- 1Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Dermatology Department, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
Background: Many observational studies have identified a link between unsaturated fatty acids and psoriasis. However, they contain reverse causality and confounding factors, and there is no definite causal study between unsaturated fatty acids and psoriasis.
Objectives: Analysis of causality between unsaturated fatty acids and psoriasis by Mendelian randomization.
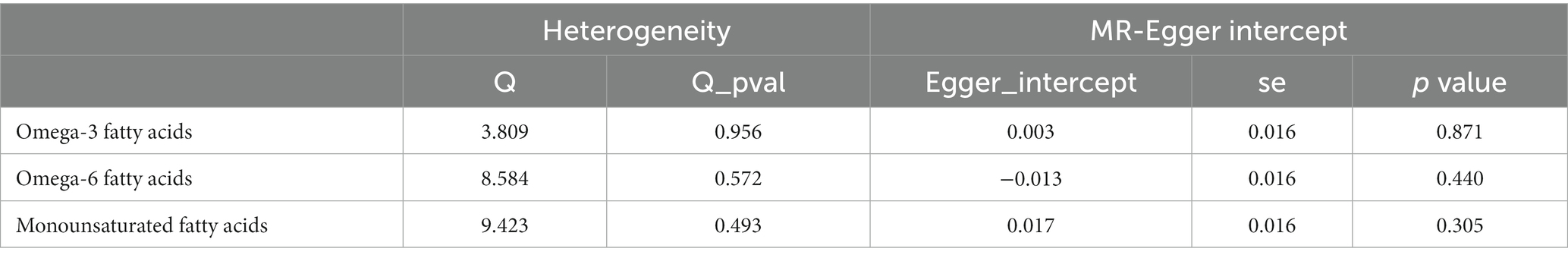
Methods: We used IEU Open GWAS Project, omega-3 PUFA and omega-6 PUFA data from 114,999 subjects, MUFA data from 13,535 subjects, and psoriasis data from 4,510 cases and 212,242 controls were included. We employed the inverse-variance weighted (IVW) method as the primary analytical approach and four additional MR methods. Moreover, we performed heterogeneity and horizontal pleiotropy assessments using Cochrane’s Q and MR-Egger intercept tests, respectively. Finally, we performed sensitivity analyses to enhance our findings’ precision and veracity.
Results: IVW results showed no causal effect of omega-3 PUFA on psoriasis (p = 0.334; OR, 0.909; 95% CI, 0.748–1.104), omega-6 PUFA cause psoriasis (p = 0.046; OR, 1.174; 95% CI, 1.003–1.374), MUFA cause psoriasis (p = 0.032; OR, 1.218; 95% CI, 1.018–1.457), no causal effect of omega-3 PUFA in psoriasis (p = 0.695; OR, 0.989; 95% CI, 0.937–1.044), no causal effect of omega-6 PUFA in psoriasis (p = 0.643; OR, 1.013; 95% CI, 0.960–1.068), psoriasis is not causal to MUFA (p = 0.986; OR, 1.000; 95% CI, 0.949–1.055). Heterogeneity, horizontal pleiotropy, and sensitivity analyses showed reliable results.
Conclusion: We found that circulating omega-6 PUFA and MUFA cause psoriasis, while omega-3 PUFA do not. Treatments that lower circulating omega-6 PUFA and MUFA are effective in psoriasis. After a better understanding of fatty acid intake and circulation, the population can be advised to regulate their diet.
Introduction
Lipids have traditionally been considered cell membrane structural components and metabolic energy sources. With the deepening of research, it has been found that lipids also have regulatory functions, which can regulate various cellular processes, and are of great significance to the health and disease states of the body (1). It is now recognized that lipids play an important role in psoriasis, and patients with psoriasis often have abnormalities in lipid expression and metabolism and lipid transporters and receptors (2). Mateusz et al. examined lipid-lowering therapy’s effects on psoriasis. In most patients, statins, fibrates, glitazones, and GLP-1 analogs, together with conventional psoriasis medications, relieved symptoms. However, there are also a few cases that are ineffective or aggravating for psoriasis (3). Dietary saturated fatty acids can aggravate the severity of psoriasis, while unsaturated fatty acids can relieve psoriasis to some extent (4, 5). Compared to biological agents with side effects and high economic burdens, it is safer and more cost-effective to improve psoriasis by regulating dietary lipids (6, 7). Existing research has mostly focused on polyunsaturated fatty acids, although monounsaturated fatty acids (MUFA) are also important to health (8). Various types of fatty acids may indirectly affect inflammation and neuronal signaling through adipose tissue, microbiome, intestine and vasculature. Clinical and epidemiological studies have shown that Diets rich in omega-6 polyunsaturated fatty acids (PUFA), saturated fatty acids (SFA), and trans fatty acids increase neuroinflammation. In contrast, diets rich in MUFA, omega-3 PUFA and sphingolipids can diminish neuroinflammation. However, the underlying regulatory mechanisms are multifactorial, making it difficult to establish causality (9).
Mendelian randomization studies have been used to study the causality between fatty acids and primary liver cancer (10), sepsis (11), bipolar disorder (12), and atopic dermatitis (13). Mendelian randomization studies eliminate reverse causation and confounding effects, yielding more rigorous results than observational experiments (14). Mendelian randomization discovered that blood lipids (15, 16), body mass index (17), smoking (18), and drinking (19) promote psoriasis. Unsaturated fatty acids and psoriasis are not yet linked causally. Thus, this study used Mendelian randomization to examine the bidirectional causality between omega-3, omega-6, and MUFA and psoriasis.
