- Department of Urology, The First Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang, China
Objective: Several epidemiological studies have linked tomato products consumption with prostate cancer risk; however, the findings yielded inconsistent results. The aim of the present meta-analysis is to summary the evidence on this association based on eligible cohort studies.
Materials and Methods: A comprehensive literature search of articles was performed in March 2021 using PubMed, ISI Web of Science, and Scopus databases. A random-effects model was used to calculate the combined relative risks (RRs) and their corresponding 95% confidence intervals (CIs). Heterogeneity across studies was assessed using Cochran's Q statistic and the I2 score.
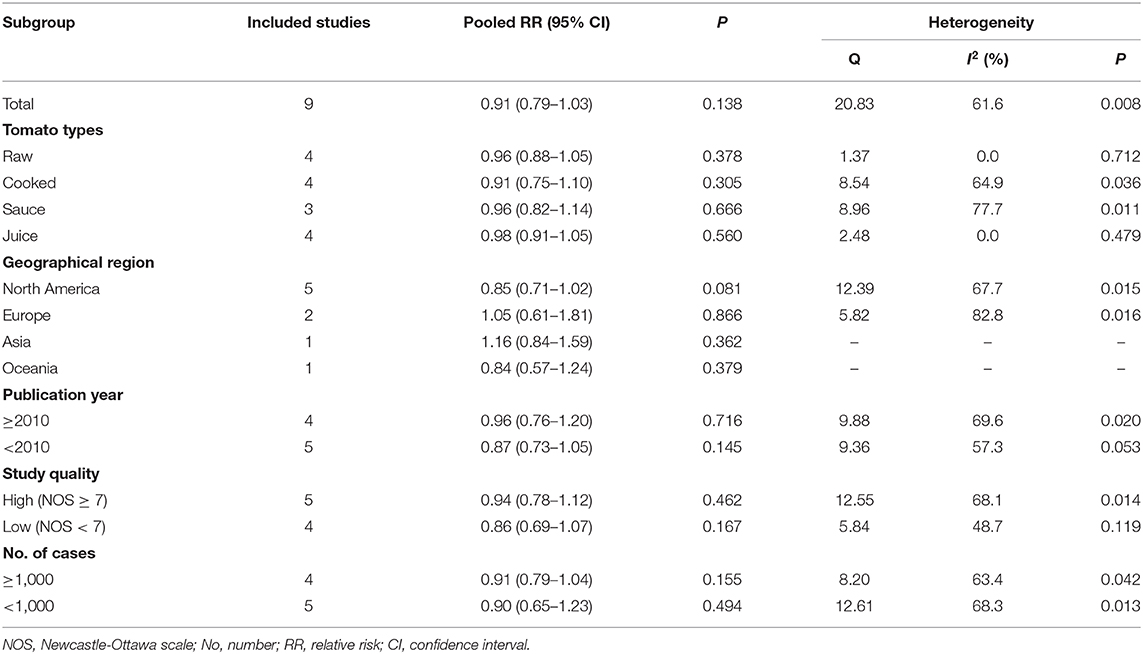
Results: A total of 10 prospective studies were finally included in our meta-analysis. There was no evidence of a significant association between tomato products consumption and prostate cancer risk (RR 0.91, 95% CI 0.79–1.03, P = 0.138). Subgroup meta-analyses were performed by tomato types, geographical region, publication year, study quality and number of cases. No significant associations were observed in any subgroups (all P > 0.05). No significant publication bias was observed using Begg's test (P = 0.602) or Egger's test (P = 0.957).
Conclusion: The results of this meta-analysis indicated that tomato consumption was not related with the risk of prostate cancer. Further prospective large-scale cohort studies are still warranted to verify our findings.
