- 1CDI (Centro Diagnostico Italiano), Milan, Italy
- 2Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
Sarcopenia has been described as a muscle disease, with multiple adverse consequences on human health. Recommendations aimed at supporting awareness, prevention, early detection and treatment of this disease are needed. This review focuses on the epidemiology, pathophysiology and early detection of elderly sarcopenia. As far as treatment is concerned, physical activity and nutritional support are specifically evaluated. An individually tailored resistance exercise training program appears to be crucial for a positive outcome of the sarcopenia prevention and treatment. The nutritional intervention is mostly based on the supplementation with high-quality proteins (i.e., whey protein) in order to increase the intake of essential amino acids and in particular of leucine. In addition, of relevant importance appears to be the supplementation with vitamin D, with omega-3 fatty acids and probiotics. This review evaluates the results of the most qualified studies on the nutritional supplementation of sarcopenic elderly subjects and shows that promising results have been achieved in community elderly subjects, or subjects followed in rehabilitation centers and in nursing homes, with additional resistance exercise programs.
1 Introduction
Sarcopenia is a muscle disease, (1) characterized by a progressive and generalized alteration of skeletal muscles, with a reduction in muscle mass and muscle strength. Sarcopenia is associated with physical disability, poor quality of life and increased mortality (2).
The prevalence of sarcopenia is high in the elderly population of both sexes. The epidemiological data show a high percentage of sarcopenia either among aging communities (5–10%) (3–5) or among elderly residents living in care homes (15–30%) (3, 5), or hospitalized in acute care wards (37%) (3, 6). For elderly patients followed in a rehabilitation setting the rate of sarcopenia goes up to 76% (7). Additionally, sarcopenia may coexist with obesity in the form of sarcopenic obesity, due to the frequent sedentary lifestyle in adult and elderly population (8). Sarcopenia is tightly related to aging, malnutrition, sedentary lifestyle, low physical activity and chronic diseases (9). It is associated with negative physical conditions because it favors clinical frailty (10, 11) and it increases falls (12) mortality, disability and institutionalization (13–15).
Although the negative effects of sarcopenia on human health and on social and healthcare costs have been widely demonstrated (16–19), this clinical problem is often under considered and undertreated, (7) (Figure 1).
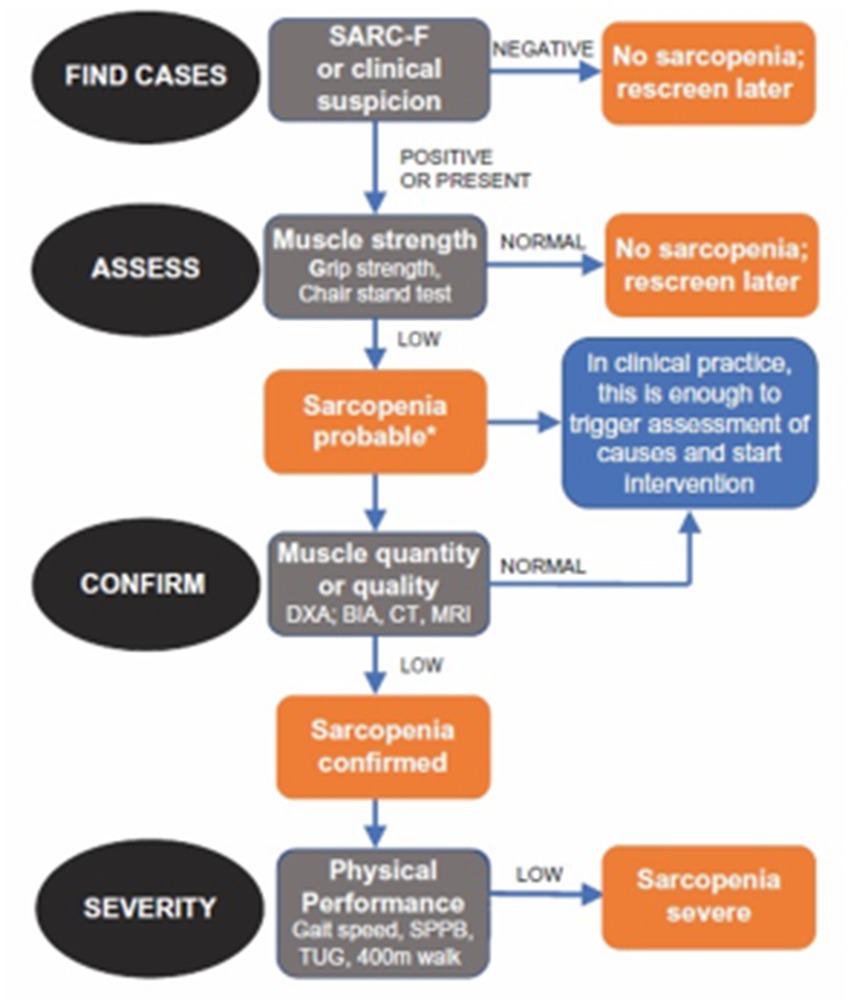
Figure 1. Diagnostic algorithm of sarcopenia produced by the European Working Group on Sarcopenia in Older People (7).
