- 1Health Management Center, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 2Department of Infectious Diseases, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 3Chongqing Key Laboratory of Precision Prevention and Control of Viral Infectious Diseases, Chongqing, China
Background: This study aims to investigate the impact of home quarantine on insulin resistance and other metabolic parameters in general populations through the changes in metabolic profile, especially Triglyceride/Glucose (TyG).
Methods: This study included participants who underwent two health checkups at the Health Management Center of the First Affiliated Hospital of the Army Medical University, before and after home quarantine, between December 2021 and February 2023. The home quarantine policy in the Chongqing area was executed between August 2022 and December 2022. Triglyceride/Glucose (TyG), TyG-body mass index (TyG-BMI), and TyG-waist circumference (TyG-WC) were calculated.
Results: A total of 19,957 cases were screened, and 2,473 participants (mean age: 39.35 ± 10.36 years, 69.8% female) were included for the final analysis. Compared to before home quarantine, the TyG (6.80 ± 0.58 vs. 6.83 ± 0.58, p < 0.001), TyG-BMI (158.30 ± 31.92 vs. 159.48 ± 31.73, p < 0.001), and TyG-WC (533.53 ± 103.15 vs. 535.78 ± 103.65, p = 0.039) increased significantly after the home quarantine. Besides, glucose, lymphocyte, and platelet decreased significantly while BMI, systolic blood pressure, diastolic blood pressure, pulse, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, uric acid, red blood cell, and monocyte also increased significantly after home quarantine (all p < 0.05).
Conclusion: Home quarantine might pose a potential negative impact on insulin resistance risk, indicating that insulin resistance and other metabolic health parameters during the home quarantine period should be monitored regularly.
Introduction
The pandemic of novel coronavirus pneumonia (COVID-19) has been a global health issue since 2020 (1) to minimize exposure to COVID-19 and avoid the adverse effects of COVID-19 infection. Many countries executed isolation policies including home quarantine, lockdown, travel restrictions, and reducing crowd gatherings. In China, due to the large population, home quarantine was the most commonly used method for self-isolation (2). As a result, China’s home quarantine initiative received positive feedback and was successful in curbing the COVID-19 transmission (2, 3). However, the home quarantine policy also leads to dramatic lifestyle changes during that period. For the majority, the physical activity, eating behaviors, and dietary habits were strictly limited——reduction of outdoor exercise, an increase of sedentary behavior, and unhealthy eating habits (4, 5), which raised significant concerns about the impact on the subjects with the chronic non-communicable disease, such as obesity (6), cardiovascular disease (CVD) (7) and mental health burden (8).
Insulin resistance is a state characterized by reduced responsiveness in insulin-targeting tissues under high insulin levels. It is proven to be associated with many diseases, including metabolic syndrome (9), Type 2 Diabetes Mellitus (T2DM), atherosclerosis, and various metabolic diseases (10). Therefore, the accurate evaluation of insulin resistance is of ultimate importance. Currently, the hyperglycemic clamp is still the gold standard for insulin resistance evaluation. However, due to its inconvinience (11), novel biomarkers including Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and Triglyceride/Glucose (TyG) Index have been extensively studied in various ethnic populations and shown great predictive value for insulin resistance (12). Recent studies have proven that TyG is superior to HOMA-IR for predicting metabolic syndrome (13). Moreover, Wu et al. revealed that TyG was associated with respiratory diseases like respiratory symptoms and chronic bronchitis, while HOMA-IR did not exhibit the correlation in the same cohort (14).
The accessibility of the TyG index makes it an important tool for the health management of insulin resistance at a population level. Apart from TyG, the TyG-body mass index (TyG-BMI), and TyG-waist circumference (TyG-WC) were other two popular indicators for evaluating insulin resistance. Zhou et al. compared TyG and TyG-BMI, and their results revealed that the TyG index appears to be a more promising indicator for risk stratification (15). TyG-WC was also widely used in the evaluation of insulin resistance like non-alcoholic fatty liver disease (NAFLD) and metabolic-associated fatty liver disease (MAFLD) (16). Several studies have already proven that the COVID-19 lockdown could affect life habits significantly and further contribute to the development of metabolic diseases (17, 18). The pandemic of the Ebola virus, COVID-19 has been controlled, but influenza A and many other viruses still possess great risk potential for public health (19, 20). Simpson et al. also proposed the concept of pathogen X, which could be any pathogen, including but not limited to viruses, bacteria, fungi, parasites, or prions (21). Therefore, home quarantine remains an applicable strategy for epidemic containment during infectious disease outbreaks and requires our further attention. However, to our knowledge, there was no study focused specifically on the change in insulin resistance status based on the hematological examination results and clinical parameters.
Herein, this study aims to investigate the impact of home quarantine measures on metabolic health by examining changes in insulin resistance and related biochemical parameters among the general population.
Methods
Study design and population
The home quarantine policy in the Chongqing area of southwestern China was executed between August 2022 and December 2022. Therefore, the before-home quarantine period was defined as December 2021–February 2022, and the after-home quarantine period was defined as December 2022–February 2023 in this study. This retrospective study screened participants who received health checkups at the Health Management Center of the First Affiliated Hospital (Southwest hospital) of the Army Medical University during both before the home quarantine period and after the home quarantine period. The inclusion criteria were: (1) participants > 18 years and < 80 years; (2) participants with at least one health checkup data both before and after the quarantine period. The exclusions were: (1) participants with known history of cardiovascular, cerebrovascular, and metabolic diseases; (2) participants diagnosed with metabolic diseases or a subclinical state of metabolic diseases in the health check-up before home quarantine, including diabetes, fatty liver disease, gout, and hyperthyroidism; (3) pregnant participants; (4) non-native residents; (5) participants with incomplete health check-up data. Personal information was anonymized during the data collection and analysis.