Introduction
Prostate cancer is the second most common cancer diagnosis made in men and the fifth leading cause of death worldwide. According to GLOBOCAN 2018 estimates, about 1.27 million new cases of prostate cancer occurred worldwide in 2018, with higher prevalence in the developed world compared with that in the developing countries (1). Prostate cancer risk has been reported to be positively associated with the following: black ethnicity, having a family history of prostate cancer, and advanced age (2). Dietary habits, including nutrients and dietary patterns, potentially affect prostate cancer pathogenesis and progression through various mechanisms mediated inflammation, antioxidant effects, and the action of sex hormones (3). Several dietary factors (4, 5) have been identified to be associated with the risk of prostate cancer, although controversial results have been reported for almost all nutrients.
Dietary tomato or lycopene intake has been shown beneficial for multiple health outcomes in humans (6). The association between tomato consumption and prostate cancer risk also has been assessed by several observational studies and meta-analyses. An early meta-analysis by Xu et al. (7) published in 2016 included a total of 24 case-control and cohort studies with 15,099 cases and reported that tomato intake may have a weak protective effect against prostate cancer. A dose-response meta-analysis published in 2018 also reported that increased tomato consumption was inversely associated with prostate cancer risk (8). However, a recent large pooled analysis failed to find a protective role of tomato consumption on prostate cancer (9). Considering these inconsistencies, we performed the present meta-analysis based on all eligible cohort studies to re-evaluate the relationship between tomato intake and the risk of prostate cancer.
Materials and Methods
Literature Search
Literature search was performed in March 2021 using PubMed, ISI Web of Science, and Scopus databases by two independent reviewers (JL and DK) with the following search algorithm: (tomato or tomatoes or lycopene or vegetable or vegetables or diet or nutrition) and (prostate cancer or prostatic cancer or prostate neoplasm or prostatic neoplasm) and (cohort or case-cohort or nested case-control or prospective or trial). The reference lists of retrieved articles and reviews were also examined to identify any additional relevant studies. This systematic review and meta-analysis was designed, performed, and reported according to the standards of quality for reporting meta-analyses (10).
Inclusion Criteria
An included study met all the following criteria: (i) the risk factor was consumption of tomato or tomato products; (ii) the outcome was the incidence of prostate cancer; (iii) study design was cohort, case-cohort, nested case-control or clinical trial; and (iv) the risk estimates with their corresponding 95% confidence intervals (CIs) were provided. If multiple studies used data from the same population, the study with the largest sample size was included in this meta-analysis. There were no restrictions on publication language, publication date or publication status.
Methodological Quality Assessment
Methodological quality of each included study was assessed using Newcastle-Ottawa Scale (NOS, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) by two independent reviewers (JL and DK). NOS is a tool used for assessing the quality of non-randomized studies included in a systematic review and/or meta-analyses. NOS contains eight items within three domain and the highest score is 9. A study with score from 7–9 was considered as high quality.
Data Collection
Data was extracted and recorded using a pre-defined form by two independent reviewers (JL and DK). The following data were collected from each study: first author's surname, publication year, country where the study was performed, study design, age, number of cases, method of exposure measurement, method of outcome assessment, fully-adjusted risk estimates with their corresponding 95% CIs, and adjusted variables in the statistical analysis. If data were not reported in the primary study, the items were designated “not applicable.” Any discrepancies were resolved by consensus.
Statistical Methods
Two studies (11, 12) provided the relative risks (RRs) for raw tomato and cooked tomato separately. In this situation, the overall RR was calculated by combined these risk estimates using a fixed effect model with inverse-variance method (13). Finally, the summary RR of all included studies was calculated using a DerSimonian and Laird random-effects model (14), which incorporates both within- and between-study variability. Stratified analyses were performed based on tomato types, study region, publication year, study quality, and sample size. One duplicate publication (15) was excluded from the main analysis but was included in subgroup analysis. Significant heterogeneity across studies was detected based on Q statistic (significant level set at 0.1) (16). The I2 score was used to assess the degree of heterogeneity (I2 <25%: small heterogeneity; I2 = 25–50%: moderate heterogeneity; I2 > 50%: large heterogeneity). A sensitivity analysis was conducted by excluding each study in turn and repeated the meta-analysis to assess the impact of each included study on the summary risk estimate. Publication bias was evaluated using Begg's test (17) and Egger's test (18).
