- 1Department of Urology, The Affiliated Nanchong Central Hospital of North Sichuan Medical College (University), Nanchong, China
- 2Department of Anesthesiology, North Sichuan Medical College (University), Nanchong, China
- 3Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
Objective: This meta-analysis aims to assess whether the prognostic nutritional index (PNI) score before treatment can be an independent biomarker of the prognosis of patients with upper tract urothelial carcinoma (UTUC).
Materials and methods: We systematically search PubMed, Embase, Scopus database, and Cochrane Library, and the search time is up to April 2021. Use STATA 16.0 software for data processing and statistical analysis.
Results: Six studies, including seven cohorts, were eventually included in our meta-analysis. The meta-analysis results showed that low PNI scores are associated with worse OS (HR: 1.92; 95% CI 1.60 to 2.30; P < 0.01), DFS/RFS/PFS (HR: 1.57; 95% CI 1.33 to 1.85; P < 0.01), and CSS/DSS (HR: 1.79; 95% CI 1.49 to 2.16; P < 0.01), which supported the PNI score as an independent prognostic biomarker for survival outcomes. The subgroup analysis and Begg’s test showed that the results were stable.
Conclusion: Based on current evidence, this meta-analysis proves that the PNI score of UTUC patients before treatment is an independent prognostic biomarker. It performs well on OS, DFS/RFS/PFS, and CSS/DSS. This conclusion needs to be verified by a prospective cohort study with larger sample size and a more rigorous design.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022338503], identifier [CRD42022338503].
Introduction
Upper tract urothelial carcinoma (UTUC) is a malignant tumor, that locates from the calyx system to the distal ureter. UTUC is relatively rare, accounting for only 5–10% of urothelial carcinoma (1, 2). Currently, the standard treatment of non-metastatic UTUC remains radical nephroureterectomy (RNU) with bladder cuff excision. However, approximately 60% of patients with UTUC are invasive at diagnosis, and the prognosis is poor (3). Previous studies show that the 5-year specific survival is < 50% for UTUC patients with pT2 or pT3 and < 10% for pT4 (2). Some preoperative and postoperative factors, such as tumor stage, tumor grade, tumor size, and lymph node involvement, were suggested to predict prognosis in UTUC (4). Nonetheless, not every UTUC patient can receive surgical treatment or undergo radical surgery (5). Thus, the potential pretreatment prognostic marker is particularly important in UTUC.
The prognostic nutritional index (PNI) was originally described by Onodera et al. (6), which were calculated by serum albumin levels and peripheral lymphocyte count (7). PNI is a simple and easily accessible index used to evaluate the perioperative immune and nutritional status and risk of post-operative complications (8). Research has shown that PNI has been validated as an independent prognostic factor for various types of cancer (8–10).
Although some studies have been published, the role of PNI as a predictor of prognosis is still controversial in UTUC (7, 11). This study aims to evaluate whether the PNI may serve as an independent prognostic biomarker for patients with upper tract urothelial carcinoma, to assist clinicians in improving the prognosis of UTUC patients.
Materials and methods
Literature search and eligibility criteria
Based on the guidelines of Preferred Reporting Items for Systematic Reviews (12), we performed a systematic search to identify studies in PubMed, Embase, Scopus database, and Cochrane Library. The latest search time was April 2022. Search terms included: “upper tract urothelial cancer,” “UTUC,” “malignant tumor,” “radical nephroureterectomy,” “treatment,” “surgical*,” “prognostic nutritional index,” “PNI,” “predict*,” “prognostic*,” “factor,” “indicators.” Combine the above search fields with logical operators to get as many search results as possible. Besides, some research references were searched manually.
The inclusion and exclusion of the study were as follows: (1) Upper tract urothelial cancer was pathologically diagnosed, and there were no other types of malignant or metastatic cancer. (2) Before treatment, the prognostic nutritional index was calculated. (3) All the patients received surgical intervention: NU or RNU and did not receive other surgical treatment during the same period. (4) The researchers followed up with the patients for a certain period and were able to obtain at least one of the over survival (OS), cancer-specific survival (CSS), disease-specific survival (DSS), recurrence-free survival (RFS), progression-free survival (PFS), or disease-free survival (DFS). (5) The effects between the low PNI group and the high PNI group on the prognosis of surgical patients were evaluated, and the hazard ratio (HR) was presented in the study. (6) The design type of included study was retrospective or prospective. Letters, case reports, reviews, repeated studies, studies unrelated to the topic, animal experiments, and research without available data were excluded.
The process of identifying studies was completed independently by two authors (CM and LG). At the same time, data extraction and quality assessment were performed for the included studies. Negotiating between the two authors resolved the differences, and a consensus result was reached.
