- 1Department of Science and Education, Shaoguan First People’s Hospital, Shaoguan, China
- 2The First Clinical College of Guangdong Medical University, Zhanjiang, China
- 3Tianjingshan Forest Farm of Guangdong Province (Guangdong Tianjingshan National Forest Park Management Office), Shaoguan, China
- 4Department of Respiratory Medicine, Shaoguan First People’s Hospital, Shaoguan, China
- 5College of Environment and Climate, Jinan University, Guangzhou, China
- 6Guangdong Provincial Observation and Research Station for Atmospheric Environment and Carbon Neutrality in Nanling Forests, Guangzhou, China
- 7Research Institute of Tropical Forestry, Chinese Academy of Forestry, Guangzhou, China
- 8Beijiangyuan National Forest Ecosystem Research Station, Nanling Mts. China, Guangzhou, China
- 9Princess Máxima Center for Pediatric Oncology, Utrecht University, Utrecht, Netherlands
- 10Department of Prevention and Health Care, Shaoguan First People’s Hospital, Shaoguan, China
Introduction: Rapid urbanization and environmental degradation have escalated health challenges such as sleep disturbances, mood disorders, and chronic stress. Forest bathing, derived from Japan’s Shinrin-yoku, offers immersive sensory engagement with nature, providing psychological and physiological benefits. Low-latitude evergreen broad-leaved forests may amplify these benefits due to phytoncides and negative ions.
Methods: In this prospective cohort study, 36 healthy young participants recruited via WeChat were assessed within Tianjing Mountain Forest in Guangdong Province. Pre- and post-intervention evaluations used standardized measures: Athens Insomnia Scale (AIS), Self-Rating Scale of Sleep (SRSS), Profile of Mood States (POMS), and Perceived Stress Scale (PSS). Routine blood tests, lymphocyte subset profiling, and immune markers (Aquaporin 9 (AQP9), Heat Shock Protein A6 (HSPA6), salivary immunoglobulin A (IgA) (salivary IgA), and lysozyme) were measured immediately, one week, and one month post-intervention.
Results: Significant improvements were observed across multiple domains. Sleep quality improved with decreased AIS scores during the intervention and lower SRSS scores up to one month post-intervention. POMS and PSS scores declined, indicating mood and stress improvements. Physiologically, transient hemoglobin and red blood cell increases occurred, with platelet normalization by one month. Immune profiling showed increased total, regulatory, and helper T cells, an improved CD4/CD8 ratio, and elevated B lymphocytes at one month. Serum AQP9 and HSPA6 levels decreased, while salivary IgA and lysozyme surged.
Discussion: These findings suggest forest bathing offers immediate and multidimensional health benefits, highlighting its potential as a non-pharmacological intervention for holistic well-being. The study advocates integrating natural spaces into urban planning and public health strategies to promote health in urbanized populations.
Clinical trial registration: This study had been registered at the China Clinical Trial Registration Center (registration number: ChiCTR2500096972; https://www.chictr.org.cn/showproj.html?proj=241942).
Introduction
In the 21st century, humanity faces a growing health crisis driven by chronic stress, environmental degradation, and lifestyle changes (Tierney et al., 2023). As urbanization accelerates and natural habitats shrink, people are increasingly disconnected from the natural world that once formed the basis of human health and wellbeing (Franckowiak et al., 2019). This disconnection has profound implications for both individual health and the sustainability of our societies. Sleep disorders (Kechter and Leventhal, 2019), emotional dysregulation (Gross, 2002), and chronic stress (Martínez Arroyo et al., 2019) have become significant threats to public health.
The concept of forest medicine, rooted in traditional practices and now supported by modern scientific evidence, offers a unique bridge between human health and environmental conservation (Tsunetsugu et al., 2010; Miyazaki et al., 2014). At its core is “Shinrin-yoku,” or forest bathing, a practice pioneered in Japan that involves immersing oneself in the forest atmosphere through the senses (Miyazaki et al., 2014). Unlike conventional exercise or tourism, forest bathing emphasizes slow, mindful engagement with nature, allowing individuals to reconnect with the natural world in a meaningful way (Lee et al., 2011).
The health benefits of forest environments have been increasingly documented across multiple disciplines. Psychologically, forest bathing has been shown to reduce anxiety, depression, and stress while enhancing mood and cognitive function (Lee et al., 2011). Physiologically, it lowers blood pressure, heart rate, and stress hormone levels while improving immune function and sleep quality (Lee et al., 2011). These effects are not merely transient but can persist for weeks after the forest experience, suggesting profound implications for preventive medicine.
The mechanisms underlying these benefits are complex and multifaceted. Forest environments contain phytoncides—volatile organic compounds released by trees—that directly influence human physiology by modulating immune responses and reducing inflammation (Li et al., 2010). The visual beauty of forests, their gentle sounds, and the tactile experience of natural surfaces all contribute to a sensory experience that calms the nervous system and reduces stress (Li et al., 2007). Additionally, the negative ions present in forest air and the microbial diversity of forest soils may play roles in enhancing human health that are only beginning to be understood (Craig et al., 2016).
However, research on the sustained benefits of forest bathing for sleep, mood, stress reduction, and immune system regulation remains limited. Most existing studies focus only on single physiological or psychological indicators, lacking dynamic monitoring across different intervention time points. This limits our understanding of forest bathing’s comprehensive effects and underlying biological mechanisms.
From an environmental perspective, forest medicine represents a powerful argument for forest conservation and sustainable management (Prüss-Üstün et al., 2016). As forests provide not only ecosystem services but also direct health benefits to humans, their protection becomes a matter of public health as well as environmental policy. This interdisciplinary approach challenges us to rethink how we value natural spaces and integrate them into our healthcare systems.
This study aims to advance our understanding of forest bathing’s health benefits by examining its effects on sleep quality, mood, stress levels, and immune function over both short and long terms. By employing rigorous scientific methods and interdisciplinary perspectives, we seek to provide evidence-based insights that can inform healthcare practices, environmental policies, and urban planning. Our findings may help establish forest bathing as a legitimate therapeutic intervention while highlighting the importance of preserving natural environments for human health.
The significance of this research extends beyond individual health outcomes. It contributes to a broader dialogue about how humanity can reconnect with nature in ways that benefit both people and the planet. As we face growing health challenges in an increasingly urbanized world, forest medicine offers a promising pathway toward holistic wellbeing that honors the intricate relationship between humans and the natural world.
Materials and methods
Participants and procedures
We recruited experimental subjects through the WeChat official account of Shaoguan First People’s Hospital. About WeChat official account advertising link can be found at: https://mp.weixin.qq.com/s/MnY0GYHERw64mRRn625mIg. Individuals who are interested in participating can register by filling out an online questionnaire. We will conduct a detailed face-to-face evaluation of young applicants who meet the basic criteria after initial screening to determine whether they will ultimately be included in the experimental study. In the end, we included 36 participants. This study was approved by Shaoguan First People’s Hospital (Approval Number: Shaoguan First People’s Hospital Medical Ethics Review Number ((2024)33). This study had been registered at the China Clinical Trial Registration Center (registration number: ChiCTR2500096972). The program complies with the Helsinki Declaration of 1975 (revised in 1983). The study was fully explained to all participants in both oral and written form. Each participant signed an informed consent form.
Inclusion criteria were as follows: (1) healthy subjects, male or female, aged 18–45, never smoking, in good health; (2) body mass index 19–26, male weight ≥ 50 kg, female weight ≥ 45 kg; and (3) fully understand and voluntarily sign the informed consent form.
