- 1Department of Thoracic Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Key Laboratory of Cardio-Thoracic Surgery, Fujian Medical University, Fuzhou, China
- 3Key Laboratory of Gastrointestinal Cancer (Fujian Medical University), Ministry of Education, Fuzhou, China
- 4Fujian Key Laboratory of Tumor Microbiology, Department of Medical Microbiology, Fujian Medical University, Fuzhou, China
- 5Fuqing City Hospital Affiliated to Fujian Medical University, Fuzhou, China
Background: Lung cancer is the most common global cancer in terms of incidence and mortality. Its main driver is tobacco smoking. The identification of modifiable risk factors isa public health priority. Green tea consumption has been examined in epidemiological studies, with inconsistent findings. Thus, we aimed to apply Mendelian randomization to clarify any causal link between green tea consumption and the risk of lung cancer.
Methods: We utilized a two-sample Mendelian randomization (MR) approach. Genetic variants served as instrumental variables. The goal was to explore a causal link between green tea consumption and different lung cancer types. Green tea consumption data was sourced from the UK Biobank dataset, and the genetic association data for various types of lung cancer were sourced from multiple databases. Our analysis included primary inverse-variance weighted (IVW) analyses and various sensitivity test.
Results: No significant associations were found between green tea intake and any lung cancer subtypes, including non-small cell lung cancer (adenocarcinoma and squamous cell carcinoma) and small cell lung cancer. These findings were consistent when applying multiple Mendelian randomization methods.
Conclusion: Green tea does not appear to offer protective benefits against lung cancer at a population level. However, lung cancer's complex etiology and green tea's potential health benefitssuggest more research is needed. Further studies should include diverse populations, improved exposure measurements and randomized controlled trials, are warranted.
Introduction
Tea is one of the most widely consumed beverages in the world, known not just for its flavors but also for its potential health benefits. It is a rich source of polyphenols, specifically a catechin called epigallocatechin-3-gallate (EGCG) (1). Prior research has unveiled the antioxidant, anti-inflammatory (2), and anticarcinogenic properties of EGCG (3, 4), opening the possibility that regular consumption of tea, particularly green tea, might offer protective effects against various health conditions, including cancer.
Lung cancer, primarily driven by tobacco smoking, remains the most common cancer globally, both in terms of incidence and mortality (5). Despite the well-established role of smoking in lung cancer development (6, 7), other environmental factors (8, 9) and lifestyle factors (10, 11) are also important, and their influence on the causes of lung cancer is still an active area of research. Given the severity and prevalence of lung cancer, identifying additional modifiable risk factors is a high priority in public health. Among these, diet has received increasing attention (12, 13), making the exploration of dietary elements like green tea crucial.
Epidemiologically, the relationship between green tea consumption and lung cancer risk has been extensively studied, but the findings have been inconsistent. Some observational studies and meta-analyses suggest a protective effect of green tea (14, 15), particularly among non-smokers (16), while others have found null associations (17, 18). These discrepancies can be attributed to several factors such as variations in tea consumption habits, differences in the preparation method and type of tea, and potential confounding by tobacco smoking and other lifestyle factors.
Mendelian randomization (MR), an epidemiological tool that leverages genetic variants as instrumental variables, offers a unique opportunity to mitigate such biases inherent to observational studies (19). In our studies, genetic variants strongly associated with an exposure, the consumption of green tea, are used to form an instrumental variable. Since these genetic variants are determined at conception, they are not prone to reverse causation, and they should be unrelated to the confounding factors that typically bias observational studies. By using genetically predicted exposures, MR studies can provide less confounded estimates of causal effects, thereby overcoming limitations of conventional epidemiological studies (20). Despite its potential, to our knowledge, the MR approach has not yet been applied to investigate the causal effect of green tea consumption on lung cancer risk.
Therefore, our findings aim to add a new perspective to the existing literature on the relationship between green tea consumption and lung cancer risk. By employing Mendelian randomization, this study seeks to clarify the potential causal links that have been previously obscured in observational studies. While our results may not directly influence the current understanding of lung cancer etiology, they provide a foundation for future research in this area, potentially guiding more targeted investigations into preventive strategies.
Methods
Study design
This study employed a two-sample Mendelian randomization (MR) approach to investigate the potential causal relationship between green tea consumption and the risk of distinct types of lung cancer, namely, non-small cell lung cancer (NSCLC) (with further division into adenocarcinoma and squamous cell carcinoma subtypes) and small cell lung cancer (SCLC) (Figure 1).
