- 1Department of Emergency Medicine, Augusta University, Augusta, GA, United States
- 2Harmonic Scientific LLC (parent company MMJ Labs LLC), Lewes, DE, United States
- 3Kaizo Clinical Research Institute, Landover, MD, United States
- 4Emory University, Atlanta, GA, United States
- 5Department of Health Policy and Administration, Penn State College, University Park, PA, United States
- 6Department of Psychology, Georgia State University, Atlanta, GA, United States
- 7Department of Statistics and Analytical Sciences, Kennesaw State University, Kennesaw, GA, United States
Background: Low back pain (LBP) is the leading cause of disability worldwide. Up to half of moderate-to-severe acute LBP (aLBP) progress to chronic (cLBP), with neuromotor, fascial, and muscle pathology contributing to inoperable mechanical disability. A novel thermomechanical stimulation (M-Stim) device delivering stochastic and targeted vibration frequencies relieved LBP in a pilot. Efficacy versus an active control, for cLBP prevention, or reversing disability was undetermined.
Methods: As part of a National Institutes of Health (NIH) double-blind, randomized controlled trial, 159 chiropractic patients with non-radiating moderate-to-severe LBP [Numeric Rating Scale (NRS) ≥4] were randomized to add either the multimodal M-Stim device or 4-lead transcutaneous electrical nerve stimulation (TENS) for 30 minutes daily to other therapies. Between June 2022 and July 2024, pain scores, analgesic use, and device adherence were recorded for 28 days, with weekly follow-up up to 6 months. Primary outcomes included PROMIS Pain Interference scores, NRS pain scores, and transition from aLBP to cLBP (Pain Interference ≥55 at 3 months). Exploratory analyses examined higher-severity subgroups, including those meeting NIH Research Task Force (RTF) criteria, obesity, longer pain duration, and an integrated analysis with common criteria for intractable inoperable mechanical cLBP.
Results: For 44 aLBP and 115 cLBP participants [mean age 42.6, 54% female, BMI 30.9 (SD 6.19), NRS 5.51 (SD 2.15)], M-Stim was noninferior to TENS for initial and 10-day relief. Over time, Linear Mixed Models (intention-to-treat) showed M-Stim significantly improved pain and disability for both aLBP and cLBP, (p < .001 to p = .024). With higher severity, 23.9% (11/46) M-Stim users reached “no disability” (PROMIS = 40.7) vs. 7.1% (2/28) TENS users [RR 0.81 (95% CI 0.66–0.99), p = 0.04]. M-Stim yielded significantly greater improvement than TENS in those with pain ≥5 years, BMI ≥30, or mechanical cLBP (all p < .05). Significantly fewer aLBP M-Stim users transitioned to cLBP at 3 months [31.8% vs. 72.7%, RR 0.44 (95% CI 0.23–0.85), NNT = 2.4, p = 0.015].
Conclusions: A multimodal M-Stim device reduced progression to cLBP significantly more than TENS. Both devices reduced pain initially, but M-Stim reduced pain and disability significantly more over time, particularly in cLBP subsets with higher severity, duration, or BMI.
Clinical Trial Registration: https://clinicaltrials.gov/study/NCT04494698, identifier NCT04494698.
Introduction
Low back pain (LBP) is the most disabling condition worldwide, responsible for over 70 million years lived with disability annually (1). Up to 80% of adults will experience acute LPB (aLBP) in their lifetimes, with up to 50% of moderate-to-severe aLBP becoming chronic (cLBP ≥3 m) (2). Opioid use, body mass index (BMI), female sex, and psychological factors are associated with chronicity. Increasingly, paraspinal muscles, postural instability and thoracolumbar fascial derangement are viewed as targets for intervention (3–5). Within days of severe muscular, ligamentous, bony, or nerve injury, compensatory multifidus and erector spinae muscular derangement begins. The injured muscles undergo a pattern of inflammation, reactive hypertrophy, hypoperfusion, and spasm (3, 6). External or pain-mediated immobilization leads to muscular fatty changes and further hypoperfusion within weeks (7, 8), with inflammatory changes in fascia causing pain and instability as pain transitions to chronic (5, 9, 10). Reflex and proprioceptive responses to pain are altered, with this neuromotor hypofunction (11) associated with ongoing functional instability and pain (12, 13). While previously these nonspecific findings in “mechanical” cLBP meant non-treatable (14), interventions preserving or improving muscle and fascia function (15) could potentially reduce acute-to-chronic transition as well as reverse the disability of mechanical cLBP.
Evidence-based guidelines recommend multimodal pain interventions for both acute exacerbations and chronic rehabilitation (16–18). Physical modalities including cold reduce inflammation. Heat reduces spasm and increases perfusion, releasing adhered fascia that painfully restricts movement (19). Exercise and yoga reduce acute inflammatory pain and improve cLBP (20), transcutaneous electrical nerve stimulation (TENS) is well-established to reduce pain intensity for aLBP via central endogenous opioid release (21, 22), and acupuncture and acupressure are well-supported (23).
Two emerging “precision physics” therapies (vibration and implanted electrical stimuli) may restore function for mechanical cLBP. In addition to blocking transmission of pain via the 200 Hz neuromodulatory frequency (24), focal mechanical stimulation (M-Stim) at other frequencies reduces LBP via various hypothesized mechanisms (25–27). Whether through pain inhibition, improving proprioception (28), or restoring neuromotor function (29), the initial common pathway likely involves newly described mechanical force ion channels repairing myofascial contributors to mechanical cLBP (25, 27, 30). Recently, twice daily electrical stimulation (E-Stim) via electrodes implanted in the multifidus muscle reduced mechanical cLBP disability from severe to moderate in 6 months (31), down to mild within 2 years (32). The authors attribute the improvement in part to improved neuromotor control (33), which is also a fast-acting effect of vibration well-described in kinesiotherapy literature (29).
An NIH-funded multimodal heat, pressure, and harmonic multifrequency vibration device (M-Stim) reduced both a/cLBP 57% after 20 minutes in a recent pilot (34). Neither the multifidus E-Stim nor the M-Stim have been tested over time against a control. This prospective, randomized active-controlled trial investigated pain, disability, and chronicity progression in moderate-to-severe LBP subjects using M-Stim or TENS. The objectives were to compare immediate and 10-day Pain Intensity using a numeric rating scale (NRS), and cLBP disability using PROMIS Pain Interference. Outcomes included 3-month resolution of aLBP and 6-month cLBP restoration of normal function (Pain Interference < 55), and exploratory outcomes in more at-risk or severely affected subjects. A neural network identified characteristics associated with M-Stim responders.
