- 1Department of Human Biology, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands
- 2Endometriosis Center, Amsterdam University Medical Centers, Academic Medical Center, Amsterdam, Netherlands
- 3Amsterdam Reproduction and Development Research Institute, Amsterdam, Netherlands
- 4Division of Gastroenterology-Hepatology, Department of Internal Medicine, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands
- 5Centre for Healthy Eating & Food Innovation (HEFI), Maastricht University, Maastricht, Netherlands
- 6Department of Obstetrics and Gynecology, Máxima Medical Center, Veldhoven, Netherlands
- 7Grow-School of Oncology and Reproduction, Maastricht University, Maastricht, Netherlands
- 8Department of Obstetrics and Gynaecology MUMC+, Maastricht, Netherlands
Endometriosis is characterized by the presence of endometrium-like tissue outside the uterus. The etiology remains largely unknown. Despite adequate treatment, patients can still experience symptoms or side effects resulting in therapy incompliance and in self-management strategies such as dietary measures is increasing. A gluten free diet is thought to be contributory in reducing endometriosis-related pain, thereby optimizing quality of life. However, data is conflicting and currently provides no evidence for causality. This narrative review aims to put the effect of dietary self-management strategies on endometriosis in a balanced perspective, especially the effect of gluten and a gluten free diet. Several studies have found a strong overlap in symptoms, metabolic and immune responses associated with endometriosis and those associated with celiac disease, ulcerative colitis, Crohn’s disease, irritable bowel syndrome and non-celiac wheat sensitivity. However, it remains unclear whether these diseases and/or disorders are causal to an increased risk of endometriosis. Some studies have found a positive effect on the risk of endometriosis, endometriosis-related symptoms and quality of life (QoL) when women either avoided certain nutrients or foods, or applied a specific nutrient supplementation. This includes the avoidance of red meat, an increasing intake of foods rich in anti-oxidants, omega-3, micronutrients and dietary fibers (e.g., fruit, vegetables) and the appliance of a gluten free diet. However, data from the available studies were generally graded of low quality and it was noted that placebo and/or nocebo effects influenced the reported positive effects. In addition, such effects were no longer seen when adjusting for confounders such as overweight, when a translation was made from in vitro to in vivo, or when the nutrients were not supplemented as isolated sources but as part of a mixed daily diet. Finally, some studies showed that long-term adherence to a gluten free diet is often associated with an impaired diet quality and nutrient intake, leading to negative health outcomes and reduced QoL. Concluding, scientific evidence on the efficacy of dietary interventions on well-defined clinical endpoints of endometriosis is lacking and recommending a gluten free diet to women solely diagnosed with endometriosis should therefore not be advised.
Introduction
Endometriosis is characterized by the presence of endometrium-like tissue outside the endometrium and myometrium, usually with an associated inflammatory process (1). The presence of intra-abdominal endometriosis can be suspected based on clinical symptoms such as dysmenorrhea, dyschezia, hematochezia, dysuria, hematuria, dyspareunia, chronic pelvic pain and infertility. Complaints are often related to the menstrual cycle and may be progressive in nature. Therefore, endometriosis can be associated with reduced mental, physical and social wellbeing leading to a lower quality of life (QoL) (2–4). Because of this, Saunders and Horne (5) recently proposed to consider endometriosis as a syndrome rather than a single disease state. The QoL impairment, the risk of work-related disability and unemployed work status as well as the related costs are high and similar to other widespread chronic inflammatory conditions such as Diabetes Mellitus Type 2, Crohn’s disease and Rheumatoid Arthritis (1, 3, 6, 7).
Because many endometriosis symptoms strongly overlap with other chronic conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD) and celiac disease (CD) (see Figure 1), early diagnosis is often missed (9–16). In the Netherlands it may take up to 10 years, with a median delay of 7.4 years between a first visit to a medical practitioner and a confirmed diagnosis of endometriosis (17). During the period of diagnostic delay, 75% of women consult 1–4 medical professionals for their symptoms because they are not satisfied with the advice and/or treatment offered (18). In general, it can be stated that clinical symptoms and the experience of the patient do not always reflect the severity of the disease (19). Treatment of endometriosis consists of four pillars: hormonal therapy, pain management, surgery and/or therapy with assisted reproductive techniques (ART). Nevertheless, applied medical and/or surgical treatment methods can be insufficient to alleviate symptoms or may be accompanied with side effects. Both can affect treatment compliance negatively (20–22). This has resulted in an increasing interest in self-management strategies among women diagnosed with endometriosis (23).
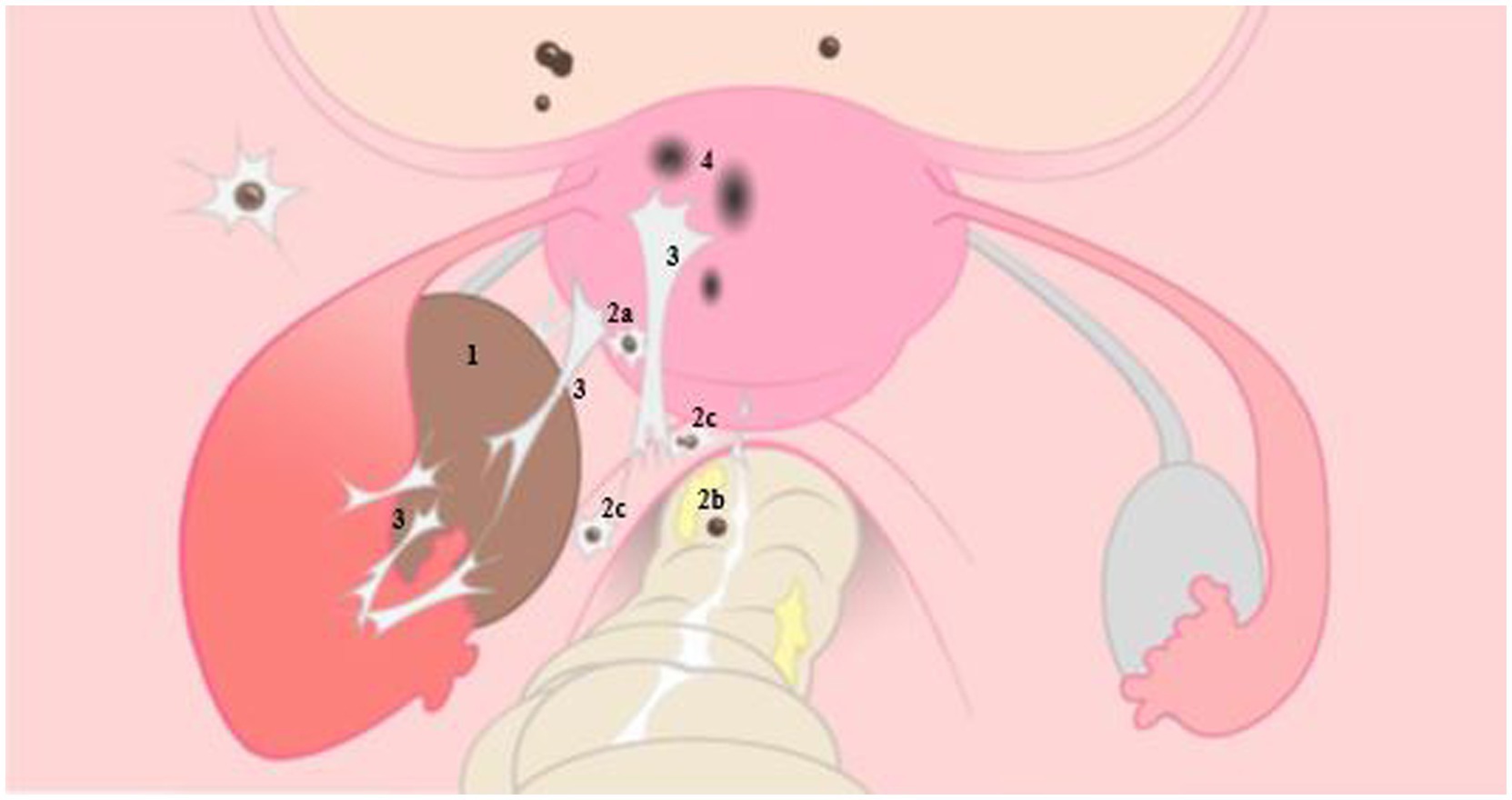
Figure 1. Illustrative representation of endometriosis on the left, with normal anatomy of the female reproductive organ on the right. 1: ovarian endometrioma, 2: deep endometriosis located on the bladder and posterior uterine wall (2a), on the rectal wall (2b) and sacro-uterine ligament (2c), 3: adhesions caused by endometriosis, 4: adenomyosis. With permission L. van der Houwen (8).
Within the realms of complementary and alternative medicine suggestions are being made, i.e., on social media but also in a number of medical publications, that a range of non-medical, often self-management strategies might result in pain relief and an improvement of wellbeing. They include relaxation exercises (e.g., yoga, breathing exercises and meditation), the use of heat, acupuncture, physiotherapy and the use of cannabidiol (CBD) oil. In addition, specific dietary measures such as a gluten free diet (GFD), the Low FODMAP diet and the so-called endometriosis diet (aspects of these are discussed further below in detail) have been suggested to help mitigate endometriosis associated pain and discomfort (1, 24–30). Women that are receptive to suggestions regarding symptom reduction often self-implement such measures (23, 26, 31–35) and recently it was shown that this may result in positive effects on QoL (36, 37). Because of this, the current European endometriosis guidelines advice healthcare providers to discuss non-medical strategies and address aspects of QoL and psychosocial wellbeing (1). However, since evidence providing plausible biochemical mechanisms explaining the perceived benefits is scarce or lacking (30) no recommendations are made in these guidelines regarding dietary strategies (1).
Associations between diet components and endometriosis have been addressed in a number of recent systematic analysis and reviews but they do not reflect causality (38–44). Plausible mechanisms related to the effects of specific food components on pathology and symptoms in vivo, that may underlie these associations, have thus far hardly been discussed thoroughly. Much of the data pointing to plausible mechanisms is obtained from in vitro and animal studies which do not necessarily reflect the human situation. Therefore, we aim to critically discuss the explaining associations between endometriosis and dietary interventions, and their challenges and limitations. We will do this by the example of three selected diet components. Supplementation with omega 3 fatty acids and red meat avoidance will be discussed in short, whereas the association between gluten related pathologies and endometriosis will be discussed in great detail.
For this narrative overview, selected original research papers were obtained using the university E-Library academic search tools PUBMED, WEB OF SCIENCE, SCOPUS and EMBASE, ENDNOTE global search tool, as well reference lists in research papers and reviews (see Appendix). Papers on randomized controlled trials, retrospective and prospective studies, systematic reviews and meta-analysis were included in this narrative review. Relevant citations in publications were cross-checked with the original content in the sources cited.
Endometriosis background
Prevalence of endometriosis
It is estimated that over 175 million women worldwide suffer from endometriosis and is thought to occur in 5%–15% in women of reproductive age (15–49 years) (45–50). With women of reproductive age representing about 38% of the global population, the currently reported endometriosis prevalence equals between 1.9% and 5.7% in the global population (51, 52). Underreporting due to diagnostic complexity and delay is very likely.
Causes and mechanisms associated with endometriosis
Due to the complexity of endometriosis and the multiple associations with other diseases and/or disorders, many hypotheses on endometriosis etiology have been put forward. The most accepted and oldest theory dating back to 1927 is the “Retrograde menstruation” theory, also referred to as the “Implantation Theory”. In this theory it is proposed that fragments of the endometrium are dragged through the Fallopian tubes into the abdominal cavity during menstruation, followed by implantation on the peritoneum. These mucosal fragments can form endometriotic lesions which in turn can spread through the abdominal cavity. However, it should be noted that the phenomenon of retrograde menstruation physiologically occurs in the majority of women with patent Fallopian tubes whereas only a fraction of women develop endometriosis (53–55). Therefore, other theories are proposed including coelomic metaplasia (which has been suggested in women with Mullerian duct defects and explains peritoneal lesions by the transformation of peritoneal mesothelium into glandular endometrium) and hematologic or lymphatic spread (which explains extra-pelvic lesions) (5, 22).
The pathogenesis of endometriosis includes local estrogen production, progestogen resistance as well as chronic local and systemic inflammation. An altered inflammatory response, usually mediated by tumor necrosis factor (TNF)-alpha expression and sustained by Interleukin (IL)-16 in the peritoneal cavity, appears to be estrogen-dependent. Post-menarchal increased estrogen levels have been shown to impact endometriosis prevalence, whereas reduced levels are clearly associated with a lower prevalence (56). In addition, estrogen contributes to the growth and proliferation of endometrial cells, both in utero and of ectopic endometriosis lesions. It also suppresses the expression of several anti-inflammatory genes (IL-8, IL-6, TNF-a) and is involved in the modulation of endometriosis-related pain by exerting neuro-modulatory functions, both through indirect and direct mechanisms, on sensory and sympathetic nerves (57). Suggestions have been put forward that estrogens from meat consumption may contribute to endometriosis risk resulting in recommendations to avoid or limit red meat consumption (58, 59). Finally, another theory concerns “Bacterial /microbial contamination and ‘Dysbiosis’1” (61–64). It is suggested that intestinal dysbiosis, for example in celiac disease patients, is associated with inflammation, impaired barrier function and microbial translocation possibly increasing the risk of endometriosis. However, thus far no plausible mechanisms have been defined to explain how dysbiosis could cause endometriosis. Below we discuss this matter in more detail. Extensive detail on endometriosis subtypes, pathogenically mechanisms and etiology pathways can be found in recent reviews (5, 55, 65–68). An illustrative representation of endometriosis is given in Figure 1.
Critical assessment and discussion on the association and cause-effect of selected dietary factors in endometriosis
Several studies have suggested there might be a causal effect between the development of endometriosis and either supplementation or avoidance of certain supplements and/or nutrients. However, these studies are often of low quality and only show associations. They do not provide proof of causality. Therefore, a number of critical aspects in the assessment of the effect of a diet or specific dietary components on health outcomes are discussed below.
Available observational studies have suggested a strong overlap between symptoms associated with endometriosis and with other chronic diseases which are also characterized by immune and inflammatory responses such as CD, ulcerative colitis, Crohn’s disease, non-celiac/wheat gluten sensitivity (NCGS/NCWS) and IBS (7, 69–73). In women diagnosed with endometriosis a 2-3fold risk to also be diagnosed with IBS has been observed (38, 39). In women suffering from IBS and being suspect of gluten related symptoms, a 5- to 6-fold risk of suffering from CD was found (74). Both a case control study (75) and a systematic review and meta-analysis (76) concluded that endometriosis was associated with autoimmune diseases including ID and IBD. However, both authors addressed there is little understanding of shared biological mechanisms and pathways between endometriosis and autoimmune diseases that would explain such association (Figure 2).
