- 1Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Clinical Nutrition, Children’s Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 3Department of Clinical Nutrition, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, China
- 4Center for Disease Control and Prevention, Zhengzhou, China
- 5Department of Nutrition and Food Hygiene, College of Public Health, Zhengzhou University, Zhengzhou, China
Background and aim: Malnutrition is a critical challenge in intensive care unit (ICU) patients, with the timing of energy sufficiency being a key yet debated factor in nutritional support. This study aimed to investigate the association between the timing of achieving energy sufficiency (defined as ≥70% of daily energy targets, 17.5 kcal/kg/day) in critically ill patients and their clinical outcomes, providing evidence-based guidance for ICU nutritional protocols.
Methods: In this prospective observational study, adult patients admitted to the ICU for ≥3 days were stratified into three groups based on the time to achieve energy sufficiency: early (≤3 days), middle (4–7 days), and late (>7 days). Clinical outcomes, including in-hospital mortality, 60-day mortality, ICU length of stay, and gastrointestinal complications, were compared across groups. Cox proportional hazards regression models were used to assess the independent association between energy sufficiency timing and mortality, while restricted cubic spline (RCS) analysis explored nonlinear dose–response relationships using days to energy sufficiency as a continuous variable. Statistical analyses were performed using SPSS 25.0 and R 4.2.3 (two-tailed tests, α = 0.05).
Results: A total of 826 critically ill patients were initially screened, with 584 meeting the predefined inclusion and exclusion criteria and ultimately enrolled in this study. The middle-group patients (achieving energy sufficiency at 4–7 days) demonstrated the lowest in-hospital mortality (15.6%) and 60-day mortality (28.5%), significantly lower than the late group (32.0 and 49.0%, respectively; p < 0.001). After adjusting for confounders (age, BMI, disease severity, etc.), both early and middle energy sufficiency remained independent protective factors against 60-day mortality (HR = 0.398 and 0.399, respectively; p < 0.001). RCS analysis revealed a nonlinear dose–response relationship: mortality decreased with delayed energy sufficiency up to day 6, after which mortality risk significantly increased (p < 0.001 for overall correlation; inflection point at day 6).
Conclusion: The timing of achieving energy sufficiency (17.5 kcal/kg/day) is significantly associated with 60-day mortality in ICU patients. Combining RCS-derived inflection point (day 6) and intergroup comparisons, the optimal window for achieving energy sufficiency appears to be 4–6 days post-ICU admission, balancing metabolic stability and tissue repair needs while avoiding early overfeeding risks.
1 Introduction
Critical illness, triggered by major trauma, extensive burns, or life-threatening diseases, induces a state of severe physiological stress characterized by hypercatabolism and insulin resistance (1). This metabolic derangement accelerates the breakdown of skeletal muscle and adipose tissue, leading to progressive wasting, functional decline, and heightened vulnerability to multi-organ failure, nosocomial infections, and critical illness–related debility (2, 3). Concurrently, factors such as disease-induced anorexia, psychological stress (e.g., depression, anxiety), and clinical interventions (e.g., tracheal intubation, preoperative fasting, gastrointestinal dysfunction) collectively restrict oral and enteral intake, often resulting in energy deficits and increasing the risk of malnutrition (4–6). Notably, malnutrition affects 30–50% of ICU patients, prolonging hospital and ICU stays while escalating the risk of complications (e.g., infections, ICU-acquired weakness) and mortality (7–9).
Malnutrition management in critically ill patients remains a major clinical challenge, with nutritional support strategies—including timing of initiation, delivery route, and macronutrient targets—being critical determinants of outcomes (10, 11). Despite guidelines from organizations like the Society of Critical Care Medicine (SCCM) and ASPEN recommending early achievement of energy goals (within 24–48 h) for high-risk patients (12), early isocaloric feeding has been associated with refeeding syndromes, including hyperglycemia, hepatic steatosis, and increased infection risk (13). While combined enteral nutrition (EN) and parenteral nutrition (PN) improves energy delivery compared to EN alone, it does not consistently affect 30-day mortality or length of stay (14). For example, Arabi et al.’s (15) RCT showed similar 90-day mortality between hypocaloric (≤14 kcal/kg/day) and full-caloric (≥70% target) feeding groups, but fewer infections in the hypocaloric arm. Subsequent studies by Heidegger et al. (16) and Allingstrup et al. (17) have reported conflicting results when comparing indirect calorimetry-guided individualized nutrition (e.g., 25 kcal/kg/day) with standardized protocols. These inconsistencies highlight unresolved questions about the optimal timing and dosage of energy delivery. To address this gap, our study hypothesized that the timing of achieving energy sufficiency (≥70% of target) would influence 60-day mortality in ICU patients. By systematically evaluating the association between energy sufficiency timing and clinical outcomes, we aim to provide evidence for personalized nutritional protocols in critical care.
2 Method
2.1 Participants
This prospective observational study enrolled adult patients admitted to the intensive care unit (ICU) of the First Affiliated Hospital of Zhengzhou University between December 2020 and October 2021. Inclusion criteria were: (1) diagnosis of critical illness; (2) aged 18–90 years; (3) ICU stay ≥72 h. Exclusion criteria included contraindications to nutritional support, severe hepatic failure, extracorporeal membrane oxygenation (ECMO) support, severe mental disorders, secondary shock, pregnancy/lactation, participation in other trials, or refusal to consent. Patients were stratified into three groups based on the time to achieve energy sufficiency (defined as ≥70% of daily energy targets, 17.5 kcal/kg/day): early (≤3 days), middle (4–7 days), and late (>7 days).
The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University (2021-KY-0023-001), and written informed consent was obtained from all participants or their legal representatives. All the data used for analysis was anonymous.