Many recent studies evaluated the nutritional approach to treat sarcopenia in the elderly (20–23).
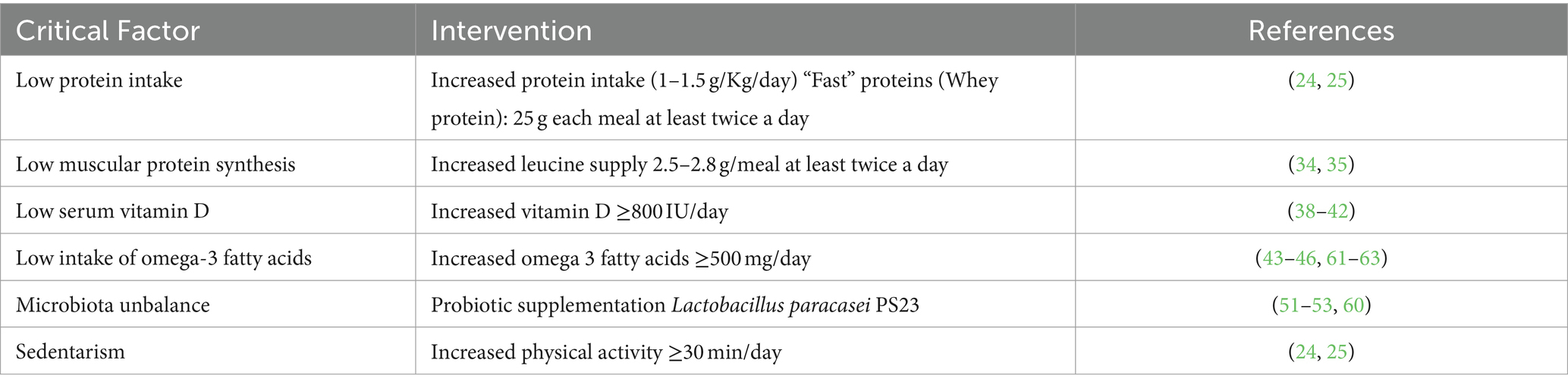
The objective of this review is to focus on a recommended muscle-targeted intervention—namely a whey protein, leucine, vitamin-D, Omega-3-fatty acid and probiotic supplementation—for the prevention or treatment of sarcopenia (9, 24, 25).
2 Pathophysiology of sarcopenia
Aging is physiologically associated with a decrease in muscle mass that represents a physiological process. This reduction is roughly 8% every ten years after the age of 40 and 15% after 70 years (3). The increase of inflammation and of anabolic resistance as well as a reduction of the protein intake and of physical activity are the main causes of this pathophysiological event (9, 24, 26, 27). The increased amino acids (AAs) splanchnic extraction represents an additional reason of their reduced availability in aging (25). In addition, after short term hospitalization or bed rest, the synthesis of muscle proteins is reduced by 30% in elderly subjects, with a loss of 1 kg of muscle mass in 3 days; whereas, after four weeks in similar conditions, young adults show a muscle loss of 0.5 kg (28).
