Abstract
Nutritional support is crucial for critically ill patients. Recent clinical studies suggest that both overfeeding during the acute phase of critical illness and overly conservative or delayed nutritional therapy can pose significant risks. Given substantial individual variability among critically ill patients, it is challenging to prescribe universally applicable and objective feeding strategies; Instead, we pointed out which nutritional interventions were harmful. We also summarized the reasons for protective nutrition, and elaborated the advantages of protective nutrition from three perspectives: gastrointestinal protection, nutritional protection and metabolic protection. In particular, it is emphasized that overfeeding will lead to metabolic disorders, such as mitochondrial dysfunction, autophagy inhibition, ketogenic inhibition, hyperglycemia, insulin resistance, etc. These detrimental processes can exacerbate one another, contributing to multiple organ dysfunction syndrome and poorer clinical outcomes. We also propose protective nutrition strategies comparable to lung protective ventilation strategies, which may benefit patients. Vigilant monitoring during nutritional implementation is also paramount, enhancing awareness of adverse events for early diagnosis and intervention to mitigate their harm.
1 Introduction
During the acute phase of critical illness, there is an increase in catabolism characterized by intense inflammatory response, glycogen and protein breakdown, and dysregulation of metabolic control (1). These multifaceted factors may contribute to malnutrition and the development of intensive care unit (ICU)-acquired weakness, which in turn can prolong mechanical ventilation and hospitalization, and even increase ICU and in-hospital mortality (2, 3). The uncontrollable hypercatabolic state during the acute phase of critical illness prompted recognition of the importance of nutrition, aiming to counteract the catabolic metabolism and thus improve the nutritional status and clinical outcomes of critically ill patients (4, 5). Previous studies have also showed that early and full nutritional therapy associated with reduced infectious complications and ICU-related complications in critically ill patients, and can even reduce 60-day mortality (6–8). However, these studies are all observational in nature, making it difficult to avoid bias. The better prognosis observed may not be attributable to full nutritional therapy, but rather to the fact that these patients had milder conditions and better feeding tolerance. Therefore, imposing a causal relationship in this context is highly misleading.
Given the uncertainty of the relationship between nutritional therapy and prognosis, several randomized controlled trials (RCTs) have been conducted in recent years to explore the true relationship between the two, and these studies have shown that early full nutrition is not beneficial and may even be harmful (9–15). This finding, contrary to previous thinking, has upended clinical nutrition practice. Based on this evidence, recent guidelines have begun to advocate progressive nutritional treatment rather than reaching nutritional goals as early as possible. However, no large RCT has shown that progressive nutrition is superior to long-term low-dose feeding.
For patients with acute respiratory distress syndrome (ARDS), lung protective ventilation strategies [low tidal volume, low plateau pressure, and appropriate positive end-expiratory pressure (PEEP)] both promote alveolar reexpansion to maintain oxygenation and prevent ventilator-induced lung injury. It has been proven to reduce mortality and ventilator days in ARDS patients (16). The gastrointestinal (GI) tract is similar to the lungs in that it seems disadvantageous to abandon or overuse, and protective use seems to be an optimal state, which not only plays the function of the organ but also avoids the damage caused by overuse. We will combine the latest research results, summarize the idea of protective nutrition, and discuss from the three aspects of why, what and how to protect.
2 Why to protect
2.1 Full nutrition is deleterious
The estimation of caloric requirements in critically ill patients typically relies on energy expenditure (EE). However, during the acute phase of critical illness, caloric needs may be lower than EE. This discrepancy arises because the body generates endogenous glucose in the state of critical illness, a process that remains unaffected by exogenous supplementation (17). Given the endogenous production of glucose, full nutrition during the acute phase can lead to overfeeding, potentially exerting negative impacts on both the body and disease recovery (18, 19).
As early as 2011, the idea of early full nutrition was challenged. The EPaNIC trial (N = 4,640) compared the outcomes of critically ill patients receiving early (<48 h) supplemental parenteral nutrition (SPN) vs. late (>48 h) SPN in the ICU (9). The results indicated that patients in the late SPN group had lower infection rates, fewer complications, shorter durations of mechanical ventilation, and better prognosis. Subsequent post-hoc analysis of the EPaNIC trial suggested that the better outcomes in the late SPN group were due to higher nutritional intake during the acute phase in the early SPN group compared to the late SPN group, and that higher early protein intake appeared a key factor influencing the observed outcomes (20). In this study, the primary factor influencing the outcomes of critically ill patients was full feeding. The adverse effects of PN were found to be dose-dependent rather than route-dependent (20). The CALORIES trial (N = 2,400) also did not observe any effect of feeding route on the 30-day mortality rate of critically ill patients (21). Furthermore, the NUTRIREA-2 trial (N = 2,410) validated this viewpoint, finding that compared to enteral nutrition (EN), isocaloric parenteral nutrition (PN) had no differential impact on the prognosis of critically ill patients with mechanical ventilation and shock, and identified a higher incidence of digestive system complications in the EN group (13). In other clinical studies, despite variations in interventions, timing, and patient populations, the negative impact of early full nutrition on GI adverse events, blood glucose levels, and mortality has been observed (10, 11, 22). In the PROTINVENT retrospective study, it was also observed that patients receiving full nutrition (protein intake >1.2 g/kg/day) exhibited poorer clinical outcomes (23). In the NUTRIREA-3 trial (N = 3,044), while no increase in mortality was observed with early standard calorie and protein supplementation (calorie: 6 kcal/kg/day, protein: 0.4 g/kg/day) compared to early low-dose nutrition (calorie: 25 kcal/kg/day, protein: 1-1.3 g/kg/day) in mechanically ventilated critically ill patients with shock, there was an associated increase in ICU length of stay, gastrointestinal adverse events, and other complications (24). Higher protein supply was found in the EFFORT trial (N = 1,301) to be particularly harmful for patients with acute kidney injury and higher organ failure scores at baseline (25). A recent PRECISe trial (N = 935) found that high enteral protein supply led to poorer health-related quality of life in critically ill patients and did not improve functional outcomes within 180 days of ICU admission (26). Interestingly, the EFFORT Trial (N = 2,088) in non-critically ill found that compared with the control group (mean calorie 1,211 kcal/day, protein 47 g/day), A higher nutrient supply (mean calorie 1,501 kcal/day, protein 57 g/day) improved clinical outcomes in patients at nutritional risk. Another RCT found that adult patients undergoing cardiac surgery with cardiopulmonary bypass who received 2.0 g/kg/day of amino acids did not experience an increased 30-day mortality compared to the placebo group. Moreover, it reduced the incidence of acute kidney injury (AKI) (27). These outcomes appeared contradictory to previous theories. However, it should be noted that the study population did not consist of critically ill patients and is weakly comparable to previously presented studies. This also suggests that strengthening nutritional support may be beneficial for patients entering the recovery phase of critical illness. Further research is warranted to elucidate nutritional therapy in critically ill patients and to provide more comprehensive evidence for nutritional practice.
2.2 Moderate nutrition may be beneficial
Due to the stress state, increased metabolic rate, and the demands of tissue repair, critically ill patients have a greater need for nutritional therapy compared to ordinary patients. The supplementation of exogenous energy and protein is likely important at some point. Failure to provide adequate and timely nutrition could lead to malnutrition and other adverse clinical outcomes (28–30). In retrospective studies, an intake below 50% was found to correlate with poorer clinical outcomes. This level of intake may result in significant calorie deficit, deplete energy reserves, reduce lean body mass, and potentially increase the risk of infectious complications (8, 31). A prospective observational study across 21 countries involving 167 intensive care units (N = 2,772) indicated that lower energy and protein delivery were associated with increased 60-day mortality and prolonged duration of mechanical ventilation in patients with mechanical ventilation (6). However, these non-interventional studies appear to overlook the true underlying reasons for the higher mortality rate in patients with low nutritional intake [greater disease severity potentially leading to increased occurrence of feeding intolerance (FI)]. Zusman et al.'s study found (N = 1,171) that 60-day mortality was lowest in severely ill patients when the percent of administered calories divided by resting energy expenditure was equal to 70% (32). The EuroPN study (N = 1,172) demonstrated that compared to lower intake levels (<10 kcal/kg, <0.8 g/kg), a daily moderate intake (10–20 kcal/kg, 0.8–1.2 g/kg) was associated with a higher likelihood of successful weaning and a reduced risk of mortality (33). Similar to the results of a meta-analysis (34), the results of this study also support the existence of a “U-shaped curve” of nutritional intake (both excessive and inadequate supply being detrimental, with moderate intake being optimal), highlighting that moderate nutritional intake is beneficial for critically ill patients. However, as critical illness recovery progresses, the inflection point of the U-shaped curve may shift rightward (indicating higher nutritional intake). The PROTINVENT study (N = 455) suggested a time-dependent relationship between protein intake and mortality, revealing that the lowest 6-month mortality rates were observed when protein intake increased from <0.8 g/kg/day on days 1–2 to 0.8–1.2 g/kg/day on days 3–5, and exceeded 1.2 g/kg/day after day 5 (23).