Dose–response meta-analysis was performed with the method described by Greenland et al. (19) and Orsini et al. (20). Only studies that provided at least 3 quantitative categories were included. When a range of tomato consumption was provided, the median or mean value was regarded as the corresponding exposure dose. If the median or mean value was not reported, we used the midpoint of each category. If the lower or upper boundary was not provided, the boundary was assumed to have the same amplitude as the adjacent category. We converted the amount of tomato intake into a uniform measurement of grams (g) per day with the following equivalencies: 148 g per serving for raw tomatoes; 60 g per serving for cooked tomatoes; 104 g per serving (1:1 ratio of raw and cooked tomato products) for tomato products (8). A potential non-linear dose–response relationship between tomato intake and prostate cancer risk was examined using restricted cubic splines with three knots at the 25th, 50th, and 75th percentiles of the distribution. A P-value for non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero (21). All statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX) with two-sided P-values (set at 0.05).
Results
Study Search and Main Characteristics of Included Studies
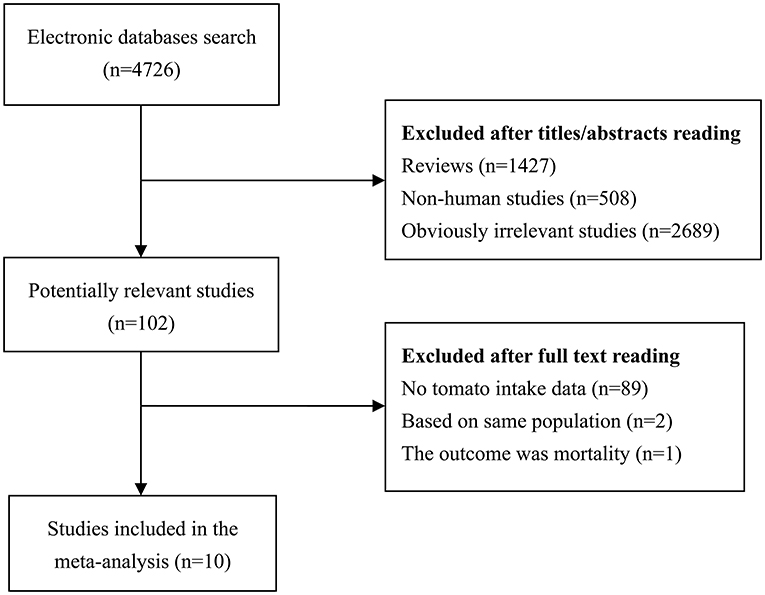
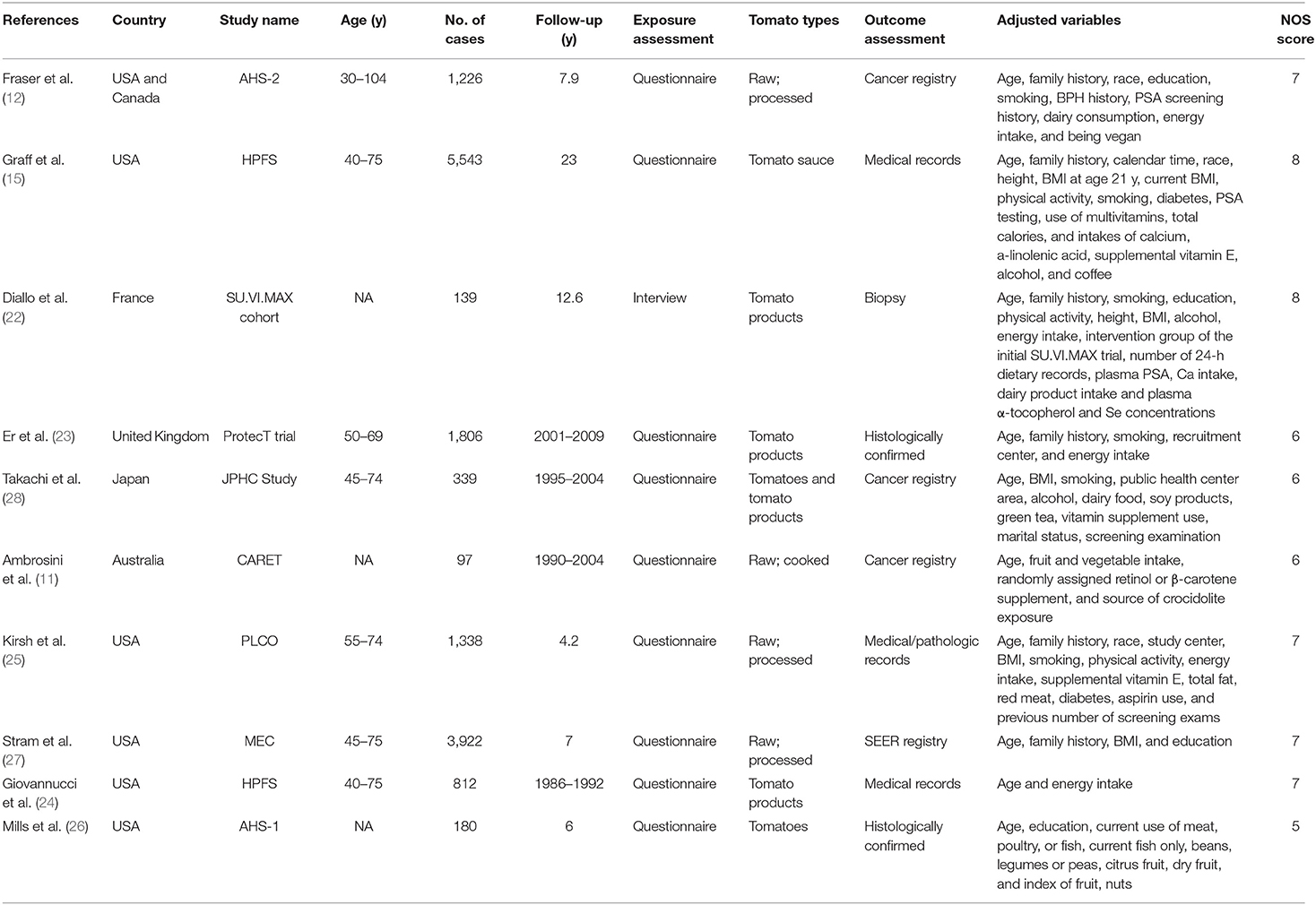
