- 1Key Laboratory of Animal Protein Deep Processing Technology of Zhejiang Province, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo, China
- 2Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 3Key Laboratory of Vector Biology and Pathogen Control of Zhejiang Province, Huzhou University, Huzhou, China
- 4Key Lab of Clean Energy and Green Circulation, College of Chemistry and Material Science, Huaibei Normal University, Huaibei, China
Identification of meat authenticity is a matter of increasing concerns due to religious, economical, legal, and public health reasons. However, little is known about the inspection of eight meat species in one tube reaction due to technological challenge of multiplex polymerase chain reaction (PCR) techniques. Here, a developed multiplex PCR method can simultaneously authenticate eight meat species including ostrich (753 bp), cat (564 bp), goose (391 bp), duck (347 bp), chicken (268 bp), horse (227 bp), dog (190 bp), and sheep (131 bp). The detectable deoxyribonucleic acid (DNA) contents for each target species was as low as 0.01 ng in both raw and heat-treated meat or target meat down to 0.1% (w/w) of total meat weight reflecting high stability of the assay in heat processing condition, indicating that this method is adequate for tracing meat origin in real-world meat products, which has been further validated by authenticity assays of commercial meat products. Overall, this method is a powerful tool for accurate evaluation of meat origin with a good application foreground.
Introduction
Some animal species such as ruminant (beef and sheep) and poultry (chicken and duck) are the popular meat resource along with the top consumption rate at all corners of the world, which can supply essential nutrients especially for the richest protein source (1, 2). However, the growing demand for animal protein has further exacerbated the incidence of meat frauds in animal protein-based foodstuffs, which has caused severe global issue (3–6). Meat adulteration, whether deliberately or unintentionally, not only breaks market rules but also violates ethical norms and religious laws. For example, pork consumption is strictly restricted in Islam and Judaism; beef is prohibited for the Hindus (7, 8). Most importantly, meat frauds risk the food safety and even threaten public health such as metabolic disorders, allergies and infectious diseases, because both inedible and edible meat products can occasionally elicit allergic reactions especially for sensitized patients (5, 6, 9–11). Based on this, meat molecular detection is important to protect consumers from being deceived, and ensure food safety in dietary practices.
Many analytical methods have been developed for meat authentication. Traditionally, protein-based methods such as enzyme-linked immunosorbent assay (ELISA) are more reliable to identify animal species, which have gained huge popularity in food business based on the simplicity and low cost (12). However, denaturation of protein under extreme thermal treatment prevents the accuracy of meat detection during food processing and let such techniques unsuitable for meat authentication especially in processed meat products (13, 14). Unlike proteins and peptides, deoxyribonucleic acid (DNA) molecules possess more stability against harsh thermal and chemical treatments (15, 16). Thus, DNA-based methods are more favorable for meat species detection in various meat samples. The polymerase chain reaction (PCR) approaches are well known DNA-based methods for detecting animal origin in foodstuffs, which include species-specific PCR, multiplex PCR, PCR-RFLP, PCR-RAPD, DNA barcoding, real-time PCR, and multiplex PCR (3). Among them, multiplex PCR assays are cost-effective and time-saving for simultaneous identification of multiple meat species. In particular, mitochondrial DNA (mtDNA) has multiple copies showing intraspecies conservation and interspecies polymorphism, which are proper target sites for species-specific primers. Moreover, DNA stability increases the chance of survival in processed products even at the condition of heat processing treatments, indicating that target sequences of mtDNA designated as target primers are adequate for meat inspection in heat processing foodstuffs. As reported, mtDNA sequences such as cytochrome b, 12S and 16S rRNA, D-loop, ATPase subunits 6 and 8, NADH dehydrogenases are common targets for designing species-specific primers of multiplex PCR (3, 17–21).
In this study, using mtDNA genes including 16S rRNA, NADH dehydrogenase subunit 1, 3, and 5, Cytochrome c oxidase subunit I, III, ATPase subunits 6, and D-loop as targets, species-specific primers for eight animal species ostrich, cat, goose, duck, chicken, horse, dog, and sheep were designed and then checked through the analyses of cross-reactivity, specificity, sensitivity, and robustness. Using the optimized PCR system, an octuplex PCR assay was ultimately developed with eight sets of species-specific primer pairs, which can inspect eight meat origin in both raw and processed meat products. To our knowledge, little is known about the molecular authentication of eight animal ingredients in one PCR reaction due to technological challenge of this multiplex PCR technique. Moreover, this method has been validated to be adequate for assessment of meat fraud incidences in commercial foodstuffs.
Materials and methods
Samples collection and deoxyribonucleic acid extraction
Fresh pure meat of 17 target species were purchased from local market, farm as well as online supermarket platform, which included 14 land animals of sheep (Ovis aries), dog (Canis lupus), horse (Equus caballus), chicken (Gallus gallus), duck (Anas platyrhynchos), turkey (Meleagris gallopavo), pigeon (Columba livia), camel (Camelus bactrianus), rabbit (Oryctolagus cuniculus), ostrich (Struthio camelus), cattle (Bos taurus), cat (Felis catus), goose (Anser cygnoides), and pig (Sus scrofa) as well as 3 aquatic species of small yellow croaker (Larimichthys polyactis), tuna (Thunnus orientalis) and black carp (Mylopharyngodon piceus). In addition, 30 commercial samples including raw and heat processing of meat balls (5), meat slices (5), kebab (3), sausages (6), jerky (5), and cutlets (4) were purchased from markets or online supermarket platform. All samples were transported under ice-chilled condition and stored at –80°C until further use for DNA extraction. Genomic DNA was isolated from each sample using a Beyotime kit (D0063, Beyotime Biotechnology Co., Ltd., Shanghai, China). The purity and concentration of extracted DNA were measured by a NanoDrop 2000 spectrophotometer (NanoDrop 2000, UV–Vis spectrophotometer, United States) (22).
