- 1Department of Neurosurgery, The Children’s Hospital of Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Department of Neurology, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital Affiliated to Tongji University School of Medicine, Shanghai, China
Background: Predicting the duration of dysphagia after acute ischemic stroke (AIS) is important for clinical treatment decisions.
Objective: The purpose of this study is to assess the swallowing function of AIS patients and to develop and validate a prognostic model for the need for nasogastric tube (NGT) in these patients.
Materials and methods: We included 554 AIS patients during 2018–2019 as the development group and had 186 AIS patients as the external validation group. The primary end point of the study was the retention of NGT in patients 1 week after admission (Functional Oral Intake Scale ≤ 4). Swallowing function and stroke-associated pneumonia (SAP) at 1 month post-onset were also the objectives of this study. The volume-viscosity swallow test (V-VST) was used to assess the patient’s impaired swallowing function. The Predictive model was built by logistic regression.
Results: Overall, a total of 104 patients required indwelling NGT at 1 week of AIS onset in development group. The final prognostic model includes 5 variables: age (OR: 1.085, 95%CI: 1.049–1.123), neutrophil-to-lymphocyte ratio (NLR) (OR: 1.332, 95%CI: 1.090–1.626), NIHSS (OR: 1.092, 95%CI: 1.025–1.164), history of drinking (OR: 2.532, 95%CI: 1.452–4.417) and stroke location (Subtentorial vs. Supratentorial, OR: 1.954, 95%CI: 1.088–3.509). The prediction model had an AUC of 0.810, while the external validation group was 0.794.
Conclusion: In stroke patients, it is very important to decide early whether to indwell a NGT. The nomogram will support decision making for NGT insertion and help these patients recover from their condition.
Introduction
Dysphagia affects about half of the estimated number of stroke patients worldwide each year (1, 2). It is one of the common complications of acute ischemic stroke (AIS), and it is associated with pneumonia, prolonged hospitalization, increased mortality, and poor long-term prognosis (2). Dysphagia can lead to inadequate oral intake, causing malnutrition, dehydration, and stroke-associated pneumonia (SAP) (3). In particular, SAP, a typical complication of dysphagia, increases the overall mortality of stroke patients (4, 5). Malnutrition may impair immunity, wound healing, muscle strength and psychological drive. Therefore, hospitals should aim to provide at least adequate nutrition to all patients. Some of these patients will benefit from oral supplementation, but others will require aggressive nutritional support. This can usually be provided through a nasogastric tube (NGT). Clinicians need to decide within the first 48 h whether an NGT should be inserted to prevent these complications (6). However, not all patients who have suffered a stroke will benefit from NGT. Patients with retained NGT often experience nasopharyngeal discomfort, and many have oral pain, thirst, difficulty swallowing, and hoarseness. Local pressure effects from NGT may lead to sinusitis, otitis media, nasal erosion and abscess formation. Short-term damage to the esophagus may include esophagitis, localized abrasions and ulcers due to gastroesophageal reflux, while long-term damage includes significant esophageal strictures (7).
The guidelines recommend feeding with NGT if oral intake is unlikely to resume within 1 week (7). Therefore, clinicians need to predict the duration of impaired swallowing so that NGT can be placed at the appropriate time. There is currently a great deal of variation in the decision to use NGT, which often depends on the subjective experience of the clinician. Nutritional support should be administered as early as possible (within 72 h after stroke), with an emphasis on early and accurate prediction of patients who will require NGT within 1 week (8).
Some studies have explored risk factors associated with long-term impaired swallowing function, including the National Institutes of Health Stroke Scale (NIHSS), SAP, and age.
Although many studies have investigated the factors influencing swallowing function in patients with AIS, it is clear that not just a single factor is involved in this process. Nomogram is a widely used tool that combines multiple predictors to predict patient outcomes. The purpose of this study is to integrate different influencing factors to predict dysphagia in patients with AIS and to give a clinical decision on the need for NGT.
Materials and methods
Patients
Between 2018 and 2019, 554 patients with AIS were included in the study as a development group. Only AIS patients who did not require immediate tracheal intubation or neurosurgical intervention were eligible for enrollment. Patients with pre-existing dysphagia or any concomitant disease that could cause dysphagia were excluded. Exclusion criteria: (1) late admission (>48 h after AIS); (2) late initial swallowing assessment (>48 h after AIS); (3) patients lost to follow-up; (4) initial swallowing assessment more than 1 day after admission; (5) Patients with prior dysphagia or stroke recurrence; (6) Patients with pre-existing chronic pneumonia or active infection or fever within 2 weeks prior to admission; (7) history of blood disorders, severe liver disease, cancer or immunosuppressive therapy; (8) Patients with significant decline in consciousness after stroke.
Dysphagia assessment
The patients meeting the eligibility criteria finished the volume-viscosity swallow test (V-VST) within 48 h of admission. As previously reported, the intention of the V-VST was to determine the impaired efficacy and safety during swallowing (9). Impaired efficacy was defined as labial seal and oral residue, while impaired safety was defined as cough and a ≥3% decrease in oxygen saturation. The last volume that is safe and effective to swallow at different viscosities will be recorded. Based on the results of the V-VST test, the patient’s swallowing function was recorded as 4 grades: Grade I: no dysphagia (safety and efficacy); Grade II: mild dysphagia (safety impaired only); Grade III: moderate dysphagia (efficacy impaired only); Grade IV: severe dysphagia (impaired safety and efficacy).
We use Functional Oral Intake Scale (FOIS) to measure oral intake. FOIS ranges from level 1 to level 7 (from cannot eat through mouth to completely unrestricted oral diet). Severe oral intake deficiency was defined as a single diet or worse (FOIS score ≤ 4), as previous studies have shown a 40% reduction in energy and protein intake and an overall inadequate intake in these individuals, and these patients will be remained NGT.