2.2 Data collection
In this study, to minimize measurement bias, a single trained researcher systematically collected data using a standardized case report form (CRF) via the electronic medical record (EMR) system. Data were prospectively extracted from patient charts, daily nutrition prescriptions, and nursing records.
2.2.1 Baseline data
The following data were recorded in the baseline: patient general information (sex, age, height and weight), nutritional status, and severity of disease. Nutritional status was evaluated using the Nutritional Risk Screening 2002 score (NRS 2002) and modified Nutrition Risk in the Critically Ill score (mNUTRIC) (7, 9); disease severity was assessed via the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) score (1, 3).
2.2.2 Nutritional exposure data
Nutritional parameters were recorded daily until hospital discharge or up to day 14 in the ICU, including:
Support modalities: oral feeding, enteral nutrition (EN), parenteral nutrition (PN), or combined EN+PN.
Energy sources: glucose dose (via PN/EN), type and dose of EN preparations, type and dose of PN preparations, and oral intake quantified by direct weighing of uneaten food (precision ± 5 g).
Energy intake calculation: Daily energy intake was computed as the sum of calories from oral feeding, EN, and PN, based on nutrient composition tables. According to the ESPEN guidelines, hypocaloric or underfeeding is an energy administration below 70% of the defined target (20–25 kcal/kg/day). So we consider a target of energy intake ≥70% of the ESPEN-recommended target as energy sufficiency (18). Patients were stratified into:
Early group: energy sufficiency achieved within ≤3 days (first 3-day average ≥17.5 kcal/kg/day).
Middle group: energy sufficiency achieved on days 4–7 (first 7-day average ≥17.5 kcal/kg/day, with days 1–3 < 17.5 kcal/kg/day).
Late group: energy sufficiency achieved after >7 days (first 14-day average ≥17.5 kcal/kg/day, with days 1–7 < 17.5 kcal/kg/day).
2.2.3 Outcome data
Primary and secondary outcomes included:
Mortality: in-hospital mortality and 60-day all-cause mortality.
Length of stay: ICU duration and total hospital stay (days).
Gastrointestinal (GI) complications: diarrhea: defined as ≥3 loose stools/day for ≥2 consecutive days; GI intolerance: documented vomiting, abdominal distension, or gastric residual volume >200 mL (5, 13).
