Abstract
Coronavirus disease 2019 (COVID-19) disrupts the intestinal micro-ecological balance, and patients often develop the intestinal disease. The gut is the largest immune organ in the human body; intestinal microbes can affect the immune function of the lungs through the gut-lung axis. It has been reported that tea polyphenols (TPs) have antiviral and prebiotic activity. In this review, we discussed TPs reduced lung-related diseases through gut-lung axis by inhibiting dysbiosis. In addition, we also highlighted the preventive and therapeutic effects of TPs on COVID-19 complications, further demonstrating the importance of research on TPs for the prevention and treatment of COVID-19 in humans. Based on this understanding, we recommend using TPs to regulate the gut microbiota to prevent or alleviate COVID-19 through the gut-lung axis.
Introduction
Human pathogenic coronavirus, including severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2, it binds to the angiotensinogen-converting enzyme 2 (ACE2), a recently discovered mono-carboxypeptidase and the first ACE homolog, and then enters the cell (1). SARS-CoV S1 contains a receptor-binding domain (RBD) that explicitly recognizes ACE2 as its receptor (2), and tea polyphenols (TPs) have been found to bind to RBD to inhibit virus invasion (3). Numerous studies have demonstrated TPs to prevent obesity, diabetes, cardiovascular disease, cancer, and antiviral activity and fight diseases caused by oxidative stress and inflammation (4, 5). For instance, when the balance between the accumulation of reactive oxygen species (ROS) and the body's antioxidant process is disturbed, oxidative stress can be induced, causing damage to cells and tissues, thus leading to various diseases (6). However, when TPs enter the body, the activity of antioxidant enzymes increases, the inhibition of lipid peroxidation, and the production of ROS in the body can be promoted to achieve the antioxidant effect (7). These effects are also likely to help alleviate a range of complications caused by the new coronavirus.
Tea is the most popular beverage besides water and the most widely used (8). In China, tea consumption has been more than 5,000 years. TPs are a mixture of phenolic compounds extracted from tea leaves. In terms of concentration, tea catechins are one of the most important bioactive substances in tea leaves, accounting for 60–80% of total polyphenols. Catechins are the main polyphenol compounds in tea, including epigallocatechin-3-gallate (EGCG), epigallocatechin-3-gallate, epicatechin-3-gallate and epicatechin, the content and activity of EGCG was the highest (9).
It is known that the dysbiosis of the human gut microbiota is associated with various health conditions, including respiratory tract infections (RTI) via the gut-lung axis. The gut microbiota is involved in various physiological responses, including nutrient absorption, energy regulation, glucose metabolism, and immune system regulation (10). Perhaps only 1.9% of the gut microbiome is heritable, while more than 20% of the biodiversity of the microbiome is derived from the environment (including diet). TPs can effectively modulate gut microbiota composition, thereby effectively improving gut microbiome and host health. For many, COVID-19 brings few symptoms, but others are life-threatening due to SARS-CoV-2. While certain gut microbes have been linked to adverse outcomes from viral infections, some researchers suggest using these bacteria as biomarkers. If gut health affects the prognosis of COVID-19, we should use it to better control and prevent COVID-19. TPs have been used in research in the fields of immunity, psychiatric diseases, cardiovascular and metabolic diseases, and have achieved certain achievements. It can be seen that it is reasonable to use tea polyphenols to regulate intestinal microecology and prevent and intervene in COVID-19 (11). Therefore, improving the nutritional status of patients and enhancing the body's immunity by regulating the microbiota is of great significance for the treatment of novel coronavirus pneumonia. In this review, we summarized the possible use of TPs to prevent viral infections. In addition, the mechanism of action of TPs against COVID-19 was discussed from the perspective of the gut-lung axis.
The Important Role of Gut Microbes in Covid-19
Patients with COVID-19 show signs of intestinal flora imbalance, which can cause intestinal damage and damage to the lung and vital organ systems in the event of a pathogenic SARS-CoV-2 infection. Therefore, it is essential to maintain a healthy gut microbiome to optimize the immune system in order to prevent excessive inflammation (12).
Intestinal Flora and the Gut-Lung Axis
Intestinal microorganisms can interact with the immune system, and the immune cells generated by the immune function between the intestine and the lungs move through the lymphatic system and blood circulation. The interaction network between the intestine and lung tissue mediated by microorganisms and immune cells is called the “gut-lung axis” (13). The imbalance of intestinal flora interacts with lung diseases and respiratory infections. When a deadly influenza virus invades, intestinal flora such as endogenous Bifidobacteria will increase, enhancing the host's resistance to influenza (14). The main bacterial phyla of the lungs are the same as the intestines, mainly Firmicutes and Bacteroides, followed by Proteobacteria and Actinomycetes, the interaction between the lung microbiota and immunity is also a two-way process (15). “Gut-lung axis” refers to the intestinal flora that can affect and regulate the immunity and function of the lungs, and may be related to acute and chronic lung diseases (16). And patients with chronic gastrointestinal inflammation and other diseases have a higher prevalence of lung diseases. Respiratory influenza infection can cause intestinal injury when lung injury occurs, and influenza infection changes the composition of intestinal microflora (17).
Patients with respiratory infections usually have intestinal dysfunction, and some COVID-19 patients experience gastrointestinal (GI) symptoms, including diarrhea and vomiting (18). The proportion of 651 COVID-19 patients with gastrointestinal symptoms was 11.4%; trends in fever and severity (severe/critical, mechanical ventilation, and ICU admission rates) were significantly higher in COVID-19 patients with gastrointestinal symptoms (19). Experiments in mice have shown that depletion or loss of the intestinal microbiota can lead to impaired immune response and worsen the prognosis of bacterial or viral respiratory infections (20). The gut-lung axis results from complex interactions between microbial components in the gut and lung flora and local and long-term immunity. Mice infected with the H1N1 flu in the nose developed lung infections, a marked change in the composition of the intestinal flora, and an increase in Bacteroides (21). Using mouse models of respiratory tract influenza infection found that respiratory tract influenza infection can cause intestinal damage and change the composition of the intestinal microbiome with the increase of Enterobacteriaceae bacteria and the decrease of Lactobacillus and Lactococcus (22). In a meta-analysis, the gut microbiota of 30 COVID-19 subjects, 24 H1N1 patients, and 30 healthy controls were evaluated. It was found that the intestinal bacterial diversity of subjects infected with SARS-CoV-2 was significantly reduced, and the relative abundance of beneficial microorganisms, such as Bifidobacterium was also reduced (23). Therefore, it is speculated that SARS-CoV-2 may indirectly affect the intestinal flora related to the intestine-pulmonary axis and damage human immunity, and it can prevent and treat lung infections caused by SARS-CoV-2 by regulating the relevant intestinal flora.
Changes in the Intestinal Flora of COVID-19 Patients
The intestinal microflora is closely associated with respiratory viral infections and causes various infections through the gut-lung axis (24). In addition, influenza infection will affect the composition of the intestinal flora, and the disorder of the intestinal flora will reduce the host's antiviral immune response, thereby aggravating the lung damage caused by these infections (25). Among them, changes in the intestinal environment and immune factors caused by actinomycetes may aggravate the damage caused by inflammatory bowel disease. Compared with healthy individuals, the fecal microbiome of COVID-19 patients has significantly changed. The baseline abundance of Coprobacillus, Clostridium ramosum, and Clostridium hatheway correlates with the severity of COVID-19; the abundance of Faecalibacterium prausnitzii (an anti-inflammatory bacteria) the degree is negatively correlated with the severity of the disease (26). Sequenced 274 feces samples (including feces from 100 COVID-19 patients) and found that members of the Bacteroidetes phylum in patients with COVID-19 were relatively abundant, and the compositional differences in the gut microbiota of COVID-19 were mainly caused by the enrichment of Ruminococcus gnavus, Ruminococcus torques and Bacteroides dorei, and the depletion of Bifidobacterium adolescentis, Faecalibacterium prausnitzii and Eubacterium rectale (27). In conclusion, the gut microbiota of SARS-CoV-2-infected patients is altered by a decrease in commensal microorganisms, a loss of bacterial diversity, and an increase in opportunistic pathogens.
