- 1Division of Hematology–Oncology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan
- 2Department of Nursing, Min-Hwei Junior College of Health Care Management, Tainan, Taiwan
- 3Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 4School of Medicine, China Medical University, Taichung, Taiwan
- 5Department of Occupational Safety and Health, China Medical University, Taichung, Taiwan
- 6Department of Public Health, China Medical University, Taichung, Taiwan
Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce mortality in patients with cancer, especially breast cancer, but their influence on second cancer risk is uncertain. This study aimed to examine whether NSAID use is associated with second cancer risk in patients with breast cancer. This population-based propensity score-matched cohort study using Taiwan’s National Health Insurance Research Database enrolled patients with newly diagnosed breast cancer (n = 7356) with and without (n = 1839) NSAID therapy from 2000 to 2009. They were followed up until the diagnosis of second cancer, death, or end of 2011. Cox proportional hazard models were used to estimate adjusted hazard ratios (aHR). The NSAID cohort had a lower incidence rate of second cancer than the non-NSAID cohort (5.57 vs. 9.19 per 1,000 person-years), with an aHR of 0.63 (95% confidence interval (CI) 0.46–0.87). When compared with the non-NSAID cohort, the second cancer incidence was lower in patients taking non-cyclooxygenase 2 inhibitors (aHR 0.67, 95% CI 0.47–0.94) and in those receiving multiple NSAIDs during follow-up (aHR 0.55, 95% CI 0.37–0.84). A dose–response relationship existed in NSAID cumulative days. The findings demonstrate that NSAID use reduces second cancer risk in a dose-dependent manner in patients with primary breast cancer.
Introduction
Breast cancer is one of the rapidly growing cancers in developed countries. In Taiwan, with the increase in breast cancer incidence and its associated medical-resource utilization (1), the chance of early breast cancer detection has increased significantly owing to the implementation of the national policy on mammography screening (2). In addition to resection surgery, patients with breast cancer usually receive adjuvant chemotherapy, radiotherapy, and hormone therapy after surgery. Chemotherapeutic drugs, hormone-based drugs, and radiation contribute to the long-term survival benefit of patients with breast cancer. However, these treatments have some inevitable long-term side effects, especially the increased risk of second cancer development (3). Therefore, with the increase in the long-term survival of patients with breast cancer, the problem of second cancer should be focused on during the follow-up of long-term survivors.
Nonsteroidal anti-inflammatory drugs (NSAIDs) include aspirin and cyclooxygenase (COX) inhibitors. COX inhibitors are further divided into COX-2 selective and non-selective (non-COX-2) classes. In recent years, aspirin and NSAIDs have been suggested to have inhibitory effects on the pathogenesis of cancerization (4, 5), and evidence in this regard has been accumulating from basic and clinical research (6–9). Some studies have revealed that the use of aspirin or NSAIDs reduce the risk of breast cancer in the general population (10, 11) and in individuals with a family history of breast cancer (12). This cancer-preventive effect has also been observed in certain populations such as patients with chronic dialysis (13), women with obesity (14), and persons without smoking habits (15). Furthermore, reduced cancer-related mortality has been shown in patients with cancer using NSAIDs (16–18). Especially, the use of NSAIDs after breast cancer diagnosis could reduce mortality in patients with breast cancer (19–21). However, to the best of our knowledge, no study has so far examined whether NSAID use has any effect on second cancer risk in patients with breast cancer.
Performing randomized clinical trials with NSAIDs is challenging because these are old patent-off drugs. Conducting clinical trials is a time-consuming and expensive affair. Unlike new anti-cancer drugs, recruiting a sufficient number of patients to participate in NSAID-related clinical trials is difficult. The Taiwan National Health Insurance Research Database (NHIRD) has collected rich information on drug use history, hospitalization history, and disease event in Taiwanese patients over the past 20 years (22), which serves as a data resource for biomedical research (23). This study aimed to investigate the association of NSAID use with the incidence of second cancer in patients with primary breast cancer by using the NHIRD data.
Materials and Methods
Data Sources
We collected data from the NHIRD, derived from Taiwan’s single-payer compulsory National Health Insurance Program, which covers up to 99% of the 23 million Taiwanese citizens. The NHIRD contains inpatient, and outpatient visit dates, medical diagnosis, expenditure, and prescription details. This retrospective cohort study used the Registry of Catastrophic Illness Patient Database (RCIPD), a part of the NHIRD. The insurants in the RCIPD were individuals with fatal illnesses, including 30 categories of diseases requiring long-term extensive care, such as cancer. The disease record system in the NHIRD was built based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). This study was approved by the Research Ethics Committee of China Medical University (CMUH-104-REC2-115-R4), and the need for informed consent was waived. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies.