The primary endpoint was persistent severe oral intake impairment (FOIS score ≤ 4) at 1 week of follow-up, which was based on the following guideline: if oral intake impairment persisted for 1 week, NGT feeding was recommended to be initiated. The secondary endpoint was the retention of NGT in patients 1 month after admission (if oral intake was still expected to be impaired 1 month after stroke, it was recommended for percutaneous endoscopic gastrostomy feeding). The third endpoint is the presence of SAP after AIS.
Stroke-associated pneumonia diagnostic criteria
SAP in the first week after stroke onset was diagnosed retrospectively on the basis of the Centers for Disease Control and Prevention’s modified criteria: “at least one of the former and one of the latter criteria met: (A) abnormal respiratory examination, pulmonary infiltrates on chest x-rays; (B) productive cough with purulent sputum, microbiological cultures from lower respiratory tract or blood cultures, leukocytosis, elevated CRP” (10).
Clinical and laboratory assessments
Laboratory examination were assessed on an empty stomach the next morning. Computed tomography (CT) examinations were completed within 24 h of admission. Repeat CT and magnetic resonance imaging (MRI) were performed prior to discharge, and stroke laterality, stroke location, affected arterial territory and number of focus are classified according to MRI. Classification of cerebrovascular events were classified according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (11).
Development of the statistical model
Baseline patient data and risk factors were statistically analyzed using SPSS. Univariate analysis was performed using chi-square test, t-test or Kruskal-Wallis rank sum test. Multivariate analysis was performed using logistic regression analysis. The nomogram is analyzed and constructed by using the R, rms package. After calculating risk factors using logistic regression analysis, we used P-values and effect values to construct nomogram and assessed the performance of the nomogram by calculating area under curve (AUC).
Validation of the statistical model
The prediction accuracy of the model was evaluated by 186 AIS patients as external validation. The receiver operating characteristic (ROC) curve was used to evaluate the accuracy of the model. Calibration plots were used to assess the agreement between predicted and practical results.
Results
Baseline data for patients in the development group are shown in Table 1. Of the 554 patients in the development group, 104 were indwelt with an NGT within 1 week of onset, with a mean age of 65.01 ± 7.97 years, including 72 males and 32 females, and a mean HIHSS score of 5.26 ± 3.25. The number of patients with good swallowing function who did not require indwelling NGT was 450, their mean age was 59.03 ± 10.68, mean NIHSS score was 3.79 ± 4.18, of which 288 were male and 162 were female.
The V-VST is used to assess the patient’s swallowing function. In univariate analysis, there were significant differences between the two groups (1 week) with respect to five variables: age, NIHSS, neutrophil-to-lymphocyte ratio (NLR), stroke location and history of drinking (Figure 1). The chi-square test showed that the probability of indwelling NGT was higher in alcohol drinkers, while subtentorial lesions alone tended to have indwelling NGT than supratentorial lesions alone (Figure 1).
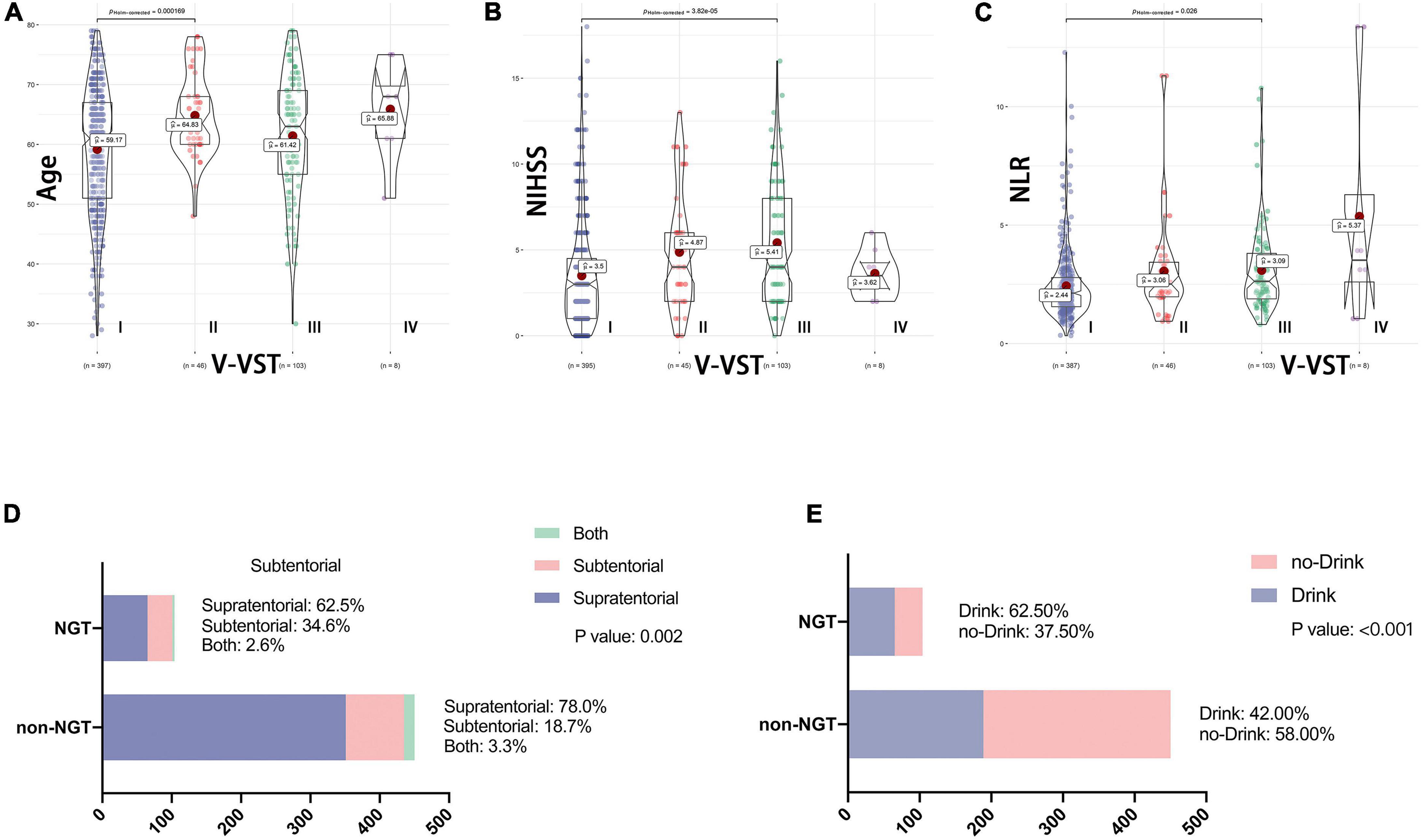
Figure 1. Association between baseline data and swallowing function. V-VST, the volume-viscosity swallow test; NIHSS, National Institutes of Health Stroke Scale on admission; NLR, Neutrophil to lymphocyte ratio; NGT, need for nasogastric tube. Figure shows the correlation analysis between the five variables which were ultimately included in the nomogram and swallowing function. (A–C) Shows the relationship between continuous variables including age, NLR and NIHSS and V-VST, all with statistically significant differences in their V-VST grade distributions. (D,E) Shows the association between categorical variables including stroke location and drinking and NGT, again with statistical differences.