Design of species-specific primers
The primers for each species were designed by targeting at mitochondria sequences including D-loop of sheep (GenBank Accession No. KP702285.1), ATPase subunits 6 of dog (MN181404.1), cytochrome c oxidase subunit I of horse (MN187574.1), NADH dehydrogenase subunit 3 of chicken (MK163565.1), NADH dehydrogenase subunit 1 of duck (MK770342.1), cytochrome c oxidase subunit III of goose (KJ124555.1), NADH dehydrogenase subunit 5 of cat (MT499915.1) and 16S rRNA of ostrich (Y12025.1). Combined the analyses of Oligo 7.0 and BLAST programs, species-specific primers were designed and optimized according to physical parameters of cross-reactivity, melting temperature, self-complementarity as well as secondary structures. The specificity of species-specific primers was confirmed by alignment against animal species including 14 land animals and 3 aquatic species as aforementioned by a ClustalW sequence alignment program and the MEGA6 software. Finally, the cross reaction was determined by species-specific primer pair against a total of 16 non-target animal species through simplex PCR assays. The information of primer sets in detail was shown in Table 1. The designed primers were synthesized by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd. (Shanghai, China) (22).
Simplex and multiplex polymerase chain reaction assays
A simplex PCR assay was constructed for target species with individual set of species-specific primers. PCR reaction of a final 25 μL volume contains 2.5 μL of 10 × EasyTaq® Buffer, 2 μL of 2.5 mM dNTPs, 0.5 μL of 5 U/μL EasyTaq DNA Polymerase, 0.5 μL of 10 μM each primer, genomic DNA and ddH2O. PCR amplification with deionized water in place of template DNA as a negative control to check any DNA contamination in each reaction system. PCR reaction was conducted by the initial denaturation at 94°C for 5 min, followed by 34 cycles of denaturation at 94°C for 30 s, annealing at 63°C for 30 s and extension at 72°C for 45 s, and a final extension at 72°C for 5 min in T100™ Thermal Cycler (Bio-Rad, Germany). For multiplex PCR assays, they were started from duplex to octuplex PCRs with optimized PCR system. PCR reaction system (25 μL) included 2.5 μL of 10 × EasyTaq® Buffer, 2 μL of 2.5 mM dNTPs, 0.5 μL of 5 U/μL EasyTaq DNA Polymerase, 0.5 μL of 10 μM each primer pair of eight species, DNA mixture of eight species at the indicated concentration and refilled ddH2O to 25 μL. PCR amplification was performed by T100™ Thermal Cycler under the same condition of PCR amplification as simplex PCR. PCR products were electrophoresed on a 5% agarose gel by using 4S GelRed Nucleic Acid Stain, and visualized by Gel DocTM XR + System with Image LabTM Software (BIO-RAD) (23).
Tests for specificity, sensitivity, and reproducibility
The specificity of each primer pair was individually checked by PCR assays against individual sample of all species (camel, pigeon, chicken, duck, horse, cattle, pork, turkey, goose, sheep, rabbit, ostrich, dog, cat, small yellow croaker, tuna, and black carp). Simplex and multiplex PCR results were run on 5% agarose gel and then visualized for the proper amplification. To determine the sensitivity of the multiplex assay, a series of PCR amplification were performed by using serial dilutions of the premixed genomic DNA templates of eight target species in one reaction. Ten concentrations of all target templates ranging from 10 to 0.01 ng were used for detecting the limit of detection (LOD). The amplified products were run on a 5% agarose gel for separation and visualization. To check the reproducibility of species-specific primers, raw meat samples for each species were deliberately subjected to heat processing treatment of boiled (97–99°C, 30 min) and microwave-cooked (750 W, 10 min) patterns. The robustness of PCR assay was evaluated with genomic DNA extracted from heat processing samples (22).
Results
Specificity of polymerase chain reaction assay
For all applied PCR systems, specificity is a prerequisite for multiplex assays. To construct a multiplex PCR assay, species-specific primers were designed through Oligo 7.0 and BLAST programs. As expected, PCR amplification with species-specific primers produced target bands with the predicted size of 753, 564, 391, 347, 268, 227, 190, and 131 bp for ostrich, cat, goose, duck, chicken, horse, dog, and sheep, respectively (Supplementary Figure 1A). As positive controls, three universal eukaryotic primer pairs targeting 18S, 16S, and 12S rRNA produced the predicted size of 99, 240, and 456 bp with similar intensities in all reaction tubes (Supplementary Figure 1B), suggesting the presence of high-quality DNA in all meat samples. As expected, the target bands were successfully amplified with target DNA isolated from a single species in the presence of premixed primers of all eight species but not seven non-target species (Supplementary Figure 1C), suggesting that primers designed for each species can specifically amplify target species. This conclusion was further supported by the assay that the target bands were generated by each set of species-specific primers in the presence of DNA mixture of all eight but not seven non-target species (Supplementary Figure 1D). In addition, cross-reaction of PCR amplification with each primer pair was further examined, which showed no cross-reactivity against sixteen non-target species indicated (data not shown). Collectively, the results demonstrated that the designed species-specific primers are adequate for food inspection.
