- 1College of Animal Science and Technology, Qingdao Agricultural University, Qingdao, China
- 2College of Veterinary Medicine, Qingdao Agricultural University, Qingdao, China
- 3School of Public Health, Zhejiang University School of Medicine, Hangzhou, China
- 4Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Bio-X Institutes, Shanghai Jiao Tong University, Shanghai, China
- 5Center for Biomedical Informatics, Harvard Medical School, Boston, MA, United States
Background: Previous studies have demonstrated that diabetes is often accompanied with lower magnesium status. However, practical details regarding the influences of magnesium intervention on hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes (T2D) need to be further investigated.
Methods: Web of Science, ScienceDirect, and PubMed were searched for relevant literatures published through April 30, 2022, and high-quality data were pooled to evaluate the effects of magnesium supplementation on glycemic, circulating lipids, and blood pressure control in T2D, and to explore the associated practical details.
Results: Pooled analyses of 24 randomized controlled trials with 1,325 T2D individuals revealed that subjects who received magnesium supplementation had statistically significant reductions in fasting plasma glucose, glycated hemoglobin, systolic blood pressure and diastolic blood pressure, with WMD values of –0.20 mM (95% CI: –0.30, –0.09), –0.22% (95% CI: –0.41, –0.03), –7.69 mmHg (95% CI: –11.71, –3.66) and –2.71 mmHg (95% CI: –4.02, –1.40), respectively. Detailed subgroup analyses demonstrated that health status of participants including age, body mass index, country, duration of disease, baseline magnesium level and baseline glycemic control condition as well as magnesium formulation, dosage and duration of intervention influenced the effects of magnesium addition. Dose-effect analysis showed that 279 mg/d for 116 d, 429 mg/d for 88 d and 300 mg/d for 120 d are the average optimal dosages and durations for improving glycemic, circulating lipids, and blood pressure controls, respectively.
Conclusion: Our findings provide clinically relevant information on the adjuvant therapy of magnesium for improving hyperglycemia, hypercholesterolemia, and hypertension in T2D.
Introduction
The diabetes prevalence is predicted to be 10.9% by 2045 worldwide, which has negative effects on the well-being of individuals (1). Type 2 diabetes (T2D) is a common metabolic disorder usually accompanied with β cell impairment, insulin resistance and hyperglycemia (2), leading to a diminished glucose control. Previous work established that T2D individuals have significant positive relations to formations of hypertension and hypercholesterolemia (3), which are the main risk factors related to cardiovascular diseases resulting in high mortality worldwide (4). Therefore, populations with T2D appear to own common syndromes of increased plasma glucose, reduced insulin sensitivity, hypertension and dyslipidemia simultaneously (5), highlighting the importance to find ways to treat these complications concurrently.
Magnesium plays a key role in many metabolisms as a cofactor of enzymatic pathways (6). Previous work showed that hypomagnesemia was reported in about 30% of diabetic patients (7). Accumulating evidence demonstrated that higher magnesium intake improved insulin release and sensibility (8, 9), dyslipidemia (10), and dysfunction of endothelial cells (11), and reduced thrombotic tendency (12) and vascular contractility (13). Therefore, clinical magnesium supplementation may be a strategy to improve the outcomes of T2D cases.
Several systematic reviews (14, 15) carried out on randomized controlled trials (RCT) were performed to examine the beneficial influences of magnesium intervention on development of T2D, but the results were less conclusive. Furthermore, meta-analysis that simultaneous investigated the influences of magnesium intervention on hyperglycemia, hypertension and hyperlipidemia in T2D is relatively limited. Thus, the effects of magnesium addition improving the parameters related to the complications of T2D, as well as the associated practical issues, require additional investigation.
We hypothesized that the dosage effects of magnesium addition on clinical outcomes of T2D depend on the disease status and mode of intervention. Therefore, in this meta-analytic study, we collected RCT data of updated studies to investigate the efficacy of magnesium supplementation on glycemic, plasma lipids and blood pressure controls in T2D, and to explore the optimal details associated with this strategy based on the patient’s health status and mode of intervention through subgroup and dose-effect analyses.
Methods
Data collection
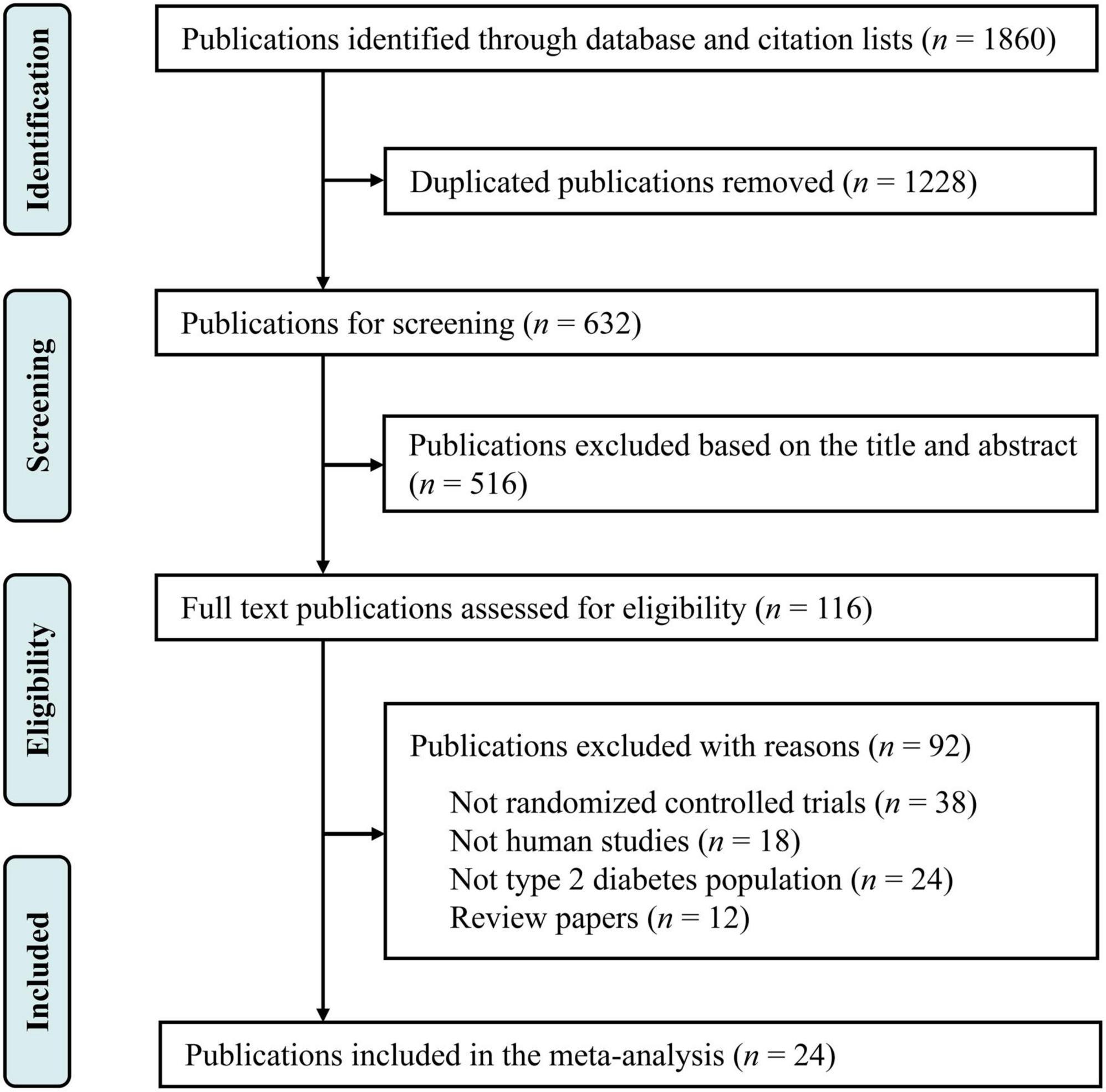
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database under CRD42022324969, and we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) instructions (16). Relevant published studies were searched in Web of Science, PubMed and ScienceDirect published through April 30, 2022, with the following keywords: (“magnesium supplementation” OR “magnesium intervention” OR “magnesium”) AND (“diabetes” OR “type 2 diabetes” OR “non-insulin-dependent diabetics”) AND [“glucose” OR “fasting plasma glucose (FPG) OR “glycemia” OR “glycemic control” OR “insulin” OR “glycated hemoglobin (HbA1c)” OR “homeostasis model assessment-insulin resistance (HOMA-IR)”] AND [“lipids” OR “total cholesterol (TC)” OR “total triglyceride (TG)” OR “low density lipoprotein cholesterol (LDL-C)” OR “high density lipoprotein cholesterol (HDL-C)”] AND [“blood pressure” OR “systolic blood pressure (SBP)” OR “diastolic blood pressure (DBP)”]. There were no limitations in the language of studies. Additional publications were further collected through reviewing the references of selected relevant publications. The selection process is summarized in Figure 1.
