- Department of General Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Background: At present, stroke ranks as the third leading cause of mortality, and hypertension is a major risk factor for stroke. Complementary assessment of inflammation level, immunity, and nutritional status is now possible using the newly developed C-reactive protein-albumin-lymphocyte (CALLY) index biomarker. One key concern in this study is that its correlation with the risk of stroke in individuals with hypertensiveness.
Methods: In this study, we used cross-sectional analyses from the National Health and Nutrition Examination Survey (NHANES) database through 2003 to 2010. The CALLY index was calculated by albumin and lymphocytes divided by C-reactive protein (CRP). In order to further analysis, the CALLY index was log-transformed to increase data normality and lessen the impact of extreme values on the analytical findings. We investigated the odds ratios and confidence intervals of the ln CALLY index and its components in connection to stroke in people with hypertension. A weighted multivariable logistic regression model was carried out. Additionally, we used weighted restricted cubic splines (RCS) and subgroup analyses to further examine the association between the CALLY index and stroke prevalence in hypertensive individuals.
Results: This study included 8,146 hypertensive participants, of whom 616 hypertensive participants had a stroke. In unadjusted modeling, we found a 39% reduction in the incidence of stroke in the hypertensive population in the highest ln CALLY quartile group (OR 0.61, 95% CI 0.46–0.82), and the negative association remained significant after adjustment for confounders. While ALB showed a robust protective impact in hypertensive people, with greater ALB levels linked to a decreased risk of stroke (OR 0.50, 95% CI 0.37–0.68), we also discovered a positive correlation between CRP and stroke risk (OR 1.13, 95% CI 1.04–1.22). A substantial correlation between the ln CALLY index and stroke risk in hypertensive individuals was also validated by subgroup analysis. The ln CALLY index and stroke risk in this sample also showed a strong linear negative connection, according to weighted restricted cubic spline (RCS) analysis.
Conclusion: There is a significant negative association between the CALLY index and stroke risk in hypertensive patients in the U.S. adults. The CALLY index may be a potential indicator for early identification of individuals at higher risk of stroke in hypertensive patients and provide potential for clinical intervention.
1 Introduction
The Global Burden of Disease study states that stroke is the third leading cause of death worldwide, after ischemic heart disease and COVID-19 (1). The global prevalence of stroke has reached 100 million people, with a mortality rate of 87.4 per 100,000 people (1). Stroke predominantly results from an interruption in cerebral blood flow, causing ischemic and hypoxic conditions within the brain tissue (2). This condition is mainly categorized into two types: ischemic stroke and hemorrhagic stroke (2). Despite notable advancements in stroke management, various complications, including motor dysfunction, cognitive-linguistic disorders, swallowing difficulties, and anxiety-depression, persist (3). These complications significantly diminish the quality of life, increase psychological burden, and impose heavy economic costs on patients, families, and society. Therefore, promptly identifying high-risk groups and proactively managing relevant risk factors are crucial for the effective prevention of stroke.
Recent studies highlight hypertension as a significant contributor to stroke risk (4). Hypertensive patients are 9.7 times more likely to experience a stroke compared to individuals with normal blood pressure (5). Previous studies have shown that a persistent, long-lasting systemic inflammatory response is caused by hypertension. This is thought to be crucial for hypertensive individuals to experience a stroke (6, 7). Patients with hypertension have higher inflammatory markers. These chemical mediators create atherosclerosis, stimulate immune cells, and harm vascular endothelial cells, and all of them enhance the risk of stroke (8–10). Additionally, these inflammatory mediators can raise C-reactive protein (CRP) levels, which has been connected to a higher risk of stroke (11, 12). However, inflammation often comes with immune system dysfunction. Especially, hypertensive patients have higher levels of CD4+ and CD8+ T cells than those with normal blood pressure. These cells release inflammatory cytokines that invade the brain, triggering vascular and nervous inflammation, which in turn raises the risk of stroke (13, 14). Additionally, malnutrition is common among stroke survivors and can significantly worsen their prognosis (15). Studies have shown that serum albumin, the most abundant protein in circulation, affects atherosclerosis by influencing inflammation, platelet aggregation, and antioxidant activity (16). Consequently, inflammation, immune response, and nutritional factors play key roles in the development of stroke in individuals with hypertension.
A study conducted by Iida et al. (17) introduced the C-reactive protein-albumin-lymphocyte index (CALLY), a comprehensive biomarker that integrates CRP, albumin levels, and lymphocyte count to assess inflammation, immunological status, and nutritional condition (18). Prior investigations have revealed that the CALLY index is a potent indicator of negative cardiovascular consequences in individuals who have experienced ST-segment elevation myocardial infarction (19). Moreover, Wu et al. (20) observed a negative correlation between the CALLY index and metabolic syndrome. Further investigations have revealed significant associations between the CALLY index and various conditions, including sarcopenia, cancer, and chronic obstructive pulmonary disease (21–24), thereby validating its role as a reliable prognostic marker. Nevertheless, the association between the CALLY index and stroke risk in hypertensive populations remains to be clarified.
In order to fill this research gap, the present study delved into the relationship between the CALLY index and the incidence of stroke in patients with confirmed hypertension using cross-sectional analysis of NHANES data. This approach aims to offer a novel framework and theoretical foundation for the early prediction and prevention of stroke in hypertensive populations.
2 Materials and methods
2.1 Data sources and study participants
The National Health and Nutrition Examination Survey, or NHANES, is a significant survey conducted in the US to evaluate the general health of Americans through questionnaires, laboratory testing, and examinations. It collects information on health status, dietary habits, nutritional intake, and chronic diseases. Based on this, we utilized the NHANES dataset from 2003 to 2010 for cross-sectional analyses.
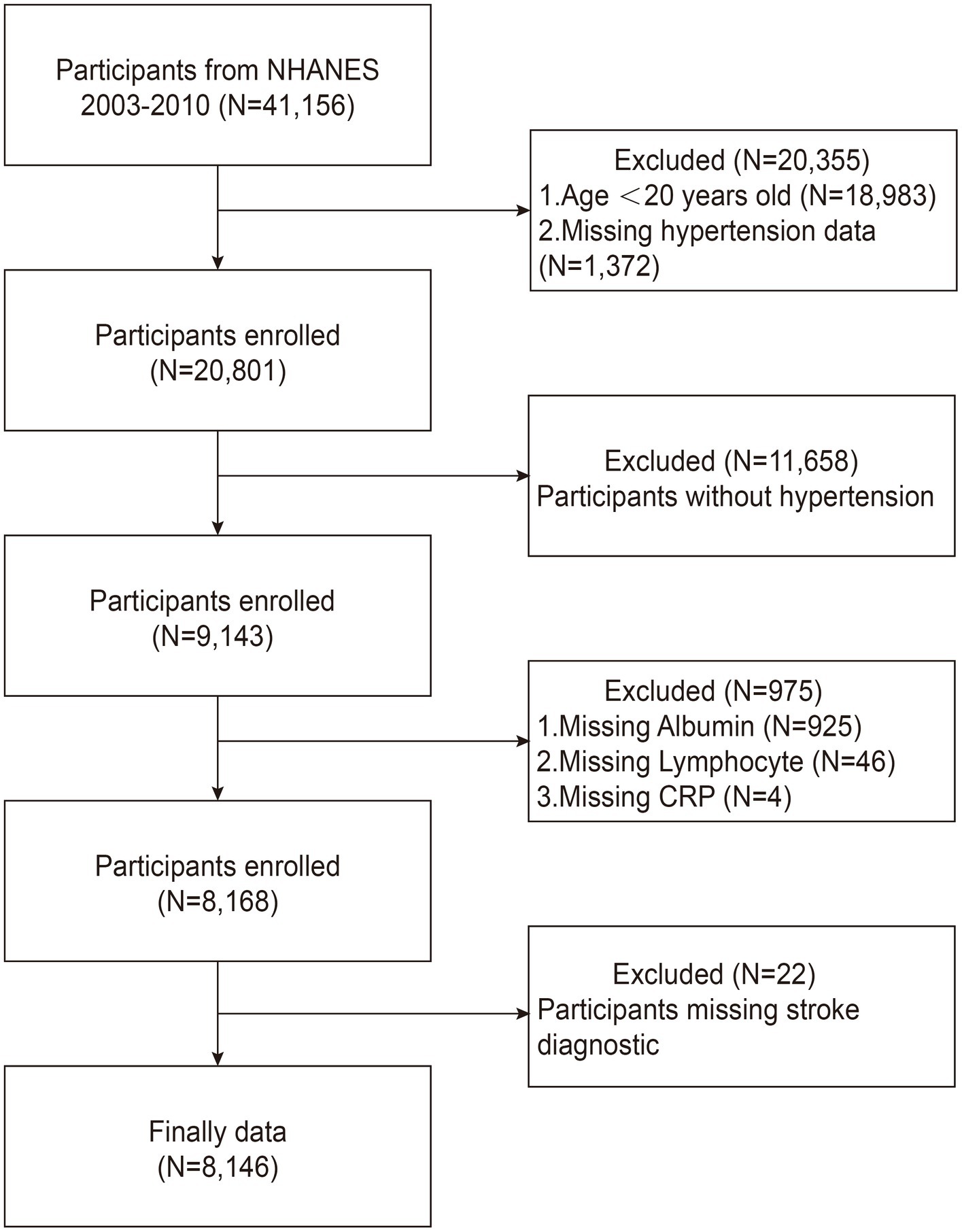
From 2003 to 2010, we included a total of 41,156 participants from the NHANES database. To guarantee the quality and dependability of the study’s findings, strict exclusion criteria were applied based on previous studies (25–27). The exclusion criteria used in our study were as follows: (1) patients younger than 20 years or lacking hypertension data (n = 20,355); (2) patients without hypertensive disorders (n = 11,658); (3) patients with missing albumin, lymphocyte, or CRP data (n = 975); and (4) patients without stroke diagnostic information (n = 22). Ultimately, 8,146 hypertensive patients were included for further analysis, including 616 stroke survivors, as shown in Figure 1.
