- Department of Nutrition and Food Science, University of Maryland, College Park, MD, United States
The intricate relationship between nutrition, the gut microbiome, and brain development has garnered significant attention in recent years, concerning its implications for child behavior and cognitive function. The gut-brain axis mediates this relationship through microbial modulation of inflammation, neuroactive compounds, and blood–brain barrier integrity, particularly during prenatal and early postnatal periods. Healthy dietary patterns such as whole foods, high-fiber foods, and minimally processed foods play a crucial role in shaping the gut microbiota, promoting microbial diversity and overall gut health. As a result, a balanced and diverse microbiome supports healthy brain function and development. Furthermore, disruptions in gut microbiota composition have been linked to various neurodevelopmental disorders in children, including autism spectrum disorder, attention deficit hyperactivity disorder, and anxiety. By integrating findings from animal models, clinical trials, and epidemiological studies, this review summarizes current advances on how early-life nutrition and gut microbiota interaction influence brain development and childhood behaviors. Ultimately, this paper underscores the potential for dietary interventions to promote optimal neurodevelopmental health and address behavioral issues in children.
1 Introduction
Early childhood, particularly the first 1,000 days, is marked by rapid growth and developmental milestones, making it a foundational phase that significantly influences cognitive, emotional, and physical well-being throughout life (1). This stage is also crucial for addressing the risk of neuropsychiatric conditions (2). During this timeframe, the brain experiences exponential growth in volume and the formation of neuronal pathways. Brain volume doubles in the first year and grows an additional 15% in the second year, reaching approximately 80% of its adult volume. Both white and gray matter volumes increase gradually over time (2). By using in vivo magnetic resonance spectroscopy, researchers studied the developmental profiles of six metabolites in five brain regions (3). They found that concentrations of N-acetyl-aspartate, creatine, and glutamate increased rapidly from birth to 3 months, coinciding with the rapid growth of axons and the formation of synapses. Notably, even in the first three months, before the introduction of solid foods, infants receive essential nutrients exclusively through breast milk or formula, both of which play a crucial role in shaping early brain development (1). This highlights the first 3 months of life as a critical period of rapid metabolite changes in brain development.
Neurodevelopment is crucial for long-term health and is significantly influenced by early-life experiences. During this stage, much of brain development is primarily guided by genetic programming (4). Also, emerging evidence highlights the role of epigenetic mechanisms, heritable changes in gene expression that do not alter the DNA sequence, in mediating the effects of environmental factors such as nutrition, stress, and microbial exposure. The epigenetic mechanism enables the reprogramming of the epigenome in response to external stimuli (5). Nutrition during early childhood is particularly crucial for providing the necessary nutrients to support healthy brain growth and development. It acts as a major epigenetic regulator (6). In utero development from conception is also crucial for the formation of the fetal central nervous system (CNS). It is essential to prioritize nutrients that can enhance neural health and brain development during early developmental stages, including macro-and micronutrients like calories, protein, fatty acids, iron, zinc, iodine, and choline for overall brain function (6). Conversely, malnutrition or poor dietary practices during these critical stages may lead to developmental delays, cognitive impairments, and an increased risk of chronic diseases later in life (6).
In utero, maternal nutrition plays an important role in supporting fetal development and influencing long-term health outcomes in the offspring, as key processes like neural tube formation and neurogenesis occur in utero and are sensitive to nutrient deficiencies. Inadequate intake of certain key nutrients during pregnancy may lead to impaired fetal programming, resulting in adverse health outcomes in adulthood (7). While nutrition in the first three months, especially through breastfeeding, supports rapid brain growth and functional maturation, it cannot fully reverse prenatal deficits (8). Some studies indicated that higher-quality maternal dietary patterns are associated with improved visual–spatial skills in early childhood and enhanced intelligence and executive function in mid-childhood (9). For example, studies have shown a positive association between specific dietary components like seafood (10), fruits (11), and nuts (12) and childhood cognitive functions and intelligence quotient (IQ). Although maternal influences on offspring neurodevelopment are well-established, emerging evidence suggests that paternal factors also contribute to early brain development and neurodevelopmental disorders. Paternal health, age, diet, and exposure to environmental toxins can all influence sperm quality and epigenetic programming (13).
The gut microbiome is a vast community of trillions of microorganisms residing in the gastrointestinal (GI) tract. This complex ecosystem plays a critical role in human health, influencing various body functions and contributing to overall well-being. Fetal gut colonization may initiate in utero, facilitated by unique microbial communities in the placenta and amniotic fluid (14). The initial colonization of the microbiome coincides with neurodevelopment at the same critical developmental windows that are vulnerable to disruption (15). The gut-brain axis (GBA) refers to the bidirectional communication network between the GI tract and the CNS. Recent research suggests that microbes and their metabolic by-products play an active role in regulating early brain development. Neurodevelopment, including myelination, neurogenesis, and microglia activation, depends on the gut microbial composition, indicating that initial colonization and microbiota maturation can have long-lasting effects on mental well-being later in life (16). However, disturbances during this crucial developmental period can adversely affect brain function, potentially leading to various neurodevelopmental and neuropsychiatric disorders (17). For example, early-life gut dysbiosis caused by maternal and postnatal factors, such as diet, stress, and infection, may lead to systemic and neuroinflammation, ultimately resulting in abnormal brain development (17). As shown in Figure 1, early-life dietary patterns and phytochemicals influence the gut microbiome composition in mothers and their offspring, further altering neuroactive metabolites that interact with the GBA via immune, neuroendocrine, and vagus nerve pathways. These interactions contribute to structural and functional brain alterations, impacting cognitive function and behavioral patterns. In summary, a balanced gut microbiome, or eubiosis, supports healthy neurodevelopment, whereas an unbalanced gut microbiome, or dysbiosis, is associated with an increased risk of neurodevelopmental disorders.
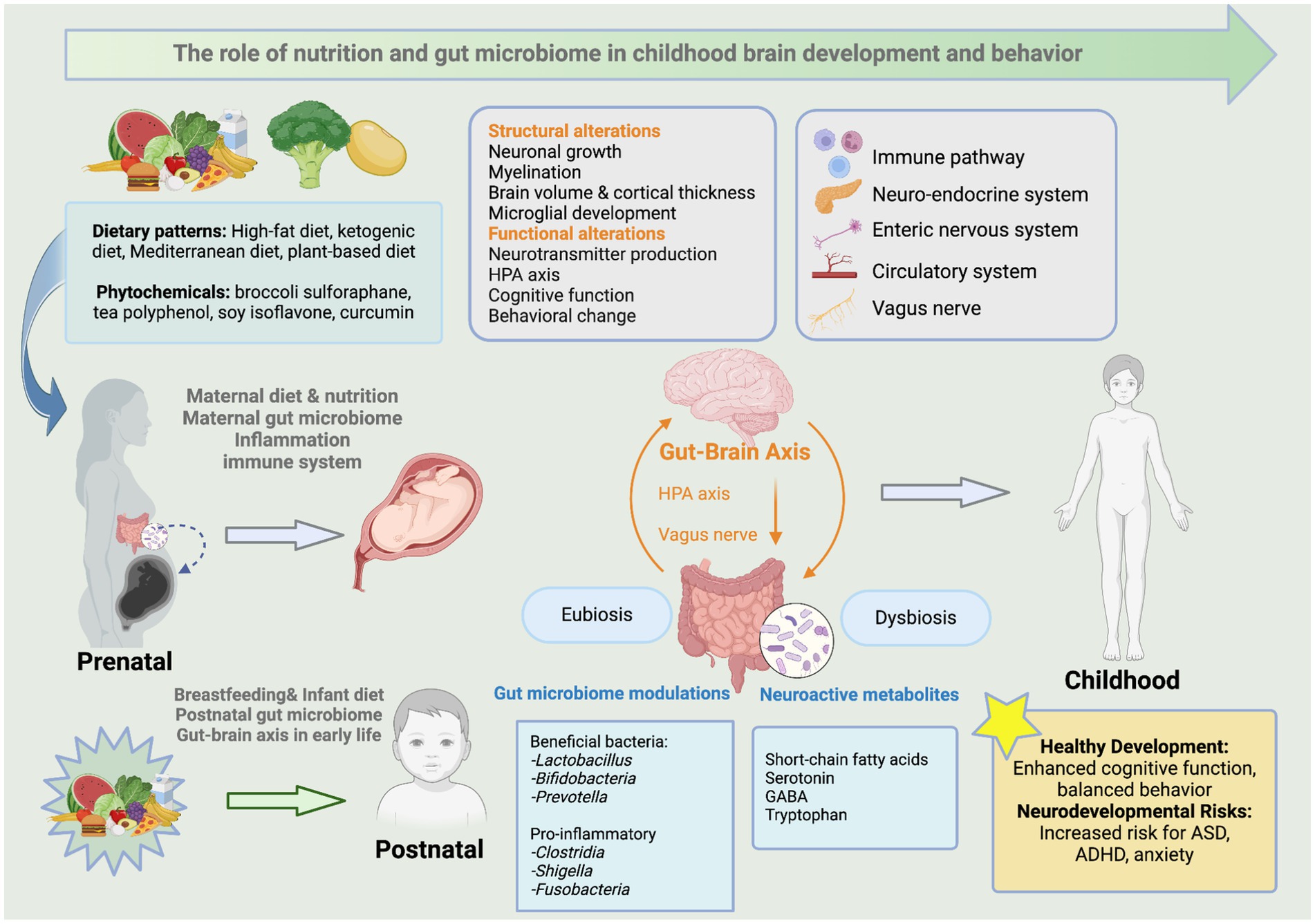
Figure 1. The role of early-life nutrition and gut microbiome in childhood brain development and behavior. This figure illustrates the impacts of early-life dietary patterns and phytochemicals on the gut microbiome composition in mothers and their offspring. This change modulates brain structure and functions through neuroactive metabolites that interact with the GBA via various pathways such as the immune, neuroendocrine, and vagus nerve. It further links microbial alterations to neurodevelopmental outcomes in children. Created with BioRender.com.
Considering the significance of early-life nutrition and the gut microbiome during the critical developmental window, it is essential to explore their roles in the GBA and the potential effects on brain development and childhood behavior. Understanding how the gut microbiome influences brain development and function through various pathways offers valuable insights into the interplay between early-life diets, microbes, cognitive functions, and behavioral well-being. It also directs future nutrition guidelines that can reduce the risk of behavioral disorders and improve mental health in children.
2 Early brain development and neurological health
2.1 Early brain development and influential factors
Brain development is a protracted process that begins as early as the third week of gestation and continues from childhood to adulthood (18). During the prenatal stage, the CNS undergoes rapid growth and differentiation, including neural tube formation, the proliferation of neural cells, and the early stages of synaptogenesis, synapse pruning, and myelination (19). By birth, the brain has established its basic structure, setting the stage for continuous growth postnatally. This dynamic and essential process lays the foundation for brain development and cognitive, emotional, and social functioning throughout life. Brain development during this period is characterized by significant synaptogenesis and myelination, which enhance neural connectivity and function. Brain gray matter, which includes neuronal cell bodies, and white matter composed of nerve fibers, continue to grow and develop throughout childhood and into later adolescence (18). Brain development from early childhood to early adulthood supports the sensory, language, social, and emotional systems critical for cognitive and behavioral functioning (19).
Although genetic factors are the primary contributors to early brain development, environmental factors, such as nutritional status and exposure to toxins, also play key roles in this process (20). Some factors include maternal exposure to viral infections, drugs, and heavy metals, which may be linked to impaired brain development as well as cognitive and behavioral disorders (21). Maternal diabetes significantly increases the risk of brain malformations in the fetus. For example, two specific cerebral malformations strongly associated with maternal diabetes are holoprosencephaly and caudal regression syndrome (20). Despite the metabolic conditions of mothers, maternal nutritional status is essential for fetal brain development. Maternal obesity has been linked to impaired neurodevelopment and the increased risk of psychiatric disorders, such as Attention Deficit Hyperactivity Disorder (ADHD) (22) and Autism Spectrum Disorder (ASD) in children (23). Likewise, a study focused on adults whose mothers were exposed to undernutrition during the Dutch Hunger Winter revealed that they performed more poorly on selective attention tasks, highlighting the long-term impact of prenatal nutrition on cognitive functioning later in life (24). Fetal growth restriction (FGR) is a condition where a fetus does not grow to its expected size during pregnancy, resulting from a combination of maternal, fetal, and placental factors. Specifically, maternal causes of FGR include poor nutrition, obesity, hypertension, smoking, and chronic diseases (25). FGR has been shown to significantly impair normal brain development, leading to reduced brain volume, head circumference, gray matter volume, total number of neurons, and myelin content, all of which are associated with brain functional deficits (25, 26).
2.2 Mental and behavioral health disorders
Early brain development builds the foundation for cognition and behavior throughout the lifetime. Disturbances, such as nutritional deficiencies, environmental toxins, stress, and infections, during this critical developmental window may lead to mental or behavioral disorders (27). Mental health disorders (MHD) are prevalent among children and adolescents and have been steadily increasing in the past decades (28). Specifically, MHD includes emotional conditions like obsessive-compulsive disorder, anxiety, and depression, as well as behavioral disorders such as oppositional defiant disorder (ODD), conduct disorder (CD), and ADHD, often accompanied by speech or language delays and other developmental disabilities such as ASD (29). Between 2016 and 2019, the most commonly diagnosed mental disorders among U. S. children aged 3–17 were ADHD, anxiety, behavior problems, and depression (30). These emotional and behavioral disorders often co-occur and bring negative impacts on daily functioning and overall well-being, leading to increased risk of morbidity and early mortality in children. Moreover, childhood emotional and behavioral disorders bring short-term and long-term complications later in their personal and professional lives (31). Disruptive behavior problems in parents, including ADHD, ODD, and CD, have been associated with an increased risk of abusive behavior toward their offspring (32). Additionally, research suggests that early onset of MHDs can increase the risk of developing chronic mental health issues in adulthood, such as severe depression and substance abuse (33). Notably, about 50% of adults with mental disorders report symptom onset during adolescence. Overall, mental and behavioral disorders are among the leading noncommunicable diseases affecting the health of children and young adults (34).
2.3 IQ and cognitive function
Early brain development, characterized by the overproduction and subsequent pruning of synapses, is crucial for establishing cognitive functions such as attention, memory, thinking, learning, and perception (35). The morphology of the frontal, mesial prefrontal, temporal, and occipital regions is linked to later motor abilities, while features of the posterior parietal regions correlate with subsequent language development (36). Additionally, the temporal and occipital regions are correlated with future cognitive performance. Similarly, the properties of white-matter microstructure are moderately associated with working memory performance in one-year-old children (37). Also, the increase in white-matter volume reflects the improvements in motor performance, sensory, and auditory information (38). These studies collectively suggest that the integrity of brain structure facilitates communication between different brain regions, thereby contributing to the development of early cognitive abilities. IQ is a composite measure that reflects a range of cognitive abilities, including memory, problem-solving, logical reasoning, and verbal skills. It is influenced by both genetic and environmental factors (39). Studies indicate that changes in brain volume during early childhood and adolescence influence IQ performance (40). Additionally, cerebral volume has been shown to be positively associated with IQ level, particularly with cortical gray matter volume in the prefrontal region of the brain (41). Nevertheless, environmental factors play significant roles in influencing intelligence during childhood development, including the home environment and parenting practices, access to education and learning resources, and healthcare and nutritional support.
3 Gut microbiome in early brain development
3.1 Roles of the gut microbiome in early development
It has been widely acknowledged that the gut microbiome shares a symbiotic relationship with the host. Disruption in the gut microbiome is associated with an increased risk of various diseases, including inflammatory bowel disorders (42), metabolic diseases such as obesity and diabetes (43), and neurological disorders. In infants, the gut microbiome has a significant influence on the development of crucial physiological functions necessary for infant growth (44). During infancy, the gut microbiome is essential for various biological functions, including the digestion and metabolism of colostrum, breast milk, formula, and transitional foods (44).
Early childhood is a critical period for establishing a gut microbiome community, which exhibits high intra-and inter-individual variability due to a combination of factors, including birth mode, feeding, and environmental exposures (45). In terms of the number of species and types, the gut microbiome composition is primarily influenced by diet, especially during the first three years of life. This period featured significant dietary pattern transitions, including breastfeeding or formula feeding, weaning, and the gradual introduction of various solid foods (46). In the first six months, breast milk also plays a foundational role in establishing the infant gut microbiome by promoting the growth of beneficial bacteria such as Bifidobacterium and Lactobacillus (47, 48).
In recent years, the association between maternal diet and infant gut microbiome composition has gained intensive attention. Primary dietary elements, such as fiber, fats, and proteins, are strongly linked to distinct gut bacteria clusters in both mothers and newborns, highlighting the significant role of maternal nutrition in shaping the early gut microbiome (49). Maternal diets also impact the diversity and bacterial composition of breast milk, primarily by influencing the profile of human milk oligosaccharides (HMOs), which are essential to the health benefits of breast milk (50, 51). HMOs predominantly affect the fat composition and immunomodulatory components in breast milk, which in turn impact the infant’s gut microbiome and immune development. The influence of maternal diets on the infant gut microbiome is further supported by animal studies, which demonstrate that maternal dietary patterns can change the offspring gut microbiome as early as the pregnancy period (52–54).
3.2 The gut-brain axis (GBA)
The GBA describes the bidirectional communication between the gut microbiome and the brain, mediated through neural, immune, and endocrine pathways. This process is facilitated by the production of neuroactive compounds, metabolites, and hormones regulated or produced by the gut microbiome (55, 56). The GBA mechanism involves several systems, including the CNS, autonomic nervous system (ANS), enteric nervous system (ENS), hypothalamic pituitary adrenal (HPA) axis, immune system, gut microbes, and their metabolites (55). The parasympathetic and sympathetic branches of ANS transmit afferent signals from the gut ENS to the CNS. Conversely, the efferent signals are transmitted from the CNS to the ENS in the intestinal wall (57). The HPA axis transmits information from the brain stem to the gut via the vagus nerve and sensory neurons (58). Together, these systems form a dynamic and interconnected network that governs physiological functions and responses to environmental stimuli, including stress, digestion, and immune regulation.
The gut microbiome can alter the level of neurotransmitter precursors or regulate the production of various neurotransmitters (59). For instance, γ-aminobutyric acid (GABA) can be produced by Lactobacilli, Bifidobacteria, and Bacteroidetes, while serotonin synthesis is supported by E. coli and specific Lactobacillus strains (60). Brain function depends on neurotransmitter-driven signals between neurons and glial cells. Excitatory neurotransmitters include glutamate, acetylcholine, and dopamine, while GABA, glycine, and serotonin are inhibitory neurotransmitters. These neurotransmitters are essential for bowel physiological regulation, mood, and the neuroendocrine system (61). Growing evidence indicates that neurotransmitters, amino acids, and microbial metabolites, such as short-chain fatty acids (SCFAs), can enter portal circulation, influencing the immune system, metabolism, and neurons in the ENS or signaling to the brain via the vagus nerve (56). The vagus nerve enables the two-way connection between the brain and the gut microbiome by exchanging signals produced by microbial metabolites, inflammatory responses, or neuroendocrine cells influenced by the gut microbiota (62).
An imbalanced gut microbiome community may profoundly interrupt the relationship between the gut and brain, leading to mental and behavioral disorders and gastrointestinal diseases (63, 64). As a result, abnormal levels of neurotransmitters can contribute to the development of neurological and psychological disorders (65). Additionally, the disrupted gut microbiome affects the gut barrier integrity, further impairing the transmission of signaling molecules such as neurotransmitters (66). SCFAs have neuroactive properties that play a key role in maintaining and regulating the integrity of the gut and the blood–brain barrier (BBB) (67).
The substantial comorbidity between psychiatric/neurological disorders and GI conditions implies the potential involvement of bidirectional communication between the gut and the CNS (68). Dysbiosis enables pathogenic microbes, bacterial metabolites, and luminal content to pass freely through the bloodstream to the CNS, leading to potential neurological conditions. For instance, these elements can impair the BBB, trigger microglial activation, and induce damage in neuronal cells (68). Increased bacterial metabolites and inflammatory cytokines in the gut and the BBB lead to neuroinflammation, contributing to brain immunological dysfunctions (69).
Germ-free (GF) animals have been widely used to examine the roles of the gut microbiome in brain functions. Studies on GF animals have shown the causal relationship between the gut microbiota and the regulation of brain function and behavior (70). These studies revealed that abnormal brain development, marked by deficits in neuronal plasticity, impaired neuroprotection, altered neurotransmission, disrupted myelination, and behavioral abnormalities, is closely associated with inadequate colonization of the early-life gut microbiome and altered GBA (71, 72). For example, GF mice demonstrated a higher incidence of anxiety-like behaviors, as well as social and cognitive conditions such as memory dysfunction and increased motor activity, than conventionally-raised mice (16, 73).
Changes in the gut microbiome due to other factors such as antibiotic use, dietary habits, and infections are also linked to the development and progression of mental and behavioral disorders (74). Early-life antibiotic use increases the abundance of Escherichia, Staphylococcus, and Clostridioides, but reduces the abundance of important SCFA producers (75). In animal studies, antibiotic treatment can cause dysbiosis of the gut microbiome, resulting in impaired spatial memory and increased anxiety-like behavior in rats (70, 76). Disruptions to the gut microbiota due to antibiotics can cause significant changes in the immune system, driving it toward a more pro-inflammatory state of the CNS (77). Research has shown that early-life antibiotic use induced changes in gut microbial diversity that directly cause neuroinflammation and alter the structure and function of the brain, contributing to mental and behavioral disorders in children (59, 78). Moreover, psychosocial factors such as insecure mother-infant attachment have also been associated with increased antibiotic use in infancy, suggesting that caregiving environments may indirectly influence gut microbial development and neurobehavioral outcomes (79).
3.3 Roles of the gut microbiome in childhood neurological conditions
Studies have shown that gut microbiota can influence various aspects of brain function, including learning, memory, social behavior, and behaviors related to anxiety and depression (80). The causal association between the gut microbial profile and mental and behavioral issues has been supported by fecal microbiome transplantation (FMT) in GF animal models (68). The GF recipient mice that underwent FMT from mice with behavioral issues developed psychiatric symptoms similar to those of the host. In addition, approximately 70–90% of patients who suffer from psychiatric disorders have comorbid GI symptoms such as constipation and diarrhea (81, 82).