The detailed process of literature search and review has been shown in Figure 1. Initial search identified 4,726 potentially relevant studies. After reading titles or abstracts, 1,427 reviews, 508 non-human studies and 2,689 obviously irrelevant studies were removed. After further full text reading, 92 studies were removed with the following reasons: no tomato intake data (n = 89), based on same population (n = 2), and the outcome was mortality (n = 1). A total of 10 prospective studies (11, 12, 15, 22–28) were finally included in our meta-analysis. These studies were published between 1989 and 2020 and from the following geographical region: Europe (n = 1), North America (n = 7), Asia (n = 1), and Oceania (n = 1). Exposure data was collected by self-administrated questionnaire, except for the Diallo et al.'s study (22), which used interview. Outcome data was confirmed histologically or collected from cancer registry/medical records. Study quality scores assessed by NOS ranged from 5 to 8, with a mean value of 6.7. The main study characteristics have been summarized in Table 1.
Summary Analysis and Study Heterogeneity
Multivariable adjusted RRs with their CIs for each included study and for the combination of all studies are presented in Figure 2. The highest consumption of tomato products was not significantly associated with the risk of prostate cancer, compared with the lowest consumption group (RR 0.91, 95% CI 0.79–1.03, P = 0.138). There was significant heterogeneity across included studies (P = 0.008 for heterogeneity, I2 = 61.6%).