The primary endpoint of this study was the indwelling NGT of patients 1 week after admission (FOIS ≤ 4). We used univariate and multivariate analysis to identify potential prognostic factors for NGT indwelling. In univariate analysis, there were significant differences in age (59.03 vs. 65.01, P < 0.001), NIHSS (3.79 vs. 5.26, P = 0.001), NLR (2.36 vs. 3.91, P < 0.001), stroke location (18.7 vs. 34.60%, P = 0.002) and drinking history (42.0 vs. 62.50%) between the two groups (1 week) (Table 1). On the basis of univariate analysis, multivariate logistic regression analysis was used to analyze the variables with statistical differences (Table 2). Finally, five variables had independent and significant effects on whether to retain NGT: age (OR: 1.085, 95% CI: 1.049–1.123, P < 0.001), NLR (OR: 1.332, 95% CI: 1.090–1.626, P: 0.005), NIHSS (OR: 1.092, 95% CI: 1.025–1.164, P: 0.007), drinking history (OR: 2.532, 95% CI: 1.452–4.417, P: 0.001) and lesion location (subtentorial vs. suprarenal, OR: 1.954, 95% CI: 1.088–3.509, P: 0.025). Based on this, we constructed the corresponding nomogram (Figure 2).
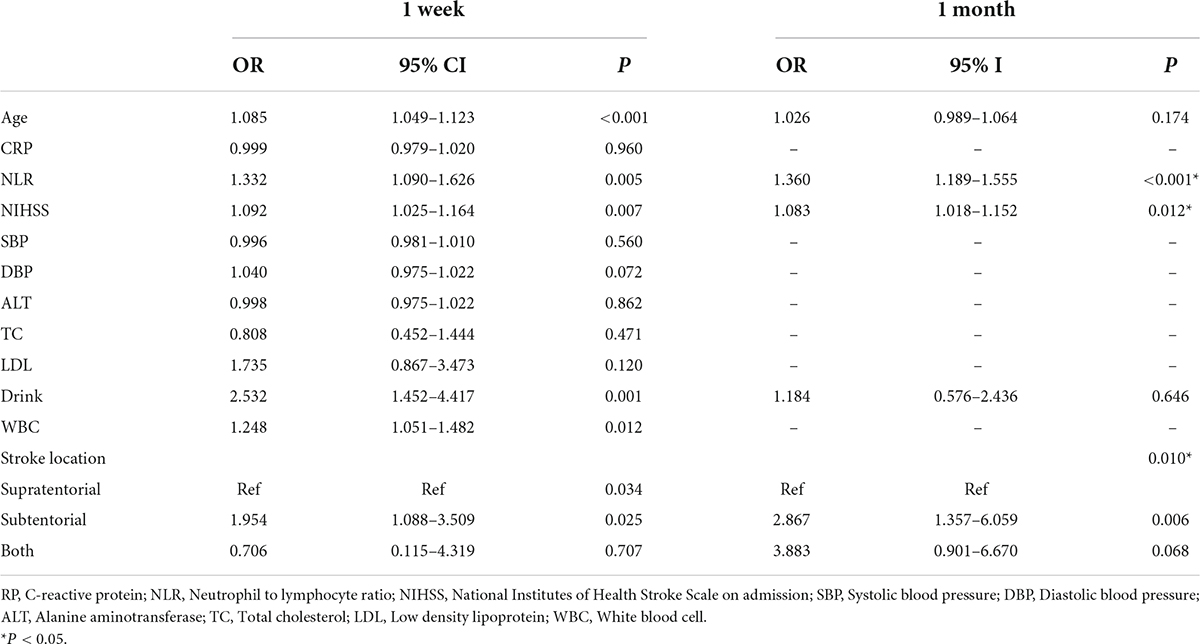
Table 2. Univariate analysis for the potential factors associated with indwelling nasogastric tube by logistic regression.

Figure 2. (A,B) The nomograms for predicting the prognosis of AIS patients. NIHSS, National Institutes of Health Stroke Scale on admission; NLR, Neutrophil to lymphocyte ratio. To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the probability axis to determine the likelihood of NGT.