2.3 Statistical methods
We first described basic information, nutritional status, disease severity, and patient outcomes in the study population, and then we analyzed differences in clinical outcomes between patients with different timing of achieving energy sufficiency. For quantitative data, comparisons between groups of multiple independent samples were analyzed using one-way analysis of variance (ANOVA) if normality was satisfied and variances were chi-squared, otherwise the Kruskal-Wallis test was used. For qualitative data, the X2 test was used for intergroup comparisons, and Fisher’s exact probability method was used if the test conditions were not met.
We used a Cox proportional hazard regression model to explore the linear relationship between different timing of achieving energy sufficiency and patient survival outcomes. However, in actual clinical practice, the efficacy of nutritional interventions may exhibit a threshold effect or inflection point phenomenon. Therefore, we employed restricted cubic spline (RCS) analysis to explore the nonlinear association between the specific number of days to achieve energy sufficiency in the ICU and 60-day mortality among patients who reached energy sufficiency. Subgroup analyses were subsequently performed to further explore whether outcomes differed significantly by age and gender. RCS were plotted using R 4.3.2, and all other analyses were performed using SPSS 25.0 with two-sided tests at a test level of α = 0.05.
3 Results
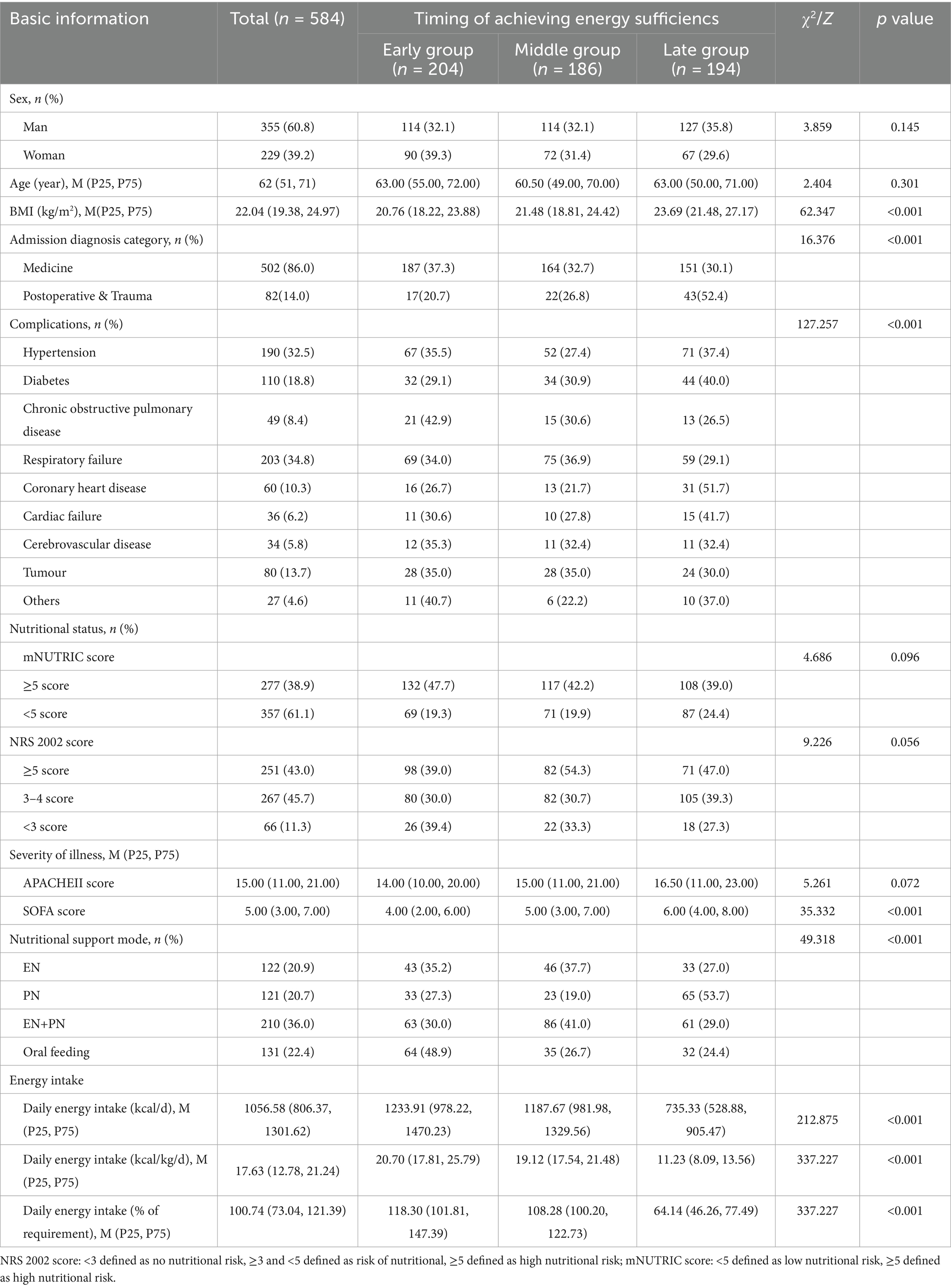
3.1 Basic information about the study subjects
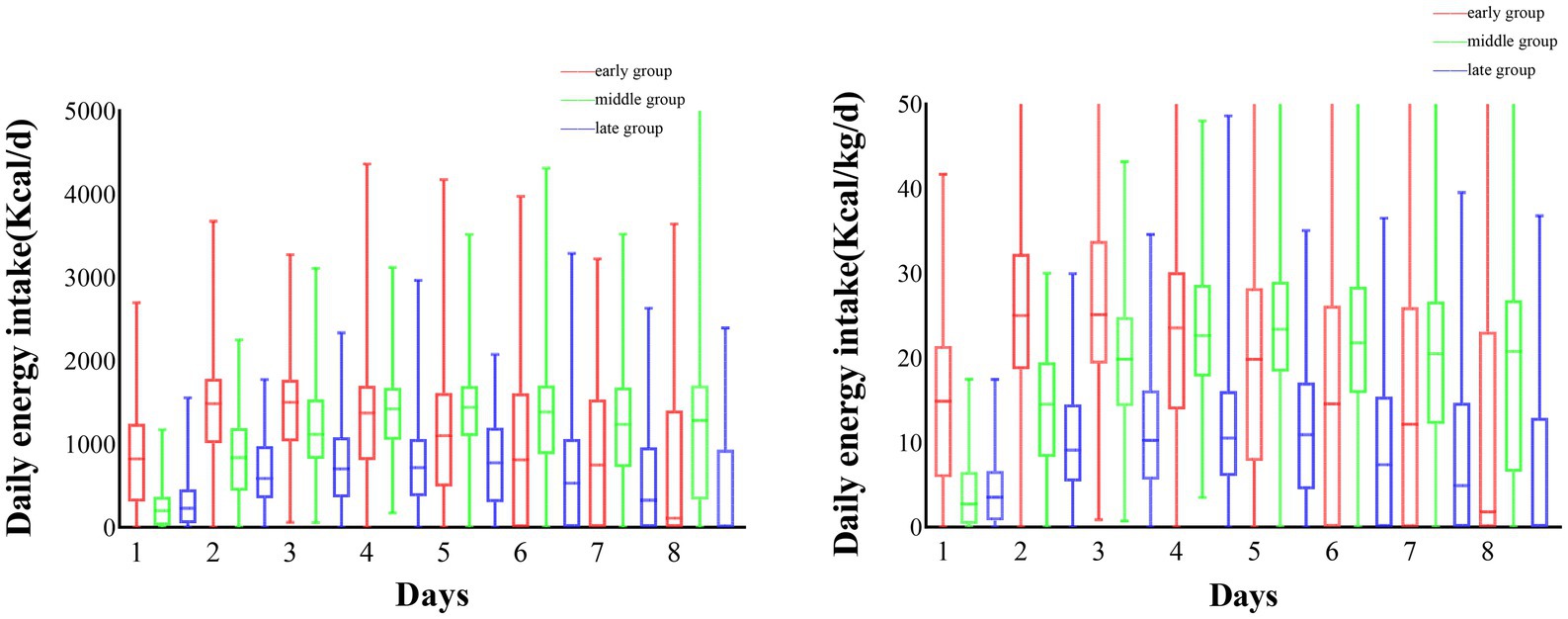
Overall, a total of 584 patients were included in this study, 204 patients in the early group, 186 in the middle group, and 194 in the late group. we compared baseline characteristics between groups at different times of achieving energy adequacy, and the results showed that there were no statistically significant differences between the three groups in terms of gender, age, mNUTRIC score, NRS 2002 score, and APACHE II score (p > 0.05), and statistically significant differences in terms of BMI, SOFA score, mode of nutritional support, and daily energy intake (p < 0.05). For specific details, please refer to Table 1. The average daily energy intake of the three groups of patients is shown in Figure 1.