2.2 Calculation of the CALLY index
The serum albumin, lymphocyte and CRP blood indices for each cycle are measured by an automated blood tester. The calculation of the CALLY index is determined by the following formula (28, 29):
To improve the normality of the data and reduce the influence of extreme values on the analytical results, the CALLY index (original variable) was log-transformed prior to analysis. Specifically, all values of the CALLY index were converted to their natural logarithmic form (ln CALLY) to better satisfy the assumptions of linear regression and enhance the robustness of the model. This transformation facilitated a distribution closer to normality and improved the linearity of the association between the CALLY index and stroke risk, thereby strengthening the reliability and interpretability of the results.
2.3 Diagnosis of hypertension
Hypertension was defined based on prior studies (30, 31), as follows: (1) a mean systolic blood pressure of at least 140 mmHg; (2) a mean diastolic blood pressure of at least 90 mmHg; (3) answer “Yes” to the following questionnaire questions: “Has a doctor or health professional ever told you that you have hypertension, also known as high blood pressure (HBP)?” or “Are you currently taking prescribed medication for high blood pressure?”
2.4 Evaluation of stroke
Stroke status of participants was determined based on questionnaire on medical history from NHANES. In the questionnaire, professionals asked, “Has a doctor or other medical professional ever told you about a stroke?” People who answered “yes” to this question were considered to have had a stroke.
2.5 Definition of covariates
The covariates considered in this study include age, sex, ethnic origin (including Mexican American, non-Hispanic White, non-Hispanic Black, other Hispanic, and other racial categories), level of education (classified as less than beneath high school, high school, and high school diploma or higher), marriage (married, unmarried), body mass index (BMI, in kg/m2) (≥30, 25–30, <25), drinking consumption (have you consumed more than 12 alcoholic beverages in the past year), smoking (never smoker, ever smoker, current smoker), and questionnaire with history of diabetes, heart failure (HF), and coronary artery heart disease (CHD). Moreover, fasting blood glucose (FBG) levels, triglycerides (TG) levels, total cholesterol (TC) levels, high-density lipoprotein cholesterol (HDL-C) levels, low-density lipoprotein cholesterol (LDL-C) levels, and waist circumference (WC) were also recorded.
2.6 Statistical analysis
Sample weights were calculated using the NHANES-recommended Full Sample 2-Year MEC Exam Weight (WTMEC2YR), divided by 4, with clustering and stratification incorporated into all analyses. Whereas classification variables are displayed as frequencies (n, %), continuous variables are displayed as mean ± standard deviation. Differences in participant characteristics across CALLY index quartiles were assessed using one-way analysis of variance (ANOVA) for continuous variables and Pearson’s chi-square test for categorical variables. The relationship between the CALLY index and stroke risk in hypertensive individuals was examined using weighted multivariate logistic regression models. The crude model was unadjusted, while Model 1 adjusted for age, race, sex, education, and marriage. Model 2 further adjusted for smoking, drinking consumption, diabetes, HF, FBG, TC, HDL-C, TG, BMI, and WC, in addition to the covariates from Model 1. Potential non-linear associations were examined through restricted cubic spline (RCS) analysis. Subgroup and interaction analyses were performed to assess heterogeneity across different strata. Missing data were imputed using random forest interpolation. R Studio was used to conduct the statistical analysis, and a two-tailed p-value threshold of less than 0.05 was used to assess significance.
2.7 Statement of morals
The National Center for Health Statistics’ (NCHS) Ethics examination Board approved the NHANES datasets used in this study after they undergone a thorough examination. Informed consent was obtained in writing from all participants prior to their involvement. Given that the study relied on the analysis of publicly available datasets, all participant data were anonymized and de-identified, thereby exempting the research from the need for ethical approval from local institutions.
3 Results
3.1 Baseline analysis
Data from 8,146 participants were analyzed, with baseline characteristics shown in Table 1. The average age of the cohort was 56.98 ± 15.40 years, with 48.96% male and 51.04% female participants, 73.93% of whom identified as non-Hispanic White. Among the participants, 616 (6.10%) had a stroke diagnosis. Those in the top quarter of the ln CALLY index were more probable to be married, overweight, male, and well-educated than those in the lowest group. Regarding lifestyle factors, the high ln CALLY group had a higher proportion of never-smokers and alcohol consumers. With respect to comorbidities, the prevalence of diabetes and heart failure was lower in the highest ln CALLY index quartile. In terms of blood biomarkers, this group showed elevated fasting blood glucose levels, C-reactive protein, total cholesterol, in contrast triglyceride levels were reduced. Additionally, this group had higher albumin concentrations, lymphocyte counts, and HDL cholesterol levels.
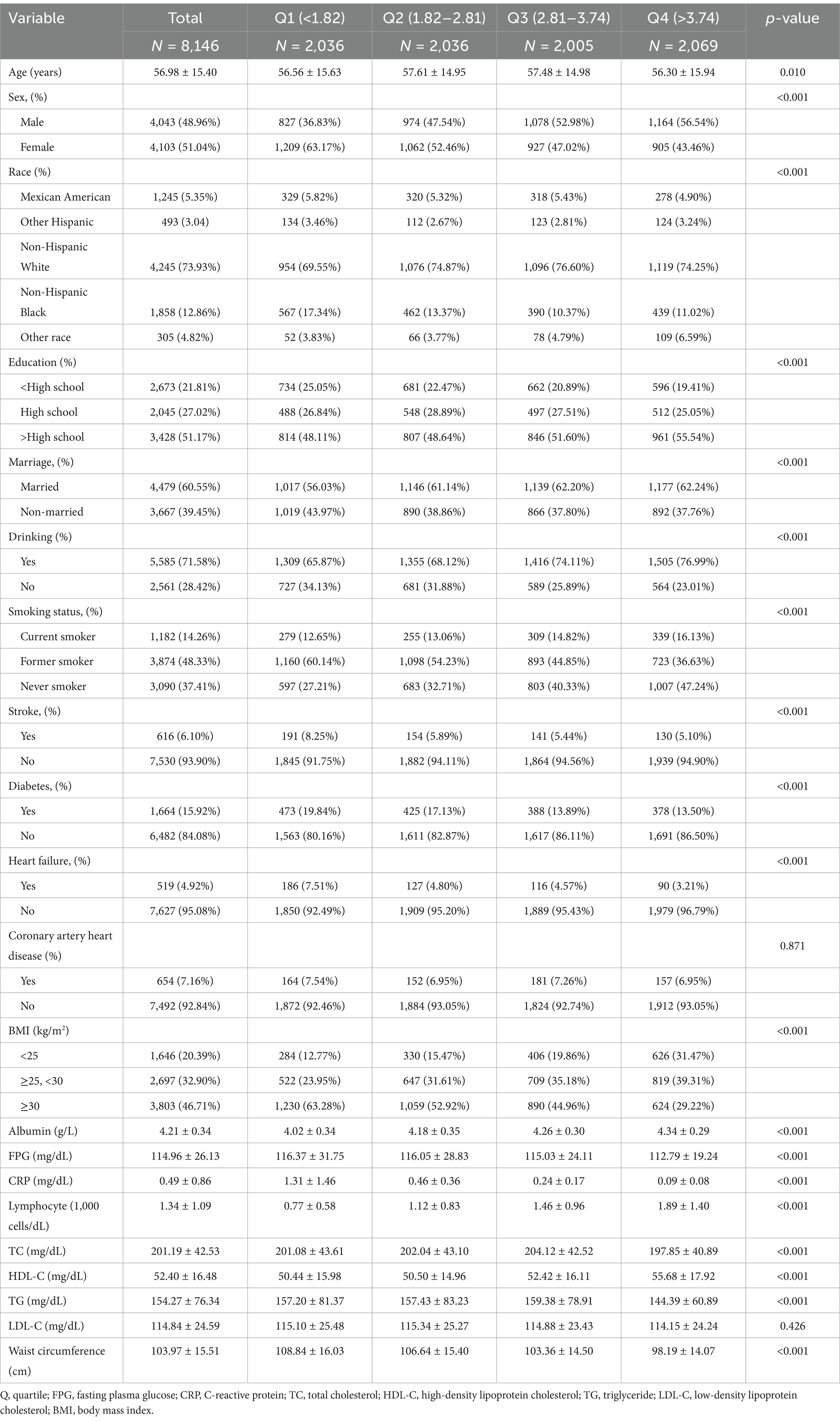
Table 1. Weighted patient demographics and baseline characteristics of participants according to ln CALLY index.