Unfortunately, there are currently no RCTs specifically comparing the effects of low-dose and moderate-dose nutrition on the prognosis of critically ill patients. However, we believe that the most appropriate feeding dose for patients may also have a time-dependent nature, with a transition from limited to progressive to open feeding strategy, which may be more suitable for critically ill patients (35). The timing of implementing this transition in feeding strategies remains unclear, and further research is needed to identify biomarkers indicating the critical illness phase. Compared with calorie, protein lacks a two-stage or multi-stage nutritional target, which may be more conducive to precision nutritional treatment for critically ill patients.
3 What to protect
3.1 Gastrointestinal protection
Early EN (<48 h) exerts a protective effect on the GI mucosal barrier, promotes gastrointestinal peristalsis, reduces bacterial translocation, and stimulates increased intestinal blood flow, thereby aiding in the maintenance of normal metabolic activity and repair capacity of the intestinal mucosa (36, 37). Under the blow of critical illness, protective nutrition may be a good match for impaired GI function, which not only plays the role of GI tract, but also avoids the deterioration of GI function. The ESPEN guidelines recommend that the feeding volume of early EN in the acute phase of critical illness should not exceed 70% of estimated EE (1, 19), and a progressive feeding approach appears to be more beneficial for the clinical outcomes of critically ill patients (18, 35).
Animal experiments indicate that low-dose EN promotes the recovery of intestinal barrier function in rats following ischemia/reperfusion injury by enhancing NF-κB/HIF-1α pathway expression (38). Furthermore, low-dose EN can also activates the JAK1-STAT6 pathway, facilitating the expression of pIgR and secretory immunoglobulin A (sIgA), thereby mitigating immune damage to the murine intestinal mucosa (39). Robles et al. summarized overfeeding in animal experiments. Overfeeding can lead to a strong and prolonged hyperphagic response, and it may reduce survival following infection (40). In addition, Zhang et al. found in a mouse model of acute pancreatitis that Short-peptide-based EN has the function of restoring ZO-1 expression, mucous layer and goblet cells, thereby reducing intestinal bacterial translocation in mice with severe acute pancreatitis (41).
Unfortunately, there is currently no large-scale RCT that has found early EN to be truly superior to delayed EN (42). There is no evidence to suggest that early EN reduces mortality in critically ill patients. The differences observed in secondary outcomes such as ICU length of stay and infections vary greatly across studies, and there remains significant heterogeneity (42). In fact, early EN may not be suitable for all critically ill patients, as initiating it too early may lead to FI. Predicting the likelihood of FI and implementing early pre-intervention (delayed EN) for high-risk patients is a more conservative strategy that may reduce the incidence of FI (43). However, delayed EN may fails to provide the trophic effects on the gut. It is clear that early EN does not have a universally positive effect on all patients; thus, an individualized assessment of the benefits and risks of early EN is warranted. Accordingly, further research in this field is warranted.
3.2 Nutritional protection
During the acute phase of critical illness, patients often experience not only impaired gastrointestinal motility and barrier function but also digestive and absorptive dysfunctions (1). A prospective cross-sectional study (N = 563) revealed that, excluding primary exocrine pancreatic insufficiency (EPI), 52.2% of critically ill patients exhibited EPI (fecal elastase −1 < 200 ug/g), with 18.3% of patients experiencing severe EPI (fecal elastase −1 < 100 ug/g). Factors such as shock, sepsis, diabetes, cardiac arrest, hyperlactatemia, invasive mechanical ventilation, and hemodialysis may all contribute to the development of EPI, severely impacting the digestive absorption capacity of critically ill patients (44). The deficiency of digestive enzymes may leads to the restriction of protein digestion in severe patients, and the protein supplemented through the EN pathway is not easily absorbed by the intestine. Therefore, there seems to be an irreconcilable contradiction between protein requirements and digestion and absorption disorders. Inappropriate nutritional support may lead to adverse gastrointestinal events such as abdominal distension and diarrhea (45).
In 2023, the Mayo Clinic defined a short peptide-based formula (PBF) as an EN formula in which protein is hydrolyzed into “2-3 peptides.” 2-3 peptides can enter intestinal epithelial cells through active transport and are the main way the body absorbs proteins. PepT1 operates through a proton-coupled mechanism, utilizing the proton gradient to drive the uptake of 2-3 peptides without the need for additional energy expenditure (46). When a proton and a peptide simultaneously bind to the external surface of PepT1, a conformational change occurs, resulting in the translocation of the bound peptide and proton into the cell. This mechanism allows PepT1 to function efficiently in the low pH environment of the intestinal lumen (46–48). Another distinction between PBF and standard polymerized formula (SPF) is that the lipid component of SPF consists predominantly of long-chain triglycerides (LCT), typically ranging from 13 to 24 carbon atoms. These LCTs necessitate pancreatic and biliary fat digestion and emulsification to be metabolized into glycerol and free fatty acids, ultimately entering enterocytes and subsequently forming chylomicrons (49). In contrast, PBF often contains significant amounts of medium-chain triglycerides (MCT), with lengths of 6–12 carbon atoms. MCTs are passively absorbed, bypassing the need for complex fat digestion and emulsification (50). It has also been shown the MCTs do not rely on the carnitine acyltransferase system to enter mitochondria for β-oxidation (51). This enables quicker metabolism of MCTs and enhances their utilization, even in conditions of protein deficiency (52). Obviously, these may be beneficial for critically ill patients. Therefore, this formula was suggested to improve EN tolerance, and it was found that the use of PBF in patients with FI may lead to improved clinical outcomes, along with a corresponding reduction in healthcare utilization and potential nursing costs (53). Due to the ileal brake mechanism, critically ill patients receiving jejunal feeding are unable to stimulate the secretion of pancreatic proteases (54). Therefore, compared to other formulas, PBF may be more suitable for patients receiving post-pyloric feeding (55). Additionally, due to mitochondrial dysfunction and tissue hypoxia in the acute phase of critical illness, lipid metabolism is impaired (56). Elevated levels of exogenous fat nutrition can exacerbate mitochondrial damage and organ dysfunction, potentially leading to poorer clinical outcomes (57–59). Early provision of low-fat nutrition may play a protective role in nutritional support.
In some clinical studies, PBF has been found to reduce the incidence of gastric retention and diarrhea, achieve nutritional adequacy more rapidly, shorten ICU length of stay, and lower readmission rates (60–64). The ASPEN guidelines recommend the use of PBF for critically ill patients with severe malabsorption, such as persistent diarrhea (4). However, due to the low quality of evidence supporting these recommendations, they are considered as having a low level of evidence. Nevertheless, based on current theoretical rationale, there is reason to believe that PBF can provide this nutritional protective effect for patients with gastrointestinal injury, although high-quality research is still needed to further validate its efficacy.
3.3 Metabolic protection
3.3.1 Metabolism in the acute phase of critical illness
During the acute phase of critical illness, particularly in the early stages, the body predominantly undergoes catabolism (1). This phase is characterized by accelerated protein and fat breakdown, increased gluconeogenesis, insulin resistance, and elevated basal and resting EE. Due to the body's self-protection mechanisms, in response to critical illness, a significant amount of protein and fat is converted into glucose to meet the metabolic demands (65). More than half of the body's energy needs are supplied through this pathway, which remains active regardless of exogenous energy supplementation (17, 19, 66). Consequently, there exists a risk of overfeeding during this phase, potentially inhibiting certain beneficial metabolic processes.