Sensitivity of multiplex polymerase chain reaction assay
Based on simplex PCR system optimized, multiplex PCRs were gradually constructed and an octuplex PCR method was ultimately developed with eight pairs of species-specific primers. Serial dilution of each meat DNA ranging from 10 to 0.01 ng was used to determine the sensitivity of this assay in one PCR reaction. As shown in Figure 1A, PCR products of ostrich, cat, goose, duck, chicken, horse, dog, and sheep were availably detected from 10 to 0.01 ng DNA. Electropherograms were drawn from the bands by using Image Lab™ Software, in which the intensities of peak patterns reflected the brightness of bands. As shown in Figure 1B, intact peaks patterns for ostrich, cat, goose, duck, chicken, horse, dog, and sheep with gradually decreased intensities of peaks were observed from lane 1 to 10, suggesting that available inspection for all target species can be achieved at the low level of 0.01 ng DNA. Therefore, LOD of this octuplex PCR method was presumably 0.01 ng DNA.
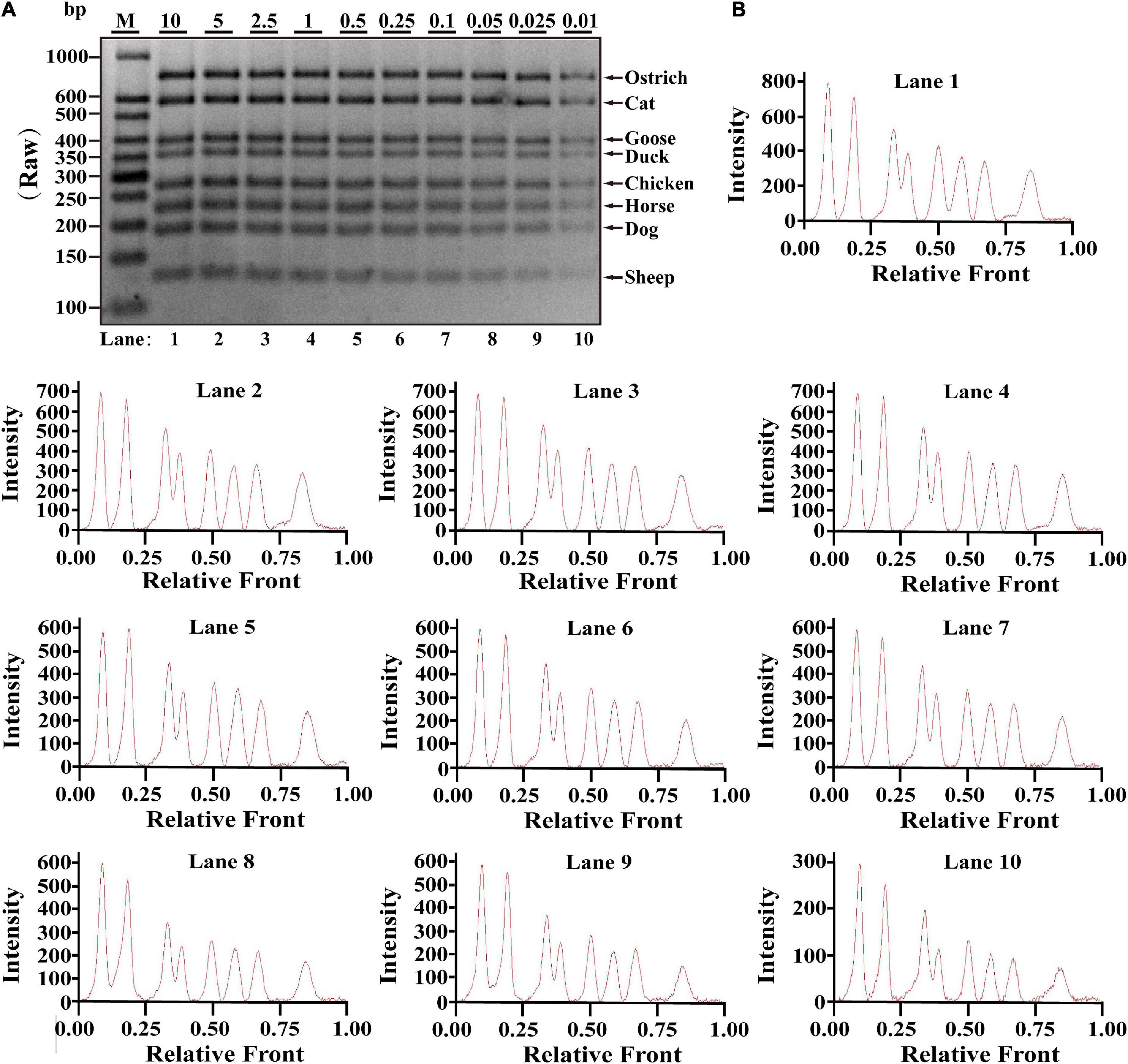
Figure 1. Validation of the sensitivity of multiplex PCR assay in raw meat samples. (A) Gel image of PCR fragments amplified by multiplex PCR using premixed DNA templates of eight species (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01 ng) with species-specific primers of eight meat species in a single PCR reaction. (B) The corresponding electropherogram of gel image represented ostrich, cat, goose, duck, chicken, horse, dog, and sheep in each lane. Lanes 1–10 are presented with labels (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01) in (A). The value of number at the horizontal line means the relative position of peaks distant from the top of agarose gel. The value of number at the vertical line means the fluorescent intensity of DNA-bound dyes using 4S GelRed Nucleic Acid Stain. Lane M is ladder DNA.