Methods
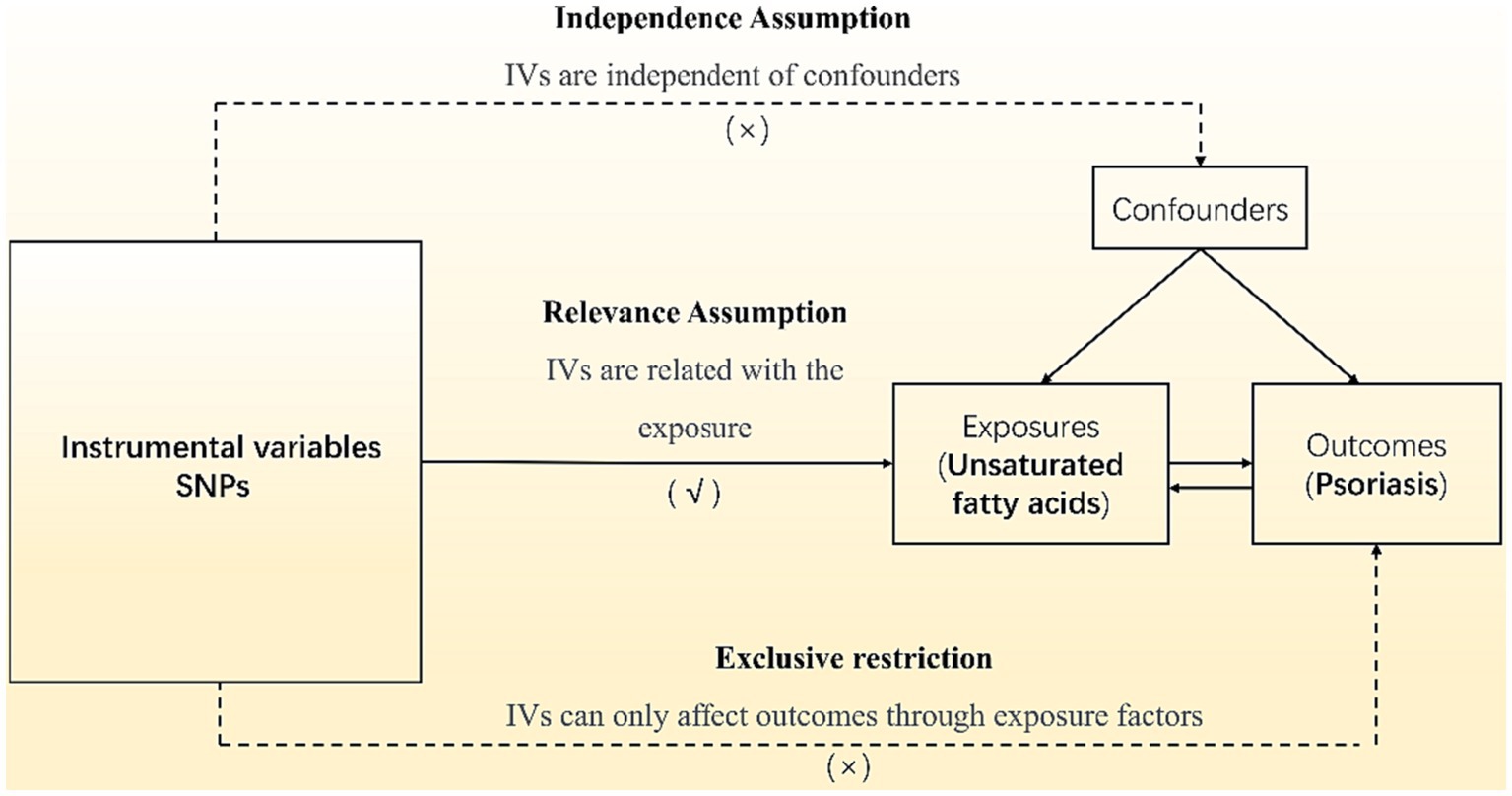
Our analyses employed summary data from published studies or available genome-wide association studies (GWAS) and did not require ethics committee approval. All subjects signed informed consent, and the GWAS institutional ethics review board approved each research. Omega-3 PUFA and omega-6 PUFA data use the datasets with ID met-d-Omega_3 and met-d-Omega_6 in the IEU Open GWAS Project, a European population containing males and females, including 114,999 research objects. The MUFA data uses the dataset ID met-c-916 in the IEU Open GWAS Project, a European population including males and females with 13,535 research subjects. The psoriasis data uses the dataset ID finn-b-L12_PSORIASIS in the IEU Open GWAS Project, a European population with 4,510 cases and 212,242 controls. Mendelian randomization studies used single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs). Regarding the inclusion criteria for SNPs, we chose a significance threshold of p ≤ 5 × 10−8 and a minor allele frequency ≥ 3% to ensure robustness and generalizability of the findings. We excluded SNPs with reported loci overlap or linkage disequilibrium R2 < 0.001 to avoid potential confounding effects. To avoid bias from weak IVs, further screening was performed with a criterion of F > 10, and palindromic SNPs were eliminated to generate the final IVs. The canonical Mendelian randomization analysis followed the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines (20). The analysis process adhered to three assumptions of Mendelian randomization studies are shown in Figure 1: (1) the correlation assumption, where the SNP is strongly associated with the exposure; (2) the exclusion assumption, where the SNP is unrelated to the outcome; and (3) the independence assumption, where the SNP is unrelated to confounding factors. Various methods, including inverse variance weighting (IVW), weighted median (WM), MR-Egger, weighted model, and simple model, were used to estimate the causal relationship between the exposure and outcome. Heterogeneity was tested using Cochran’s Q, and pleiotropy was examined through MR-Egger regression of intercept values. Additionally, PhenoScanner1 was utilized to detect links between genes and other diseases, aiding in the identification and exclusion of gene pleiotropy. All analyses were performed using R 4.1.2 software, incorporating packages such as “MR-PRESSO,” “Two-Sample MR,” and “MR.RAPS.”
