of torrential rainfall and salvage logging on post-wildfire soil properties in ne Iberian Peninsula. Catena 177, 210–218. doi: 10.1016/j.catena.2019.02.014
Franzese, J., Ghermandi, L., and Donaldo, B. (2009). Post-fire shrub recruitment in a semi-arid grassland: the role of microsites. J. Veg. Sci. 20, 251–259. doi: 10.1111/j.1654-1103.2009.05733.x
Gajaje, K., Duelang, M., Ultra, V. U. Jr., Marenga, W., and Rantong, G. (2024). Impact of fly ash deposition on plants diversity, metal content, and associated risk on grazing ruminants in the vicinity of Morupule power station, Botswana. Arid Land Res. Manag. 22, 1–25. doi: 10.1080/15324982.2024.2327989
Garbeva, P., Van Elsas, J., and Van Veen, J. (2008). Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 302, 19–32. doi: 10.1007/s11104-007-9432-0
Garland, J. L., and Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359. doi: 10.1128/aem.57.8.2351-2359.1991
Ghazoul, J., Burivalova, Z., Garcia-Ulloa, J., and King, L. A. (2015). Conceptualizing forest degradation. Trends Ecol. Evol. 30, 622–632. doi: 10.1016/j.tree.2015.08.001
Ghermandi, L., De Torres Curth, M., Franzese, J., and Gonzalez, S. (2010). Non-linear ecological processes, fires, environmental heterogeneity and shrub invasion in northwestern Patagonia. Ecol. Model. 221, 113–121. doi: 10.1016/j.ecolmodel.2009.07.026
Gómez-Rey, M. X., Couto-Vázquez, A., García-Marco, S., and González-Prieto, S. J. (2013). Impact of fire and post-fire management techniques on soil chemical properties. Geoderma 195, 155–164. doi: 10.1016/j.geoderma.2012.12.005
Gotelli, N. J., and Ellison, A. M. (2013) A primer of ecological statistics (2nd Ed.). Sinauer Associates, Inc.
Govender, N., Trollope, W. S., and Van Wilgen, B. W. (2006). The effect of fire season, fire frequency, rainfall and management on fire intensity in savanna vegetation in South Africa. J. Appl. Ecol. 43, 748–758. doi: 10.1111/j.1365-2664.2006.01184.x
Grayston, S. J., Wang, S., Campbell, C. D., and Edwards, A. C. (1998). Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 30, 369–378. doi: 10.1016/S0038-0717(97)00124-7
Guidi, G., Pera, A., Giovannetti, M., Poggio, G., and Bertoldi, M. (1988). Variations of soil structure and microbial population in a compost amended soil. Plant Soil 106, 113–119. doi: 10.1007/BF02371202
Gülci, S. (2019). The determination of some stand parameters using SfM-based spatial 3D point cloud in forestry studies: an analysis of data production in pure coniferous young forest stands. Environ. Monit. Assess. 191:495. doi: 10.1007/s10661-019-7628-4
Gupta, S., Kumar, M., and Srivastava, A. (2024). Hydraulic conductivity of soils: a comprehensive review of the impacts of chemicals, soil salinity, organic matter, and land use. IOP Conf. Ser. Earth Environ. Sci. 1327:012032. doi: 10.1088/1755-1315/1327/1/012032
Gwynn-Jones, D., Saugier, B., Roy, J., and Mooney, H. (2002). Terrestrial global productivity. Oxford: Oxford University Press.
Haritash, A., and Kaushik, C. (2009). Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J. Hazard. Mater. 169, 1–15. doi: 10.1016/j.jhazmat.2009.03.137
Hartmann, A., Schmid, M., Tuinen, D. V., and Berg, G. (2009). Plant-driven selection of microbes. Cham: Springer.
Hassan, N. E. (2024). Global warming: Causes, impacts and urgent strategies for a sustainable future: A. GSC Adv. Res. Rev. 20, 73–87.
Heard, L., and Channon, B. (1997). Guide to a native vegetation survey (agricultural region) using the biological survey of South Australia methodology. Adelaide: Department of Housing and Urban Development, South Australian Government.
Heath, R., and Heath, A. (2009). Field guide to the plants of northern Botswana including the Okavango Delta. London: Royal Botanic Gardens.