This study was approved by the ethics committee of the Southwest Hospital of Army Medical University, PLA [Approval No. (B)KY2024191]. Due to the retrospective nature of this study, the informed consents were waived by the ethics committee.
Data collection and outcome definition
The basic information (age, gender), anthropometric data (body weight, height, systolic blood pressure [SBP], diastolic blood pressure [DBP], waist circumference (WC), and hip circumference), and laboratory test were extracted directly from the electronic record. Body mass index (BMI) and Waist-Hip Ratio (WHR) accordingly. The fasting blood sample was obtained for the laboratory examinations, including White blood cells (WBC), Neutrophils (NEU), lymphocytes (LYMPH), Monocytes (MON), Eosinophils (EOS), Total Cholesterol (TC), Triglyceride (TG), High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), fasting blood Glucose (Glu) and Uric Acid (UA). The TyG, TyG-BMI, and TyG-WC indexes were calculated using the following formula (22, 23):
“Δ” represents the growth rate of each index and is calculated as:
Δ = [(Post–home quarantine value − Pre–home quarantine value)/Pre–home quarantine value] × 100%. A Δ value greater than 0 indicates positive growth, while a Δ value less than or equal to 0 indicates negative growth or no growth.
Statistical analysis
All the statistical analyses were performed using SPSS 26.0 (IBM Corp., New York, USA) and GraphPad Prism 7 (GraphPad Software, California, USA). The distribution of indicators was determined according to the Q-Q plots. Continuous data conformed to the normal distribution were expressed as mean ± standard deviation (SD) and tested by paired sample t-test. Indicators that deviated from the normal distribution were expressed as median (lower quartile, upper quartile) and compared using the paired Wilcoxon signed-rank test. Categorical data was expressed as n (%), and the chi-square test was used for comparison between groups. A two-sided p < 0.05 was considered statistically significant in this study.
Results
Comparison of characteristics before and after quarantine
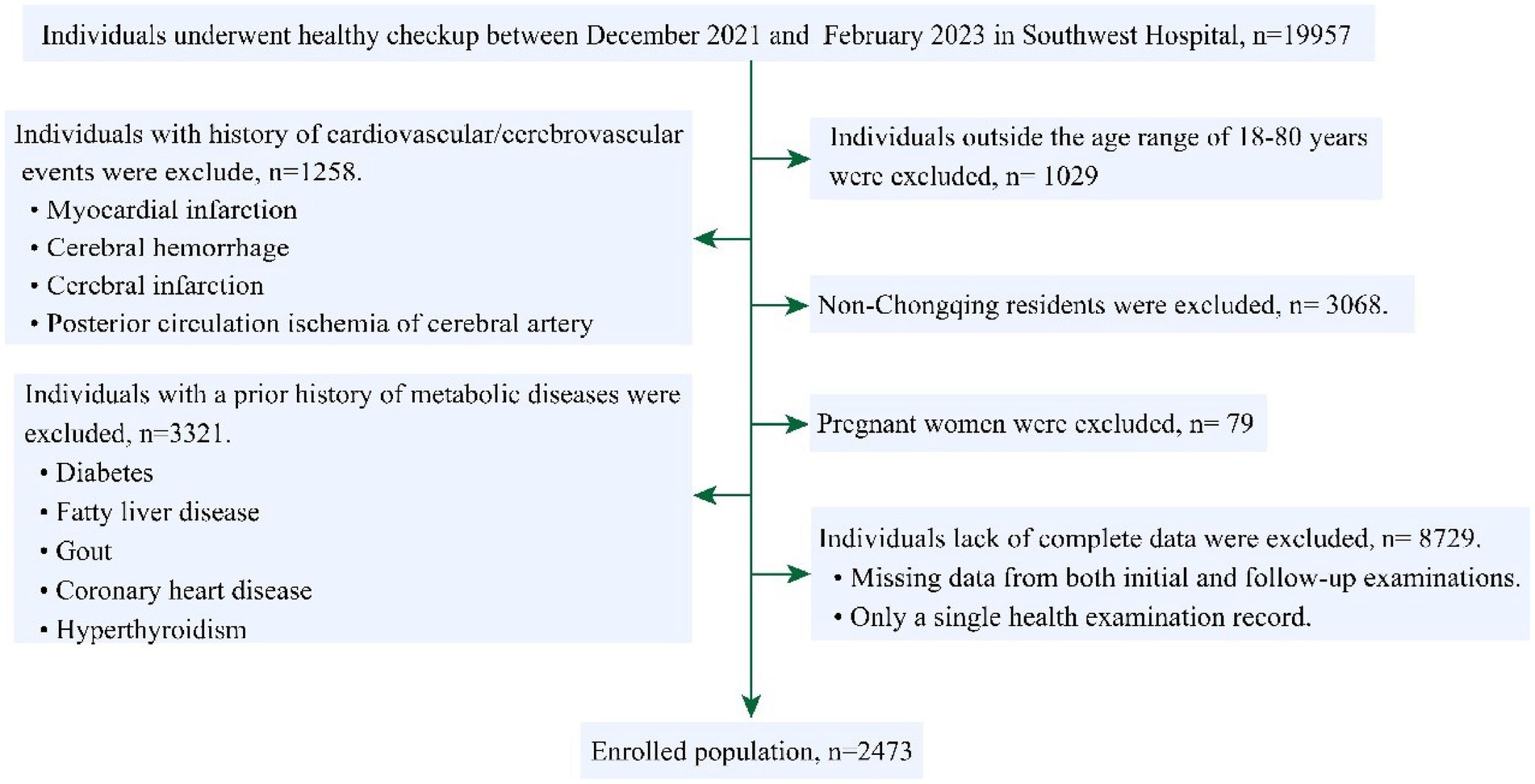
A total of 19,957 cases were screened, among them, after exclusion of patients <18 years or >80 years (n = 1,029), non-native residents (n = 3,068), with prior cardiovascular/cerebrovascular disease (n = 1,258), metabolic diseases (n = 3,321), pregnant women (n = 79), and incomplete data (n = 8,729), 2,473 participants (mean age: 39.35 ± 10.36 years, 69.8% female) were enrolled for final analysis (Figure 1). The average interval between the twice physical checkups was 379.88 ± 38.82 (days).