Criteria on included study
Studies met the following criteria were included in this meta-analysis: (i) being a parallel or cross-over design in RCT, (ii) exploring the influences of magnesium addition in T2D patients, (iii) reporting data on one or more of the following items: FPG, insulin, HDL-C, LDL-C, TC and TG concentrations along with HbA1c, HOMA-IR, SBP and DBP values; (iv) reporting the information on above items at baseline and at the end of follow-up. Exclusion criteria were (i) the study was not a RCT, (ii) the study had no control group, (iii) the study had a case-control, cross-sectional or cohort design, and (iv) the study didn’t report information related to baseline or follow-up parameters; (v) the study was a methodologic report, review, comment or abstract.
Data extraction
Detailed information was collected from the eligible studies, containing the first author’s name, study region, sample size, participant age and body mass index (BMI), duration of diabetes, dosage and duration of magnesium intervention, and plasma concentrations of magnesium, glucose, insulin, HDL-C, LDL-C, TC and TG as well as HbA1c, HOMA-IR, SBP and DBP values. The units of FBS, insulin and HbA1c were converted to mM, mU/L and percentage, respectively. Besides, the HDL-C, LDL-C, TC and TG concentrations were all collated in mg/dL.
Quality assessment
The methodologic quality of the RCTs were assessed by Jadad scale (17). Each publication was assigned a score from 0 (“poor”) to 5 (“good”) according to the criteria: (i) does the article have a randomized design? (ii) does the article have a double-blind design? (iii) does the article report withdrawals and dropouts? (iv) does the article describe the randomization procedures and they are appropriate? (v) does the study report appropriate blinding techniques? Each “yes” or “no” response will get 1 or 0 points, respectively (Supplementary Table 1).
Quantitative data synthesis
We used Stata version 14 to perform the statistical analyses. The differences of mean and SD between baseline and endpoint were calculated according to the formula: changes of mean = (measure at endpoint) – (measure at baseline); SD = squareroot [(SDbaseline)2 + (SDendpoint)2 – (2R × SDbaseline × SDendpoint)], assuming a correlation coefficient (R) = 0.5. The weighted mean difference (WMD) for continuous outcomes were computed between the magnesium and control groups using a random-effect model. Between-study heterogeneity was assessed using the I2 statistic, with 0–25%, 25.1–75%, and 75.1–100% representing a low, moderate, or high degree of heterogeneity, respectively (18). Publication bias was measured by the contour-enhanced funnel plots and Egger’s linear regression test, with threshold of significance at P < 0.05. The effects of individual studies on the pooled meta-analytic results were determined with the sensitivity analysis (19).
Subgroup and dose-effect analyses
Subgroup analyses were made according to the participant’s age and BMI, country, duration of disease, baseline magnesium level and baseline glycemic control condition as well as magnesium formulation, dosage and duration of intervention. Meta-regression analyses were used to compare the subgroup differences. In addition, dose-effect model was used to find the optimal dosage and duration of magnesium intervention with the R 4.2.0 software (The R Foundation Conference Committee, USA).
Results
Study characteristics
Our primary search identified 1,860 publications in Web of Science, PubMed and ScienceDirect (Figure 1). After removing the duplicate literatures, 116 articles were screened in detail. Among these, 92 records were removed, including 38 publications that were not RCTs, 24 publications that were not conducted in T2D populations, 18 animal trials, and 12 review papers. Finally, 24 publications were included in the final meta-analysis (Table 1).
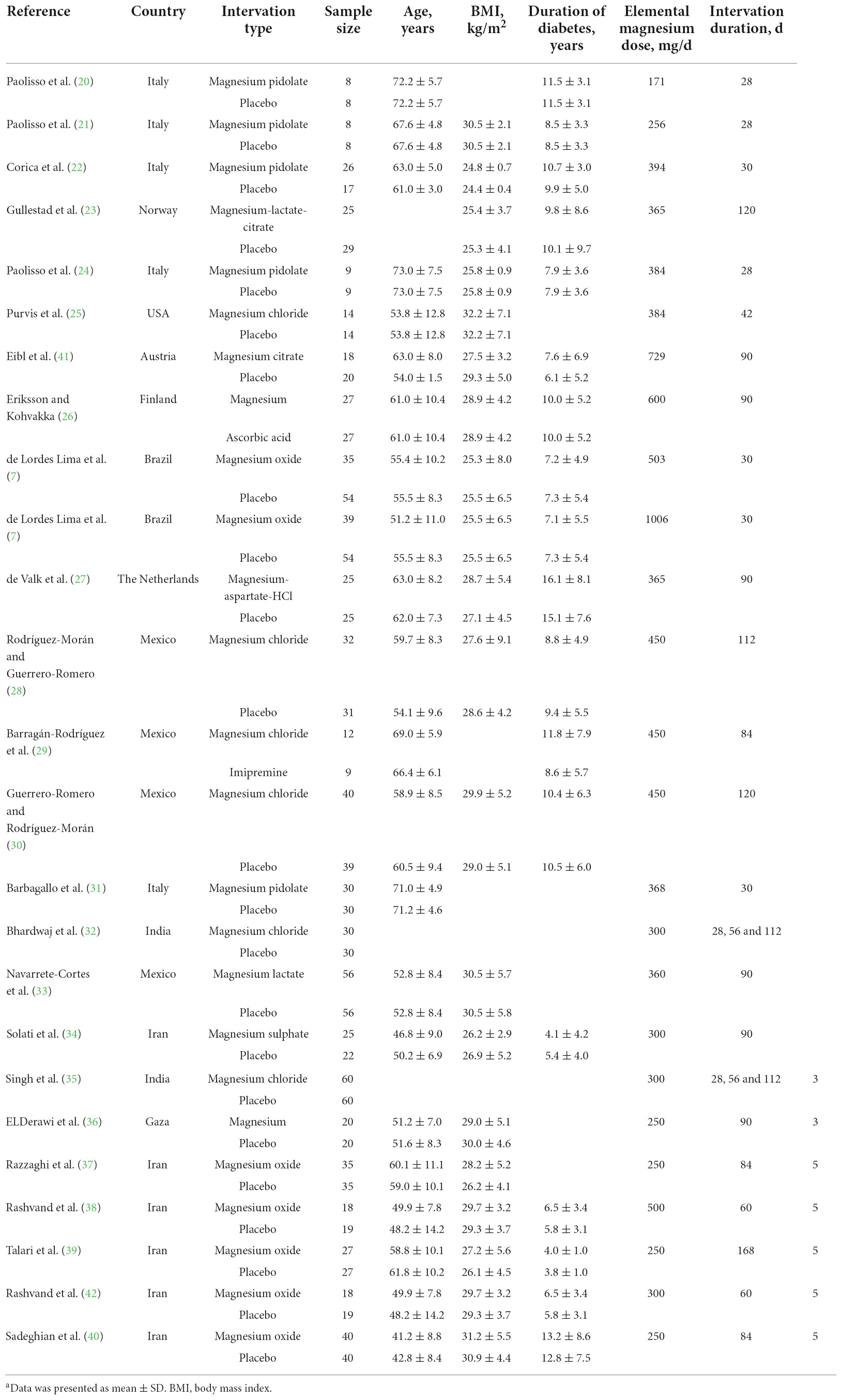
Table 1. Characteristics of included studies on magnesium intervention in type 2 diabetes mellitusa.
Effects of magnesium supplementation on glycemic control
The effect of magnesium supplementation on FPG was reported in 27 observations from 22 studies (7, 20–40). The following analysis revealed that magnesium administration decreased the FPG concentration, with a WMD value of –0.20 mM (95% CI: –0.30, –0.09; I2 = 43.5%, Figure 2A).