3.2 The association between the ln CALLY index and its components with stroke incidence in individuals with hypertension
To further investigate the relationship between the ln CALLY index and its components with stroke incidence in individuals with hypertension and to present the results more intuitively, we constructed three logistic regression models to examine the independent effects of the ln CALLY index and its components on stroke incidence. When the ln CALLY index was treated as a continuous variable (see Figure 2), we observed a significant negative correlation between stroke risk and the ln CALLY index in hypertensive individuals (OR 0.87, 95% CI 0.81–0.94). After categorizing the ln CALLY values into four quartiles, with the first quartile as the reference group, in the unadjusted model, the highest quartile of ln CALLY showed a 39% reduction in stroke incidence compared to the reference group (OR 0.61, 95% CI 0.46–0.82). In Model 2, after adjusting for demographic characteristics, blood parameters, and comorbidities, the highest quartile of ln CALLY remained negatively associated with stroke incidence, with a 30% reduction in stroke risk for each 1-unit increase in ln CALLY.
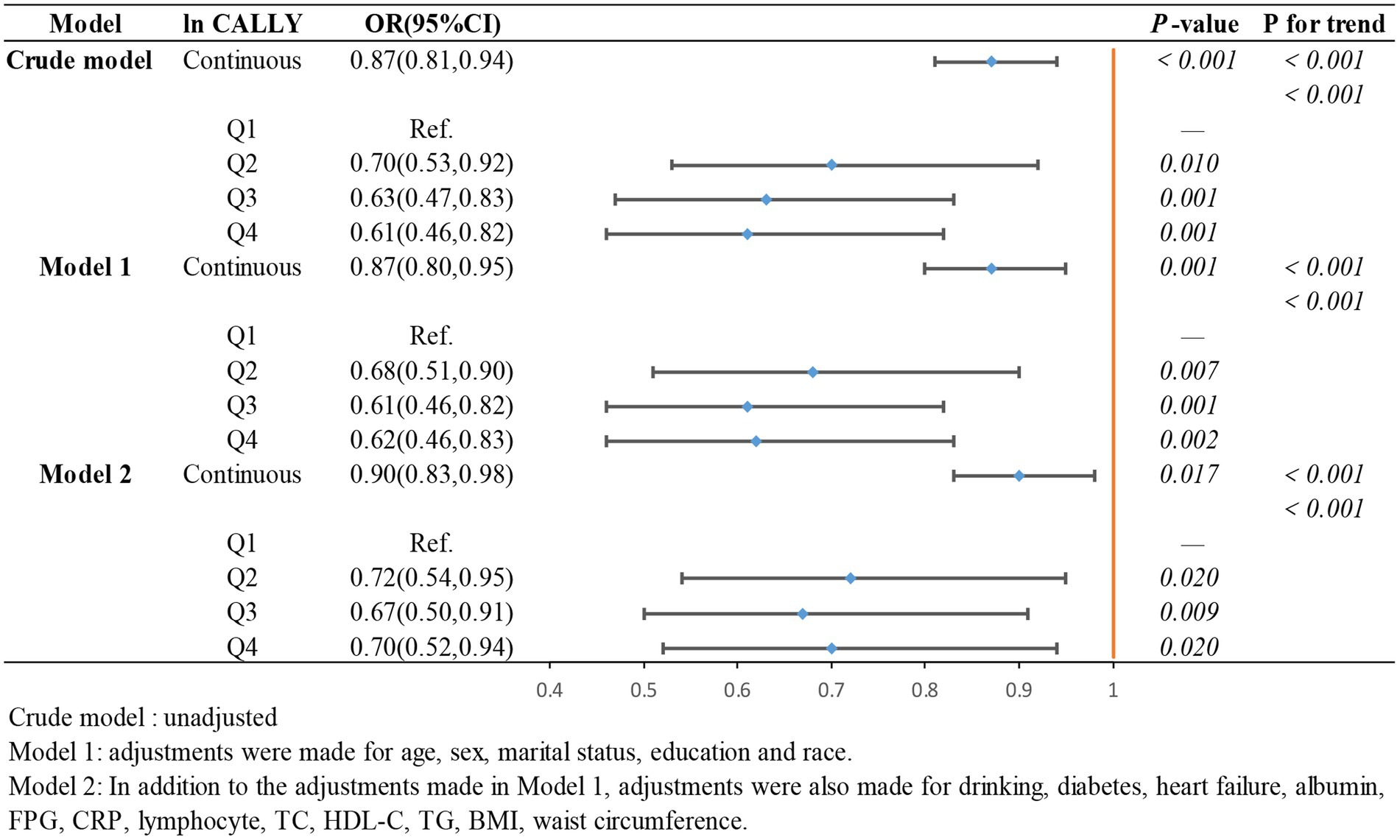
Figure 2. The association between Ln CALLY index and stroke occurrence in hypertensive participants.