3.3.2 Mitochondrial protection
During the acute phase of critical illness, varying degrees of mitochondrial dysfunction are often present (67). Mitochondria serve as the primary site for cellular energy production, and impairment of their function can lead to disturbances in cellular metabolism and organ dysfunction (68). Mitochondria are particularly sensitive to the increased oxidative stress in critical illness. Abnormalities in the function and structure of this organelle further lead to excessive generation of reactive oxygen species and decreased production of adenosine triphosphate (ATP) (69, 70). To reduce metabolic demands, mitochondrial dysfunction may progress in a manner similar to hibernation, which could aid in maintaining cellular life, though it may come at the cost of organ system failure (67). In such circumstances, the administration of full nutrients may have deleterious effects, as damaged mitochondria are unable to effectively utilize the additional energy supply, potentially leading to increased oxidative stress and cellular damage (71–73). Therefore, in clinical practice, protective nutrition may be more appropriate for severe patients during the acute phase to avoid exacerbating mitochondrial dysfunction and the associated adverse outcomes. It is not only a strategy of nutritional protection but also a strategy of organelle and organ protection. The objective of this strategy is to balance energy provision with the metabolic needs of patients by controlling nutrient intake, while simultaneously reducing the risk of exacerbating pathological processes resulting from metabolic disturbances and oxidative stress.
3.3.3 Autophagy activation
Autophagy, as a central molecular pathway, plays a crucial role in maintaining cellular and organismal homeostasis (74, 75). Through this process, cells are able to eliminate damaged or dysfunctional organelles and proteins, thereby preserving the stability of the cellular environment. In critically ill states, cells often suffer varying degrees of functional impairment, which can increase the risk of multiple organ failure. Therefore, the activation of autophagy becomes particularly important (76). Animal experiments have demonstrated the protective effects of autophagy on kidney, lung, liver, and intestinal injuries (77–80). However, artificial feeding can inhibit the activation of autophagy in critical illness, particularly at high protein/amino acid doses (18). Insufficient autophagy may further exacerbate mitochondrial dysfunction, leading to organ failure and adverse outcomes (81, 82). During the acute phase of critical illness, protective nutrition establishes a relatively starved or nutrient-restricted condition, thereby promoting the beneficial metabolic process of autophagy. This approach may potentially improve the prognosis of critically ill patients.
3.3.4 Ketogenic activation
Under fasting or starvation conditions, due to inadequate glucose supply, the body undergoes a gradual transition from glucose metabolism to lipid metabolism, accompanied by the activation of ketogenesis (83, 84). Ketogenesis primarily functions through the inhibition of histone deacetylases, reduction of oxidative stress, enhancement of mitochondrial efficiency, promotion of autophagy, and modulation of inflammation (83, 85–87). Studies have shown that ketogenesis can increase the oxidation of intramuscular triacylglycerol during exercise, thereby increasing endurance (88). Animal experiments indicate that deficiency in peroxisome proliferator-activated receptor alpha (PPARα) impairs ketogenesis activation, which is associated with increased mortality in mice following bacterial infection (89). Studies have shown that ketones can provide up to 70% of the brain's energy needs, that the uptake of ketones by the brain increases significantly during acute brain injury, and that ketone supplements have potential in the treatment of traumatic brain injury (90). Therefore, for patients at high risk of FI, opting for early fasting with late initiation of small-dose EN appears to offer greater benefits. We should probably not focus solely on the balance between the benefits of early EN and the harms of FI.
3.3.5 Blood glucose control
Factors such as nutritional therapy and stress during critical illness can lead to elevated blood glucose, but the optimal blood glucose target is still controversial (91). Several previous studies have shown that early administration of PN with tight glycemic control (TGC) between 80 and 110 mg per deciliter reduces morbidity, ICU length of stay, and mortality in surgical ICU patients (92, 93). However, in a recent RCT by Gunst et al. (N = 9,230), TGC did not reduce mortality among critically ill patients who did not receive early PN (94). Only minor benefits of TGC were observed in several secondary outcomes. The seemingly contradictory findings stemmed from the fact that delayed initiation of PN implied lower energy intake, thereby resulting in significantly less severe hyperglycemia in the liberal glucose control group compared to previous two RCTs (94). We have reason to believe that the higher mortality observed in the control groups of the previous two RCTs may have been due to overfeeding during the acute phase and more severe hyperglycemia. This could lead to glucose overload in critical organs and cells, which is associated with poorer clinical outcomes (95). Studies have shown that higher blood glucose may inhibit mitochondrial repair processes, leading to further mitochondrial dysfunction (81).
During critical illness, stress and emergency responses can increase the activity of the neuroendocrine system, promoting the release of catecholamines and cortisol. This stimulation enhances glycogenolysis and gluconeogenesis in the liver, concurrently inducing peripheral insulin resistance (IR) and inhibiting glucose entry into cells (96). Additionally, inflammatory responses during critical illness contribute to IR (97). IR is characterized by reduced effectiveness of insulin in facilitating glucose uptake and utilization, along with downregulation of insulin-dependent glucose transporter proteins in peripheral tissues, thereby leading to stress-induced hyperglycemia (98). The triglyceride-glucose (TyG) index, calculated from fasting triglyceride (TG) and fasting plasma glucose (FPG) levels, has been validated for assessing the degree of IR in patients (99–101). Moreover, the excessive use of insulin due to hyperglycemia can also result in poorer clinical outcomes (102). In one RCT (N = 6,104), the TGC group exhibited higher mortality compared to the control group, attributable to the increased administration of insulin (103). Additionally, insulin may adversely affect organ recovery and disease resolution by inhibiting autophagy and ketogenesis (104, 105). Protective nutrition can not only reduce the incidence of hyperglycemia, but also reduce the use of insulin, which may be more suitable for severe patients.
3.3.6 The interaction of metabolic processes
Overfeeding can lead to adverse effects such as mitochondrial dysfunction, reduced autophagy, ketogenesis suppression, hyperglycemia, and excessive insulin utilization. As outlined previously, these metabolic processes interact intricately. For instance, during overfeeding, elevated blood glucose levels can inhibit mitochondrial repair, thereby exacerbating mitochondrial dysfunction. Moreover, it can decrease autophagic efficiency, leading to reduced autophagy, and impair fatty acid metabolism, thereby suppressing ketogenesis. These interrelationships among metabolic processes may exacerbate organ damage and result in poorer outcomes (Figure 1). Theoretically, protective nutrition strategies are more beneficial in activating these beneficial metabolic processes to benefit critically ill patients.
Figure 1
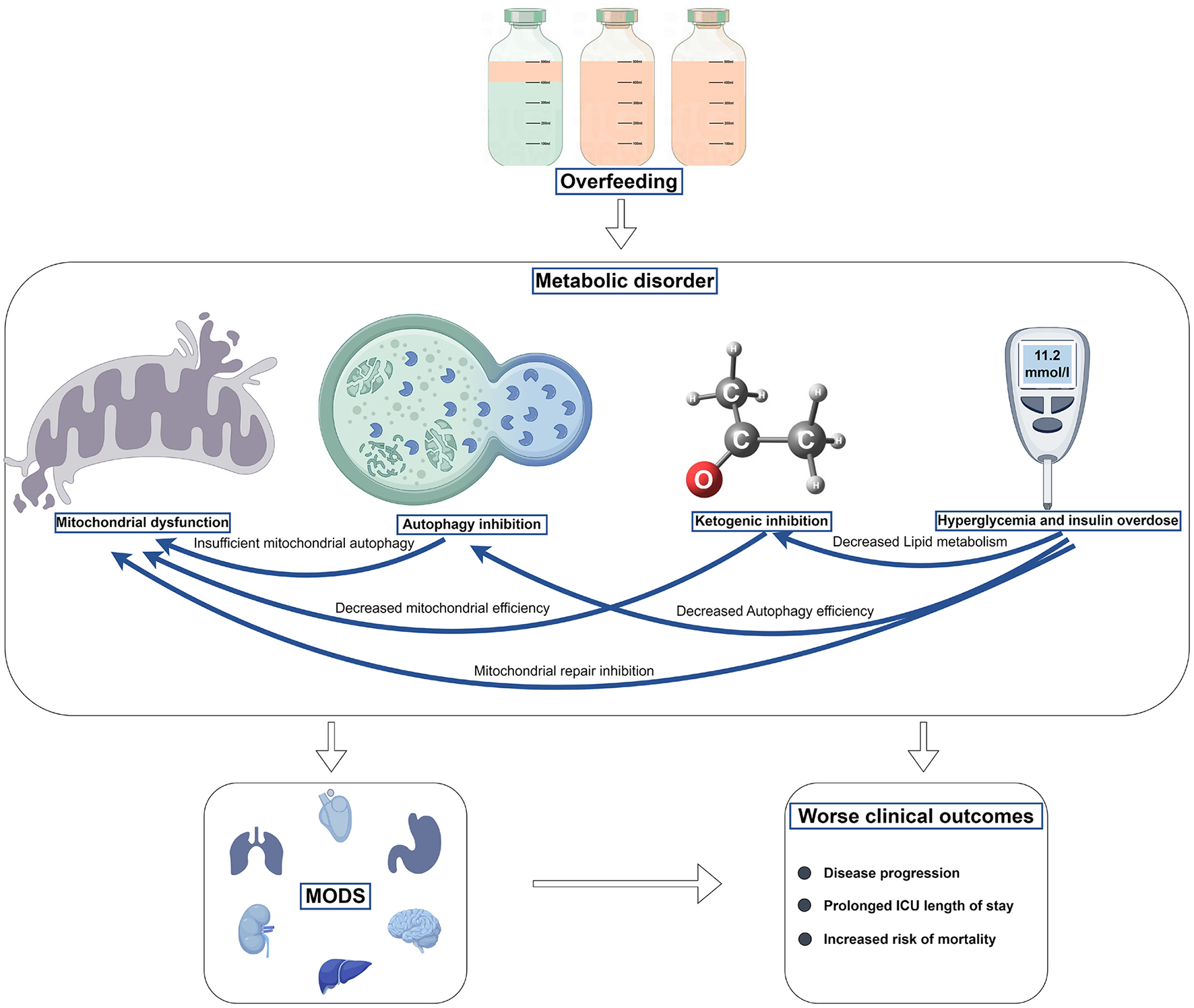
The harm of overfeeding in the acute stage of critical illness.
