- 1Department of Nutrition and Food Hygiene, School of Public Health, Nantong University, Nantong, China
- 2Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Department of Nutrition and Food Hygiene, School of Public Health, Soochow University, Suzhou, China
- 3Department of Anesthesiology, Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China
Background: The association between α-linolenic acid (ALA) and mortality is inconsistent and has not been summarized systematically.
Objective: The purpose was to conduct a meta-analysis that synthesized the results of prospective cohort studies to investigate associations between ALA intake and mortality.
Methods: We conducted a comprehensive search on PubMed, Embase, and Web of Science databases on May 1, 2021, for relevant prospective cohort studies which reported associations of ALA (assessed by dietary surveys and/or ALA concentrations in body tissues) with mortality from all-cause, cardiovascular disease (CVD), and other diseases. Multivariable-adjusted relative risks (RRs) were pooled by a random or fixed-effects model.
Results: A total of 34 prospective cohort studies, of which 17 reported dietary ALA intake, 14 for ALA biomarkers, and the remaining 3 reported both of intake and biomarkers. The studies included 6,58,634 participants, and deaths were classified into all-cause mortality (56,898), CVD mortality (19,123), and other diseases mortality (19,061). Pooled RRs of ALA intake were 0.93 (95% CI: 0.86, 1.01, I2 = 71.2%) for all-cause mortality, 0.90 (95% CI: 0.83, 0.98, I2 = 22.1%) for CVD mortality, and 0.94 (95% CI: 0.83, 1.06, I2 = 73.3%) for other diseases mortality. The two-stage random-effects dose-response analysis showed a linear relationship between dietary ALA intake and CVD-mortality and each 0.5% energy increment of ALA intake was associated with a 5% lower risk of CVD-mortality (RR: 0.95; 95% CI: 0.90, 1.00). Pooled RRs per SD increment of ALA biomarkers were 0.99 (95% CI: 0.96, 1.01, I2 = 27%) for all-cause mortality, 1.00 (95% CI: 0.98, 1.03, I2 = 0%) for CVD mortality and 0.98 (95% CI: 0.95, 1.01, I2 = 0%) for other diseases mortality.
Conclusions: This meta-analysis summarizing the available prospective cohort studies indicated that ALA intake was associated with reduced risk of mortality, especially CVD mortality. Our findings suggest that ALA consumption may be beneficial for death prevention.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO; identifier: CRD42021264532.
Introduction
Cardiovascular diseases and cancer account for two-thirds of global deaths (1). Mounting evidence showed that the n−3 polyunsaturated fatty acids (n−3 PUFAs) played a beneficial role in cardiovascular disease (CVD) and cancer risk reduction (2–4). A recent meta-analysis of randomized controlled trials (RCTs) reported that marine omega-3 supplementation significantly lowered the risk for CVD mortality (5). The n−3 PUFAs family includes alpha-linolenic acid (ALA, plant-sourced), eicosapentaenoic (EPA), and docosahexaenoic (DHA) acids (mostly seafood-sourced). However, marine fatty fish is not readily available to all populations, and concerns about mercury contamination, radioactive releases, and unsustainable ocean fisheries have been raised (6, 7). ALA is rich in flaxseed, canola, soybean, and walnuts and can be partly converted into DHA and EPA (8, 9). Plant-based ALA is more affordable and widely available globally. Thus, whether ALA can reduce the risk of all-cause or cause-specific mortality is of considerable public health importance.
Alpha-linolenic acid is associated with lowering CVD risk parameters (10). Studies showed that ALA effectively reduced blood pressure and serum oxidized low-density lipoprotein (LDL) (11, 12). A recent meta-analysis of RCTs showed that dietary ALA supplementation decreased LDL levels more than EPA or DHA (13). Although ALA decreased cardiometabolic indicators, such as LDL, a most recent meta-analysis of RCTs showed that increasing ALA intake probably made little or no difference to all-cause mortality, cardiovascular mortality, and coronary heart disease mortality (5). The meta-analysis of RCTs is considered to provide the most reliable evidence on the effectiveness of interventions, but it was found that the above-mentioned meta-analysis was based on only five RCTs, and two of these five RCTs weighted nearly 100% (81.7 and 17%) of this meta-analysis (5). Because of the insufficient number of trials, we still need to summarize the second-best hierarchy of evidence (prospective studies) to evaluate whether ALA could reduce the risk of all-cause or CVD mortality.
Cardiovascular disease is an umbrella term covering coronary heart disease (CHD), stroke, congenital heart diseases, and peripheral artery disease. In 2012, Pan et al. found that dietary ALA significantly lowered fatal CHD from six prospective cohort studies but not stroke (14), and Wei et al. updated the information and generated similar results in 2018 (15). Though dietary ALA is associated with a reduced risk of fatal CHD, the associations with other subtypes of CVD are unclear. Moreover, some meta-analyses showed that ALA increased prostate cancer risk (16–18). These disparate findings caused confusion about ALA on total mortality and cause-specific mortality (CVD-caused mortality, cancer, and other diseases-caused mortality). Thus, we systematically reviewed the literature and conducted a meta-analysis to investigate the relationship between ALA intake and total or cause-specific mortality. Given ALA intakes varied markedly by geographic and socioeconomic factors, subgroup analyses were also fully performed in this present meta-analysis.
Methods
Literature Search
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. An electronic literature search was first conducted in the databases of PubMed, Embase, and Web of Science through September 1, 2020, and re-searched on May 1, 2021, to identify studies reporting associations between ALA intake and/or biomarkers and mortality by following keywords or phrases: (“fatty acids” OR “linoleic acids” OR “α-linolenic acids” OR ALA) AND (mortality OR death) AND (prospective study OR cohort study OR follow-up study). The detailed search strategies were listed in Supplementary Table 1. We accessed either self-reported dietary surveys or ALA concentrations in body tissues and mortality from all-cause, CVD, and other diseases. The search was restricted to human studies published in the English language.
Selection of Articles
Study selection and data extraction were undertaken independently by two investigators (L-HC and QH). The titles and abstracts of literature were examined carefully one by one. After excluding articles that were obviously irrelevant or incomplete, we further read the literature in full to determine the eventual inclusion. Differences of opinion were resolved by discussion with another investigator. Studies incorporated into the final analysis need to meet the following criteria: (1) were original investigations; (2) were prospective cohort studies; and (3) with multivariable-adjusted risk estimates [hazard ratio (HR) or relative risk (RR)] for associations between ALA (dietary intakes and/or ALA concentrations) and at least one mortality outcome, including total mortality, CVD mortality, and death from other diseases (i.e., death from inflammatory diseases, non-CVD mortality, non-arrhythmic death, and cancer-specific death).
Data Extraction
The contents to be extracted included the first author, name of the cohort, geographic region, follow-up duration, year of publication, sample size, number of a total or specific death, dietary assessment method, mortality type, multivariable-adjusted risk estimates with 95% confidence intervals (CIs), and adjusted covariates in the studies. For studies that reported biomarkers of ALA, besides the above-mentioned items, ALA measurement methods and tissue types were extracted. When several endpoints of cardiovascular mortality were available, we adopted the endpoints with the broadest coverage (for example, we choose CVD mortality other than specific CVD mortality and cancer mortality other than specific cancer mortality). When several articles reported data on the same cohort, the record with the largest number of cases was used to calculate the total number of participants of this meta-analysis. Study quality was assessed by using the Newcastle-Ottawa Scale (NOS; scores ranged from 0 to 9).