Methods
Device description
Mechanical low back pain dysfunction typically affects multiple vertebral lengths of muscle and a larger area of overlying fascia (10, 19). To cover the thoracolumbar field, the multimodal M-Stim device (DuoTherm™, Harmonic Scientific LLC, Lewes, DE) is a wearable 13 × 20 cm thermoconductive metal plate held by a compressive neoprene belt stretching to 150 cm. Harmonic motor frequencies (50 Hz, 100 Hz, and 200 Hz) deliver stochastically varying patterns and beats of mechanical (vibratory) force (34). To direct the impulses, the DuoTherm device incorporates metal shaping to concentrate pressure on the paraspinal muscles while applying varying amplitudes of M-Stim to the lumbar fascia field (Figure 1). The approach was first described by Lundeberg, who found a single 100 Hz or 200 Hz motor on a 6″ × 8″ flat plate reduced low back pain more than TENS, with some vibration subjects experiencing a prolonged pain reduction of days to weeks (35, 36).
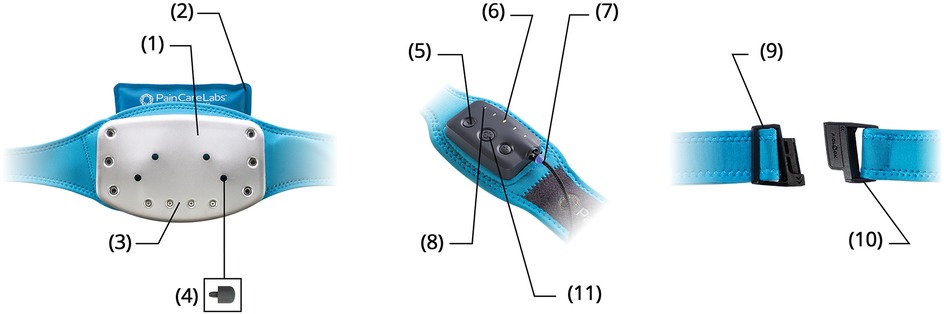
Figure 1. Contoured temperature plate (1). Natural Clay Ice/Heat Pack (2). Multi-Vibration Motor Array (3). Trigger Point Acupressure Nubs (4). Five Intensity Settings (5). LED Cycle & Intensity Display (6). Magnetic Charging Cable (7). Eight Therapy Cycles (8). Custom Fit Waistband (9). Slide-N-Lock Magnetic Buckle (10). Haptic Touch Panel (11).
Eight patterns or “therapy cycles” couple temporary nociceptor neuromodulation pain blockade with frequencies associated with different cellular repair processes. Over 100 studies of a single-motor 200 Hz device demonstrate significant a-delta nociceptor pain reduction (37, 38). The mechanism, described by Salter et al, is driven by Pacinian mechanoreceptors maximally inhibiting nociceptor firing through dorsal horn ATP release and adenosine blocking of the presynaptic spinothalamic tract (“spinal gating”) (24). Additional pain relief therapy cycle frequencies are associated with reduced calcitonin gene-related peptide (CGRP) in the dorsal root ganglia, oxytocin release inhibiting pain via periaqueductal gray pathways, and reduced delayed onset muscle soreness peripherally and centrally (27). Frequencies associated with mechanical tissue restoration [neuromotor reflex (29, 36), reduction of fatty changes (39), inflammation (40), and vasodilation (41, 42)] are coupled to reach deeper tissues with constructive interference. The 12 therapy cycles programmed for the pilot were reduced to the 8 most frequently used, relying on subject biofeedback to choose patterns (30, 40).
To enhance patient choice and add synergistic tissue and central pain benefits, heat and cold packs can be placed behind the plate to reduce spasm or inflammation. Four holes in the plate allow for different locations of a 1.5 cm silicone acupressure nub to target myofascial trigger points (43), with 5 patient-controlled amplitude settings. Having options improves self-efficacy (feeling empowered to control problems like pain) which is associated with opioid reduction and improved chronic pain management (44).
Trial design and participants
This prospective randomized double-blind active-controlled trial recruited 160 adults between 20 and 75 years verbally endorsing moderate-to-severe LBP [Numeric Rating Scale (NRS) ≥4/10] between June 2022 and December 2023, with follow-up completed July 2024. Two chiropractic clinics in Maryland and Virginia served as sites to eliminate the potential bias of onsite opioid prescribing. Enrollment was stratified by participant endorsement of chronic (cLBP) ≥3 months (n = 100) or acute (aLBP) <3-month pain (n = 60) and consenting to 6- and 3-month follow-up, respectively. Exclusion criteria included radicular pain, sickle cell disease, sensitivity to cold or vibration, a pacemaker, diabetic neuropathy or skin lesions in the low back, or inability to apply the devices as directed (Supplementary Material S1).
This trial was part of the National Institutes of Health Help End Addiction Long term (HEAL) program and was funded by the National Institute on Drug Abuse. The Kaizo Clinical Research Institutional Review Board approved the trial, which was registered with ClinicalTrials.gov NCT04494698.
Interventions
Participants were randomized to add 30-minute daily use of a prescription 8-channel 4-lead electrical stimulation TENS unit (LG Smart TENS, LGMedSupply, Cherry Hill, NJ), or multimodal 8-cycle M-Stim (DuoTherm™, Harmonic Scientific LLC, Lewes, DE) to any ongoing therapies or treatments. Pain, opioid and device use were reported daily for 28 days, and weekly for up to 6 months. (Opioid outcomes beyond first 28-day use are reported elsewhere.).
Randomization, procedure, blinding, and assessment
Prior to treatment, clinic intake staff assessed eligibility and obtained digital informed consent for a study “to evaluate the effect of an electric or mechanical stimulation device on opioid use and pain relief”. After signing the tablet, the study ID and coded device assignment were randomly generated through a link to a Qualtrics random number generator with no blocking or further stratification. While the participant recorded current pain intensity, study staff retrieved the assigned device. Participants watched the appropriate training video (Supplementary Material S3) and used the device for 30 min while completing registration data entry.