The analysis process adhered to three assumptions of Mendelian randomization studies: (1) the correlation assumption, where the SNP is strongly associated with the exposure; (2) the exclusion assumption, where the SNP is unrelated to the outcome; and (3) the independence assumption, where the SNP is unrelated to confounding factors.
Results
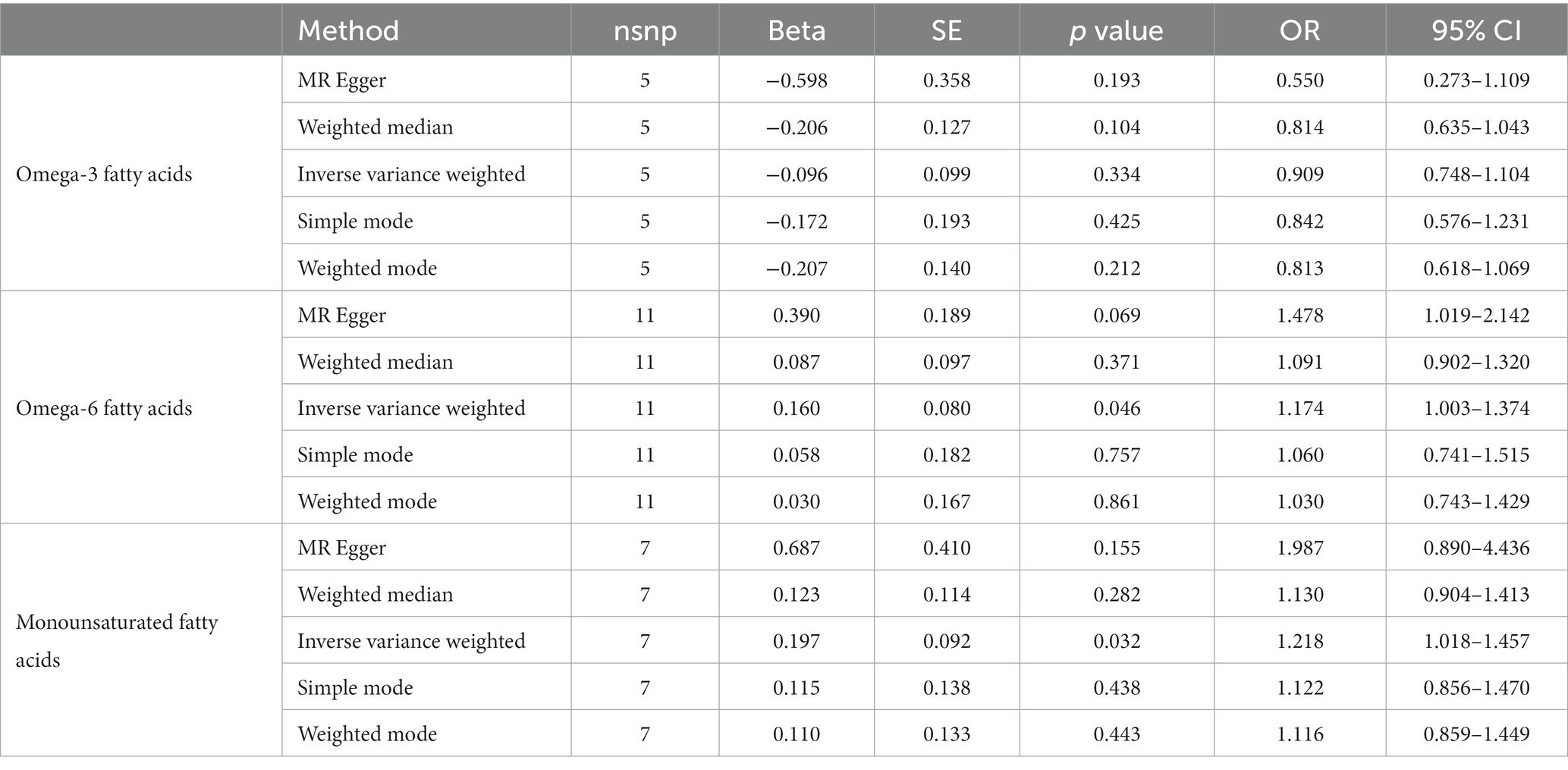
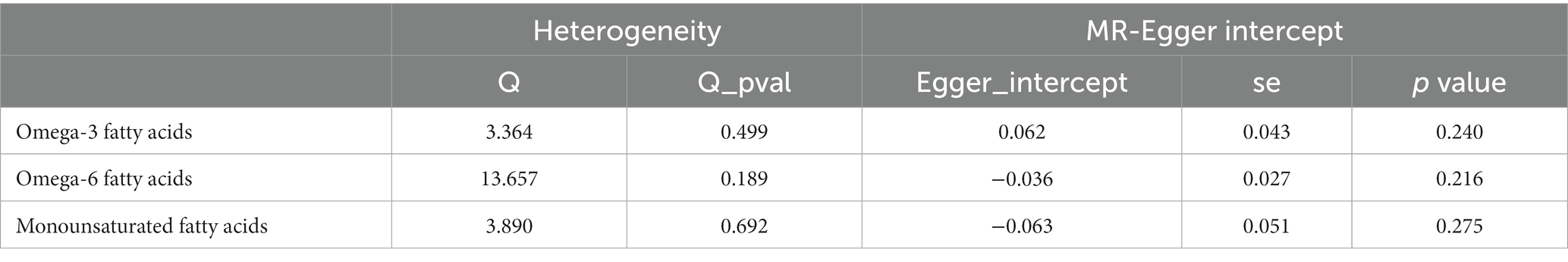
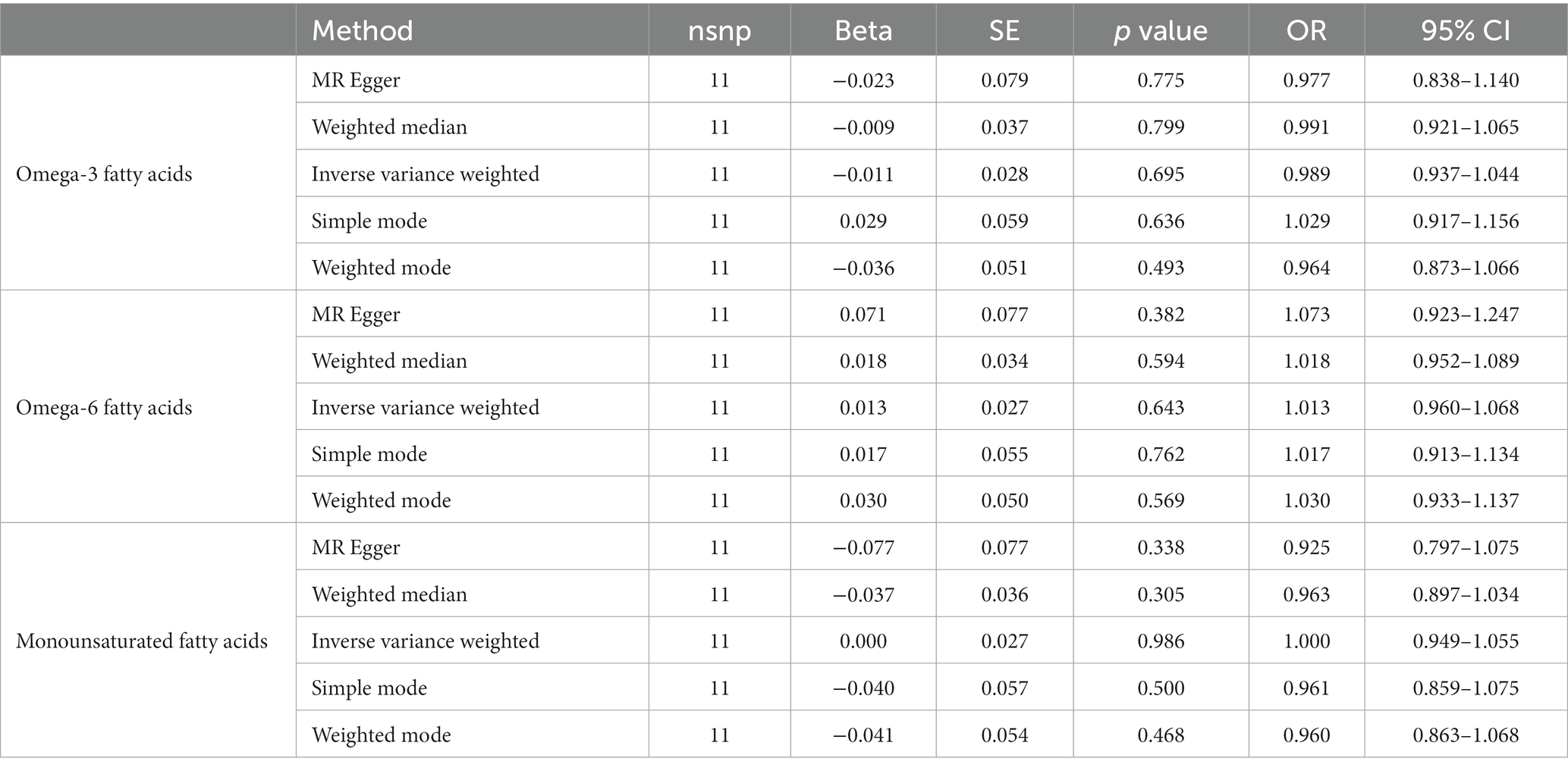
After ensuring the robustness, generalizability and Heterogeneity of SNPs while avoiding their potential confounding effects and pleiotropy, we selected 5, 11, and 7 SNPs as IVs for Mendelian randomization analysis between omega-3 PUFA, omega-6 PUFA, and MUFA and psoriasis, respectively. 