4 How to protect
How can we practice to achieve the GI protection, nutritional protection and metabolic protection that we mentioned earlier? Based on the above theoretical basis, and compared with the lung protective ventilation strategy, we put forward the protective nutrition strategy.
Low-dose feeding and low-dose protein and calories strategies are similar, utilizing limited GI function while avoiding the harms of overfeeding. These two strategies are similar to low tidal volume and limited platform pressure in lung protective ventilation strategies for ARDS patients, which take advantage of limited lung function and avoid the harm caused by excessive ventilation. On the other hand, appropriate formula may reduce the burden of GI digestion and absorption, lower the risk of a secondary blow to GI, and may decrease the occurrence of FI, although the current evidence on the selection of nutritional formulas is very weak. This strategy would be similar to appropriate PEEP, which promotes collapsed alveolar distension and improves ventilate blood flow ratio and oxygenation. It can also reduce lung damage caused by overinflated alveoli (Figure 2).
Figure 2
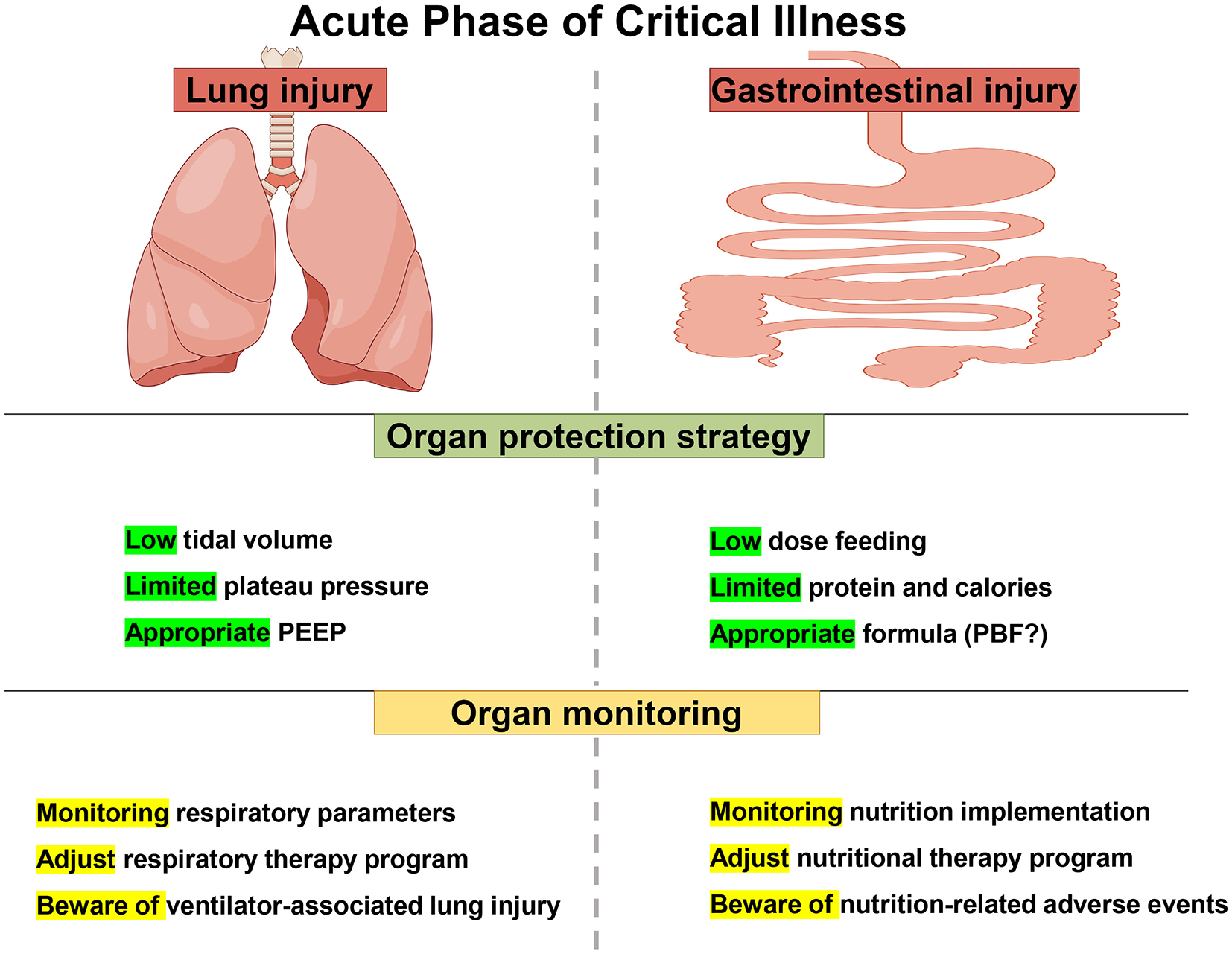
Lung protective ventilation strategies and protective nutrition strategies in acute stage of critical illness.
Current guidelines still recommend early EN for patients who are able to tolerate it, in order to achieve the benefits of EN (19). When EN alone is insufficient to meet the patient's nutritional needs, SPN can be used alongside EN to fulfill the patient's nutritional needs, although the optimal timing for SPN application remains unclear (4, 19). When a patient is unable to tolerate EN, the benefits and risks of PN should be carefully weighed, and PN may need to be provided in a timely manner, rather than abandoning nutritional therapy. The potential harm from overfeeding may be more severe than the risks associated with the nutritional route itself. It is important to focus on the daily nutrient intake and the monitoring of overfeeding.
We have to admit that it is difficult to come up with a universal strategy due to the large individual differences in critically ill patients. Although we can only come up with a general idea of nutritional treatment, following these recommendations may benefit patients.
5 What needs to be monitored
5.1 Overfeeding
The monitoring of overfeeding is a hot topic in recent years, because we recognize the harm of overfeeding and try to avoid this phenomenon in clinical nutrition practice (106). Indirect calorimetry serves as the gold standard for measuring EE, derived from measurements of VO2 and VCO2 (107). However, the measured EE does not necessarily reflect the true energy requirements of patients in the acute phase of critical illness, during which significant endogenous glucose production occurs (17). Providing nutrition equivalent to measured EE often results in overfeeding (1, 36). Currently, the clinical measurement of endogenous glucose production is complex and not widely implemented. Achieving the equation of endogenous glucose + exogenous supplementation = EE is not our ultimate goal, as optimal exogenous supplementation may also need to consider the potential risks of inhibiting fasting responses, such as autophagy and ketosis. While the ideal dose is individualized and still uncertain, identifying clinical indicators of overfeeding is crucial for guiding nutritional therapy effectively (18).
Overfeeding can manifest as hyperglycemia and increased insulin requirements, azotemia, elevated urea-to-creatinine ratio (UCR), and hypertriglyceridemia. However, these indicators are non-specific and can vary between individuals (106). Interestingly, underfeeding can also manifest as hyperuremia and an elevated blood UCR (108). UCR is also influenced by factors such as circulating fluid volume, underlying disease and therapeutic intervention, which limits its specificity as a reliable indicator of overfeeding (109, 110). This presents significant challenges in using UCR as an effective marker for overfeeding. In critically ill patients, there is an urgent need to explore and develop correction equations for UCR that accurately reflect nutritional status. The correlation between UCR and nutritional intake remains an area requiring further research. Currently, significant challenges exist in monitoring overfeeding due to the lack of specific indicators. We advocate for the concept of protective nutrition, as the harm from overfeeding may surpass the detriments of underfeeding (111). In the absence of adequate monitoring tools, the administration of small doses of nutrition during the early acute phase is recommended, at least not to cause more harm.
