- 1Division of Preventive Medicine, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, Kyoto, Japan
- 2Department of Physiology and Cell Biology, Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 3Division of Applied Life Sciences, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan
- 4Department of Life Sciences, The University of Tokyo, Tokyo, Japan
- 5Faculty of Agriculture, Department of Food Science and Human Nutrition, Setsunan University, Osaka, Japan
- 6Faculty of Agriculture, Ryukoku University, Shiga, Japan
- 7Division of Community and Family Medicine, Center for Community Medicine, Jichi Medical University, Tochigi, Japan
Background: Hepcidin-25 is a 25 amino acid hepatokine and a key regulator of iron metabolism related to iron deficiency anemia. Recent studies have suggested that an elevated hepcidin level is correlated with low energy availability. Leptin is an appetite-suppressing adipokine and has been reported to stimulate hepcidin production in animals and cultured cells. While leptin is modulated by exercise, it is known that endurance runners and sprinters practice different types of exercise. This study investigated and compared the relationships between hepcidin and leptin levels, iron status, and body fat to understand better the risk of iron deficiency anemia in endurance runners and sprinters.
Methods: Thirty-six male college track and field athletes (15 endurance runners and 21 sprinters) were recruited for this study. Dietary intake, body composition, and blood levels of ferritin, hepcidin-25, leptin, and adiponectin were measured. Correlations between hepcidin levels and ferritin, body fat, leptin, and adiponectin were evaluated using Pearson's correlation coefficient for each group.
Results: The endurance runners had lower hepcidin levels and higher leptin and adiponectin levels compared with sprinters. Ferritin was positively correlated with hepcidin-25 levels in both the endurance and sprinter groups. A positive correlation was observed between hepcidin-25 and body fat or leptin levels only in sprinters.
Conclusion: This is the first study investigating the relationship between blood levels of hepcidin and leptin in athletes. The positive correlation between hepcidin-25 and leptin was observed in sprinters but not endurance runners.
Introduction
It is well-known that iron deficiency anemia is a problem for exercise performance and health in athletes (1–3). Therefore, it is highly desirable to monitor iron deficiency in athletes in the field. Hepcidin is secreted from the liver and adipose tissue and is a primary regulator of iron metabolism (4, 5). Excess hepcidin degrades the ferroportin export channels on the surface of macrophages and the intestinal duodenum, resulting in reduced iron recycling and absorption from the intestine (4, 5). The relationships between increased hepcidin levels and risk of iron deficiency have been investigated in various health settings, and hepcidin has been suggested as a surrogate marker for iron metabolism in athletes (4–9).
Excessive exercise increases the risk of iron deficiency (3), and multiple studies have attempted to clarify the relationship between blood levels of hepcidin and energy availability (7–10). The appetite-related hormone leptin is a well-known adipokine (11, 12) reported to stimulate hepcidin production in mice and cultured cells (13, 14). Exercise training modulates leptin levels and sensitivity, as well as appetite (15–17), and leptin-induced modulation of hepcidin may affect energy availability. Leptin is lower in body fat during fasting and acute exercise, which generally depicts the state of endurance runners (18). On the other hand, eating disorders were observed more in endurance runners than other types of athletes (19), which potentially disrupt leptin dynamics (20). However, no studies have investigated the relationship between hepcidin and leptin levels in athletes.
The type of exercise performed by athletes differentially exercise time, body fat, and hormone secretion, including leptin (15–17). In track and field clubs, endurance runners and sprinters typically engage in different types of exercise. Therefore, this study investigated the relationships between hepcidin and leptin, iron status, and body fat to understand better the risk of iron deficiency anemia in endurance runners and sprinters. We hypothesize that leptin is correlated with hepcidin level in sprinters but not in endurance runners.
Materials and Methods
Participants
Thirty-six male University track and field athletes (15 endurance runners and 21 sprinters) were enrolled in this cross-sectional study. The inclusion criterion was endurance runner or sprinter, and the exclusion criteria were the athletes who have iron deficiency (ferritin <20.0 ng/mL, one sprinter) (1). All athletes belonged to the same University club, and the team was in the top tier of University teams in Japan, running 5,000 m in <16 min and 30 s, and 100 m in <11.5 s. The athletes practiced 5–6 days per week, 3 h per day. This study was carried out in accordance with the recommendations of the Declaration of Helsinki (Fortaleza 2013). The study protocol was approved by the Ethics Committee of the Institutional Review Board of Kyoto Medical Center (approval number: 2013-005), and all participants provided written informed consent. The subjects were instructed to fast for 10 h and avoid exercise for 24 h before taking the following measurements.