3.3.1 Autism spectrum disorder (ASD)
ASD is a developmental disability characterized by difficulties in social interaction and restricted, repetitive behaviors or activities. In 2020, approximately one in 36 children was diagnosed with ASD (83). Symptoms may appear in early childhood and can vary in severity, affecting language development, sensory processing, and behaviors. The etiology of ASD can be genetic and non-genetic factors (84). Genetic factors contribute to about 60% of the ASD cases, while the remaining cases may be influenced by environmental factors such as maternal nutritional and metabolic status, infection during pregnancy, and toxin exposure (84).
Several studies have reported that early-life gut dysbiosis is associated with childhood ASD (85, 86), characterized by decreased levels of SCFAs and microbial producers (82). These gut microbiome dysfunctions are closely linked to the severity of core symptoms of ASD (87). In animal models and clinical cases, ASD has been linked to elevated levels of bacterial strains such as Bilophila, Clostridium, Dorea, and Lactobacillus, along with a reduced abundance of Blautia (88). In comparison to healthy children, ASD patients have Firmicutes and Bacteroidetes as the dominant taxa, with Proteobacteria and Actinobacteria being the least abundant (88). Specific microbial alterations include a decrease in Bifidobacterium, Blautia, Prevotella, Veillonella, and Turicibacter, accompanied by an increase in Lactobacillus, Bacteroides, Desulfovibrio, and Clostridium. In particular, Clostridium is the most commonly found bacteria enriched in ASD patients, possibly due to its ability to produce exotoxins, propionate, and p-cresol, which may worsen the severity of ASD symptoms (89, 90).
3.3.2 Attention deficit hyperactivity disorder (ADHD)
ADHD, another common neurodevelopmental disorder, is characterized by symptoms such as hyperactivity, inattention, and impulsivity. Some children may experience anxiety, depression, ODD, and CD alongside ADHD (91). Based on data from 2022, around 7 million (11.4%) U. S. children have been diagnosed with ADHD (91). ADHD can be caused by genetics, non-inheritable factors, and complex interplay. Genetic predisposition plays a significant role, with about 70% heritability (92). Despite the gene variants, prenatal and perinatal influences, including maternal smoking, poor nutrition, premature birth, and exposure to environmental toxins, are associated with an increased risk of ADHD in children (92).
ADHD is also associated with alterations in gut microbiome composition caused by early-life food intake (93). Studies have found that children with a higher adherence to a healthy diet were less likely to develop ADHD, while the Western dietary pattern increased the risk (94). Moreover, essential nutrients such as omega-3 fatty acids, iron, zinc, and polyphenols play a crucial role in mitigating ADHD risk (95). Given that pediatric patients with ADHD often experience GI discomfort, the impacts of diet on both gut health and neurodevelopment may provide a key link between nutrition and ADHD symptoms (96). Microbiome studies in ADHD patients have shown decreased diversity and reduced levels of short-chain fatty acids (SCFAs), which are critical for neuroimmune signaling (59). Research suggests that within the gut microbiome of individuals with ADHD, the phylum Actinobacteria, particularly the genus Bifidobacterium, is present in significantly higher levels compared to those without ADHD, indicating a potential role of Actinobacteria in the development of ADHD symptoms (97, 98). Another study reported that ADHD patients had a significantly lower abundance of the genus Faecalibacterium, which has shown a negative association with the severity of ADHD symptoms (99). Besides, a study identified a significant increase in bacterial genes that encode cyclohexadienyl dehydratase in ADHD cases compared to controls (98). This enzyme is crucial for the synthesis of phenylalanine, a precursor to dopamine, suggesting a potential microbial influence on dopamine-related pathways in ADHD (98).
However, the results on the impacts of the gut microbiota on ADHD show mixed findings. Some studies have shown no significant differences in the diversity of the gut microbiome between ADHD patients and healthy populations (98–101). The bacterial genera Clostridiales and Porphyromonadaceae have been reported to have either elevated or reduced levels across different studies. The genus Bifidobacterium also showed high variability, with some studies showing an increase in ADHD subjects. In contrast, others report reductions, especially in species such as B. longum and B. adolescentis in infant samples (102). These inconsistencies reflect the complex roles of the gut microbiome in ADHD.
3.3.3 Depression and anxiety
Depression and anxiety are increasingly recognized as significant mental health concerns among children. Although cognitive behavioral therapy is the most common therapy for anxiety and depression in children, studies have shown that a healthy lifestyle, such as a balanced diet and physical activity, can help manage these symptoms (103).
Animal models demonstrated the effects of the gut microbiome on modulating the neurological features of depression. FMT from depressed patients to GF mice showed the impact on initiating the symptoms of depression (104, 105). Studies found that the most affected bacterial phyla include Firmicutes, Actinobacteria, and Bacteroidetes, with a notable increase in the Bacteroidetes/Firmicutes ratio in depressed individuals (105). This imbalance was marked by an increased presence of the genus Bacteroides and a decrease in the genera Blautia, Faecalibacterium, and Coprococcus (106, 107).
Preclinical studies supported the effectiveness of microbial treatment, such as probiotics supplementation and FMT, in the prevention or treatment of the onset and progression of depression (108). In animal models, the relevant symptoms in GF-mice with depression, anxiety, and a highly active HPA were attenuated by supplementing the mice with Bifidobacterium infantis and Lactobacillus, along with the modulation of GABA receptors by the vagus nerve (109, 110). One study found that children with depression showed increased bacterial richness and distinct β-diversity compared to healthy controls (111). Pro-inflammatory genera such as Streptococcus was elevated in the depression group, while anti-inflammatory genus such as Faecalibacterium was diminished, leading to alterations in the production of immunomodulatory metabolites (111). This disrupted gut microbiome community correlates with the increased level of pro-inflammatory bacteria but a decreased level of butyrate-producing bacteria (106).
3.3.4 Cognition
In addition to its influence on mental health, the gut microbiome also plays a significant role in cognitive function, particularly during critical developmental periods (108). Disturbances in microbiome development may negatively affect the development of cognitive functions (112). A study of school-aged children revealed a strong connection between full-scale IQ (FSIQ) and the gut microbiome composition. Higher FSIQ scores were linked to a greater gut microbiome α-diversity and specific taxa, including Prevotella, Dialister, Sutterella, Ruminococcus callidus, and Bacteroides uniformis (113). In another study, in children older than 18 months, several microbial species were notably enriched in those with higher cognitive function scores, including Alistipes obesi, Asaccharobacter celatus, and several SCFA-producing species, such as Eubacterium eligens and Faecalibacterium prausnitzii (114). Interestingly, the same study discovered that different gut microbial species were related to various cognitive functions. For example, Bifidobacterium pseudocatenulatum, Blautia wexlerae, and Eubacterium eligens were key predictors of expressive language, while Roseburia faecis, Streptococcus salivarius, and Fusicatenibacter saccharivorans may be linked to gross motor skills (114). Similarly, another longitudinal study analyzed bacterial composition data from infants aged 3–6 months and found that a higher abundance of Bacteroides, accompanied by a lower presence of Escherichia/Shigella and Bifidobacterium, was negatively associated with fine motor skills. Additionally, the increased abundance of Lachnospiraceae and Clostridiales taxa and reduced Bacteroides were linked to poorer communication, personal, and social skills at 3 years of age (115). Surprisingly, several studies have found that higher alpha diversity in infancy was associated with poorer performance on early learning composite, visual reception, and expressive language skills (116, 117). This finding contradicts the commonly accepted view that greater microbial diversity is beneficial, possibly due to reduced dominance of neurodevelopment-supporting bacteria. These findings suggest that a specific microbial composition, rather than greater diversity alone, may be critical for supporting early cognitive development (117).
4 Prenatal and early-life nutrition in brain development
In the prenatal period, environmental factors, including the maternal diet, toxin exposure, and stress, can significantly impact fetal brain development. Among these, maternal nutrition during pregnancy exerts a more significant impact. Nutrients and dietary compounds consumed by the mother not only support fetal growth but also contribute to shaping the initial fetal microbiome, which in turn impacts neurodevelopment (118, 119). Early pregnancy and infancy are critical phases for brain development, setting the stage for the formation of cognitive, motor, and socio-emotional functions that will continue to evolve throughout childhood and adulthood (120). Specifically, the prenatal period around 22 days after conception is considered a critical window for neural tube formation. At this stage, sufficient nutrient intake, such as folic acid, copper, and vitamin A, is essential for neural tube closure and prevents birth defects in the brain and skull (120). During this critical period, fetuses and infants are susceptible to environmental factors, especially nutritional deficiency. Therefore, sufficient nutrient supply is critical to support the rapid trajectory of key neurodevelopmental milestones, including synapse formation and myelination (121). Moreover, the postnatal periods from birth to early childhood are considered a sensitive period and “window of opportunity” where the brain continues to develop but retains some plasticity, allowing it to respond to environmental stimuli and nutrition intervention (122). The impacts of nutritional deficiencies on neurogenesis and synaptogenesis during prenatal development are often irreversible, as these critical developmental events are tightly programmed during specific phases of embryogenesis. Studies indicate that an unbalanced maternal diet or key nutrient deficiency can result in neurobehavioral delays, potentially affecting cognitive and behavioral outcomes in children (123). In contrast, nutrient deficiencies during the postnatal period may be recoverable due to the brain’s neural plasticity, allowing for functional adaptation and recovery at this stage (124).
Both macro-and micronutrients are essential for neural development. Malnutrition-induced energy deficits or excesses will not only result in stunting growth but also lead to impaired cognitive function among children (125). Any deficiency of these nutrients during the critical and sensitive window of development may cause long-term impacts on brain structure and functions (126), as well as organizational events such as neurogenesis, cell migration, differentiation, and glial cell function (127). Among the key nutrients, polyunsaturated fatty acids (PUFAs), particularly omega-3 fatty acids like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are crucial for brain development. DHA is a key structural component of neuronal cell membranes. DHA level changes can influence cell membrane-associated proteins and the function of protein receptors (128). Unlike DHA, EPA acts as a precursor for eicosanoids and a modulator of cytokines, which play roles in neurotransmission and neuromodulation (128). Brain plasticity and learning ability in mice can be impacted by omega-3 PUFA supplementation due to its effects on enhancing hippocampal levels of neurotrophic factors, reducing oxidative stress, and metabolic effects (129). In human studies, the effectiveness of omega-3 PUFA supplementation in psychiatric disorders has been reported, including ADHD, ASD, and depression, although the results remain controversial (130, 131).
Vitamin A, a fat-soluble micronutrient, can be converted to its bioactive form, retinoic acid (RA), and acts as a transcriptional regulator. RA is essential for CNS development through the regulation of neurodifferentiation and neuronal patterning. RA also maintains brain plasticity and neural stem cell production in the mature CNS. Therefore, the disrupted RA signaling may lead to motor neuron diseases and degenerative diseases (132). In mice, vitamin A deficiency not only led to physical and ocular abnormalities but also impaired long-term potentiation and depression, along with declined cognitive skills (133). Vitamin D is essential not only for bone health but also for neurodevelopment as a fat-soluble vitamin. It regulates the biosynthesis of neurotransmitters and neurotrophic factors. The abundance of vitamin D receptors in the fetal brain highlights its crucial role in early neurodevelopment (134). In mouse studies, vitamin D deficiency in mothers or the offspring impaired learning abilities and memory and increased the risk of psychiatric disorders later in life (135). Similarly, human studies found that prenatal vitamin A and D sufficiency decreased the risk of schizophrenia and other mental disorders later in life (136). Preclinical studies have explored the role of maternal folate in neurodevelopment and brain functioning in the offspring, including DNA replication, gene expression, and synthesis of phospholipids and neurotransmitters (137). Human studies further support a positive association between maternal folate intake during pregnancy and cognitive function in the offspring (138). When it comes to minerals, zinc and copper are the trace elements that are vital for neuronal functions. Zinc is essential for normal neurogenesis, neuronal migration, myelination, synaptogenesis, and regulation of neurotransmitters. Insufficient zinc intake in the postnatal period can have significant impacts on mental and cognitive development (139). Copper deficiency may lead to abnormal brain development, as evidenced by Menkes disease, a neurodegenerative disorder in infants and young children due to defective copper transport (140). The impaired metabolism and signaling of zinc and copper have been reported in brain diseases and psychiatric disorders, including ASD and major depressive disorder (141, 142). In summary, prenatal and early-life nutrition play a fundamental role in shaping brain development. Deficiencies and imbalances in these nutrients may lead to adverse neurological consequences in the long run.
5 Impacts of dietary patterns on the gut microbiome and brain development
Although many studies focus on analyzing the impacts of individual nutrients, assessing diet intake as a whole is essential for exploring the interplay between food components and nutrients that contribute to infant neurodevelopment and behavior (143). Maternal malnutrition, whether in the form of undernutrition or overnutrition, can have profound impacts on early fetal neurodevelopment and increase the risk of various neuropsychiatric conditions later in life (123). Poor maternal diet quality has been associated with lower intelligence scores (9, 144, 145), emotional and behavioral dysregulation (143, 146), and hyperactivity-inattention symptoms (147, 148). In contrast, a healthier maternal dietary pattern characterized by a higher intake of fruits, vegetables, and fish while limiting red meat and trans fats has been linked to fewer behavioral difficulties and improved executive function in the offspring (143). This section will discuss several common dietary patterns and their impact on brain development and the gut microbiome in offspring, as shown in Table 1.
5.1 High-fat diet
In the United States, a significant proportion of women of childbearing age face weight-related health challenges. Nearly 60% of women aged 20 to 39 are classified as overweight, with one-third falling into the category of obesity. Additionally, around 16% of women in this age group are diagnosed with metabolic syndrome (149). Maternal obesity and overweight can lead to gestational diabetes and other metabolic disorders that may affect fetal development (150). Maternal metabolic disruptions, including insulin resistance, elevated blood glucose, and systemic inflammation, can interfere with critical stages of fetal brain development (151). Energy-dense diets such as high-fat diets (HFD) or Western-type diets are primary dietary patterns that contribute to overweight and obesity as well as related metabolic disorders.
Maternal HFD can directly affect the intrauterine environment, which, in turn, has significant implications for infant brain development and behavior. Maternal HFD can induce increased brain lipid peroxidation, altered hippocampal neurogenesis, and heightened inflammation, which negatively affect neurodevelopmental processes, leading to an increased risk of emotional and behavioral disorders in the offspring (152). Maternal obesity-associated neurodevelopmental conditions such as ASD (153–155), ADHD (156–158), depression, anxiety (159), and cognitive functions (160) are the most common outcomes observed in cohort studies (151). Specifically, pre-pregnancy maternal obesity and diabetes have been linked to a higher risk of ASD and intellectual disabilities in the offspring. The risk increases significantly when obesity and diabetes co-occur (153). Recent studies revealed that maternal BMI is considered a potent biomarker for offspring ASD risk (161, 162). A large cohort study found that each unit increase in pre-pregnancy BMI was linked to a 3% higher odds of children having a high ADHD symptom score (22), consistent with findings from many other studies (163, 164). Few epidemiological studies focus on the association between maternal HFD and the risk of depression and anxiety in the offspring. Studies found that maternal BMI is related to a two-fold increased risk of emotion dysregulation and intensity in the offspring (163, 165). Interestingly, either being small or large for gestational age also increases the odds of depression later in life (166, 167). Besides, maternal dietary patterns contribute to cognitive functions in the offspring, with high maternal BMI specifically linked to poorer visual-motor abilities and verbal recognition (160, 168). Maternal HFD exposure has a long-term impact on brain development, resulting in brain structural changes, which can persist into adulthood. Studies found that both regional and total brain volumes were significantly affected by early-life HFD exposure, with a positive correlation between dietary fat percentage (169). Additionally, they identified several genes that are highly expressed in brain regions sensitive to early-life HFD exposure, which are related to feeding behavior and autism in both mice and humans (169).
As shown in Table 1, animal studies and human clinical trials have explored potential mechanisms linking maternal HFD intake to offspring neurodevelopmental conditions. It is believed that maternal HFD consumption impairs offspring neurodevelopment by triggering inflammation in the maternal gut, adipose tissue, and placenta, leading to elevated proinflammatory cytokine levels in both the mother and fetus (151). These increased proinflammatory cytokines include various interleukins, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), creating a state of chronic low-grade inflammation in the mother, which can negatively impact the neurodevelopment of the offspring (151, 170). For example, elevated levels of IL-6 in mothers have been associated with reduced social behaviors in the offspring (171). In contrast, elevated IL-17a has been shown to contribute to abnormalities in brain development, particularly in the cortex (172). Maternal hyperglycemia, induced by HFD, triggers excessive proinflammatory cytokine production in the placenta. Neonatal hyperglycemia also increases IL-1β, TNF-α, and toll-like receptor activity in spleen cells, causing chronic low-grade inflammation in infants. All these changes activate microglia, induce the inflammatory response in the CNS, and impair fetal and neonatal brain development (173). As a result, these proinflammatory cytokines are linked to a higher likelihood of alterations in cognition and behavioral disorders (174, 175). For example, studies in rodent models showed increased microglial activation markers and proinflammatory cytokine levels in the hippocampus of offspring exposed to maternal HFD (176). Some evidence suggests that brain development during lactation is also sensitive to HFD. Maternal HFD during lactation can cause inflammation in the offspring’s brain, leading to impaired axon formation, particularly in the hypothalamus, and reduced arcuate nucleus neuronal fiber densities (175, 177).
Recent studies reveal a potential causal link between maternal HFD-induced gut microbiome dysbiosis and offspring neurodevelopmental conditions. Maternal HFD can cause a disrupted gut microbiome, leading to gut-brain signaling dysregulation that impacts synaptic plasticity, which is essential for proper neuronal communication and development. Synaptic plasticity is considered an early site where mental disorders may begin to develop (178). Impaired synaptic function, driven by these microbiome changes, can negatively affect social behaviors in the offspring and has been linked to the onset of neurodevelopmental and psychiatric disorders (179, 180).
A growing amount of evidence implicates the potential role of the gut microbiome in neurodevelopment and behaviors. Animal studies found that microbial community alterations induced by maternal HFD consumption influence the offspring’s synaptic function and behavior (178). Specifically, they found that maternal HFD increased microbial gene activity in the maternal gut, leading to changes in quinolinic acid production and kynurenine levels, which are important for neuronal plasticity and glutamate metabolism. During adolescence, offspring exposed to maternal HFD showed heightened locomotor activity and anxiety-like behaviors, along with an increase in glutamate-related gene expression (178). Similarly, another study reported that maternal HFD caused gut dysbiosis in the offspring. A specific gut bacterial species, Lactobacillus reuteri (L. reuteri), was significantly reduced in the offspring exposed to maternal HFD. Interestingly, reintroducing L. reuteri significantly improved social interactions (181). L. reuteri has been shown to promote the production of oxytocin, a hormone involved in social bonding, trust, and emotional regulation (182). Oxytocin plays a crucial role in maternal care, pair bonding, and social recognition, making it essential for healthy social interactions (183). Moreover, beneficial microbial metabolites such as SCFAs are crucial for normal fetal brain development. In animal studies, an HFD resulted in a decreased level of butyrate-producing bacteria, like Lachnospira and Ruminococcus, contributing to lower butyrate levels in HFD-exposed mothers (184). HFD can regulate SCFA receptor expression in adipose tissue. These receptors are also found in fetal and uteroplacental tissues, suggesting their roles in maternal gut-fetal brain signaling (151). To conclude, maternal HFD and obesity can negatively impact offspring neurodevelopment by inducing neuroinflammation, oxidative stress, and altered synaptic plasticity, which may be due to HFD-induced gut microbiome dysbiosis.
5.2 Ketogenic diet
The ketogenic diet (KD) is a nutritional approach emphasizing high fat and low carbohydrate intake. KD typically contains approximately 60% fat, 30% protein, and 10% carbohydrate. By drastically reducing around 50% of carbohydrates, the body enters a metabolic state called ketosis, primarily burning fat for energy instead of glucose (185). The KD was initially used for treating pediatric epilepsy and has now become a tailored dietary regimen facilitating weight loss in obese and overweight patients (185). However, its effects on fetal neurodevelopment remain largely unknown. By following KD for a few days, several organs, including the brain, experience a shift from glycolysis to ketosis. Notably, fat is a primary component in breast milk, suggesting a neonatal preference for ketone bodies as a key energy source in early development (186, 187). Besides, astrocytes, oligodendrocytes, and neurons from the developing brain can use ketone bodies for respiration (186). Ketone bodies, a group of liver-produced fat metabolites, help to prevent the mitochondrial permeability transition and reduce the generation of reactive oxygen species (188). The functions of ketone bodies in CNS cell development have been reported (187). Studies have shown increased enzymes for ketone utilization during gestation that support early brain development, specifically lipid and white matter synthesis (189). However, excessive ketone bodies may have adverse effects on fetal brain development because of the impaired production of nucleic acids, which are crucial for cell growth and development (189). KD broadly impacts brain function, influencing multiple cellular and molecular processes. Specifically, KD can alter gene expression and modify neurotransmission. Evidence has shown that KD shifted the balance between excitatory and inhibitory signaling, which can stabilize neural networks (190). In an animal study, KD treatment showed improvements in myelin formation and white matter development, possibly attributed to the altered neurotransmitter signaling pathways (191). Additionally, KD increases levels of neurotrophic factors to support neuron growth and resilience. Protein phosphorylation is also affected by KD, leading to modified key signaling pathways involved in memory and learning (192). KD also alters amino acid metabolism, potentially enhancing the production of neurotransmitters like GABA (193). KD has been found to inhibit the mammalian target of the rapamycin (mTOR) signaling pathway, leading to anticonvulsant effects. The mTOR plays a key role in cell growth, metabolism, and autophagy, which are associated with the progression of ASD (194).
Prenatal and early postnatal KD treatment in mice led to structural alterations in the offspring’s brains (189, 195). The cortical volume showed slight bilateral enlargement, while the midbrain volume was reduced unilaterally. During the postnatal period, cortical enlargement became more pronounced, and the hypothalamus was relatively larger, whereas the hippocampus, corpus callosum, and olfactory bulb were relatively smaller (189). These alterations in brain structure may correspond to functional and behavioral changes in KD-exposed offspring, though further postnatal behavioral studies are needed to clarify these effects. Although the clinical evidence is limited due to the difficulties of implementing the KD diet in humans, maternal KD exposure has been shown to influence offspring behavior due to alterations in brain structure and neurodevelopmental processes in mice (189).