5.2 Refeeding syndrome
Refeeding syndrome (RS) refers to a spectrum of metabolic abnormalities that occur when nutritional intake is reintroduced after a period of prolonged starvation or malnutrition. It is primarily characterized by metabolic changes, electrolyte imbalances, and vitamin deficiencies following the initiation of nutritional therapy (112). Micronutrients can distribute unevenly during critical illness, rendering early measurements potentially misleading (113). In the context of refeeding syndrome, which occurs upon the rapid reinstitution of feeding after prolonged starvation, declines in micronutrient and electrolyte concentrations (e.g., vitamin B1, phosphate, potassium) can be abrupt and severe, potentially posing a fatal risk for individuals in a state of starvation or catabolic metabolism (114, 115). Micronutrients play crucial roles in metabolism, immunity, gene transcription, and other physiological processes (116). However, symptoms of micronutrient deficiencies often mimic those of critical illness, leading to frequent oversight (117). In the context of refeeding syndrome (RS, which can occur after the rapid reinstitution of feeding following prolonged starvation), micronutrient deficiencies may manifest. The current common diagnostic criterion for refeeding syndrome includes a reduction in phosphate levels to < 0.65 mmol/L, with a decrease of at least 0.16 mmol/L (118). An RCT (N = 339) by Doig et al. showed that adult critically ill patients who developed refeeding hypophosphatemia within 72 h of starting nutritional support in the ICU had better 60-day survival and longer overall survival in the restricted feeding group than in the standard feeding group (118). A retrospective study by Olthof et al. (N = 337) showed that low caloric intake was associated with a reduced risk of death at 6 months in patients with refeeding hypophosphatemia (119). Therefore, monitoring phosphate changes, early identification of refeeding hypophosphatemia, and giving targeted restrictive nutrition and correcting micronutrient and electrolyte deficiencies may improve the prognosis of severely ill patients (112). Additionally, the timely supplementation of micronutrients is crucial. The 2022 ESPEN guidelines propose recommendations for the presumed optimal intake of micronutrients (113). However, the evidence supporting these recommendations is relatively weak. Due to the uneven distribution of micronutrients caused by factors such as disease and inflammation, relying on blood micronutrient concentrations as a basis for supplementation is not accurate. Furthermore, there is no evidence of the benefits of excessive supplementation, and there may be harm (113, 120). The ideal dosage for micronutrient supplementation remains an area requiring further investigation.
5.3 Acute mesenteric ischemia
Acute mesenteric ischemia (AMI) is infrequently encountered in the ICU but carries a markedly high mortality rate (exceeding 50%) (121). Despite recent advancements in the recognition of AMI, involving imaging and interventional radiology for diagnostic assistance, there has been limited improvement in mortality (121, 122). Delayed diagnosis leading to delayed treatment likely contributes significantly to this scenario, representing a critical prognostic factor (123, 124). Early identification of AMI, prompt cessation of EN, implementation of intestinal revascularization or surgical intervention can prevent further exacerbation of intestinal ischemia, thus avoiding or delaying the occurrence of intestinal necrosis (125, 126). AMI should be ruled out when patients present with acute gastrointestinal symptoms that cannot be explained by feeding alone. A meta-analysis summarizing biomarkers for diagnosing AMI found that urinary I-FABP and D-dimer exhibit moderate predictive values in assessing transmural mesenteric ischemia. Unfortunately, none of the biomarkers reached the level of accurate prediction (122). Whether combining these biomarkers will increase diagnostic effectiveness still needs to be explored further. When there is a high suspicion of AMI, computed tomography imaging, particularly a biphasic protocol consisting of angiography and venous phase scanning, is widely utilized to confirm nonspecific clinical findings (127). Raising awareness about AMI and its early identification and diagnosis is crucial for improving the prognosis of critically ill patients.
5.4 Other gastrointestinal adverse events
Monitoring during nutritional implementation is crucial because it is a personalized therapy, and all plans are not static. We should emphasize the monitoring of the nutritional implementation process to achieve comprehensive protective nutrition. Monitoring of nutrition is similar to monitoring of respiratory therapy, and only continuous monitoring and continuous adjustment could achieve continuous protection (Figure 2).
Monitoring of GI adverse events is essential, and factors such as bowel sounds, intra-abdominal pressure, and bedside ultrasound can provide valuable indications of the patient's GI function status. Due to limited monitoring methods, our initial assessment of the GI function of critically ill patients may be erroneous, which is unavoidable. Fortunately, monitoring can play a role in timely correction, minimizing the harm caused by erroneous decisions.
6 Conclusions
Recent guidelines and RCTs have shown that overfeeding in the acute phase is detrimental to clinical outcomes in severely ill patients. Protective nutrition strategies are a synthesis of guidelines and clinical studies. We also discussed the reasons for implementing protective nutrition strategies, and discussed its potential beneficial effects on GI function, metabolic complications, and organ function. In addition, we highlight the importance of post-nutritional monitoring, which, despite its relative scarcity, is necessary to implement protective nutrition throughout. There is significant individual variability among critically ill patients, and a universal feeding strategy applicable to all patients is difficult to obtain, but the concept of protective nutrition is what we advocate.
Statements
Author contributions
YW: Conceptualization, Data curation, Investigation, Writing – original draft. YaL: Conceptualization, Investigation, Methodology, Writing – review & editing. NL: Data curation, Investigation, Methodology, Software, Writing – review & editing. YuL: Methodology, Writing – review & editing. HL: Conceptualization, Supervision, Writing – review & editing. DZ: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Wu Jieping Medical Foundation (320.6750.2022-13-2 and 320.6750.2023-13-2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Singer P Blaser AR Berger MM Alhazzani W Calder PC Casaer MP et al . ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. 10.1016/j.clnu.2018.08.037
2.
Vanhorebeek I Latronico N Van den Berghe G . ICU-acquired weakness. Intensive Care Med. (2020) 46:637–53. 10.1007/s00134-020-05944-4
3.
Kaegi-Braun N Mueller M Schuetz P Mueller B Kutz A . Evaluation of nutritional support and in-hospital mortality in patients with malnutrition. JAMA Netw Open. (2021) 4:e2033433. 10.1001/jamanetworkopen.2020.33433
4.
Taylor BE McClave SA Martindale RG Warren MM Johnson DR Braunschweig C et al . Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (sccm) and american society for parenteral and enteral nutrition (A. SPEN). Crit Care Med. (2016) 44:390–438. 10.1097/CCM.0000000000001525
5.
Singer P Berger MM Van den Berghe G Biolo G Calder P Forbes A et al . ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. (2009) 28:387–400. 10.1016/j.clnu.2009.04.024
6.
Alberda C Gramlich L Jones N Jeejeebhoy K Day AG Dhaliwal R et al . The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. (2009) 35:1728–37. 10.1007/s00134-009-1567-4
7.
Heyland DK Stephens KE Day AG McClave SA . The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. (2011) 30:148–55. 10.1016/j.clnu.2010.09.011
8.
Dvir D Cohen J Singer P . Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. (2006) 25:37–44. 10.1016/j.clnu.2005.10.010
9.
Casaer MP Mesotten D Hermans G Wouters PJ Schetz M Meyfroidt G et al . Early vs. late parenteral nutrition in critically ill adults. N Engl J Med. (2011) 365:506–17. 10.1056/NEJMoa1102662
10.
Rice TW Wheeler AP Thompson BT Steingrub J Hite RD Moss M et al . Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. (2012) 307:795–803. 10.1001/jama.2012.137
11.
Arabi YM Aldawood AS Haddad SH Al-Dorzi HM Tamim HM Jones G et al . Permissive underfeeding or standard enteral feeding in critically Ill adults. N Engl J Med. (2015) 372:2398–408. 10.1056/NEJMoa1502826
12.
Chapman M Peake SL Bellomo R Davies A Deane A Horowitz M et al . Energy-dense vs. routine enteral nutrition in the critically Ill. N Engl J Med. (2018) 379:1823–34. 10.1056/NEJMoa1811687
13.
Reignier J Boisramé-Helms J Brisard L Lascarrou JB Ait Hssain A Anguel N et al . Enteral vs. parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. (2018) 391:133–43. 10.1016/S0140-6736(17)32146-3
14.