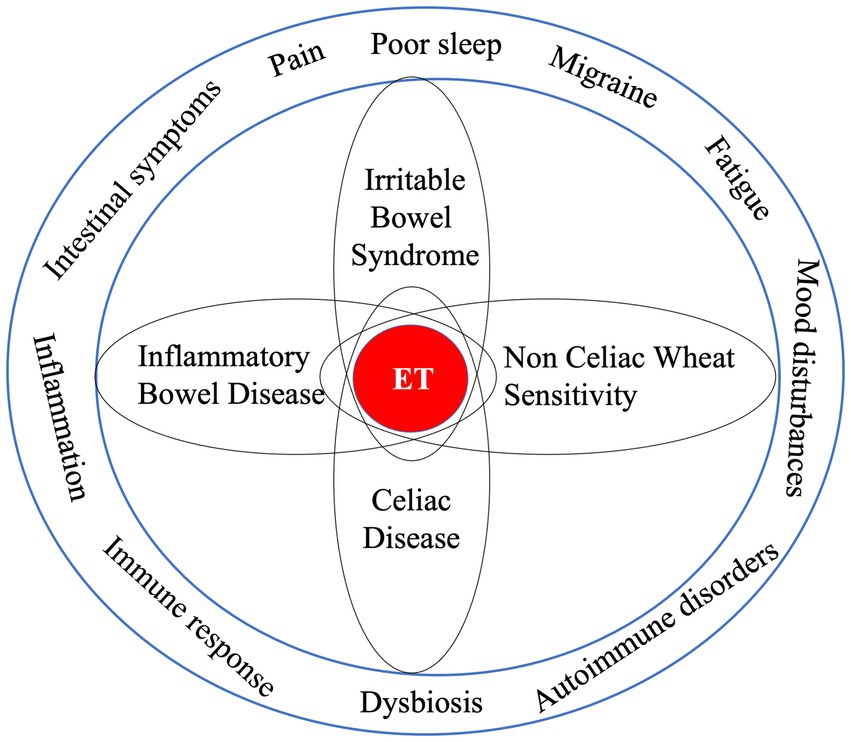
Figure 2. Endometriosis characteristics and symptoms are associated with other disease states, which raises questions about the chance of “shared” underlying pathologies as well as possible risks that one initiates and/or potentiates the other. Since associations are no proof of causality great care should be taken with the interpretation of their meaning.
Because specific food components are involved in the pathologies of CD, ulcerative colitis, Crohn’s disease, NCWS and IBS, and because these diseases are thought to be associated with endometriosis, suggestions have been made that nutritional strategies recommended in these conditions might also be relevant for women diagnosed with endometriosis. A few examples of diets suggested to be beneficial for women diagnosed with endometriosis are an anti-inflammatory diet that is rich in long chain poly-unsaturated fatty acids (PUFA) and polyphenols and low in trans-fatty acids (58, 77–79); the Low FODMAP diet (FODMAP – Fermentable Oligo-, Di-, Monosaccharides And Polyols which give rise to gas formation, osmotic fluid shift, feelings of bloating and intestinal distress) (27), a low nickel diet (80) and finally supplementation with pre-, pro- and synbiotics2 (81). In addition, it was advised to reduce or avoid red meat consumption (both processed and non-processed) because of its content of heme-iron (a potential pro-oxidant) and a suggested presence of estrogens due to hormonal treatment of cattle. Hormonal treatment of cattle is a farmers practice to stimulate growth and meat yield, in countries where this is legally allowed like the United States. However, it is forbidden in all European countries (58). The avoidance of red meat is part of the so-called endometriosis diet (37, 82). However, the extensive analysis of available data performed by Shafrir et al. (83) and by Nap and de Roos (30) concluded that “conflicting results have been reported on the associations between endometriosis and dietary components.” Therefore, there is no clear advice regarding dietary interventions in the most recent ESHRE Guideline for endometriosis (1, 30, 83).
Although pain perception may be used as a subjective clinical endpoint it is influenced by many factors, among which also positive and negative expectations, cognitive attention and mood. Nevertheless, patients who self-implement nutritional measures indicate that these result into better wellbeing and less pain perception (28, 33, 37, 40, 82, 84). Part of the positive effect of a food component on mood and QoL may be attributed to a placebo effect (a positive effect experienced just because the user thinks that intake of the component helps the condition) or nocebo effect (belief that a negative outcome occurs because a certain food component causes harm and avoiding it will result in feeling better). Both placebo and nocebo effects are based on expectations which are subject to tremendous influence of the news in social and main stream media as well as opinions of peers and health professionals in close contact. The latter was confirmed in a randomized, double blind, placebo-controlled, international multicenter study, addressing the effects of expectancy on experiencing adverse reactions of gluten intake, after exposure to foods labelled “gluten free” or “containing gluten” (85). It was shown that the combined effect of expectancy had a strong effect on overall and individual GI symptoms, reflecting a nocebo effect. In addition, a recent meta-analysis concluded that evidence of positive impact on pain perception and QoL, was derived from non-randomized controlled trials with small sample sizes. There were multiple sources of bias and when assessed using GRADE criteria the evidence was graded as low to very low-quality (41, 86, 87). Nevertheless, despite the presence of placebo/nocebo effects, Leonardi et al. plead to encourage patients and healthcare providers to more strongly consider and encourage diet treatments if this improves the patients’ wellbeing, even if these improvements may be induced by effects of expectation and belief (84). This strategy may bear certain risks because the advised diet treatment might induce changes in nutrient intake. This is important to patient’s health and is discussed later in this paper.
Association or proven cause-effect?
Endometriosis and microbiota
Although the uterus has been considered a sterile environment for a long time, it has recently been found that the endometrium harbors low quantities of resident microbiota (88). However, much less than in the vagina (89, 90). It has additionally been suggested that fecal compounds and/or microbiota may contaminate the vagina (91) leading to cervico-vaginal dysbiosis, inflammation, infection and tissue damage (92–94). It has been hypothesized that this deteriorates endometrium integrity, allowing for the migration of cells or tissue fragments with blood to other sites. It is suggested that the latter may result in tissue adherence and the initiation of endometriosis lesions (95–98). Similarly, Blander et al. argued that an increase in pro-inflammatory and a decrease in anti-inflammatory microbiota composition may result in conditions leading to increased epithelial permeability, giving rise to microbial infiltration and translocation to blood (99).3 This may in turn lead to metabolic inflammatory conditions similar to endometriosis (101, 102).
Dysbiosis in CD, NCWS, ulcerative colitis and Crohn’s disease is associated with inflammation induced impaired intestinal barrier integrity (103) and may lead to passage of microbes into blood, evidenced by the presence of flagellin (marker of microbial translocation from intestine to blood) in blood (104). Because of overlapping inflammatory metabolism and symptoms, it has been speculated that endometriosis and intestinal dysbiosis may interact. However, whether intestinal dysbiosis is causal to developing endometriosis is highly speculative and remains unproven.
Intestinal dysbiosis, estrogen cycling and endometriosis
A potential effect of the gut microbiome associated estrobolome—a collection of genes in the intestinal microbiome modulating the amount of resorption of estrogen passing from blood into the intestine - has been described. It was discussed that intestinal dysbiosis may lead to excess estrogens being reabsorbed, causing a hyper-estrogenic environment that will impact endometriosis (64, 105, 106). It has been shown that intestinal resident microbiota communities are influenced by hormonal changes, which behave cyclical. In addition, hormonal fluctuations can modulate anti-microbial peptides in the uterine mucosa and endometrial fluid, as well as influence the local endometriosis immune cells (107, 108). Thus, both the endometrium microbiota composition and the way its metabolism is changed by hormonal cycling and vice versa may theoretically play a role in endometriosis etiology.
Perrotta et al. determined the fecal microbiota composition by collecting rectal swabs of women with and without endometriosis. They found no differences in fecal microbiome composition of endometriosis patients versus their healthy controls (109). However, experimental animal studies suggest that the intestinal microbiota may lead to endometriosis and disease progression. Chadchan et al. observed that antibiotic treatment in an experimental endometriosis mouse model reduced inflammatory markers and endometriosis progression (110). An influencing role of intestinal microbiota related metabolism and/or endotoxemia is a likely explanation. Ni et al. observed that microbiota induced changes in fecal matter of experimental endometriosis mice (increased bile acid and decreased alpha-linolenic acid concentration) were related to disease progression (111). However, in this study dysbiosis and related metabolic effects occurred after the mice were treated with estrogen and endometrial fragments were transplanted. Therefore, the conclusion that dysbiosis was causal to endometriosis development by Ni et al. (111) seems unjustified. How the introduction of endometriosis in these mice has resulted in the initiation of dysbiosis remains unclear. It has been shown that diet and microbiota related increased β-glucuronidase activity can result in elevated estrogens levels whereas a dysbiosis induced decreased activity may induce decreased circulating estrogens (112), which in fact should counteract the earlier suggested positive association between dysbiosis and endometriosis. Neither dysbiosis nor dysbiosis-associated effects on estrobolome, while suggesting that these pose an increasing the risk of endometriosis, should be an argument to avoid gluten. At present, there are no in vivo cause-effect data in this respect.
As mentioned above, many associations between the consumption of certain food components and occurrence of disease symptoms are based on observational data showing correlations. However, this does not provide proof of causality. In addition, favorable effects observed after exposure to isolated food components in in vitro and in animal intervention studies do not allow direct generalization to daily human mixed diet consumption conditions. Another critical aspect is how well all factors that play a role in vivo are truly comparable to the increasingly performed in vitro tests. Oral food processing, gastric peristalsis, intestinal transit, the presence of digestion enzymes and organic acids in oral cavity, stomach and intestine (carbohydrases, proteases, gastric acid and bile) as well as the processes of absorption (transport mechanisms) that play a large role in in vivo studies, should be similar to the circumstances of in vitro studies. However, in vitro systems lack feedback from the gastrointestinal sensors in delaying gastric emptying and extending gastric acid exposure. They additionally lack a mucus layer, delaying exposure to the enterocytes for absorption, while at the same time extending intraluminal digestion. In addition, with the exception of organoids, they lack brush border membrane-associated enzyme activities which can influence the rate of digestion and absorption (113, 114).
Another point of concern is that the direct in vitro exposure of bioactive molecules to cells or cell-lines are often unrealistic with respect to the in vivo situation. The quantitative exposure (bio-accessibility) and the resulting bioavailability may be extremely low in vivo. In addition, the test molecules may undergo microbial modification in the intestine prior to absorption. For example, as a result of microbial metabolism, pro-inflammatory and immune stimulating compounds, e.g., conversion of primary to secondary bile acids (115, 116), and alternatively, anti-inflammatory compounds (e.g., derived from antioxidant-polyphenols) (117) may be formed in the intestine. In addition, post-absorption, many of the absorbed compounds will also become subject to conjugation in the liver, which changes their potential bioactivity and health effects. For this reason, total antioxidant capacity, as measured in vitro, has no relevance for the in vivo situation and its use as “favorable for health” should be discouraged (118).
Effects of isolated food components vs. mixed diet on endometriosis
Effects of isolated food compounds, as observed in in vitro studies, may differ from the effects when it is part of a mixed diet. As an example, it was long assumed that free radicals present in cigarette smoke, cause oxidative stress in the lungs and that this plays a role in the development of lung cancer. Based on in vitro data it was thought that supplementing lipophilic antioxidants such as vitamin E and carotenoids would counteract oxidative stress and decrease lung cancer risk. However, a higher lung cancer and death incidence was observed among consumers taking beta-carotene supplements, compared to the placebo group (119, 120).
Positive effects of fruit and vegetable consumption on the risk of developing endometriosis have been suggested because these are rich in antioxidants, micronutrients and dietary fibers (82, 83). However, the observational study by Schwarz et al. (121) drew a contradictory conclusion. After adjusting for a healthy eating index, positive effects associated with fruit fibers disappeared. Even more so, when pooling total vegetable and cruciferous fiber intake an increased endometriosis risk was observed (121). A recent systematic review by Nirgianakis et al. (42) reviewed 9 human and 12 animal studies addressing the effect of dietary interventions on endometriosis. The human studies were classified as supplementation with isolated dietary components (e.g., antioxidants), exclusion or avoidance of selected dietary components (e.g., red meat), or complete diet modification. It was concluded that although the selected animal studies showed promising results, they did not reflect the reality of consumption in humans (42). Accordingly, before making recommendations, effects observed in vitro or in animal model studies should always be confirmed by human intervention studies with habitual frequencies and quantities of food intake. Below we aim to put this complexity further in perspective by discussing two specific examples of diet components which, based on experimental data in animals, in vitro studies, as well as observational data, are suggested to play a role in endometriosis etiology and/or symptoms, but lack a clear cause-effect evidence: (1) omega-3 fatty acids and (2) red meat. Subsequently, dietary gluten will be discussed in great detail.
Omega-3 fatty acids
It has originally been suggested that omega 3 poly-unsaturated fatty acids [n-3 PUFA: α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], as precursors of prostaglandins, express anti-inflammatory activity (122). Observational data show that low fish oil consumption is associated with a 22% more likelihood to be diagnosed with endometriosis (78). Based on this observation supplementation is often advised to patients suffering from inflammatory diseases such as rheumatoid arthritis, IBS, IBD but also endometriosis. However, the question is whether there is a plausible mechanism by which n-3 PUFA may either reduce endometriosis initiation or endometriosis associated symptoms.