Nevertheless, KD has been shown to improve ASD core symptoms and comorbidities like seizures in mice (196). Prenatal KD reversed the abnormalities of maternal immune activation-induced ASD, while control diet-fed mice demonstrated repetitive self-directed behaviors (196). However, such an effect was only observed in male offspring, indicating potential sex-dependent differences in response to KD (196). This finding is consistent with other animal models focused on different etiologies of KD (197–199). For example, animals with ASD induced by prenatal valproic acid showed improved social behaviors after KD treatment (199). Another study found that KD enhanced brain activity related to social novelty in Engrailed 2 gene knockout mice, as indicated by increased levels of neuronal activation in specific brain regions (198). In a human study, one patient out of six showed significant improvement on the Childhood Autism Rating Scale, and the other five showed milder symptom improvements after KD treatment. Overall, KD ameliorates comorbidities such as ADHD and sleeping disorders in ASD patients (200). Other studies showed similar results, suggesting significant improvements in cognition and sociability in children with ASD who underwent a KD treatment (201–203). However, a larger scale of clinical studies is necessary to validate the effectiveness of KD treatment in pediatric patients with behavioral conditions.
Evidence on KD treatment in ADHD patients is scarce. One study using a canine model with naturally occurring epilepsy and comorbid ADHD showed that KD improved ADHD-related behaviors such as chasing, excitability, and trainability (204). In a spontaneously hypertensive rat (SHR) model, KD treatment ameliorated the relevant symptoms, similar to methylphenidate, the first-line medication for ADHD patients. Importantly, this study found that KD increased the abundance of Bacteroidota in SHR rats (205). Besides, KD has been shown to exert antidepressant and mood-stabilizing effects on managing both physical and mental symptoms, mainly due to its impact on regulating GABA and glutamate levels, which are critical for maintaining brain functions and dopamine levels (206). In addition, the shifts in brain energy metabolism induced by KD may help counteract the global cerebral hypometabolism often observed in depression and bipolar disorder. Another potential benefit of ketosis is its ability to lower intracellular sodium levels, possibly contributing to mood stabilization (207). One randomized controlled clinical trial showed that children with epilepsy on KD enhanced their behavioral and cognitive functioning, demonstrated by lower anxiety and hostility levels, higher productivity, and improved attention (208), which is consistent with other observational studies (208–211). However, the effectiveness of KD remains controversial. For example, some studies revealed a tendency of worsening emotional problems among children on KD (212).
The gut microbiome plays a vital role in mediating the therapeutic effects of KD on managing CNS disorders. An increasing amount of research supports the potent therapeutic effects of KD on neurological disorders or illnesses via remodeling the gut microbiome (213). One study used a rodent model of ASD to validate the gut microbiome disturbances in the case of ASD compared to the control group. The results showed that the total amount of bacteria was significantly reduced because of the antimicrobial-like effect of KD (214). Specifically, the gut microbiome revealed notable shifts in mice that resembled similar changes observed in humans with ASD (215). These changes included an increase in Clostridium cluster XI and Bacteroidetes and a decrease in Firmicutes species (215, 216). KD increased the Firmicutes-to-Bacteroidetes ratio, counteracting the low ratio commonly seen in ASD phenotypes. Meanwhile, KD increased the SCFA-producing bacteria, including Akkermansia muciniphila and Lactobacillus, twofold to threefold in mice following KD (215). KD intake also decreased the levels of Desulfovibrio, a harmful bacterium. Taken together, these changes in the gut microbiome support the potential protection of KD in maintaining the integrity of the BBB, which further promotes neurovascular development (213). Generally, the beneficial effects of KD are mainly attributed to its impacts on the gut microbiome by decreasing the α-diversity and species richness (213). However, this remains controversial. For example, the study in SHR rats found that KD increased the richness and diversity of the gut microbiota at the phylum, family, and genus levels (205). Additionally, KD treatment can alleviate ADHD symptoms in SHR by enhancing gut microbiota-driven amino acid and glucose metabolism (205). While KD has shown promising effects in improving ASD, ADHD, and mood disorders by stabilizing neural networks and reducing neuroinflammation, its long-term effects on fetal brain development remain unclear. More clinical studies are needed to determine the efficacy of maternal and neonatal KD in pediatric neurodevelopmental disorders.
5.3 Mediterranean diet
Mediterranean diet (MD) is a well-known healthy dietary pattern rich in unprocessed food, vegetables, fruit, whole grains, nuts, legumes, fish, and olive oil, while sparse in red meat. MD can provide potent health benefits against various human chronic diseases, such as cardiovascular diseases, diabetes, metabolic-related conditions, and cancers (217). MD stands out for its high amount of monounsaturated fatty acids, omega-3 and omega-6 PUFAs, and antioxidants, contributing to healthy neurological and behavioral development in children (218, 219). MD consumption has been related to the methylation changes in inflammation-related genes (220). In a mouse model with chronic inflammation, MD improved recognition memory, the shifts in the gut microbiome composition, and the brain proteomic profile (221). In human studies, MD consumption supplemented with olive oil and nuts improved cognition in the elderly (222).
Many studies have shown that MD adherence can lead to improved pregnancy and birth outcomes, including a reduced risk of gestational diabetes, hypertension, preterm delivery, and intrauterine fetal growth restriction (223). Recent research focused on the impacts of maternal MD on behavioral development showed that low intake of fish and high intake of processed food were related to early-onset of persistent conduct problems in children (224). A diet high in vegetables and fruits and low in meats, similar to the MD, has been shown to enhance children’s IQ development (144). Similar results support the beneficial effects of maternal MD adherence on improving the offspring’s brain development and reducing the risk of neurodevelopmental and behavioral disorders (147, 225–227). The low adherence to maternal MD may lead to higher susceptibility to anxiety, depression, ADHD, and autistic traits in children (219, 228). In contrast to MD, an unhealthy dietary pattern characterized by a high intake of processed foods and sweets has been related to an increased risk of internalizing and externalizing problems (229) as well as hyperactivity-inattention symptoms in children aged between 5 and 8 years old (147). As mentioned before, unhealthy diets such as HFD or a Western diet can cause unbalanced growth factors and neurotransmitters, leading to abnormal behavioral development in children. On the contrary, the main components of MD, such as antioxidants and beneficial fatty acids, support healthy brain development (230). Additionally, studies focused on fetal outcomes and found that those offspring exposed to maternal MD had larger total fetal brain volume, corpus callosum, and right frontal lobe, contributing to optimized brain structure and neurological functions (231). Nevertheless, direct evidence connecting MD consumption to behavioral outcomes in the offspring remains largely unexplored.
Studies have examined the impacts of MD consumption during the postnatal period. The results showed the beneficial effects of MD on improving cognitive function and school performance (232), lowering the risk of depression (233) and ADHD (234), along with metabolic protective effects on BMI, glucose, and lipid profiles in children (235). Therefore, MD has been recognized as a promising strategy for treating depression and alleviating systemic inflammation among children and adolescents (236). MD was first tested and used as an adjuvant treatment strategy among young adults with major depressive disorder in the “SMILES” trial (237). One study found that the MIND diet, a combination of MD and Dietary Approaches to Stop Hypertension (DASH) diet for Neurodegenerative Delay, was effective in improving depressive symptoms, slowing cognitive decline in the elderly, and reducing the risk of anxiety disorder in adults (238, 239). Another case–control study in Iranian children found that high adherence to the MIND diet was closely related to lower odds of ADHD (240). More longitudinal studies are required to explore the impacts of the MIND diet on brain health in children and adolescents.
While some evidence suggests the potential effects of a maternal MD on offspring neurodevelopment, findings on the specific mechanisms remain limited. MD is abundant in essential vitamins, minerals, flavonoids, polyphenols, and other phytochemicals that help reduce oxidative stress and inflammation in neural tissues (241). Additionally, epigenetic modifications may provide a possible explanation for the observed association. Studies have found that the hypomethylation at the maternally expressed 3 imprinting gene relates to adverse neurobehavioral phenotypes (230, 242). In the context of mental and behavioral problems, significant methylation changes have been observed in schizophrenia and bipolar disorder (243) and ADHD symptoms in children (244). Intriguingly, maternal MD adherence has been found to be associated with improved behavioral outcomes in the offspring, accompanied by DNA methylation changes at specific imprinted differentially methylated regions (245). More studies are needed to support the early intervention of MD for preventing or treating neurodevelopmental disorders in the offspring.
MD consumption significantly affects the gut microbiome ecosystem. Research found that MD supported the growth of beneficial bacteria such as Lactobacillus, Akkermansia, g_Erysipelatoclostridiaceae, Lachnoclostridium, Intestimonas, and Parasutterella, known for SCFA-producers that play a key role in enhancing neurocognitive and neuromotor functions (246, 247). These bacterial compositional alterations also contribute to inhibiting systemic inflammation and oxidative stress. Supportively, MD-fed mice displayed more KEGG pathways, particularly those involved in D-alanine metabolism, glycosaminoglycan breakdown, steroid hormone biosynthesis, and pathogen defense (246). MD improved gut and brain inflammatory profiles, such as reduced levels of cytokines including IL-1β, IL-6, and TNF-α, leading to enhanced neurocognitive and neuromuscular functions (246). Behavioral improvements by MD treatment were accompanied by shifts in microbiota composition, including the restored Firmicutes-to-Bacteroidota ratio and increases in Muribaculum, Rikenellaceae Alloprevotella, and especially Akkermansia, which strongly correlated with improved performance (221). Moreover, research indicates that maternal adherence to the MD influenced the fecal gut microbiome with an increased abundance of bacterial species such as Ruminococcaceae, Acidaminococcaceae, and Bacteroidaceae (248). Although many studies are exploring the effects of MD on children’s neurodevelopment, mechanistic exploration focused on the gut microbiome correlation is still insufficient. More studies in this area are needed to further validate the causal link between MD consumption and improved neurodevelopmental and behavioral outcomes in children and the gut microbiome changes.
5.4 Plant-based diet
A plant-based diet (PD) emphasizes the consumption of food derived from plants with a limited intake of animal products. Due to the rich content of antioxidants and anti-inflammatory components, as well as its low calorie content, PD is also effective in reducing age-related cognitive decline (249). Intervention trials in healthy adults found that PD adherence was associated with increased productivity and improvements in anxiety, stress, and depressive symptom scores (250, 251). However, some controversial results showed that low meat intake was related to depressive symptoms or no protective effects were found (252, 253), while others showed protective effects associated with PD intake (254, 255). One case–control study found that children in the highest quartile of the PD Index had higher energy, macronutrient, and key micronutrient intake with significantly lower odds of ADHD than the counterparty (256). Similarly, another study on preschoolers in China found that “vegetarian” or PD dietary patterns were negatively correlated with ADHD symptoms (257).
Overall, research on PD impacts on neurological and psychiatric disorders is limited as compared to MD, which has been well studied for its neuroprotective benefits (258). The potential mechanism linking PD to brain health may involve reduced systemic inflammation (259). This can be attributed to the high intake of anti-inflammatory plant compounds, such as polyphenols, flavonoids, and fiber, as well as the avoidance of pro-inflammatory molecules in animal-derived foods. While PD offers numerous health benefits, it is possible that exclusive PD can cause nutritional deficiencies, contributing to increased depressive symptoms (254). Key nutrients such as vitamin B12, iron, omega-3 fatty acids, and zinc are rich in animal-based foods and play crucial roles in neurotransmitter function, brain structure, and mood regulation (260). Therefore, infants and children, as well as their mothers, following a strict vegetarian or vegan diet during pregnancy, may be at a higher risk of impaired neurodevelopment. Nevertheless, there is little direct evidence to show the negative impacts of a maternal vegetarian or vegan diet on children’s cognitive function (261).
The microbiome composition affects the availability of neurotransmitter precursors, such as tyrosine and tryptophan, which are essential for the synthesis of dopamine and serotonin (262). The levels of SCFAs are closely linked to the intake of fruits, vegetables, and legumes. However, a strict vegan or vegetarian diet decreases SCFA levels, potentially due to weight loss and a high intake of low-fermentable non-starch polysaccharides (263). Notably, PD can lead to increased levels of Prevotella and decreased levels of Bacteroides, altering metabolic pathways involved in neurotransmitter production (258). Also, studies have reported that PD led to an increased abundance of butyrate-producing bacteria, such as Ruminococcaceae, Lachnospiraceae, Coprococcus, Roseburia, Blautia, Alistipes, and Faecalibacterium prausnitzii (264). Lower inflammation-associated PD may contribute to improved cognitive function and mental well-being (265). In summary, while PD may support brain health through anti-inflammatory and microbiome-related mechanisms, its long-term neurological effects on neurodevelopment and mental health in children remain inconclusive and require further investigation.
6 Impacts of bioactive nutrients on neurodevelopment and the gut-brain axis
6.1 Broccoli sulforaphane
Sulforaphane (SFN), a bioactive compound abundant in cruciferous vegetables such as broccoli, Brussels sprouts, and kale, has gained significant attention for its neuroprotective and chemopreventive properties (266, 267). SFN can act as an inducer of the nuclear factor erythroid 2-related factor 2 (Nrf2), regulating the antioxidant response element pathway and delivering protective effects against various neurodevelopmental diseases and behavioral disorders (266).
As shown in Table 2, preclinical research on the neuroprotective effects of SFN has yielded promising results over the past few decades. SFN plays a crucial role in supporting mitochondrial and synaptic function, which leads to reduced neuroinflammation and promotes neuroprotection. SFN exerts its bioactive effects by regulating key genes involved in cellular defense and damage repair, thereby enabling cellular activities that counteract oxidative stress, inflammation, DNA-damaging agents, and radiation (268). The anti-inflammatory properties of SFN can further mitigate cytotoxicity in the nervous system, thereby exerting neuroprotective effects (269). In a placebo-controlled, double-blind, randomized trial, a group of participants aged 13–27 with moderate to severe ASD who received SFN treatment demonstrated improvements in social interaction, abnormal behavior, and verbal communication, partially due to SFN-included protective effects on oxidative stress, mitochondrial function, lipid peroxidation, and neuroinflammation (268). These results are consistent with the outcomes of other clinical trials that focused on SNF-led neuroprotective effects in children and young adults (270–274). However, the significance of the protective effects remains uncertain (275).
In animal models, SFN has been shown to attenuate lipopolysaccharide-induced depression-like behaviors by inhibiting TNF-α in the serum and suppressing microglial activation in brain regions (276). Consistently, in another mouse model, SFN provided neuroprotective and antidepressant effects by regulating glucocorticoid receptor expression and reducing inflammation-induced apoptosis and disrupted neuroplasticity, potentially through the NF-κB and ERK/CREB/BDNF signaling pathways (277, 278). Another study confirmed the effects of repeated SFN treatments in reversing depression-like behaviors and increasing the total antioxidant capacity in animal models (279). As a lower Nrf2 level is correlated with depression, SFN may induce antidepressant effects by inducing Nrf2 activation (280). A clinical study investigated the effects of SFN supplements in depressed patients and showed improvements in depressive symptoms caused by cardiac interventions (281). In addition to its antidepressant effects, SFN has been found to alleviate symptoms of ASD in mice and humans, as supported by improved verbal and non-verbal communication scores (282). In BTBR mice, SFN reduced Th17 immune responses and oxidative stress biomarkers while enhancing antioxidant defenses. The effects of SFN may be attributed to its mediation of Nrf2 activation, facilitating the restoration of immune balance and reducing oxidative stress, which leads to improvements in ASD symptoms (283). Although many studies demonstrated promising effects of SFN on ameliorating ASD-related symptoms, evidence on other mental and behavioral disorders, as well as cognitive functions related to SFN, is still insufficient.
Early-life intake of broccoli or its bioactive derivative SFN has demonstrated robust neuroprotective effects in children. Maternal intake of broccoli SFN may prevent behavioral abnormalities in the offspring via maternal immune activation (MIA), a known factor in the development of neuropsychiatric disorders (284). In an animal study, pregnant and lactating female mice were given food pellets enriched in glucoraphanin, a precursor of SFN, which demonstrated protective effects against MIA-induced cognitive deficits in mouse offspring (284). Juvenile offspring with glucoraphanin showed improved social interactions after MIA. This study also found that glucoraphanin prevented the loss of parvalbumin immunoreactivity in the medial prefrontal cortex, a key area implicated in cognitive function. Moreover, supplementation of SFN during the juvenile or adolescent stage helped prevent phencyclidine-induced cognitive deficits in schizophrenia in adulthood (285).
Studies have focused on the changes in the gut microbiome induced by maternal SFN treatment. It was found that maternal SFN significantly increased microbial α-diversity and β-diversity in the offspring (286). Significant changes at the genus level include an increased abundance of Lactobacillus, Bifidobacterium, Ruminococcaceae UCG-014, and Intestinibacter. In contrast, genera like Oscillibacter, Enterorhabdus, Blautia, and Lachnospiraceae were less abundant in the treatment group compared to the control. Additionally, they found changes in KEGG pathways and lower proinflammatory biomarkers in plasma, such as IL-6 and TNF-α, following SFN administration (286). In ASD children, SFN supplements enriched the beneficial bacteria Bifidobacterium and Lactobacillales, along with alleviating symptoms, indicating SFN may exert its neuroprotective effects through reshaping the gut microbiome profiles (282). Nonetheless, research focused on the impact of early-life or maternal treatment with SFN or cruciferous vegetable-related foods on brain development as well as the gut microbiome-driven mechanisms remains largely unexplored. Thus, further investigations are warranted to fully understand the underlying mechanisms and long-term impacts of SFN and its enriched vegetables on neurodevelopmental and behavioral disorders in the affected populations.
6.2 Tea polyphenol
Green tea is rich in polyphenols that significantly contribute to its health-promoting properties. The primary polyphenols in green tea include epigallocatechin-3-gallate (EGCG), epicatechin, and epigallocatechin. Numerous studies have demonstrated the beneficial effects of green tea on cancer, obesity, infections, and degenerative neurological diseases (287). The green tea polyphenols also show profound neuroprotective effects on brain health and behavior, mediated through their antioxidant, anti-inflammatory, and neuroprotective activities (288). Most of these health benefits can be attributed to their potent antioxidant and anti-inflammatory effects. Tea polyphenols (TPs) can influence the CNS through various mechanisms, including the interaction with the BBB, modulation of neurotransmitter systems, and effects on the cerebrovascular system (289, 290). Recent studies have shown that TPs exert their protective effects against neurodegenerative diseases by modulating the gut microbiome (289). The active metabolites produced by TPs and the enhanced gut microbiome diversity work synergistically to reduce neuronal damage and improve cell survival. This two-way mechanism involves both the direct and indirect neuroprotective effects that can inhibit inflammation and oxidative stress, leading to overall brain health (291). Therefore, TPs are considered bioactive compounds with neuroprotective and neuromodulatory impact (292).
Research in middle-aged and older adults has demonstrated the significant benefits of TPs on brain health (293). A clinical trial in elderly Japanese individuals found that higher consumption of green tea was associated with fewer depressive symptoms (294). Another study found a negative association between green tea consumption and cognitive impairment among the middle-aged Chinese population (295). This suggests that TPs may inhibit monoamine oxidase (MAO), resulting in increased monoamine levels in glial cells —a mechanism similar to that of antidepressants (296). Similarly, TP consumption showed antidepressant effects in mouse models with behavioral and depressive disorders (296, 297). In another mouse model, TPs improved recognition ability and reduced anxiety-like behaviors by suppressing TNF-α production (298). Previous studies found improvements in locomotor activity and spatial cognition learning ability in TP-treated animals (299, 300). Moreover, EGCG treatment has been shown to mitigate neurological damage in a rat model with ASD by regulating key gene expression associated with neurodevelopment (301). Similarly, TPs-derived flavanol catechin has demonstrated the ability to ameliorate behavioral, biochemical, neurological, and molecular impairments in ASD mouse models by modulating the nitric oxide pathway (302). In a human study, healthy young participants who consumed green tea for five weeks demonstrated improved reward learning and prevented depression-related symptoms (303). Similarly, a cross-sectional study found that higher consumption of green tea is associated with a lower prevalence of depression (304).
Early-life green tea consumption may render long-term beneficial effects on brain health later in life. One animal study has shown that offspring mice exposed to prenatal green tea consumption displayed faster sensory-motor reflex responses and notable stimulation effects (288). These beneficial effects further extended to adolescence, showing increased locomotor activity, decreased anxiety and fear, and improved memory and learning abilities. However, despite its potential benefits, green tea consumption is generally not recommended for young children due to its caffeine content and possible adverse effects. Future research should investigate the potential role of isolated tea polyphenols in pediatric neurodevelopment and behavioral correction, while considering safe and appropriate dosage guidelines.
TPs modulate the intestinal microbiota composition that benefits the host health. The intestinal microbiota can transform TPs into metabolites that exhibit significant neuroprotective effects (305). Specifically, tea catechins increased the proliferation of Bifidobacterium and Lactobacillus/Enterococcus groups and reduced the abundance of Bacteroides-Prevotella, Clostridium histolyticum, and Eubacterium-Clostridium groups. Additionally, the total SCFA concentrations in cells treated with EGCG were noticeably higher compared to the control (306, 307). In a mouse model with circadian rhythm disorder, TPs restored gut dysbiosis by increasing the abundance of Akkermansia and Muribaculum while decreasing Desulfovibrio (308). Another study found similar patterns of gut microbiome changes in response to TPs. TPs can inhibit harmful bacteria like H. pylori, S. aureus, Bacteroides, and L. monocytogenes while promoting beneficial probiotics such as Bifidobacterium and Lactobacillus-Enterococcus (309). Probiotics have been found to regulate abnormal brain activity by mitigating anxiety-and depression-like behaviors via the vagus nerve. Lactobacillus supplementation has been shown to reduce anxiety in mice by enhancing GABA receptor expression (309). TPs can prevent cognitive dysfunction by strengthening the intestinal barrier via targeting neuroinflammatory pathways (308).
