- 1Department of Nutrition and Food Safety, School of Public Health, Xi'an Jiaotong University, Xi'an, China
- 2School of Public Health, Institute of Maternal, Child and Adolescent Health, Lanzhou University, Lanzhou, China
- 3Inner Mongolia Yili Industrial Group Co., Ltd., Hohhot, China
- 4Inner Mongolia Dairy Technology Research Institute Co., Ltd., Hohhot, China
- 5National Center of Technology Innovation for Dairy, Hohhot, China
- 6Key Laboratory for Disease Prevention and Control and Health Promotion of Shaanxi Province, Xi'an, China
Introduction: Breastfeeding can reduce the risk of serious illness in preterm infants. However, the influence of human milk on the gut microecology and early development of preterm infants remains unclear.
Methods: The YI Study is a prospective cohort protocol conducted in China, designed to investigate the dynamic associations among breast milk composition, infant gut microecology, and health from a mother–breastmilk–preterm infant triad perspective. From January 2023 to May 2024, a total of 50 mother–term infant dyads and 35 mother–preterm infant dyads were enrolled and followed up at six timepoints: v1 (0–7 days), v2 (8–14 days), v3 (1 month), v4 (2 months), v5 (4 months), and v6 (6 months). Data collection included questionnaires, anthropometric measurements, and biospecimens. Questionnaires (including birth medical records, environment, feeding practices and illnesses status) and anthropometric measurements were collected at all visits. Biospecimens included paired samples of breast milk and feces were obtained at each visit, and comprehensively analyzed by multi-omics techniques. We also collected heel blood at birth to examine immune status and saliva at the v6 visit to explore the role of its constituents in dietary behaviors.
Results: The average age of the mothers was 30.9 ± 3.5 years. The median gestational age was 36 (35, 36) weeks in the Preterm Group and 39 (38, 40) weeks in the Term group. The completion rate up to V6 was 82.9% in the Preterm Group and 94% in the Term Group. All samples were collected within the predefined visit windows, with a total of 452 breast milk, 465 infant feces, 227 maternal feces, 49 heel blood and 98 saliva.
Discussion: Through ultra-early, multi-temporal, multi-sample collection, combined with multi-omics technologies, the YI study will provide an opportunity to explore the dynamic association of human milk as a complex biological system with gut microecology and health in preterm infants in depth.
1 Introduction
Rapid advances in medicine and intensive care technology in recent years have significantly improved the survival rate of preterm infants. However, the incidence of serious conditions in preterm infants remains notably high (1) and the factors affecting the growth and development of premature infants should be more widely explored.
Breastfeeding is essential to establish healthy gut microbiome (2) and reduce the risk of serious diseases (3–5). However, the breastfeeding rates among preterm infants in China remain low, with less than 30% being breastfed within 6 months of delivery (6–10). In addition, although breast milk is considered the “gold standard” of nutrition for healthy infants, it may not fully meet the specific nutritional needs of preterm infants (11). Premature birth may alter biological and hormonal signals, and prompt changes in breast milk composition and gut microbiome (12–14).
Existing cohorts of term infants have established extensive databases of breast milk (15–17). Increasing research has also been devoted to providing clues to the composition of breast milk and its association with the health of preterm infants. A meta-study systematically compared the essential fatty acid content of breastmilk in preterm infants across lactation periods with that of term mothers (18). A Spanish study found that Neuropilin-1 and kallikrein-6 in breast milk were higher in mothers with preterm infants and were associated with neural development (19). Additionally, the concentration of adipokines in breast milk of very preterm infants has been found to influence the length of infants, with MFG-E8 levels being lower in the breast milk of mothers whose infants developed delayed sepsis (4). Breast milk taken by preterm infants with necrotizing enterocolitis was found to have lower HMO concentrations, which also affected the development of gut microbiota in infants (20). Besides, feeding practices, mode of delivery and maternal diet have also been found to impact on the gut microbiome of preterm infants (21–23). However, simply analyzing the individual components of breast milk from preterm mothers is not sufficient to fully understand its complexity and its impact on health (24). Human milk needs to be studied as a biological system, especially for the special population of preterm infants. The physiological heterogeneity of the mother, the complex composition of the breast milk and the specific physiology of preterm infants are all part of a co-adaptive system, and changes in each system affect the development and health of the infant. Nevertheless, there is lack of preterm cohorts with a systems biology perspective to provide evidence for the specificities of the composition and microbiota of breast milk from preterm mothers and its impact on the gut microecology and health of preterm infants.
Therefore, this study aims to longitudinally investigate the associations within the mother-breastmilk-preterm infant triad, so as to provide a scientific basis for establishing a preterm infant breast milk database and promoting health research in preterm infants.
2 Methods and analysis
2.1 Study design
The YI study is a multicenter, prospective cohort study conducted in 4 tertiary hospitals in Xi’an and Lanzhou in China, including preterm and term mother-infant dyads, followed from birth to 6 months of age. The aims of the cohort study were (Figure 1):
1. To detect the content of nutrients and bioactive components of breast milk and their dynamic patterns, and explore their impact on the establishment of gut microbiota in preterm infants during the early stages of life;
2. To reveal the dynamic interactions between breast milk and gut microecology of preterm infants at multiple time points, and to identify the key factors of interactions through multi-omics techniques;
3. To study the immune status of preterm infants after birth by testing blood immune factors. To examine the health status, developmental level and disease risk of preterm infants by multi-omics analyses of saliva;
4. To study the effects of internal factors (immune status and gene) and external factors (birth mode, maternal diet, feeding practices, overweight and obesity, metabolic diseases, passive smoking and so on) on breast milk and infant gut microecology.