Statistical Analyses
Fully adjusted RR or HR and corresponding 95% CI were recorded for meta-analyses. If the original article did not give 95% CI, we calculated the 95% CI based on RR and p-value according to the methods created by Altman et al. (19). HRs were assumed to approximate RRs (20–22). Furthermore, some studies may report ORs instead of RRs. These data can be transferred into RRs (22). For studies that listed risk estimates based on various ALA intake categories (e.g., tertiles, quartiles, quintiles, or specific thresholds), we pooled RRs by comparing the highest with the lowest categories estimates. While for those studies which reported the associations between biomarkers of ALA in body tissues and mortality, we assumed that the association was linear and pooled RRs for each SD increment. If the RRs for each SD increment is unavailable, we estimated RRs and 95% CI by dividing the lnRRs and 95% CI of the extreme tertiles, quartiles, or quintiles by 1.94, 2.30, or 2.56, and the interquartile lnRRs by 1.68 for special studies reporting RRs per interquartile increment in biomarkers (23, 24). The heterogeneity of the effect sizes among studies was tested using the I2 statistics. Either a fixed-effects or random-effects model (in the presence of heterogeneity, I2 > 50%) was used to calculate the combined effect size. Publication bias was evaluated with the Funnel plots and Egger's regression model. Stratified meta-analysis and univariate meta-analysis were used to find the source of heterogeneity. The pooled RR was statistically significant when 95% CI was not one.
We conducted a dose-response random-effects meta-analysis for each mortality type to examine impacts of the dosage of dietary ALA intake and risk of mortality [two-stage generalized least-square for the trend in Stata (25)]. Normally, the middle quintile of the ALA of each study was recorded as the median intake. If the median was not provided, we used the midpoint of higher and lower bounds of each category to approximate it, and 1.5 times of the cutoff threshold for the highest category was used when the extremum was opening range while the cutoff threshold of the lowest category divided by 1.5 was used to evaluate the minimum intake. The units of ALA intake in all references were unified as a percentage of energy, while for those studies that reported intake with other units, we unify them by conversion to facilitate calculation. We assumed that the average energy requirement is an appropriate value of ~2,000 kcal per day, and ALA daily intake is 1.5 g,.6% of energy, and total fat provides 30% of energy (26). When extracting the data of ALA intake, we noticed that unit of ALA of one article was milligram [the lowest weekly ALA consumption was 1.1 mg] (27). Because the unit of ALA intake from several articles that included Italian participants was grams [for example, the lowest quartiles were 0.695 g (28), the mean intake of ALA was 1.5 g (29)], it was suspected that the unit of ALA for this article could also be in grams (27). We tried contacting the author by email for confirmation of the unit, unfortunately, we did not get a response. Thus, we performed the dose-response analysis with both gram or milligram to compare the final results. All data were statistically performed using Stata version 11.0 (StataCorp, College Station, TX).
Results
Study Selection
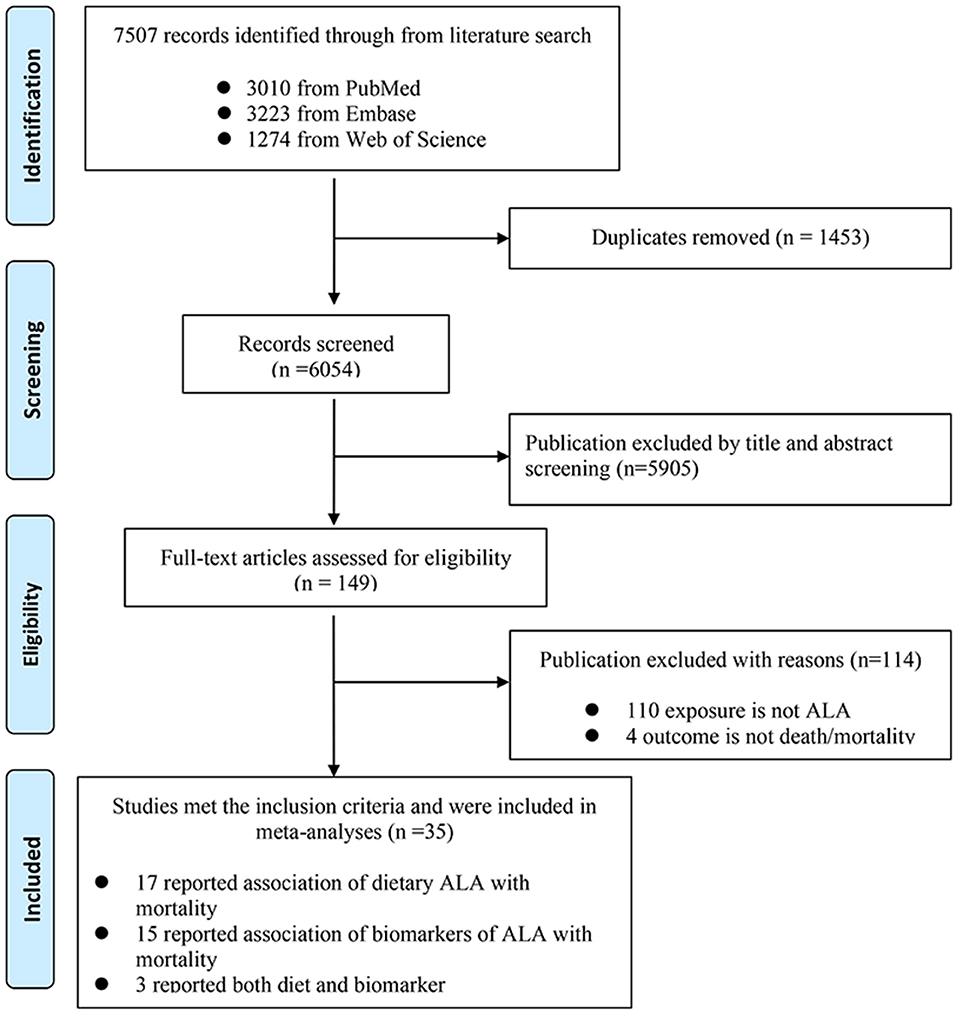
We retrieved 7,507 records in total after searching the three databases. Specifically, we retrieved 3,010 records from PubMed, 3,223 from Embase, and the remaining 1,274 from Web of Science. After browsing of titles and abstracts, 149 records were screened out for further evaluation by carefully checking the full-length articles. Finally, we obtained 34 prospective cohort articles meeting the inclusion criteria, 17 of them assessed ALA diet, 14 of them assessed ALA biomarkers, and 3 of them assessed both diet and biomarkers (Figure 1). The quality of these studies was shown in Supplementary Table 2.
Dietary ALA Intake and Mortality
The 20 articles (with 17 cohorts) about dietary ALA intake and mortality included 632,772 individuals. The subtypes of mortality are all-cause mortality (50,651 deaths) (27, 30–40), CVD mortality (16,706 deaths) (2, 30, 32, 34, 36, 37, 40–46) and cancer and/or other disease-caused mortality (17,427 deaths) (30, 34–36, 47). Median follow-up duration ranged from 3.1 to 34 years. Diet information of 17 articles were collected by using food-frequency questionnaires (FFQs) (2, 27, 31, 33–38, 40–47), two articles used 24-h dietary recall (30, 39) and one used 4-day food record (32) (Table 1).