Model performance and validation
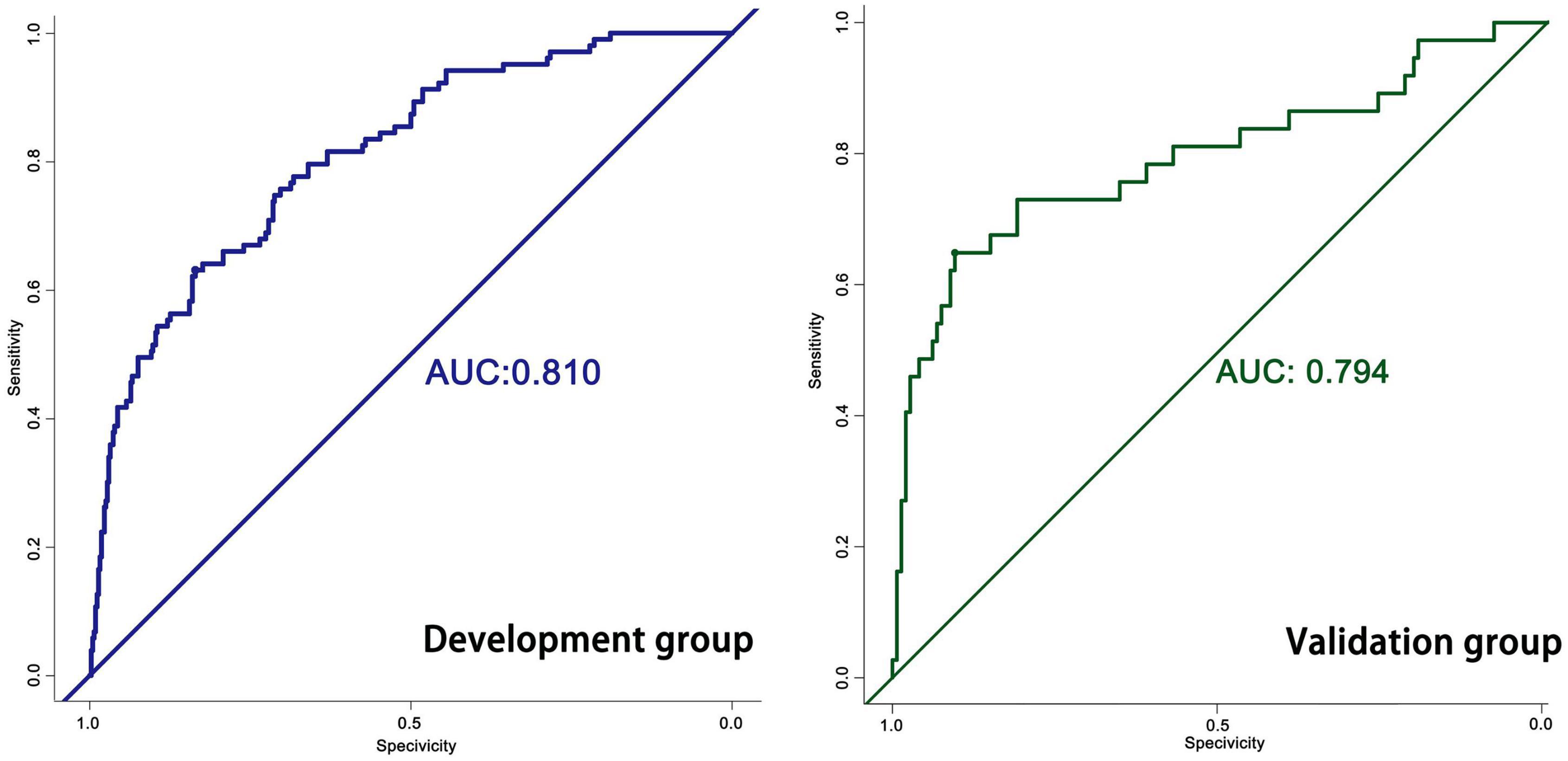
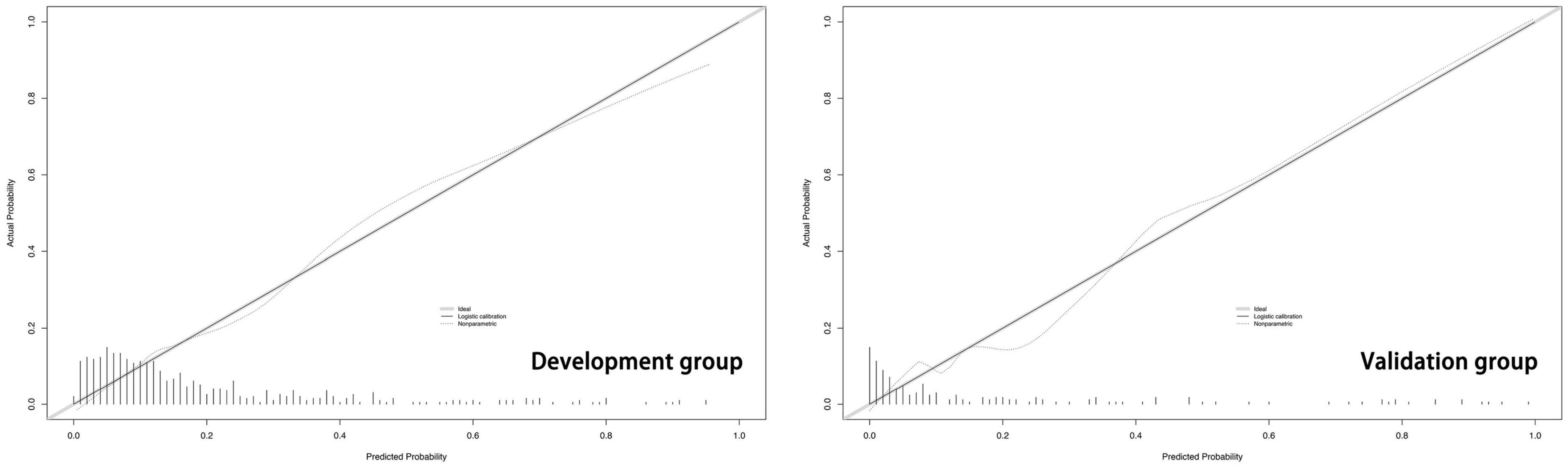
The predictive model built on the basis of 554 patients in the development group (1 week) was validated for its ability to predict accurately based on the AUC. In the development group, AUC = 0.810, which has good predictive power. An AUC of 0.794 was achieved in 186 externally validated patients, again showing excellent predictive accuracy (Figure 3). The prediction model at one month of onset achieved an AUC of 0.755. Calibration plots are likewise one of the good ways to evaluate the accuracy of the prediction model, as shown in Figure 4, calibration plots show the best agreement between the predicted and observed results.