6.3 Soy isoflavones
Soy foods have become a fundamental component in human diets, particularly among individuals following vegetarian and vegan lifestyles, due to their nutrient-dense protein content. Soybean products are rich in bioactive isoflavones, a class of phytoestrogens with a chemical structure similar to estrogen (310). There are three main isoflavone aglycones found in soybeans, including genistein, daidzein, and glycitein. Genistein is the most abundant isoflavone in soy products and has been well-studied due to its potent chemoprotective effects against various chronic human diseases (267, 310). Due to their structural similarity to estrogen, soy isoflavones can bind to estrogen receptor (ER), although their binding and activation capacity are significantly weaker compared to endogenous estrogen, 17-β estradiol (311). ERα and ERβ are the primary types of ER in mammals, among which ERβ is specifically involved in brain function (312). Interestingly, equol, a metabolite produced from isoflavones mediated by gut microbiota, may offer greater cognitive benefits due to its superior antioxidant properties, higher affinity to ERβ, longer bioavailability, and ability to enhance mitochondrial regulatory effects (313, 314). However, equol production depends on soy consumption and the presence of specific gut bacteria (315).
Studies have focused on the role of soy isoflavones in the prevention of breast cancer and other menopausal symptoms in postmenopausal women due to its phytoestrogenic effects (267). Moreover, estrogen is believed to have neuroprotective properties that play a significant role in cognitive function. There is a growing interest in exploring the beneficial effects of soy intake on cognitive functions due to its estrogen-like activity. Clinical studies have shown that soy isoflavone supplementation improves cognition and visual memory among postmenopausal women (316–318). Soy consumption has also been shown to improve memory, thinking, and behavior in Alzheimer’s patients through mechanisms such as reducing amyloid β, decreasing tau phosphorylation, and inhibiting the mitochondrial apoptotic pathway (319). Similarly, animal studies have concluded that dietary soy isoflavone supplementation improves spatial learning and memory skills in mice (320, 321).
Epidemiological studies support the important roles of estrogen in brain development and function. Therefore, early-life exposure to phytoestrogen-rich soy diets may influence early neurodevelopment in children. For example, fetuses and infants can access phytoestrogens through maternal routes via the placenta or breast milk (322). Phytoestrogens can bind to ER and activate estrogen-related signaling in the brain, which may influence the programming processes of the CNS (323). In a cohort study conducted on Chinese children, a moderate level of prenatal isoflavone exposure has been found to relate to a decreased risk of childhood neurobehavioral problems such as depression and anxiety. However, a high level of prenatal exposure negatively impacted behavioral health (324). Another study found that high prenatal isoflavone consumption decreased the risk of hyperactivity in Japanese children (325). This dose-dependent response seen in human cohort studies has been validated in animal studies, showing that a high level of prenatal soy exposure is related to an increased risk of offensive and depressed behaviors in mice (323, 326). Therefore, the dose and timing of soy isoflavone exposure may be critical in determining the outcomes of neurobehavioral development.
In animal studies, maternal exposure to soy isoflavone, daidzein, during pregnancy and lactation resulted in enhanced social affiliation and improved spatial memory associated with reduced ERα expression in the brain of the female offspring, suggesting that maternal daidzein may influence social and cognitive behaviors through, at least in part, altering ERα pathway (327). A large longitudinal study in children found that feeding soy formula during infancy was related to reduced female-typical play behavior (328). Moreover, a potential association between soy formula and autistic behaviors was found in humans (329). While the exact mechanisms remain unclear, some evidence suggests that the neuroprotective effects of isoflavones may be linked to their ability to regulate the expression of apoptosis-related genes, thereby inhibiting the mitochondrial apoptotic pathway in the brain (324, 330). Supportively, a rodent study found that the soy genistein intervention reduced apoptosis in hippocampal neurons (330).
Intensive studies have investigated the effects of soy isoflavones on the gut microbiome, bacterial metabolites, and host physiological responses. It was found that soy isoflavones can alter the composition and diversity of gut microbial communities. Additionally, soy isoflavones influence the production of bacterial metabolites such as SCFAs and neurotransmitter precursors, which play critical roles in maintaining gut and brain health (331, 332). Specifically, soy isoflavone treatment enhanced intestinal homeostasis, significantly reducing the levels of Klebsiella, Pseudomonas, Acinetobacter, Escherichia-Shigella, and Staphylococcus. Meanwhile, the relative abundance of beneficial bacteria, including Bifidobacterium, Bacteroides, Dorea, Faecalibacterium, and Lactobacillus, significantly increased (332). In another study, soy isoflavones significantly decreased the Firmicutes species and lowered the Firmicutes-to-Bacteroidetes (F/B) ratio, while increasing the compositions of Proteobacteria and Actinobacteria (333). Research indicates that memory ability is closely linked to gut microbiota composition, with Bacteroidetes and Firmicutes being the dominant phyla (334). Studies have shown that reduced microbiota diversity, decreased Actinobacteria, and an elevated F/B ratio were associated with cognitive decline, particularly in aging and neurodegenerative conditions (334). In this context, the observed reduction in the F/B ratio and microbial composition changes may correspond to improved memory retention following soy isoflavone treatment (333, 335). While extensive research has examined soy-induced gut microbiota changes and their role in aging-related cognitive decline, studies on its impact on brain development and memory function in children remain limited, underscoring a critical gap that requires further investigation.
6.4 Curcumin
Curcumin, a natural compound classified as a curcuminoid, has been used as a traditional Asian medicine due to its potent antioxidant and anti-inflammatory properties. Curcumin has been implicated in its roles in enhancing brain health and functions, validated in both in vivo and in vitro studies (336). Numerous studies indicate that curcumin could be a promising therapy for neurological disorders, as it can cross the BBB and exert its effects through Nrf2 activation and neuroinflammation suppression (337). Curcumin can also induce antidepressant effects by inhibiting the immobility period, increasing serotonin and dopamine levels, and inhibiting the MAO (338). Curcumin enhanced memory and learning abilities by boosting the cholinergic system and antioxidant activity (339). Furthermore, the beneficial effects of curcumin on long-term depression and anxiety have been linked to the upregulation of the brain-derived neurotrophic factor (BDNF) and the reduction of inflammatory factors in the brain (340). By reversing BDNF expression and extracellular signal-regulated kinase in the hippocampus, curcumin improved cognitive and behavioral parameters (341). Interestingly, curcumin also promoted the synthesis of PUFAs, such as DHA from its precursor, α-linolenic acid, and related enzymes in the liver and brain (342). Correspondingly, elevated DHA was associated with a reduction in anxiety-related behaviors in mice (343).
Beyond its impacts on neurological disorders and age-related neurodegenerative diseases, early-life curcumin supports brain development and hippocampal neurogenesis, enhancing neural plasticity and promoting neural repair (344). Specifically, maternal curcumin encourages the proliferation of embryonic cortical neural stem cells (NSCs) by activating ERK and p38 MAP kinase pathways, which are important for primary NSC proliferation (344). Besides maternal nutrition, curcumin exhibits significant neuroprotective benefits by mitigating the toxic effects of various environmental agents. For example, it counteracted Bisphenol-A induced neurogenesis impairment in developing rat brains by enhancing the proliferation and differentiation of NSCs through the Wnt/β-catenin signaling. Curcumin also alleviated mercury-induced neurodevelopmental deficits, restoring neurotransmitter balance and reducing anxiety in mouse offspring (345, 346). Similar neuroprotective effects of curcumin were observed when exposed to lead (347), valproic acid (348), fluoride (349), and alcohol (350) during pregnancy.
During the neonatal period, curcumin supplementation has been shown to improve autism-related symptoms in mouse models by increasing social interactions, reducing repetitive behaviors, and enhancing cognitive functions. Also, curcumin reversed the suppression of hippocampal neurogenesis (312). In the rat model of autism, neonatal curcumin supplementation can effectively improve birth weight and brain volume and restore several impaired parameters, including the depleted IFN-γ, serotonin, and glutamine (348). In another idiopathic mouse model of ASD, curcumin-enhanced α7-nicotinic acetylcholine receptors in CNS neurons reduced oxidative stress and alleviated ASD-like behavior, suggesting its potential as a novel pharmacological approach for ASD treatment (351). Studies regarding the effects of curcumin on ADHD are scarce. However, a preliminary behavioral study found that mice treated with curcumin exhibited less anxious and hyperactive behavior in the open field test and the social interaction test (352). Several clinical studies suggest that curcumin may be a beneficial antidepressant agent. However, these results remain inconclusive (353–356). Taken together, the neuroprotective effects of curcumin are promising, with significantly more research on its effects on depression and cognitive function than on its impact on ASD and ADHD.
Curcumin undergoes biotransformation through enzymes produced by enterocytes and hepatocytes, and is further metabolized by enzymes from the gut microbiota. These microbial enzymes can convert curcumin into bioactive derivatives, enhancing its stability, bioavailability, and therapeutic effects. Specifically, curcumin is metabolized by gut bacteria, such as E. coli and Blautia sp., producing bioactive derivatives, including tetrahydrocurcumin, demethylcurcumin, and ferulic acid (357). The pharmacological and pharmacokinetic properties of curcumin are mediated by several gut microbes, including Bifidobacteria pseudocatenulatum, Enterococcus faecalis, Bifidobacteria longum, Lactobacillus acidophilus, and Lactobacillus casei (358). On the other hand, curcumin administration improves gut microbial balance by increasing beneficial Bifidobacteria and Lactobacilli while reducing pathogenic Prevotellaceae, Coriobacterales, Enterobacteria, and Enterococci (359). In a mouse study, curcumin consumption led to a decrease in microbial diversity and richness, especially those bacteria triggering the onset of cancer and systemic diseases such as Prevotellaceae, Bacteroidaceae, and Rikenellaceae (357, 360). Curcumin and its gut microbiota-derived metabolites, particularly tetrahydrocurcumin, exhibit strong neuroprotective effects. They combat oxidative stress by enhancing antioxidant enzyme activity and modulating key pathways (PI3K/Akt, AMPK, Nrf2) involved in neuronal survival (357, 361). These findings highlight the crucial interplay between curcumin and the gut microbiota, emphasizing its role in modulating microbial composition and enhancing bioavailability through microbial metabolism. Given the neuroprotective effects and potential therapeutic applications of curcumin, future research should focus on optimizing its bioavailability and exploring its long-term impact on gut microbiota and neurological health.
6.5 Other bioactive phytochemicals
Research on bioactive phytochemicals is promising as emerging evidence highlights their potential to support neurodevelopment. In addition to the phytochemicals mentioned above, several other compounds, such as resveratrol, flavonoids, lutein, and carotenoids, play significant roles in neurodevelopment and cognitive function.
Resveratrol, commonly found in grapes, peanuts, and berries, is a natural polyphenol compound with antioxidant, anti-inflammatory, and neuroprotective properties. It can cross the BBB and regulate brain functions related to cognition, memory, and emotion, acting as a neuroprotective biofactor (362). Due to its antioxidant and anti-inflammatory effects, resveratrol has been explored in animal models of neurological diseases, implicating its beneficial effects in ASD (363). Clinical studies suggest that resveratrol, when used as an adjuvant treatment, improves ASD-related symptoms, such as irritability, hyperactivity, and non-compliance in children (364, 365). In animal models, resveratrol has demonstrated the ability to mitigate neuroinflammation in propionic acid-induced ASD mice, counteracting their neurological, sensory, behavioral, and molecular deficits. By modulating inflammatory cytokines and oxidative stress, resveratrol helps restore neuronal function and synaptic plasticity (366). Similarly, in the BTBR model of ASD, resveratrol exerted similar therapeutic effects by modifying the immune profiles (367). The pharmacological effects of resveratrol via gut microbiome modulation are also of interest. It reshaped the neurotoxin methylmercury-induced gut microbiome composition in mice. Specifically, resveratrol administration counteracted the changes caused by the toxin, restoring the Firmicutes/Bacteroidetes ratio and increasing Lactobacillus and Muribaculaceae_unclassified while reducing the abundance of the toxin-upregulated genera (368). Interestingly, resveratrol and gut microbiota have a mutual influence. Resveratrol alters microbial composition while being metabolized by certain bacteria. Bifidobacteria infantis and Lactobacillus acidophilus contribute to piceid production from resveratrol (369). Additionally, resveratrol enhances gut microbiota diversity by inhibiting Enterococcus faecalis and promoting the growth of Lactobacillus and Bifidobacterium (370). In addition to the changes in gut microbiome composition, resveratrol is also involved in the regulation of glucagon-like peptide 1 and the 5-hydroxytryptamine level, ultimately impacting brain function via the GBA (371).
Flavonoids, abundant in fruits, vegetables, and tea, support neuroprotection by reducing neuroinflammation and promoting synaptic plasticity. Their effects on the brain are attributed to their ability to protect neurons, enhance neuronal function, and promote neurogenesis. Evidence indicates that flavonoids exert neuroprotective actions by modulating key intracellular signaling pathways involved in neuronal survival, differentiation, and memory formation (372). Preclinical studies suggest that flavonoids possess antidepressant properties by reversing depression-related deficits through their antioxidant activity, neurotransmitter modulation, increased BDNF levels, and regulation of key enzyme activities (373). One intervention trial in children demonstrated positive mood effects following the consumption of a flavonoid-rich blueberry drink, likely due to the increased cerebral blood flow and inhibited MAO activity (374). A similar intervention trial in young adults found improvements in depression and gut microbiome composition (375). Beyond depression, flavonoid administration has been shown to improve ASD-related features, including socialization deficits and repetitive or stereotypic behaviors in both animal and human studies (376). Although promising, more human studies are needed to confirm the efficacy of flavonoids as a therapeutic approach. Besides, flavonoids alter gut microbiota by replacing harmful pathogens with beneficial bacteria and promoting the production of SCFAs (377). In an intervention trial of depression, flavonoids increased Bacteroidetes and Actinobacteria phyla, as well as Lachnospiraceae, Bifidobacteriaceae, and Akkermansiaceae families. At the genus level, genera like Lactobacillus, Alistipes, Roseburia, Akkermansia, Bifidobacterium, and Collinsella became more abundant, while Faecalibacterium, Streptococcus, and Eubacterium_g23 showed lower abundance (375). They also found a positive correlation between the BDNF level and the Lachnospiraceae family. These changes suggest that flavonoid treatment altered gut microbiota composition, which may play a role in its therapeutic effects on depression (375).
Carotenoids, a class of naturally occurring pigments in fruits and vegetables, play essential roles in brain health and cognitive function. Lutein and zeaxanthin, which are particularly abundant in dark leafy greens, can cross the BBB and accumulate in the brain and retina (378). In the developing brain, lutein constitutes a significantly larger proportion of total carotenoids compared to adults, underscoring its importance in early cognitive function, visual processing, and overall brain maturation during childhood (379). Chronic inflammation is associated with reduced BDNF expression, which impairs neuroplasticity and cognitive function. The anti-inflammatory capability of lutein can increase the BDNF (380). In adults, lutein and zeaxanthin levels are positively related to better cognitive function. Recent studies have shown that early consumption of lutein and zeaxanthin may improve childhood receptive vocabulary and mid-childhood executive function (381). One meta-analysis found that carotenoids are effective in improving depressive symptoms, with animal studies supporting this by showing that zeaxanthin treatment reduced the levels of IL-6, IL-1β, and TNF-α in the hippocampus (382). Evidence of the interaction between carotenoids and the gut microbiome is still lacking. One study showed that all four carotenoids, including β-carotene, lutein, lycopene, and astaxanthin, increased the abundance of Roseburia and Parasutterella and inhibited the abundance of Collinsella (383). Moreover, carotenoids with different structures have distinct impacts on the gut microbiome. For example, β-Carotene notably increased SCFA production by promoting the growth of SCFA-producing bacteria. Xanthophylls such as lutein and zeaxanthin have a more significant impact on the gut microbiome than carotenes (383).
Overall, these phytochemicals play essential roles in brain function, as shown in Table 2. Their neuroprotective properties contribute to cognitive function, emotional regulation, and neurodevelopment, making them promise for supporting brain health and alleviating neurological disorders. The effects of many other dietary compounds remain underexplored, particularly in the context of their interactions with the GBA. Further research is needed to identify and characterize these compounds and elucidate their mechanisms of action in brain and microbiome health.
7 Limitation
While this review highlights the extensive evidence about the association between early-life nutrition, the gut microbiome, and neurodevelopment, several limitations should be acknowledged. First, although maternal nutrition and childhood behaviors are well studied, paternal influences, including paternal age, diet, health status, stress, and microbiome contributions, were not explored in depth in this review. Emerging research suggests that paternal factors can also impact offspring neurodevelopment through epigenetic and microbial pathways, and their exclusion may give an incomplete picture of early-life factors of brain health (13, 384, 385). Moreover, while findings from animal studies, clinical trials, and epidemiological research suggest a compelling role for dietary interventions in supporting neurodevelopment and gut health, the evidence remains mixed, particularly in conditions such as ADHD. Inconsistent results may arise from methodological variability, differences in microbiome analysis, heterogeneous age groups, and the presence of comorbidities. These factors highlight the need for more standardized, large-scale, and longitudinal studies to better understand causal relationships.
8 Conclusion
The interplay between nutrition, the gut microbiome, and brain development plays a fundamental role in influencing child behavior and cognitive function. This review emphasizes the bidirectional communication between the gut and brain, highlighting the impacts of early-life nutrition on neurodevelopmental outcomes. Dietary patterns, as well as bioactive components in food such as tea polyphenols, broccoli-derived sulforaphane, curcumin, and soy isoflavones, have shown promising neuroprotective effects by modulating oxidative stress, neuroinflammation, and gut microbiota composition. Conversely, disruptions in microbial balance, often influenced by unhealthy dietary patterns and deficits in specific nutrients, have been linked to neurodevelopmental disorders, including ASD, ADHD, and anxiety. Clinically, current results support the potential of early-life nutrition and gut microbiome modulation to promote optimal brain development and mitigate behavioral disorders in children. A holistic approach that integrates maternal and early-life nutrition, dietary patterns, and bioactive nutrient intake may offer a promising strategy for promoting lifelong brain health, cognitive well-being, and a healthy gut system.
Author contributions
YJ: Conceptualization, Writing – original draft. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the United States Department of Agriculture, National Institute of Food and Agriculture (USDA NIFA, 2021–67017-39063 to YL), UMD Maryland Agricultural Experiment Station (MAES) Competitive Grant (MD-NFSC-243085 to YL), and startup fund supported by the Department of Nutrition and Food Science, University of Maryland.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schwarzenberg, SJ, Georgieff, MK, Committee on NutritionDaniels, S, Corkins, M, Golden, NH, et al. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. (2018) 141:e20173716. doi: 10.1542/peds.2017-3716
2. Gilmore, JH, Knickmeyer, RC, and Gao, W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. (2018) 19:123–37. doi: 10.1038/nrn.2018.1
3. Blüml, S, Wisnowski, JL, Nelson, MD, Jr. Paquette, L, Gilles, FH, Kinney, HC, et al. Metabolic maturation of the human brain from birth through adolescence: insights from in Vivo magnetic resonance spectroscopy. Cereb Cortex. (2013) 23:2944–55. doi: 10.1093/cercor/bhs283
4. Zhou, Y, Song, H, and Ming, G. Genetics of human brain development. Nat Rev Genet. (2024) 25:26–45. doi: 10.1038/s41576-023-00626-5
5. Bale, TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. (2015) 16:332–44. doi: 10.1038/nrn3818
6. Wachs, TD, Georgieff, M, Cusick, S, and McEwen, B. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience and psychological research. Ann N Y Acad Sci. (2014) 1308:89–106. doi: 10.1111/nyas.12314
7. Hanley, B, Dijane, J, Fewtrell, M, Grynberg, A, Hummel, S, Junien, C, et al. Metabolic imprinting, programming and epigenetics – a review of present priorities and future opportunities. Br J Nutr. (2010) 104:S1–S25. doi: 10.1017/S0007114510003338
8. Likhar, A, and Patil, MS. Importance of maternal nutrition in the first 1,000 days of life and its effects on child development: a narrative review. Cureus. (2022). 14:e30083. doi: 10.7759/cureus.30083
9. Mahmassani, HA, Switkowski, KM, Scott, TM, Johnson, EJ, Rifas-Shiman, SL, Oken, E, et al. Maternal diet quality during pregnancy and child cognition and behavior in a US cohort. Am J Clin Nutr. (2021) 115:128–41. doi: 10.1093/ajcn/nqab325
10. Hibbeln, JR, Spiller, P, Brenna, JT, Golding, J, Holub, BJ, Harris, WS, et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: two systematic reviews. Prostaglandins Leukot Essent Fatty Acids. (2019) 151:14–36. doi: 10.1016/j.plefa.2019.10.002
11. Bolduc, FV, Lau, A, Rosenfelt, CS, Langer, S, Wang, N, Smithson, L, et al. Cognitive enhancement in infants associated with increased maternal fruit intake during pregnancy: results from a birth cohort study with validation in an animal model. EBioMedicine. (2016) 8:331–40. doi: 10.1016/j.ebiom.2016.04.025
12. Gignac, F, Romaguera, D, Fernández-Barrés, S, Phillipat, C, Garcia Esteban, R, López-Vicente, M, et al. Maternal nut intake in pregnancy and child neuropsychological development up to 8 years old: a population-based cohort study in Spain. Eur J Epidemiol. (2019) 34:661–73. doi: 10.1007/s10654-019-00521-6
13. Janecka, M, Mill, J, Basson, MA, Goriely, A, Spiers, H, Reichenberg, A, et al. Advanced paternal age effects in neurodevelopmental disorders—review of potential underlying mechanisms. Transl Psychiatry. (2017) 7:e1019. doi: 10.1038/tp.2016.294
14. Collado, MC, Rautava, S, Aakko, J, Isolauri, E, and Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. (2016) 6:23129. doi: 10.1038/srep23129
15. Borre, YE, O’Keeffe, GW, Clarke, G, Stanton, C, Dinan, TG, and Cryan, JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. (2014) 20:509–18. doi: 10.1016/j.molmed.2014.05.002
16. Heijtz, RD, Wang, S, Anuar, F, Qian, Y, Björkholm, B, Samuelsson, A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci. (2011) 108:3047–52. doi: 10.1073/pnas.1010529108
17. Dash, S, Syed, YA, and Khan, MR. Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disorders. Front Cell Dev Biol. (2022) 10:880544. doi: 10.3389/fcell.2022.880544
18. Stiles, J, and Jernigan, TL. The basics of brain development. Neuropsychol Rev. (2010) 20:327–48. doi: 10.1007/s11065-010-9148-4
19. Tierney, AL, and Nelson, CA. Brain development and the role of experience in the early years. Zero Three. (2009) 30:9–13.