Exclusion criteria were as follows: (1) participants who participated in any drug clinical trials within 3 months prior to the trial; (2) individuals with chronic or active gastrointestinal diseases such as esophageal disease, gastritis, gastric ulcer, enteritis, active gastrointestinal bleeding, or gastrointestinal surgery within 3 years; (3) individuals with a history of cardiovascular system, endocrine system, urinary system, nervous system, respiratory system, hematology, immunology (including personal or family history of inherited immune deficiency), psychiatry, metabolic abnormalities, etc., who are still clinically significant according to researchers; (4) individuals with a history of allergies to drugs, food, or other substances; (5) individuals with a history of cancer, epilepsy, frequent headaches, and tuberculosis infection; (6) those who cannot tolerate venous puncture or have a history of dizziness or needle fainting; (7) patients who have undergone surgery within 6 months prior to the experiment, which has been determined by the researchers to affect drug absorption, distribution, metabolism, and excretion; or have undergone surgical procedures within 3 months prior to the experiment; or those who plan to undergo surgical procedures during the study period; (8) those who received the vaccine within 3 months before the experiment; (9) individuals who have used any medication within the 30 days prior to the trial; (10) blood donors or those who have lost a large amount of blood (>400 ml) within 3 months before the experiment; (11) alcoholics or frequent drinkers within the 6 months prior to the experiment, who consume more than 14 units of alcohol per week (1 unit = 360 ml of beer or 45 ml of 40% spirits or 150 ml of wine); those who are unwilling to stop drinking alcohol or using any alcoholic products during the trial period; or the alcohol breath test result is positive (>0.0 mg/100 ml); (12) individuals who consume excessive amounts of tea, coffee, and/or caffeinated beverages (8 or more cups, 1 cup = 250 ml) daily, or who do not agree to stop drinking tea, coffee, and/or caffeinated beverages during the trial period; (13) individuals who consume a diet that may affect the metabolism of the investigational drug within 7 days prior to taking the drug (including grapefruit or grapefruit products, oranges, oranges, strawberries, apricots, xanthine diet, etc.), or who the researcher believes have other diets that may affect the absorption, distribution, metabolism, and excretion of the drug, or who do not agree to stop consuming the aforementioned diet during the trial period; (14) individuals who have special dietary requirements and cannot follow a uniform diet, or those who are lactose intolerant; (15) female participants who engage in unprotected sexual activity within 2 weeks prior to the trial, or who have a pregnancy plan, sperm or egg donation plan within 6 months from screening to the end of the trial, or who refuse to use one or more non-pharmacological contraceptive measures (such as complete abstinence, contraceptive rings, partner ligation, etc.) during the trial period; (16) drug abusers or those who have used soft drugs (such as marijuana) within the past 3 months prior to the experiment, or those who have used hard drugs (such as cocaine, phencyclidine, etc.) within the past 1 year prior to the experiment; (17) for patients with creatinine clearance rate < 80 ml/min, the Cockcroft Gault formula for calculating creatinine clearance rate is CrCl = [(140 age) × body weight (kg)]/[0.814 × Scr (μ mol/L)]. When calculating creatinine clearance rate, attention should be paid to the unit of creatinine; women are calculated to be 0.85; (18) individuals with positive results in urine drug screening (morphine, methamphetamine, ketamine, methylenedioxymethamphetamine, tetrahydrocannabinol acid, and cocaine); (19) individuals with clinically significant abnormalities in physical examination, electrocardiogram, laboratory tests, color ultrasound, chest radiograph, vital signs, and laboratory related examinations (subject to clinical judgment); (20) individuals with positive screening results for infectious diseases; (21) individuals with positive results in nicotine urine testing; (22) female participants who have used oral contraceptives within 30 days prior to the trial; (23) female subjects who have used long-acting estrogen or progesterone injections or implants within 6 months prior to the trial; (24) pregnant or lactating women, or those whose blood pregnancy test is positive; and (25) participants may not be able to complete this study due to other reasons or may have other reasons deemed inappropriate by the researchers to participate in the trial.
Experimental location
The experimental site of forest bathing is located in Tianjing Mountain Forest in the western part of Ruyuan Yao Autonomous County, Guangdong Province. Wuling South Range, a tributary of the Nanling Mountains (112°53′-113°15′E, 24°39′-24°51′N). The forest coverage rate is 96.7%. The region has a mountainous climate in the subtropical monsoon climate zone, with abundant rainfall and distinct wet and dry seasons. The average annual temperature is 17∼20°C, and the daily relative humidity is 75%∼95%. The zonal vegetation is dominated by broad-leaved evergreen forests.
It is rich in botanical resources, with numerous forest levels, diverse plant types and plant varieties. There are 287 families, 1,262 genera, and 3,890 species of wild vascular plants in the jurisdiction, accounting for about 55% of the total number of identified wild vascular plants in Guangdong Province. Among them, 60 families of bryophytes, 153 genera and 351 species, 46 families of ferns, 112 genera and 363 species; 7 families of gymnosperms, 11 genera and 19 species; and 174 families of angiosperms, 986 genera and 3,157 species, making it a veritable “treasure house of plant genes.” Among them, those protected at the national level include Metasequoia, Boreal tree, Cyathea fern, which is called the living fossil of plants and the dinosaur contemporaries, and those protected at the second level include Red-winged Chihuahua, Mountain Lychee, Guangdong Pine, and so on.
During the dry season, 12 major tree species were observed. Adin maple and South Ridge arrowroot were the main emitters of isoprene, accounting for 85.3% and 95.7% of their total emissions, respectively. Some alpine dwarf trees (e.g., wigwam maple bark, mountain alum, and sclerocarpus) and many broadleaf species (e.g., muhly, southridge quebracho, wild lacustrine, and cedar) emitted monoterpenes that accounted for more than 70% of their total biogenic volatile organic compounds (BVOCs) emissions (Chen et al., 2025). Sesquiterpenes were also detected in each of the forest tree species, with the highest proportion of sesquiterpenes, 51.1%, being found in Rhododendron fortunei. Five tree species (i.e., heather, fir, clouded rhododendron, false ground maple bark, and sclerocarpus) emitted other BVOCs in proportions ranging from 3.5% to 29.5%.
Most broadleaf trees released higher monoterpenes than isoprene. In the dry season, dominant trees at low elevations mainly released isoprene, α-pinene and d-limonene, with Adin maple mainly releasing isoprene (85.3%) and α-pinene (8.7%); muhly mainly releasing α-pinene (54.3%) and isoprene (4.9%); and Nangling quebracho mainly releasing d-limonene (28.0%) and α-pinene (8.8%). At medium elevations, the dominant trees mainly released longifolium, 2-methyl-3-buten-2-ol (MBO) and d-limonene and α-pinene. Among them, wild lacustrine α-pinene emission was the largest, accounting for 82.6% of the total BVOCs emission. The emissions of α-pinene, longifolene and MBO released by heather all accounted for more than 20% of their total emissions (26.7%, 26.0%, and 29.5%, respectively). Fir also released a larger amount of α-pinene, 42.0%, and had 28.0% and 18.6% of its emissions of longifolium and d-limonene, respectively. Five-rowed wood released d-limonene and α-pinene which accounted for a larger share of the total BVOCs emissions, 54.0% and 18.2%, respectively.
At higher elevations, the dominant trees emitted mainly α-pinene and isoprene. Emissions of α-pinene were detected from all five tree species at this elevation and differed in their share of total BVOCs emissions as follows: false ground maple bark (74.0%), Sclerocarya sclerotiorum (80.2%), mountain alum (26.2%), cloudy rhododendron (14.6%), and Southridge arundinacea (3.6%). It is worth noting that isoprene emissions from Southridge arrowroot accounted for 95.7% of total BVOCs emissions, while cis-β-octadiene emissions from mountain alum accounted for 53.8% of total BVOCs emissions.
Measures
Athens insomnia scale
The Athens insomnia scale (AIS) (Zhou et al., 2017) is a self-assessment scale for assessing insomnia symptoms, with subjective perception of sleep as the main rating, developed by a research group in Athens, Greece, in 2000, and is an internationally recognized self-assessment scale for sleep quality. The AIS consists of 8 items, each of which is rated on a scale of 0–3, with 0 indicating “no problem” and 3 indicating “serious problem,” and the total score ranging from 0 to 24: 0–5: no insomnia; 6–9: mild insomnia; 10–15: moderate insomnia; 10–15: moderate insomnia; 10–15: moderate insomnia; and 10–15: moderate insomnia. Score: moderate insomnia; 16–24 points: severe insomnia.
Self-rating scale of sleep
The self-rating scale of sleep (SRSS) is a self-rating scale developed by Prof. Li Jianming, Executive Director of the Chinese Mental Health Association, for assessing the sleep status of an individual. SRSS has been used to cover eight regions of the country and a number of industries, including workers, farmers, the People’s Liberation Army, college students and the service industry and other 13,273 normal people of different ages, sexes and occupations, and the coefficient of validity is 0.5625, with a P-value of <0.0001, which indicates that the scale has good reliability and validity (Li et al., 2000). The SRSS consists of 10 items, each of which is scored on a 5-point scale (1–5), with higher scores indicating more severe sleep problems.
Profile of mood states
Profile of mood states (POMS) is primarily used to assess an individual’s emotional state over a specific period of time and is a self-report questionnaire (Lira et al., 1977). The POMS scale contains 65 adjectives describing different emotional states on a 5-point scale (0 = almost none, 1 = somewhat, 2 = moderately, 3 = quite a bit, 4 = very much). The score for each mood factor was derived by summing the scores for the corresponding adjectives. The total Mood State Score was calculated by subtracting the sum of the scores for the five negative mood factors from the sum of the scores for the two positive mood factors (vitality and sense of self-esteem) and adding a constant (100). Higher scores indicate that the individual’s emotional state is more negative.
Perceived stress scale
The perceived stress scale (PSS) was developed by Cohen et al. (1983). In 1983 to measure an individual’s subjective perception of stress in life situations. Traditional stress research has relied on objective events (such as the Life Events Scale) to assess stress levels, but these methods have ignored individuals’ subjective perceptions of events. The PSS was developed based on the theoretical assumption that an individual’s perception of stress (rather than the event itself) is the key factor influencing health and behavior. The PSS consists of 14 items that measure the level of stress an individual has experienced in the past month, and scores from all the items are summed up to give a total score ranging from 0 to 56 points. The higher the score, the more stress the individual feels.