Higgins, S. I., Bond, W. J., and Trollope, W. S. (2000). Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J. Ecol. 88, 213–229. doi: 10.1046/j.1365-2745.2000.00435.x
Hu, M., and Wan, S. (2024). Effects of burning and nitrogen addition on foliar stoichiometry and nutrient resorption in a subtropical–temperate ecotonal forest. For. Ecol. Manag. 572:122284. doi: 10.1016/j.foreco.2024.122284
Jayaramaiah, R. H., Martins, C. S., Egidi, E., Macdonald, C. A., Wang, J.-T., Liu, H., et al. (2025). Soil function-microbial diversity relationship is impacted by plant functional groups under climate change. Soil Biol. Biochem. 200:109623. doi: 10.1016/j.soilbio.2024.109623
Jhariya, M., Bargali, S., Swamy, S., and Oraon, P. (2013). Herbaceous diversity in proposed mining area of Rowghat in Narayanpur District of Chhattisgarh, India. J. Plant Dev. Sci. 5, 385–393.
Jhariya, M., and Oraon, P. (2012). Regeneration status and species diversity along the fire gradients in tropical deciduous forest of Chhattisgarh. J. Plant Dev. Sci. 4, 49–54.
Jhariya, M. K., and Raj, A. (2014). Effects of wildfires on flora, fauna and physico-chemical properties of soil-An overview. J. Appl. Nat. Sci. 6, 887–897. doi: 10.31018/jans.v6i2.550
Ji, S., Li, G., Cong, J., Xu, H., Han, D., and Gao, C. (2025). Prescribed burning effects on carbon and nutrient cycling processes in peatlands of greater Khingan Mountains, Northeast China. Sci. Total Environ. 963:178441. doi: 10.1016/j.scitotenv.2025.178441
Johnson, D. B., Yedinak, K. M., Sulman, B. N., Berry, T. D., Kruger, K., and Whitman, T. (2024). Effects of fire and fire-induced changes in soil properties on post-burn soil respiration. Fire Ecol. 20:90. doi: 10.1186/s42408-024-00328-1
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). Organisms as ecosystem engineers. Ecosystem management: Selected readings. Cham: Springer.
Kanagaraj, S., Selvaraj, M., Das Kangabam, R., and Munisamy, G. (2017). Assessment of tree species diversity and its distribution pattern in Pachamalai reserve Forest, Tamil Nadu. J. Sustain. For. 36, 32–46. doi: 10.1080/10549811.2016.1238768
Kauffman, J. B., Till, K. M., and Shea, R. W. (1992). Biogeochemistry of deforestation and biomass burning. Washington, DC: Acs Publications.
Keeley, J. E., and Fotheringham, C. (2000). Role of fire in regeneration from seed. Seeds: The ecology of regeneration in plant communities. Wallingford: Cabi Publishing.
Keeley, J. E., and Zedler, P. H. (2009). Large, high-intensity fire events in southern California shrublands: debunking the fine-grain age patch model. Ecol. Appl. 19, 69–94. doi: 10.1890/08-0281.1
Kgosikoma, O., Mojeremane, W., and Harvie, B. A. (2012). Pastoralists’ perception and ecological knowledge on savanna ecosystem dynamics in semi-arid Botswana. Ecol. Soc. 17:11. Available at: https://www.jstor.org/stable/26269215
Kikstra, J. S., Nicholls, Z. R., Smith, C. J., Lewis, J., Lamboll, R. D., Byers, E., et al. (2022). The IPCC sixth assessment report wgiii climate assessment of mitigation pathways: from emissions to global temperatures. Geosci. Model Dev. 15, 9075–9109. doi: 10.5194/gmd-15-9075-2022
Kittur, B., Swamy, S., Bargali, S., and Jhariya, M. K. (2014). Wildland fires and moist deciduous forests of Chhattisgarh, India: divergent component assessment. J. For. Res. 25, 857–866. doi: 10.1007/s11676-014-0471-0
Kumar, A., Singh, S., Gaurav, A. K., Srivastava, S., and Verma, J. P. (2020). Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 11:1216. doi: 10.3389/fmicb.2020.01216
Kuzyakov, Y., and Blagodatskaya, E. (2015). Microbial hotspots and hot moments in soil: concept & review. Soil Biol. Biochem. 83, 184–199. doi: 10.1016/j.soilbio.2015.01.025
Lambers, H., Chapin Iii, F. S., and Pons, T. L. (2008). Plant physiological ecology. Cham: Springer Science & Business Media.