Supporting that the effects of a single food component may differ from the effect of the component when being part of a mixed diet, studies have shown that the effects of n-3 PUFA supplementation may differ for consuming fatty fish as a source of omega 3. Observational study outcomes are conflicting (51, 121, 123), the biological plausibility is lacking and there is debate on the efficacy to reduce inflammation in vivo (43, 123–126). Su et al. performed a meta-analysis and concluded that there was no benefit of ALA on reducing blood inflammatory markers (44). In contrast, they surprisingly found that ALA supplementation may increase inflammation. Nodler et al. (127) performed a double-blind placebo-controlled study on the effects of n-3 PUFA supplementation for 6 months in adolescent girls and young women with endometriosis. Increased n-3 PUFA intake resulted in a reduction in pain scores not different from placebo treatment. It is discussed that “caution must be applied given the widespread direct marketing of these supplements to girls and women with endometriosis implying a beneficial impact on symptoms. A strong placebo effect was evident in multiple outcome measures, suggesting that participation itself, and not the supplements, conferred improvement even at six months” (127). Despite the observed association between higher levels of fish oil consumption and reduced endometriosis risk (78), evidence to conclude that n-3 PUFA supplementation causally helps to reduce endometriosis initiation or symptoms is lacking. The differing results discussed above reflect that the overall composition of the diet (including other factors influencing inflammatory or anti-inflammatory potential) plays a critical role. In addition, the physical condition of the individual (presence of obesity, associated decreased microbiota diversity and presence of insulin resistance) as well as smoking behavior will also influence the effects of diet and of study outcomes (128).
Red meat
A prospective cohort study by Yamamoto et al. (58) investigated the effect of meat consumption on endometriosis risk and found a positive association. They suggested that this may at least partially be caused by heme-iron (acting as pro-oxidant, inflammation inducer) and steroid hormones present in meat. However, other studies did not confirm these findings and suggested that this may only occur when consuming seven or more red meat servings per week, which, with habitual food consumption may only happen in extreme cases (129). These contradicting findings require a critical assessment of potential sources of bias that may influence data interpretation. For example, high meat consumers generally are overweight (130), which is associated with low-grade chronic inflammation (131). Montonen et al. noted that an elevation of inflammation markers (hs-CRP) in high meat consumers was no longer significant after adjusting for overweight (132). In addition, obesity in women, in particular abdominal obesity, is associated with elevated serum and adipose tissue estradiol levels (60, 133–135). It is possible that the effect observed by Yamamoto et al. was due to overweight, which they did not correct for in their analysis (58). Furthermore, Yamamoto et al. referred to other studies suggesting that hormones from steroid hormone-treated animals, present in consumption meat, may play a role in endometriosis risk (58, 136, 137). However, the cited study of Andersson and Skakkebaek (136) concerned pre-pubertal young children whereas in the other study Brinkman et al. (137) studied postmenopausal women. In both cases endogenous estrogen production was low. It is thought that the impact of estrogens from meat consumption on circulating estrogen levels will differ much from that in women of reproductive age, because they have a much higher endogenous estrogen level (137). Concluding, none of the studies available at present quantified estrogen levels in meat (and as a result of consumption) and there is no conclusive data that (red) meat consumption is causally involved in endometriosis etiology and symptomatology.
Do adverse effects of gluten interact with endometriosis?
Both untreated (newly diagnosed) celiac disease and confirmed endometriosis are associated with inflammation, immune responses and dysbiosis. This raises the question whether other disease states showing similar and overlapping associations may mutually and interactively worsen endometriosis pathology. For example, is it plausible that inflammation and immune response signaling molecules released from intestinal sites due to gluten exposure potentiate inflammation of endometriosis lesions at other sites? To put this matter in a correct perspective, background of gluten, gluten related pathologies and effects of GFD are discussed step by step below.
What is gluten?
The main storage proteins of all cereal grains, with the exception of oats and rice, are “prolamins”. This term is based on the fact that these proteins are rich in proline and glutamine. Gluten is comprised of two prolamin fractions, gliadin and glutenin. Figure 3 represents an approximate composition of wheat and wheat protein. Glutenin is particularly important for the formation of an elastic network during dough kneading, subsequently enabling the entrapment of fermentation gases and rising of the dough. Gliadin is the more important fragment, arising from protein digestion (peptides), that can cause adverse reactions in the intestines (see further below). Gluten contributes to about 70–80% of the total grain protein content in common (bread) wheat, ~78% in spelt wheat, ~70% in emmer wheat (all 3 used in bread making) and ~ 77%, in durum wheat (mostly used for pasta making) (141, 142).
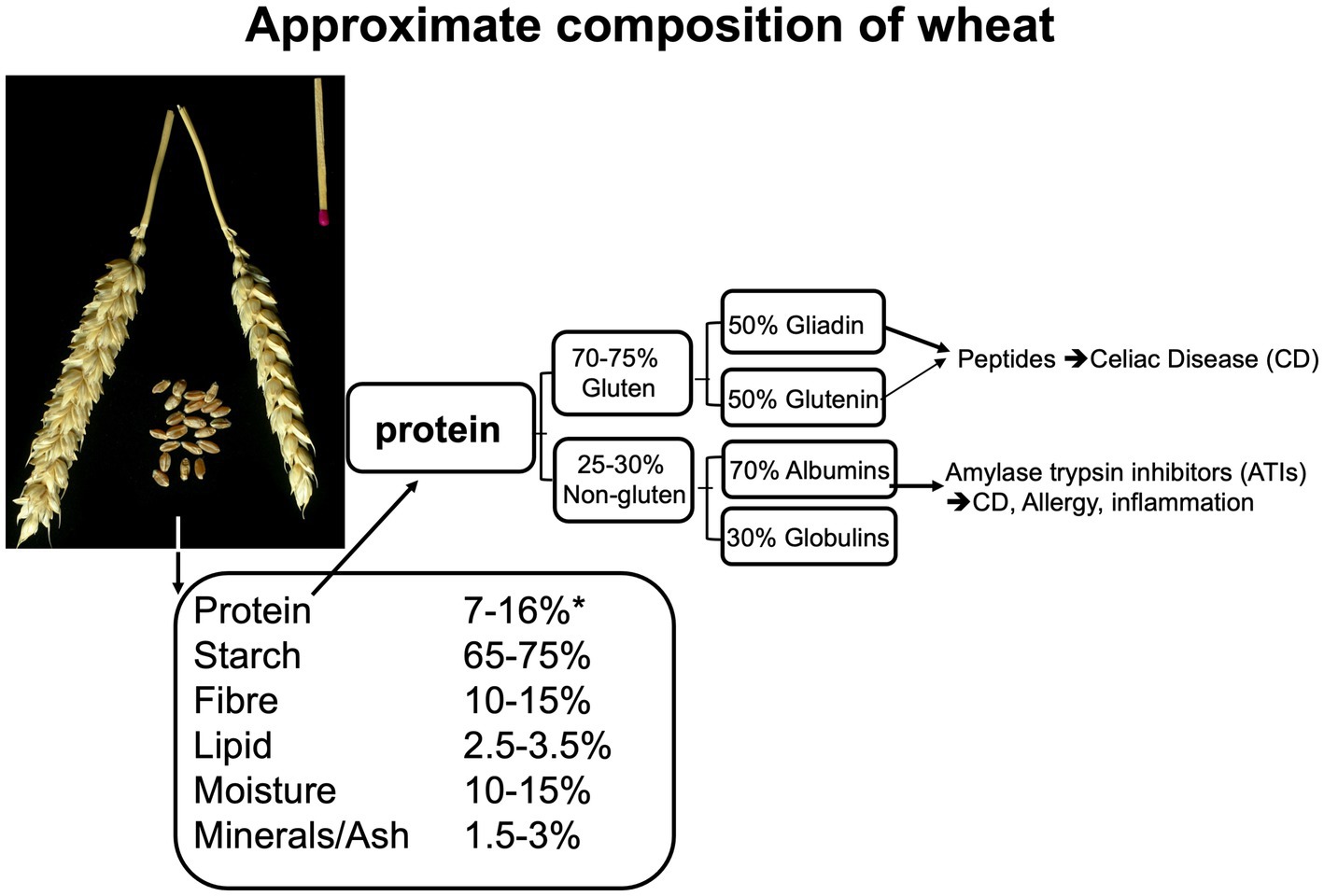
Figure 3. Composition of the wheat kernel and of wheat protein, based on data from (138, 139), adapted from Brouns and Shewry (140).
Based on the measurement of “true ileal digestibility” it has been suggested that the degree of digestion of wheat protein in humans is ≈90%–95%, similar to the range of digestion of most plant proteins (143). Specific sections of the gluten protein contain proline-rich sequences which cannot be degraded by the human proteases pepsin, trypsin and chymotrypsin. This results in a small fraction of peptides that potentially may cause immune and inflammatory responses in the intestines in susceptible individuals (see next section for detail). The storage proteins in barley (hordein, ~50%–55% of total protein) and in rye (secalin, ~47%–50%) are molecular closely related to gluten and are therefore considered as “gluten proteins” (141, 144). Because avenin proteins in oats do not resemble gluten, oat is generally considered to be a gluten free grain. However, oat-based foods may become contaminated with gluten containing grains or flours in the agri-food chain.
In social media it is often suggested that ancient wheat types contain less gluten and are safer for individuals suffering from gluten disorders. However, an extensive compositional analysis of wheat types showed that over time the protein content slightly declined while starch content increased. Similar to these observations, recent analysis showed that the “ancient” wheat types spelt, emmer and einkorn have higher contents of gluten compared to common wheat (bread wheat) (142). Along with the higher protein content, the amount of gliadin and digestion resistant gliadin derived immuno-reactive peptides involved in the etiology of celiac disease is also higher in these wheats (142, 145–147).
Gluten disorders pathology and prevalence
Undigested gluten peptides (size of 10–40 amino acid residues), exposed to the intestinal epithelium, can pass the luminal side of intestinal epithelium and enter the lamina propria via the transcellular or paracellular route. Subsequently, peptide deamidation by the enzyme “tissue transglutaminase” (tTG) takes place. In addition, dendritic cells recognize specific amino acid sequences on the surface of the antigen (called epitopes) which can lead to binding to HLA-DQ2 and HLA-DQ8 molecules on antigen-presenting cells and subsequent initiation of immune and inflammatory responses (148). This condition ultimately leads to increased numbers of intraepithelial lymphocytes and villous damage. The latter causes digestion-absorption problems that are found in up to 38% of patients with CD, where one or more nutritional deficiencies (calories, dietary fiber, vitamins and minerals) are seen. This might result in diarrhea, steatorrhea and weight loss (149, 150). Extensive detail on CD etiology and pathology can be found in other reviews (70, 151, 152). Different features of gluten/wheat exposure in CD, wheat allergy (WA) NCWS and of endometriosis are presented in Table 1.
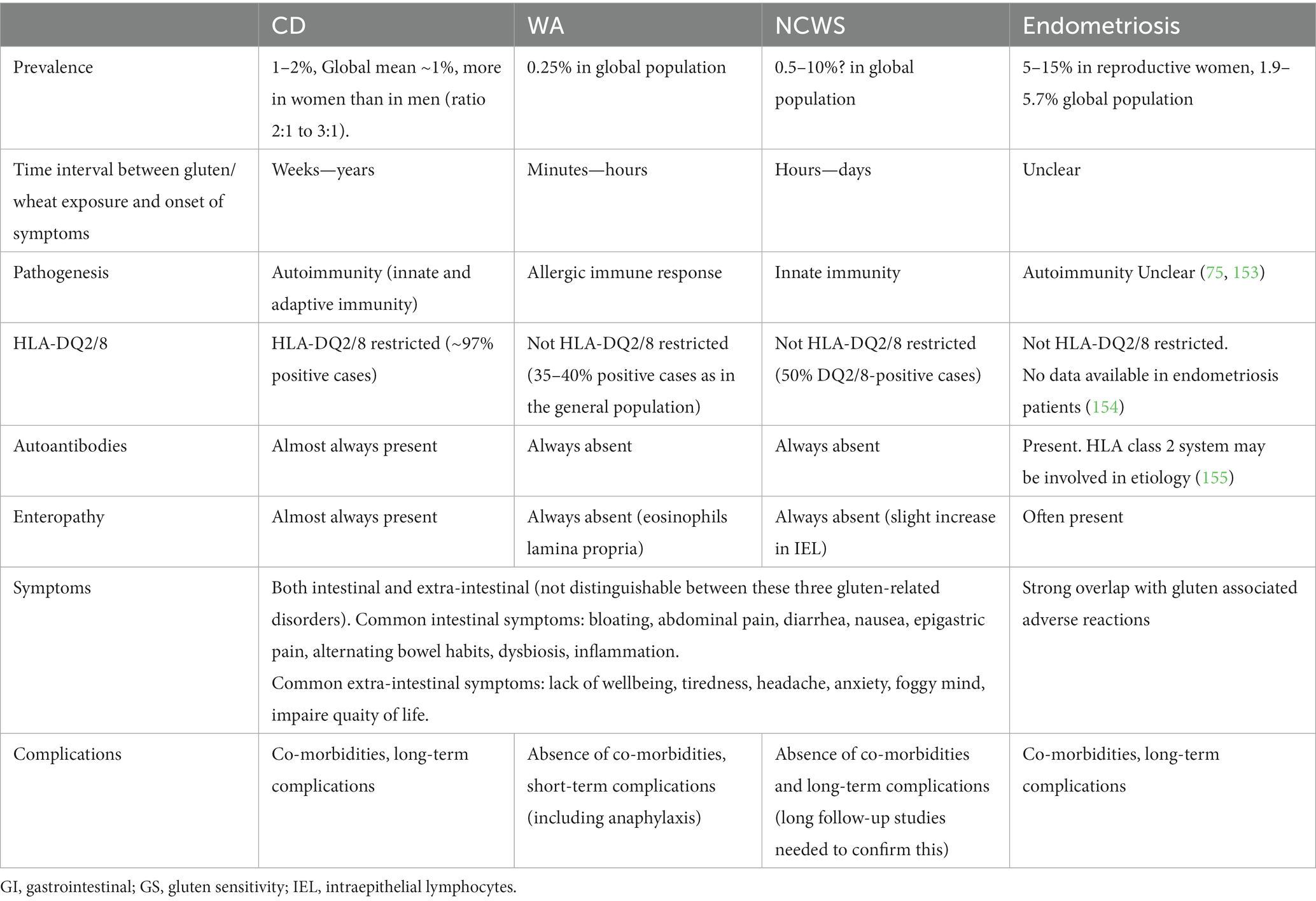
Table 1. Features of gluten/wheat exposure in celiac cisease (CD), wheat allergy (WA), non-celiac wheat sensitivity (NCWS), formally often named non-celiac gluten sensitivity (NCGS) and of endometriosis.
Important to notice is that CD only develops in genetically predisposed individuals, expressing the gene haplotypes HLA-DQ2 or DQ8 (156, 157). Approximately 25%–40% of the general population (the exact numbers differ per country that was studied) is predisposed and expresses these gene haplotypes (158, 159). However, based on confirmed diagnosis it is estimated that only about 4%–6% of these predisposed individuals actually develop CD. A global mean population prevalence of about 1% is mostly cited. The fact that the majority of predisposed individuals do not develop CD implies that additional environmental non-gluten factors might play a role in the disease initiation (160).
