- 1Huck Institutes of the Life Sciences, Pennsylvania State University, State College, PA, United States
- 2Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA, United States
- 3Materials Research Institute, The Pennsylvania State University, University Park, PA, United States
- 4Department of Plant Pathology and Environmental Microbiology, The Pennsylvania State University, University Park, PA, United States
Beech Leaf Disease (BLD), caused by the foliar nematode Litylenchus crenatae, has emerged as a swiftly spreading forest disease across North America. This previously unknown nematode and the disease it causes have been devastating for North American beech forests, posing serious threats to biodiversity and ecosystem stability. The rapid spread of BLD and other recent forest pests serves as cautionary examples, illustrating how quickly a pathogen can establish itself in new non-native regions, leading to significant ecological and economic consequences. The destruction brought by this nematode in just a decade highlights the potentially disastrous effects of BLD if it spreads to other vital beech forest areas, particularly in European countries and China, where beech trees are essential for industries and markets that depend on such hardwood species. The loss of beech forests could compel industries to depend more on alternative raw materials that are significantly less sustainable and environmentally friendly, leading to supply chain instability and major price fluctuations. Urgent actions, such as implementing quarantine regulations for the BLD nematode, international research collaboration in areas such as breeding BLD-resistant beech trees, developing economically feasible large-scale tree treatments, and enhancing BLD screening through Artificial Intelligence—driven image—based diagnosis tools and geospatial surveillance approaches, are essential to prevent BLD from escalating into a global forest crisis.
1 Introduction- growing nematode threats to forests
Nematodes are the most abundant and widespread multicellular animals on Earth, inhabiting nearly every environment from oceans and deserts to Arctic soils, where they play vital ecological roles (Van Den Hoogen et al., 2019; Bongers and Ferris, 1999; Gardner, 2001). With an estimated over twenty thousand species and staggering population densities reaching billions per acre in some soils, these microscopic worms impact everything from nutrient cycling to environmental monitoring (Gardner, 2001; Yeates, 2007; Kantor et al., 2025). Yet, despite their ecological and economic importance, nematodes remain largely unknown outside of scientific circles. Their diversity, adaptability, and global presence make them critical to understanding ecosystem health in a changing climate.
Plant-parasitic nematodes in particular can be highly destructive, threatening food security, environmental ecosystems, and economic stability. Their resilience and ability to multiply rapidly make it extremely difficult, if not impossible, to mitigate their effects once they become established and widespread. Compounding the challenge is the fact that nematology remains a relatively under—researched field (Khan, 2012). In forestry, the subtle, late-emerging symptoms of nematode infestations, combined with the difficulty of accessing forest sites for timely damage assessment, often allow these pests to cause extensive and largely undetected destruction (Khan, 2012; Kantor et al., 2025). Considering that global forest cover has already declined by approximately 40% since the advent of agriculture over 11,000 years ago (Khan, 2012), largely due to land clearing for farming, fuel, and urban expansion, the emergence of nematodes as an additional threat to forest health adds a troubling new dimension to an already concerning trend.
One in particular, the pinewood nematode (Bursaphelenchus xylophilus), has wreaked havoc on global forestry with significant consequences for pine forests and global pinewood-related trade. The pinewood nematode is considered the causal agent of Pine Wilt Disease (PWD), which affects several species of conifers (Bergdahl, 1988). This nematode was first introduced from North America to Asia in the early twenteeth century and subsequently to Europe (Portugal), demonstrating a highly destructive capability against pine trees. In Japan, it was confirmed as the causal agent for PWD in 1971. The Japanese government started investing in control programs in 1980, but by 1984, the pinewood nematode had already inflicted severe damage, affecting about 25% of the country's pine forests, especially in coastal areas where it became an epidemic (Bergdahl, 1988). Europe followed with Finland first placing an embargo on all raw softwood shipments from North America, Japan, Canada, and other countries known to have the disease. By 1988, multiple countries had imposed or considered restrictions on the importation of coniferous wood known to have PWD, including Sweden, Norway, South Korea, (imposed) and UK, Australia. As a result, millions of dollars worth of wood trade have been lost by U.S. chipwood industries (Bergdahl, 1988).
Today, the pinewood nematode is widely distributed and its presence has been confirmed in eastern Asia (Japan, Korea, Taiwan, and China), Europe (Portugal and Spain), and North America (U.S., Canada, Mexico) (Back et al., 2024). According to the European and Mediterranean Plant Protection Organization (EPPO), the pinewood nematode is classified as a top-priority quarantine organism due to its severe pathogenic impact. Symptoms such as yellowing and reddish browning of the pine needles are visible around 3 weeks after infection by the nematode, but it can also show signs of infection after a few months (Back et al., 2024). PWD can cause tree mortality within a relatively short period (1-3 months), leading to significant economic losses, including an estimated 2 million m3 of forest being lost each year in Japan alone (Mota and Vieira, 2004; Back et al., 2024). In Korea, where pine is the dominant tree species, yearly disease management costs have varied between $6.9 and 9.5 million per year (Shin, 2008). In Portugal (Europe), the costs of trying to eradicate this disease have amounted to a staggering €80 million from 1999-−2009, without accounting for direct losses from tree mortality (Forestry Commission, 2017). North America has faced significant economic losses as well due to the Europe-imposed import ban of 1993, with estimated impacts reaching up to $150 million in the U.S. and as much as $700 million in Canada (Carnegie et al., 2018).
A century later, we face a new invasive forest nematode threat to North America's native beech forests, the Beech Leaf Disease (BLD) causing nematode, potentially originating from Asia. While different nematode species cause BLD and PWD affecting different tree species, the rapid spread and severe consequences observed with PWD highlight how quickly a nematode-tree interaction can escalate once established.
2 Background on beech leaf disease and its causal agent
About 15% of approximately 27,000 species of described nematodes are Plant-Parasitic Nematodes (PPNs) (Decraemer and Hunt, 2006; Kantor et al., 2025). In the fall of 2017, nematodes collected from diseased American beech (Fagus grandifolia) leaves and submitted by the Ohio Department of Agriculture to the USDA in Beltsville, Maryland, led to the identification of a foliar nematode, Litylenchus crenatae (L. crenatae), a species originally described in Japan (Kanzaki et al., 2019). In 2018, Ewing et al. acknowledged BLD's “rapid spread and variability in environmental conditions” and that it was unlikely that “BLD is an abiotic disorder” but “rather a disease caused by a microbe” (Ewing et al., 2018). Carta et al. (2020) were the first to provide a detailed overview of L. crenatae and connect it to BLD. Since then, multiple articles have been published (Supplementary Table S1) connecting the disease with the nematode as the causal agent (Marra and LaMondia, 2020; Kantor et al., 2022a; Vieira et al., 2023; Burke et al., 2025). Today, L. crenatae is widely viewed as the causal agent for BLD. Supplementary Table S1 outlines what we consider relevant BLD publications since Ewing's 2018 review to date (June 2025). Earlier publications focused on providing a general overview of BLD and its causal agent, L. crenatae, followed by the first reports of the disease, BLD identifications, and species descriptions. The most recent BLD research has been expanding into transmission vectors and disease distribution, plant-nematode interactions, microbiome, and management approaches (Supplementary Table S1).