Various new substances that could improve the muscle anabolism with modulation of androgen receptors or of the myostatin–activin process are being studied (27). Myostatin and activin are negative regulators of muscle growth and are now used as markers of loss of muscle mass, that is of sarcopenia (29). In any case, it must be emphasized that the adequate nutritional intake is the fundamental requirement to ensure the optimization of the muscle mass and of its function in the elderly. The scientific evidence available today indicates that an appropriate intake of proteins, an adequate physical exercise and vitamin D supplementation when it is deficient, are the fundamental nutritional requirements to achieve this goal (9, 24, 25). For people older than 65 years it is recommended a daily intake of 1–1.2 g/kg of proteins every day, with a higher amount of proteins (1.2–1.5 g/kg/day) when an inflammatory pathology is present. Proteins should mostly be of high quality, such as whey proteins, with high content of essential amino acids (EAAs) and rich in leucine (24, 25). At each meal, the intake of proteins of high quality should vary from 25 to 30 grams, with a leucine intake ranging from 2.8 to 3 grams and this should be provided at least twice a day (24, 30, 31). The minimum daily intake of leucine should be of 78.5 mg/kg (24, 30, 31). The high presence of EAAs and the fast digestion process of whey protein ensure that this protein source guarantees a marked anabolic efficacy (32) leucine has proven to be particularly effective in favorably modulating protein turnover and anabolism (30, 33).
Specific foods for special medical purposes (FSMP) should be provided when the food intake is inadequate. Various studies showed that oral supplementation with whey protein rich FSMP is followed by the greatest post prandial concentration of plasma AAs and by the greatest muscle protein synthesis, when compared with any other protein source (34, 35). This effect is demonstrated either with additional supplementation of leucine or not, either with additional increase of the energy supply or not and either with additional physical exercise or not (34, 35).
Beta-hydroxy beta-methylbutyrate (HMB), is a metabolite of leucine and it has been shown to be useful in the attenuation of the muscular mass loss, muscle strength and muscle function (36). The pharmacokinetics of leucine are characterized by a fast absorption and distribution, while HMB shows a slow metabolism with longer promotion of muscle protein synthesis (MPS) and lower breakdown rates (36).
The supplementation with essential amino acids also appears of great interest since these amino acids are rapidly assimilated by the digestive system without consumption of energy, that is of adenosine triphosphate (ATP), according to the blood /cell cytoplasm gradient. The more rapid the increase in concentration in the blood, the faster EAAS enter the cell. Finally, the intake of essential amino acids in free form is a more efficient anabolic stimulus than the intake of an equal quantity in the form of proteins (37).
Vitamin D has been demonstrated to be effective in various ways on muscle recovery and MPS (38) and its synergic effect with leucine in stimulating protein anabolism has been well described (39). Therefore, by virtue of the frequent finding of vitamin D deficiency in elderly subjects, a supplementation of at least 800–1,000 IU every day of vitamin D should be provided in sarcopenic elderly subjects (40–42). Also, omega-3 fatty acids can reduce muscle loss and favor muscle synthesis (43) on muscle mass has been shown in various animal models and in vitro (44–46) as well as in human experiments, regardless of the anti-inflammatory action (47).
Recently, a gut-muscle correlation has been described (48). Gut microbiota could mediate the correlation between nutrition and aging by regulating the host’s immune function, metabolism, insulin activity and gene expression (49, 50), and gut microbiota has been correlated with physical performance of elderly subjects (51, 52). Various studies have shown a correlation between gut microbiota composition and physical performance in the older population (51, 52). Aged rats supplemented with Lactobacillus paracasei PS23 showed a reduced muscle loss (53) and reduced cognitive decline (54). Therefore, a probiotic supplementation could be useful to favorably influence gut microbiota in elderly subjects with sarcopenia.
3 Efficacy trials
Among the numerous studies on the efficacy of FSMP useful for muscular mass synthesis, the most qualified clinical trials, with randomized and controlled study protocols and high number of recruited patients, are here reported.
In the PROVIDE study, malnourished older patients with sarcopenia living independently were randomized to vitamin D and leucine-enriched whey protein nutritional supplement or to an isocaloric control product, given twice daily for 13 weeks. Although the trial did not reach a significant between group difference in SPPB (Short Performance Physical Battery) and grip strength, the chair stand test as well as the appendicular muscle mass showed a significant improvement in patients supplemented with the muscle targeted food for special medical purposes (MT-FSMP) (55).
In the randomized study performed by Chanet et al. to evaluate the effect of a standardized breakfast supplemented with vitamin D and leucine enriched whey protein or a non-caloric placebo in healthy older men, a significant benefit towards appendicular lean mass was observed only in the test group at the end of a 6 week intervention (56).