Blood routine test
We systematically observed the dynamic changes of physiological indicators in the subjects over a short period of time, and carried out standardized routine blood tests under strictly controlled experimental conditions with multiple venous blood collections from the subjects in a fasting state. In the routine blood tests, we monitored a number of indicators: hemoglobin concentration directly reflects the oxygen-carrying capacity of the blood, red blood cell count (RBC) is used to assess the total number of circulating red blood cells, and platelet count reflects the body’s coagulation function, whose abnormal changes can provide important clues for the diagnosis of hemorrhagic or thrombotic diseases. In addition, basophil count, as a specific marker of immune-inflammatory response, can reflect systemic lupus erythematosus disease activity (Liang et al., 2015), which is of unique reference value for analyzing the state of immune response and inflammatory pathogenesis.
Lymphocyte subsets
Lymphocyte subpopulations are an important component of the immune system, playing a key role in maintaining the body’s immune homeostasis, fending off pathogen invasion, and monitoring abnormal cells in the body. In this trial, we measured a variety of lymphocyte subpopulations, including total T-lymphocytes, suppressor T-lymphocytes, assisted T lymphocytes, CD4/CD8 ratio, B lymphocytes, and natural killer cells. Specifically: (1) total T lymphocytes, as the core component of cellular immunity, are responsible for recognizing and clearing infectious pathogens or malignant target cells; (2) suppressor T lymphocytes play a role in the regulation of immune homeostasis through the secretion of inhibitory cytokines (e.g., IL-10 and TGF-β) and the inhibition of effector T cell activity by direct contact (Sakaguchi et al., 2008); (3) assisted T lymphocytes as the core regulator of adaptive immunity, it secretes cytokines to assist the activation of B lymphocytes, promotes the differentiation of cytotoxic T lymphocytes, and regulates the intensity of immune response (Murphy and Reiner, 2002); (4) the CD4/CD8 ratio reflects the homeostatic state of immune regulation, and its abnormality is closely related to the development of a variety of immune-related diseases (e.g., autoimmune diseases and infectious diseases) Pantaleo et al. (1993); (5) B lymphocytes mediate humoral immune responses by differentiating into plasma cells that secrete specific antibodies; and (6) natural killer cells, as important effector cells of intrinsic immunity, can kill viral and tumor cells without sensitization and regulate the immune response by secreting cytokines (Gong et al., 2021).
Immune system components
In addition, we also collected other related immune indicators, such as aquaporin 9 (AQP9), heat shock 70-kDa protein 6, Salivary immunoglobulin A (IgA), and lysozyme. AQP9, as an important member of the aquaporin family of proteins, has been shown to be involved in regulating the water metabolism and activation status of the cells in the monocyte-macrophage system in recent studies. In lipopolysaccharide (LPS)-stimulated macrophages, upregulation of AQP9 expression promotes cytokine secretion and enhances the inflammatory response. This protein plays a key role in intrinsic immunity by regulating intracellular osmotic pressure homeostasis and influencing phagocytosis and polarization direction of macrophages (Rump and Adamzik, 2024). Heat shock 70-kDa protein 6 acts as a stress-induced molecular chaperone and is involved in protein folding, assembly, translocation, and degradation to protect cells from stress damage (Radons, 2016). SIgA is the first line of mucosal immunity, and specific immune defenses against Streptococcus caries mutans are mainly provided by salivary secreted IgA antibodies, which play a central role in oral immune defense (Russell et al., 1999). Lysozyme, as an effector molecule of the innate immune system, plays an antibacterial role by hydrolyzing peptidoglycan of the bacterial cell wall (Callewaert and Michiels, 2010). In addition to its direct bactericidal function, lysozyme improves and enhances the phagocytosis and digestion ability of macrophages (Huan et al., 1995).
Research design and experimental procedure
This study utilized a prospective cohort study design to improve the sensitivity and accuracy of the experiment by using its own control. The effects of the forest environment on subjects’ sleep, mood and immune function were investigated.
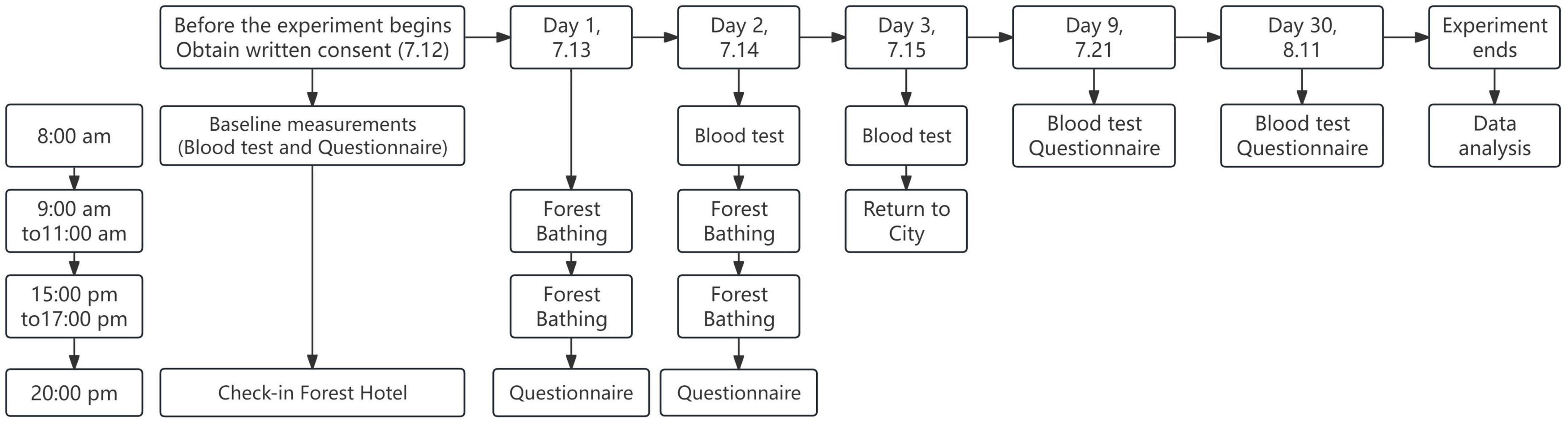
The steps of the experiment are shown in Figure 1. The day before the experiment began, all participants were required to remain fasted, complete blood sample collection and carefully fill out the questionnaire. At 9:00 on the first day of the experiment, participants walked 2.5 km along the recreational trail in the forest park to experience forest bathing activities. At 15:00, both groups repeated the morning walk again, maintaining the same exercise intensity and method. At 20:00, participants completed the questionnaire. On the second and third days of the experiment, participants were asked to collect blood samples on an empty stomach in the morning, repeat the forest bathing walk on the first day of the experiment at the same time in the morning and afternoon, and complete the questionnaire in the evening. On the fourth day of the experiment, 1 week after the experiment, and 1 month after the experiment participants were only asked to complete the blood test and questionnaire.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9.0 software. For the analysis of count data across five groups, the normality of the data distribution was first assessed using appropriate statistical tests (e.g., Shapiro–Wilk or Kolmogorov–Smirnov tests). For data that conformed to a normal distribution, a repeated measures one-way ANOVA was conducted, with the Geisser–Greenhouse correction applied to account for potential violations of the sphericity assumption. Post hoc comparisons were performed using the Holm–Sidák’s multiple comparisons test, with individual variances calculated for each pairwise comparison to identify specific group differences. In cases where the data did not meet the assumptions of normality, the Friedman test, a non-parametric alternative, was employed to assess differences among the five groups. If the Friedman test indicated significant differences, Dunn’s multiple comparisons test was used as a post hoc procedure to determine which specific groups differed from each other, with appropriate adjustments for multiple testing to control the family-wise error rate. All tests were two-tailed, and a significance level of P < 0.05 was considered statistically significant.
Results
Effects of forest bathing on sleep, mood, and stress
In this 3-day forest bathing study, four assessment tools were administered—AIS, SRSS, brief POMS, and PSS —at baseline, during the intervention, on the day of completion, 1 week post-intervention, and 1 month post-intervention to capture temporal changes in subjective experiences.
For AIS (Figure 2a), a significant reduction in scores was observed during the intervention. The average score decreased from 6.1 at baseline to 4.7 on July 13, and further to 3.5 on July 14. The improvement observed on July 14 was highly statistically significant (P < 0.0001), indicating that short-term forest bathing has a positive effect on sleep quality. However, during the follow-up period, scores gradually increased, reaching 4.8 1 week after the intervention and 5.2 1 month post-intervention. These follow-up scores were not significantly different from the baseline value, suggesting that the beneficial effects of forest bathing on insomnia were predominantly short-term.