Quality evaluation
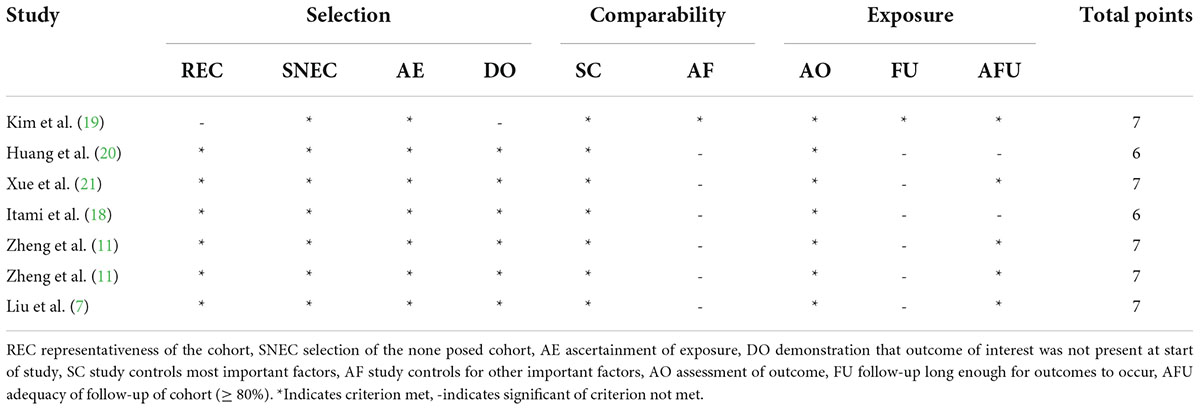
Based on the results of the identifying process, we used the NOS scale to assess the quality of included studies (13). The scale includes three question areas for selection, comparability, and exposure. The scale ranged from zero to nine stars, and studies with a score of six stars or more were considered high quality.
Data extraction
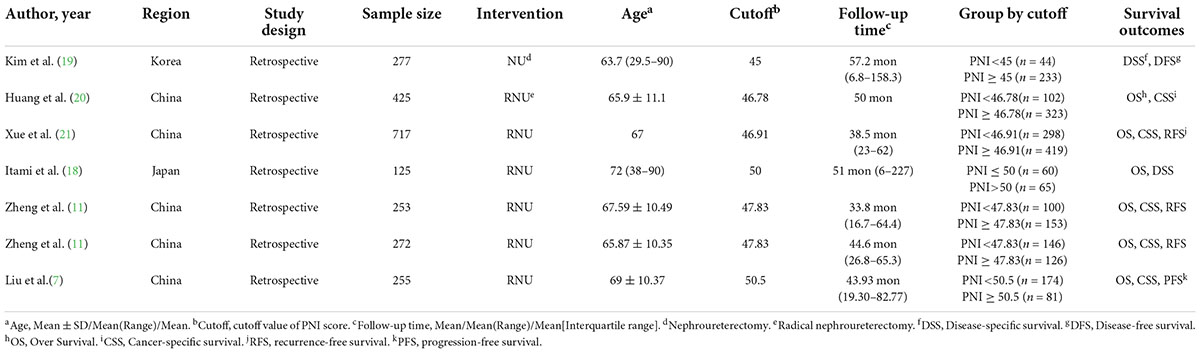
The researchers used the standard table to extract the following information from included studies: first author’s name, publication year, region, study design, sample size, intervention, mean age, cutoff value, follow-up time, survival statistics, hazard ratio (HR) and 95% confidence intervals (95% CI).
Data analysis
Data analysis was done by using Stata version 16.0 (StataCorp LP, University City, Texas, United States). Using the HR and its 95% CI of the multivariate analysis in each study to assess the importance of the PNI score for the prognosis of UTUC patients. In the meta-analysis, when the effect index is HR, the risk ratio is usually taken as the logarithm as the effect value (14). Therefore, we enter commands in the Stata 16 software to find the logarithmic values of HR, the upper limit of HR’s 95% CI, and the lower limit of HR’s 95% CI, and then perform the meta-analysis. The others can be extracted directly from the original study without conversion. We performed the Q test and χ2 test to value the heterogeneity between the included literatures. If I2 > 50%, the differences between the studies are considered significant, and random effect models are used (15). In addition, a sensitivity analysis is also carried out on this basis (16). We did subgroup analyses for each survival statistic based on the cutoff value. Begg’s test was used to test for publication bias between studies, and P < 0.05 was considered biased (17).