Outcome assessments were completed by the participants, who were blinded to which device powered the study hypotheses. The protocol statistician (KS) and study coordinator (JS) knew device assignments and had access to data, but did not conduct analysis. The PI(AB) was blinded to allocation and all data during enrollment, accessing data only after study completion. The analyzing statisticians (JW, OT) were blinded to device assignment until completion of primary analysis. Success of participant blinding was tested at 3 months with prompts, “select if you think you received… control or treatment” and “How confident are you?”
Measures
Registration data included the NIH Minimum Data set for low back pain studies (45), (Supplementary Material S3), including demographic information, work and lawsuit status, opioid use for back pain, Sullivan Pain Catastrophizing scale [0(none)-52(extreme)] (46), and Patient-Reported Outcomes Measurement Information System (PROMIS®) (47)Physical Function (4a), Depression (4a), and Pain Interference (8a) (disability) scales. PROMIS responses from 1(low)-5(high) are normed to a United States average T-score where M = 50 SD = 10. For Pain Interference the summed responses from 8 questions yield possible T-scores from no disability (40.7) to completely disabled (77), where mild disability ≥55. (www.healthmeasures.net) We also collected back pain etiology and a 13-intervention inventory of prior treatments including cannabis and gabapentin. (Supplementary Material S3) Clinic staff documented treatments received the day of enrollment, with subjects reporting thereafter.
Text and email prompts reminded participants to record pain (NRS), opioid use, treatments, and device use daily for 28 days, with Pain Interference weekly throughout follow-up. To collect heterogeneous opioid information, we created a skip-logic data collection instrument algorithm [34 dose-per-pill options, 15 opioid formulations (e.g., hydrocodone, hydromorphone), and 6 pill sources], translated to milligrams of morphine equivalents (MME) (Supplementary Material S2, DOSE Tool).
Outcomes
Outcomes of interest included 30 min and 10-day changes in Pain Intensity using a 0–10 NRS with a clinically significant difference of 2. Pain Interference (disability) were compared by 10 (one SD) and 20 (2 SD) point improvements, resolution to below mild disability T ≥ 55, and elimination of disability (T = 40.7) (48). For spine and LBP, a minimum clinically important difference (MCID) is 8–9 (49). To compare PROMIS Pain Interference to the Oswestry Disability Index (ODI) specifically for LBP, T-scores of 57.7–65.4 correspond to “moderate” LBP disability (ODI of 31–40), and 65.7–71.5 crosswalks to an ODI of “severe” (41–60) (50). Resolution of cLBP disability and avoiding aLBP progression to cLBP were defined by lack of ongoing mild Pain Interference (T ≥ 55) at 6 and 3 months respectively (48).
Exploratory cLBP disability outcomes included subsets with BMI > 30, ongoing pain duration ≥5 years, and two definitions of greater severity. In 2015, Deyo et al. with the NIH Research Task Force (RTF) on Low Back Pain (45) found 7-day average Pain Intensity, PROMIS Physical Function(4a), Depression, and four Pain Interference(4a) questions as most predictive of greater disability, with scores >27 deemed moderate and 36–48 severe in a population being evaluated for spinal surgery. For “intractable inoperable mechanical cLBP”, the FDA defined intractable as attempting 3 or more interventions without pain resolution, and the common severity criteria with Gilligan and Decker's implanted multifidus stimulator studies (31, 32, 51), including pain >6 months, pain more than half the days each week, LBP worse than other pain, and a Pain Interference T-score ≥60 [crosswalked to an Oswestry Disability Index (ODI) of 25] (50), with exclusion criteria of ongoing lawsuit or “psychiatric unacceptability” (PROMIS Depression T-Score ≥60, Pain Catastrophizing ≥50).
Finally, to determine factors associated with improved function after M-Stim, we developed an ensemble machine learning approach combining multiple neural networks. We integrated characteristics from the Low Back Pain Minimum Data Set along with factors associated with LBP chronicity. To predict outcomes, we implemented an ensemble strategy averaging predictions from twenty individual neural networks. The model incorporated early stopping to prevent overfitting. SHAP (SHapley Additive exPlanations) analysis identified key predictive features (Supplementary Material S6).
Sample size calculation
Sample sizes were initially calculated for the opioid study against standard care: 60 aLBP/opioid naive subjects for initiation outcomes and 100 cLBP with or without chronic opioid use for change over time, based on an effect size of.6 for an implanted spinal cord stimulator on opioid use reduction (52). To estimate power of this intended recruitment, for cLBP pain intensity focal vibration has a SMD of −1.07, while vibration studies for disability show contradictory effect sizes (25). Using the focal vibration effect size estimate of 1.0, a two-tailed test of pain intensity for acute patients with 27 in each group with attrition of 10% (60 enrolled) would give a power of 0.95, or establish noninferiority against TENS (effect size 0.69) (53). Using an effect size of 0.5 for change in disability, 64 participants would be needed for each group with power of 0.8 and significance set at 0.05, indicating the acute group was anticipated to be underpowered for disability. G*Power (54).
Analysis
Intention-to-treat analysis included summary statistics (means, standard deviations, proportions) calculated using T-tests and relative risks using Chi-squared tests or Fisher's Exact for small cell numbers. For overall pain changes over time, a linear mixed-effects model (LMM) with full-information maximum likelihood followed intention-to-treat principles, with missing data assumed to be at random. NRS Pain Intensity and PROMIS Pain Interference differences at 3- and 6-month time points were calculated for completing participants, and using last outcome carried forward (LCF) imputation assuming data were missing at random. When screening verbal pain intensity or duration differed from recorded registration data (e.g., enrolled as “acute” with 3-month follow-up but later endorsing ongoing LBP for 5 years), registration data was used for categorization, but subjects were not re-consented to extend the study duration. A linear regression assessed interaction with any factors differing by enrollment groups.
The one-tailed significance level was set at 0.025 for noninferiority tests and two-tailed at.05 for comparison statistics, reporting 95% confidence intervals. Data analysis was performed using STATANow/SE 18.5 and MedCalc https://www.medcalc.org/calc/fisher.php (Version 23.2.1; accessed April 14, 2025).