Study Subjects
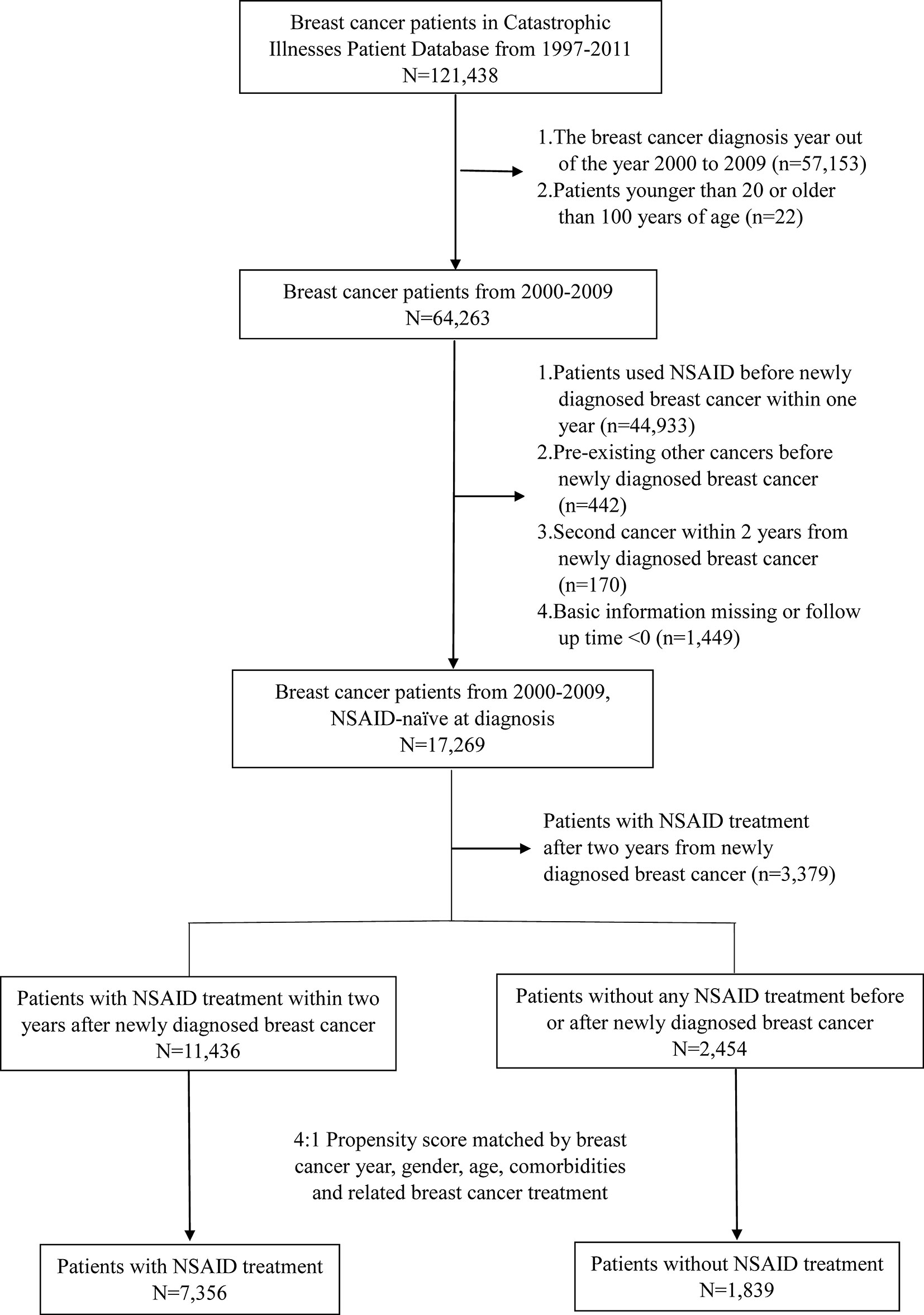
A population-based cohort study was conducted to explore the association of NSAID use with second cancer risk in patients with primary breast cancer. We established a breast cancer cohort of 64,263 women (aged ≥20 years) with newly diagnosed breast cancer (ICD-9-CM 174) from 2000 to 2009.
The exclusion criteria of the study were as follows: preexisting cancers other than breast cancer, history of using NSAIDs before the diagnosis of breast cancer, and second cancer within 2 years of newly diagnosed breast cancer. Cancer takes time to develop; hence, we determined that the second cancer must occur at least two years after the primary breast cancer. This definition was intended to clearly define that these two events did not occur simultaneously. A total of 17,269 patients with newly diagnosed breast cancer who were NSAID-naïve at diagnosis and did not have other preexisting cancers were selected. Patients with NSAID treatment 2 years after the newly diagnosed breast cancer were further excluded (n = 3,379). In this study, the NSAID cohort was defined as patients with NSAID treatment within 730 days (2 years) of the initial date of breast cancer diagnosis to avoid immortal time. Day 731 after the diagnosis date of breast cancer was defined as the index date. The flowchart is shown in Figure 1.
Covariates
The distribution of age and baseline comorbidities was compared between patients with and without NSAID treatment (n = 11,436 and 2,454, respectively) (Table 1). Sixteen baseline comorbidities were identified in our study, including congestive heart failure (ICD-9-CM codes 398.91, 425, and 428), dementia (ICD-9-CM codes 290.0-290.4, 294.1, and 331.0-331.2), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, and 496), rheumatic disease (ICD-9-CM odes 710, 714, 725, and A430), peptic ulcer disease (ICD-9-CM codes 531-534), cirrhosis (ICD-9-CM codes 571, A347), hypertension (ICD-9-CM codes 401-405, A260, and A269), diabetes (ICD-9-CM code 250), hypercholesterolemia (ICD-9-CM code 272.0), hyperlipidemia (ICD-9-CM codes 272.2 and 272.4), ischemic heart disease (ICD-9-CM codes 410-414 and 429), atrial fibrillation and flutter (ICD-9-CM code 427.3), cardiovascular disease (ICD-9-CM codes 410-414, 428, 430-438, and 440-448), peripheral vascular disease (ICD-9-CM code 440-444), chronic kidney disease (ICD-9-CM codes 580-589 and A350), and depression (ICD-9-CM codes 296.2, 296.3, 296.82, 300.4, 309.0, 309.1, 309.28, and 311). The criterion of at least one inpatient or two outpatient admissions for such diseases before the initial date of breast cancer diagnosis was used to enhance diagnostic precision.
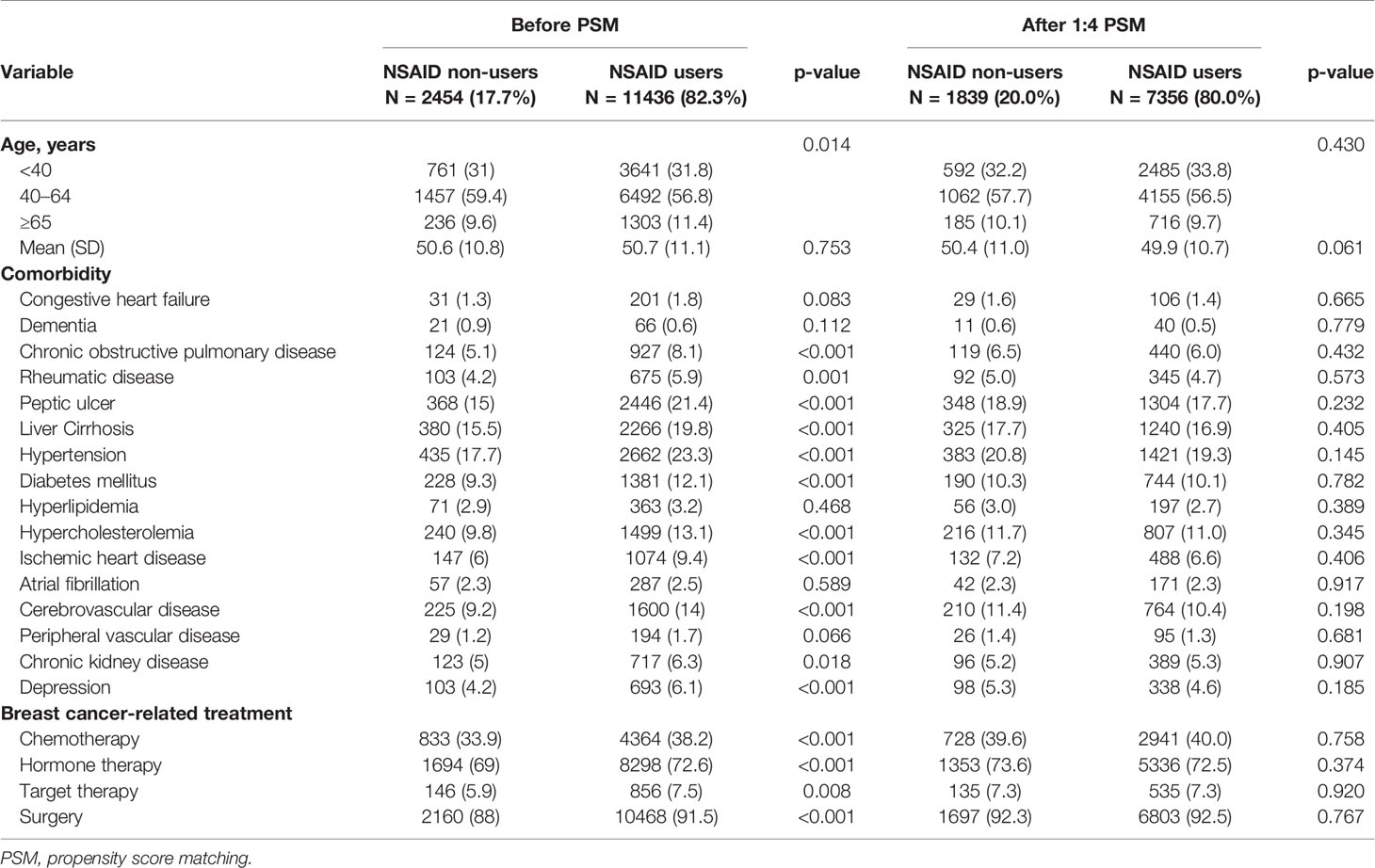
Table 1 Distribution of age, comorbidity, breast cancer-related treatment, and second cancer between the non-NSAID and NSAID cohorts.