Patients with metabolic and GI are considered to be at moderate to high risk of SARS-CoV-2 infection, suggesting that gut dysbiosis directly affects the severity of COVID-19 (28). RNA metagenomics sequencing was performed on the continuous fecal virus extracts of 15 COVID-19 hospitalized patients. Feces with high SARS-CoV-2 infectivity have higher microbiome functions, and demonstrated the increased relative abundance of Collinsella aerofaciens, C. tanakaei, Morganella morganii, and Streptococcus infants (29). Based on COVID-19 patient data, a blood proteomics risk score was constructed, and it was found that gut microbial characteristics can highly predict the susceptibility and severity of COVID-19 (30). Therefore, intestinal microbial characteristics and related metabolites can be used as potential prevention/treatment targets for intervention, especially for those who are susceptible to SARS-CoV-2 infection.
Gut Flora Regulates Immunity Through the Gut-Lung Axis
The gastrointestinal tract hosts a complex and highly diverse microbial ecosystem that interacts with the host to ensure the establishment and persistence of immune homeostasis (31). These complex microbial communities provide important genomic and enzymatic capabilities and play critical roles in the immune system's induction, development, and function, protection from pathogens and sustained tolerance to innocuous antigens, and protection of the ecology of the microbiota (32). The gut microbiome is the protective agent during pneumococcal pneumonia, and the gut microbiome enhances primary alveolar macrophage function (20). In an acute lung infection model, oral administration of segmented filamentous bacteria stimulates pulmonary T helper cell responses and reduces S. pneumoniae infection and mortality (33). Studies have shown that patients with COVID-19 have lower levels of probiotics (such as Lactobacillus and Bifidobacterium) (34). Because of the critical role of the intestinal flora and its metabolites in regulating the host's immune and inflammatory response, the regulation of the intestinal flora can be used to prevent and treat COVID-19 and related diseases (such as viral and/or bacterial pneumonia, acute respiratory infections, or flu) has attracted considerable attention.
The interaction between the gastrointestinal tract and the respiratory tract is achieved through a common mucosal immunity. There is persistent crosstalk between the intestine and the pulmonary mucosa through the mesenteric lymphatic system and the pulmonary lymph nodes (35). Short chain fatty acids (SCFAs) induce the expression of dendritic cell and macrophage pattern recognition receptors and regulate cytokine secretion and antibody synthesis (sIgA and IgM) (36). Dysbiosis in the lung affects the immune system and decreased immune cell recruitment leads to increased viral load in the lungs and reduced IFN-α and -β production, which negatively affects T cell priming (37). Germ-free mice display defects in several specific immune cell populations, such as impaired innate lymphocyte function, lack of IgA-producing plasma cells, and, more generally, increased susceptibility to infection (38). Using each of 53 separate bacterial species to single-colonize mice, it was found that the diversity of microbes in the gut ensures the ability of the microbiota to produce consistent immune regulation (39). When circumventing responses to pathogenic infections such as coronaviruses, a healthy gut microbiome may be vital to maintain an optimal immune system, preventing a cascade of excessive immune responses that ultimately damage the lungs and vital organ systems (Figure 1).
Figure 1
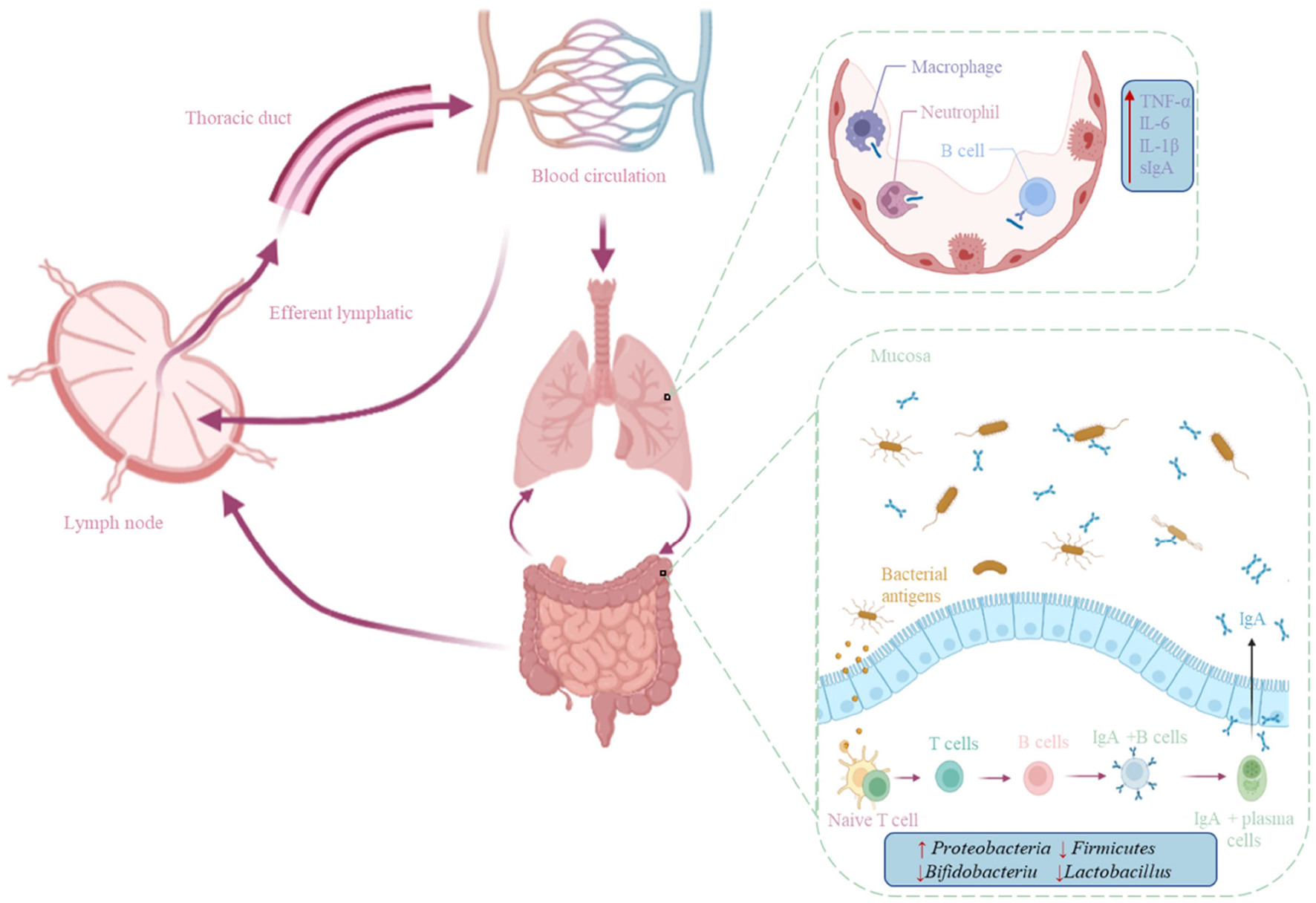
Mechanisms of the gut-lung axis in the immune system. The previous crosstalk between the gut and lung mucosa is through the mesenteric lymphatic system and the lymph nodes in the lungs, and immune cells generated by the immune function between the gut and the lungs move through the lymphatic system and blood circulation.