The second endpoint was the patient’s NGT retention at 1 month of onset. One month after onset is an important time point, the guidelines suggest that percutaneous endoscopic gastrostomy may be a reasonable option if swallowing disorders are likely to persist beyond 1 month (7). We re-analyzed the 5 variables: age, NIHSS, NLR, stroke location and drinking history to assess whether these indicators were equally applicable to predict prognosis at 1 month. After multifactorial logistic regression analysis, patient’s NLR (OR: 1.360, 95% CI: 1.189–1.555, P < 0.001), NIHSS (OR: 1.083, 95% CI: 1.018–1.152, P: 0.012) and stroke location (subtentorial vs. suprarenal: OR: 2.867, 95%CI: 1.357–6.059, P: 0.001) retained their effective predictive power. In contrast, age and drinking history lost their previous predictive effects. Accordingly, we redrew the corresponding nomogram at 1 month after onset (Figure 2).
Patients’ stroke-associated pneumonia at 1 month onset
The third endpoint of the study was the presence of SAP in patients with AIS. Previous studies have shown that patients with post-stroke dysphagia have a threefold higher risk of developing pneumonia than those who do not have dysphagia (2). Therefore, we tried to clarify whether the 5 variables of age, NIHSS, NLR, stroke location and drinking history had an effect on SAP after AIS. As shown in Figure 5, the age (65.86 vs. 59.39), NIHSS (5.38 vs. 3.76), and NLR (3.79 vs. 2.51) were statistically different between the two groups; the stroke location was statistically different by chi-square test, while drinking did not appear to be associated with the development of SAP.
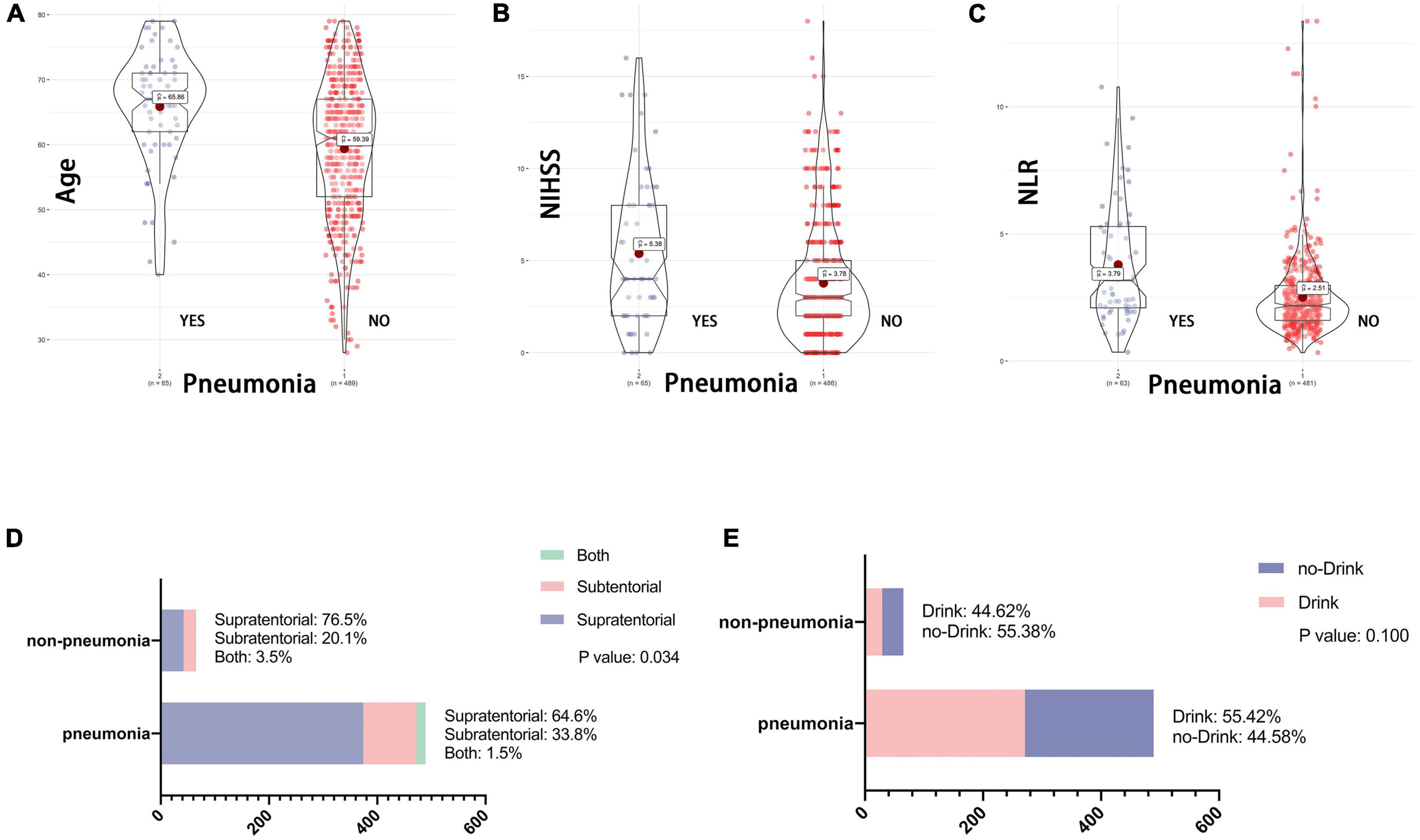
Figure 5. (A–E) Association between baseline data and SAP. NIHSS, National Institutes of Health Stroke Scale on admission; NLR, Neutrophil to lymphocyte ratio. One of the endpoint of this study is the presence of stroke associated SAP after AIS. As with the variables included in Figure 1, whether the patient drank at 1 month of follow-up had no effect on the occurrence of SAP. While patient age, NLR, NHISS and stroke location remained statistically different between the two groups.