Breast cancer-related treatments, including chemotherapy, hormone therapy, target therapy, and surgery, were also identified. Chemotherapy was defined as the administration of one of the following drugs: docetaxel, paclitaxel, doxorubicin, and epirubicin, which are essential components in various chemotherapeutic regimens. Hormone therapy was defined as the administration of one of the following hormone-based drugs: tamoxifen, anastrozole, letrozole, and exemestane. Target therapy was defined as treatment with trastuzumab. Surgical treatment was defined as having undergone any of the following procedures: partial mastectomy (ICD-9-CM code 85.2-85.25), total mastectomy, and modified radical mastectomy (ICD-9-CM code 85.4-85.48).
Study Cohorts With Propensity Score Matching (PSM)
Patients with NSAID therapy within 2 years were older and had a higher proportion of baseline comorbidity, chemotherapy, hormone therapy, target therapy, and surgery than those without NSAID therapy (Table 1). In this study, 1:4 PSM was performed to identify the non-NSAID cohort. The propensity score was estimated by logistic regression for the controlling of breast cancer year, age, and baseline comorbidities. Following PSM, 7,356 patients with and 1,839 patients without NSAID treatment were included in this study. Both cohorts were followed up until the occurrence of a secondary cancer (ICD-9-CM 140–208, except for breast cancer), death, or end of 2011.
In the NSAID cohort, NSAID products and their dosage were retrieved based on the treatment history during the follow-up. Three different NSAIDs were specified in the study cohort, namely, aspirin, COX-2 inhibitors, and non-COX-2 inhibitors. The NSAID dosage was grouped based on cumulative days: <7, 8–28, 29–90, and >90 days.
Statistical Analysis
Categorical variables were presented as numbers (percentage), and continuous variables were expressed as means (standard deviation). Cox proportional hazards regression model analyses were performed to assess the hazard ratio (HR) of second cancer for the NSAID cohort in comparison with the non-NSAID cohort. Death could be a competing risk for second cancer; hence, competing risk analysis was conducted by using Fine and Gray’s proportional hazards regression analysis (24) to estimate the subdistribution HR (SHR) and 95% confidence interval (CI). Age- and comorbidity-stratified SHR of second cancer were also estimated for the NSAID cohort. Data management and statistical analyses were performed using SAS software 9.4 (SAS Institute, Cary, NC, USA). The significance level was set at P < 0.05 using two-tailed tests.
Results
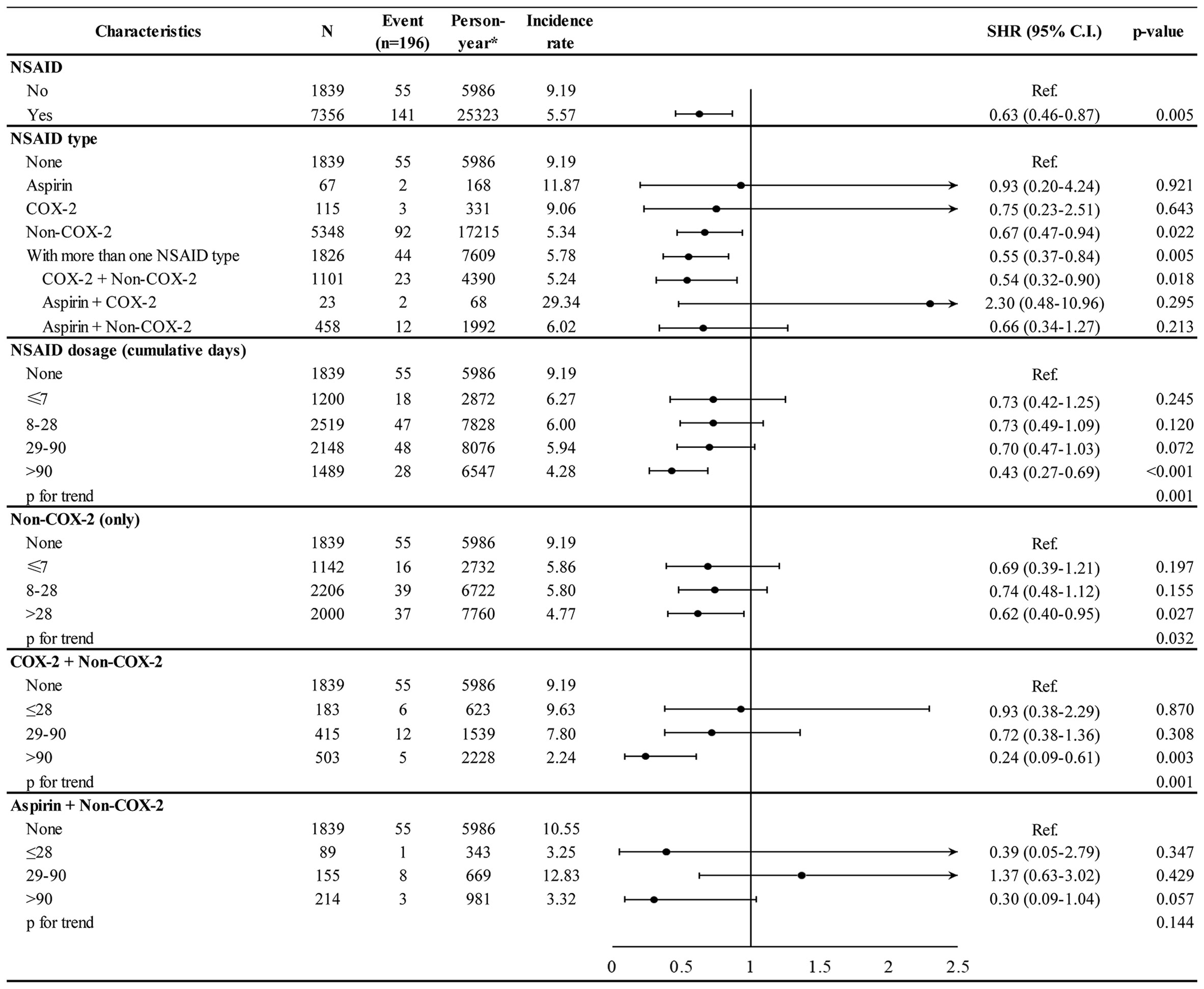
After the PSM, the baseline characteristics were well balanced between the NSAID and non-NSAID cohorts (Table 1). The incidence rate per 1,000 person-years for second cancer was 5.57 in the NSAID cohort and 9.19 in the non-NSAID cohort (Figure 2). After adjusting for age, comorbidities, and breast cancer-related treatment, the adjusted SHR of NSAID use was 0.63 (95% CI 0.46–0.87) for second cancer incidence.
