- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 3Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 4Songqiao Hospital of Wanquan Town, Wenzhou, China
Background: The exact pathogenic mechanism of the painful diabetic peripheral neuropathy (DPN) is poorly understood. Our study aimed to evaluate the association amongst vitamin D status, inflammatory cytokines, and painful DPN.
Methods: A total of 483 patients were divided into three groups, i.e., diabetes without DPN (no-DPN, n = 86), diabetes with painless DPN (painless DPN, n = 176) and diabetes with painful DPN (painful DPN, n = 221) groups. Basic information and laboratory results were collected. The concentrations of vitamin D (25-hydroxyvitamin D), high-sensitivity C-reactive protein, interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were also measured.
Results: The prevalence of severe vitamin D deficiency (<10 ng/mL) was more common in the painful DPN group than in the painless DPN and no-DPN groups (25.8,12.5, and 8.1%, respectively, P < 0.01). Cases in the painful DPN group had significantly higher concentrations of IL-6 (P < 0.01) and TNF-α (P < 0.01) than those in the two other groups. The multivariate logistic analysis showed that severe vitamin D deficiency, IL-6, and TNF-α were independent risks for painful DPN after adjusting for confounding factors. Furthermore, the vitamin D status had significantly negative correlations with IL-6 (r = −0.56, P < 0.01) and TNF-α (r = −0.47, P < 0.01) levels.
Conclusion: Severe vitamin D deficiency was an independent risk factor for the painful DPN. Severe vitamin D deficiency status may play a role in the painful DPN pathogenesis through elevated IL-6 and TNF-α levels.
Introduction
China has the world's largest diabetes epidemic, which continues to increase. In China, the latest publication has presented that the prevalence of diabetes has increased to 12.8% nationwide (1). A total of 693 million people are estimated to have diabetes by 2045 (2). An estimated 60–70% of patients with diabetes mellitus (DM) suffer from neuropathies, and the diabetic peripheral neuropathy (DPN) is common amongst patients with diabetes (3). About 25% of people with diabetic neuropathy also develop painful DPN (4, 5). Major neuropathic symptoms include burning, aching and electric shock-like pains, resulting in a huge burden on patients' health and the society (6, 7). Painful DPN is a remarkable economic burden for patients, families and the society (5, 7). Despite recent advances, the pathogenesis of painful DPN remains incomplete. Previous studies indicate an association between vitamin D deficiency and the painful DPN. Mohammad et al. have reported that the vitamin D status (deficient, insufficient, and sufficient vitamin D) and the neuropathic pain in patients with DM have no correlation (8). Shillo et al. have demonstrated that after adjustment for age, weight, activity score and sunlight exposure, people with painful DPN have significantly lower serum vitamin D level compared with those with painless DPN and DM without DPN (9). A recent study has suggested that vitamin D deficiency is related to painful DPN (10). Furthermore, Basit et al. have conducted a clinical trial to assess the effects of a single intramuscular dose of 600,000 IU vitamin D3 on the symptoms of painful DPN (11). Results show that vitamin D is an efficacious treatment for DPN. Another study also shows that the visual analog scale in patients with painful DPN after treatment with 2000 IU vitamin D3 decreases to 50%. A recent review is performed to investigate the effects of vitamin D supplementation on the signs and symptoms of DPN (12). Results show that vitamin D supplementation can improve subjective and objective pain scores in painful DPN. However, the mechanisms underlying the effect remain unclear.
Vitamin D is believed to maintain a balance between inflammation and immunosuppression (13). Observational studies show that elevated inflammation in chronic conditions, including osteoarthritis (14), hypertensive disorders in pregnancy (15), and diabetic foot infection (16), is linked to vitamin D deficiency. An animal experiment also shows that the increased levels of IL-1β and TNF-α are related to painful DPN in rats (17). Yanik's et al. findings suggest that IL-10 can reduce the pain behavior in an animal model of painful DPN (18).
The present study is based on the hypothesis that the vitamin D status plays a role in the painful DPN pathogenesis through elevated inflammation. To test our hypothesis, we have measured the vitamin D status and the circulating levels of hs-CRP, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ in patients with painful DPN and evaluated the relationship between vitamin D and the abovementioned cytokines.