Potential Applications of TPS to Alleviate Covid-19
The gut-lung axis plays an important role in SARS-CoV-2 infection, so targeting the gut-lung axis to treat COVI-19 is particularly important. TPs are considered to be multifunctional bioactive molecules, which have antiviral effects in addition to antibacterial and intestinal flora regulation to enhance immune function (40). Therefore, TPs are considered to have potential preventive and therapeutic effects on COVID-19.
Antibacterial Effect of TPs
In addition to various pharmacological effects such as antioxidant, lowering blood sugar, and immune regulation, TPs also have potent antibacterial effects, especially for Gram-positive and Gram-negative bacteria (41). Although the current research results on the antibacterial mechanism of TPs are not very clear, researchers generally believe that the mechanism involves many aspects, such as destroying the cell wall membrane structure, interfering with cell growth and division, and inducing oxidative stress (42). Catechins can inhibit bacterial toxins directly by binding to bacterial toxins or indirectly by preventing bacterial toxin secretion or promoting bacterial protease breakdown (43).
The influence of the catechin structure in TPs on the antibacterial effect mainly includes: (1) The complexation of the ortho-phenolic hydroxyl group with the metal ion. The metal ions in bacteria are partly prosthetic groups of enzymes and partly essential bacteria elements. Multiple ortho-phenolic hydroxyl groups of TPs molecules can be used as multi-base ligands to undergo complex reactions with iron, calcium, and other ions to produce precipitation. Induce oxidative stress while depriving bacteria of essential nutrients, thereby affecting bacterial activity, growth, and reproduction (44, 45); (2) The phenolic hydroxyl group and benzene ring structure are combined with proteins. The phenolic hydroxyl group and benzene ring structure of TPs can be combined with bacterial proteins through hydrogen bonds or hydrophobicity, affecting the physiological functions of proteins, thereby inhibiting bacterial infection and metabolic activity (46, 47); (3) The effect of polymerization degree on the bacteriostatic effect of catechins. For example, compared with catechin monomers, its oligomers have higher antibacterial properties, which may be attributed to the polymers having more phenolic hydroxyl groups and benzene ring structures and having a more substantial binding ability with proteins (48). However, compared with catechin oligomers, catechin polymers and polymers have weaker bacteriostatic effects, which may be because the molecular weight of catechin polymers increases with the increase of the degree of polymerization, making it challenging to penetrate bacterial cell membranes (49). In addition, affected by the steric hindrance effect of macromolecules, the activity of the phenolic hydroxyl group is weakened, resulting in a decrease in the antibacterial ability.
Regulation of Gut Microbes by TPs
TPs can promote the growth of beneficial bacteria in the intestinal tract and inhibit the growth of pathogenic microorganisms in the intestinal tract from regulating the composition of intestinal flora (50). Intestinal microbes are an essential component of the intestinal environment. Intestinal microbes can enhance the function of the intestinal barrier by interacting with the body's metabolism to produce various metabolites and promote mucosal immune homeostasis (51). The intestinal mucosal barrier is a defense system against external infections and self-maintenance and plays an essential role in maintaining intestinal homeostasis and body health. Zhang et al. (52) studied TPs' therapeutic and preventive effects on ileal injury and intestinal flora disorder. The results showed that TPs could reduce inflammatory and oxidative stress markers, increase the levels of antioxidant enzymes and tight junction proteins, effectively improve the intestinal flora imbalance, reduce the damage to the intestinal mucosa and boost the body's immunity.
Ten volunteers who did not drink green tea drank it for 10 consecutive days, and the proportion of Bifidobacteria showed an overall increasing trend (53). A mouse model was established to explore the regulatory effect of TPs on the intestinal flora. The study found that after feeding green TPs, specific bacterial communities such as Bacteroidetes and Proteobacteria still increased, and Firmicutes showed a decreasing trend (54). This result indicates that TPs in green tea can improve the diversity of intestinal flora and regulate the composition of flora, thereby improving and maintaining the ecological balance of intestinal flora, which is beneficial to human health. The addition of TPs to calf feed reduces Clostridium perfringens in the gut, which is associated with a lower incidence of digestive and respiratory diseases (55). Green tea consumption decreased relative abundance at the phylum level of Bacteroidetes. In addition, SCFAs-producing bacteria, including Faecalibacterium, Coprococcus, and Bifidobacterium longum, increased, while species from Prevotella decreased. And SCFAs are important factors regulating cytokine secretion and antibody synthesis (56). In conclusion, the protective effect of TPs on intestinal microflora has been supported by a large number of experimental results. Therefore, the reconstruction of immune homeostasis through the normalization of the intestinal microbiome is considered an effective method to treat COVID-19.
Inhibitory Mechanism of TPs on SARS-CoV-2
Recent studies have demonstrated that TPs, particularly EGCG, inhibit coronavirus enzymes as well as coronavirus replication in vitro (57). However, laboratory and clinical studies have been performed to study the efficacy of green tea consumption in COVID-19 treatment, and the results are promising. SARS-CoV-2 has a high affinity for ACE2, which acts as a receptor for the spike glycoprotein on the surface of coronaviruses to facilitate virus entry. Nrf2 is a cytoprotective transcription factor that regulates the expression of a wide range of genes involved in detoxification, inflammatory, immune and antiviral responses (58). EGCG, via activating Nrf2, can suppress ACE2 (a cellular receptor for SARS-CoV-2) and TMPRSS2 (the cell entry that mediates the virus) (59). 3CL protease is required for the maturation of SARS-CoV-2, and numerous experiments have demonstrated that TPs (EGCG and theaflavins) have inhibitory effects on SARS-CoV-2 3CL protease (60). Mice with COVID-19 had lower levels of coronavirus RNA in their lungs when fed EGCG and TPs containing more than 60% catechins (61). Results demonstrated that EGCG treatment decreases viral RNA and viral protein production in the media, therefore, EGCG can inhibit coronavirus replication.
Data from docking simulations and in vitro assays suggest that EGCG is capable of inhibiting the SARS-Cov-2 major protease activity and thus can be used to interfere with SARS-Cov-2 infection (62). In a recent study reviewing the antiviral activities of EGCG and theaflavins, the authors suggest that both polyphenols are able to interact with receptors present in the structure of the SARS-CoV-2 virus, thereby inhibiting its replication. In particular, theaflavin-3,3'-digallate (TF3) can be employed as prophylactic agents due to their capacity to bind spike RBD the main binding domain of the S protein located on the S1 subunit of the SARS-CoV-2 virus; and TF3 can directly bind to viral M protease and ACE2 receptors, helping to fight SARS-CoV-2. EGCG can be used as a potential preventive agent because of its ability to dock various active sites of the SARS-CoV-2 virus (63).
Evidence suggests that patients infected with RNA viruses are in a chronic oxidative stress state, which is induced by the activation of phagocytes to produce and release ROS, and leads to the depletion of antioxidant defense systems (64). The increase in reactive oxygen species and the loss of antioxidant defense mechanisms increase the incidence of SARS-CoV-2 infection and the risk of immune dysfunction and death (65). Catechins activate antioxidant enzymes, and the antioxidant power of human plasma increases with the continued intake of green tea. These antioxidant defense systems also protect against oxidative damage in the brain, long-term intake of green tea catechins may be important because cells are often exposed to oxidative stress (66). TPs inhibits certain enzymes involved in reactive oxygen species ROS production by upregulating other endogenous antioxidant enzymes (such as glutathione peroxidase, superoxide dismutase and catalase); while promoting heme oxygenation enzyme 1 expression to reduce ROS production (67). Therefore, it is necessary to supplement dietary antioxidants to improve immunity when managing COVID-19. The use of exogenous antioxidants such as TPs can significantly influence the clinical outcome of COVID-19 by improving patients' health, speeding up the immune process, and thus shortening hospital stay.