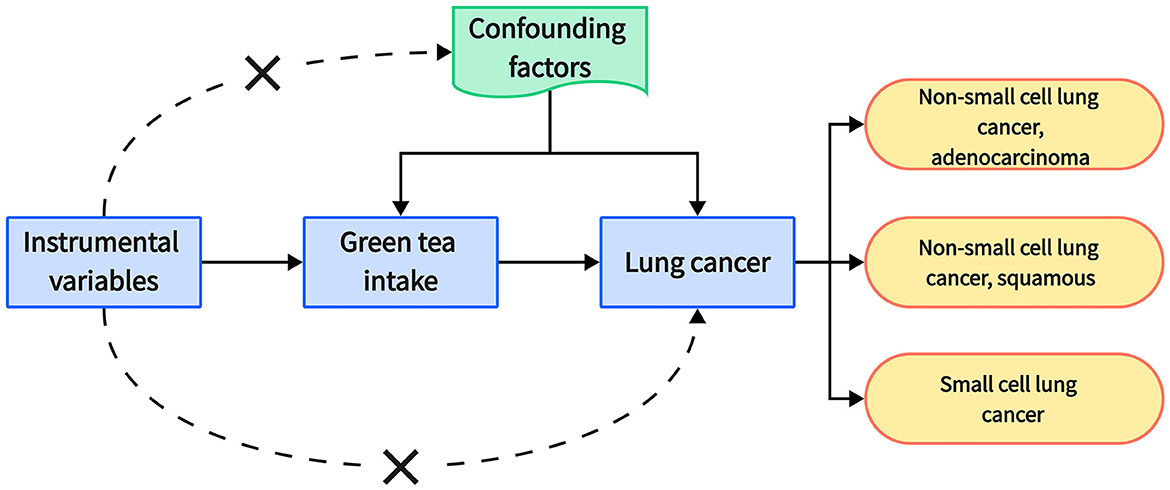
Figure 1. Schematic representation of Mendelian randomization analysis framework. It illustrates the sequence of analysis: starting with instrumental variables that are genetic variants associated with green tea intake, the pathway leads to lung cancer, highlighting subtypes such as adenocarcinoma and squamous non-small cell lung cancer, and small cell lung cancer. Confounding factors are denoted as being separate from the causal pathway. This visual underscores the exclusion of confounding variables in the Mendelian randomization approach and delineates the hypothesized influence of green tea consumption on lung cancer subtypes.
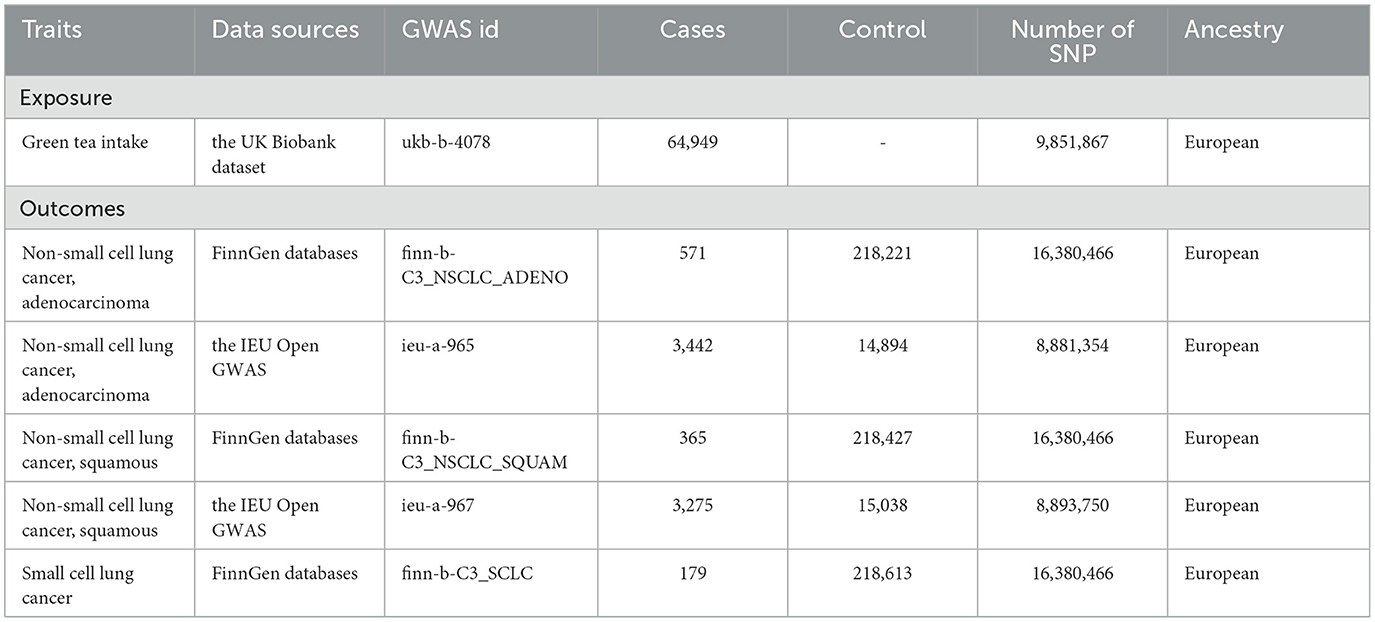
The detailed characteristics of the data implemented in this study are comprehensively captured in Table 1. Meanwhile, the step-by-step procedure of the SNP selection process, integral to our analysis, along with the derived results are graphically represented in Figure 2.