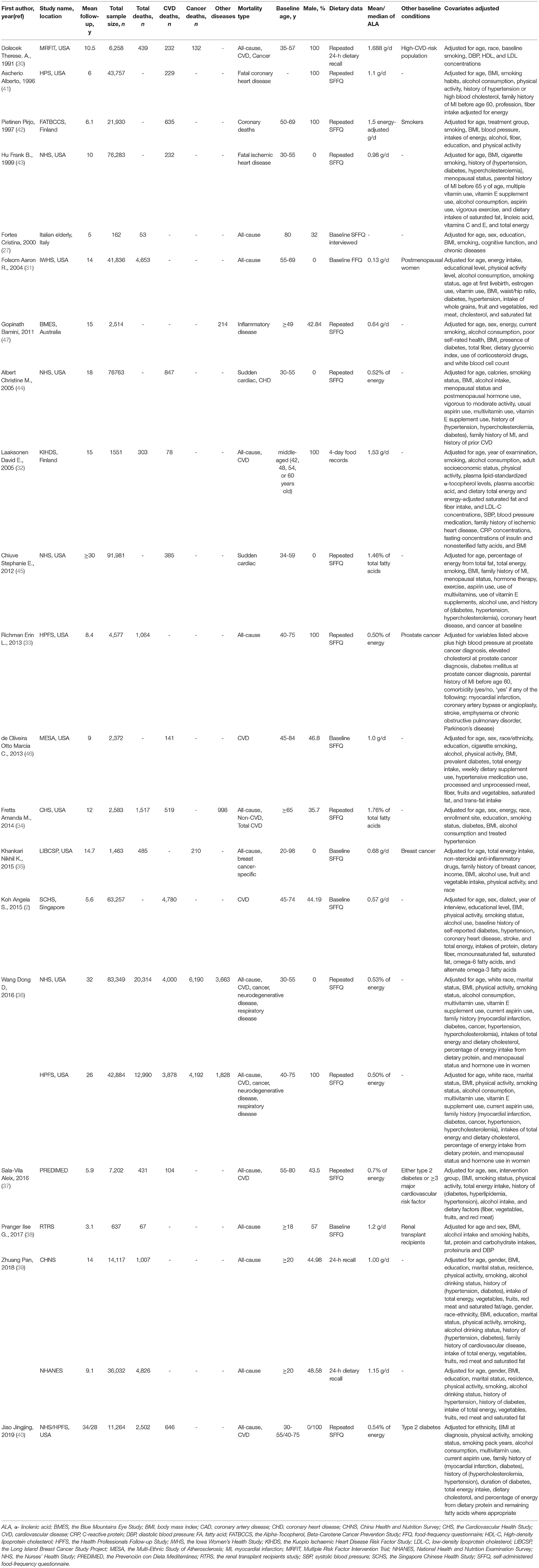
Table 1. Characteristics of prospective cohort studies that evaluated the association between dietary α-linolenic acid (ALA) intake and mortality from all-cause, cardiovascular disease (CVD) and other diseases.
We excluded the article by Laaksonen et al. for ALA and total mortality analyses because they did not give RR and 95% CI (32). Articles by Wang et al. (36). and Jiao et al. (40) used the Nurses' Health Study (NHS) to study CVD, but they took different sub-population of the cohort [Wang et al. (36) excluded participants who had a history of diabetes, CVD, or cancer at baseline; Jiao et al. (40) selected participants with type 2 diabetes at baseline for her research]. A similar situation for articles by Wang et al. (36) and Richman et al. (33) was that they both employed the Health Professionals Follow-up Study (HPFS); Wang et al. (36) excluded cancer at baseline, while Richman et al. (33) picked men with non-metastatic prostate cancer as a baseline to his analysis. Thus, in our meta-analysis, we treated them as different studies and included these articles. For some articles that included more than one cohort study, we treated them as separate analyses. Finally, 11 articles with 13 analyses were pooled and the result showed that intake of ALA was not associated with all-cause mortality (Figure 2A, RR = 0.93; 95% CI: 0.86, 1.01; I2 = 71.2%, p < 0.001). Several articles using the data of NHS to study fatal ischemic heart disease (43) or sudden cardiac death or CHD (44) and CVD mortality (36), we extracted data with the umbrella term for CVD mortality other than specific CVD mortality. The pooled RR for ALA and CVD mortality was 0.9 (Figure 2B, 95% CI: 0.83, 0.98; I2= 22.1%, p = 0.003), which showed that intake of ALA was associated with a lower risk of CVD-mortality. The pooled RR was 0.94 (Figure 2C, 95% CI: 0.83, 1.06; I2= 73.3%, p < 0.001) for cancer and other diseases which caused mortality. Because there was heterogeneity, we reported all the above results from the random-effects model. Considering the baseline characteristics of participants, geographical location, study quality, dietary assessment methods, sex, and follow-up years of these cohorts may influence the results, we performed stratified meta-analyses (Supplementary Table 3). The results showed that ALA intake was associated with lower risk of total mortality in European countries (RR = 0.70, 95% CI: 0.55, 0.89) and in studies with repeated FFQ method for ALA intake assessment (RR = 0.90, 95% CI: 0.82, 0.99). The CVD-mortality protective effects of ALA were more evident in general population, in studies with repeated FFQ method for ALA intake assessment, and in studies with fewer males. For other disease-caused mortality, there were no significant associations with any subgroups analyses (Supplementary Table 3).
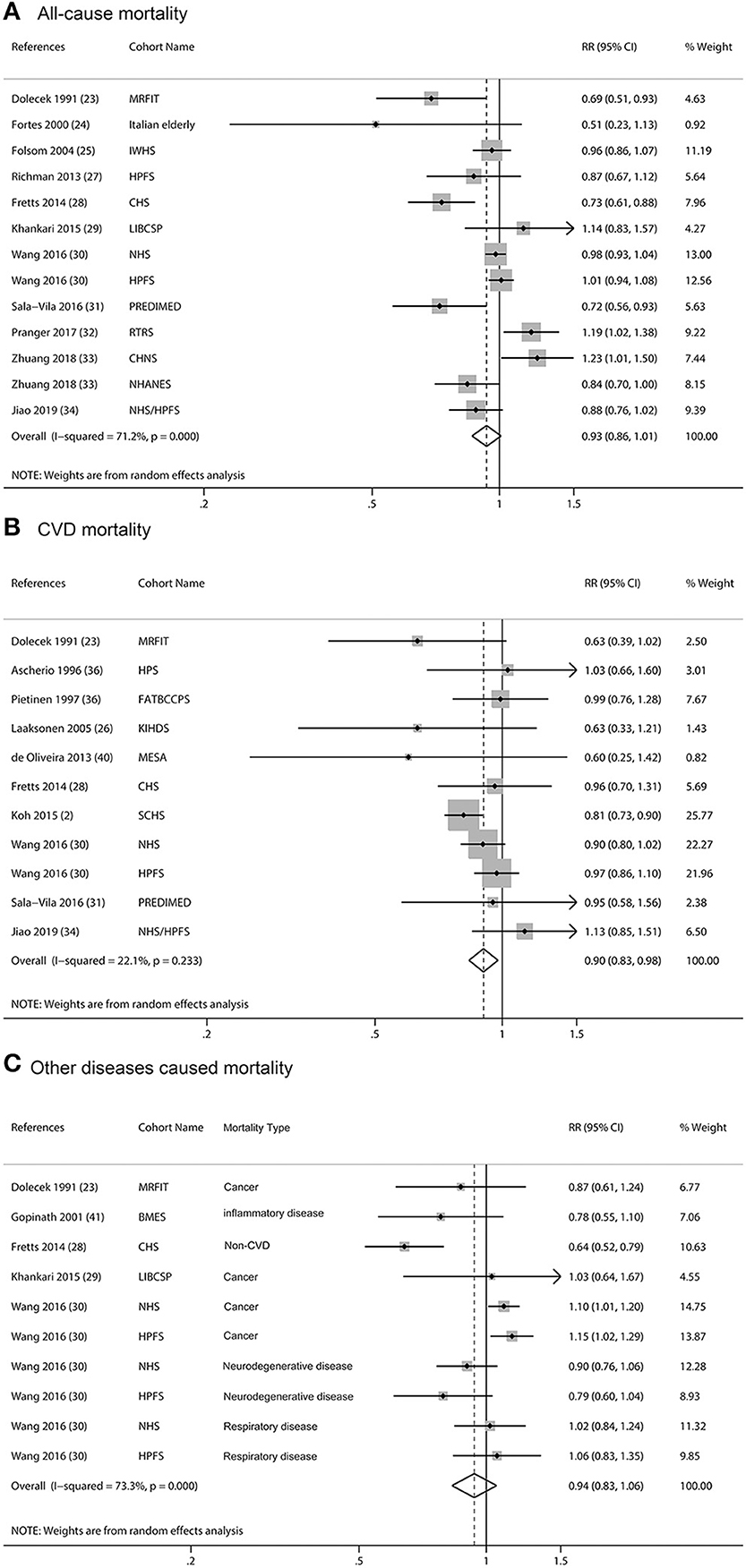
Figure 2. Meta-analysis of the associations between dietary α-linolenic acid (ALA) intake and mortality from all cause (A), cardiovascular disease (CVD) (B), and other diseases (C) in prospective cohort studies. CI, confidence interval. The horizontal lines denote the 95% CIs, some of which extend beyond the limits of the scales. The square is proportional to the weight of each study. The diamond represents the overall pooled estimate of the relative risk.