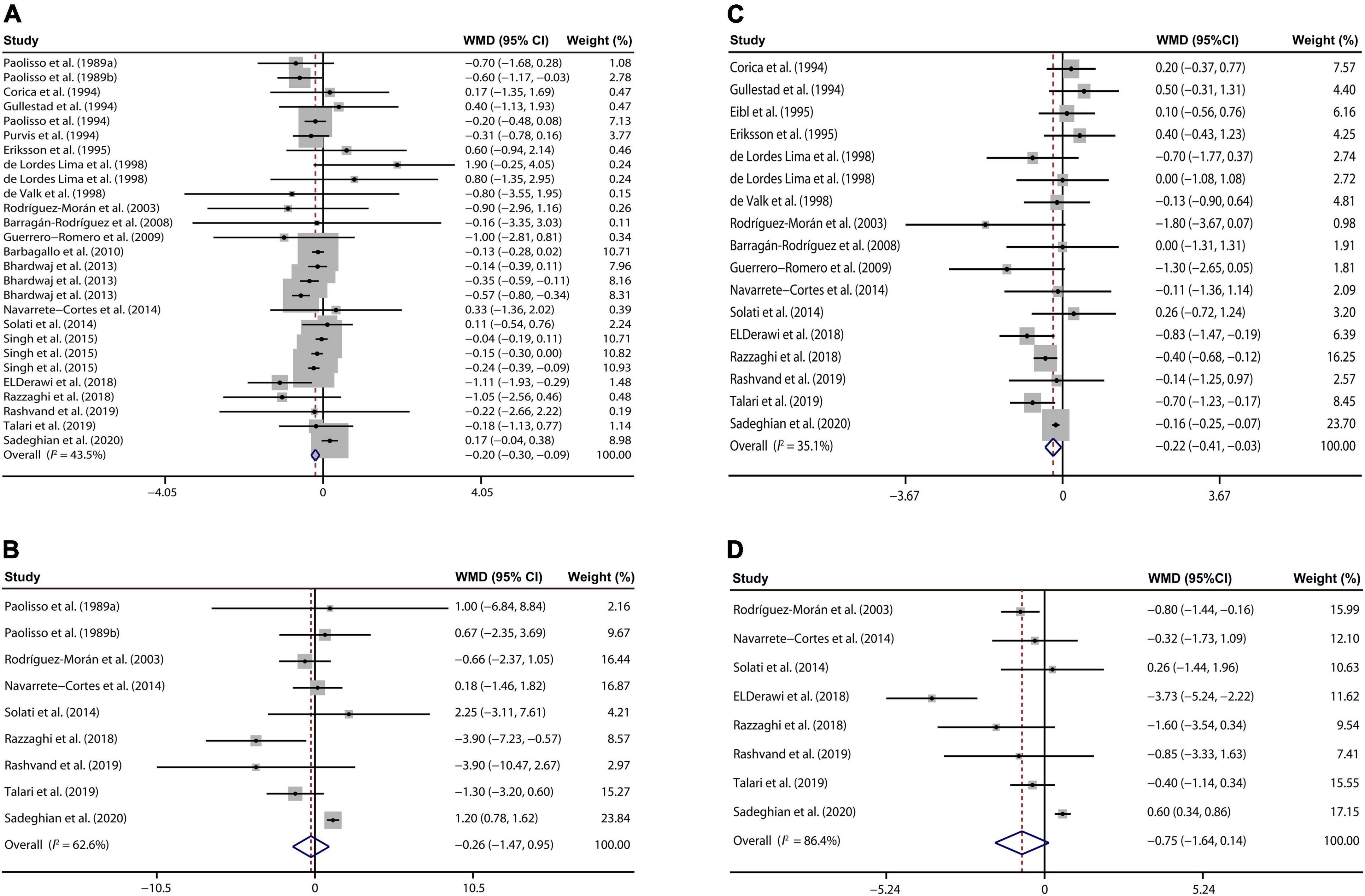
Figure 2. Forest plots for the effects of magnesium supplementation on FPG (A), insulin (B), HbA1c (C), and HOMA-IR (D) compared to controls in pooled analysis. For each study, the solid black circles represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the overall WMD determined with a random-effect model. FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance; WMD, weighted mean difference.
Nine studies (20, 21, 28, 33, 34, 37–40) involving 495 T2D patients presented that oral magnesium had no significant influence on plasma insulin concentration relative to the control group (WMD: –0.26 mU/L; 95% CI: –1.47, 0.95; I2 = 62.6%, Figure 2B).
Our meta-analysis of 17 interventions (7, 22, 23, 26–30, 33, 34, 36–41) found a significant reduction in HbA1c of T2D populations received magnesium addition relative to the control treatment (WMD: –0.22%; 95% CI: –0.41, –0.03; I2 = 35.1%, Figure 2C).
We also explored whether magnesium administration regulates insulin sensitivity through analyzing the HOMA-IR data from the 8 studies (28, 33, 34, 36–40). However, no significant difference in HOMA-IR was found between the intervention and control group (WMD: –0.75; 95% CI: –1.64, 0.14; I2 = 86.4%, Figure 2D).
Effects of magnesium supplementation on lipid metabolism
Ten eligible publications (22, 26–28, 33, 34, 37, 39, 41, 42) suggested that there was no remarkable decrease in TC of T2D cases between the magnesium and control groups (WMD: –0.42 mg/dL; 95% CI: –7.49, 6.66; I2 = 0%, Figure 3A).
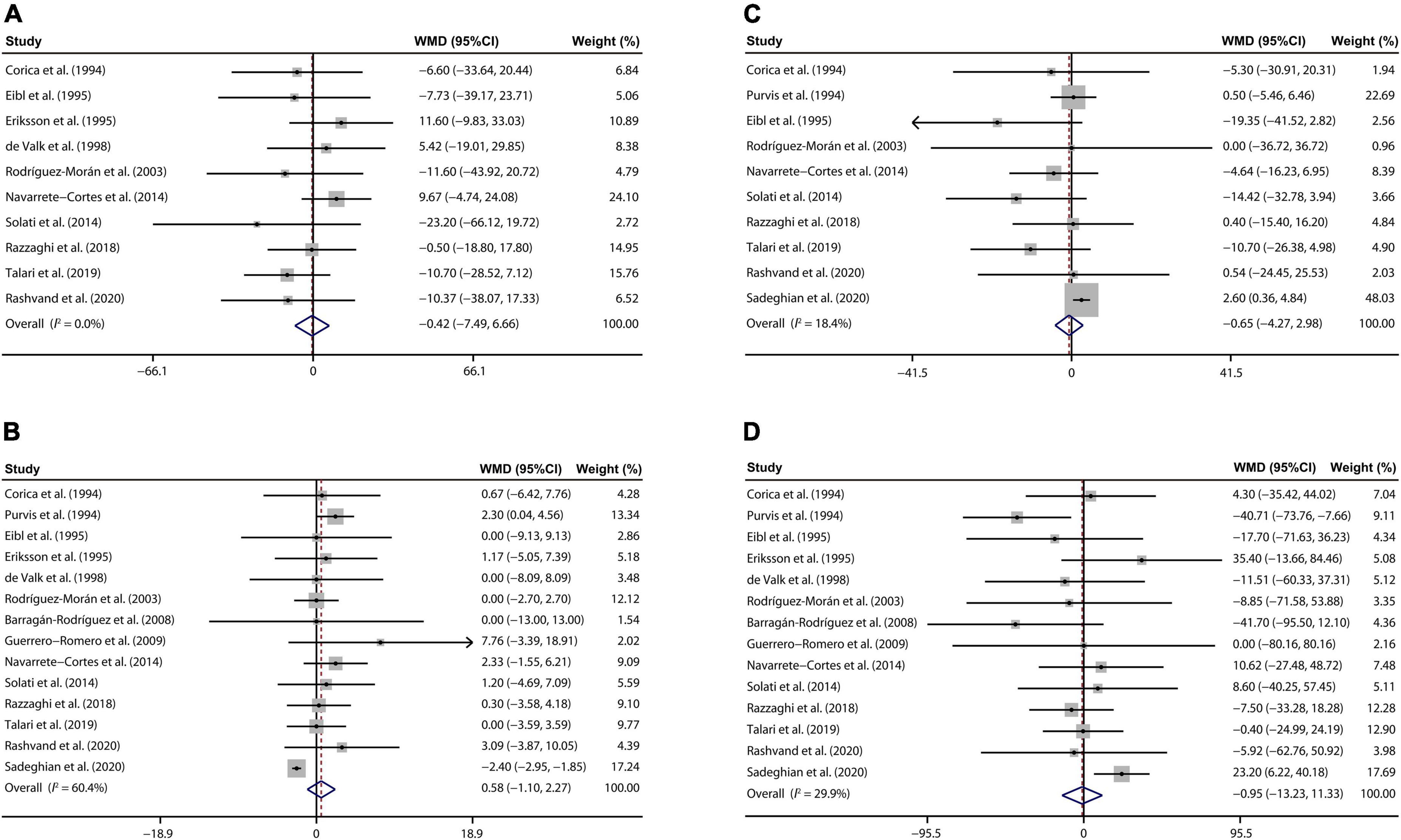
Figure 3. Forest plots for the effects of magnesium supplementation on TC (A), HDL-C (B), LDL-C (C), and TG (D) compared to controls in pooled analysis. For each study, the solid black circles represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the overall WMD determined with a random-effect model. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, total triglyceride; WMD, weighted mean difference.
