- Responsible and Inclusive Technologies Research, Exploratory Sciences Division, IBM Thomas J. Watson Research Center, Yorktown Heights, NY, United States
Pain research traverses many disciplines and methodologies. Yet, despite our understanding and field-wide acceptance of the multifactorial essence of pain as a sensory perception, emotional experience, and biopsychosocial condition, pain scientists and practitioners often remain siloed within their domain expertise and associated techniques. The context in which the field finds itself today—with increasing reliance on digital technologies, an on-going pandemic, and continued disparities in pain care—requires new collaborations and different approaches to measuring pain. Here, we review the state-of-the-art in human pain research, summarizing emerging practices and cutting-edge techniques across multiple methods and technologies. For each, we outline foreseeable technosocial considerations, reflecting on implications for standards of care, pain management, research, and societal impact. Through overviewing alternative data sources and varied ways of measuring pain and by reflecting on the concerns, limitations, and challenges facing the field, we hope to create critical dialogues, inspire more collaborations, and foster new ideas for future pain research methods.
Introduction
In 2020, the International Association for the Study of Pain (IASP) updated its over 40-year-old definition of pain, officially conceptualizing it as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.”1 Along with this definition, six key elements were added to further contextualize the experience of pain, including: the simultaneous influence of biology, psychology, and society on pain perception, pain's differentiation from nociception, pain's learned elements, pain's ability to be both adaptive and maladaptive, that subjective pain reports should always be respected, and that there are multiple ways to show or be in pain. While terminology updates may not seem monumental, these changes were seen by many as crucial in moving pain research forward, representing a field-wide consensus of just how multifactorial, bidirectional, and personal the experience of pain is, something that had been debated, contested, or ignored for years (1–4).
Yet despite this acceptance and a growing commitment to studying and treating pain more holistically, the pain field is still relatively fractured, with scientists and clinicians largely remaining within and relying on their domain expertise and utilizing practices and techniques which don't always reflect the multidimensional or nuanced nature of pain. This dearth of collaboration and the continuation of disciplinary siloes is detrimental and ultimately unsustainable in the context in which the field finds itself today. Perhaps foremost in mind is the ongoing COVID-19 pandemic, which not only subjected millions of pain patients to postponed procedures, reduced access to medications, and exacerbated pain levels (5–9) but also further laid bare the rampant systemic inequity within many healthcare systems, effects which were compounded by discrimination within pain management more specifically2 (10, 11). Simultaneously, the speed of translating methods and data into digital formats is increasing not only the amount of data gathered but also the diversity of data streams collected—there is a need to be able to makes sense of all this information quickly to share insights and scale associated innovations in pain management. In turn, machine learning and other data science methods are increasingly being relied upon both in basic research and clinical settings. Together, the rising adoption of digital infrastructures, an increasing reliance on machine learning and big data, and an existing backdrop of the global pandemic and ongoing pain care disparities all necessitate a fundamental change in the ways we conceptualize, measure, monitor, analyze, and manage pain, as well as how and where we collaborate across research and care efforts.
It is with this in mind that we write this review. As researchers in the field of pain neuroscience and responsible technology, we ask—what does it mean to conduct pain research conscientiously and to create accountable pain science and pain-related technology that produces impacts which are minimally harmful to society's most vulnerable and mutually beneficial to the multiple constituents involved. We hold the responsibility to not only educate our fellow researchers and clinical colleagues on the technical advancements in existing and emerging pain methods, but also on their limitations and potential negative consequences. In this way, the paper is as much a call-to-action as it is an overview of pain research. We first summarize the state-of-the-art across emerging or strengthening areas of pain methods. Within each area, we describe the different data signals and sources that attempt to measure various aspects of the pain experience (biological, psychological, social) at different times, in different places, and for different applications. Next, we touch on protocol, technical, and human-centered considerations for each area, highlighting the critical assumptions or foreseeable ethical, legal, or social issues (12) surrounding the methodological advancements or associated technologies. We conclude by reflecting on the possible implications of these techniques given their overlapping strengths and weaknesses, and identify places where multi-, cross-, inter-, or anti-disciplinary approaches and collaborations could provide novel solutions, generate new research findings, and/or create new standards of care or practice.
State-of-the-Art in Pain Methods
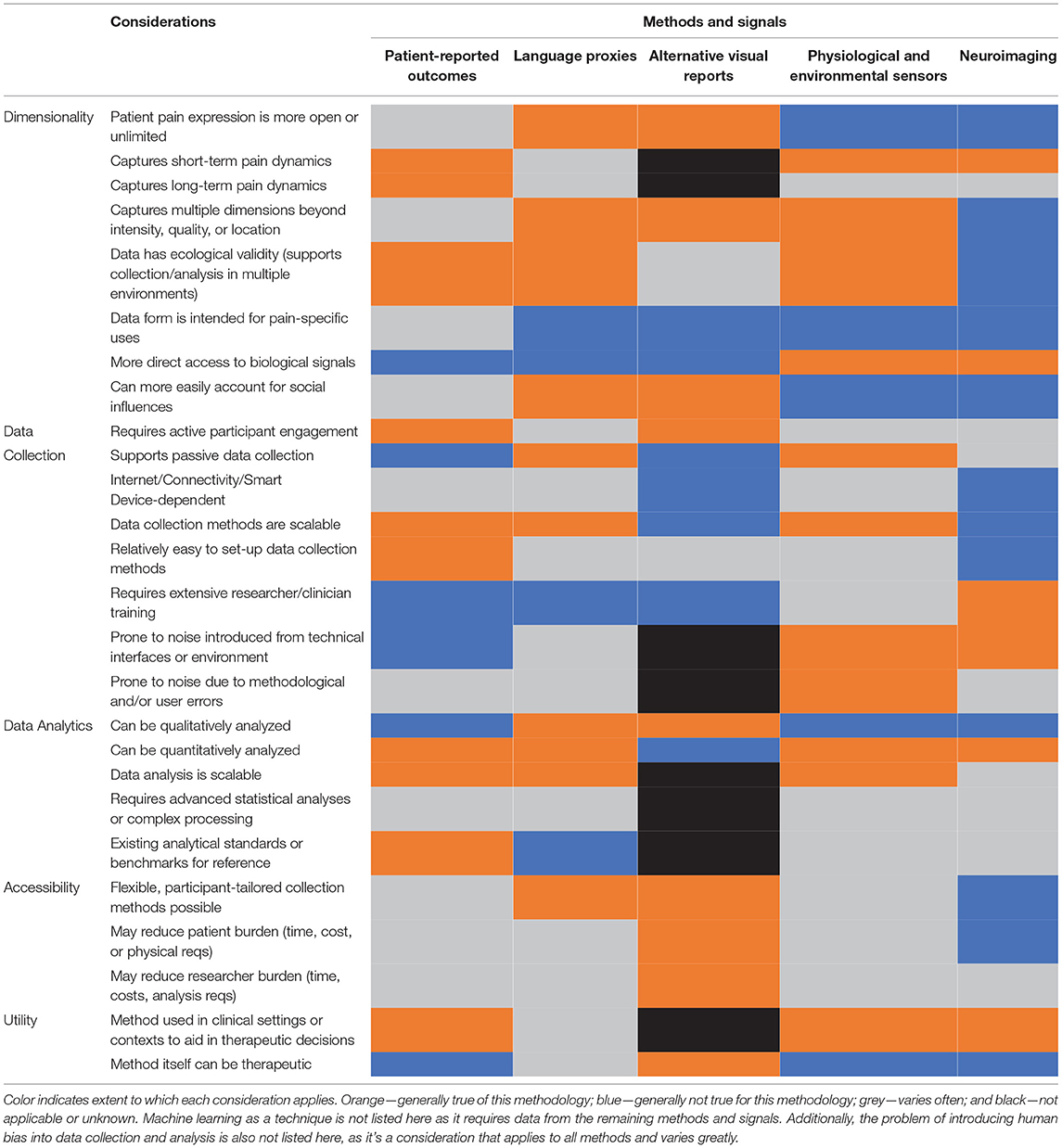
The following represent state-of-the-art (SOTA) methods that are either emerging in pain research or are producing new findings and foundational discoveries in the pain field. Importantly, we aim to include methods that together encompass the multidimensional nature of pain, from the biological and physiological aspects (neuroimaging and physiological sensors) to the psychological and cognitive aspects (self-report and language) all the way to the social and ecological aspects (visual reports and environmental sensors), finishing with machine learning as an overarching method that can analyze the different data types generated. This review is by no means exhaustive and does not include critical findings or research in the areas of sociology, anthropology, or epidemiology, which we consider greatly important to the pain field but outside the scope of this paper. Additionally, the methods reviewed were chosen based on the authors' previous research experiences, publications, and subject matter expertise. For a high-level summary and comparison of some of the key considerations across all methods, see Table 1.
Reporting Pain—Self-Reports and Patient-Reported Outcomes
Patient reported outcome measures (PROs, PROMs, or self-reports) are often considered “the gold standard” for measuring acute and chronic pain. They include familiar condition-agnostic questionnaires, such as but not limited to the numeric pain rating scale (NRS or NPRS) (13), the visual analogue scale (VAS) (14), the McGill Pain Questionnaire (MPQ) (15), various versions of facial pain scales (16), the Gracely Pain Scale (17), and the Brief Pain Inventory (BPI) (18), as well as subsets of questions from much larger batteries like the National Institutes of Health Patient Reported Outcome Measurement Information System [PROMIS] (19). There are also PROs that focus specifically on designated pain conditions, such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for arthritis (20) or the Oswestry Disability Index (ODI) for low back pain (21). There have already been numerous reviews and comparisons of these scales (22–26). Rather than repeat these efforts, we focus instead on methodological considerations for their implementation, such as where and how they are administered.
Until recently, many PROs have been implemented cross-sectionally, at clinic or research visits, occurring every few weeks to months or more infrequently on the order of years. Their administration has also largely been limited to written or verbal responses (e.g., completing questions on a physical form or answering aloud in front of a staff member); this might happen once during a visit or several times in succession as part of provoked pain paradigms, psychophysics experiments, or quantitative sensory testing (27–29). This infrequent in-person approach might make sense for specific pain conditions or highly-controlled research questions but does not easily measure pain fluctuations (30) or pain perturbation by environmental, psychological, or socioeconomic factors (31–34). Likewise, many existing PROs are unidimensional or only focus on preconceived features of importance in the pain experience (e.g., pain intensity, pain location, or pain qualia), even though quality of life improvements may be higher priorities for many patients (35). In the instances where sleep, mobility, sociability, mental health, social support, or sexual health are measured, they are often collected as separate reports, creating much longer batteries, a reaction which might increase response burden or administrative burden (36–39). Finally, the context of administration can greatly influence self-report reliability—the environmental setting (in a clinic or research center), social setting (in front of a clinician or researcher), and temporal requirements (retrospective summaries or momentary one-offs) can all introduce a variety of cognitive and recall biases into pain reports (40). It is well-known that there are discrepancies between momentary pain assessments and pain memories3 (41, 42) due to heuristic strategies like the peak-end rule and cognitive phenomena like the recency effect. Additionally, patients' incentives, cultural display rules, social desirability, and confirmation bias can all introduce intentional or unintentional over- or under-reporting of pain dimensions (43–46).
To improve pain reports, recent methods have focused on increasing the frequency, diversity, and contextual validity of measurements via the use of ecological momentary assessments [EMAs (47)], where PROs are collected semi-continuously from a patient's natural environment as part of their day-to-day routines (48, 49). This kind of sampling can be done relatively easily due to the nearly ubiquitous use of internet, smart phones, tablets, and computers, as well as the implementation of apps and electronic data capture systems that seamlessly integrate cloud storage, content repositories, security measures, and data collection methods. Although EMAs have been used for a number of years within the healthcare space (50, 51), we have seen a recent uptake in these methods due to their relevance, utility, and need during the pandemic, especially in the realm of pain (7, 52, 53). Researchers have now been able to better study the temporal dynamics of pain (54), as well as find more nuanced relationships between pain intensity, location, descriptions, and flares with sleep disruption, psychological symptoms, personality characteristics, and concomitant activities or medication use (1–4)4. From a scientific and clinical perspective, care providers and researchers can more efficiently track their patients longitudinally without the need for increased visits (reducing care burden and study costs), and likewise, patients can participate in studies, clinical trials, and pain treatments more easily, reducing financial and time commitments by not traveling to on-site locations at designated or restrictive times.
While some of the in-clinic recall biases mentioned above are minimized or avoided using EMAs, biases related to reporting due to incentives (e.g., qualifying for treatment or passing eligibility criteria) or social desirability can still inflate or deflate self-reports, and cognitive or psychological elements such as current mood can still influence how a person rates their pain in a moment (55). Likewise, prior experience with self-report measures, patient preferences, and clinical history can all influence how people complete momentary assessments at home (40). Moreover, new biases or cognitive phenomena can also be introduced using this method depending upon implementation–studies have shown that the simple act of rating pain every day or receiving feedback about self-reports can change the qualities of pain time-courses and treatment responses due to increased awareness, reporting fatigue, habituation, or increased reporting accuracy [for one example, see (56)].
Protocol and Technical Considerations
Issues of compensation and accessibility are important to consider with any kind of PRO. At in-person visits, patients are often compensated for their time filling out these questionnaires and completing study requirements, as well as ideally reimbursed for travel. In contrast, there might be assumptions that EMAs require less work or less time due to decreased travel or increased convenience. However, at-home methods might be more intensive, since patients might be expected to rate more frequently or consistently within a given time frame, and they could also be perceived as more invasive from a privacy perspective. At-home PROs also have considerably more accessibility requirements, and there might be considerable upfront costs or training to implement an EMA system in a way that serves as many patients as possible (47). For example, patients need to have access to a computer or smart device, as well as internet or a data plan. If any or all of these are not available, teams must decide whether it is monetarily feasible to provide participants with a smart phone and data plan at no cost to them for the study duration. Additional flexibility in terms of user interface design or content collection may need to be implemented, particularly for patients who may require bigger text, read-aloud functions, or different UI interaction methods (e.g., voice control or buttons instead of sliders to accommodate hand mobility differences) (57). Adjusting frequency or time of PRO deployment or reporting to better fit with participants needs might be necessary to accommodate work and family time (58).
Human-Centered Considerations
It is well-documented that self-reports of pain are influenced by and change significantly based on participants' gender, age, ethnicity, and culture (44, 59, 60). For example, depending on age, previous experiences with pain, and communication ability, utilizing scales with numbers and words alone may not be the best way of measuring patients' pain. In lieu of this, pain drawings—which show the front and back of the body in its entirety—may be a better option for PROs as they allow people to visually indicate where on their body they are experiencing pain. These include simple pain maps where a person circles areas of the body or shades certain dermatomes or myotomes (61, 62), as well as more layered versions that incorporate colors, numbers, or words to indicate intensity and qualia (1–5). Importantly, body maps can be used longitudinally (63) to show how pain somatotopy fluctuates in time, with efforts to digitalize these into EMAs of their own (64). Similarly, the use of pictograms to illustrate pain experiences and sensations has also emerged—these consist of static images or cartoons that often show a person's facial expressions along with body language and additional context about pain location or source, and they have been helpful in studying pain in children as well as those with literacy, speech, or cognitive differences (65–67).
Critical Assumptions
Across nearly all numeric PROs measuring intensity, there is a discrepancy between the underlying neurophysiological pain response and the outward reporting of pain. While there is ample evidence that human perceptions of pain and pain relief are not linear—e.g., in cases like wind-up, hyperalgesia, offset analgesia, temporal summation, or sensitization (68–72)—the large majority of PROs essentially force people to translate this complex non-linear perception onto a linear scale. From a technical perspective, this limitation might be partly addressed via post-hoc statistics or a-priori models using non-linear functions. However, this may also represent a foundational disconnect within pain research and methods more generally. At the very least, it likely points to a need for a re-designed way to assess even the most fundamental dimensions of pain, like intensity5.
PROs have been and continue to be a fundamental research tool in pain methods, as they allow for a relatively easy way to communicate various aspects of the pain experience across different time scales and potentially across different contexts. Due to their prevalence and long history of use in the field, many also have a rich literature base and existing set of standard practices and benchmarks [e.g., minimal detectable change (MDC) scores, international population norms, etc.] that researchers can draw upon for comparison and analysis. However, as others have cautioned (73, 74), they should not be placed on a methodological pedestal as somehow being better or less problematic than other methods overviewed here. Critically, the reliance on numbers, pre-selected word anchors, and forced descriptions of qualia or intensity can change how people report their pain (75) and/or profoundly limit patients' ability to share their unique pain stories and define what pain is like for them (76). To measure pain more authentically, we need to understand pain using the person's own language, relying on patients' voices and narratives to define and extract meaningful information about their experiences.
Listening to and Reading Pain—Language as a Proxy of Personal Pain Experience
Elaine Scarry said, “Physical pain has no voice, but when it at last finds a voice, it begins to tell a story” (77). In the last decade, the utilization of written or spoken language as a signal of underlying physical and mental health, indicator of neurological or cognitive conditions, or metric to quantify or categorize neuropsychological states has gained momentum. Previous studies have shown language's utility in identifying ingested drugs and dosages (78), quantifying depression severity (79), dissociating and predicting mental illnesses (80–82), and tracking changes in neurological disease symptoms (83–85). Given many chronic pain conditions are also neurodegenerative (86) and linked to changes in cognitive functioning or emotional processing, researchers are investigating whether quantitative language features can also measure pain qualities, although qualitative research investigating pain and language has been around for some time (77, 87–90). Unlike questionnaires, collecting patient language about their own experience is far less limiting, and whereas numeric PROs could be influenced by various cognitive processes, voice acoustic properties and lower-level linguistic and syntactic structures are harder to consciously influence in the same way (91, 92).
Prior work investigating the relationship between speech characteristics and pain have shown some promising links, primarily in the domain of acoustics and pain intensity. Breathiness (93), Mel frequency cepstrum coefficients (MFCCs) (93, 94), loudness (94), fundamental frequency (95, 96), formants (95), jitter (95), and speech rate (97) have all been associated with induced acute, perturbed, simulated, and/or chronic pain intensity in laboratory settings (98). Additionally, a number of content-related features from spoken and written language have also shown potential in assessing pain intensity, emotional pain severity, pain-related emotions, and changes in perceived qualia; these features have included personal pronoun usage, sentiment, verbosity, and psycholinguistic structure of words (99–106). Other aspects of the pain experience—such as quality of life metrics, pain interference, and even diagnostic category—have been evaluated using higher-level linguistic features, such as metaphor usage (107–109). Quantitative language metrics like these might be able to serve as an alternative to numeric ratings or as “proxies” of pain intensity or quality. This might be useful for both patients and pain practitioners—it could increase the number and frequency of pain data collection points (since many people talk throughout the day as part of their normal interactions) while simultaneously reducing collection burden (by decreasing the number of pain ratings needed).
Researchers have recently shown language's utility in understanding and measuring a variety of dimensions of the pain experience outside of qualia or intensity. Quantitative language features from semi-structured interviews have been used to quantify placebo response in chronic pain patients (110), and large-scale text-mining of electronic medical records has been utilized to detect pain disparities in underserved communities (111). Similarly, social media posts have been analyzed to longitudinally track patients and identify new pain phenotypes (112), geospatially monitor and characterize opioid use (113), conceptualize how pain is socially communicated (114, 115), track local and federal pain treatment policies (116), and discover population-level increases in pain conditions and symptoms (117).