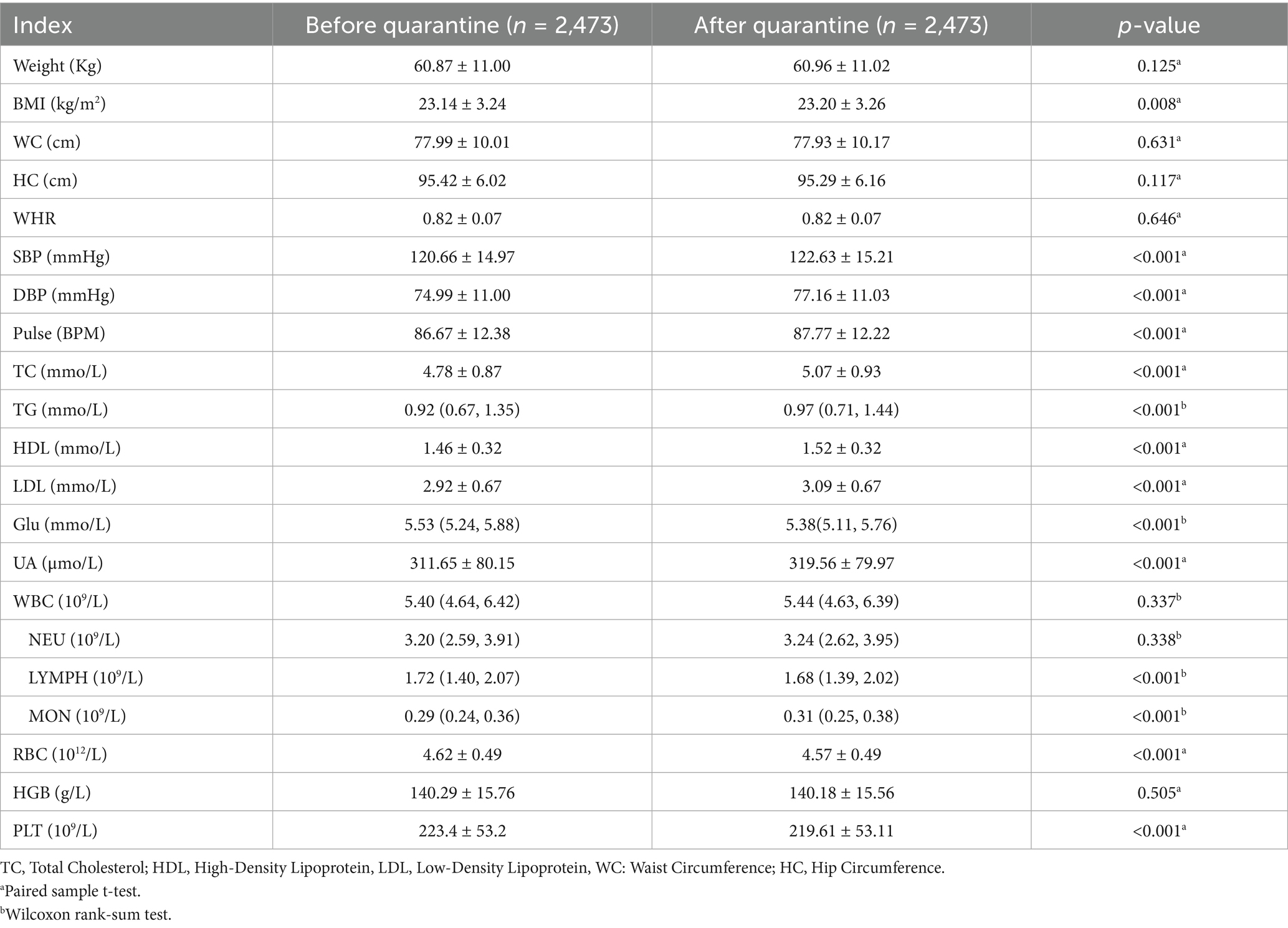
Compared with the characteristics before quarantine, there were significantly lower Glu (5.38 [5.11, 5.76] vs. 5.53[5.24, 5.88], p < 0.001), LYMPH (1.68 [1.39, 2.02] vs. 1.72 [1.40, 2.07], p < 0.001), RBC (4.57 ± 0.49 vs. 4.62 ± 0.49, p < 0.001), and PLT (219.61 ± 53.11 vs. 223.4 ± 53.2, p < 0.001); while higher in BMI (23.20 ± 3.26 vs. 23.14 ± 3.24, p = 0.008), SBP (122.63 ± 15.21 vs. 120.66 ± 14.97, p < 0.001), DBP (77.16 ± 11.03 vs. 74.99 ± 11.00, p < 0.001), Pulse (87.77 ± 12.22 vs. 86.67 ± 12.38, p < 0.001), TC (5.07 ± 0.93 vs. 4.78 ± 0.87, p < 0.001), TG (0.97 [0.71, 1.44] vs. 0.92 [0.67, 1.35], p < 0.001), HDL (1.52 ± 0.32 vs. 1.46 ± 0.32, p < 0.001), LDL (3.09 ± 0.67 vs. 2.92 ± 0.67, p < 0.001), UA (319.56 ± 79.97 vs. 311.65 ± 80.15, p < 0.001), MON (0.31 [0.25, 0.38] vs. 0.29 [0.24, 0.36], p < 0.001) (Table 1).