We evaluated potential dose-response associations among dietary ALA intake and risk of all-cause of mortality, CVD-mortality, and other diseases mortality. The median ALA intake categories ranged from 0.07 to 1.08% of energy. The two-stage random-effects dose-response analysis showed a non-linear relationship between dietary ALA intake and all-cause mortality or other diseases mortality (Figures 3A,C). As mentioned in the Methods section, it was suspected that the unit of ALA of one article might be grams (27). We compared the ALA data with a unit of gram or milligram for our dose-response analysis and found that it did not change the final results. There was a linear relationship between dietary ALA intake and CVD-mortality and each 0.5% energy increment of ALA intake was associated with a 5% lower risk of CVD-mortality (RR: 0.95; 95% CI: 0.90, 1.00; Figure 3B).
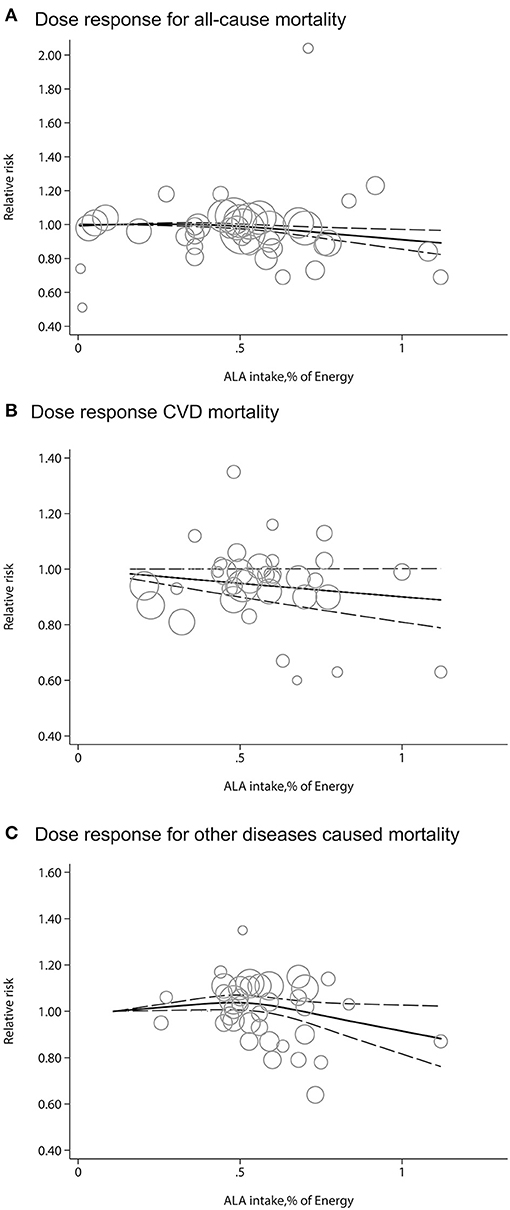
Figure 3. Dose-response meta-analysis for associations between dietary ALA intake and mortality from all causes (A), CVD (B) and other diseases (C) in prospective cohort studies. The pooled relative risk (RR) trend by ALA intake dosage (solid line) and its 95% CIs (dashed lines) was obtained by a random-effects dose-response meta-analysis. Circles represent RRs according to ALA categories from each study, inversely proportional to the variance of log RRs. CVD, cardiovascular disease.
Biomarkers of ALA and Mortality
There were 17 articles that reported biomarkers of ALA, including 32,368 participants, of which 8,067 were all-cause mortality (28, 32, 34, 48–57), 3,146 died from CVD (28, 32, 46, 48–51, 56, 58, 59), and 2,632 were died from other diseases (34, 48, 49, 60). The median follow-up periods were from 3 to 33.7 years. ALA concentrations were measured by gas chromatography or gas-liquid chromatography in different body tissues, including three studies on cholesteryl esters from serum (54, 56, 58), three studies on erythrocyte (48, 49, 51), five studies on phospholipids from plasma (34, 46, 53, 55) or serum (60), one study on adipose tissue (50), one study on whole blood (57), and the remaining four on total serum (28, 32, 52, 59) (Table 2). NOS scores of these studies were from 5 to 9 (Supplementary Table 2).
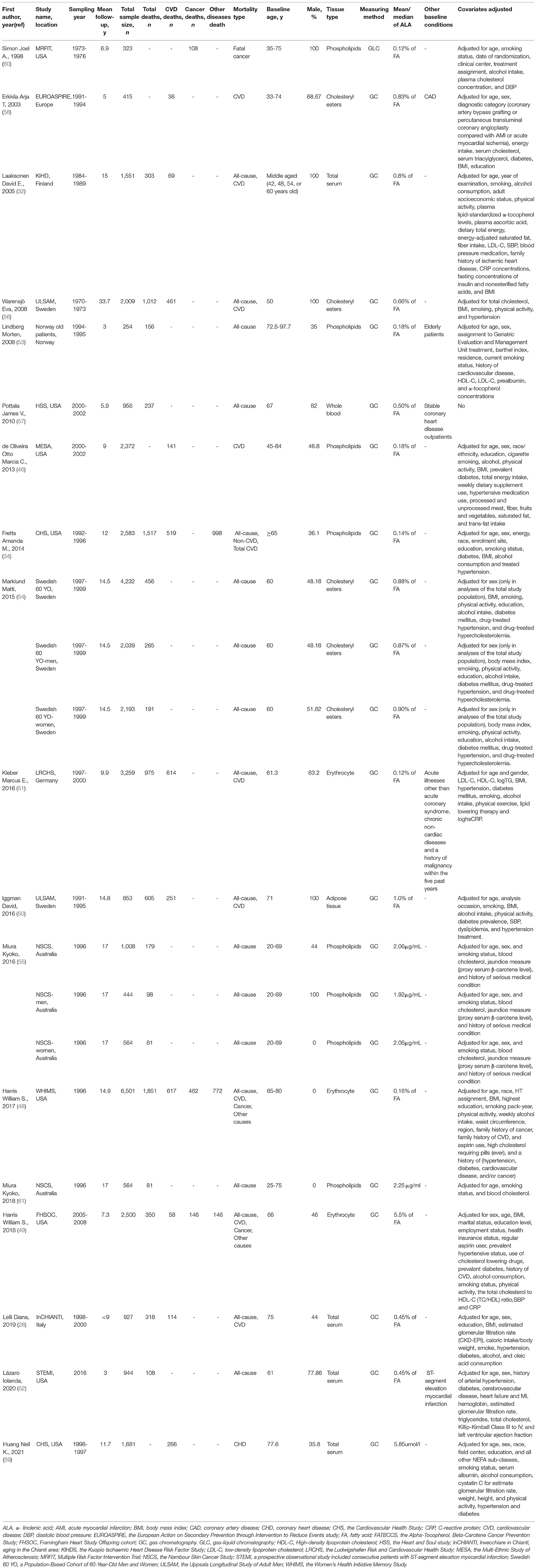
Table 2. Characteristics of prospective cohort studies that evaluated the association between biomarkers of ALA in body tissues and mortality from all-cause, CVD and other diseases.