Methods
Study Design and Participants
Between April 2018 and August 2019, patients with type 2 diabetes mellitus (T2DM) were recruited from the Department of Endocrinology at the Affiliated Hospital of Wenzhou Medical University. All participants were consecutive patients. T2DM was diagnosed using 75 g oral glucose tolerance tests in accordance with the American Diabetes Association's criteria. The exclusion criteria included type 1 diabetes, hepatic failure, chronic renal impairment, inflammatory diseases, malignancy, hyperparathyroidism, recent vitamin D supplementation, central nervous system diseases, and causes of polyneuropathy other than diabetes. All participants in our study were divided into three groups, i.e., diabetes without DPN (no-DPN, n = 86), diabetes with painless DPN (painless DPN, n = 176), and diabetes with painful DPN (painful DPN, n = 221) groups.
Data Collection
A detailed clinical history, including age, gender, diabetes duration, smoking, hypertension, and antidiabetic medication usage, was obtained from participants' medical records and through self-reporting. The body mass index (BMI) was calculated using the formula: BMI = weight (kg)/height (m2).
Blood samples were collected from the antecubital vein in the overnight fasting state. Blood samples were used for estimating renal and liver functions, plasma glucose (by using standard enzymatic methods), glycated hemoglobin (HbA1c, by using high-performance liquid chromatography) and lipid profiles (by using standard enzymatic methods), including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG). The early-morning spot urine specimen was collected using immunoturbidimetry and the standard enzymatic method (Wako, Osaka, Japan) to assess the urinary albumin-to-creatinine ratio (UACR).
Assessment of DPN
DPN was assessed using the Michigan Neuropathy Screening Instrument (MNSI), a validated screening tool for DPN. The MNSI included two separate assessments: a 15-item self-administered questionnaire and a structured examination of feet (MNSIE) that scored for abnormalities of appearance, presence of ulcers, vibration perception and ankle reflexes. All MNSIEs in our study were performed by a trained professional to reduce interviewer variability. The threshold for DPN was established via previous validation studies in adults and used a score of more than 2 on the MNSIE out of a total score of 8. The details of the examination are described in the previous study (19, 20).
Assessment of the Neuropathic Pain
The neuropathic pain was diagnosed when the DN4 questionnaire gave a value ≥4. The DN4 questionnaire is a patient-reported symptom-based 10-item approach consisting of sensory descriptors and signs related to the bedside examination. This questionnaire was developed and validated by the French Neuropathic Pain Group. The cutoff value of 4/10 represented the highest percentage of correctly diagnosing patients (86.0%) and had sensitivity and specificity of 82.9 and 89.9%, respectively (20).
Measurement of the Serum Vitamin D
25-hydroxyvitamin D was measured through the electrochemiluminescence immunoassay by using the Roche Modular E170 Analyzer (Roche Diagnostics GmbH, Switzerland). The vitamin D status of participants were classified in accordance with the Endocrine Society's clinical practice guidelines (sufficient vitamin D level, >30 ng/mL; insufficient vitamin D level, 20–30 ng/mL; deficient vitamin D level, 10–19.9 ng/mL and severe vitamin D deficiency, <10 ng/mL) (21).
Serum Cytokine Levels
Serum samples collected for detection of cytokine concentrations were stored at −70°C. The serum concentrations of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ in the samples were evaluated using the Luminex xMAP (Ceger Biotechnology Co., Ltd., China) in accordance with the manufacturer's instructions. Serum levels were measured in pg/mL. The hs-CRP was measured using the highly sensitive nephelometric assay (Ceger Biotechnology Co., Ltd., China).
