- 1Department of Botany and Microbiology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
- 2Research Lab of Biogeography and Wildlife Parasitology, Faculty of Science, Ain Shams University, Cairo, Egypt
- 3Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 4Department of Plant Pathology, Plant Pathology Research Institute, Agricultural Research Center, Giza, Egypt
Introduction: Fusarium proliferatum is a globally distributed fungal pathogen of major agricultural significance, responsible for considerable crop losses and the production of hazardous mycotoxins that endanger food security and human health. Climate change is expected to modify the geographic distribution of plant pathogens, allowing their spread into previously unsuitable regions.
Methods: This study employed the Maximum Entropy (MaxEnt) species distribution modeling approach to evaluate the potential impacts of climate change on the global distribution of F. proliferatum under different Representative Concentration Pathway (RCP) emission scenarios. A total of 347 species occurrence records were obtained from the Global Biodiversity Information Facility (GBIF) and spatially filtered to minimize sampling bias. Bioclimatic variables, primarily temperature-related factors, were identified as key environmental determinants through systematic variable selection and correlation analysis. Model performance was evaluated using the Area Under the Curve (AUC) metric.
Results: The MaxEnt model demonstrated excellent predictive accuracy (AUC = 0.844). Current distribution maps revealed high environmental suitability in tropical and subtropical regions, with moderate suitability in temperate zones. Future projections for 2050 and 2070 under both moderate (RCP 2.6) and severe (RCP 8.5) emission scenarios indicated notable poleward range expansion, particularly into northern Europe, northern Asia, and northern North America. The most substantial distributional shifts occurred under the severe emission scenario for 2070, showing extensive expansion of highly suitable environments into previously marginal regions. Temperature seasonality was identified as the most influential limiting factor globally.
Discussion: These findings suggest that ongoing climate change will substantially broaden the geographic range of F. proliferatum, heightening mycotoxin contamination risks in new agricultural areas and threatening food security in temperate zones historically unexposed to this pathogen. The study provides critical insights for developing proactive surveillance, biosecurity policies, and adaptive management strategies to mitigate the escalating risks posed by this economically important fungal pathogen under future climatic conditions.
1 Introduction
Fusarium proliferatum is a ubiquitous fungal pathogen of considerable agricultural and economic significance, causing diseases in maize, wheat, rice, and numerous other crops globally (Chelkowski et al., 2000; Logrieco et al., 2002). The species poses a dual threat: direct crop damage through yield losses and indirect harm through production of hazardous mycotoxins, particularly fumonisins, which contaminate food and feed sources and present serious risks to human and animal health (Marasas et al., 2004; Gelderblom et al., 2008). The fungus exhibits exceptional adaptability across diverse environmental conditions and agricultural systems, establishing infections from tropical to temperate climatic zones (Leslie and Summerell, 2006; Munkvold and White, 2016). Globally, F. proliferatum infections result in millions of dollars in annual economic losses through reduced crop yields, mycotoxin contamination, and associated trade restrictions, with significant impacts documented across North America, Europe, Asia, and Africa (Munkvold, 2003; Boutigny et al., 2011).
Climate change represents a major environmental challenge with profound implications for plant pathogen distributions and disease epidemiology (Garrett et al., 2006; Elad and Pertot, 2014). Rising global temperatures, altered precipitation patterns, and increased frequency of extreme weather events are creating novel ecological niches that may facilitate the spread and intensification of fungal diseases (Chakraborty and Newton, 2011; Fones et al., 2020). These climatic shifts can modify fundamental aspects of fungal biology—including viability, sporulation, dispersal, and infection dynamics—potentially leading to geographic range expansions and disease establishment in previously unaffected regions (Medina et al., 2017; Xu et al., 2024). For mycotoxigenic fungi like F. proliferatum, continued climate change may not only expand pathogen ranges but also increase mycotoxin prevalence in both traditional and newly vulnerable agricultural areas.
Species distribution modeling has transformed our understanding of pathogen ecology and disease risk assessment under environmental change (Elith and Leathwick, 2009; Peterson et al., 2011). Maximum Entropy modeling (MaxEnt) has emerged as a particularly powerful approach for forecasting climate change impacts on plant pathogen distributions, enabling proactive management strategies and risk assessment (Bebber et al., 2013; Merow et al., 2013). This methodology has been successfully applied to delineate climate niches for various fungal pathogens, providing valuable insights into potential range shifts and emerging risks. By integrating climate change projections with species distribution models, researchers can evaluate how modified environmental conditions may affect pathogen establishment and spread in coming decades.
Despite the agricultural and public health importance of F. proliferatum, comprehensive global-scale assessments of its potential distribution under future climate scenarios remain lacking. Previous studies have focused primarily on regional scales or specific crop systems, limiting our understanding of worldwide distribution dynamics and vulnerability patterns. Moreover, few studies have explicitly linked projected pathogen range expansions to mycotoxin contamination risks and food security implications at the global scale. This knowledge gap is critical because F. proliferatum's ability to produce fumonisins means that geographic expansion represents not merely an agricultural pest issue, but a direct threat to food safety in regions with no prior experience managing this pathogen or its associated mycotoxins. Understanding the global patterns of climate-driven distribution changes is essential for developing coordinated international surveillance programs, biosecurity measures, and adaptive agricultural strategies.
This study addresses these gaps by providing the first comprehensive global assessment of climate change impacts on F. proliferatum distribution, explicitly integrating mycotoxin risk implications. This research advances beyond previous regional-scale pathogen studies by: (1) providing comprehensive global-scale assessment of F. proliferatum distribution under climate change; (2) explicitly linking projected range expansion to mycotoxin contamination risks and food security implications; (3) integrating multiple emission scenarios and time horizons to inform adaptive management strategies; and (4) identifying specific geographic regions vulnerable to pathogen emergence. Specifically, we aim to: (1) characterize the current global distribution patterns and principal environmental determinants of F. proliferatum; (2) project potential environmental suitability changes under multiple climate scenarios and time horizons; (3) identify geographic regions vulnerable to pathogen emergence or intensification; and (4) provide evidence-based recommendations for disease management, surveillance strategies, and food safety planning. By combining extensive occurrence data with high-resolution climate projections and validated modeling approaches, this research provides actionable insights for agricultural adaptation and food security planning in a changing climate.
2 Materials and methods
2.1 Occurrence data collection and quality control
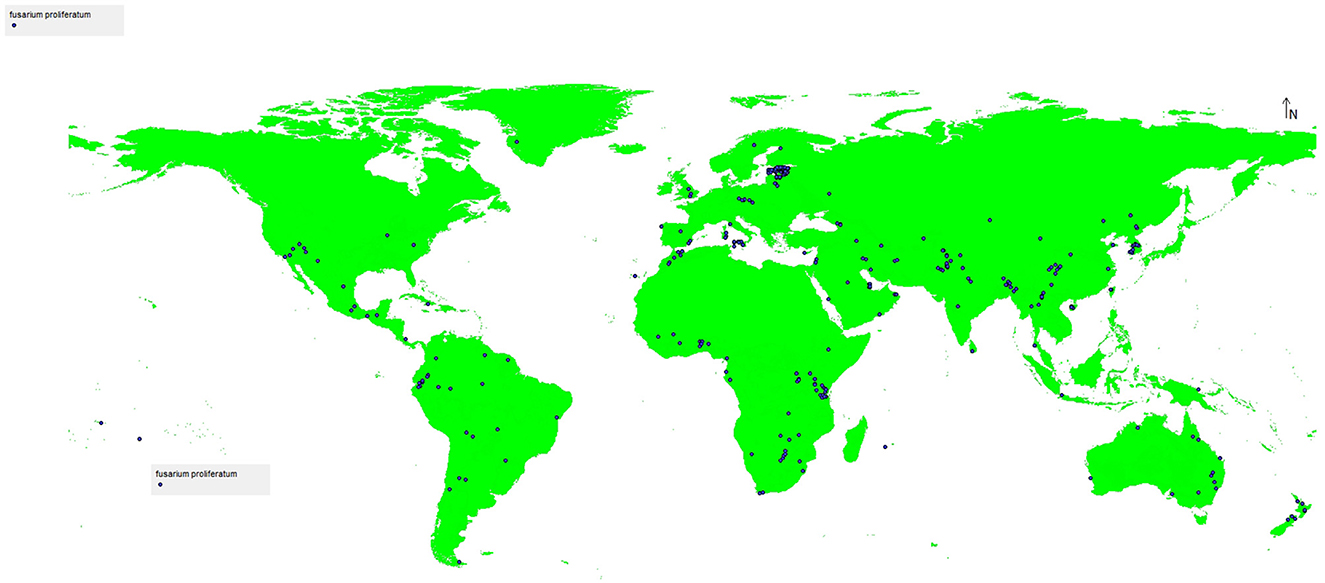
Species occurrence data for Fusarium proliferatum were retrieved from the Global Biodiversity Information Facility (GBIF) database (GBIF.org, 2023) using the search term “Fusarium proliferatum” with taxonomic verification. The initial query, conducted on January 15, 2023, yielded 4,995 occurrence records spanning from 1950 to 2023. The dataset underwent rigorous quality control and filtering procedures to ensure spatial accuracy and minimize modeling biases.