In another controlled study, 130 sarcopenic elderly subjects were randomly supplemented with one serving per day containing 22 grams of whey protein, 10.9 grams of essential amino acids (including 4 grams of leucine), 100 IU of vitamin D or an isocaloric quantity of maltodextrin for 90 days. A personalized program of moderate-intensity physical activity was planned at the same time for all subjects. The intervention group showed a significantly higher increase in muscle mass, handgrip strength and physical performance, when compared with the control group (57). In addition, the intervention group showed lower CRP values and improved quality of life scores.
The IRIS randomized study evaluated the efficacy of a whey protein-based nutritional formula, enriched with leucine and vitamin D, twice daily, in addition to a standard hospital diet and a physical exercise rehabilitation program, in older in-patients with sarcopenia and compared it with an isocaloric control formula supplemented for 8 weeks (57). The four meters gait speed as well as the chair stand test, the TUG (timed up and go test), the SPPB (short physical performance battery), the Barthel index, the handgrip strength, the ADL (activity daily living), the QoL (quality of life) and appendicular muscle mass were significantly improved only in the intervention group. Moreover, CRP levels, healthcare resource consumption, and length of stay in hospital were lowered only in the intervention group (58).
Sarcopenia associated with obesity (8) is an interesting topic because weight loss is beneficial in overweight older subjects, but it may be associated with loss of skeletal muscle mass and sarcopenia. Verreijen et al. showed that a 13 week weight loss program was followed by a similar weight loss and fat mass loss in older obese subjects supplemented with whey protein, leucine and vitamin D as compared to the control group; but the intervention group showed an increase in appendicular muscle mass, whereas the control group evidenced a decrease in the same variable (59).
The effect on muscle mass of a two-month randomized intervention in sarcopenic elderly subjects with a MT-FSMP composed of 500 mg of omega-3 fatty acids, 2.5 g of leucine and probiotic Lactobacillus paracasei PS23 (30 billion, freeze dried), versus a placebo control group, has been evaluated by Rondanelli et al. (60). The obtained data showed a significant increase of appendicular lean mass, of the Tinetti scale, of the SPPB total score and of the handgrip strength in the intervention group, while the control group did not show any difference. Moreover, the comparison between the two studied groups demonstrated a significant decrease of the visceral adipose tissue and a significant increase of valine, leucine, isoleucine, and total amino acids only in the test group (60).
4 Discussion and conclusion
Several studies have been conducted with different muscle targeted foods for special medical purposes in older subjects with sarcopenia. The obtained results demonstrate that the intervention with these products, possibly in combination with a physical exercise program, promotes muscle protein synthesis and promotes the increase of muscle mass and muscle strength, and improves the physical performance of elderly sarcopenic subjects (Table 1). In addition, multiple studies show that these interventions prevent the muscle mass loss in subjects at high risk of becoming sarcopenic (Table 1).
The efficacy of MT-FSMP is higher in association with physical activity and in particular with resistance exercise programs (57–59, 64, 65) have also been shown in subjects who do not increase physical activity (65–67). This is a great advantage for individuals who, for various reasons, have difficulty in carrying out rehabilitative physical activity programs. The same muscular benefits have been demonstrated in elderly obese individuals who need to implement nutritional measures aimed at reducing body weight (59, 68, 69). The efficacy of MT-FSMP has been shown in heterogeneous patient populations, (70, 71) and these nutritional interventions showed to reduce healthcare resource consumption in rehabilitation (58). To detect the recovery of muscle mass, the minimum duration treatment should be 4–8 weeks. Future research should evaluate the efficacy of long-term supplementation. Data on its tolerability up to 6 months have been provided (65, 72, 73). Moreover, this nutritional intervention could prevent the oxidation of dietary proteins as a source of energy (74). The effect of combined, multifactorial interventions (MT-FSMP and physical activity) would be desirable due to their synergistic effects. It has to be stated that, according to the triage theory of Bruce Ames, it could be advisable to supplement all the population with clinical signs of sarcopenia (75).
In conclusion, available data indicate that a muscle-targeted oral nutritional supplementation constitutes an effective treatment of sarcopenia and should be offered as a first-line therapeutic intervention in these subjects. The positive outcome of the nutritional intervention may be additionally increased when a targeted resistance exercise program is added. This intervention appears useful also to prevent sarcopenia in high-risk elderly subjects.