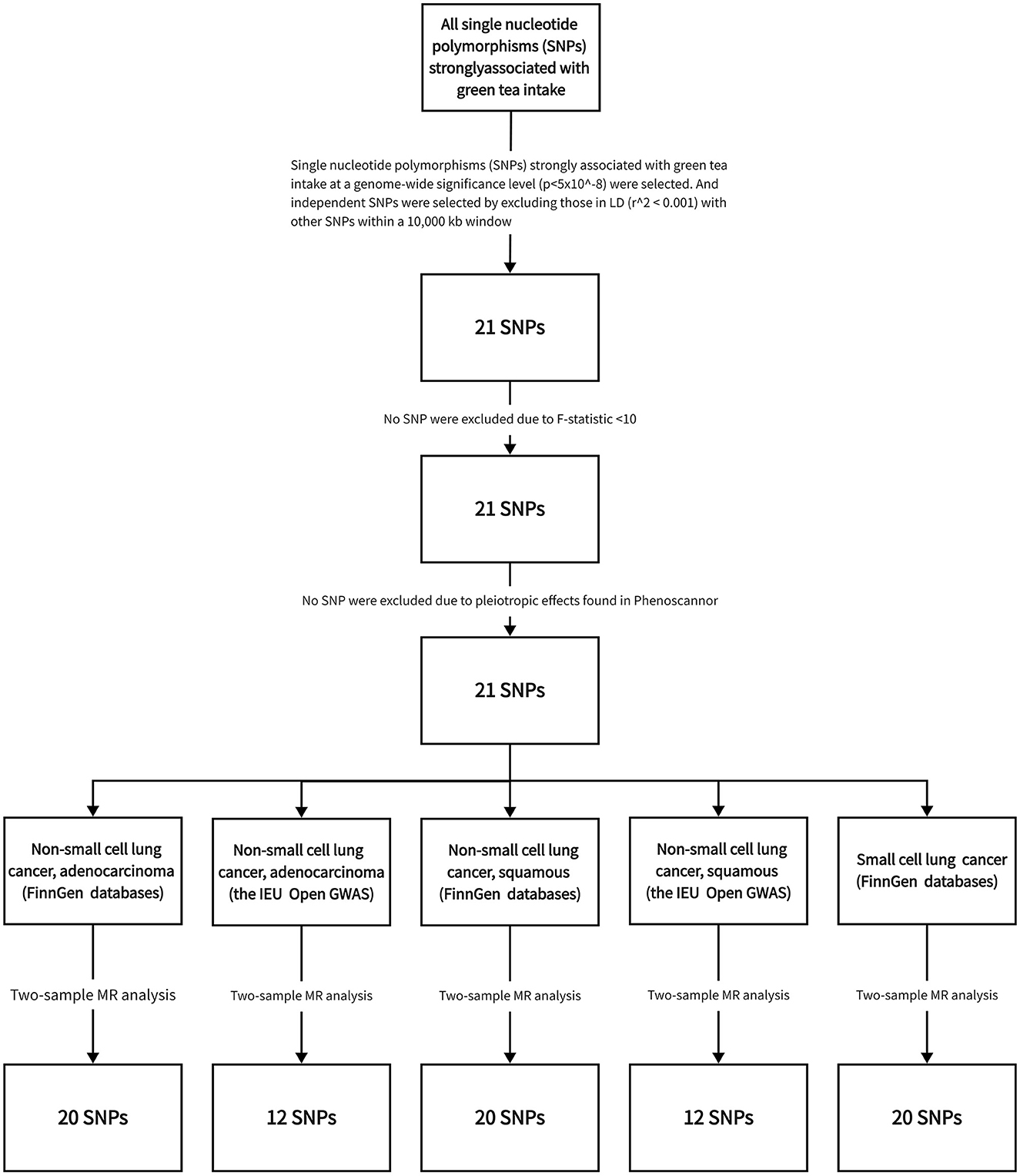
Figure 2. Flowchart illustrating the methodology of Mendelian randomization analysis utilized in the current investigation.