Body Composition
Body weight, body fat content, and skeletal muscle mass were measured using an Inbody 720 analyzer (Biospace, Seoul, Korea). The body fat and skeletal muscle were estimated using the impedance method by measuring the voltage drop initiated from a current as it passes between electrodes (21, 22).
Questionnaire of Track and Field History and Dietary Record
Track and field history was assessed via a questionnaire. Daily intake of energy and nutrients was determined using the brief-type self-administered diet history questionnaire (BDHQ) (23, 24). The BDHQ is a four-page, fixed-portion questionnaire that estimates the dietary intake of 46 food and beverage items during the past month. Details of the BDHQ, including the food and beverage items queried and the methods used, are provided elsewhere (23, 24).
Blood Analysis
Blood samples were collected from the antecubital vein between 9:30 and 10:30 am. Peripheral blood tests were performed to determine red blood cell (RBC) counts, hemoglobin (Hb) and hematocrit (Hct) levels, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration. To quantify the active form of hepcidin, hepcidin-25, serum samples were mixed with synthetic human hepcidin (Peptide Institute, Osaka, Japan) as an internal standard and applied to a reverse-phase PLRP-S column (5 mm, 300 Å, 150 × 3 × 2.1 mm; Varian, Inc, Palo Alto, CA, USA). Levels of hepcidin-25 in the eluate were measured using a 4000 QTRAP liquid chromatography tandem mass spectrometry system (Applied Biosystems, Foster City, CA) (25). A LABOSPECT 008α automatic colorimetric analyzer (Hitachi High-Technologies Corporation, Schaumburg, IL, USA) was used to measure serum levels of iron. To test levels of iron stored in the body, we measured the concentrations of ferritin in serum using an iatro ferritin kit (LSI Medience Corporation, Tokyo, Japan). The leptin levels were quantified using a human leptin radioimmunoassay kit (EMD Millipore Corporation, Billerica, MA) (26). The adipokine adiponectin was measured using a human adiponectin latex kit (LSI Medience Corporation) (27).
Statistical Analysis
Data were expressed as means ± SD. The normal distribution was tested using the Shapiro-Wilk test. The hepcidin-25 level was log-transformed since it is not normal distribution in the endurance runner group. Differences between groups were analyzed using the independent Student's or Mann- Whitney U test. The effect size was calculated by cohen's d value. The correlation analysis was used in a Pearson's correlation coefficient, respectively. A multivariate regression analysis was performed to evaluate the relationship between the log hepcidin-25 and leptin or body fat, independent of ferritin levels. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics software, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
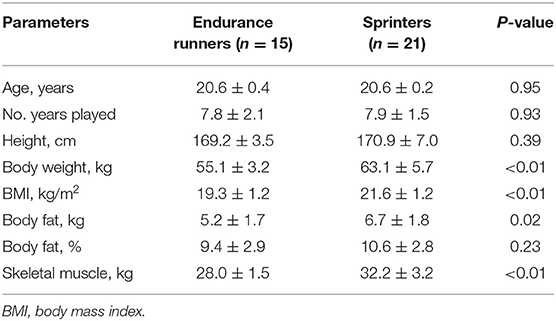
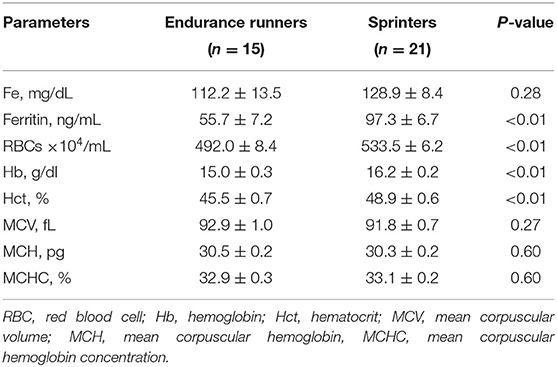
The anthropometric characteristics of the study subjects are shown in Table 1. The participants had an average age of 20.1 ± 0.2 years and had engaged in track and field events for an average of 7.8 ± 0.3 (range, 4–11) years. As expected from previous studies (28, 29), the sprinters had a significantly higher body weight, body mass index, and lean mass and body fat compared with endurance runners (P < 0.05). There were no significant differences between endurance runners vs. sprinters for absolute intake of energy (2,387 ± 85.5 vs. 2,209 ± 136 kcal/day; P = 0.34), protein (81.3 ± 3.7 vs. 81.9 ± 5.7 g/day; P = 0.94), fat (68.7 ± 4.3 vs. 64.3 ± 4.2 g/day; P = 0.48), carbohydrates (336.6 ± 11.5 vs. 311.6 ± 25.0 g/day, P = 0.45), and iron (8.1 ± 2.0 vs. 8.6 ± 3.0 mg/day; P = 0.64). Anemia-related blood test parameters are shown in Table 2. As previously reported (1, 30, 31), endurance runners had lower concentrations of ferritin and Hb, numbers of RBCs, and % Hct compared with sprinters (P < 0.05).