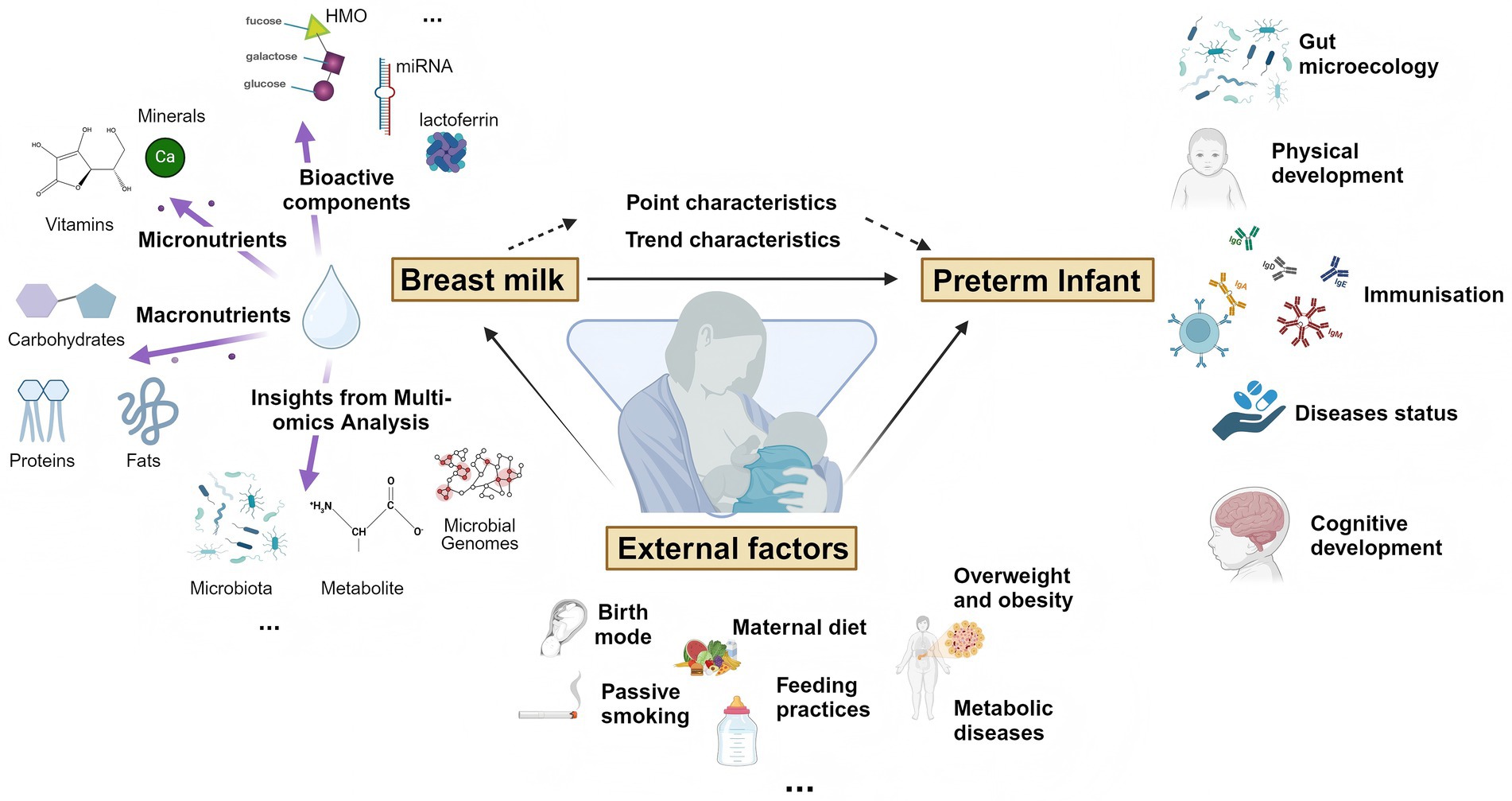
Figure 1. Visualization chart for YI study aims. Created with BioRender.com.
The study was approved by the Research Ethics Committee of Xi’an Jiaotong University (XJTU2022-1486) and registered with the Chinese Clinical Trial Registry (ChiCTR2200064473). All participants were informed of the study’s purpose, procedures, potential benefits and risks prior to enrollment, and written informed consents were obtained during the initial survey. This study was conducted in accordance with the principles in the Declaration of Helsinki.
2.2 Participant selection
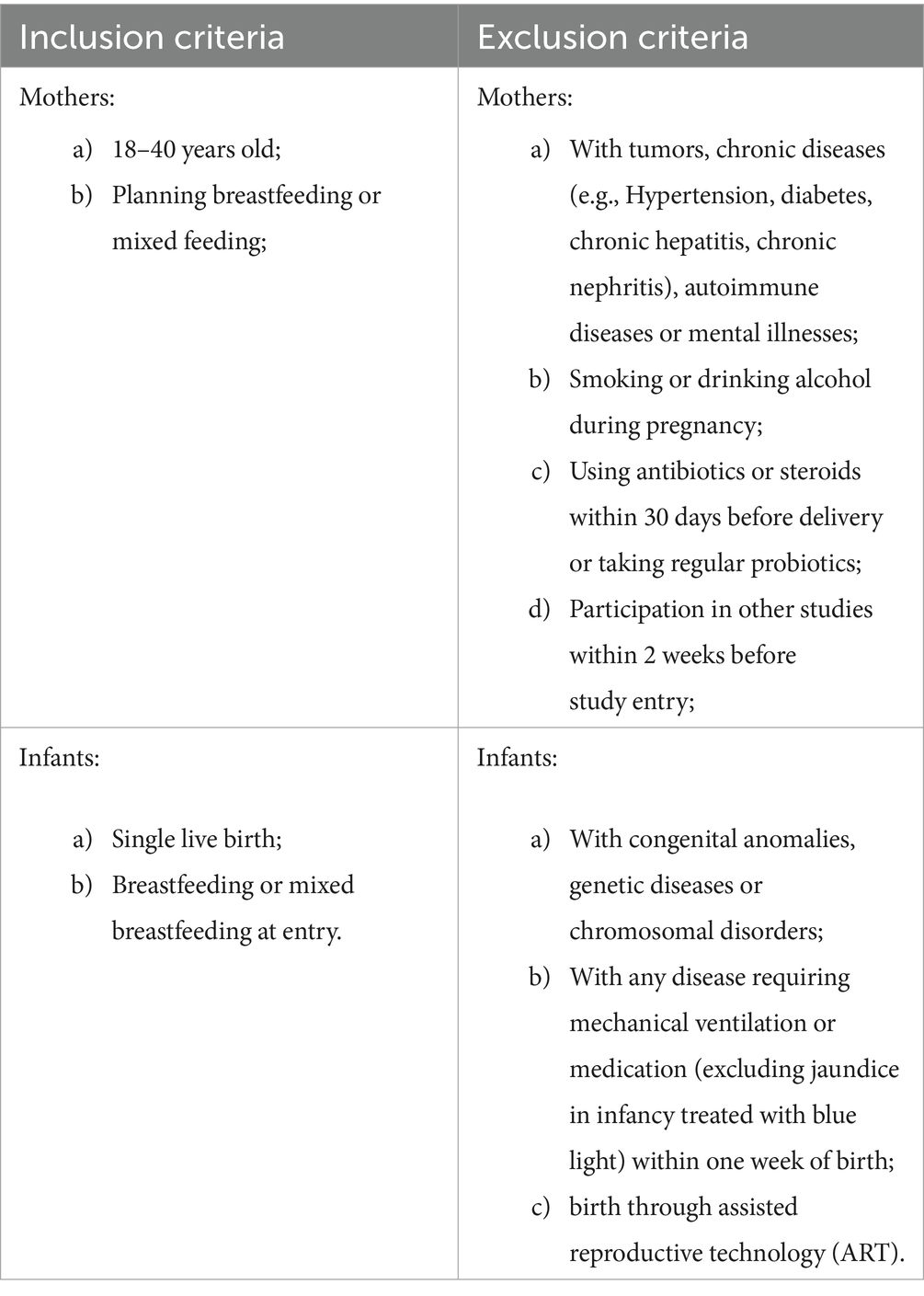
Mother-infant dyads were recruited from the four centers for antenatal care and delivery. Mothers were screened about 4 weeks before the expected date of delivery and infants were screened within 7 days of delivery, with only mother-infant dyads both fitting the criteria being included in the study. Based on gestational age, dyads were divided into Preterm Group (born at 32–36+6 weeks of gestational age) and Term Group (born at 37–41 weeks of gestational age) (see Table 1).
2.3 Follow up
All mother–infant dyads were followed up immediately after birth, with a total of six visits up to the six-month age of the infant: v1 (0–7 days), v2 (8–14 days), v3 (1 month±1 week), v4 (2 months±1 week), v5 (4 months±2 weeks), and v6 (6 months±2 weeks). The collection of biospecimens, questionnaires and anthropometric measurements was conducted following the theory of the mother-breastmilk-infant triad, as detailed in Figure 2.