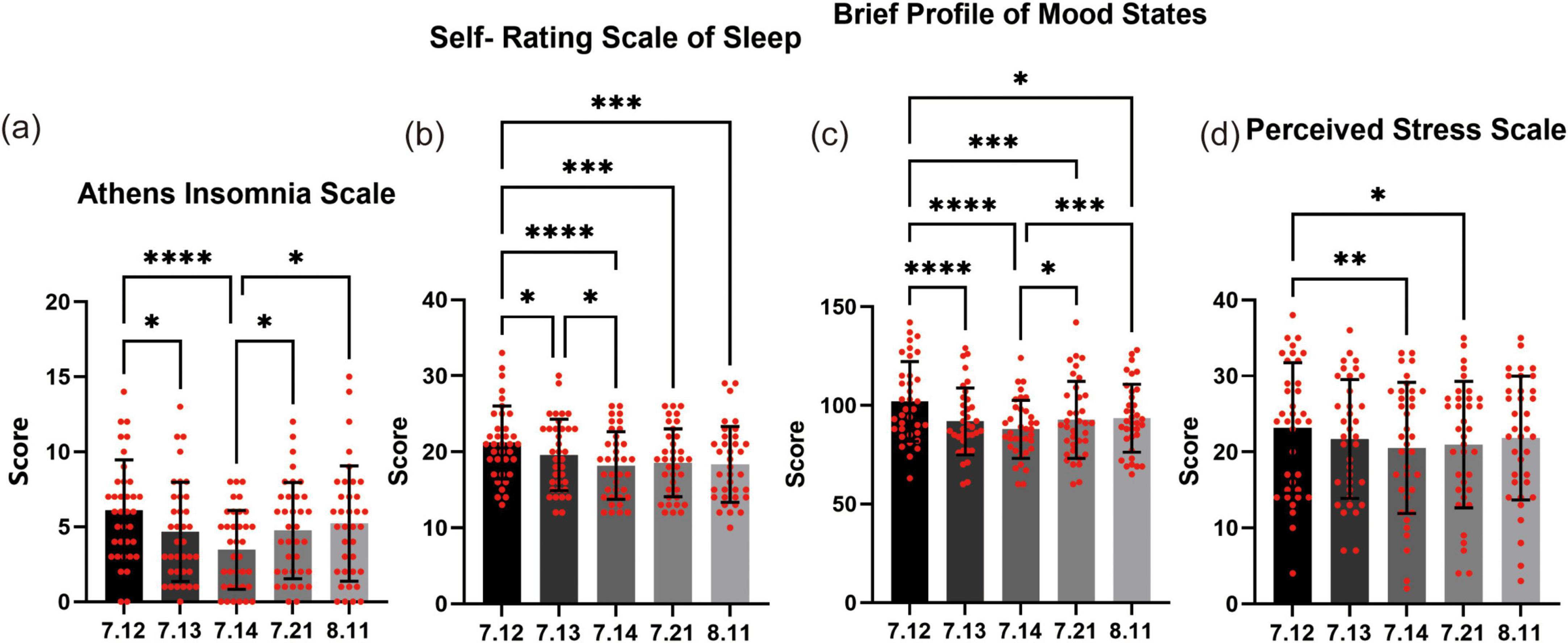
Figure 2. Effects of forest bathing on sleep, mood, and stress. (a) Athens insomnia scale (AIS): at baseline (July 12), the average score was 6.1, which significantly decreased to 4.7 on July 13 and further to 3.5 on July 14. Although scores showed a slight rebound during follow-up—4.8 on July 21 and 5.2 on August 11—they did not differ significantly from the baseline level. (b) Self-rating scale of sleep (SRSS): the baseline score of 21.2 decreased progressively to 19.6 on July 13, 18.2 on July 14, and remained low at 18.6 on July 21 and 18.3 on August 11. (c) Profile of mood states (POMS): participants’ mood scores improved from a baseline of 102.0 on July 12 to 91.9 on July 13 and 87.9 on July 14. Although the scores slightly increased to 92.7 on July 21 and 93.5 on August 11, they remained significantly lower than the baseline value. (d) Perceived stress scale (PSS): the average stress score decreased from 23.2 at baseline to 21.7 on July 13 and 20.5 on July 14. Follow-up assessments yielded scores of 21.0 on July 21 and 21.8 on August 11, with the July 21 value showing a significant improvement compared to baseline. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Self-rating scale of sleep (Figure 2b) revealed a sustained positive effect of forest bathing on sleep quality. At baseline, the average score was 21.2, which significantly decreased to 19.6 on July 13, and further to 18.2 on July 14. On subsequent assessments, the scores remained lower than baseline, with an average of 18.6 on July 21 and 18.3 on August 11. One-way ANOVA confirmed that all post-intervention scores were significantly lower than the pre-intervention score, with the improvement on July 14 being particularly noteworthy (P < 0.001). These results suggest that the beneficial effects of forest bathing on sleep can be maintained for at least 1 month.
For POMS (Figure 2c), the results reveal a consistent pattern of mood improvement associated with forest bathing. At baseline (July 12), the average mood score was 102.0, indicating a relatively less favorable state. As the intervention progressed, participants’ scores decreased significantly—dropping to 91.9 on July 13 and further to 87.9 on July 14—with these changes reaching statistical significance (P < 0.0001). This rapid improvement underscores the short-term efficacy of forest bathing in enhancing mood. Importantly, the positive effects were sustained even after the intervention ended. On July 21, 1 week post-intervention, the average mood score was 92.7, and by August 11 it was 93.5. Although these follow-up scores reflect a slightly attenuated improvement compared to the immediate post-intervention values, they remained significantly lower than the baseline. Statistical analyses confirmed that the improvements observed at all post-intervention time points were significant relative to baseline.
During the forest bathing intervention, participants’ perceived stress PSS levels showed a marked decline (Figure 2d). At baseline (July 12), the average stress score was 23.2. As the intervention progressed, the scores decreased to 21.7 on July 13 and further to 20.5 on July 14. This reduction, particularly between July 12 and July 14, was statistically significant (P < 0.01), demonstrating the rapid and pronounced stress-relieving effect of forest bathing. Importantly, the reduction in stress was maintained after the intervention. On July 21, 1 week post-intervention, the average stress score was 21.0, and by August 11 it was 21.8. Although these follow-up scores indicate a slight attenuation compared to the immediate post-intervention levels, they remained significantly lower than the baseline. Notably, the comparison between the scores on July 21 and July 14 reached statistical significance (P < 0.05), underscoring that the beneficial effects of forest bathing on stress relief can persist for at least 1 week.
Effects of forest bathing on blood routine test-related immune indicators
The hemoglobin concentration (HGB) (Figure 3a) showed a significant increase on July 14 compared to July 12 (P < 0.01). Similarly, HGB on July 21 was also higher than that on July 12, and this difference was statistically significant (P < 0.05). However, by August 11, the decrease in HGB did not show a statistically significant difference compared to the level at the beginning of the experiment.
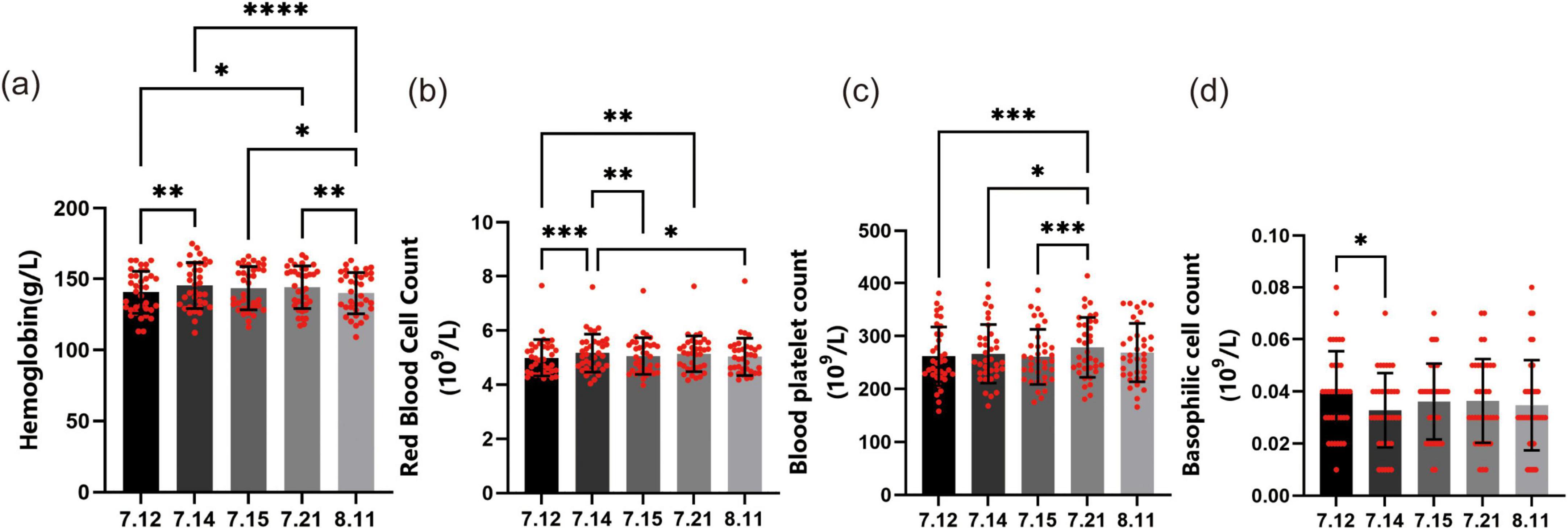
Figure 3. Effects of forest bathing on blood routine test-related immune indicators. (a) Hemoglobin concentration (HGB): on July 12, the average HGB was recorded. It significantly increased on July 14 and July 21 compared to the baseline. (b) Red blood cell count (RBC): similar to HGB, the RBC significantly increased on July 14 and July 21 compared to July 12. (c) Platelet count (PLT): during the 3-day forest bathing period, PLT fluctuated daily but remained relatively stable. One week after the experiment, the count showed a significant upward trend compared to the 3-day period during the experiment. (d) Basophil count: on July 12, the average basophil count was 0.039 (109/L). It significantly decreased to 0.033 (109/L) on July 14. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The trend of RBC (Figure 3b) changes was very similar to that of HGB. The RBC on July 14 showed a significant increase compared to July 12, with the difference being statistically significant (P < 0.001). Similarly, RBC on July 21 was also significantly higher than that on July 12 (P < 0.01). However, by August 11, RBC had decreased and returned to the pre-forest bathing level, with no significant difference compared to July 12.