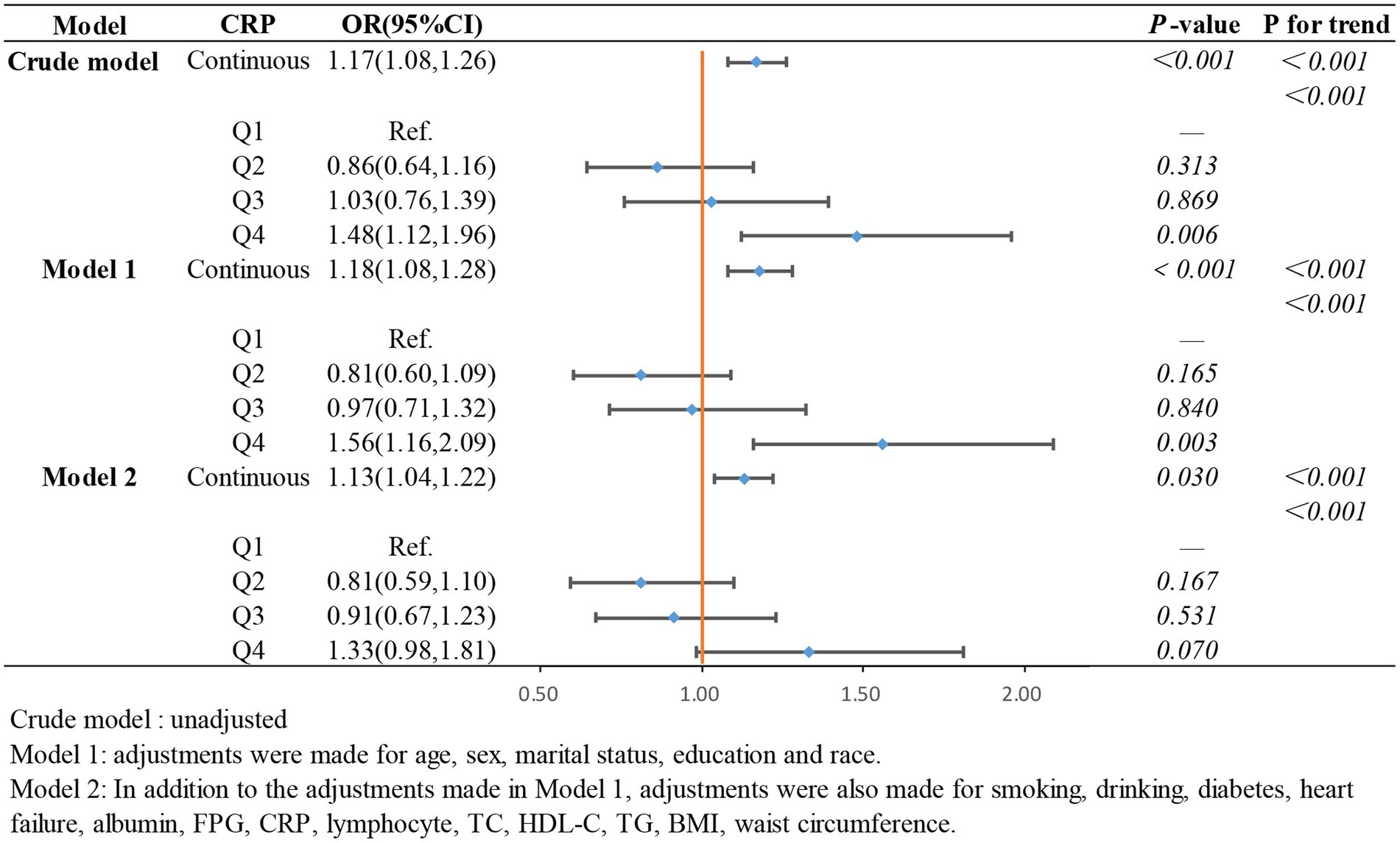
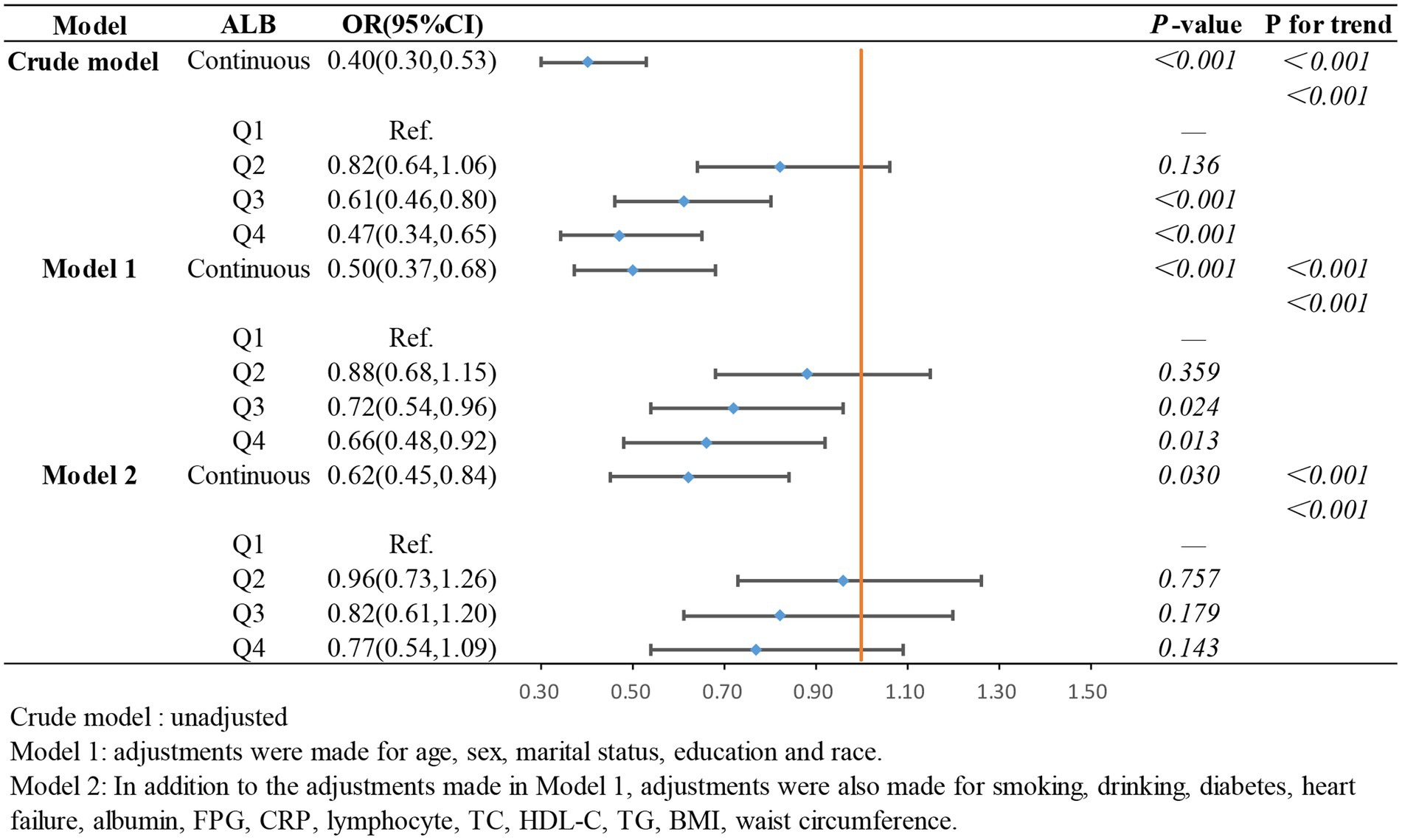
In the weighted logistic regression analysis further exploring the relationship between the components of ln CALLY and stroke risk in hypertensive individuals, Figure 3 shows that CRP is positively correlated with stroke risk in Model 2 (OR 1.13, 95% CI 1.04–1.22). Additionally, we found that in Model 2, the OR for the continuous variable decreased from 1.18 to 1.13 compared to the unadjusted model, indicating a slight attenuation of this relationship after adjusting for confounders. In contrast, Figure 4 shows that ALB exerts a strong protective effect in hypertensive individuals, with higher ALB levels associated with lower stroke risk (OR 0.50, 95% CI 0.37–0.68). Figure 5 demonstrates that lymphocyte count, as a continuous variable, is not significantly associated with stroke risk in hypertensive individuals. Trend analysis across all models (p trend <0.001) consistently reveals a significant upward trend.