The association of second cancer risk with NSAID type and dosage is shown in Figure 2. When compared with the non-NSAID cohort, the second cancer incidence was significantly lower in patients taking non-COX-2 inhibitors (adjusted SHR 0.67, 95% CI 0.47–0.94) and in those receiving multiple NSAIDs during follow-up (adjusted SHR 0.55, 95% CI 0.37–0.84). Higher cumulative days of NSAID use was associated with a lower incidence of second cancer in a dose–response manner (p for trend 0.001), and the adjusted SHR was 0.43 (95% CI 0.27–0.69) in patients using NSAID for >90 days. The dose–response relationship was also observed for a specific NSAID type. Patients using non-COX-2 inhibitors for >28 days and COX-2 along with non-COX-2 inhibitors for >90 days had a lower incidence of second cancer, with adjusted SHR of 0.62 (95% CI 0.40–0.95) and 0.24 (95% CI 0.09–0.61), respectively.
The association of comorbidity with second cancer is shown in Supplementary Figure 1. Patients with liver cirrhosis (adjusted SHR 1.52, 95% CI 1.06–2.18) had a higher HR for second cancer. Furthermore, the association of NSAID treatment with second cancer stratified by age and comorbidities is depicted in Figure 3. NSAID treatment was associated with a lower second cancer incidence in patients with hypertension (adjusted SHR 0.37, 95% CI 0.20–0.68) but not in those without hypertension (adjusted SHR 0.74, 95% CI 0.51–1.10), and the hypertension–NSAID interaction reached a borderline significance (p = 0.077).
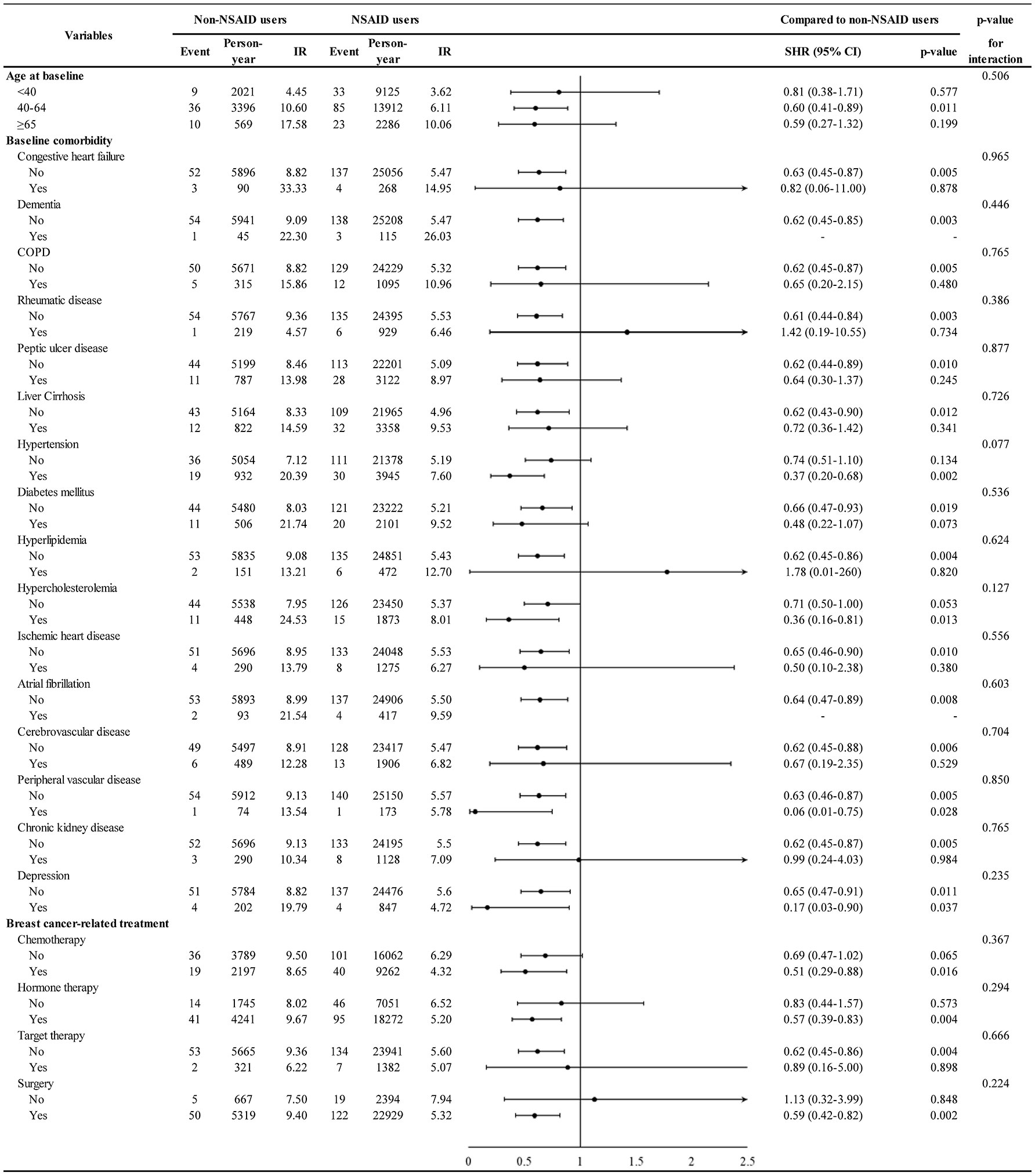
Figure 3 Incidence and hazard ratio of second cancer for NSAID stratified by age, comorbidity, and breast cancer-related treatment.