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., and Crowley, D. (2011). Biochar effects on soil biota–a review. Soil Biol. Biochem. 43, 1812–1836. doi: 10.1016/j.soilbio.2011.04.022
Levine, J. S., Cofer, W. R. Iii, Cahoon, D. R. Jr., and Winstead, E. L. (1995). A driver for global change. Environ. Sci. Technol. 29, 120A–125A. doi: 10.1021/es00003a746
Liu, J., Qiu, L., Wang, X., Wei, X., Gao, H., Zhang, Y., et al. (2018). Effects of wildfire and topography on soil nutrients in a semiarid restored grassland. Plant Soil 428, 123–136. doi: 10.1007/s11104-018-3659-9
Ludwig, F., De Kroon, H., Berendse, F., and Prins, H. H. (2004). The influence of savanna trees on nutrient, water and light availability and the understorey vegetation. Plant Ecol. 170, 93–105. doi: 10.1023/B:VEGE.0000019023.29636.92
Maabong, K. E., and Mphale, K. (2021). Wildfires in Botswana and their frequency of occurrence. Atmospheric Clim. Sci. 11, 689–696. doi: 10.4236/acs.2021.114040
Machida, W. S., Gomes, L., Moser, P., Castro, I. B., Miranda, S. C., Da Silva-Junior, M. C., et al. (2021). Long term post-fire recovery of woody plants in savannas of Central Brazil. For. Ecol. Manag. 493:119255. doi: 10.1016/j.foreco.2021.119255
Manjunath, M., Kumar, U., Yadava, R. B., Rai, A. B., and Singh, B. (2018). Influence of organic and inorganic sources of nutrients on the functional diversity of microbial communities in the vegetable cropping system of the Indo-Gangetic plains. C. R. Biol. 341, 349–357. doi: 10.1016/j.crvi.2018.05.002
McCune, B., and Grace, J. B. (2002). Analysis of ecological communities. Gleneden Beach, Oregon, USA: MjM Software, 304.
Memoli, V., Santorufo, L., Santini, G., Ruggiero, A. G., Giarra, A., Ranieri, P., et al. (2022). The combined role of plant cover and fire occurrence on soil properties reveals response to wildfire in the Mediterranean basin. Eur. J. Soil Biol. 112:103430. doi: 10.1016/j.ejsobi.2022.103430
Moleele, N., Ringrose, S., Matheson, W., and Vanderpost, C. (2002). More woody plants? The status of bush encroachment in Botswana’s grazing areas. J. Environ. Manag. 64, 3–11. doi: 10.1006/jema.2001.0486
Molina, M., Fuentes, R., Calderón, R., Escudey, M., Avendaño, K., Gutiérrez, M., et al. (2007). Impact of forest fire ash on surface charge characteristics of Andisols. Soil Sci. 172, 820–834. doi: 10.1097/ss.0b013e31814cee44
Moore, C. E., Meacham-Hensold, K., Lemonnier, P., Slattery, R. A., Benjamin, C., Bernacchi, C. J., et al. (2021). The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. J. Exp. Bot. 72, 2822–2844. doi: 10.1093/jxb/erab090
Mubyana-John, T., Wutor, V., Ringrose, S., and Yeboah, S. (2007). Fire and its influence on microbial community structure and soil biochemical properties in the Okavango Delta, Botswana. Scientific research and Essay, 2, 47–54.
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Muñoz, I., Schmidt, J., and Weidema, B. P. (2024). The indirect global warming potential of methane oxidation in the Ipcc’s sixth assessment report. Environ. Res. Lett. 19:121002. doi: 10.1088/1748-9326/ad8edd
Nannipieri, P., Ascher, J., Ceccherini, M., Landi, L., Pietramellara, G., and Renella, G. (2003). Microbial diversity and soil functions. Eur. J. Soil Sci. 54, 655–670. doi: 10.1046/j.1351-0754.2003.0556.x
Nannipieri, P., Ascher, J., Ceccherini, M. T., Landi, L., Pietramellara, G., Renella, G., et al. (2008). Effects of root exudates in microbial diversity and activity in rhizosphere soils. Molecular mechanisms of plant and microbe coexistence. Cham: Springer.