Results
Participants
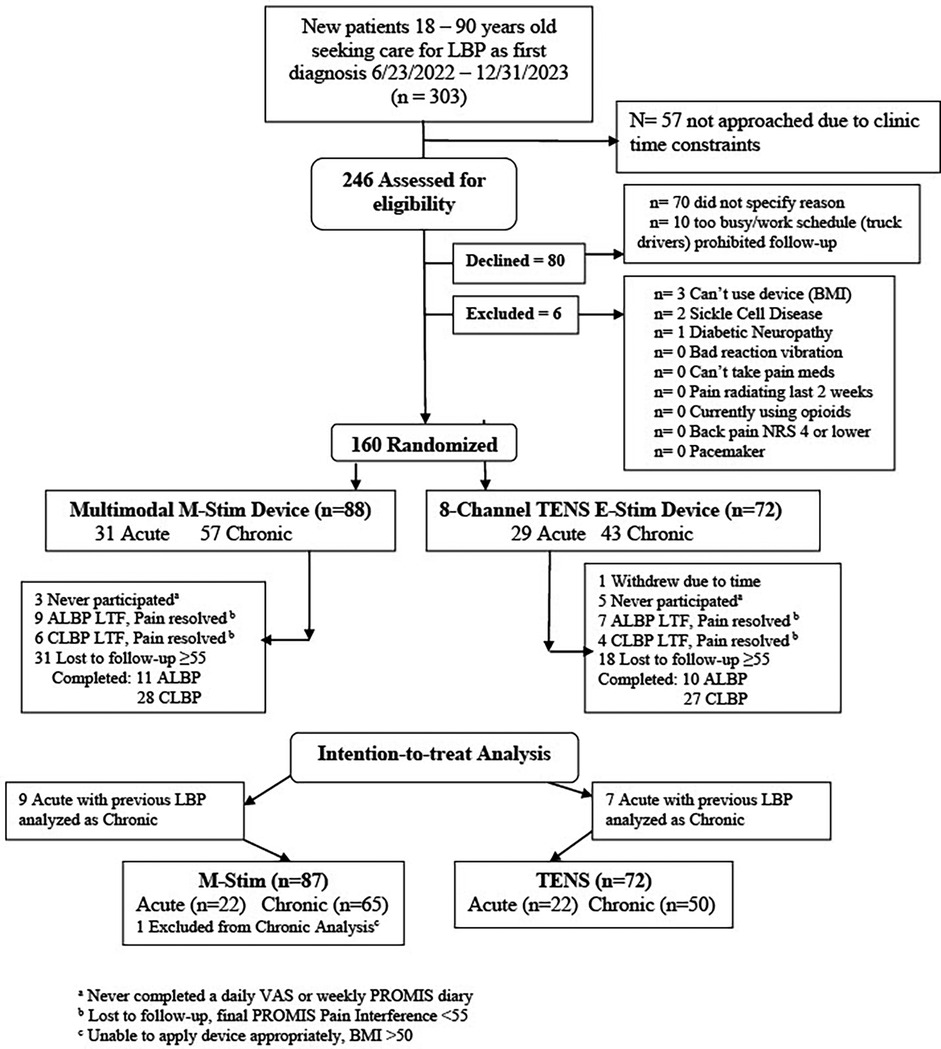
We enrolled 160 participants, of whom 159 were eligible (M-Stim = 87, TENS = 72); one enrolled M-Stim subject (BMI = 60) was unable to apply the device. (Figure 2).
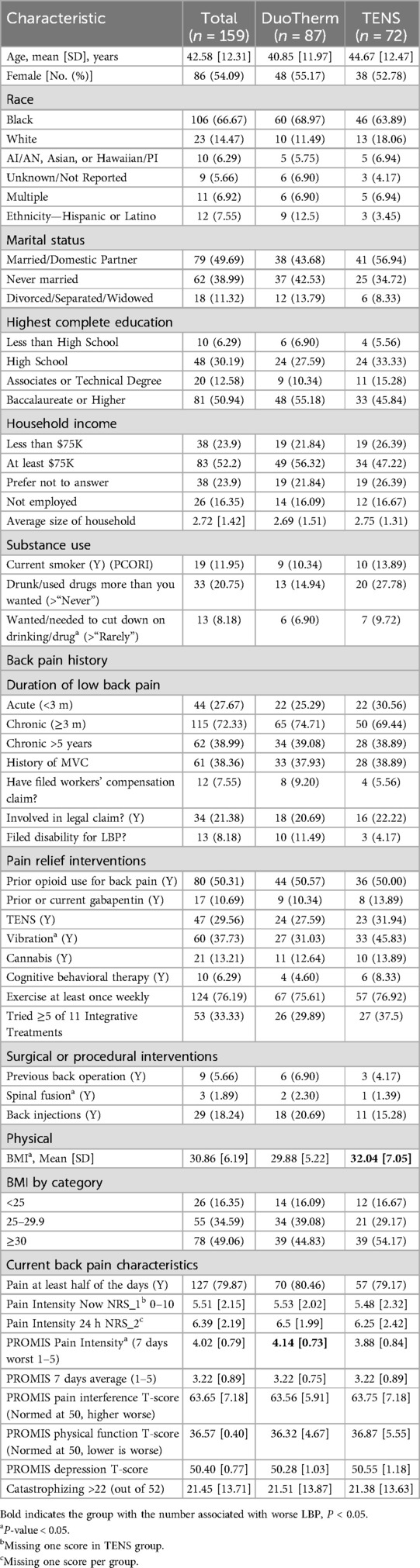
The majority were female (54.7%), non-Hispanic (94.3%), and Black or Multiple Race (72.9%). The average age was 41.1 years (SD = 12.2), BMI 30.9(SD6.19) and 31% had a household income less than $75,000. Average initial pain intensity was 5.51(SD2.15) on the 0–10 NRS, with a PROMIS Pain Interference T-Score of 63.65(SD7.18), (high moderate severity); 115(72%) had chronic pain for at least 3 months, 55% used opioids for their pain, and 112 cLBP and 39 aLBP met the definition of intractable pain (unresponsive to 3 or more interventions). (Table 1) Of M-Stim participants, 74(85%) completed 28 days, similar to the TENS users 60(83%); overall 73.6% completed 3 months and 55/100 enrolled as cLBP completed 6 months. One participant per group completed no diary entries; 3 M-stim and 5 TENS participants completed no PROMIS disability entries. Diary entries by TENS users [mean 29.4(SD11.74)] reported 68.1 min and 2.02 device uses per day, while M-Stim diaries [29.3(SD10.5)] averaged 65.5 min and 2.15 device uses per day. Average diary entry and device use frequency did not differ by intervention, duration, or baseline pain.
