- 1Department of Kinesiological Anthropology and Methodology, Faculty of Kinesiology, University of Zagreb, Zagreb, Croatia
- 2Department of Endocrinology, Diabetes, Metabolism and Clinical Pharmacology, Clinical Hospital Dubrava, Zagreb, Croatia
- 3Department of Pharmacology, Faculty of Medicine, University of J. J. Strossmayer Osijek, Osijek, Croatia
- 4Department of Endocrinology, Clinical Hospital Center Osijek, Osijek, Croatia
- 5Department of Gastroenterology and Hepatology, University Hospital Center Zagreb, Zagreb, Croatia
- 6School of Medicine, University of Zagreb, Zagreb, Croatia
- 7Faculty of Kinesiology, University of Zagreb, Zagreb, Croatia
Along with the increase in obesity and type 2 diabetes, the non-alcoholic fatty liver disease (NAFLD) incidence is escalating, thus becoming a leading cause of liver cirrhosis and a significant burden of liver-related outcomes. Since there is no pharmacotherapy available to address the NAFLD, the most effective solutions seem to be lifestyle changes centered on physical activity. Exercise could mediate its beneficial effects directly on the liver and indirectly via extrahepatic pathways, forming a dose-response relationship with NAFLD in terms of prevalence and disease severity. Health-enhancing physical activity (HEPA) levels are mainly needed to exert beneficial effects in obese subjects, while even a small amount of exercise can be beneficial for lean individuals to prevent NAFLD. This mini-review addresses three major points regarding physical activity and NAFLD: prevention, treatment, and extrahepatic benefits, offering recommendations on type and intensity of exercise in liver disease.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is an umbrella term inclosing a spectrum of clinical and pathological fatty liver disease entities which may lead to cirrhosis and hepatocellular carcinoma (HCC) (1). The prevalence of NALFD is increasing worldwide and is estimated at around 25% (2). However, the true prevalence of NALFD seems to be much higher, given the global rise of metabolic syndrome due to changes in eating habits and inclination toward a sedentary lifestyle.
Metabolic syndrome has become a growing morbidity cluster epidemic resulting in a sharp rise in obesity, type 2 diabetes mellitus (T2DM), hypertension, and dyslipidemia. Its liver manifestation—NAFLD has become the most common cause of the chronic liver disease (2). Moreover, the prevalence of NAFLD among patients with T2DM is even higher, 56%, while the overall prevalence of non-alcoholic steatohepatitis (NASH), a progressive form of NAFLD, reaches 37% (3). Finally, the incidence of NAFLD-related HCC, accompanied by life-threatening complications, is continuously increasing (4). Furthermore, lean individuals with NAFLD share the same severe histological phenotype as obese subjects and are associated with metabolic syndrome and an increased risk of all-cause mortality (5).
A recent meta-analysis assessing RCTs with dietary interventions but without any added physical activity tried to establish the effect of different dietary modifications on intrahepatic lipid content (IHL), liver fibrosis, and liver function in patients with NAFLD. The study showed Mediterranean diet without energy restriction leads to significant reduction of IHL. However, it is important to note that the diet without exercise did not lead to significant changes in liver enzymes, lipid profile, fasting glucose or insulin, or homeostatic assessment for insulin resistance. On the other hand, hypocaloric diet with foods high in unsaturated fatty acids significantly decreases ALT and AST, but its effects on steatosis remain to be established (6).
NAFLD development in obese and non-obese individuals is closely related to a sedentary lifestyle and a western diet (7). Physical activity, especially structured exercise, has been shown to improve hepatic steatosis and is the core treatment during the whole NAFLD disease spectrum. Physical activity has an essential role in weight reduction and maintenance, influences healthier body composition, reduces hepatic steatosis and NAFLD-associated cardiovascular and malignant burden (8). Importantly, modest weight gain in lean individuals has deleterious effects on metabolic disturbances primarily through increased visceral adipose tissue (9, 10). Bodyweight and waist circumference reduction achieved through lifestyle intervention are independent predictors of NAFLD resolution in lean patients (11).