Neary, D. G., Klopatek, C. C., Debano, L. F., and Ffolliott, P. F. (1999). Fire effects on belowground sustainability: a review and synthesis. For. Ecol. Manag. 122, 51–71. doi: 10.1016/S0378-1127(99)00032-8
Németh, I., Molnár, S., Vaszita, E., and Molnár, M. (2021). The biolog EcoPlate™ technique for assessing the effect of metal oxide nanoparticles on freshwater microbial communities. Nano 11:1777. doi: 10.3390/nano11071777
Novara, A., Gristina, L., Rühl, J., Pasta, S., D'angelo, G., La Mantia, T., et al. (2013). Grassland fire effect on soil organic carbon reservoirs in a semiarid environment. Solid Earth 4, 381–385. doi: 10.5194/se-4-381-2013
Nyamadzawo, G., Gwenzi, W., Kanda, A., Kundhlande, A., and Masona, C. (2013). Understanding the causes, socio-economic and environmental impacts, and management of veld fires in tropical Zimbabwe. Fire Sci. Rev. 2, 1–13. doi: 10.1186/2193-0414-2-2
Oddi, F., Dudinszky, N., and Ghermandi, L. (2010). Spatial dynamics of Fabiana imbricata shrublands in northwestern Patagonia in relation to natural fires. Nat. Hazards Earth Syst. Sci. 10, 957–966. doi: 10.5194/nhess-10-957-2010
Pellegrini, A. F., Hedin, L. O., Staver, A. C., and Govender, N. (2015). Fire alters ecosystem carbon and nutrients but not plant nutrient stoichiometry or composition in tropical savanna. Ecology 96, 1275–1285. doi: 10.1890/14-1158.1
Peng, S.-L., Ge, Z.-W., Liu, G.-C., and Mao, L.-F. (2022a). Environmental drivers of soil microbial activity and diversity along an elevational gradient. J. Mt. Sci. 19, 1336–1347. doi: 10.1007/s11629-021-7083-x
Peng, Z., Liang, C., Gao, M., Qiu, Y., Pan, Y., Gao, H., et al. (2022b). The neglected role of micronutrients in predicting soil microbial structure. NPJ Biofilms Microb. 8:103. doi: 10.1038/s41522-022-00363-3
Prescott, C. E., and Grayston, S. J. (2013). Tree species influence on microbial communities in litter and soil: current knowledge and research needs. For. Ecol. Manag. 309, 19–27. doi: 10.1016/j.foreco.2013.02.034
Prieto, I., Armas, C., and Pugnaire, F. I. (2012). Water release through plant roots: new insights into its consequences at the plant and ecosystem level. New Phytol. 193, 830–841. doi: 10.1111/j.1469-8137.2011.04039.x
Raison, R. J. (1979). Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformations: a review. Plant and soil, 51, 73–108.
Reisinger, A. (2024). Why addressing methane emissions is a non-negotiable part of effective climate policy. Front. Sci. 2:1451011.
Riishojgaard, L. P., and Tarasova, O. (2024). Reducing methane emissions—the “easy” way to keep 1.5 alive? Front. Sci. 2:1462219. doi: 10.3389/fsci.2024.1462219
Romero-Matos, J., Cánovas, C. R., Macías, F., Pérez-López, R., León, R., Millán-Becerro, R., et al. (2023). Wildfire effects on the hydrogeochemistry of a river severely polluted by acid mine drainage. Water Res. 233:119791. doi: 10.1016/j.watres.2023.119791
Roodt, V. (2015). Grasses & grazers of Botswana and the surrounding savanna. Midrand: Penguin Random House South Africa.