Statistical Analysis
The SPSS 21.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Data were presented as mean ± SD, percentages or median values with interquartile range. Differences between groups were tested using ANOVA. Count data were tested using χ2 tests. The Spearman correlations were used for the bivariate analysis of the association between vitamin D and clinical/biochemical parameters. The multivariate logistic analysis was performed using identified independent variables, and odds ratios (OR) between the comparison groups were obtained with 95% confidence interval (CI). All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
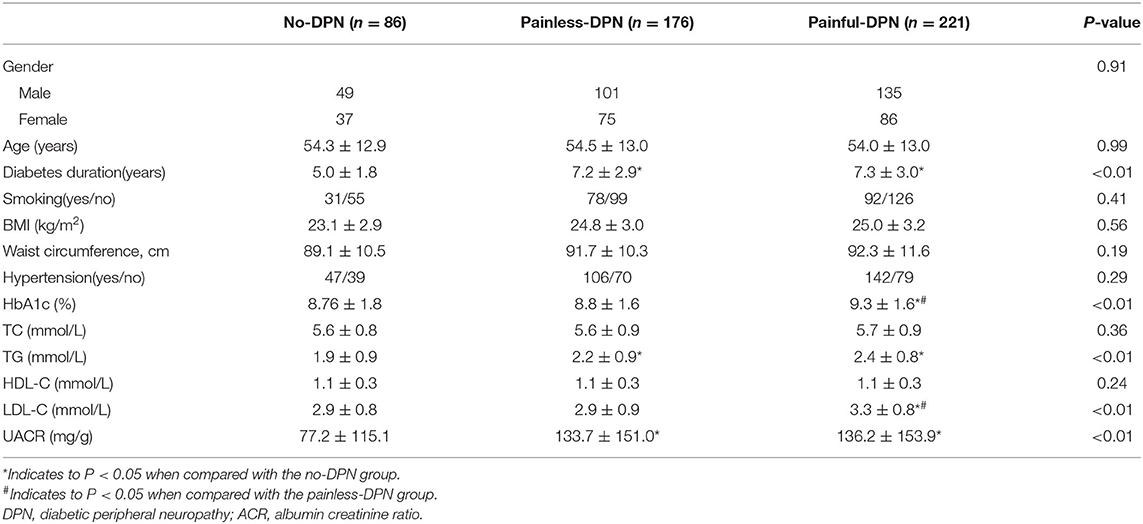
A total of 483 patients (age = 28–79 years) were recruited for our study. Table 1 shows the baseline characteristics. No significant difference in gender, age, BMI, the proportion of smokers and hypertension was found amongst the no-DPN, painless DPN and painful DPN groups (all P > 0.05). No significant difference in the levels of TC and HDL-C was observed amongst the three groups (all P > 0.05). The levels of UACR and TG and the diabetes duration in the painless and painful DPN groups were significantly higher than those in the no-DPN group (all P < 0.01). The levels of HbA1c and LDL-C in the painful DPN group were significantly higher compared with those in the no-DPN and the painless DPN groups (all P < 0.01).
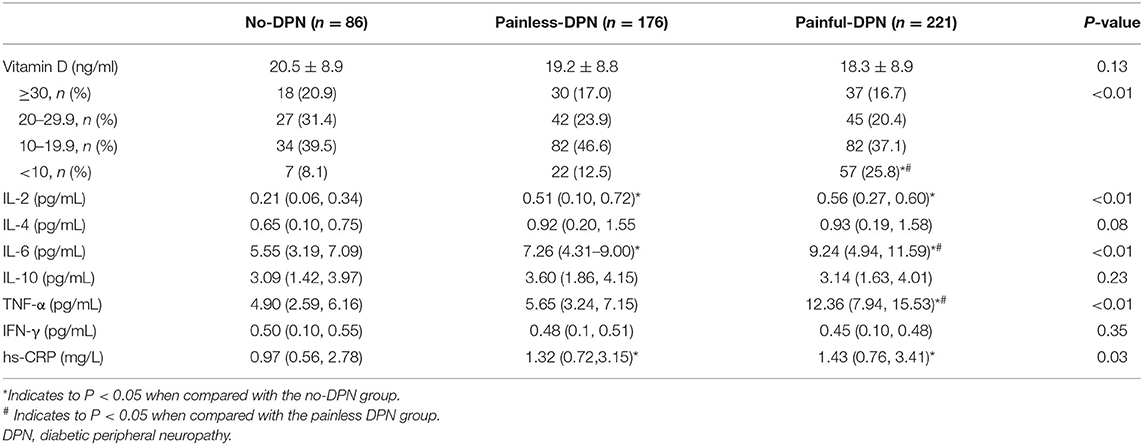
No significant difference in vitamin D level was found amongst the three groups (P > 0.05, Table 2). A significant difference in the prevalence of severe vitamin D deficiency was found amongst the three groups. The prevalence of severe vitamin D deficiency was detected in 8.1, 12.5, and 25.8% of no-DPN, painless DPN and painful DPN participants, respectively (P < 0.01). No significant difference was observed in IL-4, IL-10, and IFN-γ levels amongst the three groups. The painful DPN group had significantly higher IL-6 and TNF-α levels compared with the no-DPN and the painless DPN groups (all P < 0.01). The painless and the painful DPN groups had significantly higher serum hs-CRP and IL-2 levels compared with the no-DPN group (P = 0.03 and P < 0.01, respectively).