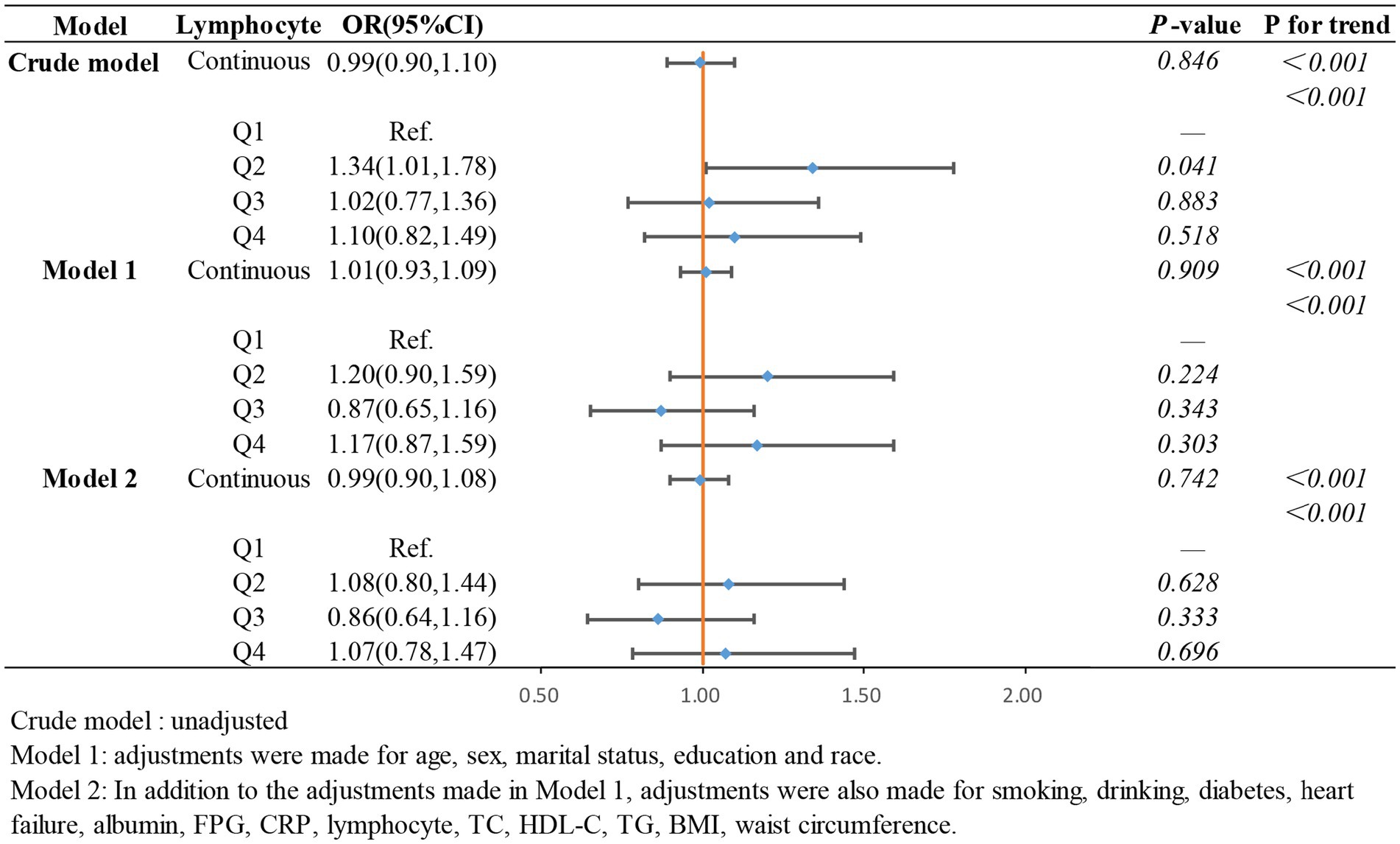
Figure 5. The association between lymphocyte count and stroke occurrence in hypertensive participants.
Furthermore, the weighted restricted cubic spline (RCS) analysis was used to visualize the relationship between the ln CALLY index and stroke incidence in hypertensive individuals, as shown in Figure 6. The RCS curve analysis indicates a significant linear negative correlation between ln CALLY and stroke incidence (p < 0.0003). As ln CALLY increases, stroke incidence in hypertensive individuals decreases significantly. However, when the ln CALLY value reaches 3.737, further increases in ln CALLY lead to a plateau in the negative correlation.
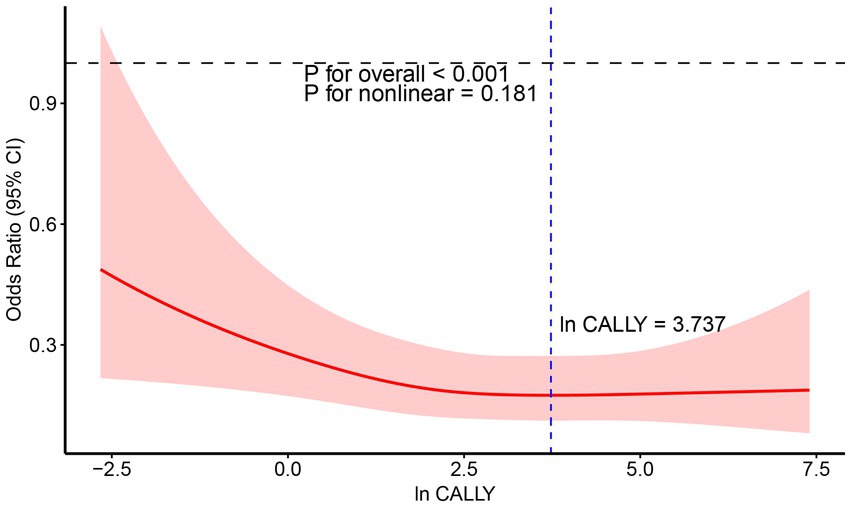
Figure 6. RCS analysis of the relationship between Ln CALLY index and stroke occurrence in hypertensive participants.
3.3 Subgroup analyses
To assess the stability of the relationship between the ln CALLY index and stroke risk in hypertensive individuals across different subgroups, we conducted subgroup analyses and tested for interaction effects. In the subgroup analysis using ln CALLY as a continuous variable (see Figure 7), we found that individuals with BMI ≥30 showed a significantly lower stroke risk with higher ln CALLY values (OR = 0.83, 95% CI: 0.73–0.93, p = 0.002), suggesting that a higher ln CALLY index may have a stronger protective effect in the high BMI population. Additionally, the negative correlation between ln CALLY and stroke risk was more pronounced in males (OR = 0.84, 95% CI: 0.74–0.95, p = 0.007), indicating that an increase in ln CALLY may be associated with a reduction in stroke risk in men. In contrast, no significant relationship was observed between ln CALLY and stroke risk in females (p = 0.507).

Figure 7. Subgroups analysis of the association between ln CALLY index and stroke in hypertensive populations pectoris.
However, in the subgroup analysis using ln CALLY quartiles (see Figure 8), we found that the interaction between gender and ln CALLY quartiles significantly influenced stroke risk. Specifically, males in the Q2, Q3, and Q4 groups had a significantly lower stroke risk compared to the reference group (Q1), with p-values of 0.038, <0.001, and 0.011, respectively. In contrast, no statistically significant differences were found between the quartiles and the reference group in females (p-values of 0.177, 0.725, and 0.394). These results suggest that the association between ln CALLY and stroke differs by gender. Furthermore, in the analysis of ln CALLY index quartiles, we observed a stronger negative correlation between the fourth quartile and the first quartile in individuals who were married, consumed alcohol, had no diabetes, had no heart failure, and had BMI ≥30.
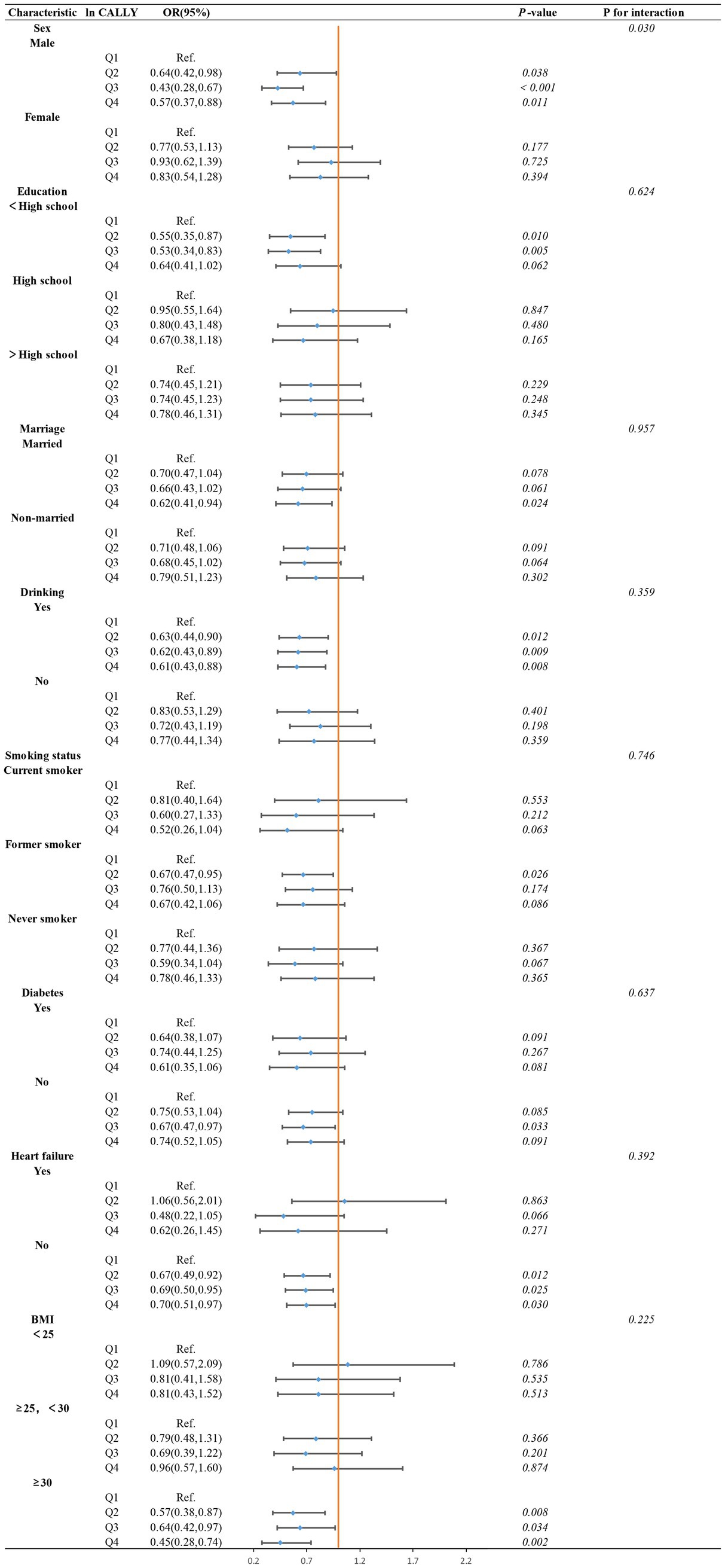
Figure 8. Subgroups analysis of the association between ln CALLY index quartiles and stroke in hypertensive populations pectoris.