Genome-Wide Association Study procedure
To investigate the causal relationships between green tea consumption and lung cancer subtypes, we utilized a Genome-Wide Association Study (GWAS) approach. GWAS enables the comprehensive examination of common, low-penetrance genetic variants and their association with specific phenotypes, in this case, lung cancer and its subtypes (21).
In this study, we carried out a Genome-Wide Association Study (GWAS) to identify single nucleotide polymorphisms (SNPs) that are associated with green tea consumption and its potential effect on lung cancer risk. The GWAS was performed following a well-structured workflow, which is based on established guidelines and procedures. Samples were collected from volunteers participating in the UK Biobank, following informed consent and ethical approval. Genotyping was done using the Affymetrix Axiom UK BiLEVE array, and subsequent quality control measures were applied (22).
Exposure data and instrumental variable selection
The exposure of interest in our study was green tea consumption. We sourced data from the UK Biobank dataset (Green tea intake Dataset: ukb-b-4078), which consists of 64,949 participants of European descent, both males and females, who reported their green tea intake.
From this dataset, we identified single nucleotide polymorphisms (SNPs) strongly associated with green tea intake at a genome-wide significance level (p < 5x10∧-8) in the genome-wide association study (GWAS). These SNPs were employed as instrumental variables (IVs) for green tea consumption, providing a means of approximating the randomized exposure necessary for our Mendelian randomization analysis.
To ensure the validity of our IVs and to avoid bias introduced by the co-inheritance of genetic variants, we conducted linkage disequilibrium (LD) pruning. We selected independent SNPs by excluding those in LD (r∧2 < 0.001) with other SNPs within a 10,000 kb window. This stringent LD threshold and window size ensured the selected SNPs were independent and reduced the likelihood of biased MR estimates due to correlated instruments.
We further refined the selection of our IVs through a series of steps. Firstly, we calculated the F-statistic for each SNP to measure the strength of the IVs. All SNPs yielded an F-statistic of > 10, indicating a low risk of weak instrument bias; thus, no SNP was excluded at this step.
Next, we scrutinized the selected SNPs using the PhenoScanner database to assess whether these SNPs were associated with established lung cancer risk factors (P < 5 × 10–8), such as age and smoking. SNPs associated with these confounders were also excluded to minimize confounding bias.
The resulting set of SNPs, which were independently and robustly associated with green tea intake and not with known confounders, were then employed as instrumental variables in our subsequent Mendelian randomization analyses.
Outcome data
We sourced genetic association data for various types of lung cancer from multiple databases to evaluate the potential impact of green tea consumption on lung cancer risk.
The first dataset involved non-small cell lung cancer (NSCLC), specifically adenocarcinoma, from the FinnGen study (Dataset: finn-b-C3_NSCLC_ADENO). This dataset included 571 cases and 218,221 controls, all of European descent and comprising both males and females.
In addition, we used the lung adenocarcinoma dataset from the IEU Open GWAS database (Dataset: ieu-a-965), comprising a European population of both sexes with 3,442 cases and 14,894 controls.
We extracted data for NSCLC of the squamous cell subtype from the FinnGen study (Dataset: finn-b-C3_NSCLC_SQUAM). This dataset involved a European population of both sexes, with 365 cases and 218,427 controls.
Also, the squamous cell lung cancer dataset from the IEU Open GWAS database (Dataset: ieu-a-967) was used, including 3,275 cases and 15,038 controls from a European population of both sexes.
Lastly, we used the small cell lung cancer (SCLC) dataset from the FinnGen study (Dataset: finn-b-C3_SCLC). This encompassed a European population of both sexes, with 179 cases and 218,613 controls.
Each of these datasets provided SNP-wise association summary statistics from respective genome-wide association studies (GWAS). These data were used for the Mendelian randomization analysis to examine the putative causal relationships.
Mendelian randomization analysis
We conducted a two-sample MR using several approaches. The main analysis was performed using the inverse variance-weighted (IVW) method. The MR-Egger regression, weighted median method, and weighted mode-based estimator were employed for sensitivity analyses. These methods provide different assumptions about the pleiotropy of the genetic instruments and are used to test the robustness of the findings.
The MR-Egger regression can provide a valid causal estimate even when all genetic variants are invalid instruments, as long as the instrument strength independent of direct effect (InSIDE) assumption holds. The weighted median method can provide a correct estimate if at least 50% of the weight in the analysis comes from valid instruments. The weighted mode-based estimator will give a consistent estimate if the largest number of similar individual-instrument causal effect estimates comes from valid instruments.
Statistical analysis
Methodology overview: Mendelian randomization analysis
In this study, we employed a two-sample Mendelian Randomization (MR) approach to investigate the potential causal relationship between green tea consumption and the risk of developing various types of lung cancer.