Of participants initially presenting with aLBP assigned to 3-month follow-up, 16 recorded prior low back pain in their registration dataset (acute-on-chronic) and were analyzed for change over time with cLBP participants. Baseline pain and disability in enrolled acute and chronic participants verified appropriateness of allocation, with acute-on-chronic more closely resembling chronic (Missing Data, Supplementary Material S4).
Pain intensity outcomes
Initial 30-min and 10-day NRS acute pain relief were similar for both aLBP and cLBP, with M-Stim noninferior to TENS (aLBP 95%CI1.02,0.98, p < .0001, cLBP 95%CI0.58,0.72, P < .0001). Using Linear Mixed Model (LMM) intention-to-treat analysis for 44 aLBP and 115 cLBP participants, NRS pain intensity decreased significantly more rapidly over time for M-Stim as compared to TENS. (Figure 3) Both M-Stim and TENS aLBP participants and chronic M-Stim participants averaged final pain intensity below 2.5 (Supplementary Material S7).
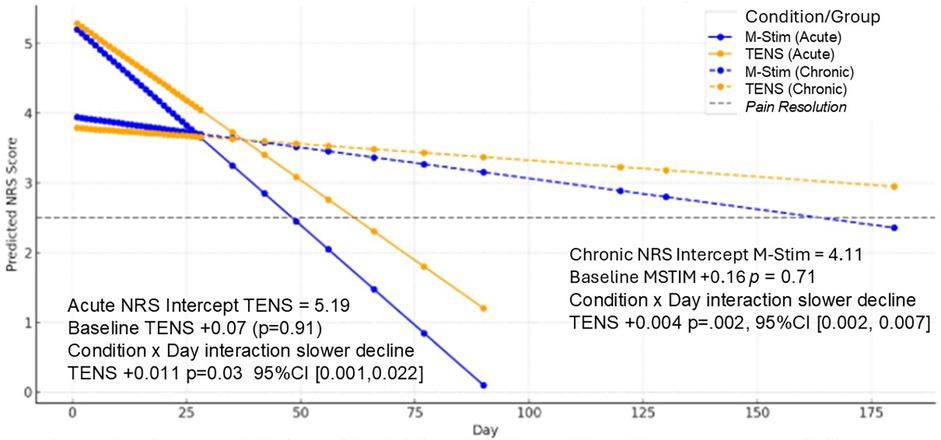
Figure 3. LMM change in NRS pain intensity over time. Baseline average NRS did not differ statistically for aLBP or cLBP. All NRS scores decreased significantly over the course of follow-up, with TENS decreasing less and significantly more slowly than M-Stim. All average predicted NRS endpoints showed resolution of NRS Pain Intensity except cLBP TENS subjects.
Chronic low back pain and disability outcomes
Using LMM intention-to-treat analysis for 115 cLBP participants, pain interference decreased significantly more rapidly over time for M-Stim (n = 65) as compared to TENS (n = 50), with a Condition × Week interaction of +0.18 (0.02, 0.34, p = .024) (Figure 4a).
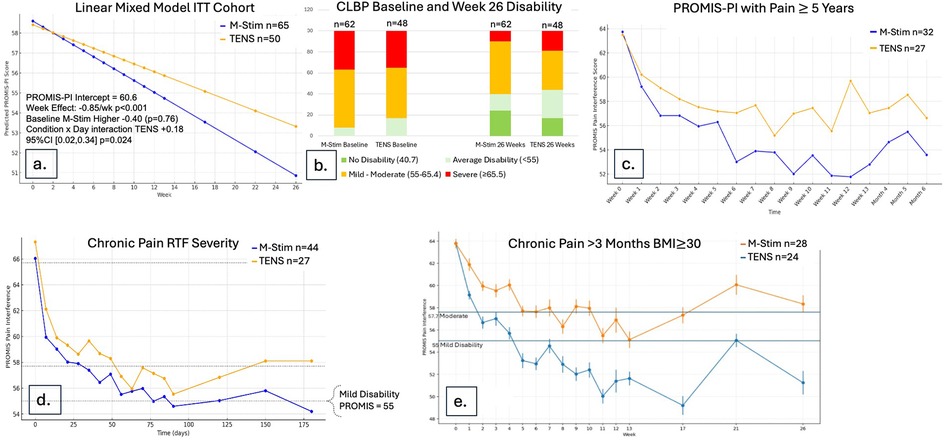
Figure 4. Disability reduction over time for cLBP cohort. (a) Using a Linear Mixed Model in ITT cohort, M-Stim regained function more rapidly. (b) Proportions of disability category for those completing ≥1 diary. (c) Mean disability scores by month for those with pain ≥5 years and completing ≥1 diary. (d) Disability scores RTF Moderate-to-Severe for those completing ≥1 diary. (e) Disability scores with 95%CI for those with BMI > 30 and completing ≥1 diary.