The platelet count (PLT) (Figure 3c) fluctuated daily during the forest bathing period but remained at a relatively stable level overall. Through one-way ANOVA test, we found that the PLT changed every day during the 3-day forest bathing, but there was no significant statistical difference. However, 1 week after the experiment, PLT showed a significant upward trend and had statistical differences compared to the PLT during the 3-day period of the experiment, with P values of less than 0.001, less than 0.05, and less than 0.001, respectively. One month after the experiment, we observed that PLT returned to a level similar to that during the experiment, indicating that the increase in PLT was temporary and did not last beyond 1 month.
On July 12, the average basophil count (Figure 3d) of the participants was 0.039 (109/L). This value decreased to 0.033 (109/L) on July 14, which was a significant decrease (P < 0.05). Subsequently, the value slightly increased to 0.036 (109/L) on July 15 and remained at 0.036 (109/L) on July 21. By August 11, the basophil count had slightly decreased to 0.034 (109/L). These changes may be related to environmental changes during the forest bathing period, such as changes in temperature and humidity, which may affect the body’s immune response and thus the number of basophils. However, except for the significant decrease on July 14, the subsequent changes in the count did not show any statistically significant differences.
Effects of forest bathing on lymphocyte subsets
Total T lymphocytes (Figure 4a), which are the primary immune cells in the body and include helper T cells and suppressor T cells, showed significant changes during and after the forest bathing intervention. The total T lymphocyte count of the participants started at 1,336.1 on July 12, 2024. During the forest bathing period and in the follow-up, there was a remarkable increase in the count. By 11 August 2024, it had reached 1,598.9. The increase was statistically significant when compared to the baseline value on July 12. Specifically, the differences on July 15 (P < 0.05), July 21 (P < 0.01), and August 11 (P < 0.001) were all significant. These findings suggest that forest bathing may have a positive effect on enhancing the overall activity of T cells.
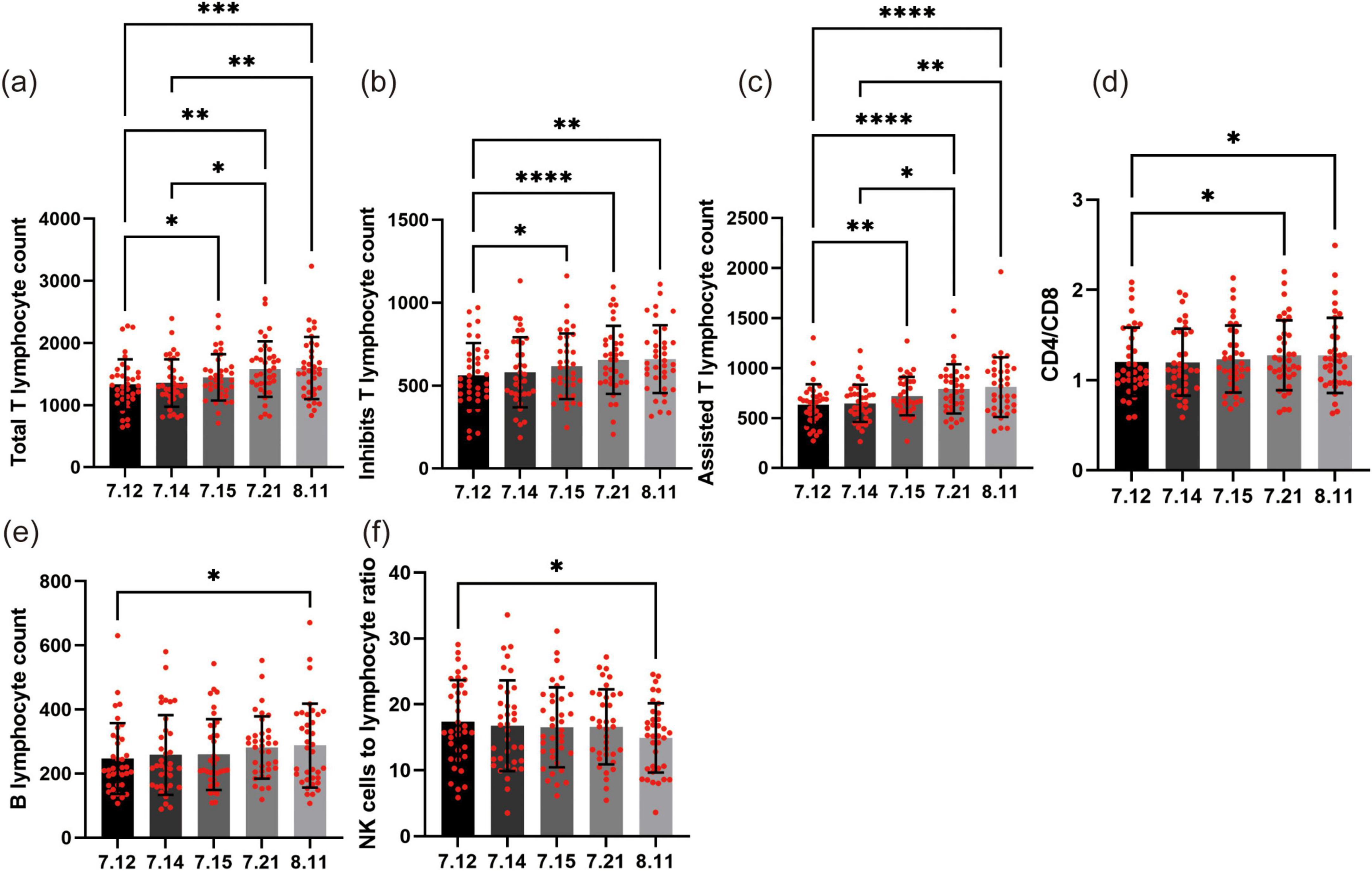
Figure 4. Effects of forest bathing on lymphocyte subsets. (a) Total T lymphocyte count: starting at 1,336.1 on July 12, the total T lymphocyte count increased significantly during and after forest bathing, reaching 1,598.9 by August 11. Significant increases were observed on July 15, July 21, and August 11. (b) Suppressor T lymphocyte count: starting at 562.1 on July 12, the suppressor T cell count increased to 659.7 by August 11. (c) Helper inducer T cell count: starting at 635.1 on July 12, the helper inducer T cell count increased to 810.8 by August 11. Significant increases were observed on July 15, July 21, and August 11. (d) CD4/CD8 ratio: starting at 1.20 on July 12, the CD4/CD8 ratio increased to 1.28 by August 11. (e) B lymphocyte count: starting at 247.0 on July 12, the B lymphocyte count increased to 287.4 by August 11. The increase was not statistically significant during the forest bathing period but was significant when comparing August 11 to July 12. (f) NK cell to lymphocyte ratio: starting at 17.3 on July 12, the NK cell to lymphocyte ratio decreased to 15.0 by August 11. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Suppressor T lymphocytes (Figure 4b), also known as regulatory T cells, play a crucial role in controlling immune responses and preventing over – active immune reactions from damaging the body. The count of these cells in the participants showed an upward trend during and after the forest bathing intervention. Starting at 562.1 (106/L) on July 12, the suppressor T cell count increased to 659.7 (106/L) by August 11. The increase was most significant 1 week after the intervention (P < 0.0001). Although the growth rate slowed down after 1 month, the increase remained statistically significant (P < 0.01). These findings indicate that forest bathing may help regulate the balance of the immune system and enhance the function of suppressor T cells. This is consistent with the observed increase in total T lymphocyte count, suggesting that forest bathing has a comprehensive effect on modulating the immune system.