Needham DM Dinglas VD Morris PE Jackson JC Hough CL Mendez-Tellez PA et al . Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic vs. full enteral feeding EDEN trial follow-up. Am J Respir Crit Care Med. (2013) 188:567–76. 10.1164/rccm.201304-0651OC
15.
Deane AM Little L Bellomo R Chapman MJ Davies AR Ferrie S et al . Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET). A randomized controlled trial. Am J Respir Crit Care Med. (2020) 201:814–22. 10.1164/rccm.201909-1810OC
16.
Brower RG Matthay MA Morris A Schoenfeld D Thompson BT Wheeler A . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. 10.1056/NEJM200005043421801
17.
Fraipont V Preiser JC . Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr. (2013) 37:705–13. 10.1177/0148607113505868
18.
van Zanten ARH De Waele E Wischmeyer PE . Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. (2019) 23:368. 10.1186/s13054-019-2657-5
19.
Singer P Blaser AR Berger MM Calder PC Casaer M Hiesmayr M et al . ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. (2023) 42:1671–89. 10.1016/j.clnu.2023.07.011
20.
Casaer MP Wilmer A Hermans G Wouters PJ Mesotten D Van den Berghe G . Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. (2013) 187:247–55. 10.1164/rccm.201206-0999OC
21.
Harvey SE Parrott F Harrison DA Bear DE Segaran E Beale R et al . Trial of the route of early nutritional support in critically ill adults. N Engl J Med. (2014) 371:1673–84. 10.1056/NEJMoa1409860
22.
Pardo E Lescot T Preiser JC Massanet P Pons A Jaber S et al . Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. (2023) 27:7. 10.1186/s13054-022-04298-1
23.
Koekkoek W van Setten CHC Olthof LE Kars J van Zanten ARH . Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr. (2019) 38:883–90. 10.1016/j.clnu.2018.02.012
24.
Reignier J Plantefeve G Mira JP Argaud L Asfar P Aissaoui N et al . Low vs. standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med. (2023) 11:602–12. 10.1016/S2213-2600(23)00092-9
25.
Heyland DK Patel J Compher C Rice TW Bear DE Lee ZY et al . The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet. (2023) 401:568–76. 10.1016/S0140-6736(22)02469-2
26.
Bels JLM Thiessen S van Gassel RJJ Beishuizen A De Bie Dekker A Fraipont V et al . Effect of high vs. standard protein provision on functional recovery in people with critical illness (PRECISe): an investigator-initiated, double-blinded, multicentre, parallel-group, randomised controlled trial in Belgium and the Netherlands. Lancet. (2024) 404:659–69. 10.1016/S0140-6736(24)01304-7
27.
Landoni G Monaco F Ti LK Baiardo Redaelli M Bradic N Comis M et al . A Randomized trial of intravenous amino acids for kidney protection. N Engl J Med. (2024) 391:687–98. 10.1056/NEJMoa2403769
28.
Hoffmann M Schwarz CM Fürst S Starchl C Lobmeyr E Sendlhofer G et al . Risks in management of enteral nutrition in intensive care units: a literature review and narrative synthesis. Nutrients. (2020) 13:82. 10.3390/nu13010082
29.
Lew CCH Yandell R Fraser RJL Chua AP Chong MFF Miller M . Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review [formula: see text]. JPEN J Parenter Enteral Nutr. (2017) 41:744–58. 10.1177/0148607115625638
30.
Pohlenz-Saw JAE Merriweather JL Wandrag L . (Mal)nutrition in critical illness and beyond: a narrative review. Anaesthesia. (2023) 78:770–8. 10.1111/anae.15951
31.
Villet S Chiolero RL Bollmann MD Revelly JP Cayeux RNM Delarue J et al . Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. (2005) 24:502–9. 10.1016/j.clnu.2005.03.006
32.
Zusman O Theilla M Cohen J Kagan I Bendavid I Singer P . Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. (2016) 20:367. 10.1186/s13054-016-1538-4
33.
Matejovic M Huet O Dams K . Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. (2022). 10.21203/rs.3.rs-1338063/v1
34.
Davies ML Chapple LS Chapman MJ Moran JL Peake SL . Protein delivery and clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care Resusc. (2017) 19:117–27. 10.1016/S1441-2772(23)00783-4
35.
Wang Y Li Y Li Y Li H Zhang D . Enteral feeding strategies in patients with acute gastrointestinal injury: From limited to progressive to open feeding. Nutrition. (2023) 117:112255. 10.1016/j.nut.2023.112255
36.
Preiser JC Arabi YM Berger MM Casaer M McClave S Montejo-González JC et al . A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit Care. (2021) 25:424. 10.1186/s13054-021-03847-4
37.
Al-Dorzi HM Arabi YM . Nutrition support for critically ill patients. JPEN J Parenter Enteral Nutr. (2021) 45:47–59. 10.1002/jpen.2228
38.
Wu C Wang X Jiang T Li C Zhang L Gao X et al . Partial enteral nutrition mitigated ischemia/reperfusion-induced damage of rat small intestinal barrier. Nutrients. (2016) 8:502. 10.3390/nu8080502
39.
Sun H Bi J Lei Q Wan X Jiang T Wu C et al . Partial enteral nutrition increases intestinal sIgA levels in mice undergoing parenteral nutrition in a dose-dependent manner. Int J Surg. (2018) 49:74–9. 10.1016/j.ijsu.2017.12.011
40.
Ranea-Robles P Lund J Clemmensen C . The physiology of experimental overfeeding in animals. Mol Metab. (2022) 64:101573. 10.1016/j.molmet.2022.101573
41.
Zhang J Yu WQ Wei T Zhang C Wen L Chen Q et al . Effects of short-peptide-based enteral nutrition on the intestinal microcirculation and mucosal barrier in mice with severe acute pancreatitis. Mol Nutr Food Res. (2020) 64:e1901191. 10.1002/mnfr.201901191
42.
Fuentes Padilla P Martínez G Vernooij RW Urrútia G Roqué IFM Bonfill Cosp X . Early enteral nutrition (within 48 hours) vs. delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. (2019) 2019:CD012340. 10.1002/14651858.CD012340.pub2
43.
Wang Y Li Y Wang H Li H Li Y Zhang L et al . Development and validation of a nomogram for predicting enteral feeding intolerance in critically ill patients (NOFI): Mixed retrospective and prospective cohort study. Clin Nutr. (2023) 42:2293–301. 10.1016/j.clnu.2023.10.003
44.
Wang S Ma L Zhuang Y Jiang B Zhang X . Screening and risk factors of exocrine pancreatic insufficiency in critically ill adult patients receiving enteral nutrition. Crit Care. (2013) 17:R171. 10.1186/cc12850
45.
Xing J Zhang Z Ke L Zhou J Qin B Liang H et al . Enteral nutrition feeding in Chinese intensive care units: a cross-sectional study involving 116 hospitals. Crit Care. (2018) 22:229. 10.1186/s13054-018-2159-x
46.
Viennois E Pujada A Zen J Merlin D . Function, regulation, and pathophysiological relevance of the POT superfamily, specifically PepT1 in inflammatory bowel disease. Compr Physiol. (2018) 8:731–60. 10.1002/j.2040-4603.2018.tb00024.x
47.
Liang R Fei YJ Prasad PD Ramamoorthy S Han H Yang-Feng TL et al . Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. (1995) 270:6456–63. 10.1074/jbc.270.12.6456
48.
Merlin D Si-Tahar M Sitaraman SV Eastburn K Williams I Liu X et al . Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. (2001) 120:1666–79. 10.1053/gast.2001.24845
49.
Łoś-Rycharska E Kieraszewicz Z Czerwionka-Szaflarska M . Medium chain triglycerides (MCT) formulas in paediatric and allergological practice. Prz Gastroenterol. (2016) 11:226–31. 10.5114/pg.2016.61374
50.
Flickinger BD . Utilizing biotechnology in producing fats and oils with various nutritional properties. J AOAC Int. (2007) 90:1465–9. 10.1093/jaoac/90.5.1465
51.
Limketkai BN Zucker SD . Hyperammonemic encephalopathy caused by carnitine deficiency. J Gen Intern Med. (2008) 23:210–3. 10.1007/s11606-007-0473-0
52.
Watanabe S Tsujino S . Applications of medium-chain triglycerides in foods. Front Nutr. (2022) 9:802805. 10.3389/fnut.2022.802805
53.