The association of NSAID treatment with different types of second cancer is shown in Figure 4. The NSAID cohort demonstrated lower HR for hepatic, biliary tract, gallbladder, and pancreatic cancers (adjusted SHR 0.26, 95% CI 0.09–0.76) than the non-NSAID cohort. However, NSAID use did not have a protective effect in case of a subsequent gynecologic cancer (adjusted SHR 0.84, 95% CI 0.38–1.88). The statistical power of such second cancer stratified analyses could be limited because of the small sample size for each specific second cancer.
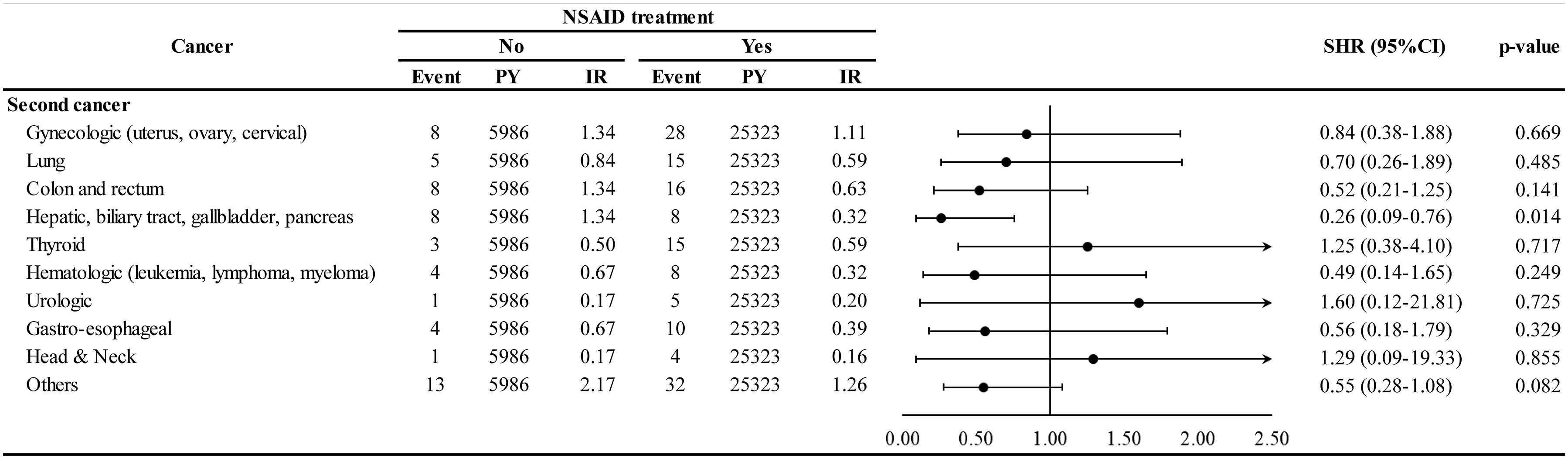
Figure 4 Incidence and hazard ratio of specific second cancer for NSAID. PY, person-years; IR, incidence rate, per 1000-per years; SHR, subdistribution hazard ratio; CI, confidence interval.
Discussion
In this nationwide population-based cohort study of patients with primary breast cancer, PSM was used to establish the NSAID and non-NSAID cohorts that were similar in terms of baseline characteristics, and their subsequent second cancer risk was investigated. This study also evaluated the sub-classifications of NSAIDs, their combined use, and NSAID dosage in cumulative days with respect to the patients’ carcinogenicity. This study showed that the NSAID cohort had a lower incidence of second cancer than the non-NSAID cohort. The risk was particularly lower for patients undergoing non-COX-2 treatments, those receiving non-COX-2 along with COX-2 during the follow-up, and those receiving NSAID dosage for a cumulative period of >90 days.
In our study, drug subgroup analysis unexpectedly revealed that the protective effect of NSAID is often derived from non-COX-2 drugs. The use of aspirin and COX-2 drugs had no lowering effect on the incidence of second cancer in patients with primary breast cancer. Aspirin was the most disputed drug among all NSAIDs. The reason for the increased attention was that aspirin exerted a protective effect in the normal population based on the findings of clinical studies (25–27). However, this drug could not reduce breast cancer-specific mortality in patients with primary breast cancer, as reported by many clinical studies (28–30). Strasser-Weippl et al. also showed that COX-2 combined with aspirin has no effect on the disease-free survival of patients with breast cancer (31). Our study suggested that non-COX-2 and COX-2 drugs have different effects in reducing the incidence of second cancer in patients with breast cancer. Such inconsistent findings might reflect the actual drug consumption frequency among the people in Taiwan (aspirin N = 67, COX-2 N = 115, and non-COX-2 N = 5348). The fact that >90% of the patients were in the non-COX2 cohort made the number of patients in the other two cohorts (aspirin and COX-2) too small to reveal their statistical effect. Furthermore, many studies have shown that non-selective COX-2 drugs have better anti-cancer activity than selective COX-2 agents (12, 32, 33).
Another important study finding was that using more than one NSAID during the follow-up had variable effects in reducing the incidence of second cancer as the use of COX-2 along with non-COX-2 drugs during the follow-up could reduce second cancer risk. However, aspirin combined with either COX-2 or non-COX-2 drug did not reduce second cancer risk. He et al. also found that the use of aspirin combined with COX-2 reduces COX-2 chemopreventive effects in colorectal cancer (34). Additionally, gastrointestinal studies have demonstrated that the concomitant use of aspirin and COX-2 reduces the gastrointestinal benefit of COX-2 (35, 36). Our study reveals that the NSAID dosage is proportional to the reduction in the incidence of second cancer risk. Other studies have also demonstrated that the cancer-preventive effect of NSAID is dose-dependent (7, 13, 16), including an intake duration of at least 90 days (6, 37–39), 180 days (15, 39), or longer (years) (14, 33).