Discussion
Dysphagia is a common complication after AIS, and we utilized 740 AIS patients to develop a clinical prediction model to predict the likelihood of retaining an NGT within 1 week to guide decision making for NGT feeding in those with dysphagia after AIS. The model incorporates clinical data that are readily available and commonly used in clinical work, making this predictive model easy to use and generalize.
In previous studies, some factors associated with swallowing function in patients with AIS have been explored. Bravata et al. (12) found that using the NIHSS to predict dysphagia had better performance than the nursing admission dysphagia screening tool. Jeyaseelan et al. (13). found the NIHSS to be more sensitive than a 3-sip water test. In Toscano’s study, the NHISS without dysphagia was 4.98 ± 3.50 and with dysphagia was 15.77 ± 7.79 (14). In the study by Henke et al., it was found that the NIHSS cutoff for whether or not post-stroke dysphagia occurred was 4.5, and the mean NIHSS score for all patients was 3 (15). Similar to the study by Henke et al., in our study the mean NIHSS was 3.97 and by calculating the Youden Index we obtained the same cutoff score of 4.5. However, the mean NIHSS for patients with dysphagia in their study was 8 and ours was 5.3. This may be related to selection bias in our study. In addition to this, different definitions of dysphagia criteria may have contributed to this discrepancy. However, the main problem with NIHSS is the lack of sufficient specificity, which correlates with negative predictive values below 40% and positive predictive values of about 90%. This means that patients above the threshold are highly likely to have swallowing problems, but those below the threshold cannot be classified as dysphagia. Therefore, the NIHSS should not be used as the only screening parameter to detect dysphagia, but rather as one of the items to provide clinical decision making. In this study, NIHSS was used as part of the model to participate in the assessment of the likelihood of patients retaining NGT. NIHSS was an independent predictor both at 1 week and at 1 month of onset.
Alcohol consumption is related to dysphagia after AIS. Heavy alcohol consumption in a normal diet reduces the pressure of the lower esophageal sphincter, slowing esophageal motility and gastric emptying, thus promoting acid reflux (16). In our study, although a history of drinking was a risk factor for retention of NGT at 1 week, this association was not present at 1 month.
There are conflicting findings regarding the location of the lesion and dysphagia after AIS. Previous studies had shown that the risk of SAP was associated with brainstem lesions, whereas the relationship between cortical stroke and SAP or dysphagia had not been confirmed (17, 18). In the study by Suntrup et al. (19), there was a significant difference in the percentage of local infarcts in different brain regions between patients presenting and not presenting with specific functional impairment. In particular, lesions of the pre- and post-central gyri, opercular region, supramarginal gyrus and respective subcortical white matter tracts in the right hemisphere are associated with dysphagia, and postcentral lesions are particularly associated with severe swallowing disorders. The swallowing process remains controversial in terms of the degree of lateralization. Several studies have shown that swallowing exhibits asymmetries in the cerebral hemispheres (20, 21). Studies have shown differences in swallowing physiology between right- and left-sided unilateral stroke patients, with pharyngeal damage being more severe in patients with right-hemisphere strokes (22). The only area that predicts severe dysphagia is the right postcentral gyrus, the primary sensory area for swallowing (23). Although these studies were meticulous and interesting, we analyzed the location of the lesion in the modeling group of 554 patients in three aspects of stroke laterality, stroke location and affected arterial territory, and found that stroke laterality (left or right, bilateral) and affected arterial territory (anterior cerebral, middle cerebral, posterior cerebral, vertebral, basilar, or multiple) had no effect on patient retention of NGT. Similar to conventional wisdom, our study found a higher probability of dysphagia in subtentorial lesions than in supratentorial (OR: 1.954, 95%CI: 1.088–3.509, P: 0.025).
In one of our previous studies, NLR had a stronger and statistically significant predictive effect in predicting the prognosis of patients with AIS compared with white blood cells, neutrophils or lymphocytes alone and neutrophils plus lymphocytes (24). As mentioned earlier, among peripheral circulating leukocytes, neutrophils are considered to be an important mediator of ischemic brain injury (25). Experimental evidence suggests that some specific lymphocyte subtypes play a key role in the inflammatory response to chemical injury and are major cerebral protective immunomodulators (26). NLR reflects the balance between neutrophil and lymphocyte levels and their differential immune activity, and NLR has been used as a potential predictor of mortality in patients with AIS (27). This study found that NLR was strongly associated with SAP (3.79 vs. 2.51, p < 0.001). This shares a common view with the study by Lan et al. (28). in which NLR was an independent risk factor for AIS-associated infection (OR: 1.16, 95%CI: 1.01–1.33, P: 0.034). Thus, high NLR levels strongly predict a poor prognosis for patients with AIS, both the higher incidence of NGT retention rate and higher SAP were strongly associated with high NLR levels.
The third endpoint of this study was SAP, which we found to be associated with age, NIHSS, NLR, and stroke location. Here we have to think about the question: does the different way of screening for dysphagia have an impact on SAP? In fact, different methods of determining dysphagia will have a significant impact on the occurrence of SAP. An accurate swallowing screening test may be crucial in lowering the incidence of SAP. Indeed, recent studies reported a higher SAP incidence in stroke patients who failed a high-sensitive screening for dysphagia compared to those who passed the screening (4, 29). In addition, the high-sensitivity screening method is more effective in reducing the incidence of SAP than the low-sensitivity screening method. In the study of Jannini et al. found that among patients screened by dysphagia, none of the patients screened by the high-sensitivity method developed SAP compared to 31.82% in the low-sensitivity group (30). Patients screened with low-sensitivity protocols and those who fail to screen are at increased relative risk of SAP and poorer clinical outcomes of stroke due to missed diagnosis of dysphagia.