Our meta-analysis of 14 RCTs (22, 25–30, 33, 34, 37, 39–42) demonstrated that magnesium treatment had no significant effects on serum HDL-C concentrations than those with placebo treatment (WMD: 0.58 mg/dL; 95% CI: –1.10, 2.27; I2 = 60.4%, Figure 3B).
There were 10 studies reporting the influence of magnesium addition on serum LDL-C levels (22, 25, 28, 33, 34, 37, 39–42). Further analysis indicated that no prominent changes were found in LDL-C concentrations of T2D patients after magnesium administration than the control group (WMD: –0.65 mg/dL; 95% CI: –4.27, 2.98; I2 = 18.4%, Figure 3C).
At last, 14 studies (22, 25–30, 33, 34, 37, 39–42) involving 776 T2D persons demonstrated that circulating TG concentrations were not disturbed by the magnesium intervention (WMD: –0.95 mg/dL; 95% CI: –13.23, 11.33; I2 = 29.9%, Figure 3D).
Effects of magnesium supplementation on blood pressure
Our analysis of 8 eligible publications (25–31, 34) demonstrated that magnesium treatment contributed to reducing the SBP, with a WMD value of –7.69 mmHg (95% CI: –11.71, –3.66; I2 = 36.7%, Figure 4A). On the other hand, a meta-analysis of 8 studies (25–31, 34) indicated that DBP of T2D patient was prominently decreased by the magnesium intervention (WMD: –2.71 mmHg; 95% CI: –4.02, –1.40; I2 = 0%, Figure 4B).
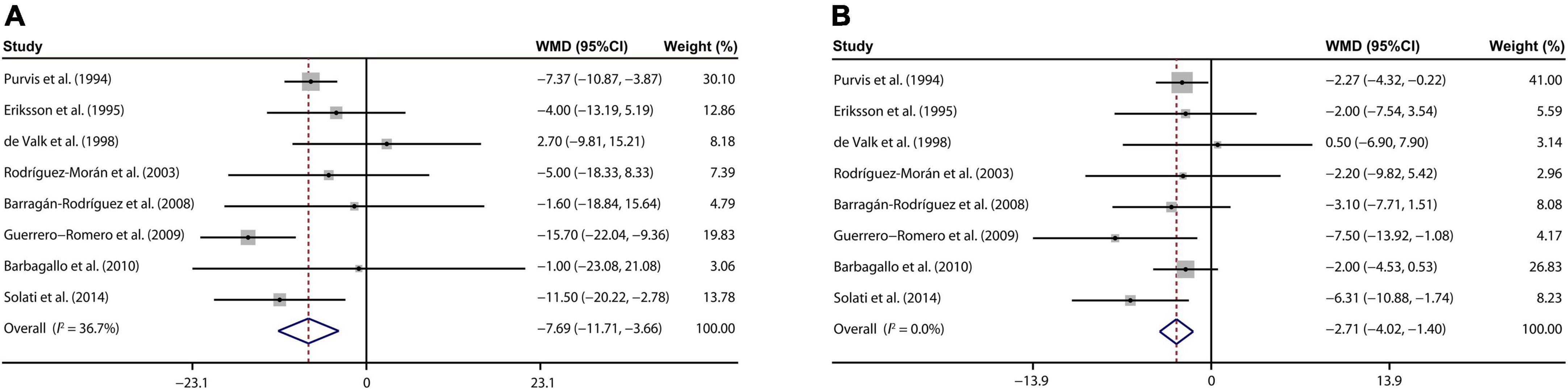
Figure 4. Forest plots for the effects of magnesium supplementation on SBP (A) and DBP (B) compared to controls in pooled analysis. For each study, the solid black circles represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the overall WMD determined with a random-effect model. DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Subgroup analyses of magnesium supplementation effects on glycemic control
Subgroup analyses about the use of magnesium for 4 glycemic indicators presented that magnesium treatment in patients with hypomagnesemia (plasma magnesemia ≤0.74 mM) (P = 0.020) or for a duration of ≥ 90 d (P = 0.013) exhibited a stronger effect on reducing FPG of T2D cases than respective other subgroups (Table 2).
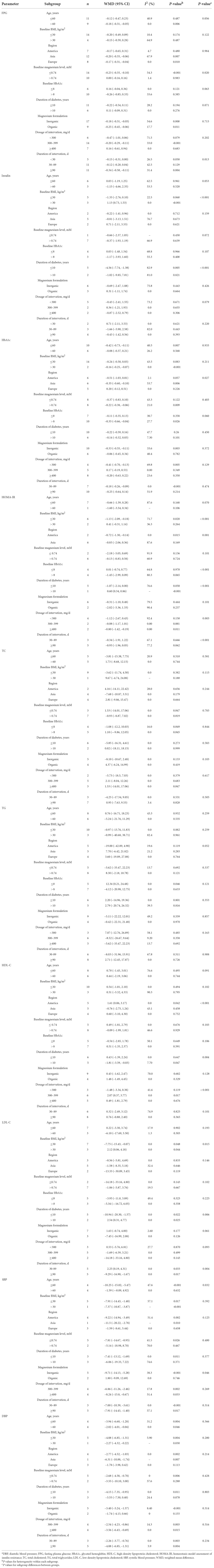
Table 2. Subgroup analyses for the effects of magnesium supplementation on glycemic, lipid and blood pressure parameters in type 2 diabetes mellitus patientsa.
As shown in Table 2, the influences of magnesium addition on insulin concentration were stronger among >30 than those BMI ≤ 30 kg/m2 (P < 0.001). In addition, T2D person whose duration of diabetes ≤10 years had a greater decline in circulating insulin concentration after magnesium supplementation than that had a longer course of disease (P < 0.001).
Compared with the American and European populations, our analysis revealed that magnesium administration exerted a significant effect on HbA1c in Asian T2D persons (P = 0.027, Table 2). However, the other factors were not significant determinants of between-study heterogeneity for HbA1c change during the magnesium treatment (P > 0.05).
Subgroup analyses revealed that magnesium addition at ≥ 400 mg/d dosage (P = 0.003) or for ≥90 d duration (P < 0.001) had a greater effect on HOMA-IR in T2D persons those are from America (P = 0.001), or with BMI ≤ 30 kg/m2 (P < 0.001), or with better baseline glycemic control (HbA1c > 8, P < 0.001) or with diabetes ≤ 10 years (P < 0.001) compared with the respective subgroups (Table 2).
Subgroup analyses of magnesium supplementation effects on lipid metabolism
Subgroup analyses based on the human health status and operational details of intervention revealed no significant differences in the influences of magnesium intervention on circulating TC and TG concentrations in T2D cases (P > 0.05, Table 2).
As shown in Table 2, our analysis revealed that magnesium application at a dosage of 300–399 mg/d (P < 0.001) exerted a more positive effect on increasing the serum HDL-C concentrations in T2D patients those were from America (P < 0.001), or with diabetes ≤10 years (P = 0.004). Subjects with BMI ≤ 30 kg/m2 (P = 0.015) or diagnosed as diabetes less than 10 years (P = 0.006) had lower plasma LDL-C concentrations after magnesium treatment. Furthermore, duration of administration is also a potential source of heterogeneity for the influences of magnesium on LDL-C variety (P = 0.004).
Subgroup analyses of magnesium supplementation effects on blood pressure
The effect of magnesium addition on SBP was greater for subject’s age ≤60 than those age > 60 years (P = 0.032). Besides, the effect of magnesium intervention on SBP was stronger for inorganic supplements than for organic supplements (P = 0.046). However, factors about the human baseline metabolic status and mode of intervention had no effects on the influence of magnesium on DBP (P > 0.05).
Dose-effect analyses
The dose-effect analyses showed that the optimal dosages of magnesium addition for FPG, insulin, HbA1c and HOMA-IR were 171, 218, 476 and 250 mg/d, respectively (Figures 5A–D). The optimal durations of magnesium administration for FPG, insulin, HbA1c and HOMA-IR were 124, 132, 111 and 95 d, respectively (Figures 5E–H).