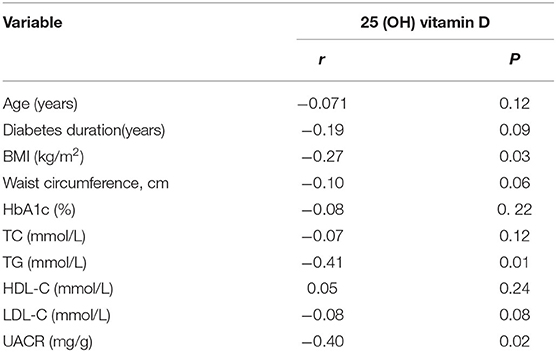
The Spearman correlations between the vitamin D and clinical and biochemical parameters are shown in Table 3. The BMI (r = −0.27, P = 0.03), TG (r = −0.41, P = 0.01) and UACR (r = −0.40, P = 0.02) showed significantly inverse correlations with vitamin D. Vitamin D was not significantly correlated with waist circumference, diabetes duration, and HbA1c.
The Spearman correlation analyses showed that the vitamin D level was negatively associated with IL-6 (r = −0.56, P < 0.01), TNF-α (r = −0.47, P < 0.01), IL-2 (r = 0.29, P < 0.01), and hs-CRP (r = −0.09, P = 0.02) levels (Figure 1). A negative correlation was identified between the vitamin D level and the pain score DN4 (r = −0.20, P < 0.01).
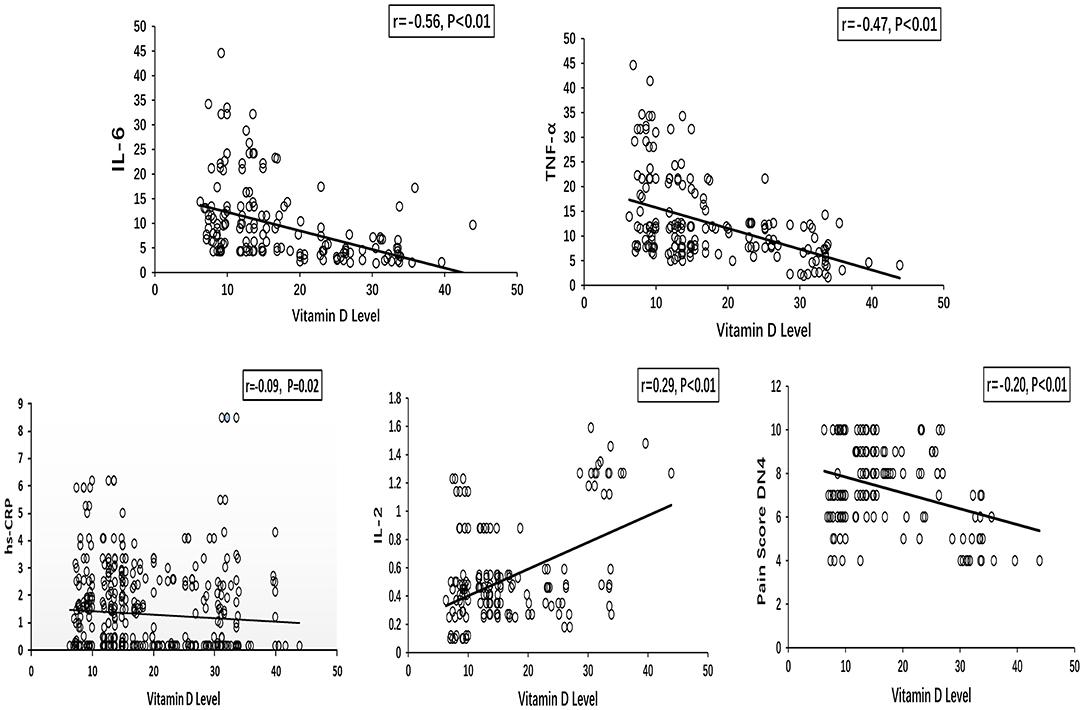
Figure 1. Spearman correlations between vitamin D level and inflammatory cytokines (IL-6, TNF-α, hs-CRP, and IL-2), pain score DN4 in painful DPN group. DPN, diabetic peripheral neuropathy.
Multivariate logistic models were constructed to examine the risk factors for painful DPN. The univariable analysis showed that severe vitamin D deficiency, being male and HbA1c, TG, LDL-C, UACR, IL-6, and TNF-α levels were significantly associated with the risk of painful DPN.
As shown in Figure 2, the multivariate analysis after adjustment for confounding factors, the severe vitamin D deficiency was still strongly associated with painful DPN (OR = 2.46, 95% CI, 1.39–4.48, P = 0.01). IL-6 (OR = 1.45, 95% CI, 1.12–2.32, P = 0.02) and TNF-α levels were also significantly associated with the presence of painful DPN after adjustment (OR = 1.37, 95% CI, 1.29–1.49, P < 0.01; Figure 2).