Protocol and Technical Considerations
To capture acoustic properties of language, speech is required. Speech can be elicited actively—via responses to short narrative or monolog free-speech prompts6, dialogue and semi-structured open-ended interviews (118–121), descriptions of pictures (122), reading passages (123), or verbal motor tasks like phonoarticulatory diadochokinesis (124)—or passively, by randomly sampling voice snippets from the environment (125, 126). Speech data can be recorded using professional sound equipment or with simple hand-held recorders, as well as via built-in microphones in phones and computers. Alternatively, one can also collect non-spoken language or transcribe collected speech into text—in these instances, participants might be asked to write or type their responses. They might also consent to let investigators use prior or on-going free text in the form of social media posts or direct messages, data which can also be obtained via publicly-available datasets, licenses, or crowd-sourcing (127).
The methods chosen and features calculated need to be designed with the specific application or pain condition in mind, as many factors will influence their utility and feasibility. For example, open-ended monolog prompts might be appropriate for adults with pain but may not be easily understood or fully answered by children; similarly, for some populations, speech may be easier or more natural to elicit than typing a response, which might be better for participants who text often or for participants who might find texting more convenient than talking during the workday. Likewise, the use of off-the-shelf or personal recording devices might make sense for larger clinical trials or at-home assessments to scale data collection and analysis, but for smaller studies interested in vocal characteristics of pain, investing in recording equipment and sound-proofing might result in higher quality acoustic data. Additional study design choices could also dictate how burdensome methods like these could be for patients—outside of elicitation methods and frequency, elements such as randomization without repetition and varied prompt or picture topics might also need to be employed to sustain engagement and reduce boredom [for some examples, see (128)].
There are also many procedural and analytic considerations. Hand transcription of voice responses and qualitative coding of language using methods like grounded theory (129) or interpretative phenomenology analysis (130) might work well for smaller studies, but these approaches are not always feasible for larger studies—they may not scale with data collection or analysis needs, nor are they easily reproducible across studies or applicable for acoustic designs. If participants are collecting language using at-home methods, there will likely be additional sources of noise (e.g., additional voices, background TV, movement) (131), which affects transcription accuracy and acoustic feature calculation. These methods will require participant training to minimize these effects, as well as various pre-processing software to filter out these interferences (132–134). In the absence of paid-for transcription services, researchers can also utilize Automatic-Speech-Recognition (ASR) to transcribe spoken language into text for easier data processing (135). However, ASR's transcription quality is only as good as its underlying acoustic models and associated training data (136). Reducing gender and racial biases in ASR and large language models is an area of on-going investigation7 (137, 138); instead of relying strictly on population-based language models for transcription, practitioners can pre-train models on small samples of transcribed individual speech from reading passages [e.g., the Rainbow Passage (139)] to improve accuracy and reduce word error rate (140). In addition to issues of scaling collection and transcription, researchers will also need to think about how to scale analyses of such a rich data source. For this, natural language processing (NLP) (141, 142) is commonly used, deploying sub-methods like named entity recognition or topic modeling to classify differences in pain (143–145).
Human-Centered Considerations
The language we use to represent pain changes as a function of our neuropsychological development, our experiences with pain, and relatedly with age (146–148)—findings or methods that hold true to one age group may not be applicable to another. Similarly, gender identity, associated norms or social conditioning, and racial or ethnic community practices and values can also influence if and how people talk about pain (149–153). Likewise, culture, religious beliefs, and native language also influence how pain is verbalized, described, and conceptualized (154–162). All these elements can affect patient engagement with the method itself, as well as downstream analyses, interpretations of the data, and validation of any results.
Critical Assumptions
Utilizing patients' language as the “ground truth” is in line with the basic premise that pain is inherently subjective and highlights the necessity of respecting patient's reports. However, the utilization of patient language holds normative and even ableist assumptions about the best forms of communication or the legitimacy of different communication sources. There may be some people who prefer not to communicate through talking or may be limited in their ability to use written or spoken language due to cognitive differences or neurodivergence more broadly. There is some research showing existing PROs and language-based measures fall short for people with Autism or Asperger's who may communicate pain differently8 (163). This is particularly troublesome given this community may experience more acute and chronic pain than other members of the neurodivergent community or their neurotypical counterparts. Additionally, many of the language models used today carry assumptions about the acoustic tones and rhythms found in “typical” conversational speech. This could negatively impact pain patients with speech impediments (164) or those whose language patterns do not match that of model creators (typically White, English-speaking men) (165, 166)9, and could exclude or delegitimize the use of lyrics, rhymes, or singing, all of which can also be used to communicate pain (167).
Thus, although the recent use of language proxies for pain have been an important development in the field—allowing for more intuitive, social, and patient-centric measurements of broader experiences in ways that are relatively flexible and often scalable—there is still work to be done to make this method inclusive. Moreover, sometimes language as a signal simply is not enough, as many authors have already eloquently described; for example, Virginia Woolf said, “…let a sufferer try to describe a pain in his head to his doctor and language at once runs dry (168)” and Emily Dickinson said, “Pain has an element of blank—it cannot recollect when it began or if there were a time when it was not” (169). In moments when pain is particularly intense or unrelenting, people in pain might not be able to speak. Additionally, non-verbal utterances like cries, gasps, or groans may be produced instead of words, and methods and techniques which can both capture these vocalizations and dissociate them from other noises and language features would be useful and appropriate (170).
Seeing Pain—Use of Visual and Alternative Pain Reports
As an alternative to quantitative or language-based assessments, there are also self-reports which try to bypass traditional forms of reporting (or use words and numbers sparingly as additional components to the primary report, which is visual in nature). While face scales, pictograms, and body maps mentioned previously might be considered visual reports to a certain degree and are important from an accessibility standpoint, they have pitfalls surrounding representative assumptions as to what base bodies look like (e.g., average size with two legs and two arms) with no way to easily account for physical differences due to weight, genetics, or corporeal disruptions from illness or trauma. Likewise, facial scales have assumptions of what reference faces are comprised of (e.g., two eyes, two ears, a nose, and a mouth) and what base facial expressions look like. They also reiterate beliefs about the existence of universal expressions or emotions (171, 172)—an idea that is still debated (173, 174)—as well hold assumptions that everyone can intuitively understand facial expressions or easily infer meaning from them, which isn't true (175). In this section, we focus on different methods that try to visually depict aspects of the patient's pain experience, driven primarily by their embodied and situated perspective.
Visual arts have long been utilized in community and healthcare spaces as therapeutic resources, places of resistance to the biomedical model, and forms of communication and community building (176, 177). Visual art can take many forms—painting, drawing, handwork (e.g., pottery, sculpture, sewing, or basket weaving), movement, cinematography, photography, and acting. Many if not all these genres have been explored in the context of pain's physical and psychological forms (178–180), and there are an increasing number of research studies and grassroots efforts aimed at utilizing self-created art to depict and share patient's subjective pain experience. For example, there have been methods that ask patients to paint or draw what their pain looks or feels like as a form of expression (181–183); there are also online communities of pain patients who collect and showcase art related to their pain, with the specific goal of improving communication with care providers10. Others have used photography and mixed media to capture various aspects of people's pain (76, 184), as well as clay work and sculptures (185) and patient co-created animations (186, 187)11. There is also evidence of the ability of visual arts to communicate aspects of pain in theater and performance settings (188), particularly in the realm of dance and movement. Research in dance-movement therapy (DMT) is beginning to show that certain kinds of movement-based exercises can communicate pain in new and beneficial ways. For example, the DMT exercise called “mirroring” asks patients to communicate their pain through movements or postures, during or after which they then visualize these movements mirrored back to them by another participant (who might be another pain patient, a therapist, a loved one, or care provider). Semi-structured open-ended movement displays like these have been shown to improve empathy toward people in pain, transfer bodily understanding and awareness to those not experiencing pain, and potentially cause analgesia12. Similarly, there has also been an increase in the use of cinema, film, video recordings, and documentaries to try to communicate multiple dimensions of pain (and do so multi-modally through stories, art, and depictions of people in pain and their environments)13. Early qualitative research has shown that viewing films of pain or pain performances may impact how care providers think about or conceptualize their patient's experience (189). Methods like these have been able to elicit elements of pain not typically asked about in the clinic, on questionnaires, or within interviews such as feeling disconnected from one's body, a loss of identity, feeling powerlessness, or fuller senses of suffering14 (189, 190). They have also been transformed into re-usable and clinically-impactful tools that patients can use to have more personalized conversations with their care providers (75, 186).
The use of visual or alternative pain reports like these is particularly exciting for a couple reasons. First, they challenge the pain field to consider pain displays that are not only defined by an individual in pain but also by larger communities experiencing pain (shifting ideas about self-reports to community-reports). They also encourage the pain field to consider the use and benefits of participatory methods whereby patients co-design or even dictate what pain measures and metrics look like, in some ways challenging the notion of what it means to be a “pain expert” or pain researcher (191). Second, they open the door to novel pain measurement tools that could be back-translated into clinical use via converting traditional or language-based PROs into something visual. For example, clay has been used to sculpt expressions of emotions or re-communicate body language (192)—what would this look like in the context of PROs that use faces or body maps as primary pain measures? Could patients use clay to show how their bodies feel, where the pain is located, and/or what the consequences of pain are? Similarly, using visual or mixed methods approaches in body map contexts might be able to capture pain elements more intuitively and include those outside of intensity or location, such as pain directionality up or down, pain radiation outwards, or pain fluctuation from a source. What would it look like to visually record the completing of static pain drawings in real time as a method to measure the temporal evolution of multiple aspects of spontaneous or on-going pain? More investigation is surely warranted in being able to translate, validate, or mix visual and non-visual reports and methods.
Protocol and Technical Considerations
Because these forms of expressing pain rely on patient-specific communication and can also be time-intensive to create or perform, it makes it difficult to scale or re-apply data collection methods across different populations, cultures, and even across sample sizes of patients (like in the context of larger clinical trials). Likewise, the subjective and intensely personal nature of these methods creates tensions between the need for unique qualitative data and interpretations on the one hand, and current scientific, technical, and medical requirements of reproducible data and quantifiable results on the other. It's challenging to be able to validate these methods across new patient cohorts or new contexts, and even more challenging to form quantifiable measurements of the data (and do so in ways that don't reproduce the pitfalls of other methods or exploit, reduce, or ignore patient experience). While there are a lot of unknowns in this realm, we note that this line of inquiry remains essentially limited to the social sciences and relatively unexplored in the biomedical pain sciences. It would be worthwhile forming and funding interdisciplinary research collaborations between quantitative and qualitative experts, such as but not limited to pain scientists, clinicians, social scientists, artists, and patients.
Human-Centered Considerations
The visual methods summarized here all try to center the human-experience of pain as expressed by individuals or even collective communities. On their own or in certain therapeutic environments, they can be empowering and disrupting. But within the context of the current healthcare system complex and under the biomedical gaze, they can still perpetuate harmful stereotypes and assumptions, namely that pain is always shown or eventually can be “known” or made obvious (189). Notably, this assumption is found across all the methods reviewed here and is not unique to visual reports, although they illustrate it well. The reliance on appearance or the presumed outward expression of pain in some contexts can function as a form of forced assimilation, as it does not always acknowledge the hidden aspect of suffering and ignores that many pain patients are forced or prefer to hide their pain at work or at home, or present as “normal” in order to function in society [a medical form of “passing” or “covering” (193, 194)]. Due to the assumption that significant or severe pain will eventually be “seen,” there are often incongruences between verbal reports, elicited behavior, and felt internal reality which might cause biased pain assessments or withheld pain treatments. As a community, pain patients already must deal with numerous stigmas surrounding hidden disabilities, perceived burden and guilt, labor and productivity norms, and assumed malingering (195–200). Care should be taken to reduce, minimize, or prevent (if possible) additional stigmatization through the method used or data collected.
Critical Assumptions
Alternative visual reports have played an important role in the pain field, as they have not only allowed for fuller expressions of the pain experience but also largely challenged the traditional status quo of “acceptable ways” to communicate pain. However, there are numerous instances where pain cannot be verbalized, displayed easily, or even “known” at all due to injury, disease, age, neurological difference, or cognitive impairment (e.g., in advanced stages of dementia, in minimally conscious individuals or comatose patients, or in those with severe communication difficulties from ALS or locked-in syndrome). There exist clinician-reported outcomes of pain (CROs) (201, 202) as well as third-person reported outcomes (203–205), which ask care team members, providers, and loved ones to rate their perceptions of the patient's pain. However, these are fundamentally limited since there might be no way for a patient to concur with the pain assessment, advocate for themselves, or rebut care decisions. Additional measures which don't rely on self-reports, self-displays, or others' reports of pain may also be useful and clinically relevant.
Sensing Pain—Measuring Pain Through Physiological and Environmental Sensors
The multidimensional nature of pain lends itself to being studied multimodally using various body, environmental, and ambient sensors (206). These are often part of a much larger system of wireless and connected devices that collect, transfer, store, and analyze data over a network (commonly referred to as the Internet of Things, IoT). IoT and sensor-based methods can be used to collect more frequent and fine-grained assessments within a growing digital health ecosystem (207). As with EMAs, these tools may reduce some of the reporting or cognitive biases found in clinical or research settings and may better contextualize pain within a person's natural environment. These methods also have the potential to reduce patient burden by not requiring frequent or active reporting, since many sensors can collect data entirely passively (208). Additionally, the use of sensors might make participating in clinical research more accessible—instead of incurring costs (time, energy, and money) to go to a visit, the clinic or research center is brought to the patient (208).
There are multiple studies showing physiological and environmental sensor utility for pain in “smart home” environments (209–212); importantly, many of these studies pair cross-sectional PROs, crowd-sourced reports, or longitudinal EMAs with sensor data (213). Some devices are used to measure specific aspects of pain sensation, perception, or psychology, while others are used to capture quality of life. The former typically fall under the category of body sensors, which capture physiological or biometric signals from the body. Wrist-worn devices and epidermal patches are often used to calculate heart rate variability (HRV), which has been correlated to acute changes in or sustained alterations to sympathetic and parasympathetic tone due to primary (214, 215), secondary (216), and chronic (217, 218) pain, as well as psychological stress (219).
Quality of life measures tend to utilize environmental or ambient sensors, although wearables are used as well. These might include passive infrared motion and magnetic door sensors to monitor movement and label certain “activities of daily living” (ADLs) based on room entry (e.g., cooking, bathing, entering/exiting the home, sleeping). Existing smart home devices like internet-enabled thermostats, humidity detectors, and light switches can also be integrated into data collection to better label certain ADL events (210, 220). Additionally, built-in device accelerometers can be used and integrated to monitor mobility via actigraphy, step counts, and time spent in different three-dimensional positions (221–223)15. Other wearable and environmental sensors are used to quantify activity levels (224, 225), gait, balance, or movement quality (226–230), and posture (231, 232), all of which might be applicable for a pain patient depending on their condition or at-home treatment or rehabilitation regimens (220, 233).
The use of sleep sensors have also been implemented, since pain itself and common pain medications like opioids are known to disrupt sleep patterns (234–236). Sleep sensors can take many forms (237, 238), including portable polysomnography sensor systems embedded into head caps, smart apps that capture movements or noise, infrared cameras that capture body position, or pressure platforms put under or into mattresses, pillows, or cushions (239–244). Finally, facial expression monitoring of pain intensity is also an active area of inquiry (245–249), part of a growing subarea of IoT and digital patient monitoring (237, 250) that utilizes multi-camera systems and video recordings to measure “emitted” facial expressions in real-time or retrospectively (251–253). These measures have been primarily based on automatic recognition of facial action coding system units (FACS) (254) and associated sentiment (255), although some analyze audio and visual signals together (256–259).
Nearly all these devices, methods, or signals have been used in critical, clinically complicated, or end-of-life situations. HRV has been used as an indicator of nociceptive pain in people who were minimally conscious or in a vegetative state (260), smart watches have been implemented to monitor movement and manage pain symptoms for patients in palliative care (261), EEG reactivity signals have been used to infer pain in comatose patients (262) and people with cognitive impairments (263), and pain facial expression has been measured in patients with dementia (264), intellectual or developmental disabilities (265), and infants (266). Although this review is focused on human pain methods, we also note that some of these sensor-based methods are also utilized to measure pain in animals, including automatic facial recognition (AFR) (267–270).
Protocol and Technical Considerations
When implementing sensor-based measurements, choosing which devices to use or program is not trivial, as there are hundreds on the market. Moreover, many direct-to-consumer devices claim to accomplish similar things or perform similarly to SOTA devices without openly shared data or validation studies (271–273). Additionally, many devices come with their own out-of-the-box or off-the-shelf output metrics that are automatically computed for the user or consumer; care should be taken as to whether or not to take these metrics at face value (since many calculations are “black box” or proprietary and thus cannot be easily examined for noise, privacy, reliability, or accuracy)16 (274, 275). Likewise, researcher access to raw, unprocessed data and researcher ability to pre- and post-process these kinds of large and noisy data streams need to be considered.
There are also many layers of complexity to implement multiple device collection congruently and privately, particularly if these methods are deployed outside of a clinical or research setting. The sensing system itself, the network connection, data communication and transference, data storage location and requirements (e.g., de-centralized or centralized), data management and security, applications, data standard compliance, and even data analytics (e.g., on the cloud, federated learning, or locally on approved devices) (207, 276) will all affect starting implementation and maintenance costs. Similarly, the reliance on technology for all measurements has some inherent risks for data collection—data quality, accuracy, detection rate, and obtainment can all be influenced by environmental, connectivity, and human factors such as: low light conditions, occlusion, power outages, wearing and charging compliance, fitting issues, or devices going out of network or range of upload hubs. Securing additional secondary methods for collecting data (e.g., diary methodology or questionnaires) should be considered, as well as backup device storage and queued data transfer abilities.
Human-Centered Considerations
Adaptability is an important design element to consider when deciding on devices and sensors to use. For example, wrist-worn devices need to have adjustable bands to be able to accommodate varying wrist sizes, and different materials should be considered based on skin sensitivities, skin fragility, or skin allergies; the same is true for patches and wireless EEGs, since adhesive materials can be abrasive or irritating to skin (277) or may not adequately capture signals depending on hair type or head fit (278). Patient living conditions and preferences should also be considered, since placement of sensors and co-use by cohabitants also matter (e.g., bed sensors may not be appropriate for people with partners, children, or pets that co-sleep with them, or for patients who sleep in non-traditional locations or environments) (279–282). Importantly, sensor methods might create new forms of participant burden (283, 284). Setting up devices for proper data collection and the need to charge devices might contribute to confusion or additional work for patients (285, 286)—having systems, methods, or devices which can reduce manual set-up, automate data uploads, or maximize battery life will aid in their long-term feasibility and engagement. As discussed with EMAs, there is also the presumption of access—considerations regarding interface design, digital literacy, and digital inequity must all be taken seriously. Time costs associated with patient or caregiver training, and monetary costs for scaled set-up at home or within a clinical infrastructure also should be accounted for up front. As with other methods, consideration of demographic and comorbid health conditions and abilities is also necessary. Gender, age, body mass index, baseline health, exercise regimens, and other factors can influence many of these physiological signals. For instance, HRV as a signal changes as a function of age, activity level, and sex (287, 288), and sensors or technologies themselves can also work differentially depending on patient variables including skin color (289, 290).