One study was not included in the meta-analysis because it did not adjust covariates and had no association between mortality and blood ALA (57). One article was excluded because of the duplicate report with previous articles and less adjusted covariates comparing with the previous article (61). The pooled multivariable-adjusted RRs, which were standardized for each SD increment in ALA concentrations, were 0.99 (95% CI: 0.96, 1.01, I2= 27%, p = 0.187) for all-cause mortality, 1.00 (95% CI: 0.98, 1.03, I2= 0.0%, p = 0.579) for CVD mortality, and 0.98 (95% CI: 0.95, 1.01, I2= 0.0%, p = 1.000) for other diseases caused mortality. None of the individual associations were statistically significant (Figure 4). Subgroup meta-analyses according to baseline characteristics of participants, geographical location, study quality, dietary assessment methods, sex, and follow-up years consistently showed that ALA concentrations were not associated with any type of mortality (Supplementary Table 4).
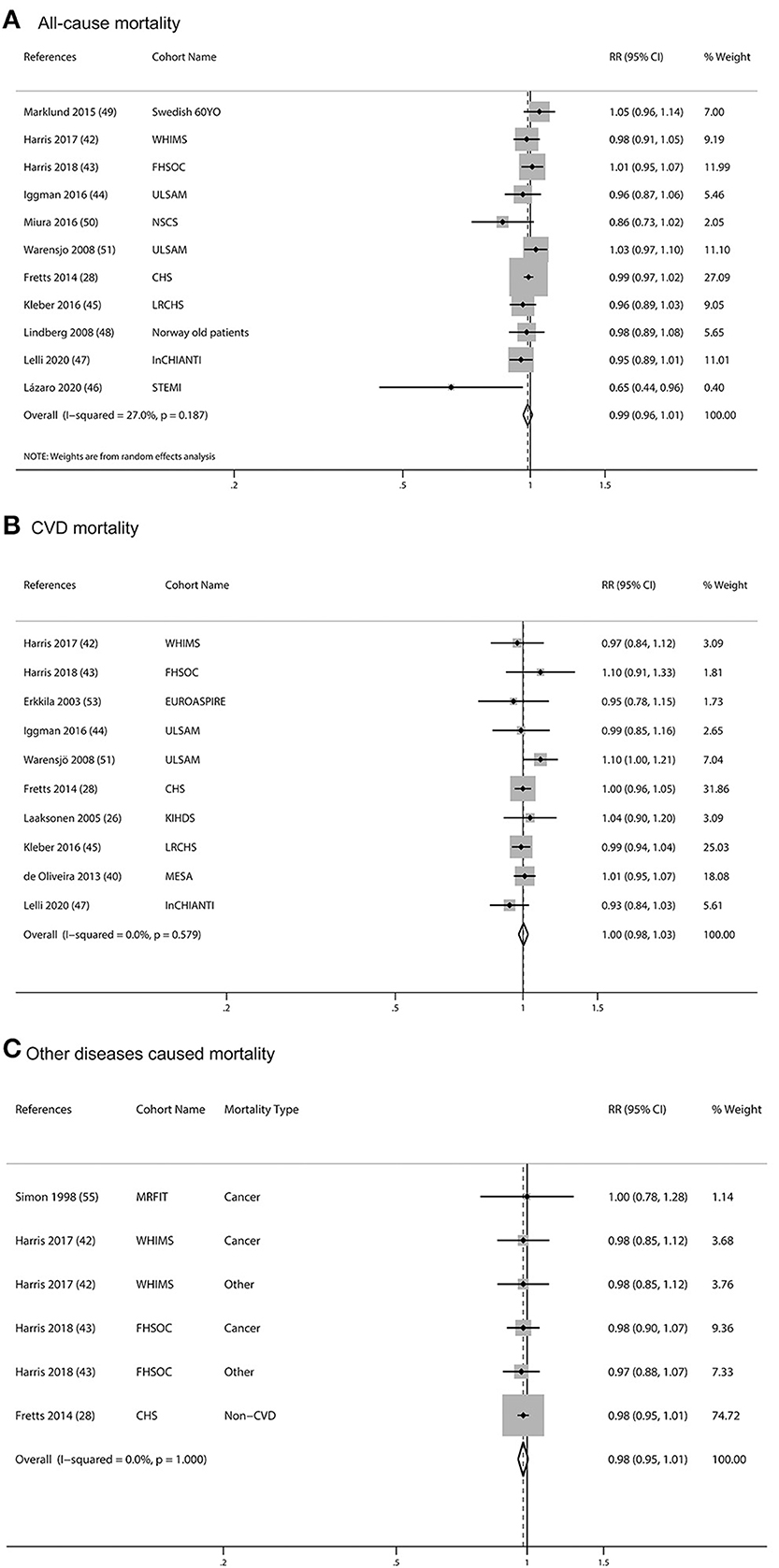
Figure 4. Meta-analysis of the associations between per SD increment biomarkers of ALA in body tissue and mortality from all-cause (A), CVD (B), and other diseases (C) in prospective cohort studies. CI, confidence interval. The horizontal lines denote the 95% CIs, some of which extend beyond the limits of the scales. The square is proportional to the weight of each study. The diamond represents the overall pooled estimate of the relative risk.
Assessment of Publication Bias
Both the Funnel plot and Egger's test showed there is no publication bias for ALA intake and/or biomarkers with total, CVD, and other diseases mortality (dietary studies, p ≥ 0.061; biomarker studies, p ≥ 0.145) (Supplementary Figure).
Discussion
In this meta-analysis of dietary and biomarker studies of ALA and cause-specific mortality, it was found that overall ALA exposure was associated with a modestly lower risk of CVD-mortality but not with all-causes of mortality or other diseases-caused mortality. ALA intake was associated with a lower risk of total mortality in European countries. There was an inverse linear relationship between dietary ALA and CVD-mortality. Every 0.5% increase in energy intake was associated with a 5% reduction in CVD-mortality. Moreover, the CVD-mortality protective effects of ALA were more evident in the general population and in studies with a higher proportion of women. The protective trend of combined risk estimates was generally similar for ALA biomarker concentrations, but the results were not statistically significant. Overall, these data support the potential benefits of ALA in the prevention of premature death.