Changes in metabolic parameters
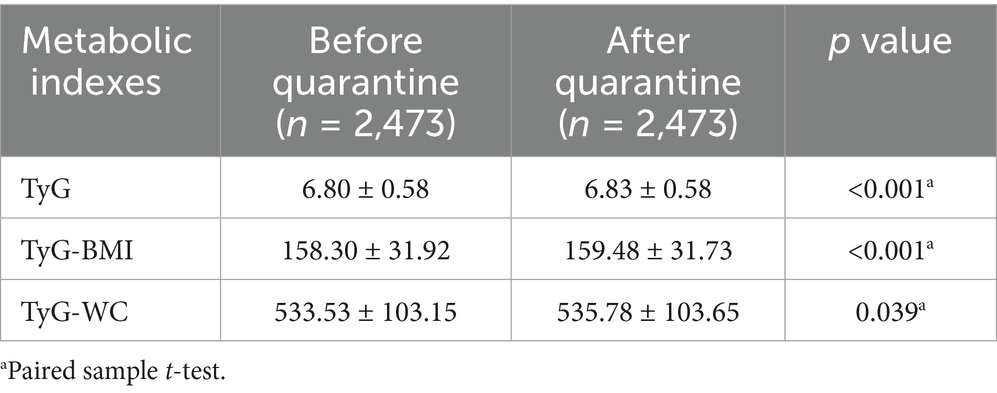
Statistical analysis revealed significant post-quarantine elevations across three metabolic indices: the TyG demonstrated an increment from 6.80 ± 0.58 to 6.83 ± 0.58 (p < 0.001), TyG-BMI increased from 158.30 ± 31.92 to 159.48 ± 31.73 (p < 0.001), and TyG-WC showed progression from 533.53 ± 103.15 to 535.78 ± 103.65 (p = 0.039) (Table 2).
Subgroup analysis of TyG changes
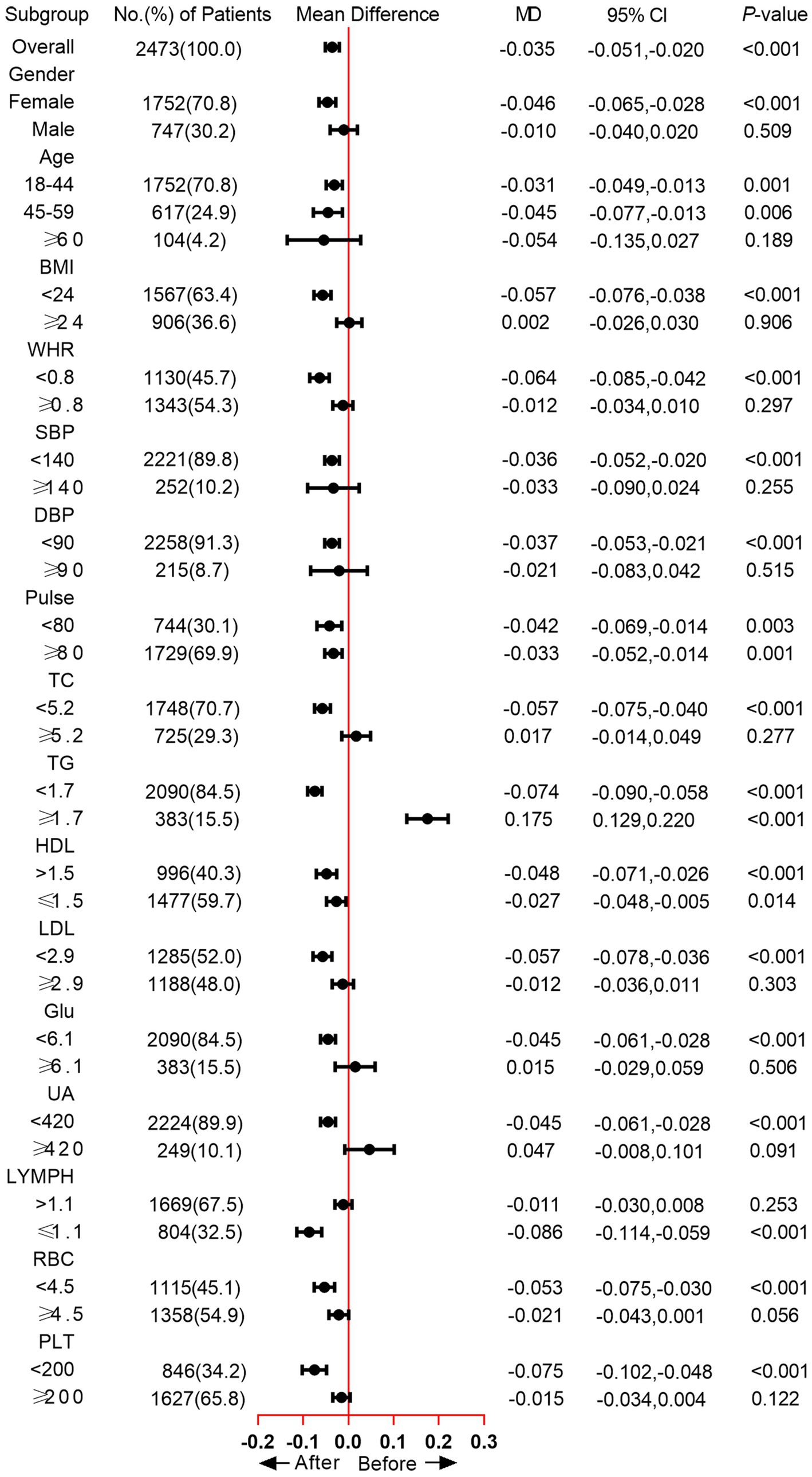
The subgroup analysis showed that the TyG was significantly increased in the female subgroup (mean difference [MD] = −0.046, p < 0.001), while no significant difference was observed in male participants. As for Age subgroups, significantly increased TyG was found in participants between 18 and 44 (MD = −0.031, p = 0.001) and 45–59 (MD = −0.045, p = 0.006). In the BMI < 24 subgroup, the MD of TyG was −0.057, and the p value was < 0.001. WHR < 0.8 group also exhibited increased TyG after home quarantine (MD = −0.064, p < 0.001). Moreover, we also analyzed the change of TyG in blood pressure, TC, TG, HDL, LDL, Glu, UA, LYMPH, RBC, and PLT subgroups, except for the TG ≥ 1.7 subgroup, an increased or unchanged TyG was observed in all other subgroups (Figure 2). When comparing the positive growth percentage of indicators, there were significant differences in PLT, HGB, NEU, UA, LDL, TC, and BMI between genders (Supplementary Figure 1).
Discussion
The present study demonstrated that home quarantine was associated with significant elevations in insulin resistance indices, specifically the TyG index, TyG-BMI, and TyG-WC. Furthermore, distinct variation patterns were observed in other metabolic parameters. These findings collectively suggest that quarantine measures may exert substantial effects on physiological homeostasis, particularly regarding insulin resistance characteristics.
The home quarantine significantly altered lifestyles, characterized by reduced physical activity, increased sedentary behavior, and unhealthy eating habits for the majority of individuals (4, 5). Changes in dietary structure, physical inactivity, and psychological pressure may explain weight gain and metabolic impairment. Fowler revealed that relative physical activity level was associated with HOMA-IR (24). Eriksson et al. demonstrated that psychological distress was associated with insulin resistance, significantly higher HOMA-IR was observed in patients with psychological distress (25), Robinson showed that metabolic syndrome and psychological burden may have independent effects on non-communicable diseases risk (26), although no previous study directly focused on psychological status and insulin resistance during the COVID-19 lockdown, many studies revealed that psychological stress was increased during the quarantine period, which could contribute to the worsening of insulin resistance indices (27, 28). Elevated psychological stress can activate