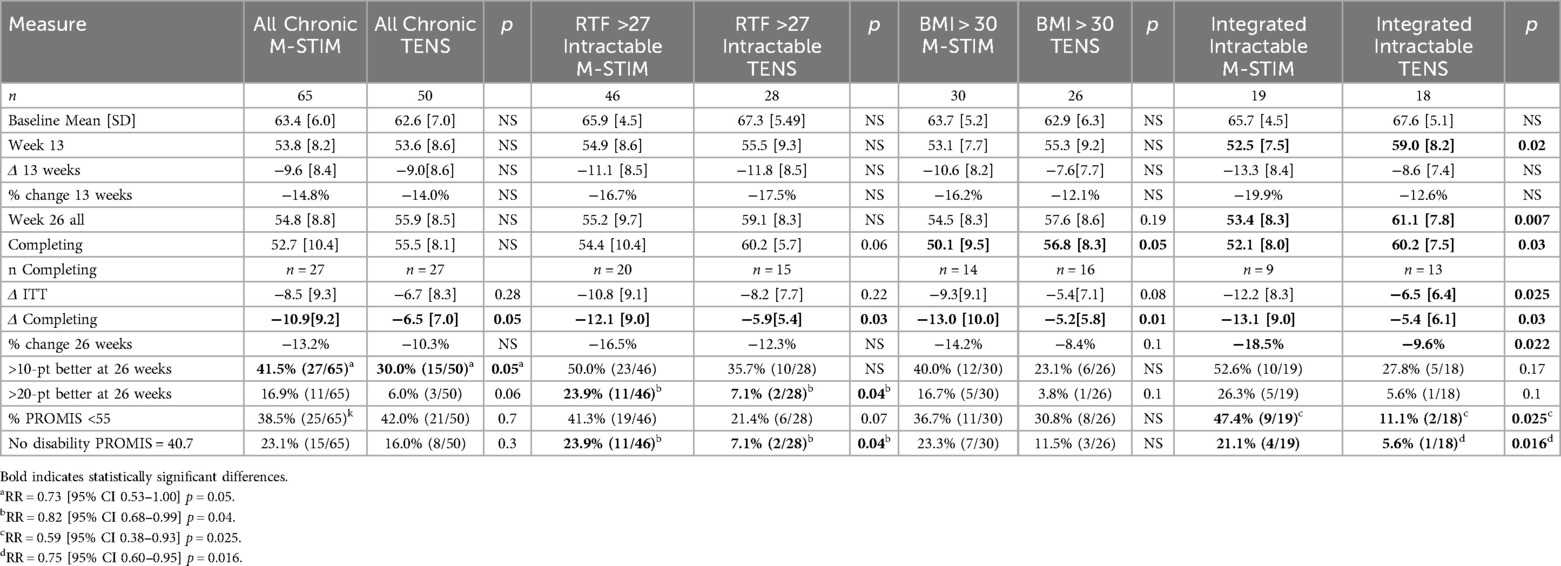
Of those recording at least one follow-up response, M-Stim (n = 62) subjects had worse initial disability. (Figure 4b) Significantly more M-Stim subjects had >1 standard deviation of improvement (27/65 versus 15/50) p = 0.05 (Table 2). For those with pain 5 or more years, M-Stim had significantly improved Pain Interference beginning at week 10 [+4.5 (95%CI 0.28, 8.73) p = 0.036], with 12/32 reaching average pain compared to 4/27 using TENS [RR 0.73 (95%CI 0.54, 1) p = 0.051]. (Figure 4c) Significantly more M-Stim subjects meeting RTF severity criteria achieved zero disability [23.9% vs. 7.1%, RR = 0.82 (95% CI 0.68, .99) p = 0.042] (Figure 4d). For those with BMI >30 by ITT, M-Stim users averaged lower pain over time (Figure 4e, NS), with significantly reduced pain interference for those completing the trial (−13) versus TENS (−5.2, p = 0.01) (Table 2).
Integrated analysis with common criteria
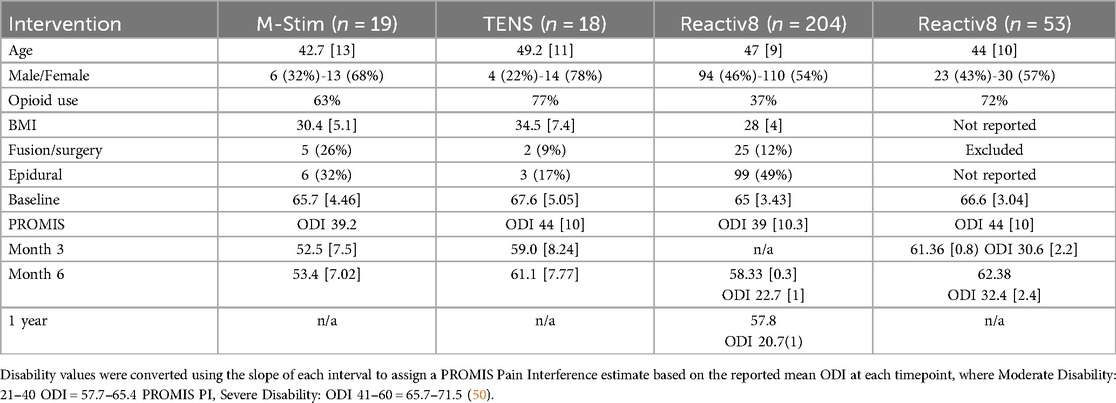
After initiation of the study, research connecting cLBP to dysfunction of the multifidus and erector spinae muscles (6, 55, 56) suggested a plausible mechanism by which multimodal M-Stim impacted function. To explore this hypothesis, we conducted an integrated analysis of intractable inoperable mechanical cLBP in the initial (31) and 1 year follow-up (51) studies of implanted multifidus E-Stim device studies (ReActiv8, Mainstay Medical, Dublin, Ireland, $27,000), using common inclusion and exclusion criteria. (Table 3).
M-Stim restored normal function for 47.4% by week 13 [Mean 52.5 (7.5)], with improvement persisting and significant at week 26 [47.4% resolution v 11.1%, RR = 0.59 (95% CI 0.38–0.93) p = 0.025] (Table 2). Final Pain Interference was in the normal range for M-Stim [53.4 (8.5)] and moderate for TENS [61.1 (7.8)] (Supplementary Material S7).
Acute disability and progression to chronic pain
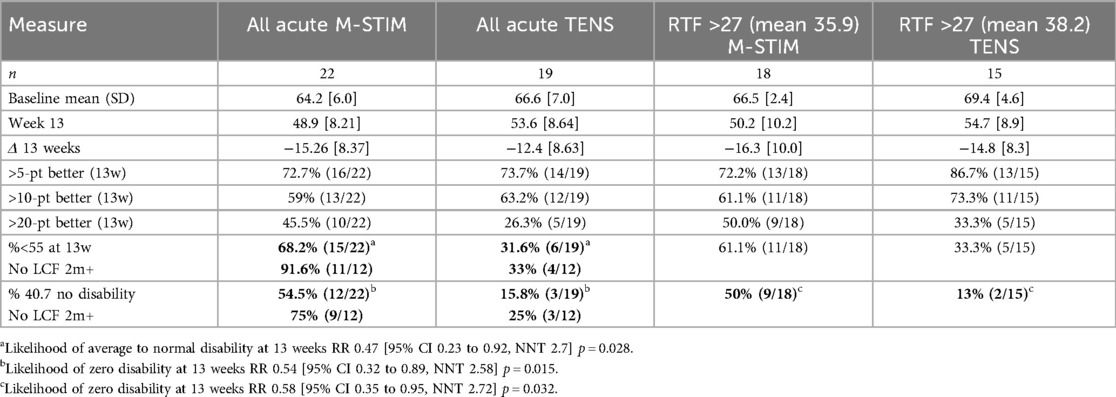
For the 22 aLBP participants in each intervention group, 16 listed motor vehicle collisions as the etiology of pain. Disability improved significantly more rapidly for aLBP M-Stim users, with a LMM condition × week interaction for TENS +0.63 95%CI [0.30, 0.95], p < 0.001. (Figure 5) Defining progression as persistent Pain Interference of mild or greater (≥55–77) at 13 weeks after enrollment, significantly fewer aLBP M-Stim users (7/22) than those using TENS (16/22) transitioned to cLBP. [RR = 0.44 (95% CI 0.23–0.85) p = .015, NNT = 2.4]. (Figure 6) In the RTF severity subset, M-Stim users were significantly more likely to report complete resolution of disability (T-Score = 40.7) at 13 weeks than TENS users (Table 4).