There has been considerable interest in identifying the potential beneficial effects of seafood omega-3 fatty acids, particularly EPA and DHA, on cause-specific mortality. Several meta-analyses of RCTs consistently showed that seafood omega-3 fatty acids played a protective role on CVD-mortality (5, 62, 63). Given the concerns about contamination of oily fish and fish oil, the interest has spread to plant-based omega-3 fatty acids. However, the association between plant-based omega-3 and cause-specific mortality remains unclear. A recent meta-analysis summarized a few intervention trials that showed increasing ALA intake was probably made little or no difference to all-cause mortality (5). However, this meta-analysis generated the results mainly from two large trials [the Alpha Omega Trial (64) and the Norwegian vegetable oil experiment (65)]. The Alpha Omega Trial observed a non-significant 3% reduction in death from any cause with ALA supplementation, as compared with placebo and EPA-DHA only. The results might be complicated by an improvement in cardioprotective drug treatment during the clinical trial. The Norwegian vegetable oil experiment was conducted in Norway >30 years ago and the background fish and fish-oil intake were already quite high in recruited population, which possibly masked any potential benefit of additional ALA. Thus, there is a lack of enough evidence from RCTs to confirm the effect of ALA on CVD-mortality. The prospective cohort study design is the best available scientific method for measuring the effects of ALA on mortality now. By combing the results of 13 prospective studies, our meta-analysis suggests that ALA consumption may be associated with lower CVD-mortality, and each 0.5% energy increment of ALA intake was associated with a 5% lower risk of CVD-mortality. Based on 2,000 kcal per day, 0.5% energy was equivalent to 1.1 g of ALA. Flaxseed oil contained the highest amount of ALA (56%) (66). Thus, the 1.1-g increment of ALA intake is reachable by an appropriate substitute, such as flaxseed oil. Our results were partly supported by previous studies, which found that ALA intake was inversely associated with fatal CHD—every 1 g/day increase in ALA intake was associated with a 10 or 12% decrease in fatal CHD risk (14, 15). Moreover, the CVD-mortality protective effects of ALA tended to be stronger in the general population. This suggested that ALA played prevention but not treatment role in CVD-mortality. In addition, ALA showed a more robust inverse association with CVD mortality in studies with men <50%, as ALA is converted to EPA in a very small proportion after consumption and absorption. Moreover, the conversion is mediated by estrogen and appears to be greater in women (67). Animal models showed that female rats had significantly higher mRNA expression of Δ5 and Δ6 desaturases, which are key enzymes involved in the endogenous synthesis of longer-chain fatty acids (68). Subcutaneous injection of estrogen had been demonstrated to result in higher expression of Δ6 desaturase and elongase enzymes (69). In the human study, Burdge et al. had also identified significant differences in the conversion of ALA between women and men. Women had a significantly greater capacity than men to synthesize EPA and DHA from ALA. The estimated mean net conversion rate of ALA to EPA in women was 21% compared to 8% in men (70). Besides CVD, we also evaluated the relationship of ALA with other disease-caused mortality and total mortality. We found a non-significant reduction trend toward other disease-caused by mortality and total mortality.
There are several methods to measure ALA exposure. However, the best exposure metric for ALA remains unclear. Dietary ALA intakes were measured by different methods (FFQ, repeated FFQ, 24-h dietary recall, and 4-d dietary records) in various studies. Self-reported dietary intake may cause recall bias. In our subgroup analyses, ALA was significantly associated with a lower risk of total mortality in studies with repeated FFQ methods for ALA intake assessment. One plausible explanation is that repeated measurements could reduce potential bias and represent true long-term intake levels. A recent pooled study showed that the blood ALA was not associated with total and cause-specific mortality (71). Researchers have resorted to the use of biomarkers to measure the “true intake” in an objective way. Usually, nutrient biomarkers in biological tissues are generally considered better than traditional dietary intake recording methods. However, this remains unsure for ALA, as ALA is partially converted to EPA mentioned previously (67). Moreover, ALA is transformed to oxylipins by cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes, which are circulating bioactive lipids (72). In addition, the most available blood biomarker concentrations of ALA reflect short-term exposures, rather than longer periods, which may be most relevant for the risk of chronic diseases (73). The correlation between ALA consumption with biomarkers concentration of ALA in circulating blood is low (average correlation of 0.24 for blood concentrations) (73). Furthermore, temporal variability or stability of ALA from tissues should also be concerned. Studies showed that reproducibility was lower for some of the plasma fatty acids over 1-3 years (74). Thus, ALA in blood concentrations may not be the ideal measurement of ALA. That is why our biomarker results are not consistent with the intake results. The strength of our study is that we assessed associations with cause-specific mortality of both dietary intake and biomarkers of ALA. In addition, compared with simple pooled studies where data are combined without being weighted, meta-analysis, where data from individual studies are weighted first, then combined, thereby avoids Simpson's paradox of simple pooling (75).
Some limitations are needed to mention. Although major confounding factors were adjusted, diet studies were limited by the use of too many dietary variables and the inability to precisely determine the intake of ALA. For example, intake of ALA may also concomitantly consume trans-ALA, which may obscure the protective effects of ALA (76). In the current meta-analysis, despite extensive efforts were put, such as meta-regression and subgroup analyses were performed, we did not identify these variations as statistically significant sources of heterogeneity, which may limit the validity of the overall combined results. The solution is that we combined the results by random-effect model, which is more stable.
In conclusion, this meta-analysis summarized the available prospective cohort studies that indicated that ALA intake was associated with reduced risk of mortality, especially CVD mortality. Our findings suggest that ALA consumption may be beneficial for the prevention of premature death and encourage consumption of ALA from vegetable oils, such as soybean and linseed oil, nuts, or ALA-enriched foods to meet dietary reference intake.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
L-HC, QH, and GL conceived and designed the study. L-HC and QH performed the statistical analysis and drafted the first version of the manuscript. LZ, L-QQ, HZ, and GX wrote sections of the manuscript. L-HC was the guarantor and has full access to all of the data in the study and takes responsibility for the integrity of the data, and the accuracy of the data analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81803307); 2017 Chinese Nutrition Society (CNS) Nutrition Research Foundation-DSM Research Fund (grant number 2017-040); the Medical Clinical Science and Technology Development Fund of Jiangsu University (grant number JLY2021054); the Scientific Research Project Contract of Jiangsu Provincial Health Commission (grant number Z2020059); and the Social Development Special Fund of Kunshan (grant number KS1931).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.743852/full#supplementary-material
Supplementary Figure. Funnel plots for testing publication bias. α-linolenic acid (ALA) intake and mortality from all causes (A), Cardiovascular Disease (CVD) (B), and other diseases (C) in prospective cohort studies; ALA biomarker and mortality from all causes (D), CVD (E) and other diseases (F) in prospective cohort studies.