Results
Description of studies
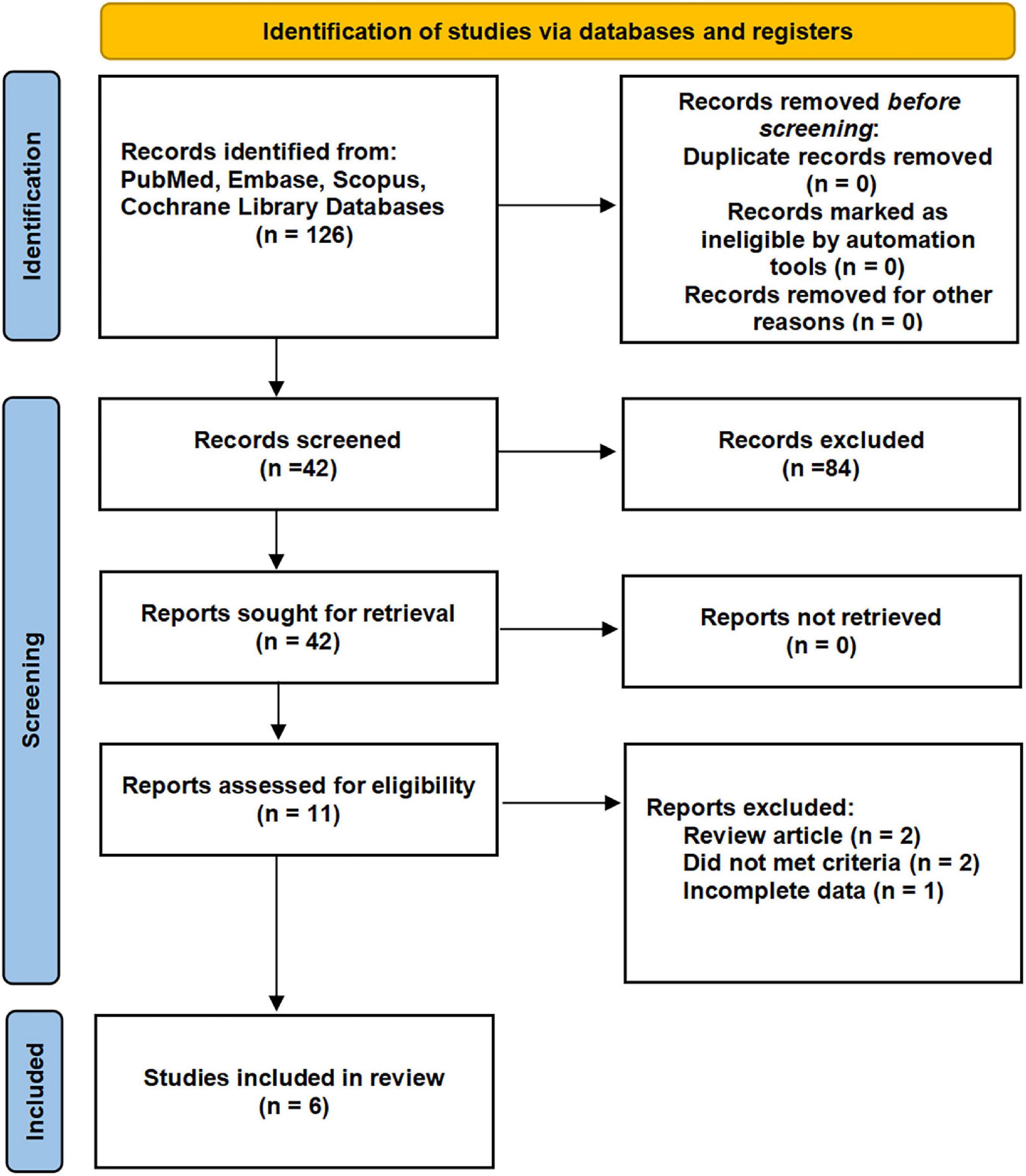
By the search process, 214 studies were screened from the established database, and two studies were searched manually. Six studies, including seven cohorts, were eventually included in our meta-analysis (7, 11, 18–21). The detailed systematic search process is shown in Figure 1. The baseline data of the included studies are given in Table 1, including age, region, type of study design, sample size, surgical type, cutoff value, follow-up time, grouping, and survival outcomes. Six studies, including 2,324 patients, were published between 2015 and 2022. The included studies were all retrospective studies.
Quality assessment
According to the scoring rules of the NOS scale, we assessed the quality of the studies. The quality scores of the included studies are recorded in Table 2. The quality scores of all included studies are ≥ 6 stars and are considered high quality.
Survival outcomes
The relationship between over survival and prognostic nutritional index score
Five studies, including six cohorts, revealed the correlation between preoperative PNI score and OS (7, 11, 18, 20, 21). According to the results of the heterogeneity test, there was no heterogeneity among the studies (I2 = 0%), and a fixed effects model was used to combine the effect size of each study. The outcomes of the meta-analysis demonstrated that lower preoperative PNI scores were associated with poorer OS (HR: 1.92; 95% CI 1.60 to 2.30; P < 0.01 Figure 2A).

Figure 2. Forest plot and meta-analysis. (A) Forest plot and meta-analysis of the relationship between over survival (OS) and prognostic nutritional index (PNI) score. (B) Forest plot and meta-analysis of the relationship between disease-free survival/recurrence-free survival/progression-free survival, and prognostic nutritional index score. (C) Forest plot and meta-analysis of the relationship between cancer-specific survival/disease-specific survival, and prognostic nutritional index score.
The relationship between disease-free survival/recurrence-free survival/progression-free survival, and prognostic nutritional index score
A total of five eligible studies revealed the prognostic role of pre-treatment PNI score on DFS/RFS/PFS in patients with UTUC (7, 11, 19, 21). Since there was no heterogeneity among studies (I2 = 0%), we used a fixed effects model to perform the meta-analysis. The ultimate result showed that the lower the preoperative PNI score of UTUC patients, the decreased their DFS/RFS/PFS (HR: 1.57; 95% CI 1.33 to 1.85; P < 0.01 Figure 2B).