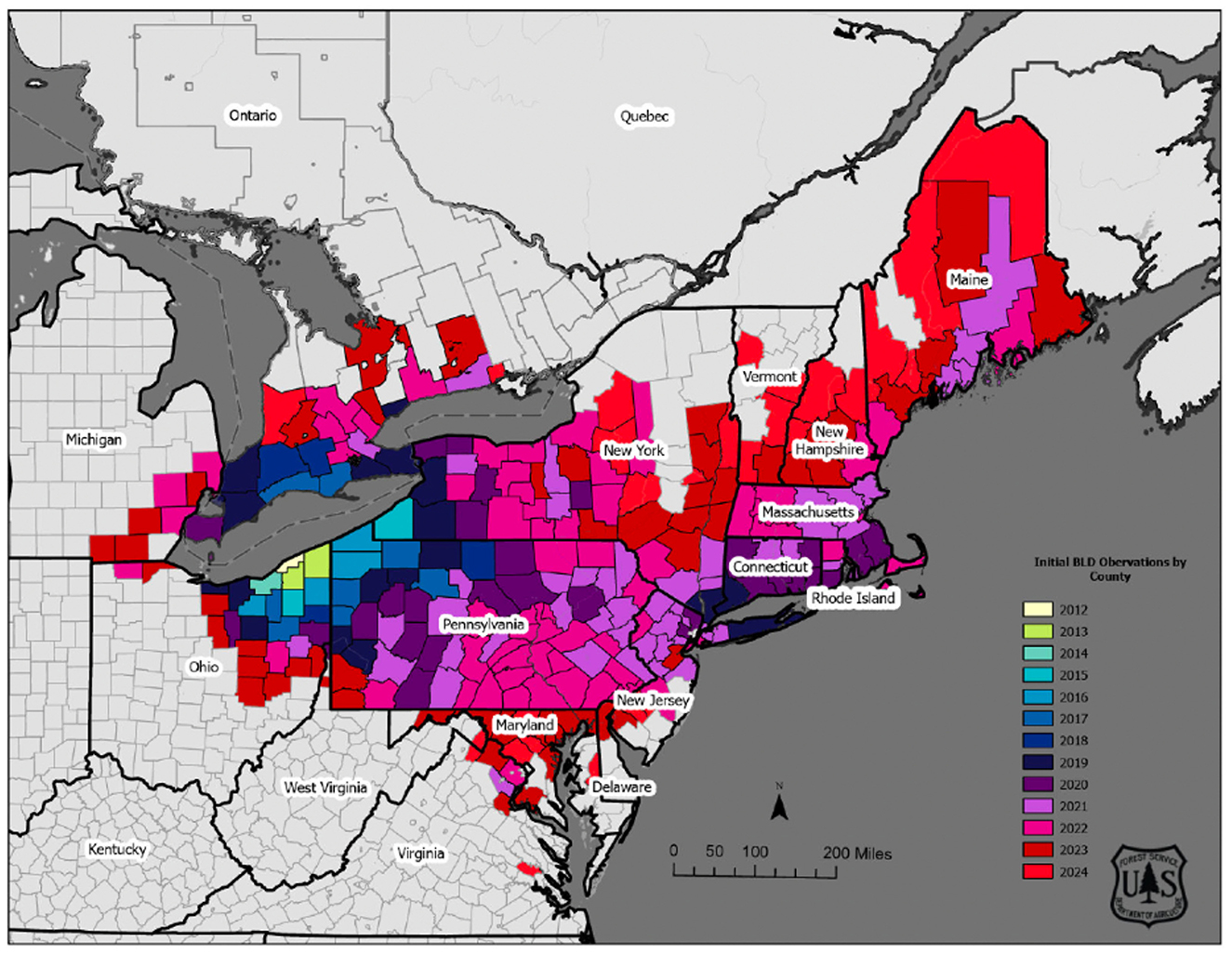
While the first reports by state/country (Connecticut, Virginia, Michigan, Ohio, Canada, New York, Maine etc.) provide the known geography of this disease and its spread, the narrative is currently incomplete as most of these reports (and other publications) tend to use data collected from nearby areas where nematology experts studying L. crenatae exist. Despite this limitation, data collected from these states, coupled with data from experimental plots (see Figure 1), provide sufficient evidence to conclude that BLD is an emerging forest crisis spreading rapidly across North America (Ewing et al., 2018; Kantor et al., 2022b). From Ohio, BLD has since expanded (Figure 1) to fifteen additional U.S. states and Ontario, Canada (Ewing et al., 2018; Zhao et al., 2023).
The fast spread of this disease (much faster than the PWD) is both unsettling and unprecedented. Based on current literature, its rapid progression can happen through multiple transmission channels, such as import of live plant or tree materials, windborne rain, humidity, or carried by insects, spiderwebs, beetles, caterpillars, birds, river streams, etc. (Carta et al., 2020; Goraya et al., 2024; Fitza et al., 2024). Unlike its co-evolved interaction with the Japanese beech, L. crenatae can induce disease in other core beech species native to Europe (F. sylvatica) and other parts of Asia (F. engleriana and F. orientalis) (Burke et al., 2025; Colbert-Pitts et al., 2025), thereby reinforcing its potential as a serious threat to global forests.
Once symptoms appear, BLD can be easily identified with the naked eye by examining the leaves (Figure 2A). Depending on the disease severity, the symptoms include interveinal darkening and swelling, crinkling, tissue necrosis, leaf curling, and irregularly thickened leaves. Diseased trees exhibit thinned crowns and branch dieback (Figure 2B) (Carta et al., 2020; Reed et al., 2020).

Figure 2. Close look at BLD-infected leaves (A). BLD-infected tree- image from under the canopy of a highly infected tree with severe leaf loss and partial branch dieback (B).
Given this nematode's rapid spread and severe impact in North America, its potential establishment in Europe and other parts of Asia could have catastrophic effects on the worldwide forest ecosystems and the beech wood-dependent economy. The following two sections explore potential ecological and economic consequences of BLD.
3 Ecological value of beech and potential impact of beech leaf disease
The diversity of species within an ecosystem often depends on the foundation species, such as dominant trees, which play a defining role (Cale et al., 2013). Beech trees, known for their longevity (up to 400 years), are vital components of old-growth forests, which harbor some of the richest biodiversity. Although the biodiversity of American beech is not widely documented, a substantial body of research on biodiversity in European beech forests is available (Brunet et al., 2010). Therefore, this paper will primarily highlight the ecological importance of beech and briefly explore key areas where beech species contribute to forest ecosystems.
In northeastern North America, the American beech (Fagus grandifolia Ehrh.) is a dominant canopy species in sugar maple-beech-yellow birch and beech-sugar maple forests (Tubbs and Houston, 1990; Cale et al., 2013; Myers et al., 2023; Shepherd et al., 2025). Less prized from an economic standpoint, the American beech trees play a critical ecological role in the northeastern forests of North America, where they are essential for maintaining ecosystem health and sustaining a wide range of species (Myers et al., 2023). Beech is a primary source of hard mast, sustaining wildlife populations. It provides crucial resources for wildlife, including food sources for pollinators and other insects, beech nuts for mammals and birds, and nesting sites (shelter) for various species. American beech masts alone support over forty wildlife species, including black bears, deer, birds, and small mammals (Jakubas et al., 2004; Storer et al., 2005).
Beech trees grow in mixed forests alongside hardwood species like sugar maple, red maple, yellow birch, black cherry, white ash, green ash, tulip poplar, and northern red oak, as well as evergreen softwood species like white pine or hemlock. These forests are home to a biodiversity of species adapted to historically old forests. Overall, beech forests are estimated to harbor as many as 10,000 species of animals [United Nations Educational Scientific and Cultural Organization (UNESCO), 2025], having a significant ecological impact. In Europe, beech (mostly Fagus sylvatica) trees (including pure beech forests and mixed stands) dominate forest ecosystems (Brunet et al., 2010) and are celebrated for their ecological and cultural significance. According to the UNESCO World Heritage, the “Ancient and Primeval Beech Forests of the Carpathians and Other Regions of Europe,” a World Heritage property spanning ninety four beech forests in eighteen countries, is on par with other global landmarks such as “the Great Barrier Reef, the Galápagos Islands, or the Grand Canyon, and cultural sites like the Taj Mahal, Machu Picchu, or Stonehenge.” Despite their dominance by a single species (i.e., F. sylvatica), these beech forests serve as critical habitats for thousands of species of flora, fauna, and fungi in Europe. By enriching soil quality and preventing erosion, these trees also contribute significantly to forest stability.
The literature primarily focuses on the European beech due to its value as a dominant species (Cesarz et al., 2013; Čerevková et al., 2021; Van Den Hoogen et al., 2019) and its economic importance as a source of timber, wood pulp, firewood, and a recreational area. As an example, Griess and Knoke (2013) demonstrated F. sylvatica's ecological value in increasing spruce's survivability when used in mixed-species stands, offering significant environmental and financial advantages even when a small amount of admixed broadleaves (below 10 % points) is used.
Beech also has a role in forest flammability, which indirectly impacts biodiversity. It is not a naturally fire-resistant tree and has a low tolerance to fire. However, beech forests rarely burn thanks to their typical forest structure. They are large, old, form a uniform stand, have a compact litter layer with low oxygen content, and minimal understory vegetation. In addition, beech is a shade-tolerant hardwood with traits such as thin leaves to maximize light capture in low-light environments (Evans and Poorter, 2001), remaining a significant component of the canopy by capturing single-tree openings formed by a fallen tree (Wiggins et al., 2004; Barden, 1980). The shade tolerance of beech trees and their ability to form dense, moisture-retaining fuel beds may help mitigate the spread of wildfires, even as abiotic change exacerbates drought conditions and fire risk.
The dense beech canopies and high shade tolerance provide the uniqueness of this specific ecosystem. Beech forests are cornerstone ecosystems that support extensive biodiversity, yet the full implications of BLD are not well understood. Very little has been published on nematode-related forest biodiversity. The existing literature primarily focuses on the positive role of nematodes in breaking down organic matter and regulating soil microbial communities, as well as their role in managing carbon and nutrient cycling across ecosystems, and serving as reliable indicators of biological activity in soils (Van Den Hoogen et al., 2019).
Because nematodes possess an extraordinary ability to adapt to a wide range of environments, they have an evolutionary advantage for the survival and development of their species. Their feeding in particular has negative impacts on forest health. As PPNs feed mainly on plant roots, they cause damage that impairs the plant's ability to absorb water and nutrients from the soil (Bernard et al., 2018; Kantor et al., 2024).