Author contributions
AG: Conceptualization, Writing – original draft. GB: Writing – review & editing. FM: Writing – review & editing. MR: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, amino acids; EAA, essential amino acids; FSMP, foods for special medical purposes; HMB, beta-hydroxy beta-methylbutyrate; MPS, muscle protein synthesis; ATP, adenosine triphosphate; SPPB, short performance physical battery; MT-FSMP, muscle targeted foods for special medical purposes; CRP, C-reactive protein; TUG, timed up and go test; SPPB, short physical performance battery; ADL, activity daily living; QoL, quality of life.
References
2. Santilli, V, Bernetti, A, Mangone, M, and Paoloni, M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. (2014) 11:177–80.
3. Cruz-Jentoft, AJ, Landi, F, Schneider, SM, Zuniga, C, Arai, H, Boirie, Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing. (2014) 43:748–59. doi: 10.1093/ageing/afu115
4. Landi, F, Calvani, R, Tosato, M, Martone, AM, Fusco, D, Sisto, A, et al. Age-related variations of muscle mass, strength, and physical performance in community-dwellers: results from the Milan EXPO survey. J Am Med Dir Assoc. (2017) 18:88.e17–24. doi: 10.1016/j.jamda.2016.10.007
5. Volpato, S, Bianchi, L, Cherubini, A, Landi, F, Maggio, M, Savino, E, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. (2014) 69:438–46. doi: 10.1093/gerona/glt149
6. Ligthart-Melis, GC, Luiking, YC, Kakourou, A, Cederholm, T, Maier, AB, and de van der Schueren MAE,. Frailty, sarcopenia, and malnutrition frequently (co-)occur in hospitalized older adults: a systematic review and meta-analysis. J Am Med Dir Assoc. (2020) 21:1216–28. doi: 10.1016/j.jamda.2020.03.006
7. Wojzischke, J, van Wijngaarden, J, van den Berg, C, Cetinyurek-Yavuz, A, Diekmann, R, Luiking, Y, et al. Nutritional status and functionality in geriatric rehabilitation patients: a systematic review and meta-analysis. Eur Geriatr Med. (2020) 11:195–207. doi: 10.1007/s41999-020-00294-2
8. Barazzoni, R, Bischoff, SC, Boirie, Y, Busetto, L, Cederholm, T, Dicker, D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. (2018) 37:1787–93. doi: 10.1016/j.clnu.2018.04.018
9. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
10. Landi, F, Calvani, R, Cesari, M, Tosato, M, Martone, AM, Bernabei, R, et al. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med. (2015) 31:367–74. doi: 10.1016/j.cger.2015.04.005
11. Cruz-Jentoft, AJ, Landi, F, Topinková, E, and Michel, J-P. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. (2010) 13:1–7. doi: 10.1097/MCO.0b013e328333c1c1
12. Yeung, SSY, Reijnierse, EM, Pham, VK, Trappenburg, MC, Lim, WK, Meskers, CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2019) 10:485–500. doi: 10.1002/jcsm.12411
13. Xu, J, Wan, CS, Ktoris, K, Reijnierse, EM, and Maier, AB. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
14. Wang, DXM, Yao, J, Zirek, Y, Reijnierse, EM, and Maier, AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. (2020) 11:3–25. doi: 10.1002/jcsm.12502
15. Landi, F, Calvani, R, Ortolani, E, Salini, S, Martone, AM, Santoro, L, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. (2017) 28:1569–76. doi: 10.1007/s00198-017-3929-z
16. Veronese, N, Stubbs, B, Volpato, S, Zuliani, G, Maggi, S, Cesari, M, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. (2018) 19:981–988.e7. doi: 10.1016/j.jamda.2018.06.007
17. Welsh, CE, Celis-Morales, CA, Ho, FK, Brown, R, Mackay, DF, Lyall, DM, et al. Grip strength and walking pace and cardiovascular disease risk prediction in 406,834 UK biobank participants. Mayo Clin Proc. (2020) 95:879–88. doi: 10.1016/j.mayocp.2019.12.032
18. Janssen, I, Shepard, DS, Katzmarzyk, PT, and Roubenoff, R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. (2004) 52:80–5. doi: 10.1111/j.1532-5415.2004.52014.x