Overall, TPs have antiviral solid and antioxidation properties that may help reduce the risk of developing severe COVID-19 symptoms; these findings highlight the potential for TPs to prevent and treat COVID-19 (Figure 2).
Figure 2
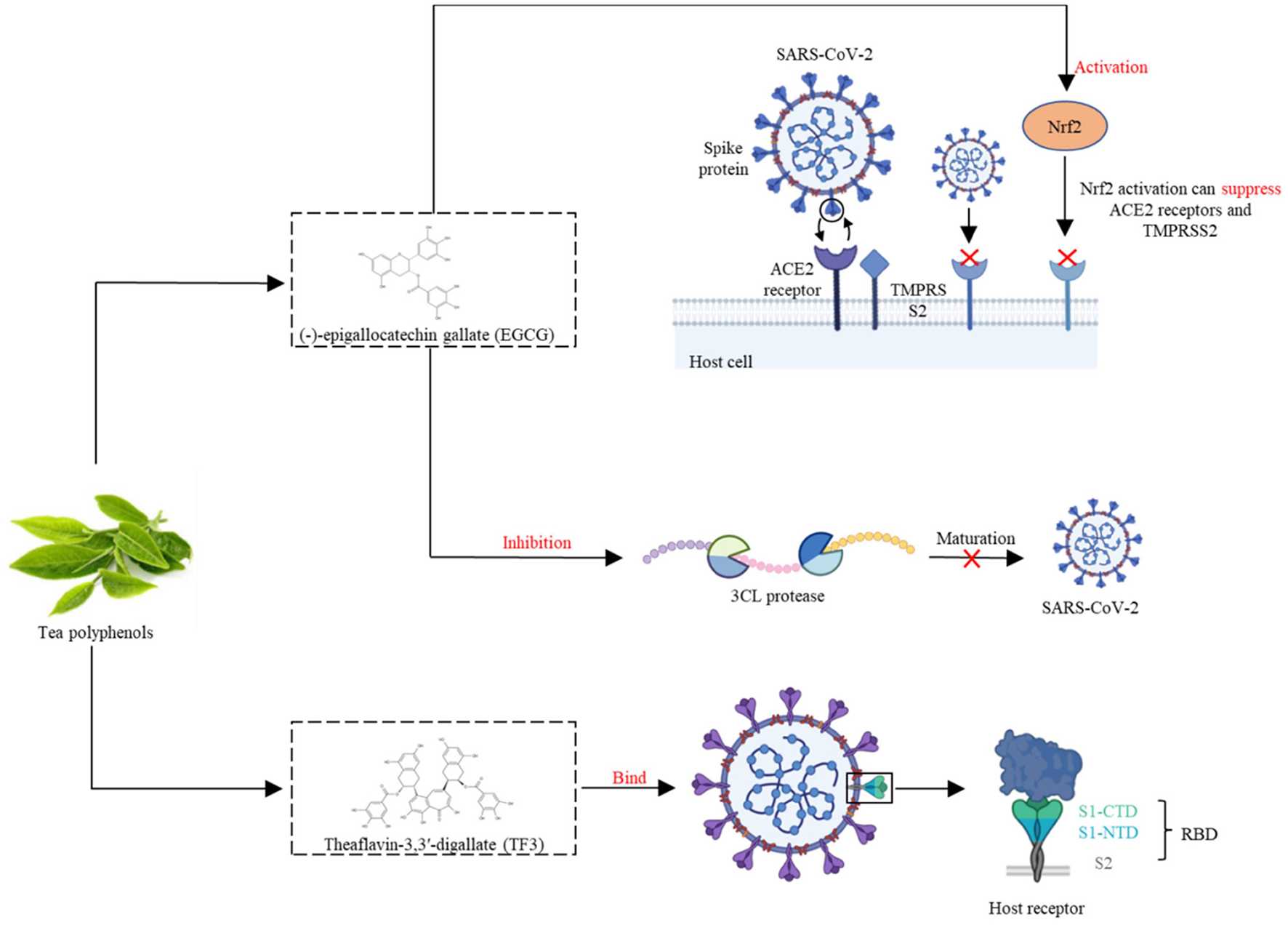
EGCG, via activating Nrf2, can suppress ACE2 receptors and TMPRSS2 during SARS-CoV-2 infection. TF3 can bind the SARS-CoV-2 spike receptor-binding domain, and help to fight SARS-CoV-2.
Reduction of Covid-19 Comorbidity Risk by TPS
In a retrospective study of 1,591 severely ill patients with COVID-19, hypertension was the most common comorbidity (49%), followed by cardiovascular disease (21%), hypercholesterolemia (18%), and diabetes (17%) (68). Patients with COVID-19 can develop complications of lung disease (cough, decreased lung diffusivity, sleep apnea, and pulmonary fibrosis), cardiovascular disease (diabetes, arrhythmia, and myocarditis), and neurological disorder (depression, anxiety, and attention disorders) (69). TP can effectively prevent and treat some complications.
Pulmonary Fibrosis
COVID-19 patients may have the sequelae of pulmonary fibrosis, with symptoms such as dry cough, fatigue, and dyspnea, leading to weight loss, worsening physical condition, long-term disability, and affecting the patient's quality of life (70). EGCG strongly inhibited neutrophil, inhibited reactive oxygen species activity and inhibited apoptosis of activated neutrophil, enhanced the regression of pulmonary inflammation model, and significantly reduced subsequent fibrosis (71). EGCG reduces NF-κB, TNF-α, and IL-1β, and this blockade may be critical for the upregulation of proinflammatory and fibrotic cytokine genes in models of pulmonary fibrosis (72). But so far, there are no clear and reliable data on the frequency and severity of pulmonary fibrosis in COVID-19 patients.
During treatment of pulmonary fibrotic rats with EGCG, the rats exhibited reduced inflammation, alveolar damage, and vascular congestion, which were associated with the membrane-stabilizing and antioxidant properties of EGCG, demonstrating that EGCG can act as a potential anti-fibrotic drug (73). Oral administration of green tea extract (equivalent to EGCG doses of 300–400 mg/kg) in drinking water to mice almost wholly prevented interstitial and peribronchial fibrosis, >99% reduction in interstitial and peribronchial fibrosis and ~50% reduction in perivascular fibrosis (74). After EGCG (600 mg given orally for 14 days) treatment in 20 patients with pulmonary fibrosis, reverses profibrotic biomarkers in their diagnostic biopsies and serum samples, EGCG treatment was associated with a reduction in fibrogenesis (75). These inhibitory activities of EGCG in rodent models and humans suggest that EGCG may be beneficial for preventing and treating pulmonary fibrosis in COVID-19 patients.
Diabetes
High-risk patients with severe COVID-19 or death have a variety of characteristics, including advanced age and masculinity, as well as potential health problems such as cardiovascular disease, obesity, and diabetes (76). Preliminary studies have found that diabetes increases the risk of infection with SARS-CoV-2 and increases the severity of COVID-19 (77). In human monocytes, an increase in glucose levels leads to an increase in SARS-COV-2 replication, which is maintained by glycolysis through the production of mitochondrial reactive oxygen species and activation of hypoxia-inducible factor 1α (78). Therefore, high blood sugar may support virus proliferation. Patients with diabetes usually have higher levels of SARS-CoV-2 infection than patients without diabetes. ACE2 knockout mice are more susceptible to high-fat diet-induced pancreatic beta-cell dysfunction than wild-type mice (79); and SARS-CoV infection can lead to hyperglycemia in people with no history of diabetes (80). This finding suggests that coronaviruses might specifically damage islets, potentially leading to hyperglycemia.