The prevalence of CD in the global population is about 1%–2%. Santoro et al. observed CD in 5 (2.2%) out of 223 women with confirmed endometriosis whereas only 2 cases of CD were found in the 246 control subjects (0.8%) (161). In addition, Aguiar et al. observed a CD prevalence of 2.5% in a cohort of 120 women with laparoscopically confirmed endometriosis, compared to a prevalence of 0.66% in their control group (162). Based on these observations one may conclude that the actual prevalence of CD among endometriosis patients is only slightly higher than in the general population. Finally, the largest survey thus far was performed in Sweden (163). In this study, using biopsy reports from all Swedish pathology departments, 11,097 women with confirmed CD were identified. None of them had a diagnosis of endometriosis before study entry. These patients were matched with data of 54,992 controls not suffering from CD. All individuals were followed up for a period of at least 5 years. During this period 118 individuals with CD and 399 matched controls developed endometriosis. When restricted to women of reproductive age at study entry (16–45 years), the mean increased risk was 35% (HR 1.35; 95% CI: 1.07–1.69). The authors concluded that endometriosis seems to be associated with previously diagnosed CD. Potential explanations included shared etiological factors and CD-mediated inflammation. How intestinal inflammation would cause initiation of endometriosis remains unclear. However, contrary to previous beliefs, in a recent study by Schwartz et al. (121), gluten intake was associated with a significantly lower risk of endometriosis diagnosis. Although an explanation for this inverse association was unclear, the results provided some evidence that eating gluten-containing foods did not increase risk of endometriosis diagnosis.
A reason often cited for recommending a GFD to endometriosis patients is the study conducted by Marziali et al. (26). They reported that endometriosis symptoms decreased after 12 months of adherence to a GFD. An important aspect of this study is that none of the patients had been tested for CD when being enrolled in the study. When suffering from CD, a GFD will reduce inflammation and immune response related symptoms, which are known to overlap with endometriosis-related symptoms. It may be that individuals showing improvement of symptoms were, at least partly, undiagnosed CD patients. In addition, 2 weeks after starting a GFD, 88 study participants had withdrawn because of side effects caused by a GFD, such as abdominal symptoms due to other food intolerances (which were not specified). Only the 207 patients who showed improvement of symptoms were allowed to continue the study. This approach may have caused undesired skewing into the direction of the favourable effects of a GFD on endometriosis-related symptoms which were seen in 156 participants whereas 51 participants showed no effect. In addition, based on similar effects seen in patients suffering from gluten/wheat sensitivity, as discussed above, strong nocebo effects related to gluten intake and placebo effects due to avoidance of gluten may have been present. Recently it was shown that, compared to non-patients, women suffering from endometriosis were more likely to self-implement a GFD, similar to observations in patients with IBS. In addition, it was six times more likely that women who implemented a GFD also made other dietary changes in the past years (164). An overview observed associations between gluten, CD and endometriosis is given in Table 2.
Is dysbiosis in celiac disease a cause or a consequence of the disease?
Zafeiropoulou et al. showed that the presence of specific bacteria was CD dependent despite the fact that adherence to a GFD had altered intestinal microbiota composition (166). Recently it was shown that the abundance of several species, pathways and metabolites was altered at the time of CD diagnosis, characterized by complex patterns of increased pro-inflammatory species and decreased protective/anti-inflammatory species (167). El Mouzan et al. studied 40 children with newly diagnosed CD, 20 healthy controls, and 19 non-CD controls. They observed a significant difference in fecal microbial composition of the CD children, among which increased Bacteroides and decreased Lactobacillus genus counts (168). Palmieri et al. collected fecal specimens from 46 CD individuals following a GFD for at least 2 years and 30 specimens from healthy controls. The microbial composition of the CD subjects was decreased in Bifidobacterium longum genus and several species belonging to the Lachnospiraceae family. Also, Bacteroides genus was found to be more abundant. In contrast to other suggestions, microbial profiles of the CD patients were consistently “non-dysbiotic” (169). After a systematic review of cross-sectional studies by Kaliciak et al. it was concluded that due to a lack of taxonomic uniformity between studies and the enormous inter-individual differences in microbiota composition, a comparison of microbial communities between CD patients, CD-GFD patients and untreated CD patients was impossible (170). Vacca et al. came to a similar conclusion (171). These recent observations suggest that unfavorable intestinal microbiota changes and associated metabolism can result from various environmental conditions preceding the diagnosis of CD. Examples of these are self-implemented measures to help alleviate experienced gastrointestinal discomfort and pain (e.g., FODMAP avoidance resulting in low fiber intake, GFD, use of nonsteroidal anti-inflammatory drugs and of antibiotics). Although by adhering to a GFD a partial normalization may occur, the effects of GFD on microbiota changes will not only depend on the avoidance of gluten but also significantly on the compositional quality of the GFD (see further below for more detail).
Non celiac gluten sensitivity
When hearing or reading from influencers and celebrities on social media that gluten in general can cause health-related harm, one may impose a self-belief that gluten is the cause of a range of intestinal symptoms and general malaise and that these symptoms will disappear after adhering to a GFD. This, as well as associated gluten-free marketing claims, have led to an increasing belief that ‘living gluten-free’ is equal to a healthy lifestyle (172). Within the general population the prevalence of individuals believing that gluten causes harm, resulting in them avoiding gluten, may range from a few percent up to 15% of the general population (172–174). However, the actual number of individuals having symptoms after consuming gluten or wheat containing foods, while not suffering from CD, wheat allergy or dermatitis herpetiformis, appear to be much lower.
For example, Capannolo et al. studied 392 persons (307 females and 85 males) selected from an IBS cohort. They had contacted the gastroenterology unit because they experienced gastrointestinal symptoms, particularly after consuming gluten. During their initial CD screening, it was found that the positive predictive value of the gluten-related symptoms (defined as the probability that someone with symptoms actually suffers due to gluten/wheat exposure) was only 7% (74). To put the latter in a correct prevalence perspective, 7% of an IBS cohort (usually representing 10%–12% of total population) would equal a NCWS prevalence of close to 1% in the total population. Molina-Infante and Caroccio analyzed data from 10 double-blind, placebo-controlled, gluten-challenge trials, covering data from 1.312 adults. They observed gluten specific effects in 38 of 231 self-reported NCWS patients, confirming the low prevalence of proven gluten/wheat disorders in people reporting symptoms after gluten/wheat consumption. In addition, a 40% rate of nocebo effects was observed (175). However, it should be noted that the percentage of individuals who were newly diagnosed to suffer from CD during the initial study patient entry screening was 6.63% in the study of Capannolo et al. (74). This percentage is much higher than the general population prevalence of about 1% and warrants diagnosis to confirm or reject the presence of CD in both IBS and endometriosis patients.
Effects of non-gluten compounds on symptoms overlapping with endometriosis
In studies applying challenge tests with gluten or FODMAP it was observed that individuals with self-reported gluten sensitivity reacted primarily to the rapidly fermentable fructans present in grains. Not gluten but fermentation of FODMAP was found to be a significant trigger of IBS and NCWS symptoms that showed an overlap with endometriosis symptoms (176–178). Crawley et al. (178) performed a double-blind placebo-controlled gluten-food challenge with equal cross-over, in a population cohort of 1,266 participants (aged 15–21 years). They studied participants who reported gluten induced gastrointestinal symptoms, as indicated by self-reported questionnaires. They found that, compared to placebo, adding gluten to the diet neither induced gastrointestinal symptoms nor worsened mental health. A high nocebo effect was noted. In fact, their gastrointestinal symptoms reported were similar to IBS symptoms which may be caused by multiple factors (178). These findings do not exclude the possibility that a small subset of individuals reporting sensitivity to gluten/wheat may suffer from an intestinal non-classical type of food allergy (not associated with immunoglobulin E). In this condition, systemic immune activation and compromised intestinal epithelial barrier integrity have been found (104, 179). It is thought that amylase trypsine inhibitors (ATIs), present in wheat and in commercially used wheat gluten isolate, may play a crucial role here (180–182).
Specific dietary measures and their potential consequences
Gluten free diet
Following a life-long GFD is challenging, costly and can be socially isolating. Children and adults with CD report reduced QoL because of the lifelong dietary restrictions (183). The availability of gluten free food (all food without wheat, barley, rye and for food production technological reasons added gluten isolate) is still very limited. In addition, commercial gluten free food is often of doubtful composition. The use of flours from corn, rice, potato and tapioca, in exchange for gluten containing sources, results in a reduced content of micronutrients, dietary fiber and protein and a higher glycemic response. In addition, there is a higher use of saturated fat, trans-fat and salt in the processing of gluten free foods (150, 184, 185) and often gluten free labeled foods do still contain significant amounts of gluten (186, 187).
A low dietary fiber intake is of particular concern (150) because it will minimize fiber fermenting microbiota diversity (in particular those from Bifidobacterium and Lactobacillus genera (188)) and metabolism which play a key role in immunity and health (169). Based on a population-based study with 124,447 participants, Littlejohns et al. concluded that GFD followers have a poorer self-reported health and a higher prevalence of blood and immune disorders and undesired digestive conditions (164). Because adherence to a GFD and/or FODMAP diet has potential risks of low fiber intakes, special attention to educate and guide endometriosis patients to help select appropriate fiber rich foods is needed. For extensive reviews of these aspects (see 189–191).
Low FODMAP diet
For patients suffering from IBS and other diseases associated with IBS-like symptoms it is often recommended to reduce the consumption of FODMAP. Within this category of rapidly fermentable carbohydrates, fructans present in grain-based foods such as bread may lead to a significant daily FODMAP intake (192). However, the recommendation to avoid these may result in an important reduction of overall fiber intake, unless compensated by consumption of other high fiber sources (193). It has been shown that chronic low intake of soluble, well-fermentable fiber (diet low in plant-based foods) is associated with small intestinal and colonic microbiome disturbances, decreased intestinal mucus layer thickness and impaired barrier function/increased permeability. This is based both on a study where a gnotobiotic mouse model where mice were colonized with synthetic human intestinal microbiota was applied and an extensive systematic review including only human studies (194, 195). More importantly, low fiber intake causes a reduced saccharolytic fermentation in the colon, resulting in low production of short chain fatty acids (SCFA) - most importantly butyrate, propionate and acetate. These SCFA are essential for immunity and health protection of the intestine. A reduced SCFA production is known as a pro-inflammatory condition and is associated with an increased risk of IBD, intestinal cancer, reduced insulin sensitivity, intestinal tract associated immune compatibility, impaired intestinal barrier function and/or enhanced permeability. In addition, during reduction of saccharolytic fermentation, an increased protein fermentation and formation of hydrogen sulfide, ammonia, and p-cresol and branched-chain fatty acids takes place. These changes are known to promote intestinal inflammation and are also implicated in the etiology of intestinal cancers (196, 197). Hill et al. concluded that a low FODMAP strategy should be implemented with care due to the psychological and nutritional risks of a restrictive diet (198). Before recommending a complex and restrictive diet, such as the low FODMAP diet or a GFD, a positive medical diagnosis is a must (165, 199). When in post diagnostic guidance the patient is properly educated and guided long-term by nutrition experts, these risks can be minimized (200).
Limitations of available studies
Currently available systematic reviews and meta-analyses were all done in the same period (2020–2021), thus considered to have the same available database. In general study outcomes presented and discussed in these reviews showed positive, negative or no associations between the consumption of certain foods and/or nutrients and prevalence of endometriosis, or the perception of pain and QoL. Most intervention studies were of relatively short duration, did not have appropriate control conditions, were subject to significant bias and resulted in low to very low scores of evidence quality. The latter was also concluded in systematic reviews of observational data. As a result, interpretations about the effects of specific foods and/or food components on endometriosis were equivocal (128, 129, 132, 144). More recently, Nap and the Roos concluded in their critical review that evidence regarding the efficacy of dietary interventions in women with endometriosis is conflicting and effects of dietary interventions on specific types of endometriosis (e.g., superficial peritoneal endometriosis, ovarian endometrioma and deep infiltrating endometriosis) remains unknown. In addition, it is conceivable that certain co-morbidities (e.g., obesity) may also influence the effect of dietary interventions. Moreover, they stated that evidence regarding plausible biochemical mechanisms, resulting in the perceived effects of the dietary intervention, is scarce or lacking (30). As result, it is impossible to make evidence-based recommendations for dietary measures at present, to help reduce the initiation of endometriosis and its management once diagnosed. Current healthy eating guidelines, which include avoiding trans fatty acids, limiting alcohol, meat and salt consumption should also be followed by endometriosis patients (see further below). According to the conclusions presented in Table 3 there seems to be consensus that there is a clear need for better quality studies.
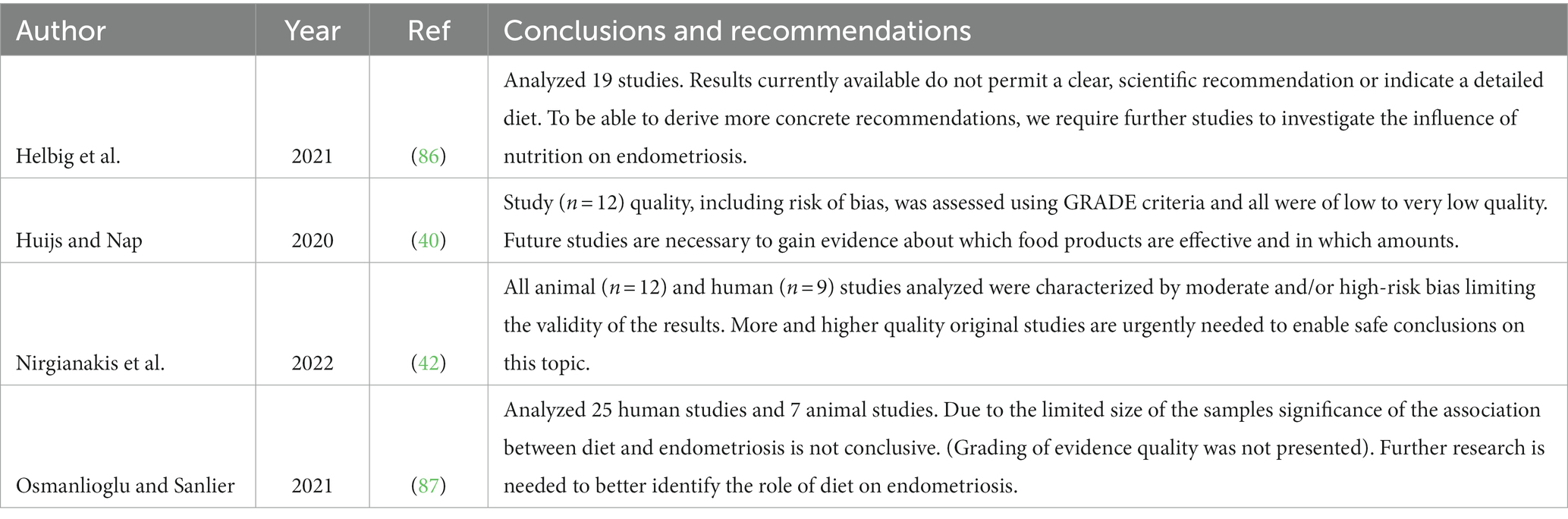
Table 3. Conclusions and recommendations related to moderate-high risks of bias and low to very low-quality evidence in observational and intervention studies evaluated in systematic reviews/meta-analysis.