As the primary organism linked to BLD (Carta et al., 2020), L. crenatae's devastating impact on North American beech forests combined with the apparent absence of natural resistance in F. grandifolia has raised significant concerns about its potential to spread to other regions worldwide (Vieira et al., 2023; Kantor et al., 2024). Most recently, Colbert-Pitts et al. (2025) demonstrated the high reproductivity of L. crenatae in F. sylvatica by showing its ability to impair leaf function and reduce overall tree performance and forest health. These recent findings elevate the risk of BLD as a global forest threat from a theoretical concern to a tangible, emerging global threat to European forest ecosystems. If inadvertently spread to the native regions of F. sylvatica, BLD will threaten this intricate web of biodiversity associated with abiotic variables, and it is likely to extend its range further and exacerbate infection rates. The resulting ecological impacts could be profound and widespread.
4 Economic importance of beech and potential impact of beech leaf disease
The decline of beech forests can also have far-reaching economic implications. This paper considers three parameters for beech trees' monetary value, namely: hardwood industry, carbon sequestration, and a less common parameter in the economy of beech- its value to the textiles industry.
As the number of infected trees increases, the hardwood products industry could face significant financial losses. In Europe, particularly in central, eastern, and southeastern regions, beech wood holds substantial commercial value, and it is one of the most important commercial hardwood species, valued for its physical and mechanical properties. Beech wood has around 250 known uses and is one of Europe's most diversely utilized tree species (Durrant et al., 2016). However, since feudal times, many of these ancient European beech forests have been converted into agricultural land for anthropogenic purposes. The Carpathian Mountains are the second-largest mountain range and home to Europe's most extensive old-growth and virgin temperate forests, primarily composed of beech and mixed beech forests (Kholiavchuk et al., 2023). The European beech dominates the Carpathian forests, occupying over 53%, followed by Norway spruce (30%), oak 15% and silver fir 2.4% (Kholiavchuk et al., 2023).
In the U.S., beech wood is considered to have low but not insignificant economic value. It is used for fuel and as a building material (e.g., flooring, furniture) (Shepherd et al., 2025). The estimated economic and environmental cost of the loss of beech in Ohio alone is $225 million. Beech constitutes more than 25% of the forests in Vermont. Sugar maple/beech/yellow birch is the most significant single forest type in Pennsylvania, at 3.1 million acres, accounting for 59% of the area in the maple/beech/birch forest-type group and 18% of forest land overall (USDA Forest Service Cleveland Metroparks, 2024).
Since BLD research has been rapidly expanding over the past decade, several reports of BLD-related beech mortality have been published based on localized plot assessments in the U.S. and Canada (Shepherd et al., 2025; Reed et al., 2022). Recently, Reed et al. (2022) surveyed 646 live American beech trees from seventeen locations in Ontario, Canada, and two hundred and forty eight live American beech trees from the U.S. BLD was identified in fourteen of these seventeen Canada locations, with twenty five of the thirty four plots having trees with symptoms in seventeen of the thirty U.S. plots. Interestingly, the authors remark that BLD symptoms were already more widely distributed than another beech pest, the Beech Bark Disease (BBD), that has been first detected decades before BLD (McLaughlin and Greifenhagen, 2012; Cale et al., 2017; Shepherd et al., 2025). Comparatively, BBD is caused by a non-native scale insect and a fungal pathogen (Witter et al., 2004), and has been documented to kill up to 50% of trees within 10 years, with higher rates recorded in certain areas (Kibbe and Bonello, 2023). Long-term monitoring at these sites near the origin of BLD showed a substantial rise in American beech mortality over the 9 years following the disease's arrival, compared to pre-BLD conditions. While direct causation could not be conclusively established in this 2022 study, the analysis revealed a strong correlation between the emergence of BLD and rising mortality rates. Notably, beech death increased exponentially after the disease appeared, with saplings being the most susceptible. In addition, tree growth rates within the study plots declined significantly after BLD was first detected (Reed et al., 2022).
In a recent study (Shepherd et al., 2025), the sharp increase in American beech mortality in Ohio study sites, especially from 2021 to 2023, coincides with a noticeable decline in tree growth. Of the 263 trees tracked between 2011 and 2023, 29.6% died, with most (96%) after BLD was first reported in 2014, with mortality accelerating exponentially. Growth, measured by percent change in Diameter At Breast Height (DBH), also declined significantly. From 2011 to 2016, before BLD became widespread, the average growth rate was 1.77%, but dropped to just 0.46% between 2017 and 2022 (p < 0.01) (Shepherd et al., 2025).
This reduction in growth, combined with high mortality rates, signals a serious threat to the ecological and commercial value of beech trees. During the pre-BLD period, soil treatments influenced growth, with control and TSP plots showing higher rates than those treated with lime. However, once BLD symptoms became prevalent, soil amendments no longer had a meaningful effect (Shepherd et al., 2025). This suggests that BLD overrides typical soil-related growth dynamics, posing long-term risks for forest productivity.
Most recently, Canada has been making efforts to highlight the economic potential of beech as a more affordable alternative to birch in the wood flooring market, compelling the hardwood flooring industry to adapt by incorporating American beech into wood supplies for sawmills in western Quebec (Bernard et al., 2018; Myers et al., 2023). The introduction and spread of L. crenatae pose a growing threat to the beech supply chain in Canada, particularly in Ontario. Between 2010 and 2019, approximately 47% of all beech products imported into Canada were live trees intended for propagation, a known high-risk pathway for nematode transmission. Notably, over 90% of all beech imports originated from the United States, with the remainder coming from European countries including Italy, Germany, the Netherlands, and Poland. Of the live beech tree imports, 88.63% came from the U.S., while 11.36% originated from the Netherlands, with Ontario receiving imports from both sources. Within Ontario, nursery surveys revealed that most beech seedlings are sourced from western North America, primarily Oregon, British Columbia, and Washington, before being cultivated and sold locally. Genetic analyses confirmed the presence of a genetically uniform nematode population across sampled locations, consistent with a recent, single-source introduction. The distribution of BLD symptoms in Ontario, concentrated around early detection sites and decreasing radially, suggests a temporal and spatial gradient of spread, likely exacerbated by nursery trade routes and environmental conditions favorable to nematode development (Fitza et al., 2024). This pattern highlights the urgent need for coordinated monitoring of nursery stock movements and nematode populations to prevent further disruption to the beech supply chain and to limit economic and ecological losses.
Carbon sequestration
While beech trees are not the most efficient carbon assimilators when compared to other deciduous trees, they have long lifespans, large biomass, and they grow very tall (up to 130 feet tall and 5 feet wide), making them significant carbon sinks during their lifetime (Fraser et al., 2023). According to information from the Forest Ecology Network, maple/beech/birch stands have the highest carbon density per acre, storing approximately 550 million metric tons. In terms of carbon sequestration function, the average value of a pure beech stand is about $320/ha/year (Badeban et al., 2015).
Recent studies (Fletcher et al., 2024) have shown that BLD can lower the carbon assimilation rate, with trees exhibiting a progressive increase in carbon allocation to symptomatic leaves. This response, coupled with decreased leaf gas exchange essential for photosynthesis, depletes stored carbon, compromising the tree's long-term growth potential and, as a result, carbon sequestration.
Beech trees also play a vital role in the textile industry, serving as a primary source of cellulose for producing viscose fibers. Beech trees contain around 40-50% cellulose (Fengel and Wegener, 2011). The high cellulose content and specific wood structure of beech trees make them an ideal raw material for manufacturing strong, soft, and durable viscose fibers. The viscose industry relies heavily on beech trees as a sustainable and renewable source of cellulose, providing employment opportunities and generating significant revenue for the textile industry.
The global textile market remains dominated by cotton (25%) and polyester (55%), both of which have significant drawbacks (Figure 3). Cotton requires vast amounts of water, leading to aquifer depletion and limited new land, which suggests its market share may drop to 23-24% in the next decade (Chapagain et al., 2006). Polyester, being an oil-based material, consumes high energy and contributes to plastic pollution, with microfibers released during washing contaminating oceans and land. The remaining 20% of the market comprises various materials, with Man-Made Cellulosic Fibers (MMCF) experiencing rapid growth (8%). Derived from dissolving wood pulp (DWP) from hardwoods like Eucalyptus, Birch, Aspen, Acacia, Maple, and Beech, or softwoods such as Pine, Spruce, Fir, Hemlock, and Larch, these fibers are processed into viscose, modal, lyocell, or acetate, offering softness, breathability, and moisture-wicking properties. MMCFs are biodegradable, renewable, and require less water and energy than other fibers.