By comparing the subgroup analyses of ln CALLY index as both a continuous and categorical variable, we identified an interesting phenomenon. The interaction analysis of the continuous variable subgroup showed no significant interaction effects across subgroups (p for interaction >0.05). However, the interaction effects in the categorical variable subgroup analysis revealed a statistically significant gender interaction (p for interaction <0.05), with no significant interactions observed in other subgroups (p for interaction >0.05). This phenomenon may be due to the clearer group distinctions provided by the categorical variable (quartiles), which helps to observe effect variations within specific groups, making the gender effect more prominent. In contrast, in the continuous variable analysis, the relationship between gender and the ln CALLY index was treated as linear, which may not capture the complex interaction between gender and the ln CALLY index.
4 Discussion
Our study performed a cross-sectional analysis of 8,146 adult hypertensive patients to examine the association of the ln CALLY index with stroke prevalence. The results showed that among people with hypertension, the incidence of stroke decreased by 39% for every unit increase in the ln CALLY index. After limiting for potential confounders, this inverse connection persisted, suggesting that the CALLY index may serve as a protective factor in predicting the risk of stroke in this population. We further completed subgroup analyses and interaction tests, which showed a more documented relationship. In addition, significant gender differences were found in the subgroup analyses, with males being more affected.
The CALLY Index is a combined measure that reflects inflammation, immune function, and nutritional status. Hypertension induces systemic chronic inflammation through the classical inflammasome activation pathway (32). Long-term hypertension leads to vascular sclerosis, endothelial injury, and necrosis (33), which release damage-associated molecular patterns (DAMPs). These molecules further activate the NLRP3 inflammasome, promoting the release of inflammatory factors, thereby exacerbating neurovascular inflammation and promoting the development of atherosclerosis (34, 35). Additionally, upon binding to Toll-like receptors, DAMPs promote the release of pro-inflammatory cytokines such as IL-1β (36, 37). The release of these inflammatory factors increases the body’s inflammatory response, which in turn raises the risk of stroke in individuals with hypertension (11, 38). CRP, a well-established biomarker of inflammation (39), has been shown to predict cardiovascular events in a prospective study involving 27,939 American women, with a 30-year follow-up period (40). A Mendelian randomization study highlighted a clear causal relationship between CRP and stroke (41). Furthermore, a randomized double-blind trial demonstrated that individuals with elevated CRP levels had a 4.7-fold increased risk of recurrent stroke compared to those without elevated CRP (42). These findings suggest that elevated CRP promotes stroke occurrence, aligning with our study’s observation of an association between CRP and stroke risk in individuals with hypertension.
During the activation of inflammation, the immune system plays a critical role. DAMPs bind to Toll-like receptors, triggering the innate immune response and further activating T lymphocytes and B lymphocytes (43). T lymphocytes secrete pro-inflammatory cytokines that exacerbate vascular inflammation and cerebral tissue damage. On the other hand, B lymphocytes, produce autoantibodies that amplify immune responses and inflammation, further elevating stroke risk (44). Tregs (regulatory T lymphocytes) release anti-inflammatory cytokines, including IL-10 and TGF-β, reducing inflammation, minimizing brain tissue damage, and enhancing functional recovery. Tregs also inhibit immune responses by binding to dendritic cells through CTLA-4 receptors, suppressing dendritic cell activation, thereby suppressing immune responses and mitigating inflammation, which reduces stroke risk (45, 46). As indicated by a bidirectional Mendelian randomization study, both B lymphocytes and T lymphocytes have bidirectional effects on stroke risk (47). In our study, no clear positive or negative correlation was found between lymphocyte count and stroke. Further research is needed to explore and validate this potential association.
Nutrition is essential for cardiovascular health, and serum albumin, as an important nutritional marker, exhibits anti-inflammatory and antioxidant functions (48). First, albumin contains abundant thiol groups, acting as an antioxidant to scavenge reactive oxygen and nitrogen species (49). Second, low serum albumin leads to impaired fibrinolysis and reduced platelet aggregation, promoting the formation of atherosclerotic plaques or blood clots, thus increasing the risk of stroke (50). Finally, low serum albumin levels decrease red blood cell deformability, increasing blood viscosity and further elevating stroke risk (51). A study from the NHANES database involving 17,303 participants identified a negative correlation between serum albumin levels and stroke risk (15). Additionally, a cohort study from Japan found that hypoalbuminemia is associated with an increased risk of stroke (52). Our study also explored the relationship between albumin (ALB) levels and stroke risk in individuals with hypertension, and similarly demonstrated a negative correlation between serum albumin levels and stroke risk.
The CHA2DS2-VASc score has become a widely accepted tool for the clinical assessment and prediction of stroke risk. A prospective study involving 1,494 patients demonstrated that the CHA2DS2-VASc score offers superior clinical utility in forecasting acute ischemic stroke (53). Furthermore, a study of 62,227 patients noted that the CHA2DS2-VASc score independently predicted the occurrence of acute ischemic stroke (54). This scoring system incorporates clinical factors such as age, sex, and medical history, allowing for real-time assessment that is both cost effective and straightforward to implement. However, it primarily relies on long-term clinical characteristics rather than reflecting the patient’s current physiological state. This limits its sensitivity in younger populations and its ability to detect subtle short-term clinical fluctuations. The Framingham score is another tool used for stroke risk evaluation (55), and a study tracking 27,748 stroke-free individuals over 5.6 years revealed a positive correlation between higher Framingham scores and increased stroke risk (56). While the Framingham score is simple, affordable, and effective for long-term risk assessment, it also has notable limitations in capturing dynamic clinical conditions and assessing short-term stroke risk. In contrast, the CALLY index provides real-time physiological status through hematological markers, evaluating immune, nutritional, and inflammatory status from multiple dimensions, offering a high degree of objectivity.
Subgroup analyses in this study further indicated a more pronounced correlation of the CALLY index with the risk of stroke in hypertensive men (p for interaction = 0.0304). A meta-analysis of 59 studies from 19 countries across five continents found that stroke incidence is 33% higher in men compared to women (57). This difference may be attributed to the effects of sex hormones, which modulate cerebrovascular reactivity. Specifically, estrogen enhances endothelial nitric oxide synthase (eNOS) activity, increasing nitric oxide (NO) production, promoting vasodilation, improving vascular compliance, reducing arterial blood pressure, and minimizing endothelial cell damage, which collectively reduces stroke risk (58). Furthermore, estrogen inhibits the onset of atherosclerosis by suppressing the proliferation of vascular smooth muscle cells, reducing lipoprotein(a) accumulation, and decreasing the adhesion and differentiation of monocytes, thereby mitigating vascular wall damage and inflammatory responses (59). Additionally, the smaller size of women’s arteries and hearts may result in less vascular damage under similar hemodynamic conditions, contributing to a lower incidence of stroke in women (60, 61).
The CALLY index, as a reliable biomarker, offers clinicians a preliminary means to assess stroke risk in hypertensive patients. In comparison to traditional risk assessment methods, the CALLY index provides superior ease of use and real-time applicability, offering robust scientific support for early intervention and treatment. Research indicates that fluctuations in the CALLY index are closely linked to patients’ immune, inflammatory, and nutritional status, thereby justifying the adjustment of treatment strategies based on CALLY index levels, particularly in the domains of inflammation, immunity, and nutrition therapy. Our findings advocate for the implementation of a risk stratification approach to hypertension management in clinical practice. Evidence suggests that patients with a low CALLY index face a significantly heightened stroke risk. Consequently, for hypertensive patients exhibiting low CALLY index levels, proactive early interventions, including routine monitoring and optimal blood pressure control, should be prioritized, alongside personalized treatment strategies. This approach facilitates early identification of high-risk individuals and ensures timely, targeted interventions to reduce stroke incidence.