For SAP, we still have the following concerns. Although SAPs usually occur within the first 7 days after a stroke episode, in half of the cases of SAPs, patients are not affected by dysphagia. In this case, half of the SAPs may appear after 7 days due to immunosuppression and oropharyngeal pathogen colonization associated with tube feeding, especially in the ICU, where the incidence of SAP is as high as 38% (31).
Based on the above, it is likely that a significant proportion of patients who develop SAP are not associated with dysphagia. The body enters a state of immunosuppression to protect the damaged brain tissue, hence attenuating the autoimmune reaction in the brain. However, this stroke-induced immunosuppression predisposes the body to pathogen invasion, leading to stroke-induced immunosuppression syndrome (SIDS) and infection (32, 33). As mentioned in our article, NLR can be used to estimate the degree of immunosuppression that occurs after such strokes, with higher NLR suggesting more pronounced immunosuppression.
Bacterial colonization is especially common in the critically ill for various reasons, including frequently impaired host defenses, the presence of invasive devices that form a nidus for colonization, and the administration of often long-term or repeated courses of antibiotics. Therefore, this fraction of patients with severe SAP may not be caused by dysphagia, but by colonization with colonizing pathogenic bacteria. This part of the SAP patients deserves a deeper investigation in the future.
The present study still has some limitations. First, our study cohort excluded patients who were lost to follow-up. Most patients at day 7 were still hospitalized or able to be followed up on an outpatient basis and could be followed up face-to-face. However, at the 1 month follow-up, most patients were followed up with a telephone questionnaire, which may have caused bias. Many patients with severe stroke were not included in the study, resulting in bias. Second, this study included patients who were hospitalized in large hospitals, and it remains to be studied whether the findings can be generalized downward to lower-level hospitals. Thirdly, we assessed the patient’s degree of dysphagia by V-VST and determined whether the patient needed an indwelling NGT by FOIS. However, this is not the gold standard for assessing dysphagia. Here, instrumental assessment of dysphagia, such as video fluoroscopy or fiberoptic endoscopic evaluation of swallowing, is clearly superior (34). Although instrumental assessment is considered the gold standard, it is also more complex and expensive compared to clinical assessment, making it less widely applicable. Non-instrumental assessment is a shortcoming of this study, while assessment by V-VST has its own advantages. V-VST is a widely validated method for assessing dysphagia that is easy to perform, inexpensive, and has good patient cooperation, making it easy to obtain clinical data. Finally, the present study failed to include hemorrhagic stroke, and subsequent studies may be able to remedy this regret. Factors such as leukoaraiosis, post stroke cognitive impairment, and other factors that may affect dysphagia were also excluded (mainly contrary to the principle of model simplicity). These interesting variables will be studied in a follow-up study.
Conclusion
We have developed a simple model by utilizing easily accessible clinical variables, which can well assess the possibility of indwelling NGT in AIS patients within 1 week after the onset of AIS, and timely insertion of NGT for patients who may need it to supply adequate nutrition. The model allows for personalized clinical decision making by identifying those individuals who may benefit from NGT feeding.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study was approved by the Ethics Committee of the Shanghai Fourth People’s Hospital and complied with the Helsinki Declaration. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
PZ, Z-LC, and ZS designed the study. WZ and WS interpreted the data. PZ wrote the manuscript. PZ, WZ, and WS performed the statistical analyses. JW, ZZ, CL, and NW screened and extracted the data. ZS and Z-LC supervised the study. All authors have made an intellectual contribution to the manuscript and approved the submission.
Funding
This work was supported by the Hongkou District Clinical Medicine Outstanding Young Talents Training Plan” (HKYQ2022-05).
Acknowledgments
We acknowledge all the authors who participated in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. (2007) 6:1106–14. doi: 10.1016/S1474-4422(07)70291-0
2. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
3. Dziewas R, Beck AM, Clave P, Hamdy S, Heppner HJ, Langmore SE, et al. Recognizing the Importance of dysphagia: stumbling blocks and stepping stones in the twenty-first century. Dysphagia. (2017) 32:78–82. doi: 10.1007/s00455-016-9746-2
4. Suntrup-Krueger S, Minnerup J, Muhle P, Claus I, Schröder JB, Marian T, et al. The effect of improved dysphagia care on outcome in patients with acute stroke: trends from 8-Year data of a large stroke register. Cerebrovasc Dis. (2018) 45:101–8. doi: 10.1159/000487811
5. Dziewas R, Michou E, Trapl-Grundschober M, Lal A, Arsava EM, Bath PM, et al. European Stroke organisation and european society for swallowing disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J. (2021) 6:LXXXIX–CXV. doi: 10.1177/23969873211039721
6. Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. (2005) 365:764–72. doi: 10.1016/S0140-6736(05)17983-5
7. Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. (2003) 52:vii1–12. doi: 10.1136/gut.52.suppl_7.vii1
8. Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. (2001) 140:280–9. doi: 10.1007/s002210100813