Qualitative dietary guidelines for endometriosis patients
A regular consumption of whole grain foods is strongly associated with significant reductions in the risks of diabetes, cardiovascular disease, intestinal inflammatory disorders, intestinal dysbiosis, colon cancer as well as a more favorable weight management. The quantity of dietary fibers and associated bioactive molecules play an important role in this (102, 201–212). These observations have resulted in a globally supported recommendation of food authorities to regularly consume whole grains as part of a healthy diet and lifestyle. Accordingly, there are good reasons to advise the consumption of grains (most of which do contain gluten and FODMAP) to endometriosis patients when there is no diagnosis of biopsy-proven gluten disorders and/or IBS-related FODMAP fermentation distress.
In general, women with endometriosis should be recommended to follow the qualitative dietary guidelines as adviced by the World Health Organisation (WHO), encompassing restriction of salt, red and processed meat products, reduction of added sugars, syrups and refined carbohydrate/starch sources. In addition, regular intake of whole grains, fruits/vegetables, choice of fat sources rich in mono/polyunsaturted fatty acids and a reduction of foods containg trans fatty acids (209, 213–216). These recommendations are associated with reduced diet related inflammation and significant reductions in chronic disease. Education of endometriosis patients in this respect will help to avoid implementation of self-treatment strategies, some of which in long term may result in negative health outcomes, particulalry when not professionally guided.
Conclusion
Endometriosis is a multifactorial disorder of still largely unknown etiology. Many etiological and causality hypotheses have been put forward. Yet, none of these give a satisfying full evidence-based explanation for the various manifestations of the disease. A role of nutrition related metabolic, inflammatory and immune factors has been proposed to play a role in the etiology and the symptomatology but this is largely based on assumptions rather than good quality evidence. Endometriosis can be associated with multiple symptoms that strongly overlap with those of CD, Crohn’s Disease, NCWS and IBS. They typically share common pathological factors such as inflammation, immune responses, microbiota dysbiosis and impaired epithelial barrier function. In addition, chronic symptoms of poor sleep, chronic fatigue, changed mood, anxiety, migraine and depression are encountered. This, in addition to high recurrence rates, often lead to patient treatment unsatisfaction and the search for alternative, non-medical strategies to help relief symptoms. Among these are diet related recommendations with the target to help reduce inflammation and pain.
Patients reported that by applying dietary interventions, they felt better and their QoL improved. However, a careful and critical analysis of the scientific evidence regarding the observed positive effect of dietary interventions on symptoms and QoL points to a strong influence of placebo and nocebo effects. Observational data linking diet factors to the risk of endometriosis are conflicting and form no evidence of causality. Effects of nutrients and/or food components largely depend on molecular characteristics, which may differ significantly within a certain class. The generalizing that a certain class, e.g., “dietary antioxidants”, helps reduce oxidative stress and inflammation, and is therefore beneficial to reduce endometriosis symptoms, remains unproven. Claims that the inclusion or exclusion of certain nutritional components is beneficial for endometriosis prevention and management are largely based on data from experimental in vitro or animal studies that suggest positive effects. However, these do generally not reflect habitual diet practices and could be misleading.
There is currently no data available of longer-term nutritional intervention studies using established biomarkers of well-defined clinical endpoints of pathology and symptoms. The fact that grains containing gluten, ATIs and FODMAP are involved in the etiology of CD, NCWS and WA, of which symptomatology and a number of pathological factors overlap with endometriosis, does not provide evidence of a causal role in endometriosis etiology. In addition, there is no convincing evidence that the prevalence of CD, NCWS or IBS is increased in endometriosis patients. Given the potential impact that adhering to a life-long GFD may have on QoL, nutritional status, intestinal microbiota composition and potentially health, a GFD recommendation to endometriosis patients should be discouraged, unless a patient has been tested and positively diagnosed to suffer also from a gluten related disorder.
Author contributions
FB: wrote the initial draft of the manuscript and conceptualized and prepared figures. AH, VM, JM, DK, and KV: wrote parts belonging to their expertise. FB, AH, DK, KV, MB, JM, and VM: contributed to revising and editing the manuscript. All authors have revised the final version of the manuscript and approved of the submitted manuscript.
Funding
Open access costs for this publication were funded by Maastricht University and Amsterdam UMC. Maastricht university receives funding from the Dutch Government “TKI- Top Knowledge Institute,” ref. nr TKI1601P01 joint funded by Agri-Food chain businesses who donated unrestricted research grants for the international research consortium “Well On Wheat?,” addressing the study of grain based foods and gluten related disorders. Donating industries have no influence on study execution, interpretation/publishing of data and conclusions drawn (https://www.wellonwheat.org).
Conflict of interest
The authors report to have received funding for attending/speaking at conferences and/or other funding that might be perceived as potential conflict of interest, as follows: FB: travel and speaker fees in the period 2018–2020 for various conferences addressing health aspects of cereals and bread with special reference to gluten disorders in Brussels, Paris, Cappadocia, Athens, Munich, Istanbul, Wageningen, Leuven and Barcelona. AH: travel grant from Merck KGaA to visit the congress of the European Society of Human Reproduction and Embryology (ESHRE) 2022 in Milano. DK: research funding from Allergan, Will Pharma, Grunenthal, ZonMw, MDLS, UEG, Horizon 2020, Rome Foundation, all outside of submitted work. His institution has received speaker’s fee from Dr. Falk on his behalf, outside of submitted work. JM: research funding from Maxima Medical Center and ZonMw, both outside of submitted work. VM: travel and speaker’s fees from Guerbet as well as research grants from Guerbet, Merck and Ferring, all outside of submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Dysbiosis is a lasting unfavorable change in the composition/diversity of resident microbiota communities and their metabolism, relative to that found in healthy individuals (60).
2. ^Prebiotics: fermentable carbohydrates that are preferably fermented by health beneficial microbiota such as lactobacilli and bifidobacteria. Probiotics: food supplements supplying health beneficial live microbiota. Synbiotics: supplement containing pre- and probiotics.
3. ^Microbial translocation is defined as the passage of both viable and nonviable microbes and microbial products such as endotoxin, across an anatomically intact intestinal barrier (100).
References
1. Becker, CM, Bokor, A, Heikinheimo, O, Horne, A, Jansen, F, Kiesel, L, et al. Eshre guideline: endometriosis. Hum Reprod Open. (2022) 2022:hoac009. doi: 10.1093/hropen/hoac009
2. van Barneveld, E, Manders, J, van Osch, FHM, van Poll, M, Visser, L, van Hanegem, N, et al. Depression, anxiety, and correlating factors in endometriosis: a systematic review and meta-analysis. J Womens Health (Larchmt). (2022) 31:219–30. doi: 10.1089/jwh.2021.0021
3. van Poll, M, van Barneveld, E, Aerts, L, Maas, JWM, Lim, AC, de Greef, BTA, et al. Endometriosis and sexual quality of life. Sex Med. (2020) 8:532–44. doi: 10.1016/j.esxm.2020.06.004
4. Minko, A, Turon-Skrzypinska, A, Ryl, A, Bargiel, P, Hilicka, Z, Michalczyk, K, et al. Endometriosis-a multifaceted problem of a modern woman. Int J Environ Res Public Health. (2021) 18:8177. doi: 10.3390/ijerph18158177
5. Saunders, PTK, and Horne, AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cells. (2021) 184:2807–24. doi: 10.1016/j.cell.2021.04.041
6. Simoens, S, Dunselman, G, Dirksen, C, Hummelshoj, L, Bokor, A, Brandes, I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. (2012) 27:1292–9. doi: 10.1093/humrep/des073
7. Zondervan, KT, Becker, CM, Koga, K, Missmer, SA, Taylor, RN, and Vigano, P. Endometriosis. Nat Rev Dis Primers. (2018) 4:9. doi: 10.1038/s41572-018-0008-5
8. Lvd, Houwen. Endometriosis associated subfertility. Surgical treatment and assisted reproduction techniques. Vrije Universiteit Amsterdam (2019).
9. Anglesio, MS, Papadopoulos, N, Ayhan, A, Nazeran, TM, Noe, M, Horlings, HM, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. (2017) 376:1835–48. doi: 10.1056/NEJMoa1614814
10. Badipatla, KR, Vupputuri, A, Niazi, M, Blaise, MN, and Nayudu, SK. Colonic endometriosis: dig deeper for diagnosis. Gastroenterology Res. (2017) 10:59–62. doi: 10.14740/gr760e
11. Dimoulios, P, Koutroubakis, IE, Tzardi, M, Antoniou, P, Matalliotakis, IM, and Kouroumalis, EA. A case of sigmoid endometriosis difficult to differentiate from colon cancer. BMC Gastroenterol. (2003) 3:18. doi: 10.1186/1471-230X-3-18
12. Kim, JS, Hur, H, Min, BS, Kim, H, Sohn, SK, Cho, CH, et al. Intestinal endometriosis mimicking carcinoma of rectum and sigmoid colon: a report of five cases. Yonsei Med J. (2009) 50:732–5. doi: 10.3349/ymj.2009.50.5.732
13. Muthyala, T, Sikka, P, Aggarwal, N, Suri, V, Gupta, R, and Nahar, U. Endometriosis presenting as carcinoma colon in a perimenopausal woman. J Midlife Health. (2015) 6:122–4. doi: 10.4103/0976-7800.165592
14. Nisenblat, V, Bossuyt, PM, Farquhar, C, Johnson, N, and Hull, ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. (2016) 2:CD009591. doi: 10.1002/14651858.CD009591.pub2
15. Samet, JD, Horton, KM, Fishman, EK, and Hruban, RH. Colonic endometriosis mimicking colon cancer on a virtual colonoscopy study: a potential pitfall in diagnosis. Case Rep Med. (2009) 2009:379578. doi: 10.1155/2009/379578
16. Worley, MJ, Welch, WR, Berkowitz, RS, and Ng, SW. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. (2013) 14:5367–79. doi: 10.3390/ijms14035367
17. Staal, AH, van der Zanden, M, and Nap, AW. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Investig. (2016) 81:321–4. doi: 10.1159/000441911
18. Greene, R, Stratton, P, Cleary, SD, Ballweg, ML, and Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. (2009) 91:32–9. doi: 10.1016/j.fertnstert.2007.11.020
19. Kor, E, Mostafavi, SRS, Mazhin, ZA, Dadkhah, A, Kor, A, Arvanagh, SH, et al. Relationship between the severity of endometriosis symptoms (dyspareunia, dysmenorrhea and chronic pelvic pain) and the spread of the disease on ultrasound. BMC Res Notes. (2020) 13:546. doi: 10.1186/s13104-020-05388-5
20. Facchin, F, Barbara, G, Saita, E, Mosconi, P, Roberto, A, Fedele, L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. (2015) 36:135–41. doi: 10.3109/0167482X.2015.1074173
21. Becker, CM, Gattrell, WT, Gude, K, and Singh, SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. (2017) 108:125–36. doi: 10.1016/j.fertnstert.2017.05.004
22. Zondervan, KT, Becker, CM, and Missmer, SA. Endometriosis. N Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
23. Armour, M, Sinclair, J, Chalmers, KJ, and Smith, CA. Self-management strategies amongst Australian women with endometriosis: a National Online Survey. BMC Complement Altern Med. (2019) 19:17. doi: 10.1186/s12906-019-2431-x
24. Bellini, M, Tonarelli, S, Nagy, AG, Pancetti, A, Costa, F, Ricchiuti, A, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. (2020) 12:148. doi: 10.3390/nu12010148
26. Marziali, M, Venza, M, Lazzaro, S, Lazzaro, A, Micossi, C, and Stolfi, VM. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. (2012) 67:499–504.