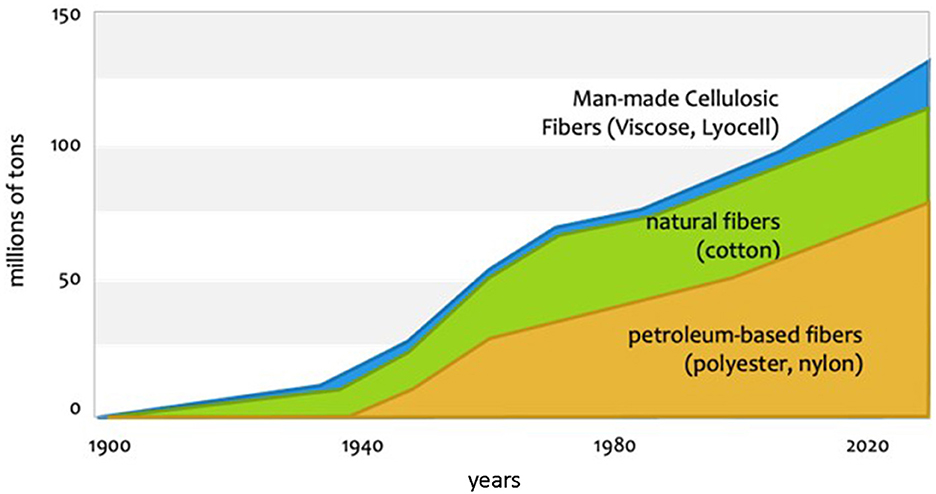
Figure 3. Global textile production for major fibers over the last century shows the dominance of cotton and polyester, while man-made cellulosic fibers are experiencing significant growth as well.
Cellulose is primarily derived from wood pulp and cotton, making its production costs highly sensitive to the availability and pricing of these raw materials. Disruptions in timber supply can cause sharp increases in cellulose prices. In Europe and Asia, beech trees are integral to the production of high-quality viscose fibers, a more sustainable alternative to cotton and polyester. Beech tree-derived viscose fibers are used to produce a wide range of textiles, including clothing, upholstery, and industrial fabrics. The decline of beech trees could disrupt cellulose supply chains, leading to price volatility and increased reliance on less sustainable materials such as petroleum-based fibers.
Trade policies, tariffs, and political instability in countries that produce cellulose can further contribute to price volatility. Moreover, changes in global oil prices and inflation can affect both the cost of manufacturing and transporting cellulose. The U.S. is a net importer of wood pulp, making it vulnerable to any disruptions to the supply chain. All of North America's manufacturing contribution is 15% while the manufacturing of cellulosic products is primarily concentrated in Asia. Any supply chain and trade disruptions may lead to a reduction in cellulose production. Over the past few decades, the prices of dissolving wood pulp (DWP) have consistently risen; however, the global market experiences significant fluctuations due to major events. For instance, a cotton harvest failure propelled the price to $1900/t in the 2010s, while COVID-19 caused it to plummet to $600/t in 2020. Assuming the current price of $1000/t for 0.425 Mt/yr of North American DWP, which represents approximately 6% of the global production of 7 million tons, the market is valued at $425 million annually. Its global market value in 2025 is $8,328 million, and is projected to experience further growth [Bulk Chemicals Dissolving Wood Pulp (DWP) Analysis Report, 2025]. The price may be influenced in the future by the decline of beech trees caused by BLD; however, it is almost impossible to forecast the level of such impact because the market remains highly complex with an integrated global supply chain. While beech trees serve purposes in timber and pulp production, the price of timber is set at $20-40/t, which is significantly less than that of DWP. Assessing the actual economic impact presents significant challenges, warranting a separate review of this subject.
5 Current gaps and potential solutions for managing BLD
5.1 Economic feasibility of existing treatments
Research into the early detection, mitigation, and management of BLD is advancing rapidly in response to its swift spread. Recent studies in laboratory and private field-grown beech nurseries from Ohio have shown promising results on BLD control. For example, foliar applications of fluopyram reduced live nematode counts by over 90% in European beech trees (Loyd et al., 2024). In addition, drawing on previous success with chemical injectable treatments for other tree diseases, such as Dutch elm disease and sycamore anthracnose, researchers have successfully tested thiabendazole and other benzimidazole compounds on symptomatic BLD beech as root flare injections (Loyd et al., 2024, 2025). These compounds offer new modes of action and application techniques to reduce nematode infection, particularly for large or tall beech trees, where foliar treatments may be impractical or where minimizing environmental exposure is crucial (Loyd et al., 2024).
However, both types of treatments for BLD present significant challenges for large-scale forest management due to high costs, logistical constraints, and potentially adverse effects on the forest environment and associated biodiversity. For example, Arbotect 20-S is the current industry standard fungicide used to treat individual trees. However, its large-scale application is not currently economically feasible. The macroinjection treatment with Arbotect 20-S used to provide therapy to beech with BLD requires specialized equipment (~$730 for a kit), significant time to uptake solution (on average 45-120 m per tree), and trained personnel to administer the treatment. This product must be diluted in high-quality deionized or distilled water to avoid precipitation and inactivity of the active ingredient. The uptake time depends on the evapotranspiration rates of the tree since the injection solution volume is significant (i.e., 50 oz/in DBH). For example, a 20 “DBH tree would require about 8 gallons of solution to be taken up and translocated throughout the canopy. The cost of this treatment can vary based on several factors, including the condition of the tree, environmental conditions that affect uptake time, the region of the country, and the number of trees being treated, among others. Anecdotally, the cost of treating an individual tree could range anywhere from $15 to $35/per DBH, so a 20” tree would range in price from $300 to $700 according to sources within the arboriculture industry.
In time, these treatments may become more economically feasible and could form the foundation for efficient nematode management. However, at present, they are not a viable solution for addressing BLD on a large scale.
5.2 Lessons learned from the past
Lessons from the management of another beech forest disease, the Beech Bark Disease (BBD), could offer valuable insights for BLD management. BBD, which was caused by an introduced insect-disease complex involving the soft-bodied scale Cryptococcus fagisuga and species of Neonectria fungi, led to severe degradation of beech forests, transforming them into stunted stands with diminished commercial and ecological value (Loo et al., 2005). Despite extensive research and development efforts, the eradication of BBD proved unfeasible in large natural areas because Neonectria cannot functionally be removed, scale insects disperse widely after arrival, can hide in the bark crevices, and reproduce clonally (Myers et al., 2023). There are also other labor, financial, and environmental constraints (Wiggins et al., 2004). Management strategies focused on biological, chemical, physical, and even cultural controls proved successful only on a small scale, requiring repeated treatments (Dracup and MacLean, 2018; Myers et al., 2023). Initial cultural efforts focused on reducing beech thickets by removing understory beech trees to support other healthy tree species' development or completely removing overstory BBD-susceptible F. grandifolia, followed by herbicide applications (Ostrofsky and McCormack, 1986), were replaced with efforts to collect genetic material from BLD-resistant beech from larger geographic areas and breeding genetically diverse BLD-resistant beech (Reed et al., 2022).
The regenerative capacity of beech trees, which can reproduce both asexually (via root or stump sprouts) and sexually, supports the potential for breeding programs aimed at developing resistance to pests (Loo et al., 2005). So far, conventional vegetative propagation techniques have not been very successful with American beech. Although this species commonly exhibits root sprouting after disturbance, efforts to root cuttings and employ micropropagation have achieved only limited success, with challenges in subsequent plant development, particularly during overwintering (Loo et al., 2005) and reliable transfer to soil (Hazubska-Przybyl et al., 2015).
Advanced biotechnological methods offer promising alternatives for conservation, particularly for tree species that suffer from poor seed sets, low germination rates, recalcitrant seeds, and a reliance on vegetative propagation. Techniques for the in vitro medium-to-long-term storage of plant embryonic axes, pollen, and propagules are increasingly applied in tree species conservation (Yadav et al., 2024). In this context, developing BLD-resistant germplasm through biotechnology holds potential for future management efforts. The overall success of plant biotechnology projects largely depends on the ability to regenerate whole plants from in vitro cultures (Benson, 2000). Benson (2000) defines in vitro recalcitrance as the general inability of plant cells, tissues, and organs to respond to tissue culture manipulations. Although this concept is broadly applicable, beech trees are particularly challenging because of their slow growth and the inherent difficulties in initiating and maintaining tissue cultures. This recalcitrance complicates the development of reliable in vitro propagation protocols for beech, thereby lengthening the process of breeding and restoration programs. For comparison, early efforts by Koch and Carey (2014) to survey for BBD-resistant beech candidates and produce resistant—cross seed are promising but take a significant amount of time. As a result, while an up-and-coming future solution, breeding for BLD resistance is still not a viable option for this disease's short to medium-term management.
6 Future directions in BLD research and management
6.1 Early detection and research priorities
The rapid spread of BLD in North America signals the potential for similar outbreaks in other beech-growing regions of the world, including Europe and parts of Asia such as China. Large-scale management remains challenging due to the absence of effective forest—wide treatments. Until practical biological, chemical, physical, and cultural solutions become available, early detection, diagnostics, prevention, and continued investment in research and expertise (especially nematology, plant pathology, biotechnology, and geospatial sciences) remain the most viable approaches for managing the disease. Priorities include identifying susceptible tree populations, conducting field surveys, and investigating host-pathogen interactions, disease progression, and biotic and abiotic factors. These foundational insights will inform science-based strategies to mitigate beech-related ecological and economic damage.