The relationship between cancer-specific survival/disease-specific survival, and prognostic nutritional index score
Six studies, including seven cohorts, showed the correlation between preoperative PNI score and CSS/DSS (7, 11, 18–21). Given the heterogeneity test outcome (I2 = 0%), we used the fixed effects model. Our results suggested that a lower level of preoperative PNI was associated with decreased CSS/DSS (HR: 1.79; 95% CI 1.49 to 2.16; P < 0.01 Figure 2C).
Subgroup analysis
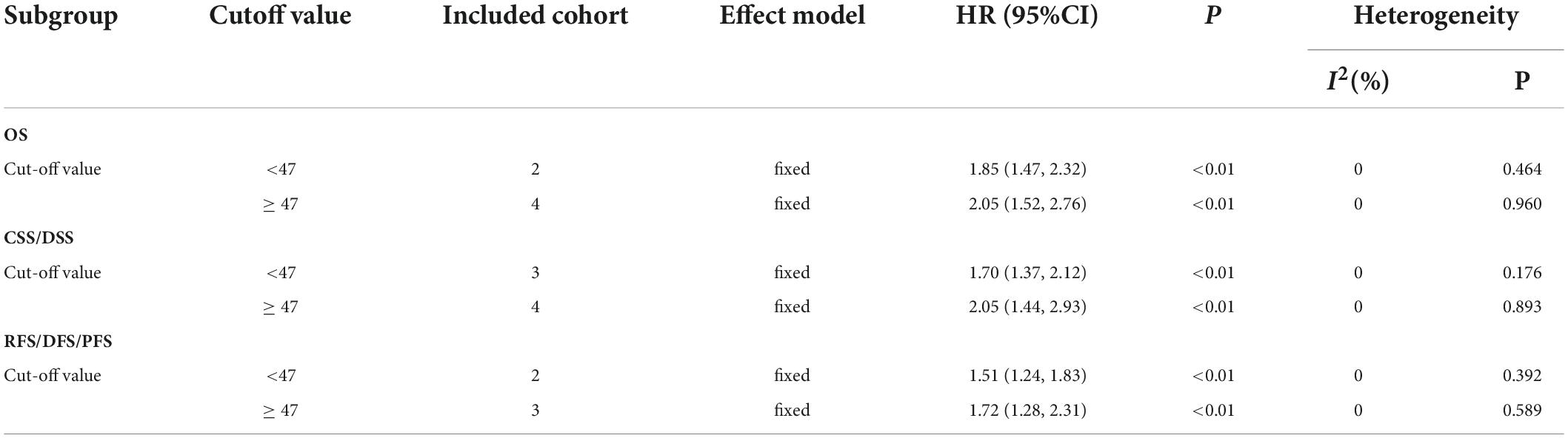
Owing to the lack of sufficient data, subgroup analysis was only performed in terms of cutoff value. Stratified analysis by the size of cutoff value also showed that a low pre-treatment PNI score was associated with the worse OS, DFS/RFS/PFS, and CSS/DSS (Table 3).
Sensitivity analysis
Sensitivity analysis was performed by excluding one single study once a time and recalculating the effect size of the remaining part. It reflected the impact of the individual on the whole. The result of our sensitivity analysis showed that no single study significantly influenced the pooled HR and 95% CI. This meant that our results were stable (Figure 3).
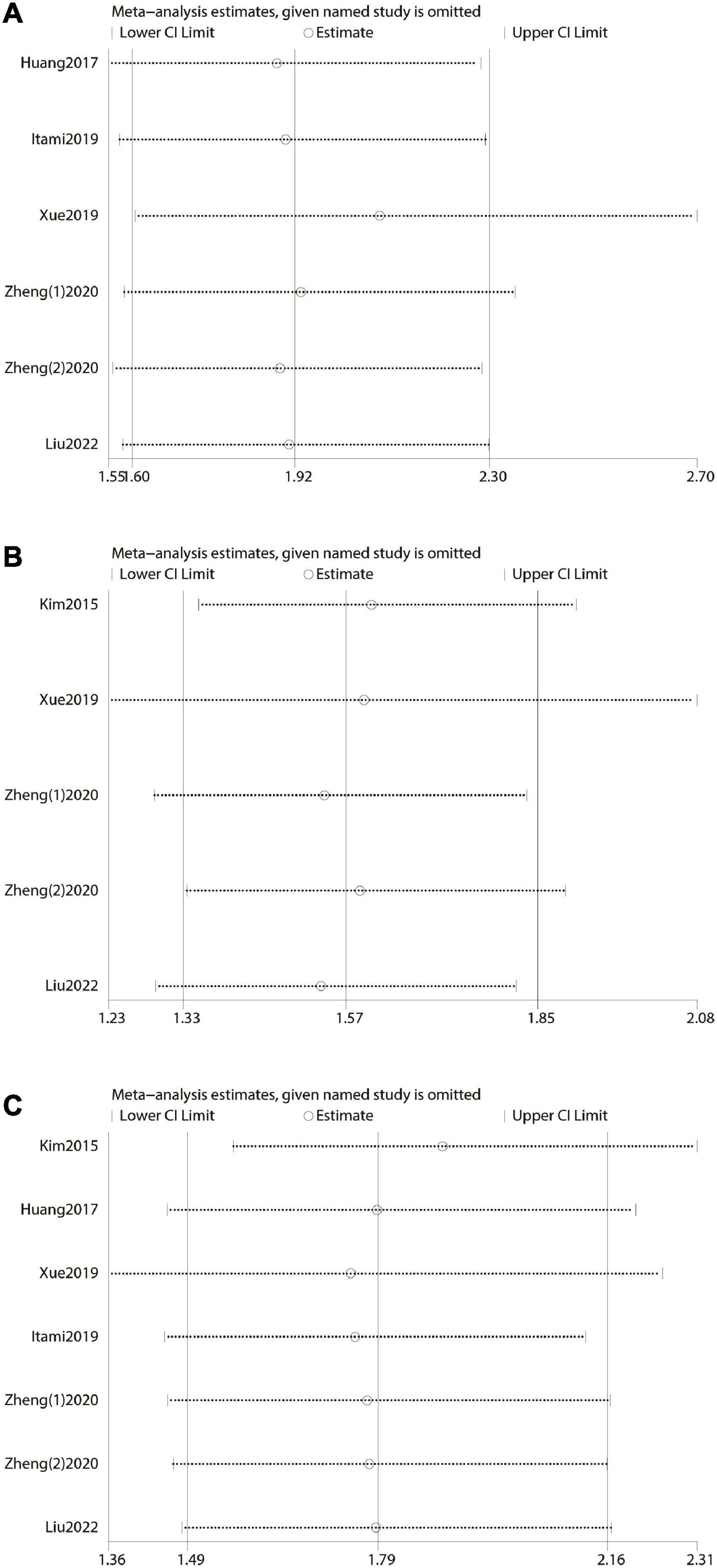
Figure 3. Forest plot and sensitivity analysis. (A) Forest plot and sensitivity analysis of the relationship between over survival (OS) and prognostic nutritional index (PNI) score. (B) Forest plot and sensitivity analysis of the relationship between disease-free survival/recurrence-free survival/progression-free survival, and prognostic nutritional index score. (C) Forest plot and sensitivity analysis of the relationship between cancer-specific survival/disease-specific survival, and prognostic nutritional index score.
Publication bias
In terms of OS or DFS/RFS/PFS or CSS/DSS, Publication bias was evaluated by Begg’s test. The P values of them were all above 0.05, showing no significant publication bias was found (Figure 4). That is to say, the results of our meta-analysis were reliable based on the available articles.