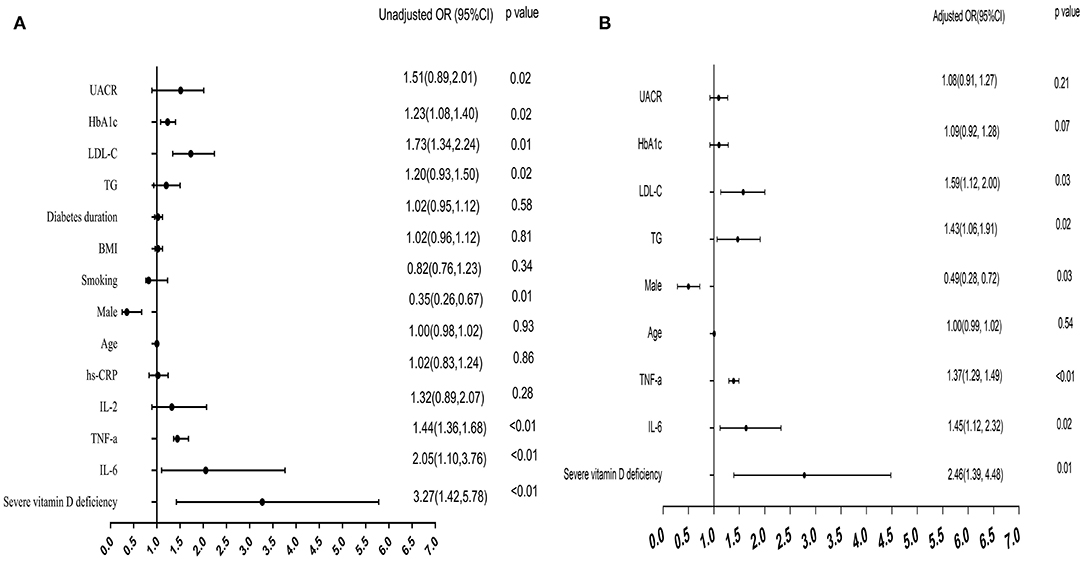
Figure 2. Forest plots for multivariate logistic analysis of variables for painful DPN. (A) Univariate logistic analysis: unadjusted odds ratio (OR) for each variable. (B) multivariate analysis: adjusted OR after adjustment for age, gender, HbA1c, TG, LDL-C, and UACR. DPN, diabetic peripheral neuropathy.
Discussion
The 2001–2004 National Health and Nutrition Examination Survey has shown that the vitamin D deficiency (<30 ng/ml) is associated with self-reported peripheral neuropathy symptoms in American adults with diabetes (22). He et al. have found that the vitamin D deficiency is evidently associated with DPN, and vitamin D level <16.01 ng/mL predicts more than two-fold risk of the presence of DPN (23). Stephanotis et al. have reported that patients with vitamin D deficiency (<20 ng/mL) have a higher risk of symptomatic DPN (OR = 2.04, 95% CI = 0.99–4.02) compared with patients with 25-OH-D level of 30–40 ng/mL (24). Our study shows no significant difference in the prevalence of vitamin D deficiency (10–19.9 ng/mL) amongst the three groups (P = 0.053). The present study reveals that 25.8% of patients with painful DPN have severe vitamin D deficiency, whereas 8.1 and 12.5% of patients in the DM and the painless DPN groups have severe vitamin D deficiency. Our findings demonstrate a higher frequency of severe vitamin D deficiency in the painful DPN group than in the DM and the painless DPN groups (P < 0.01).
A negative correlation is identified between the vitamin D level and the pain score DN4 (r = −0.20, P < 0.01). The vitamin D level is lowest in people with the highest reported pain score. After adjusting for age, sex, smoking, BMI, diabetes duration, LDL-C, HbA1c, and UACR, severe vitamin D deficiency is an independent risk factor for painful DPN. Our findings suggest that severe vitamin D deficiency may have an important role in the pathogenesis of painful DPN.