11 SNPs were used as IVs for Mendelian randomization analysis of unsaturated fatty acids in psoriasis (Supplementary Table S1). IVW results showed no causal effect of omega-3 PUFA on psoriasis (p = 0.334; OR, 0.909; 95% CI, 0.748–1.104), omega-6 PUFA cause psoriasis (p = 0.046; OR, 1.174; 95% CI, 1.003–1.374), MUFA cause psoriasis (p = 0.032; OR, 1.218; 95% CI, 1.018–1.457), The results of MR-Egger, weighted median, simple mode, and weighted mode analyses also showed the same trend (Figure 2; Table 1). Global test of MRPRESSO analysis, scatter plot, Leave-one-out analysis, heterogeneity analysis and pleiotropic analysis showed that omega-6 PUFA and MUFA have causal effects on psoriasis, which is credible (Supplementary Figures S1–S4, Table S2; Table 2). IVW results showed psoriasis not causal to omega-3 PUFA (p = 0.695; OR, 0.989; 95% CI, 0.937–1.044), psoriasis is not causal for omega-6 PUFA (p = 0.643; OR, 1.013; 95% CI, 0.960–1.068), psoriasis is not causal to MUFA (p = 0.986; OR, 1.000; 95% CI, 0.949–1.055). MR-Egger, weighted median, simple mode, and weighted mode analyses also show the same trend (Figure 3; Table 3). The results of the Global test of MRPRESSO, qualitative and pleiotropic analysis showing no causality of psoriasis to omega-3 PUFA, omega-6 PUFA and MUFA are credible (Supplementary Table S3; Table 4).
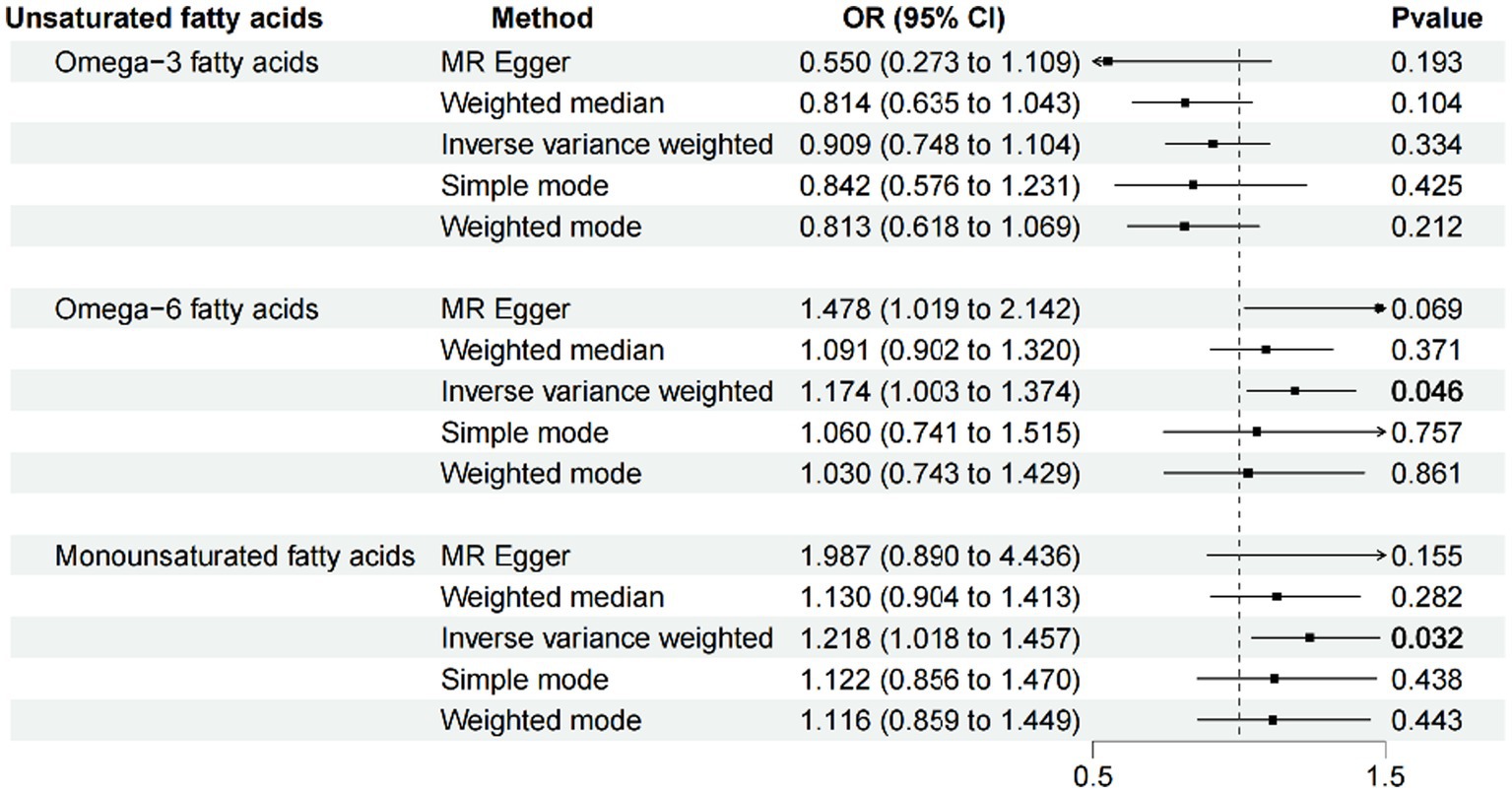
Figure 2. MR results (forward). IVW results showed no causal effect of omega-3 PUFA on psoriasis (p = 0.334; OR, 0.909; 95% CI, 0.748–1.104), omega-6 PUFA cause psoriasis (p = 0.046; OR, 1.174; 95% CI, 1.003–1.374), MUFA cause psoriasis (p = 0.032; OR, 1.218; 95% CI, 1.018–1.457), The results of MR-Egger, weighted median, simple mode, and weighted mode analyses also showed the same trend.