27. Moore, JS, Gibson, PR, Perry, RE, and Burgell, RE. Endometriosis in patients with irritable bowel syndrome: specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. (2017) 57:201–5. doi: 10.1111/ajo.12594
28. Vennberg Karlsson, J, Patel, H, and Premberg, A. Experiences of health after dietary changes in endometriosis: a qualitative interview study. BMJ Open. (2020) 10:e032321. doi: 10.1136/bmjopen-2019-032321
29. Clower, L, Fleshman, T, Geldenhuys, WJ, and Santanam, N. Targeting oxidative stress involved in endometriosis and its pain. Biomol Ther. (2022) 12:1055. doi: 10.3390/biom12081055
30. Nap, A, and de Roos, N. Endometriosis and the effects of dietary interventions: what are we looking for? Reprod Fertil. (2022) 3:C14–22. doi: 10.1530/RAF-21-0110
31. Buggio, L, Barbara, G, Facchin, F, Frattaruolo, MP, Aimi, G, and Berlanda, N. Self-management and psychological-sexological interventions in patients with endometriosis: strategies, outcomes, and integration into clinical care. Int J Women’s Health. (2017) 9:281–93. doi: 10.2147/IJWH.S119724
32. MacKichan, F, Paterson, C, Henley, WE, and Britten, N. Self-care in people with long term health problems: a community based survey. BMC Fam Pract. (2011) 12:53. doi: 10.1186/1471-2296-12-53
33. Mardon, AK, Leake, HB, Hayles, C, Henry, ML, Neumann, PB, Moseley, GL, et al. The efficacy of self-management strategies for females with endometriosis: a systematic review. Reprod Sci. (2022) 30:390–407. doi: 10.1007/s43032-022-00952-9
34. O’Hara, R, Rowe, H, and Fisher, J. Self-management in condition-specific health: a systematic review of the evidence among women diagnosed with endometriosis. BMC Womens Health. (2019) 19:80. doi: 10.1186/s12905-019-0774-6
35. Seear, K. The third shift: health, work and expertise among women with endometriosis. Health Sociol Rev. (2009) 18:194–206. doi: 10.5172/hesr.18.2.194
36. O’Hara, R, Rowe, H, and Fisher, J. Self-management factors associated with quality of life among women with endometriosis: a cross-sectional Australian survey. Hum Reprod. (2021) 36:647–55. doi: 10.1093/humrep/deaa330
37. van Haaps, AW, Wijbers, J, Schreurs, A, and Mijatovic, V. A better quality of life could be achieved by applying the endometriosis diet: a cross-sectional study in Dutch endometriosis patients. Reprod Biomed Online. (2022) 46:623–30. doi: 10.1016/j.rbmo.2022.12.010
38. Chiaffarino, F, Cipriani, S, Ricci, E, Mauri, PA, Esposito, G, Barretta, M, et al. Endometriosis and irritable bowel syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet. (2021) 303:17–25. doi: 10.1007/s00404-020-05797-8
39. Nabi, MY, Nauhria, S, Reel, M, Londono, S, Vasireddi, A, Elmiry, M, et al. Endometriosis and irritable bowel syndrome: a systematic review and meta-analyses. Front Med (Lausanne). (2022) 9:914356. doi: 10.3389/fmed.2022.914356
40. Huijs, E, and Nap, A. The effects of nutrients on symptoms in women with endometriosis: a systematic review. Reprod Biomed Online. (2020) 41:317–28. doi: 10.1016/j.rbmo.2020.04.014
41. Sverrisdottir, UA, Hansen, S, and Rudnicki, M. Impact of diet on pain perception in women with endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2022) 271:245–9. doi: 10.1016/j.ejogrb.2022.02.028
42. Nirgianakis, K, Egger, K, Kalaitzopoulos, DR, Lanz, S, Bally, L, and Mueller, MD. Effectiveness of dietary interventions in the treatment of endometriosis: a systematic review. Reprod Sci. (2022) 29:26–42. doi: 10.1007/s43032-020-00418-w
43. Ajabnoor, SM, Thorpe, G, Abdelhamid, A, and Hooper, L. Long-term effects of increasing omega-3, omega-6 and total polyunsaturated fats on inflammatory bowel disease and markers of inflammation: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. (2021) 60:2293–316. doi: 10.1007/s00394-020-02413-y
44. Su, H, Liu, R, Chang, M, Huang, J, Jin, Q, and Wang, X. Effect of dietary alpha-linolenic acid on blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. (2018) 57:877–91. doi: 10.1007/s00394-017-1386-2
45. Ávalos Marfil, A, Barranco Castillo, E, Martos García, R, Mendoza Ladrón de Guevara, N, and Mazheika, M. Epidemiology of endometriosis in Spain and Its autonomous communities: A large, nationwide study. Int J Environ Res Public Health. (2021) 18:7861. doi: 10.3390/ijerph18157861
46. Sarria-Santamera, A, Orazumbekova, B, Terzic, M, Issanov, A, Chaowen, C, and Asunsolo-Del-Barco, A. Systematic review and meta-analysis of incidence and prevalence of endometriosis. Healthcare (Basel). (2020) 9:29. doi: 10.3390/healthcare9010029
47. Zhang, S, Gong, TT, Wang, HY, Zhao, YH, and Wu, QJ. Global, regional, and national endometriosis trends from 1990 to 2017. Ann N Y Acad Sci. (2021) 1484:90–101. doi: 10.1111/nyas.14468
48. Feng, J, Zhang, S, Chen, J, Yang, J, and Zhu, J. Long-term trends in the incidence of endometriosis in China from 1990 to 2019: a joinpoint and age-period-cohort analysis. Gynecol Endocrinol. (2021) 37:1041–5. doi: 10.1080/09513590.2021.1975675
49. Rowlands, IJ, Abbott, JA, Montgomery, GW, Hockey, R, Rogers, P, and Mishra, GD. Prevalence and incidence of endometriosis in Australian women: a data linkage cohort study. BJOG. (2021) 128:657–65. doi: 10.1111/1471-0528.16447
50. El-Toukhy, T. Prevalence of endometriosis: how close are we to the truth? BJOG. (2021) 128:666. doi: 10.1111/1471-0528.16466
53. Sampson, JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. (1927) 3:93–110.43.
54. Kruitwagen, RF, Poels, LG, Willemsen, WN, de Ronde, IJ, Jap, PH, and Rolland, R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril. (1991) 55:297–303. doi: 10.1016/S0015-0282(16)54119-3
55. Koninckx, PR, Ussia, A, Adamyan, L, Tahlak, M, Keckstein, J, Wattiez, A, et al. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract Res Clin Obstet Gynaecol. (2021) 71:14–26. doi: 10.1016/j.bpobgyn.2020.08.005
56. Machado-Linde, F, Pelegrin, P, Sanchez-Ferrer, ML, Leon, J, Cascales, P, and Parrilla, JJ. 2-Methoxyestradiol in the pathophysiology of endometriosis: focus on angiogenesis and therapeutic potential. Reprod Sci. (2012) 19:1018–29. doi: 10.1177/1933719112446080
57. Liang, Y, and Yao, S. Potential role of estrogen in maintaining the imbalanced sympathetic and sensory innervation in endometriosis. Mol Cell Endocrinol. (2016) 424:42–9. doi: 10.1016/j.mce.2016.01.012
58. Yamamoto, A, Harris, HR, Vitonis, AF, Chavarro, JE, and Missmer, SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. (2018) 219:178 e1–178.e10. doi: 10.1016/j.ajog.2018.05.034
60. Peterson, CM, Johnstone, EB, Hammoud, AO, Stanford, JB, Varner, MW, Kennedy, A, et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO study. Am J Obstet Gynecol. (2013) 208:451 e1–451 e11. doi: 10.1016/j.ajog.2013.02.040
61. Laschke, MW, and Menger, MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. (2016) 215:68.e1–4. doi: 10.1016/j.ajog.2016.02.036
62. Khan, KN, Fujishita, A, Hiraki, K, Kitajima, M, Nakashima, M, Fushiki, S, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. (2018) 17:125–33. doi: 10.1002/rmb2.12083
63. Garcia-Penarrubia, P, Ruiz-Alcaraz, AJ, Martinez-Esparza, M, Marin, P, and Machado-Linde, F. Hypothetical roadmap towards endometriosis: prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum Reprod Update. (2020) 26:214–46. doi: 10.1093/humupd/dmz044
64. Jiang, I, Yong, PJ, Allaire, C, and Bedaiwy, MA. Intricate connections between the microbiota and endometriosis. Int J Mol Sci. (2021) 22:5644. doi: 10.3390/ijms22115644
65. Taylor, HS, Kotlyar, AM, and Flores, VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397:839–52. doi: 10.1016/S0140-6736(21)00389-5
66. Gruber, TM, and Mechsner, S. Pathogenesis of endometriosis: the origin of pain and subfertility. Cells. (2021) 10:1381. doi: 10.3390/cells10061381
67. Maenhoudt, N, De Moor, A, and Vankelecom, H. Modeling endometrium biology and disease. J Pers Med. (2022) 12:1048. doi: 10.3390/jpm12071048
68. Horne, AW, and Missmer, SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. (2022) 379:e070750. doi: 10.1136/bmj-2022-070750
69. Lebwohl, B, Ludvigsson, JF, and Green, PH. State of the art review: celiac disease and non-celiac gluten sensitivity. The BMJ. (2015) 351:h4347. doi: 10.1136/bmj.h4347
70. Leonard, MM, Sapone, A, Catassi, C, and Fasano, A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. (2017) 318:647–56. doi: 10.1001/jama.2017.9730
71. Brouns, F, van Rooy, G, Shewry, P, Rustgi, S, and Jonkers, D. Adverse reactions to wheat or wheat components. Compr Rev Food Sci Food Saf. (2019) 18:1437–52. doi: 10.1111/1541-4337.12475
72. Carroccio, A, Giambalvo, O, Blasca, F, Iacobucci, R, D’Alcamo, A, and Mansueto, P. Self-reported non-celiac wheat sensitivity in high school students: demographic and clinical characteristics. Nutrients. (2017) 9:771. doi: 10.3390/nu9070771
73. Ek, M, Roth, B, Bengtsson, M, and Ohlsson, B. Gastrointestinal symptoms in women with endometriosis and microscopic colitis in comparison to irritable bowel syndrome: a cross-sectional study. Turk J Gastroenterol. (2021) 32:819–27. doi: 10.5152/tjg.2020.19583
74. Capannolo, A, Viscido, A, Barkad, MA, Valerii, G, Ciccone, F, Melideo, D, et al. Non-celiac gluten sensitivity among patients perceiving gluten-related symptoms. Digestion. (2015) 92:8–13. doi: 10.1159/000430090
75. Porpora, MG, Scaramuzzino, S, Sangiuliano, C, Piacenti, I, Bonanni, V, Piccioni, MG, et al. High prevalence of autoimmune diseases in women with endometriosis: a case-control study. Gynecol Endocrinol. (2020) 36:356–9. doi: 10.1080/09513590.2019.1655727
76. Shigesi, N, Kvaskoff, M, Kirtley, S, Feng, Q, Fang, H, Knight, JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:486–503. doi: 10.1093/humupd/dmz014
77. Hopeman, MM, Riley, JK, Frolova, AI, Jiang, H, and Jungheim, ES. Serum polyunsaturated fatty acids and endometriosis. Reprod Sci. (2015) 22:1083–7. doi: 10.1177/1933719114565030
78. Missmer, SA, Chavarro, JE, Malspeis, S, Bertone-Johnson, ER, Hornstein, MD, Spiegelman, D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. (2010) 25:1528–35. doi: 10.1093/humrep/deq044
79. Saguyod, JJU, As, K, Velarde, MC, and Simmer, RCM. Diet and endometriosis-revisiting the linkages to inflammation. J Endometr Pelvic Pain Disord. (2018) 10:51–8. doi: 10.1177/2284026518769022
80. Borghini, R, Porpora, MG, Casale, R, Marino, M, Palmieri, E, Greco, N, et al. Irritable bowel syndrome-like disorders in endometriosis: prevalence of nickel sensitivity and effects of a low-nickel diet. An open-label pilot study. Nutrients. (2020) 12:341. doi: 10.3390/nu12020341
81. Bilal, M, Ashraf, S, and Zhao, X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and synbiotics. Front Nutr. (2022) 9:931458. doi: 10.3389/fnut.2022.931458
82. Krabbenborg, I, de Roos, N, van der Grinten, P, and Nap, A. Diet quality and perceived effects of dietary changes in Dutch endometriosis patients: an observational study. Reprod Biomed Online. (2021) 43:952–61. doi: 10.1016/j.rbmo.2021.07.011
83. Shafrir, AL, Farland, LV, Shah, DK, Harris, HR, Kvaskoff, M, Zondervan, K, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. (2018) 51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001
84. Leonardi, M, Hicks, C, El-Assaad, F, El-Omar, E, and Condous, G. Endometriosis and the microbiome: a systematic review. BJOG. (2020) 127:239–49. doi: 10.1111/1471-0528.15916
85. de Graaf, MCG, Croden, F, Lawton, CL, Winkens, B, Hesselink, MAM, van Rooy, G, et al. The effect of consumer expectancy versus actual gluten intake on gastrointestinal symptoms in non-coeliac gluten sensitivity. Gastroenterology-DDW. (2023) 164:S-408–9. doi: 10.1016/S0016-5085(23)01944-3
86. Helbig, M, Vesper, AS, Beyer, I, and Fehm, T. Does nutrition affect endometriosis? Geburtshilfe Frauenheilkd. (2021) 81:191–9. doi: 10.1055/a-1207-0557
87. Osmanlioglu, S, and Sanlier, N. The relationship between endometriosis and diet. Hum Fertil (Camb). (2021):1–16. doi: 10.1080/14647273.2021.1995900
88. Koedooder, R, Mackens, S, Budding, A, Fares, D, Blockeel, C, Laven, J, et al. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum Reprod Update. (2019) 25:298–325. doi: 10.1093/humupd/dmy048
89. Winters, AD, Romero, R, Gervasi, MT, Gomez-Lopez, N, Tran, MR, Garcia-Flores, V, et al. Does the endometrial cavity have a molecular microbial signature? Sci Rep. (2019) 9:9905. doi: 10.1038/s41598-019-46173-0
90. O’Callaghan, JL, Turner, R, Dekker Nitert, M, Barrett, HL, Clifton, V, and Pelzer, ES. Re-assessing microbiomes in the low-biomass reproductive niche. BJOG. (2020) 127:147–58. doi: 10.1111/1471-0528.15974
91. Ata, B, Yildiz, S, Turkgeldi, E, Brocal, VP, Dinleyici, EC, Moya, A, et al. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. (2019) 9:2204. doi: 10.1038/s41598-019-39700-6
92. Lin, WC, Chang, CY, Hsu, YA, Chiang, JH, and Wan, L. Increased risk of endometriosis in patients with lower genital tract infection: a nationwide cohort study. Medicine (Baltimore). (2016) 95:e2773. doi: 10.1097/MD.0000000000002773
93. Cheong, HC, Yap, PSX, Chong, CW, Cheok, YY, Lee, CYQ, Tan, GMY, et al. Diversity of endocervical microbiota associated with genital Chlamydia Trachomatis infection and infertility among women visiting obstetrics and gynecology clinics in Malaysia. PLoS One. (2019) 14:e0224658. doi: 10.1371/journal.pone.0224658