the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system, leading to increased cortisol and catecholamine secretion, which in turn impairs insulin signaling, reduces glucose uptake in peripheral tissues, and promotes hepatic gluconeogenesis—mechanisms known to exacerbate insulin resistance (29, 30). In addition, stress-related behavioral changes, such as reduced physical activity, altered sleep patterns, and unhealthy dietary habits, may further aggravate insulin resistance (30, 31).
While a study from Poland found that reduced intake of vegetables, fruits, and legumes during the epidemic blockade, increased their intake of meat, dairy products, and fast food, leading to weight gain (32). Moreover, Pinto revealed that excessive and prolonged sedentary behaviors can lead to insulin resistance and other diseases. A more direct evidence was reported by Gonzalez et al., they revealed a worse insulin resistance status after the lockdown period (33). Besides, other studies demonstrated that the disruption of lifestyle and unhealthy behaviors altered circadian biology and exposed people to health problems such as weight gain/obesity (34), dyslipidaemia (35), and cardiometabolic health (36), which have been proven to be associated with metabolic abnormalities (37).
TyG, TyG-BMI, and TyG-WC as novel metabolic parameters have received extensive research focus. The relation between TyG-related parameters and COVID-19 infection was also found in several previous studies. Ren et al. reported an increased risk of COVID-19 severity and morbidity and higher TyG (38). Another study performed by Chang et al. showed that a high TyG index was associated with an increased risk for severe COVID-19 complications (39). For the TyG-BMI and TyG-WC, although the relevant reports were much less compared to the TyG index, Shabestari still showed a positive association between the TyG-BMI index and the risk of COVID-19 (40). The current evidence collectively underscores that dysregulated TyG indices, including TyG-BMI and TyG-WC, demonstrate consistent associations with adverse COVID-19 outcomes. Notably, while COVID-19 infection status was not systematically documented in our cohort, the ubiquitous nature of viral exposure during the study period may contribute to observed TyG elevations through indirect pathophysiological mechanisms. This investigation provides novel insights by identifying quantifiable increases in TyG parameters, specifically during home quarantine—a previously unexamined temporal window in metabolic research. Interestingly, we observed that HDL increased after home quarantine, which was in alignment with the findings of Ojo et al. (41), while both Valenzise and Bonfrate reported that HDL level decreased after the lockdown period, especially in females (6, 42), this might be related to changes in diet or lifestyle during the quarantine period. However, this interesting finding should be further confirmed in other population-based studies.