19. Norman, K, and Otten, L. Financial impact of sarcopenia or low muscle mass – a short review. Clin Nutr. (2019) 38:1489–95. doi: 10.1016/j.clnu.2018.09.026
20. Gielen, E, Beckwée, D, Delaere, A, De Breucker, S, Vandewoude, M, Bautmans, I, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. (2021) 79:121–47. doi: 10.1093/nutrit/nuaa011
21. Martínez-Arnau, FM, Fonfría-Vivas, R, and Cauli, O. Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients. (2019) 11:2504. doi: 10.3390/nu11102504
22. Wright, J, and Baldwin, C. Oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people: a systematic review and meta-analysis. Clin Nutr. (2018) 37:1879–91. doi: 10.1016/j.clnu.2017.09.004
23. Martin-Cantero, A, Reijnierse, EM, Gill, BMT, and Maier, AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta-analysis. Nutr Rev. (2021) 79:315–30. doi: 10.1093/nutrit/nuaa064
24. Bauer, J, Biolo, G, Cederholm, T, Cesari, M, Cruz-Jentoft, AJ, Morley, JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
25. Morley, JE, Argiles, JM, Evans, WJ, Bhasin, S, Cella, D, Deutz, NEP, et al. Nutritional recommendations for the management of Sarcopenia. J Am Med Dir Assoc. (2010) 11:391–6. doi: 10.1016/j.jamda.2010.04.014
26. Katsanos, CS, Kobayashi, H, Sheffield-Moore, M, Aarsland, A, and Wolfe, RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. (2005) 82:1065–73. doi: 10.1093/ajcn/82.5.1065
27. Reginster, J-Y, Beaudart, C, Al-Daghri, N, Avouac, B, Bauer, J, Bere, N, et al. Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clin Exp Res. (2021) 33:3–17. doi: 10.1007/s40520-020-01663-4
28. Kortebein, P, Ferrando, A, Lombeida, J, Wolfe, R, and Evans, WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. (2007) 297:1769. doi: 10.1001/jama.297.16.1772-b
29. Park, SS, Kwon, E-S, and Kwon, K-S. Molecular mechanisms and therapeutic interventions in sarcopenia. Osteoporos Sarcopenia. (2017) 3:117–22. doi: 10.1016/j.afos.2017.08.098
30. Borack, MS, and Volpi, E. Efficacy and safety of leucine supplementation in the elderly. J Nutr. (2016) 146:2625S–9S. doi: 10.3945/jn.116.230771
31. Szwiega, S, Pencharz, PB, Rafii, M, Lebarron, M, Chang, J, Ball, RO, et al. Dietary leucine requirement of older men and women is higher than current recommendations. Am J Clin Nutr. (2021) 113:410–9. doi: 10.1093/ajcn/nqaa323
32. Boirie, Y, and Guillet, C. Fast digestive proteins and sarcopenia of aging. Curr Opin Clin Nutr Metab Care. (2018) 21:37–41. doi: 10.1097/MCO.0000000000000427
33. Xu, Z, Tan, Z, Zhang, Q, Gui, Q, and Yang, Y. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr. (2015) 113:25–34. doi: 10.1017/S0007114514002475
34. Naclerio, F, and Seijo, M. Whey protein supplementation and muscle mass: current perspectives. Nutr Diet Suppl. (2019) 11:37–48. doi: 10.2147/NDS.S166195
35. Cribb, PJ, Williams, AD, Carey, MF, and Hayes, A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. (2006) 16:494–509. doi: 10.1123/ijsnem.16.5.494
36. Engelen, MPKJ, and Deutz, NEP. Is β-hydroxy β-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations? Curr Opin Clin Nutr Metab Care. (2018) 21:207–13. doi: 10.1097/MCO.0000000000000459
37. Rondanelli, M, Aquilani, R, Verri, M, Boschi, F, Pasini, E, Perna, S, et al. Plasma kinetics of essential amino acids following their ingestion as free formula or as dietary protein components. Aging Clin Exp Res. (2017) 29:801–5. doi: 10.1007/s40520-016-0605-7
38. Garcia, M, Seelaender, M, Sotiropoulos, A, Coletti, D, and Lancha, AH. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. (2019) 60:66–9. doi: 10.1016/j.nut.2018.09.031
39. Salles, J, Chanet, A, Giraudet, C, Patrac, V, Pierre, P, Jourdan, M, et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res. (2013) 57:2137–46. doi: 10.1002/mnfr.201300074
40. Beaudart, C, Buckinx, F, Rabenda, V, Gillain, S, Cavalier, E, Slomian, J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. (2014) 99:4336–45. doi: 10.1210/jc.2014-1742