Our study showed that NSAID treatment is associated with a lower second cancer incidence in patients aged 40–64 years (SHR = 0.60, p-value = 0.011) as well as in patients aged >65 years (SHR = 0.59, though not reached statistical significance) (Figure 3). This result implies that both pre-menopausal and post-menopausal women with breast cancer can derive chemopreventive benefit by using NSAID. Previous studies have also shown that NSAID exhibits its chemopreventive effect in pre- and post-menopausal women (12, 25, 32); nonetheless, some studies have shown that post-menopausal women could particularly benefit from the NSAID anti-cancer effect (26, 27, 33).
This study observed that liver cirrhosis is associated with increased second cancer incidence (see Supplementary Figure 1). Globally, Taiwan was a country with a high prevalence of hepatitis B and hepatitis C (40), which caused a higher incidence of liver cirrhosis and lead to an increased cancer incidence (41, 42). In our study, the protective influence of NSAID on second cancers was more predominant in patients with hypertension. Such patients have been well-educated to take antihypertensive drugs periodically, and previous studies have proposed that antihypertensive drugs might increase cancer risk (43–45). The interactive effect between NSAIDs and antihypertensive drugs needs to be explored further.
Although NSAID use decreased second cancer in many organ systems, only hepatic, biliary tract, and pancreatic cancers showed significance (Figure 4). Previous studies in Taiwan have also found that NSAID use decreased hepatocellular carcinoma risk in patients with chronic hepatitis B and infection (6, 16, 37).
A key strength of this study is the novelty of examining the association between NSAID use and second cancer incidence in patients with primary breast cancer. The use of a nationwide representative cohort design with large sample sizes and the application of PSM to consider a range of comorbidities and breast cancer-related treatments and to balance their distributions in the two cohorts with and without NSAID therapy are other important advantages. Furthermore, this study has provided empirical evidence for the association of NSAID use with second cancer risk reduction in a dose–response manner.
However, this study also has several limitations. First, the study is relatively old and has a relatively short study period, i.e., from 1997 to 2011. It takes at least 4–5 years for second cancer to develop in primary breast cancer survivors. We selected women with newly diagnosed breast cancer from 2000 to 2009 and then followed them up in 2011. A longer follow-up period might allow more cases of primary breast cancer to fit into our study and allow more second cancer events to appear, making statistical evidence more meaningful. Second, the patient’s tumor, lymph node, metastasis stage, body mass index, and family history could not be obtained because of the limitation of the NHIRD. This study only explored recordable risk factors based on reliable clinical classifications. Although the distributions of these factors, including covariates, were significantly different between the two groups (patients with and without NSAID therapy), they were well balanced after PSM. Third, on the pathologic subtypes of primary breast cancer was not available. We assumed indirectly that patients who ever received hormone therapy were estrogen-receptor or progesterone-receptor positive, and those who received target therapy had human epidermal growth factor receptor 2 overexpression. The triple negative breast cancer might have been missed in this type of analysis. Finally, a causal relationship between NSAID consumption and second cancer risk could not be inferred directly owing to the observational nature of our study.
In conclusion, with a large collection of approximately 10,000 patients with breast cancer in Taiwan, this population-based cohort study showed that those taking non-COX-2 inhibitors and those receiving multiple NSAIDs during follow-up had a lower second cancer incidence in a dose-dependent manner. To the best of our knowledge, this study is the first to investigate the relationship between NSAID use and second cancer incidence in patients with primary breast cancer. Studies with a prospective design, larger sample size, and longer follow-up period are needed to further verify our findings.
Data Availability Statement
The data set for this article are not publicly available because public availability of the data set is restricted by local regulations to protect privacy. Any researcher interested in accessing the data set can submit an application form to the Ministry of Health and Welfare requesting access.
Ethics Statement
This study was approved by the Research Ethics Committee of China Medical University (CMUH-104-REC2-115-R4), and the need for informed consent was waived. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Study concept and design: Y-CL and S-HW. Acquisition of data: S-HW and Y-JP. Analysis and interpretation of data: all authors. Drafting of the manuscript: Y-CL, S-HW, and Y-JP. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: S-HW, M-CL, and C-CL. Obtaining funding: Y-CL, S-HW, and Y-JP. Administrative, technical, or material support: Y-CL, S-HW, and Y-JP. Study supervision: S-HW and Y-JP. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Ditmanson Medical Foundation Chia-Yi Christian Hospital (Grant No. R104-9), China Medical University Hospital, Taiwan (DMR-109-161), China Medical University, Taiwan (CMU108-MF-62; CMU108-S-23; CMU109-MF-111; CMU110-S-22), Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), and Tseng-Lien Lin Foundation, Taichung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Yu-Fen Li for her help with the analysis of the NHIRD.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.756143/full#supplementary-material