The results of our study also showed a lower level of LYMPH, EOS, and BAS in the general population after long-term isolation at home. This finding is in correlation with a previous study, patients with COVID-19 in China showed lower lymphocyte counts, higher leukocyte counts, and neutrophil-lymphocyte ratio (NLR), as well as lower percentages of MON, EOS, and BAS (43). This evidence revealed the potential association between blood cell count change and SARS-COV-2 infection. We further found elevated UA, BMI, TC, LDL, and Glu levels during the quarantine. More importantly, similar findings were present in children and adolescents, Morelli et al. showed that COVID-19 lockdown had a negative impact on adolescents’ metabolic and inflammatory profile (44), while Dong et al. analyzed the metabolic health of Chinese children and concluded that long-term lockdown due to COVID-19 outbreak might cause adverse impact on children’s metabolism and lead to increased risk of cardiovascular diseases (45).
Still, this study has certain limitations. First, although the sample size is sufficient, this is a single-center study, which may restrict the generalizability of the results to other regions or healthcare settings in China. Large-scale validation is required for future clinical applications. Second, limited parameters were included in this study. The dietary habits, daily activity information, and other insulin resistance-related parameters (e.g., HOMA-IR) were not recorded in this study. Third, all the participants underwent home quarantine, no control participants were included in this study. Fourth, the absence of COVID-19 infection data represents an additional limitation. In this retrospective design, the before–home quarantine period was defined as December 2021 to February 2022 to match the post-quarantine period and reduce seasonal bias; however, this may introduce potential selection bias and omit changes occurring between March and July 2022. Finally, due to the retrospective nature of this study, we did not survey the diet habits of the participants, we acknowledged that diet habits could affect the metabolic status, and in the future prospective study, we would take these factors into consideration.
Home quarantine may exacerbate insulin resistance risk, highlighting the necessity for regular monitoring of metabolic parameters (e.g., insulin resistance indices, and glycemic control markers) during isolation periods. This clinical vigilance could facilitate timely interventions to mitigate progression towards diabetes mellitus and cardiovascular complications, particularly in individuals with pre-existing metabolic vulnerabilities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the ethics committee of the Southwest Hospital of Army Medical University, PLA [Approval No. (B)KY2024191]. Due to the retrospective nature of this study, the informed consents were waived by the ethics committee.
Author contributions
YZ: Writing – original draft, Data curation. WC: Writing – original draft. LR: Writing – original draft, Data curation, Formal analysis. JW: Writing – original draft, Formal analysis. JX: Writing – original draft. SL: Writing – original draft. QM: Writing – review & editing. ZC: Writing – review & editing. HL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Army Medical University (grant number 4175ZA383) and the Chongqing Municipal Health Commission (grant number 20205NCPZX01).
Acknowledgments
The authors would like to thank all individuals who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1610474/full#supplementary-material
References
1. Dong, E, Du, H, and Gardner, L. An interactive web-based dashboard to track Covid-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
2. Kraemer, MUG, Yang, CH, Gutierrez, B, Wu, CH, Klein, B, Pigott, DM, et al. The effect of human mobility and control measures on the Covid-19 epidemic in China. Science (New York, NY). (2020) 368:493–7. doi: 10.1126/science.abb4218
3. Zhang, R, Wang, Y, Lv, Z, and Pei, S. Evaluating the impact of stay-at-home and quarantine measures on Covid-19 spread. BMC Infect Dis. (2022) 22:648. doi: 10.1186/s12879-022-07636-4
4. Luciano, F, Cenacchi, V, Vegro, V, and Pavei, G. Covid-19 lockdown: physical activity, sedentary behaviour and sleep in Italian medicine students. Eur J Sport Sci. (2021) 21:1459–68. doi: 10.1080/17461391.2020.1842910
5. Karageorghis, CI, Bird, JM, Hutchinson, JC, Hamer, M, Delevoye-Turrell, YN, Guerin, SMR, et al. Physical activity and mental well-being under Covid-19 lockdown: a cross-sectional multination study. BMC Public Health. (2021) 21:988. doi: 10.1186/s12889-021-10931-5
6. Bonfrate, L, Di Ciaula, A, Khalil, M, Farella, I, Chirico, R, Vilahur, G, et al. Gender-dependent impact of Covid-19 lockdown on metabolic and psychological aspects. Intern Emerg Med. (2023) 18:385–95. doi: 10.1007/s11739-022-03173-9
7. Lippi, G, Henry, BM, and Sanchis-Gomar, F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (Covid-19). Eur J Prev Cardiol. (2020) 27:906–8. doi: 10.1177/2047487320916823