To determine the availability of this method in real-word foodstuffs, model sheep adulteration was constructed by simultaneous addition of seven meat tissues including ostrich, cat, goose, duck, chicken, horse and dog at 0.1, 0.25, 0.5, 1, 2.5, 5, or 10% of total weight, respectively. Next, the samples were subjected to genomic DNA extraction for multiplex PCR amplification. the specific amplicons for each species were clearly displayed even at target meat percentage of 0.1% (Supplementary Figures 2A,B).
Validation of reproducible multiplex assay under heat-treated meat materials
Since heat-processing treatments might cause DNA degradation, validation of PCR assay in terms of stability is essential for heat-treated samples prior to applying the technique on commercially processed food products, which was evaluated using DNA extracted from heat-processing samples of boiled and microwave-cooked treatments, respectively. For boiling meat samples, eight target bands were obviously observed at the range of 0.01–10 ng DNA; meanwhile, intact peak patterns for eight species were found in lanes 1–10 (Figures 2A,B), drawing a conclusion that LOD of this method was 0.01 ng DNA for boiling meat tissues. Using the same multiplex PCR system, template DNA from microwave-cooking meat tissues was used for assessing the availability of this multiplex PCR technique. Combined the analyses of agarose gel and electropherogram for microwave-cooking samples in Figures 3A,B, LOD of this method was about 0.01 ng DNA similar to that of boiling meat tissues. Taken together, the threshold value for inspection of heat processing meat was about 0.01 ng DNA.
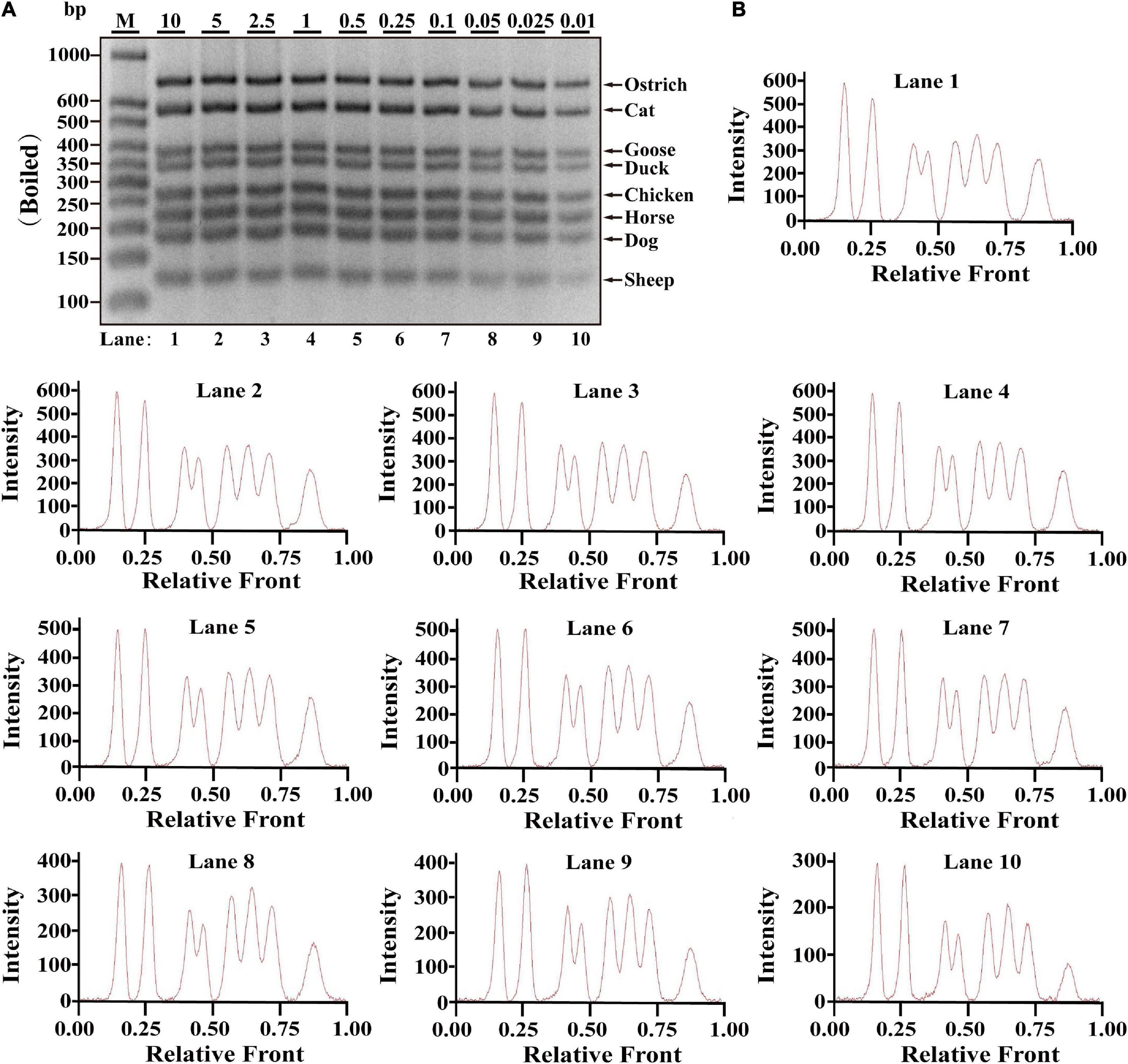
Figure 2. Validation of the sensitivity of multiplex PCR assay in boiling meat samples. (A) Gel image of PCR fragments amplified by multiplex PCR using premixed DNA templates of eight species (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01 ng) with species-specific primers of eight meat species in a single PCR reaction. (B) The corresponding electropherogram of gel image represented ostrich, cat, goose, duck, chicken, horse, dog, and sheep in each lane. Lanes 1–10 are presented with labels (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01) in (A). The value of number at the horizontal line means the relative position of peaks distant from the top of agarose gel. The value of number at the vertical line means the fluorescent intensity of DNA-bound dyes using 4S GelRed Nucleic Acid Stain. Lane M is ladder DNA.