Figure 3. MR results (reverse). IVW results showed psoriasis not causal to omega-3 PUFA (p = 0.695; OR, 0.989; 95% CI, 0.937–1.044), psoriasis is not causal for omega-6 PUFA (p = 0.643; OR, 1.013; 95% CI, 0.960–1.068), psoriasis is not causal to MUFA (p = 0.986; OR, 1.000; 95% CI, 0.949–1.055). MR-Egger, weighted median, simple mode, and weighted mode analyses also show the same trend.
Discussion
Our findings support earlier research demonstrating omega-3 PUFAs do not cause psoriasis. Omega-3 PUFA is mainly categorized into three representative lipids: α-linoleic acid (ALA), docosahexaenoic acids (DHA), and eicosapentaenoic acid (EPA) (21). RNA sequencing data showed that the ALA metabolic pathway was significantly altered in psoriatic skin compared with normal skin (22). After dietary intervention in psoriatic mice, DHA significantly reduced circulating pro-inflammatory cytokines and bioactive lipid mediators and altered macrophage phenotypes and lipid oxidation genes. However, EPA did not exhibit this function (23). An analysis of data from the National Health and Nutrition Examination Survey (NHANES) found a potential association between daily dietary intake of eicosatetraenoic acid and a lower risk of psoriasis among U.S. adults. As the main representative lipids of omega-3 PUFA, ALA and DHA have opposite associations with psoriasis, while EPA has not been reported to have an association with psoriasis. We speculate that their effects are superimposed on each other, which ultimately leads to the lack of causality between omega-3 PUFA and psoriasis.
Contrarily, there is no such significant correlation between EPA and DHA (24). Supplementation with omega 3 PUFA alone does not significantly reduce autoimmune disease in a national, randomized, double-blind, placebo-controlled trial of older adults in the United States (25). Systematic reviews and meta-analyses also showed no association between omega-3 PUFA supplementation and improvement in psoriasis (26, 27).
Some omega-3 PUFA metabolites have anti-inflammatory properties, according to conventional wisdom. However, partially generated metabolites of omega-6 PUFA increase skin inflammation by boosting the signaling of other chemical mediators, such as cytokines and chemokines (28). However, there are still ambiguities or contradictions in the results of many studies. We cannot yet categorize omega-3 and omega-6 PUFA as good or bad for illnesses (29). Our study predicted at the genetic level that omega-6 PUFAs would cause psoriasis. This is consistent with the clinical observation that a large amount of omega-6 PUFA is detected in the blood and skin of patients with psoriasis. Omega-6 PUFA, an inflammatory mediator, is thought to cause psoriasis (30, 31).
There are few studies on MUFA, and most studies speculate that MUFA can reduce the performance of psoriasis. For example, human and animal dietary studies have shown that replacing SFA intake with MUFAs activates beneficial anti-inflammatory mechanisms (M2 macrophage polarization, adipocyte IL-10 secretion, and NLRP3 inflammasome inhibition) and reverses the harmful effects of SFAs on adipose tissue, liver tissue, and β-cells (32). Animal experiments have shown that MUFA subtype omega-9 carrying and cooperating with phosphodiesterase inhibitors can inhibit activated neutrophils, thereby reducing psoriasis-like lesions in mice (33). According to clinical observations, patients with low MUFA intake had higher PASI scores and C-reactive protein levels than those with high intake (34). However, our results show that MUFA can lead to psoriasis. Obese and non-obese psoriasis patients with or without arthritis have greater serum MUFAs than healthy people (35, 36).
Our data also suggest that omega-3 and omega-6 PUFA, and MUFA do not cause reverse psoriasis. In conclusion, omega-6 PUFAs and MUFAs genetically cause psoriasis. However, there are still some shortcomings in this study. The unsaturated fatty acid data we used for analysis were derived from serum polyunsaturated fatty acids. Although circulating fatty acids are closely related to fatty acid intake, the two cannot be completely equal. Therefore, the effect of intervening fatty acid intake on psoriasis deserves more study. This study employed European data; thus, further research is needed to determine if the results apply to other races. This study only studied at the level of omega-3 PUFA, omega-6 PUFA and MUFA. Subsequent research should be conducted with more subdivided components. Mendelian randomization is an emerging study strategy that reduces confounding and reverse causality, but the analysis is completely dependent on the gene level, and its reliability is still being tested.