Computational tools and software
The statistical analyses were performed using R software, version 4.2.1. For the MR analyses, we utilized two specific R packages: “MendelianRandomization” and “TwoSampleMR”.
Primary analysis approach: Inverse Variance-Weighted method
As part of our primary analysis, the Inverse Variance-Weighted (IVW) method was applied. This method aggregates the estimated effects of individual genetic variants, weighted by their precision, to provide an overall causal effect estimate.
Sensitivity analyses
MR-Egger Regression: We used this method as it allows for a valid causal inference even in the presence of pleiotropic effects, provided that the InSIDE (Instrument Strength Independent of Direct Effect) assumption is met.
Weighted Median Method: This method can yield a correct causal estimate when at least 50% of the weight in the analysis comes from valid instrumental variables.
Weighted Mode-Based Estimator: This method will deliver a consistent causal estimate if the largest number of similar individual-instrument causal effect estimates originates from valid instrumental variables.
Results
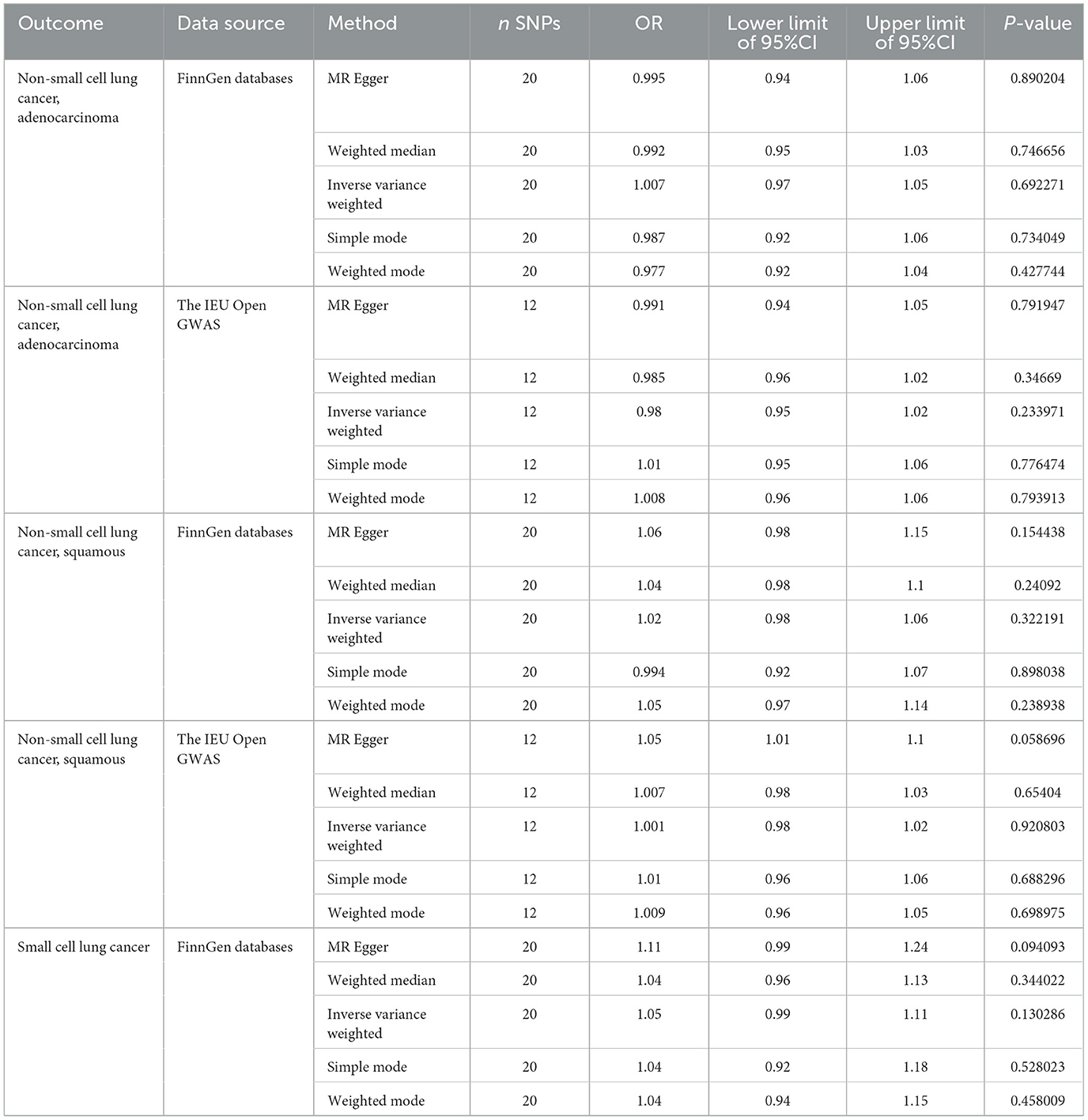
Our comprehensive Mendelian Randomization analysis probed the relationship between green tea intake and various subtypes of lung cancer (Table 2).