41. Dhesi, JK. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. (2004) 33:589–95. doi: 10.1093/ageing/afh209
42. Yang, A, Lv, Q, Chen, F, Wang, Y, Liu, Y, Shi, W, et al. The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J Cachexia Sarcopenia Muscle. (2020) 11:678–89. doi: 10.1002/jcsm.12545
43. Troesch, B, Eggersdorfer, M, Laviano, A, Rolland, Y, Smith, AD, Warnke, I, et al. Expert opinion on benefits of long-chain Omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. (2020) 12:2555. doi: 10.3390/nu12092555
44. Jeromson, S, Mackenzie, I, Doherty, MK, Whitfield, PD, Bell, G, Dick, J, et al. Lipid remodeling and an altered membrane-associated proteome may drive the differential effects of EPA and DHA treatment on skeletal muscle glucose uptake and protein accretion. Am J Physiol Endocrinol Metabolism. (2018) 314:E605–19. doi: 10.1152/ajpendo.00438.2015
45. Kamolrat, T, and Gray, SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun. (2013) 432:593–8. doi: 10.1016/j.bbrc.2013.02.041
46. Gray, SR, and Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr Opin Clin Nutr Metab Care. (2018) 21:104–9. doi: 10.1097/MCO.0000000000000441
47. Di Girolamo, FG, Situlin, R, Mazzucco, S, Valentini, R, Toigo, G, and Biolo, G. Omega-3 fatty acids and protein metabolism. Curr Opin Clin Nutr Metab Care. (2014) 17:145–50. doi: 10.1097/MCO.0000000000000032
48. Ticinesi, A, Lauretani, F, Milani, C, Nouvenne, A, Tana, C, Del Rio, D, et al. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut-muscle Axis? Nutrients. (2017) 9:1303. doi: 10.3390/nu9121303
49. Sonnenburg, JL, and Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature. (2016) 535:56–64. doi: 10.1038/nature18846
50. Schroeder, BO, and Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. (2016) 22:1079–89. doi: 10.1038/nm.4185
51. Claesson, MJ, Jeffery, IB, Conde, S, Power, SE, O’Connor, EM, Cusack, S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) 488:178–84. doi: 10.1038/nature11319
52. Jeffery, IB, Lynch, DB, and O’Toole, PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. (2016) 10:170–82. doi: 10.1038/ismej.2015.88
53. Chen, L-H, Huang, S-Y, Huang, K-C, Hsu, C-C, Yang, K-C, Li, L-A, et al. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging. (2019) 11:756–70. doi: 10.18632/aging.101782
54. Huang, S-Y, Chen, L-H, Wang, M-F, Hsu, C-C, Chan, C-H, Li, J-X, et al. Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients. (2018) 10:894. doi: 10.3390/nu10070894
55. Bauer, JM, Verlaan, S, Bautmans, I, Brandt, K, Donini, LM, Maggio, M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. (2015) 16:740–7. doi: 10.1016/j.jamda.2015.05.021
56. Chanet, A, Verlaan, S, Salles, J, Giraudet, C, Patrac, V, Pidou, V, et al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. (2017) 147:2262–71. doi: 10.3945/jn.117.252510
57. Rondanelli, M, Klersy, C, Terracol, G, Talluri, J, Maugeri, R, Guido, D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. (2016) 103:830–40. doi: 10.3945/ajcn.115.113357
58. Rondanelli, M, Cereda, E, Klersy, C, Faliva, MA, Peroni, G, Nichetti, M, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle. (2020) 11:1535–47. doi: 10.1002/jcsm.12532
59. Verreijen, AM, Verlaan, S, Engberink, MF, Swinkels, S, de Vogel-van den Bosch, J, and Weijs, PJM. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. (2015) 101:279–86. doi: 10.3945/ajcn.114.090290
60. Rondanelli, M, Gasparri, C, Barrile, GC, Battaglia, S, Cavioni, A, Giusti, R, et al. Effectiveness of a novel food composed of leucine, Omega-3 fatty acids and probiotic Lactobacillus paracasei PS23 for the treatment of sarcopenia in elderly subjects: a 2-month randomized double-blind placebo-controlled trial. Nutrients. (2022) 14:4566. doi: 10.3390/nu14214566