Conclusion
We found that circulating omega-6 PUFA and MUFA cause psoriasis, while omega-3 PUFA do not. Treatments that lower circulating omega-6 PUFA and MUFA are effective in psoriasis. After a better understanding of fatty acid intake and circulation, the population can be advised to regulate their diet.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved and employed by the summary data from published studies or available genome-wide association studies (GWAS) and did not require ethics committee approval. All subjects signed informed consent, and the GWAS institutional ethics review board approved each research. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JL: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. QS: Conceptualization, Investigation, Writing – original draft. CG: Investigation, Methodology, Writing – original draft. YW: Investigation, Writing – original draft. YM: Investigation, Writing – original draft. YZ: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding supported by Tianjin Municipal Health Commission Research Project of Western and Integrative Medicine (grant number 2021001), Research project of Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital (grant number 2022010).
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1280962/full#supplementary-material
Footnotes
References
1. Dyall, SC, Balas, L, Bazan, NG, Brenna, JT, Chiang, N, da Costa Souza, F, et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. (2022) 86:101165. doi: 10.1016/j.plipres.2022.101165
2. Nowowiejska, J, Baran, A, and Flisiak, I. Aberrations in lipid expression and metabolism in psoriasis. Int J Mol Sci. (2021) 22:6561. doi: 10.3390/ijms22126561
3. Matwiejuk, M, Mysliwiec, H, Jakubowicz-Zalewska, O, Chabowski, A, and Flisiak, I. Effects of Hypolipidemic drugs on psoriasis. Metabolites. (2023) 13:493. doi: 10.3390/metabo13040493
4. Herbert, D, Franz, S, Popkova, Y, Anderegg, U, Schiller, J, Schwede, K, et al. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J Invest Dermatol. (2018) 138:1999–2009. doi: 10.1016/j.jid.2018.03.1522
5. Nakamizo, S, Honda, T, and Kabashima, K. Saturated fatty acids as possible key amplifiers of psoriatic dermatitis. J Invest Dermatol. (2018) 138:1901–3. doi: 10.1016/j.jid.2018.07.004
6. Honda, T, and Kabashima, K. Current understanding of the role of dietary lipids in the pathophysiology of psoriasis. J Dermatol Sci. (2019) 94:314–20. doi: 10.1016/j.jdermsci.2019.05.003
7. Madden, SK, Flanagan, KL, and Jones, G. How lifestyle factors and their associated pathogenetic mechanisms impact psoriasis. Clin Nutr. (2020) 39:1026–40. doi: 10.1016/j.clnu.2019.05.006
8. Gibson, RA. Musings about the role dietary fats after 40 years of fatty acid research. Prostaglandins Leukot Essent Fatty Acids. (2018) 131:1–5. doi: 10.1016/j.plefa.2018.01.003
9. Custers, EEM, and Kiliaan, AJ. Dietary lipids from body to brain. Prog Lipid Res. (2022) 85:101144. doi: 10.1016/j.plipres.2021.101144
10. Liu, Y, He, J, Cai, L, and Leng, A. The effects of fatty acids on primary liver Cancer: a two-sample Mendelian randomization study. Nutr Cancer. (2023) 75:1560–70. doi: 10.1080/01635581.2023.2216914
11. Lei, P, Xu, W, Wang, C, Lin, G, Yu, S, and Guo, Y. Mendelian randomization analysis reveals causal associations of polyunsaturated fatty acids with Sepsis and mortality risk. Infect Dis Ther. (2023) 12:1797–808. doi: 10.1007/s40121-023-00831-z
12. Zhang, M, Li, X, and Dong, L. Assessment of causal relationships between omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: a 2-sample bidirectional mendelian randomization study. Food Funct. (2023) 14:6200–11. doi: 10.1039/d3fo00265a
13. Lin, JY, Ma, LJ, Yuan, JP, Yu, P, and Bai, BX. Causal effects of fatty acids on atopic dermatitis: a Mendelian randomization study. Front Nutr. (2023) 10:1083455. doi: 10.3389/fnut.2023.1083455
14. Davies, NM, Holmes, MV, and Davey, SG. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
15. Zhang, ZY, Jian, ZY, Tang, Y, and Li, W. The relationship between blood lipid and risk of psoriasis: univariable and multivariable Mendelian randomization analysis. Front Immunol. (2023) 14:1174998. doi: 10.3389/fimmu.2023.1174998
16. Xiao, Y, Jing, D, Tang, Z, Peng, C, Yin, M, Liu, H, et al. Serum lipids and risk of incident psoriasis: a prospective cohort study from the UK biobank study and Mendelian randomization analysis. J Invest Dermatol. (2022) 142:3192–3199.e12. doi: 10.1016/j.jid.2022.06.015
17. Budu-Aggrey, A, Brumpton, B, and Tyrrell, J. Evidence of a causal relationship between body mass index and psoriasis: a mendelian randomization study. PLoS Med. (2019) 16:e1002739. doi: 10.1371/journal.pmed.1002739