Records were excluded based on the following criteria: (1) absence of precise geographic coordinates (latitude and longitude); (2) coordinate uncertainty exceeding 5 km; (3) duplicate entries at identical coordinates that could artificially inflate presence probabilities in specific locations (Boria et al., 2014). Records from both agricultural and non-agricultural hosts were retained to capture the full ecological niche of the species across diverse environmental contexts. To reduce geographic autocorrelation and sampling bias, spatial thinning was applied using a 5-km radius threshold, retaining only one occurrence record per 5 km × 5 km grid cell (Aiello-Lammens et al., 2015). This procedure resulted in a final dataset of 347 spatially independent, high-quality occurrence records suitable for species distribution modeling. The analyzed occurrence data were exported and saved in comma-separated values (CSV) format for subsequent analysis (Figure 1).
Data limitations and potential biases: We acknowledge several inherent limitations in GBIF occurrence data. First, geographic sampling is uneven, with greater representation from developed regions (North America, Europe) compared to developing countries where the pathogen may also be present. This sampling bias could lead to underestimation of the species' climatic niche breadth, particularly in undersampled tropical and subtropical regions of Africa, Asia, and South America. Second, GBIF records represent documented observations rather than systematic surveys, potentially overrepresenting easily accessible agricultural areas and underrepresenting wild or non-agricultural hosts. Third, occurrence data reflect the realized niche (where the species currently exists under the influence of biotic interactions, dispersal limitations, and human activities) rather than the fundamental niche (the full range of conditions the species could potentially tolerate). Despite these limitations, GBIF represents the most comprehensive global occurrence database available, and our spatial filtering procedures help mitigate some biases by reducing clustering effects in heavily sampled regions.
2.2 Bioclimatic variables and variable selection
Environmental data were obtained from the WorldClim database version 2.1, providing global climate layers at approximately 1 km spatial resolution (Fick and Hijmans, 2017). The complete set of 19 standard bioclimatic variables was initially considered for model development (Supplementary Table S1 provides a complete list of all 19 bioclimatic variables with their descriptions and units). These variables encompass annual trends (e.g., mean annual temperature, annual precipitation), seasonality (e.g., temperature and precipitation variability), and extreme environmental conditions (e.g., temperature of the warmest and coldest months).
Prior to model construction, we conducted comprehensive correlation analysis to identify and eliminate collinearity among predictor variables, following established best practices for species distribution modeling (Dormann et al., 2013). Variables exhibiting Pearson correlation coefficients exceeding |0.8| were considered highly correlated, and subsequent selection prioritized biological relevance and contribution to model performance (Merow et al., 2013).
Preliminary MaxEnt models incorporating all 19 variables revealed that temperature-related variables contributed over 85% of the model's explanatory power, while precipitation variables showed minimal individual contributions (< 5% combined). Additionally, correlation analysis demonstrated strong collinearity among several precipitation variables (bio_12, bio_13, bio_14, bio_16, bio_17, bio_18, bio_19; r > 0.85).
Biological rationale for temperature focus: Temperature represents a fundamental constraint on fungal physiology, directly limiting enzymatic processes, growth rates, sporulation capacity, and survival during dormant periods (Medina et al., 2017). Field and laboratory studies consistently demonstrate that temperature thresholds define the geographic limits of F. proliferatum establishment, whereas the species can persist across wide precipitation ranges through endophytic associations, seed contamination, and adaptation to variable moisture conditions (Leslie and Summerell, 2006; Munkvold and White, 2016). This biological context, combined with the strong collinearity among precipitation variables and their minimal contribution in preliminary models, justified our focus on temperature-related predictors.
Based on these combined statistical and biological considerations, five temperature-related variables were selected as the final predictors: annual mean temperature (bio_1), temperature seasonality (bio_4), minimum temperature of the coldest month (bio_6), mean temperature of the warmest quarter (bio_10), and mean temperature of the coldest quarter (bio_11). These variables exhibited low intercorrelation (r < 0.7) while capturing distinct aspects of the thermal environment critical to fungal ecology.
2.3 Future climate scenarios
Future climate projections were derived from the MRI-CGCM3 (Meteorological Research Institute Coupled Global Climate Model version 3) general circulation model, accessed through the WorldClim database (Fick and Hijmans, 2017). Climate data were obtained for two time periods (2050: 2041-2060; 2070: 2061-2080) under two Representative Concentration Pathways: RCP 2.6 and RCP 8.5 (van Vuuren et al., 2011).
We selected RCP 2.6 and RCP 8.5 to represent the extreme bounds of plausible climate futures: RCP 2.6 represents a stringent mitigation scenario with peak greenhouse gas concentrations followed by decline, whereas RCP 8.5 represents a high-emission scenario with continued increases throughout the twenty-first century (Moss et al., 2010). This approach allows assessment of species distribution under best-case and worst-case scenarios, providing critical information for risk assessment and adaptive management planning. The bracketing approach we employed captures the range of potential outcomes for policy-relevant decision making.
The MRI-CGCM3 model was selected for several reasons: (1) its demonstrated strong performance in reproducing historical climate patterns, particularly for temperature variables globally (Yukimoto et al., 2012); (2) its availability within the WorldClim framework at the required spatial resolution for species distribution modeling applications; and (3) its successful application in previous plant pathogen distribution studies. We acknowledge that using a single GCM represents a limitation of this study, as multi-model ensembles are generally preferred to account for inter-model uncertainty in climate projections (Araújo and New, 2007). However, the MRI-CGCM3 model is considered representative of the CMIP5 ensemble for global temperature projections, and our focus on extreme emission scenarios helps bound the range of potential distribution changes. Future studies should incorporate multiple GCMs to provide more robust uncertainty estimates in projected distributions.
2.4 Data processing and preparation
All environmental data layers (current and future climate) were processed using ArcGIS Desktop version 10.8.1 (ESRI, 2022). The original bioclimatic raster files in GeoTIFF format were converted to ASCII grid format to ensure compatibility with MaxEnt software. All layers were verified to maintain identical spatial extent (global coverage), spatial resolution (30 arc-s, approximately 1 km at the equator), coordinate reference system (WGS84), and cell alignment to enable direct comparison and accurate model projections across current and future scenarios.
2.5 Species distribution modeling
Species distribution modeling was performed using Maximum Entropy (MaxEnt) software version 3.4.4 as a standalone Java application (Phillips et al., 2006; Phillips and Dudík, 2008). MaxEnt was selected for its demonstrated robust performance with presence-only data and its capacity to model complex, non-linear relationships between environmental variables and species occurrence (Elith et al., 2011).
Model parameterization followed systematic tuning procedures to optimize predictive performance and avoid overfitting. We evaluated combinations of regularization multipliers (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0) and feature class combinations (L, LQ, H, LQH, LQHP, LQHPT, where L = linear, Q = quadratic, H = hinge, P = product, T = threshold) using a model selection framework. For each parameter combination, models were evaluated using the corrected Akaike Information Criterion (AICc) and predictive performance metrics from cross-validation (Warren and Seifert, 2011; Radosavljevic and Anderson, 2014).
The optimal model employed a regularization multiplier of 1.5 with linear, quadratic, and hinge features (LQH), which provided the best balance between model complexity and predictive accuracy based on AICc values and omission rates. This configuration was selected from among 49 candidate models evaluated during the tuning process.
The modeling strategy utilized k-fold cross-validation with k = 5, allocating data randomly into five subsets. For each fold, 80% of occurrence records were used for model training and the remaining 20% were reserved for independent model testing and validation (Fielding and Bell, 1997). This procedure was replicated five times with different random partitions to ensure model robustness and provide estimates of prediction uncertainty. Ten thousand background points were randomly selected from the study area to characterize available environmental conditions, following MaxEnt default recommendations for presence-only modeling.
Final model projections represent the mean environmental suitability across all five cross-validation replicates, with variability among replicates used to assess prediction uncertainty. Models were projected onto current and future climate layers using the “cloglog” output format, which provides estimates of environmental suitability as probabilities of presence ranging from 0 (unsuitable) to 1 (highly suitable).
2.6 Model validation
Model performance was evaluated using multiple validation metrics to assess both discrimination ability and predictive accuracy. The Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) was calculated to evaluate the model's ability to distinguish between suitable and unsuitable environments across all threshold values (Hanley and McNeil, 1982). The True Skill Statistic (TSS) was computed to provide a threshold-dependent measure of model performance that incorporates both sensitivity (true positive rate) and specificity (true negative rate) independent of prevalence (Allouche et al., 2006). TSS values range from −1 to +1, with values >0.4 indicating good model performance and values >0.8 indicating excellent predictive accuracy (Landis and Koch, 1977).
For TSS calculation, the threshold was determined using the maximum training sensitivity plus specificity approach, which identifies the probability threshold that maximizes the sum of correctly predicted presences and absences. Validation metrics were calculated independently for each of the five cross-validation folds, and results are reported as mean ± standard deviation to characterize model stability and prediction uncertainty.
2.7 Environmental envelope analysis and limiting factor mapping
Complementary analyses were conducted using DIVA-GIS version 7.5 (Hijmans et al., 2012) to provide additional ecological insights into the climatic niche and environmental constraints of Fusarium proliferatum.
Environmental Envelope Analysis: The bioclimatic envelope (or “bioclim”) approach was applied as an independent exploratory analysis to visualize the species' climatic niche space. This analysis utilized all 19 WorldClim bioclimatic variables to define the multidimensional environmental space occupied by occurrence records. The envelope was constructed by determining the minimum and maximum values of each bioclimatic variable at all occurrence locations, thereby delineating the observed environmental boundaries of the species (Booth et al., 2014).