9. Guillén-Solà A, Marco E, Martínez-Orfila J, Donaire Mejías MF, Depolo Passalacqua M, Duarte E, et al. Usefulness of the volume-viscosity swallow test for screening dysphagia in subacute stroke patients in rehabilitation income. NeuroRehabilitation. (2013) 33:631–8. doi: 10.3233/NRE-130997
10. De Raedt S, De Vos A, Van Binst AM, De Waele M, Coomans D, Buyl R, et al. High natural killer cell number might identify stroke patients at risk of developing infections. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e71. doi: 10.1212/NXI.0000000000000071
11. Treatment Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The publications committee for the trial of ORG 10172 in acute stroke treatment (TOAST) investigators. J Am Med Assoc JAMA. (1998) 279:1265–72. doi: 10.1001/jama.279.16.1265
12. Bravata DM, Daggett VS, Woodward-Hagg H, Damush T, Plue L, Russell S, et al. Comparison of two approaches to screen for dysphagia among acute ischemic stroke patients: nursing admission screening tool versus National Institutes of Health stroke scale. J Rehabil Res Dev. (2009) 46:1127–34. doi: 10.1682/jrrd.2008.12.0169
13. Jeyaseelan RD, Vargo MM, Chae J. National institutes of health stroke scale (NIHSS) as an early predictor of poststroke dysphagia. PM R. (2015) 7:593–8. doi: 10.1016/j.pmrj.2014.12.007
14. Toscano M, Viganò A, Rea A, Verzina A, Sasso D’Elia T, Puledda F, et al. Sapienza global bedside evaluation of swallowing after stroke: the GLOBE-3S study. Eur J Neurol. (2019) 26:596–602. doi: 10.1111/ene.13862
15. Henke C, Foerch C, Lapa S. Early screening parameters for dysphagia in acute ischemic stroke. Cerebrovasc Dis. (2017) 44:285–90. doi: 10.1159/000480123
16. Surdea-Blaga T, Negrutiu DE, Palage M, Dumitrascu DL. Food and gastroesophageal reflux disease. Curr Med Chem. (2019) 26:3497–511. doi: 10.2174/0929867324666170515123807
17. Momosaki R, Abo M, Kakuda W, Uruma G. Which cortical area is related to the development of dysphagia after stroke? A single photon emission computed tomography study using novel analytic methods. Eur Neurol. (2012) 67:74–80. doi: 10.1159/000333778
18. Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, et al. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. (2007) 8:62. doi: 10.1186/1471-2202-8-62
19. Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur J Neurol. (2015) 22:832–8. doi: 10.1111/ene.12670
20. Mistry S, Verin E, Singh S, Jefferson S, Rothwell JC, Thompson DG, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol. (2007) 585:525–38. doi: 10.1113/jphysiol.2007.144592
21. Li WQ, Lin T, Li X, Jing YH, Wu C, Li MN, et al. TMS brain mapping of the pharyngeal cortical representation in healthy subjects. Brain Stimul. (2020) 13:891–9. doi: 10.1016/j.brs.2020.02.031
22. Wilmskoetter J, Martin-Harris B, Pearson WG Jr., Bonilha L, Elm JJ, Horn J, et al. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiol Behav. (2018) 194:144–52. doi: 10.1016/j.physbeh.2018.05.010
23. Teismann IK, Steinsträter O, Warnecke T, Suntrup S, Ringelstein EB, Pantev C, et al. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. (2009) 10:71. doi: 10.1186/1471-2202-10-71
24. Zhang WB, Zeng YY, Wang F, Cheng L, Tang WJ, Wang XQ. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. (2020) 12:2428–39. doi: 10.18632/aging.102752
25. Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. (2015) 46:2916–25. doi: 10.1161/STROKEAHA.115.010620
26. Liesz A, Zhou W, Na SY, Hämmerling GJ, Garbi N, Karcher S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. (2013) 33:17350–62. doi: 10.1523/JNEUROSCI.4901-12.2013
27. Gokhan S, Ozhasenekler A, Mansur Durgun H, Akil E, Ustündag M, Orak M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci. (2013) 17:653–7.
28. Lan Y, Sun W, Chen Y, Miao J, Li G, Qiu X, et al. Nomogram including neutrophil-to-lymphocyte ratio for the prediction of stroke-associated infections. Front Neurol. (2020) 11:574280. doi: 10.3389/fneur.2020.574280
29. Joundi RA, Martino R, Saposnik G, Giannakeas V, Fang J, Kapral MK. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke. (2017) 48:900–6. doi: 10.1161/STROKEAHA.116.015332
30. Jannini TB, Ruggiero M, Viganò A, Comanducci A, Maestrini I, Giuliani G, et al. The role of the Sapienza GLObal Bedside Evaluation of Swallowing after Stroke (GLOBE-3S) in the prevention of stroke-associated pneumonia (SAP). Neurol Sci. (2022) 43:1167–76. doi: 10.1007/s10072-021-05449-y
31. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
32. Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. (2017) 45:299–307. doi: 10.1007/s15010-016-0972-1
33. Santos Samary C, Pelosi P, Leme Silva P, Rieken Macedo Rocco P. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care. (2016) 20:391. doi: 10.1186/s13054-016-1573-1
Keywords: swallowing function, dysphagia, acute ischemic stroke, nasogastric tube, stroke associated pneumonia
Citation: Zhang P, Zhang W, Shi W, Weng J, Zhang Z, Lin C, Wang N, Shen Z and Chen Z-L (2022) Swallowing function after acute ischemic stroke: Development and validation of a novel clinical prognostic model. Front. Nutr. 9:970253. doi: 10.3389/fnut.2022.970253
Received: 15 June 2022; Accepted: 02 September 2022;
Published: 05 October 2022.
Edited by:
Roser Granero, Universitat Autònoma de Barcelona, SpainReviewed by:
Massimiliano Toscano, Sapienza University of Rome, ItalyAntenucci Roberto, Azienda USL Piacenza, Italy
Copyright © 2022 Zhang, Zhang, Shi, Weng, Zhang, Lin, Wang, Shen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhipeng Shen, c2hlbnpwQHpqdS5lZHUuY24=; Zhi-Lin Chen, Y2hlbnpoaWxpbjIwMTZAMTYzLmNvbQ==
†These authors share first authorship
 Peiliang Zhang1†
Peiliang Zhang1† Wenbo Zhang
Wenbo Zhang Jianbin Weng
Jianbin Weng Zhi-Lin Chen
Zhi-Lin Chen