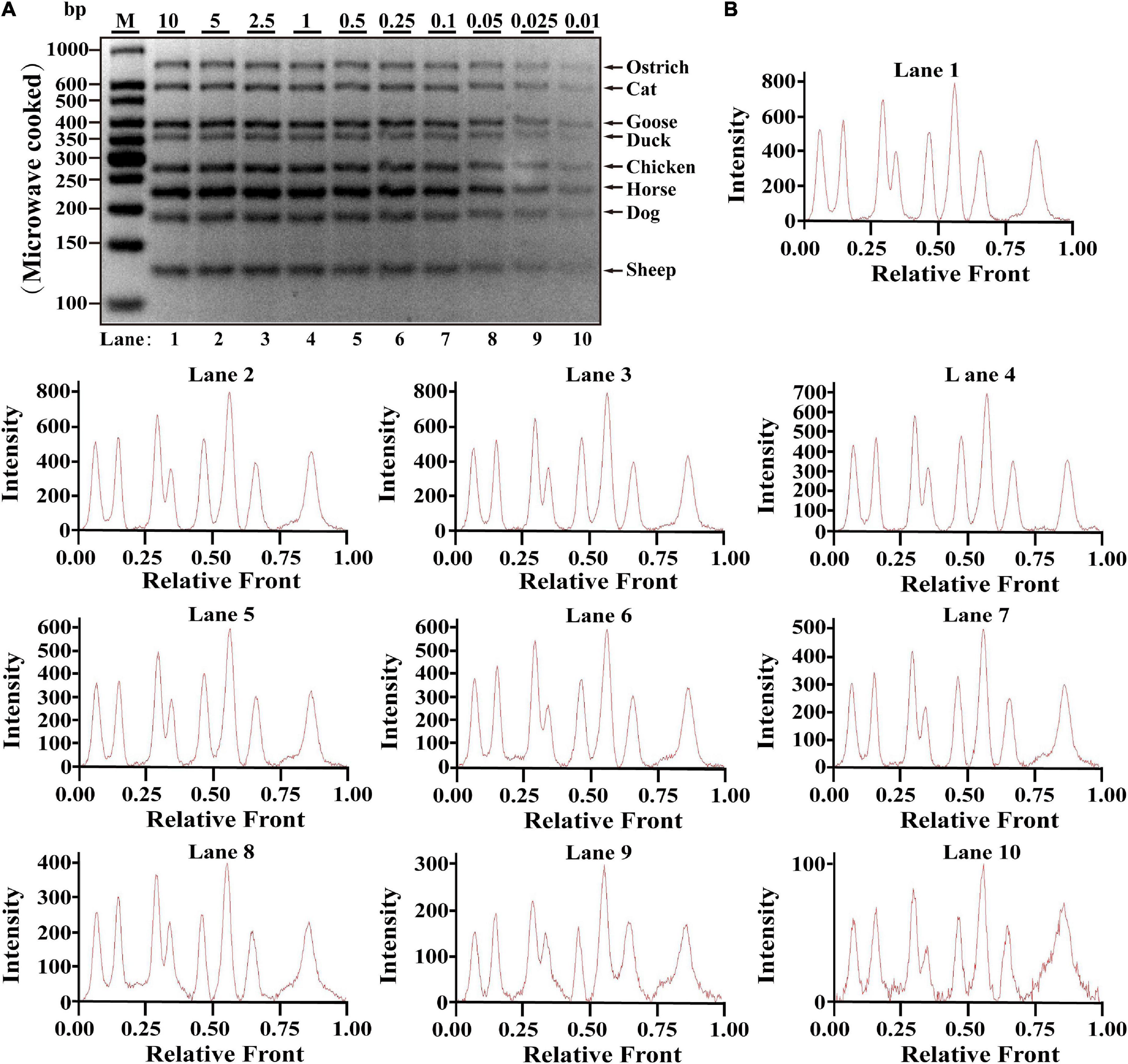
Figure 3. Validation of the sensitivity of multiplex PCR assay in microwave-cooking meat samples. (A) Gel image of PCR fragments amplified by multiplex PCR using premixed DNA templates of eight species (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01 ng) with species-specific primers of eight meat species in a single PCR reaction. (B) The corresponding electropherogram of gel image represented ostrich, cat, goose, duck, chicken, horse, dog, and sheep in each lane. Lanes 1–10 are presented with labels (10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, and 0.01) in (A). The value of number at the horizontal line means the relative position of peaks distant from the top of agarose gel. The value of number at the vertical line means the fluorescent intensity of DNA-bound dyes using 4S GelRed Nucleic Acid Stain. Lane M is ladder DNA.