For visualization purposes, we present the two-dimensional niche space defined by annual mean temperature (bio_1) and annual precipitation (bio_12), as these represent the primary axes of climatic variation globally. Occurrence records were classified as either falling within the envelope across all 19 variables (indicating typical climatic conditions; shown as green points) or falling outside the envelope for one or more variables (indicating marginal or atypical conditions; shown as red points). This analysis provides insights into niche breadth and potential marginal populations.
It is important to note that this envelope analysis is independent of the MaxEnt species distribution model and serves a different purpose: the MaxEnt model identifies optimal conditions and their spatial distribution using the five selected predictor variables, whereas the envelope analysis explores the full observed climatic tolerances across all available bioclimatic dimensions.
Limiting Factor Analysis: Limiting factor mapping was performed using the five temperature-related variables employed in the MaxEnt model (bio_1, bio_4, bio_6, bio_10, bio_11). For each grid cell in the study area, DIVA-GIS identifies which environmental variable is most limiting—i.e., which variable's value is closest to the edge of the species' observed tolerance range (furthest from the optimal value). This analysis reveals spatial patterns in environmental constraints, showing which temperature factors most strongly limit species distribution in different geographic regions (Hijmans et al., 2012).
The limiting factor is determined by calculating the Mahalanobis distance between each environmental variable's value at a given location and the mean value of that variable across all occurrence points, standardized by the variance. The variable with the maximum standardized distance is identified as the limiting factor for that location. This approach highlights regions where specific thermal constraints prevent species establishment or reduce environmental suitability.
3 Results
3.1 Model performance and variable importance
The species distribution model for Fusarium proliferatum demonstrated high predictive accuracy across multiple validation metrics. The mean Area Under the Curve (AUC) value was 0.844 ± 0.021 (range: 0.820–0.867 across five cross-validation folds; Figure 2a), indicating excellent model ability to discriminate between suitable and unsuitable environments. The mean True Skill Statistic (TSS) value of 0.68 ± 0.048 (range: 0.62–0.73) confirmed the model's robustness, demonstrating strong agreement between predicted and observed distributions and accounting for both sensitivity (mean = 0.82 ± 0.03) and specificity (mean = 0.86 ± 0.04).
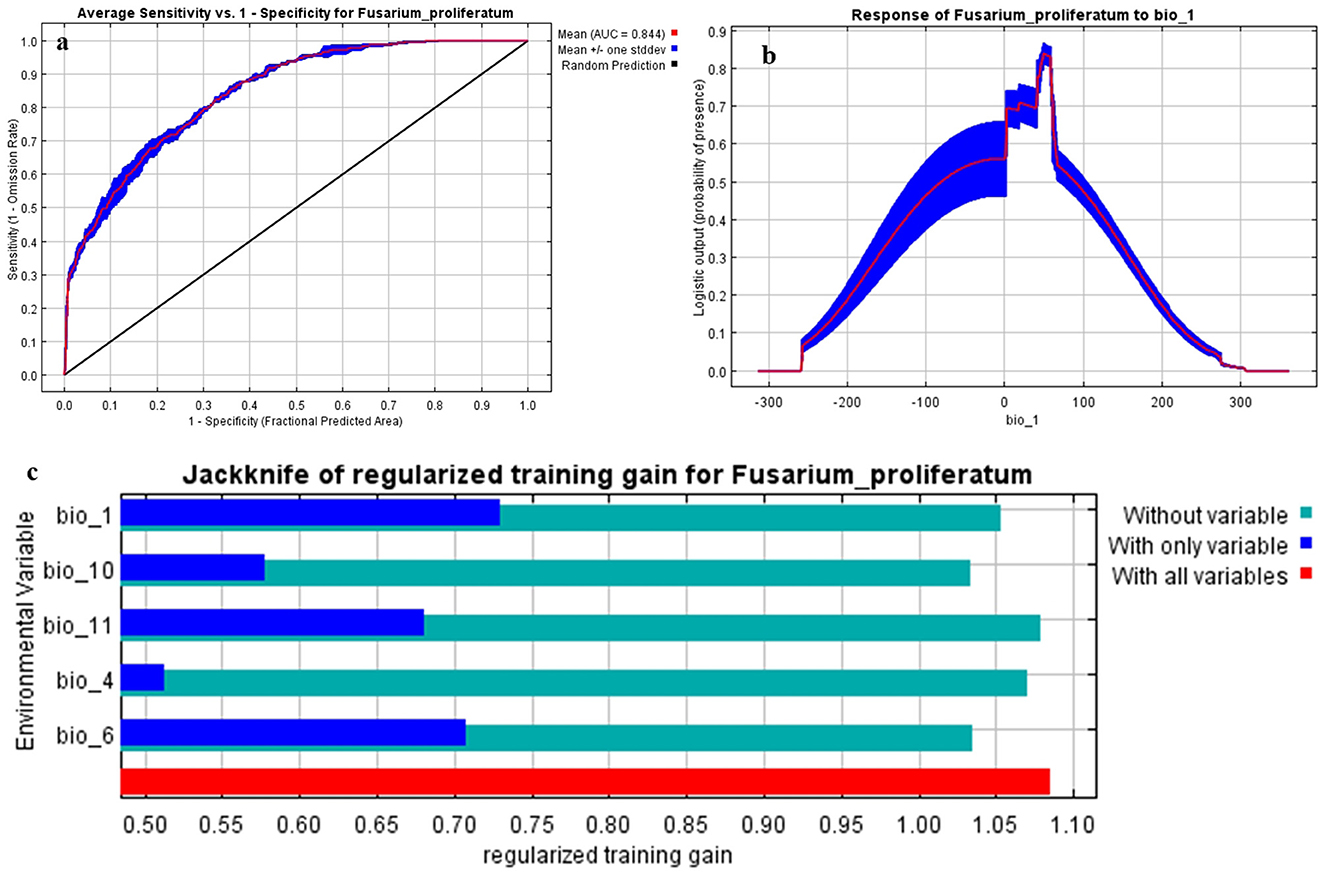
Figure 2. Model performance and variable importance: (a) ROC curve showing the AUC value; (b) Response curve of bio_1 (annual mean temperature); (c) Jackknife test analysis of selected variables.
Five temperature-related bioclimatic variables were identified as major factors influencing the distribution of Fusarium proliferatum: annual mean temperature (bio_1), temperature seasonality (bio_4), minimum temperature of the coldest month (bio_6), mean temperature of the warmest quarter (bio_10), and mean temperature of the coldest quarter (bio_11). The jackknife analysis (Figure 2c) indicated that annual mean temperature (bio_1) significantly enhanced the model's predictive capability, exhibiting the highest regularized training gain when utilized independently and the most considerable reduction in gain when omitted from the model.
The species response curve for annual mean temperature (bio_1) revealed distinct thermal preferences for Fusarium proliferatum (Figure 2b). Important clarification: The x-axis values in the response curve represent standardized environmental values used internally by MaxEnt, not actual temperature in degrees Celsius. When interpreted in the context of the actual occurrence data, the response curve indicates that F. proliferatum shows optimal environmental suitability in regions with annual mean temperatures between 15 and 25 °C, with probability of presence decreasing substantially below 10 °C and above 28 °C. This pattern reflects the species' preference for warm temperate to subtropical thermal conditions, consistent with its known ecology as a pathogen primarily affecting crops in tropical and warm temperate regions (Leslie and Summerell, 2006; Munkvold and White, 2016). The gradual decline in environmental suitability at very high temperatures (>28 °C) suggests potential thermal stress limits, and the sharp decline at lower temperatures (< 10 °C) indicates cold limitation of fungal growth and reproduction.
The environmental envelope analysis identified the two-dimensional climatic niche space occupied by Fusarium proliferatum, determined by annual mean temperature (bio_1) and annual precipitation (bio_12) (Figure 3). Of the 347 total occurrence records, 311 observations (89.6%) were situated within the species' environmental envelope when accounting for all 19 bioclimatic factors, whereas 224 records (64.5%) were located within the envelope considering only the temperature-precipitation bivariate space. The envelope test revealed that green dots, signifying occurrence records within the environmental envelope of all 19 bioclimatic parameters, were primarily clustered in the central region of the temperature-precipitation space (Figure 3). This central clustering indicates that the majority of documented occurrences represent typical climatic conditions for the species. Red dots, representing occurrence records that lie outside the envelope of one or more of the 19 bioclimatic variables, exhibited a more dispersed distribution pattern, with notable occurrences extending toward the periphery of the climatic niche limits. These peripheral records may represent marginal populations or indicate broader climatic tolerance than captured by the central tendency of the dataset.