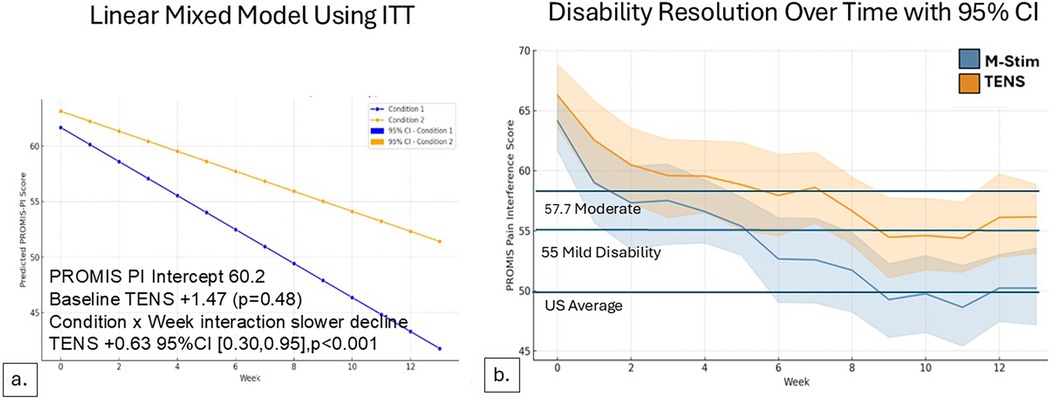
Figure 5. Disability reduction over time for acute pain cohort. For those with LBP duration <3 months, Pain Interference Disability Scores decreased over time. (a) LMM model using intention to treat, M-Stim users had more rapid resolution of disability. (b) Reduction with weekly SD for aLBP with ≥1 diary.
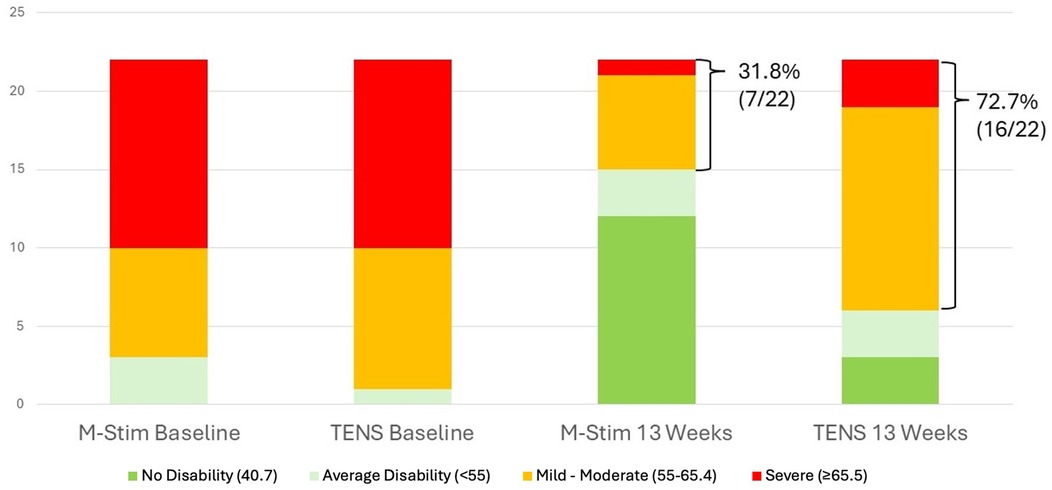
Figure 6. Acute pain baseline vs. Week 13 Disability. The relative risk of persistent pain at 13 weeks was significantly lower in the M-Stim group [RR = 0.44 (95% CI 0.23–0.85) p = 0.015, NNT = 2.4].
Neural network and device use
Features most strongly associated with improved function were a history of motor vehicle collision, thermal therapy application concurrent with device (only available with M-Stim), and greater device usage frequency. Factors associated with reduced improvement included higher BMI, prior opioid use for LBP, and higher initial pain scores. Model performance was evaluated using mean absolute error and R², demonstrating improved predictive accuracy over baseline. (Supplementary Material S6) Average device time per use and use per day did not differ between groups or responders within groups. In the M-Stim group, responders tended to use the device more walking or working than in bed (p = .11), while non-responders were less likely to use any thermal interventions (Figure 7).