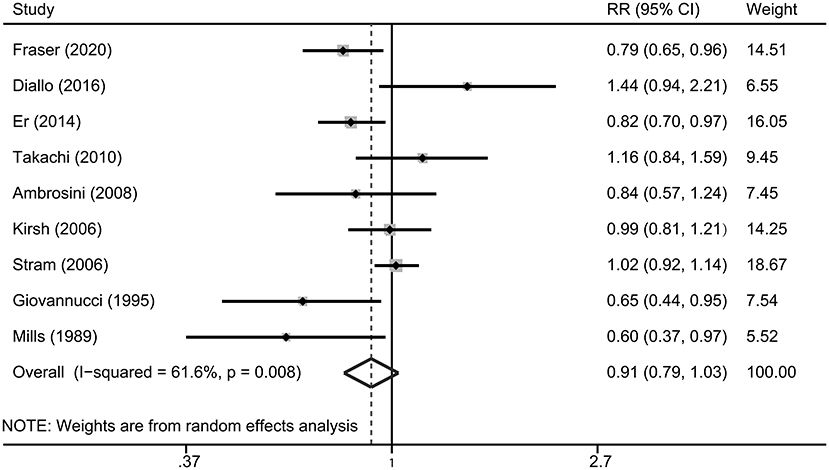
Figure 2. A forest plot showing risk estimates of the association between tomato consumption and prostate cancer risk.
Subgroup Analysis
Subgroup meta-analyses were performed by tomato types, geographical region, publication year, study quality and number of cases. No significant associations were observed in any subgroups (Table 2, all P > 0.05).
Sensitivity Analysis and Publication Bias Analysis
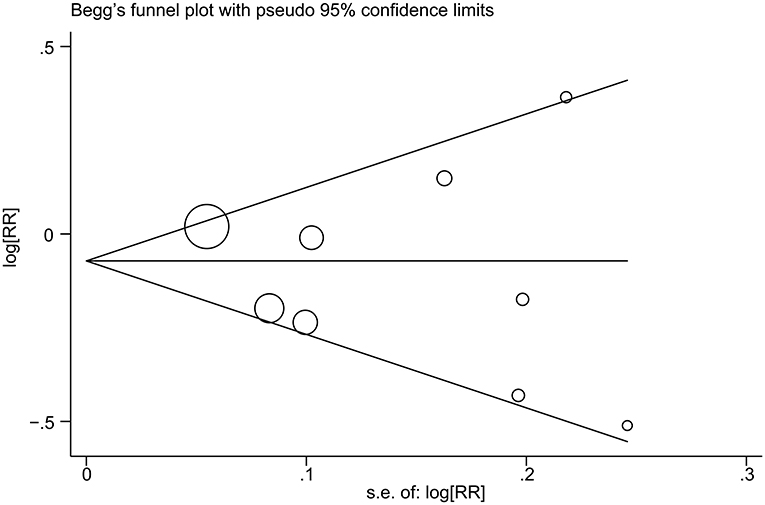
The impact of individual study on the summary RR was assessed by repeating the meta-analysis after removing each study in turn. The study-specific RRs ranged from a low of 0.88 (95% CI 0.78–1.00) to a high of 0.93 (95% CI 0.80–1.07) by removing the study by Diallo et al. (22) and the study by Fraser et al. (12), respectively (Figure 3). Similar results were obtained when excluding two studies (11, 12) that reported the RRs for raw tomato and cooked tomato separately (pooled RR = 0.93, 95% CI 0.80–1.09). No significant publication bias was observed using Begg's test (P = 0.602, Figure 4) or Egger's test (P = 0.957).