Physical Acitivty in Context of Liver Disease
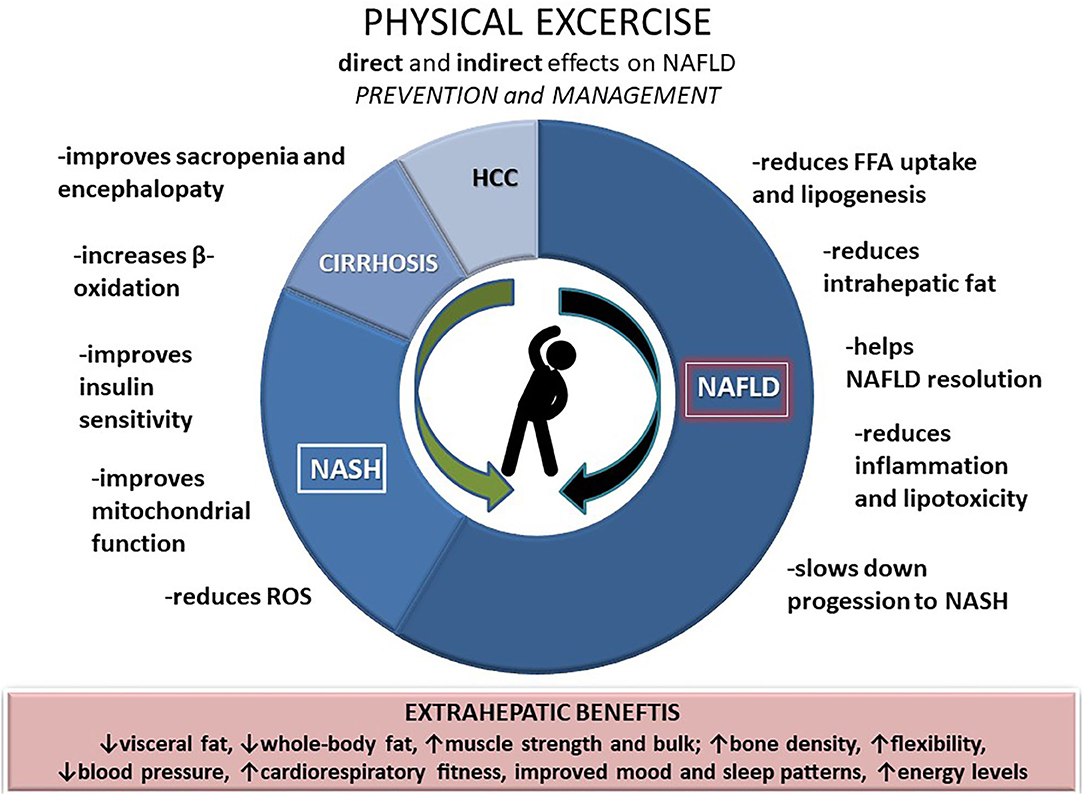
Health-enhancing physical activity defined as either vigorous activity at least 3 days/week and accumulating at least 1,500 metabolic equivalents (METs)-minutes per week (MET-min/week) or seven or more days/week of any combination of walking, moderate, or vigorous activities accumulating at least 3,000 MET-min/week has been recently independently (after adjusted for confounders such as diet and obesity) associated with a lower risk of both NAFLD and lean NAFLD in the Asian population, while the risk of lean NAFLD was significantly lower even in minimally active lean individuals compared inactive lean individuals (adjusted OR, 0.8; 95% CI, 0.6–0.98) (12). Skeletal muscle as an endocrine organ secretes cytokines and myokines, through which, while working/contracting, it communicates with liver and adipose tissue, among others, and is involved in an anti-inflammatory response (13). In addition, physical activity (1,500 MET-min/week of vigorous or 3,000 MET-min/week of intermediate activity) significantly lowers the ALT levels and improves the hepatocellular injury in individuals with NAFLD (8). Although research regarding the effects of exercise on NAFLD is relatively recent, both experimental and clinical data support its importance, especially that of vigorous intensity, which effectively decreases intrahepatic lipid content and slows down the progression to NASH (14). In a small RCT including 24 individuals with biopsy-proven NASH, the benefit was seen from 12-week cycling and resistance training to decrease hepatic triglyceride content, plasma triglyceride levels, and visceral fat. However, no effects were reported concerning BMI, liver enzymes, or inflammation and fibrosis, suggesting weight managing strategies should be incorporated in NASH treatment (15). In patients with cirrhosis, exercise can acutely increase portal pressure, but it has positive health effects in the long term. Moreover, physical activity can improve the aerobic capacity, which is decreased in patients with advanced cirrhosis and adds to anyhow high mortality burden (16). In addition, by increasing skeletal muscle mass physical activity improves sarcopenia and reduces the risk of encephalopathy (17–20). Evidence regarding the effects of exercise on HCC risk is still scarce, but epidemiological studies suggest a lower risk in patients who regularly and vigorously exercise (21).