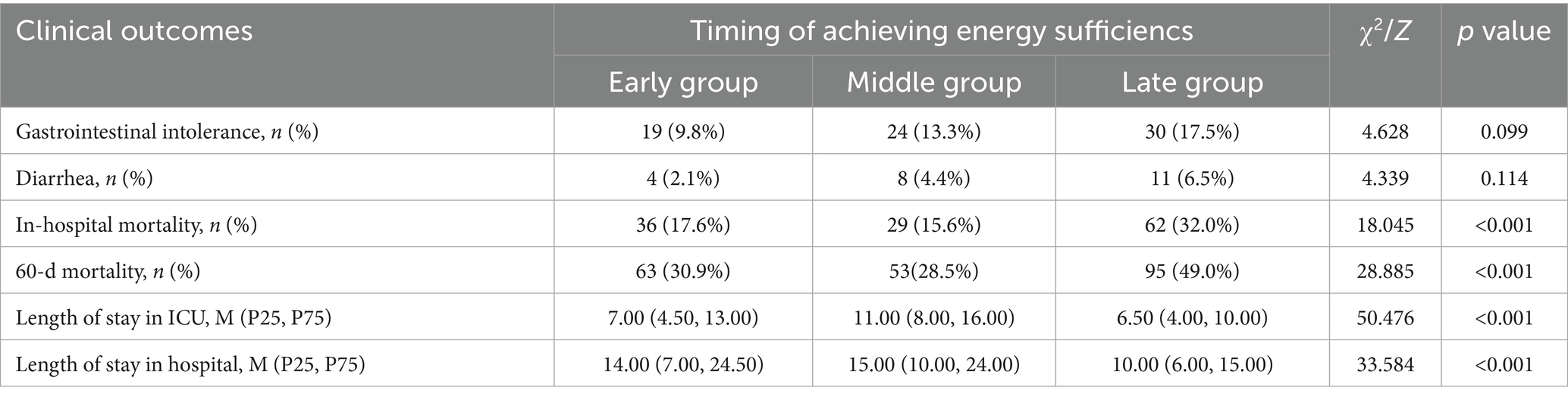
3.2 Relationship between timing of achieving energy sufficiency and clinical outcomes
We compared clinical outcomes between groups at different times of achieving energy sufficiency, as shown in Table 2. There were no statistically significant differences in the incidence of gastrointestinal intolerance and diarrhea among the three groups (p > 0.05), and there were statistically significant differences between in-hospital mortality and 60d mortality (p < 0.001). Patients in the late group exhibited the highest in-hospital mortality and 60-day mortality, followed by those in the early group, while patients in the middle group had the lowest mortality rates. In terms of ICU length of stay and hospital duration, mid-term group patients had the longest ICU and hospital stays, followed by early group patients, with late group patients demonstrating the shortest durations.
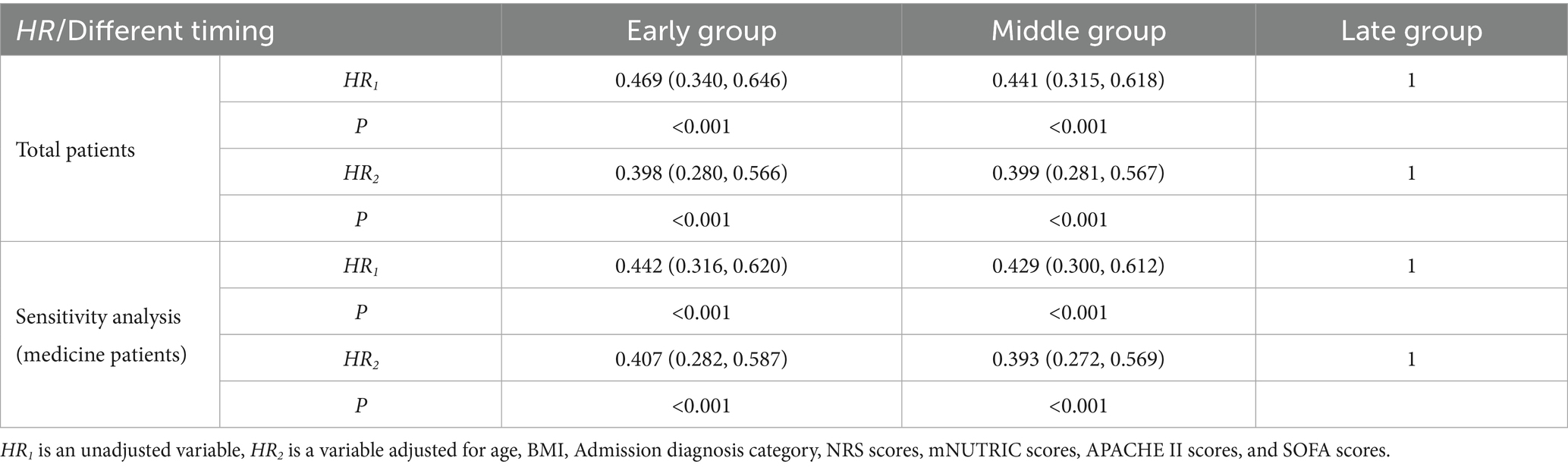
After that, we included the timing of achieving energy sufficiency as a variable in the COX regression model and the results are shown in Table 3 (Including sensitivity analysis). Using the late group as a reference, achieving energy sufficiency in the early (HR = 0.469, 95% CI: 0.340, 0.646, p < 0.001) and middle (HR = 0.441, 95% CI: 0.315, 0.618, p < 0.001) periods were both protective factors for 60-day mortality. After adjusting for confounders such as sex, age, BMI, nutritional status, and disease severity, early group and middle group remained protective factors for 60d death.