Drinking 3–4 cups of tea per day (600–900 mg/day) is often considered to prevent personal obesity metabolic syndrome or reduce disease risk (81). In mice fed a high-fat (60% calorie) diet; we found that EGCG (0.32% of the diet) significantly reduced weight gain, body fat, and visceral fat at 16 weeks (82). A retrospective study of 17,413 Japanese adults aged 40–65 showed compared with people who drank <1 cup of green tea a week, drinking more than six cups a day reduced the risk of diabetes by 33% (83). TPs in reducing plasma cholesterol levels, prevention of hypertension and improving endothelial function in the role of helping to prevent cardiovascular diseases. Weight loss and improved metabolic health may help better cope with COVID-19, whether regular drinking tea (and the required amount) can reduce the risk of COVID-19 infection and related syndromes requires a large number of experiments to prove.
Depression
In addition to posing a significant threat to physical health, the COVID-19 pandemic also poses a threat to the population's mental health due to increased fear and uncertainty; and disruption to social and economic systems. The prevalence of depression in the general population during the COVID-19 outbreak is 25% (84). Alterations in the composition of the gut microbiota can increase the permeability of the gut barrier, activate systemic inflammatory and immune responses, modulate the release and efficacy of monoamine neurotransmitters, alter the activity of the hypothalamic-pituitary-adrenal axis and function, and alters the abundance of brain-derived neurotrophic factor (BDNF) (85). A deficiency of BDNF may lead to neuroplasticity impairment and depression. The mechanism of the anti-depression effect of TPs is related to the inhibition of HPA axis hyperactivity by reducing serum corticosterone and ACTH levels (86). TPs also have an anti-anxiety effect (similar to anti-anxiety drugs) at lower doses.
An investigation involving 2,011 Finnish general individuals found that daily consumption of tea was negatively correlated with the risk of depression (87). In addition, participants who drank ≥4 cups of green tea per day had a 51% lower prevalence of depressive symptoms compared to those who drank ≤ 1 cup of green tea per day (88). The antidepressant mechanism of TPs may be related to scavenging brain free radicals, regulating monoamine neurotransmitters in the brain tissue of depressed animals, and increasing the activity of brain antioxidant enzymes. Via establishing a mouse model of depression, it was found that the content of 5-HT and norepinephrine in the brain tissue of normal mice was significantly higher than that of depressed mice (89). After TPs were given, the content of 5-HT and norepinephrine in the brain tissue was significantly higher than that in the original depression mice (90). The antidepressant effect of TPs has been proven, prompt prevention of mental health status is also necessary for COVID-19 patients, whether regular drinking tea can reduce the risk of COVID-19 infection and related syndromes needs to be further investigated (Table 1, Figure 3).
Table 1
| Comorbidity | Risk | Experimental model | Results | Reference |
|---|---|---|---|---|
| Pulmonary fibrosis | 33% | EGCG doses of 300–400 mg/kg | >99% reduction in interstitial and peribronchial fibrosis and ~50% reduction in perivascular fibrosis | (74) |
| Diabetes | 17% | Drink ≥ 6 cups a day | 33% lower risk of diabetes | (83) |
| Depression | 25% | Drink ≥ 4 cups a day | 51% lower prevalence of depressive symptoms | (88) |
The reduction of COVID-19 comorbidity risk by TPs.
Figure 3
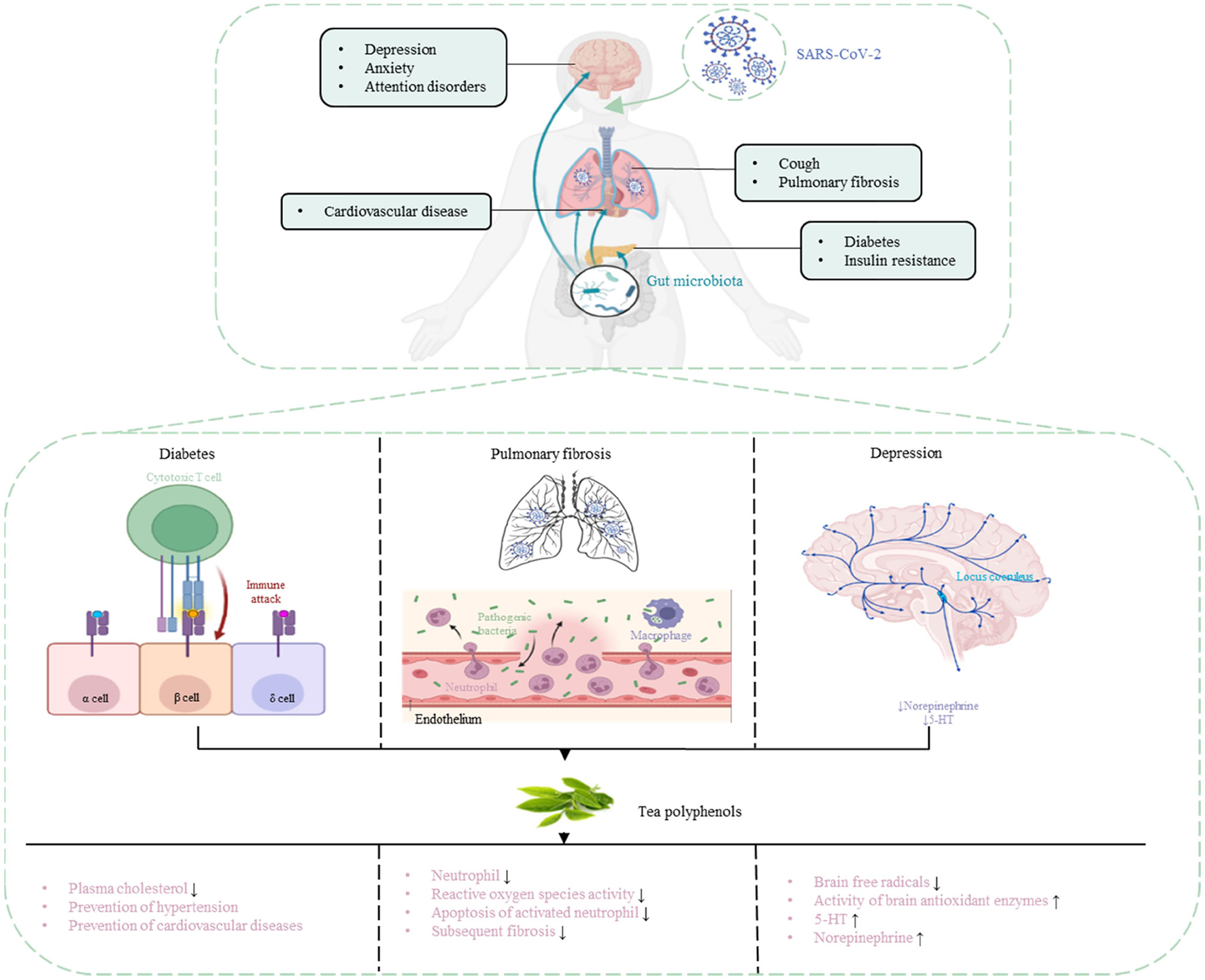
Prevention and treatment of COVID-19 complications with TPs.
Dosage and Instructions for Correct Use of TPS
Tea is rich in polyphenols, inexpensive, readily available, and most importantly, safe for long-term use regardless of the patient's age (91). However, it is essential to note that taking EGCG containing preparations during pregnancy may increase the risk of fatal leukemia (92). According to the review of toxicological evidence, the liver is the target organ, and hepatotoxicity is the critical effect, strongly associated with certain dosing conditions (such as mode of administration, fasting) and positively correlated with catechin and EGCG content (93). In the case of oral therapy, changes in hepatotoxicity and serum lipid profiles were evident only at the highest dose of 108 mg/kg/p.o. However, EGCG treatment to achieve appreciable plasma concentrations may concomitantly increase serum lipids, thereby increasing the severity of the liver injury (94). At present, we have not accurately explained the association mechanism between EGCG, liver, and blood lipids, which must be clarified further. Regular consumption of green tea appears to be safe, but high doses of green tea extract in dietary supplements may affect drug metabolism and efficacy (95). Because these products contain concentrated bioactive agents, the doses consumed extensively exceed the doses available from food. Compared to the control group (about 10 mg per day), short-term (3 days) overconsumption of green tea catechins (about 8 grams per day) resulted in a significant increase in liver enzyme activity (by 35–80%) (96). Interestingly, short-term overdose of green tea extract has a more significant effect on drug biotransformation enzymes than long-term small doses. There is no clear information on how much catechins should be taken to achieve the best results. Therefore, it is necessary to evaluate the specific amount of catechin and its possible adverse effects.