20. Gressens, P, Mesples, B, Sahir, N, Marret, S, and Sola, A. Environmental factors and disturbances of brain development. Semin Neonatol. (2001) 6:185–94. doi: 10.1053/siny.2001.0048
21. Jash, S, and Sharma, S. Pathogenic infections during pregnancy and the consequences for fetal brain development. Pathogens. (2022) 11:193. doi: 10.3390/pathogens11020193
22. Rodriguez, A, Miettunen, J, Henriksen, TB, Olsen, J, Obel, C, Taanila, A, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes. (2008) 32:550–7. doi: 10.1038/sj.ijo.0803741
23. Reynolds, LC, Inder, TE, Neil, JJ, Pineda, RG, and Rogers, CE. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J Perinatol. (2014) 34:688–92. doi: 10.1038/jp.2014.80
24. de Rooij, SR, Wouters, H, Yonker, JE, Painter, RC, and Roseboom, TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci. (2010) 107:16881–6. doi: 10.1073/pnas.1009459107
25. Miller, SL, Huppi, PS, and Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. (2016) 594:807–23. doi: 10.1113/JP271402
26. Tolsa, CB, Zimine, S, Warfield, SK, Freschi, M, Rossignol, AS, Lazeyras, F, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. (2004) 56:132–8. doi: 10.1203/01.PDR.0000128983.54614.7E
27. Spenrath, MA, Clarke, ME, and Kutcher, S. The science of brain and biological development: implications for mental Health Research, practice and policy. J Can Acad Child Adolesc Psychiatry. (2011) 20:298–304.
28. Monaco, AP. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur J Hum Genet. (2021) 29:387–95. doi: 10.1038/s41431-020-00726-4
29. Ogundele, MO. Behavioural and emotional disorders in childhood: a brief overview for paediatricians. World J Clin Pediatr. (2018) 7:9–26. doi: 10.5409/wjcp.v7.i1.9
30. Data and statistics on children’s Mental Health [Internet]. Centers for Disease Control and Prevention. (2025). Available at: https://www.cdc.gov/children-mental-health/data-research/index.html (Accessed May 27, 2025).
31. Offerman, ECP, Asselman, MW, Bolling, F, Helmond, P, Stams, G-JJM, and Lindauer, RJL. Prevalence of adverse childhood experiences in students with emotional and behavioral disorders in special education schools from a multi-informant perspective. Int J Environ Res Public Health. (2022) 19:3411. doi: 10.3390/ijerph19063411
32. Smith, JD, Dishion, TJ, Shaw, DS, Wilson, MN, Winter, CC, and Patterson, GR. Coercive family process and early-onset conduct problems from age 2 to school entry. Dev Psychopathol. (2014) 26:917–32. doi: 10.1017/S0954579414000169
33. Copeland, WE, Shanahan, L, Costello, EJ, and Angold, A. Childhood and adolescent psychiatric disorders as predictors of Young adult disorders. Arch Gen Psychiatry. (2009) 66:764–72. doi: 10.1001/archgenpsychiatry.2009.85
34. Kessler, RC, Berglund, P, Demler, O, Jin, R, Merikangas, KR, and Walters, EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. (2005) 62:593–602. doi: 10.1001/archpsyc.62.6.593
35. Huttenlocher, PR, and Dabholkar, AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. (1997) 387:167–78. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z
36. Spann, MN, Bansal, R, Rosen, TS, and Peterson, BS. Morphological features of the neonatal brain support development of subsequent cognitive, language, and motor abilities. Hum Brain Mapp. (2014) 35:4459–74. doi: 10.1002/hbm.22487
37. Short, SJ, Elison, JT, Goldman, BD, Styner, M, Gu, H, Connelly, M, et al. Associations between white matter microstructure and infants’ working memory. NeuroImage. (2013) 64:156–66. doi: 10.1016/j.neuroimage.2012.09.021
38. Paus, T, Zijdenbos, A, Worsley, K, Collins, DL, Blumenthal, J, Giedd, JN, et al. Structural maturation of neural pathways in children and adolescents: in Vivo study. Science. (1999) 283:1908–11. doi: 10.1126/science.283.5409.1908
39. Lange, N, Froimowitz, MP, Bigler, ED, and Lainhart, JEBrain Development Cooperative Group. Associations between IQ, Total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol. (2010) 35:296–317. doi: 10.1080/87565641003696833
40. Giedd, JN, Blumenthal, J, Jeffries, NO, Castellanos, FX, Liu, H, Zijdenbos, A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. (1999) 2:861–3. doi: 10.1038/13158
41. Reiss, AL, Abrams, MT, Singer, HS, Ross, JL, and Denckla, MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. (1996) 119:1763–74. doi: 10.1093/brain/119.5.1763
42. Quaglio, AEV, Grillo, TG, De Oliveira, ECS, Di Stasi, LC, and Sassaki, LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. (2022) 28:4053–60. doi: 10.3748/wjg.v28.i30.4053
43. Fan, Y, and Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
44. Yang, I, Corwin, EJ, Brennan, PA, Jordan, S, Murphy, JR, and Dunlop, A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res. (2016) 65:76–88. doi: 10.1097/NNR.0000000000000133
45. Stewart, CJ, Ajami, NJ, O’Brien, JL, Hutchinson, DS, Smith, DP, Wong, MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562:583–8. doi: 10.1038/s41586-018-0617-x
46. Di Profio, E, Magenes, VC, Fiore, G, Agostinelli, M, La Mendola, A, Acunzo, M, et al. Special diets in infants and children and impact on gut Microbioma. Nutrients. (2022) 14:3198. doi: 10.3390/nu14153198
47. Lyons, KE, Ryan, CA, Dempsey, EM, Ross, RP, and Stanton, C. Breast Milk, a source of beneficial microbes and associated benefits for infant health. Nutrients. (2020) 12:1039. doi: 10.3390/nu12041039
48. Savage, JH, Lee-Sarwar, KA, Sordillo, JE, Lange, NE, Zhou, Y, O'Connor, GT, et al. Diet during pregnancy and infancy and the infant intestinal microbiome. J Pediatr. (2018) 203:47–54.e4. doi: 10.1016/j.jpeds.2018.07.066
49. García-Mantrana, I, Selma-Royo, M, González, S, Parra-Llorca, A, Martínez-Costa, C, and Collado, MC. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. (2020) 11:962–78. doi: 10.1080/19490976.2020.1730294
50. Taylor, R, Keane, D, Borrego, P, and Arcaro, K. Effect of maternal diet on maternal Milk and breastfed infant gut microbiomes: a scoping review. Nutrients. (2023) 15:1420. doi: 10.3390/nu15061420
51. Boudry, G, Charton, E, le Huerou-Luron, I, Ferret-Bernard, S, le Gall, S, Even, S, et al. The relationship between breast Milk components and the infant gut microbiota. Front Nutr. (2021) 8:629740. doi: 10.3389/fnut.2021.629740
52. Ma, J, Prince, AL, Bader, D, Hu, M, Ganu, R, Baquero, K, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. (2014) 5:3889. doi: 10.1038/ncomms4889
53. Babu, ST, Niu, X, Raetz, M, Savani, RC, Hooper, LV, and Mirpuri, J. Maternal high-fat diet results in microbiota-dependent expansion of ILC3s in mice offspring. JCI Insight. (2018) 3:e99223. doi: 10.1172/jci.insight.99223
54. Chen, M, Li, S, Arora, I, Yi, N, Sharma, M, Li, Z, et al. Maternal soybean diet on prevention of obesity-related breast cancer through early-life gut microbiome and epigenetic regulation. J Nutr Biochem. (2022) 110:109119. doi: 10.1016/j.jnutbio.2022.109119
55. Carabotti, M, Scirocco, A, Maselli, MA, and Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. (2015) 28:203–9.
56. Morais, LH, Schreiber, HL, and Mazmanian, SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
57. McCorry, LK. Physiology of the autonomic nervous system. Am J Pharm Educ. (2007) 71:78. doi: 10.5688/aj710478
58. Rusch, JA, Layden, BT, and Dugas, LR. Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol. (2023) 14:1130689. doi: 10.3389/fendo.2023.1130689
59. Silva, YP, Bernardi, A, and Frozza, RL. The role of Short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. (2020) 11:25. doi: 10.3389/fendo.2020.00025
60. Dicks, LMT. Gut Bacteria and neurotransmitters. Microorganisms. (2022) 10:1838. doi: 10.3390/microorganisms10091838
61. Yano, JM, Yu, K, Donaldson, GP, Shastri, GG, Ann, P, Ma, L, et al. Indigenous Bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
62. Bresesti, I, Salvatore, S, Valetti, G, Baj, A, Giaroni, C, and Agosti, M. The microbiota-gut Axis in premature infants: physio-pathological implications. Cells. (2022) 11:379. doi: 10.3390/cells11030379
63. Cryan, JF, and Dinan, TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
64. Raskov, H, Burcharth, J, Pommergaard, H-C, and Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. (2016) 7:365–83. doi: 10.1080/19490976.2016.1218585
65. Chen, Y, Xu, J, and Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. (2021) 13:2099. doi: 10.3390/nu13062099
66. Sharon, G, Sampson, TR, Geschwind, DH, and Mazmanian, SK. The central nervous system and the gut microbiome. Cell. (2016) 167:915–32. doi: 10.1016/j.cell.2016.10.027
67. Aburto, MR, and Cryan, JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis. Nat Rev Gastroenterol Hepatol. (2024) 21:222–47. doi: 10.1038/s41575-023-00890-0
68. Settanni, CR, Ianiro, G, Bibbò, S, Cammarota, G, and Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: current evidence on fecal microbiota transplantation. Prog Neuro-Psychopharmacol Biol Psychiatry. (2021) 109:110258. doi: 10.1016/j.pnpbp.2021.110258
69. Park, JC, and Im, S-H. The gut-immune-brain axis in neurodevelopment and neurological disorders. Microbiome Res Rep. (2022) 1:23. doi: 10.20517/mrr.2022.11
70. Fröhlich, EE, Farzi, A, Mayerhofer, R, Reichmann, F, Jačan, A, Wagner, B, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun. (2016) 56:140–55. doi: 10.1016/j.bbi.2016.02.020
71. Salami, M. Interplay of good Bacteria and central nervous system: cognitive aspects and mechanistic considerations. Front Neurosci. (2021) 15:613120. doi: 10.3389/fnins.2021.613120
72. Luczynski, P, McVey Neufeld, K-A, Oriach, CS, Clarke, G, Dinan, TG, and Cryan, JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. (2016) 19:pyw020. doi: 10.1093/ijnp/pyw020
73. Gareau, MG, Wine, E, Rodrigues, DM, Cho, JH, Whary, MT, Philpott, DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. (2011) 60:307–17. doi: 10.1136/gut.2009.202515
74. Loh, JS, Mak, WQ, Tan, LKS, Ng, CX, Chan, HH, Yeow, SH, et al. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. (2024) 9:37–53. doi: 10.1038/s41392-024-01743-1
75. Lynch, CMK, Cowan, CSM, Bastiaanssen, TFS, Moloney, GM, Theune, N, van de Wouw, M, et al. Critical windows of early-life microbiota disruption on behaviour, neuroimmune function, and neurodevelopment. Brain Behav Immun. (2023) 108:309–27. doi: 10.1016/j.bbi.2022.12.008
76. Wang, T., Hu, X, Liang, S, Li, W, Wu, X, Wang,, et al., Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes. (2015) 6:707–717. doi: 10.3920/BM2014.0177
77. Erny, D, Hrabě de Angelis, AL, Jaitin, D, Wieghofer, P, Staszewski, O, David, E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
78. Minter, MR, Zhang, C, Leone, V, Ringus, DL, Zhang, X, Oyler-Castrillo, P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. (2016) 6:30028. doi: 10.1038/srep30028
79. Fuertes, M, Gonçalves, JL, Faria, A, Lopes-dos-Santos, P, Conceição, IC, and Dionisio, F. Maternal sensitivity and mother-infant attachment are associated with antibiotic uptake in infancy. J Health Psychol. (2022) 27:2197–210. doi: 10.1177/1359105320941245
80. Warner, BB. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res. (2019) 85:216–24. doi: 10.1038/s41390-018-0191-9
81. Soltysova, M, Tomova, A, and Ostatnikova, D. Gut microbiota profiles in children and adolescents with psychiatric disorders. Microorganisms. (2022) 10:2009. doi: 10.3390/microorganisms10102009
82. Adams, JB, Johansen, LJ, Powell, LD, Quig, D, and Rubin, RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterol. (2011) 11:22. doi: 10.1186/1471-230X-11-22
83. Maenner, MJ. Prevalence and characteristics of autism Spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. (2023) 72:1–14. doi: 10.15585/mmwr.ss7202a1
84. Ostrowski, J, Religioni, U, Gellert, B, Sytnik-Czetwertyński, J, and Pinkas, J. Autism Spectrum disorders: etiology, epidemiology, and challenges for public health. Med Sci Monit Int Med J Exp Clin Res. (2024) 30:e944161. doi: 10.12659/MSM.944161
85. Bundgaard-Nielsen, C, Knudsen, J, Leutscher, PDC, Lauritsen, MB, Nyegaard, M, Hagstrøm, S, et al. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: a systematic literature review. Gut Microbes. (2020) 11:1172–87. doi: 10.1080/19490976.2020.1748258
86. Fattorusso, A, Di Genova, L, Dell’Isola, GB, Mencaroni, E, and Esposito, S. Autism Spectrum disorders and the gut microbiota. Nutrients. (2019) 11:521. doi: 10.3390/nu11030521
87. Gorrindo, P, Williams, KC, Lee, EB, Walker, LS, McGrew, SG, and Levitt, P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. (2012) 5:101–8. doi: 10.1002/aur.237
88. Alamoudi, MU, Hosie, S, Shindler, AE, Wood, JL, Franks, AE, and Hill-Yardin, EL. Comparing the gut microbiome in autism and preclinical models: a systematic review. Front Cell Infect Microbiol. (2022) 12:905841. doi: 10.3389/fcimb.2022.905841
89. Liu, F, Li, J, Wu, F, Zheng, H, Peng, Q, and Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Transl Psychiatry. (2019) 9:43. doi: 10.1038/s41398-019-0389-6
90. Frye, RE, Rose, S, Slattery, J, and MacFabe, DF. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microb Ecol Health Dis. (2015) 26:27458. doi: 10.3402/mehd.v26.27458
91. Danielson, ML, Claussen, AH, Bitsko, RH, Katz, SM, Newsome, K, Blumberg, SJ, et al. ADHD prevalence among U.S. children and adolescents in 2022: diagnosis, severity, co-occurring disorders, and treatment. J Clin Child Adolesc Psychol Off J Soc Clin Child Adolesc Psychol Am Psychol Assoc Div. (2024) 53:343–60. doi: 10.1080/15374416.2024.2335625
92. Thapar, A, Cooper, M, Jefferies, R, and Stergiakouli, E. What causes attention deficit hyperactivity disorder? Arch Dis Child. (2012) 97:260–5. doi: 10.1136/archdischild-2011-300482
93. Cassidy-Bushrow, AE, Sitarik, AR, Johnson, CC, Johnson-Hooper, TM, Kassem, Z, Levin, AM, et al. Early-life gut microbiota and attention deficit hyperactivity disorder in preadolescents. Pediatr Res. (2023) 93:2051–60. doi: 10.1038/s41390-022-02051-6
94. Shareghfarid, E, Sangsefidi, ZS, Salehi-Abargouei, A, and Hosseinzadeh, M. Empirically derived dietary patterns and food groups intake in relation with attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. Clin Nutr ESPEN. (2020) 36:28–35. doi: 10.1016/j.clnesp.2019.10.013
95. Lange, KW, Nakamura, Y, and Reissmann, A. Diet and food in attention-deficit hyperactivity disorder. J Future Foods. (2022) 2:112–8. doi: 10.1016/j.jfutfo.2022.03.008
96. Ming, X, Chen, N, Ray, C, Brewer, G, Kornitzer, J, and Steer, RA. A gut feeling: a hypothesis of the role of the microbiome in attention-deficit/hyperactivity disorders. Child Neurol Open. (2018) 5:86799. doi: 10.1177/2329048X18786799
97. Wang, Q, Yang, Q, and Liu, X. The microbiota–gut–brain axis and neurodevelopmental disorders. Protein Cell. (2023) 14:762–75. doi: 10.1093/procel/pwad026
98. Aarts, E, Ederveen, THA, Naaijen, J, Zwiers, MP, Boekhorst, J, Timmerman, HM, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. (2017) 12:e0183509. doi: 10.1371/journal.pone.0183509
99. Jiang, H, Zhou, YY, Zhou, GL, Li, YC, Yuan, J, Li, XH, et al. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav Brain Res. (2018) 347:408–13. doi: 10.1016/j.bbr.2018.03.036
100. Wan, L, Ge, W-R, Zhang, S, Sun, Y-L, Wang, B, and Yang, G. Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder. Front Neurosci. (2020) 14:127. doi: 10.3389/fnins.2020.00127
101. Szopinska-Tokov, J, Dam, S, Naaijen, J, Konstanti, P, Rommelse, N, Belzer, C, et al. Investigating the gut microbiota composition of individuals with attention-deficit/hyperactivity disorder and association with symptoms. Microorganisms. (2020) 8:406. doi: 10.3390/microorganisms8030406
102. Cickovski, T, Mathee, K, Aguirre, G, Tatke, G, Hermida, A, Narasimhan, G, et al. Attention deficit hyperactivity disorder (ADHD) and the gut microbiome: An ecological perspective. PLoS One. (2023) 18:e0273890. doi: 10.1371/journal.pone.0273890
103. Khalid, S, Williams, CM, and Reynolds, SA. Is there an association between diet and depression in children and adolescents? A systematic review. Br J Nutr. (2016) 116:2097–108. doi: 10.1017/S0007114516004359
104. Kelly, JR, Borre, Y, O' Brien, C, Patterson, E, el Aidy, S, Deane, J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
105. Zheng, P, Zeng, B, Zhou, C, Liu, M, Fang, Z, Xu, X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. (2016) 21:786–96. doi: 10.1038/mp.2016.44
106. Liu, L, Wang, H, Zhang, H, Chen, X, Zhang, Y, Wu, J, et al. Toward a deeper understanding of gut microbiome in depression: the promise of clinical applicability. Adv Sci. (2022) 9:e2203707. doi: 10.1002/advs.202203707
107. Simpson, CA, Diaz-Arteche, C, Eliby, D, Schwartz, OS, Simmons, JG, and Cowan, CSM. The gut microbiota in anxiety and depression – a systematic review. Clin Psychol Rev. (2021) 83:101943. doi: 10.1016/j.cpr.2020.101943
108. Mohajeri, MH, La Fata, G, Steinert, RE, and Weber, P. Relationship between the gut microbiome and brain function. Nutr Rev. (2018) 76:481–96. doi: 10.1093/nutrit/nuy009
109. Sudo, N, Chida, Y, Aiba, Y, Sonoda, J, Oyama, N, Yu, XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
110. Bravo, JA, Forsythe, P, Chew, MV, Escaravage, E, Savignac, HM, Dinan, TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
111. Ling, Z, Cheng, Y, Chen, F, Yan, X, Liu, X, Shao, L, et al. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: a case-control study. Front Immunol. (2022) 13:964910. doi: 10.3389/fimmu.2022.964910
112. Callaghan, B. Nested sensitive periods: how plasticity across the microbiota-gut-brain axis interacts to affect the development of learning and memory. Curr Opin Behav Sci. (2020) 36:55–62. doi: 10.1016/j.cobeha.2020.07.011
113. Lapidot, Y, Maya, M, Reshef, L, Cohen, D, Ornoy, A, Gophna, U, et al. Relationships of the gut microbiome with cognitive development among healthy school-age children. Front Pediatr. (2023) 11:1198792. doi: 10.3389/fped.2023.1198792
114. Bonham, KS, Fahur Bottino, G, McCann, SH, Beauchemin, J, Weisse, E, Barry, F, et al. Gut-resident microorganisms and their genes are associated with cognition and neuroanatomy in children. Sci Adv. (2023) 9:eadi0497. doi: 10.1126/sciadv.adi0497
115. Sordillo, JE, Korrick, S, Laranjo, N, Carey, V, Weinstock, GM, Gold, DR, et al. Association of the Infant gut Microbiome with Early Childhood Neurodevelopmental Outcomes: An ancillary study to the VDAART randomized clinical trial. JAMA Netw Open. (2019) 2:e190905. doi: 10.1001/jamanetworkopen.2019.0905
116. Jiang, H, Ling, Z, Zhang, Y, Mao, H, Ma, Z, Yin, Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
117. Carlson, AL, Xia, K, Azcarate-Peril, MA, Goldman, BD, Ahn, M, Styner, MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. (2018) 83:148–59. doi: 10.1016/j.biopsych.2017.06.021
118. Reynolds, LP, Borowicz, PP, Caton, JS, Crouse, MS, Dahlen, CR, and Ward, AK. Developmental programming of fetal growth and development. Vet Clin North Am Food Anim Pract. (2019) 35:229–47. doi: 10.1016/j.cvfa.2019.02.006
119. Vohr, BR, Poggi Davis, E, Wanke, CA, and Krebs, NF. Neurodevelopment: the impact of nutrition and inflammation during preconception and pregnancy in low-resource settings. Pediatrics. (2017) 139:S38–49. doi: 10.1542/peds.2016-2828F
120. Prado, EL, and Dewey, KG. Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. doi: 10.1111/nure.12102
121. Dobbing, J. Vulnerable periods in developing brain In: J Dobbing, editor. Brain, behaviour, and Iron in the infant diet. London: Springer (1990). 1–17. doi: 10.1007/978-1-4471-1766-7_1
122. Thompson, RA, and Nelson, CA. Developmental science and the media. Early brain development. Am Psychol. (2001) 56:5–15. doi: 10.1037/0003-066x.56.1.5
123. Cortés-Albornoz, MC, García-Guáqueta, DP, Velez-van-Meerbeke, A, and Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: a scoping review. Nutrients. (2021) 13:3530. doi: 10.3390/nu13103530
124. Rosales, FJ, Reznick, JS, and Zeisel, SH. Understanding the role of nutrition in the Brain & Behavioral Development of T oppositional defiant disorder (ODD)lers and preschool children: identifying and overcoming methodological barriers. Nutr Neurosci. (2009) 12:190–202. doi: 10.1179/147683009X423454
125. Kar, BR, Rao, SL, and Chandramouli, BA. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct. (2008) 4:31. doi: 10.1186/1744-9081-4-31
126. Georgieff, MK. Nutrition and the developing brain: nutrient priorities and measurement2. Am J Clin Nutr. (2007) 85:614S–20S. doi: 10.1093/ajcn/85.2.614S
127. Cormack, BE, Harding, JE, Miller, SP, and Bloomfield, FH. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients. (2019) 11:2029. doi: 10.3390/nu11092029
128. Peet, M, and Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. (2005) 65:1051–9. doi: 10.2165/00003495-200565080-00002
129. Wu, A, Ying, Z, and Gomez-Pinilla, F. Dietary Omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. (2004) 21:1457–67. doi: 10.1089/neu.2004.21.1457
130. Bozzatello, P, Brignolo, E, De Grandi, E, and Bellino, S. Supplementation with Omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med. (2016) 5:67. doi: 10.3390/jcm5080067
131. Lange, KW. Omega-3 fatty acids and mental health. Glob Health J. (2020) 4:18–30. doi: 10.1016/j.glohj.2020.01.004
132. Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. (2007) 8:755–65. doi: 10.1038/nrn2212
133. Misner, DL, Jacobs, S, Shimizu, Y, de Urquiza, AM, Solomin, L, Perlmann, T, et al. Vitamin a deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci. (2001) 98:11714–9. doi: 10.1073/pnas.191369798
134. Eyles, D, Burne, T, and McGrath, J. Vitamin D in fetal brain development. Semin Cell Dev Biol. (2011) 22:629–36. doi: 10.1016/j.semcdb.2011.05.004
135. Yates, N. J., Tesic, D, Feindel, KW, Smith, JT, Clarke, MW, Wale, C, et al., Vitamin D is crucial for maternal care and offspring social behaviour in rats. J Endocrinol. (2018). 237:73–85. doi: 10.1530/JOE-18-0008
136. Freedman, R, Hunter, SK, and Hoffman, MC. Prenatal primary prevention of mental illness by micronutrient supplements in pregnancy. Am J Psychiatry. (2018) 175:607–19. doi: 10.1176/appi.ajp.2018.17070836
137. Naninck, EFG, Stijger, PC, and Brouwer-Brolsma, EM. The importance of maternal folate status for brain development and function of offspring. Adv Nutr. (2019) 10:502–19. doi: 10.1093/advances/nmy120
138. Caffrey, A, McNulty, H, Rollins, M, Prasad, G, Gaur, P, Talcott, JB, et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial. BMC Med. (2021) 19:73. doi: 10.1186/s12916-021-01914-9
139. Golub, MS, Keen, CL, Gershwin, ME, and Hendrickx, AG. Developmental zinc deficiency and behavior. J Nutr. (1995) 125:2263S–71S. doi: 10.1093/jn/125.suppl_8.2263S
140. Tümer, Z, and Møller, LB. Menkes disease. Eur J Hum Genet. (2010) 18:511–8. doi: 10.1038/ejhg.2009.187
141. Zieminska, E, Ruszczynska, A, Augustyniak, J, Toczylowska, B, and Lazarewicz, JW. Zinc and copper brain levels and expression of neurotransmitter receptors in two rat ASD models. Front Mol Neurosci. (2021) 14:656740. doi: 10.3389/fnmol.2021.656740
142. Liu, X, Zhong, S, Li, Z, Chen, J, Wang, Y, Lai, S, et al. Serum copper and zinc levels correlate with biochemical metabolite ratios in the prefrontal cortex and lentiform nucleus of patients with major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. (2020) 99:109828. doi: 10.1016/j.pnpbp.2019.109828
143. Cendra-Duarte, E, Canals, J, Becerra-Tomás, N, Jardí, C, Martín-Luján, F, and Arija, V. Maternal dietary patterns and offspring behavioral problems. Pediatr Res. (2024):1–10. doi: 10.1038/s41390-024-03462-3
144. Freitas-Vilela, AA, Pearson, RM, Emmett, P, Heron, J, Smith, ADAC, Emond, A, et al. Maternal dietary patterns during pregnancy and intelligence quotients in the offspring at 8 years of age: findings from the ALSPAC cohort. Matern Child Nutr. (2017) 14:e12431. doi: 10.1111/mcn.12431
145. Mou, Y, Jansen, PW, Sun, H, White, T, and Voortman, T. Diet quality during pregnancy, adolescent brain morphology, and cognitive performance in a population-based cohort. Am J Clin Nutr. (2024) 120:1125–33. doi: 10.1016/j.ajcnut.2024.08.018
146. Pina-Camacho, L, Jensen, SK, Gaysina, D, and Barker, ED. Maternal depression symptoms, unhealthy diet and child emotional–behavioural dysregulation. Psychol Med. (2015) 45:1851–60. doi: 10.1017/S0033291714002955
147. Galera, C, Heude, B, Forhan, A, Bernard, JY, Peyre, H, Van der Waerden, J, et al. Prenatal diet and children’s trajectories of hyperactivity-inattention and conduct problems from 3 to 8 years: the EDEN mother-child cohort. J Child Psychol Psychiatry. (2018) 59:1003–11. doi: 10.1111/jcpp.12898
148. Borge, TC, Biele, G, Papadopoulou, E, Andersen, LF, Jacka, F, Eggesbø, M, et al. The associations between maternal and child diet quality and child ADHD – findings from a large Norwegian pregnancy cohort study. BMC Psychiatry. (2021) 21:139. doi: 10.1186/s12888-021-03130-4