Mohamed Elfadil O Shah RN Hurt RT Mundi MS . Peptide-based formula: clinical applications and benefits. Nutr Clin Pract. (2023) 38:318–28. 10.1002/ncp.10961
54.
Kaushik N Pietraszewski M Holst JJ O'Keefe SJ . Enteral feeding without pancreatic stimulation. Pancreas. (2005) 31:353–9. 10.1097/01.mpa.0000183374.11919.e5
55.
Silk DB . The evolving role of post-ligament of Trietz nasojejunal feeding in enteral nutrition and the need for improved feeding tube design and placement methods. JPEN J Parenter Enteral Nutr. (2011) 35:303–7. 10.1177/0148607110387799
56.
Preiser JC Ichai C Orban JC Groeneveld AB . Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54. 10.1093/bja/aeu187
57.
Yang Y Su S Zhang Y Wu D Wang C Wei Y et al . Effects of different ratios of carbohydrate-fat in enteral nutrition on metabolic pattern and organ damage in burned rats. Nutrients. (2022) 14:3653. 10.3390/nu14173653
58.
Shields BA VanFosson CA Pruskowski KA Gurney JM Rizzo JA Cancio LC . High-carbohydrate vs high-fat nutrition for burn patients. Nutr Clin Pract. (2019) 34:688–94. 10.1002/ncp.10396
59.
Wischmeyer PE San-Millan I . Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. (2015) 19 Suppl 3:S6. 10.1186/cc14724
60.
Wang YQ Li YH Li YT Li HX Zhang D . Comparisons between short-peptide formula and intact-protein formula for early enteral nutrition initiation in patients with acute gastrointestinal injury: a single-center retrospective cohort study. Ann Transl Med. (2022) 10:573. 10.21037/atm-22-1837
61.
Baldwin C Noble C . Long term effects of early nutritional support with new enterotropic peptide-based formula vs. standard enteral formula in HIV-infected patients: randomised prospective trial Chlebowski R T, Beall G, Grovenor M, Lillington L, Weintraub N, Ambler C, Richards E W, Abbruzzese B C, McCamish M A and Cope F O Nutrition 1993; 9 (6): 507-512. Clin Nutr. (1994) 13:197. 10.1016/0261-5614(94)90105-8
62.
Hamaoui E Lefkowitz R Olender L Krasnopolsky-Levine E Favale M Webb H et al . Enteral nutrition in the early postoperative period: a new semi-elemental formula vs. total parenteral nutrition. JPEN J Parenter Enteral Nutr. (1990) 14:501–7. 10.1177/0148607190014005501
63.
LaVallee C Seelam P Balakrishnan S Lowen C Henrikson A Kesting B et al . Real-world evidence of treatment, tolerance, healthcare utilization, and costs among postacute care adult patients receiving enteral peptide-based diets in the United States. JPEN J Parenter Enteral Nutr. (2021) 45:1729–35. 10.1002/jpen.2074
64.
Mundi MS Velapati S Kuchkuntla AR Hurt RT . Reduction in healthcare utilization with transition to peptide-based diets in intolerant home enteral nutrition patients. Nutr Clin Pract. (2020) 35:487–94. 10.1002/ncp.10477
65.
Al-Yousif N Rawal S Jurczak M Mahmud H Shah FA . Endogenous glucose production in critical illness. Nutr Clin Pract. (2021) 36:344–59. 10.1002/ncp.10646
66.
Iapichino G Radrizzani D Armani S Noto A Spanu P Mistraletti G . Metabolic treatment of critically ill patients: energy balance and substrate disposal. Minerva Anestesiol. (2006) 72:533–41.
67.
McClave SA Wischmeyer PE Miller KR van Zanten ARH . Mitochondrial dysfunction in critical illness: implications for nutritional therapy. Curr Nutr Rep. (2019) 8:363–73. 10.1007/s13668-019-00296-y
68.
Brand MD Nicholls DG . Assessing mitochondrial dysfunction in cells. Biochem J. (2011) 435:297–312. 10.1042/BJ20110162
69.
McKeever L Bonini M Braunschweig C . Feeding during phases of altered mitochondrial activity: a theory. JPEN J Parenter Enteral Nutr. (2018) 42:855–63. 10.1002/jpen.1010
70.
Andréasson C Ott M Büttner S . Mitochondria orchestrate proteostatic and metabolic stress responses. EMBO Rep. (2019) 20:e47865. 10.15252/embr.201947865
71.
Moonen H Van Zanten ARH . Mitochondrial dysfunction in critical illness during acute metabolic stress and convalescence: consequences for nutrition therapy. Curr Opin Crit Care. (2020) 26:346–54. 10.1097/MCC.0000000000000741
72.
Flower L Page A Puthucheary Z . Should nutrition therapy be modified to account for mitochondrial dysfunction in critical illness?JPEN J Parenter Enteral Nutr. (2021) 45:60–5. 10.1002/jpen.2190
73.
Supinski GS Schroder EA Callahan LA . Mitochondria and critical illness. Chest. (2020) 157:310–22. 10.1016/j.chest.2019.08.2182
74.
Klionsky DJ Petroni G Amaravadi RK Baehrecke EH Ballabio A Boya P et al . Autophagy in major human diseases. EMBO J. (2021) 40:e108863. 10.15252/embj.2021108863
75.
Li W He P Huang Y Li YF Lu J Li M et al . Selective autophagy of intracellular organelles: recent research advances. Theranostics. (2021) 11:222–56. 10.7150/thno.49860
76.
Vanhorebeek I Casaer M Gunst J . Nutrition and autophagy deficiency in critical illness. Curr Opin Crit Care. (2023) 29:306–14. 10.1097/MCC.0000000000001056
77.
Komatsu M Waguri S Ueno T Iwata J Murata S Tanida I et al . Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. (2005) 169:425–34. 10.1083/jcb.200412022
78.
Masiero E Agatea L Mammucari C Blaauw B Loro E Komatsu M et al . Autophagy is required to maintain muscle mass. Cell Metab. (2009) 10:507–15. 10.1016/j.cmet.2009.10.008
79.
Shutong L Yu J Jia W Huafei D Shifan Y Huili W et al . HO-1/autophagic flux axis alleviated sepsis-induced acute lung injury via inhibiting NLRP3 inflammasome. Cell Signal. (2022) 100:110473. 10.1016/j.cellsig.2022.110473
80.
Cao YY Qiao Y Wang ZH Chen Q Qi YP Lu ZM et al . The polo-like kinase 1-mammalian target of rapamycin axis regulates autophagy to prevent intestinal barrier dysfunction during sepsis. Am J Pathol. (2023) 193:296–312. 10.1016/j.ajpath.2022.11.008
81.
Gunst J Derese I Aertgeerts A Ververs EJ Wauters A Van den Berghe G et al . Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. (2013) 41:182–94. 10.1097/CCM.0b013e3182676657
82.
Brealey D Brand M Hargreaves I Heales S Land J Smolenski R et al . Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. (2002) 360:219–23. 10.1016/S0140-6736(02)09459-X
83.
Newman JC Verdin E . β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. (2017) 37:51–76. 10.1146/annurev-nutr-071816-064916
84.
Steinhauser ML Olenchock BA O'Keefe J Lun M Pierce KA Lee H et al . The circulating metabolome of human starvation. JCI Insight. (2018) 3. 10.1172/jci.insight.121434
85.
Paoli A Tinsley GM Mattson MP De Vivo I Dhawan R Moro T . Common and divergent molecular mechanisms of fasting and ketogenic diets. Trends Endocrinol Metab. (2024) 35:125–41. 10.1016/j.tem.2023.10.001
86.
Gunst J Casaer MP Langouche L Van den Berghe G . Role of ketones, ketogenic diets and intermittent fasting in ICU. Curr Opin Crit Care. (2021) 27:385–9. 10.1097/MCC.0000000000000841
87.
Singer M . The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. (2014) 5:66–72. 10.4161/viru.26907
88.
Cox PJ Kirk T Ashmore T Willerton K Evans R Smith A et al . Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. (2016) 24:256–68. 10.1016/j.cmet.2016.07.010
89.
Paumelle R Haas JT Hennuyer N Baugé E Deleye Y Mesotten D et al . Hepatic PPARα is critical in the metabolic adaptation to sepsis. J Hepatol. (2019) 70:963–73. 10.1016/j.jhep.2018.12.037
90.
White H Venkatesh B . Clinical review: ketones and brain injury. Crit Care. (2011) 15:219. 10.1186/cc10020
91.
Gunst J Verbruggen SC . Insulin resistance in critical illness: consequences for nutrition therapy and glucose management. Curr Opin Crit Care. (2023) 29:286–92. 10.1097/MCC.0000000000001055
92.