According to the United States Department of Agriculture (USDA), the average total catechin and EGCG per 100 mL of brewed green tea was 126.6 mg and 77.8 mg, respectively, based on 1 g tea leaf 100 mL infusion (93). Therefore, every 240 mL serving of brewed green tea provides about 304 mg of total catechin and 187 mg of EGCG. Thirty-six healthy male volunteers took 800 mg of EGCG orally for 10 days and were tested for safety, tolerability, and plasma kinetic behavior; the researchers found that the dose was safe and well-tolerated (97). For adults with normal liver function, the safe intakes limit of 338 mg of EGCG per day in solid form (under-eating or fasting conditions) may be considered. The observed safe level of EGCG equivalent dose (ingestion or fasting) for green tea preparations consumed in beverage form is 704 mg/day (93). In a randomized, double-blind trial of 200 healthcare workers, six capsules per day (including 378 mg of catechin and 270 mg of EGCG) for 5 months were better at preventing the flu virus than a placebo (98).
Numerous experiments are still needed to confirm the specific drug administration (green tea beverage, powdered green tea extract, catechin mixture, catechin alone), dose regimen (different doses, different duration of treatment), and administration pathway management (oral in diet, oral in a beverage) before determining the use of TPs for the treatment of COVID-19.
Summary and Future Direction
Many experiments have confirmed the safety of tea, and an appropriate amount of TPs will not cause harm to the human body. EGCG is one of the most important catechins in tea, enhancing the body's antiviral ability and gradually being regarded as a potential therapeutic agent for novel coronavirus infection. Although numerous epidemiological and clinical studies have shown that TPs have preventive and therapeutic effects on COVID-19, we lack specific dosages of TPs as dietary supplements or nutraceuticals for the prevention and treatment of COVID-19. To obtain more specific information, well-designed extensive cohort studies and human intervention trials are necessary.
Funding
This work was sponsored by Zhejiang Provincial Key Research and Development Program (2020C02037) and the Ningbo Natural Science Foundation (2021J107).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
LX: conceptualization, validation, and writing–original draft. C-TH: supervision. YL: validation and writing–original draft. ZW: editing. XZ: supervision, writing–review, and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Shang J Wan Y Luo C Ye G Geng Q Auerbach A et al . Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. (2020) 117:11727–34. 10.1073/pnas.2003138117
2.
Li F Li W Farzan M Harrison SC . Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. (2005) 309:1864–8. 10.1126/science.1116480
3.
Jena AB Kanungo N Nayak V Chainy G Dandapat J . Author correction: catechin and curcumin interact with S protein of SARS-Cov2 and ACE2 of human cell membrane: Insights from computational studies. Sci Rep. (2021) 11:8482. 10.1038/s41598-021-88218-3
4.
Ghidoli M Colombo F Sangiorgio S Landoni M Giupponi L Nielsen E et al . Food containing bioactive flavonoids and other phenolic or sulfur phytochemicals with antiviral effect: can we design a promising diet against COVID-19?Front Nutr. (2021) 8:661331. 10.3389/fnut.2021.661331
5.
Chowdhury P Barooah AK . Tea bioactive modulate innate immunity: in perception to COVID-19 pandemic. Front Immunol. (2020) 11:590716. 10.3389/fimmu.2020.590716
6.
Mao X Gu C Chen D Yu B He J . Oxidative stress-induced diseases and tea polyphenols. Oncotarget. (2017) 8:81649–61. 10.18632/oncotarget.20887
7.
Yan Z Zhong Y Duan Y Chen Q Li F . Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr. (2020) 6:115–23. 10.1016/j.aninu.2020.01.001
8.
Pan MH Tung YC Yang G Li S Ho CT . Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. (2016) 7:4481–91. 10.1039/C6FO01168C
9.
Guo TT Song D Cheng L Zhang X . Interactions of tea catechins with intestinal microbiota and their implication for human health. Food Sci Biotechnol. (2019) 28:1617–25. 10.1007/s10068-019-00656-y
10.
Claus SP Ellero SL Berger B Krause L Bruttin A Molina J et al . Colonization-induced host-gut microbial metabolic interaction. MBio. (2011) 2:e00271–10. 10.1128/mBio.00271-10
11.
Liu YC Li XY Shen L . Modulation effect of tea consumption on gut microbiota. Appl Microbiol Biotechnol. (2020) 104:981–7. 10.1007/s00253-019-10306-2
12.
He LH Ren LF Li JF Wu YN Li X Zhang L . Intestinal flora as a potential strategy to fight SARS-CoV-2 infection. Front Microbiol. (2020) 11:1388. 10.3389/fmicb.2020.01388
13.
Dang AT Marsland BJ . Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. (2019) 12:843–50. 10.1038/s41385-019-0160-6
14.
Hung YP Lee CC Lee JC Tsai PJ Ko WC . Gut dysbiosis during COVID-19 and potential effect of probiotics. Microorganisms. (2021) 9:1605. 10.3390/microorganisms9081605
15.
He Y Wen Q Yao FF Xu D Huang YC Wang JS . Gut-lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol. (2017) 43:81–95. 10.1080/1040841X.2016.1176988
16.
Piersigilli F Van Grambezen B Hocq C Danhaive O . Nutrients and microbiota in lung diseases of prematurity: The placenta-gut-lung triangle. Nutrients. (2020) 12:469. 10.3390/nu12020469
17.
Huang J Zhang J Wang X Jin Z Zhang P Su H et al . Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front Nutr. (2022) 9:821900. 10.3389/fnut.2022.821900
18.
Livanos AE Jha D Cossarini F Gonzalez-Reiche AS Tokuyama M Aydillo T et al . Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. (2021) 160:2435–50. 10.1053/j.gastro.2021.02.056
19.
Jin X Lian JS Hu JH Gao J Zheng L Zhang YM et al . Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. (2020) 69:1002–9. 10.1136/gutjnl-2020-320926
20.
Schuijt TJ Lankelma JM Scicluna BP Melo F Roelofs JH Boer JD et al . The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. (2016) 65:575–83. 10.1136/gutjnl-2015-309728
21.
Groves HT Cuthbertson L James P Moffatt MF Cox M Tregoning JS . Respiratory disease following viral lung infection alters the murine gut microbiota. Front Immunol. (2018) 9:182. 10.3389/fimmu.2018.00182
22.
Wang J Li FQ Wei HM Lian ZX Sun R Tian ZG . Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. (2014) 211:2397–410. 10.1084/jem.20140625
23.
Gu S Chen YF Wu ZJ Chen YB Gao H Lv L et al . Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. (2020) 71:2669–78. 10.1093/cid/ciaa709
24.
Huttenhower C Gevers D Knight R Abubucker S Badger JH . Structure, function and diversity of the healthy human microbiome. Nature. (2012) 486:207–14. 10.1038/nature11234
25.
Marsland BJ Trompette A Gollwitzer ES . The gut-lung axis in respiratory disease. Ann Am Thorac Soc. (2015) 12:S150–6. 10.1513/AnnalsATS.201503-133AW
26.