Exercise and Nafld: What is Known on the Mechanism(S)
In many of the published studies, the effect of exercise on improvement of liver fat content was seen even in patients who did not achieve the weight loss therefore suggesting the direct effects on liver (22, 23). Although this direct relation is still largely elusive, the available evidence implies different metabolic and molecular pathways which are involved in the reduction of hepatic fat induced by exercise.
One of the most prominent and studied mechanisms is certainly related to insulin resistance (IR). Mechanistically, IR in peripheral tissues such as adipose tissue results in an incomplete suppression of lipase, leading to enhanced lipolysis and release of free fatty acids (FFAs), which are taken up by the liver (24). Therefore, an improvement in IR might reduce the FFA flux to the liver. Moreover, IR in skeletal muscle causes the glucose transport to the liver, which is the fuel for FFA de novo synthesis (25). The main transcription factor controlling liver fatty acid metabolism, sterol regulatory element-binding protein 1 (SREBP-1), which is elevated in the NASH can be decreased by either 12-week aerobic exercise of high intensity or resistance training through the increase of AMPK, leading to reduction of de novo lipogenesis in hepatocytes (26, 27). Moreover, exercise might also induce epigenetic mechanisms such as reduction of DNA hypermethylation which positively effects de novo lipogenesis (28).
In addition, exercise might also influence liver fatty acid metabolism by increasing expression of peroxisome proliferator-activated receptor-gama (PPAR-gama), in a similar way as the thiazolidinediones (29). Besides, animal models and small scale studies suggest exercise impacts liver mitochondrial function, and can influence inflammation through up-regulation of antioxidant enzymes and anti-inflammatory markers (24, 30).
The Role of Physical Activity in the Nafld Prevention
Sitting for ≥3 h per day has been associated with increased all-cause mortality (relative risk 1.30; 95% CI 1.06–1.56), and sedentary behavior, in general, was reported higher in people predisposed to develop obesity T2DM, NAFLD, and metabolic syndrome (31). There is a strong association between increased hepatic triglyceride content and each hour spent sedentary during a day, while prospective cohort studies identified sedentary behavior as an independent risk factor for NAFLD development and potentially progression (32, 33). A large prospective randomized Da Quing study including 110,660 men and women with glucose impairment showed exercise was associated with 46% (P < 0.0005) reduction of risk in developing diabetes, irrespective of baseline glucose levels and body mass index (BMI), suggesting a vital role of physical activity in the prevention of metabolic disorders associated with insulin resistance (34). The results of the HELENA study suggest that high cardiorespiratory fitness (CRF) might have protective effects on liver enzyme levels in adolescents with high waist circumference and that the exercise focusing on increasing CRF and decreasing abdominal fat might be a good tool in the prevention and treatment of NAFLD during adolescence (35). A study by Sung and co-workers following 169,347 men and women by ultrasound for 5 years provided the first longitudinal epidemiological data supporting the role of exercise in both the prevention and treatment of NAFLD. During follow-up, out of 126,811 adults without NAFLD at baseline, 23% developed NAFLD at follow-up. On the other hand, of the 42,536 individuals with NAFLD at baseline, 34% of cases resolved. After adjusting for potential confounders, any moderate to vigorous exercise level was associated with a reduced risk of new NAFLD and resolution of already present NAFLD. The most significant benefits were seen while exercising 5 days per week and in the case of increasing the frequency of exercise bouts over time (36). Similar results were confirmed by another more recent longitudinal follow-up study where people who were already active or became physically active during the course of follow-up were less likely to develop NAFLD compared with those that remained inactive (OR = 0.75, p = 0.03 and 0.75, p = 0.04, respectively), irrespective of BMI (37).