3.3 Dose–response relationship between specific timing of achieving energy sufficiency and 60-day mortality
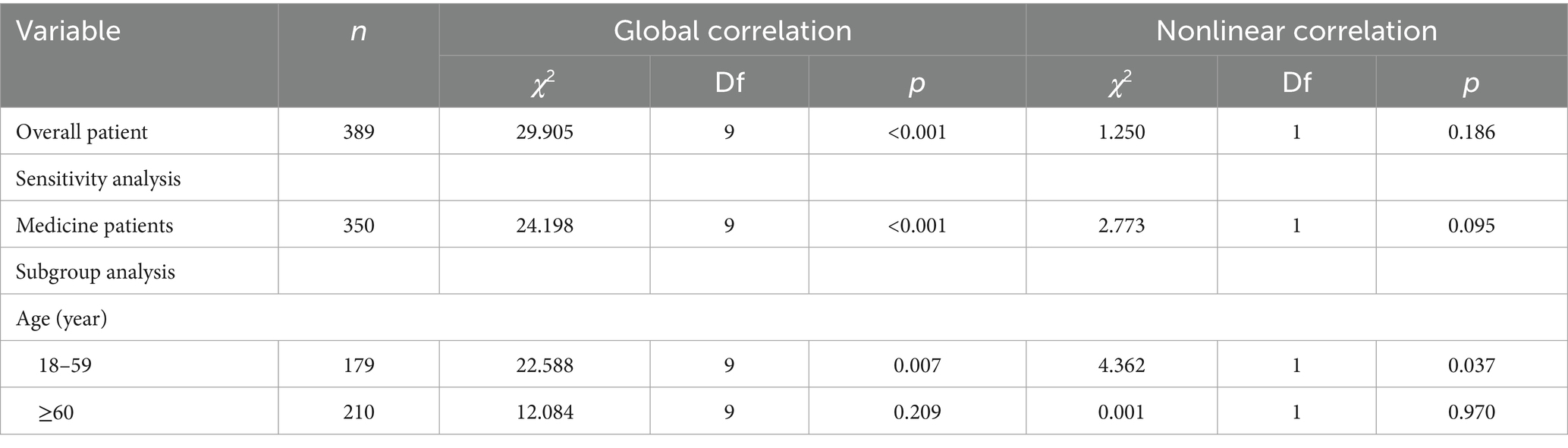
There was an overall correlation between the specific timing of patient achieving energy sufficiency in the ICU and 60-day death, but there was no nonlinear association (Ptotal < 0.001, Pnonlinear = 0.186), as shown in Table 4. The specific trend of the association is shown in Figure 2. The graph suggests that the later the patient achieves energy sufficiency from ICU admission to day 6, the lower the 60d risk of death, and the later the time after day 6, the higher the 60d risk of death. The sensitivity analysis for medicine patients also showed the same result.
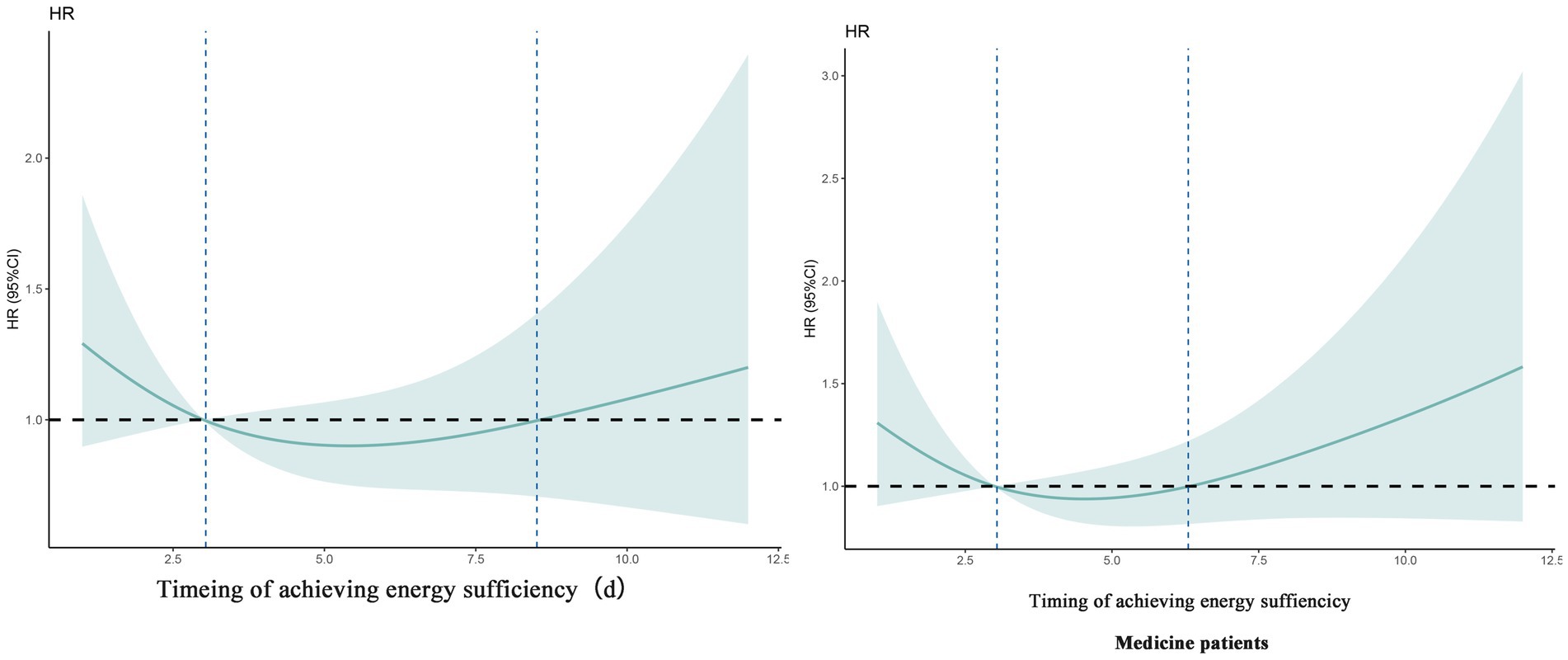
Figure 2. Dose–response relationship between specific time of achieving energy sufficiency and 60-day mortality (overall patient and sensitivity analysis).