References
1. Chang HT, Shi HY, Wang BW, Yeh SJ. Breast Cancer Incidence and Predictors of Surgical Outcome: A Nationwide Longitudinal Study in Taiwan. Clin Oncol (R Coll Radiol) (2017) 29(6):362–9. doi: 10.1016/j.clon.2017.01.005
2. Yen AM, Tsau HS, Fann JC, Chen SL, Chiu SY, Lee YC, et al. Population-Based Breast Cancer Screening With Risk-Based and Universal Mammography Screening Compared With Clinical Breast Examination: A Propensity Score Analysis of 1429890 Taiwanese Women. JAMA Oncol (2016) 2(7):915–21. doi: 10.1001/jamaoncol.2016.0447
3. Lu YC, Lu CL, Chen YY, Chen PT, Lin MS, Chen W, et al. Trend of Incidence of Second Primary Malignancies Following Breast Cancer in Taiwan: A 12-Year Nationwide Cohort Study. Breast J (2016) 22(3):360–2. doi: 10.1111/tbj.12582
4. Cai Y, Yousef A, Grandis JR, Johnson DE. NSAID Therapy for PIK3CA-Altered Colorectal, Breast, and Head and Neck Cancer. Adv Biol Regul (2020) 75:100653. doi: 10.1016/j.jbior.2019.100653
5. Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical Pharmacology of non-Steroidal Anti-Inflammatory Drugs: A Review. Antiinflamm Antiallergy Agents Med Chem (2012) 11(1):52–64. doi: 10.2174/187152312803476255
6. Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, et al. Association of Daily Aspirin Therapy With Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. JAMA Intern Med (2019) 179(5):633–40. doi: 10.1001/jamainternmed.2018.8342
7. Kuo CN, Pan JJ, Huang YW, Tsai HJ, Chang WC. Association Between Nonsteroidal Anti-Inflammatory Drugs and Colorectal Cancer: A Population-Based Case-Control Study. Cancer Epidemiol Biomarkers Prev (2018) 27(7):737–45. doi: 10.1158/1055-9965.EPI-17-0876
8. Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, et al. Associations Between Aspirin Use and the Risk of Cancers: A Meta-Analysis of Observational Studies. BMC Cancer (2018) 18(1):288. doi: 10.1186/s12885-018-4156-5
9. Pennock ND, Martinson HA, Guo Q, Betts CB, Jindal S, Tsujikawa T, et al. Ibuprofen Supports Macrophage Differentiation, T Cell Recruitment, and Tumor Suppression in a Model of Postpartum Breast Cancer. J Immunother Cancer (2018) 6(1):98. doi: 10.1186/s40425-018-0406-y
10. Takkouche B, Regueira-Mendez C, Etminan M. Breast Cancer and Use of Nonsteroidal Anti-Inflammatory Drugs: A Meta-Analysis. J Natl Cancer Inst (2008) 100(20):1439–47. doi: 10.1093/jnci/djn324
11. Clarke CA, Canchola AJ, Moy LM, Neuhausen SL, Chung NT, Lacey JV Jr, et al. Regular and Low-Dose Aspirin, Other Non-Steroidal Anti-Inflammatory Medications and Prospective Risk of HER2-Defined Breast Cancer: The California Teachers Study. Breast Cancer Res (2017) 19(1):52. doi: 10.1186/s13058-017-0840-7
12. Kim S, Shore DL, Wilson LE, Sanniez EI, Kim JH, Taylor JA, et al. Lifetime Use of Nonsteroidal Anti-Inflammatory Drugs and Breast Cancer Risk: Results From a Prospective Study of Women With a Sister With Breast Cancer. BMC Cancer (2015) 15:960. doi: 10.1186/s12885-015-1979-1
13. Ou SM, Chen YT, Chao PW, Lee YJ, Liu CJ, Yeh CM, et al. Nonsteroidal Anti-Inflammatory Drug Use Is Associated With Cancer Risk Reduction in Chronic Dialysis Patients. Kidney Int (2013) 84(1):198–205. doi: 10.1038/ki.2013.79
14. Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, et al. Aspirin, Nonsteroidal Anti-Inflammatory Drugs, Paracetamol and Risk of Endometrial Cancer: A Case-Control Study, Systematic Review and Meta-Analysis. Int J Cancer (2013) 132(5):1146–55. doi: 10.1002/ijc.27717
15. Daugherty SE, Pfeiffer RM, Sigurdson AJ, Hayes RB, Leitzmann M, Schatzkin A, et al. Nonsteroidal Antiinflammatory Drugs and Bladder Cancer: A Pooled Analysis. Am J Epidemiol (2011) 173(7):721–30. doi: 10.1093/aje/kwq437
16. Yeh CC, Lin JT, Jeng LB, Ho HJ, Yang HR, Wu MS, et al. Nonsteroidal Anti-Inflammatory Drugs Are Associated With Reduced Risk of Early Hepatocellular Carcinoma Recurrence After Curative Liver Resection: A Nationwide Cohort Study. Ann Surg (2015) 261(3):521–6. doi: 10.1097/SLA.0000000000000746
17. Verdoodt F, Dehlendorff C, Friis S, Kjaer SK. Non-Aspirin NSAID Use and Ovarian Cancer Mortality. Gynecol Oncol (2018) 150(2):331–7. doi: 10.1016/j.ygyno.2018.06.018
18. Lin JL, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, et al. Relationship Between Aspirin Use of Esophageal, Gastric and Colorectal Cancer Patient Survival: A Meta-Analysis. BMC Cancer (2020) 20(1):638. doi: 10.1186/s12885-020-07117-4
19. Leite AM, Macedo AVS, Jorge AJL, Martins WA. Antiplatelet Therapy in Breast Cancer Patients Using Hormonal Therapy: Myths, Evidence and Potentialities - Systematic Review. Arquivos Brasileiros Cardiologia (2018) 111(2):205–12. doi: 10.5935/abc.20180138
20. Huang XZ, Gao P, Sun JX, Song YX, Tsai CC, Liu J, et al. Aspirin and Nonsteroidal Anti-Inflammatory Drugs After But Not Before Diagnosis Are Associated With Improved Breast Cancer Survival: A Meta-Analysis. Cancer Causes Control (2015) 26(4):589–600. doi: 10.1007/s10552-015-0539-y
21. Kwan ML, Habel LA, Slattery ML, Caan B. Nsaids and Breast Cancer Recurrence in a Prospective Cohort Study. Cancer Causes Control (2007) 18(6):613–20. doi: 10.1007/s10552-007-9003-y
22. Sung SF, Hsieh CY, Hu YH. Two Decades of Research Using Taiwan’s National Health Insurance Claims Data: Bibliometric and Text Mining Analysis on Pubmed. J Med Internet Res (2020) 22(6):e18457. doi: 10.2196/18457
23. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s National Health Insurance Research Database: Past and Future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/CLEP.S196293
24. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94:496–509. doi: 10.1080/01621459.1999.10474144
25. Bertrand KA, Bethea TN, Gerlovin H, Coogan PF, Barber L, Rosenberg L, et al. Aspirin Use and Risk of Breast Cancer in African American Women. Breast Cancer Res (2020) 22(1):96. doi: 10.1186/s13058-020-01335-1
26. Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, et al. Effect of Aspirin and Other Nsaids on Postmenopausal Breast Cancer Incidence by Hormone Receptor Status: Results From a Prospective Cohort Study. Breast Cancer Res Treat (2011) 126(1):149–55. doi: 10.1007/s10549-010-1074-x
27. Cao Y, Tan A. Aspirin Might Reduce the Incidence of Breast Cancer: An Updated Meta-Analysis of 38 Observational Studies. Med (Baltimore) (2020) 99(38):e21917. doi: 10.1097/MD.0000000000021917
28. Mc Menamin UC, Cardwell CR, Hughes CM, Murray LJ. Low-Dose Aspirin Use and Survival in Breast Cancer Patients: A Nationwide Cohort Study. Cancer Epidemiol (2017) 47:20–7. doi: 10.1016/j.canep.2016.12.008
29. Murray LJ, Cooper JA, Hughes CM, Powe DG, Cardwell CR. Post-Diagnostic Prescriptions for Low-Dose Aspirin and Breast Cancer-Specific Survival: A Nested Case-Control Study in a Breast Cancer Cohort From the UK Clinical Practice Research Datalink. Breast Cancer Res (2014) 16(2):R34. doi: 10.1186/bcr3638
30. Barron TI, Murphy LM, Brown C, Bennett K, Visvanathan K, Sharp L. De Novo Post-Diagnosis Aspirin Use and Mortality in Women With Stage I-III Breast Cancer. Cancer Epidemiol Biomarkers Prev (2015) 24(6):898–904. doi: 10.1158/1055-9965.EPI-14-1415
31. Strasser-Weippl K, Higgins MJ, Chapman JW, Ingle JN, Sledge GW, Budd GT, et al. Effects of Celecoxib and Low-Dose Aspirin on Outcomes in Adjuvant Aromatase Inhibitor-Treated Patients: CCTG MA. 27. J Natl Cancer Inst (2018) 110(9):1003–8. doi: 10.1093/jnci/djy017
32. Dierssen-Sotos T, Gomez-Acebo I, de Pedro M, Perez-Gomez B, Servitja S, Moreno V, et al. Use of Non-Steroidal Anti-Inflammatory Drugs and Risk of Breast Cancer: The Spanish Multi-Case-Control (MCC) Study. BMC Cancer (2016) 16(1):660. doi: 10.1186/s12885-016-2692-4
33. Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT. Nonsteroidal Anti-Inflammatory Drugs and the Risk of Skin Cancer: A Population-Based Case-Control Study. Cancer (2012) 118(19):4768–76. doi: 10.1002/cncr.27406
34. He P, Yang C, Ye G, Xie H, Zhong W. Risks of Colorectal Neoplasms and Cardiovascular Thromboembolic Events After the Combined Use of Selective COX-2 Inhibitors and Aspirin With 5-Year Follow-Up: A Meta-Analysis. Colorectal Dis (2019) 21(4):417–26. doi: 10.1111/codi.14556
35. Yuan JQ, Yang M, Threapleton DE, Qi XS, Ye DQ, Mao C, et al. Systematic Review With Meta-Analysis: The Gastrointestinal Benefits of COX-2 Selective Inhibitors With Concomitant Use of Low-Dose Aspirin. Aliment Pharmacol Ther (2016) 44(8):785–95. doi: 10.1111/apt.13776
36. Lanas A, Garcia-Rodriguez LA, Arroyo MT, Gomollon F, Feu F, Gonzalez-Perez A, et al. Risk of Upper Gastrointestinal Ulcer Bleeding Associated With Selective Cyclo-Oxygenase-2 Inhibitors, Traditional Non-Aspirin Non-Steroidal Anti-Inflammatory Drugs, Aspirin and Combinations. Gut (2006) 55(12):1731–8. doi: 10.1136/gut.2005.080754
37. Lee TY, Hsu YC, Tseng HC, Lin JT, Wu MS, Wu CY. Association of Daily Aspirin Therapy With Hepatocellular Carcinoma Risk in Patients With Chronic Hepatitis C Virus Infection. Clin Gastroenterol Hepatol (2020) 18(12):2784–2792.e7. doi: 10.1016/j.cgh.2020.04.036
38. Rahme E, Ghosn J, Dasgupta K, Rajan R, Hudson M. Association Between Frequent Use of Nonsteroidal Anti-Inflammatory Drugs and Breast Cancer. BMC Cancer (2005) 5:159. doi: 10.1186/1471-2407-5-159
39. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective Reduction of Gastric Cancer Risk With Regular Use of Nonsteroidal Anti-Inflammatory Drugs in Helicobacter Pylori-Infected Patients. J Clin Oncol (2010) 28(18):2952–7. doi: 10.1200/JCO.2009.26.0695
40. Su SY, Lee WC. Mortality Trends of Liver Diseases From 1981 to 2016 and the Projection to 2035 in Taiwan: An Age-Period-Cohort Analysis. Liver Int (2019) 39(4):770–6. doi: 10.1111/liv.14027
41. Hung TH, Liang CM, Hsu CN, Tai WC, Tsai KL, Ku MK, et al. Association Between Complicated Liver Cirrhosis and the Risk of Hepatocellular Carcinoma in Taiwan. PloS One (2017) 12(7):e0181858. doi: 10.1371/journal.pone.0181858
42. Chien J, Liu J, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, et al. Risk and Predictors of Hepatocellular Carcinoma for Chronic Hepatitis B Patients With Newly Developed Cirrhosis. J Gastroenterol Hepatol (2016) 31(12):1971–7. doi: 10.1111/jgh.13422
43. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, et al. Antihypertensive Drugs and Risk of Cancer: Network Meta-Analyses and Trial Sequential Analyses of 324,168 Participants From Randomised Trials. Lancet Oncol (2011) 12(1):65–82. doi: 10.1016/S1470-2045(10)70260-6
44. Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin Converting Enzyme Inhibitors and Risk of Lung Cancer: Population Based Cohort Study. BMJ (2018) 363:k4209. doi: 10.1136/bmj.k4209
45. Harding JL, Sooriyakumaran M, Anstey KJ, Adams R, Balkau B, Brennan-Olsen S, et al. Hypertension, Antihypertensive Treatment and Cancer Incidence and Mortality: A Pooled Collaborative Analysis of 12 Australian and New Zealand Cohorts. J Hypertens (2016) 34(1):149–55. doi: 10.1097/HJH.0000000000000770
Keywords: NSAID, breast cancer, second cancer, cohort study, risk reduction
Citation: Lu Y-C, Chen P-T, Lin M-C, Lin C-C, Wang S-H and Pan Y-J (2021) Nonsteroidal Anti-Inflammatory Drugs Reduce Second Cancer Risk in Patients With Breast Cancer: A Nationwide Population-Based Propensity Score-Matched Cohort Study in Taiwan. Front. Oncol. 11:756143. doi: 10.3389/fonc.2021.756143
Received: 10 August 2021; Accepted: 04 November 2021;
Published: 24 November 2021.
Edited by:
Antonino Musolino, University of Parma, ItalyReviewed by:
Shereen Elazzazy, Hamad Medical Corporation, QatarMassimo Ambroggi, Guglielmo da Saliceto Hospital, Italy
Copyright © 2021 Lu, Chen, Lin, Lin, Wang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Heng Wang, d2FuZ3NoQG1haWwuY211LmVkdS50dw==; Yi-Jiun Pan, cGFueWlqaXVuQG1haWwuY211LmVkdS50dw==
 Yin-Che Lu
Yin-Che Lu Pin-Tzu Chen1
Pin-Tzu Chen1 Mei-Chen Lin
Mei-Chen Lin Shi-Heng Wang
Shi-Heng Wang