Non-small cell lung cancer, adenocarcinoma
Initially, we evaluated the association between green tea consumption and non-small cell lung cancer, specifically adenocarcinoma, using two independent datasets from FinnGen databases and the IEU Open GWAS. Our primary IVW analysis consistently indicated a non-significant association for both datasets (OR = 1.007, 95% CI: 0.97 to 1.05, p = 0.69; OR = 0.981, 95% CI: 0.95 to 1.02, p = 0.23 respectively). This non-significant effect was corroborated by several alternative MR techniques including MR Egger, Weighted Median, Simple Mode, and Weighted Mode, reinforcing the robustness of our results.
Non-small cell lung cancer, squamous
For squamous cell lung cancer, another subtype of non-small cell lung cancer, our primary IVW analysis revealed a similar pattern of non-significant associations across datasets from FinnGen databases and the IEU Open GWAS (OR = 1.022, 95% CI: 0.98–1.06, p = 0.32; OR = 1.001, 95% CI: 0.98–1.02, p = 0.92 respectively). The robustness of these findings was reinforced by additional MR methods, suggesting a lack of a strong causal effect of green tea intake on the risk of squamous cell lung cancer.
Small cell lung cancer
Regarding small cell lung cancer, our IVW analysis did not indicate a significant association between green tea intake and disease risk (OR = 1.048, 95% CI: 0.99–1.11, p = 0.13). Additional MR methods, including MR Egger, Weighted Median, Simple Mode, and Weighted Mode, echoed these non-significant findings.
Collectively, our comprehensive Mendelian Randomization analysis, encompassing multiple lung cancer subtypes and employing a suite of MR methodologies, consistently suggested a lack of significant causal relationships between green tea intake and lung cancer risk. However, the complexity of cancer etiology and potential pleiotropic effects of genetic instruments warrant a cautious interpretation of our findings.
Discussion
In the current Mendelian randomization study, we sought to clarify the potential causal relationship between green tea consumption and lung cancer risk, which, to our knowledge, represents the first attempt to explore this relationship from a genetic epidemiology standpoint. We systematically examined different subtypes of lung cancer, including non-small cell lung cancer with adenocarcinoma and squamous cell carcinoma subtypes, and small cell lung cancer. Through both primary inverse-variance weighted analyses and various sensitivity analyses, our results consistently showed no significant associations between green tea intake and lung cancer risk.
Our conclusions stand in stark contrast to a considerable volume of prior research, which has observed a potential protective effect of green tea consumption against lung cancer, particularly among non-smokers (14, 16). This finding aligns with Wang et al.'s meta-analysis of case-control and cohort studies (23). In this meticulous assessment of daily tea consumers vs. infrequent tea drinkers, a statistically significant decreased risk of lung cancer was noted among daily consumers, suggesting a dose-response relationship. The observed protective effect was particularly striking in Asian populations, potentially indicating genetic or lifestyle interactions.
Despite these persuasive findings emerging from observational studies and the synthesis of multiple individual studies in meta-analyses, our Mendelian randomization analysis does not corroborate these protective associations. This dichotomy underscores the complexity of diet-cancer relationships and the methodological challenges in their robust examination (24, 25). It highlights the importance of diverse research methodologies, such as Mendelian randomization, in producing comprehensive and reliable insights, which might sometimes run counter to the prevailing narrative based on observational studies.
The discrepancy can be attributed to the inherent limitations of observational studies, which are subject to confounding bias and reverse causation. For instance, individuals who regularly consume green tea might adopt other healthier lifestyle habits such as regular exercise, balanced diet, and abstaining from smoking, which independently reduce lung cancer risk. Conversely, Mendelian randomization analysis leverages genetic variants as instrumental variables, which are not influenced by these lifestyle factors, thereby providing more robust and unbiased causal effect estimates.
While our Mendelian randomization analysis did not reveal a significant protective effect of green tea intake against lung cancer, we believe it is essential to highlight the substantial body of research that underscores the potential health benefits of green tea. Notably, green tea is enriched with polyphenols, particularly a catechin known as epigallocatechin-3-gallate (EGCG), recognized for its antioxidant, anti-inflammatory, and anticarcinogenic properties (1–4, 26). These properties have been substantiated by numerous in vitro and in vivo studies (27–29), which offer compelling evidence of the potential anti-cancer attributes of green tea and EGCG. These bioactive compounds are implicated in several crucial anticancer mechanisms, such as arresting the cell cycle, inducing apoptosis in cancer cells, and inhibiting angiogenesis, processes that could stymie the progression and proliferation of cancer cells. While our study does not confirm the protective effect of green tea consumption on lung cancer at a population level, the biological plausibility for green tea's anticancer potential cannot be entirely dismissed.