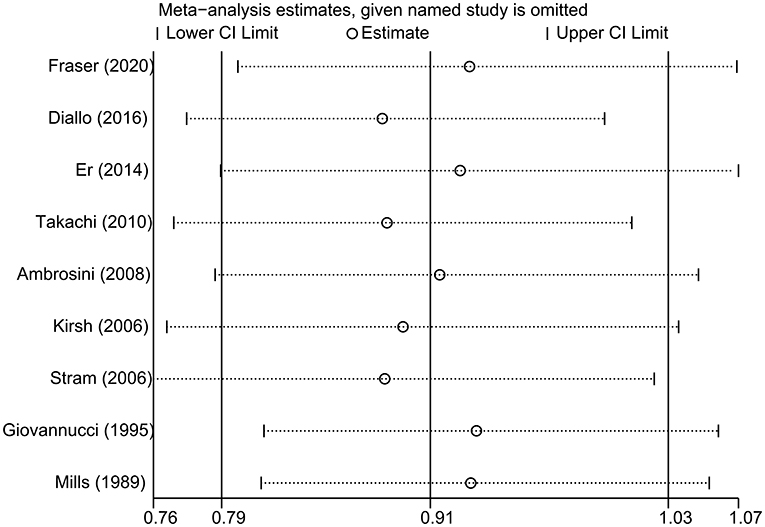
Figure 3. Sensitivity analysis was conducted by omitting each study in turn and repeated the meta-analysis.
Dose-Response Meta-Analysis for the Association Between Tomato Intake and Prostate Cancer Risk
The number of studies eligible for the dose–response analysis was five (22, 24–26, 28), four (11, 12, 24, 25), and thee (11, 12, 25) for tomato products, raw tomatoes, and cooked tomatoes, respectively. There was no evidence of non-linearity. All P-values for non-linear assessment were >0.05. The pooled RRs for a 20 g/day increase in tomato intake were 0.99 (95% CI 0.97–1.01), 0.99 (95% CI 0.97–1.01), and 0.98 (95% CI 0.96–1.00), respectively (Figure 5).
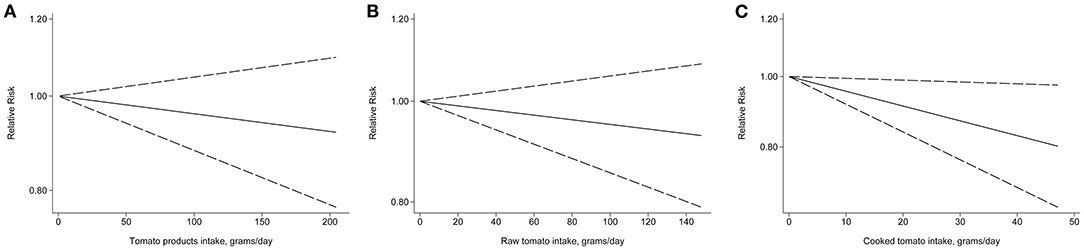
Figure 5. Relative risk for prostate cancer by doses of tomato intake based on the results of the dose–response meta-analyses. Solid line represents the estimated relative risks and the dotted lines represent the 95% confidence intervals. (A) Tomato products; (B) Raw tomato; (C) Cooked tomato.
Discussion
The current meta-analysis systematically summarized the results of 10 cohort studies including a total of 15,402 cases. The pooled results of this study indicated that the consumption of tomato products was not associated with the risk of prostate cancer. In addition, no significant associations were observed in any individual tomato products, including raw tomatoes, cooked tomatoes, tomato sauces, and tomato juice, in subgroup analyses. Although a borderline significant association between cooked tomato intake and prostate cancer risk was observed in dose-response analysis, this result should be interpreted with caution as only three studies were eligible and the findings may have been due to chance.