The Role of Physical Activity in the Nafld Treatment
Growing evidence highlights the need for physical activity in reducing the body weight (at best >10%) in order to improve liver histology and reduce fibrosis in NAFLD patients (38, 39). Weight loss achieved through physical activity improves hepatic and peripheral insulin sensitivity, but physical activity, regardless of the effects on body mass, also directly decreases the pro-inflammatory and oxidative stress markers and improves liver enzymes. According to the data from a recently published systemic review encompassing 24 exercise-only studies in NAFLD, structured exercise leads to a 20–30% relative reduction in hepatic steatosis, independent of weight loss (40). In addition, exercise might also affect the gut microbiota and modulate the liver inflammatory response and NASH progression (41) (Figure 1).
There is currently a gap in knowledge of the type, duration, and/or intensity of physical activity that would bring the best results for patients with NAFLD. It seems that both aerobic and anaerobic training for at least 4 months decrease to the same extent the overall adipose tissue, hepatic fat, and BMI, while no data exists on their potentially differential effects on liver histology (22). On the other hand, liver histology tends to depend on the exercise intensity and, according to some data, improves more with high-intensity activity (22, 42).
Extrahepatic Benefits of Physical Activity and how it Affects Nafld Prognosis
In a randomized control trial recruiting NAFLD patients, exercise was associated with improvement of endothelial function, evaluated by flow-mediated dilatation of the brachial artery (43). Mentioned NO-dependent vascular dilatation is an important protective mechanism for cardiovascular health. Moreover, with its effect on muscle mass, physical activity reduces the risk of sarcopenia and improves cardiorespiratory fitness, which is low, especially in patients with advanced liver disease such as cirrhosis (19). Physical activity improves insulin sensitivity on the peripheral tissues and the liver and improves glucose metabolism (or glycemic control in clinically manifest diabetes), slowing down NAFLD progression and reducing overall cardiovascular risk. Moreover, it reduces systemic inflammation, lowers arterial blood pressure, and improves dyslipidemia (44). The established beneficial effect of physical activity on cardiovascular health reported in the general population is also applicable for the NAFLD patients, and given that cardiovascular disease remains the leading cause of death in NAFLD patients, encouraging regular exercise should be advocated and prescribed to all NAFLD patients, and during the entire disease course (45).
Recommendations for Physical Activity in the Nafld Patients
Physical activity and specially structured exercises offer benefits independent of weight loss and represent the core treatments for NAFLD patients. Both aerobic and resistance training effectively reduce hepatic steatosis and reduce the NAFLD-associated cardiovascular risk (46). The exercise program should be tailored to a patient's preference and capacity, depending on physical fitness level, stage of the liver disease, and other comorbidities. High-intensity interval training (HIIT) is an attractive exercise modality for treating patients with NAFLD, especially those who lack time to exercise, while it reduces visceral adipose tissue, intrahepatic fat, and fibrosis (47). General recommendations include 150 min of weekly accumulated moderate-intensity aerobic exercise, accompanied by strength and endurance training at least two to three times weekly, avoiding consecutive days and including 8–10 exercises using the major muscle groups, with 10–15 repetitions in a moderate to high intensity. In addition, just reducing or breaking up sedentary time by few minutes of walking should also be a therapeutic target for patients who cannot attend the structured exercise programs. To assure the therapeutic effects, attention should be paid to patients' compliance to exercise and attain exercise goals (48, 49).
As majority of NAFLD patients are obese, special attention must be put on an exercise program which would be doable and also lead to a meaningful weight loss (10%) and improvements in cardiorespiratory fitness to provide health benefits (39). Current literature supports the evidence that both aerobic and anaerobic exercise with a duration of 20–60 min per session when performed in moderate intensity and practiced 4–7 days weekly for at least 6 months (with and without diet restriction) can lead to improvements in liver histology and therefore reversal of liver damage in NASH patients (50), while recently published study on overweight and obese patients supports the beneficial role of aerobic exercise regardless of dose and intensity (low-intensity/ high-volume, high-intensity/low-volume, low-intensity/low-volume) on the reduction of liver fat content (42). Therefore, recent guidelines emphasize the importance of exercise but leave the choice of training to be individually tailored according to patients' preferences and likelihood of adherence to exercise program in the long term (51).