149. Ervin, RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Rep. (2009) 13:1–7.
150. Godfrey, KM, Reynolds, RM, Prescott, SL, Nyirenda, M, Jaddoe, VWV, Eriksson, JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. (2017) 5:53–64. doi: 10.1016/S2213-8587(16)30107-3
151. Urbonaite, G, Knyzeliene, A, Bunn, FS, Smalskys, A, and Neniskyte, U. The impact of maternal high-fat diet on offspring neurodevelopment. Front Neurosci. (2022) 16:909762. doi: 10.3389/fnins.2022.909762
152. Tozuka, Y, Kumon, M, Wada, E, Onodera, M, Mochizuki, H, and Wada, K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int. (2010) 57:235–47. doi: 10.1016/j.neuint.2010.05.015
153. Li, M, Fallin, MD, Riley, A, Landa, R, Walker, SO, Silverstein, M, et al. The Association of Maternal Obesity and Diabetes with Autism and other developmental disabilities. Pediatrics. (2016) 137:e20152206. doi: 10.1542/peds.2015-2206
154. Wang, Y, Tang, S, Xu, S, Weng, S, and Liu, Z. Maternal body mass index and risk of autism Spectrum disorders in offspring: a Meta-analysis. Sci Rep. (2016) 6:34248. doi: 10.1038/srep34248
155. Krakowiak, P, Walker, CK, Bremer, AA, Baker, AS, Ozonoff, S, Hansen, RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. (2012) 129:e1121:–e1128. doi: 10.1542/peds.2011-2583
156. Chan, AYL, Gao, L, Hsieh, MHC, Kjerpeseth, LJ, Avelar, R, Banaschewski, T, et al. Maternal diabetes and risk of attention-deficit/hyperactivity disorder in offspring in a multinational cohort of 3.6 million mother–child pairs. Nat Med. (2024) 30:1416–23. doi: 10.1038/s41591-024-02917-8
157. Buss, C, Entringer, S, Davis, EP, Hobel, CJ, Swanson, JM, Wadhwa, PD, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One. (2012) 7:e37758. doi: 10.1371/journal.pone.0037758
158. Fernandes, C, Grayton, H, Poston, L, Samuelsson, AM, Taylor, PD, Collier, DA, et al. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. (2012) 17:1159–60. doi: 10.1038/mp.2011.164
159. Van Lieshout, RJ, Robinson, M, and Boyle, MH. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can J Psychiatry Rev Can Psychiatr. (2013) 58:151–9. doi: 10.1177/070674371305800305
160. Widen, EM, Kahn, LG, Cirillo, P, Cohn, B, Kezios, KL, and Factor-Litvak, P. Prepregnancy overweight and obesity are associated with impaired child neurodevelopment. Matern Child Nutr. (2017) 14:e12481. doi: 10.1111/mcn.12481
161. Bilder, DA, Bakian, AV, Viskochil, J, Clark, EAS, Botts, EL, Smith, KR, et al. Maternal prenatal weight gain and autism Spectrum disorders. Pediatrics. (2013) 132:e1276–e1283. doi: 10.1542/peds.2013-1188
162. Surén, P, Gunnes, N, Roth, C, Bresnahan, M, Hornig, M, Hirtz, D, et al. Parental obesity and risk of autism Spectrum disorder. Pediatrics. (2014) 133:e1128:–e1138. doi: 10.1542/peds.2013-3664
163. Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. (2010) 51:134–43. doi: 10.1111/j.1469-7610.2009.02133.x
164. Fuemmeler, BF, Zucker, N, Sheng, Y, Sanchez, CE, Maguire, R, Murphy, SK, et al. Pre-pregnancy weight and symptoms of attention deficit hyperactivity disorder and executive functioning behaviors in preschool children. Int J Environ Res Public Health. (2019) 16:667. doi: 10.3390/ijerph16040667
165. Rizzo, T, Silverman, B, Metzger, B, and Cho, N. Behavioral adjustment in children of diabetic mothers. Acta Paediatr. (1997) 86:969–74. doi: 10.1111/j.1651-2227.1997.tb15181.x
166. Mola, CLD, França, GVAD, Quevedo, L, and Horta, BL. Low birth weight, preterm birth and small for gestational age association with adult depression: systematic review and meta-analysis. Br J Psychiatry. (2014) 205:340–7. doi: 10.1192/bjp.bp.113.139014
167. Colman, I, Ataullahjan, A, Naicker, K, and Van Lieshout, RJ. Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry Rev Can Psychiat. (2012) 57:422–8. doi: 10.1177/070674371205700705
168. Monthé-Drèze, C, Rifas-Shiman, SL, Gold, DR, Oken, E, and Sen, S. Maternal obesity and offspring cognition: the role of inflammation. Pediatr Res. (2019) 85:799–806. doi: 10.1038/s41390-018-0229-z
169. Fernandes, DJ, Spring, S, Roy, AR, Qiu, LR, Yee, Y, Nieman, BJ, et al. Exposure to maternal high-fat diet induces extensive changes in the brain of adult offspring. Transl Psychiatry. (2021) 11:149–9. doi: 10.1038/s41398-021-01274-1
170. Andriani, K, Margetaki, K, Kampouri, M, Koutra, K, Bitsios, P, Chalkiadaki, G, et al. Association between high levels of inflammatory markers and cognitive outcomes at 4 years of age: the Rhea mother-child cohort study, Crete, Greece. Cytokine. (2019) 117:1–7. doi: 10.1016/j.cyto.2019.01.010
171. Gumusoglu, SB, Fine, RS, Murray, SJ, Bittle, JL, and Stevens, HE. The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav Immun. (2017) 65:274–83. doi: 10.1016/j.bbi.2017.05.015
172. Fujitani, M, Miyajima, H, Otani, Y, and Liu, X. Maternal and adult interleukin-17A exposure and autism Spectrum disorder. Front Psych. (2022) 13:836181. doi: 10.3389/fpsyt.2022.836181
173. Vuong, B, Odero, G, Rozbacher, S, Stevenson, M, Kereliuk, SM, Pereira, TJ, et al. Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J Neuroinflammation. (2017) 14:80. doi: 10.1186/s12974-017-0859-9
174. Chez, MG, Dowling, T, Patel, PB, Khanna, P, and Kominsky, M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. (2007) 36:361–5. doi: 10.1016/j.pediatrneurol.2007.01.012
175. Kang, SS, Kurti, A, Fair, DA, and Fryer, JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J Neuroinflammation. (2014) 11:156. doi: 10.1186/s12974-014-0156-9
176. Bilbo, SD, and Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. (2010) 24:2104–15. doi: 10.1096/fj.09-144014
177. Vogt, MC, Paeger, L, Hess, S, Steculorum, SM, Awazawa, M, Hampel, B, et al. Neonatal insulin action impairs hypothalamic Neurocircuit formation in response to maternal high-fat feeding. Cell. (2014) 156:495–509. doi: 10.1016/j.cell.2014.01.008
178. Ratsika, A, Codagnone, MG, Bastiaanssen, TFS, Hoffmann Sarda, FA, Lynch, CMK, Ventura-Silva, AP, et al. Maternal high-fat diet-induced microbiota changes are associated with alterations in embryonic brain metabolites and adolescent behaviour. Brain Behav Immun. (2024) 121:317–30. doi: 10.1016/j.bbi.2024.07.020
179. Taoufik, E, Kouroupi, G, Zygogianni, O, and Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: an overview of induced pluripotent stem-cell-based disease models. Open Biol. (2018) 8:180138. doi: 10.1098/rsob.180138
180. Wang, X, Christian, KM, Song, H, and Ming, G. Synaptic dysfunction in complex psychiatric disorders: from genetics to mechanisms. Genome Med. (2018) 10:9. doi: 10.1186/s13073-018-0518-5
181. Buffington, SA, Prisco, GVD, Auchtung, TA, Ajami, NJ, Petrosino, JF, and Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. (2016) 165:1762–75. doi: 10.1016/j.cell.2016.06.001
182. Varian, BJ, Poutahidis, T, DiBenedictis, BT, Levkovich, T, Ibrahim, Y, Didyk, E, et al. Microbial lysate upregulates host oxytocin. Brain Behav Immun. (2016) 61:36–49. doi: 10.1016/j.bbi.2016.11.002
183. Donaldson, ZR, and Young, LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. (2008) 322:900–4. doi: 10.1126/science.1158668
184. Gohir, W, Kennedy, KM, Wallace, JG, Saoi, M, Bellissimo, CJ, Britz-McKibbin, P, et al. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J Physiol. (2019) 597:3029–51. doi: 10.1113/JP277353
185. Masood, W, Annamaraju, P, Khan Suheb, MZ, and Uppaluri, KR. Ketogenic Diet In: StatPearls. Treasure Island, FL: StatPearls Publishing (2024)
186. Edmond, J, Auestad, N, Robbins, RA, and Bergstrom, JD. Ketone body metabolism in the neonate: development and the effect of diet. Fed Proc. (1985) 44:2359–64.
187. Barry, D, Ellul, S, Watters, L, Lee, D, Haluska, R Jr, and White, R. The ketogenic diet in disease and development. Int J Dev Neurosci. (2018) 68:53–8. doi: 10.1016/j.ijdevneu.2018.04.005
188. Kim, DY, Davis, LM, Sullivan, PG, Maalouf, M, Simeone, TA, Brederode, J, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. (2007) 101:1316–26. doi: 10.1111/j.1471-4159.2007.04483.x
189. Sussman, D, Ellegood, J, and Henkelman, M. A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse. BMC Pregnancy Childbirth. (2013) 13:198. doi: 10.1186/1471-2393-13-198
190. Cheng, CM, Kelley, B, Wang, J, Strauss, D, Eagles, DA, and Bondy, CA. A ketogenic diet increases brain insulin-like growth Factor receptor and glucose transporter gene expression. Endocrinology. (2003) 144:2676–82. doi: 10.1210/en.2002-0057
191. Mychasiuk, R, and Rho, JM. Genetic modifications associated with ketogenic diet treatment in the BTBRT+Tf/J mouse model of autism spectrum disorder. Autism Res. (2017) 10:456–71. doi: 10.1002/aur.1682
192. Ziegler, DR, Araújo, E, Rotta, LN, Perry, ML, and Gonçalves, C-A. A ketogenic diet increases protein phosphorylation in brain slices of rats. J Nutr. (2002) 132:483–7. doi: 10.1093/jn/132.3.483
193. Yudkoff, M, Daikhin, Y, Melø, TM, Nissim, I, Sonnewald, U, and Nissim, I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. (2007) 27:415–30. doi: 10.1146/annurev.nutr.27.061406.093722
194. McDaniel, SS, Rensing, NR, Thio, LL, Yamada, KA, and Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. (2011) 52:e7–e11. doi: 10.1111/j.1528-1167.2011.02981.x
195. Sussman, D, van Eede, M, Wong, MD, Adamson, SL, and Henkelman, M. Effects of a ketogenic diet during pregnancy on embryonic growth in the mouse. BMC Pregnancy Childbirth. (2013) 13:109. doi: 10.1186/1471-2393-13-109
196. Ruskin, DN, Murphy, MI, Slade, SL, and Masino, SA. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS One. (2017) 12:e0171643. doi: 10.1371/journal.pone.0171643
197. Ruskin, DN, Svedova, J, Cote, JL, Sandau, U, Rho, JM, Kawamura, M, et al. Ketogenic diet improves Core symptoms of autism in BTBR mice. PLoS One. (2013) 8:e65021. doi: 10.1371/journal.pone.0065021
198. Verpeut, JL, DiCicco-Bloom, E, and Bello, NT. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult engrailed 2 null mice. Physiol Behav. (2016) 161:90–8. doi: 10.1016/j.physbeh.2016.04.001
199. Castro, K, Baronio, D, Perry, IS, Riesgo, S, and Gottfried, C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci. (2017) 20:343–50. doi: 10.1080/1028415X.2015.1133029
200. Spilioti, M, Evangeliou, AE, Tramma, D, Theodoridou, Z, Metaxas, S, Michailidi, E, et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD). Front Hum Neurosci. (2013) 7:858. doi: 10.3389/fnhum.2013.00858
201. Lee, RWY, Corley, MJ, Pang, A, Arakaki, G, Abbott, L, Nishimoto, M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. (2018) 188:205–11. doi: 10.1016/j.physbeh.2018.02.006
202. Evangeliou, A, Vlachonikolis, I, Mihailidou, H, Spilioti, M, Skarpalezou, A, Makaronas, N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. (2003) 18:113–8. doi: 10.1177/08830738030180020501
203. El-Rashidy, O, El-Baz, F, El-Gendy, Y, Khalaf, R, Reda, D, and Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. (2017) 32:1935–41. doi: 10.1007/s11011-017-0088-z
204. Packer, RMA, Law, TH, Davies, E, Zanghi, B, Pan, Y, and Volk, HA. Effects of a ketogenic diet on ADHD-like behavior in dogs with idiopathic epilepsy. Epilepsy Behav. (2016) 55:62–8. doi: 10.1016/j.yebeh.2015.11.014
205. Liu, Y, Yang, C, Meng, Y, Dang, Y, and Yang, L. Ketogenic diet ameliorates attention deficit hyperactivity disorder in rats via regulating gut microbiota. PLoS One. (2023) 18:e0289133. doi: 10.1371/journal.pone.0289133
206. Operto, FF, Matricardi, S, Pastorino, GMG, Verrotti, A, and Coppola, G. The ketogenic diet for the treatment of mood disorders in comorbidity with epilepsy in children and adolescents. Front Pharmacol. (2020) 11:578396. doi: 10.3389/fphar.2020.578396
207. El-Mallakh, RS, and Paskitti, ME. The ketogenic diet have mood-stabilizing properties. Med Hypotheses. (2001) 57:724–6. doi: 10.1054/mehy.2001.1446
208. IJff, DM, Postulart, D, Lambrechts, DAJE, Majoie, MHJM, de Kinderen, RJA, Hendriksen, JGM, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy Behav. (2016) 60:153–7. doi: 10.1016/j.yebeh.2016.04.033
209. Pulsifer, MB, Gordon, JM, Brandt, J, Vining, EP, and Freeman, JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol. (2001) 43:301–6. doi: 10.1017/S0012162201000573
210. Kinsman, SL, Vining, EPG, Quaskey, SA, Mellits, D, and Freeman, JM. Efficacy of the ketogenic diet for intractable seizure disorders: review of 58 cases. Epilepsia. (1992) 33:1132–6. doi: 10.1111/j.1528-1157.1992.tb01770.x
211. Nordli, DR Jr, Kuroda, MM, Carroll, J, Koenigsberger, DY, Hirsch, LJ, Bruner, HJ, et al. Experience with the ketogenic diet in infants. Pediatrics. (2001) 108:129–33. doi: 10.1542/peds.108.1.129
212. Lambrechts, DAJE, Bovens, MJM, de la Parra, NM, Hendriksen, JGM, Aldenkamp, AP, and Majoie, MJM. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurol Scand. (2013) 127:103–8. doi: 10.1111/j.1600-0404.2012.01686.x
213. Rawat, K, Singh, N, Kumari, P, and Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev Neurosci. (2021) 32:143–57. doi: 10.1515/revneuro-2020-0078
214. Klein, MS, Newell, C, Bomhof, MR, Reimer, RA, Hittel, DS, Rho, JM, et al. Metabolomic modeling to monitor host responsiveness to gut microbiota manipulation in the BTBRT+tf/j mouse. J Proteome Res. (2016) 15:1143–50. doi: 10.1021/acs.jproteome.5b01025
215. Newell, C, Bomhof, MR, Reimer, RA, Hittel, DS, Rho, JM, and Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. (2016) 7:37. doi: 10.1186/s13229-016-0099-3
216. Finegold, SM, Dowd, SE, Gontcharova, V, Liu, C, Henley, KE, Wolcott, RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. (2010) 16:444–53. doi: 10.1016/j.anaerobe.2010.06.008
217. Martini, D. Health benefits of Mediterranean diet. Nutrients. (2019) 11:1802. doi: 10.3390/nu11081802
218. Miyake, Y, Tanaka, K, Okubo, H, Sasaki, S, and Arakawa, M. Maternal consumption of vegetables, fruit, and antioxidants during pregnancy and risk for childhood behavioral problems. Nutrition. (2020) 69:110572. doi: 10.1016/j.nut.2019.110572
219. Cendra-Duarte, E, Canals, J, Iglesias-Vázquez, L, Jardí, C, Martín-Luján, F, and Arija, V. Adherence to the Mediterranean diet during pregnancy and behavioural problems at 4 years of age. Matern Child Nutr. (2024) 20:e13700. doi: 10.1111/mcn.13700
220. Arpón, A, Riezu-Boj, JI, Milagro, FI, Marti, A, Razquin, C, Martínez-González, MA, et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J Physiol Biochem. (2016) 73:445–55. doi: 10.1007/s13105-017-0552-6
221. Pontifex, MG, Connell, E, le Gall, G, Lang, L, Pourtau, L, Gaudout, D, et al. A novel Mediterranean diet-inspired supplement ameliorates cognitive, microbial, and metabolic deficits in a mouse model of low-grade inflammation. Gut Microbes. (2024) 16:2363011. doi: 10.1080/19490976.2024.2363011
222. Martínez-Lapiscina, EH, Clavero, P, Toledo, E, Estruch, R, Salas-Salvadó, J, San Julián, B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. (2013) 84:1318–25. doi: 10.1136/jnnp-2012-304792
223. Xu, J, Wang, H, Bian, J, Xu, M, Jiang, N, Luo, W, et al. Association between the maternal Mediterranean diet and perinatal outcomes: a systematic review and Meta-analysis. Adv Nutr. (2023) 15:100159. doi: 10.1016/j.advnut.2023.100159
224. Mesirow, MS, Cecil, C, Maughan, B, and Barker, ED. Associations between prenatal and early childhood fish and processed food intake, conduct problems, and co-occurring difficulties. J Abnorm Child Psychol. (2017) 45:1039–49. doi: 10.1007/s10802-016-0224-y
225. Lv, S, Qin, R, Jiang, Y, Lv, H, Lu, Q, Tao, S, et al. Association of Maternal Dietary Patterns during gestation and offspring neurodevelopment. Nutrients. (2022) 14:730. doi: 10.3390/nu14040730
226. Rijlaarsdam, J, Cecil, CAM, Walton, E, Mesirow, MSC, Relton, CL, Gaunt, TR, et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J Child Psychol Psychiatry. (2017) 58:19–27. doi: 10.1111/jcpp.12589
227. Che, X, Gross, SM, Wang, G, Hong, X, Pearson, C, Bartell, T, et al. Impact of consuming a Mediterranean-style diet during pregnancy on neurodevelopmental disabilities in offspring: results from the Boston birth cohort. Precis Nutr. (2023) 2:e00047. doi: 10.1097/PN9.0000000000000047
228. Steenweg-de Graaff, J, Tiemeier, H, Steegers-Theunissen, RP, Hofman, A, Jaddoe, VW, Verhulst, FC, et al. Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clin Nutr Edinb Scotl. (2014) 33:115–21. doi: 10.1016/j.clnu.2013.03.002
229. Jacka, FN, Ystrom, E, Brantsaeter, AL, Karevold, E, Roth, C, Haugen, M, et al. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: a prospective cohort study. J Am Acad Child Adolesc Psychiatry. (2013) 52:1038–47. doi: 10.1016/j.jaac.2013.07.002
230. House, JS, Mendez, M, Maguire, RL, Gonzalez-Nahm, S, Huang, Z, Daniels, J, et al. Periconceptional maternal Mediterranean diet is associated with favorable offspring behaviors and altered CpG methylation of imprinted genes. Front Cell Dev Biol. (2018) 6:107. doi: 10.3389/fcell.2018.00107
231. Nakaki, A, Crovetto, F, Urru, A, Piella, G, Borras, R, Comte, V, et al. Effects of Mediterranean diet or mindfulness-based stress reduction on fetal and neonatal brain development: a secondary analysis of a randomized clinical trial. Am J Obstet Gynecol MFM. (2023) 5:101188. doi: 10.1016/j.ajogmf.2023.101188
232. Naveed, S, Lakka, T, and Haapala, EA. An overview on the associations between health behaviors and brain health in children and adolescents with special reference to diet quality. Int J Environ Res Public Health. (2020) 17:953. doi: 10.3390/ijerph17030953
233. Zielińska, M, Łuszczki, E, Michońska, I, and Dereń, K. The Mediterranean diet and the Western diet in adolescent depression-current reports. Nutrients. (2022) 14:4390. doi: 10.3390/nu14204390