Figure 1 shows the serum levels of the hepcidin-25 and adipokines leptin and adiponectin in the endurance runner and sprinter groups. The serum levels of hepcidin-25 varied (range, 1.1–18.8 ng/ml) even though the participants belonged to the same club. The hepcidin-25 level (6.1 ± 5.3 vs. 9.9 ± 4.7 ng/ml; d = 0.76, P = 0.03), and log-hepcidin-25 levels (0.65 ± 0.37 vs. 0.93 ± 0.25 ng/ml; d = 0.89, P < 0.01) were lower in the endurance runners compared with sprinters. Leptin levels were higher in endurance runners compared with sprinters (4.2 ± 0.9 vs. 3.6 ± 0.9 ng/ml; d = 0.67, P = 0.03), and adiponectin levels (10.4 ± 3.4 vs. 8.1 ± 2.8 ng/ml; d = 0.74, P = 0.03) between these groups.
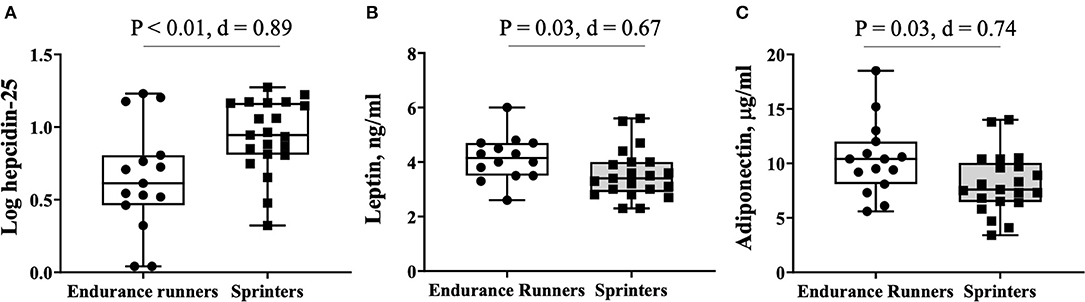
Figure 1. Comparison of hepatokine and adipokine levels between endurance runners and sprinters. (A) log hepcidin-25, (B) leptin, (C) adiponectin.
Correlation analyses were performed to examine the relationship between hepcidin levels and adipokine levels (Figure 2). It was reported that hepcidin-25 levels were significantly and positively correlated with the amount of body fat and serum ferritin levels (5, 32, 33). Therefore, we investigated the relationship between hepcidin-25 and these parameters in endurance runners and sprinters. Levels of serum ferritin and hepcidin-25 were positively correlated in both the endurance runner and sprinter groups (Figure 2A). A positive correlation between hepcidin-25 and body fat was observed in sprinters but not in the endurance runners (Figure 2B). We investigated the relationship between serum levels of leptin and hepcidin-25 because leptin has been shown to correlate with body fat and appetite and stimulate hepcidin production (11–14). A significant correlation between leptin and hepcidin concentrations was observed in sprinters but not in endurance runners (Figure 2C), while there was no correlation observed between adiponectin and hepcidin in either group (Figure 2D). Furthermore, multivariate regression analysis showed that the leptin levels were correlated with hepcidin-25 levels independent of ferritin in sprinters but not observed in endurance runners (Table 3).