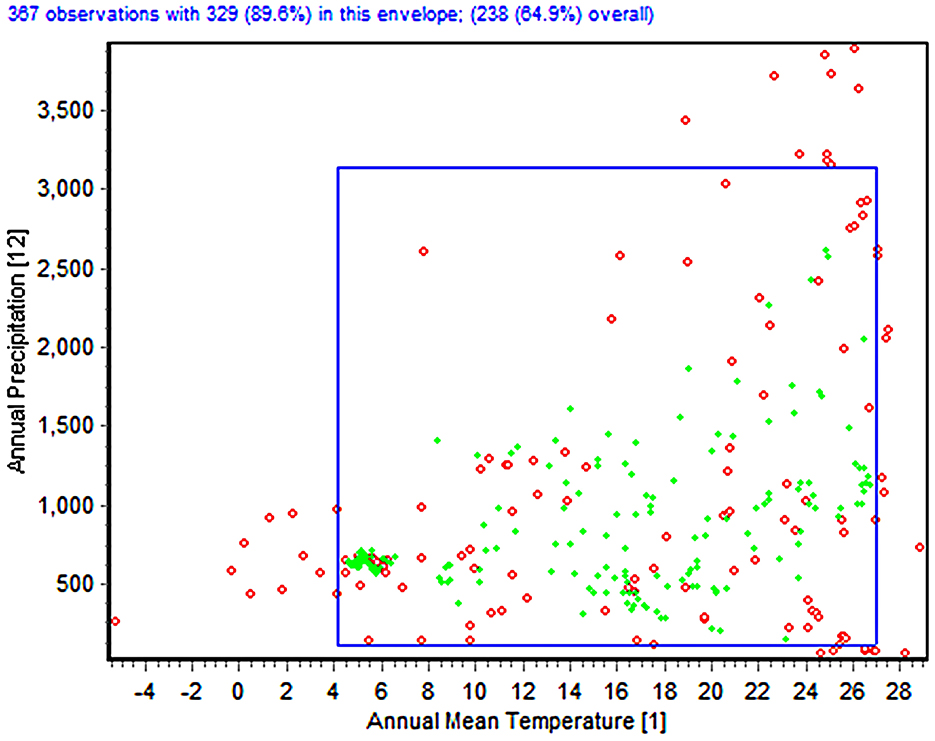
Figure 3. Two-dimensional niche analysis (the envelope test) of bio_1 (annual mean temperature) and bio_12 (annual precipitation). Green dots represent records that occur within the envelope for this test and for all the bioclimatic variables; red dots indicate records that occur outside the envelope either for this test or, if within the envelope for this test, outside the envelope for any other bioclimatic variables.
3.2 Current global distribution of Fusarium proliferatum
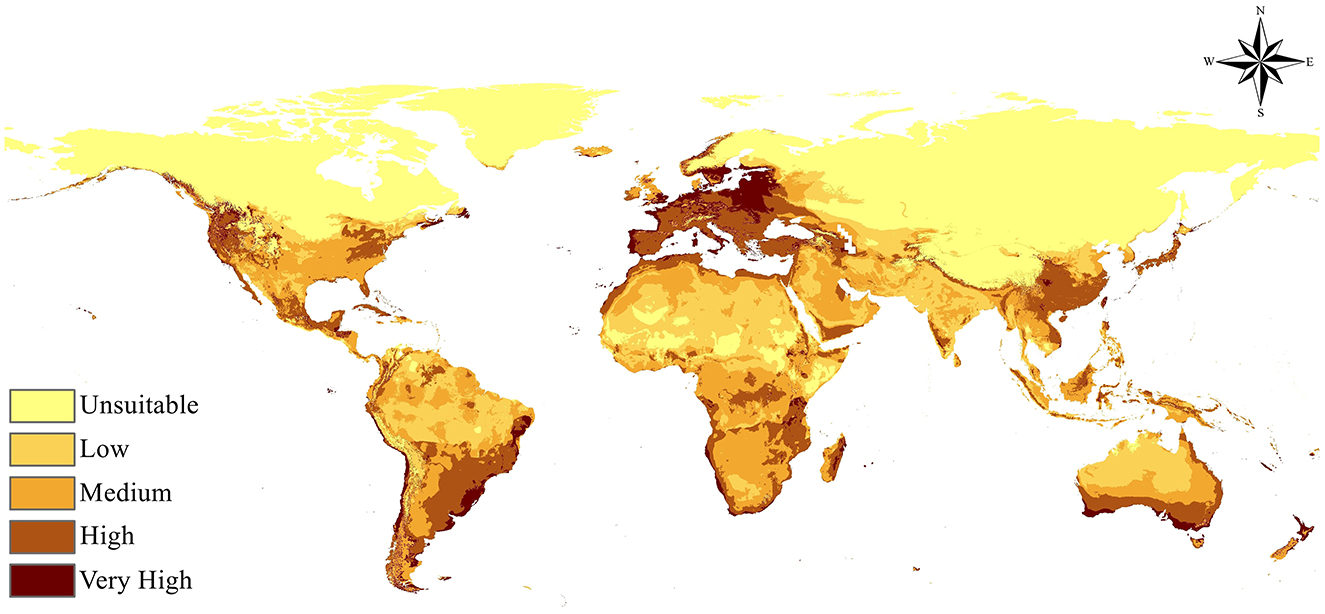
The MaxEnt species distribution model accurately forecasted the present global distribution of Fusarium proliferatum, highlighting specific patterns of environmental suitability across several geographical regions (Figure 4). The model output reveals a distinct gradient of suitability classes, with areas of very high environmental suitability primarily located in tropical and subtropical regions including South America, Central America, sub-Saharan Africa, Southeast Asia, and portions of South Asia. The eastern portions of Europe, particularly in temperate zones, exhibit moderate environmental suitability, suggesting favorable environmental circumstances for pathogen presence in these areas. The Atlantic coastal areas of South America, especially Brazil, Argentina, and Uruguay, show exceptional environmental suitability, and the southern portions of Australia also present highly favorable circumstances.
Regions of high and medium environmental suitability encompass transitional zones, including segments of the southeastern United States, Mediterranean Europe, and parts of eastern Asia, indicating areas conducive to pathogen survival and moderate disease pressure under present climatic conditions. Northern regions including Scandinavia, northern Russia, northern Canada, and Alaska currently exhibit low to unsuitable conditions. Arid regions such as the Sahara Desert, Arabian Peninsula, and central Australia show consistently low environmental suitability due to extreme environmental conditions.
Current mycotoxin risk distribution: The present distribution pattern indicates that mycotoxin contamination risk is concentrated in tropical and subtropical agricultural regions where maize, rice, and other susceptible crops are extensively cultivated. High-risk areas include major grain-producing regions of Brazil, Argentina, sub-Saharan Africa, India, Southeast Asia, and southern China. Temperate regions of Europe, northern North America, and northern Asia currently experience limited risk, providing a baseline against which future changes can be assessed.
3.3 Future projections of Fusarium proliferatum distribution
3.3.1 RCP 2.6 projection for 2050
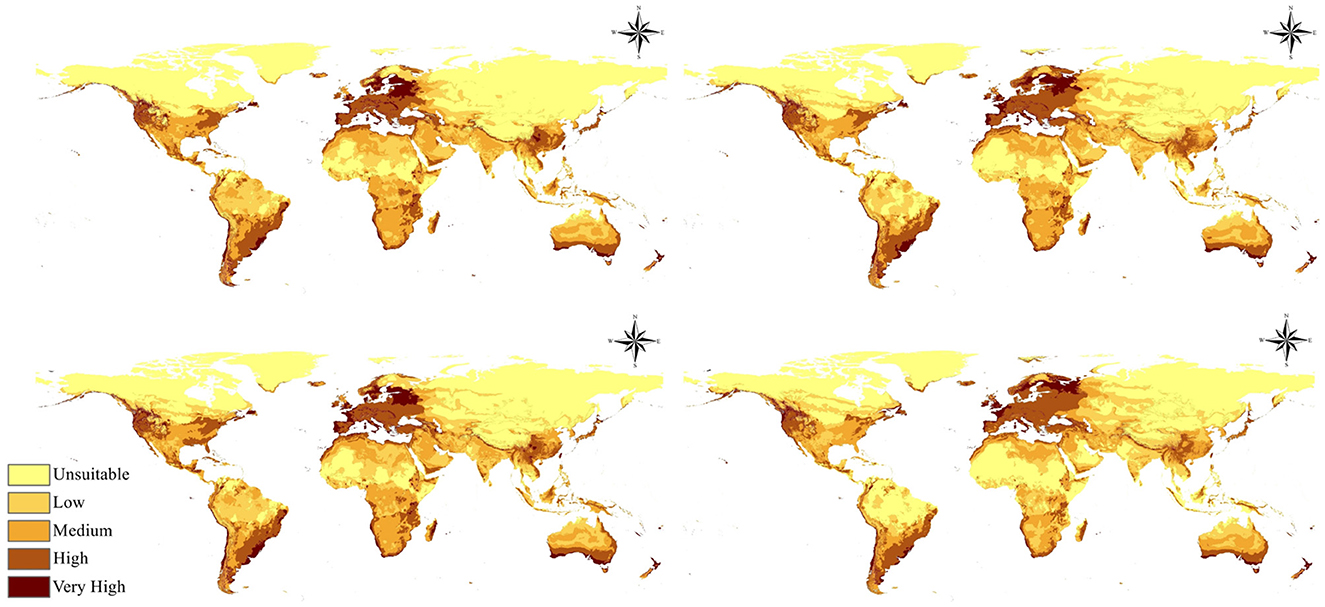
Under the moderate mitigation scenario (RCP 2.6) anticipated for 2050, modeling of Fusarium proliferatum distribution indicated clear patterns of environmental suitability expansion across several global regions (Figures 5a, 6a). The species exhibited potential for modest proliferation into traditionally inhospitable northern latitudes, particularly in northern Europe, northern Asia, and certain regions of northern North America. Zones of medium to high environmental suitability were primarily concentrated in temperate countries, with notable potential for expansion in Eastern Europe, Central Asia, and portions of the northern United States.