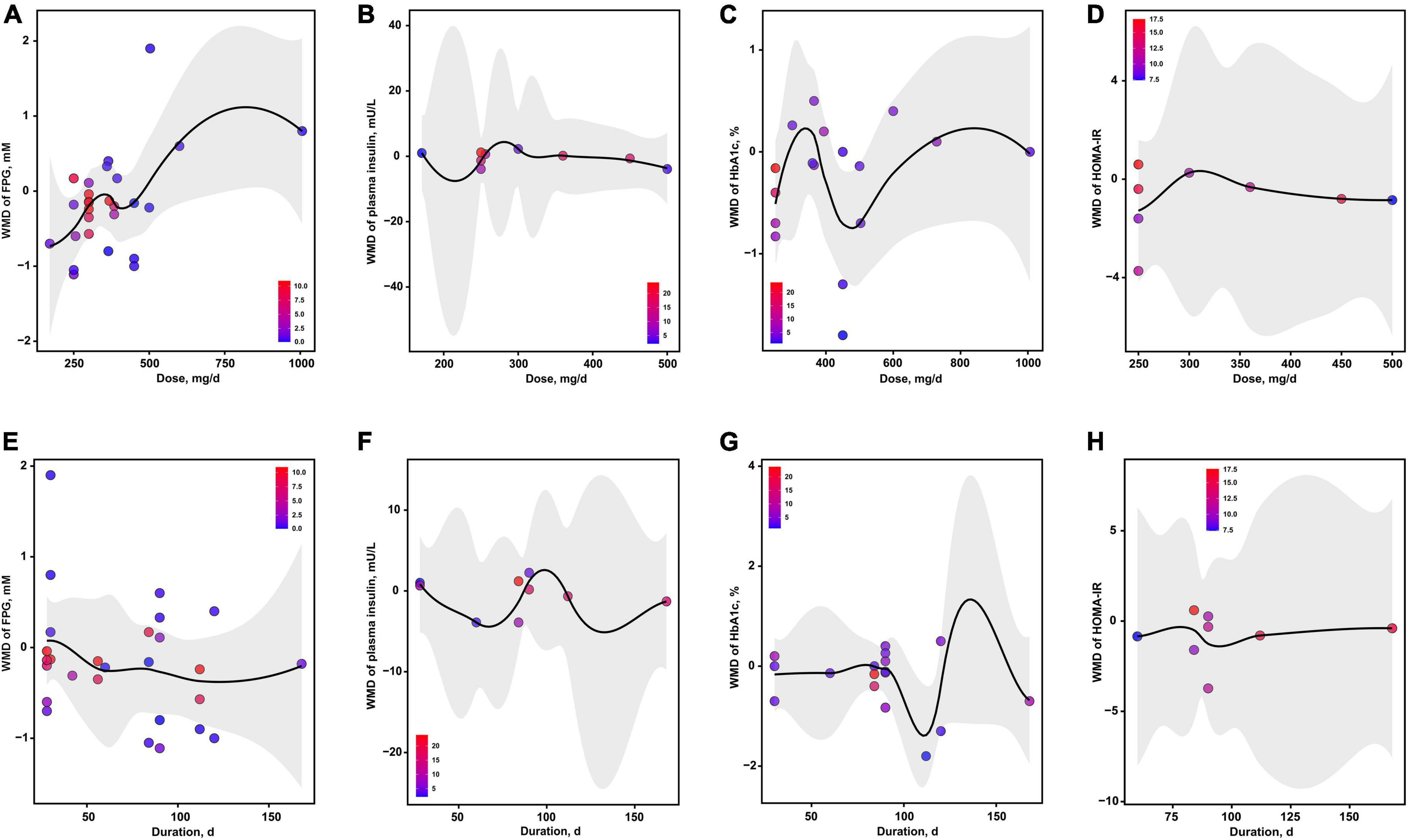
Figure 5. The effects of dosage or duration of magnesium intervention on glycemic control for FPG (A,E), insulin (B,F), HbA1c (C,G), and HOMA-IR (D,H), respectively. FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance.
The dose-effects of magnesium intervention on 4 serum lipids indicators were shown in Figure 6. The optimal dosages of magnesium supplementation that may mediate TC, TG, HDL-C and LDL-C were 300, 438, 250 and 729 mg/d, respectively (Figures 6A–D). In addition, the optimal durations of magnesium supplementation that may mediate TC, TG, HDL-C and LDL-C were 128, 46, 81 and 98 d, respectively (Figures 6E–H).
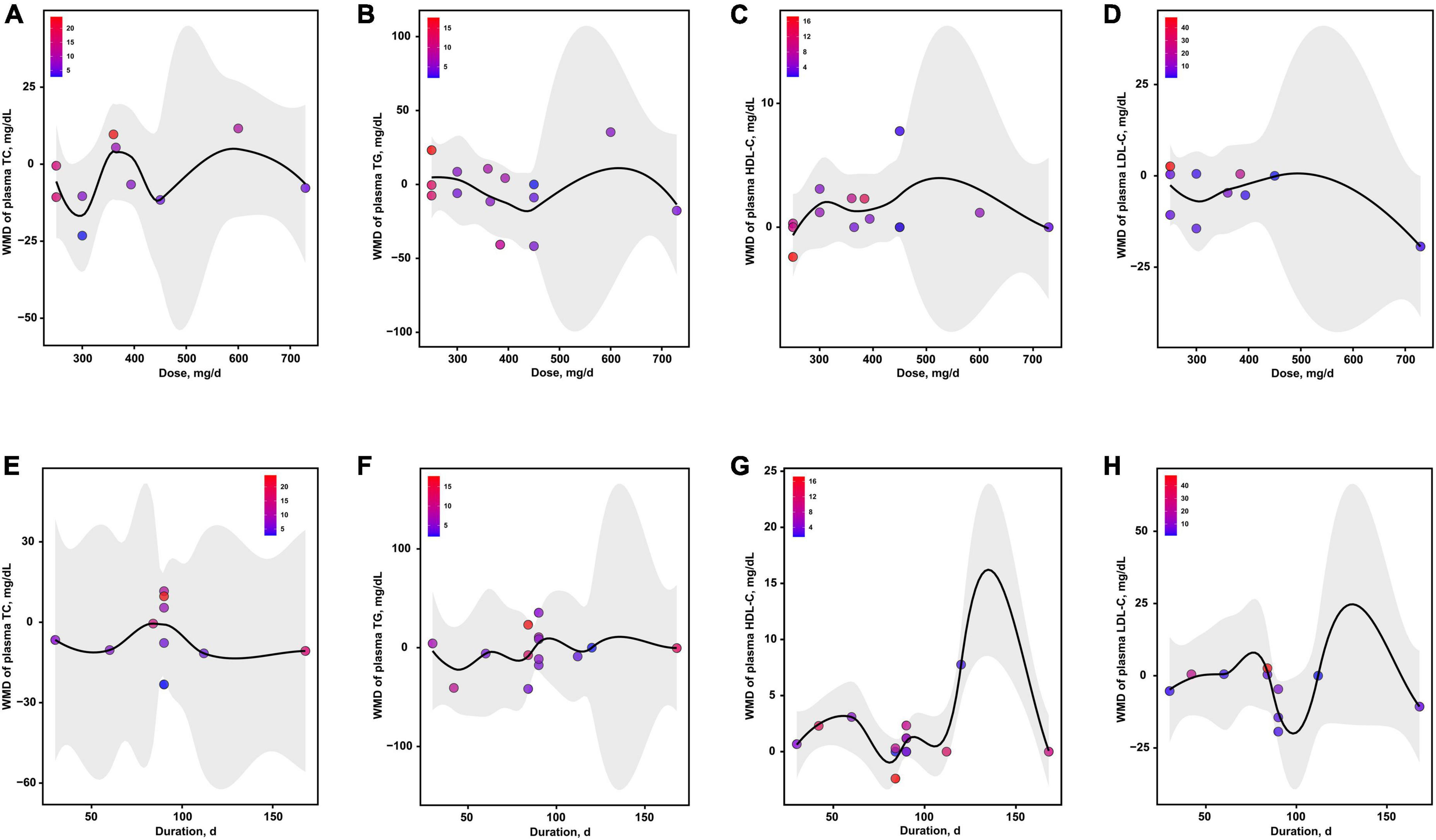
Figure 6. The effects of dosage or duration of magnesium intervention on lipid metabolism for TC (A,E), TG (B,F), HDL-C (C,G), and LDL-C (D,H), respectively. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, total triglyceride.
As presented in Figure 7, the optimal dosages of magnesium addition for SBP and DBP were both 300 mg/d, respectively (Figures 7A,B). The optimal durations of magnesium supplementation for SBP and DBP were both 120 d, respectively (Figures 7C,D).