The findings of our study were not completely consistent with the previous meta-analyses (7, 8, 29, 30). The earliest meta-analysis by Etminan et al. (29) published in 2004 found that higher consumption of raw tomato or cooked tomato products was significantly related with a lower risk of prostate cancer. The study by Xu et al. (7) analyzed a total of 24 observational studies and reported that tomato intake was associated with a reduced risk of prostate cancer (RR 0.86, 95% CI 0.75–0.98, P = 0.019). The mostly recent meta-analysis published in 2018 (8) suggested a dose-response relationship with prostate cancer risk for total tomato consumption and for cooked tomatoes and sauces based on thirty observational studies. It worth to mention that the majority of studies included in these meta-analyses were case-control studies, which were prone to selection and recall bias. The evidence from cohort studies on the association of tomato intake with prostate cancer risk was relatively weak. For example, in the subgroup analysis of Xu et al.'s study (7), the RR for cohort studies was 0.96 (95% CI 0.84–1.10, P = 0.579). An early meta-analysis based on three prospective studies also found no significant association between raw or cooked tomato intake and prostate cancer risk (30).
One of the main mechanisms that has been proposed to explain the favorable effect of tomato intake on cancer prevention involves lycopene, which are found in high amounts in tomato products (31). A recent study indicated that lycopene is a promising chemotherapy drug by inhibiting prostate cancer progression via the inflammatory response signaling (32). Wang et al. (33) found that dietary lycopene consumption and its blood level were all associated with the risk of prostate cancer based on a systematic review and dose-response meta-analysis. Grainger et al. (34) performed a phase 2 dose-escalating study and firstly confirmed the phytoene and phytofluene in prostate tissue after a dietary intervention. However, Rowles et al. (35) found that tomato or lycopene had no significant impact on the emergence of castration-resistant prostate cancer in a murine model. Therefore, the role of tomato and lycopene intake on the incidence of prostate cancer is still inconsistent. Our meta-analysis based on published cohort studies supported that there was no clear association between tomato consumption and prostate cancer risk.
Our meta-analysis has several strengths. First, the present study had large sample size and thus enhanced the statistical power. Second, this meta-analysis only included cohort studies, which avoided the recall and selection biases. Third, based on the NOS scores, the methodological quality of the included studies was generally high. Finally, no obvious publication bias was observed across studies, indicating that the results were less likely to prone to biases.
Several limitations should also be acknowledged. First, the number of eligible studies was relatively limited and most of the included studies were performed in USA, which limited the generality of the findings of our study. Second, a certain degree of heterogeneity existed, which may distort the summary risk estimate. Finally, the types of tomato product and the cut-off points were various among the included studies, which might also impact the current analysis.
In conclusion, the results of this study indicated that tomato consumption was not related with the risk of prostate cancer. Further prospective large-scale cohort studies are still warranted to verify our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JL and DK contributed to the conception or design of the work. JL, DK, and QH contributed to the acquisition, analysis, or interpretation of data for the work. JL and DK drafted the manuscript. QH critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, et al. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. (2017) 117:1562–71. doi: 10.1038/bjc.2017.312
3. Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. (2020) 211447. doi: 10.3390/ijms21041447
4. Xu X, Cheng Y, Li S, Zhu Y, Zheng X, Mao Q, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr. (2014) 53:1615–23. doi: 10.1007/s00394-014-0667-2
5. Zhu Y, Li Q, Xu X. Dietary inflammatory index and the risk of prostate cancer: a dose-response meta-analysis. Eur J Clin Nutr. (2020) 74:1001–8. doi: 10.1038/s41430-019-0500-3
6. Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, et al. Tomato and lycopene and multiple health outcomes: umbrella review. Food Chem. (2021) 343:128396. doi: 10.1016/j.foodchem.2020.128396
7. Xu X, Li J, Wang X, Wang S, Meng S, Zhu Y, et al. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep. (2016) 6:37091. doi: 10.1038/srep37091
8. Rowles JL 3rd, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis. (2018) 21:319–36. doi: 10.1038/s41391-017-0005-x
9. Petimar J, Wilson KM, Wu K, Wang M, Albanes D, van den Brandt PA, et al. A pooled analysis of 15 prospective cohort studies on the association between fruit, vegetable, and mature bean consumption and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. (2017) 26:1276–87. doi: 10.1158/1055-9965.epi-16-1006
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9, W64.