References
1. Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151-210. doi: 10.1016/S0140-6736(17)32152-9
2. Koh AS, Pan A, Wang R, Odegaard AO, Pereira MA, Yuan JM, et al. The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol. (2015) 22:364-72. doi: 10.1177/2047487313517576
3. Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. (2008) 52:988-96. doi: 10.1016/j.jacc.2008.06.018
4. Yang B, Wang FL, Ren XL, Li D. Biospecimen long-chain N-3 PUFA and risk of colorectal cancer: a meta-analysis of data from 60,627 individuals. PLoS ONE. (2014) 9:e110574. doi: 10.1371/journal.pone.0110574
5. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochr Database Syst Rev. (2020) 3:Cd003177. doi: 10.1002/14651858.CD003177.pub5
6. Dabrowska J, Sobota M, Swiader M, Borowski P, Moryl A, Stodolak R, et al. Marine waste-sources, fate, risks, challenges and research needs. Int J Environ Res Public Health. (2021) 18:433. doi: 10.3390/ijerph18020433
7. Wennberg M, Strömberg U, Bergdahl IA, Jansson JH, Kauhanen J, Norberg M, et al. Myocardial infarction in relation to mercury and fatty acids from fish: a risk-benefit analysis based on pooled Finnish and Swedish data in men. Am J Clin Nutr. (2012) 96:706-13. doi: 10.3945/ajcn.111.033795
8. Damude HG, Kinney AJ. Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids. (2007) 42:179-85. doi: 10.1007/s11745-007-3049-1
9. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. (2006) 83:1467S-76S. doi: 10.1093/ajcn/83.6.1467S
10. Parikh M, Netticadan T, Pierce GN. Flaxseed: its bioactive components and their cardiovascular benefits. Am J Physiol Heart Circ Physiol. (2018) 314:H146-59. doi: 10.1152/ajpheart.00400.2017
11. Khalesi S, Irwin C, Schubert M. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J Nutr. (2015) 145:758-65. doi: 10.3945/jn.114.205302
12. Hashimoto M, Tanabe Y, Hossain S, Matsuzaki K, Ohno M, Kato S, et al. Intake of alpha-linolenic acid-rich perilla frutescens leaf powder decreases home blood pressure and serum oxidized low-density lipoprotein in Japanese adults. Molecules. (2020) 25:2099. doi: 10.3390/molecules25092099
13. Chen H, Deng G, Zhou Q, Chu X, Su M, Wei Y, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid versus α-linolenic acid supplementation on cardiometabolic risk factors: a meta-analysis of randomized controlled trials. Food Funct. (2020) 11:1919-32. doi: 10.1039/C9FO03052B
14. Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, et al. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. (2012) 96:1262-73. doi: 10.3945/ajcn.112.044040
15. Wei J, Hou R, Xi Y, Kowalski A, Wang T, Yu Z, et al. The association and dose-response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br J Nutr. (2018) 119:83-9. doi: 10.1017/S0007114517003294
16. Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. (2004) 134:919-22. doi: 10.1093/jn/134.4.919
17. Carayol M, Grosclaude P, Delpierre C. Prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. Cancer Causes Control. (2010) 21:347-55. doi: 10.1007/s10552-009-9465-1
18. Simon JA, Chen YH, Bent S. The relation of alpha-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr. (2009) 89:1558s-64s. doi: 10.3945/ajcn.2009.26736E
19. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. (2011) 343:d2090. doi: 10.1136/bmj.d2090
20. Yang Y, Li W, Zhu H, Pan XF, Hu Y, Arnott C, et al. Prognosis of unrecognised myocardial infarction determined by electrocardiography or cardiac magnetic resonance imaging: systematic review and meta-analysis. BMJ. (2020) 369:m1184. doi: 10.1136/bmj.m1184
21. Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. (2020) 370:m2297. doi: 10.1136/bmj.m2297
22. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diab Obes Metab. (2021) 23:1746-53. doi: 10.1111/dom.14388
23. Li J, Guasch-Ferré M, Li Y, Hu FB. Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. (2020) 112:150-67. doi: 10.1093/ajcn/nqz349
24. Li W, Huang A, Zhu H, Liu X, Huang X, Huang Y, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Austr. (2020) 213:374-9. doi: 10.5694/mja2.50781
25. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. (2006) 6:40-57. doi: 10.1177/1536867X0600600103
26. Zhao X, Xiang X, Huang J, Ma Y, Sun J, Zhu D. Studying the evaluation model of the nutritional quality of edible vegetable oil based on dietary nutrient reference intake. ACS Omega. (2021) 6:6691-8. doi: 10.1021/acsomega.0c05544
27. Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology. (2000) 11:440-5. doi: 10.1097/00001648-200007000-00013
28. Lelli D, Antonelli Incalzi R, Ferrucci L, Bandinelli S, Pedone C. Association between PUFA intake and serum concentration and mortality in older adults: a cohort study. Clin Nutr. (2019) 39:510-5. doi: 10.1016/j.clnu.2019.02.030
29. Bidoli E, La Vecchia C, Montella M, Maso LD, Conti E, Negri E, et al. Nutrient intake and ovarian cancer: an Italian case-control study. Cancer Causes Control. (2002) 13:255-61. doi: 10.1023/A:1015047625060
30. Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet. (1991) 66:205-16. doi: 10.1159/000419291
31. Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. (2004) 160:1005-10. doi: 10.1093/aje/kwh307
32. Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. (2005) 165:193-9. doi: 10.1001/archinte.165.2.193
33. Richman EL, Kenfield SA, Chavarro JE, Stampfer MJ, Giovannucci EL, Willett WC, et al. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern Med. (2013) 173:1318-26. doi: 10.1001/jamainternmed.2013.6536
34. Fretts AM, Mozaffarian D, Siscovick DS, Sitlani C, Psaty BM, Rimm EB, et al. Plasma phospholipid and dietary alpha-linolenic acid, mortality, CHD and stroke: the Cardiovascular Health Study. Br J Nutr. (2014) 112:1206-13. doi: 10.1017/S0007114514001925
35. Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: a population-based follow-up study on Long Island, New York. Cancer. (2015) 121:2244-52. doi: 10.1002/cncr.29329
36. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. (2016) 176:1134-45. doi: 10.1001/jamainternmed.2016.2417
37. Sala-Vila A, Guasch-Ferré M, Hu FB, Sánchez-Tainta A, Bulló M, Serra-Mir M, et al. Dietary α-Linolenic Acid, Marine ω-3 Fatty Acids, and Mortality in a Population With High Fish Consumption: Findings From the PREvención con DIeta MEDiterránea (PREDIMED) Study. J Am Heart Assoc. (2016) 5:e002077. doi: 10.1161/JAHA.116.002077
38. Pranger IG, Gruppen EG, van den Berg E, Soedamah-Muthu SS, Navis G, Gans ROB, et al. Intake of n-3 fatty acids and long-term outcome in renal transplant recipients: a post hoc analysis of a prospective cohort study. Br J Nutr. (2016) 116:2066-73. doi: 10.1017/S0007114516004207
39. Zhuang P, Wang W, Wang J, Zhang Y, Jiao J. Polyunsaturated fatty acids intake, omega-6/omega-3 ratio and mortality: findings from two independent nationwide cohorts. Clin Nutr. (2019) 38:848-55. doi: 10.1016/j.clnu.2018.02.019
40. Jiao J, Liu G, Shin HJ, Hu FB, Rimm EB, Rexrode KM, et al. Dietary fats and mortality among patients with type 2 diabetes: analysis in two population based cohort studies. BMJ. (2019) 366:l4009. doi: 10.1136/bmj.l4009
41. Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: Cohort follow up study in the United States. BMJ. (1996) 313:84-90. doi: 10.1136/bmj.313.7049.84
42. Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The alpha-tocopherol, beta-carotene cancer prevention Study. Am J Epidemiol. (1997) 145:876-87. doi: 10.1093/oxfordjournals.aje.a009047