6.2 Advancing genomic tools and resistance breeding
Traditional forest tree breeding has improved growth and resistance in tree species, but its progress is constrained by the low pace of natural selection and the limits of existing genetic diversity (Naidoo et al., 2019). Advances in genomic technologies, especially CRISPR and host-induced RNA interference (RNAi), may offer precise tools to introduce targeted genetic changes, overcoming these inherent constraints. Recent efforts to sequence the genome of L.crenatae mark a promising step toward understanding its biology, though annotations and full release are still pending. Wolf and Vieira (2024) characterized for the first time the bud scale morphology of two different cultivars of F. sylvatica infected with L. crenatae. Their work provided insight into the dynamic processes occurring during L. crenatae infection (Wolf and Vieira, 2024). Comparative insights from nematodes like B. xylophilus (pine wood nematode), whose genome has been available since 2011 (Kikuchi et al., 2011), show how genomic tools can facilitate studies of parasitism, population structure, and virulence (Palomares-Rius et al., 2015). In agriculture, technologies such as CRISPR/Cas-based genome editing have already shown success in creating nematode-resistant crops by targeting susceptibility genes—for example, OsHPP04 in rice (Huang et al., 2023) and GmSNAP02 in soybeans (Usovsky et al., 2023). In addition to CRISPR/Cas, additional studies reported the successful use of RNAi-based approaches to control nematode infections by targeting various classes of genes (Joshi et al., 2022). In forestry, Cao et al. (2024) provides a detailed overview of CRISPR applications and its potential to enhance disease resistance (Cao et al., 2024). These developments highlight the potential for similar strategies in forest species.
For BLD specifically, researchers are exploring genetic resistance in F. grandifolia aided by molecular markers and observations of “lingering” beech trees that remain relatively healthy in areas heavily affected by BLD Holden Forests and Gardens.Beech Leaf Disease. Marker-assisted selection (MAS) could accelerate the development of resistant germplasm (Collard and Mackill, 2008). This approach has been effective in other tree species. Additional lessons can be learned from the management of the BBD, where clusters of disease—free American beech have been observed in areas heavily impacted by BBD, suggesting the existence of heritable resistance traits (Koch et al., 2007). When resistant tree clusters lack sufficient variation for long-term success (Duval et al., 2025), planting disease—resistant trees with high genetic diversity may help overcome inbreeding limitations.
6.3 Geospatial technologies for BLD monitoring and modeling
When sufficient manpower and expertise are not available, geospatial technologies offer scalable solutions for early monitoring and BLD modeling. Geospatial methodologies, tools, and solutions have been successfully applied in forestry for monitoring tree growth, forest health, disease progression, and pest severity (Das et al., 2024). Remote Sensing (RS), which involves detecting and monitoring an area's physical characteristics by measuring its reflected and emitted radiation from satellite or aircraft, has already proven effective in differentiating nematode infestation from drought stress at different time intervals, allowing for early disease detection and predictive modeling of nematode infestations (Kantor et al., 2025; Susič et al., 2018). Since BLD triggers characteristic anatomical and physiological differences between asymptomatic and symptomatic leaves (Ewing et al., 2018), these differences can lead to distinct spectral signatures, such as changes in color (dark green interveinal banding) and shape (crinkling). Hyperspectral Imaging (HSI), a method of detection from a distance that captures data across numerous spectral bands, can provide a detailed analysis of plant health based on these parameters (Kantor et al., 2025). Multispectral imaging, although less detailed than HIS (uses fewer spectral bands), can also effectively identify stress responses in plants (Kuska et al., 2022).
In the United States, the U.S. Department of Agriculture (USDA) Forest Service actively monitors and maps the spread of BLD through systematic field surveys conducted at monitoring plots. These observations, analyzed through Geographic Information Systems (GIS) platforms, support early detection, change detection, and development of spatial risk models (Das et al., 2024; Zhao et al., 2023; Fearer et al., 2022; Goraya et al., 2024). In addition to identifying infection spots, forest service specialists assess key parameters such as Live Crown Ratio (LCR), Diameter at Breast Height (DBH), canopy density, discoloration, defoliation, dieback, branch breakage, tree mortality, etc. This integrated monitoring system provides a replicable framework for other regions at risk.
6.4 Community engagement and public surveillance
Given that BLD symptoms are relatively easy to identify because of their unique characteristics (i.e., dark interveinal banding on their leaves), efforts to increase awareness at the community level could further aid in early disease detection. Incorporating BLD identification into youth camps, school curricula, and other forestry education programs could empower volunteers to participate in surveillance and data collection. Public engagement not only facilitates data ground truthing but also strengthens early warning systems and fosters broader community awareness of forest health threats.
6.5 Artificial intelligence (AI) and machine learning (ML) for BLD diagnostics
As more data becomes available, the implementation of artificial intelligence (AI)-based methods such as Machine Learning (ML), Deep Learning (DL), and Convolutional Neural Networks (CNNs) will offer scalable solutions for rapid, image-based disease identification, and outbreak prediction. These technologies can detect subtle visual cues in symptomatic trees and integrate sensor-based and historical data for predictive modeling of BLD outbreaks. AI offers speed, scalability, early detection, and reduced chemical reliance (Tammina et al., 2024), advantages over traditional visual or microscopic diagnostic methods. However, current limitations include the lack of high-quality, labeled datasets for training models, insufficient computational infrastructure, and gaps in cross-disciplinary collaboration between nematologists and computer scientists. Addressing these challenges will be essential to unlocking the full potential of AI/ML in nematode diagnostics.
7 International research collaboration to prevent BLD global spread
The rapid spread of BLD in North America emphasizes the need for international research and biosecurity cooperation to prevent its introduction elsewhere. Although BLD is currently listed on the European and Mediterranean Plant Protection Organization (EPPO) Alert List, it has not yet been added to the quarantine list, leaving European forests vulnerable to inadvertent transmission. Europe has taken some precautionary steps, including the Plant health status of Fagus spp. (FAGUSTAT) project undertaken as part of the Euphresco initiative, which surveyed the health status of Fagus spp. across six countries in 2021(Belgium, the Netherlands, Romania, Slovenia, the United Kingdom, and Ireland). While the surveys found no evidence of L. crenatae, they highlighted both the effectiveness of early surveillance and the practical challenges of international research collaborations, such as the inability to acquire viable inoculum or culture the nematode under laboratory conditions (Viaene et al., 2022). These limitations hinder the study of BLD's pathogenicity and life cycle in European contexts. Furthermore, climate similarities between countries like Ireland and affected areas in the U.S. and Canada (Bourkea and McGeea, 2024) heighten the risk of BLD establishment if the disease were introduced. Although EU regulations restrict the import of Fagus plants from non-EU countries, the nematode's ability to overwinter in dormant buds and the possibility of a broader, unidentified host range pose residual risks, particularly if asymptomatic infections go undetected. These concerns make international research and policy-making collaborations essential for understanding BLD's phytopathology and mitigating its transmission potential. Ongoing meetings and research collaborations between North American and European researchers can foster timely knowledge exchange, ensuring coordinated responses to such emerging threats (Viaene et al., 2022). Future inclusion of BLD on the EPPO quarantine list could help standardize surveillance, improve early detection systems, and support stronger containment protocols. As shown in Figure 4, the consequences of inaction are significant: BLD threatens biodiversity, disrupts ecosystems, and impacts key economic sectors. Given limited large-scale treatment options and low natural resistance in beech trees, investments in advanced diagnostic tools such as genomics, AI-based imaging, and geospatial modeling alongside breeding programs for resistant cultivars are crucial. Ultimately, a unified international strategy combining regulatory, scientific, and technological efforts is necessary to safeguard global beech forests from destructive threats such as BLD.