11. Ambrosini GL, de Klerk NH, Fritschi L, Mackerras D, Musk B. Fruit, vegetable, vitamin A intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis. (2008) 11:61–6. doi: 10.1038/sj.pcan.4500979
12. Fraser GE, Jacobsen BK, Knutsen SF, Mashchak A, Lloren JI. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2. Cancer Causes Control. (2020) 31:341–51. doi: 10.1007/s10552-020-01279-z
13. Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. (1955) 19:251–3.
15. Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. (2016) 103:851–60. doi: 10.3945/ajcn.115.118703
16. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
19. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9.
20. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
21. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
22. Diallo A, Deschasaux M, Galan P, Hercberg S, Zelek L, Latino-Martel P, et al. Associations between fruit, vegetable and legume intakes and prostate cancer risk: results from the prospective Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr. (2016) 115:1579–85. doi: 10.1017/s0007114516000520
23. Er V, Lane JA, Martin RM, Emmett P, Gilbert R, Avery KN, et al. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (ProtecT) trial. Cancer Epidemiol Biomarkers Prev. (2014) 23:2066–77. doi: 10.1158/1055-9965.epi-14-0322
24. Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. (1995) 87:1767–76. doi: 10.1093/jnci/87.23.1767
25. Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. (2006) 15:92–8. doi: 10.1158/1055-9965.epi-05-0563
26. Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. (1989) 64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6
27. Stram DO, Hankin JH, Wilkens LR, Park S, Henderson BE, Nomura AM, et al. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: the multiethnic cohort study* (United States). Cancer Causes Control. (2006) 17:1193–207. doi: 10.1007/s10552-006-0064-0
28. Takachi R, Inoue M, Sawada N, Iwasaki M, Sasazuki S, Ishihara J, et al. Fruits and vegetables in relation to prostate cancer in Japanese men: the Japan Public Health Center-Based Prospective Study. Nutr Cancer. (2010) 62:30–9. doi: 10.1080/01635580903191502
29. Etminan M, Takkouche B, Caamaño-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. (2004) 13:340–5.
30. Chen J, Song Y, Zhang L. Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. J Nutr Sci Vitaminol. (2013) 59:213–23. doi: 10.3177/jnsv.59.213
31. Mirahmadi M, Azimi-Hashemi S, Saburi E, Kamali H, Pishbin M, Hadizadeh F. Potential inhibitory effect of lycopene on prostate cancer. Biomed Pharmacother. (2020) 129:110459. doi: 10.1016/j.biopha.2020.110459
32. Jiang LN, Liu YB, Li BH. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J Androl. (2018) 21:80–5. doi: 10.4103/aja.aja_70_18
33. Wang Y, Cui R, Xiao Y, Fang J, Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS ONE. (2015) 10:e0137427. doi: 10.1371/journal.pone.0137427
34. Grainger EM, Moran NE, Francis DM, Schwartz SJ, Wan L, Thomas-Ahner J, et al. A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J Nutr. (2019) 149:26–35. doi: 10.1093/jn/nxy232
Keywords: prostate cancer, tomato, cohort, meta-analysis, risk
Citation: Luo J, Ke D and He Q (2021) Dietary Tomato Consumption and the Risk of Prostate Cancer: A Meta-Analysis. Front. Nutr. 8:625185. doi: 10.3389/fnut.2021.625185
Received: 16 November 2020; Accepted: 08 April 2021;
Published: 04 May 2021.
Edited by:
Giuseppe Grosso, NNEdPro Global Centre for Nutrition and Health, United KingdomReviewed by:
Jose Paulo Andrade, Universidade Do Porto, PortugalAgnieszka Micek, Jagiellonian University, Poland
Copyright © 2021 Luo, Ke and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Luo, MTIwMjA0OUB6anUuZWR1LmNu
 Jie Luo
Jie Luo Dandan Ke
Dandan Ke