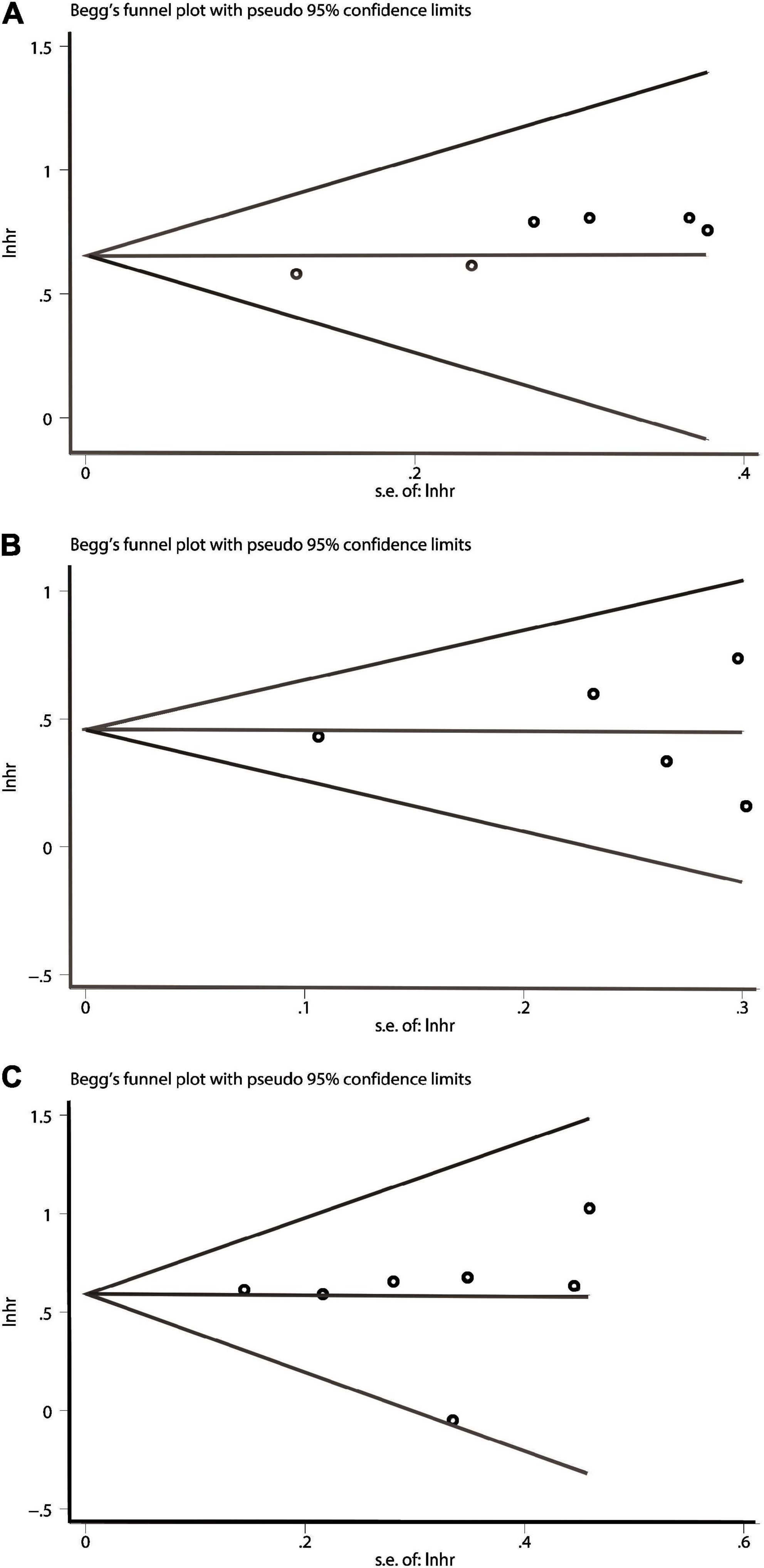
Figure 4. Begg’s test for publication bias. (A) Over survival (OS). (B) Disease-free survival/recurrence-free survival/progression-free survival (DFS/RFS/PFS). (C) cancer-specific survival/disease-specific survival (CSS/DSS).
Discussion
Although RNU was the standard treatment for UTUC, approximately one-third of UTUC patients who undergo surgery will experience early recurrence, and 80% of them will eventually die from UTUC (22). The current pre-operative prognostic indicters, such as c-reactive protein (23), fibrinogen (24), pre-treatment lymphocyte-monocyte ratio (25), and pre-treatment neutrophil-to-lymphocyte ratio (26), are helpful to the prediction of survival outcomes of UTUC patients, but it only focuses on inflammatory conditions. As is well-known, the nutritional status of tumor patients is closely related to their prognosis (27). Based on body mass index, serum albumin, and preoperative weight loss, Gregg et al. developed a simple model to predict 90-day mortality and 3-year OS in patients with bladder cancer (28). Moreover, a study conducted by Huang et al. (29) showed that decreased preoperative pre-albumin levels as an independent prognostic factor for CSS and OS in patients with UTUC.
Prognostic nutritional index (PNI) was a simple and accessible preoperative indicator that could provide a comprehensive and objective assessment of the inpatient’s condition. Due to the particularity of the PNI score composition, it could reflect the body’s protein metabolism and immune function, which were usually associated with the body’s nutritional status and immune response. Several retrospective studies have reported that PNI may be one of the potential predictors of postoperative survival outcomes in UTUC patients (7, 18). Consequently, we performed a meta-analysis to evaluate the impact of PNI on the prognosis outcomes in UTUC patients after surgical treatment.