8. Wang, Y, Shi, L, Que, J, Lu, Q, Liu, L, Lu, Z, et al. The impact of quarantine on mental health status among general population in China during the Covid-19 pandemic. Mol Psychiatry. (2021) 26:4813–22. doi: 10.1038/s41380-021-01019-y
9. Roberts, CK, Hevener, AL, and Barnard, RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. (2013) 3:1–58. doi: 10.1002/j.2040-4603.2013.tb00484.x
10. Sampath Kumar, A, Maiya, AG, Shastry, BA, Vaishali, K, Ravishankar, N, Hazari, A, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 62:98–103. doi: 10.1016/j.rehab.2018.11.001
11. dade Cassia Silva C,, Zambon, MP, Vasques, ACJ, Camilo, DF, de Góes Monteiro Antonio, MÂR, and Geloneze, B. The threshold value for identifying insulin resistance (Homa-Ir) in an admixed adolescent population: a hyperglycemic clamp validated study. Arch Endocrinol Metab. (2023) 67:119–25. doi: 10.20945/2359-3997000000533
12. Tahapary, DL, Pratisthita, LB, Fitri, NA, Marcella, C, Wafa, S, Kurniawan, F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of Homa-Ir and tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581
13. Son, DH, Lee, HS, Lee, YJ, Lee, JH, and Han, JH. Comparison of triglyceride-glucose index and Homa-Ir for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. (2022) 32:596–604. doi: 10.1016/j.numecd.2021.11.017
14. Wu, TD, Fawzy, A, Brigham, E, McCormack, MC, Rosas, I, Villareal, DT, et al. Association of triglyceride-glucose index and lung health: a population-based study. Chest. (2021) 160:1026–34. doi: 10.1016/j.chest.2021.03.056
15. Zhou, Z, Liu, Q, Zheng, M, Zuo, Z, Zhang, G, Shi, R, et al. Comparative study on the predictive value of Tg/Hdl-C, Tyg and Tyg-Bmi indices for 5-year mortality in critically ill patients with chronic heart failure: a retrospective study. Cardiovasc Diabetol. (2024) 23:213. doi: 10.1186/s12933-024-02308-w
16. Xue, Y, Xu, J, Li, M, and Gao, Y. Potential screening indicators for early diagnosis of Nafld/Mafld and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol (Lausanne). (2022) 13:951689. doi: 10.3389/fendo.2022.951689
17. Martinez-Ferran, M, de la Guía-Galipienso, F, Sanchis-Gomar, F, and Pareja-Galeano, H. Metabolic impacts of confinement during the Covid-19 pandemic due to modified diet and physical activity habits. Nutrients. (2020) 12:12061549. doi: 10.3390/nu12061549
18. Oncina-Cánovas, A, Compañ-Gabucio, L, Vioque, J, Ruiz-Canela, M, Corella, D, Salas-Salvadó, J, et al. More adult women than men at high Cardiometabolic risk reported worse lifestyles and self-reported health status in the Covid-19 lockdown. Nutrients. (2024) 16:2000. doi: 10.3390/nu16132000
19. Feldmann, H, Sprecher, A, and Geisbert, TW. Ebola. N Engl J Med. (2020) 382:1832–42. doi: 10.1056/NEJMra1901594
20. Ciminski, K, Chase, GP, Beer, M, and Schwemmle, M. Influenza a viruses: understanding human host determinants. Trends Mol Med. (2021) 27:104–12. doi: 10.1016/j.molmed.2020.09.014
21. Simpson, S, Kaufmann, MC, Glozman, V, and Chakrabarti, A. Disease x: accelerating the development of medical countermeasures for the next pandemic. Lancet Infect Dis. (2020) 20:e108–15. doi: 10.1016/S1473-3099(20)30123-7
22. Duan, S, Yang, D, Xia, H, Ren, Z, Chen, J, and Yao, S. Cardiometabolic index: a new predictor for metabolic associated fatty liver disease in Chinese adults. Front Endocrinol (Lausanne). (2022) 13:1004855. doi: 10.3389/fendo.2022.1004855
23. Yu, XR, Du, JL, Jiang, M, Ren, Y, Zhang, FL, Kong, FL, et al. Correlation of Tyg-Bmi and Tyg-Wc with severity and short-term outcome in new-onset acute ischemic stroke. Front Endocrinol (Lausanne). (2024) 15:1327903. doi: 10.3389/fendo.2024.1327903
24. Fowler, JR, Tucker, LA, Bailey, BW, and LeCheminant, JD. Physical activity and insulin resistance in 6,500 Nhanes adults: the role of abdominal obesity. J Obes. (2020) 2020:3848256. doi: 10.1155/2020/3848256
25. Eriksson, MCM, Lundgren, J, Hellgren, M, Li, Y, Björkelund, C, Lindblad, U, et al. Association between low internal health locus of control, psychological distress and insulin resistance. An exploratory study. PLoS One. (2023) 18:e0285974. doi: 10.1371/journal.pone.0285974
26. Robinson, E, Daly, M, and Putra, I. The psychological burden associated with metabolic syndrome: evidence from UK and US older adults. Obes Sci Pract. (2024) 10:e780. doi: 10.1002/osp4.780
27. Low, RST, Overall, NC, Chang, VT, Henderson, AME, and Sibley, CG. Emotion regulation and psychological and physical health during a Nationwide Covid-19 lockdown. Emotion. (2021) 21:1671–90. doi: 10.1037/emo0001046
28. Altieri, M, and Santangelo, G. The psychological impact of Covid-19 pandemic and lockdown on caregivers of people with dementia. Am J Geriatr Psychiatry. (2021) 29:27–34. doi: 10.1016/j.jagp.2020.10.009
29. Sharma, VK, and Singh, TG. Chronic stress and diabetes mellitus: interwoven pathologies. Curr Diabetes Rev. (2020) 16:546–56. doi: 10.2174/1573399815666191111152248
30. Tsigos, C, Kyrou, I, Kassi, E, and Chrousos, GP. Stress: endocrine physiology and pathophysiology. In: KR Feingold, SF Ahmed, B Anawalt, MR Blackman, A Boyce, and G Chrousos, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. (2000).