Assisted T lymphocytes (Figure 4c), also known as helper T cells, play a crucial role in activating other immune cells such as B cells and CD8+ T cells. Our data showed a gradual increase in the count of assisted T cells in the participants during and after the forest bathing intervention. Starting at 635.1 on July 12, the count increased to 810.8 by August 11. The increase was statistically significant, particularly when compared to the baseline value on July 12. Specifically, the differences on July 15 (P < 0.01), July 21 (P < 0.0001), and August 11 (P < 0.0001) were all significant. These findings suggest that forest bathing may help enhance the activity of assisted T cells, and this effect appears to last for at least 1 month. This is consistent with the observed increase in total T lymphocyte count and suppressor T cell count, further supporting the potential of forest bathing to modulate the immune system in a positive way.
CD4 and CD8 are two distinct types of T cells that play crucial roles in immune responses. Changes in the CD4/CD8 ratio (Figure 4d) can reflect the balance of the immune system. Similarly, the CD4/CD8 ratio showed a similar trend. Starting at 1.20 on July 12, the ratio increased to 1.28 by August 11. The increase was statistically significant 1 week and 1 month after the intervention (P < 0.05), but the change during the forest bathing period was not significant. This suggests that forest bathing may help improve the balance of the immune system, but the effect may take some time to become apparent.
B lymphocytes (Figure 4e) are essential members of the immune system, responsible for producing antibodies to combat pathogens. During the forest bathing period, the B lymphocyte count of the participants showed a slight upward trend. Specifically, starting at 247.0 on July 12, the count gradually increased to 287.4 by August 11. Although the increase was continuous, it did not reach statistical significance during the forest bathing period. However, when comparing the data from August 11 to that from July 12, the increase was statistically significant (P < 0.05). This suggests that forest bathing may have a positive impact on B lymphocyte count after 1 month.
Natural killer cells are the front-line warriors of the immune system, capable of rapidly identifying and destroying virus-infected cells or cancer cells. Our study results showed that during the forest bathing period, the NK cell count of the participants did not show significant changes. From 341.2 on July 12 to 330.3 on August 11, although there were fluctuations in the values, these changes did not reach statistical significance. This means that at least in the short term, forest bathing seems to have no significant impact on the number of NK cells. However, when we analyzed the ratio of NK cells to lymphocytes (Figure 4f), we found some interesting trends. On July 12, the average ratio of NK cells to lymphocytes in the participants was 17.3, and by August 11, this ratio had decreased to 15.0. This downward trend was statistically significant (P < 0.05), indicating that after 1 month, the proportion of NK cells to lymphocytes had decreased. This change may imply that forest bathing has a subtle impact on certain aspects of the immune system, although this effect may not be apparent in the short term.
Effects of forest bathing on immune system components
Aquaporin 9, a major water channel protein highly expressed in human leukocytes, is localized at the leading edge of migrating cells and is essential for normal cell motility through its regulation of water flux (Ishibashi et al., 2009). Elevated AQP9 expression is a known marker of chronic inflammation and has been proposed as a potential biomarker for several autoimmune diseases (Mesko et al., 2010). At baseline, the average Serum AQP9 (Figure 5a) concentration was 64.4 pg/ml. Following the commencement of forest bathing, a marked decrease was observed, with levels declining to 51.9 pg/ml on July 14 and further to 38.8 pg/ml on July 15. This pronounced reduction suggests that forest bathing may elicit an immediate and potent effect on lowering Serum AQP9, potentially reflecting diminished inflammation or other beneficial immune responses. After cessation of the forest bathing sessions, Serum AQP9 levels began to rebound, increasing to 45.0 pg/ml on July 21 and 59.4 pg/ml on August 11. Although these later values remained below the baseline, the partial recovery indicates that the acute impact of forest bathing on AQP9 may not be sustained over an extended period.
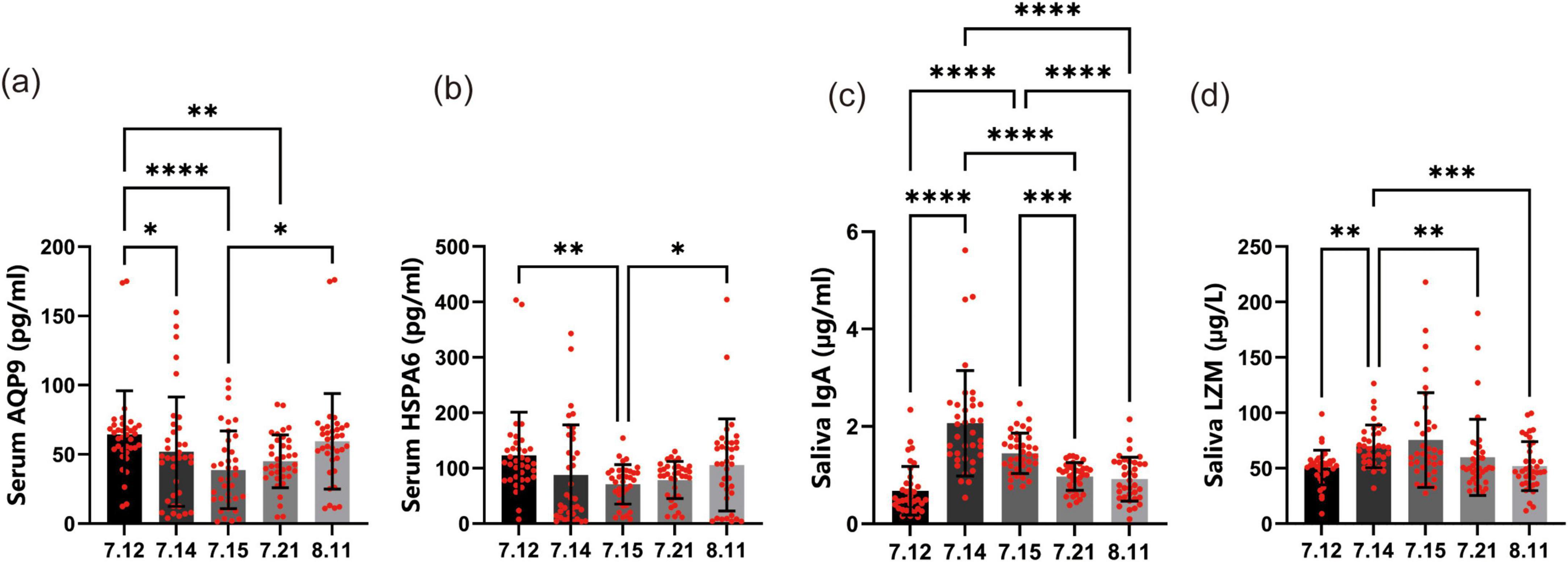
Figure 5. Effects of forest bathing on immune system components. (a) Serum aquaporin 9 (AQP9) levels: at baseline (July 12), the average Serum AQP9 level was 64.4 pg/ml. During the forest bathing period, there was a significant decrease in Serum AQP9 levels, with values of 51.9 pg/ml on July 14 and 38.8 pg/ml on July 15. After the intervention, the levels partially recovered to 45.0 pg/ml on July 21 and 59.4 pg/ml on August 11. (b) Serum heat shock protein A6 (HSPA6) levels: at baseline (July 12), the average Serum HSPA6 level was 123.3 pg/ml. During the forest bathing period, there was a notable decrease in Serum HSPA6 levels, with values of 87.6 pg/ml on July 14 and 71.0 pg/ml on July 15. After the intervention, the levels partially recovered to 78.6 pg/ml on July 21 and 105.8 pg/ml on August 11. (c) Salivary immunoglobulin A (IgA) levels: at baseline (July 12), the average Salivary IgA level was 0.68 μg/ml. During the forest bathing period, there was a significant increase in Salivary IgA levels, with values of 2.1 μg/ml on July 14 and 1.4 μg/ml on July 15. After the intervention, the levels declined to 1.0 μg/ml on July 21 and 0.9 μg/ml on August 11. (d) Salivary lysozyme (LZM) levels: at baseline (July 12), the average Salivary LZM level was 50.7 μg/L. During the forest bathing period, there was a significant increase in Salivary LZM levels, with values of 69.8 μg/L on July 14 and 75.4 μg/L on July 15. After the intervention, the levels declined to 59.9 μg/L on July 21 and 52.0 μg/L on August 11. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Heat shock 70-kDa protein 6, a member of the HSP70 family (Leung et al., 1990), is primarily localized in the cytoplasm and extracellular exosomes (Radons, 2016). Elevated HSPA6 expression has been implicated in the progression of various malignancies, including gastric, lung, and liver cancers (Su et al., 2021). In addition, HSPA6 plays a critical role in internal ribosome entry site (IRES)-mediated translation, and its knockdown has been shown to reduce luciferase activity driven by viral IRES elements (e.g., enterovirus A71, coxsackievirus A16, echovirus 9, encephalomyocarditis virus, and hepatitis C virus). These findings suggest that HSPA6 may facilitate the function of essential cellular proteins required for viral IRES activity (Su et al., 2021). At baseline, the average Serum HSPA6 level (Figure 5b) was 123.3 pg/ml. A marked decrease was observed during the forest bathing period, with levels dropping to 87.6 pg/ml on July 14 and further to 71.0 pg/ml on July 15. This significant reduction indicates that forest bathing may exert an immediate and potent effect on lowering Serum HSPA6 levels, potentially reflecting reduced cellular stress or an enhanced immune response. Following the intervention, Serum HSPA6 levels partially rebounded, increasing to 78.6 pg/ml on July 21 and 105.8 pg/ml by August 11. Although these values remained below the baseline, the partial recovery suggests that the acute effects of forest bathing on HSPA6 may not be sustained over the long term.