This meta-analysis provided an evidence-based medicine analysis of six published studies exploring the prognostic and survival indicators of PNI in patients with UTUC. Our results showed that low PNI scores are associated with worse OS, DFS/RFS/PFS, and CSS/DSS, which supported the PNI score as an independent prognostic biomarker for survival outcomes.
Increasing evidence shows that the presence of nutritional deficiencies and systematic inflammatory response might play an important position in the development and progress of human cancers (30). Albumin is the main component of serum proteins, reflecting the nutritional status of the human body to a certain extent. It could regulate inflammatory reaction and exert antioxidant effects against carcinogens (31). In addition, low albumin levels reflect nutritional deficiencies, which could lead to reduced immune function and poor anticancer response (32). Recently, studies have shown that preoperative low albumin is an independent predictor of poor prognosis in patients with malignant tumors (33, 34). A study involving 214 glioblastoma patients have shown that serum albumin levels correlated with OS (HR = 0.966; 95% CI 0.938 to 0.995, P = 0.023) (35). Another study indicated that compared with those with hypoalbuminemia, vulvar cancer patients with normal albumin levels had a longer 5-year OS (58.6 vs. 17.1%, P = 0.004) (36). Furthermore, albumin levels are related to the systemic inflammatory response (37). Previous studies have found that albumin synthesis was reduced with the release of tumor necrosis factor. Under inflammatory conditions, the increased permeability of the vascular endothelium leads to albumin escape (38). Ishizuka et al. found that the relationship between hypoalbuminemia and poor postoperative outcome in patients with colorectal cancer was associated with increased inflammation (39). These studies proved the vital role of serum albumin as a nutritional indicator in cancer and inflammation, which supported the conclusions of this meta-analysis.