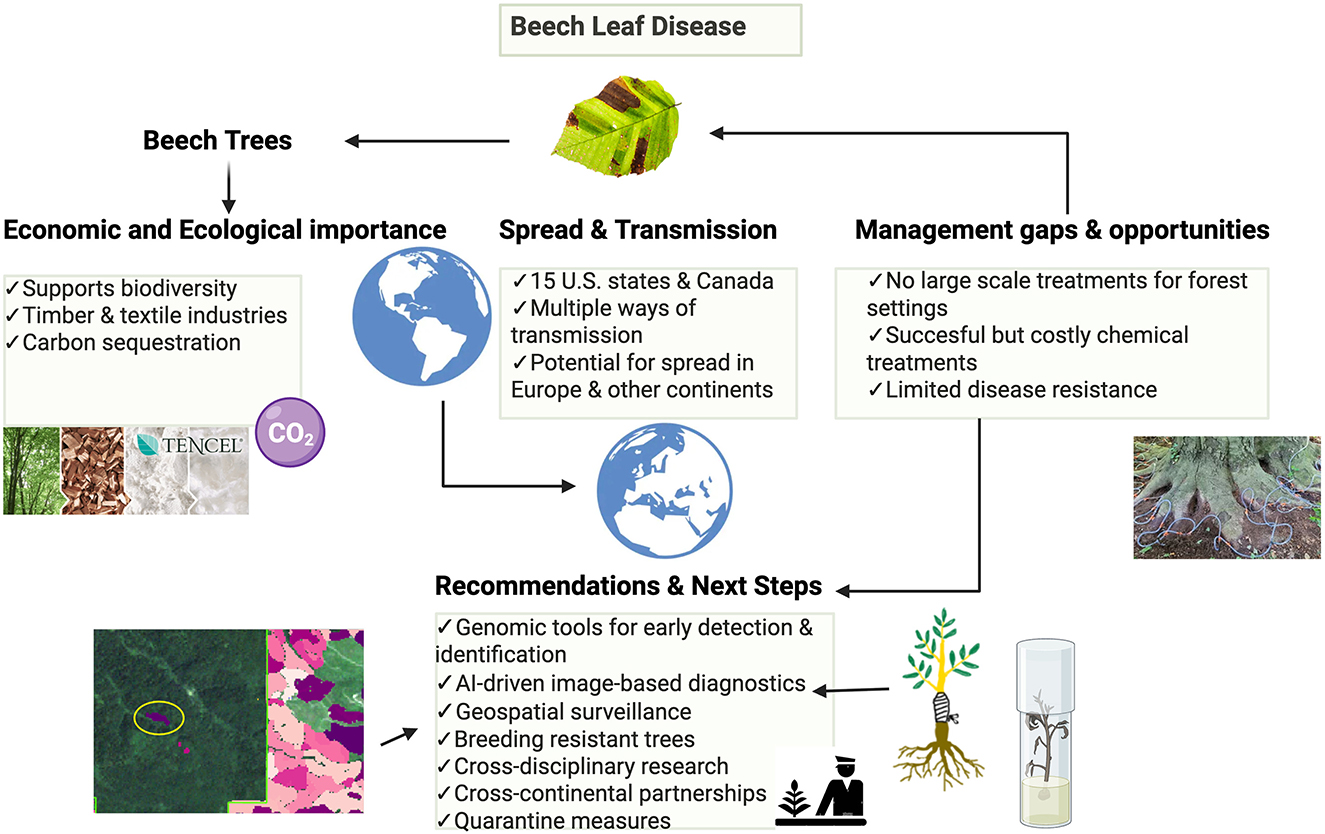
Figure 4. Beech leaf disease: threats, transmission and strategic responses. Created with biorender.com.
Author contributions
CK: Writing – review & editing, Writing – original draft, Methodology, Visualization, Validation, Data curation, Supervision, Conceptualization. MD: Writing – review & editing, Writing – original draft, Data curation, Validation, Methodology. MK: Writing – review & editing, Data curation, Writing – original draft, Validation, Methodology, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially supported by the International Programs of the U.S. Forest Service, Department of Agriculture, under the 23-JV-11132762-200 (MK), by the McIntire-Stennis Cooperative Forestry Program, and the U.S. Department of Agriculture's National Institute of Food and Agriculture (NIFA) project no. PEN05052 (MK) and the NIFA Federal Appropriations under project PEN04828 (MK). MD thanks the Huck Endowment at Pennsylvania State University for the support.
Acknowledgments
We thank Kathleen Demchak for her valuable advice, which prompted further investigation into BLD's impact on textiles. We also express our gratitude to Dr. Sergey Malkov for the helpful discussion on man-made cellulosics. Also, we would like to acknowledge the valuable input received from Paulo Vieira (USDA-ARS) during the initial draft of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1606260/full#supplementary-material
References
Back, M. A., Bonifácio, L., Inácio, M. L., Mota, M., and Boa, E. (2024). Pine wilt disease: a global threat to forestry. Plant Pathol. 73, 1026–1041. doi: 10.1111/ppa.13875
Badeban, Z., Mashayekhi, Z., Zebardast, L., and Mobrghee, N. (2015). Economic valuation of carbon sequestration function in the mixed and pure beech stands (case study: Kheyrud forests). Environ. Res. 5, 147–156.
Barden, L. S. (1980). Tree replacement in a cove hardwood forest of the southern Appalachians. Oikos 35, 16–19. doi: 10.2307/3544722
Benson, E. E. (2000). Special symposium: In vitro plant recalcitrance in vitro plant recalcitrance: an introduction. In Vitro Cell. Dev. Biol. Plant 36, 141–148. doi: 10.1007/s11627-000-0029-z
Bergdahl, D. R. (1988). Impact of pinewood nematode in North America: present and future. J Nematol. 20, 260–265.
Bernard, A., Gélinas, N., Duchateau, E., Durocher, C., and Achim, A. (2018). American beech in value-added hardwood products: Assessing consumer preferences. BioResources, 13, 6893–6910. doi: 10.15376/biores.13.3.6893-6910
Bongers, T., and Ferris, H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224–228. doi: 10.1016/S0169-5347(98)01583-3
Bourkea, A., and McGeea, C F. (2024). Department of agriculture, food and the Marine, Ireland. express pest risk analysis: Litylenchus crenatae subsp. Mccannii, Conor Francis McGee.
Brunet, J., Fritz, Ö., and Richnau, G. (2010). Biodiversity in European beech forests-a review with recommendations for sustainable forest management. Ecol. Bull. 53, 77–94.
Bulk Chemicals Dissolving Wood Pulp (DWP) Analysis Report (2025). Market to grow by a CAGR of 2.6 to 2033, Driven by Government incentives, Popularity of Virtual Assistants, and Strategic Partnerships. Available online at: https://www.archivemarketresearch.com/reports/dissolving-wood-pulp-dwp-78033#. (Accessed June 15, 2025)
Burke, D. J., Colbert-Pitts, M., Macy, T., Carrino-Kyker, S. R., and Martin, D. (2025). The presence and distribution of nematode Litylenchus crenatae ssp. mccannii, the causative agent of beech leaf disease, in forest stands across Ohio. Environ. Monit. Assess. 197:478. doi: 10.1007/s10661-025-13886-x
Cale, J. A., Garrison-Johnston, M. T., Teale, S. A., and Castello, J. D. (2017). Beech bark disease in North America: over a century of research revisited. For. Ecol. Manage. 394, 86–103. doi: 10.1016/j.foreco.2017.03.031
Cale, J. A., McNulty, S. A., Teale, S. A., and Castello, J. D. (2013). The impact of beech thickets on biodiversity. Biol. Invasions 15, 699–706. doi: 10.1007/s10530-012-0319-5
Cao, H. X., Michels, D., Vu, G. T. H., and Gailing, O. (2024). Applications of CRISPR technologies in forestry and molecular wood biotechnology. Int. J. Mol. Sci. 25:11792. doi: 10.3390/ijms252111792
Carnegie, A. J., Venn, T., Lawson, S., Nagel, M., Wardlaw, T., Cameron, N., et al. (2018). An analysis of pest risk and potential economic impact of pine wilt disease to Pinus plantations in Australia. Aus. For. 81, 24–36. doi: 10.1080/00049158.2018.1440467
Carta, L. K., Handoo, Z. A., Li, S., Kantor, M., Bauchan, G., McCann, D., et al. (2020). Beech Leaf Disease symptoms caused by newly recognized nematode subspecies Litylenchus crenatae mccannii (Anguinata) described from Fagus grandifolia in North America. For. Pathol. 50:e12580. doi: 10.1111/efp.12580
Čerevková, A., Renčo, M., Miklisová, D., and Gömöryová, E. (2021). Soil nematode communities in managed and natural temperate forest. Diversity 13:327. doi: 10.3390/d13070327
Cesarz, S., Ruess, L., Jacob, M., Jacob, A., Schaefer, M., and Scheu, S. (2013). Tree species diversity versus tree species identity: driving forces in structuring forest food webs as indicated by soil nematodes. Soil Biol. Biochem. 62, 36–45. doi: 10.1016/j.soilbio.2013.02.020
Chapagain, A. K., Hoekstra, A. Y., Savenije, H. H., and Gautam, R. (2006). The water footprint of cotton consumption: an assessment of the impact of worldwide consumption of cotton products on the water resources in the cotton producing countries. Ecol. Econ. 60, 186–203. doi: 10.1016/j.ecolecon.2005.11.027
Colbert-Pitts, M., Kantor, M. R., Jansen, A., Burke, D. J., and Vieira, P. (2025). Cellular dynamics of Beech Leaf Disease on Fagus sylvatica. Plant Pathol. 74, 1389–1406. doi: 10.1111/ppa.14101
Collard, B. C., and Mackill, D. J. (2008). Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Transact. R. Soc. B Biol. Sci. 363, 557–572. doi: 10.1098/rstb.2007.2170
Das, P., Rahimzadeh-Bajgiran, P., Livingston, W., McIntire, C. D., and Bergdahl, A. (2024). Modeling forest canopy structure and developing a stand health index using satellite remote sensing. Ecol. Inform. 84:102864. doi: 10.1016/j.ecoinf.2024.102864
Decraemer, W., and Hunt, D. J. (2006). “Structure and classification,” in Plant Nematology. CABI Wallingford: UK. 3-32. doi: 10.1079/9781845930561.0003
Dracup, E. C., and MacLean, D. A. (2018). Partial harvest to reduce occurrence of American beech affected by beech bark disease: 10 year results. For. Int. J. For. Res. 91, 73–82. doi: 10.1093/forestry/cpx033
Durrant, T. H., de Rigo, D., and Caudullo, G. (2016). “Fagus sylvatica in Europe: distribution, habitat, usage and threats,” in European Atlas of Forest. Tree Species, eds. J. San-Miguel-Ayanz, D. Rigo, G. Cadullo, T. Houstan Durant and A. Mauri (Office of the European Union: Luxembourg), 94–95.