Salivary IgA is a pivotal component of the oral immune system, preventing pathogen adhesion to epithelial surfaces and helping maintain a balanced oral microbiota (Sun et al., 2016). At baseline, the average salivary IgA concentration (Figure 5c) was 0.7 μg/ml. During the forest bathing period, levels increased markedly, reaching 2.1 μg/ml on July 14 and 1.4 μg/ml on July 15, suggesting that forest bathing may exert an immediate and potent effect on enhancing oral immune function. Following cessation of the forest bathing sessions, salivary IgA levels partially declined, measuring 1.0 μg/ml on July 21 and 0.9 μg/ml on August 11. Although these values did not revert to the baseline, their partial recovery indicates that the acute elevation of salivary IgA observed during the intervention may not be fully sustained over the long term.
Lysozyme is a crucial component of the oral immune system, playing a vital role in protecting the oral cavity from bacterial infections (Mukherjee et al., 2008). It is a small molecule enzyme that can hydrolyze the cell walls of bacteria, particularly Gram-positive bacteria, thereby destroying them. LZM is widely present in human saliva and other secretions, and its levels are closely related to the occurrence and development of oral diseases such as dental caries and periodontitis. In our study, the levels of salivary LZM (Figure 5d) showed significant changes over the course of the forest bathing intervention. At baseline, the average salivary LZM level was 50.7 μg/L. During the forest bathing period, there was a notable increase in salivary LZM levels. On July 14, the level rose to 69.8 μg/L, and on July 15, it further increased to 75.4 μg/L. This significant increase suggests that forest bathing may have an immediate and potent effect on enhancing salivary LZM production, which could be associated with improved oral immune function. After the forest bathing period, the levels of salivary LZM began to decline slightly. On July 21, the level decreased to 59.92 μg/L, and by August 11, it had further decreased to 52.0 μg/L. Although the levels did not return to the baseline value, the partial recovery indicates that the effect of forest bathing on salivary LZM may not be sustained over the long term.
Overall, these findings suggest that forest bathing has a significant impact on Serum AQP9, Serum HSPA6, salivary IgA, and salivary LZM levels, with the most pronounced effects observed in the early stages of the intervention.
Discussion
Our comprehensive study reveals that forest bathing provides multifaceted health benefits mediated through interconnected psychological, physiological, and immunological pathways. These findings not only affirm previous research but also provide novel insights, particularly highlighting the unique contributions of low-latitude evergreen broad-leaved forests dominated by Safflower lotus.
Forest bathing engages multiple sensory pathways—such as visual, auditory, and olfactory stimuli—which together induce relaxation by modulating the central and autonomic nervous systems (Tsunetsugu et al., 2010). Growing evidence has shown that such exposure to forest environments can effectively reduce negative emotions and stress while enhancing psychological wellbeing (Morita et al., 2007; Park et al., 2010). Consistent with these findings, our study demonstrated significant improvements in mood and perceived stress during the intervention, with reductions in POMS and PSS scores sustained up to 1 month post-intervention. Furthermore, by applying AIS and SRSS for the first time in a forest bathing context, we observed notable improvements in sleep quality. These results suggest that forest bathing can provide rapid psychological benefits that last over time, likely building a basis for broader physiological and immunological effects. It serves as a practical and evidence-based intervention helping people recover emotionally and reduce stress, and offers new views on its lasting impacts on sleep and mental health.
The physiological and immunological benefits observed in this study provide multifaceted evidence for the health-promoting effects of forest bathing. Notably, we evaluated hematological indicators such as HGB and RBCs, which showed transient increases during the intervention—likely reflecting an acute response to physical activity and elevated oxygen demand. These returned to baseline levels post-intervention, suggesting a short-term physiological adaptation.
On the immune level, we incorporated lymphocyte subset analysis to complement existing literature focused on NK cell activity (Li et al., 2008). Our findings revealed sustained increases in total T lymphocytes, including both helper and suppressor subsets, indicating a potential enhancement in immune regulation. Stress-related molecular markers also demonstrated notable changes. Serum levels of AQP9 and HSPA6 declined following forest exposure, suggesting reductions in chronic inflammation and cellular stress. These molecular shifts further support the stress-relieving and anti-inflammatory properties of forest environments. Additionally, we assessed oral immune markers, including salivary IgA and LZM. Salivary IgA increased markedly during forest bathing, indicating enhanced mucosal immune defense. Although levels declined after the intervention, they remained above baseline. Salivary LZM showed a similar pattern, with significant elevation during the intervention and partial reduction afterward. These results point to an immediate but moderately sustained boost in oral immune function, consistent with the role of IgA and LZM in pathogen defense (Sun et al., 2016; Mukherjee et al., 2008). Taken together, the integration of cellular, molecular, and mucosal immune indicators presents a comprehensive view of forest bathing’s multidimensional effects. These findings deepen our understanding of how nature exposure may support immune resilience through both systemic and local pathways.
The unique environment of low-latitude ever green broad-leaved forests, distinct from the high-latitude coniferous forests typically studied, may contribute to these findings. The specific phytoncides released by Safflower lotus (Chen et al., 2025), along with the unique climate and biodiversity of this ecosystem, could influence the observed health outcomes. This environmental context may explain discrepancies with previous studies conducted in different forest types (Li, 2022). Our findings underscore the potential of forest bathing as a non-pharmacological intervention for promoting holistic health. The transient yet significant improvements in multiple health domains highlight its relevance in preventive medicine and public health strategies. Urban planners and policymakers should consider integrating forest spaces into urban designs to leverage these health benefits.
This study has several limitations. First, although we used cold-chain transport and on-site centrifugation to preserve sample integrity, the long and winding route from the forest to the laboratory may still have caused some sample degradation, such as hemolysis or protein breakdown. Second, the sample size was limited by site capacity and logistical conditions. While statistical power was acceptable, larger studies in better-equipped settings would strengthen generalizability. Third, participants were healthy young adults, which limits applicability to other populations. Future studies should include diverse groups, such as those with chronic illnesses or mental health conditions. Fourth, the study was conducted in a single forest type—a low-latitude evergreen broad-leaved forests. Comparisons with other forest environments are needed to understand ecological influences on health outcomes. Lastly, the study duration was short. Long-term research is needed to assess sustained effects and explore underlying mechanisms.