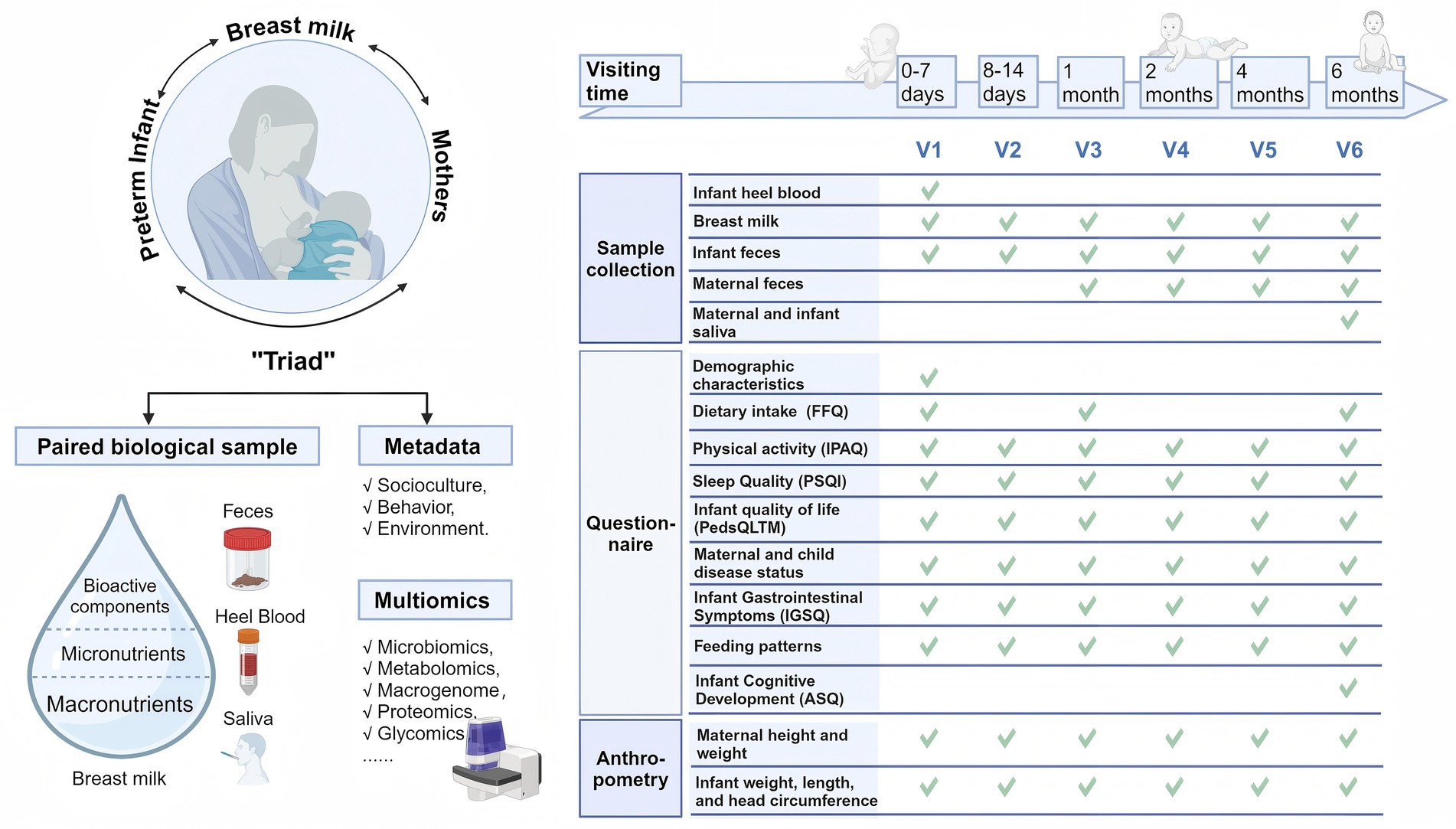
Figure 2. The principle for cohort establishment and time points for dyads data and sample collection. Created with BioRender.com.
2.4 Date collection
2.4.1 Questionnaires
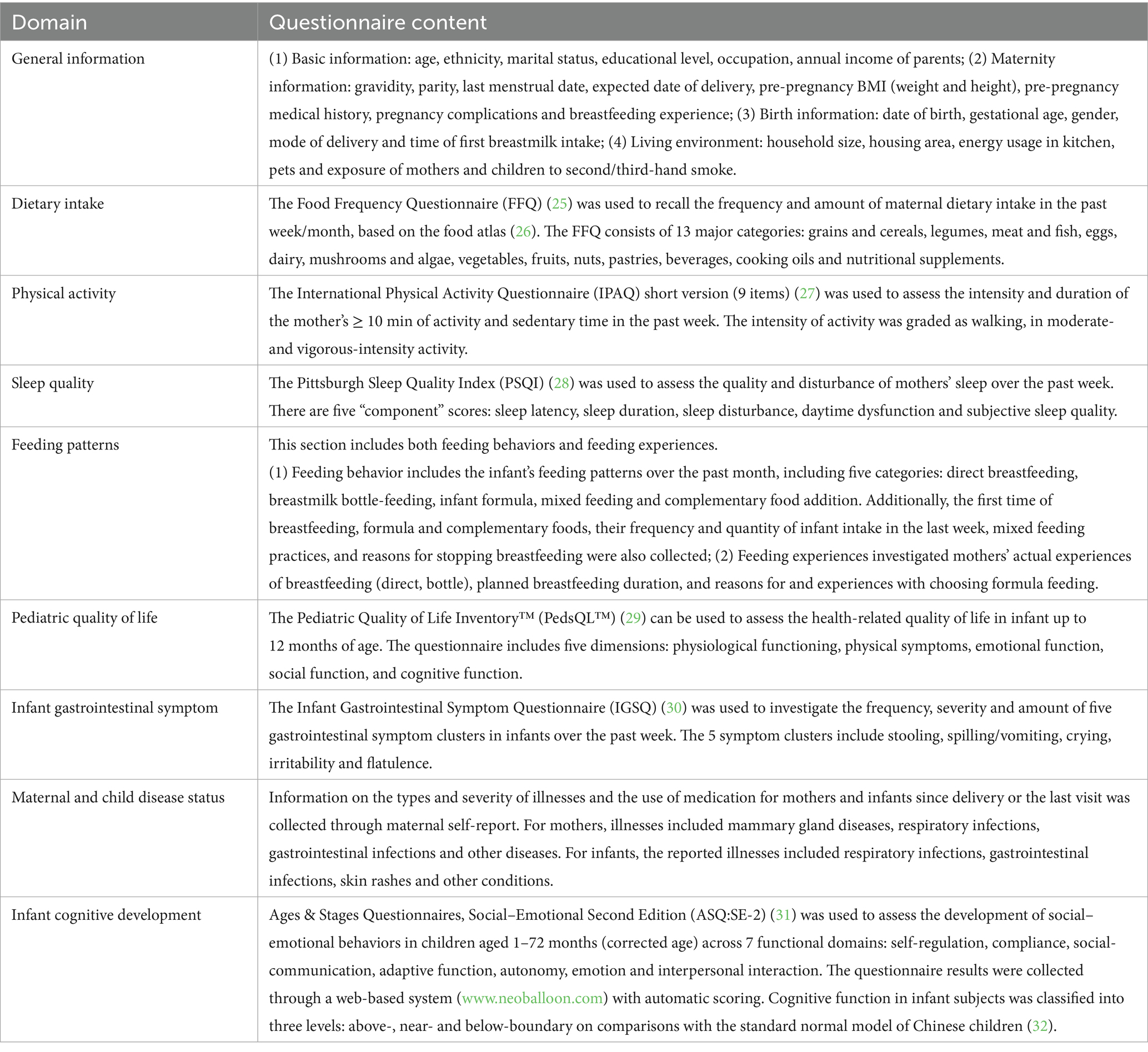
Information was collected at different time points using a set of validated questionnaires, which were administered through face-to-face interviews, as shown in Table 2.