A key strength of this study is the use of the NHANES database, which ensures a nationally representative sample and strengthens the generalizability of the findings. In addition, we introduced the CALLY index as a novel, non-invasive biomarker that could improve stroke risk assessment in hypertensive individuals. However, some limitations of our study have to be recognized. First, the precision of the data may be impacted by the NHANES design, which permits the gathering of some measurements only at a single time point. Additionally, excluding people with insufficient information may result in selection prejudice. Second, as this study relies on the NHANES database, it is not possible to accurately determine whether participants with chronic diseases were also experiencing acute infections. Acute infections can cause an elevation in CRP levels, potentially compromising the stability of the CALLY index and its ability to predict outcomes. This is a limitation of our study. Finally, we cannot conclude causal links because of the retrospective and cross-sectional form of this study, which leaves our observations vulnerable to inherent biases. Notwithstanding these drawbacks, the results have clinical significance. Our research indicates a preliminary inverse relationship between the CALLY index and the risk of stroke in hypertensive populations, larger, multi-center clinical trials are required to validate its precision and wider application.
5 Conclusion
Our analysis suggests that in U.S. adults, the CALLY index is negatively correlated with stroke risk in hypertensive patients. The CALLY index may provide additional value in identifying individuals at higher stroke risk among hypertensive populations. Additional prospective studies are required to corroborate our findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XF: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization. DQ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization. JZ: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. YL: Data curation, Methodology, Validation, Writing – original draft. XL: Writing – original draft. WT: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. SL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors appreciate the National Health and Nutrition Examination Survey for supplying the data and acknowledge the selfless dedication of all participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
2. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
3. GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. (2024) 23:973–1003. doi: 10.1016/S1474-4422(24)00369-7
4. Pandian, JD, Sylaja, PN, Lackland, DT, Babu, V, Kumar Paramasivan, N, Sebastian, I, et al. World stroke organization and world hypertension league position statement on hypertension control strategies in prevention and management of stroke. Int J Stroke. (2025) 20:151–65. doi: 10.1177/17474930241309276
5. Li, J, Jiang, S, Zhang, Y, Tang, G, Wang, Y, Mao, G, et al. H-type hypertension and risk of stroke in Chinese adults: a prospective, nested case-control study. J Transl Int Med. (2015) 3:171–8. doi: 10.1515/jtim-2015-0027
6. Sylvester, MA, and Brooks, HL. Sex-specific mechanisms in inflammation and hypertension. Curr Hypertens Rep. (2019) 21:53. doi: 10.1007/s11906-019-0959-2
7. Low, A, Mak, E, Rowe, JB, Markus, HS, and O’Brien, JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. (2019) 53:100916. doi: 10.1016/j.arr.2019.100916
8. Buonacera, A, Stancanelli, B, and Malatino, L. Stroke and hypertension: an appraisal from pathophysiology to clinical practice. Curr Vasc Pharmacol. (2019) 17:72–84. doi: 10.2174/1570161115666171116151051
9. Deussen, A, and Kopaliani, I. Targeting inflammation in hypertension. Curr Opin Nephrol Hypertens. (2023) 32:111–7. doi: 10.1097/MNH.0000000000000862
10. Dinh, QN, Drummond, GR, Sobey, CG, and Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. (2014) 2014:406960. doi: 10.1155/2014/406960
11. Sesso, HD, Buring, JE, Rifai, N, Blake, GJ, Gaziano, JM, and Ridker, PM. C-reactive protein and the risk of developing hypertension. JAMA. (2003) 290:2945–51. doi: 10.1001/jama.290.22.2945
12. Na, S, Kim, T, Koo, J, Hong, YJ, and Kim, S-H. Vessel wall enhancement and high-sensitivity CRP as prognostic markers in intracranial atherosclerotic stroke: a prospective cohort study. Eur Stroke J. (2025). 3:23969873251317341. doi: 10.1177/23969873251317341
13. Itani, HA, McMaster, WGJ, Saleh, MA, Nazarewicz, RR, Mikolajczyk, TP, Kaszuba, AM, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. (2016) 68:123–32. doi: 10.1161/HYPERTENSIONAHA.116.07237
14. Jayaraj, RL, Azimullah, S, Beiram, R, Jalal, FY, and Rosenberg, GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. (2019) 16:142. doi: 10.1186/s12974-019-1516-2
15. Wang, Y, Zhuang, Y, Huang, H, Ke, J, Lin, S, and Chen, F. Association of serum albumin levels and stroke risk in adults over 40 years: a population-based study. Medicine. (2023) 102:e34848. doi: 10.1097/MD.0000000000034848
16. Ding, C, Wang, H, Huang, X, Hu, L, Shi, Y, Li, M, et al. Association between serum albumin and peripheral arterial disease in hypertensive patients. J Clin Hypertens. (2020) 22:2250–7. doi: 10.1111/jch.14071
17. Iida, H, Tani, M, Komeda, K, Nomi, T, Matsushima, H, Tanaka, S, et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB. (2022) 24:101–15. doi: 10.1016/j.hpb.2021.06.414
18. Müller, L, Hahn, F, Mähringer-Kunz, A, Stoehr, F, Gairing, SJ, Michel, M, et al. Immunonutritive scoring for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: evaluation of the CALLY index. Cancers. (2021) 13:5018. doi: 10.3390/cancers13195018
19. Ji, H, Luo, Z, Ye, L, He, Y, Hao, M, Yang, Y, et al. Prognostic significance of C-reactive protein-albumin-lymphocyte (CALLY) index after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Int Immunopharmacol. (2024) 141:112860. doi: 10.1016/j.intimp.2024.112860
20. Wu, L, Han, D, Xue, Y, He, S, Ma, Z, Su, S, et al. Association between the C-reactive protein-albumin-lymphocyte index and metabolic syndrome: evidence from the 2003–2010 national health and nutrition examination survey. Diabetol Metab Syndr. (2025) 17:39. doi: 10.1186/s13098-025-01609-8
21. Jia, P, Shen, F, Zhao, Q, Wu, X, Sun, K, Wang, X, et al. Association between C-reactive protein-albumin-lymphocyte index and overall survival in patients with esophageal cancer. Clin Nutr. (2025) 45:212–22. doi: 10.1016/j.clnu.2024.12.032
22. Zhu, D, Lin, Y-D, Yao, Y-Z, Qi, X-J, Qian, K, and Lin, L-Z. Negative association of C-reactive protein-albumin-lymphocyte index (CALLY index) with all-cause and cause-specific mortality in patients with cancer: results from NHANES 1999–2018. BMC Cancer. (2024) 24:1499. doi: 10.1186/s12885-024-13261-y
23. Ding, Y, Liu, Y, Yu, J, Cai, C, Fu, L, Zhu, J, et al. The association between the CALLY index and all-cause mortality in patients with COPD: results from the cohort study of NHANES 2007–2010. Int J Chron Obstruct Pulmon Dis. (2025) 20:159–69. doi: 10.2147/COPD.S485036
24. Li, Y, Wei, Q, Ke, X, Xu, Y, Xu, B, Zhang, K, et al. Higher CALLY index levels indicate lower sarcopenia risk among middle-aged and elderly community residents as well as hospitalized patients. Sci Rep. (2024) 14:24591. doi: 10.1038/s41598-024-75164-z
25. Williams, B, Mancia, G, Spiering, W, Agabiti Rosei, E, Azizi, M, Burnier, M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