Application of multiplex polymerase chain reaction assay on commercial meat products
The applicability of this assay was checked with a total of 30 popular consumption products in food items of meat balls, meat slices, kebab, sausages, cutlets, and jerky. As shown in Supplementary Table 1, most samples contained the same meat origin as declared on the label. However, some products had contaminated with extra ingredients unlabeled. As illustrated, 3 of 10 (30%) sheep samples, 3 of 10 (30%) horse samples, and 2 of 10 (20%) ostrich samples had been validated to adulterate with meat ingredients unlisted. From the survey, some mislabeling samples often contaminated poultry meat such as duck, chicken, and goose, which were undeclared on the product labels. The mislabeling of meat products may be intentionally contaminated with cheaper meats for economic profit or unintentionally cross-contaminated in the production chain. For unintentional contamination, the sensitive method is necessary to monitor a little amount of meat contamination. From this survey, the proposed technique can be availably applied to monitor and control meat contamination.
Discussion
Multiplex PCR techniques are highly favorable for the inspection of multiple targets in a single platform, which have gained huge popularity in food business based on the simplicity and low cost through simple agarose gel analysis (24–26). However, mutual interference of components such as templates and primers would become more complex with the increase of more primers and multiplicity of multiplex PCR reaction, which often cause lower efficiency and even the failure of amplification (8), indicating that multiplex PCRs are often subjected to technological challenge. Through the survey of multiplex PCRs recently published in Supplementary Table 2, multiplex PCRs generally detect less than eight animal origin in one-tube reaction platform. To our knowledge, there is only one report for monitoring eight meat ingredients through one-tube multiplex PCR method with the help of universal primers (27). Here, this proposed method can identify eight meat origin by eight sets of highly specific primers without extra universal primers in one tube reaction.
The specificity of primers is a prerequisite for multiplex PCR assays. To obtain a specific multiplex PCR assay, the primers should specifically match the target species, and have huge mismatches with non-targets (19, 28). Accordingly, the feature of primers is crucial for accurate authentication of meat species. By Oligo 7.0 and BLAST programs, target primers showed more stringent specificity and shared similar melting temperature to that of other targets ensuring to anneal with target templates under the same set of PCR conditions. As reported, even a single base pair that mismatches at the 3′ end of the primers with target DNA might interfere with the efficiency of PCR amplification (29). In this regard, target primers were stringently evaluated on base mismatches within primer annealing sites and then were aligned in silico against 16 other non-target species as mentioned. The sequences of each primer pair completely match with target species. Furthermore, the specificity of target primers was confirmed based on species-specific amplification of PCR assays. Notably, species-specific primers produced target bands with differential intensities under the same PCR condition, indicating that target primers have different amplification efficiency in this multiplex PCR system.
Referring to multiplex PCR assays (19, 24, 30), serial dilution of each meat DNA ranging from 10 to 0.01 ng was used to determine LOD of this method in one PCR reaction. Since it generated weaker bands for some species at the concentration of 0.01 ng DNA (Figures 1–3), lower DNA levels of each meat such as 1 pg or 0.01 pg were not further tested for LOD. Compared to LOD of multiplex PCR assays varying from 1 pg to 0.32 ng (Supplementary Table 1), LOD of this method is as low as 0.01 ng DNA in various meat samples of raw, boiled and microwave-cooked meat materials, suggesting that this method is qualified for monitoring meat resource. However, over-representation of certain species in an unknown sample might disguise the low presence of another one and generate a false-negative result. Most importantly, determination of LOD was accomplished by three independent experiments. In addition, economic benefits are a critical factor for the substitution of expensive and high-quality meat with inferior and low-cost ones and therefore the amounts of adulterated ingredients should be easily detected in real-word foodstuffs. The reproducibility assay reflected high stability of the method even for samples undergoing harsh heat-processing condition, which provides substantial evidences for the availability of the application of this PCR assay on commercial meat products (Figures 2, 3). Consistent with other reports (30–32), this multiplex PCR method demonstrates that meat fraud with cheap or poor-quality meat has become a commonplace in real-world foodstuffs (Supplementary Table 1), suggesting that this method is indeed adequate for identification of meat species in actual adulteration event due to its high sensitivity. Overall, the proposed octuplex PCR technique is a powerful tool for accurate evaluation of meat origin, which is crucial to safeguard consumers from meat fraud and contributes to establish discipline in food business.