Critical Assumptions
Sensor-based measurements of pain are important resources for studying and assessing patients' experiences. Their contribution to the pain field has largely been their ability to collect multiple kinds of data through often passive or minimally burdensome methods, allowing for a much larger breadth of use and the discovery of correlations with other ecological events, physiological signals, or patient activities. However, these measurements also have the potential to perpetuate harmful suppositions about what pain “is.” For example, many of these measures likely follow the recent trend of calling pain a “fifth vital sign”—while pain perception and report should indeed be considered vital to a person's health and well-being, the comparison to a vital sign immediately conjures up mechanisms akin to strictly biological measures like heart rate and blood pressure (72). In some ways, a hyper or a singular focus on these kinds of metrics can work to erase the comprehensive and multidimensional conceptualization of pain that IASP has put forth. This framing ignores the intensely social aspect of pain communication by solely placing the data of interest as something emitted by the patient, as opposed to also being witnessed by others. Likewise, there is often an unsaid assumptions that sensor-based metrics are more objective or less noisy than self-reported metrics, and some even argue that they work to reduce the kinds of human biases seen when measuring pain (291). However, as mentioned, sensors are subject to numerous points of human and environmental interference that can introduce sources of noise. Moreover, we also know that the decisions of which kinds of sensors to use are not neutral (292) and that the underlying sensor data that accompanying algorithms are trained on are not representative. This is easily seen in automatic facial recognition, where pain inference accuracy remains questionable at best (293–295). Setting aside the technology and methods for a moment, we already know that humans aren't reliably accurate at recognizing the emotional expressions of others17 (172). This effect is seen not only in day-to-day life, but also concerningly within clinical settings, where the facial and bodily expressions of women and people of color are notoriously under-valued, minimized, or ignored altogether (296–303). These existing human errors and pervasive prejudices means that the data sources and the data labels that go into methods like these are in turn quite biased, as are interpretations of the results, which has implications not only for downstream use but also how to even define accuracy in the first place.
There are additional issues related to privacy, surveillance, and consent that are seen using these methods. While most of the data generated are biometric in a generic sense (generated by biology), some data can also be considered biometric in the legal sense (reasonably identifying of an individual), which raises important ethical and legal concerns surrounding protecting privacy, identity, and sensitive health data. This is particularly relevant for passive data collection methods, where someone might easily forget or not notice that a device is on and upload information they may not have intended or wanted to. In addition to privacy concerns, long-term psychological consequences of “the quantified self,” informed and flexible consent (including the right to be forgotten), transparency of data collection and use, and autonomy in decisions made and interpretations formed from these data are all critical issues to think about (304–311).
Imaging Pain—Neuroimaging Measurements of Pain Sensation and Perception
Outside of wearable and environmental sensors, neuroimaging has also been used to study additional physiological and biological components of pain. There are various technologies for imaging and measuring brain structure and function as it relates to pain. Chronic pain research, in particular, has benefitted tremendously from these tools, strengthening evidence that acute and chronic pain mechanisms are distinct (312, 313) and shifting the field's focus of attention from somatosensory processing and “the pain matrix” (314) toward brain circuitry related to reward and decision making (30, 313, 315), emotion (316, 317), and memory (41). The novel findings and confirmatory evidence offered in these studies have fostered innovative research, and substantiated new targets for treating chronic pain with active pharmaceuticals (318) and placebo (319, 320).
Yet, there is still much to understand about the data created by these technologies. The physical properties of magnetic resonance imaging (MRI), for example, are well-known, but the physiological basis the signals detected is still an active area of research: cortical thickness (321, 322), synchronous activity in various brain circuits (323, 324), and white matter integrity (325) all change with experiences of pain, but the causal links remain hidden. Likewise, electroencephalography (EEG), nearly a century old technology, produces data whose source mechanisms are still not fully known: why does the brain produce specific oscillatory frequencies, what structures and processes account for those frequencies, and why do they change with pain? Of course, answering these questions could create fundamental shifts in how researchers understand and study pain. But in the meantime, analyses constantly evolve and adapt to account for changing knowledge and technological advances. Thus, while neuroimaging can produce exciting research findings and useful insights that may one day lead to more effective clinical pain treatments, there are several practical issues to consider.
Protocol and Technical Considerations
Each imaging modality comes with unique advantages and limitations. Temporal resolution is highest in modalities that more directly measure brain electrical activity (like EEG) and are much better at capturing fast neural events, but because these measures are most often taken at the surface of the scalp, detecting signals from pain-relevant deeper brain structures is challenging (326). On the other hand, fMRI is more suited to pain-relevant subcortical sites and has considerably greater spatial resolution, but signal detection is dependent on less-direct measures of neural activity and are orders of magnitude slower. Any interpretation about pain/neuronal relationships using this method must take into account blood oxygenation, hemodynamics, and other sources of physiological activity mixed within the signal (327). Electrocorticography (ECoG) has both high spatial and temporal resolution, as well as direct measures of neural activity, but it requires highly invasive surgery, and thus can only be used under special clinical circumstances (328). Additionally, each modality is susceptible to various sources of noise like breathing, heartbeat, eye and body movements, and electrical activity from the surrounding environment. These confounds may be accounted for using a range of signal processing methods (329–331), although there is no agreed-upon field standard.
The limits of these methods to specify and distinguish physiological and cognitive functions relevant for pain remain questionable, particularly for MRI. The “pain matrix,” for example, a highly studied brain circuit which exhibits coactivation of somatosensory, cingulate, and insular cortices with painful stimuli, has mostly been disregarded as pain-specific and is now more commonly recognized as a network which responds to “salient” stimuli, painful or not (314). Similarly, putatively more pain-specific and granular sets of brain regions (332, 333) have shown little to no improvement in distinguishing pain. A recent study showed that using only small fractions of these maps resulted in almost no loss in researchers' ability to decode perceptual pain states (334). Further, the general notion that high spatial resolution in fMRI is even capable of meaningfully explaining cognitive functions is being tested18. These results further support the need for cross-disciplinary research, suggesting that pain is too complex to be adequately understood using current neuroimaging methods. A harsher take might be that popular neuroimaging methods altogether cannot meaningfully describe fundamental functional properties of the brain (335), and that funding may be more appropriately allocated to methods with greater promise.
Human-Centered Considerations
Pain research necessarily involves some degree of vulnerability, and this is only compounded when imaging is involved. In attempts to establish experimental control, clinical pain studies are often less than inclusive, with exclusion criteria that may disqualify people with comorbidities, clinical or drug use histories, or who are subject to various other social circumstances. Imaging creates even tighter restrictions: experiments can be lengthy and require multiple visits at special facilities, which can make it difficult for those who need to accommodate work or dependent care schedules, and those without reliable transportation or adequate funds to travel. The imaging process itself can be physically uncomfortable for people who cannot stay still or in certain positions for long periods of time, or those who suffer from anxiety in closed spaces. For MRI experiments, magnetic material must be removed and/or absent from the body during scanning, which makes it impossible for people with certain implants, work histories or medical/cosmetic procedures, or those who prefer not to remove certain items from their body, to participate. Likewise, to obtain stronger EEG signals, researchers may choose to exclude people with coarse and curly hair in order to get closer contact between the sensors and the scalp (278). Enforcing all these criteria, of course, may leave researchers with findings that cannot be generalized outside of a select, homogeneous, subpopulation with limited pain experiences.
The clunky setup of many neuroimaging devices may also prohibit measuring everyday experiences of clinical pain. For example, research participants may not feel their pain while seated or laying in certain positions. Pain may be sporadic and unpredictable, which makes longitudinal tracking or early-stage detection of disease status difficult. Data collection sessions may also be too short to capture slow pain dynamics which can occur over hours, days, or longer. Attempts to evoke feelings of pain while collecting data may impose an unnatural pain experience, and reporting pain during data collection may also affect pain perception (336). For these and other reasons, current neuroimaging methods are not scalable and further limits those who may wish to participate in research. Mobile neurotechnologies may ease some of these restrictions19, but signal quality from these devices has yet to be fully validated by the research community.
Neuroimaging experiments can also range widely in financial cost. While EEG experiments are relatively cheap, the going rate for a single hour of MRI scanning may be hundreds to thousands of dollars. This price is compounded by a pervasive fascination with big data. “Scale thinking,” or the desire to process and own an ever-growing volume of work, not only frames how industry tends to think about its problems (337) but also influences the research community, as evidenced in efforts to conduct larger and larger neuroimaging datasets like the Human Brain Project20 and Human Connectome Project21. The discoveries and solutions yielded from these efforts, however, may not be proportional to the investment. In a recent simulation, increasing participants by orders of magnitude resulted in only incremental gains in predictive performance22. While not disqualifying, results like this one raise questions about what society should expect from these technologies, whether certain methods may reap larger benefits from scientific funding, and at what point these decisions should be made (and who should be making them).
Critical Assumptions
As mentioned, neuroimaging has been a vital method in the field of pain research, providing us with a non-invasive way to interrogate and better understand underlying neurological patterns in pain perception or chronification. Yet, while the brain is undoubtedly key to understanding the mechanisms of pain, neuroimaging researchers should resist promoting neuroessentialist views that reduce the pain experience to mere brain or spinal cord signals. Doing so risks diminishing the importance of measurement from other methods, may misdirect more practical efforts to diagnose and treat pain (338), and may proliferate a social dependence on scientific authority (339).
Analyzing Pain—the Role of Machine Learning in Pain Data and Pain Research
Pain researchers in all the fields and methods presumably generate data in volumes or complexities that may become exceedingly difficult to manage. Machine learning (ML) can ease some of those difficulties, helping researchers to process data, as well as explain, predict, and understand various aspects of pain itself. By identifying patterns in data without relying on explicit programming or rules, machine learning allows researchers to further investigate those patterns which might not otherwise surface. Different aspects of data cleaning, clustering, or modeling can be done with machine learning, and it is becoming a standard tool across many scientific disciplines.
Most machine learning tasks come in two different forms, supervised and unsupervised. A “supervised” machine learning model may be designed to classify pain responses, attempting to determine a person's analgesic response likelihood (319) or the presence or absence of pain (340, 341), or it may be designed as a regression problem to indicate how a person's pain rating will change over time. This pattern-finding process is part of “training” a machine learning model, which ultimately shapes the model's statistical properties and its resulting output scores. “Unsupervised” machine learning, on the other hand, identifies patterns in data without the need for labels. An example of this might be to find predominant themes in text data from patient interviews, or for segmenting patient populations into different groups. Both supervised and unsupervised methods can be carried out using algorithms with varying degrees of complexity, each of which may be more appropriate than others for handling specific data types, dimensions, and quantities. While other sections of this review specify protocol or technical considerations for their respective methods, a full accounting of those considerations is beyond the scope of this section due to the breadth of algorithms available, and the nuances which may vary according to the kind of pain data being used. Importantly, comprehensive coverage of those considerations have been reported elsewhere (342–344). Instead, in this section we concentrate on the intersecting human-centered considerations and critical assumptions within machine learning, particularly around one binding requirement for all its forms: the need for a large amount of data. The growing incentive to generate pain data is a key reason for the growth of machine learning in pain research, and vice versa.
Human-Centered Considerations and Critical Assumptions
The healthcare industry generates data in tremendous volumes, and is projected to be one of the fastest-growing data producers in the world by 202523. Various types of patient data attract machine learning researchers to healthcare, but the availability of clinical images in particular is pushing radiology toward the forefront of adopting and integrating machine learning into clinical practice (345–347). One recent study using convolutional neural networks (a popular kind of machine learning used for visual object recognition) demonstrated researchers' ability to identify non-standard patterns of knee pathology, and predicted patients' subjective pain reports 61% better than traditional measures (110). Additionally, using this non-standard approach, researchers were able to account for racial disparities in pain perception at 5x the rate than previously accomplished. This finding is particularly important considering growing concern around imbalanced community representation in medical data: it shows that machine learning can uncover pain-related pathologies to the benefit of marginalized communities, despite their historical exclusion in healthcare.
This is a rather uncommon result in machine learning literature, however, which typically results in an elevated risk for maintaining or amplifying existing societal disparities due to the systemic inequities under which data is created, collected, and used (292, 348). This problem is often framed in terms of bias—applying bias correction through data preprocessing methods24 (349) or adjusting model parameters through procedural fairness25 are then frequently suggested as ways to mitigate these risks. Bias, however, can affect machine learning at many stages of development and implementation, and this can be much harder to address with technical approaches which can't account for existing societal inequities and power imbalances (350, 351). An example of this is shown in a recent study that corrected a healthcare algorithm which disproportionately assigned Black patients lower risk scores, despite having worse health outcomes than White patients with the same score (352). Here, the old algorithm used a financial variable as a proxy for health outcome, which differentially corresponded to communities due to differences in needs, access, and trust in healthcare institutions (353, 354). A corrected machine learning model was created through more rigorous evaluation and the use of a dependent variable which more faithfully represented patients' actual health. The result was a reduced disparity of scores across racialized groups.
Of course, designing a machine learning model like this requires more knowledge than is available in a database. It requires a model evaluation process that extends beyond calculating conventional performance metrics, and an ability to critically assess the presumed impartiality of data. This is particularly pertinent in pain research, as pain is neither an objective measure, nor is it measured or treated equally across different patient and societal communities (300). Accordingly, attempts to objectively encode it for the purpose of scaling research or medical solutions through machine learning continues to pose a risk of entrenching societal harms (337)—more data does not guarantee more meaningful discovery or better predictions. Contextual knowledge of data is also needed, as is transparency about the analytics—without either, scientists can end up making the pain experience worse for patients navigating an already unfair system26.
This point is perhaps most germane to the open-source machine learning and data science communities who are known for a culture of sharing, while at the same time publishing highly controversial work in areas where data is plentiful, but subject matter expertise or critique is lacking. The quick surge of machine learning enthusiasts offering their tech expertise in the beginning of the COVID-19 pandemic is a prime example: in a study evaluating 232 COVID-19 predictive models, it was found that none of them were clinically useful, and almost all of them were “poorly reported,” had a “high risk of bias,” and reported performance that was “probably optimistic” (355). This result may be due to the conventional approach of judging machine learning model quality around benchmark metrics that often don't translate well in real-world practice (356, 357). Machine learning models built on the online availability of face data, for example, have spurred studies making claims about the ability to computationally model one's trustworthiness (358), sexual orientation (359), and criminality27, all of which are based on the debunked pseudoscience of physiognomy (360). Facial emotion recognition, as well, stands against long-held observations that emotion and affect are too complex to be captured by facial expression alone (172). Yet, facial expression data remains a valuable commodity for businesses offering affect recognition solutions28, as well as a popular data source for machine learning studies, including those attempting to measure pain (293, 361, 362).
However, open-source data sharing, and the communities that partake, can and do create positive opportunities for the pain research field (363). As of writing this paper, Kaggle, a popular data science challenge forum, had 964 notebooks under the search for “pain”29; GitHub hosts a list of open-source pain databases30; and OpenPain31 hosts the largest set of brain images from academic pain studies, available to anyone. The culture of sharing in machine learning presents relatively low barriers to those interested in studying pain (given sufficient time and internet access), opening the field to fresh enthusiasm and unique perspectives. This benefit can be reciprocal as well—pain research might also inspire new machine learning algorithms for single-shot and transfer learning, for example32.
The machine learning field is quickly changing and becoming a central tool for many scientific disciplines—in fact, multiple studies cited in other sections of this review have used it in some form. In some ways, as a tool and as an academic discipline, its use and study can foster the collaborative research we advocate for throughout this paper. But pain researchers must not take for granted that machine learning is a sufficient or superior way to explain pain mechanisms or predict its occurrence, particularly in the absence of contextual knowledge of the complex nature of pain.
Discussion
The methods overviewed here cover a wide range of possible technologies and processes that exist today in the pain field. As they move from research settings into applied settings, they have the potential to help better personalize pain treatment, increase access to pain care, improve clinical trial accuracy, and influence or transform many aspects of the digital health space. However, in additional to the promising advantages and existing strengths of these methods, each also has a considerable number of concerns, limitations, or critical issues which need to be acknowledged and addressed.
All technologies and methodologies are simultaneously situated within and productive of multi-layered contexts of creation and use. The context of creation represents the ideas, people, processes, and data that are brought into or created within the design, implementation, and initial analyses of a given project. In the pain field, this is largely within the domain of academic, medical, or industry research, and it includes the physical hardware (e.g., neuroimaging scanners, sensors, paper forms, smart devices), the software and digital infrastructure (e.g., algorithms, internet, data capture systems, apps), raw or preprocessed data forms (e.g., acoustic files, images, transcriptions, reported outcomes, derived features), data sources (e.g., pain patients themselves, care givers, crowd- or-open sourced datasets), and data practitioners (e.g., the researchers designing the study or collecting and analyzing the data). In contrast, the context of use is largely situated within medical practice and the healthcare and wellness industry complex (e.g., hospitals, pharmaceutical and medical device companies, biomedical startups, insurance companies), and it includes clinical practitioners (e.g., pain physicians or other care providers), end-users and consumers (e.g., pain patients, pain communities, care givers, clinicians, device representatives), and the application or purpose itself (e.g., monitoring, treating, communicating). Importantly, both creation and use intersect with ongoing and historical social contexts as well.
When reviewing the contexts of creation and use within pain research, four distinct but inter-related problems consistently emerge: (1) a lack of representative datasets, (2) a tendency toward scientific reductionism and essentialism, (3) harmful assumptions around scientific objectivity and associated expertise or legitimacy, and (4) the potential for unchecked application or use of findings. As we have seen, large language models and sensor datasets are known to be non-representative; the same is also true for many neuroimaging datasets (364–367). This has implications for downstream contexts of use, as it could introduce bias into models or findings, potentially making future treatment decisions unfair, exacerbating existing pain care disparities, or even resulting in harm or death. Another observation is that all methods are inherently limited in the extent to which they can adequately capture pain experience—they all run the risk of oversimplifying the pain experience, whether elevating one dimension over another or ignoring an aspect altogether. For example, in both sensor- and neuroimaging-based methods, we see researchers referring to measures as “biomarkers” of pain, which tends to reduce pain into a unimodal phenomenon, one based in or best known through underlying biology instead of psychosocial factors. Likewise numeric, language-based, and visual PROs might better capture more of the cognitive elements of pain or even some of the social dynamics but may fail to acknowledge important physiological mechanisms.
We also see a tendency for pain researchers to view pain as “an object” and patients as sources of data as opposed to experts. This reproduces unequitable power dynamics and problematic assumptions about what can be measured, what should be measured, and whose measurements matter. For example, brain features, physiological data, or facial recognition data are commonly referred to as “objective measures of pain” (368–372)33. This terminology positions the collected measures of the body as somehow better because they are presumed to be less subjective or more “neutral” since patient's self-report is removed. It is worth spending more time on this presumption of objectivity due to its pervasiveness across pain methods and scientific practice. As scientists, we are taught that production of knowledge is only valid if our findings are “intelligible” or known, and that almost any unknown can—through rigorous methods, quantitative metrics, a bit of intellect, and hard work—eventually be made intelligible (373). We are also taught that through the scientific method we are somehow magically able to remove ourselves from the context of our science, echoing the argument of neutrality in that “good science” is not influenced by subjective phenomena like values, opinions, experiences, or emotions. But these ideas are simply that—just ideas. They represent notions and assumptions that aren't guaranteed or necessary truths (292, 372, 374, 375). This line of reasoning and particular use of language matters for pain research not only because the notion of objectivity is completely antithetical to the IASP definition of pain, but also because it can create a false hierarchy of data value and expertise. The notion of objective pain metrics may work against patient self-reports by appearing less noisy, less biased, or even more trustworthy. In turn, this can result in bias against patients, missed opportunities for treatment, or potentially dangerous overtreatment if a purported “objective measure” contradicts a patient's “subjective statement.” Perhaps more fundamentally, the intersectionality of pain as a biological, psychological, social, and cultural reality that can be simultaneously hidden and seen or embodied and disembodied, calls into question whether it can even be “measured” in the first place (360, 374, 376).