26. Xu, Z, Tang, J, Chen, X, Jin, Y, Zhang, H, and Liang, R. Associations of C-reactive protein-albumin-lymphocyte (CALLY) index with cardiorenal syndrome: insights from a population-based study. Heliyon. (2024) 10:e37197. doi: 10.1016/j.heliyon.2024.e37197
27. Chen, J, Luo, C, Tan, D, and Li, Y. J-shaped associations of pan-immune-inflammation value and systemic inflammation response index with stroke among American adults with hypertension: evidence from NHANES 1999–2020. Front Neurol. (2024) 15:1417863. doi: 10.3389/fneur.2024.1417863
28. Feng, J, Wang, L, Yang, X, and Chen, Q. Clinical significance of preoperative CALLY index for prognostication in patients with esophageal squamous cell carcinoma undergoing surgery. Sci Rep. (2024) 14:713. doi: 10.1038/s41598-023-51109-w
29. Takeda, Y, Sugano, H, Okamoto, A, Nakano, T, Shimoyama, Y, Takada, N, et al. Prognostic usefulness of the C-reactive protein-albumin-lymphocyte (CALLY) index as a novel biomarker in patients undergoing colorectal cancer surgery. Asian J Surg. (2024) 47:3492–8. doi: 10.1016/j.asjsur.2024.03.054
30. Chen, L, Zhang, J, Zhou, N, Weng, J-Y, Bao, Z-Y, and Wu, L-D. Association of different obesity patterns with hypertension in US male adults: a cross-sectional study. Sci Rep. (2023) 13:10551. doi: 10.1038/s41598-023-37302-x
31. Cai, Y, Chen, M, Zhai, W, and Wang, C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005–2018. BMC Public Health. (2022) 22:481. doi: 10.1186/s12889-022-12942-2
32. Bai, B, Yang, Y, Wang, Q, Li, M, Tian, C, Liu, Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. (2020) 11:776. doi: 10.1038/s41419-020-02985-x
33. Boutouyrie, P, Chowienczyk, P, Humphrey, JD, and Mitchell, GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. (2021) 128:864–86. doi: 10.1161/CIRCRESAHA.121.318061
34. Dinarello, CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. (2011) 41:1203–17. doi: 10.1002/eji.201141550
35. Xu, Y-J, Zheng, L, Hu, Y-W, and Wang, Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. (2018) 476:28–37. doi: 10.1016/j.cca.2017.11.005
36. McCarthy, CG, Goulopoulou, S, Wenceslau, CF, Spitler, K, Matsumoto, T, and Webb, RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. (2014) 306:H184–96. doi: 10.1152/ajpheart.00328.2013
37. Przykaza, Ł. Understanding the connection between common stroke comorbidities, their associated inflammation, and the course of the cerebral ischemia/reperfusion cascade. Front Immunol. (2021) 12:782569. doi: 10.3389/fimmu.2021.782569
38. Cirillo, P, Golino, P, Calabrò, P, Calì, G, Ragni, M, De Rosa, S, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. (2005) 68:47–55. doi: 10.1016/j.cardiores.2005.05.010
39. Zhong, C, Zhu, Z, Wang, A, Xu, T, Bu, X, Peng, H, et al. Multiple biomarkers covering distinct pathways for predicting outcomes after ischemic stroke. Neurology. (2019) 92:e295–304. doi: 10.1212/WNL.0000000000006717
40. Ridker, PM, Moorthy, MV, Cook, NR, Rifai, N, Lee, I-M, and Buring, JE. Inflammation, cholesterol, lipoprotein(a), and 30-year cardiovascular outcomes in women. N Engl J Med. (2024) 391:2087–97. doi: 10.1056/NEJMoa2405182
41. Yang, T, Xie, W, Hu, F, and Cai, B. Causal relationship between C-reactive protein and ischemic stroke caused by atherosclerosis: a Mendelian randomization study. J Stroke Cerebrovasc Dis. (2024) 33:107873. doi: 10.1016/j.jstrokecerebrovasdis.2024.107873
42. Wang, G, Jing, J, Li, J, Pan, Y, Yan, H, Meng, X, et al. Association of elevated hs-CRP and multiple infarctions with outcomes of minor stroke or TIA: subgroup analysis of CHANCE randomised clinical trial. Stroke Vasc Neurol. (2021) 6:80–6. doi: 10.1136/svn-2020-000369
43. Kirabo, A, Fontana, V, de Faria, APC, Loperena, R, Galindo, CL, Wu, J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. (2014) 124:4642–56. doi: 10.1172/JCI74084
44. Mikolajczyk, TP, and Guzik, TJ. Adaptive immunity in hypertension. Curr Hypertens Rep. (2019) 21:68. doi: 10.1007/s11906-019-0971-6
45. Meng, X, Yang, J, Dong, M, Zhang, K, Tu, E, Gao, Q, et al. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. (2016) 13:167–79. doi: 10.1038/nrcardio.2015.169
46. Bravo-Alegria, J, McCullough, LD, and Liu, F. Sex differences in stroke across the lifespan: the role of T lymphocytes. Neurochem Int. (2017) 107:127–37. doi: 10.1016/j.neuint.2017.01.009
47. Deng, X, Hou, S, Wang, Y, Yang, H, and Wang, C. Genetic insights into the relationship between immune cell characteristics and ischemic stroke: a bidirectional Mendelian randomization study. Eur J Neurol. (2024) 31:e16226. doi: 10.1111/ene.16226
48. Don, BR, and Kaysen, G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
49. Quinlan, GJ, Martin, GS, and Evans, TW. Albumin: biochemical properties and therapeutic potential. Hepatology. (2005) 41:1211–9. doi: 10.1002/hep.20720
50. Gresele, P, Deckmyn, H, Huybrechts, E, and Vermylen, J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. (1984) 33:2083–8. doi: 10.1016/0006-2952(84)90577-x
51. Joles, JA, Willekes-Koolschijn, N, and Koomans, HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. (1997) 52:761–70. doi: 10.1038/ki.1997.393
52. Li, J, Imano, H, Yamagishi, K, Cui, R, Muraki, I, Umesawa, M, et al. Serum albumin and risks of stroke and its subtypes—the circulatory risk in communities study (CIRCS). Circ J. (2021) 85:385–92. doi: 10.1253/circj.CJ-20-0384
53. Su, C-H, Tsao, T-F, Chen, A-C, Chang, K-W, Yang, Y-S, Ueng, K-C, et al. CHA2 DS2—VASc scores for outcome prediction in acute ischaemic stroke. Eur J Clin Investig. (2018) 48:e12884. doi: 10.1111/eci.12884
54. Su, C-H, Lo, C-H, Chen, H-H, Tsai, C-F, Yip, H-T, Hsu, K-C, et al. CHA2DS2-VASc score as an independent outcome predictor in patients hospitalized with acute ischemic stroke. PLoS One. (2022) 17:e0270823. doi: 10.1371/journal.pone.0270823
55. Wolf, PA, D’Agostino, RB, Belanger, AJ, and Kannel, WB. Probability of stroke: a risk profile from the Framingham study. Stroke. (1991) 22:312–8. doi: 10.1161/01.str.22.3.312
56. McClure, LA, Kleindorfer, DO, Kissela, BM, Cushman, M, Soliman, EZ, and Howard, G. Assessing the performance of the Framingham Stroke Risk Score in the reasons for geographic and racial differences in stroke cohort. Stroke. (2014) 45:1716–20. doi: 10.1161/STROKEAHA.114.004915
57. Appelros, P, Stegmayr, B, and Terént, A. Sex differences in stroke epidemiology: a systematic review. Stroke. (2009) 40:1082–90. doi: 10.1161/STROKEAHA.108.540781
58. Krause, DN, Duckles, SP, and Pelligrino, DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. (2006) 101:1252–61. doi: 10.1152/japplphysiol.01095.2005
59. Nathan, L, and Chaudhuri, G. Estrogens and atherosclerosis. Annu Rev Pharmacol Toxicol. (1997) 37:477–515. doi: 10.1146/annurev.pharmtox.37.1.477
60. Haast, RAM, Gustafson, DR, and Kiliaan, AJ. Sex differences in stroke. J Cereb Blood Flow Metab. (2012) 32:2100–7. doi: 10.1038/jcbfm.2012.141
Keywords: Cally index, stroke, nutritional, inflammation, NHANES
Citation: Fan X, Qing D, Zhao J, Luo Y, Li X, Tan W and Liu S (2025) The relationship between CALLY index and stroke in hypertensive patients: insights from NHANES. Front. Nutr. 12:1592641. doi: 10.3389/fnut.2025.1592641
Edited by:
Hipólito Nzwalo, University of Algarve, PortugalReviewed by:
Ibrahim Taskin Rakici, Health Sciences University Istanbul Training and Research Hospital, TürkiyeMathew John, Jubilee Mission Medical College and Research Institute, India
Copyright © 2025 Fan, Qing, Zhao, Luo, Li, Tan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiping Liu, bGl1c3A0NTZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xingfu Fan
Xingfu Fan Di Qing†
Di Qing† Jin Zhao
Jin Zhao Shiping Liu
Shiping Liu