Additionally, our study shows that inflammatory cytokines, such as hs-CRP, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, participate in the development and the progression of painful DPN. Our results reveal that the levels of IL-6 and TNF-α in patients in the painful DPN group are significantly higher compared with those in the no-DPN and the painless DPN groups (all P < 0.01). In addition, after adjusting for age, sex, smoking, BMI and diabetes duration, the levels of LDL-C, HbA1c, UACR, IL-6, and TNF-α are independent risk factors for painful DPN. A report from the Cooperative Health Research in the Region of Augsburg (KORA) F4/FF4 cohort with a mean follow-up of 6.5 years shows that IL-6 (OR = 1.31,95% CI, 1.00–1.71) and TNF-α (OR = 1.31, 95% CI, 1.03–1.67) levels are related to the incident distal sensorimotor polyneuropathy after adjusting for known distal sensorimotor polyneuropathy risk factors (25). Rosane et al. have reported that TNF-α can lead to the apoptosis of Schwann cells with subsequent damage to the peripheral nerve (26). TNF-α also improves the channel function in sensory neurons and leads to peripheral nerve injury, thereby inducing pain (27). Nadi et al. have found that exercises can significantly reduce TNF-α and CRP levels and have a consequent improvement in imbalance, pain and tingling (28). Our findings are in accordance with some previous reports. Machiavelli et al. have found that the serum IL-6 level increases in more than 40% of patients with DM and that the elevated IL-6 level has a significantly negative correlation with small and large nerve fiber functions and neuropathic pain (29). Backryd et al. have also demonstrated that patients with neuropathic pain have a higher level of IL-6 (30). A study by Angst et al. has also been reported that increased IL-6 concentration was noted in the patients with painful DPN (31).Data also show that IL-6 is a proinflammatory cytokine that has a significant effect on glial cells and neurons and trigger the onset of DPN in motion (32). Ma et al. have performed SC144 to block the IL-6-mediated signal transduction and demonstrate the function of IL-6 in the mechanical hyperalgesia induced by DM. Their data show that the administration of SC144 can effectively alleviate the mechanical hyperalgesia in STZ animals compared with controls (33). A recent study has also found a significant decrease in the neuropathy severity, a decrease in the IL-6 level and an increase in the IL-10 level after treatment with cholecalciferol (40,000 IU/week) for 24 weeks in patients with T2DM and DPN (34).
Data from the present study confirm that the vitamin D metabolite has strong associations with IL-6 (r = −0.56, P < 0.01) and TNF-α (r = −0.47, P < 0.01). The significantly negative correlations of vitamin D with inflammatory cytokines are consistent with those observed in previous studies. Tiwari et al. have evaluated the serum vitamin D level in 112 patients with diabetic foot infection and in 109 patients with diabetes but without foot infection. Tiwari et al. have found a significantly positive correlation with severe vitamin deficiency and high cytokine levels in patients with diabetes especially those with foot infection. Laird et al. have reported that the concentrations of IL-6 and CRP in individuals with vitamin D deficiency (<10 ng/mL) are significantly higher compared with those with sufficient vitamin D status (> 30 ng/mL) after adjusting for age, sex and BMI (P < 0.05) (35). Several mechanisms may explain the relationship of inflammatory cytokines with the vitamin D status (13, 36, 37).Vitamin D modulates nuclear transcription factors implicated in the cytokine generation and action by interacting with vitamin D response elements in the promoter region of cytokine genes. Moreover, vitamin D can activate the nuclear factor-β and regulate encoding proinflammatory cytokines. Calcitriol regulates the macrophage production of inflammatory factors via the calcium-dependent mechanism.
Some limitations exist in the present study. First, this study is a single-center cross-sectional study with a small number of cases. Second, the vitamin D level is affected by various factors. Seasonal variations in sun exposure and dietary sources of vitamin D are not analyzed. Further long-term prospective cohort or interventional studies are needed to confirm the causality in painful DPN.
In conclusion, our data suggest that severe vitamin D deficiency may play an important role in the progression of painful DPN. Furthermore, our analyses show that severe vitamin D deficiency is associated with altered inflammatory cytokines in painful DPN. In conclusion, severe vitamin D deficiency plays a role in painful DPN pathogenesis through elevated inflammation IL-6 and TNF-α levels. However, future studies with large number of subjects are needed to confirm these findings.
Standard Biosecurity and Institutional Safety Procedures
All the biosafety measurements have been adopted and the institutional safety procedures are adhered. The laboratory of our institution has biosafety level 2 (BSL-2) standard where all standards and protocols are adopted as per the guidelines of Clinical and Laboratory Standards Institute (CLSI).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Wenzhou Medical College. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CZ and SF designed and conceived the research. CS, NX, and YH recruited participants and collected data. LD and GX analyzed the data and interpreted the results. ZQ and CZ drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The paper was supported by Wenzhou Science and Technology Bureau (Grant No. Y20190548) and the Natural Science Foundation of Zhejiang Province (Grant No. LGD21H070001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge our colleagues from the Endocrinology Department and the Neurology Department at the First Affiliated Hospital of Wenzhou Medical University for their assistance in the present study.