2.4.2 Anthropometry
All measurements were carried out by two trained investigators. The anthropometry of the mother included height and weight. The weight of the mothers was measured statically with a calibrated weighing scale (Yiqing, Senssun, Zhongshan, China) without shoes and heavy clothing and recorded with an accuracy of 0.1 kg. The height of the mother was recorded in centimeters to an accuracy of 0.1 cm.
Infancy anthropometry included length, weight and head circumference. Length and weight were measured by the electronic infant scale (iR-Baby, Senssun, Zhongshan, China) with an accuracy of 0.1 cm and 5 g, respectively. The scale was placed horizontally on the floor and the child was placed on the scale in a supine position for length and weight readings. Head circumference was measured using a soft tape (Wentai, Foshan, China), positioned around the head at the midpoints of the two eyebrow arches and the occipital tuberosity (the largest protuberance) with an accuracy of 0.1 cm.
2.4.3 Biospecimens
Heel blood of infants at birth, breast milk (all visits), feces (all visits) and saliva (at the v6 visit) of mothers and infants were collected. All samples were transported at −20° after sampling and transported back to the central laboratory within 10 h for storage at −80°C.
2.4.3.1 Heel blood
Under aseptic conditions, the clinical researchers placed the neonate in the supine position, and stabilized and engorged the infant’s plantar puncture site. After skin disinfection, a sterile needle was used to puncture the plantar foot swiftly in either a straight or oblique puncture. A drop of blood was deposited onto an FTA card, with a total volume of 2–3 mL. After the dried blood spot was made, placed the FTA card immediately into a sterile EP tube and stored at room temperature.
2.4.3.2 Human milk
The collections were all conducted between 9 a.m. and 11 a.m. The sampled breast was required to be neither breastfed nor pumped for at least 2 h before sampling, and no skin care products, cosmetics, or other reagents were applied for 24 h before collection. The breast milk on the sampled side was required to be completely excreted and mixed thoroughly in a sterile breastmilk bottle, and then taken in volumes of 10 mL (0–14 days) or 40–50 mL (≥1 month) transferred to 1 mL, 2 mL, 5 mL, and 10 mL tubes, respectively.
2.4.3.3 Infant and maternal fecal sample collection
Infant fecal samples were collected by scraping feces from the central part of the stool that had not touched the diaper with moderate hardness. Samples were stored in collection boxes filled to approximately 1/3 to 2/3 of their volume.
Maternal feces were self-collected by participants following standardized procedures. Prior to sampling, participants were instructed to urinate, flush the toilet, and place the plastic bidet-style stool collection box in a clean toilet seat. Participants used clean spoons to scrape feces from the middle and inside of feces that had not touched the toilet or external surfaces with moderate consistency. A volume equivalent to 1/3 to 2/3 of the collection cassette was retained.
Additionally, an extra portion of fecal samples was collected into microplastic-free storage tubes at each sampling event. Both infant and maternal fecal samples were collected using disposable stainless steel spoons and stored in stainless steel collection tubes. The scraping position and procedure adhered to the standard sample collection protocol.
2.4.3.4 Infant and maternal saliva collection
Infant saliva was collected by researchers wearing disposable medical gloves when infants were emotionally stable and had not been fed for 2 h.
A sterile cotton swab was gently rubbed along the inside of the infant’s mouth for 2–3 min. Once at least 1/3 of the swab was saturated, store it in a collection tube with a tight-fitting lid.
Maternal saliva was self-collected by participants after fasting for at least 30 min and rinsing with water before collection. A sterile cotton swab was placed in the mouth for 2–3 min (without chewing) and stored in the collection tube with the lid closed after at least 1/3 of the swab was soaked.
2.5 Quality control
All investigators were trained in Good Clinical Practice (GCP) guidelines and were guided by a well-established program survey operations manual. All on-site processes of the questionnaires and samples were conducted with the assistance and supervision of the investigators. The collection and administration of questionnaires and biological samples for each subject were also the responsibility of the designated investigator (assigned by the center director).
Within 1 week after the end of the survey, all paper-based information was digitized using the research electronic data capture (REDCap (33)) tools at the School of Health, Xi’an Jiaotong University, and entered by the researcher in charge of the on-site survey. The REDCap system provides the first line of quality defense for the consistency, completeness, and logicality of the research data, and ensures data security. System permissions restricted investigators to access only the questionnaires they entered, whereas the center manager had access to all questionnaires. The center manager provides the second line of defense for the integrity and logic of the data. Logical corroboration was performed both within individual questionnaires and across different questionnaires for the same subject. Paper-based records (questionnaires and sampling information forms) were uniformly preserved by the center manager and sealed after verification and entry. All recorded information remained traceable to the original documents.
2.6 Laboratory test
Dried blood spot samples were analyzed using the OLINK Target 96 Inflammation panel, which analyzed the levels of 92 proteins in blood samples primarily associated with immune and inflammatory biological processes, based on proximity extension assay (PEA) technology.
Micronutrients in breast milk: vitamins were analyzed by liquid chromatography-mass spectrometry (LC–MS) and minerals by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Lactose in breast milk was analyzed by high-performance liquid chromatography with refractive index detection (HPLC-RID). Total protein was examined by Kjeldahl method; proteins were analyzed by LC–MS method targeting specific peptide fragments; and 16 amino acids were examined by automatic amino acid analysis (AAA), which is based on ion exchange chromatography coupled with ninhydrin chromogenic reaction. The lipids in breast milk, including fatty acids, phospholipids, glycolipids and structural lipids, were examined. Fatty acids were detected by gas chromatography with flame ionisation detection (GC-FID); phospholipids were measured by high-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD); glycolipids were tested by liquid chromatography–tandem mass spectrometry (LC–MS/MS); structural lipids were measured by silver ion normal-phase liquid chromatography with evaporative light scattering detection (Ag+-NP-LC-ELSD). The HMOs in breast milk were measured by liquid chromatography-high resolution mass spectrometry (LC-HRMS). Osmolality in breast milk was analyzed using freezing point osmometry (FPO).
The collected breast milk, fecal and saliva samples were analyzed in a multi-omics approach. 16S rRNA gene sequencing involves amplifying the hypervariable V3-V4 region of the 16S rRNA gene using polymerase chain reaction (PCR) and sequencing the amplicons with high-throughput sequencing platforms, to detect the diversity, abundance and taxonomic composition of microbiota. Metagenomic analysis involves random fragmentation of all microbial genomic DNA in the sample, followed by sequencing using high-throughput platforms, to analyze composition, functional genes and metabolic potential of the microbial community. Using untargeted metabolomics, LC–MS/MS was employed to analyze polar and non-polar metabolites, while gas chromatography-tandem mass spectrometry (GC–MS) was used for volatile and low molecular weight metabolites, to discover potential biomarkers and elucidates metabolic pathway alterations.