Conclusion
This study provides a reliable, low-cost, and rapid approach, which offers unambiguous detection and discrimination of eight animal species. Compared to multiplex PCRs documented, the detectable mtDNA contents for each target species were as low as 0.01 ng DNA in various meat materials so that the proposed multiplex PCR is highly promising for meat authentication in actual adulteration event, which is also easily implemented by simple agarose gel analysis without specific sophisticated equipment. The availability of the method has been corroborated by the application of multiplex PCR on commercial meat products. Therefore, molecular authentication or molecular traceability of meat origins through this multiplex PCR technique has provided an accurate evaluation of meat ingredients in real-world foodstuffs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SW, QL, and ZC: conception and design of the investigation and work. CY, GZ, SZ, YG, and ZC: completion of the experiments. CY, GZ, DP, QX, SW, QL, and ZC: evaluation and analysis of the results. CY, GZ, SW, QL, and ZC: manuscript writing. CY, GZ, SZ, YG, SW, QL, and ZC: final approval of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 31901668), the Natural Science Foundation of Zhejiang Province of China (No. LY22C200002), and the Natural Science Foundation of Ningbo (No. 2021J108).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.979977/full#supplementary-material
References
1. Uddin SMK, Hossain MAM, Chowdhury ZZ, Johan MRB. Short targeting multiplex PCR assay to detect and discriminate beef, buffalo, chicken, duck, goat, sheep and pork DNA in food products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2021) 38:1273–88. doi: 10.1080/19440049.2021.1925748
2. Hossain MAM, Ali ME, Abd Hamid SB, Mustafa S, Desa MNM. Targeting double genes in multiplex PCR for discriminating bovine, buffalo and porcine materials in food chain. Food Control. (2017) 73:175–84. doi: 10.1016/j.foodcont.2016.08.008
3. Li YC, Liu SY, Meng FB, Liu DY, Zhang Y, Wang W, et al. Comparative review and the recent progress in detection technologies of meat product adulteration. Compr Rev Food Sci Food Saf. (2020) 19:2256–96. doi: 10.1111/1541-4337.12579
4. Uddin SMK, Hossain MM, Chowdhury ZZ, Johan MR. Detection and discrimination of seven highly consumed meat species simultaneously in food products using heptaplex PCR-RFLP assay. J Food Composit Anal. (2021) 100:103938.
5. Wang WJ, Liu JJ, Zhang QD, Zhou X, Liu B. Multiplex PCR assay for identification and quantification of bovine and equine in minced meats using novel specific nuclear DNA sequences. Food Control. (2019) 105:29–37. doi: 10.1016/j.foodcont.2019.05.016
6. Li JC, Li JP, Liu RX, Wei YX, Wang SW. Identification of eleven meat species in foodstuff by a hexaplex real-time PCR with melting curve analysis. Food Control. (2021) 121:107599.
7. Li TT, Jalbani YM, Zhang GL, Zhao ZY, Wang ZY, Zhao XY, et al. Detection of goat meat adulteration by real-time PCR based on a reference primer. Food Chem. (2019) 277:554–7. doi: 10.1016/j.foodchem.2018.11.009
8. Li JC, Li JP, Xu SG, Xiong SY, Yang JN, Chen X, et al. A rapid and reliable multiplex PCR assay for simultaneous detection of fourteen animal species in two tubes. Food Chem. (2019) 295:395–402. doi: 10.1016/j.foodchem.2019.05.112
9. Safdar M, Junejo Y, Arman K, Abasiyanik MF. A highly sensitive and specific tetraplex PCR assay for soybean, poultry, horse and pork species identification in sausages: development and validation. Meat Sci. (2014) 98:296–300. doi: 10.1016/j.meatsci.2014.06.006
10. Ashaolu TJ, Yupanqui CT. Suppressive activity of enzymatically-educed soy protein hydrolysates on degranulation in IgE-antigen complex-stimulated RBL-2H3 cells. Funct Foods Health Dis. (2017) 7:545–61.
11. Tanabe S, Miyauchi E, Muneshige A, Mio K, Sato C, Sato M. PCR method of detecting pork in foods for verifying allergen labeling and for identifying hidden pork ingredients in processed foods. Biosci Biotech Biochem. (2007) 71:1663–7. doi: 10.1271/bbb.70075
12. Zhao L, Hua MZ, Li S, Liu J, Zheng W, Lu X. Identification of donkey meat in foods using species-specific PCR combined with lateral flow immunoassay. RSC Adv. (2019) 9:26552–8. doi: 10.1039/c9ra05060d
13. Asensio L, Gonzalez I, Garcia T, Martin R. Determination of food authenticity by enzyme-linked immunosorbent assay (ELISA). Food Control. (2008) 19:1–8. doi: 10.1016/j.foodcont.2007.02.010
14. Sentandreu MA, Sentandreu E. Authenticity of meat products: tools against fraud. Food Res Int. (2014) 60:19–29. doi: 10.1016/j.foodres.2014.03.030
15. Mansouri M, Khalilzadeh B, Barzegari A, Shoeibi S, Isildak S, Bargahi N, et al. Design a highly specific sequence for electrochemical evaluation of meat adulteration in cooked sausages. Biosens Bioelectron. (2020) 150:111916. doi: 10.1016/j.bios.2019.111916
16. Mokhtar NFK, El Sheikha AF, Azmi NI, Mustafa S. Potential authentication of various meat-based products using simple and efficient DNA extraction method. J Sci Food Agric. (2020) 100:1687–93. doi: 10.1002/jsfa.10183