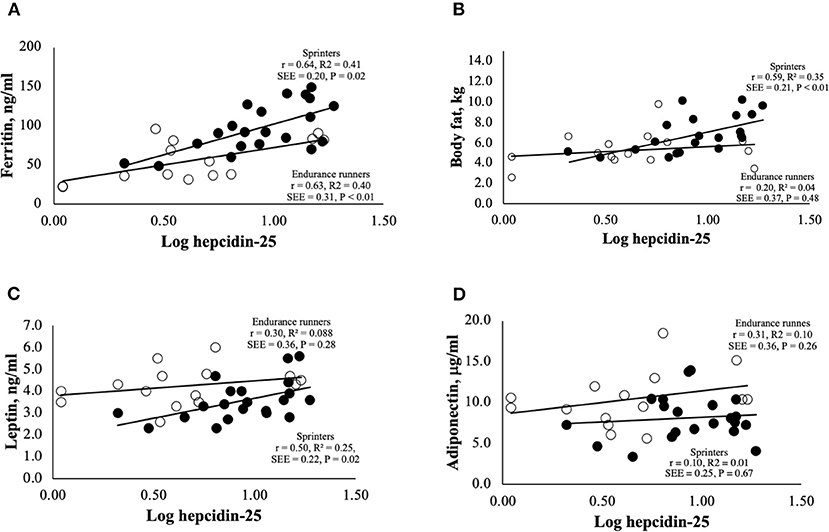
Figure 2. Correlations between serum levels of hepcidin-25 and (A) ferritin, (B) body fat, (C) leptin, and (D) adiponectin for endurance runners and sprinters. The hepcidin-25 value was log transformed, to keep normal distribution. Correlations were determined using Pearson's correlation coefficient. The open and closed circles represent the endurance runners and sprinters, respectively. SSE, Standard error of estimate.
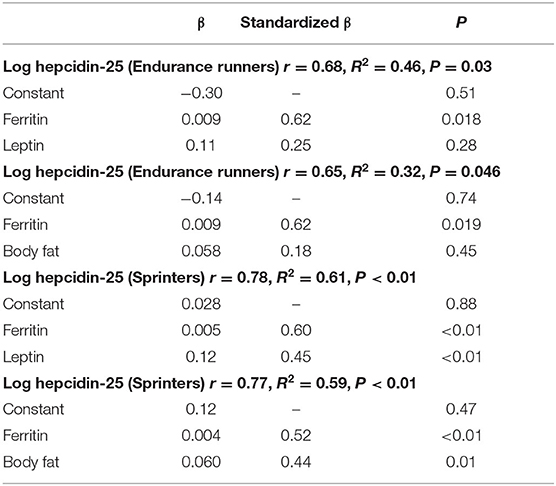
Table 3. Hepcidin-25 level is correlated with leptin and body fat in sprinters but not in endurance runners.
Discussion
The main finding of the present study was that we discovered a correlation between the serum levels of hepcidin and leptin in male college-level sprinters but not endurance runners. This is the first study to show a relationship between hepcidin and leptin concentrations in athletes.
Although the mechanism underlying the association between hepcidin and leptin levels in the blood is unknown, there have been mouse and cell culture studies that indicated a relationship between these two proteins (13, 14). Serum hepcidin levels are low in leptin-deficient (ob/ob) mice. Interestingly, leptin receptor-deficient (db/db) mice have higher leptin levels than ob/ob mice, but low hepcidin levels (13), which suggests that leptin receptors may stimulate hepcidin production or secretion. Indeed, the administration of recombinant leptin to ob/ob mice for 2 weeks showed a significant increase in the levels of serum hepcidin and hepatic hepcidin mRNA (Hamp) in the liver (13). Furthermore, leptin stimulation increased hepcidin mRNA in cultured human HuH7 hepatoma cells (14).
The relationship between hepcidin and leptin levels observed in the sprinter group may be due to the stimulation of the leptin receptor. However, in the present study, there was no correlation between serum levels of hepcidin and leptin in endurance runners. Basically, endurance runners have a higher energy expenditure during daily training (e.g., 10–20 km of running/day, ~500–1,000 kcal/day) than sprinters. Besides, the practice without diet tends to be higher observed in long-distance runners (34). Since we did not estimate accurate dietary intake (e.g., 3-day weighted-food records) and energy expenditure, we did not estimate the energy valance or availability. However, compared to sprinters, a lower body fat amount, BMI, and anemia-related parameters (35, 36) in endurance runners may indicate the lower energy availability. In athletes, low energy availability may cause iron deficiency anemia (1–3, 8). The chronic stress from excessive exercise may disrupt leptin metabolism (15, 37–40). Leptin levels are not only decreased during chronic changes in body fat (37) but also during short-term changes corresponding to energy states (38–40), such as fasting and dietary restriction. In terms of iron metabolism, it was reported that the treatment of 3T3L-1 adipocytes with iron decreased the expression of leptin mRNA (37). Our data showed that endurance runners had lower iron status and higher leptin levels compared with sprinters regardless of the amount of body fat. Therefore, it is speculated that there was no relationship between the hepcidin and leptin levels in endurance runners because of disruption of energy and iron status induced by vigorous endurance-related exercise.