Conclusion
In conclusion, our study demonstrates that forest bathing in low-latitude evergreen broad-leaved forests offers significant and multi-dimensional health benefits. By elucidating the interconnected psychological, physiological, and immunological mechanisms, we strengthen the scientific rationale for integrating forest medicine into public health strategies. As urbanization continues to expand, the conservation and utilization of natural environments will become increasingly vital, offering a sustainable means for enhancing human health and facilitating reconnection with nature in modern society.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Shaoguan First People’s Hospital Medical Ethics Review Number ((2024)33). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XD: Writing – original draft, Software, Data curation, Validation, Investigation, Methodology. DL: Data curation, Writing – original draft, Investigation, Software, Validation. JO: Writing – review and editing, Supervision, Resources, Funding acquisition. HX: Supervision, Conceptualization, Project administration, Writing – original draft. ZT: Writing – review and editing, Investigation, Supervision, Validation. LQ: Supervision, Writing – review and editing. JY: Writing – review and editing, Supervision. HW: Supervision, Writing – review and editing, Validation. DG: Writing – review and editing, Supervision, Validation. ZL: Supervision, Writing – review and editing, Methodology. XW: Validation, Supervision, Writing – review and editing. YX: Resources, Writing – review and editing, Supervision, Project administration. XF: Supervision, Writing – review and editing, Methodology. JT: Visualization, Validation, Writing – review and editing, Supervision. MW: Supervision, Writing – review and editing, Validation. BZ: Resources, Writing – review and editing, Investigation, Formal Analysis. HP: Funding acquisition, Supervision, Writing – review and editing. YP: Supervision, Project administration, Writing – review and editing, Validation. QW: Writing – review and editing, Project administration, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research work was supported by the Shaoguan Science and Technology Program (210811164532141).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1619569/full#supplementary-material
References
Callewaert, L., and Michiels, C. W. (2010). Lysozymes in the animal kingdom. J. Biosci. 35, 127–160. doi: 10.1007/s12038-010-0015-5
Chen, X., Gong, D., Lin, Y., Xu, Q., Wang, Y., Liu, S., et al. (2025). Emission characteristics of biogenic volatile organic compounds in a subtropical pristine forest of southern China. J. Environ. Sci. 148, 665–682. doi: 10.1016/j.jes.2023.09.041
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. doi: 10.2307/2136404
Craig, J. M., Logan, A. C., and Prescott, S. L. (2016). Natural environments, nature relatedness and the ecological theater: Connecting satellites and sequencing to shinrin-yoku. J. Physiol. Anthropol. 35:1. doi: 10.1186/s40101-016-0083-9
Franckowiak, G. A., Perdicas, M., and Smith, G. A. (2019). Spatial ecology of coyotes in the urbanizing landscape of the Cuyahoga Valley, Ohio. PLoS One 4:e0227028. doi: 10.1371/journal.pone.0227028
Gong, Y., Klein Wolterink, R., Wang, J., Bos, G., and Germeraad, W. (2021). Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 14:73. doi: 10.1186/s13045-021-01083-5
Gross, J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology 39, 281–291. doi: 10.1017/s0048577201393198
Huan, J., Han, Y., and Chen, Y. (1995). [Effect of lysostaphin on phagocyte function in burn mice]. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi = Chinese J. Plastic Surg. Burns 11, 255–257. doi: 10.3760/cma.j.issn.1000-7806.1995.04.107
Ishibashi, K., Hara, S., and Kondo, S. (2009). Aquaporin water channels in mammals. Clin. Exp. Nephrol. 13, 107–117. doi: 10.1007/s10157-008-0118-6
Kechter, A., and Leventhal, A. M. (2019). Longitudinal association of sleep problems and distress tolerance during adolescence. Behav. Med. 45, 240–248. doi: 10.1080/08964289.2018.1514362
Lee, J., Park, B. J., Tsunetsugu, Y., Ohira, T., Kagawa, T., and Miyazaki, Y. (2011). Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 125, 93–100. doi: 10.1016/j.puhe.2010.09.005
Leung, T., Rajendran, M., Monfries, C., Hall, C., and Lim, L. (1990). The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B’) and isolation of its cDNA and genomic DNA. Biochem. J. 267, 125–132. doi: 10.1042/bj2670125
Li, J., Yin, S., and Duan, J. (2000). Analysis rating of sleep state of 13273 normal persons. China J. Health Psychol. 8:351. doi: 10.13342/j.cnki.cjhp.2000.03.059
Li, Q. (2022). Effects of forest environment (Shinrin-yoku/Forest bathing) on health promotion and disease prevention -the Establishment of “Forest Medicine”. J. Biol. Regul. Homeost Agents 24, 157–165. doi: 10.1265/ehpm.22-00160
Li, Q., Morimoto, K., Kobayashi, M., Inagaki, H., Katsumata, M., Hirata, Y., et al. (2010). A day trip to a forest park increases human natural killer activity and the expression of anti-cancer proteins in male subjects. Int. J. Immunopathol. Pharmacol. 21, 117–127. doi: 10.1177/039463200802100113
Li, Q., Morimoto, K., Nakadai, A., Inagaki, H., Katsumata, M., Shimizu, T., et al. (2008). Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 21, 117–127. doi: 10.1177/039463200802100113
Li, Q., Morimoto, K., Nakadai, A., Inagaki, H., Katsumata, M., Shimizu, T., et al. (2007). Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 20, 3–8. doi: 10.1177/03946320070200S20
Liang, P., Tang, Y., Fu, S., Lv, J., Liu, B., Feng, M., et al. (2015). Basophil count, a marker for disease activity in systemic lupus erythematosus. Clin. Rheumatol. 34, 891–896. doi: 10.1007/s10067-014-2822-9
Lira, F. T., White, M. J., and Finch, A. J. (1977). Anxiety and mood states in delinquent adolescents. J. Pers. Assess. 41, 532–536. doi: 10.1207/s15327752jpa4105_12
Martínez Arroyo, O., Andreu Vaíllo, Y., Martínez López, P., and Galdón Garrido, M. (2019). Emotional distress and unmet supportive care needs in survivors of breast cancer beyond the end of primary treatment. Support Care Cancer 27, 1049–1057. doi: 10.1007/s00520-018-4394-8
Mesko, B., Poliska, S., Szegedi, A., Szekanecz, Z., Palatka, K., Papp, M., et al. (2010). Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med. Genomics 3:15. doi: 10.1186/1755-8794-3-15
Miyazaki, Y., Ikei, H., and Song, C. (2014). [Forest medicine research in Japan]. Nihon Eiseigaku Zasshi. Jpn. J. Hygiene 69, 122–135. doi: 10.1265/jjh.69.122
Morita, E., Fukuda, S., Nagano, J., Hamajima, N., Yamamoto, H., Iwai, Y., et al. (2007). Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 121, 54–63. doi: 10.1016/j.puhe.2006.05.024
Mukherjee, S., Vaishnava, S., and Hooper, L. (2008). Multi-layered regulation of intestinal antimicrobial defense. Cell. Mol. Life Sci. 65, 3019–3027. doi: 10.1007/s00018-008-8182-3
Murphy, K. M., and Reiner, S. L. (2002). The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944. doi: 10.1038/nri954
Pantaleo, G., Graziosi, C., and Fauci, A. S. (1993). The immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328, 327–335. doi: 10.1056/NEJM199302043280508
Park, B. J., Tsunetsugu, Y., Kasetani, T., Kagawa, T., and Miyazaki, Y. (2010). The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prevent. Med. 15, 18–26. doi: 10.1007/s12199-009-0086-9
Prüss-Üstün, A., Corvalán, C. F., Bos, R., and Neira, M. P. (2016). Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks. Available online at: https://apps.who.int/iris/handle/10665/204585. (Accessed November 21, 2021).
Radons, J. (2016). The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 21, 379–404. doi: 10.1007/s12192-016-0676-6
Rump, K., and Adamzik, M. (2024). Aquaporins in sepsis- an update. Front. Immunol. 15:1495206. doi: 10.3389/fimmu.2024.1495206
Russell, M., Hajishengallis, G., Childers, N., and Michalek, S. (1999). Secretory immunity in defense against cariogenic mutans streptococci. Caries Res. 33, 4–15. doi: 10.1159/000016490
Sakaguchi, S., Yamaguchi, T., Nomura, T., and Ono, M. (2008). Regulatory T cells and immune tolerance. Cell 133, 775–787. doi: 10.1016/j.cell.2008.05.009
Su, Y.-S., Hwang, L.-H., and Chen, C.-J. (2021). Heat shock protein A6, a novel HSP70, is induced during enterovirus A71 infection to facilitate internal ribosomal entry site-mediated translation. Front. Microbiol. 12:664955. doi: 10.3389/fmicb.2021.664955
Sun, H., Chen, Y., Zou, X., Li, Q., Li, H., Shu, Y., et al. (2016). Salivary secretory immunoglobulin (SIgA) and lysozyme in malignant tumor patients. BioMed Res. Int. 2016:8701423. doi: 10.1155/2016/8701423
Tierney, B., Van den Abbeele, P., Al-Ghalith, G., Verstrepen, L., Ghyselinck, J., Calatayud, M., et al. (2023). Capacity of a microbial synbiotic to rescue the in vitro metabolic activity of the Gut Microbiome following Perturbation with alcohol or antibiotics. Appl. Environ. Microbiol. 89:e0188022. doi: 10.1128/aem.01880-22
Tsunetsugu, Y., Park, B.-J., and Miyazaki, Y. (2010). Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prevent. Med. 15, 27–37. doi: 10.1007/s12199-009-0091-z
Keywords: sleep improvement, mood regulation, immune modulation, nature therapy, forest bathing
Citation: Dai X, Liao D, Ouyang J, Xiao H, Tian Z, Qiu L, Yang J, Wang H, Gong D, Li Z, Wang X, Xu Y, Fang X, Tan J, Wang M, Zhou B, Peng H, Peng Y and Wu Q (2025) Forest bathing enhances sleep, mood, and immunity: insights from low-latitude evergreen broad-leaved forests. Front. For. Glob. Change 8:1619569. doi: 10.3389/ffgc.2025.1619569
Received: 29 April 2025; Accepted: 16 June 2025;
Published: 09 July 2025.
Edited by:
Guangyu Wang, The University of British Columbia, CanadaReviewed by:
Slawomir Murawiec, Harmonia Luxmed Medical Center, PolandNatalia Korcz, Forest Research Institute (IBL), Poland
Copyright © 2025 Dai, Liao, Ouyang, Xiao, Tian, Qiu, Yang, Wang, Gong, Li, Wang, Xu, Fang, Tan, Wang, Zhou, Peng, Peng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huagui Peng, bmxodWFndWlAMTYzLmNvbQ==; Yihua Peng, cGVuZ3lpaHVhMTIzQDE2My5jb20=; Qiang Wu, c2d3dXFpYW5nQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
 Xiangheng Dai
Xiangheng Dai Dongteng Liao2†
Dongteng Liao2† Hao Wang
Hao Wang Daocheng Gong
Daocheng Gong Xu Wang
Xu Wang Qiang Wu
Qiang Wu