3 Result
From January 2023 to May 2024, a total of 50 mother-infant dyads of term infants and 35 mother-infant dyads of preterm infants were finally enrolled according to the inclusion and exclusion criteria (Figure 3). In the Preterm Infant Group, one mother was unable to attend the v2 visit due to work obligations; six mother-infant dyads dropped out between v3 and v6, giving a completion rate of 82.9% at v6. In the Term Infant Group, the completion rate remained 100% until v3, after which three mother-infant dyads withdrew, with a final completion rate of 94% at v6. All samples were collected within the predefined visit windows, with a total of 452 breast milk samples, 465 infant fecal samples, 227 maternal fecal samples, 49 heel blood samples and 98 saliva samples.
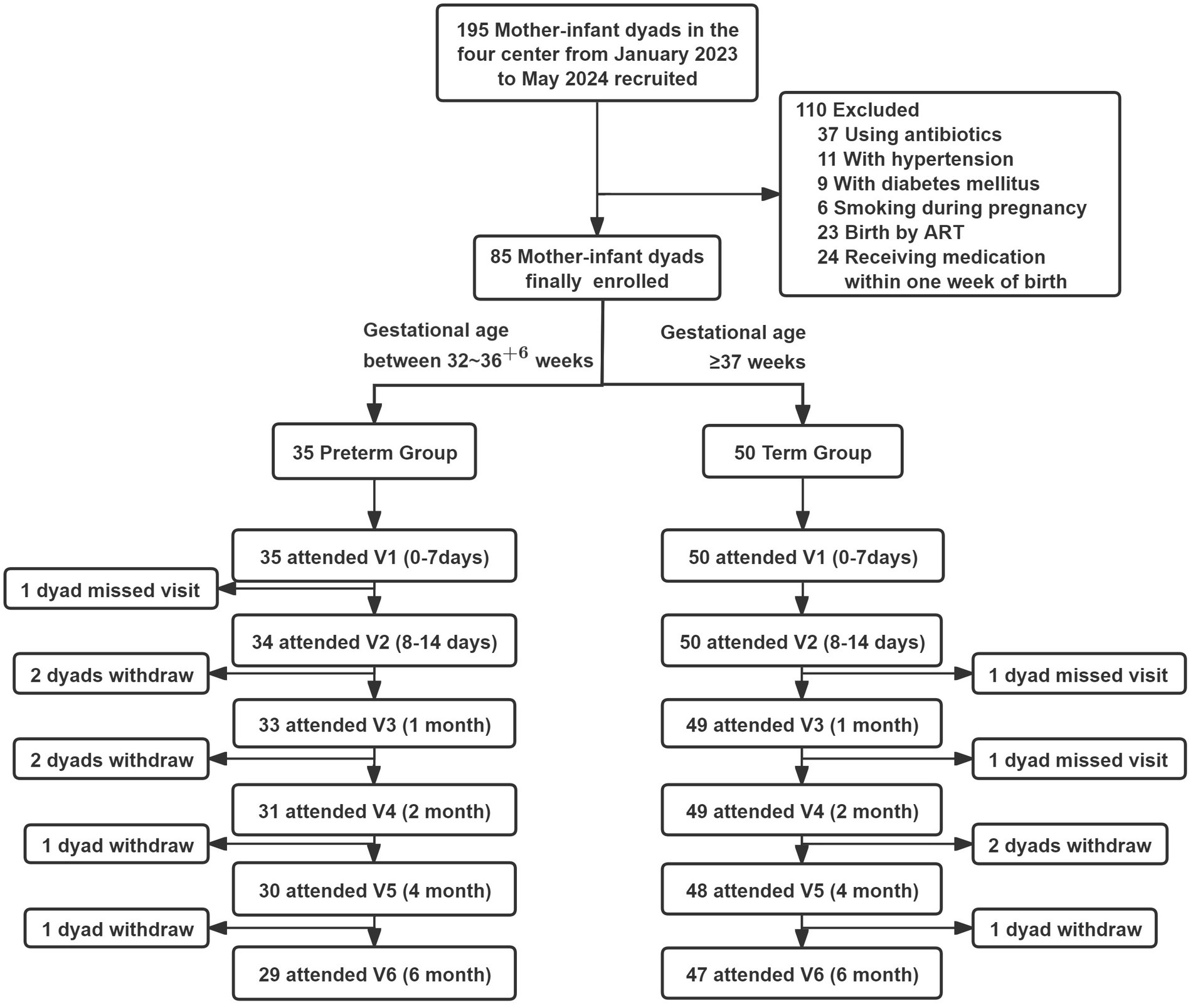
Figure 3. Flow chart of participants missed visit, defined as a follow-up visit that was skipped due to the participants left the place of the follow-up visit, but still attended the subsequent follow-up visits. Withdraw, means that participants completely quit the study.
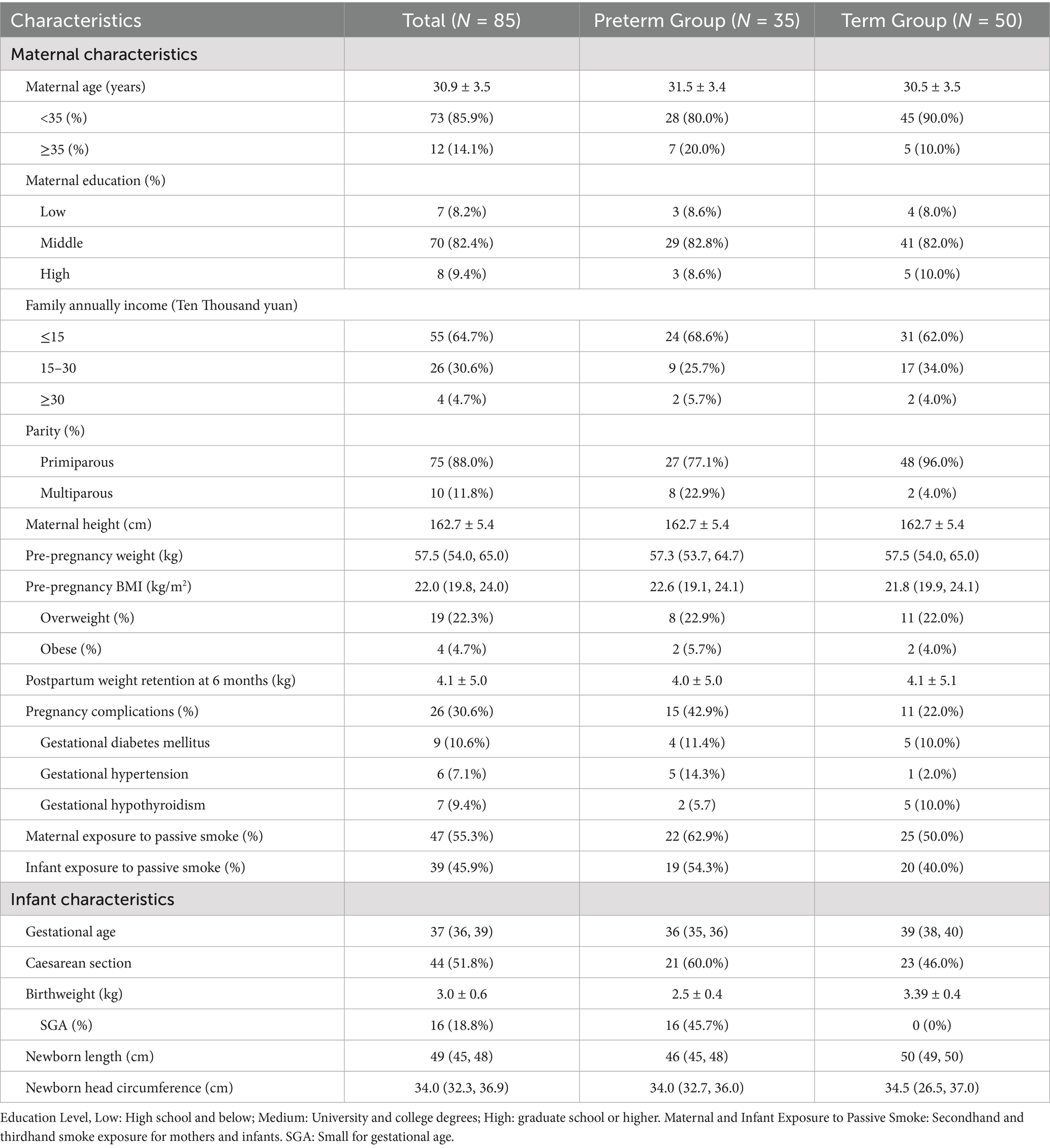
Baseline characteristics of mothers and infants in the cohort are presented in Table 3. The average age of the mothers was 30.9 ± 3.5. In the Preterm Group, the proportions of advanced maternal age (20%), multiparous women (22.9%), pregnancy complications (42.9%), and passive smoking exposure in both infants and mothers were higher than those in the Term Group. The median gestational age was 36 (35, 36) weeks in the Preterm Group and 39 (38, 40) weeks in the Term group. In the Preterm Group, 60% were delivered by cesarean section and 45.7% were small for gestational age (SGA).