94. Wessels, JM, Dominguez, MA, Leyland, NA, Agarwal, SK, and Foster, WG. Endometrial microbiota is more diverse in people with endometriosis than symptomatic controls. Sci Rep. (2021) 11:18877. doi: 10.1038/s41598-021-98380-3
95. Khan, KN, Kitajima, M, Hiraki, K, Yamaguchi, N, Katamine, S, Matsuyama, T, et al. Escherichia Coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. (2010) 94:2860-3 e1-3. doi: 10.1016/j.fertnstert.2010.04.053
96. Khan, KN, Kitajima, M, Inoue, T, Tateishi, S, Fujishita, A, Nakashima, M, et al. Additive effects of inflammation and stress reaction on toll-like receptor 4-mediated growth of endometriotic stromal cells. Hum Reprod. (2013) 28:2794–803. doi: 10.1093/humrep/det280
97. Khan, KN, Kitajima, M, Yamaguchi, N, Fujishita, A, Nakashima, M, Ishimaru, T, et al. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Hum Reprod. (2012) 27:3417–24. doi: 10.1093/humrep/des331
98. Tai, FW, Chang, CY, Chiang, JH, Lin, WC, and Wan, L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. (2018) 7:379. doi: 10.3390/jcm7110379
99. Blander, JM, Longman, RS, Iliev, ID, Sonnenberg, GF, and Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. (2017) 18:851–60. doi: 10.1038/ni.3780
100. Alexander, JW, Boyce, ST, Babcock, GF, Gianotti, L, Peck, MD, Dunn, DL, et al. The process of microbial translocation. Ann Surg. (1990) 212:496–510; discussion 1-2. doi: 10.1097/00000658-199010000-00012
101. Menees, S, and Chey, W. The gut microbiome and irritable bowel syndrome. F1000Res. (2018) 7:1029. doi: 10.12688/f1000research.14592.1
102. Wang, Y, Cao, Y, Lebwohl, B, Song, M, Sun, Q, Green, PHR, et al. Gluten intake and risk of digestive system cancers in 3 large prospective cohort studies. Clin Gastroenterol Hepatol. (2021) 20:1986–1996.e11. doi: 10.1016/j.cgh.2021.11.016
103. Bascunan, KA, Araya, M, Roncoroni, L, Doneda, L, and Elli, L. Dietary gluten as a conditioning factor of the gut microbiota in celiac disease. Adv Nutr. (2020) 11:160–74. doi: 10.1093/advances/nmz080
104. Uhde, M, Ajamian, M, Caio, G, De Giorgio, R, Indart, A, Green, PH, et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut. (2016) 65:1930–7. doi: 10.1136/gutjnl-2016-311964
105. Othman, ER, Markeb, AA, Khashbah, MY, Abdelaal, II, ElMelegy, TT, Fetih, AN, et al. Markers of local and systemic estrogen metabolism in endometriosis. Reprod Sci. (2021) 28:1001–11. doi: 10.1007/s43032-020-00383-4
106. Salliss, ME, Farland, LV, Mahnert, ND, and Herbst-Kralovetz, MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. (2021) 28:92–131. doi: 10.1093/humupd/dmab035
107. Crha, KVP, Zakova, J, Jeseta, M, Pilka, R, Vodicka, J, and Crha, I. The role of uterine microbiome and epithelial-mesenchymal transition in endometrial function. Med J Cell Biol. (2019) 7:146–51. doi: 10.2478/acb-2019-0020
108. Agostinis, C, Mangogna, A, Bossi, F, Ricci, G, Kishore, U, and Bulla, R. Uterine immunity and microbiota: a shifting paradigm. Front Immunol. (2019) 10:2387. doi: 10.3389/fimmu.2019.02387
109. Perrotta, AR, Borrelli, GM, Martins, CO, Kallas, EG, Sanabani, SS, Griffith, LG, et al. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: a pilot study. Reprod Sci. (2020) 27:1064–73. doi: 10.1007/s43032-019-00113-5
110. Chadchan, SB, Cheng, M, Parnell, LA, Yin, Y, Schriefer, A, Mysorekar, IU, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod. (2019) 34:1106–16. doi: 10.1093/humrep/dez041
111. Ni, Z, Sun, S, Bi, Y, Ding, J, Cheng, W, Yu, J, et al. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am J Reprod Immunol. (2020) 84:e13307. doi: 10.1111/aji.13307
112. Flores, R, Shi, J, Fuhrman, B, Xu, X, Veenstra, TD, Gail, MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. (2012) 10:253. doi: 10.1186/1479-5876-10-253
113. Bolte, G, Wolburg, H, Beuermann, K, Stocker, S, and Stern, M. Specific interaction of food proteins with apical membranes of the human intestinal cell lines Caco-2 and T84. Clin Chim Acta. (1998) 270:151–67. doi: 10.1016/S0009-8981(97)00218-0
114. Foltz, M, van der Pijl, PC, and Duchateau, GS. Current in vitro testing of bioactive peptides is not valuable. J Nutr. (2010) 140:117–8. doi: 10.3945/jn.109.116228
115. Ridlon, JM, Kang, DJ, Hylemon, PB, and Bajaj, JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. (2014) 30:332–8. doi: 10.1097/MOG.0000000000000057
116. Yang, R, and Qian, L. Research on gut microbiota-derived secondary bile acids in cancer progression. Integr Cancer Ther. (2022) 21:15347354221114100. doi: 10.1177/15347354221114100
117. Plamada, D, and Vodnar, DC. Polyphenols-gut microbiota interrelationship: a transition to a new generation of prebiotics. Nutrients. (2021) 14:137. doi: 10.3390/nu14010137
118. Pompella, A, Sies, H, Wacker, R, Brouns, F, Grune, T, Biesalski, HK, et al. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition. (2014) 30:791–3. doi: 10.1016/j.nut.2013.12.002
119. Koo, LC. Diet and lung cancer 20+ years later: more questions than answers? Int J Cancer. (1997) 71:22–9. doi: 10.1002/(sici)1097-0215(1997)10+<22::aid-ijc7>3.0.co;2-b
120. Zhao, H, and Jin, X. Causal associations between dietary antioxidant vitamin intake and lung cancer: a Mendelian randomization study. Front Nutr. (2022) 9:965911. doi: 10.3389/fnut.2022.965911
121. Schwartz, NRM, Afeiche, MC, Terry, KL, Farland, LV, Chavarro, JE, Missmer, SA, et al. Glycemic index, glycemic load, fiber, and gluten intake and risk of laparoscopically confirmed endometriosis in premenopausal women. J Nutr. (2022) 152:2088–96. doi: 10.1093/jn/nxac107
122. Calder, PC. New evidence in support of the cardiovascular benefit of long-chain N-3 fatty acids. Ital Heart J. (2003) 4:427–9.
123. Harbige, LS. Fatty acids, the immune response, and autoimmunity: a question of N-6 essentiality and the balance between N-6 and N-3. Lipids. (2003) 38:323–41. doi: 10.1007/s11745-003-1067-z
124. Pischon, T, Hankinson, SE, Hotamisligil, GS, Rifai, N, Willett, WC, and Rimm, EB. Habitual dietary intake of N-3 and N-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. (2003) 108:155–60. doi: 10.1161/01.CIR.0000079224.46084.C2
125. Innes, JK, and Calder, PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. (2018) 132:41–8. doi: 10.1016/j.plefa.2018.03.004
126. Parazzini, F, Vigano, P, Candiani, M, and Fedele, L. Diet and endometriosis risk: a literature review. Reprod Biomed Online. (2013) 26:323–36. doi: 10.1016/j.rbmo.2012.12.011
127. Nodler, JL, DiVasta, AD, Vitonis, AF, Karevicius, S, Malsch, M, Sarda, V, et al. Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2020) 112:229–36. doi: 10.1093/ajcn/nqaa096
128. Mozaffari, H, Daneshzad, E, Larijani, B, Bellissimo, N, and Azadbakht, L. Dietary intake of fish, N-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. (2020) 59:1–17. doi: 10.1007/s00394-019-01901-0
129. Trabert, B, Peters, U, De Roos, AJ, Scholes, D, and Holt, VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. (2011) 105:459–67. doi: 10.1017/S0007114510003661
130. Wang, Y, and Beydoun, MA. Meat consumption is associated with obesity and central obesity among us adults. Int J Obes. (2009) 33:621–8. doi: 10.1038/ijo.2009.45
131. Calder, PC, Ahluwalia, N, Brouns, F, Buetler, T, Clement, K, Cunningham, K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. (2011) 106:S5–S78. doi: 10.1017/S0007114511005460
132. Montonen, J, Boeing, H, Fritsche, A, Schleicher, E, Joost, HG, Schulze, MB, et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. (2013) 52:337–45. doi: 10.1007/s00394-012-0340-6
133. Hediger, ML, Hartnett, HJ, and Louis, GM. Association of endometriosis with body size and figure. Fertil Steril. (2005) 84:1366–74. doi: 10.1016/j.fertnstert.2005.05.029
134. Gao, M, Allebeck, P, Mishra, GD, and Koupil, I. Developmental origins of endometriosis: a Swedish cohort study. J Epidemiol Community Health. (2019) 73:353–9. doi: 10.1136/jech-2018-211811
135. Hetemaki, N, Mikkola, TS, Tikkanen, MJ, Wang, F, Hamalainen, E, Turpeinen, U, et al. Adipose tissue estrogen production and metabolism in premenopausal women. J Steroid Biochem Mol Biol. (2021) 209:105849. doi: 10.1016/j.jsbmb.2021.105849
136. Andersson, AM, and Skakkebaek, NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol. (1999) 140:477–85. doi: 10.1530/eje.0.1400477
137. Brinkman, MT, Baglietto, L, Krishnan, K, English, DR, Severi, G, Morris, HA, et al. Consumption of animal products, their nutrient components and postmenopausal circulating steroid hormone concentrations. Eur J Clin Nutr. (2010) 64:176–83. doi: 10.1038/ejcn.2009.129
138. Zorb, C, Ludewig, U, and Hawkesford, MJ. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. (2018) 23:1029–37. doi: 10.1016/j.tplants.2018.08.012
139. Kucek, LK, Veenstra, LD, Amnuaycheewa, P, and Sorrells, ME. A grounded guide to gluten: how modern genotypes and processing impact wheat sensitivity. Compr Rev Food Sci Food Saf. (2015) 14:285–302. doi: 10.1111/1541-4337.12129
140. Brouns, F, and Shewry, PR. Do gluten peptides stimulate weight gain in humans? Nutr Bull. (2022) 47:186–198. doi: 10.1111/nbu.12558
141. Schalk, K, Lexhaller, B, Koehler, P, and Scherf, KA. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS One. (2017) 12:e0172819. doi: 10.1371/journal.pone.0172819
142. Geisslitz, S, Longin, CFH, Scherf, KA, and Koehler, P. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods. (2019) 8:409. doi: 10.3390/foods8090409
143. Bos, C, Juillet, B, Fouillet, H, Turlan, L, Dare, S, Luengo, C, et al. Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr. (2005) 81:87–94. doi: 10.1093/ajcn/81.1.87
144. Gellrich, C, Schieberle, P, and Wieser, H. Biochemical characterization and quantification of the storage protein (secalin) types in Rye flour. Cereal Chem. (2003) 80:102–9. doi: 10.1094/CCHEM.2003.80.1.102
145. Gregorini, A, Colomba, M, Ellis, HJ, and Ciclitira, PJ. Immunogenicity characterization of two ancient wheat alpha-gliadin peptides related to coeliac disease. Nutrients. (2009) 1:276–90. doi: 10.3390/nu1020276
146. Colomba, MS, and Gregorini, A. Are ancient durum wheats less toxic to celiac patients? A study of α-gliadin from Graziella Ra and Kamut. Sci World J. (2012) 2012:837416. doi: 10.1100/2012/837416
147. Prandi, B, Tedeschi, T, Folloni, S, Galaverna, G, and Sforza, S. Peptides from gluten digestion: a comparison between old and modern wheat varieties. Food Res Int. (2017) 91:92–102. doi: 10.1016/j.foodres.2016.11.034
148. Sollid, LM, Qiao, S-W, Anderson, RP, Gianfrani, C, and Koning, F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by Hla-Dq molecules. Immunogenetics. (2012) 64:455–60. doi: 10.1007/s00251-012-0599-z
149. Theethira, TG, Dennis, M, and Leffler, DA. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev Gastroenterol Hepatol. (2014) 8:123–9. doi: 10.1586/17474124.2014.876360
150. Cardo, A, Churruca, I, Lasa, A, Navarro, V, Vazquez-Polo, M, Perez-Junkera, G, et al. Nutritional imbalances in adult celiac patients following a gluten-free diet. Nutrients. (2021) 13:2877. doi: 10.3390/nu13082877
151. Fasano, A, Sapone, A, Zevallos, V, and Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology. (2015) 148:1195–204. doi: 10.1053/j.gastro.2014.12.049
152. De Giorgio, R, Volta, U, and Gibson, PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. (2016) 65:169–78. doi: 10.1136/gutjnl-2015-309757
153. Begum, W, Rai, S, Banerjee, S, Bhattacharjee, S, Mondal, MH, Bhattarai, A, et al. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. (2022) 12:9139–53. doi: 10.1039/D2RA00378C
154. Ishii, K, Takakuwa, K, Kashima, K, Tamura, M, and Tanaka, K. Associations between patients with endometriosis and Hla class ii; the analysis of Hla-Dqb1 and Hla-Dpb1 genotypes. Hum Reprod. (2003) 18:985–9. doi: 10.1093/humrep/deg192
155. Greenbaum, H, Galper, BL, Decter, DH, and Eisenberg, VH. Endometriosis and autoimmunity: can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun Rev. (2021) 20:102795. doi: 10.1016/j.autrev.2021.102795
156. Fasano, A, and Catassi, C. Clinical practice. Celiac disease. N Engl J Med. (2012) 367:2419–26. doi: 10.1056/NEJMcp1113994
157. Lebwohl, B, Sanders, DS, and Green, PHR. Coeliac disease. Lancet. (2018) 391:70–81. doi: 10.1016/S0140-6736(17)31796-8
158. Catassi, C, Gatti, S, and Lionetti, E. World perspective and celiac disease epidemiology. Dig Dis. (2015) 33:141–6. doi: 10.1159/000369518
159. Lionetti, E, Gatti, S, Pulvirenti, A, and Catassi, C. Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol. (2015) 29:365–79. doi: 10.1016/j.bpg.2015.05.004
160. Auricchio, R, and Troncone, R. Can celiac disease be prevented? Front Immunol. (2021) 12:672148. doi: 10.3389/fimmu.2021.672148
161. Santoro, L, Campo, S, D’Onofrio, F, Gallo, A, Covino, M, Campo, V, et al. Looking for celiac disease in Italian women with endometriosis: a case control study. Biomed Res Int. (2014) 2014:236821. doi: 10.1155/2014/236821
162. Aguiar, FM, Melo, SB, Galvao, LC, Rosa-e-Silva, JC, dos Reis, RM, and Ferriani, RA. Serological testing for celiac disease in women with endometriosis. A pilot study. Clin Exp Obstet Gynecol. (2009) 36:23–5.