This is not, of course, to say that we cannot or should not study pain but rather that as a field, we need to be critical of the assumptions of our work, the questions we ask, and the goals of our research applications. We also need to re-center pain patients in our processes and see their lived experiences as valid forms of expertise. Given all this, researchers should keep in mind that amassing multimodal data alone or through collaboration (as we suggest below) is not a solution by itself. In the end, a primary goal in pain research has been to try to impose order on an experience which has remained difficult to define, in the hopes of creating more scalable pain diagnoses and treatments. While diversifying data sources may help represent pain more realistically or wholistically, attempts to scale subjectivity and ambiguity or ignore them (i.e., pain prediction models) runs a risk of disadvantaging data outliers (376) and delegitimizing the pain of those who do not fit the model. Researchers, in seeking to use or build large data sets, should also be wary of “scale thinking,” as data amount or data diversity is not necessarily proportional to model performance (377), and can be unintentionally extractive (337).
Finally, pain research is often done without much thought to widespread application. However, many of the methods utilized, datasets and models created, or associated findings might be relevant to actors outside direct patient care, and this raises serious ethical questions about the use of pain data, methods, and tools. For instance, pain researchers might be comfortable if the data they collected or the predictive models they built were used to help physicians better triage pain care or reduce the likelihood of patients being exposed to detrimental medicinal side effects. However, would they still feel comfortable if the same dataset was shared with insurance companies to infer pre-existing chronic pain conditions and potentially prevent some pain patients from receiving care? Would they let their algorithms be used in legal cases to determine legitimacy of workman's compensation claims? Would they want their amassed EMA, sensor, and language data being sold to third party companies whose intentions are unknown? Would they advocate for application in the absence of robust and repeated validation? Although these concerns are limited within the academic space due to Institutional Review Board data sharing requirements, in industry research spaces where client needs and business potential might sometimes take precedence over basic research considerations, concerns around dual- or multi-use technology or repurposing of data and algorithms are particularly relevant.
So where does this leave us, as researchers, as clinicians, and as an entire field? On the one hand, there are multiple take-aways and concrete actions that individual practitioners can do to improve their research today. For example, researchers can engage in reflective and reflexive praxis to combat the positivity bias that is rampant in the pain field, due to the idea that any research that is intended to minimize pain or suffering must automatically be beneficial. Additionally, labs can engage in data practices which combat individual and systemic biases in healthcare and medical pain research, including but not limited to: using simulated or randomized data sets to blind researchers during analyses (378, 379) conducting fairness audits or applying de-biasing techniques, and aiming to collect data from a diverse and importantly representative set of patients to account for differences due to biology as well as socioeconomic indicators of health. The latter means fundamentally shifting some of the processes and ideas that go into creating eligibility requirements or advertising for clinical studies, as well as focusing on and dedicating resources toward building and fostering trust from and within patient communities who have been previously harmed or invalidated by medical research (380). Funding agencies may also consider allocating additional resources in grant awards so that recipients are able to adapt to patient needs in the contexts of accessibility, digital literacy, and digital equity. Researchers can also be more intentional and careful about the language used in their grants and publications. Practically, this might be as simple as being upfront and transparent about limitations like under-powered samples or degrees of freedom (381). Critically, this would look like reframing study designs, labels, and interpretations to confront systemic biases and stereotypes in pain research [for a concrete example of anti-racist pain methods, see (382)]. Regarding methodologies and applications of the findings, researchers and clinicians can take the time to do thorough informed consent discussions so that patients and participants understand the kinds of data being collected, how they are collected, and their intended use. Practitioners can also combat unintended dual-, multi-, or repeated-use or media contortion of results or outcomes via pre-registration (383), as well as by setting and communicating clear limits for use and advocating for validation, replication, and testing to combat hype or overselling of findings (381).
On the other hand, this review signifies the urgency to move beyond individuals, labs, or specific disciplinary or technical fixes, and advocates for multimodal and multidisciplinary methods. Because each method discussed contains an aspect another does not, it paves the way for interesting and innovative collaborations that combine different strengths and mitigate different weaknesses Moreover, this review shows the importance and, we'd argue, necessity for including pain patients and social scientists in pain research processes (from design to interpretation and application). There already exist many examples of multidisciplinary collaborations, participatory methods, and/or multimodal outcomes that pain practitioners can look toward for inspiration and re-use, including painimations (185, 186), photovoice (384), pain cards (75, 183, 385), cross-culture approaches (386, 387), and more (388). Of course, this is not to say that collaboration will be easy or straight forward. It can be challenging to know when and how to best integrate different collection methods, measurement requirements, or data types. As with each individual method, the specific research question and context (e.g., the pain condition at hand, clinical standards or procedural requirements, resource allocation, existing expertise, etc.) will all shape decisions around if and how to combine forces. This is an area of active exploration and investigation, and to date, there do not exist any guidelines or best practices for collaboration within, or integration of, pain research methods.
Conclusions
In this paper, we aimed to not only summarize the benefits and limitations of a breadth of existing and emerging pain methods, but also show their relationships to larger sociotechnical considerations and provide points of potential intersection in techniques and measures. It is our hope that this review can contribute to the larger pain field by functioning both as a mirror and a north star via the identification of “pain points” and gaps in our thinking and practice, the outlining of potentially mitigative actions, and the encouragement of cross- and multi-disciplinary collaborations amongst knowledge creators and care providers. As a field, if we care about upholding and advocating for the definition of pain put forth by IASP, we cannot continue to operate under the guise of research or clinical practice as usual. We cannot continue to ignore systemic inequities in pain care, as these biases end up in our data and circle back to inform future pain practice via our models and results. This cycle must stop. We cannot continue to function under the assumption that some forms of data are more valid than others, that some forms of research methods are more valuable than others, or that we can treat various pain metrics as if they can be easily aggregated, combined, or compared without considerable forethought and attention to context. The transformation of the field will likely involve a balance of qualitative and quantitative practices and tools, as well as empirical and theoretical modes of inquiry, and will necessitate a need for multiple ways of thinking about, communicating, and approaching pain that break traditional disciplinary, skill, and knowledge boundaries. What the future pain field will look like remains unknown, but ultimately and critically, it is up to all of us to build it.
Positionality Statement
SB is a White, cis-gender female, able-bodied, ace researcher for a tech company. Her primary research background is in cognitive neuroscience and bioethics, with 14 years in the pain field. Her epistemic and methodological lenses are influenced by patient-centered care practices and design, as well as feminist and critical disability theory. As a researcher trained in both biological and social sciences, she wants to encourage the habit of critical self-reflection and reflexivity within scientific research and the transparent sharing of backgrounds, experiences, and values that may influence how researchers approach, frame, conduct, and analyze their work. AB is a White and Filipino cis-gender male from the Midwest who has worked in tech as a data scientist for 4 years. He is a former neuroscientist with a background in theoretical neuroscience and conscious perception.
Author Contributions
SB outlined and organized the manuscript. SB and AB co-drafted, co-wrote, and co-edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
SB and AB are paid employees of IBM Research.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank our Responsible and Inclusive Technology research colleagues, who although were not involved in the writing of this manuscript, have critically helped shape the ways that we think about and approach pain science, methods, and tools.
Footnotes
1. ^International Association for the Study of Pain (IASP). Definition of pain. In: Terminology (2020). Available online at: https://www.iasp-pain.org/resources/terminology/.
2. ^O'Donnell J, Alltucker K. Medical Bias: From Pain Pills to COVID-19, Racial Discrimination in Health Care Festers. USA Today (2020). Available online at: https://www.usatoday.com/story/news/health/2020/06/14/festering-racial-bias-health-care-factor-covid-19-disparities/5320187002/.
3. ^Stein NL. Memory for Everyday and Emotional Events (1997). Available online at: http://site.ebrary.com/id/10806640.
4. ^Some examples in Abstracts from the North American Neuromodulation Society's 2021 Virtual Meeting, January 15–16, 2021. Neuromodulation: technology at the neural interface. (2021) 24:e1–276.
5. ^Barron D. The Problem With Pain Scores. Scientific American (2021). Available online at: https://www.scientificamerican.com/article/the-problem-with-pain-scores/.
6. ^Boyd Z, Elliot Z, Fruehwald J, Hall-Lew L, Lawrence D. An evaluation of sociolinguistic elicitation methods. In: The 18th International Conference of the Phonetic Sciences. Glaskow (2015). Available online at: https://www.internationalphoneticassociation.org/icphs-proceedings/ICPhS2015/Papers/ICPHS0800.pdf.
7. ^OpenAI. Better Language Models and Their Implications (2019). Available online at: https://openai.com/blog/better-language-models/.
8. ^Eveleth R. Beyond the Smiley-Face Pain Scale. The Atlantic (2015). Available online at: https://www.theatlantic.com/health/archive/2015/01/beyond-the-smiley-face-pain-scale/384049/.
9. ^Additional media coverage examples: https://www.brookings.edu/research/detecting-and-mitigating-bias-in-natural-language-processing/ and https://www.technologyreview.com/2021/05/20/1025135/ai-large-language-models-bigscience-project/.
10. ^Example: painexhibit.org.
11. ^Example: https://painimation.pitt.edu.
12. ^Hughes V. Using Dance/Movement Therapy and Laban Movement Analysis to Build a Better Model of Rehabilitation for Chronic Pain; 2018. Available: https://digitalcommons.slc.edu/cgi/viewcontent.cgi?article=1041&context=dmt_etd.
13. ^Some Examples: Unrest, On a Scale of 1 to 10, Tipping the Pain Scale, and This Might Hurt.
14. ^Nicholls DA, Groven KS, Kinsella EA, Anjum RL. Mobilizing Knowledge in Physiotherapy: Critical Reflections on Foundations and Practices (2021). Available online at: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=2628279.
15. ^Rauck R. Predicting pain now and in the future through personalized physiologic mobility metrics. In: Poster presented at; 2021 Jan 15 North American Neuromodulation Society, Virtual Meeting (2021).
16. ^See two examples: (1) Wetsman N. Data From Health Apps Offers Opportunities and Obstacles to Researchers. The Verge (2019). Available online at: https://www.theverge.com/2019/7/3/20681254/data-health-apps-clue-period-tracking-sleep-fitness-research.
(2) Wetsman N. Apple Watch's Data ‘Black Box' Poses Research Problems. The Verge (2021). Available online at: https://www.theverge.com/2021/7/27/22594178/apple-watch-data-research-heart-rate-reliability.
17. ^van Zalk N, Lalitharatne TD, Tan Y, Nanayakkara T. Lack of Universality in Pain Recognition from Animated Facial Expressions. Open Science Framework (2021). Available online at: https://osf.io/9ukqy.
18. ^Nakai T, Nishimoto S. Preserved representations and decodability of diverse cognitive functions across the cortex, cerebellum, and subcortex. Neuroscience. (2021) [https://doi.org/10.1101/2021.12.09.471939].
19. ^Example: https://www.kernel.com.
20. ^Example: https://www.humanbrainproject.eu.
21. ^Example: https://www.humanconnectomeproject.org.
22. ^Schulz M-A, Bzdok D, Haufe S, Haynes J-D, Ritter K. Performance reserves in brain-imaging-based phenotype prediction. Neuroscience. (2022). [https://doi.org/10.1101/2022.02.23.481601].
23. ^Reinsel D, Gantz J, Rydning J. The Digitization of the World From Edge to Core. IDC (Data Age 2025). Report No.: #US44413318 (2018). Available online at: https://www.seagate.com/files/www-content/our-story/trends/files/idc-seagate-dataage-whitepaper.pdf.
24. ^Lum K, Isaac W. To predict and serve? Significance Mag. (2016) 13:14–9.
25. ^Barocas S, Hardt M, Narayanan A. Fairness and Machine Learning. fairmlbook.org (2019). Available online at: http://ww.fairmlbook.org.
26. ^For a recent example, see: https://www.wired.com/story/opioid-drug-addiction-algorithm-chronic-pain/.
27. ^Fussell S. An Algorithm That ‘Predicts' Criminality Based on a Face Sparks a Furor. WIRED (2020). Available online at: https://www.wired.com/story/algorithm-predicts-criminality-based-face-sparks-furor/.
28. ^Example: https://www.affectiva.com.
29. ^Example: https://www.kaggle.com.
30. ^Example: https://github.com/philippwerner/pain-database-list.
31. ^Example: https://www.openpain.org.
32. ^Mohamed S, Oft D. Pain and machine learning. In: Workshop on Biological and Artificial Reinforcement Learning (2020). Available online at: https://deepmind.com/research/publications/2020/Pain-and-Machine-Learning.
33. ^Examples in media include: https://www.healthline.com/health-news/mental-scientists-objectively-measure-pain-for-the-first-time-041213, https://www.apa.org/monitor/2017/11/measure-pain, and https://www.sciencedaily.com/releases/2011/09/110913172623.ht.
References
1. Blanchard C, Blanchard R, Fellous J, Guimaraes F, Irwin W, LeDoux J, et al. The brain decade in debate: III neurobiology of emotion. Brazilian J Med Biol Res. (2001) 34:283–93. doi: 10.1590/S0100-879X2001000300001
2. Coakley S, Shelemay KK, editors. Pain and Its Transformations: The Interface of Biology and Culture. Cambridge, MA: Harvard University Press (2007). p. 439.
3. Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. (2007) 8:71–80. doi: 10.1038/nrn2042
4. Gilam G, Gross JJ, Wager TD, Keefe FJ, Mackey SC. What is the relationship between pain and emotion? Bridging Constructs Commun Neuron. (2020) 107:17–21. doi: 10.1016/j.neuron.2020.05.024
5. Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. (2020) 161:1694–7. doi: 10.1097/j.pain.0000000000001950
6. Nieto R, Pardo R, Sora B, Feliu-Soler A, Luciano JV. Impact of COVID-19 lockdown measures on spanish people with chronic pain: an online study survey. JCM. (2020) 9:3558. doi: 10.3390/jcm9113558
7. Puntillo F, Giglio M, Brienza N, Viswanath O, Urits I, Kaye AD, et al. Impact of COVID-19 pandemic on chronic pain management: looking for the best way to deliver care. Best Pract Res Clin Anaesthesiol. (2020) 34:529–37. doi: 10.1016/j.bpa.2020.07.001
8. Shanthanna H, Strand NH, Provenzano DA, Lobo CA, Eldabe S, Bhatia A, et al. Caring for patients with pain during the COVID−19 pandemic: consensus recommendations from an international expert panel. Anaesthesia. (2020) 75:935–44. doi: 10.1111/anae.15076
9. Perez J, Niburski K, Stoopler M, Ingelmo P. Telehealth and chronic pain management from rapid adaptation to long-term implementation in pain medicine: a narrative review. PR9. (2021) 6:e912. doi: 10.1097/PR9.0000000000000912
10. Ezenwa MO, Fleming MF. Racial disparities in pain management in primary care. J Health Dispar Res Pract. (2012) 5:12–26.
11. Fetta J, Evans H. The impact of discrimination in pain management: strategies to improve pain outcomes. Topics Pain Manag. (2021) 37:1–8. doi: 10.1097/01.TPM.0000798016.46041.a2
12. Parker LS, Sankar PL, Boyer J, Jean McEwen JD, Kaufman D. Normative and conceptual ELSI research: what it is, and why it's important. Genet Med. (2019) 21:505–9. doi: 10.1038/s41436-018-0065-x
13. Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. (2003) 3:310–6. doi: 10.1111/j.1530-7085.2003.03034.x
14. Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. (1985) 5:145–8. doi: 10.1007/BF00541514
15. Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. (2009) 144:35–42. doi: 10.1016/j.pain.2009.02.007
16. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale – revised: toward a common metric in pediatric pain measurement. Pain. (2001) 93:173–83. doi: 10.1016/S0304-3959(01)00314-1
17. Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. (1978) 5:5–18. doi: 10.1016/0304-3959(78)90020-9
18. Cleeland C, Ryab K. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med. (1994) 23:129–38.
19. Askew RL, Cook KF, Keefe FJ, Nowinski CJ, Cella D, Revicki DA, et al. A PROMIS measure of neuropathic pain quality. Value Health. (2016) 19:623–30. doi: 10.1016/j.jval.2016.02.009
20. Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain. (2002) 100:55–64. doi: 10.1016/S0304-3959(02)00239-7
21. Fairbank J, Pynsent P. The Oswestry disability index. Spine. (2000) 25:2940–53. doi: 10.1097/00007632-200011150-00017
22. Haefeli M, Elfering A. Pain assessment. Eur Spine J. (2006) 15:S17–24. doi: 10.1007/s00586-005-1044-x
23. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales: pain rating scales. J Clin Nurs. (2005) 14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x
24. Pathak A, Sharma S, Jensen MP. The utility and validity of pain intensity rating scales for use in developing countries. PR9. (2018) 3:e672. doi: 10.1097/PR9.0000000000000672
25. Burckhardt CS, Jones KD. Adult measures of pain: the McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short-Form McGill Pain Questionnaire (SF-MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven-Yale Multidisciplinary Pain Inventory (WHYMPI). Arthritis Rheumat. (2003) 49:S96–104. doi: 10.1002/art.11440
26. Pogatzki-Zahn E, Schnabel K, Kaiser U. Patient-reported outcome measures for acute and chronic pain: current knowledge and future directions. Curr Opin Anaesthesiol. (2019) 32:616–22. doi: 10.1097/ACO.0000000000000780
27. Price DD, Riley JL, Wade JB. Psychophysical approaches to measurement of the dimensions and stages of pain. In: Handbook of Pain Assessment. 2nd ed. New York, NY: The Guilford Press (2001). p. 53–75.
28. Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. (2007) 129:256–9. doi: 10.1016/j.pain.2007.03.030
29. De Vita MJ, Buckheit K, Gilmour CE, Moskal D, Maisto SA. Development of a novel brief quantitative sensory testing protocol that integrates static and dynamic pain assessments: test-retest performance in healthy adults. Pain Med. (2022) 23:347–51. doi: 10.1093/pm/pnab290
30. Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. (2006) 26:12165–73. doi: 10.1523/JNEUROSCI.3576-06.2006
31. Fagerlund AJ, Iversen M, Ekeland A, Moen CM, Aslaksen PM. Blame it on the weather? The association between pain in fibromyalgia, relative humidity, temperature and barometric pressure. PLoS ONE. (2019) 14:e0216902. doi: 10.1371/journal.pone.0216902
32. Grabovac I, Dorner TE. Association between low back pain and various everyday performances: activities of daily living, ability to work and sexual function. Wien Klin Wochenschr. (2019) 131:541–9. doi: 10.1007/s00508-019-01542-7
33. Vadivelu N, Kai AM, Kodumudi G, Babayan K, Fontes M, Burg MM. Pain and psychology—a reciprocal relationship. Ochsner J. (2017) 17:173.