References
1. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ. (2020) 369:m997. doi: 10.1136/bmj.m997
2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
3. Witzel II, Jelinek HF, Khalaf K, Lee S, Khandoker AH, Alsafar H. Identifying common genetic risk factors of diabetic neuropathies. Front Endocrinol. (2015) 6:88. doi: 10.3389/fendo.2015.00088
4. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. (2011) 34:2220–4. doi: 10.2337/dc11-1108
5. Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. (2015) 29:212–7. doi: 10.1016/j.jdiacomp.2014.10.013
6. Baute V, Zelnik D, Curtis J, Sadeghifar F. Complementary and alternative medicine for painful peripheral neuropathy. Curr Treat Options Neurol. (2019) 21:44. doi: 10.1007/s11940-019-0584-z
7. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. (2005) 30:374–85. doi: 10.1016/j.jpainsymman.2005.04.009
8. Alkhatatbeh M, Abdul-Razzak KK. Neuropathic pain is not associated with serum vitamin D but is associated with female gender in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. (2019) 7:e000690. doi: 10.1136/bmjdrc-2019-000690
9. Shillo P, Selvarajah D, Greig M, Gandhi R, Rao G, Wilkinson ID, et al. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet Med. (2019) 36 44-51. doi: 10.1111/dme.13798
10. Alam U, Petropoulos IN, Ponirakis G, Ferdousi M, Asghar O, Jeziorska M, et al. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab Res Rev. (2021) 37:e3361. doi: 10.1002/dmrr.3361
11. Basit A, Basit KA, Fawwad A, Shaheen F, Fatima N, Petropoulos IN, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. (2016) 4:e000148. doi: 10.1136/bmjdrc-2015-000148
12. Yammine K, Wehbe R, Assi C. A systematic review on the efficacy of vitamin D supplementation on diabetic peripheral neuropathy. Clin Nutr. (2020) 39:2970–4. doi: 10.1016/j.clnu.2020.01.022
13. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. (2012) 71:50–61. doi: 10.1017/S0029665111001650
14. Zheng S, Wang B, Han W, Zhu Z, Wang X, Jin X, et al. Vitamin D supplementation and inflammatory and metabolic biomarkers in patients with knee osteoarthritis: post hoc analysis of a randomised controlled trial. Br J Nutr. (2018) 120:41–8. doi: 10.1017/S0007114518001174
15. Adela R, Borkar RM, Mishra N, Bhandi MM, Vishwakarma G, Varma BA, et al. Lower serum vitamin d metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Front Immunol. (2017) 8:273. doi: 10.3389/fimmu.2017.00273
16. Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. (2014) 112:1938–43. doi: 10.1017/S0007114514003018
17. Zhu GC, Chen YW, Hung CH. Neural mobilization attenuates mechanical allodynia and decreases proinflammatory cytokine concentrations in rats with painful diabetic neuropathy. Phys Ther. (2018) 98:214–22. doi: 10.1093/ptj/pzx124
18. Yanik BM, Dauch JR, Cheng HT. Interleukin-10 reduces neurogenic inflammation and pain behavior in a mouse model of type 2 diabetes. J Pain Res. (2020) 13:3499–512. doi: 10.2147/JPR.S264136
19. Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. (2012) 29:937–44. doi: 10.1111/j.1464-5491.2012.03644.x
20. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. (2005) 114:29–36. doi: 10.1016/j.pain.2004.12.010
21. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
22. Soderstrom LH, Johnson SP, Diaz VA, Mainous GA III. Association between vitamin D and diabetic neuropathy in a nationally representative sample_ results from 2001-2004 NHANES. Diabet Med. (2012) 29:50–5. doi: 10.1111/j.1464-5491.2011.03379.x
23. He R, Hu Y, Zeng H, Zhao J, Zhao J, Chai Y, et al. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. (2017) 33:1–8. doi: 10.1002/dmrr.2820
24. Esteghamati A, Fotouhi A, Faghihi-Kashani S, Hafezi-Nejad N, Heidari B, Sheikhbahaei S, et al. Non-linear contribution of serum vitamin D to symptomatic diabetic neuropathy: a case-control study. Diabetes Res Clin Pract. (2016) 111:44–50. doi: 10.1016/j.diabres.2015.10.018