The relationship between inflammation and cancer was first described in the mid-19th century (40). In recent years, there has been increasing evidence of an association between inflammation, which is thought to be a pivotal event in the early development of cancer, and poor oncological prognosis (41, 42). Lymphocytes are common inflammatory cells in the tumor microenvironment and play an important anti-tumor effect in the immune system (42). In the advanced stage, tumor cells could destroy lymphocytes by editing proapoptotic ligands, and eventually achieve immune escape. In addition, the anti-tumor immune response mediated by CD8+ T lymphocytes also has an important role in the treatment of tumors. However, it doesn’t work endlessly. Some cancer-associated cells, such as fibroblasts, macrophages, and regulatory T cells, might produce an immune barrier to counteract the immune function of T cells, leading to a decrease in the number of T lymphocytes, tumor cell proliferation, and metastasis (43).
To our knowledge, this is the first meta-analysis to focus on the prognostic value of PNI in UTUC patients, and we followed PRISM guidelines strictly to perform this meta-analysis. However, some limitations cannot be avoided. First, the included studies are all retrospective studies, and the level of evidence is low. Second, the included studies are limited to East Asia, making the research results less universal. Third, due to the small number of studies available, not enough information is available to perform subgroup analysis to identify high-risk populations.
Conclusion
In conclusion, this meta-analysis revealed that the preoperative PNI is a potential independent biomarker of the postoperative prognosis of UTUC patients. A low PNI score predicts worse OS, DFS/RFS/PFS, and CSS/DSS in patients. Therefore, the clinician can individualize disease management for patients based on the PNI score for better treatment outcomes. This conclusion requires a larger sample size and a more rigorously designed prospective study to prove it.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL conceived and designed the experiments. CM, LP, and KL analyzed the data. LG, KL, and JL contributed reagents, materials, and analysis. CM, LG, and FY wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by Sichuan Science and Technology Program under Grant number: 2020YFS0320.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2021) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
2. Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. (2012) 62:100–14. doi: 10.1016/j.eururo.2012.02.030
3. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. (2009) 115:1224–33. doi: 10.1002/cncr.24135
4. Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. (2017) 35:379–87. doi: 10.1007/s00345-016-1928-x
5. Krabbe LM, Bagrodia A, Westerman ME, Gayed BA, Haddad AQ, Sagalowsky AI, et al. Molecular profile of urothelial carcinoma of the upper urinary tract: are pelvicalyceal and ureteral tumors different? World J Urol. (2016) 34:105–12. doi: 10.1007/s00345-015-1584-6
6. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
7. Liu J, Wu P, Lai S, Song X, Wang M, Wang X, et al. Clinicopathological and prognostic significance of preoperative prognostic nutritional index in patients with upper urinary tract urothelial carcinoma. Nutr Cancer. (2022) 74:2964–74. doi: 10.1080/01635581.2022.2049829
8. Hu X, Wang YH, Lia T, Yang ZQ, Shao YX, Yang WX, et al. Prognostic value of preoperative prognostic nutritional index in patients with renal cell carcinoma after nephrectomy. Clin Chim Acta. (2020) 509:210–6. doi: 10.1016/j.cca.2020.06.025
9. Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. (2021) 25:421–7. doi: 10.1007/s11605-019-04492-7
10. Hayasaka K, Shiono S, Suzuki K, Endoh M, Okada Y. Postoperative prognostic nutritional index as a prognostic factor after non-small cell lung cancer surgery. Gen Thorac Cardiovasc Surg. (2020) 68:1163–71. doi: 10.1007/s11748-020-01366-7
11. Zheng Y, Yu D, Yu Z, Zhao D, Chen Y, Chen W, et al. Association of preoperative systemic immune-inflammation index and prognostic nutritional index with survival in patients with upper tract urothelial carcinoma. J Cancer. (2020) 11:5665–77. doi: 10.7150/jca.44915
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
14. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:243.0.co;2-8
15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
16. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
17. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
18. Itami Y, Miyake M, Tatsumi Y, Gotoh D, Hori S, Morizawa Y, et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int J Clin Oncol. (2019) 24:533–45. doi: 10.1007/s10147-018-01381-y
19. Kim M, Moon KC, Choi WS, Jeong CW, Kwak C, Kim HH, et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World J Urol. (2015) 33:1439–57. doi: 10.1007/s00345-015-1484-9
20. Huang J, Yuan Y, Wang Y, Chen Y, Kong W, Xue W, et al. Preoperative prognostic nutritional index is a significant predictor of survival in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Urol Oncol. (2017) 35:.e1–671. doi: 10.1016/j.urolonc.2017.07.028