Zuo T Zhang F Lui GCY Yeoh YK Li AYL Zhan H et al . Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. (2020) 159:944–55. 10.1053/j.gastro.2020.05.048
27.
Yeoh TK Zuo T Lui CG Zhang F Liu Q Li AY et al . Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. (2021) 70:698–706. 10.1136/gutjnl-2020-323020
28.
Vodnar DC Mitrea L Teleky BE Szabo K Călinoiu LF Nemeş SA et al . Coronavirus disease (COVID-19) caused by (SARS-CoV-2) infections: a real challenge for human gut microbiota. Front Cell Infect Microbiol. (2020) 10:575559. 10.3389/fcimb.2020.575559
29.
Zuo T Liu Q Zhang F Lui GCY Tso E Yeoh YK et al . Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. (2021) 70:276–84. 10.1136/gutjnl-2020-322294
30.
Gou WL Fu YQ Yue L Chen GD Cai X Shuai ML et al . Gut microbiota, inflammation, and molecular signatures of host response to infection. J Genet Genomics. (2021) 48:792–802. 10.1016/j.jgg.2021.04.002
31.
Littman DR Pamer EG . Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. (2011) 10:311–23. 10.1016/j.chom.2011.10.004
32.
Zeppa SD Agostini D Piccoli G Stocchi V Sestili P . Gut microbiota status in COVID-19: an unrecognized player?Front Cell Infect Microbiol. (2020) 10:576551. 10.3389/fcimb.2020.576551
33.
Gauguet S D'Ortona S Ahnger-Pier K Duan B Surana NK Lu R et al . Intestinal microbiota of mice influences resistance to staphylococcus aureus pneumonia. Infect Immun. (2015) 83:4003–14. 10.1128/IAI.00037-15
34.
Cristofori F Dargenio VN Dargenio C Miniello VL Barone M Francavilla R . Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. (2021) 12:578386. 10.3389/fimmu.2021.578386
35.
Baradaran Ghavami S Pourhamzeh M Farmani M Raftar S Shahrokh S Shpichka A et al . Cross-talk between immune system and microbiota in COVID-19. Expert Rev Gastroenterol Hepatol. (2021) 15:1281–94. 10.1080/17474124.2021.1991311
36.
Frieman M Heise M Baric R . SARS coronavirus and innate immunity. Virus Res. (2008) 133:101–12. 10.1016/j.virusres.2007.03.015
37.
Abt MC Osborne LC Monticelli LA Doering TA Alenghat T Sonnenberg GF et al . Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. (2012) 37:158–70. 10.1016/j.immuni.2012.04.011
38.
Surana NK Kasper DL . Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest. (2014) 124:4197–203. 10.1172/JCI72332
39.
Geva-Zatorsky N Sefik E Kua L Pasman L Tan TG Ortiz-Lopez A et al . Mining the human gut microbiota for immunomodulatory organisms. Cell. (2017) 168:928–43. 10.1016/j.cell.2017.01.022
40.
Hong MY Cheng L Liu YN Wu ZF Zhang P Zhang X et al . natural plant source-tea polyphenols, a potential drug for improving immunity and combating virus. Nutrients. (2022) 14:550. 10.3390/nu14030550
41.
Cui Y Oh YJ Lim J Youn M Lee I Pak HK et al . AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against gram-positive and gram-negative bacteria. Food Microbiol. (2012) 29:80–7. 10.1016/j.fm.2011.08.019
42.
Arakawa H Maeda M Okubo S Shimamura T . Role of hydrogen peroxide in bactericidal action of catechin. Biol Pharm Bull. (2004) 27:277–81. 10.1248/bpb.27.277
43.
Renzetti A Betts JW Fukumoto K Rutherford RN . Antibacterial green tea catechins from a molecular perspective: mechanisms of action and structure-activity relationships. Food Funct. (2020) 11:9370–96. 10.1039/D0FO02054K
44.
Manna MS Sahaa P Ghoshal AK . Iron complexation of pharmaceutical catechins through selective separation. RSC Adv. (2014) 4:26247–50. 10.1039/C4RA03683B
45.
Kim HS Quon MJ Kim JA . New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. (2014) 2:187–95. 10.1016/j.redox.2013.12.022
46.
Hu J Huang Y Xiong M Luo S Chen Y Li Y . The effects of natural flavonoids on lipoxygenase-mediated oxidation of compounds with a benzene ring structure–a new possible mechanism of flavonoid anti-chemical carcinogenesis and other toxicities. Int J Toxicol. (2006) 25:295–301. 10.1080/10915810600746122
47.
Musial C Kuban-Jankowska A Gorska-Ponikowska M . Beneficial properties of green tea catechins. Int J Mol Sci. (2020) 21:1744. 10.3390/ijms21051744
48.
Matsui T . Condensed catechins and their potential health-benefits. Eur J Pharmacol. (2015) 765:495–502. 10.1016/j.ejphar.2015.09.017
49.
Liu X Le Bourvellec C Guyot S Renard C . Reactivity of flavanols: their fate in physical food processing and recent advances in their analysis by depolymerization. Compr Rev Food Sci Food Saf . (2021) 20:4841-4880. 10.1111/1541-4337.12797
50.
Li J Chen C Yang H Yang X . Tea polyphenols regulate gut microbiota dysbiosis induced by antibiotic in mice. Food Res Int. (2021) 141:110153. 10.1016/j.foodres.2021.110153
51.
Soderholm AT Pedicord VA . Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunol. (2019) 158:267–80. 10.1111/imm.13117
52.
Zhang L Gui S Wang J Chen Q Zeng J Liu A et al . Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J Funct Foods. (2020) 64:103654. 10.1016/j.jff.2019.103654
53.
Jin JS Touyama M Hisada T Benno Y . Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol Immunol. (2012) 56:729–39. 10.1111/j.1348-0421.2012.00502.x
54.
Guo XJ Cheng M Zhang X Cao JX Wu ZF Weng PF . Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int J Food Sci Technol. (2017) 52:1723–30. 10.1111/ijfs.13479
55.
Ishihara N Chu DC Akachi S Juneja LR . Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest Prod Sci. (2001) 68:217–29. 10.1016/S0301-6226(00)00233-5
56.
Chen T Yang CS . Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: implications on health effects. Crit Rev Food Sci Nutr. (2020) 60:2691–709. 10.1080/10408398.2019.1654430
57.
Hong S Seo SH Woo SJ Kwon Y Song M Ha NC . Epigallocatechin gallate inhibits the uridylate-specific endoribonuclease nsp15 and efficiently neutralizes the SARS-CoV-2 strain. J Agric Food Chem. (2021) 69:5948–54. 10.1021/acs.jafc.1c02050
58.
Mendonca P Soliman KFA . Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants. (2020) 9:659. 10.3390/antiox9080659
59.
Zhang ZC Zhang XC Bi KY He YF Yan WJ Yang CS et al . Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci Technol. (2021) 114:11–24. 10.1016/j.tifs.2021.05.023
60.
Du A Zheng R Disoma C Li SQ Chen ZP Li SJ et al . Epigallocatechin-3-gallate, an active ingredient of traditional Chinese medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int J Biol Macromol. (2021) 176:1–12. 10.1016/j.ijbiomac.2021.02.012
61.
Park R Jang M Park YI Park Y Jung W Park J et al . Epigallocatechin Gallate (EGCG), a green tea polyphenol, reduces coronavirus replication in a mouse model. Viruses. (2021) 13:2533. 10.3390/v13122533
62.
Zhu Y Xie DY . Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front Plant Sci. (2020) 11:601316. 10.3389/fpls.2020.601316
63.
Mhatre S Srivastava T Naik S Patravale V . Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine. (2021) 85:153286. 10.1016/j.phymed.2020.153286
64.