43. Hu FB, Stampfer MJ, Manson JAE, Rimm EB, Wolk A, Colditz GA, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. (1999) 69:890-7. doi: 10.1093/ajcn/69.5.890
44. Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. (2005) 112:3232-8. doi: 10.1161/CIRCULATIONAHA.105.572008
45. Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, et al. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr. (2012) 96:498-507. doi: 10.3945/ajcn.112.040287
46. de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of atherosclerosis. J Am Heart Assoc. (2013) 2:e000506. doi: 10.1161/JAHA.113.000506
47. Gopinath B, Buyken AE, Flood VM, Empson M, Rochtchina E, Mitchell P. Consumption of polyunsaturated fatty acids, fish, and nuts and risk of inflammatory disease mortality. Am J Clin Nutr. (2011) 93:1073-9. doi: 10.3945/ajcn.110.009977
48. Harris WS, Luo J, Pottala JV, Espeland MA, Margolis KL, Manson JE, et al. Red blood cell polyunsaturated fatty acids and mortality in the Women's Health Initiative Memory Study. J Clin Lipidol. (2017) 11:250-9.e5. doi: 10.1016/j.jacl.2016.12.013
49. Harris WS, Tintle NL, Etherton MR, Vasan RS. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: the Framingham Heart Study. J Clin Lipidol. (2018) 12:718-27.e6. doi: 10.1016/j.jacl.2018.02.010
50. Iggman D, Arnlov J, Cederholm T, Riserus U. Association of adipose tissue fatty acids with cardiovascular and all-cause mortality in elderly men. JAMA Cardiol. (2016) 1:745-53. doi: 10.1001/jamacardio.2016.2259
51. Kleber ME, Delgado GE, Lorkowski S, Maerz W, von Schacky C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. (2016) 252:175-81. doi: 10.1016/j.atherosclerosis.2016.06.049
52. Lázaro I, Rueda F, Cediel G, Ortega E, García-García C, Sala-Vila A, et al. Circulating omega-3 fatty acids and incident adverse events in patients with acute myocardial infarction. J Am Coll Cardiol. (2020) 76:2089-97. doi: 10.1016/j.jacc.2020.08.073
53. Lindberg M, Saltvedt I, Sletvold O, Bjerve KS. Long-chain n-3 fatty acids and mortality in elderly patients. Am J Clin Nutr. (2008) 88:722-9. doi: 10.1093/ajcn/88.3.722
54. Marklund M, Leander K, Vikström M, Laguzzi F, Gigante B, Sjögren P, et al. Polyunsaturated fat intake estimated by circulating biomarkers and risk of cardiovascular disease and all-cause mortality in a population-based cohort of 60-year-old men and women. Circulation. (2015) 132:586-94. doi: 10.1161/CIRCULATIONAHA.115.015607
55. Miura K, Hughes MCB, Ungerer JP, Green AC. Plasma eicosapentaenoic acid is negatively associated with all-cause mortality among men and women in a population-based prospective study. Nutr Res. (2016) 36:1202-9. doi: 10.1016/j.nutres.2016.09.006
56. Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. (2008) 88:203-9. doi: 10.1093/ajcn/88.1.203
57. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease The Heart and Soul Study. Circ Cardiovasc Qual Outcomes. (2010) 3:406-12. doi: 10.1161/CIRCOUTCOMES.109.896159
58. Erkkila AT, Lehto S, Pyorala K, Uusitupa MIJ. n-3 fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. (2003) 78:65-71. doi: 10.1093/ajcn/78.1.65
59. Huang NK, BuŽková P, Matthan NR, Djoussé L, Hirsch CH, Kizer JR, et al. Associations of serum nonesterified fatty acids with coronary heart disease mortality and nonfatal myocardial infarction: the CHS (Cardiovascular Health Study) Cohort. J Am Heart Assoc. (2021) 10:e019135. doi: 10.1161/JAHA.120.019135
60. Simon JA, Fong J, Bernert JT Jr, Browner WS. Serum fatty acids and the risk of fatal cancer. MRFIT Research Group. Multiple risk factor intervention trial. Am J Epidemiol. (1998) 148:854-8. doi: 10.1093/oxfordjournals.aje.a009710
61. Miura K, Hughes MCB, Ungerer JPJ, Smith DD, Green AC. Absolute versus relative measures of plasma fatty acids and health outcomes: example of phospholipid omega-3 and omega-6 fatty acids and all-cause mortality in women. Eur J Nutr. (2018) 57:713-22. doi: 10.1007/s00394-016-1358-y
62. Kim J, Hoang T, Kim JM, Bu SY, Choi JH, Park E, et al. All-cause mortality and cardiovascular death between statins and omega-3 supplementation: a meta-analysis and network meta-analysis from 55 randomized controlled trials. Nutrients. (2020) 12:3203. doi: 10.3390/nu12103203
63. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. (2019) 8:e013543. doi: 10.1161/JAHA.119.013543
64. Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. (2010) 363:2015-26. doi: 10.1056/NEJMoa1003603
65. Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K. A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. The Norwegian vegetable oil experiment of 1965-66. Scand J Clin Lab Invest Supplement. (1968) 105:1-20. doi: 10.1080/00365516809168196
66. Richardson CE, Hennebelle M, Otoki Y, Zamora D, Yang J, Hammock BD, et al. Lipidomic analysis of oxidized fatty acids in plant and algae oils. J Agric Food Chem. (2017) 65:1941-51. doi: 10.1021/acs.jafc.6b05559
67. Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. (2011) 94:1914s-9s. doi: 10.3945/ajcn.110.000893
68. Extier A, Langelier B, Perruchot MH, Guesnet P, Van Veldhoven PP, Lavialle M, et al. Gender affects liver desaturase expression in a rat model of n-3 fatty acid repletion. J Nutr Biochem. (2010) 21:180-7. doi: 10.1016/j.jnutbio.2008.10.008
69. Kim D, Choi JE, Park Y. Low-linoleic acid diet and oestrogen enhance the conversion of α-linolenic acid into DHA through modification of conversion enzymes and transcription factors. Br J Nutr. (2019) 121:137-45. doi: 10.1017/S0007114518003252
70. Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. (2002) 88:411-20. doi: 10.1079/BJN2002689
71. Harris WS, Tintle NL, Imamura F, Qian F, Korat AVA, Marklund M, et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun. (2021) 12:2329. doi: 10.1038/s41467-021-22370-2
72. Du Y, Taylor CG, Aukema HM, Zahradka P. Role of oxylipins generated from dietary PUFAs in the modulation of endothelial cell function. Prostaglandins Leukot Essent Fatty Acids. (2020) 160:102160. doi: 10.1016/j.plefa.2020.102160
73. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress Lipid Res. (2008) 47:348-80. doi: 10.1016/j.plipres.2008.03.003
74. Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomark Prevent. (2010) 19:938-46. doi: 10.1158/1055-9965.EPI-09-1318
75. Bravata DM, Olkin I. Simple pooling versus combining in meta-analysis. Evaluat Health Profess. (2001) 24:218-30. doi: 10.1177/01632780122034885
Keywords: α-linolenic acid (ALA), dietary polyunsaturated acid, biomarkers, mortality, cardiovascular disease
Citation: Chen L-H, Hu Q, Li G, Zhang L, Qin L-Q, Zuo H and Xu G (2021) Dietary Intake and Biomarkers of α-Linolenic Acid and Mortality: A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 8:743852. doi: 10.3389/fnut.2021.743852
Received: 19 July 2021; Accepted: 04 October 2021;
Published: 03 November 2021.
Edited by:
Alessandra Bordoni, University of Bologna, ItalyReviewed by:
Philip Calder, University of Southampton, United KingdomYuli Huang, Southern Medical University, China
Copyright © 2021 Chen, Hu, Li, Zhang, Qin, Zuo and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Hua Chen, bGhjaGVuQG50dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Li-Hua Chen
Li-Hua Chen Qingjing Hu
Qingjing Hu Guijie Li1
Guijie Li1 Li Zhang
Li Zhang Hui Zuo
Hui Zuo