Duval, H., Heurtevin, L., Dlalah, N., Caravel, C., Callot, C., and Van Ghelder, C. (2025). Identification and expression of the peach TNL RMia genes for the resistance to the root-knot nematode, Meloidogyne incognita. Sci. Hortic. 343:114081. doi: 10.1016/j.scienta.2025.114081
Evans, J., and Poorter, H. J. P. C. (2001). Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 24, 755–767. doi: 10.1046/j.1365-3040.2001.00724.x
Ewing, C. J., Hausman, C. E., Pogacnik, J., Slot, J., and Bonello, P. (2018). Beech leaf disease: an emerging forest epidemic. Forest Pathol. 49:12488. doi: 10.1111/efp.12488
Fearer, C. J., Conrad, A. O., Marra, R. E., Georskey, C., Villari, C., Slot, J., et al. (2022). A combined approach for early in-field detection of beech leaf disease using near-infrared spectroscopy and machine learning. Front. For. Glob. Change 5:934545. doi: 10.3389/ffgc.2022.934545
Fengel, D., and Wegener, G. (2011). Wood: Chemistry, Ultrastructure, Reactions 2nd Edn. Walter de Gruyter, Berlin.
Fitza, K. N., Allison, J., Slippers, B., Chingandu, N., and Reed, S. E. (2024). Diversity and potential sources of introduction of the beech leaf nematode (Litylenchus crenatae mccannii) to Ontario, Canada. Can. J. Plant Pathol. 46, 356–366. doi: 10.1080/07060661.2024.2312150
Fletcher, L. R., Borsuk, A. M., Fanton, A. C., Johnson, K. M., Richburg, J., Zailaa, J., et al. (2024). Anatomical and physiological consequences of beech leaf disease in Fagus grandifolia L. For. Pathol. 54:e12842. doi: 10.1111/efp.12842
Forestry Commission (2017). Contingency plan for the pine wood nematode (Bursaphelenchus xylophilus) and its longhorn beetle (Monochamus spp.) vectors. Available online at: https://cdn.forestresearch.gov.uk/2022/02/pwncontingencyplan21november2017.pdf (Accessed June 7, 2025).
Fraser, J. S., Knapp, L. S. P., Graham, B., Jenkins, M. A., Kabrick, J., Saunders, M., et al. (2023). Carbon dynamics in old-growth forests of the Central Hardwoods Region, USA. For. Ecol. Manag. 537:120958. doi: 10.1016/j.foreco.2023.120958
Gardner, S. L. (2001). Worms, Nematoda. Faculty Publications from the Harold W. Manter Laboratory of Parasitology. doi: 10.1016/B0-12-226865-2/00286-8
Goraya, M., Kantor, C., Vieira, P., Martin, D., and Kantor, M. (2024). Deciphering the vectors: unveiling the local dispersal of Litylenchus crenatae ssp. mccannii in the American beech (Fagus grandifolia) forest ecosystem. PloS ONE 19:e0311830. doi: 10.1371/journal.pone.0311830
Griess, V. C., and Knoke, T. (2013). Bioeconomic modeling of mixed Norway spruce—European beech stands: economic consequences of considering ecological effects. Eur. J. For. Res. 132, 511–522. doi: 10.1007/s10342-013-0692-3
Hazubska-Przybyl, T., Chmielarz, P., and Bojarczuk, K. (2015). In vitro responses of various explants of Fagus sylvatica. Dendrobiology 73, 135-144. doi: 10.12657/denbio.073.014
Holden Forests and Gardens.Beech Leaf Disease. Available online at: https://holdenfg.org/beech-leaf-disease.com (Accessed June 5 2025).
Huang, Q., Lin, B., Cao, Y., Zhang, Y., Song, H., Huang, C., et al. (2023). CRISPR/Cas9-mediated mutagenesis of the susceptibility gene OsHPP04 in rice confers enhanced resistance to rice root-knot nematode. Front. Plant Sci. 14:1134653. doi: 10.3389/fpls.2023.1134653
Jakubas, W. J., McLaughlin, C. R., Jensen, P. G., and McNulty, S. A. (2004). “Alternate year beechnut production and its influence on bear and marten populations,” in Proceedings of the Beech Bark Disease Symposium. USDA Forest Service General Technical Report NE-331. eds. C.A. Evans, and J.A. Lucas. Saranac Lake, New York,NY: United state of America.
Joshi, I., Kohli, D., Pal, A., Chaudhury, A., Sirohi, A., and Jain, P. K. (2022). Host delivered-RNAi of effector genes for imparting resistance against root-knot and cyst nematodes in plants. Physiol. Mol. Plant Pathol. 118:101802. doi: 10.1016/j.pmpp.2022.101802
Kantor, C., Eisenback, J. D., and Kantor, M. (2024). Biosecurity risks to human food supply associated with plant-parasitic nematodes. Front. Plant Sci. 15:1404335. doi: 10.3389/fpls.2024.1404335
Kantor, C., Teixeira, M., Kantor, M., and Gleason, C. (2025). tiny invaders, big trouble: emerging nematode threats in the United States. Phytopathology 115, 587-595. doi: 10.1094/PHYTO-09-24-0290-IA
Kantor, M., Handoo, Z., Carta, L., and Li, S. (2022a). First report of beech leaf disease, caused by Litylenchus crenatae mccannii, on American beech (Fagus grandifolia) in Virginia. Plant Dis. 106:1764. doi: 10.1094/PDIS-08-21-1713-PDN
Kantor, M., Handoo, Z., Kantor, C., and Carta, L. (2022b). Top ten most important US-regulated and emerging plant-parasitic nematodes. Horticulturae 8:208. doi: 10.3390/horticulturae8030208
Kanzaki, N., Ichihara, Y., Aikawa, T., Ekino, T., and Masuya, H. (2019). Litylenchus crenatae. sp. (Tylenchomorpha: Anguinidae), a leaf gall nematode parasitising Fagus crenata Blume. Nematology 21, 5–22. doi: 10.1163/15685411-00003190
Khan, M. R. (2012). Nematodes, an emerging threat to global forests: assessment and management. Plant Pathol. J. 11, 99–113. doi: 10.3923/ppj.2012.99.113
Kholiavchuk, D., Gurgiser, W., and Mayr, S. (2023). Carpathian forests: past and recent developments. Forests 15:65. doi: 10.3390/f15010065
Kibbe, E., and Bonello, E. (2023). Beech Bark Disease. Available online at: https://treessc.org/beech-bark-disease/ (Accessed June 6, 2025).
Kikuchi, T., Cotton, J. A., Dalzell, J. J., Hasegawa, K., Kanzaki, N., McVeigh, P., et al. (2011). Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 7:e1002219. doi: 10.1371/journal.ppat.1002219
Koch, J. L., and Carey, D. W. (2014). A technique to screen American beech for resistance to the beech scale insect (Cryptococcus fagisuga Lind.). JoVE 87:51515. doi: 10.3791/51515
Koch, J. L., Mason, M. E., and Carey, D. W. (2007). Advances in breeding American beech for resistance to beech bark disease. tree improvement in the 21st century: planning for the future. Proceedings of the 3rd Northern Forest Genetics Association Meeting Staff Paper Series No. 194(2007), 22-28.
Kuska, M. T., Heim, R. H., Geedicke, I., Gold, K. M., Brugger, A., and Paulus, S. (2022). Digital plant pathology: a foundation and guide to modern agriculture. J. Plant Dis. Protect. 129, 457–468. doi: 10.1007/s41348-022-00600-z
Loo, J., Ramirez, M., and Krasowski, M. (2005). “American beech vegetative propagation and genetic diversity” in Beech Bark Disease: Proceedings of the Beech Bark Disease Symposium, edn. C.A. Evans, J.A. Lucas, and M. J. Twery. (Newtown Square, PA: US), 106-112.