There were some limitations to this study. First, we did not evaluate leptin receptor sensitivity in our subjects. However, there is no way to evaluate the tissue leptin receptor in humans. Second, we did not evaluate exercise activity and did not estimate total energy intake using BDHQ; therefore we could not estimate energy availability. The doubly labeled water method would be needed to investigate detailed energy expenditure in a day. We evaluated dietary intake using a questionnaire. However, this method may not be appropriate for individual athletes. Indeed, according to our BDHQ data, our subjects consumed ~2,000 kcal/day of energy, which is the average recommended intake for a sedentary individual. To investigate the relationship between hepcidin levels and energy state, 3-day food intake records will be needed in future studies.
In conclusion, the positive correlation between serum levels of hepcidin-25 and leptin in sprinters and lack of correlation in endurance runners. Further studies are warranted to investigate the relationships between blood leptin and hepcidin levels and energy availability in athletes. This will provide useful insight on how to prevent sport-related anemia through hepcidin level monitoring.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Institutional Review Board of Kyoto Medical Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SN and NS designed the study. SN, HT, NA, KI, and NS collected and assembled of data. SN performed the statistical analysis and prepared the manuscript. HT, AI, MF, KK, KI, and NS did the trial management and helped to draft the manuscript with its critical review. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
Funding
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (17J11622, 17K18244, 19K11512, and 20K11409).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the track and field athletes who participated in this study and the track and field general manager for his assistance in recruiting participants. We also thank Saki Hirai, Sayaka Higuchi, Natsuki Uchiyama, and Nene Enomoto for their help in data collection.
References
1. Parks RB, Hetzel SJ, Brooks MA. Iron Deficiency and Anemia among Collegiate Athletes: A Retrospective Chart Review. Med Sci Sports Exerc. (2017) 49:1711–5. doi: 10.1249/MSS.0000000000001259
2. Beard J, Tobin B. Iron status and exercise. Am J Clin Nutr. (2000) 72:594S−7S. doi: 10.1093/ajcn/72.2.594S
3. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. (2007) 39:1867–72. doi: 10.1249/mss.0b013e318149f111
4. Hare DJ. Hepcidin: a real-time biomarker of iron need. Metallomics. (2017) 9:606–8. doi: 10.1039/C7MT00047B
5. Badenhorst CE, Black KE, O'Brien WJ. Hepcidin as a prospective individualized biomarker for individuals at risk of low energy availability. Int J Sport Nutr Exerc Metab. (2019) 31:1–11. doi: 10.1123/ijsnem.2019-0006
6. Sandström G, Rödjer S, Jacobsson S, Nelson D, Börjesson M. Increased level of serum hepcidin in female adolescent athletes. Clin J Sport Med. (2018) 28:180–3. doi: 10.1097/JSM.0000000000000423
7. Ishibashi A, Maeda N, Sumi D, Goto K. Elevated serum hepcidin levels during an intensified training period in well-trained female long-distance runners. Nutrients. (2017) 9:E277. doi: 10.3390/nu9030277
8. Ishibashi A, Kojima C, Tanabe Y, Iwayama K, Hiroyama T, Tsuji T, et al. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol Rep. (2020) 8:e14494. doi: 10.14814/phy2.14494
9. Hennigar SR, Berryman CE, Kelley AM, Anderson BJ, Young AJ, McClug JP, et al. High-altitude acclimatization suppresses hepcidin expression during serve energy deficit. High Alt Med Biol. (2020) 21:232–6. doi: 10.1089/ham.2019.0109