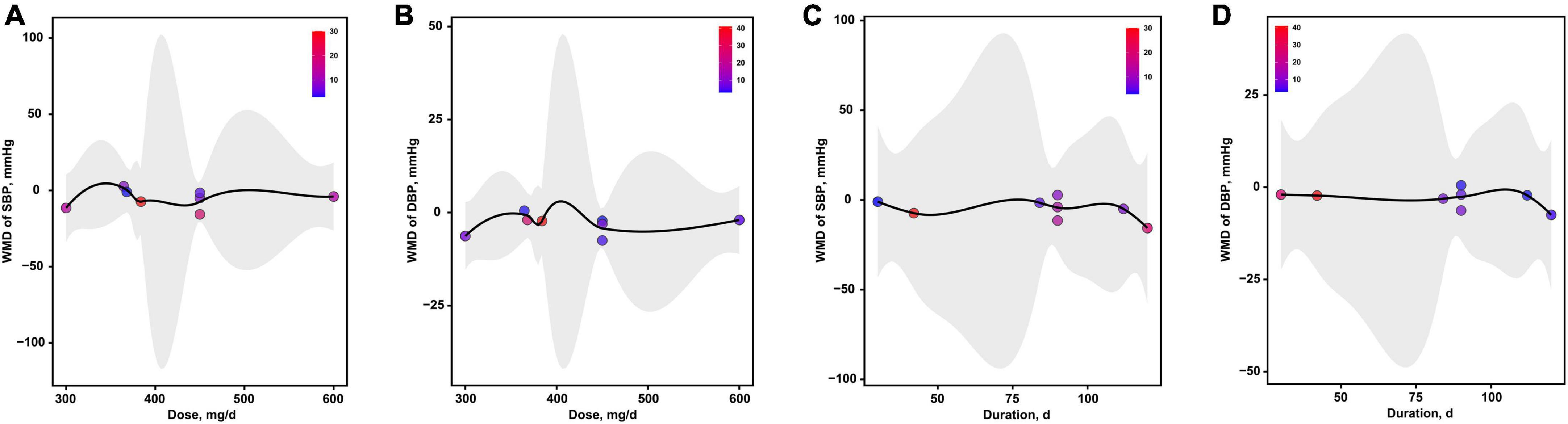
Figure 7. The effects of dosage or duration of magnesium intervention on blood pressure for SBP (A,C) and DBP (B,D), respectively. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Publication bias and sensitivity analysis
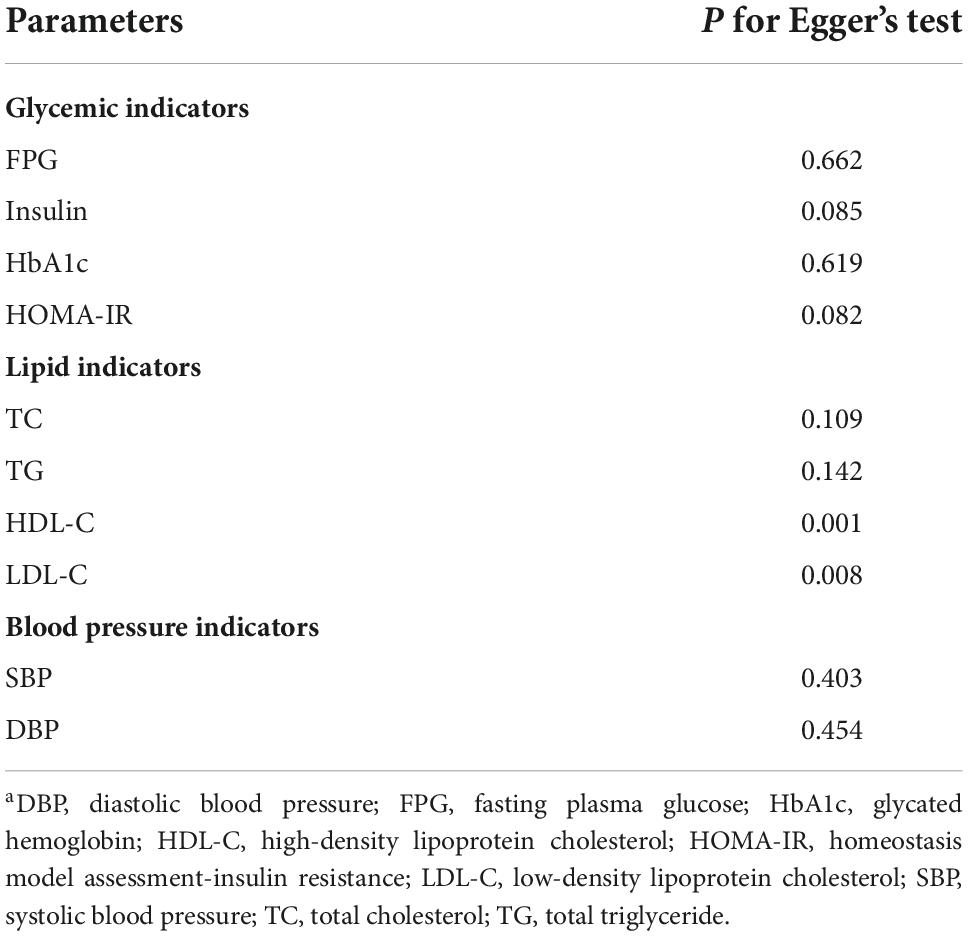
As shown in Table 3 and Supplementary Figures 1–3, no significant publication bias were found for the effects of intervention on FPG, insulin, HbA1c, HOMA-IR, SBP and DBP (all P > 0.05). For the effects of magnesium supplementation on improving the levels of serum lipids, significant publication bias were found for HDL-C and LDL-C (P = 0.001 and P = 0.008, respectively), but not for TC and TG (P = 0.109 and P = 0.142, respectively). Furthermore, sensitivity analyses showed that no single study had an effect on the pooled effect size (Supplementary Figures 4–6).
Discussion
We analyzed the data from 24 RCTs with 1,325 cases across 11 countries, which offered the most up-to-date evidence demonstrating the effects and operational details of oral magnesium on improving hyperglycemia, hypercholesterolemia, and hypertension in T2D patients.
The increased prevalence of hypomagnesaemia identified in diabetic cases informs the design and development of magnesium supplementation to ameliorate the status of T2D patients (43). Similar to previous review that magnesium supplementation for 1 to 4 months reduced FPG (14), and contrary to the meta-analysis done by Chua et al. (44) that routine magnesium intervention had no effects on HbA1c, our updated findings revealed that oral magnesium both significantly decreased the FPG and increased the HbA1c in T2D persons, highlighting the important role of magnesium in improving the short- and long-term glycemic control. Magnesium may improve glucose metabolism via several pathways. One possible explanation for this observation is that Mg2+ may adjust for the rate of glucokinase activity and then regulated the glucose utilization (45). In addition, binding of MgATP, an adenine nucleotide, to the sulfonylurea receptor 1 affects the opening of the ATP-sensitive K+ channel that controls the membrane depolarization and subsequent exocytosis of insulin-containing granules (45), which further mediated the circulating glucose concentration. At last, it is established that magnesium may play key roles on other parameters closely related to glucose metabolism, including body composition, general health, and sleep quality (46). Further subgroup analysis showed that baseline magnesium concentration is a main factor contributing to the heterogeneity on the effects of magnesium on FPG. Blaine et al. (47) pointed out that higher magnesium supply rapidly elevates its renal output, suggesting that basal magnesium status may be associated with the efficacy of magnesium administration. Previous work indicated that magnesium deficiency decreased insulin sensitivity throughout blocking insulin pathways to trigger the acute phaseresponse (48). Although our overall ungrouped analysis showed no significant effects of magnesium on HOMA-IR, subgroup analyses demonstrated that patient’s health status and mode of intervention are both the remarkable determinants of heterogeneity, suggesting that the clinical application of magnesium addition for increasing insulin sensitivity should be flexible specific to each person’s settings.
Subgroup analysis in current study showed that magnesium administration at 300-399 mg/d dosage led to an increase in plasma HDL-C concentrations in patients with T2D from America, which was supported by prevenient research that the beneficial effect of magnesium on dyslipidemia partly resulted from the generation of HDL by depressing HMG-CoA reductase and stimulation of lecithin cholesterol acyl transferase (49). On the other hand, a recent study showed that magnesium supplementation reduced lipid deposition in hepatocytes by activating autophagy by activating the AMPK-mTOR pathway, indicating a relationship between magnesium and lipid accumulation (50). Furthermore, Sales et al. (51) observed that the increased insulin sensitivity occurred after magnesium addition in our current study may also help to improve the dyslipidemia. The present results showed that magnesium supplementation also lessened the hyperglycemia of T2D patients whose baseline BMI ≤ 30 kg/m2 or durations of diabetes ≤10 years through decreasing the plasma LDL-C concentrations. Ample evidence indicated that dietary intake of divalent cations including magnesium promoted fecal excretion of fat (52), implying that the reduced serum LDL-C after magnesium intervention may be in part due to the inhibition of absorption. It is noteworthy that magnesium addition may also increase serum LDL-C concentrations in obese T2D cases with longer duration of diabetes, which implied that the clinical application of magnesium supplementation in relieving hyperglycemia should be performed in early stage of diabetes specific to each patient’s metabolic status.