Ryan, C. M., and Williams, M. (2011). How does fire intensity and frequency affect miombo woodland tree populations and biomass? Ecol. Appl. 21, 48–60. doi: 10.1890/09-1489.1
Sankaran, M., Ratnam, J., and Hanan, N. P. (2004). Tree–grass coexistence in savannas revisited–insights from an examination of assumptions and mechanisms invoked in existing models. Ecol. Lett. 7, 480–490. doi: 10.1111/j.1461-0248.2004.00596.x
Sankaran, M., Ratnam, J., and Hanan, N. (2008). Woody cover in African savannas: the role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 17, 236–245. doi: 10.1111/j.1466-8238.2007.00360.x
Schafer, J. L., and Mack, M. C. (2010). Short-term effects of fire on soil and plant nutrients in palmetto Flatwoods. Plant Soil 334, 433–447. doi: 10.1007/s11104-010-0394-2
Scholes, R. J., and Archer, S. R. (1997). Tree-grass interactions in savannas. Annu. Rev. Ecol. Syst. 28, 517–544. doi: 10.1146/annurev.ecolsys.28.1.517
Sebata, A. (2017). Ecology of woody plants in African savanna ecosystems. Plant Ecol. Trad. Approaches Recent Trends 10:69865. doi: 10.5772/intechopen.69865
Sheuyange, A., Oba, G., and Weladji, R. B. (2005). Effects of anthropogenic fire history on savanna vegetation in northeastern Namibia. J. Environ. Manag. 75, 189–198. doi: 10.1016/j.jenvman.2004.11.004
Silva, D. M., Batalha, M. A., and Cianciaruso, M. V. (2013). Influence of fire history and soil properties on plant species richness and functional diversity in a neotropical savanna. Acta Botanica Brasilica 27, 490–497. doi: 10.1590/S0102-33062013000300005
Silvério, D. V., Brando, P. M., Bustamante, M. M., Putz, F. E., Marra, D. M., Levick, S. R., et al. (2019). Fire, fragmentation, and windstorms: a recipe for tropical forest degradation. J. Ecol. 107, 656–667. doi: 10.1111/1365-2745.13076
Simpson, K. J., Ripley, B. S., Christin, P. A., Belcher, C. M., Lehmann, C. E., Thomas, G. H., et al. (2016). Determinants of flammability in savanna grass species. J. Ecol. 104, 138–148. doi: 10.1111/1365-2745.12503
Singh, A., and Dubey, S. K. (2012). Temporal variation in methanogenic community structure and methane production potential of tropical rice ecosystem. Soil Biol. Biochem. 48, 162–166. doi: 10.1016/j.soilbio.2012.01.022
Smit, G. (2004). An approach to tree thinning to structure southern African savannas for long-term restoration from bush encroachment. J. Environ. Manag. 71, 179–191. doi: 10.1016/j.jenvman.2004.02.005
Souchie, F. F., Pinto, J. R. R., Lenza, E., Gomes, L., Maracahipes-Santos, L., and Silvério, D. V. (2017). Post-fire resprouting strategies of woody vegetation in the Brazilian savanna. Acta Botanica Brasilica 31, 260–266. doi: 10.1590/0102-33062016abb0376
Su, X., Shiogama, H., Tanaka, K., Tachiiri, K., Hajima, T., Watanabe, M., et al. (2024). Reductions in atmospheric levels of non-Co2 greenhouse gases explain about a quarter of the 1998-2012 warming slowdown. Commun. Earth Environ. 5:594. doi: 10.1038/s43247-024-01723-x
Taupedi, S. B., and Ultra, V. U. Jr. (2022). Morupule fly ash as amendments in agricultural soil in Central Botswana. Environ. Technol. Innov. 28:102695. doi: 10.1016/j.eti.2022.102695
Thukral, A. K., Bhardwaj, R., Kumar, V., and Sharma, A. (2019). New indices regarding the dominance and diversity of communities, derived from sample variance and standard deviation. Heliyon 5:e02606. doi: 10.1016/j.heliyon.2019.e02606
Tilman, D. (1999). The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474. doi: 10.2307/176540
Trollope, W. (1984). Fire in savanna. Ecological effects of fire in south African ecosystems. Cham: Springer.
Trollope, W., Trollope, L., and Hartnett, D. (2002). Fire behaviour a key factor in the fire ecology of African grasslands and savannas. Millpress Science Publishers.
Tüfekçioğlu, A., Küçük, M., Sağlam, B., Bilgili, E., and Altun, L. (2010). Soil properties and root biomass responses to prescribed burning in young corsican pine (Pinus nigra Arn.) stands. Journal of Environmental Biology, 31:369.
Turpault, M.-P., Gobran, G., and Bonnaud, P. (2007). Temporal variations of rhizosphere and bulk soil chemistry in a Douglas fir stand. Geoderma 137, 490–496. doi: 10.1016/j.geoderma.2006.10.005
Ulfah, M., Fajri, S., Nasir, M., Hamsah, K., and Purnawan, S. (2019). Diversity, evenness and dominance index reef fish in Krueng Raya water, Aceh Besar. IOP Conf. Series Earth Environ. Sci. 348:012074.