The strengths of our study are multifaceted. We adopted a stringent selection process for the genetic instruments for green tea intake, ensuring they were strongly associated with the exposure and were not linked to known confounders or in linkage disequilibrium. By employing several MR methodologies, each providing different assumptions and robustness to pleiotropy, we were able to provide a comprehensive and rigorous assessment of the causal effect.
Despite the novel insights offered by our study, we must recognize and address several potential limitations. Primarily, our reliance on self-reported green tea consumption data might be subjected to reporting bias. Participants could inadvertently overstate or understate their green tea consumption, which could introduce errors into our analysis. Implementing more objective and standardized measures of green tea intake, such as biomarkers, might help to attenuate this potential bias in future research.
Further, we must consider the inherent variability in green tea consumption across different populations. Variations in tea brewing methods, types of green tea consumed, and the specific green tea components consumed could introduce heterogeneity into our exposure measurement. For instance, brewing temperature and duration, as well as the part of the tea plant used, can significantly influence the concentration of polyphenols and other bioactive compounds in the tea (30, 31). Future studies might benefit from capturing this variability more accurately, possibly by incorporating data on specific tea brewing and consumption practices.
Another potential limitation pertains to the demographic specificity of our study cohort. Our analysis was rooted in data from populations of European descent, which could limit the generalizability of our findings. Genetic differences, dietary habits (32), and lifestyle factors (33) vary widely across different ethnic and geographic groups (34), and these factors might interact with green tea consumption in influencing lung cancer risk. Consequently, caution should be exercised in extrapolating our findings to other populations. Future research could aim to replicate our findings in diverse ethnic and demographic groups, thereby enhancing the global applicability and robustness of our conclusions.
Taken together, while our study provides valuable insights into the relationship between green tea consumption and lung cancer risk, the above limitations underscore the need for continued exploration in this area.
Conclusion
In this Mendelian Randomization study, we rigorously examined the causal link between green tea consumption and various lung cancer subtypes, employing robust statistical methodologies. Our analyses, across multiple sensitivity tests, consistently show no significant causal effect of green tea intake on lung cancer risk, including adenocarcinoma, squamous cell, and small cell lung cancers.
Though our results indicate a null relationship with lung cancer risk, they should not negate the potential health benefits of bioactive compounds in green tea, such as EGCG. The complex etiology of lung cancer and the potential pleiotropic effects of our genetic instruments warrant cautious interpretation. For future studies, we recommend larger and more diverse cohorts, as well as improved instrumental variables to address confounding factors. Such investigations are vital for a nuanced understanding of green tea's role in lung cancer etiology.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://gwas.mrcieu.ac.uk/, https://www.finngen.fi/en.
Author contributions
JL: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. YL: Data curation, Methodology, Validation, Writing – original draft. JJ: Data curation, Methodology, Validation, Writing – original draft. LG: Resources, Writing – review & editing. ZS: Validation, Writing – review & editing. CY: Data curation, Writing – review & editing. PL: Data curation, Writing – review & editing. MK: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a shared parent affiliation with the author(s) to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. (2011) 82:1807–21. doi: 10.1016/j.bcp.2011.07.093
2. Singh NA, Mandal AK, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J. (2016) 15:60. doi: 10.1186/s12937-016-0179-4
3. Thangapazham RL, Singh AK, Sharma A, Warren J, Gaddipati JP, Maheshwari RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. (2007) 245:232–41. doi: 10.1016/j.canlet.2006.01.027
4. Xu XY, Zhao CN, Cao SY, Tang GY, Gan RY Li HB. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit Rev Food Sci Nutr. (2020) 60:1693–705. doi: 10.1080/10408398.2019.1588223
5. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
6. Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. (2013) 368:351–64. doi: 10.1056/NEJMsa1211127