Contrary to the study done by Song et al. (14) and in line with previous findings reported by Asbaghi et al. (53) and Zhang et al. (54), our pooled results with a greater number of RCTs proved that magnesium supplementation induced a profound decline in blood pressure of T2D patients. Wu et al. (55) further found that every 0.1 mmol/L increment in circulating serum magnesium level was associated with a 4% reduction in hypertension incidence. Accumulating evidence illustrated that magnesium released intracellular sodium and calcium stores through triggering membrane -Na+/K+ -ATPase and thereby decreases the blood hypertension through reduces the peripheral vascular resistance (56). Secondly, magnesium reduces hypertension may also attribute to its influences on the abundance of osteopontin, matrix Gla protein, and receptor potential melastin 7 (TRPM7), which were collectively observed to depress the vascular calcification (57). Thirdly, magnesium can also decrease vascular tone by releasing the nitric oxide (NO) from the coronary endothelium as well as resisting the influences of vasoconstrictor molecules such as calcium, bradykinin, or serotonin (58). Similar to previous research by Asbaghi et al. (53), our data exhibited that supplementation with inorganic magnesium had more positive effects on reducing blood pressure than the organic formulation. Magnesium is absorbed by both passive diffusion and active transport (59). Given that lower magnesium intakes may elevate the role of active process, it is possible that the amount of magnesium intake may regulate the magnesium absorption from compounds with different properties (60). However, the bioavailability of magnesium from inorganic and organic supplements, including absorption, retention or urinary excretion, needs to be further assessed.
With respect to the contradictory results from former trials, our updated meta-analysis increased the statistical power to systematically examine the effects of oral magnesium on indicators of glycemic control, lipid metabolism and blood pressure that related to complications in T2D individuals (61, 62). On this foundation, we followed subgroup and dose-effect analyses to explore the potential factors influencing the effects of magnesium administration, which provided a key reference for clinical application of this strategy in T2D patients. However, several limitations merit consideration. First, some of the included publications had small groups, such as <30 persons/group). Second, magnesium formulation, dosage and duration varied across different RCTs, which induced differential results and resulted in difficulties in assessing the real effect of magnesium supplementation. Third, some RCTs offered limited consideration to alimentary magnesium intake that may influence the effects of magnesium treatment. Nevertheless, the likelihood of this bias has been evaluated through the subgroup analysis based on the baseline circulating magnesium levels.
Conclusion
In summary, our findings indicated that magnesium supplementation had profitable effects on serum glucose, lipids, and pressure controls. Subgroup analyses revealed that magnesium administration in patients with hypomagnesemia or for a duration of ≥90 d exhibited a stronger effect on decreasing FPG, while intervention at ≥400 mg/d dosage or for ≥90 d duration had a greater effect on HOMA-IR in T2D persons with BMI ≤ 30 kg/m2 or baseline HbA1c > 8% or duration of diabetes ≤10 years. Subjects with BMI ≤ 30 kg/m2 or diagnosed as diabetes less than 10 years had lower plasma LDL-C concentrations, and American T2D patients received a dosage of 300–399 mg/d had greater HDL-C concentrations after oral magnesium supplementation. In addition, the inorganic magnesium supplements were more beneficial for lowering the SBP of younger T2D populations. At last, 279 mg/d for 116 d, 429 mg/d for 88 d and 300 mg/120 d are the average optimal dosage and duration for improving glycemic, circulating lipids, and blood pressure controls, respectively. Taken together, our study provide clinically relevant information on the adjuvant therapy of magnesium for T2D. In the future, guidelines for clinical practice of magnesium supplementation including dosage and duration according to each individual’s health status as well as the chronic safety of magnesium addition at high dosages should be further assessed in large multinational prospective RCTs, and the causal effects would be explored (3, 63–67).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MX conceived this study and supervised this research. LX and XL conceived the study, searched the literature, and performed data extraction. XL conducted the primary statistical analysis. XW provided statistical expertise. LX wrote the manuscript. LX, MX, and XW contributed by critically revising the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (32102553) and the National Key Basic Research Program of China (2020YFC2008002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1020327/full#supplementary-material
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Nie Q, Hu J, Gao H, Fan L, Chen H, Nie S. Polysaccharide from Plantago asiatica L attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocolloid. (2017) 86:34–42. doi: 10.1016/j.foodhyd.2017.12.026
3. Wang X, Fang X, Zheng W, Zhou J, Song Z, Xu M, et al. Genetic support of a causal relationship between iron status and type 2 diabetes: a Mendelian randomization study. J Clin Endocrinol Metab. (2021) 106:e4641–51. doi: 10.1210/clinem/dgab454
4. Xu MQ, Ye Z, Hu FB, He L. Quantitative assessment of the effect of angiotensinogen gene polymorphisms on the risk of coronary heart disease. Circulation. (2007) 116:1356–66. doi: 10.1161/CIRCULATIONAHA.107.728857
5. Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: an update of meta-analysis. Crit Rev Food Sci Nutr. (2021) 61:1670–88. doi: 10.1080/10408398.2020.1764488
6. Paolisso G, Scheen A, D’Onofrio F, Lefèbvre P. Magnesium and glucose homeostasis. Diabetologia. (1990) 33:511–4. doi: 10.1007/BF00404136
7. de Lordes Lima M, Cruz T, Pousada JC, Rodrigues LE, Barbosa K, Canguçu V. The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care. (1998) 21:682–6. doi: 10.2337/diacare.21.5.682
8. Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. (2003) 24:39–52. doi: 10.1016/S0098-2997(02)00090-0
9. Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care. (2004) 27:59–65. doi: 10.2337/diacare.27.1.59
10. Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. (2005) 28:1438–44. doi: 10.2337/diacare.28.6.1438
11. Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. (2003) 24:107–36. doi: 10.1016/S0098-2997(02)00094-8
12. Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. (2002) 238:163–79. doi: 10.1023/A:1019998702946
13. Seelig MS, Heggtveit HA. Magnesium interrelationships in ischemic heart disease: a review. Am J Clin Nutr. (1974) 27:59–79. doi: 10.1093/ajcn/27.1.59
14. Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. (2006) 23:1050–6. doi: 10.1111/j.1464-5491.2006.01852.x
15. Simental-Mendía LE, Sahebkar A, Rodríguez-Morán M, Guerrero-Romero F. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res. (2016) 111:272–82. doi: 10.1016/j.phrs.2016.06.019
16. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
18. Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. (2012) 31:3805–20. doi: 10.1002/sim.5453
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
20. Paolisso G, Sgambato S, Pizza G, Passariello N, Varricchio M, D’Onofrio F. Improved insulin response and action by chronic magnesium administration in aged NIDDM subjects. Diabetes Care. (1989) 12:265–9. doi: 10.2337/diacare.12.4.265
21. Paolisso G, Passariello N, Pizza G, Marrazzo G, Giunta R, Sgambato S, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh). (1989) 121:16–20. doi: 10.1530/acta.0.1210016
22. Corica F, Allegra A, Di Benedetto A, Giacobbe MS, Romano G, Cucinotta D, et al. Effects of oral magnesium supplementation on plasma lipid concentrations in patients with non-insulin-dependent diabetes mellitus. Magnes Res. (1994) 7:43–7.
23. Gullestad L, Jacobsen T, Dolva LO. Effect of magnesium treatment on glycemic control and metabolic parameters in NIDDM patients. Diabetes Care. (1994) 17:460–1. doi: 10.2337/diacare.17.5.460
24. Paolisso G, Scheen A, Cozzolino D, Di Maro G, Varricchio M, D’Onofrio F, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab. (1994) 78:1510–4. doi: 10.1210/jcem.78.6.8200955
25. Purvis JR, Cummings DM, Landsman P, Carroll R, Barakat H, Bray J, et al. Effect of oral magnesium supplementation on selected cardiovascular risk factors in non-insulin-dependent diabetics. Arch Fam Med. (1994) 3:503–8. doi: 10.1001/archfami.3.6.503
26. Eriksson J, Kohvakka A. Magnesium and ascorbic acid supplementation in diabetes mellitus. Ann Nutr Metab. (1995) 39:217–23. doi: 10.1159/000177865
27. de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring type 2 diabetic patients. Diabet Med. (1998) 15:503–7. doi: 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M
28. Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care. (2003) 26:1147–52. doi: 10.2337/diacare.26.4.1147
29. Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res. (2008) 21:218–23.
30. Guerrero-Romero F, Rodríguez-Morán M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind, placebo-controlled clinical trial. J Hum Hypertens. (2009) 23:245–51. doi: 10.1038/jhh.2008.129
31. Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. (2010) 23:131–7.