Rowaiye AB Onuh OA Oli AN Okpalefe OA Oni S Nwankwo EJ . The pandemic COVID-19: a tale of viremia, cellular oxidation and immune dysfunction. Pan Afr Med J. (2020) 36:188. 10.11604/pamj.2020.36.188.23476
65.
Delgado-Roche L Mesta F . Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. (2020) 51:384–7. 10.1016/j.arcmed.2020.04.019
66.
Haque AM Hashimoto M Katakura M Tanabe Y Hara Y Shido O . Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. (2006) 136:1043–7. 10.1093/jn/136.4.1043
67.
Yahfoufi N Alsadi N Jambi M Matar C . The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. (2018) 10:1618. 10.3390/nu10111618
68.
Grasselli G Zangrillo A Zanella A Antonelli M Cabrini L Castelli A et al . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. (2020) 323:1574–81. 10.1001/jama.2020.5394
69.
Li QQ Wiele TV . Gut microbiota as a driver of the interindividual variability of cardiometabolic effects from tea polyphenols. Crit Rev Food Sci Nutr. (2021) 13:1–27. 10.1080/10408398.2021.1965536
70.
Lechowicz K Drozdzal S Machaj F Rosik J Szostak B Zegan-Barańska M et al . COVID-19: The potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. (2020) 9:1917. 10.3390/jcm9061917
71.
You H Wei L Sun WL Wang L Yang ZL Liu Y et al . The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int J Mol Med. (2014) 34:92–102. 10.3892/ijmm.2014.1745
72.
Sriram N Kalayarasan S Sudhandiran G . Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pharmacol Res. (2009) 22:221–36. 10.1016/j.pupt.2008.12.010
73.
Sriram N Kalayarasan S Sudhandiran G . Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem Biol Interact. (2009) 180:271–80. 10.1016/j.cbi.2009.02.017
74.
Donà M Dell AI Calabrese F Benelli R Morini M et al . Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. (2003) 170:4335–41. 10.4049/jimmunol.170.8.4335
75.
Chapman HA Wei Y Montas G Leong D Golden JA Trinh BN et al . Reversal of TGFβ1-driven profibrotic state in patients with pulmonary fibrosis. N Engl J Med. (2020) 382:1068–70. 10.1056/NEJMc1915189
76.
Holman N Knighton P Kar P Keefe J Curley M Weaver A et al . Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. (2020) 8:823–33. 10.1016/S2213-8587(20)30271-0
77.
Chen Y Yang D Cheng B Chen J Peng A Yang C et al . Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. (2020) 43:1399–407. 10.2337/dc20-0660
78.
Lim S Bae JH Kwon HS Nauck MA . COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat Rev Endocrinol. (2021) 17:11–30. 10.1038/s41574-020-00435-4
79.
Lu CL Wang Y Yuan L Li Y Li XY . The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects the function of pancreatic β cells by improving the function of islet microvascular endothelial cells. Int J Mol Med. (2014) 34:1293–300. 10.3892/ijmm.2014.1917
80.
Yang JK Lin SS Ji XJ Guo LM . Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. (2010) 47:193–9. 10.1007/s00592-009-0109-4
81.
Yang CS Zhang ZY . Studies on the prevention of cancer and cardiometabolic diseases by tea: Issues on mechanisms, effective doses, and toxicities. J Agric Food Chem. (2019) 67:5446–56. 10.1021/acs.jafc.8b05242
82.
Bose M Lambert JD Ju J Reuhl KR Shapses SA Yang CS . The major green tea polyphenol, (–)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. (2008) 138:1677–83. 10.1093/jn/138.9.1677
83.
Iso H Date C Wakai K Fukui M Tamakoshi A . The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. (2006) 144:554–62. 10.7326/0003-4819-144-8-200604180-00005
84.
Bueno-Notivol J Gracia-García P Olaya B Lasheras I López-Antón R Santabárbara J . Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int J Clin Health Psychol. (2021) 21:100196. 10.1016/j.ijchp.2020.07.007
85.
Liu Y Wu Z Cheng L Zhang X Yang H . The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of TPs relieving depression. Food Funct. (2021) 12:7651–63. 10.1039/D1FO01091C
86.
Zhu WL Shi HS Wei YM Wang SJ Sun CY et al . Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol Res. (2012) 65:74–80. 10.1016/j.phrs.2011.09.007
87.
Hintikka J Tolmunen T Honkalampi K Haatainen K Koivumaa-Honkanen H Tanskanen A et al . Daily tea drinking is associated with a low level of depressive symptoms in the Finnish general population. Eur J Epidemiol. (2005) 20:359–63. 10.1007/s10654-005-0148-2
88.
Pham NM Nanri A Kurotani K Kuwahara K Kume A Sato M et al . Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr. (2014) 17:625–33. 10.1017/S1368980013000360
89.
Sun QY Cheng L Zhang X Wu ZF Weng PF . The interaction between tea polyphenols and host intestinal microorganisms: an effective way to prevent psychiatric disorders. Food Funct. (2021) 12:952–62. 10.1039/D0FO02791J
90.
Liu Y Jia GG Gou LS Sun LY Fu XB Lan N et al . Antidepressant-like effects of tea polyphenols on mouse model of chronic unpredictable mild stress. Pharmacol Biochem Behav. (2013) 104:27–32. 10.1016/j.pbb.2012.12.024
91.
Tang GY Meng X Gan RY Zhao CN Liu Q Feng YB et al . Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. (2019) 20:6196. 10.3390/ijms20246196
92.
Lambert JD Sang S Yang CS . Possible controversy over dietary polyphenols: benefits vs. risks. Chem Res Toxicol. (2007) 20:583–5. 10.1021/tx7000515
93.
Hu J Webster D Cao J Shao A . The safety of green tea and green tea extract consumption in adults-Results of a systematic review. Regul Toxicol Pharmacol. (2018) 95:412–33. 10.1016/j.yrtph.2018.03.019
94.
Ramachandran B Jayavelu S Murhekar K Rajkumar T . Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicol Rep. (2016) 3:336–45. 10.1016/j.toxrep.2016.03.001
95.
Boušová I Matoušková P Bártíková H Szotáková B Hanušová V Tománková V et al . Influence of diet supplementation with green tea extract on drug-metabolizing enzymes in a mouse model of monosodium glutamate-induced obesity. Eur J Nutr. (2016) 55:361–71. 10.1007/s00394-015-0856-7
96.
Bonkovsky HL . Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann Intern Med. (2006) 144:68–71. 10.7326/0003-4819-144-1-200601030-00020
97.
Ullmann U Haller J Decourt JD Girault J Spitzer V Weber P . Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res. (2004) 74:269–78. 10.1024/0300-9831.74.4.269
98.
Matsumoto K Yamada H Takuma N Niino H Sagesaka YM . Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: a randomized controlled trial. BMC Complement Altern Med. (2011) 11:15. 10.1186/1472-6882-11-15
Summary
Keywords
tea polyphenols, COVID-19, gut microbiota, gut-lung axis, antiviral
Citation
Xu L, Ho C-T, Liu Y, Wu Z and Zhang X (2022) Potential Application of Tea Polyphenols to the Prevention of COVID-19 Infection: Based on the Gut-Lung Axis. Front. Nutr. 9:899842. doi: 10.3389/fnut.2022.899842
Received
19 March 2022
Accepted
25 March 2022
Published
14 April 2022
Volume
9 - 2022
Edited by
Minhao Xie, Nanjing University of Finance and Economics, China
Reviewed by
Zhenjun Zhu, Jinan University, China; Yanhui Han, University of Massachusetts Amherst, United States
Updates
Copyright
© 2022 Xu, Ho, Liu, Wu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Tang Ho ctho@sebs.rutgers.eduXin Zhang zhangxin@nbu.edu.cn
This article was submitted to Food Chemistry, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.