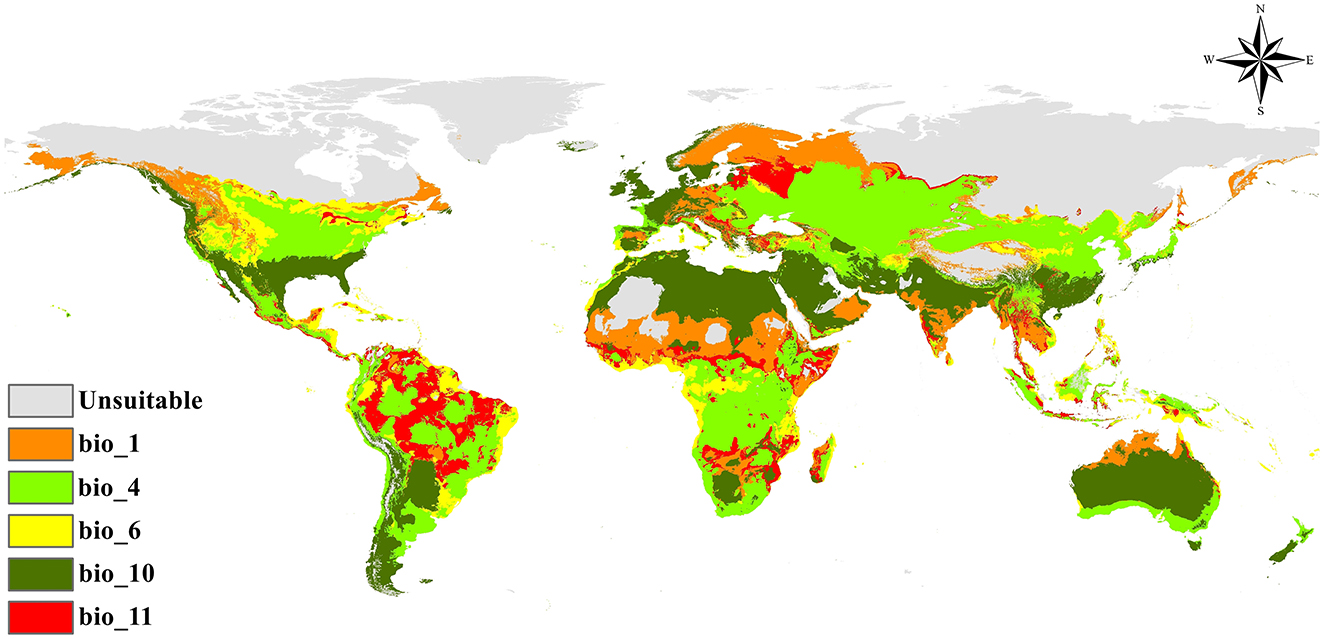
Figure 5. Predicted future distribution of environmental suitability of Fusarium proliferatum: (a) RCP 2.6 for 2050; (b) RCP 8.5 for 2050; (c) RCP 2.6 for 2070; (d) RCP 8.5 for 2070.
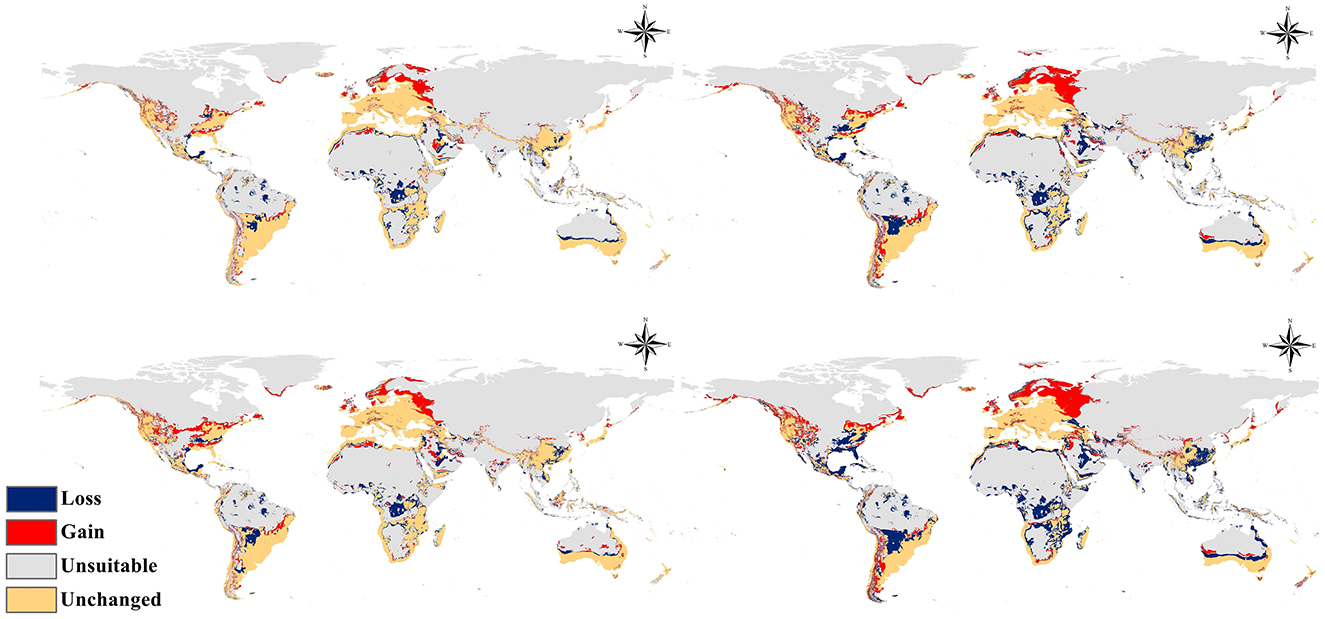
Figure 6. Calibration maps showing the change in range of Fusarium proliferatum: (a) RCP 2.6 for 2050; (b) RCP 8.5 for 2050; (c) RCP 2.6 for 2070; (d) RCP 8.5 for 2070.
Tropical and subtropical regions exhibited consistent environmental suitability patterns, with areas of high suitability persisting throughout portions of Central America, northern South America, sub-Saharan Africa, and Southeast Asia. The Arctic regions and extreme northern territories remained predominantly unsuitable for species establishment, and arid areas in North Africa, the Middle East, and central Australia exhibited persistently low suitability potential. This moderate scenario suggests that aggressive climate mitigation could constrain the magnitude of poleward expansion, maintaining many northern regions as marginal rather than optimal environments.
Mycotoxin risk implications: Under this scenario, fumonisin contamination risk begins to extend into southern portions of northern Europe and parts of the northern United States and southern Canada, requiring initial development of monitoring capacity in these regions.
3.3.2 RCP 8.5 projection for 2050
The high-emission scenario (RCP 8.5) for 2050 anticipated more substantial distributional changes for Fusarium proliferatum compared to the moderate RCP 2.6 scenario. Zones of high environmental suitability markedly expanded in temperate climates, especially in northern Europe, northern Asia, and the northern tier of North America. The species showed increased colonization potential in areas previously identified as having lower suitability ratings, including portions of Eastern Europe, northern China, and the northern Great Plains of North America (Figures 5b, 6b).
Tropical regions retained their elevated environmental suitability rating, with certain locations exhibiting enhanced favorable conditions. However, some equatorial regions showed variable patterns, with certain areas potentially experiencing reduced favorability due to anticipated temperature extremes. The expansion under RCP 8.5 by 2050 was notably more pronounced than under RCP 2.6, with larger geographic areas transitioning from unsuitable or low environmental suitability to moderate and high suitability categories.
Mycotoxin risk implications: The more aggressive expansion under RCP 8.5 introduces fumonisin risk to extensive temperate agricultural zones, including major wheat and maize producing regions of northern Europe and northern Asia that currently lack monitoring infrastructure.
3.3.3 RCP 2.6 projection for 2070
Long-term forecasts for 2070 under the RCP 2.6 scenario suggested a sustained but moderate increase in suitable environments for Fusarium proliferatum. The species exhibited enduring colonization capability in northern latitudes, transforming formerly marginal regions into more stable medium and high suitability zones. European regions showed notable consistency in sustaining favorable conditions, and certain areas of northern Asia and North America displayed a gradual expansion of suitable environments (Figures 5c, 6c).
Tropical and subtropical regions maintained relatively stable environmental suitability patterns compared to the 2050 projections, indicating the stabilization of favorable conditions in these locations. Desert regions and polar territories largely remained unsuitable, with minimal alterations in environmental suitability categories. The progression from 2050 to 2070 under RCP 2.6 showed continued but measured expansion, suggesting that sustained climate mitigation efforts could limit the rate and extent of poleward spread.
Mycotoxin risk implications: By 2070 under moderate mitigation, fumonisin monitoring becomes necessary across most of temperate Europe and southern portions of northern Asia and North America, requiring established regulatory frameworks and testing capacity.
3.3.4 RCP 8.5 projection for 2070
The RCP 8.5 scenario for 2070 forecasted substantial distributional alterations for Fusarium proliferatum, representing the most dramatic projected changes among all scenarios examined. Highly favorable environments expanded extensively into formerly marginal and unsuitable areas. Temperate zones in the Northern Hemisphere exhibited significant enhancements in environmental suitability, particularly in Scandinavia, northern Russia, northern Canada, and Alaska (Figures 5d, 6d).