34. Maly A, Vallerand AH. Neighborhood, socioeconomic, and racial influence on chronic pain. Pain Manag Nurs. (2018) 19:14–22. doi: 10.1016/j.pmn.2017.11.004
35. Ferrell BR. The impact of pain on quality of life. A decade of research. Nurs Clin North Am. (1995) 30:609–24.
36. Gastfriend DR, Donovan D, Lefebvre R, Murray KT. Developing a baseline assessment battery: balancing patient time burden with essential clinical and research monitoring. J Stud Alcohol Suppl. (2005) 94–103. doi: 10.15288/jsas.2005.s15.94
37. Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. (2011) 14:1101–8. doi: 10.1016/j.jval.2011.06.003
38. Atkinson TM, Schwartz CE, Goldstein L, Garcia I, Storfer DF Li Y, et al. Perceptions of response burden associated with completion of patient-reported outcome assessments in oncology. Value Health. (2019) 22:225–30. doi: 10.1016/j.jval.2018.07.875
39. Eisele G, Vachon H, Lafit G, Kuppens P, Houben M, Myin-Germeys I, et al. The effects of sampling frequency and questionnaire length on perceived burden, compliance, and careless responding in experience sampling data in a student population. Assessment. (2022) 29:136–51. doi: 10.1177/1073191120957102
40. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. (2015) 16:403–18. doi: 10.1038/nrn3976
41. Berger SE, Vachon-Presseau É, Abdullah TB, Baria AT, Schnitzer TJ, Apkarian AV. Hippocampal morphology mediates biased memories of chronic pain. Neuroimage. (2018) 166:86–98. doi: 10.1016/j.neuroimage.2017.10.030
42. Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for pain: Relation between past and present pain intensity. Pain. (1985) 23:375–80. doi: 10.1016/0304-3959(85)90007-7
43. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. JMDH. (2016) 9:211–7. doi: 10.2147/JMDH.S104807
44. Peacock S, Patel S. Cultural influences on pain. Rev Pain. (2008) 1:6–9. doi: 10.1177/204946370800100203
45. Schmidt RF, Willis WD, editors. Underreporting of pain. In: Encyclopedia of Pain. Berlin; Heidelberg: Springer Berlin Heidelberg (2007). p. 2591. Available online at: http://link.springer.com/10.1007/978-3-540-29805-2/4685 (accessed March 8, 2022).
46. Boring BL, Walsh KT, Nanavaty N, Ng BW, Mathur VA. How and why patient concerns influence pain reporting: a qualitative analysis of personal accounts and perceptions of others' use of numerical pain scales. Front Psychol. (2021) 12:663890. doi: 10.3389/fpsyg.2021.663890
47. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. (2008) 4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415
48. Pagé MG, Gauvin L, Sylvestre MP, Nitulescu R, Dyachenko A, Choinière M. An ecological momentary assessment study of pain intensity variability: ascertaining extent, predictors, and associations with quality of life, interference and health care utilization among individuals living with chronic low back pain. J Pain. (2022). doi: 10.1016/j.jpain.2022.01.001. [Epub ahead of print].
49. Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, et al. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. (2005) 52:3670–4. doi: 10.1002/art.21407
50. Stone AA. The Science of Real-Time Data Capture: Self-Reports in Health Research. Oxford; New York, NY: Oxford University Press (2007). Available online at: http://site.ebrary.com/id/10271669 (accessed March 9, 2022).
51. Doherty K, Balaskas A, Doherty G. The design of ecological momentary assessment technologies. Interact Comput. (2020) 32:257–78. doi: 10.1093/iwcomp/iwaa019
52. Eccleston C, Blyth FM, Dear BF, Fisher EA, Keefe FJ, Lynch ME, et al. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. (2020) 161:889–93. doi: 10.1097/j.pain.0000000000001885
53. Huckins JF, daSilva AW, Wang W, Hedlund E, Rogers C, Nepal SK, et al. Mental health and behavior of college students during the early phases of the COVID-19 pandemic: longitudinal smartphone and ecological momentary assessment study. J Med Internet Res. (2020) 22:e20185. doi: 10.2196/20185
54. Stone AA, Obbarius A, Junghaenel DU, Wen CKF, Schneider S. High-resolution, field approaches for assessing pain: ecological Momentary Assessment. Pain. (2021) 162:4–9. doi: 10.1097/j.pain.0000000000002049
55. Treister R, Lawal OD, Shecter JD, Khurana N, Bothmer J, Field M, et al. Accurate pain reporting training diminishes the placebo response: results from a randomised, double-blind, crossover trial. PLoS ONE. (2018) 13:e0197844. doi: 10.1371/journal.pone.0197844
56. Rouzaud Laborde C, Cenko E, Mardini MT, Nerella S, Kheirkhahan M, Ranka S, et al. Satisfaction, usability, and compliance with the use of smartwatches for ecological momentary assessment of knee osteoarthritis symptoms in older adults: usability study. JMIR Aging. (2021) 4:e24553. doi: 10.2196/24553
57. Chandler KD, Hodge CJ, McElvaine K, Olschewski EJ, Melton KK, Deboeck P. Challenges of ecological momentary assessments to study family leisure: participants' perspectives. J Leis Res. (2022) 53:159–65. doi: 10.1080/00222216.2021.2001398
58. Lazaridou A, Elbaridi N, Edwards RR, Berde CB. Pain assessment. In: Essentials of Pain Medicine. Elsevier (2018). p. 39–46.e1. Available online at: https://linkinghub.elsevier.com/retrieve/pii/B978032340196800005X doi: 10.1016/B978-0-323-40196-8.00005-X (accessed February 27, 2022).
59. Sharma S, Ferreira-Valente AC, Williams AC, Abbott JH, Pais-Ribeiro J, Jensen MP. Group differences between countries and between languages in pain-related beliefs, coping, and catastrophizing in chronic pain: a systematic review. Pain Med. (2020) 21:1847–62. doi: 10.1093/pm/pnz373
60. Murphy DR, Hurwitz EL, Gerrard JK, Clary R. Pain patterns and descriptions in patients with radicular pain: does the pain necessarily follow a specific dermatome? Chiropr Man Ther. (2009) 17:9. doi: 10.1186/1746-1340-17-9
61. Hildebrandt J. Prediction of psychosocial factors by pain drawing in patients with chronic back pain: editorial. Pain Med. (2014) 15:1067–9. doi: 10.1111/pme.12370
62. Shaballout N, Neubert TA, Boudreau S, Beissner F. From paper to digital applications of the pain drawing: systematic review of methodological milestones. JMIR Mhealth Uhealth. (2019) 7:e14569. doi: 10.2196/14569
63. Shaballout N, Aloumar A, Neubert TA, Dusch M, Beissner F. Digital pain drawings can improve doctors' understanding of acute pain patients: survey and pain drawing analysis. JMIR Mhealth Uhealth. (2019) 7:e11412. doi: 10.2196/11412
64. Stones C. Positively picturing pain? Using patient-generated pictures to establish affective visual design qualities. Int J Design. (2013) 7:85–97.
65. de Knegt NC, Schuengel C, Lobbezoo F, Visscher CM, Evenhuis HM, Boel JA, et al. Comprehension of pictograms for pain quality and pain affect in adults with Down syndrome. J Intellect Dev Disabil. (2016) 41:222–32. doi: 10.3109/13668250.2016.1176129
66. Stones C, Knapp P, Closs SJ. Creating a better picture of chronic pain: improving pain pictogram designs through systematic evaluation of user responses. Br J Pain. (2016) 10:177–85. doi: 10.1177/2049463716657365
67. Petre B, Tetreault P, Mathur VA, Schurgin MW, Chiao JY, Huang L, et al. A central mechanism enhances pain perception of noxious thermal stimulus changes. Sci Rep. (2017) 7:3894. doi: 10.1038/s41598-017-04009-9
68. Finan PH, Hessler EE, Amazeen PG, Butner J, Zautra AJ, Tennen H. Oscillations in daily pain prediction accuracy. Nonlinear Dyn Psychol Life Sci. (2010) 14:27–46.
69. Dillon DJ. Temporal summation of pain from radiant stimulation. Percept Psychophys. (1971) 10:109–11. doi: 10.3758/BF03214328
70. Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. (1999) 79:75–82. doi: 10.1016/S0304-3959(98)00154-7
71. Cecchi GA, Huang L, Hashmi JA, Baliki M, Centeno MV, Rish I, et al. Predictive dynamics of human pain perception. PLoS Comput Biol. (2012) 8:e1002719. doi: 10.1371/journal.pcbi.1002719
72. Schiavenato M, Craig KD. Pain assessment as a social transaction: beyond the “Gold Standard”. Clin J Pain. (2010) 26:667–76. doi: 10.1097/AJP.0b013e3181e72507
73. Twycross A, Voepel-Lewis T, Vincent C, Franck LS, von Baeyer CL. A debate on the proposition that self-report is the gold standard in assessment of pediatric pain intensity. Clin J Pain. (2015) 31:707–12. doi: 10.1097/AJP.0000000000000165
74. Lena F, Pappaccogli M, Santilli M, Torre M, Modugno N, Perrotta A. How does semantic pain and words condition pain perception? A short communication. Neurol Sci. (2022) 43:691–6. doi: 10.1007/s10072-021-05577-5
75. Padfield D, Zakrzewska JM. Encountering Pain: Hearing, seeing, speaking (2021). doi: 10.14324/111.9781787352636
76. Scarry E. The Body in Pain: The Making and Unmaking of the World. New York, NY: Oxford university press (1987).
77. Agurto C, Cecchi GA, Norel R, Ostrand R, Kirkpatrick M, Baggott MJ, et al. Detection of acute 3,4-methylenedioxymethamphetamine (MDMA) effects across protocols using automated natural language processing. Neuropsychopharmacology. (2020) 45:823–32. doi: 10.1038/s41386-020-0620-4
78. Alpert M, Pouget ER, Silva RR. Reflections of depression in acoustic measures of the patient's speech. J Affect Disord. (2001) 66:59–69. doi: 10.1016/S0165-0327(00)00335-9
79. Mota NB, Furtado R, Maia PPC, Copelli M, Ribeiro S. Graph analysis of dream reports is especially informative about psychosis. Sci Rep. (2015) 4:3691. doi: 10.1038/srep03691
80. Corcoran CM, Carrillo F, Fernández-Slezak D, Bedi G, Klim C, Javitt DC, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. (2018) 17:67–75. doi: 10.1002/wps.20491
81. Cecchi G, Corcoran C. O23 automated analysis of recent-onset and prodromal schizophrenia. Schizophr Bull. (2018) 44:S76. doi: 10.1093/schbul/sby015.193
82. Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the Nun Study. JAMA. (1996) 275:528–32. doi: 10.1001/jama.275.7.528
83. Eyigoz E, Mathur S, Santamaria M, Cecchi G, Naylor M. Linguistic markers predict onset of Alzheimer's disease. EClinicalMedicine. (2020) 28:100583. doi: 10.1016/j.eclinm.2020.100583
84. Norel R, Agurto C, Rice JJ, Ho BK, Cecchi GA. Speech-based identification of L-DOPA ON/OFF state in Parkinson's Disease subjects. Neuroscience. (2018) doi: 10.1101/420422
85. Apkarian AV, Scholz J. Shared mechanisms between chronic pain and neurodegenerative disease. Drug Discov Today Dis Mech. (2006) 3:319–26. doi: 10.1016/j.ddmec.2006.09.006
86. Kleinman A. The Illness Narratives: Suffering, Healing, and the Human Condition. New York, NY: Basic Books (1989). p. 284.
87. Wittgenstein L, Anscombe GEM. Philosophical Investigations: the English Text of the Third Edition. 3 ed. Englewood Cliffs, NJ: Prentice Hall (2000). p. 250.
88. Biro D. The Language of Pain: Finding Words, Compassion, and Relief. 1st ed. New York, NY: W.W. Norton (2010). p. 256.
89. Frank AW. The Wounded Storyteller: Body, Illness, and Ethics. 2nd ed. Chicago, IL: The University of Chicago Press (2013). p. 253.
90. Xu M, Tachibana RO, Okanoya K, Hagiwara H, Hashimoto R-I, Homae F. Unconscious and distinctive control of vocal pitch and timbre during altered auditory feedback. Front Psychol. (2020) 11:1224. doi: 10.3389/fpsyg.2020.01224
91. Zlatev J, Blomberg J. Language may indeed influence thought. Front Psychol. (2015) 6:1631. doi: 10.3389/fpsyg.2015.01631
92. Oshrat Y, Bloch A, Lerner A, Cohen A, Avigal M, Zeilig G. Speech prosody as a biosignal for physical pain detection. In: Proceedings of Speech Prosody 8. Boston, MA (2016).
93. Thiam P, Kessler V, Amirian M, Bellmann P, Layher G, Zhang Y, et al. Multi-modal pain intensity recognition based on the SenseEmotion database. In: IEEE Transactions on Affective Computing, vol. 12, p. 743–760. doi: 10.1109/TAFFC.2019.2892090
94. Rasmussen-Barr E, Äng B, Arvidsson I, Nilsson-Wikmar L. Graded exercise for recurrent low-back pain: a randomized, controlled trial with 6-, 12-, and 36-month follow-ups. Spine. (2009) 34:221–8. doi: 10.1097/BRS.0b013e318191e7cb
95. Porter FL, Miller RH, Marshall RE. Neonatal pain cries: effect of circumcision on acoustic features and perceived urgency. Child Dev. (1986) 57:790. doi: 10.2307/1130355
96. Roy N, Volinn E, Merrill RM, Chapman CR. Speech motor control and chronic back pain: a preliminary investigation. Pain Med. (2009) 10:164–71. doi: 10.1111/j.1526-4637.2007.00393.x
97. Raine J, Pisanski K, Simner J, Reby D. Vocal communication of simulated pain. Bioacoustics. (2019) 28:404–26. doi: 10.1080/09524622.2018.1463295
98. Cinciripini PM, Floreen A. An assessment of chronic pain behavior in a structured interview. J Psychosom Res. (1983) 27:117–23. doi: 10.1016/0022-3999(83)90087-9
99. Rude S, Gortner EM, Pennebaker J. Language use of depressed and depression-vulnerable college students. Cogn Emot. (2004) 18:1121–33. doi: 10.1080/02699930441000030
100. Holmes D, Alpers GW, Ismailji T, Classen C, Wales T, Cheasty V, et al. Cognitive and emotional processing in narratives of women abused by intimate partners. Viol Against Women. (2007) 13:1192–205. doi: 10.1177/1077801207307801
101. Lascaratou C. The Language of Pain: Expression or Description?. Converging Evidence in Language and Communication Research. Amsterdam: John Benjamins Publishing Company (2007). Available online at: http://www.jbe-platform.com/content/books/9789027292056 (accessed June 15, 2020).
102. Wilson D, Williams M, Butler D. Language and the pain experience. Physiother Res Int. (2009) 14:56–65. doi: 10.1002/pri.424
103. Tausczik YR, Pennebaker JW. The psychological meaning of words: LIWC and computerized text analysis methods. J Lang Soc Psychol. (2010) 29:24–54. doi: 10.1177/0261927X09351676
104. Rowbotham S, Wardy AJ, Lloyd DM, Wearden A, Holler J. Increased pain intensity is associated with greater verbal communication difficulty and increased production of speech and co-speech gestures. PLoS ONE. (2014) 9:e110779. doi: 10.1371/journal.pone.0110779
105. Borelli E, Crepaldi D, Porro CA, Cacciari C. The psycholinguistic and affective structure of words conveying pain. PLoS ONE. (2018) 13:e0199658. doi: 10.1371/journal.pone.0199658
106. Disney SJ. Figurative language in describing pain and lifestyle impact. English Stud. (2020) 101:1009–29. doi: 10.1080/0013838X.2020.1847891
107. Bullo S, Hearn JH. Parallel worlds and personified pain: a mixed-methods analysis of pain metaphor use by women with endometriosis. Br J Health Psychol. (2021) 26:271–88. doi: 10.1111/bjhp.12472
108. Munday I, Kneebone I, Rogers K, Newton-John T. The language of pain: is there a relationship between metaphor use and adjustment to chronic pain? Pain Med. (2021) pnaa467. doi: 10.1093/pm/pnaa467
109. Berger SE, Branco P, Vachon-Presseau E, Abdullah TB, Cecchi G, Apkarian AV. Quantitative language features identify placebo responders in chronic back pain. Pain. (2021) 162:1692–704. doi: 10.1097/j.pain.0000000000002175
110. Pierson E, Cutler DM, Leskovec J, Mullainathan S, Obermeyer Z. An algorithmic approach to reducing unexplained pain disparities in underserved populations. Nat Med. (2021) 27:136–40. doi: 10.1038/s41591-020-01192-7
111. Heintzelman NH, Taylor RJ, Simonsen L, Lustig R, Anderko D, Haythornthwaite JA, et al. Longitudinal analysis of pain in patients with metastatic prostate cancer using natural language processing of medical record text. J Am Med Inform Assoc. (2013) 20:898–905. doi: 10.1136/amiajnl-2012-001076
112. Sarker A, Gonzalez-Hernandez G, Ruan Y, Perrone J. Machine learning and natural language processing for geolocation-centric monitoring and characterization of opioid-related social media chatter. JAMA Netw Open. (2019) 2:e1914672. doi: 10.1001/jamanetworkopen.2019.14672
113. Tighe PJ, Goldsmith RC, Gravenstein M, Bernard HR, Fillingim RB. The painful tweet: text, sentiment, and community structure analyses of tweets pertaining to pain. J Med Internet Res. (2015) 17:e84. doi: 10.2196/jmir.3769
114. Deng H, Wang Q, Turner DP, Sexton KE, Burns SM, Eikermann M, et al. Sentiment analysis of real-world migraine tweets for population research. Cephalalgia Rep. (2020) 3:251581631989886. doi: 10.1177/2515816319898867
115. Graves RL, Goldshear J, Perrone J, Ungar L, Klinger E, Meisel ZF, et al. Patient narratives in Yelp reviews offer insight into opioid experiences and the challenges of pain management. Pain Manag. (2018) 8:95–104. doi: 10.2217/pmt-2017-0050
116. Fiok K, Karwowski W, Gutierrez E, Saeidi M, Aljuaid AM, Davahli MR, et al. A study of the effects of the COVID-19 pandemic on the experience of back pain reported on Twitter® in the United States: a natural language processing approach. IJERPH. (2021) 18:4543. doi: 10.3390/ijerph18094543
117. Haanstra TM, Hanson L, Evans R, van Nes FA, De Vet HCW, Cuijpers P, et al. How do low back pain patients conceptualize their expectations regarding treatment? Content analysis of interviews. Eur Spine J. (2013) 22:1986–95. doi: 10.1007/s00586-013-2803-8
118. De Sola H, Maquibar A, Failde I, Salazar A, Goicolea I. Living with opioids: a qualitative study with patients with chronic low back pain. Health Expect. (2020) 23:1118–28. doi: 10.1111/hex.13089
119. Goksör C, Mannerkorpi K, Bergenheim A. Experiences with an educational program for patients with chronic widespread pain: a qualitative interview study. Scand J Pain. (2021) 22:279–87. doi: 10.1515/sjpain-2021-0080
120. Rossen CB, Høybye MT, Jørgensen LB, Bruun LD, Hybholt L. Disrupted everyday life in the trajectory of low back pain: a longitudinal qualitative study of the cross-sectorial pathways of individuals with low back pain over time. Int J Nurs Stud Adv. (2021) 3:100021. doi: 10.1016/j.ijnsa.2021.100021
121. Cooper PV. Discourse production and normal aging: performance on oral picture description tasks. J Gerontol. (1990) 45:P210–4. doi: 10.1093/geronj/45.5.P210
122. Patel R, Connaghan K, Franco D, Edsall E, Forgit D, Olsen L, et al. “The Caterpillar”: a novel reading passage for assessment of motor speech disorders. Am J Speech Lang Pathol. (2013) 22:1–9. doi: 10.1044/1058-0360(2012/11-0134)
123. Padovani M, Gielow I, Behlau M. Phonarticulatory diadochokinesis in young and elderly individuals. Arq Neuro Psiquiatr. (2009) 67:58–61. doi: 10.1590/S0004-282X2009000100015
124. Casillas M, Cristia A. A step-by-step guide to collecting and analyzing long-format speech environment (LFSE) recordings. Collabra Psychol. (2019) 5:24. doi: 10.1525/collabra.209
125. Cha N, Kim A, Park CY, Kang S, Park M, Lee JG, et al. Hello there! is now a good time to talk?: Opportune moments for proactive interactions with smart speakers. Proc ACM Interact Mob Wearable Ubiquitous Technol. (2020) 4:1–28. doi: 10.1145/3411810
126. Hosio SJ, Karppinen J, Takala EP, Takatalo J, Goncalves J, van Berkel N, et al. Crowdsourcing treatments for low back pain. In: Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems. Montreal, QC: ACM (2018). p. 1–12. Available online at: https://dl.acm.org (accessed February 27, 2022).