Loyd, A. L., Borden, M. A., Littlejohn, C. A., Rigsby, C. M., Brantley, B., Ware, M., et al. (2025). Thiabendazole as a therapeutic root flare injection for Beech Leaf Disease Management. AUF 51, 215–225. doi: 10.48044/jauf.2025.007
Loyd, A. L., Cowles, R. S., Borden, M. A., LaMondia, J. A., Mitkowski, N., Faubert, H., et al. (2024). Exploring novel management methods for Beech Leaf Disease, an emerging threat to forests and landscapes. J. Environ. Horticult. 42, 1–13. doi: 10.24266/0738-2898-42.1.1
Marra, R. E., and LaMondia, J. A. (2020). First report of beech leaf disease, caused by the foliar nematode, Litylenchus crenatae mccannii, on American beech (Fagus grandifolia) in Connecticut. Plant Dis. 104:2527. doi: 10.1094/PDIS-02-20-0442-PDN
McLaughlin, J. A., and Greifenhagen, S. (2012). “Beech bark disease in Ontario: a primer and management recommendations,” in Forest Research Note 71. Ontario Ministry of Natural Resources, Ontario Forest Research Insitute, Vol. 71 (Sault Ste. Marie, ON, Canada), 1–8.
Mota, M., and Vieira, P. (2004). The pinewood nematode, Bursaphelenchus xylophilus in “Proceedings of an international workshop. University of Évora, Portugal, August 20-22, 2001 (Vol. 1),” Brill. doi: 10.1163/9789047413097_004
Myers, A. L., Storer, A. J., Dickinson, Y. L., and Bal, T. L. (2023). A review of propagation and restoration techniques for American beech and their current and future application in mitigation of beech bark disease. Sustainability 15:7490. doi: 10.3390/su15097490
Naidoo, S., Slippers, B., Plett, J. M., Coles, D., and Oates, C. N. (2019). The road to resistance in forest trees. Front. Plant Sci. 10:273. doi: 10.3389/fpls.2019.00273
Ostrofsky, W. D., and McCormack, M. L. Jr (1986). Silvicultural management of beech and the beech bark disease. North. J. Appl. For. 3, 89–91. doi: 10.1093/njaf/3.3.89
Palomares-Rius, J. E., Tsai, I. J., Karim, N., Akiba, M., Kato, T., Maruyama, H., et al. (2015). Genome-wide variation in the pinewood nematode Bursaphelenchus xylophilus and its relationship with pathogenic traits. BMC Genomics 16, 1–13. doi: 10.1186/s12864-015-2085-0
Reed, S. E., Greifenhagen, S., Yu, Q., Hoke, A., Burke, D. J., Carta, L. K., et al. (2020). Foliar nematode, Litylenchus crenatae ssp. mccannii, population dynamics in leaves and buds of beech leaf disease-affected trees in Canada and the US. For. Pathol. 50:e12599. doi: 10.1111/efp.12599
Reed, S. E., Volk, D., Martin, D. K., Hausman, C. E., Macy, T., Tomon, T., et al. (2022). The distribution of beech leaf disease and the causal agents of beech bark disease (Cryptoccocus fagisuga, Neonectria faginata, N. ditissima) in forests surrounding Lake Erie and future implications. For. Ecol. Manage. 503:19753. doi: 10.1016/j.foreco.2021.119753
Shepherd, B. L., Burke, D. J., and Stuble, K. L. (2025). Fagus grandifolia growth and mortality a decade after the emergence of Beech leaf disease. Trees For. People 20:100836. doi: 10.1016/j.tfp.2025.100836
Shin, S. C. (2008). “Pine wilt disease in Korea,” in Pine Wilt Disease, 1st Edn, eds. B.G. Zhao, K. Futai, J. R. Sutherland, and Y. Takeuchi, Y. (Springer, Tokyo), 26–32.
Storer, A. J., Rosemier, J. N., Beachy, B. L., and Flaspohler, D. J. (2005). “Potential effects of beech bark disease and decline in beech abundance on birds and small mammals” in Beech Bark Disease: Proceedings of the Beech Bark Disease Symposium, eds. C. A. Evans, J. A. Lucas, and M. J. Twery. (Newtown Square, PA: US. Department of Agriculture, Forest Service), 72–78.
Susič, N., Žibrat, U., Širca, S., Strajnar, P., Razinger, J., Knapič, M., et al. (2018). Discrimination between abiotic and biotic drought stress in tomatoes using hyperspectral imaging. Sens. Actuators B Chem. 273, 842–852. doi: 10.1016/j.snb.2018.06.121
Tammina, M. R., Sumana, K., Singh, P. P., Lakshmi, T. V., and Pande, S. D. (2024). “Prediction of plant disease using artificial intelligence,” in Microbial Data Intelligence and Computational Techniques for Sustainable Computing, (Singapore: Springer Nature), 25-48. doi: 10.1007/978-981-99-9621-6_2
Tubbs, C. H., and Houston, D. R. (1990). “American beech,” in Silvics of North America: 2. Hardwoods, Agriculture Handbook, eds. R. M. Burns, and B. H. Honkala (Washington DC: United States of America)
United Nations Educational Scientific and Cultural Organization (UNESCO) (2025). Ancient and Primeval Beech Forests of the Carpathians and Other Regions of Europe. Available online at: https://whc.unesco.org/en/list/1133/ (Accessed March 15, 2025).
Usovsky, M., Gamage, V. A., Meinhardt, C. G., Dietz, N., Triller, M., Basnet, P., et al. (2023). Loss-of-function of an α-SNAP gene confers resistance to soybean cyst nematode. Nat. Commun. 14:7629. doi: 10.1038/s41467-023-43295-y
Van Den Hoogen, J., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D. A., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572,.194–198. doi: 10.1038/s41586-019-1418-6
Viaene, N., Ebrahimi, N., Haegeman, A., Douda, O., van Bruggen, A., Ogris, N., et al. (2022). “FAGUSTAT: investigating beech leaf disease, a threat to beech trees and forests in Europe,” in 7th International Congress of Nematology (Warsaw).
Vieira, P., Kantor, M. R., Medina-Mora, C. M., Sakalidis, M. L., and Handoo, Z. A. (2023). First report of the beech leaf disease nematode Litylenchus crenatae mccannii (Nematoda: Anguinidae) in Michigan. Plant Pathol. Environ. Microbiol. 107:2266. doi: 10.1094/PDIS-10-22-2468-PDN
Wiggins, G. J., Grant, J. F., Windham, M. T., Vance, R. A., Rutherford, B., Klein, R., et al. (2004). Associations between causal agents of the beech bark disease complex [Cryptococcus fagisuga (Homoptera: Cryptococcidae) and Nectria spp.] in the Great Smoky Mountains National Park. Environ. Entomol. 33, 1274–1281. doi: 10.1603/0046-225X-33.5.1274
Witter, J., Stoyenoff, J., Petrillo, H., Yocum, J., and Cohen, J. (2004). Effects of beech bark disease on trees and ecosystems. Beech Bark Dis. 1001:28.
Wolf, E., and Vieira, P. (2024). Rapid assessment of beech leaf disease in Fagus sylvatica buds. For. Pathol. 54:e12858. doi: 10.1111/efp.12858
Yadav, S., Priya, K., Dhiman, R., Godara, S., Kandari, P., and Thakur, A. (2024). “Long-and medium-term storage of germplasm for conservation of tree species. in Biotechnological Approaches for Sustaining Forest Trees and Their Products, eds. D. Thomas, M. Razdan, and A. Kumar (Singapore: Springer Nature Singapore) 241-275. doi: 10.1007/978-981-97-4363-6_10
Yeates, G. W. (2007). Abundance, diversity, and resilience of nematode assemblages in forest soils. Can. J. For. Res. 37, 216-225. doi: 10.1139/x06-172
Keywords: beech leaf disease, nematodes, Litylenchus crenatae, Europe, ecology, management, textiles
Citation: Kantor C, Demirel MC and Kantor M (2025) Unveiling the threat of beech leaf disease: lessons from North America. Front. For. Glob. Change 8:1606260. doi: 10.3389/ffgc.2025.1606260
Received: 04 April 2025; Accepted: 28 August 2025;
Published: 19 September 2025.
Edited by:
Bernard Slippers, University of Pretoria, South AfricaReviewed by:
Lee Robertson, National Institute of Agricultural and Food Research and Technology, SpainAshish Kumar Singh, Indian Agricultural Research Institute (ICAR), India
Copyright © 2025 Kantor, Demirel and Kantor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mihail Kantor, bXBrNjE0OEBwc3UuZWR1
†ORCID: Melik C. Demirel orcid.org/0000-0003-0466-7649
 Camelia Kantor
Camelia Kantor Melik C. Demirel1,2,3†
Melik C. Demirel1,2,3† Mihail Kantor
Mihail Kantor