7. Lareau S, Slatore C, Smyth R. Lung cancer. Am J Respir Crit Care Med. (2021) 204:P21–P2. doi: 10.1164/rccm.20411P21
8. Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. (2013) 14:813–22. doi: 10.1016/S1470-2045(13)70279-1
9. Markowitz SB, Levin SM, Miller A, Morabia A. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American insulator cohort. Am J Respir Crit Care Med. (2013) 188:90–6. doi: 10.1164/rccm.201302-0257OC
10. Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. (2014) 100:693–700. doi: 10.3945/ajcn.113.079194
11. Cannioto R, Etter JL, LaMonte MJ, Ray AD, Joseph JM, Al Qassim E, et al. Lifetime physical inactivity is associated with lung cancer risk and mortality. Cancer Treat Res Commun. (2018) 14:37–45. doi: 10.1016/j.ctarc.2018.01.001
12. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
13. Wei X, Zhu C, Ji M, Fan J, Xie J, Huang Y, et al. Diet and risk of incident lung cancer: a large prospective cohort study in UK biobank. Am J Clin Nutr. (2021) 114:2043–51. doi: 10.1093/ajcn/nqab298
14. Yuan JM. Green tea and prevention of esophageal and lung cancers. Mol Nutr Food Res. (2011) 55:886–904. doi: 10.1002/mnfr.201000637
15. Lee AH, Liang W, Hirayama F, Binns CW. Association between green tea consumption and lung cancer risk. J Prev Med Public Health. (2010) 43:366–7. doi: 10.3961/jpmph.2010.43.4.366
16. Huang CC, Lai CY, Lin IH, Tsai CH, Tsai SM, Lam KL, et al. Joint effects of cigarette smoking and green tea consumption with miR-29b and DNMT3B mRNA expression in the development of lung cancer. Genes. (2022) 13:836. doi: 10.3390/genes13050836
17. Tang N, Wu Y, Zhou B, Wang B, Yu R. Green tea, black tea consumption and risk of lung cancer: a meta-analysis. Lung Cancer. (2009) 65:274–83. doi: 10.1016/j.lungcan.2008.12.002
18. Yu C, Tang H, Guo Y, Bian Z, Yang L, Chen Y, et al. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a population-based cohort study. Ann Intern Med. (2018) 168:489–97. doi: 10.7326/M17-2000
19. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
20. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. (2017) 26:2333–55. doi: 10.1177/0962280215597579
21. Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. (2017) 101:5–22. doi: 10.1016/j.ajhg.2017.06.005
22. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
23. Wang L, Zhang X, Liu J, Shen L, Li Z. Tea consumption and lung cancer risk: a meta-analysis of case-control and cohort studies. Nutrition. (2014) 30:1122–7. doi: 10.1016/j.nut.2014.02.023
24. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
25. Bohan Brown MM, Brown AW, Allison DB. Nutritional epidemiology in practice: learning from data or promulgating beliefs? Am J Clin Nutr. (2013) 97:5–6. doi: 10.3945/ajcn.112.052472
26. Chu C, Deng J, Man Y, Qu Y. Green tea extracts Epigallocatechin-3-gallate for different treatments. Biomed Res Int. (2017) 2017:5615647. doi: 10.1155/2017/5615647
27. Du GJ, Zhang Z, Wen XD Yu C, Calway T, Yuan CS, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. (2012) 4:1679–91. doi: 10.3390/nu4111679
28. Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. (2010) 62:931–7. doi: 10.1080/01635581.2010.509536
29. Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. (2004) 62:204–11. doi: 10.1301/nr.2004.may.204-211
30. Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Wozniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. (2020) 60:626–59. doi: 10.1080/10408398.2018.1546669
31. Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. (2018) 11:39. doi: 10.3390/nu11010039
32. Contaldo F, Santarpia L, Cioffi I, Pasanisi F. Nutrition transition and cancer. Nutrients. (2020) 12:795. doi: 10.3390/nu12030795
33. Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. (2017) 75:405–19. doi: 10.1093/nutrit/nux012
Keywords: lung cancer, green tea, Mendelian randomization, polyphenols, epigallocatechin-3-gallate (EGCG), Genome-Wide Association Study (GWAS)
Citation: Lu J, Lin Y, Jiang J, Gao L, Shen Z, Yang C, Lin P and Kang M (2024) Investigating the potential causal association between consumption of green tea and risk of lung cancer: a study utilizing Mendelian randomization. Front. Nutr. 11:1265878. doi: 10.3389/fnut.2024.1265878
Received: 24 July 2023; Accepted: 05 February 2024;
Published: 19 February 2024.
Edited by:
Junmin Zhang, Lanzhou University, ChinaReviewed by:
Deepak Kumar Verma, Indian Institute of Technology Kharagpur, IndiaJianbo Lin, First Affiliated Hospital of Fujian Medical University, China
Anna M. Witkowska, Medical University of Bialystok, Poland
Copyright © 2024 Lu, Lin, Jiang, Gao, Shen, Yang, Lin and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingqiang Kang, bWluZ3FpYW5nX2thbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jieming Lu
Jieming Lu Ye Lin1,2†
Ye Lin1,2† Mingqiang Kang
Mingqiang Kang