61. Smith, GI, Julliand, S, Reeds, DN, Sinacore, DR, Klein, S, and Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. (2015) 102:115–22. doi: 10.3945/ajcn.114.105833
62. McGlory, C, Calder, PC, and Nunes, EA. The influence of Omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front Nutr. (2019) 6:e144. doi: 10.3389/fnut.2019.00144
63. Robinson, SM, Reginster, JY, Rizzoli, R, Shaw, SC, Kanis, JA, Bautmans, I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. (2018) 37:1121–32. doi: 10.1016/j.clnu.2017.08.016
64. Barichella, M, Cereda, E, Pinelli, G, Iorio, L, Caroli, D, Masiero, I, et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology. (2019) 93:e485–96. doi: 10.1212/WNL.0000000000007858
65. Dimori, S, Leoni, G, Fior, L, and Gasparotto, F. Clinical nutrition and physical rehabilitation in a long-term care setting: preliminary observations in sarcopenic older patients. Aging Clin Exp Res. (2018) 30:951–8. doi: 10.1007/s40520-017-0859-8
66. Bothwell, LE, Greene, JA, Podolsky, SH, and Jones, DS. Assessing the gold standard—lessons from the history of RCTs. N Engl J Med. (2016) 374:2175–81. doi: 10.1056/NEJMms1604593
67. Liberman, K, Njemini, R, Luiking, Y, Forti, LN, Verlaan, S, Bauer, JM, et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res. (2019) 31:845–54. doi: 10.1007/s40520-019-01208-4
68. Memelink, RG, Pasman, WJ, Bongers, A, Tump, A, van Ginkel, A, Tromp, W, et al. Effect of an enriched protein drink on muscle mass and glycemic control during combined lifestyle intervention in older adults with obesity and type 2 diabetes: a double-blind RCT. Nutrients. (2020) 13:64. doi: 10.3390/nu13010064
69. Pasman, WJ, Memelink, RG, de Vogel-van den Bosch, J, Begieneman, MPV, van den Brink, WJ, Weijs, PJM, et al. Obese older type 2 diabetes mellitus patients with muscle insulin resistance benefit from an enriched protein drink during combined lifestyle intervention: the PROBE study. Nutrients. (2020) 12:2979. doi: 10.3390/nu12102979
70. Lewis, R, Gómez Álvarez, CB, Rayman, M, Lanham-New, S, Woolf, A, and Mobasheri, A. Strategies for optimising musculoskeletal health in the 21st century. BMC Musculoskelet Disord. (2019) 20:164. doi: 10.1186/s12891-019-2510-7
71. Volkert, D, Beck, AM, Cederholm, T, Cruz-Jentoft, A, Goisser, S, Hooper, L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. (2019) 38:10–47. doi: 10.1016/j.clnu.2018.05.024
72. Bauer, JM, Mikušová, L, Verlaan, S, Bautmans, I, Brandt, K, Donini, LM, et al. Safety and tolerability of 6-month supplementation with a vitamin D, calcium and leucine-enriched whey protein medical nutrition drink in sarcopenic older adults. Aging Clin Exp Res. (2020) 32:1501–14. doi: 10.1007/s40520-020-01519-x
73. Oesen, S, Halper, B, Hofmann, M, Jandrasits, W, Franzke, B, Strasser, E-M, et al. Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly—a randomized controlled trial. Exp Gerontol. (2015) 72:99–108. doi: 10.1016/j.exger.2015.08.013
74. Luiking, YC, Abrahamse, E, Ludwig, T, Boirie, Y, and Verlaan, S. Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: a randomized trial. Clin Nutr. (2016) 35:48–58. doi: 10.1016/j.clnu.2015.02.013
Keywords: elderly sarcopenia, leucine, muscle mass, muscle protein synthesis, muscle strength, omega-3 fatty acids, vitamin D, whey protein
Citation: Giacosa A, Barrile GC, Mansueto F and Rondanelli M (2024) The nutritional support to prevent sarcopenia in the elderly. Front. Nutr. 11:1379814. doi: 10.3389/fnut.2024.1379814
Edited by:
Giuseppe Poli, Department of Clinical and Biological Sciences, ItalyCopyright © 2024 Giacosa, Barrile, Mansueto and Rondanelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Attilio Giacosa, YXR0aWxpby5naWFjb3NhQGdtYWlsLmNvbQ==
 Attilio Giacosa
Attilio Giacosa Gaetan Claude Barrile
Gaetan Claude Barrile Francesca Mansueto2
Francesca Mansueto2 Mariangela Rondanelli
Mariangela Rondanelli