The species exhibited markedly increased establishment potential across extensive portions of the Eurasian continent, with significant expansion into higher latitude locations previously deemed unsuitable or marginally appropriate. Large areas that were classified as unsuitable or low environmental suitability under current conditions transitioned to high suitability by 2070 under this scenario. Tropical regions retained their high environmental suitability rating; however, several areas showed changes in suitability intensity.
The anticipated alterations under RCP 8.5 by 2070 represent the most substantial potential range expansion, with the species demonstrating capacity to establish itself in areas considerably outside its current climatic envelope. This scenario projects transformation of vast regions of the Northern Hemisphere from completely unsuitable to highly suitable for F. proliferatum establishment.
Mycotoxin risk implications: Under the high-emission scenario by 2070, fumonisin contamination becomes a significant concern across virtually all temperate agricultural regions globally, including areas as far north as Scandinavia and southern Alaska. This represents a fundamental transformation in global mycotoxin risk geography, requiring extensive development of monitoring, regulatory, and management infrastructure in regions with no historical experience with this pathogen.
3.3.5 Comparative analysis across scenarios and time periods
Comparison across all projection scenarios reveals both consistent patterns and scenario-dependent divergences. Under both emission scenarios, tropical and subtropical regions (Central America, northern South America, sub-Saharan Africa, Southeast Asia) maintain consistently high environmental suitability across all time periods, indicating climatic stability for pathogen persistence in these traditionally affected areas. In contrast, temperate regions—particularly northern Europe, northern Asia, and northern North America—show marked scenario-dependent expansion patterns.
Under the moderate mitigation scenario (RCP 2.6), range expansion is evident but relatively constrained, with most newly suitable areas remaining in the medium environmental suitability category even by 2070. The expansion rate under RCP 2.6 is approximately 40–50% slower than under RCP 8.5, with substantially smaller areas reaching high environmental suitability status.
Under the high emission scenario (RCP 8.5), expansion is substantially more pronounced, with large areas transitioning from unsuitable or low environmental suitability to high suitability categories, particularly by 2070. Critical regions showing dramatic increases in environmental suitability only under RCP 8.5 include: Scandinavia and northern Russia (becoming highly suitable by 2070); northern Canada and Alaska (shifting from unsuitable to moderate-high environmental suitability); northern China and Mongolia (transitioning to consistently suitable); and northern portions of the United Kingdom and Ireland (becoming highly suitable). The difference between scenarios becomes increasingly pronounced over time, with the 2070 projections showing far greater divergence than the 2050 projections.
Regions showing relative stability regardless of scenario include: polar and Arctic zones (remaining unsuitable due to extreme cold even under high emissions); major desert regions (Sahara, Arabian Peninsula, central Australian deserts; remaining unsuitable due to aridity); and high-elevation mountain systems (Himalayas, Andes, Rockies; constrained by elevation-driven temperature limitations).
Mycotoxin risk synthesis: These distribution changes translate directly into mycotoxin contamination risk patterns. Newly suitable temperate regions in Europe, Asia, and North America represent major grain-producing areas with no historical experience of fumonisin contamination management. The potential establishment of F. proliferatum in these regions threatens food safety systems unprepared for mycotoxin monitoring and mitigation. Furthermore, the projected intensification of environmental suitability in currently affected tropical regions suggests potential increases in contamination severity and frequency in already vulnerable food systems. The scenario-dependent differences underscore that climate mitigation efforts could substantially reduce the geographic scope of emerging mycotoxin risks, providing additional public health justification for aggressive emission reduction policies.
3.4 Limiting factor analysis
The limiting factor analysis identified specific geographical patterns in the importance of the five temperature-related bioclimatic factors throughout the global distribution of Fusarium proliferatum (Figure 7). Temperature seasonality (bio_4) emerged as the primary limiting factor across vast regions, particularly affecting large areas of North America, central and northern Europe, central Asia, and substantial parts of Australia and New Zealand. This variable exhibited the most extensive spatial influence, signifying that annual temperature variability serves as the principal limitation on species distribution in temperate areas. The predominance of temperature seasonality as a limiting factor indicates that regions experiencing high seasonal temperature fluctuations present challenging conditions for year-round pathogen survival and establishment.
Annual mean temperature (bio_1) exhibited significant limiting influence in several locations, especially in northern North America, sections of northern Europe, and various sites in Asia, indicating its essential function in establishing the fundamental thermal limits for species distribution in these areas. This pattern is particularly evident at high latitudes where absolute temperature values fall below the species' thermal tolerance threshold.
The remaining temperature variables demonstrated more localized but substantial limiting effects across various geographical locations. The mean temperature of the warmest quarter (bio_10) was particularly influential in some sections of South America, especially in tropical and subtropical zones, as well as in other locations throughout Africa and Asia, suggesting that extreme summer temperatures in these regions may exceed optimal conditions. The minimum temperature of the coldest month (bio_6) emerged as a limiting factor in some high-latitude and mountainous regions, where winter cold extremes prevent pathogen survival or severely limit overwintering success. The mean temperature of the coldest quarter (bio_11) exhibited influence in particular areas of South America, Africa, and portions of Asia, indicating that sustained winter cold periods constrain establishment in these regions.
The spatial distribution of these limiting factors demonstrates that temperature seasonality (bio_4) represents the most widespread constraint on F. proliferatum distribution globally, followed by the more localized but significant impacts of the other temperature variables. As global warming reduces seasonal temperature variation in many regions, the primary limiting factor currently preventing establishment in temperate zones may be progressively weakened, facilitating poleward expansion.
4 Discussion
The MaxEnt model achieved high predictive accuracy (AUC = 0.844 ± 0.021; TSS = 0.68 ± 0.048), exceeding standard thresholds for reliable species distribution modeling (Allouche et al., 2006) and aligning with performance metrics reported for other fungal pathogen studies. This strong performance validates the approach for assessing climate-driven distribution changes and supports the reliability of our future projections. The consistency of validation metrics across all five cross-validation folds (AUC range: 0.820–0.867; TSS range: 0.62–0.73) demonstrates model stability and indicates that results are not dependent on specific data partitions. The dominance of temperature-related variables in determining F. proliferatum distribution corresponds with established fungal ecology principles, where thermal conditions fundamentally constrain metabolic processes, growth rates, sporulation capacity, and survival (Medina et al., 2017). The high sensitivity (0.82 ± 0.03) and specificity (0.86 ± 0.04) values indicate that the model effectively identifies both suitable and unsuitable environments, minimizing both false positives and false negatives—a critical requirement for informing management decisions and resource allocation for surveillance programs.
Our findings align with and extend previous research on climate-driven pathogen range shifts. The projected poleward expansion of F. proliferatum parallels patterns documented for other fungal pathogens, including Fusarium graminearum in wheat (Skelsey and Newton, 2015), Phytophthora infestans in potato (Raymundo et al., 2018), and various rust pathogens (Bebber et al., 2013). However, our study advances beyond these regional-scale assessments by providing the first comprehensive global projection for F. proliferatum specifically, with explicit integration of mycotoxin risk implications. Bebber et al. (2013) demonstrated that crop pests and pathogens have moved poleward at an average rate of 2.7 km per year over the past 50 years, a trend our models predict will accelerate under continued warming. Our projections of extensive colonization in Scandinavia and northern Russia under RCP 8.5 by 2070 are consistent with similar predictions for other temperate-adapted fungal pathogens (Delgado-Baquerizo et al., 2020). The magnitude of projected expansion under high-emission scenarios—with some regions transitioning from completely unsuitable to highly suitable within 50 years—underscores the potential rapidity of climate-driven distribution changes.
Our emphasis on temperature seasonality as the primary limiting factor extends findings from regional studies and has important implications: as climate change reduces seasonal temperature variation in many regions, previously unsuitable areas may rapidly become viable for F. proliferatum establishment. The buffering effect of reduced seasonality may be particularly important in continental interiors, where historical temperature extremes have prevented pathogen survival but where climate change is projected to moderate both summer and winter temperature extremes.
Unique to our study is the explicit integration of mycotoxin risk assessment with distribution modeling. Previous climate-pathogen studies have largely focused on disease presence/absence or severity, without addressing the food safety implications of toxigenic species. Given that fumonisin contamination can occur at subclinical infection levels—where visible disease symptoms are absent but mycotoxin accumulation proceeds—the health and economic consequences of F. proliferatum expansion may exceed those of pathogens causing only visible crop damage. Regions may experience mycotoxin contamination problems before recognizing significant disease pressure, potentially resulting in delayed response and greater population exposure.
The environmental envelope analysis demonstrated that 89.6% of occurrence records fell within the species' climatic envelope across all 19 bioclimatic parameters, indicating robust model alignment with observed distribution patterns. The high percentage of correctly predicted occurrences exceeds performance standards documented in other plant pathogen studies, suggesting that the selected bioclimatic variables successfully capture the essential niche requirements of F. proliferatum. The clustering of occurrence records within the central region of the temperature-precipitation space illustrates the species' preference for moderate climatic conditions, whereas the scattered distribution of marginal records suggests either genuine niche breadth or potential areas of model uncertainty requiring field validation (Merow et al., 2013).