127. Cedeno D, Kelley C, Tilley D, Smith W, Thomas S, Vallejo A, et al. Basic research. Pain Pract. (2020) 20:7–95. doi: 10.1111/papr.12886
128. Lennox Thompson B, Gage J, Kirk R. Living well with chronic pain: a classical grounded theory. Disabil Rehabil. (2020) 42:1141–52. doi: 10.1080/09638288.2018.1517195
129. Robinson V, King R, Ryan CG, Martin DJ. A qualitative exploration of people's experiences of pain neurophysiological education for chronic pain: the importance of relevance for the individual. Man Ther. (2016) 22:56–61. doi: 10.1016/j.math.2015.10.001
130. Howell P. Effect of speaking environment on speech production and perception. JHES. (2008) 11:51–7. doi: 10.1618/jhes.11.51
131. Brown C, Snodgrass T, Kemper SJ, Herman R, Covington MA. Automatic measurement of propositional idea density from part-of-speech tagging. Behav Res Methods. (2008) 40:540–5. doi: 10.3758/BRM.40.2.540
133. Eyben F, Wöllmer M, Schuller B. Opensmile: the munich versatile and fast open-source audio feature extractor. In: Proceedings of the International Conference on Multimedia - MM '10. Firenze: ACM Press (2010). p. 1459. Available online at: http://dl.acm.org/citation.cfm?doid=1873951.1874246 (accessed March 30, 2021).
134. Alharbi S, Alrazgan M, Alrashed A, Alnomasi T, Almojel R, Alharbi R, et al. Automatic speech recognition: systematic literature review. IEEE Access. (2021) 9:131858–76. doi: 10.1109/ACCESS.2021.3112535
135. Kumar Y, Singh N. A comprehensive view of automatic speech recognition system - a systematic literature review. In: 2019 International Conference on Automation, Computational and Technology Management (ICACTM). London: IEEE (2019). p. 168–73. Available online at: https://ieeexplore.ieee.org/document/8776714/ (accessed February 27, 2022).
136. Koenecke A, Nam A, Lake E, Nudell J, Quartey M, Mengesha Z, et al. Racial disparities in automated speech recognition. Proc Natl Acad Sci USA. (2020) 117:7684–9. doi: 10.1073/pnas.1915768117
137. Markl N, Lai C. Context-sensitive evaluation of automatic speech recognition: considering user experience & language variation. In: Proceedings of the First Workshop on Bridging Human–Computer Interaction and Natural Language Processing. Association for Computational Linguistics (2021). p. 34–40. Available online at: https://aclanthology.org/2021.hcinlp-1.6.pdf
138. Fairbanks G. Voice and Articulation Drillbook. 2nd ed. New York, NY: Harper and Row (1960). p. 124–39. Available online at: https://www.york.ac.uk/media/languageandlinguistics/documents/currentstudents/linguisticsresources/Standardised-reading.pdf
139. Yi C, Zhou S, Xu B. Efficiently fusing pretrained acoustic and linguistic encoders for low-resource speech recognition. IEEE Signal Process Lett. (2021) 28:788–92. doi: 10.1109/LSP.2021.3071668
140. Carlson LA, Hooten WM. Pain—linguistics and natural language processing. Mayo Clin Proc Innov Qual Outcomes. (2020) 4:346–7. doi: 10.1016/j.mayocpiqo.2020.01.005
141. Demner-Fushman D, Elhadad N, Friedman C. Natural language processing for health-related texts. In: Shortliffe EH, Cimino JJ, editors. Biomedical Informatics. Cham: Springer International Publishing (2021). p. 241–72. Available online at: https://link.springer.com/10.1007/978-3-030-58721-5_8 (accessed February 27, 2022).
142. Tighe PJ, Sannapaneni B, Fillingim RB, Doyle C, Kent M, Shickel B, et al. Forty-two million ways to describe pain: topic modeling of 200,000 pubmed pain-related abstracts using natural language processing and deep learning–based text generation. Pain Med. (2020) 21:3133–60. doi: 10.1093/pm/pnaa061
143. Nunes DAP, de Matos DM, Gomes JF, Neto F. Chronic Pain and Language: A Topic Modelling Approach to Personal Pain Descriptions. (2021). Available online at: https://arxiv.org/abs/2109.00402 (accessed February 26, 2022).
144. Alambo A, Andrew R, Gollarahalli S, Vaughn J, Banerjee T, Thirunarayan K, et al. Measuring pain in sickle cell disease using clinical text. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). Montreal, QC: IEEE (2020). p. 5838–41. Available online at: https://ieeexplore.ieee.org/document/9175599/ (accessed March 11, 2022).
145. Gagliese L, Melzack R. Age-related differences in the qualities but not the intensity of chronic pain. Pain. (2003) 104:597–608. doi: 10.1016/S0304-3959(03)00117-9
146. Stanford EA, Chambers CT, Craig KD. A normative analysis of the development of pain-related vocabulary in children. Pain. (2005) 114:278–84. doi: 10.1016/j.pain.2004.12.029
147. Craig KD, Stanford EA, Fairbairn NS, Chambers CT. Emergent pain language communication competence in infants and children. Enfance. (2006) 58:52. doi: 10.3917/enf.581.0052
148. Strong J, Mathews T, Sussex R, New F, Hoey S, Mitchell G. Pain language and gender differences when describing a past pain event. Pain. (2009) 145:86–95. doi: 10.1016/j.pain.2009.05.018
149. McEwen K, Young K. Ballet and pain: reflections on a risk-dance culture. Qual Res Sport Exerc Health. (2011) 3:152–73. doi: 10.1080/2159676X.2011.572181
150. Jaworska S, Ryan K. Gender and the language of pain in chronic and terminal illness: a corpus-based discourse analysis of patients' narratives. Soc Sci Med. (2018) 215:107–14. doi: 10.1016/j.socscimed.2018.09.002
151. Cleland JA, Palmer JA, Venzke JW. Ethnic differences in pain perception. Phys Ther Rev. (2005) 10:113–22. doi: 10.1179/108331905X55749
152. Losin EAR, Woo CW, Medina NA, Andrews-Hanna JR, Eisenbarth H, Wager TD. Neural and sociocultural mediators of ethnic differences in pain. Nat Hum Behav. (2020) 4:517–30. doi: 10.1038/s41562-020-0819-8
153. Moore RA, Dworkin SF. Ethnographic methodologic assessment of pain perceptions by verbal description. Pain. (1988) 34:195–204. doi: 10.1016/0304-3959(88)90166-2
154. Nortjé N, Albertyn R. The cultural language of pain: a South African study. South Afr Fam Pract. (2015) 57:24–7. doi: 10.1080/20786190.2014.977034
155. Rodrigues-de-Souza DP, Palacios-Ceña D, Moro-Gutiérrez L, Camargo PR, Salvini TF, Alburquerque-Sendín F. Socio-cultural factors and experience of chronic low back pain: a spanish and brazilian patients' perspective. A qualitative study. PLoS ONE. (2016) 11:e0159554. doi: 10.1371/journal.pone.0159554
156. Lor M, Vang X, Rabago D, Brown RL, Backonja M. “It Hurts as If…”: pain-associated language, visual characterization, and storytelling in hmong adults. Pain Med. (2020) 21:1690–702. doi: 10.1093/pm/pnz268
157. Gianola M, Llabre MM, Losin EAR. Effects of language context and cultural identity on the pain experience of Spanish–English bilinguals. Affec Sci. (2021) 2:112–27. doi: 10.1007/s42761-020-00021-x
158. Perović M, Jacobson D, Glazer E, Pukall C, Einstein G. Are you in pain if you say you are not? Accounts of pain in Somali–Canadian women with female genital cutting. Pain. (2021) 162:1144–52. doi: 10.1097/j.pain.0000000000002121
159. Hollingshead NA, Vrany EA, Hsueh L, Stewart JC, Hirsh AT. Language use and generation status are associated with chronic pain differences in Mexican Americans. J Immigrant Minority Health. (2022) 24:342–50. doi: 10.1007/s10903-021-01160-4
160. Taylor MA, Glowacki EM. The language of women's pain: ideology and critical cultural competencies in pain literacy. Front Commun. (2020) 5:36. doi: 10.3389/fcomm.2020.00036
161. Moraes EB de, Dal Fabbro DR, Oliveira LB de, Leão ER. Pain management of amazon indigenous peoples: a community-based study. JPR Vol. (2021) 14:1969–80. doi: 10.2147/JPR.S298219
162. Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. Sci World J. (2013) 2013:1–20. doi: 10.1155/2013/916178
163. Feng S, Kudina O, Halpern BM, Scharenborg O. Quantifying Bias in Automatic Speech Recognition. (2021). Available online at: https://arxiv.org/abs/2103.15122 (accessed March 12, 2022).
164. Zhang S, Zhang X, Zhang W, Søgaard A. Sociolectal analysis of pretrained language models. In: Proceedings of the 2021 Conference on Empirical Methods in Natural Language Processing. Punta Cana: Association for Computational Linguistics (2021). p. 4581–8. Available online at: https://aclanthology.org/2021.emnlp-main.375 [accessed March 14, 2022).
165. Bender EM, Gebru T, McMillan-Major A, Shmitchell S. On the dangers of stochastic parrots: can language models be too big?  . In: Proceedings of the 2021 ACM Conference on Fairness, Accountability, and Transparency. Virtual Event Canada: ACM (2021). p. 610–23. Available online at: https://dl.acm.org (accessed March 14, 2022).
. In: Proceedings of the 2021 ACM Conference on Fairness, Accountability, and Transparency. Virtual Event Canada: ACM (2021). p. 610–23. Available online at: https://dl.acm.org (accessed March 14, 2022).
166. Feig E. Cathartic poetry: healing through narrative. Perm J. (2018) 22:17–196. doi: 10.7812/TPP/17-196
168. Dickinson E, Wetzsteon R. The Collected Poems of Emily Dickinson. New York, NY: Barnes & Noble Classics (2003). p. 361.
169. Lautenbacher S, Salinas-Ranneberg M, Niebuhr O, Kunz M. Phonetic characteristics of vocalizations during pain. PR9. (2017) 2:e597. doi: 10.1097/PR9.0000000000000597
170. Ekman P. Are there basic emotions? Psychol Rev. (1992) 99:550–3. doi: 10.1037/0033-295X.99.3.550
171. Ekman P, Friesen WV. A new pan-cultural facial expression of emotion. Motiv Emot. (1986) 10:159–68. doi: 10.1007/BF00992253
172. Heaven D. Why faces don't always tell the truth about feelings. Nature. (2020) 578:502–4. doi: 10.1038/d41586-020-00507-5
173. Jackson JC, Watts J, Henry TR, List JM, Forkel R, Mucha PJ, et al. Emotion semantics show both cultural variation and universal structure. Science. (2019) 366:1517–22. doi: 10.1126/science.aaw8160
174. Eveleth R. Beyond the Smiley-Face Pain Scale. The Atlantic. (2015). Available online at: https://www.theatlantic.com/health/archive/2015/01/beyond-the-smiley-face-pain-scale/384049/
175. Stuckey HL, Nobel J. The connection between art, healing, and public health: a review of current literature. Am J Public Health. (2010) 100:254–63. doi: 10.2105/AJPH.2008.156497
176. Junge MB. History of art therapy. In Gussak DE, Rosal ML, editors. The Wiley Handbook of Art Therapy. Hoboken, NJ: Wiley Black (2016). p. 7–16.
177. Camic PM. Playing in the mud: health psychology, the arts and creative approaches to health care. J Health Psychol. (2008) 13:287–98. doi: 10.1177/1359105307086698
178. Hamel J. Somatic Art Therapy Alleviating Pain Trauma Through Art. Milton: Taylor & Francis Group (2021). Available online at: http://public.eblib.com/choice/PublicFullRecord.aspx?p=6527649 (accessed March 12, 2022).
179. Angheluta A, Lee B. Art therapy for chronic pain: applications and future directions. Can J Counsel Psychother. (2011) 45:112–31.
180. Kirkham JA, Smith JA, Havsteen-Franklin D. Painting pain: an interpretative phenomenological analysis of representations of living with chronic pain. Health Psychol. (2015) 34:398–406. doi: 10.1037/hea0000139
181. Phillips J, Ogden J, Copland C. Using drawings of pain-related images to understand the experience of chronic pain: a qualitative study. Br J Occup Ther. (2015) 78:404–11. doi: 10.1177/0308022614562791
182. Hass-Cohen N, Bokoch R, Goodman K, Conover KJ. Art therapy drawing protocols for chronic pain: quantitative results from a mixed method pilot study. Arts Psychother. (2021) 73:101749. doi: 10.1016/j.aip.2020.101749
183. Padfield D, Omand H, Semino E, Williams AC de C, Zakrzewska JM. Images as catalysts for meaning-making in medical pain encounters: a multidisciplinary analysis. Med Humanities. (2018) 44:74–81. doi: 10.1136/medhum-2017-011415
184. Crocker T, Carr SMD. Clay Work and Body Image in Art Therapy: Using Metaphor and Symbolism to Heal. New York, NY: Routledge (2021).
185. Rao N, Perdomo S, Jonassaint CA. Novel method for digital pain assessment using abstract animations: human-centered design approach. JMIR Hum Factors. (2022) 9:e27689. doi: 10.2196/27689
186. Jonassaint CR, Rao N, Sciuto A, Switzer GE, De Castro L, Kato GJ, et al. Abstract animations for the communication and assessment of pain in adults: cross-sectional feasibility study. J Med Internet Res. (2018) 20:e10056. doi: 10.2196/10056
187. Graver D. Violent theatricality: displayed enactments of aggression and pain. Theatre J. (1995) 47:43. doi: 10.2307/3208805
188. McGarry LM, Russo FA. Mirroring in dance/movement therapy: potential mechanisms behind empathy enhancement. Arts Psychother. (2011) 38:178–84. doi: 10.1016/j.aip.2011.04.005
189. Toye F, Jenkins S. ‘It makes you think' – exploring the impact of qualitative films on pain clinicians. Br J Pain. (2015) 9:65–9. doi: 10.1177/2049463714549776
190. Hartblay C. Disability expertise: claiming disability anthropology. Curr Anthropol. (2020) 61:S26–36. doi: 10.1086/705781
191. Kennedy I. Patients are experts in their own field. BMJ. (2003) 326:1276–7. doi: 10.1136/bmj.326.7402.1276
192. Agyei EO, Dika MD, Donkor K, Amoanyi R. Usage of clay in depicting facial expressions. IDA: Int Des Art J. (2021) 3:174–83.
193. Evans HD. Uncovering: making disability identity legible. DSQ. (2017) 37. doi: 10.18061/dsq.v37i1.5556
194. Prince MJ. Persons with invisible disabilities and workplace accommodation: findings from a scoping literature review. JVR. (2017) 46:75–86. doi: 10.3233/JVR-160844
195. De Ruddere L, Craig KD. Understanding stigma and chronic pain: a-state-of-the-art review. Pain. (2016) 157:1607–10. doi: 10.1097/j.pain.0000000000000512
196. Carr DB. Patients with pain need less stigma, not more. Pain Med. (2016) 17:1391–3. doi: 10.1093/pm/pnw158
197. Tuck NL, Johnson MH, Bean DJ. You'd better believe it: the conceptual and practical challenges of assessing malingering in patients with chronic pain. J Pain. (2019) 20:133–45. doi: 10.1016/j.jpain.2018.07.002
198. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. (2012) 13:715–24. doi: 10.1016/j.jpain.2012.03.009
199. Serbic D, Pincus T. The relationship between pain, disability, guilt and acceptance in low back pain: a mediation analysis. J Behav Med. (2017) 40:651–8. doi: 10.1007/s10865-017-9826-2
200. Serbic D, Evangeli M, Probyn K, Pincus T. Health-related guilt in chronic primary pain: a systematic review of evidence. Br J Health Psychol. (2022) 27:67–95. doi: 10.1111/bjhp.12529
201. Lam E, Yee C, Wong G, Popovic M, Drost L, Pon K, et al. A systematic review and meta-analysis of clinician-reported versus patient-reported outcomes of radiation dermatitis. Breast. (2020) 50:125–34. doi: 10.1016/j.breast.2019.09.009
202. Wolfensberger A, Vuistiner P, Konzelmann M, Plomb-Holmes C, Léger B, Luthi F. Clinician and patient-reported outcomes are associated with psychological factors in patients with chronic shoulder pain. Clin Orthopaed Relat Res. (2016) 474:2030–9. doi: 10.1007/s11999-016-4894-0
203. Sharp TJ, Nicholas MK. Assessing the significant others of chronic pain patients: the psychometric properties of significant other questionnaires. Pain. (2000) 88:135–44. doi: 10.1016/S0304-3959(00)00312-2
204. Swanson DW, Maruta T. The family's viewpoint of chronic pain. Pain. (1980) 8:163–6. doi: 10.1016/0304-3959(88)90003-6
205. Block AR, Boyer SL. The spouse's adjustment to chronic pain: cognitive and emotional factors. Soc Sci Med. (1984) 19:1313–7. doi: 10.1016/0277-9536(84)90018-2
206. Aung MSH, Alquaddoomi F, Hsieh CK, Rabbi M, Yang L, Pollak JP, et al. Leveraging multi-modal sensing for mobile health: a case review in chronic pain. IEEE J Sel Top Signal Process. (2016) 10:962–74. doi: 10.1109/JSTSP.2016.2565381
207. Kelly JT, Campbell KL, Gong E, Scuffham P. The internet of things: impact and implications for health care delivery. J Med Internet Res. (2020) 22:e20135. doi: 10.2196/20135
208. Farahani B, Firouzi F, Chakrabarty K. Healthcare IoT. In: Firouzi F, Chakrabarty K, Nassif S, editors. Intelligent Internet of Things. Cham: Springer International Publishing (2020). p. 515–45. Available online at: http://link.springer.com/10.1007/978-3-030-30367-9_11 (accessed March 9, 2022).