10. Hennigar SR, McClung JP, Hatch-McChesney A, Allen JT, Wilson MA, Carrigan CT, et al. Energy deficit increases hepcidin and exacerbates declines in dietary iron absorption following strenuous physical activity: a randomized-controlled cross-over trial. Am J Clin Nutr. (2020) 10:nqaa289. doi: 10.1093/ajcn/nqaa289
11. Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol. (2000) 143:293–311. doi: 10.1530/eje.0.1430293
12. Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. (2014) 223:T83–96. doi: 10.1530/JOE-14-0358
13. Yamamoto K, Kuragano T, Kimura T, Nanami M, Hasuike Y, Nakanishi T. Interplay of adipocyte and hepatocyte: leptin upregulates hepcidin. Biochem Biophys Res Commun. (2018) 495:1548–54. doi: 10.1016/j.bbrc.2017.11.103
14. Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. (2007) 137:2366–70. doi: 10.1093/jn/137.11.2366
15. Bobbert T, Mai K, Brechtel L, Schulte HM, Weger B, Pfeiffer AFH, et al. Leptin and endocrine parameters in marathon runners. Int J Sports Med. (2012) 33:244–8. doi: 10.1055/s-0031-1291251
16. Tremblay A, Dutheil F, Drapeau V, Metz L, Lesour B, Chapier R, et al. Long-term effects of high-intensity resistance and endurance exercise on plasma leptin and ghrelin in overweight individuals: the RESOLVE Study. Appl Physiol Nutr Metab. (2019) 44:1172–9. doi: 10.1139/apnm-2019-0019
17. Middelbeek RJW, Motiani P, Brandt N, Nigro P, Zheng J, Virtanen KA, et al. Exercise intensity regulates cytokine and klotho responses in men. Nutr Diabetes. (2021) 11:5. doi: 10.1038/s41387-020-00144-x
18. Fernandes MF, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T. Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab. (2015) 22:741–9. doi: 10.1016/j.cmet.2015.08.003
19. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. (2004). 14:25–32. doi: 10.1097/00042752-200401000-00005
20. Yen JY, Lin HC, Lin PC, Liu TL, Long CY, Ko CH. Leptin and ghrelin concentrations and eating behaviors during the early and late luteal phase in women with premenstrual dysphoric disorder. Psychoneuroendocrinology. (2020). 118:104713. doi: 10.1016/j.psyneuen.2020.104713
21. Bailey BW, Lecheminant G, Hope T, Bell M, Tucker LA. A comparison of the agreement, internal consistency, and 2-day test stability of the InBody 720, GE iDXA, and BOD POD® gold standard for assessing body composition. Meas Phys Educ Exerc Sci. (2017) 3:231–8. doi: 10.1080/1091367X.2017.1422129
22. Nirengi S, Fujibayashi M, Furuno S, Uchibe A, Kawase Y, Sukino S, et al. Nonalcoholic fatty liver disease in University Rugby football players. Front Endocrinol. (2018) 9:341. doi: 10.3389/fendo.2018.00341
23. Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. (2012) 22:151–9. doi: 10.2188/jea.JE20110075
24. Shiraishi M, Haruna M, Matsuzaki M, Murayama R, Sasaki S. Availability of two self-administered diet history questionnaires for pregnant Japanese women: a validation study using 24-hour urinary markers. J Epidemiol. (2017) 27:172–9. doi: 10.1016/j.je.2016.05.005
25. Uehata T, Tomosugi N, Shoji T, Sakaguchi Y, Suzuki A, Kaneko T, et al. Serum hepcidin-25 levels and anemia in non-dialysis chronic kidney disease patients: a cross-sectional study. Nephrol Dial Transplant. (2012) 27:1076–83. doi: 10.1093/ndt/gfr431
26. Belva F, De Schepper J, Roelants M, Tournaye H, Bonduelle M, Provyn S. Body fat content, fat distribution and adipocytokine production and their correlation with fertility markers in young adult men and women conceived by intracytoplasmic sperm injection (ICSI). Clin Endocrinol. (2018) 88:985–92. doi: 10.1111/cen.13571
27. Kobayashi H, Otsuka H, Yanai M, Haketa A, Hara M, Hishiki M, et al. Adiponectin is not associated with renalfunction decline in community-dwelling elderly adults. Medicine. (2018) 97:e10847. doi: 10.1097/MD.0000000000010847
28. Maughan RJ, Watson JS, Weir J. Relationships between muscle strength and muscle cross-sectional area in male sprinters and endurance runners. Eur J Appl Physiol Occup Physiol. (1983) 50:309–8. doi: 10.1007/BF00423237