4 Discussion
To the best of our knowledge, the YI study is the first prospective multimodal cohort study dedicated to investigating the associations between the complex components of breastmilk, gut microecology, and the health of preterm infants within the systems biology framework of the mother-breastmilk-preterm infant triad. By integrating data from multiple levels and time points, the YI study aims to comprehensively elucidate the critical role of breast milk in establishing gut microecology and promoting the health of preterm infants during the early stages of life, which provides the possibility of developing intervention strategies for this vulnerable population.
Breast milk establishes an efficient pathway for delivering nutrients and biologically active components, facilitating the vertical transmission of specific microbiome to infants (34–36). Importantly, breastmilk is not an isolated entity; its components are influenced by internal factors (lactating parent) and external factors (socioeconomic, cultural, behavioral and environmental contexts), all embedded within the mother-breastmilk-infant triad (37). From a systems biology perspective, the YI study cohort collected comprehensive metadata at multiple time points encompassing maternal demographic characteristics, environmental exposures, lifestyle factors (diet, sleep, exercise) and feeding practices. Paired biological samples were also systematically collected and the growth, development and health of infants have been tracked. By combining genomics, metabolomics, proteomics, microbiomics and other multi-omics technologies, multidimensional explorations of the complex interactions between breast milk and infant gut microecology can be realized in this cohort. The study design of YI study allows investigation of the effects of external factors on the triad, and the identification of bioactive breastmilk components that regulate preterm infant health. Notably, several critical areas remain underexplored in preterm infants, such as the association between bioactive components or microbiota in breast milk and the establishment of gut microecology, as well as the link between maternal nutrition and physiological status with breast milk characteristics and infant growth phenotypes, which could all be explored under this study design. The integration of diverse data types holds the potential to overcome the limitations of traditional research.
In addition, comprehensive and longitudinal data were incorporated in the study through ultra-early and multi-temporal follow-ups, covering colostrum, transitional milk, and various stages of maturation. Colostrum is the first colonizer of the infant intestine (34) and contains high concentrations of HMOs, secretory immunoglobulin A (IgA), lactoferrin, lysozyme and microRNA (miRNA) (38–40), which play critical roles in supporting gut microecology and immune system development (41–44). Compared to colostrum from term infants, that from preterm mothers has been reported to contain higher levels of immune components to address physiological immaturity (45, 46); however, these findings remain inconsistent (47) and lack evidence from Chinese populations. Studies have shown substantial microbial sharing between maternal breast milk and newborns, including the vertical transmission of Bifidobacterium bifidum (48). Nonetheless, these studies have focused on term infants and lacked systematic data on colostrum and transitional milk. The YI study addressed these gaps by providing longitudinal evidence on preterm infant health, offering dynamic data and associations across developmental stages. All samples and data have been meticulously recorded following standardized workflows and formats. Currently, analyses of lipids, proteins, amino acids and HMOs in breast milk, as well as microbiome and metabolome analyses of breast milk and feces have been completed, with results expected to be available next year.
There are several limitations. Firstly, medical interventions for preterm infants during hospitalization may influence the study purpose. We recorded the illnesses and medications of the mother-infant dyads both during hospitalization and after discharge, allowing for the application of complex statistical models to control for potential confounders and mitigate their effects. Second, the specificity of the study subjects led to a relatively small sample size, which impacted the generalizability and statistical power of the findings. Nevertheless, the high compliance of this study (>80%) enhances the quality of the data, partially offsetting the limitations associated with sample size. Third, the follow-up duration was relatively short, extending only to 6 months of age, compared to other long-term birth cohort studies such as the Shanghai Birth Cohort (SBC) and the Finnish Health and Early Life Microbiota (HELMi) cohort (49–51). However, the homogeneity of infant food sources during the first 6 months of life facilitates the exploration of the cohort’s primary purposes. Beyond 6 months, the introduction of diverse complementary foods introduces additional confounding factors that may complicate the analysis. Finally, all participants in this study were recruited from Xi’an and Lanzhou, which may limit the representativeness of the findings for preterm mother-infant dyads across broader geographic and demographic contexts.
5 Conclusion
This protocol outlines a longitudinal dynamic cohort of the mother-breastmilk-preterm infant triad. Through ultra-early, multi-temporal, multi-sample collection, combined with multi-omics analyses, the YI study will provide an opportunity to explore the dynamic association of breast milk as a complex biological system with gut microecology and health in preterm infants in depth.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Xi’an Jiaotong University (XJTU2022-1486). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XW: Data curation, Project administration, Writing – original draft. YF: Data curation, Investigation, Project administration, Writing – original draft. YL: Data curation, Methodology, Writing – review & editing. YT: Data curation, Methodology, Writing – review & editing. SZ: Investigation, Supervision, Writing – review & editing. JL: Investigation, Supervision, Writing – review & editing. JZha: Investigation, Supervision, Writing – review & editing. FL: Data curation, Methodology, Writing – review & editing. JZho: Data curation, Methodology, Writing – review & editing. TL: Project administration, Resources, Writing – review & editing. SD: Project administration, Resources, Writing – review & editing. IS: Project administration, Resources, Writing – review & editing. LS: Project administration, Supervision, Writing – review & editing. XL: Project administration, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Huhhot Science & Technology Plan (No. 2021-National Center of Technology innovation for Dairy-4).
Acknowledgments
We thank the personnel of all hospitals in Xi’an and Lanzhou for the help of recruiting subjects. In addition, we thank all participants for their contributions to our study and Yili for supporting the conduct of our cohort!