Van den Berghe G Wilmer A Hermans G Meersseman W Wouters PJ Milants I et al . Intensive insulin therapy in the medical ICU. N Engl J Med. (2006) 354:449–61. 10.1056/NEJMoa052521
93.
van den Berghe G Wouters P Weekers F Verwaest C Bruyninckx F Schetz M et al . Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. 10.1056/NEJMoa011300
94.
Gunst J Debaveye Y Güiza F Dubois J De Bruyn A Dauwe D et al . Tight blood-glucose control without early parenteral nutrition in the ICU. N Engl J Med. (2023) 389:1180–90. 10.1056/NEJMoa2304855
95.
Gunst J Van den . Berghe G. Blood glucose control in the ICU: don't throw out the baby with the bathwater! Intensive Care Med. (2016) 42:1478–81. 10.1007/s00134-016-4350-3
96.
Morley JE Thomas DR Wilson MM . Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. (2006) 83:735–43. 10.1093/ajcn/83.4.735
97.
Stumpf F Keller B Gressies C Schuetz P . Inflammation and nutrition: friend or foe?Nutrients. (2023) 15:1159. 10.3390/nu15051159
98.
Lheureux O Preiser JC . Role of nutrition support in inflammatory conditions. Nutr Clin Pract. (2017) 32:310–7. 10.1177/0884533617695242
99.
Guerrero-Romero F Simental-Mendía LE González-Ortiz M Martínez-Abundis E Ramos-Zavala MG Hernández-González SO et al . The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. 10.1210/jc.2010-0288
100.
Yang Z Gong H Kan F Ji N . Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22:232. 10.1186/s12933-023-01971-9
101.
Cai W Xu J Wu X Chen Z Zeng L Song X et al . Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22:138. 10.1186/s12933-023-01864-x
102.
Herlein JA Morgan DA Phillips BG Haynes WG Sivitz WI . Antecedent hypoglycemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology. (2006) 147:2781–8. 10.1210/en.2005-1247
103.
Finfer S Chittock DR Su SY Blair D Foster D Dhingra V et al . Intensive vs. conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. 10.1056/NEJMoa0810625
104.
Gunst J . Recovery from critical illness-induced organ failure: the role of autophagy. Crit Care. (2017) 21:209. 10.1186/s13054-017-1786-y
105.
Gunst J De Bruyn A Casaer MP Vander Perre S Langouche L Van den Berghe G . Impact of tight glucose control on circulating 3-hydroxybutyrate in critically ill patients. Crit Care. (2021) 25:373. 10.1186/s13054-021-03772-6
106.
Berger MM Reintam-Blaser A Calder PC Casaer M Hiesmayr MJ Mayer K et al . Monitoring nutrition in the ICU. Clin Nutr. (2019) 38:584–93. 10.1016/j.clnu.2018.07.009
107.
De Waele E van Zanten ARH . Routine use of indirect calorimetry in critically ill patients: pros and cons. Crit Care. (2022) 26:123. 10.1186/s13054-022-04000-5
108.
Gunst J Kashani KB Hermans G . The urea-creatinine ratio as a novel biomarker of critical illness-associated catabolism. Intensive Care Med. (2019) 45:1813–5. 10.1007/s00134-019-05810-y
109.
Regolisti G Rebora P Occhino G Lieti G Molon G Maloberti A et al . Elevated serum urea-to-creatinine ratio and in-hospital death in patients with hyponatremia hospitalized for COVID-19. Biomedicines. (2023) 11:1555. 10.3390/biomedicines11061555
110.
Page A Flower L Prowle J Puthucheary Z . Novel methods to identify and measure catabolism. Curr Opin Crit Care. (2021) 27:361–6. 10.1097/MCC.0000000000000842
111.
Gunst J Casaer MP Preiser JC Reignier J Van den Berghe G . Toward nutrition improving outcome of critically ill patients: how to interpret recent feeding RCTs?Crit Care. (2023) 27:43. 10.1186/s13054-023-04317-9
112.
da Silva JSV Seres DS Sabino K Adams SC Berdahl GJ Citty SW et al . ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. (2020) 35:178–95. 10.1002/ncp.10474
113.
Berger MM Shenkin A Schweinlin A Amrein K Augsburger M Biesalski HK et al . ESPEN micronutrient guideline. Clin Nutr. (2022) 41:1357–424. 10.1016/j.clnu.2022.02.015
114.
Friedli N Stanga Z Sobotka L Culkin A Kondrup J Laviano A et al . Revisiting the refeeding syndrome: results of a systematic review. Nutrition. (2017) 35:151–60. 10.1016/j.nut.2016.05.016
115.
Boateng AA Sriram K Meguid MM Crook M . Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. (2010) 26:156–67. 10.1016/j.nut.2009.11.017
116.
Koekkoek KWA Berger MM . An update on essential micronutrients in critical illness. Curr Opin Crit Care. (2023) 29:315–29. 10.1097/MCC.0000000000001062
117.
Casaer MP Bellomo R . Micronutrient deficiency in critical illness: an invisible foe?Intensive Care Med. (2019) 45:1136–9. 10.1007/s00134-019-05678-y
118.
Doig GS Simpson F Heighes PT Bellomo R Chesher D Caterson ID et al . Restricted vs. continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. (2015) 3:943–52. 10.1016/S2213-2600(15)00418-X
119.
Olthof LE Koekkoek W van Setten C Kars JCN van Blokland D van Zanten ARH . Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr. (2018) 37:1609–17. 10.1016/j.clnu.2017.08.001
120.
Reintam Blaser A Alhazzani W Belley-Cote E Møller MH Adhikari NKJ Burry L et al . Intravenous vitamin C therapy in adult patients with sepsis: a rapid practice guideline. Acta Anaesthesiol Scand. (2023) 67:1423–31. 10.1111/aas.14311
121.
Tamme K Reintam Blaser A Laisaar KT Mändul M Kals J Forbes A et al . Incidence and outcomes of acute mesenteric ischaemia: a systematic review and meta-analysis. BMJ Open. (2022) 12:e062846. 10.1136/bmjopen-2022-062846
122.
Reintam Blaser A Starkopf J Björck M Forbes A Kase K Kiisk E et al . Diagnostic accuracy of biomarkers to detect acute mesenteric ischaemia in adult patients: a systematic review and meta-analysis. World J Emerg Surg. (2023) 18:44. 10.1186/s13017-023-00512-9
123.
Hess B Cahenzli M Forbes A Burgos R Coccolini F Corcos O et al . Management of acute mesenteric ischaemia: results of a worldwide survey. Clin Nutr ESPEN. (2023) 54:194–205. 10.1016/j.clnesp.2022.12.022
124.
Tolonen M Lemma A Vikatmaa P Peltola E Mentula P Björkman P et al . The implementation of a pathway and care bundle for the management of acute occlusive arterial mesenteric ischemia reduced mortality. J Trauma Acute Care Surg. (2021) 91:480–8. 10.1097/TA.0000000000003305
125.
Reintam Blaser A Mändul M Björck M Acosta S Bala M Bodnar Z et al . Incidence, diagnosis, management and outcome of acute mesenteric ischaemia: a prospective, multicentre observational study (AMESI Study). Crit Care. (2024) 28:32. 10.1186/s13054-024-04807-4
126.
Bala M Catena F Kashuk J De Simone B Gomes CA Weber D et al . Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg. (2022) 17:54. 10.1186/s13017-022-00443-x
127.
Yu H Kirkpatrick IDC . An Update on Acute Mesenteric Ischemia. Can Assoc Radiol J. (2023) 74:160–71. 10.1177/08465371221094280
Summary
Keywords
critical illness, intensive care, enteral nutrition, feeding intolerance, feeding strategy, refeeding syndrome, acute mesenteric ischemia
Citation
Wang Y, Li Y, Li N, Li Y, Li H and Zhang D (2025) Protective nutrition strategy in the acute phase of critical illness: why, what and how to protect. Front. Nutr. 12:1555311. doi: 10.3389/fnut.2025.1555311
Received
04 January 2025
Accepted
17 April 2025
Published
09 May 2025
Volume
12 - 2025
Edited by
Akio Shimizu, Mie University, Japan
Reviewed by
Giuseppe Pasolini, Azienda Provinciale per i Servizi Sanitari (APSS), Italy
Hilal Sipahioglu, Kayseri Education and Research Hospital, Türkiye
Updates
Copyright
© 2025 Wang, Li, Li, Li, Li and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zhang zhangdong@jlu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.