In addition, we performed subgroup analyses which showed a linear association between timing of achieving energy sufficiency and 60-day death in men patients (Ptotal = 0.009, Pnonlinear = 0.930), whereas there was a significant non-linear association between timing of achieving energy sufficiency and 60-day death in patients aged 18–59 years (Ptotal = 0.007, Pnonlinear = 0.037), and the trend of the association is shown in Figure 3.
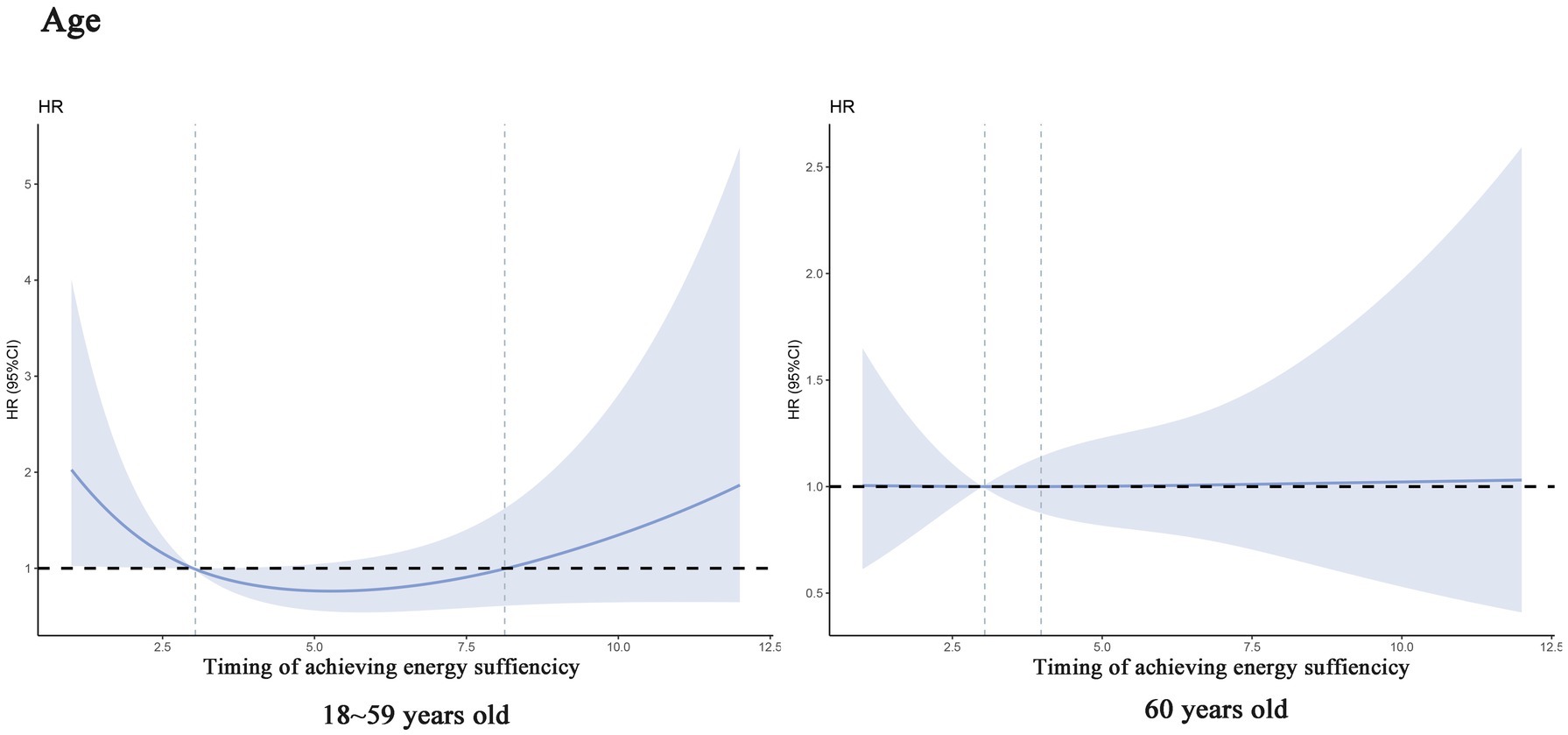
Figure 3. Dose–response relationship between specific time of achieving energy sufficiency and 60-day mortality (subgroup analysis).
4 Discussions
In this study, 584 critically ill patients were categorized into early, middle, and late groups based on the timing of achieving energy sufficiency (≥70% of the ESPEN-recommended target, 17.5 kcal/kg/day). Intergroup comparison revealed that middle-group patients exhibited the lowest in-hospital mortality (15.6%) and 60-day mortality (28.5%), significantly lower than the late group (32.0 and 49.0%, respectively; p < 0.001). While the middle group had longer ICU and hospital stays, this may reflect a balance between metabolic stabilization and active tissue repair. Mechanistically, initiating nutritional support at 4–7 days aligns with the decline of the acute inflammatory phase (e.g., reduced IL-6 and TNF-α levels), which restores insulin sensitivity and enhances energy utilization for healing rather than catabolic processes (2, 19). This timing also avoids the risks of early overfeeding, such as hyperglycemia and hepatic steatosis, while preventing irreversible muscle wasting (typically occurring after 7–10 days of inadequate nutrition) (13, 19).