31. Agorastos, A, and Chrousos, GP. The neuroendocrinology of stress: the stress-related continuum of chronic disease development. Mol Psychiatry. (2022) 27:502–13. doi: 10.1038/s41380-021-01224-9
32. Sidor, A, and Rzymski, P. Dietary choices and habits during Covid-19 lockdown: experience from Poland. Nutrients. (2020) 12:1657. doi: 10.3390/nu12061657
33. López-González, ÁA, Altisench Jané, B, Masmiquel Comas, L, Arroyo Bote, S, González San Miguel, HM, and Ramírez Manent, JI. Impact of Covid-19 lockdown on non-alcoholic fatty liver disease and insulin resistance in adults: a before and after pandemic lockdown longitudinal study. Nutrients. (2022) 14:2795. doi: 10.3390/nu14142795
34. Cena, H, Fiechtner, L, Vincenti, A, Magenes, VC, De Giuseppe, R, Manuelli, M, et al. Covid-19 pandemic as risk factors for excessive weight gain in pediatrics: the role of changes in nutrition behavior. A narrative review. Nutrients. (2021) 13:4255. doi: 10.3390/nu13124255
35. Auriemma, RS, Pirchio, R, Liccardi, A, Scairati, R, Del Vecchio, G, Pivonello, R, et al. Metabolic syndrome in the era of Covid-19 outbreak: impact of lockdown on Cardiometabolic health. J Endocrinol Investig. (2021) 44:2845–7. doi: 10.1007/s40618-021-01563-y
36. Mattioli, AV, Sciomer, S, Cocchi, C, Maffei, S, and Gallina, S. Quarantine during Covid-19 outbreak: changes in diet and physical activity increase the risk of cardiovascular disease. Nutr Metab Cardiovasc Dis. (2020) 30:1409–17. doi: 10.1016/j.numecd.2020.05.020
37. Ndrepepa, G. Uric acid and cardiovascular disease. Clin Chim Acta. (2018) 484:150–63. doi: 10.1016/j.cca.2018.05.046
38. Ren, H, Yang, Y, Wang, F, Yan, Y, Shi, X, Dong, K, et al. Association of the Insulin Resistance Marker Tyg Index with the severity and mortality of Covid-19. Cardiovasc Diabetol. (2020) 19:58. Epub 2020/05/13. doi: 10.1186/s12933-020-01035-2
39. Chang, Y, Jeon, J, Song, TJ, and Kim, J. Association of Triglyceride-Glucose Index with prognosis of Covid-19: a population-based study. J Infect Public Health. (2022) 15:837–44. Epub 2022/07/03. doi: 10.1016/j.jiph.2022.06.014
40. Shabestari, M, Azizi, R, and Ghadiri-Anari, A. Type 2 diabetes and susceptibility to Covid-19: a machine learning analysis. BMC Endocr Disord. (2024) 24:221. doi: 10.1186/s12902-024-01758-3
41. Ojo, O, Wang, XH, Ojo, OO, Orjih, E, Pavithran, N, Adegboye, ARA, et al. The effects of Covid-19 lockdown on Glycaemic control and lipid profile in patients with type 2 diabetes: a systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:1095. doi: 10.3390/ijerph19031095
42. Valenzise, M, D'Amico, F, Cucinotta, U, Lugarà, C, Zirilli, G, Zema, A, et al. The lockdown effects on a pediatric obese population in the Covid-19 era. Ital J Pediatr. (2021) 47:209. doi: 10.1186/s13052-021-01142-0
43. Qin, C, Zhou, L, Hu, Z, Zhang, S, Yang, S, Tao, Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (Covid-19) in Wuhan, China. Clin Infect Dis. (2020) 71:762–8. doi: 10.1093/cid/ciaa248
44. Augimeri, G, Fiorillo, M, Caparello, G, Ceraudo, F, Avolio, E, Morelli, C, et al. Impact of Covid-19 lockdown on metabolic/inflammatory profile in adolescents: cellular studies and predictive biomarkers. J Clin Endocrinol Metab. (2024) 109:711–21. doi: 10.1210/clinem/dgad603
Keywords: home quarantine, insulin resistance, triglyceride, glucose, body mass index, metabolic biomarkers
Citation: Zhang Y, Chen W, Ran L, Wang J, Xia J, Li S, Mao Q, Chen Z and Liu H (2025) Changes in insulin resistance and other metabolic parameters during home quarantine amid the COVID-19 pandemic among the general population. Front. Nutr. 12:1610474. doi: 10.3389/fnut.2025.1610474
Edited by:
Mauro Serafini, University of Teramo, ItalyReviewed by:
Sanny Frisca, Universitas Katolik Musi Charitas, IndonesiaRanveer Singh Jadon, All India Institute of Medical Sciences, India
Copyright © 2025 Zhang, Chen, Ran, Wang, Xia, Li, Mao, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Liu, bGl1aHVpbWluQHRtbXUuZWR1LmNu; Zongtao Chen, Y2hlbnpvbmd0YW9AdG1tdS5lZHUuY24=; Qing Mao, cWluZ21hb0B0bW11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yuting Zhang
Yuting Zhang Wenting Chen2,3†
Wenting Chen2,3† Zongtao Chen
Zongtao Chen Huimin Liu
Huimin Liu