18. Näslund-Koch, C, Vedel-Krogh, S, Bojesen, SE, and Skov, L. Smoking is an independent but not a causal risk factor for moderate to severe psoriasis: a Mendelian randomization study of 105,912 individuals. Front Immunol. (2023) 14:1119144. doi: 10.3389/fimmu.2023.1119144
19. Wei, J, Zhu, J, Xu, H, Zhou, D, Elder, JT, Tsoi, LC, et al. Alcohol consumption and smoking in relation to psoriasis: a Mendelian randomization study. Br J Dermatol. (2022) 187:684–91. doi: 10.1111/bjd.21718
20. Skrivankova, VW, Richmond, RC, Woolf, BAR, Davies, NM, Swanson, SA, VanderWeele, TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
21. Sawada, Y, Saito-Sasaki, N, and Nakamura, M. Omega 3 fatty acid and skin diseases. Front Immunol. (2021) 11:623052. doi: 10.3389/fimmu.2020.623052
22. Gao, Y, Yi, X, and Ding, Y. Combined transcriptomic analysis revealed AKR1B10 played an important role in psoriasis through the dysregulated lipid pathway and Overproliferation of keratinocyte. Biomed Res Int. (2017) 2017:8717369–10. doi: 10.1155/2017/8717369
23. Sorokin, AV, Arnardottir, H, Svirydava, M, Ng, Q, Baumer, Y, Berg, A, et al. Comparison of the dietary omega-3 fatty acids impact on murine psoriasis-like skin inflammation and associated lipid dysfunction. J Nutr Biochem. (2023) 117:109348. doi: 10.1016/j.jnutbio.2023.109348
24. Zhan, J, Tang, X, Wang, F, and Han, J. Association between daily dietary Eicosatetraenoic acid intake and the lower risk of psoriasis in American adults. Clin Cosmet Investig Dermatol. (2021) 14:1541–9. doi: 10.2147/CCID.S333288
25. Hahn, J, Cook, NR, Alexander, EK, Friedman, S, Walter, J, Bubes, V, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
26. Upala, S, Yong, WC, Theparee, T, and Sanguankeo, A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: a systematic review. Int J Rheum Dis. (2017) 20:442–50. doi: 10.1111/1756-185X.13051
27. Yang, SJ, and Chi, CC. Effects of fish oil supplement on psoriasis: a meta-analysis of randomized controlled trials. BMC Complement Altern Med. (2019) 19:354. doi: 10.1186/s12906-019-2777-0
28. Honda, T, Kabashima, K, and Kunisawa, J. Exploring the roles of prostanoids, leukotriens, and dietary fatty acids in cutaneous inflammatory diseases: insights from pharmacological and genetic approaches. Immunol Rev. (2023) 317:95–112. doi: 10.1111/imr.13193
29. Balić, A, Vlašić, D, Žužul, K, Marinović, B, and Bukvić, MZ. Omega-3 versus Omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. (2020) 21:741. doi: 10.3390/ijms21030741
30. Sorokin, AV, Domenichiello, AF, Dey, AK, Yuan, ZX, Goyal, A, Rose, SM, et al. Bioactive lipid mediator profiles in human psoriasis skin and blood. J Invest Dermatol. (2018) 138:1518–28. doi: 10.1016/j.jid.2018.02.003
31. Simard, M, Morin, S, Ridha, Z, and Pouliot, R. Current knowledge of the implication of lipid mediators in psoriasis. Front Immunol. (2022) 13:961107. doi: 10.3389/fimmu.2022.961107
32. Ravaut, G, Légiot, A, Bergeron, KF, and Mounier, C. Monounsaturated fatty acids in obesity-related inflammation. Int J Mol Sci. (2020) 22:330. doi: 10.3390/ijms22010330
33. Lin, CY, Hsu, CY, Elzoghby, AO, Alalaiwe, A, Hwang, TL, and Fang, JY. Oleic acid as the active agent and lipid matrix in cilomilast-loaded nanocarriers to assist PDE4 inhibition of activated neutrophils for mitigating psoriasis-like lesions. Acta Biomater. (2019) 90:350–61. doi: 10.1016/j.actbio.2019.04.002
34. Barrea, L, Macchia, PE, Tarantino, G, di Somma, C, Pane, E, Balato, N, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. (2015) 13:303. doi: 10.1186/s12967-015-0658-y
35. Myśliwiec, H, Baran, A, Harasim-Symbor, E, Myśliwiec, P, Milewska, AJ, Chabowski, A, et al. Serum fatty acid profile in psoriasis and its comorbidity. Arch Dermatol Res. (2017) 309:371–80. doi: 10.1007/s00403-017-1748-x
Keywords: psoriasis, polyunsaturated fatty acids, omega-3, omega-6, monounsaturated fatty acids, Mendelian randomization
Citation: Li J, Shen Q, Guo C, Wang Y, Ma Y and Zhang Y (2024) Causality of unsaturated fatty acids and psoriasis a Mendelian randomization study. Front. Nutr. 11:1280962. doi: 10.3389/fnut.2024.1280962
Edited by:
Yan Huang, University of Arkansas, United StatesReviewed by:
Anandita Pal, University of North Carolina at Chapel Hill, United StatesEmi Nishida, Okazaki City Hospital, Japan
Copyright © 2024 Li, Shen, Guo, Wang, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, bml1bml1enk3Mzc1QGFsaXl1bi5jb20=
 Junchen Li
Junchen Li Qian Shen
Qian Shen Chenqi Guo
Chenqi Guo Yingdong Wang1
Yingdong Wang1