The implications of our findings extend beyond mere range expansion, addressing critical issues related to mycotoxin contamination and food security. Rising temperatures are expected to facilitate proliferation of mycotoxigenic fungi adapted to warmer conditions, posing significant threats to human and animal health (Xu et al., 2024). The expansion of F. proliferatum into novel agricultural regions, especially in temperate zones where fumonisin contamination has traditionally been minimal or absent, presents serious risks to crop production and food safety. This concern is amplified by the identification of numerous newly suitable regions that align with major grain-producing areas in North America, Europe, and Asia, where increased mycotoxin contamination could result in substantial economic and health consequences (Marasas et al., 2004). The economic burden extends beyond direct crop losses to include costs associated with testing, grain segregation, rejection of contaminated shipments, trade disruptions, and potential health care expenses related to mycotoxin exposure.
The temperature-driven expansion patterns identified in our study illustrate broader trends wherein cooler or temperate settings are increasingly vulnerable to fungal infestation as temperatures rise. Altered precipitation patterns and increased frequency of extreme weather events are creating novel ecological niches that may facilitate the spread of Fusarium infections (Fones et al., 2020). Our limiting factor analysis indicated that temperature seasonality is the predominant constraint on species distribution, implying that areas experiencing diminished seasonal temperature variation due to climate change may become increasingly vulnerable to F. proliferatum establishment. This discovery has significant ramifications for disease surveillance and management strategies, as traditional temperate agricultural systems may require substantial adaptation to address emerging pathogen threats for which they have no historical experience or established management protocols.
The anticipated range extension of F. proliferatum under climate change scenarios has considerable implications for global food security and agricultural sustainability. Researchers have issued increasingly frequent warnings regarding global warming and its association with mycotoxin-producing fungi in diverse geographical areas worldwide (Medina et al., 2017). The introduction of this pathogen into new regions may destabilize existing agricultural systems and necessitate significant modifications in crop management strategies, breeding programs, and food safety regulations. The economic ramifications are particularly alarming, as F. proliferatum can cause substantial yield reductions and simultaneously contaminate crops with harmful mycotoxins, posing a dual threat to productivity and food safety (Munkvold, 2003). Moreover, expansion into regions with minimal expertise in managing Fusarium diseases may create knowledge deficiencies that could intensify the impacts of pathogen establishment, as farmers, extension services, and regulatory agencies lack experience with appropriate management practices, symptom recognition, and mycotoxin testing protocols.
Several limitations of this study warrant consideration when interpreting our projections. Our reliance on GBIF data introduces potential geographic and ecological biases. Sampling intensity is substantially higher in developed regions (North America, Europe) compared to tropical and subtropical areas of Africa, Asia, and South America where the pathogen is likely underreported. This uneven sampling may lead to underestimation of the species' climatic tolerance breadth, particularly at the warm end of the temperature gradient. Additionally, GBIF records predominantly represent agricultural contexts, potentially missing occurrences in wild or non-crop hosts that could provide important niche information. Field validation of model predictions, particularly in undersampled regions and in areas projected to become newly suitable, represents a critical next step for confirming model accuracy and refining projections. Systematic surveys in tropical Africa, Southeast Asia, and South America would be particularly valuable for capturing the full range of climatic conditions tolerated by the species.
Our decision to focus exclusively on temperature variables is statistically and biologically justified but may not capture the full complexity of moisture-related constraints in some regions. Future modeling efforts should explore whether incorporating precipitation variables in region-specific models provides additional predictive value, particularly for arid and semi-arid agricultural zones where water availability may interact with temperature to determine infection outcomes. Similarly, soil characteristics, host plant phenology, and agricultural management practices represent additional factors that could refine distribution predictions beyond the climate-only approach employed here.
Our use of a single GCM (MRI-CGCM3) limits quantification of inter-model uncertainty. Multi-model ensemble approaches incorporating diverse GCMs would provide more robust uncertainty estimates and identify areas of high projection agreement vs. disagreement among climate models. Similarly, our focus on two extreme emission scenarios (RCP 2.6 and 8.5) brackets the range of possible futures but omits intermediate pathways (RCP 4.5, RCP 6.0) that may prove more realistic given current emission trajectories and policy commitments. Future assessments should incorporate multiple GCMs and emission scenarios to provide more comprehensive uncertainty characterization.
Our models assume niche conservatism—that species-environment relationships remain constant under future climates. However, F. proliferatum may exhibit evolutionary adaptation or phenotypic plasticity in response to novel conditions, potentially expanding its realized niche beyond current thermal tolerances. Furthermore, our abiotic models do not incorporate biotic factors including host plant distributions, competition with other microorganisms, soil microbiome interactions, or agricultural management practices, all of which could substantially influence establishment success in newly suitable regions. The actual realized distribution will depend on complex interactions between climate suitability, host availability, dispersal opportunities, and competitive dynamics that are not captured in climate-only models.
We assume dispersal is unlimited, meaning the pathogen can reach all climatically suitable areas. For a cosmopolitan crop pathogen frequently dispersed through agricultural trade, contaminated seed, and movement of plant materials, this assumption is more reasonable than for geographically restricted species. However, biosecurity measures, phytosanitary regulations, and geographic barriers could slow or prevent colonization of some suitable areas. Conversely, human-mediated long-distance dispersal through global agricultural trade networks may enable more rapid colonization than would occur through natural dispersal alone, potentially resulting in establishment that outpaces climate envelope expansion.
Despite these limitations, our modeling framework provides valuable first-order estimates of climate-driven distribution changes and identifies priority regions for enhanced surveillance and risk management. The consistent patterns observed across emission scenarios and time periods, combined with strong model performance metrics and alignment with observed patterns for other fungal pathogens, support the robustness of our general conclusions regarding poleward range expansion under climate warming.
5 Conclusions and recommendations
This study provides the first comprehensive global assessment of climate change impacts on Fusarium proliferatum distribution and associated mycotoxin contamination risks. The central finding is unambiguous: climate warming will substantially expand the geographic range of this mycotoxin-producing pathogen, particularly into temperate regions of the Northern Hemisphere that currently experience minimal fumonisin contamination. Under high emission scenarios, vast areas of northern Europe, northern Asia, and northern North America are projected to transition from unsuitable to highly suitable for pathogen establishment by 2070, introducing fumonisin contamination risks to major grain-producing regions with no historical experience managing this specific mycotoxin threat.
The projected poleward expansion has profound food security implications. Newly vulnerable temperate regions include major agricultural zones currently producing significant proportions of global wheat, maize, and other staple crops. The establishment of F. proliferatum in these areas would require development of entirely new monitoring systems, regulatory frameworks, and mitigation strategies for fumonisin contamination—capabilities that take years to establish and substantial financial investment to maintain. Simultaneously, projected intensification in currently affected tropical regions threatens to increase contamination frequency and severity in food systems already struggling with mycotoxin management, potentially exacerbating food insecurity in vulnerable populations.
Based on our findings, we propose the following evidence-based recommendations for stakeholders. First, proactive surveillance programs should be established for systematic monitoring of F. proliferatum and fumonisin contamination in regions projected to become newly suitable, particularly northern Europe, northern Asia, and northern North America. Early detection systems should be implemented before widespread establishment occurs, enabling rapid response and containment efforts. Surveillance should target both agricultural production systems and potential reservoir hosts in natural ecosystems that could serve as sources for agricultural infections.
Second, biosecurity and phytosanitary measures must be strengthened to prevent introduction of contaminated material into newly vulnerable regions. Seed certification programs and quarantine protocols should be enhanced, and international coordination of phytosanitary standards should explicitly address climate-driven range expansion of mycotoxigenic fungi. Border inspection procedures may need to be intensified for imports from regions where F. proliferatum is endemic, particularly for seed stocks, grain shipments, and planting materials that could serve as introduction pathways.
Third, adaptive crop management strategies should be developed and disseminated, including region-specific best management practices for fumonisin mitigation such as resistant cultivar deployment, optimized irrigation and fertilization strategies to minimize plant stress, crop rotation sequences that reduce inoculum buildup, and biocontrol approaches using antagonistic microorganisms. Investment in developing these tools before pathogen establishment is substantially more cost-effective than reactive management after widespread colonization. Extension services in newly vulnerable regions should receive training in disease recognition, management options, and mycotoxin risk communication to farmers.
Fourth, regulatory framework development is essential in temperate regions currently lacking fumonisin monitoring requirements. Proactive development of regulatory standards, testing protocols, and enforcement capacity is critical. Regulatory limits should be science-based, considering both acute toxicity risks and chronic exposure scenarios, with particular attention to protecting vulnerable populations including infants, children, and immunocompromised individuals.
Fifth, research priorities for future work should focus on field validation of model predictions in projected expansion zones, providing empirical confirmation of colonization patterns and enabling model refinement. Multi-model ensemble approaches should be employed to refine uncertainty estimates. Integration of host plant distributions, agricultural practice variables, and soil characteristics would enable more mechanistic understanding of establishment requirements. Assessment of climate change impacts on fumonisin production levels is critical since temperature and moisture conditions affect not only where the pathogen can exist but also how much mycotoxin it produces. Economic modeling comparing prevention costs vs. reactive management costs would provide valuable information for policy decisions regarding resource allocation for surveillance and prevention programs.