17. Fajardo V, Gonzalez I, Rojas M, Garcia T, Martin R. A review of current PCR-based methodologies for the authentication of meats from game animal species. Trends Food Sci Tech. (2010) 21:408–21. doi: 10.1016/j.tifs.2010.06.002
18. Kumar A, Kumar RR, Sharma BD, Gokulakrishnan P, Mendiratta SK, Sharma D. Identification of species origin of meat and meat products on the DNA Basis: a review. Crit Rev Food Sci. (2015) 55:1340–51. doi: 10.1080/10408398.2012.693978
19. Ali ME, Razzak MA, Abd Hamid SB, Rahman MM, Al Amin M, Abd Rashid NR, et al. Multiplex PCR assay for the detection of five meat species forbidden in Islamic foods. Food Chem. (2015) 177:214–24. doi: 10.1016/j.foodchem.2014.12.098
20. Galal-Khallaf A. Multiplex PCR and 12S rRNA gene sequencing for detection of meat adulteration: a case study in the Egyptian markets. Gene. (2021) 764:145062. doi: 10.1016/j.gene.2020.145062
21. Thanakiatkrai P, Dechnakarin J, Ngasaman R, Kitpipit T. Direct pentaplex PCR assay: an adjunct panel for meat species identification in Asian food products. Food Chem. (2019) 271:767–72. doi: 10.1016/j.foodchem.2018.07.143
22. Cai ZD, Zhong GW, Liu QQ, Yang XQ, Zhang XX, Zhou S, et al. Molecular authentication of twelve meat species through a promising two-tube hexaplex polymerase chain reaction technique. Front Nutr. (2022) 9:813962. doi: 10.3389/fnut.2022.813962
23. Yaman BN, Celik PA, Mutlu MB, Cabuk AA. Combinational analysis of acidophilic bacterial diversity of an iron-rich environment. Geomicrobiol J. (2020) 37:877–89. doi: 10.1080/01490451.2020.1795320
24. Iqbal M, Saleem MS, Imran M, Khan WA, Ashraf K, Zahoor MY, et al. Single tube multiplex PCR assay for the identification of banned meat species. Food Addit Contam B Surveill. (2020) 13:284–91. doi: 10.1080/19393210.2020.1778098
25. Wang WJ, Wang XK, Zhang QD, Liu ZH, Zhou X, Liu B. A multiplex PCR method for detection of five animal species in processed meat products using novel species-specific nuclear DNA sequences. Eur Food Res Technol. (2020) 246:1351–60. doi: 10.1007/s00217-020-03494-z
26. Mafra I, Ferreira IMPLVO, Oliveira MBPP. Food authentication by PCR-based methods. Eur Food Res Technol. (2008) 227:649–65. doi: 10.1007/s00217-007-0782-x
27. Liu WW, Tao J, Xue M, Ji JG, Zhang YH, Zhang LJ, et al. A multiplex PCR method mediated by universal primers for the identification of eight meat ingredients in food products. Eur Food Res Technol. (2019) 245:2385–92. doi: 10.1007/s00217-019-03350-9
28. Murugaiah C, Noor ZM, Mastakim M, Bilung LM, Selamat J, Radu S. Meat species identification and Halal authentication analysis using mitochondrial DNA. Meat Sci. (2009) 83:57–61. doi: 10.1016/j.meatsci.2009.03.015
29. Wu J-H, Hong P-Y, Liu W-T. Quantitative effects of position and type of single mismatch on single base primer extension. J Microbiol Methods. (2009) 77:267–75. doi: 10.1016/j.mimet.2009.03.001
30. Hou B, Meng XR, Zhang LY, Guo JY, Li SW, Jin H. Development of a sensitive and specific multiplex PCR method for the simultaneous detection of chicken, duck and goose DNA in meat products. Meat Sci. (2015) 101:90–4. doi: 10.1016/j.meatsci.2014.11.007
31. Mane BG, Mendiratta SK, Tiwari AK. Polymerase chain reaction assay for identification of chicken in meat and meat products. Food Chem. (2009) 116:806–10. doi: 10.1016/j.foodchem.2009.03.030
32. Yacoub HA, Sadek MA. Identification of fraud (with pig stuffs) in chicken-processed meat through information of mitochondrial cytochrome b. Mitochondrial DNA A DNA Mapp Seq Anal. (2017) 28:855–9. doi: 10.1080/24701394.2016.1197220
33. Vaithiyanathan S, Vishnuraj MR, Reddy GN, Kulkarni VV. Application of DNA technology to check misrepresentation of animal species in illegally sold meat. Biocatal Agric Biotech. (2018) 16:564–8. doi: 10.1016/j.bcab.2018.10.012
Keywords: multiplex PCR, meat species, molecular authenticity, primer specificity, commercial foodstuffs
Citation: Yang C, Zhong G, Zhou S, Guo Y, Pan D, Wang S, Liu Q, Xia Q and Cai Z (2022) Detection and characterization of meat adulteration in various types of meat products by using a high-efficiency multiplex polymerase chain reaction technique. Front. Nutr. 9:979977. doi: 10.3389/fnut.2022.979977
Received: 28 June 2022; Accepted: 29 August 2022;
Published: 16 September 2022.
Edited by:
Fatih Öz, Atatürk University, TurkeyReviewed by:
Hermann Broll, Bundesinstitut für Risikobewertung (BfR), GermanyFrancisco Javier Navas González, University of Córdoba, Spain
Ziyi Zheng, University of Electronic Science and Technology of China, China
Copyright © 2022 Yang, Zhong, Zhou, Guo, Pan, Wang, Liu, Xia and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Wang, d2FuZ3NoYUB6amh1LmVkdS5jbg==; Qianqian Liu, Y3N1bGl1cWlhbkBmb3htYWlsLmNvbQ==; Zhendong Cai, emhlbmRvbmdjYWlAaG90bWFpbC5jb20=; Y2FpemhlbmRvbmdAbmJ1LmVkdS5jbg==
†These authors have contributed equally to this work
 Caijiao Yang1†
Caijiao Yang1† Guowei Zhong
Guowei Zhong Song Zhou
Song Zhou Daodong Pan
Daodong Pan Sha Wang
Sha Wang Qiang Xia
Qiang Xia Zhendong Cai
Zhendong Cai