25. J Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, Koenig W. Proinflammatory cytokines predict the incidence and progression of distal sensorimotor polyneuropathy: KORA F4/FF4 Study. Diabetes Care. (2017) 40:569–76. doi: 10.2337/dc16-2259
26. Oliveira RB, Sampaio EP, Aarestrup F, Teles RM, Silva TP, Oliveira AL, et al. Cytokines and Mycobacterium leprae induce apoptosis in human schwann cells. J Neuropathol Exp Neurol. (2005) 60:882–90. doi: 10.1097/01.jnen.0000182982.09978.66
27. He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. (2010) 1512:266–79. doi: 10.1016/j.pain.2010.06.005
28. Nadi M, Bambaeichi E, Marandi SM. Comparison of the effect of two therapeutic exercises on the inflammatory and physiological conditions and complications of diabetic neuropathy in female patients. Diabetes Metab Syndr Obes. (2019) 12:1493–501. doi: 10.2147/DMSO.S206454
29. Magrinelli F, Briani C, Romano M, Ruggero S, Toffanin E, Triolo G, et al. The association between serum cytokines and damage to large and small nerve fibers in diabetic peripheral neuropathy. J Diabetes Res. (2015) 2015:547834. doi: 10.1155/2015/547834
30. Backryd E, Ghafouri B, Larsson B, Gerdle B. Plasma pro-inflammatory markers in chronic neuropathic pain: a multivariate, comparative, cross-sectional pilot study. Scand J Pain. (2016) 10:1–5. doi: 10.1016/j.sjpain.2015.06.006
31. Angst DBM, da Silva Vieira JS, Cobas RA, Vilas-Boas Hacker MA, Pitta IJR, Giesel LM, et al. Cytokine levels in neural pain in leprosy. Front Immunol. (2020) 11:23. doi: 10.3389/fimmu.2020.00023
32. Zhou J, Zhou S. Inflammation: therapeutic targets for diabetic neuropathy. Mol Neurobiol. (2014) 49:536–46. doi: 10.1007/s12035-013-8537-0
33. Ma XQ, Qin J, Li HY, Yan XL, Zhao Y, Zhang LJ. Role of exercise activity in alleviating neuropathic pain in diabetes via inhibition of the pro-inflammatory signal pathway. Biol Res Nurs. (2019) 21:14–21. doi: 10.1177/1099800418803175
34. Karonova T, Stepanova A, Bystrova A, Jude EB. High-dose vitamin D supplementation improves microcirculation and reduces inflammation in diabetic neuropathy patients. Nutrients. (2020) 12:2518. doi: 10.3390/nu12092518
35. Laird E, McNulty H, Ward M, Hoey L, McSorley E, Wallace JMW, et al. Vitamin D deficiency is associated with inflammation in older irish adults. J Clin Endocrinol Metab. (2014) 99:1807–15. doi: 10.1210/jc.2013-3507
36. Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: a randomized controlled clinical trial. J Clin Endocrinol Metab. (2014) 99:E2485–93. doi: 10.1210/jc.2014-1977
Keywords: type 2 diabetes mellitus, severe vitamin D deficiency, inflammatory cytokine, painful diabetic peripheral neuropathy, IL-6, TNF–α
Citation: Xiaohua G, Dongdong L, Xiaoting N, Shuoping C, Feixia S, Huajun Y, Qi Z and Zimiao C (2021) Severe Vitamin D Deficiency Is Associated With Increased Expression of Inflammatory Cytokines in Painful Diabetic Peripheral Neuropathy. Front. Nutr. 8:612068. doi: 10.3389/fnut.2021.612068
Received: 30 September 2020; Accepted: 18 February 2021;
Published: 10 March 2021.
Edited by:
Alessio Molfino, Sapienza University of Rome, ItalyReviewed by:
Maria Gisella Cavallo, Sapienza University of Rome, ItalyRayaz A. Malik, Weill Cornell Medicine-Qatar, Qatar
Copyright © 2021 Xiaohua, Dongdong, Xiaoting, Shuoping, Feixia, Huajun, Qi and Zimiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Zimiao, emltaWFvY2hlbkAxNjMuY29t; Zhou Qi, emhvdXFpX3d6bWNAeWVhaC5uZXQ=
†These authors have contributed equally to this work
 Gong Xiaohua
Gong Xiaohua Luo Dongdong
Luo Dongdong Niu Xiaoting
Niu Xiaoting Chen Shuoping
Chen Shuoping Shen Feixia1
Shen Feixia1 Yang Huajun
Yang Huajun Zhou Qi
Zhou Qi Chen Zimiao
Chen Zimiao