Conflict of interest
TL, SD, and IS were employed by Inner Mongolia Yili Industrial Group Co., Ltd. TL was employed by Inner Mongolia Dairy Technology Research Institute Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Healy, DB, Ryan, CA, Ross, RP, Stanton, C, and Dempsey, EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. (2021) 7:22–33. doi: 10.1038/s41564-021-01025-4
2. Escalante, KS, Bai-Tong, SS, Allard, SM, Ecklu-Mensah, G, Sanchez, C, Song, SJ, et al. The impact of breastfeeding on the preterm infant’s microbiome and metabolome: a pilot study. Pediatr Res. (2025) 97:1227–1236. doi: 10.1038/s41390-024-03440-9
3. Miller, J, Tonkin, E, Damarell, RA, McPhee, AJ, Suganuma, M, Suganuma, H, et al. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients. (2018) 10:707. doi: 10.3390/nu10060707
4. Balcells-Esponera, C, Borràs-Novell, C, López-Abad, M, Serra, IC, Puig, AB, Renau, MI, et al. Bioactive peptides in preterm human milk: impact of maternal characteristics and their association to neonatal outcomes. Biofactors. (2023) 50:135–44. doi: 10.1002/biof.1997
5. Altobelli, E, Angeletti, PM, Verrotti, A, and Petrocelli, R. The impact of human Milk on necrotizing enterocolitis: A systematic review and Meta-analysis. Nutrients. (2020) 12:1322. doi: 10.3390/nu12051322
6. CDRF. Report on National Survey into factors influencing breastfeeding. (2019). Available at: https://cdrf-en.cdrf.org.cn/jjhdt/5273.htm (Accessed September 9, 2024).
7. UNICEF. The state of the world’s children 2019 statistical tables (UNICEF data). (2019) Available at: https://data.unicef.org/resources/dataset/sowc-2019-statistical-tables/ (Accessed September 9, 2024).
8. Jiang, X, and Jiang, H. Factors associated with post NICU discharge exclusive breastfeeding rate and duration amongst first time mothers of preterm infants in Shanghai: a longitudinal cohort study. Int Breastfeed J. (2022) 17:34. doi: 10.1186/s13006-022-00472-x
9. Wang, Y, Briere, C-E, Xu, W, and Cong, X. Factors affecting breastfeeding outcomes at six months in preterm infants. J Hum Lact. (2018) 35:80–9. doi: 10.1177/0890334418771307
10. Peng, W, Jiang, S, Li, S, Xia, S, Chen, S, Yang, Y, et al. Human milk feeding status of preterm infants in neonatal intensive care units in China. J Hum Lact. (2020) 36:283–90. doi: 10.1177/0890334419901265
11. Arslanoglu, S, Boquien, C-Y, King, C, Lamireau, D, Tonetto, P, Barnett, D, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) working group on human milk fortification. Front Pediatr. (2019) 7:76. doi: 10.3389/fped.2019.00076
12. Renzo, GCD, Tosto, V, and Giardina, I. The biological basis and prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. (2018) 52:13–22. doi: 10.1016/j.bpobgyn.2018.01.022
13. Krebs, NF, Belfort, MB, Meier, PP, Mennella, JA, O'Connor, DL, Taylor, SN, et al. Infant factors that impact the ecology of human milk secretion and composition—a report from “breastmilk ecology: genesis of infant nutrition (BEGIN)” working group 3. Am J Clin Nutr. (2023) 117:S43–60. doi: 10.1016/j.ajcnut.2023.01.021
14. Asbury, MR, Butcher, J, Copeland, JK, Unger, S, Bando, N, Comelli, EM, et al. Mothers of preterm infants have individualized breast milk microbiota that changes temporally based on maternal characteristics. Cell Host Microbe. (2020) 28:669–682.e4. doi: 10.1016/j.chom.2020.08.001
15. Prentice, P, Acerini, CL, Eleftheriou, A, Hughes, IA, Ong, KK, and Dunger, DB. Cohort profile: the Cambridge baby growth study (CBGS). Int J Epidemiol. (2015) 45:35–35g. doi: 10.1093/ije/dyv318
16. Wu, J, Gan, J, Zeng, G, Luo, X, Yang, N, Zhang, Z, et al. Investigation of human Milk as a biological system in a multicenter mother–infant cohort: protocol design and cohort profile of the Phoenix study. Nutrients. (2024) 16:2892. doi: 10.3390/nu16172892
17. García-Mantrana, I, Alcántara, C, Selma-Royo, M, Boix-Amorós, A, Dzidic, M, Gimeno-Alcañiz, J, et al. MAMI: a birth cohort focused on maternal-infant microbiota during early life. BMC Pediatr. (2019) 19:140. doi: 10.1186/s12887-019-1502-y
18. Floris, LM, Stahl, B, Abrahamse-Berkeveld, M, and Teller, IC. Human milk fatty acid profile across lactational stages after term and preterm delivery: A pooled data analysis. Prostaglandins Leukot Essent Fat Acids. (2020) 156:102023. doi: 10.1016/j.plefa.2019.102023
19. Freiría-Martínez, L, Iglesias-Martínez-Almeida, M, Rodríguez-Jamardo, C, Rivera-Baltanás, T, Comís-Tuche, M, Rodrígues-Amorím, D, et al. Proteomic analysis of exosomes derived from human mature milk and colostrum of mothers with term, late preterm, or very preterm delivery. Anal Methods. (2023) 15:4905–17. doi: 10.1039/D3AY01114C
20. Masi, AC, Embleton, ND, Lamb, CA, Young, G, Granger, CL, Najera, J, et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. (2020) 70:2273–82. doi: 10.1136/gutjnl-2020-322771
21. Matharu, D, Ponsero, AJ, Lengyel, M, Meszaros-Matwiejuk, A, Kolho, K-L, de Vos, WM, et al. Human milk oligosaccharide composition is affected by season and parity and associates with infant gut microbiota in a birth mode dependent manner in a Finnish birth cohort. EBioMedicine. (2024) 104:105182. doi: 10.1016/j.ebiom.2024.105182
22. Engevik, MA, Stripe, LK, Baatz, JE, Wagner, CL, and Chetta, KE. Identifying single-strain growth patterns of human gut microbes in response to preterm human milk and formula. Food Funct. (2022) 13:5571–89. doi: 10.1039/D2FO00447J
23. Embleton, ND, Sproat, T, Uthaya, S, Young, GR, Garg, S, Vasu, V, et al. Effect of an exclusive human milk diet on the gut microbiome in preterm infants. JAMA Netw Open. (2023) 6:e231165. doi: 10.1001/jamanetworkopen.2023.1165
24. Donovan, SM, Aghaeepour, N, Andres, A, Azad, MB, Becker, M, Carlson, SE, et al. Evidence for human milk as a biological system and recommendations for study design—a report from “breastmilk ecology: genesis of infant nutrition (BEGIN)” working group 4. Am J Clin Nutr. (2023) 117:S61–86. doi: 10.1016/j.ajcnut.2022.12.021
25. Cui, Q, Xia, Y, Wu, Q, Chang, Q, Niu, K, and Zhao, Y. Validity of the food frequency questionnaire for adults in nutritional epidemiological studies: A systematic review and meta-analysis. Critical reviews in N.A science and. Nutrition. (2021) 63:1670–88. doi: 10.1080/10408398.2021.1966737
26. Ding, Y, Yang, Y, Li, F, Shao, Y, Sun, Z, Zhong, C, et al. Development and validation of a photographic atlas of food portions for accurate quantification of dietary intakes in China. J Hum Nutr Diet. (2021) 34:604–15. doi: 10.1111/jhn.12844
27. CL, C, AL, M, Sjöström, M, AE, B, ML, B, BE, A, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
28. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
29. Varni, JW, Limbers, CA, Neighbors, K, Schulz, K, Lieu, JEC, Heffer, RW, et al. The peds QL™ infant scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. (2010) 20:45–55. doi: 10.1007/s11136-010-9730-5
30. Riley, AW, Trabulsi, J, Yao, M, Bevans, KB, and DeRusso, PA. Validation of a parent report questionnaire. Clin Pediatr (Phila). (2015) 54:1167–74. doi: 10.1177/0009922815574075
31. Squires, J, Bricker, D, and Twombly, E. Ages & stages questionnaires: social-emotional, second edition (ASQ: SE-2). Baltimore: Paul H. Brookes Publishing Co., Inc. (2015).