Multivariate Cox regression confirmed that both early (≤3 days) and middle (4–7 days) energy sufficiency were independent protective factors against 60-day mortality (adjusted HR = 0.398 and 0.399, respectively; p < 0.001). Restricted cubic spline (RCS) analysis further revealed a nonlinear dose–response relationship, with 60-day mortality decreasing up to day 6 and significantly increasing thereafter (p < 0.001 for overall correlation), supporting a critical threshold at day 6. This finding aligns with Heidegger et al.’s (16) RCT, where initiating full-energy parenteral nutrition on day 4 reduced hospital-acquired infections, and Matejovic et al.’s (20) study showing that energy deficits after day 5 were strongly associated with mortality.
Notably, our results contrast with a Chinese study reporting no impact of early energy sufficiency on outcomes (21). This discrepancy may arise from study design differences, such as inclusion of predominantly surgical patients (with lower metabolic stress than medical ICU patients), use of higher energy targets (25 kcal/kg/day vs. our 17.5 kcal/kg/day), or reliance on enteral nutrition alone (17, 21, 22). Indeed, the middle group in our study had a higher proportion of combined enteral + parenteral nutrition (46.2%), which may optimize energy delivery while preserving gut barrier function (10, 14).
Subsequently, we conducted a subgroup analysis stratified by age, and the results showed that patients <60 years showed a stronger association between delayed energy sufficiency and mortality, while the trend was relatively flat in patients over 60 years of age. This phenomenon may be related to the differences in physiological reserves, metabolic characteristics, and disease stress response in different age groups. <60-year-old patients are more likely to be in the prime of life, and the stress response triggered by critical illnesses is more intense, manifesting itself in higher stress-induced resting energy expenditure (REE, up to 50–100% above baseline) and faster rate of muscle catabolism (3). At this time, insufficient energy intake can rapidly exacerbate malnutrition, leading to decreased immune function and organ dysfunction, which can lead to a bad outcomes (19). In contrast, ≥60-year-old patients often have sarcopenia, a low basal metabolic rate, and may be in a chronic inflammatory state, exhibit “pseudo-normalized” energy requirements due to sarcopenia and chronic inflammation, blunting short-term mortality impacts (23).
Despite numerous observational studies and randomized controlled trials (RCTs) conducted over the past decade, the optimal timing and dosage of energy delivery in critically ill patients remain unclear. Based on our findings, we recommend providing patients with at least 17.5 kcal/kg/day of energy within 4–6 days after ICU admission. This study uniquely integrates energy intake timing with ICU stay duration to analyze their combined impact on 60-day mortality, offering novel evidence and directions for future research. Notably, while we observed a significant correlation between the timing of achieving energy sufficiency and 60-day mortality, this association should be interpreted as a potential marker of overall care quality or recovery trends rather than a definitive causal relationship. In clinical practice, nutritional strategies must be comprehensively evaluated alongside other medical interventions to account for individual variability in metabolic responses and disease severity.
Our study has several notable limitations that should be considered when interpreting the results. First, as an observational study, it inherently carries susceptibility to residual confounding, despite our application of multivariable regression models to adjust for known covariates. For instance, unmeasured factors—such as pre-ICU nutritional status, genetic predispositions to metabolic responses, and the impact of specific ICU interventions (e.g., sedation protocols, mechanical ventilation parameters)—may potentially influence both the timing of achieving energy sufficiency and clinical outcomes. Second, defining energy sufficiency as achieving 70% of the ESPEN-recommended target (17.5 kcal/kg/day) represents a simplified approach that may not capture the individualized nutritional requirements of critically ill patients. Our study did not account for dynamic fluctuations in energy expenditure caused by factors such as fever, sepsis, or surgical procedures. Third, the single-center design of the study may restrict the generalizability of our findings, as patient demographics, treatment protocols, and nutritional practices can differ across diverse healthcare settings.
To address these limitations, several promising future research directions deserve dedicated exploration. First, randomized controlled trials (RCTs) are pivotal to establish a causal link between the timing of achieving energy sufficiency and clinical outcomes. Such trials should systematically compare contrasting nutritional strategies such as early initiation versus delayed escalation of full-energy support—while employing sophisticated methodologies to control for confounding variables, thereby strengthening the validity of causal conclusions. Second, the advancement of personalized nutrition protocols represents a critical priority. Future studies could integrate real-time assessments of energy expenditure (e.g., via indirect calorimetry) and dynamic biomarker monitoring (e.g., serum prealbumin, C-reactive protein) to tailor nutritional interventions to the unique metabolic profiles of individual patients. Especially considering the heterogeneity in metabolic responses among critically ill populations, as it would enable adaptive nutritional support that aligns with fluctuating physiological demands (e.g., fever, sepsis, or surgical trauma). Additionally, multicenter studies with large, diverse cohorts are indispensable to enhance the generalizability of these findings. By enrolling patients across geographically and demographically varied healthcare settings, such research could validate the optimal window for achieving energy sufficiency (e.g., 4–6 days post-ICU admission) in populations with differing baseline characteristics, comorbidities, and institutional protocols. This would mitigate the single-center limitation of the present study and foster the development of universally applicable nutritional guidelines.