Author Contributions
MC: drafted and wrote and reviewed the manuscript. IB-C and AM: collected data and wrote and reviewed the manuscript. VC: critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. American gastroenterological association; american association for the study of liver diseases; american college of gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. (2012) 142:1592–609. doi: 10.1053/j.gastro.2012.04.001
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
3. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
4. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Global nonalcoholic steatohepatitis council. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. (2019) 17:748–55.e3. doi: 10.1016/j.cgh.2018.05.057
5. Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. (2019) 37:65–72. doi: 10.2337/cd18-0026
6. Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. (2021) 8:716783. doi: 10.3389/fnut.2021.716783
7. Bilic-Curcic I, Cigrovski Berkovic M, Virovic-Jukic L, Mrzljak A. Shifting perspectives - interplay between non-alcoholic fatty liver disease and insulin resistance in lean individuals. World J Hepatol. (2021) 13:80–93. doi: 10.4254/wjh.v13.i1.80
8. Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. (2016) 14:1398–411. doi: 10.1016/j.cgh.2016.04.036
9. Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. (2012) 56:1145–51. doi: 10.1016/j.jhep.2011.12.011
10. Chang Y, Ryu S, Sung E, Woo HY, Cho SI, Yoo SH, et al. Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut. (2009) 58:1419–25. doi: 10.1136/gut.2008.161885
11. Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. (2018) 69:1349–56 doi: 10.1016/j.jhep.2018.08.011
12. Jang DK, Lee JS, Lee JK, Kim YH. Independent association of physical activity with nonalcoholic fatty liver disease and alanine aminotransferase levels. J Clin Med. (2019) 8:1013. doi: 10.3390/jcm8071013
13. Catoire M, Kersten S. The search for exercise factors in humans. FASEB J. (2015) 29:1615–28. doi: 10.1096/fj.14-263699
14. Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. (2016) 63:1026–40. doi: 10.1002/hep.28132
15. Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. (2017) 15:96–102. doi: 10.1016/j.cgh.2016.07.031
16. Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. (2016) 7:e180. doi: 10.1038/ctg.2016.38
17. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. (2014) 12:1920–6.e2. doi: 10.1016/j.cgh.2014.04.016
18. Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the sportdiet study. Hepatology. (2017) 65:1293–305. doi: 10.1002/hep.28992
19. Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. (2012) 10:166–73:173.e1. doi: 10.1016/j.cgh.2011.08.028
20. Duarte-Rojo A, Ruiz-Margain A, Montano-Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. (2018) 24:122–39. doi: 10.1002/lt.24958
21. Behrens G, Matthews CE, Moore SC, Freedman ND, McGlynn KA, Everhart JE, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP diet and health study. Eur J Epidemiol. (2013) 28:55–66. doi: 10.1007/s10654-013-9767-1
22. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. (2013) 58:1287–95. doi: 10.1002/hep.26393
23. Keating SE, Hackett DA, Parker HM, Way KL, O'Connor HT, Sainsbury A, et al. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: a randomized controlled trial. Hepatol Res. (2017) 47:622–31. doi: 10.1111/hepr.12781
24. Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. (2006) 63:1393–409. doi: 10.1007/s00018-006-6600-y
25. Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci USA. (2011) 108:13705–9. doi: 10.1073/pnas.1110105108
26. Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. (2015) 61:1205–15. doi: 10.1002/hep.27544
27. Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. (2017) 7:43029. doi: 10.1038/srep43029
28. Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi JH, et al. High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics. (2017) 12:55–69. doi: 10.1080/15592294.2016.1261239
29. Wu H, Jin M, Han D, Zhou M, Mei X, Guan Y, et al. Protective effects of aerobic swimming training on high-fat diet induced nonalcoholic fatty liver disease: regulation of lipid metabolism via PANDER-AKT pathway. Biochem Biophys Res Commun. (2015) 458:862–8. doi: 10.1016/j.bbrc.2015.02.046
30. Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. (2019) 19:994–1003. doi: 10.1080/17461391.2019.1571114
31. Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS ONE. (2013) 8:e80000. doi: 10.1371/journal.pone.0080000
32. Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. (2012) 57:157–66. doi: 10.1016/j.jhep.2012.02.023
33. Bowden Davies KA, Sprung VS, Norman JA, Thompson A, Mitchell KL, Harrold JOA, et al. Physical activity and sedentary time: association with metabolic health and liver fat. Med Sci Sports Exerc. (2019) 51:1169–77. doi: 10.1249/MSS.0000000000001901
34. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. (1997) 20:537–44. doi: 10.2337/diacare.20.4.537
35. Medrano M, Labayen I, Ruiz JR, Rodríguez G, Breidenassel C, Castillo M, et al. Cardiorespiratory fitness, waist circumference and liver enzyme levels in European adolescents: the HELENA cross-sectional study. J Sci Med Sport. (2017) 20:932–6. doi: 10.1016/j.jsams.2017.04.006
36. Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol. (2016) 65:791–7. doi: 10.1016/j.jhep.2016.05.026
37. Gerage AM, Ritti-Dias RM, Balagopal PB, Conceição RDO, Umpierre D, Santos RD, et al. Physical activity levels and hepatic steatosis: a longitudinal follow-up study in adults. J Gastroenterol Hepatol. (2018) 33:741–6. doi: 10.1111/jgh.13965
38. Rodriguez B, Torres DM, Harrison SA. Physical activity: an essential component of lifestyle modification in NAFLD. Nat Rev Gastroenterol Hepatol. (2012) 9:726–31. doi: 10.1038/nrgastro.2012.200
39. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. (2015) 149:367–78.e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005
40. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. (2017) 66:142–52. doi: 10.1016/j.jhep.2016.08.023
41. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) 63:1913–20. doi: 10.1136/gutjnl-2013-306541
42. Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. (2015) 63:174–82. doi: 10.1016/j.jhep.2015.02.022
43. Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. (2014) 307:H1298–306. doi: 10.1152/ajpheart.00306.2014
44. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American college of sports medicine. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
45. Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. (1990) 132:612–28. doi: 10.1093/oxfordjournals.aje.a115704
46. Keating SE, Adams LA. Exercise in NAFLD: Just do it. J Hepatol. (2016) 65:671–3. doi: 10.1016/j.jhep.2016.06.022
47. Hamasaki H. Perspectives on interval exercise interventions for non-alcoholic fatty liver disease. Medicines. (2019) 6:83. doi: 10.3390/medicines6030083
48. Carels RA, Darby LA, Rydin S, Douglass OM, Cacciapaglia HM, O'Brien WH. The relationship btween self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Ann Behav Med. (2005) 30:182–90. doi: 10.1207/s15324796abm3003_2
49. Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. (2019) 1:468–79. doi: 10.1016/j.jhepr.2019.10.008
50. Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. (2013) 6:249–59. doi: 10.1177/1756283X13484078
51. European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
Keywords: non-alcoholic fatty liver disease, sedentary activities, physical activity, aerobic exercise, high-intensity interval training, strength training
Citation: Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A and Cigrovski V (2021) NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 8:734859. doi: 10.3389/fnut.2021.734859
Received: 01 July 2021; Accepted: 08 September 2021;
Published: 05 October 2021.
Edited by:
Speranta Iacob, Fundeni Clinical Institute, RomaniaReviewed by:
Sidney B. Peres, State University of Maringá, BrazilDaniel Vasile Balaban, Carol Davila University of Medicine and Pharmacy, Romania
Dario Coletti, Sapienza University of Rome, Italy
Copyright © 2021 Cigrovski Berkovic, Bilic-Curcic, Mrzljak and Cigrovski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Mrzljak, YW5uYS5tcnpsamFrQGdtYWlsLmNvbQ==
 Maja Cigrovski Berkovic
Maja Cigrovski Berkovic Ines Bilic-Curcic
Ines Bilic-Curcic Anna Mrzljak
Anna Mrzljak Vjekoslav Cigrovski7
Vjekoslav Cigrovski7