32. Huichao, X, Nicolette, W, Xiaoyan, B, Ruoshui, W, Chieh-Yu, C, Luis, A, et al. Validity studies of a parent-completed social-emotional measure in a representative sample in China. Appl Dev Sci. (2022) 26:689–703. doi: 10.1080/10888691.2021.1977642
33. Yan, M, Zhao, P, Wu, L, Xu, K, Yan, H, Zeng, L, et al. Method of double data entry and quality control by REDCap system. Zhonghua Liu Xing Bing Xue Za Zhi. (2021) 42:918–922. doi: 10.3760/cma.j.cn112338-20200415-00574
34. Milani, C, Duranti, S, Bottacini, F, Casey, E, Turroni, F, Mahony, J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. (2017) 81:e00036-17. doi: 10.1128/MMBR.00036-17
35. Davis, MY, Zhang, H, Brannan, LE, Carman, RJ, and Boone, JH. Rapid change of fecal microbiome and disappearance of Clostridium difficile in a colonized infant after transition from breast milk to cow milk. Microbiome. (2016) 4:53. doi: 10.1186/s40168-016-0198-6
36. Pacheco, AR, Barile, D, Underwood, MA, and Mills, DA. The impact of the Milk Glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci. (2015) 3:419–45. doi: 10.1146/annurev-animal-022114-111112
37. Bode, L, Raman, AS, Murch, SH, Rollins, NC, and Gordon, JI. Understanding the mother-breastmilk-infant “triad.”. Science. (2020) 367:1070–2. doi: 10.1126/science.aaw6147
38. Lu, Y, Liu, J, Jia, Y, Yang, Y, Chen, Q, Sun, L, et al. Mass spectrometry analysis of changes in human Milk N/O-Glycopatterns at different lactation stages. J Agric Food Chem. (2019) 67:10702–12. doi: 10.1021/acs.jafc.9b02034
39. Eker, F, Akdaşçi, E, Duman, H, Yalçıntaş, YM, Canbolat, AA, Kalkan, AE, et al. Antimicrobial properties of colostrum and milk. Antibiotics. (2024) 13:251. doi: 10.3390/antibiotics13030251
40. Li, M, Chen, J, Shen, X, Abdlla, R, Liu, L, Yue, X, et al. Metabolomics-based comparative study of breast colostrum and mature breast milk. Food Chem. (2022) 384:132491. doi: 10.1016/j.foodchem.2022.132491
41. Schirmbeck, GH, Sizonenko, S, and Sanches, EF. Neuroprotective role of Lactoferrin during early brain development and injury through lifespan. Nutrients. (2022) 14:2923. doi: 10.3390/nu14142923
42. Lewis, ZT, Totten, SM, Smilowitz, JT, Popovic, M, Parker, E, Lemay, DG, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. (2015) 3:13. doi: 10.1186/s40168-015-0071-z
43. Lodge, CJ, Lowe, AJ, Milanzi, E, Bowatte, G, Abramson, MJ, Tsimiklis, H, et al. Human milk oligosaccharide profiles and allergic disease up to 18 years. J Allergy Clin Immunol. (2021) 147:1041–8. doi: 10.1016/j.jaci.2020.06.027
44. Mantis, NJ, Rol, N, and Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. (2011) 4:603–11. doi: 10.1038/mi.2011.41
45. Kristensen-Cabrera, AI, Sherman, JP, and Lee, HC. A prospective clinical study of primo-lacto: A closed system for colostrum collection. PLoS One. (2018) 13:e0206854. doi: 10.1371/journal.pone.0206854
46. Garofoli, F, Civardi, E, Pisoni, C, Angelini, M, and Ghirardello, S. Anti-inflammatory and anti-allergic properties of colostrum from mothers of full-term and preterm babies: the importance of maternal lactation in the first days. Nutrients. (2023) 15:4249. doi: 10.3390/nu15194249
47. Castellote, C, Casillas, R, Ramírez-Santana, C, Pérez-Cano, FJ, Castell, M, Moretones, MG, et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human Milk. J Nutr. (2011) 141:1181–7. doi: 10.3945/jn.110.133652
48. Duranti, S, Lugli, GA, Mancabelli, L, Armanini, F, Turroni, F, James, K, et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. (2017) 5:66. doi: 10.1186/s40168-017-0282-6
49. Li, L, Li, K, Zhou, X, and Knowles, RL. Maximising the potential of Chinese birth cohort studies: a systematic review of mother–baby cohorts in mainland China. Public Health. (2024) 227:119–30. doi: 10.1016/j.puhe.2023.11.035
50. Zhang, J, Tian, Y, Wang, W, Ouyang, F, Xu, J, Yu, X, et al. Cohort profile: the Shanghai birth cohort. Int J Epidemiol. (2019) 48:21–21g. doi: 10.1093/ije/dyy277
Keywords: preterm infants, human milk, gut microecology, early life health, systems biology
Citation: Wang X, Feng Y, Li Y, Tian Y, Zhang S, Li J, Zhang J, Liu F, Zhou J, Li T, Duan S, Szeto IM-Y, Su L and Luo X (2025) The influence of human milk composition and its microbiome on the gut microecology and early growth and development of preterm infants (the YI study): protocol design and cohort profile. Front. Nutr. 12:1566376. doi: 10.3389/fnut.2025.1566376
Edited by:
Francisco José Pérez-Cano, University of Barcelona, SpainReviewed by:
Ana Griselda Binetti, CONICET Instituto de Lactología Industrial (INLAIN), ArgentinaMiljana Z. Jovandaric, University of Belgrade, Serbia
Copyright © 2025 Wang, Feng, Li, Tian, Zhang, Li, Zhang, Liu, Zhou, Li, Duan, Szeto, Su and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqin Luo, bHVveGlhb3FpbjIwMTJAbWFpbC54anR1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xinyue Wang
Xinyue Wang Yan Feng
Yan Feng Yilan Li
Yilan Li Yuluyuan Tian
Yuluyuan Tian Simin Zhang
Simin Zhang Jinglin Li
Jinglin Li Jiahui Zhang1
Jiahui Zhang1 Feng Liu
Feng Liu Jiahe Zhou
Jiahe Zhou Ting Li
Ting Li Sufang Duan
Sufang Duan Xiaoqin Luo
Xiaoqin Luo