5 Conclusion
Our findings suggest that the timing of energy sufficiency (70% of energy goal, i.e., 17.5 kcal/kg/d) in critically ill patients is closely related to 60-day mortality, and the optimal window for achieving energy sufficiency is 4–6 days. This finding provides key evidence for the precise formulation of nutritional support protocols in the ICU.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XY: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YL: Formal analysis, Validation, Writing – review & editing. XH: Data curation, Formal analysis, Writing – review & editing. QL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Téblick, A, Langouche, L, and Van den Berghe, G. Anterior pituitary function in critical illness. Endocr Connect. (2019) 8:R131–r143. doi: 10.1530/EC-19-0318
2. Van den Berghe, G. The 2016 ESPEN sir David Cuthbertson lecture: interfering with neuroendocrine and metabolic responses to critical illness: from acute to long-term consequences. Clin Nutr. (2017) 36:348–54. doi: 10.1016/j.clnu.2016.10.011
3. Preiser, JC, Ichai, C, Orban, JC, and Groeneveld, AB. Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54. doi: 10.1093/bja/aeu187
4. Gunst, J, and Van den Berghe, G. Parenteral nutrition in the critically ill. Curr Opin Crit Care. (2017) 23:149–58. doi: 10.1097/MCC.0000000000000385
5. Lee, ZY, Ibrahim, NA, and Mohd-Yusof, BN. Prevalence and duration of reasons for enteral nutrition feeding interruption in a tertiary intensive care unit. Nutrition. (2018) 53:26–33. doi: 10.1016/j.nut.2017.11.014
6. Marik, PE, and Annane, D. Reduced cortisol metabolism during critical illness. N Engl J Med. (2013) 369:480–1. doi: 10.1056/NEJMc1306703
7. Lew, CCH, Yandell, R, Fraser, RJL, Chua, AP, Chong, MFF, and Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review [formula: see text]. JPEN J Parenter Enteral Nutr. (2017) 41:744–58. doi: 10.1177/0148607115625638
8. Hill, A, Elke, G, and Weimann, A. Nutrition in the intensive care unit-a narrative review. Nutrients. (2021) 13:2851. doi: 10.3390/nu13082851
9. Mogensen, KM, Robinson, MK, Casey, JD, Gunasekera, NS, Moromizato, T, Rawn, JD, et al. Nutritional status and mortality in the critically ill. Crit Care Med. (2015) 43:2605–15. doi: 10.1097/CCM.0000000000001306
10. Elke, G, van Zanten, AR, Lemieux, M, McCall, M, Jeejeebhoy, KN, Kott, M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. (2016) 20:117. doi: 10.1186/s13054-016-1298-1
11. Verhage, AH, and van Vliet, AC. Clinical practice of nutritional support in Dutch intensive care units: a survey. Eur J Intern Med. (2002) 13:496–9. doi: 10.1016/s0953-6205(02)00163-2
12. McClave, SA, Taylor, BE, Martindale, RG, Warren, MM, Johnson, DR, Braunschweig, C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (a.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863
13. Chapple, LS, Weinel, L, Ridley, EJ, Jones, D, Chapman, MJ, and Peake, SL. Clinical sequelae from overfeeding in Enterally fed critically ill adults: where is the evidence? JPEN J Parenter Enteral Nutr. (2020) 44:980–91. doi: 10.1002/jpen.1740
14. Hill, A, Heyland, DK, Ortiz Reyes, LA, Laaf, E, Wendt, S, Elke, G, et al. Combination of enteral and parenteral nutrition in the acute phase of critical illness: an updated systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. (2022) 46:395–410. doi: 10.1002/jpen.2125
15. Arabi, YM, Aldawood, AS, Haddad, SH, Al-Dorzi, HM, Tamim, HM, Jones, G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. (2015) 372:2398–408. doi: 10.1056/NEJMoa1502826
16. Heidegger, CP, Berger, MM, Graf, S, Zingg, W, Darmon, P, Costanza, MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. (2013) 381:385–93. doi: 10.1016/S0140-6736(12)61351-8
17. Allingstrup, MJ, Kondrup, J, Wiis, J, Claudius, C, Pedersen, UG, Hein-Rasmussen, R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-Centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. (2017) 43:1637–47. doi: 10.1007/s00134-017-4880-3
18. Singer, P, Blaser, AR, Berger, MM, Calder, PC, Casaer, M, Hiesmayr, M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. (2023) 42:1671–89. doi: 10.1016/j.clnu.2023.07.011
19. Cerra, FB, Benitez, MR, Blackburn, GL, Irwin, RS, Jeejeebhoy, K, Katz, DP, et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest. (1997) 111:769–78. doi: 10.1378/chest.111.3.769
20. Matejovic, M, Huet, O, Dams, K, Elke, G, Vaquerizo Alonso, C, Csomos, A, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. (2022) 26:143. doi: 10.1186/s13054-022-03997-z
21. Zhou, X, Fang, H, Hu, C, Xu, J, Wang, H, Pan, J, et al. Effect of hypocaloric versus standard enteral feeding on clinical outcomes in critically ill adults - a meta-analysis of randomized controlled trials with trial sequential analysis. Med Intensiva (Engl Ed). (2021) 45:211–25. doi: 10.1016/j.medin.2019.10.003
22. Gunst, J, Casaer, MP, Preiser, J-C, Reignier, J, and Van den Berghe, G. Toward nutrition improving outcome of critically ill patients: how to interpret recent feeding RCTs? Crit Care. (2023) 27:43. doi: 10.1186/s13054-023-04317-9
Keywords: energy sufficiency timing, critical illness, nutritional support, 60-day mortality, restricted cubic spline analysis energy sufficiency timing, restricted cubic spline analysis
Citation: Yue X, Zhu X, Li Y, Huang X and Lyu Q (2025) Relationship between timing of achieving energy sufficiency and clinical outcomes in critically ill patients. Front. Nutr. 12:1565394. doi: 10.3389/fnut.2025.1565394
Edited by:
Marilia Seelaender, University of São Paulo, BrazilReviewed by:
Alessandro Domenico Quercia, Nephrology and Dialysis ASLCN1, ItalyYuchang Wang, Huazhong University of Science and Technology, China
Dina Khodeer, Suez Canal University, Egypt
Copyright © 2025 Yue, Zhu, Li, Huang and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanjun Lyu, THZxdWFuanVuNjY2QDE2My5jb20=
 Xiangfeng Yue1
Xiangfeng Yue1 Xiaoxi Zhu
Xiaoxi Zhu Quanjun Lyu
Quanjun Lyu