163. Stephansson, O, Falconer, H, and Ludvigsson, JF. Risk of endometriosis in 11,000 women with celiac disease. Hum Reprod. (2011) 26:2896–901. doi: 10.1093/humrep/der263
164. Littlejohns, TJ, Chong, AY, Allen, NE, Arnold, M, Bradbury, KE, Mentzer, AJ, et al. Genetic, lifestyle, and health-related characteristics of adults without celiac disease who follow a gluten-free diet: a population-based study of 124,447 participants. Am J Clin Nutr. (2021) 113:622–9. doi: 10.1093/ajcn/nqaa291
165. Caserta, D, Matteucci, E, Ralli, E, Bordi, G, and Moscarini, M. Celiac disease and endometriosis: an insidious and worrisome association hard to diagnose: a case report. Clin Exp Obstet Gynecol. (2014) 41:346–8. doi: 10.12891/ceog16842014
166. Zafeiropoulou, K, Nichols, B, Mackinder, M, Biskou, O, Rizou, E, Karanikolou, A, et al. Alterations in intestinal microbiota of children with celiac disease at the time of diagnosis and on a gluten-free diet. Gastroenterology. (2020) 159:2039–2051.e20. doi: 10.1053/j.gastro.2020.08.007
167. Leonard, MM, Valitutti, F, Karathia, H, Pujolassos, M, Kenyon, V, Fanelli, B, et al. Microbiome signatures of progression toward celiac disease onset in at-risk children in a longitudinal prospective cohort study. Proc Natl Acad Sci U S A. (2021) 118:e2020322118. doi: 10.1073/pnas.2020322118
168. El Mouzan, M, Al-Hussaini, A, Serena, G, Assiri, A, Al Sarkhy, A, Al Mofarreh, M, et al. Microbiota profile of new-onset celiac disease in children in Saudi Arabia. Gut Pathog. (2022) 14:37. doi: 10.1186/s13099-022-00493-1
169. Palmieri, O, Castellana, S, Bevilacqua, A, Latiano, A, Latiano, T, Panza, A, et al. Adherence to gluten-free diet restores alpha diversity in celiac people but the microbiome composition is different to healthy people. Nutrients. (2022) 14:2452. doi: 10.3390/nu14122452
170. Kaliciak, I, Drogowski, K, Garczyk, A, Kopec, S, Horwat, P, Bogdanski, P, et al. Influence of gluten-free diet on gut microbiota composition in patients with coeliac disease: a systematic review. Nutrients. (2022) 14:2083. doi: 10.3390/nu14102083
171. Vacca, M, Porrelli, A, Calabrese, FM, Lippolis, T, Iacobellis, I, Celano, G, et al. How metabolomics provides novel insights on celiac disease and gluten-free diet: a narrative review. Front Microbiol. (2022) 13:859467. doi: 10.3389/fmicb.2022.859467
172. Jansson-Knodell, CL, and Rubio-Tapia, A. The fashionable gluten-free diet-wear with caution. Am J Clin Nutr. (2021) 113:491–2. doi: 10.1093/ajcn/nqaa371
173. Aziz, I, Lewis, NR, Hadjivassiliou, M, Winfield, SN, Rugg, N, Kelsall, A, et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol. (2014) 26:33–9. doi: 10.1097/01.meg.0000435546.87251.f7
174. van Gils, T, Nijeboer, P, IJ, CE, Sanders, DS, Mulder, CJ, and Bouma, G. Prevalence and characterization of self-reported gluten sensitivity in the Netherlands. Nutrients. (2016) 8:714. doi: 10.3390/nu8110714
175. Molina-Infante, J, and Carroccio, A. Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. (2017) 15:339–48. doi: 10.1016/j.cgh.2016.08.007
176. Biesiekierski, JR, Peters, SL, Newnham, ED, Rosella, O, Muir, JG, and Gibson, PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. (2013) 145:320–8 e1-3. doi: 10.1053/j.gastro.2013.04.051
177. Skodje, GI, Sarna, VK, Minelle, IH, Rolfsen, KL, Muir, JG, Gibson, PR, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. (2018) 154:529–39 e2. doi: 10.1053/j.gastro.2017.10.040
178. Crawley, C, Savino, N, Halby, C, Sander, SD, Andersen, AN, Arumugam, M, et al. The effect of gluten in adolescents and young adults with gastrointestinal symptoms: a blinded randomised cross-over trial. Aliment Pharmacol Ther. (2022) 55:1116–27. doi: 10.1111/apt.16914
179. Fritscher-Ravens, A, Pflaum, T, Mosinger, M, Ruchay, Z, Rocken, C, Milla, PJ, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology. (2019) 157:109–118.e5. doi: 10.1053/j.gastro.2019.03.046
180. Junker, Y, Zeissig, S, Kim, SJ, Barisani, D, Wieser, H, Leffler, DA, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. (2012) 209:2395–408. doi: 10.1084/jem.20102660
181. Geisslitz, S, Shewry, P, Brouns, F, America, AHP, Caio, GPI, Daly, M, et al. Wheat ATIs: characteristics and role in human disease. Front Nutr. (2021) 8:667370. doi: 10.3389/fnut.2021.667370
182. Geisslitz, S, Weegels, P, Shewry, P, Zevallos, V, Masci, S, Sorrells, M, et al. Wheat amylase/trypsin inhibitors (ATIs): occurrence, function and health aspects. Eur J Nutr. (2022) 61:2873–80. doi: 10.1007/s00394-022-02841-y
183. Ludvigsson, JF, Card, T, Ciclitira, PJ, Swift, GL, Nasr, I, Sanders, DS, et al. Support for patients with celiac disease: a literature review. United European Gastroenterol J. (2015) 3:146–59. doi: 10.1177/2050640614562599
184. Taetzsch, A, Das, SK, Brown, C, Krauss, A, Silver, RE, and Roberts, SB. Are gluten-free diets more nutritious? An evaluation of self-selected and recommended gluten-free and gluten-containing dietary patterns. Nutrients. (2018) 10:1881. doi: 10.3390/nu10121881
185. Vici, G, Belli, L, Biondi, M, and Polzonetti, V. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. (2016) 35:1236–41. doi: 10.1016/j.clnu.2016.05.002
186. Verma, AK, Gatti, S, Galeazzi, T, Monachesi, C, Padella, L, Baldo, GD, et al. Gluten contamination in naturally or labeled gluten-free products marketed in Italy. Nutrients. (2017) 9:115. doi: 10.3390/nu9020115
187. Falcomer, AL, Santos Araujo, L, Farage, P, Santos Monteiro, J, Yoshio Nakano, E, and Puppin, ZR. Gluten contamination in food services and industry: a systematic review. Crit Rev Food Sci Nutr. (2020) 60:479–93. doi: 10.1080/10408398.2018.1541864
188. So, D, Whelan, K, Rossi, M, Morrison, M, Holtmann, G, Kelly, JT, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. (2018) 107:965–83. doi: 10.1093/ajcn/nqy041
189. Niland, B, and Cash, BD. Health benefits and adverse effects of a gluten-free diet in non-celiac disease patients. Gastroenterol Hepatol (N Y). (2018) 14:82–91.
190. Marciniak, M, Szymczak-Tomczak, A, Mahadea, D, Eder, P, Dobrowolska, A, and Krela-Kazmierczak, I. Multidimensional disadvantages of a gluten-free diet in celiac disease: a narrative review. Nutrients. (2021) 13:643. doi: 10.3390/nu13020643
191. Sergi, C, Villanacci, V, and Carroccio, A. Non-celiac wheat sensitivity: rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: a review. BMC Gastroenterol. (2021) 21:5. doi: 10.1186/s12876-020-01568-6
192. Whelan, K, Abrahmsohn, O, David, GJ, Staudacher, H, Irving, P, Lomer, MC, et al. Fructan content of commonly consumed wheat, Rye and gluten-free breads. Int J Food Sci Nutr. (2011) 62:498–503. doi: 10.3109/09637486.2011.553588
193. Brouns, F, D’elzenne, N, and Gibson, G. The dietary fibers-FODMAPS controversy. Cereal Foods World. (2017) 62:98–103. doi: 10.1094/CFW-62-3-0098
194. Desai, MS, Seekatz, AM, Koropatkin, NM, Kamada, N, Hickey, CA, Wolter, M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cells. (2016) 167:1339–53 e21. doi: 10.1016/j.cell.2016.10.043
195. Moayyedi, P, Quigley, EM, Lacy, BE, Lembo, AJ, Saito, YA, Schiller, LR, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. (2014) 109:1367–74. doi: 10.1038/ajg.2014.195
196. Verbeke, KA, Boobis, AR, Chiodini, A, Edwards, CA, Franck, A, Kleerebezem, M, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. (2015) 28:42–66. doi: 10.1017/S0954422415000037
197. Diether, NE, and Willing, BP. Microbial fermentation of dietary protein: an important factor in diet(−)microbe(−)host interaction. Microorganisms. (2019) 7:19. doi: 10.3390/microorganisms7010019
198. Hill, P, Muir, JG, and Gibson, PR. Controversies and recent developments of the low-FODMAP diet. Gastroenterol Hepatol (N Y). (2017) 13:36–45.
199. Itzlinger, A, Branchi, F, Elli, L, and Schumann, M. Gluten-free diet in celiac disease-forever and for all? Nutrients. (2018) 10:1796. doi: 10.3390/nu10111796
200. O’Keeffe, M, Jansen, C, Martin, L, Williams, M, Seamark, L, Staudacher, HM, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil. (2018) 30:e13154. doi: 10.1111/nmo.13154
201. Maki, KC, Palacios, OM, Koecher, K, Sawicki, CM, Livingston, KA, Bell, M, et al. The relationship between whole grain intake and body weight: results of meta-analyses of observational studies and randomized controlled trials. Nutrients. (2019) 11:1245. doi: 10.3390/nu11061245
202. Barrett, EM, Batterham, MJ, Ray, S, and Beck, EJ. Whole grain, bran and cereal fibre consumption and CVD: a systematic review. Br J Nutr. (2019) 121:914–37. doi: 10.1017/S000711451900031X
203. Huang, T, Xu, M, Lee, A, Cho, S, and Qi, L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. (2015) 13:59. doi: 10.1186/s12916-015-0294-7
204. Wu, H, Flint, AJ, Qi, Q, van Dam, RM, Sampson, LA, Rimm, EB, et al. Association between dietary whole grain intake and risk of mortality: two large prospective studies in us men and women. JAMA Intern Med. (2015) 175:373–84. doi: 10.1001/jamainternmed.2014.6283
205. Ley, SH, Hamdy, O, Mohan, V, and Hu, FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. (2014) 383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9
206. Lillioja, S, Neal, AL, Tapsell, L, and Jacobs, DR. Whole grains, type 2 diabetes, coronary heart disease, and hypertension: links to the aleurone preferred over indigestible fiber. Biofactors. (2013) 39:242–58. doi: 10.1002/biof.1077
207. Aune, D, Giovannucci, E, Boffetta, P, Fadnes, LT, Keum, N, Norat, T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. doi: 10.1093/ije/dyw319
208. Harvard. Harvard nutrition source-what should you eat-whole grains (2021). Available at: https://www.hsph.harvard.edu/nutritionsource/what-should-you-eat/whole-grains/.
210. Wang, W, Li, J, Chen, X, Yu, M, Pan, Q, and Guo, L. Whole grain food diet slightly reduces cardiovascular risks in obese/overweight adults: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2020) 20:82. doi: 10.1186/s12872-020-01337-z
211. Tullio, V, Gasperi, V, Catani, MV, and Savini, I. The impact of whole grain intake on gastrointestinal tumors: a focus on colorectal, gastric, and esophageal cancers. Nutrients. (2020) 13:81. doi: 10.3390/nu13010081
212. Gaesser, GA. Whole grains, refined grains, and cancer risk: a systematic review of meta-analyses of observational studies. Nutrients. (2020) 12:3756. doi: 10.3390/nu12123756
213. NORDIC. Nordic nutrition recommendations 2012. (2012). Available at: http://dx.doi.org/10.6027/Nord2014-002
214. Oberritter, H, Schäbethal, K, von Ruesten, A, and Boeing, H. The DGE-nutrition circle – representation and fundamentals of the food-based recommendations of the German Nutrition Society. Ernaehrungs Umschau Int. (2013) 60:24–9. doi: 10.4455/eu.2013.004
216. USDA. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. 9th Edition. (2020).
Appendix
For this narrative overview, research and review papers were obtained using the university E-Library academic search tools PUBMED, WEB OF SCIENCE, SCOPUS, EMBASE and ENDNOTE global search tool. Search terms used were: endometriosis, diet, dietary intervention, nutrient supplementation, self-management, adjunct therapy, inflammation, immune response, microbiota, dysbiosis, irritable bowel syndrome, inflammatory bowel disease, celiac disease, intestinal permeability, leaky gut, gluten, FODMAP, gluten sensitivity, non-celiac wheat sensitivity, QoL migraine, mood, anxiety, sleep, fatigue, nocebo effects, placebo effects, polyunsaturated fatty acids, PUFA, omega 3, short chain fatty acids, SCFA, meat, fish, food estrogens, whole grain, dietary fiber, antioxidants, polyphenols, gluten free diet. In research papers and scientific reviews the reference lists were checked for additional sources and relevant interpretations and related citations were cross-checked with the content/data in the original sources cited.
Keywords: endometriosis, diet associations, diet recommendations, omega-3, red meat, gluten-free
Citation: Brouns F, Van Haaps A, Keszthelyi D, Venema K, Bongers M, Maas J and Mijatovic V (2023) Diet associations in endometriosis: a critical narrative assessment with special reference to gluten. Front. Nutr. 10:1166929. doi: 10.3389/fnut.2023.1166929
Edited by:
Antonio Simone Laganà, University of Palermo, ItalyReviewed by:
Nicole Clemence Roy, University of Otago, New ZealandMaria Grazia Porpora, Sapienza University of Rome, Italy
Copyright © 2023 Brouns, Van Haaps, Keszthelyi, Venema, Bongers, Maas and Mijatovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fred Brouns, ZnJlZC5icm91bnNAbWFhc3RyaWNodHVuaXZlcnNpdHkubmw=
 Fred Brouns
Fred Brouns Annelotte Van Haaps
Annelotte Van Haaps Daniel Keszthelyi
Daniel Keszthelyi Koen Venema
Koen Venema Marlies Bongers
Marlies Bongers Jacques Maas7,8
Jacques Maas7,8 Velja Mijatovic
Velja Mijatovic