209. Byrom B, McCarthy M, Schueler P, Muehlhausen W. Brain monitoring devices in neuroscience clinical research: the potential of remote monitoring using sensors, wearables, and mobile devices. Clin Pharmacol Ther. (2018) 104:59–71. doi: 10.1002/cpt.1077
210. Fritz RL, Wilson M, Dermody G, Schmitter-Edgecombe M, Cook DJ. Automated smart home assessment to support pain management: multiple methods analysis. J Med Internet Res. (2020) 22:e23943. doi: 10.2196/23943
211. Naranjo-Hernández D, Reina-Tosina J, Roa LM. Sensor technologies to manage the physiological traits of chronic pain: a review. Sensors. (2020) 20:365. doi: 10.3390/s20020365
212. Argüello Prada EJ. The Internet of Things (IoT) in pain assessment and management: an overview. Inform Med Unlocked. (2020) 18:100298. doi: 10.1016/j.imu.2020.100298
213. Kraft R, Schlee W, Stach M, Reichert M, Langguth B, Baumeister H, et al. Combining mobile crowdsensing and ecological momentary assessments in the healthcare domain. Front Neurosci. (2020) 14:164. doi: 10.3389/fnins.2020.00164
214. Appelhans BM, Luecken LJ. Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol Psychol. (2008) 77:174–82. doi: 10.1016/j.biopsycho.2007.10.004
215. Garland EL. Pain processing in the human nervous system. Prim Care Clin Off Pract. (2012) 39:561–71. doi: 10.1016/j.pop.2012.06.013
216. Karri J, Zhang L, Li S, Chen YT, Stampas A, Li S. Heart rate variability: a novel modality for diagnosing neuropathic pain after spinal cord injury. Front Physiol. (2017) 8:495. doi: 10.3389/fphys.2017.00495
217. Berry ME, Chapple IT, Ginsberg JP, Gleichauf KJ, Meyer JA, Nagpal ML. Non-pharmacological intervention for chronic pain in veterans: a pilot study of heart rate variability biofeedback. Glob Adv Health Med. (2014) 3:28–33. doi: 10.7453/gahmj.2013.075
218. Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. (2016) 157:7–29. doi: 10.1097/j.pain.0000000000000360
219. Chen J, Abbod M, Shieh JS. Pain and stress detection using wearable sensors and devices—a review. Sensors. (2021) 21:1030. doi: 10.3390/s21041030
220. Grant S, Whitehouse MR, Blom AW, Judge A, Craddock I, Gooberman-Hill R. Using home sensing technology to assess outcome and recovery after joint replacement – findings from the hip and knee study of a sensor platform of healthcare in a residential environment. Osteoarthr Cartil. (2018) 26:S342–3. doi: 10.1016/j.joca.2018.02.681
221. Kheirkhahan M, Nair S, Davoudi A, Rashidi P, Wanigatunga AA, Corbett DB, et al. A smartwatch-based framework for real-time and online assessment and mobility monitoring. J Biomed Inform. (2019) 89:29–40. doi: 10.1016/j.jbi.2018.11.003
222. Bruser C, Kortelainen JM, Winter S, Tenhunen M, Parkka J, Leonhardt S. Improvement of force-sensor-based heart rate estimation using multichannel data fusion. IEEE J Biomed Health Inform. (2015) 19:227–35. doi: 10.1109/JBHI.2014.2311582
223. Bai J, Di C, Xiao L, Evenson KR, LaCroix AZ, Crainiceanu CM, et al. An activity index for raw accelerometry data and its comparison with other activity metrics. PLoS ONE. (2016) 11:e0160644. doi: 10.1371/journal.pone.0160644
224. Porciuncula F, Roto AV, Kumar D, Davis I, Roy S, Walsh CJ, et al. Wearable movement sensors for rehabilitation: a focused review of technological and clinical advances. PMR. (2018) 10(Suppl. 2):S220–32. doi: 10.1016/j.pmrj.2018.06.013
225. Patterson JT, Wu HH, Chung CC, Bendich I, Barry JJ, Bini SA. Wearable activity sensors and early pain after total joint arthroplasty. Arthroplasty Today. (2020) 6:68–70. doi: 10.1016/j.artd.2019.12.006
226. Li Z, Brown M, Wu J, Song C, Lin F, Langan J, et al. Development and evaluation of a multimodal sensor motor learning assessment. In: 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN). Las Vegas, NV: IEEE;(2018). p. 185–8. Available online at: http://ieeexplore.ieee.org/document/8329689/ (accessed March 6, 2022).
227. Qiu S, Wang H, Li J, Zhao H, Wang Z, Wang J, et al. Towards wearable-inertial-sensor-based gait posture evaluation for subjects with unbalanced gaits. Sensors. (2020) 20:1193. doi: 10.3390/s20041193
228. Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. (1996) 64:231–40. doi: 10.1016/0304-3959(95)00115-8
229. Stone E, Skubic M. Passive, in-home gait measurement using an inexpensive depth camera: initial results. In: Proceedings of the 6th International Conference on Pervasive Computing Technologies for Healthcare. San Diego, CA: IEEE (2012). Available online at: http://eudl.eu/doi/10.4108/icst.pervasivehealth.2012.248731 (accessed March 12, 2022).
230. Campbell G, Skubic M. Balance and gait impairment: sensor-based assessment for patients with peripheral neuropathy. CJON. (2018) 22:316–25. doi: 10.1188/18.CJON.316-325
231. Matuska S, Paralic M, Hudec RA. Smart system for sitting posture detection based on force sensors and mobile application. Mobile Inform Syst. (2020) 2020:1–13. doi: 10.1155/2020/6625797
232. Ramalingam M, Puviarasi R, Shern QC, Chinnavan E. Designing IoT based posture monitoring system. In: 2021 6th International Conference on Inventive Computation Technologies (ICICT). Coimbatore: IEEE (2021). p. 209–14. Available online at: https://ieeexplore.ieee.org/document/9358527/ (accessed March 6, 2022).
233. Patel S, Park H, Bonato P, Chan L, Rodgers M, A. review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. (2012) 9:21. doi: 10.1186/1743-0003-9-21
234. Tang NKY, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. (2012) 35:675-87A. doi: 10.5665/sleep.1830
235. Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain. Chest. (2018) 154:1249–59. doi: 10.1016/j.chest.2018.07.019
236. Robertson JA, Purple RJ, Cole P, Zaiwalla Z, Wulff K, Pattinson KTS. Sleep disturbance in patients taking opioid medication for chronic back pain. Anaesthesia. (2016) 71:1296–307. doi: 10.1111/anae.13601
237. Sathyanarayana S, Satzoda RK, Sathyanarayana S, Thambipillai S. Vision-based patient monitoring: a comprehensive review of algorithms and technologies. J Ambient Intell Human Comput. (2018) 9:225–51. doi: 10.1007/s12652-015-0328-1
238. Liu W, Ploderer B, Hoang T. In bed with technology: challenges and opportunities for sleep tracking. In: Proceedings of the Annual Meeting of the Australian Special Interest Group for Computer Human Interaction. Parkville, VIC: ACM (2015). p. 142–51. Available online at: https://dl.acm.org/doi/10.1145/2838739.2838742 (accessed March 12, 2022).
239. Waltisberg D, Arnrich B, Tröster G. Sleep quality monitoring with the smart bed. In: Holzinger A, Ziefle M, Röcker C, editors. Pervasive Health. Human–Computer Interaction Series. London: Springer London (2014). p. 211–27. Available online at: http://link.springer.com/10.1007/978-1-4471-6413-5_9 (accessed March 6, 2022).
240. Kwasnicki RM, Cross GWV, Geoghegan L, Zhang Z, Reilly P, Darzi A, et al. A lightweight sensing platform for monitoring sleep quality and posture: a simulated validation study. Eur J Med Res. (2018) 23:28. doi: 10.1186/s40001-018-0326-9
241. Guerrero-Mora G, Elvia P, Bianchi AM, Kortelainen J, Tenhunen M, Himanen SL, et al. Sleep-wake detection based on respiratory signal acquired through a Pressure Bed Sensor. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. San Diego, CA: IEEE (2012). p. 3452–5. Available online at: https://ieeexplore.ieee.org/document/6346708/ (accessed March 30, 2021).
242. Kortelainen JM, Mendez MO, Bianchi AM, Matteucci M, Cerutti S. Sleep staging based on signals acquired through bed sensor. IEEE Trans Inform Technol Biomed. (2010) 14:776–85. doi: 10.1109/TITB.2010.2044797
243. Lin CH, Lin CH, Chen LC, Wei SH, Sung WH, Lu LH, et al. Chronic low back pain is associated with impaired bed turning ability: evaluation by a mobility detection system. Clinical Biomechanics. (2022) 92:105572. doi: 10.1016/j.clinbiomech.2022.105572
244. Ong AA, Gillespie MB. Overview of smartphone applications for sleep analysis. World J Otorhinolaryngol Head Neck Surg. (2016) 2:45–9. doi: 10.1016/j.wjorl.2016.02.001
245. Prkachin KM. The consistency of facial expressions of pain: a comparison across modalities. Pain. (1992) 51:297–306. doi: 10.1016/0304-3959(92)90213-U
246. Prkachin KM. Assessing pain by facial expression: facial expression as nexus. Pain Res Manag. (2009) 14:53–8. doi: 10.1155/2009/542964
247. Khan R, Meyer A, Konik H, Bouakaz S. Pain detection through shape and appearance features. In: 2021 IEEE International Conference on Multimedia and Expo (ICME). (2013). p. 1–6.
248. Werner P, Al-Hamadi A, Niese R, Walter S, Gruss S, Traue H. Towards pain monitoring: facial expression, head pose, a new database, an automatic system and remaining challenges. In: Proceedings of the British Machine Vision Conference. Bristol (2013).
249. Werner P, Al-Hamadi A, Walter S. Analysis of facial expressiveness during experimentally induced heat pain. In: 2017 Seventh International Conference on Affective Computing and Intelligent Interaction Workshops and Demos (ACIIW). San Antonio, TX: IEEE (2017). p. 176–80. Available online at: http://ieeexplore.ieee.org/document/8272610/ (accessed March 6, 2022).
250. Arif-Rahu M, Grap MJ. Facial expression and pain in the critically ill non-communicative patient: state of science review. Intensive Crit Care Nurs. (2010) 26:343–52. doi: 10.1016/j.iccn.2010.08.007
251. Sikka K, Dhall A, Bartlett MS. Classification and weakly supervised pain localization using multiple segment representation. Image Vis Comput. (2014) 32:659–70. doi: 10.1016/j.imavis.2014.02.008
252. Hammal Z, Cohn JF. Automatic detection of pain intensity. In: Proceedings of the 14th ACM International Conference on Multimodal interaction - ICMI '12. Santa Monica, CA: ACM Press (2012). p. 47. Available online at: http://dl.acm.org/citation.cfm?doid=2388676.2388688 (accessed March 12, 2022).
253. Lautenbacher S, Hassan T, Seuss D, Loy FW, Garbas JU, Schmid U, et al. Automatic coding of facial expressions of pain: are we there yet? Pain Res Manag. (2022) 2022:1–8. doi: 10.1155/2022/6635496
254. Lucey P, Cohn J, Lucey S, Matthews I, Sridharan S, Prkachin KM. Automatically detecting pain using facial actions. In: 2009 3rd International Conference on Affective Computing and Intelligent Interaction and Workshops. Amsterdam: IEEE (2009). p. 1–8. Available online at: http://ieeexplore.ieee.org/document/5349321/ (accessed March 8, 2022).
255. Ghosh A, Umer S, Khan MK, Rout RK, Dhara BC. Smart sentiment analysis system for pain detection using cutting edge techniques in a smart healthcare framework. Cluster Comput. (2022) 1–17. doi: 10.1007/s10586-022-03552-z
256. Thiam P, Kessler V, Walter S, Palm G, Schwenker F. Audio-visual recognition of pain intensity. In: Schwenker F, Scherer S, editors. Multimodal Pattern Recognition of Social Signals in Human-Computer-Interaction. Lecture Notes in Computer Science; vol. 10183. Cham: Springer International Publishing (2017). p. 110–26. Available online at: http://link.springer.com/10.1007/978-3-319-59259-6_10 (accessed February 27, 2022).
257. Ren Z, Cummins N, Han J, Schnieder S, Krajewski J, Schuller B. Speech Communication: 13. ITG-FachtagungSprachkommunikation; 10-12. Oktober 2018 in Oldenburg. Berlin: VDE Verlag GmbH (2018). p. 1.
258. Manfredi PL, Breuer B, Meier DE, Libow L. Pain assessment in elderly patients with severe dementia. J Pain Symptom Manage. (2003) 25:48–52. doi: 10.1016/S0885-3924(02)00530-4
259. Horgas A, Miller L. Pain assessment in people with dementia. AJN Am J Nurs. (2008) 108:62–70. doi: 10.1097/01.NAJ.0000325648.01797.fc
260. Riganello F, Chatelle C, Schnakers C, Laureys S. Heart rate variability as an indicator of nociceptive pain in disorders of consciousness? J Pain Symptom Manage. (2019) 57:47–56. doi: 10.1016/j.jpainsymman.2018.09.016
261. Nwosu AC, Quinn C, Samuels J, Mason S, Payne TR. Wearable smartwatch technology to monitor symptoms in advanced illness. BMJ Support Palliat Care. (2018) 8:237. doi: 10.1136/bmjspcare-2017-001445
262. Tsetsou S, Novy J, Oddo M, Rossetti AO. EEG reactivity to pain in comatose patients: importance of the stimulus type. Resuscitation. (2015) 97:34–7. doi: 10.1016/j.resuscitation.2015.09.380
263. Pu L, Lion KM, Todorovic M, Moyle W. Portable EEG monitoring for older adults with dementia and chronic pain - a feasibility study. Geriatr Nurs. (2021) 42:124–8. doi: 10.1016/j.gerinurse.2020.12.008
264. Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain. (2007) 133:221–8. doi: 10.1016/j.pain.2007.09.007
265. Defrin R, Benromano T, Pick CG. Specific behavioral responses rather than autonomic responses can indicate and quantify acute pain among individuals with intellectual and developmental disabilities. Brain Sci. (2021) 11:253. doi: 10.3390/brainsci11020253
266. Oster H. Automated facial analysis of infant pain expressions: progress and future directions. Lancet Digital Health. (2021) 3:e613–4. doi: 10.1016/S2589-7500(21)00207-7
267. Lu Y, Mahmoud M, Robinson P. Estimating sheep pain level using facial action unit detection. In: 2017 12th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2017). Washington, DC: IEEE (2017). p. 394–9. Available online at: http://ieeexplore.ieee.org/document/7961768/ (accessed March 13, 2022).
268. Dalla Costa E, Pascuzzo R, Leach MC Dai F, Lebelt D, Vantini S, et al. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS ONE. (2018) 13:e0200339. doi: 10.1371/journal.pone.0200339
269. Mota-Rojas D, Marcet-Rius M, Ogi A, Hernández-Ávalos I, Mariti C, Martínez-Burnes J, et al. Current advances in assessment of dog's emotions, facial expressions, and their use for clinical recognition of pain. Animals. (2021) 11:3334. doi: 10.3390/ani11113334
270. Finka LR, Luna SP, Brondani JT, Tzimiropoulos Y, McDonagh J, Farnworth MJ, et al. Geometric morphometrics for the study of facial expressions in non-human animals, using the domestic cat as an exemplar. Sci Rep. (2019) 9:9883. doi: 10.1038/s41598-019-46330-5
271. Carrier B, Barrios B, Jolley BD, Navalta JW. Validity and reliability of physiological data in applied settings measured by wearable technology: a rapid systematic review. Technologies. (2020) 8:70. doi: 10.3390/technologies8040070
272. Goldsack JC, Coravos A, Bakker JP, Bent B, Dowling AV, Fitzer-Attas C, et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit Med. (2020) 3:55. doi: 10.1038/s41746-020-0260-4
273. Patel V, Orchanian-Cheff A, Wu R. Evaluating the validity and utility of wearable technology for continuously monitoring patients in a hospital setting: systematic review. JMIR Mhealth Uhealth. (2021) 9:e17411. doi: 10.2196/17411
274. Bhat S, Ferraris A, Gupta D, Mozafarian M, DeBari VA, Gushway-Henry N, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med. (2015) 11:709–15. doi: 10.5664/jcsm.4840
276. Mbunge E, Muchemwa B, Jiyane S, Batani J. Sensors and healthcare 50: transformative shift in virtual care through emerging digital health technologies. Glob Health J. (2021) 5:169–77. doi: 10.1016/j.glohj.2021.11.008
277. Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. (2020) 31:283–6. doi: 10.1097/DER.0000000000000575
278. Etienne A, Laroia T, Weigle H, Afelin A, Kelly SK, Krishnan A, et al. Novel electrodes for reliable EEG recordings on coarse and curly hair. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). Montreal, QC: IEEE (2020). p. 6151–4. Available online at: https://ieeexplore.ieee.org/document/9176067/ (accessed March 12, 2022).
279. Brown C, Wang Y, Carr E. Undercover dogs: pet dogs in the sleep environment of patients with chronic pain. Soc Sci. (2018) 7:157. doi: 10.3390/socsci7090157
280. Yue S, He H, Wang H, Rahul H, Katabi D. Extracting multi-person respiration from entangled RF signals. Proc ACM Interact Mob Wearable Ubiquitous Technol. (2018) 2:1–22. doi: 10.1145/3214289
281. Duong L, Andargie M, Chen J, Giakoumidis N, Eid M. Aegis: a biofeedback adaptive alarm system using vibrotactile feedback. In: 2014 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings. Montevideo: IEEE (2014). p. 293–8. Available online at: http://ieeexplore.ieee.org/document/6860755/ (accessed March 12, 2022).
282. Scott M. Relief of nocturnal intractable low back and sciatic pain by “chair sleep.” JAMA. (1966) 196:738. doi: 10.1001/jama.1966.03100210108037
283. Wannheden C, Stenfors T, Stenling A, von Thiele Schwarz U. Satisfied or frustrated? A qualitative analysis of need satisfying and need frustrating experiences of engaging with digital health technology in chronic care. Front Public Health. (2021) 8:623773. doi: 10.3389/fpubh.2020.623773
284. Urban M. ‘This really takes it out of you!' The senses and emotions in digital health practices of the elderly. Digital Health. (2017) 3:205520761770177. doi: 10.1177/2055207617701778
285. Beukenhorst AL, Howells K, Cook L, McBeth J, O'Neill TW, Parkes MJ, et al. Engagement and participant experiences with consumer smartwatches for health research: longitudinal, observational feasibility study. JMIR Mhealth Uhealth. (2020) 8:e14368. doi: 10.2196/14368
286. Brick TR, Mundie J, Weaver J, Fraleigh R, Oravecz Z. Low-burden mobile monitoring, intervention, and real-time analysis using the wear-IT framework: example and usability study. JMIR Form Res. (2020) 4:e16072. doi: 10.2196/16072
287. Voss A, Schroeder R, Heitmann A, Peters A, Perz S. Short-term heart rate variability—influence of gender and age in healthy subjects. PLoS ONE. (2015) 10:e0118308. doi: 10.1371/journal.pone.0118308
288. Antelmi I, De Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. (2004) 93:381–5. doi: 10.1016/j.amjcard.2003.09.065
289. Colvonen PJ, DeYoung PN, Bosompra NOA, Owens RL. Limiting racial disparities and bias for wearable devices in health science research. Sleep. (2020) 43:zsaa159. doi: 10.1093/sleep/zsaa159
290. Shcherbina A, Mattsson C, Waggott D, Salisbury H, Christle J, Hastie T, et al. Accuracy in Wrist-Worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. JPM. (2017) 7:3. doi: 10.3390/jpm7020003
291. Sulaiman M, Bajar N, Yaacob S, Yahya F. The application of facial expression recognitionin reducing inaccuracy in pain scale intensity identification. Open Int J Inform. (2021) 9:29–38. Retrieved from: https://oiji.utm.my/index.php/oiji/article/view/157
292. Green B. Data science as political action: grounding data science in a politics of justice. J Soc Comput. (2021) 2:249–65. doi: 10.23919/JSC.2021.0029
293. Kaltwang S, Rudovic O, Pantic M. Continuous pain intensity estimation from facial expressions. In: Bebis G, Boyle R, Parvin B, Koracin D, Fowlkes C, Wang S, et al., editors. Advances in Visual Computing. Hutchison D, Kanade T, Kittler J, Kleinberg JM, Mattern F, Mitchell JC, et al., editors. Lecture Notes in Computer Science; vol. 7432. Berlin; Heidelberg: Springer Berlin Heidelberg (2012). p. 368–77. Available online at: http://link.springer.com/10.1007/978-3-642-33191-6_36 (accessed March 13, 2022).