234. Ríos-Hernández, A, Alda, JA, Farran-Codina, A, Ferreira-García, E, and Izquierdo-Pulido, M. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. (2017) 139:e20162027. doi: 10.1542/peds.2016-2027
235. Nagata, JM, Bashir, A, Weinstein, S, al-Shoaibi, AAA, Shao, IY, Ganson, KT, et al. Social epidemiology of the Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet among early adolescents: the adolescent brain cognitive development study. Pediatr Res. (2024) 96:230–6. doi: 10.1038/s41390-023-02959-7
236. Arouca, A, Michels, N, Moreno, LA, González-Gil, EM, Marcos, A, Gómez, S, et al. Associations between a Mediterranean diet pattern and inflammatory biomarkers in European adolescents. Eur J Nutr. (2018) 57:1747–60. doi: 10.1007/s00394-017-1457-4
237. Jacka, FN, O’Neil, A, Opie, R, Itsiopoulos, C, Cotton, S, Mohebbi, M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. (2017) 15:23. doi: 10.1186/s12916-017-0791-y
238. Huang, L, Tao, Y, Chen, H, Chen, X, Shen, J, Zhao, C, et al. Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet and cognitive function and its decline: a prospective study and Meta-analysis of cohort studies. Am J Clin Nutr. (2023) 118:174–82. doi: 10.1016/j.ajcnut.2023.04.025
239. Torabynasab, K, Shahinfar, H, Jazayeri, S, Effatpanah, M, Azadbakht, L, and Abolghasemi, J. Adherence to the MIND diet is inversely associated with odds and severity of anxiety disorders: a case–control study. BMC Psychiatry. (2023) 23:330. doi: 10.1186/s12888-023-04776-y
240. Bayranj, Z, et al. The relation between MIND diet with odds of attention-deficit/hyperactivity disorder in Iranian children: a case-control study. Child Neuropsychol. 1–15. doi: 10.1080/09297049.2024.2375493
241. Franco, GA, Interdonato, L, Cordaro, M, Cuzzocrea, S, and Paola, RD. Bioactive compounds of the Mediterranean diet as nutritional support to fight neurodegenerative disease. Int J Mol Sci. (2023) 24:7318. doi: 10.3390/ijms24087318
242. Fuemmeler, BF, Lee, CT, Soubry, A, Iversen, ES, Huang, Z, Murtha, AP, et al. DNA methylation of regulatory regions of imprinted genes at birth and its relation to infant temperament. Genet Epigenet. (2016) 8:59–67. doi: 10.4137/GEG.S40538
243. Xiao, Y, Camarillo, C, Ping, Y, Arana, TB, Zhao, H, Thompson, PM, et al. The DNA Methylome and transcriptome of different brain regions in schizophrenia and bipolar disorder. PLoS One. (2014) 9:e95875. doi: 10.1371/journal.pone.0095875
244. van Mil, NH, Steegers-Theunissen, RPM, Bouwland-Both, MI, Verbiest, MMPJ, Rijlaarsdam, J, Hofman, A, et al. DNA methylation profiles at birth and child ADHD symptoms. J Psychiatr Res. (2014) 49:51–9. doi: 10.1016/j.jpsychires.2013.10.017
245. Gonzalez-Nahm, S, Mendez, M, Robinson, W, Murphy, SK, Hoyo, C, Hogan, V, et al. Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ Epigenetics. (2017) 3:7. doi: 10.1093/eep/dvx0007
246. Park, G, Kadyan, S, Hochuli, N, Pollak, J, Wang, B, Salazar, G, et al. A modified Mediterranean-style diet enhances brain function via specific gut-microbiome-brain mechanisms. Gut Microbes. (2024) 16:2323752. doi: 10.1080/19490976.2024.2323752
247. Barber, TM, Kabisch, S, Pfeiffer, AFH, and Weickert, MO. The effects of the Mediterranean diet on health and gut microbiota. Nutrients. (2023) 15:2150. doi: 10.3390/nu15092150
248. Sasaki, T, Kawamura, M, Okuno, C, Lau, K, Riel, J, Lee, MJ, et al. Impact of maternal Mediterranean-type diet adherence on microbiota composition and epigenetic programming of offspring. Nutrients. (2023) 16:47. doi: 10.3390/nu16010047
249. Joseph, J, Cole, G, Head, E, and Ingram, D. Nutrition, brain aging, and neurodegeneration. J Neurosci. (2009) 29:12795–801. doi: 10.1523/JNEUROSCI.3520-09.2009
250. Agarwal, U, Mishra, S, Xu, J, Levin, S, Gonzales, J, and Barnard, ND. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: the GEICO study. Am J Health Promot. (2015) 29:245–54. doi: 10.4278/ajhp.130218-QUAN-72
251. Beezhold, B, Radnitz, C, Rinne, A, and DiMatteo, J. Vegans report less stress and anxiety than omnivores. Nutr Neurosci. (2015) 18:289–96. doi: 10.1179/1476830514Y.0000000164
252. Li, X, Cao, HJ, Xie, SY, Li, KC, Tao, FB, Yang, LS, et al. Adhering to a vegetarian diet may create a greater risk of depressive symptoms in the elderly male Chinese population. J Affect Disord. (2019) 243:182–7. doi: 10.1016/j.jad.2018.09.033
253. Haghighatdoost, F, Mahdavi, A, Mohammadifard, N, Hassannejad, R, Najafi, F, Farshidi, H, et al. The relationship between a plant-based diet and mental health: evidence from a cross-sectional multicentric community trial (LIPOKAP study). PLoS One. (2023) 18:e0284446. doi: 10.1371/journal.pone.0284446
254. Walsh, H, Lee, M, and Best, T. The association between vegan, vegetarian, and omnivore diet quality and depressive symptoms in adults: a cross-sectional study. Int J Environ Res Public Health. (2023) 20:3258. doi: 10.3390/ijerph20043258
255. Lee, MF, Eather, R, and Best, T. Plant-based dietary quality and depressive symptoms in Australian vegans and vegetarians: a cross-sectional study. BMJ Nutr Prev Health. (2022) 4:479–86. doi: 10.1136/bmjnph-2021-000332
256. Darand, M, Hassanizadeh, S, Martami, F, Shareghfarid, E, Hosseinpour-Niazi, S, and Hosseinzadeh, M. A plant-based dietary score and attention deficit/hyperactivity disorder in Iranian children: a case-control study. J Affect Disord. (2022) 313:27–31. doi: 10.1016/j.jad.2022.06.006
257. Yan, S, Cao, H, Gu, C, Ni, L, Tao, H, Shao, T, et al. Dietary patterns are associated with attention-deficit/hyperactivity disorder (ADHD) symptoms among preschoolers in mainland China. Eur J Clin Nutr. (2018) 72:1517–23. doi: 10.1038/s41430-018-0131-0
258. Medawar, E, Huhn, S, Villringer, A, and Veronica Witte, A. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry. (2019) 9:226. doi: 10.1038/s41398-019-0552-0
259. Askari, M, Daneshzad, E, Darooghegi Mofrad, M, Bellissimo, N, Suitor, K, and Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: a systematic review and meta-analysis of observational studies. Crit Rev Food Sci Nutr. (2022) 62:261–71. doi: 10.1080/10408398.2020.1814991
260. Gómez-Pinilla, F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. (2008) 9:568–78. doi: 10.1038/nrn2421
261. Crozier, SR, Godfrey, KM, Calder, PC, Robinson, SM, Inskip, HM, Baird, J, et al. Vegetarian diet during pregnancy is not associated with poorer cognitive performance in children at age 6–7 years. Nutrients. (2019) 11:3029. doi: 10.3390/nu11123029
262. Strasser, B, Gostner, JM, and Fuchs, D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. (2016) 19:55–61. doi: 10.1097/MCO.0000000000000237
263. Hill, MJ. Cereals, cereal fibre and colorectal cancer risk: a review of the epidemiological literature. Eur J Cancer Prev. (1998) 7:S5–S10. doi: 10.1097/00008469-199805000-00002
264. Sidhu, SRK, Kok, CW, Kunasegaran, T, and Ramadas, A. Effect of plant-based diets on gut microbiota: a systematic review of interventional studies. Nutrients. (2023) 15:1510. doi: 10.3390/nu15061510
265. Sutliffe, JT, Wilson, LD, de Heer, HD, Foster, RL, and Carnot, MJ. C-reactive protein response to a vegan lifestyle intervention. Complement Ther Med. (2015) 23:32–7. doi: 10.1016/j.ctim.2014.11.001
266. Klomparens, EA, and Ding, Y. The neuroprotective mechanisms and effects of sulforaphane. Brain Circ. (2019) 5:74–83. doi: 10.4103/bc.bc_7_19
267. Jiang, Y, and Li, Y. Nutrition intervention and microbiome modulation in the Management of Breast Cancer. Nutrients. (2024) 16:2644. doi: 10.3390/nu16162644
268. Singh, K, Connors, SL, Macklin, EA, Smith, KD, Fahey, JW, Talalay, P, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci. (2014) 111:15550–5. doi: 10.1073/pnas.1416940111
269. Shapiro, TA, Fahey, JW, Dinkova-Kostova, AT, Holtzclaw, WD, Stephenson, KK, Wade, KL, et al. Safety, tolerance, and metabolism of broccoli sprout Glucosinolates and Isothiocyanates: a clinical phase I study. Nutr Cancer. (2006) 55:53–62. doi: 10.1207/s15327914nc5501_7
270. Bent, S, Lawton, B, Warren, T, Widjaja, F, Dang, K, Fahey, JW, et al. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol Autism. (2018) 9:35. doi: 10.1186/s13229-018-0218-4
271. Evans, S, and Jude Fuller, D. Initial outcomes from an autism treatment demonstration. Clin Med Investig. (2016) 1:16–9. doi: 10.15761/CMI.1000103
272. Zimmerman, A, Diggins, E, Connors, S, and Singh, K. Sulforaphane treatment of children with autism Spectrum disorder (ASD) – a Progress report (N1.002). Neurology. (2018) 90:2. doi: 10.1212/WNL.90.15_supplement.N1.002
273. Momtazmanesh, S, Amirimoghaddam-Yazdi, Z, Moghaddam, HS, Mohammadi, MR, and Akhondzadeh, S. Sulforaphane as an adjunctive treatment for irritability in children with autism spectrum disorder: a randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin Neurosci. (2020) 74:398–405. doi: 10.1111/pcn.13016
274. Ou, J, Smith, RC, Tobe, RH, Lin, J, Arriaza, J, Fahey, JW, et al. Efficacy of Sulforaphane in treatment of children with autism Spectrum disorder: a randomized double-blind placebo-controlled multi-center trial. J Autism Dev Disord. (2024) 54:628–41. doi: 10.1007/s10803-022-05784-9
275. Magner, M, Thorová, K, Župová, V, Houška, M, Švandová, I, Novotná, P, et al. Sulforaphane treatment in children with autism: a prospective randomized double-blind study. Nutrients. (2023) 15:718. doi: 10.3390/nu15030718
276. Zhang, J, Yao, W, Dong, C, Yang, C, Ren, Q, Ma, M, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. (2017) 39:134–44. doi: 10.1016/j.jnutbio.2016.10.004
277. Yu, J, Guo, M, Gong, Y, Yang, Y, Zhang, J, and Ren, L. Protective effect of sulforaphane against depressive behavior in mice through glucocorticoid receptor antagonism. Food Biosci. (2024) 61:104705. doi: 10.1016/j.fbio.2024.104705
278. Wu, S, Gao, Q, Zhao, P, Gao, Y, Xi, Y, Wang, X, et al. Sulforaphane produces antidepressant-and anxiolytic-like effects in adult mice. Behav Brain Res. (2016) 301:55–62. doi: 10.1016/j.bbr.2015.12.030
279. Pańczyszyn-Trzewik, P, Stachowicz, K, Misztak, P, Nowak, G, and Sowa-Kućma, M. Repeated Sulforaphane treatment reverses depressive-like behavior and exerts antioxidant effects in the olfactory bulbectomy model in mice. Pharmaceuticals (Basel). (2024) 17:762. doi: 10.3390/ph17060762
280. Yao, W, Zhang, JC, Ishima, T, Dong, C, Yang, C, Ren, Q, et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. (2016) 6:30659. doi: 10.1038/srep30659
281. Ghazizadeh-Hashemi, F, Bagheri, S, Ashraf-Ganjouei, A, Moradi, K, Shahmansouri, N, Mehrpooya, M, et al. Efficacy and safety of sulforaphane for treatment of mild to moderate depression in patients with history of cardiac interventions: a randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin Neurosci. (2021) 75:250–5. doi: 10.1111/pcn.13276
282. Yang, J, He, L, Dai, S, Zheng, H, Cui, X, Ou, J, et al. Therapeutic efficacy of sulforaphane in autism spectrum disorders and its association with gut microbiota: animal model and human longitudinal studies. Front Nutr. (2024) 10:1294057. doi: 10.3389/fnut.2023.1294057
283. Nadeem, A, Ahmad, SF, al-Harbi, NO, Attia, SM, Bakheet, SA, Ibrahim, KE, et al. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T+tf/J mice. Behav Brain Res. (2019) 364:213–24. doi: 10.1016/j.bbr.2019.02.031
284. Fujita, Y, Fujita, A, Ishima, T, Hirai, A, Suzuki, S, Suganuma, H, et al. Dietary intake of glucoraphanin during pregnancy and lactation prevents the behavioral abnormalities in the offspring after maternal immune activation. Neuropsychopharmacol Rep. (2020) 40:268–74. doi: 10.1002/npr2.12112
285. Shirai, Y, Fujita, Y, Hashimoto, R, Ohi, K, Yamamori, H, Yasuda, Y, et al. Dietary intake of Sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS One. (2015) 10:e0127244. doi: 10.1371/journal.pone.0127244
286. Wei, Y, Chang, L, Liu, G, Wang, X, Yang, Y, and Hashimoto, K. Long-lasting beneficial effects of maternal intake of sulforaphane glucosinolate on gut microbiota in adult offspring. J Nutr Biochem. (2022) 109:109098. doi: 10.1016/j.jnutbio.2022.109098
287. Pervin, M, Unno, K, Ohishi, T, Tanabe, H, Miyoshi, N, and Nakamura, Y. Beneficial effects of Green tea Catechins on neurodegenerative diseases. Mol J Synth Chem Nat Prod Chem. (2018) 23:1297. doi: 10.3390/molecules23061297
288. Ajarem, J, Rashedi, GA, Mohany, M, and Allam, A. Neurobehavioral changes in mice offspring exposed to green tea during fetal and early postnatal development. Behav Brain Funct. (2017) 13:10. doi: 10.1186/s12993-017-0128-1
289. Xu, L, Wang, R, Liu, Y, Zhan, S, Wu, Z, and Zhang, X. Effect of tea polyphenols on the prevention of neurodegenerative diseases through gut microbiota. J Funct Foods. (2023) 107:105669. doi: 10.1016/j.jff.2023.105669
290. Zhang, Z, Zhang, Y, Li, J, Fu, C, and Zhang, X. The neuroprotective effect of tea polyphenols on the regulation of intestinal Flora. Molecules. (2021) 26:3692. doi: 10.3390/molecules26123692
291. Ohishi, T, Fukutomi, R, Shoji, Y, Goto, S, and Isemura, M. The beneficial effects of principal polyphenols from Green tea, coffee, Wine, and curry on obesity. Molecules. (2021) 26:453. doi: 10.3390/molecules26020453
292. Molino, S, Dossena, M, Buonocore, D, Ferrari, F, Venturini, L, Ricevuti, G, et al. Polyphenols in dementia: from molecular basis to clinical trials. Life Sci. (2016) 161:69–77. doi: 10.1016/j.lfs.2016.07.021
293. Mancini, E, Beglinger, C, Drewe, J, Zanchi, D, Lang, UE, and Borgwardt, S. Green tea effects on cognition, mood and human brain function: a systematic review. Phytomedicine. (2017) 34:26–37. doi: 10.1016/j.phymed.2017.07.008
294. Niu, K, Hozawa, A, Kuriyama, S, Ebihara, S, Guo, H, Nakaya, N, et al. Green tea consumption is associated with depressive symptoms in the elderly12. Am J Clin Nutr. (2009) 90:1615–22. doi: 10.3945/ajcn.2009.28216
295. Zhang, R, Zhang, L, Li, Z, Zhang, P, Song, H, Yao, DA, et al. Green tea improves cognitive function through reducing AD-pathology and improving anti-oxidative stress capacity in Chinese middle-aged and elderly people. Front Aging Neurosci. (2022) 14:919766. doi: 10.3389/fnagi.2022.919766
296. Zhu, W-L, Shi, HS, Wei, YM, Wang, SJ, Sun, CY, Ding, ZB, et al. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol Res. (2012) 65:74–80. doi: 10.1016/j.phrs.2011.09.007
297. Liu, Y, Jia, G, Gou, L, Sun, L, Fu, X, Lan, N, et al. Antidepressant-like effects of tea polyphenols on mouse model of chronic unpredictable mild stress. Pharmacol Biochem Behav. (2013) 104:27–32. doi: 10.1016/j.pbb.2012.12.024
298. Yang, H, Xie, J, Mu, W, Ruan, X, Zhang, J, Yao, L, et al. Tea polyphenols protect learning and memory in sleep-deprived mice by promoting AMPA receptor internalization. Neuroreport. (2020) 31:857–64. doi: 10.1097/WNR.0000000000001462
299. Michna, L, Lu, Y-P, Lou, Y-R, Wagner, GC, and Conney, AH. Stimulatory effect of oral administration of green tea and caffeine on locomotor activity in SKH-1 mice. Life Sci. (2003) 73:1383–92. doi: 10.1016/S0024-3205(03)00468-5
300. Haque, AM, Hashimoto, M, Katakura, M, Tanabe, Y, Hara, Y, and Shido, O. Long-term Administration of Green tea Catechins Improves Spatial Cognition Learning Ability in Rats1. J Nutr. (2006) 136:1043–7. doi: 10.1093/jn/136.4.1043
301. Ozdemir, O., “The green tea polyphenol EGCG modulates NGF, BDNF, and CAMKII-α pathways to alleviate neurological damage in autism-induced rats. EBSCOhost.” (2025). Available online at: https://openurl.ebsco.com/contentitem/doi:10.32383%2Fappdr%2F131024?sid=ebsco:plink:crawler&id=ebsco:doi:10.32383%2Fappdr%2F131024
302. Mehta, R, Bhandari, R, and Kuhad, A. Effects of catechin on a rodent model of autism spectrum disorder: implications for the role of nitric oxide in neuroinflammatory pathway. Psychopharmacology. (2021) 238:3249–71. doi: 10.1007/s00213-021-05941-5
303. Zhang, Q, Yang, H, Wang, J, Li, A, Zhang, W, Cui, X, et al. Effect of green tea on reward learning in healthy individuals: a randomized, double-blind, placebo-controlled pilot study. Nutr J. (2013) 12:84. doi: 10.1186/1475-2891-12-84
304. Pham, NM, Nanri, A, Kurotani, K, Kuwahara, K, Kume, A, Sato, M, et al. Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr. (2014) 17:625–33. doi: 10.1017/S1368980013000360
305. Zhang, Y, Cheng, L, and Zhang, X. Interactions of tea polyphenols with intestinal microbiota and their effects on cerebral nerves. J Food Biochem. (2021) 45:e13575. doi: 10.1111/jfbc.13575
306. Zhang, X., Zhu, X., Sun, Y., Hu, B., Sun, Y., Jabbar, S., et al., “Fermentation in vitro of EGCG, GCG and EGCG3" Me isolated from oolong tea by human intestinal microbiota,” Food Res Int, vol. 54, no., pp. 1589–1595, (2013). doi: 10.1016/j.foodres.2013.10.005
307. Khairudin, MAS, Mhd Jalil, AM, and Hussin, N. Effects of polyphenols in tea (Camellia sinensis sp.) on the modulation of gut microbiota in human trials and animal studies. Gastroenterol Insights. (2021) 12:202–16. doi: 10.3390/gastroent12020018
308. Song, Z, Zhang, X, Hong, M, Wu, Z, Luo, S, and Cheng, K. Oolong tea polyphenols affect the inflammatory response to improve cognitive function by regulating gut microbiota. J Funct Foods. (2023) 105:105584. doi: 10.1016/j.jff.2023.105584
309. Hong, M, Zhang, R, Liu, Y, Wu, Z, and Weng, P. The interaction effect between tea polyphenols and intestinal microbiota: role in ameliorating neurological diseases. J Food Biochem. (2022) 46:e13870. doi: 10.1111/jfbc.13870
310. Kim, I-S. Current perspectives on the beneficial effects of soybean Isoflavones and their metabolites for humans. Antioxidants. (2021) 10:1064. doi: 10.3390/antiox10071064
311. Ziaei, S, and Halaby, R. Dietary Isoflavones and breast Cancer risk. Medicines. (2017) 4:18. doi: 10.3390/medicines4020018