29. Tyka AK, Chwastowski M, Cison T, Palka T, Tyka A, Szygula Z, et al. Effect of creatine malate supplementation on physical performance, body composition and selected hormone levels in spinters and long-distance runners. Acta Physiol Hung. (2015) 102:114–22. doi: 10.1556/APhysiol.102.2015.1.12
30. Ciekot-Sołtysiak M, Kusy K, Podgórski T, Zieliński J. Training-induced annual changes in red blood cell profile in highly-trained endurance and speed-power athletes. J Sports Med Phys Fitness. (2018) 58:1859–66. doi: 10.23736/S0022-4707.17.07819-7
31. Coates A, Mountjoy M, Burr J. Incidence of Iron Deficiency and Iron Deficient Anemia in Elite Runners and Triathletes. Clin J Sport Med. (2017) 27:493–8. doi: 10.1097/JSM.0000000000000390
32. Auguet T, Aragonès G, Berlanga A, Martínez S, Sabench F, Binetti J, et al. Hepcidin in morbidly obese women with non-alcoholic fatty liver disease. PLoS ONE. (2017) 12:e0187065. doi: 10.1371/journal.pone.0187065
33. Andrews Guzmán M, Arredondo Olguín M. Association between ferritin, high sensitivity C-reactive protein (hsCRP) and relative abundance of Hepcidin mRNA with the risk of type 2 diabetes in obese subjects. Nutr Hosp. (2014) 30:577–84. doi: 10.3305/nh.2014.30.3.7647
34. Heikura IA, Stellingwerff T, Mero AA, Uusitalo AL, Burke LM. A mismatch between athlete practice and current sports nutrition guidelines among elite female and male middle- and long-distance athletes. Int J Sport Nutr Exerc Metab. (2017) 27:351–60. doi: 10.1123/ijsnem.2016-0316
35. Finn EE, Tenforde AS, Fredericson M, Golden NH, Carson TL, Karvonen-Gutierrez CA. Markers of low iron status are associated with female athlete triad risk factors. Med Scie Sports Exerc. (2021). doi: 10.1249/MSS.0000000000002660
36. O'Leary TJ, Wardle SL, Greeves JP. Energy deficiency in soldiers: the risk of the athlete triad and relative energy deficiency in sport syndromes in the military. Front Nutr. (2020) 7:142. doi: 10.3389/fnut.2020.00142
37. Gao Y, Li Z, Gabrielsen JS, Simcox JA, Lee SH, Jones D, et al. Adipocyte iron regulates leptin and food intake. J Clin Invest. (2015) 125:3681–91. doi: 10.1172/JCI81860
38. Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. (1996) 81:4162–5. doi: 10.1210/jcem.81.11.8923877
39. Kolaczynski JW, Considine RV, Ohannesian JP, Marco C, Opentanova I, Nyce MR, et al. Response of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. (1996) 45:1511–5. doi: 10.2337/diab.45.11.1511
40. Caldeira RS, Panissa VLG, Inoue DS, Campos EZ, Monteiro PA, Giglio BM, et al. Impact to short-term high intensity intermittent training on different storages of body fat, leptin and soluble leptin receptor levels in physically active non-obese men: a pilot investigation. Clin Nutr ESPEN. (2018) 28:186–2. doi: 10.1016/j.clnesp.2018.08.005
Keywords: track and field, iron metabolism, adipokine, college athlete, body fat, diet
Citation: Nirengi S, Taniguchi H, Ishibashi A, Fujibayashi M, Akiyama N, Kotani K, Ishihara K and Sakane N (2021) Comparisons Between Serum Levels of Hepcidin and Leptin in Male College-Level Endurance Runners and Sprinters. Front. Nutr. 8:657789. doi: 10.3389/fnut.2021.657789
Received: 24 January 2021; Accepted: 05 May 2021;
Published: 31 May 2021.
Edited by:
Tyler Farney, Texas A&M University Kingsville, United StatesReviewed by:
Caio Victor Sousa, Northeastern University, United StatesKrzysztof Kusy, Poznan University of Physical Education, Poland
Copyright © 2021 Nirengi, Taniguchi, Ishibashi, Fujibayashi, Akiyama, Kotani, Ishihara and Sakane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naoki Sakane, bnNha2FuZUBnZjYuc28tbmV0Lm5lLmpw
 Shinsuke Nirengi
Shinsuke Nirengi Hirokazu Taniguchi3
Hirokazu Taniguchi3 Kazuhiko Kotani
Kazuhiko Kotani Kengo Ishihara
Kengo Ishihara Naoki Sakane
Naoki Sakane