32. Bhardwaj A, Maheswar K, Singh J, Kumar V. A study of magnesium supplementation on fasting blood glucose in patients of diabetes mellitus type-2. IJPRBS. (2013) 2:111–3.
33. Navarrete-Cortes A, Ble-Castillo JL, Guerrero-Romero F, Cordova-Uscanga R, Juárez-Rojop IE, Aguilar-Mariscal H, et al. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes Res. (2014) 27:48–56. doi: 10.1684/mrh.2014.0361
34. Solati M, Ouspid E, Hosseini S, Soltani N, Keshavarz M, Dehghani M. Oral magnesium supplementation in type II diabetic patients. Med J Islam Repub Iran. (2014) 28:67.
35. Singh YR, Verma S, Agrawal D, Singh B, Bhardwaj A, Agrawal GA. A study of magnesium supplementation on glycemic control in patients of type-2 diabetes mellitus. Indian J Clin Anat Physiol. (2015) 2:26–30.
36. ELDerawi WA, Naser IA, Taleb MH, Abutair AS. The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients. (2018) 11:44. doi: 10.3390/nu11010044
37. Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. (2018) 181:207–15. doi: 10.1007/s12011-017-1056-5
38. Rashvand S, Mobasseri M, Tarighat-Esfanjani A. The effects of choline and magnesium co-supplementation on metabolic parameters, inflammation, and endothelial dysfunction in patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. (2019) 38:714–21. doi: 10.1080/07315724.2019.1599745
39. Talari HR, Zakizade M, Soleimani A, Bahmani F, Ghaderi A, Mirhosseini N, et al. Effects of magnesium supplementation on carotid intima-media thickness and metabolic profiles in diabetic haemodialysis patients: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2019) 121:809–17. doi: 10.1017/S0007114519000163
40. Sadeghian M, Azadbakht L, Khalili N, Mortazavi M, Esmaillzadeh A. Oral magnesium supplementation improved lipid profile but increased insulin resistance in patients with diabetic nephropathy: a double-blind randomized controlled clinical trial. Biol Trace Elem Res. (2020) 193:23–35. doi: 10.1007/s12011-019-01687-6
41. Eibl NL, Kopp HP, Nowak HR, Schnack CJ, Hopmeier PG, Schernthaner G. Hypomagnesemia in type II diabetes: effect of a 3-month replacement therapy. Diabetes Care. (1995) 18:188–92. doi: 10.2337/diacare.18.2.188
42. Rashvand S, Mobasseri M, Tarighat-Esfanjani A. Effects of choline and magnesium concurrent supplementation on coagulation and lipid profile in patients with type 2 diabetes mellitus: a pilot clinical trial. Biol Trace Elem Res. (2020) 194:328–35. doi: 10.1007/s12011-019-01802-7
43. Barbagallo M, Dominguez LJ. Magnesium and type 2 diabetes. World J Diabetes. (2015) 6:1152–7. doi: 10.4239/wjd.v6.i10.1152
44. Chua FB, Cinco JE, Paz-Pacheco E. Efficacy of magnesium supplementation on glycemic control in type 2 diabetes patients: a meta-analysis. J ASEAN Fed Endocr Soc. (2017) 32:38–45. doi: 10.15605/jafes.032.01.07
45. Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. (2016) 65:3–13. doi: 10.2337/db15-1028
46. Veronese N, Dominguez LJ, Pizzol D, Demurtas J, Smith L, Barbagallo M. Oral magnesium supplementation for treating glucose metabolism parameters in people with or at risk of diabetes: a systematic review and meta-analysis of double-blind randomized controlled trials. Nutrients. (2021) 13:4074. doi: 10.3390/nu13114074
47. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. (2015) 10:1257–72. doi: 10.2215/CJN.09750913
48. Guerrero-Romero F, Rodríguez-Morán M. The Role of Magnesium in the Pathogenesis of Insulin Resistance. Insulin Resistance: Symptoms, Causes and Treatment. New York, NY: Nova Science Publishers Inc (2010). p. 167–82.
49. Verma H, Garg R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: a systematic review and meta-analysis. J Hum Nutr Diet. (2017) 30:621–33. doi: 10.1111/jhn.12454
50. Chen S, Luo S, Zou B, Xie J, Li J, Zeng Y. Magnesium supplementation stimulates autophagy to reduce lipid accumulation in hepatocytes via the AMPK/mTOR pathway. Biol Trace Elem Res. (2022). doi: 10.1007/s12011-022-03438-6
51. Sales CH, Santos AR, Cintra DE, Colli C. Magnesium-deficient high-fat diet: effects on adiposity, lipid profile and insulin sensitivity in growing rats. Clin Nutr. (2014) 33:879–88. doi: 10.1016/j.clnu.2013.10.004
52. Gacs G, Barltrop D. Significance of CA-soap formation for calcium absorption in the rat. Gut. (1977) 18:64–8. doi: 10.1136/gut.18.1.64
53. Asbaghi O, Hosseini R, Boozari B, Ghaedi E, Kashkooli S, Moradi S. The effects of magnesium supplementation on blood pressure and obesity measure among type 2 diabetes patient: a systematic review and meta-analysis of randomized controlled trials. Biol Trace Elem Res. (2021) 199:413–24. doi: 10.1007/s12011-020-02157-0
54. Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. (2016) 68:324–33. doi: 10.1161/HYPERTENSIONAHA.116.07664
55. Wu J, Xun P, Tang Q, Cai W, He K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Nutr J. (2017) 16:60. doi: 10.1186/s12937-017-0280-3
56. Fischer PW, Giroux A. Effects of dietary magnesium on sodium-potassium pump action in the heart of rats. J Nutr. (1987) 117:2091–5. doi: 10.1093/jn/117.12.2091
57. Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. (2010) 56:453–62. doi: 10.1161/HYPERTENSIONAHA.110.152058
58. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95:1–46. doi: 10.1152/physrev.00012.2014
59. de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. (2012) 5:i15–24. doi: 10.1093/ndtplus/sfr164
60. Bertinato J, Plouffe LJ, Lavergne C, Ly C. Bioavailability of magnesium from inorganic and organic compounds is similar in rats fed a high phytic acid diet. Magnes Res. (2014) 27:175–85. doi: 10.1684/mrh.2014.0374
61. Jiang L, Wang K, Lo K, Zhong Y, Yang A, Fang X, et al. Sex-specific association of circulating ferritin level and risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab. (2019) 104:4539–51. doi: 10.1210/jc.2019-00495
62. Xu M, Lin Z. Genetic influences of dopamine transport gene on alcohol dependence: a pooled analysis of 13 studies with 2483 cases and 1753 controls. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1255–60. doi: 10.1016/j.pnpbp.2010.11.001
63. Hou L, Xu M, Yu Y, Sun X, Liu X, Liu L, et al. Exploring the causal pathway from ischemic stroke to atrial fibrillation: a network Mendelian randomization study. Mol Med. (2020) 26:7. doi: 10.1186/s10020-019-0133-y
64. Zhang F, Baranova A, Zhou C, Cao H, Chen J, Zhang X, et al. Causal influences of neuroticism on mental health and cardiovascular disease. Hum Genet. (2021) 140:1267–81. doi: 10.1007/s00439-021-02288-x
65. Zhang F, Rao S, Cao H, Zhang X, Wang Q, Xu Y, et al. Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J Clin Invest. (2022) 132:e145942. doi: 10.1172/JCI145942
66. Li H, Wang X, Lu X, Zhu H, Li S, Duan S, et al. Co-expression network analysis identified hub genes critical to triglyceride and free fatty acid metabolism as key regulators of age-related vascular dysfunction in mice. Aging (Albany NY). (2019) 11:7620–38. doi: 10.18632/aging.102275
Keywords: blood pressure, glycemic control, serum lipids, magnesium supplementation, optimal details, type 2 diabetes
Citation: Xu L, Li X, Wang X and Xu M (2023) Effects of magnesium supplementation on improving hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes: A pooled analysis of 24 randomized controlled trials. Front. Nutr. 9:1020327. doi: 10.3389/fnut.2022.1020327
Received: 16 August 2022; Accepted: 09 November 2022;
Published: 18 January 2023.
Edited by:
Kaikai Li, Huazhong Agricultural University, ChinaReviewed by:
Dario Rahelic, Merkur University Hospital, CroatiaJinming Peng, Zhongkai University of Agriculture and Engineering, China
Copyright © 2023 Xu, Li, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingqing Xu, bWluZ3Fpbmd4dUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Lianbin Xu
Lianbin Xu Xiuli Li
Xiuli Li Xinhui Wang
Xinhui Wang Mingqing Xu4,5*
Mingqing Xu4,5*