294. Hong JW, Song KT. Facial expression recognition under illumination variation. In: 2007 IEEE Workshop on Advanced Robotics and Its Social Impacts. Hsinchu: IEEE (2007). p. 1–6. Available online at: https://ieeexplore.ieee.org/document/4531421/ (accessed March 13, 2022).
295. Martinez B, Valstar MF. Advances, challenges, and opportunities in automatic facial expression recognition. In: Kawulok M. Celebi ME, Smolka B, editors. Advances in Face Detection and Facial Image Analysis. Cham: Springer International Publishing (2016). p. 63–100. Available online at: http://link.springer.com/10.1007/978-3-319-25958-1_4 (accessed March 13, 2022).
296. Lloyd EP, Hugenberg K. Beyond bias: response bias and interpersonal (in)sensitivity as a contributors to race disparities. Eur Rev Soc Psychol. (2021) 32:201–34. doi: 10.1080/10463283.2020.1820699
297. Samulowitz A, Gremyr I, Eriksson E, Hensing G. “Brave Men” and “Emotional Women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag. (2018) 2018:1–14. doi: 10.1155/2018/6358624
298. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. (2013) 28:1504–10. doi: 10.1007/s11606-013-2441-1
299. Schäfer G, Prkachin KM, Kaseweter KA, Williams AC de C. Health care providers' judgments in chronic pain: the influence of gender and trustworthiness. Pain. (2016) 157:1618–25. doi: 10.1097/j.pain.0000000000000536
300. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci USA. (2016) 113:4296–301. doi: 10.1073/pnas.1516047113
301. Lee P, Le Saux M, Siegel R, Goyal M, Chen C, Ma Y, et al. Racial and ethnic disparities in the management of acute pain in US emergency departments: meta-analysis and systematic review. Am J Emerg Med. (2019) 37:1770–7. doi: 10.1016/j.ajem.2019.06.014
302. Bougie O, Yap Ma I, Sikora L, Flaxman T, Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG Int J Obstet Gy. (2019) 126:1104–15. doi: 10.1111/1471-0528.15692
303. King NB, Fraser V. Untreated pain, narcotics regulation, and global health ideologies. PLoS Med. (2013) 10:e1001411. doi: 10.1371/journal.pmed.1001411
304. Fabbrini F, Celeste E. The right to be forgotten in the digital age: the challenges of data protection beyond borders. German Law J. (2020) 21:55–65. doi: 10.1017/glj.2020.14
305. Gasser U, Ienca M, Scheibner J, Sleigh J, Vayena E. Digital tools against COVID-19: taxonomy, ethical challenges, and navigation aid. Lancet Digital Health. (2020) 2:e425–34. doi: 10.1016/S2589-7500(20)30137-0
306. Townsend D, Knoefel F, Goubran R. Privacy versus autonomy: a tradeoff model for smart home monitoring technologies. Annu Int Conf IEEE Eng Med Biol Soc. (2011) 2011:4749–52. doi: 10.1109/IEMBS.2011.6091176
307. Sun N, Esom K, Dhaliwal M, Amon JJ. Human rights and digital health technologies. Health Hum Rights. (2020) 22:21–32.
308. Al Knawy B, McKillop MM, Abduljawad J, Tarkoma S, Adil M, Schaper L, et al. Successfully implementing digital health to ensure future global health security during pandemics: a consensus statement. JAMA Netw Open. (2022) 5:e220214. doi: 10.1001/jamanetworkopen.2022.0214
309. Lupton D. The digitally engaged patient: self-monitoring and self-care in the digital health era. Soc Theory Health. (2013) 11:256–70. doi: 10.1057/sth.2013.10
310. Martinez-Martin N, Luo Z, Kaushal A, Adeli E, Haque A, Kelly SS, et al. Ethical issues in using ambient intelligence in health-care settings. Lancet Digital Health. (2021) 3:e115–23. doi: 10.1016/S2589-7500(20)30275-2
311. Ajana B. Digital health and the biopolitics of the Quantified Self. Digital Health. (2017) 3:205520761668950. doi: 10.1177/2055207616689509
312. Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. (2013) 136:2751–68. doi: 10.1093/brain/awt211
313. Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. (2010) 66:149–60. doi: 10.1016/j.neuron.2010.03.002
314. Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. (2011) 54:2237–49. doi: 10.1016/j.neuroimage.2010.09.084
315. Berger SE, Baria AT, Baliki MN, Mansour A, Herrmann KM, Torbey S, et al. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res Notes. (2014) 7:739. doi: 10.1186/1756-0500-7-739
316. Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. (2016) 139(Pt. 7):1958–70. doi: 10.1093/brain/aww100
317. Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tétreault P, Ghantous M, et al. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res. (2016) 95:605–12. doi: 10.1177/0022034516638027
318. Reckziegel D, Tétreault P, Ghantous M, Wakaizumi K, Petre B, Huang L, et al. Sex-specific pharmacotherapy for back pain: a proof-of-concept randomized trial. Pain Ther. (2021) 10:1375–400. doi: 10.1007/s40122-021-00297-2
319. Vachon-Presseau E, Berger SE, Abdullah TB, Huang L, Cecchi GA, Griffith JW, et al. Brain and psychological determinants of placebo pill response in chronic pain patients. Nat Commun. (2018) 9:3397. doi: 10.1038/s41467-018-05859-1
320. Tétreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol. (2016) 14:e1002570. doi: 10.1371/journal.pbio.1002570
321. May A. Chronic pain may change the structure of the brain. Pain. (2008) 137:7–15. doi: 10.1016/j.pain.2008.02.034
322. Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. (2012) 153:1602–9. doi: 10.1016/j.pain.2012.03.012
323. Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS ONE. (2014) 9:e106133. doi: 10.1371/journal.pone.0106133
324. Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang PC, Huang L, et al. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep. (2016) 6:34853. doi: 10.1038/srep34853
325. Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, et al. Brain white matter structural properties predict transition to chronic pain. Pain. (2013) 154:2160–8. doi: 10.1016/j.pain.2013.06.044
326. Jatoi MA, Kamel N, Malik AS, Faye I, Begum T. A survey of methods used for source localization using EEG signals. Biomed Signal Process Control. (2014) 11:42–52. doi: 10.1016/j.bspc.2014.01.009
327. Logothetis NK. What we can do and what we cannot do with fMRI. Nature. (2008) 453:869–78. doi: 10.1038/nature06976
328. Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. In: Progress in Brain Research. Elsevier (2006). p. 275–95. Available online at: https://linkinghub.elsevier.com/retrieve/pii/S0079612306590193 (accessed March 13, 2022).
329. Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. Neuroimage. (2017) 154:128–49. doi: 10.1016/j.neuroimage.2016.12.018
330. Islam MK, Rastegarnia A, Yang Z. Methods for artifact detection and removal from scalp EEG: a review. Clin Neurophysiol. (2016) 46:287–305. doi: 10.1016/j.neucli.2016.07.002
331. Puce A, Hämäläinen M, A. Review of issues related to data acquisition and analysis in EEG/MEG studies. Brain Sci. (2017) 7:58. doi: 10.3390/brainsci7060058
332. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. (2013) 368:1388–97. doi: 10.1056/NEJMoa1204471
333. Liang M, Su Q, Mouraux A, Iannetti GD. Spatial patterns of brain activity preferentially reflecting transient pain and stimulus intensity. Cerebral Cortex. (2019) 29:2211–27. doi: 10.1093/cercor/bhz026
334. Jabakhanji R, Vigotsky AD, Bielefeld J, Huang L, Baliki MN, Iannetti G, et al. Limits of decoding mental states with fMRI. Cortex. (2022) 149:101–22. doi: 10.1016/j.cortex.2021.12.015
335. Jonas E, Kording KP. Could a neuroscientist understand a microprocessor? PLoS Comput Biol. (2017) 13:e1005268. doi: 10.1371/journal.pcbi.1005268
336. Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. (2006) 27:715–21. doi: 10.1002/hbm.20213
337. Hanna A, Park TM. Against Scale: Provocations Resistances to Scale Thinking. (2020). Available online at: https://arxiv.org/abs/2010.08850 (accessed March 13, 2022).
338. Schultz W. Neuroessentialism: theoretical and clinical considerations. J Human Psychol. (2018) 58:607–39. doi: 10.1177/0022167815617296
339. Dumit J. Picturing Personhood: Brain Scans Biomedical Identity. Princeton University Press (2004). Available online at: https://www.degruyter.com/document (accessed March 13, 2022).
340. Gholami B, Haddad W, Tannenbaum A. Agitation and pain assessment using digital imaging. Annu Int Conf IEEE Eng Med Biol Soc. (2009) 2009:2176–9. doi: 10.1109/IEMBS.2009.5332437
341. Littlewort G, Bartlett M, Lee K. Faces of pain: automated measurement of spontaneousallfacial expressions of genuine and posed pain. In: Proceedings of the Ninth International Conference on Multimodal Interfaces. New York, NY: ACM (2007). p. 15–21.
342. Matsangidou M, Liampas A, Pittara M, Pattichi CS, Zis P. Machine learning in pain medicine: an up-to-date systematic review. Pain Ther. (2021) 10:1067–84. doi: 10.1007/s40122-021-00324-2
343. Jensen EK, Bäckryd E, Hilden J, Werner MU. Trajectories in severe persistent pain after groin hernia repair: a retrospective analysis. Scand J Pain. (2021) 21:70–80. doi: 10.1515/sjpain-2020-0104
344. Lötsch J, Ultsch A. Machine learning in pain research. Pain. (2018) 159:623–30. doi: 10.1097/j.pain.0000000000001118
345. Kim B, Koopmanschap I, Mehrizi MHR, Huysman M, Ranschaert E. How does the radiology community discuss the benefits and limitations of artificial intelligence for their work? A systematic discourse analysis. Eur J Radiol. (2021) 136:109566. doi: 10.1016/j.ejrad.2021.109566
346. Coppola F, Faggioni L, Regge D, Giovagnoni A, Golfieri R, Bibbolino C, et al. Artificial intelligence: radiologists' expectations and opinions gleaned from a nationwide online survey. Radiol Med. (2021) 126:63–71. doi: 10.1007/s11547-020-01205-y
347. Huisman M, Ranschaert E, Parker W, Mastrodicasa D, Koci M, Pinto de. Santos D, et al. An international survey on AI in radiology in 1,041 radiologists and radiology residents part 1: fear of replacement, knowledge, and attitude. Eur Radiol. (2021) 31:7058–66. doi: 10.1007/s00330-021-07781-5
348. Lum K, Isaac W. To predict and serve? Significance. (2016) 13:14–9. doi: 10.1111/j.1740-9713.2016.00960.x
349. Dodge J, Liao QV, Zhang Y, Bellamy RKE, Dugan C. Explaining models: an empirical study of how explanations impact fairness judgment. In: Proceedings of the 24th International Conference on Intelligent User Interfaces. Marina del Ray, CA: ACM (2019). p. 275–85. Available online at: https://dl.acm.org (accessed March 13, 2022).
350. Chen IY, Pierson E, Rose S, Joshi S, Ferryman K, Ghassemi M. Ethical machine learning in healthcare. Annu Rev Biomed Data Sci. (2021) 4:123–44. doi: 10.1146/annurev-biodatasci-092820-114757
351. Wiens J, Saria S, Sendak M, Ghassemi M, Liu VX, Doshi-Velez F, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med. (2019) 25:1337–40. doi: 10.1038/s41591-019-0548-6
352. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. (2019) 366:447–53. doi: 10.1126/science.aax2342
353. Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. (2003) 118:358–65. doi: 10.1016/S0033-3549(04)50262-5
354. Bajaj SS, Stanford FC. Beyond tuskegee — vaccine distrust and everyday racism. N Engl J Med. (2021) 384:e12. doi: 10.1056/NEJMpv2035827
355. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. (2020) 369:m1328. doi: 10.1136/bmj.m1328
356. Raji ID, Bender EM, Paullada A, Denton E, Hanna A. AI the Everything in the Whole Wide World Benchmark. (2021). Available online at: https://arxiv.org/abs/2111.15366 (accessed March 13, 2022).
357. Paullada A, Raji ID, Bender EM, Denton E, Hanna A. Data and its (dis)contents: a survey of dataset development and use in machine learning research. Patterns. (2021) 2:100336. doi: 10.1016/j.patter.2021.100336
358. Safra L, Chevallier C, Grèzes J, Baumard N. Tracking historical changes in trustworthiness using machine learning analyses of facial cues in paintings. Nat Commun. (2020) 11:4728. doi: 10.1038/s41467-020-18566-7
359. Wang Y, Kosinski M. Deep neural networks are more accurate than humans at detecting sexual orientation from facial images. J Pers Soc Psychol. (2018) 114:246–57. doi: 10.1037/pspa0000098
360. Birhane A, Guest O. Towards decolonising computational sciences. Kvinder Køn Forskning. (2020) 29:60–73. doi: 10.7146/kkf.v29i2.124899
361. Bargshady G, Zhou X, Deo RC, Soar J, Whittaker F, Wang H. Enhanced deep learning algorithm development to detect pain intensity from facial expression images. Expert Syst Appl. (2020) 149:113305. doi: 10.1016/j.eswa.2020.113305
362. El Morabit S, Rivenq A, Zighem ME, Hadid A, Ouahabi A, Taleb-Ahmed A. Automatic pain estimation from facial expressions: a comparative analysis using off-the-shelf CNN architectures. Electronics. (2021) 10:1926. doi: 10.3390/electronics10161926
363. Keefe FJ, Ballantyne J, Blyth F, Coghill RC, Dickenson A, Dionne CE, et al. Publishing the best basic and applied pain science: open science and PAIN. Pain. (2018) 159:405–6. doi: 10.1097/j.pain.0000000000001166
364. Paus T. Population neuroscience: why and how. Hum Brain Mapp. (2010) 31:891–903. doi: 10.1002/hbm.21069
365. Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, et al. What is a representative brain? Neuroscience meets population science. Proc Natl Acad Sci USA. (2013) 110:17615–22. doi: 10.1073/pnas.1310134110
366. LeWinn KZ, Sheridan MA, Keyes KM, Hamilton A, McLaughlin KA. Sample composition alters associations between age and brain structure. Nat Commun. (2017) 8:874. doi: 10.1038/s41467-017-00908-7
367. Charpentier CJ, Faulkner P, Pool ER, Ly V, Tollenaar MS, Kluen LM, et al. How representative are neuroimaging samples? Large-scale evidence for trait anxiety differences between fMRI and behaviour-only research participants. Soc Cogn Affect Neurosci. (2021) 16:1057–70. doi: 10.1093/scan/nsab057
368. Posada–Quintero HF, Kong Y, Chon KH. Objective pain stimulation intensity and pain sensation assessment using machine learning classification and regression based on electrodermal activity. Am J Physiol Regul Integr Compar Physiol. (2021) 321:R186–96. doi: 10.1152/ajpregu.00094.2021
369. Li D, Puntillo K, Miaskowski C, A. Review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. (2008) 9:2–10. doi: 10.1016/j.jpain.2007.08.009
370. Xu X, Huang Y. Objective pain assessment: a key for the management of chronic pain. F1000Res. (2020) 9:35. doi: 10.12688/f1000research.20441.1
371. Wagemakers SH, van der Velden JM, Gerlich AS, Hindriks-Keegstra AW, van Dijk JFM, Verhoeff JJC, et al. Systematic review of devices and techniques that objectively measure patients' pain. Pain Physician. (2019) 22:1–13. doi: 10.36076/ppj/2019.22.1
373. Bird F. A defense of objectivity in the social sciences, rightly understood. Sustainabil Sci Pract Policy. (2020) 16:83–98. doi: 10.1080/15487733.2020.1785679
374. Morales J, Bax A, Firestone C. Sustained representation of perspectival shape. Proc Natl Acad Sci USA. (2020) 117:14873–82. doi: 10.1073/pnas.2000715117
375. John S. Objectivity in Science. Cambridge Elements. Elements in the Philosophy of Science. Cambridge; New York, NY: Cambridge University Press (2021). p. 66.
376. Birhane A. The impossibility of automating ambiguity. Artif Life. (2021) 27:44–61. doi: 10.1162/artl_a_00336
377. Schulz MA, Bzdok D, Haufe S, Haynes JD, Ritter K. Performance reserves in brain-imaging-based phenotype prediction. Neuroscience. (2022) doi: 10.1101/2022.02.23.481601
378. MacCoun R, Perlmutter S. Blind analysis: hide results to seek the truth. Nature. (2015) 526:187–9. doi: 10.1038/526187a
379. Nuzzo R. How scientists fool themselves – and how they can stop. Nature. (2015) 526:182–5. doi: 10.1038/526182a
380. Wolfson D, Lynch TJ. Increasing trust in health care. Am J Manag Care. (2021) 27:520–2. doi: 10.37765/ajmc.2021.88790
381. Lee H, Lamb SE, Bagg MK, Toomey E, Cashin AG, Moseley GL. Reproducible and replicable pain research: a critical review. Pain. (2018) 159:1683–9. doi: 10.1097/j.pain.0000000000001254
382. Letzen JE, Mathur VA, Janevic MR, Burton MD, Hood AM, Morais CA, et al. Confronting racism in pain research: reframing study designs. J Pain. (2022). doi: 10.1016/j.jpain.2022.01.010. [Epub ahead of print].
383. Nosek BA, Beck ED, Campbell L, Flake JK, Hardwicke TE, Mellor DT, et al. Preregistration is hard, and worthwhile. Trends Cogn Sci. (2019) 23:815–8. doi: 10.1016/j.tics.2019.07.009
384. Baker TA, Wang CC. Photovoice: use of a participatory action research method to explore the chronic pain experience in older adults. Qual Health Res. (2006) 16:1405–13. doi: 10.1177/1049732306294118
385. Padfield D, Zakrzewska JM. Face2face: sharing the photograph within medical pain encounters—a means of democratisation. In: Gonzalez-Polledo E, Tarr J, editors. Painscapes. London: Palgrave Macmillan UK (2018). p. 205–28. Available online at: http://link.springer.com/10.1057/978-1-349-95272-4_10 (accessed March 12, 2022).
386. Padfield D, Wickenden M. Being in pain: using images and participatory methods to explore intercultural understanding of pain. In: Kumar M, Welikala T, editors. Teaching and Learning in Higher Education: The Context of Being, Interculturality and New Knowledge Systems. Emerald Publishing Limited (2021). p. 145–58. Available online at: https://www.emerald.com/insight/content (accessed March 8, 2022).
387. Latimer M, Sylliboy JR, Francis J, Amey S, Rudderham S, Finley GA, et al. Co-creating better healthcare experiences for First Nations children and youth: the FIRST approach emerges from Two-Eyed seeing. Paediatr Neo Pain. (2020) 2:104–12. doi: 10.1002/pne2.12024
Keywords: pain, ecological momentary assessment, language, visual reports, Internet of Things, neuroimaging, machine learning, multidisciplinary
Citation: Berger SE and Baria AT (2022) Assessing Pain Research: A Narrative Review of Emerging Pain Methods, Their Technosocial Implications, and Opportunities for Multidisciplinary Approaches. Front. Pain Res. 3:896276. doi: 10.3389/fpain.2022.896276
Received: 14 March 2022; Accepted: 12 May 2022;
Published: 02 June 2022.
Edited by:
Zhiguo Zhang, Shenzhen University, ChinaReviewed by:
Ming Zhang, Chinese Academy of Sciences (CAS), ChinaLili Zhou, Shanghai University of Sport, China
Xuejing Lu, Institute of Psychology (CAS), China
Copyright © 2022 Berger and Baria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara E. Berger, c2FyYS5lLmJlcmdlckBpYm0uY29t
 Sara E. Berger
Sara E. Berger Alexis T. Baria
Alexis T. Baria