Ultra, V. U. (2020). Growth and yield of lemongrass (Cymbopogon citratus) in fly ash with nutrient amendments and mycorrhiza for three-ratoon period. Int. J. Phytoremediation 22, 1551–1561. doi: 10.1080/15226514.2020.1786005
Ultra, V. U. Jr., Han, S.-H., and Kim, D.-H. (2013). Soil properties and microbial functional structure in the rhizosphere of Pinus densiflora (S. And Z.) exposed to elevated atmospheric temperature and carbon dioxide. J. For. Res. 18, 149–158. doi: 10.1007/s10310-012-0333-6
UltraJr, V. (2020). Fly ash and compost amendments and mycorrhizal inoculation enhanced the survival and growth of Delonix regia in cu-Ni mine tailings. Philippine Journal of Science. 149, 479–489.
Van Auken, O. W. (2000). Shrub invasions of north American semiarid grasslands. Annu. Rev. Ecol. Syst. 31, 197–215. doi: 10.1146/annurev.ecolsys.31.1.197
Van Rooyen, N., and Van Rooyen, M. W. (2019). Flowering plants of the southern Kalahari. London: Ekotrust.
Van Wyk, B. (2013). Field guide to trees of southern Africa. Midrand: Penguin Random House South Africa.
Verma, S., and Jayakumar, S. (2012). Impact of forest fire on physical, chemical and biological properties of soil: a review. Proc. Int. Acad. Ecol. Environ. Sci. 2:168.
Weil, R. R., Brady, N. C., and Weil, R. R. (2017). The nature and properties of soils. London: Pearson.
Wienk, C. L., Sieg, C. H., and Mcpherson, G. R. (2004). Evaluating the role of cutting treatments, fire and soil seed banks in an experimental framework in ponderosa pine forests of the Black Hills, South Dakota. For. Ecol. Manag. 192, 375–393. doi: 10.1016/j.foreco.2004.02.004
Woods, J., and Sekhwela, M. (2003). The vegetation resources of Botswana's savannas: an overview. S. Afr. Geogr. J. 85, 69–79. doi: 10.1080/03736245.2003.9713786
Xu, Y., Sun, J., Lin, Q., Ma, J., Shi, Y., and Lou, K. (2012). Effects of a surface wildfire on soil nutrient and microbial functional diversity in a shrubbery. Acta Ecol. Sin. 32, 258–264. doi: 10.1016/j.chnaes.2012.07.007
Yadav, A. N., and Islam, T. (2023). Insights in microbe and virus interactions with plants: 2022. Front. Microbiol. 14:1327245. doi: 10.3389/fmicb.2023.1327245
Zak, J. C., Willig, M. R., Moorhead, D. L., and Wildman, H. G. (1994). Functional diversity of microbial communities: a quantitative approach. Soil Biol. Biochem. 26, 1101–1108. doi: 10.1016/0038-0717(94)90131-7
Keywords: wildfire, rhizosphere, carbon substrate utilization, microbial metabolic activity, savanna, biogeochemical
Citation: Marenga W, Mapoka M, Ultra VU, Rantong G, Nduna B and Tsidu GM (2025) Short-term impacts of wildfire on vegetation recovery, soil chemical properties and community-level physiological profiling in a savanna ecosystem of Botswana. Front. For. Glob. Change. 8:1605456. doi: 10.3389/ffgc.2025.1605456
Edited by:
Nadia S. Santini, Universidad Nacional Autónoma de México (UNAM), MexicoReviewed by:
Anaitzi Rivero-Villar, Scientific Research Center of Yucatán (CICY), MexicoEduardo Oyague, Universidad Nacional Intercultural de la Selva Central Juan Santos Atahualpa, Peru
Copyright © 2025 Marenga, Mapoka, Ultra, Rantong, Nduna and Tsidu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Willie Marenga, bXcxNzEwMDE3OUBiaXVzdC5hYy5idw==; Venecio U. Ultra, dWx0cmF2QGJpdXN0LmFjLmJ3
 Willie Marenga
Willie Marenga Mpaphidzi Mapoka
Mpaphidzi Mapoka Venecio U. Ultra1*
Venecio U. Ultra1* Batendi Nduna
Batendi Nduna