312. Shughrue, PJ, Lane, MV, and Merchenthaler, I. Comparative distribution of estrogen receptor-α and-β mRNA in the rat central nervous system. J Comp Neurol. (1997) 388:507–25. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6
313. Sekikawa, A, Ihara, M, Lopez, O, Kakuta, C, Lopresti, B, Higashiyama, A, et al. Effect of S-equol and soy Isoflavones on heart and brain. Curr Cardiol Rev. (2019) 15:114–35. doi: 10.2174/1573403X15666181205104717
314. Muthyala, RS, Ju, YH, Sheng, S, Williams, LD, Doerge, DR, Katzenellenbogen, BS, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. (2004) 12:1559–67. doi: 10.1016/j.bmc.2003.11.035
315. Mayo, B, Vázquez, L, and Flórez, AB. Equol: a bacterial metabolite from the Daidzein Isoflavone and its presumed beneficial health effects. Nutrients. (2019) 11:2231. doi: 10.3390/nu11092231
316. Kritz-Silverstein, D, Von Mühlen, D, Barrett-Connor, E, and Bressel, MAB. Isoflavones and cognitive function in older women: the SOy and postmenopausal health in aging (SOPHIA) study. Menopause. (2003) 10:196–202. doi: 10.1097/00042192-200310030-00004
317. Cheng, P-F, Chen, JJ, Zhou, XY, Ren, YF, Huang, W, Zhou, JJ, et al. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause. (2015) 22:198–206. doi: 10.1097/GME.0000000000000290
318. Cui, C, Birru, RL, Snitz, BE, Ihara, M, Kakuta, C, Lopresti, BJ, et al. Effects of soy isoflavones on cognitive function: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2020) 78:134–44. doi: 10.1093/nutrit/nuz050
319. Ye, S, Wang, TT, Cai, B, Wang, Y, Li, J, Zhan, JX, et al. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural Regen Res. (2017) 12:1479–84. doi: 10.4103/1673-5374.215260
320. Lund, TD, West, TW, Tian, LY, Bu, LH, Simmons, DL, Setchell, KDR, et al. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. (2001) 2:20. doi: 10.1186/1471-2202-2-20
321. Pan, Y, Anthony, M, Watson, S, and Clarkson, TB. Soy phytoestrogens improve radial arm maze performance in Ovariectomized retired breeder rats and do not attenuate benefits of 17β-estradiol treatment. Menopause. (2000) 7:230–5. doi: 10.1097/00042192-200007040-00004
322. Weber, KS, Setchell, KDR, and Lephart, ED. Maternal and perinatal brain aromatase: effects of dietary soy phytoestrogens. Dev Brain Res. (2001) 126:217–21. doi: 10.1016/S0165-3806(00)00138-3
323. Rosenfeld, CS. Effects of phytoestrogens on the developing brain, gut microbiota, and risk for neurobehavioral disorders. Front Nutr. (2019) 6:142. doi: 10.3389/fnut.2019.00142
324. Zhu, L, Chen, Y, Miao, M, Liang, H, Xi, J, Wang, Y, et al. Prenatal exposures to isoflavones and neurobehavioral development in children at 2 and 4 years of age: a birth cohort study. Ecotoxicol Environ Saf. (2023) 262:115176. doi: 10.1016/j.ecoenv.2023.115176
325. Miyake, Y, Tanaka, K, Okubo, H, Sasaki, S, Tokinobu, A, and Arakawa, M. Maternal consumption of soy and isoflavones during pregnancy and risk of childhood behavioural problems: the Kyushu Okinawa maternal and child health study. Int J Food Sci Nutr. (2021) 72:1118–27. doi: 10.1080/09637486.2021.1904844
326. Wisniewski, AB, Cernetich, A, Gearhart, JP, and Klein, SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. (2005) 84:327–34. doi: 10.1016/j.physbeh.2004.12.008
327. Yu, C, Tai, F, Wu, R, Song, Z, Zhang, X, and An, X. Maternal exposure to daidzein alters behaviour and oestrogen receptor α expression in adult female offspring. Behav Pharmacol. (2010) 21:283–91. doi: 10.1097/FBP.0b013e32833aec1a
328. Adgent, MA, Daniels, JL, Edwards, LJ, Siega-Riz, AM, and Rogan, WJ. Early-life soy exposure and gender-role play behavior in children. Environ Health Perspect. (2011) 119:1811–6. doi: 10.1289/ehp.1103579
329. Westmark, CJ. Soy infant formula may be associated with autistic behaviors. Autism-Open Access. (2013) 3:20727. doi: 10.4172/2165-7890.1000120
330. Peng, Y, Jiang, B, Wu, H, Dai, R, and Tan, L. Effects of genistein on neuronal apoptosis, and expression of Bcl-2 and Bax proteins in the hippocampus of ovariectomized rats★. Neural Regen Res. (2012) 7:2874–81. doi: 10.3969/j.issn.1673-5374.2012.36.004
331. Hou, Q, Huang, J, Zhao, L, Pan, X, Liao, C, Jiang, Q, et al. Dietary genistein increases microbiota-derived short chain fatty acid levels, modulates homeostasis of the aging gut, and extends healthspan and lifespan. Pharmacol Res. (2023) 188:106676. doi: 10.1016/j.phrs.2023.106676
332. Chen, P, Sun, J, Liang, Z, Xu, H, du, P, Li, A, et al. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res Int. (2022) 152:110868. doi: 10.1016/j.foodres.2021.110868
333. Yeh, K-C, Hung, C-F, Lee, H-L, Hsieh, T-Y, and Wang, S-J. Soybean meal extract preserves memory ability by increasing presynaptic function and modulating gut microbiota in rats. Mol Neurobiol. (2022) 59:1649–64. doi: 10.1007/s12035-021-02669-3
334. Shen, L, Liu, L, and Ji, H-F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis. (2017) 56:385–90. doi: 10.3233/JAD-160884
335. Ticinesi, A, Nouvenne, A, Tana, C, Prati, B, and Meschi, T. Gut microbiota and microbiota-related metabolites as possible biomarkers of cognitive aging In: PC Guest, editor. Reviews on biomarker studies in aging and anti-aging research. Cham: Springer International Publishing (2019). 129–54.
336. Benameur, T, Giacomucci, G, Panaro, MA, Ruggiero, M, Trotta, T, Monda, V, et al. New promising therapeutic avenues of curcumin in brain diseases. Molecules. (2021) 27:236. doi: 10.3390/molecules27010236
337. Garodia, P, Hegde, M, Kunnumakkara, AB, and Aggarwal, BB. Curcumin, inflammation, and neurological disorders: How are they linked? Integr Med Res. (2023) 12:100968. doi: 10.1016/j.imr.2023.100968
338. Kulkarni, SK, Bhutani, MK, and Bishnoi, M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology. (2008) 201:435–42. doi: 10.1007/s00213-008-1300-y
339. Pan, R, Qiu, S, Lu, D, and Dong, J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J. (2008) 121:832–9. doi: 10.1097/00029330-200805010-00015
340. Choi, G-Y, Kim, HB, Hwang, ES, Lee, S, Kim, MJ, Choi, JY, et al. Curcumin alters neural plasticity and viability of intact hippocampal circuits and attenuates behavioral despair and COX-2 expression in chronically stressed rats. Mediat Inflamm. (2017) 2017:6280925–9. doi: 10.1155/2017/6280925
341. Liu, D, Wang, Z, Gao, Z, Xie, K, Zhang, Q, Jiang, H, et al. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. (2014) 271:116–21. doi: 10.1016/j.bbr.2014.05.068
342. Wu, A, Noble, EE, Tyagi, E, Ying, Z, Zhuang, Y, and Gomez-Pinilla, F. Curcumin boosts DHA in the brain: implications for the prevention of anxiety disorders. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2015) 1852:951–61. doi: 10.1016/j.bbadis.2014.12.005
343. Bhatia, HS, Agrawal, R, Sharma, S, Huo, Y-X, Ying, Z, and Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS One. (2011) 6:e28451. doi: 10.1371/journal.pone.0028451
344. Kim, SJ, Son, TG, Park, HR, Park, M, Kim, MS, Kim, HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult Hippocampus. J Biol Chem. (2008) 283:14497–505. doi: 10.1074/jbc.M708373200
345. Tiwari, SK, Agarwal, S, Tripathi, A, and Chaturvedi, RK. Correction to: Bisphenol-a mediated inhibition of hippocampal neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol Neurobiol. (2019) 56:6660–2. doi: 10.1007/s12035-019-01685-8
346. Filardi, T, Varì, R, Ferretti, E, Zicari, A, Morano, S, and Santangelo, C. Curcumin: could this compound be useful in pregnancy and pregnancy-related complications? Nutrients. (2020) 12:3179. doi: 10.3390/nu12103179
347. Saleh, HA, El-Aziz, GSA, Mustafa, HN, El-Fark, M, Tashkandi, JM, and Alzahrani, AH. Beneficial effects of curcumin Inmaternal and fetal Oxidativestress and brain damage induced by gestational Lead administration. Biomed Pharmacol J. (2018) 11:871–87. doi: 10.13005/bpj/1444
348. Al-Askar, M, Bhat, RS, Selim, M, Al-Ayadhi, L, and El-Ansary, A. Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complement Altern Med. (2017) 17:259. doi: 10.1186/s12906-017-1763-7
349. Kumar, NK, Nageshwar, M, and Reddy, KP. Protective effect of curcumin on hippocampal and behavior changes in rats exposed to fluoride during pre-and post-natal period. Basic Clin Neurosci. (2020) 11:289–99. doi: 10.32598/bcn.11.2.1189.1
350. Cantacorps, L, Montagud-Romero, S, and Valverde, O. Curcumin treatment attenuates alcohol-induced alterations in a mouse model of foetal alcohol spectrum disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. (2020) 100:109899. doi: 10.1016/j.pnpbp.2020.109899
351. Jayaprakash, P, Isaev, D, Shabbir, W, Lorke, DE, Sadek, B, and Oz, M. Curcumin potentiates α7 nicotinic acetylcholine receptors and alleviates autistic-like social deficits and brain oxidative stress status in mice. Int J Mol Sci. (2021) 22:7251. doi: 10.3390/ijms22147251
352. de Sousa Macedo, LLB, Antunes, FTT, de Andrade Alvarenga, W, Batista, MCC, de Moura, MSB, Farias, MNL, et al. Curcumin for attention-deficit–hyperactivity disorder: a systematic review and preliminary behavioral investigation. Naunyn Schmiedeberg's Arch Pharmacol. (2022) 395:803–13. doi: 10.1007/s00210-022-02236-0
353. Lopresti, AL, Maes, M, Maker, GL, Hood, SD, and Drummond, PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. (2014) 167:368–75. doi: 10.1016/j.jad.2014.06.001
354. Lopresti, AL, Maes, M, Meddens, MJM, Maker, GL, Arnoldussen, E, and Drummond, PD. Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur Neuropsychopharmacol. (2015) 25:38–50. doi: 10.1016/j.euroneuro.2014.11.015
355. Panahi, Y, Badeli, R, Karami, G-R, and Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted Curcuminoids in major depressive disorder. Phytother Res. (2015) 29:17–21. doi: 10.1002/ptr.5211
356. Bergman, J, Miodownik, C, Bersudsky, Y, Sokolik, S, Lerner, PP, Kreinin, A, et al. Curcumin as an add-on to Antidepressive treatment: a randomized, double-blind, placebo-controlled, pilot clinical study. Clin Neuropharmacol. (2013) 36:73–7. doi: 10.1097/WNF.0b013e31828ef969
357. Di Meo, F, Margarucci, S, Galderisi, U, Crispi, S, and Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients. (2019) 11:2426. doi: 10.3390/nu11102426
358. Scazzocchio, B, Minghetti, L, and D’Archivio, M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients. (2020) 12:2499. doi: 10.3390/nu12092499
359. Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: a review of mutual influence. J Nutr Metab. (2018) 2018:e1367984:1–11. doi: 10.1155/2018/1367984
360. Shen, L, Liu, L, and Ji, H-F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res. (2017) 61:1361780. doi: 10.1080/16546628.2017.1361780
361. Wei, G, Chen, B, Lin, Q, Li, Y, Luo, L, He, H, et al. Tetrahydrocurcumin provides neuroprotection in experimental traumatic brain injury and the Nrf2 signaling pathway as a potential mechanism. Neuroimmunomodulation. (2018) 24:348–55. doi: 10.1159/000487998
362. Albani, D, Polito, L, Signorini, A, and Forloni, G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. (2010) 36:370–6. doi: 10.1002/biof.118
363. Malaguarnera, M, Khan, H, and Cauli, O. Resveratrol in autism Spectrum disorders: behavioral and molecular effects. Antioxidants. (2020) 9:188. doi: 10.3390/antiox9030188
364. Hendouei, F, Sanjari Moghaddam, H, Mohammadi, MR, Taslimi, N, Rezaei, F, and Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: a double-blind and placebo-controlled randomized trial. J Clin Pharm Ther. (2020) 45:324–34. doi: 10.1111/jcpt.13076
365. Rafeiy-Torghabeh, M, Ashraf-Ganjouei, A, Moradi, K, Bagheri, S, Mohammadi, M-R, and Akhondzadeh, S. Resveratrol adjunct to methylphenidate improves symptoms of attention-deficit/hyperactivity disorder: a randomized, double-blinded, placebo-controlled clinical trial. Eur Child Adolesc Psychiatry. (2021) 30:799–807. doi: 10.1007/s00787-020-01562-z
366. Bhandari, R, and Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem Int. (2017) 103:8–23. doi: 10.1016/j.neuint.2016.12.012
367. Bakheet, SA, Alzahrani, MZ, Nadeem, A, Ansari, MA, Zoheir, KMA, Attia, SM, et al. Resveratrol treatment attenuates chemokine receptor expression in the BTBR T + tf/J mouse model of autism. Mol Cell Neurosci. (2016) 77:1–10. doi: 10.1016/j.mcn.2016.09.004
368. Chen, F, Zhang, L, Liu, Y, Zhang, A, and Wang, W. Resveratrol alleviates perinatal methylmercury-induced neurobehavioral impairments by modulating the gut microbiota composition and neurotransmitter disturbances. Environ Toxicol. (2024) 39:329–40. doi: 10.1002/tox.23973
369. Basholli-Salihu, M, Schuster, R, Mulla, D, Praznik, W, Viernstein, H, and Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst Eng. (2016) 39:1879–85. doi: 10.1007/s00449-016-1662-1
370. Qiao, Y, Sun, J, Xia, S, Tang, X, Shi, Y, and Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. (2014) 5:1241–9. doi: 10.1039/C3FO60630A
371. Chung, JY, Jeong, J-H, and Song, J. Resveratrol modulates the gut-brain Axis: focus on glucagon-like Peptide-1, 5-HT, and gut microbiota. Front Aging Neurosci. (2020) 12:588044. doi: 10.3389/fnagi.2020.588044
372. Vauzour, D, Vafeiadou, K, Rodriguez-Mateos, A, Rendeiro, C, and Spencer, JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. (2008) 3:115–26. doi: 10.1007/s12263-008-0091-4
373. Pannu, A, Sharma, PC, Thakur, VK, and Goyal, RK. Emerging role of flavonoids as the treatment of depression. Biomol Ther. (2021) 11:1825. doi: 10.3390/biom11121825
374. Khalid, S, Barfoot, KL, May, G, Lamport, DJ, Reynolds, SA, and Williams, CM. Effects of acute blueberry flavonoids on mood in children and Young adults. Nutrients. (2017) 9:158. doi: 10.3390/nu9020158
375. Park, M, Choi, J, and Lee, H-J. Flavonoid-rich Orange juice intake and altered gut microbiome in Young adults with depressive symptom: a randomized controlled study. Nutrients. (2020) 12:1815. doi: 10.3390/nu12061815
376. Savino, R, Medoro, A, Ali, S, Scapagnini, G, Maes, M, and Davinelli, S. The emerging role of flavonoids in autism Spectrum disorder: a systematic review. J Clin Med. (2023) 12:3520. doi: 10.3390/jcm12103520
377. Wang, H, Zhao, T, Liu, Z, Danzengquzhen,, Cisangzhuoma,, Ma, J, et al. The neuromodulatory effects of flavonoids and gut microbiota through the gut-brain axis. Front Cell Infect Microbiol. (2023) 13:1197646. doi: 10.3389/fcimb.2023.1197646
378. Johnson, EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly12345. Am J Clin Nutr. (2012) 96:1161S–5S. doi: 10.3945/ajcn.112.034611
379. Johnson, EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. (2014) 72:605–12. doi: 10.1111/nure.12133
380. Stringham, NT, Holmes, PV, and Stringham, JM. Lutein supplementation increases serum brain-derived neurotrophic Factor (BDNF) in humans. FASEB J. (2016) 30:689.3–3. doi: 10.1096/fasebj.30.1_supplement.689.3
381. Mahmassani, HA, Switkowski, KM, Johnson, EJ, Scott, TM, Rifas-Shiman, SL, Oken, E, et al. Early childhood lutein and Zeaxanthin intake is positively associated with early childhood receptive vocabulary and mid-childhood executive function but no other cognitive or behavioral outcomes in project viva. J Nutr. (2022) 152:2555–64. doi: 10.1093/jn/nxac188
382. Yu, Q, Xue, F, Li, Z, Li, X, Ai, L, Jin, M, et al. Dietary intake of carotenoids and risk of depressive symptoms: a systematic review and Meta-analysis. Antioxidants (Basel). (2022) 11:2205. doi: 10.3390/antiox11112205
383. Dai, Z, Li, Z, Shi, E, Nie, M, Feng, L, Chen, G, et al. Study on the interaction between four typical carotenoids and human gut microflora using an in vitro fermentation model. J Agric Food Chem. (2022) 70:13592–601. doi: 10.1021/acs.jafc.2c03464
384. Shi, Q, and Qi, K. Developmental origins of health and disease: impact of paternal nutrition and lifestyle. Pediatr Investig. (2023) 7:111–31. doi: 10.1002/ped4.12367
385. Chan, JC, Nugent, BM, and Bale, TL. Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biol Psychiatry. (2018) 83:886–94. doi: 10.1016/j.biopsych.2017.10.005
Glossary
ADHD - Attention Deficit Hyperactivity Disorder
ASD - Autism Spectrum Disorder
ANS - Autonomic Nervous System
BBB - Blood–Brain Barrier
CNS - Central Nervous System
CD - Conduct Disorder
DASH - Dietary Approaches to Stop Hypertension
DHA - Docosahexaenoic Acid
EPA - Eicosapentaenoic Acid
ENS - Enteric Nervous System
EGCG - Epigallocatechin-3-Gallate
FGR - Fetal Growth Restriction
FMT - Fecal Microbiome Transplantation
FSIQ - Full-Scale IQ
GABA - γ-Aminobutyric Acid
GF - Germ-Free
GI - Gastrointestinal
GBA - Gut-Brain Axis
HFD - High-Fat Diet
HPA - Hypothalamic Pituitary Adrenal
HMOs - Human Milk Oligosaccharides
IQ - Intelligence Quotient
IFN-γ - Interferon-Gamma
KD - Ketogenic Diet
MD - Mediterranean Diet
MHD - Mental Health Disorders
MAO - Monoamine Oxidase
mTOR - Mammalian Target of the Rapamycin
Nrf2 - Nuclear Factor Erythroid 2-Related Factor 2
NSCs - Neural Stem Cells
ODD - Oppositional Defiant Disorder
PUFAs - Polyunsaturated Fatty Acids
PD - Plant-Based Diet
RA - Retinoic Acid
SCFAs - Short-Chain Fatty Acids
SHR - Spontaneously Hypertensive Rats
SFN - Sulforaphane
TP - Tea Polyphenol
TNF-α - Tumor Necrosis Factor-Alpha
Keywords: brain development, cognitive functions, behavior, maternal nutrition, gut microbiome, bioactive compounds
Citation: Jiang Y and Li Y (2025) The role of nutrition and gut microbiome in childhood brain development and behavior. Front. Nutr. 12:1590172. doi: 10.3389/fnut.2025.1590172
Edited by:
Agnieszka Kozioł-Kozakowska, Jagiellonian University Medical College, PolandReviewed by:
Vinod Kumar Nathan, Central Food Technological Research Institute (CSIR), IndiaMarina Fuertes, Instituto Politécnico de Lisboa, Portugal
Ghizlane Bendriss, Weill Cornell Medicine-Qatar, Qatar
Copyright © 2025 Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Li, cm9zZWxpMTVAdW1kLmVkdQ==
 Yue Jiang
Yue Jiang Yuanyuan Li
Yuanyuan Li