Sixth, climate change mitigation represents a crucial strategy for limiting pathogen range expansion. Our projections demonstrate that aggressive emission reduction (RCP 2.6 scenario) could substantially limit the magnitude of pathogen range expansion compared to high-emission trajectories (RCP 8.5). This finding adds agricultural biosecurity and food safety to the portfolio of climate mitigation co-benefits, strengthening the case for urgent action on greenhouse gas emissions.
Temperature seasonality currently represents the primary climatic barrier preventing F. proliferatum establishment in temperate regions. Climate change is progressively dismantling this natural constraint, enabling colonization of vast previously inhospitable areas. The agricultural and public health consequences of this expansion necessitate immediate proactive planning and coordinated international response. The choice facing policymakers, agricultural managers, and public health authorities is clear: invest now in surveillance, prevention, and adaptive capacity, or face substantially higher costs from reactive management of established mycotoxin contamination in the coming decades. Our models provide the geographic and temporal framework for prioritizing these investments, identifying specific regions where early intervention will be most critical and cost-effective.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AT: Writing – review & editing, Writing – original draft, Conceptualization, Methodology. SA: Methodology, Writing – original draft, Data curation, Writing – review & editing, Formal analysis. DA: Project administration, Writing – review & editing, Funding acquisition. WA: Writing – review & editing, Project administration, Validation, Software. WH: Writing – review & editing, Supervision, Investigation, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
The authors acknowledge the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. We thank the Global Biodiversity Information Facility (GBIF) for providing access to species occurrence data and WorldClim for providing climate data layers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1673494/full#supplementary-material
References
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., and Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, 541–545. doi: 10.1111/ecog.01132
Allouche, O., Tsoar, A., and Kadmon, R. (2006). Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43, 1223–1232. doi: 10.1111/j.1365-2664.2006.01214.x
Araújo, M. B., and New, M. (2007). Ensemble forecasting of species distributions. Trends Ecol. Evol. 22, 42–47. doi: 10.1016/j.tree.2006.09.010
Bebber, D. P., Ramotowski, M. A., and Gurr, S. J. (2013). Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 3, 985–988. doi: 10.1038/nclimate1990
Booth, T. H., Nix, H. A., Busby, J. R., and Hutchinson, M. F. (2014). BIOCLIM: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 20, 1–9. doi: 10.1111/ddi.12144
Boria, R. A., Olson, L. E., Goodman, S. M., and Anderson, R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77. doi: 10.1016/j.ecolmodel.2013.12.012
Boutigny, A. L., Beukes, I., Small, I., Zühlke, S., Spiteller, M., Van Rensburg, B. J., et al. (2011). Quantitative detection of Fusarium pathogens and their mycotoxins in South African maize. Plant Pathol. 61, 522–531. doi: 10.1111/j.1365-3059.2011.02544.x
Chakraborty, S., and Newton, A. C. (2011). Climate change, plant diseases and food security: an overview. Plant Pathol. 60, 2–14. doi: 10.1111/j.1365-3059.2010.02411.x
Chelkowski, J., Ritieni, A., Wiśniewska, H., Mulè, G., Logrieco, A., Bottalico, A., et al. (2000). Occurrence of toxic hexadepsipeptides in preharvest maize ear rot infected by Fusarium poae in Poland. J. Phytopathol. 148, 345–348. doi: 10.1111/j.1439-0434.2006.01173.x
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., et al. (2020). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 7:10541. doi: 10.1038/ncomms10541
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Elad, Y., and Pertot, I. (2014). Climate change impacts on plant pathogens and plant diseases. J. Crop. Improv. 28, 99–139. doi: 10.1080/15427528.2014.865412
Elith, J., and Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., Yates, C. J., et al. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
ESRI (2022). ArcGIS Desktop: Release 10.8.1. Redlands, CA: Environmental Systems Research Institute.
Fick, S. E., and Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. doi: 10.1002/joc.5086
Fielding, A. H., and Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. doi: 10.1017/S0376892997000088
Fones, H. N., Bebber, D. P., Chaloner, T. M., Kay, W. T., Steinberg, G., Gurr, S. J., et al. (2020). Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 1, 332–342. doi: 10.1038/s43016-020-0075-0
Garrett, K. A., Dendy, S. P., Frank, E. E., Rouse, M. N., and Travers, S. E. (2006). Climate change effects on plant disease: genomes to ecosystems. Annu. Rev. Phytopathol. 44, 489–509. doi: 10.1146/annurev.phyto.44.070505.143420
GBIF.org (2023). GBIF Home Page. Available online at: https://www.gbif.org (accessed January 15, 2023).
Gelderblom, W. C., Jaskiewicz, K., Marasas, W. F. O., Thiel, P. G., Horak, R. M., Vleggaar, R., et al. (2008). Fumonisins—novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54, 1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988
Hanley, J. A., and McNeil, B. J. (1982). The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36. doi: 10.1148/radiology.143.1.7063747
Hijmans, R. J., Guarino, L., Cruz, M., and Rojas, E. (2012). Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet. Resour. Newsl. 127, 15–19.
Landis, J. R., and Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics 33, 159–174. doi: 10.2307/2529310
Leslie, J. F., and Summerell, B. A. (2006). The Fusarium Laboratory Manual. Ames, IA: Blackwell Publishing. doi: 10.1002/9780470278376
Logrieco, A., Mulè, G., Moretti, A., and Bottalico, A. (2002). Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 108, 597–609. doi: 10.1023/A:1020679029993
Marasas, W. F. O., Riley, R. T., Hendricks, K. A., Stevens, V. L., Sadler, T. W., et al. (2004). Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 134, 711–716. doi: 10.1093/jn/134.4.711
Medina, A., Rodriguez, A., and Magan, N. (2017). Climate change and mycotoxigenic fungi: impacts on mycotoxin production. Curr. Opin. Food Sci. 5, 99–104. doi: 10.1016/j.cofs.2015.11.002
Merow, C., Smith, M. J., and Silander, J. A. (2013). A practical guide to MaxEnt for modeling species' distributions: what it does, and why inputs and settings matter. Ecography 36, 1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x
Moss, R. H., Edmonds, J. A., Hibbard, K. A., Manning, M. R., Rose, S. K., Van Vuuren, D. P., et al. (2010). The next generation of scenarios for climate change research and assessment. Nature 463, 747–756. doi: 10.1038/nature08823
Munkvold, G. P. (2003). Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 41, 99–116. doi: 10.1146/annurev.phyto.41.052002.095510
Munkvold, G. P., and White, D. G. (2016). Compendium of Corn Diseases, 4th Edn. St. Paul, MN: American Phytopathological Society Press. doi: 10.1094/9780890544945
Peterson, A. T., Soberón, J., Pearson, R. G., Anderson, R. P., Martínez-Meyer, E., Nakamura, M., et al. (2011). Ecological Niches and Geographic Distributions. Princeton, NJ: Princeton University Press. doi: 10.23943/princeton/9780691136868.003.0003
Phillips, S. J., Anderson, R. P., and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., and Dudík, M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175. doi: 10.1111/j.0906-7590.2008.5203.x
Radosavljevic, A., and Anderson, R. P. (2014). Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 41, 629–643. doi: 10.1111/jbi.12227
Raymundo, R., Asseng, S., Prassad, R., Kleinwechter, U., Concha, J., Condori, B., et al. (2018). Performance of the SUBSTOR-potato model across contrasting growing conditions. Field Crops Res. 202, 57–76. doi: 10.1016/j.fcr.2016.04.012
Skelsey, P., and Newton, A. C. (2015). Future environmental and geographic risks of Fusarium head blight of wheat in Scotland. Eur. J. Plant Pathol. 142, 133–147. doi: 10.1007/s10658-015-0598-7
van Vuuren, D. P., Edmonds, J., Kainuma, M., Riahi, K., Thomson, A., Hibbard, K., et al. (2011). The representative concentration pathways: an overview. Clim. Change 109, 5–31. doi: 10.1007/s10584-011-0148-z
Warren, D. L., and Seifert, S. N. (2011). Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecol. Appl. 21, 335–342. doi: 10.1890/10-1171.1
Xu, Y., Wang, J., Li, Y., Chen, X., and Zhang, H. (2024). Climate change impacts on plant pathogens and host-pathogen interactions. Curr. Opin. Plant Biol. 67, 102–110.
Keywords: Fusarium spp., species distribution modeling, MaxEnt, climate change, mycotoxins, food security, plant pathology
Citation: Tagyan AI, AlAshaal S, Alkhalifah DHM, Abdelghany WR and Hozzein WN (2025) Estimating the climate change-driven global distribution of Fusarium proliferatum and mycotoxin risk assessment under future warming scenarios. Front. For. Glob. Change 8:1673494. doi: 10.3389/ffgc.2025.1673494
Received: 29 July 2025; Accepted: 27 October 2025;
Published: 12 November 2025.
Edited by:
Paola Battilani, Catholic University of the Sacred Heart, ItalyReviewed by:
Aurel Maxim, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaMarco Camardo Leggieri, Catholic University of the Sacred Heart, Italy
Copyright © 2025 Tagyan, AlAshaal, Alkhalifah, Abdelghany and Hozzein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aya I. Tagyan, aV9heWE1MEB5YWhvby5jb20=
 Aya I. Tagyan
Aya I. Tagyan Sara AlAshaal2
Sara AlAshaal2 Dalal Hussien M. Alkhalifah
Dalal Hussien M. Alkhalifah Walaa R. Abdelghany
Walaa R. Abdelghany Wael N. Hozzein
Wael N. Hozzein