21. Xue W, Tan P, Xu H, Yang L, Wei Q. Impact of the preoperative prognostic nutritional index on survival outcomes in upper tract urothelial carcinomas. Cancer Med. (2019) 8:2971–8. doi: 10.1002/cam4.2161
22. Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. (2012) 61:818–25. doi: 10.1016/j.eururo.2012.01.021
23. Luo Y, Fu SJ, She DL, Xiong HU, Yang LI. Preoperative C-reactive protein as a prognostic predictor for upper tract urothelial carcinoma: a systematic review and meta-analysis. Mol Clin Oncol. (2015) 3:924–8. doi: 10.3892/mco.2015.553
24. Pichler M, Dalpiaz O, Ehrlich GC, Stojakovic T, Martin Hernandez JM, Mannweiler S, et al. Validation of the preoperative plasma fibrinogen level as a prognostic factor in a European cohort of patients with localized upper tract urothelial carcinoma. J Urol. (2014) 191:920–5. doi: 10.1016/j.juro.2013.10.073
25. Luo Z, Jiao B, Huang T, Zhao H, Zhang G. What is the role of the preoperative blood-based inflammation biomarkers in the prognosis of upper tract urothelial carcinoma with radical nephroureterectomy? A single-centre retrospective study. Technol Cancer Res Treat. (2022) 21:15330338221095667. doi: 10.1177/15330338221095667
26. Vartolomei MD, Mathieu R, Margulis V, Karam JA, Roupret M, Lucca I, et al. Promising role of preoperative neutrophil-to-lymphocyte ratio in patients treated with radical nephroureterectomy. World J Urol. (2017) 35:121–30. doi: 10.1007/s00345-016-1848-9
27. Mbeutcha A, Roupret M, Kamat AM, Karakiewicz PI, Lawrentschuk N, Novara G, et al. Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World J Urol. (2017) 35:337–53. doi: 10.1007/s00345-016-1826-2
28. Gregg JR, Cookson MS, Phillips S, Salem S, Chang SS, Clark PE, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. (2011) 185:90–6. doi: 10.1016/j.juro.2010.09.021
29. Huang J, Wang Y, Yuan Y, Chen Y, Kong W, Chen H, et al. Preoperative serum pre-albumin as an independent prognostic indicator in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Oncotarget. (2017) 8:36772–9. doi: 10.18632/oncotarget.13694
30. Tong T, Guan Y, Xiong H, Wang L, Pang J. A meta-analysis of glasgow prognostic score and modified glasgow prognostic score as biomarkers for predicting survival outcome in renal cell carcinoma. Front Oncol. (2020) 10:1541. doi: 10.3389/fonc.2020.01541
31. Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. (2014) 61:396–407. doi: 10.1016/j.jhep.2014.04.012
33. Liu M, Wang L. Prognostic significance of preoperative serum albumin, albumin-to-globulin ratio, and prognostic nutritional index for patients with glioma: a meta-analysis. Medicine (Baltimore). (2020) 99:e20927. doi: 10.1097/MD.0000000000020927
34. Omura S, Taguchi S, Miyagawa S, Matsumoto R, Samejima M, Ninomiya N, et al. Prognostic significance of the albumin-to-globulin ratio for upper tract urothelial carcinoma. BMC Urol. (2020) 20:133. doi: 10.1186/s12894-020-00700-8
35. Han S, Huang Y, Li Z, Hou H, Wu A. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer. (2015) 15:108. doi: 10.1186/s12885-015-1125-0
36. Bekos C, Polterauer S, Seebacher V, Bartl T, Joura E, Reinthaller A, et al. Pre-operative hypoalbuminemia is associated with complication rate and overall survival in patients with vulvar cancer undergoing surgery. Arch Gynecol Obstet. (2019) 300:1015–22. doi: 10.1007/s00404-019-05278-7
37. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers (Basel). (2020) 12:1986. doi: 10.3390/cancers12071986
38. Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. (2017) 83:1220–7. doi: 10.1177/000313481708301123
39. Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. (2007) 246:1047–51. doi: 10.1097/SLA.0b013e3181454171
40. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
41. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. (2016) 2016:6058147. doi: 10.1155/2016/6058147
42. Sung HH, Jeon HG, Jeong BC, Seo SI, Jeon SS, Choi HY, et al. Clinical significance of prognosis using the neutrophil-lymphocyte ratio and erythrocyte sedimentation rate in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. (2015) 115:587–94. doi: 10.1111/bju.12846
Keywords: prognostic nutritional index, upper tract urothelial carcinoma, prognostic biomarker, meta-analysis, PNI
Citation: Meng C, Gan L, Li K, Yi F, Peng L, Li J and Li Y (2022) Prognostic nutritional index before surgical treatment may serve as a prognostic biomarker for patients with upper tract urothelial carcinoma: A systematic review and meta-analysis. Front. Nutr. 9:972034. doi: 10.3389/fnut.2022.972034
Received: 17 June 2022; Accepted: 26 August 2022;
Published: 23 September 2022.
Edited by:
Kalliopi-Anna Poulia, Agricultural University of Athens, GreeceCopyright © 2022 Meng, Gan, Li, Yi, Peng, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Li, bGl5dW54aWFuZzM2OUAxMjYuY29t
†These authors have contributed equally to this work
 Chunyang Meng1†
Chunyang Meng1† Lei Peng
Lei Peng Jinze Li
Jinze Li Yunxiang Li
Yunxiang Li