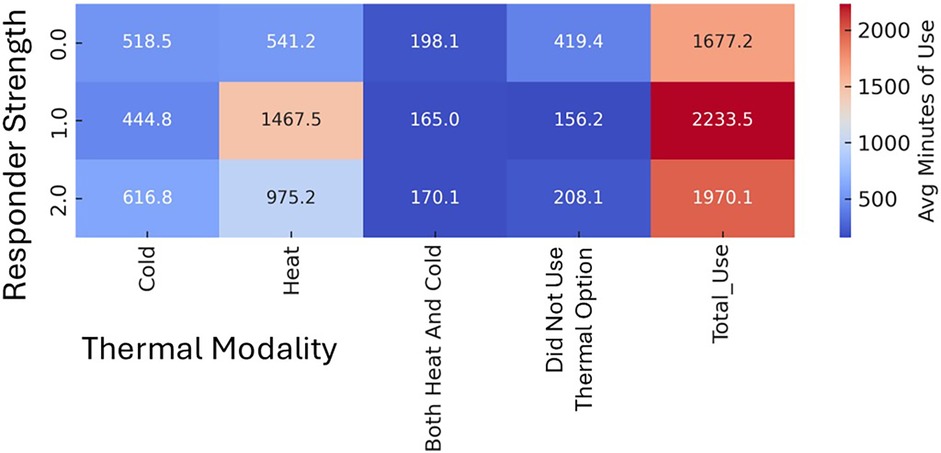
Figure 7. Thermal Use patterns in chronic participants by responder Status. Strong Responder (2.0) = zero (PROMIS = 40.7) disability AND >10-pt Improvement OR t-score below Moderate Disability AND >20-pt Improvement OR initial t-score Severe AND >15-pt Improvement. Mild Responder (1.0) has PROMIS ≤50 AND >5-pt improvements OR less than Moderate Disability AND >10-pt improvement OR initial Severe AND >10-pt Improvement. All others (0.0) are non-responders.
Blinding assessment and opioid use
The most common initial response in both groups was to answer the question regarding the study purpose without answering the allocation question “do you think you were in the treatment or control group?”. Considering this combination an “I don’t know” response, allocation guesses were made by 56 in the M-Stim group (8 “Control”, 29 Don't Know, 19 “Treatment”) and 49 using TENS (12 “Control”, 26 Don't Know, 11 “Treatment”). Using Bang's Blinding Index, M-Stim BBI = −.032 (95%CI −0.54, −0.06) and TENS BBI = −0.51 (95%CI −0.71, −0.24), both significantly against their allocation groups. Using the James Index, M-Stim = 0.54, TENS = 0.71, where values greater than 0.5 indicate adequate blinding.
First 28-day opioid use was reduced in the M-Stim group by 44.6% (32.33 milligram morphine equivalents, p = 0.02), but increased in the TENS group. There were no differences in other analgesic use, and no reported adverse events.
Discussion
In those seeking treatment at a chiropractic office for moderate-to-severe LBP, disability improved significantly more rapidly and to a greater extent for participants allocated to M-Stim for acute or chronic pain. ALBP participants assigned to the M-Stim device were significantly less likely to transition to cLBP. M-Stim's improvement of function was significant for participants with greater severity and cLBP duration, with the strongest results for the subset of intractable inoperable mechanical cLBP who had failed other interventions. This study supports the importance of multimodal options for pain management and suggests a novel role of harmonic M-Stim frequencies to restore function for those suffering from chronic mechanical LBP.
LBP is the leading cause of disability worldwide (57) and directly contributes $200 billion in annual US healthcare costs (58–61). As the most common reason for ambulatory opioid prescribing in the US (62), LBP was a logical target for the NIH Help End Addiction LongTerm (HEAL) initiative: 5% of those prescribed opioids become chronic users (63), and averting one case of OUD is estimated to save US taxpayers $325,125 (64). Current LBP guidelines support first-line multimodal interventions rather than opioids (65, 66), but physician pain management education focuses on pharmaceuticals, not integrative options (67). This study verifies that M-Stim reduces acute LBP pain at least as well as TENS. The establishment of a single FDA-approved multimodal pain device at point-of-care could promote options other than opioid prescribing.
Early physical exercise, heat, acupuncture and physiotherapy prevent cLBP progression (17, 23, 68), but are difficult to implement rapidly. In a 76-clinic US initiative to enhance early intervention, progression of moderate-to-severe aLBP to cLBP was unchanged at 40% (2). DuoTherm combines multiple elements of these preventative modalities: somatosensory heat and pressure for central and neural effects, with mechanical force-based frequencies associated with vasodilation, reduced muscle damage, and neuromotor reflex improvement for tissue effects. If the findings for acute pain are replicated, early initiation could have a significant impact for back injuries. In 2022 the 67,510 low back pain injuries in transportation workers cost an average $39,000 and 9 days off work (72); at $5,000, the M-Stim intervention would save over $2.2B per year. As LBP affects 60% of Americans each year (1), preventing 13% from progressing to cLBP could help 7.8 million people at a savings of $2,000 per patient per year in direct costs (73).
M-Stim and TENS were similar in efficacy for acute pain intensity, but intensity is less predictive of opioid use and pain chronicity than lack of coping strategies (74). In addition to central and local tissue effects, having multiple options when initial pain relief fades enhances self-efficacy and reduces pain catastrophizing (44, 69–71). The contribution of different relief modalities, different stochastic patterns of mechanical force emphasizing different frequencies, and thermal variations to self-efficacy vs. cellular mechanisms should be explored in future work.
Effective M-Stim mechanisms may differ for cLBP. The efficacy of implanted muscular rather than spinal cord stimulation for these patients supports that ongoing nociceptive cLBP requires paraspinal muscle rehabilitation rather than neuromodulatory or inflammatory blockade. The (75) outsized role of fascia in nociception and inflammation, and the discovery of reparative cellular proteins activated by mechanical force are all now active areas of investigation. Multimodal M-Stim was more likely to improve function in severely impacted cLPB subsets. Given the myriad effects of focal vibration (repeated mechanical force) recently described in the literature, hypotheses include direct analgesia (27), neuromuscular recovery (29), and low back pain specific effects (25) through targeting paraspinal muscles and fascia.
Paraspinal muscles and mechanical Low back pain
For 90% of LBP patients, imaging shows no nerve or bony target for intervention (14). Systematic reviews of the spine-stabilizing multifidus and erector spinae muscles (6, 55, 56), their overlying fascia (5, 10, 19), and the role of neuromotor control now support a common pathway to ongoing LBP initially hypothesized in 2007 by Langevin et al. (76) Muscle injury causes local inflammation, with cold reducing pain transmission and cytokine release (77, 78). Stabilizing muscles are recruited, with resultant edema and increased metabolic requirements exceeding local blood supply. Pain from the resulting ischemia and ongoing spasm (79) may respond to heat at this phase. Within a week of bracing or voluntary immobility due to pain, fatty infiltration of the muscles is seen (8), further reducing blood flow and increasing pain from ceramide production (7). Altered neuromotor responses develop rapidly in the presence of inflammation (3), leading to maladaptive motor control contributing to reinjury (80, 81) Ongoing relative ischemia and dysfunction of the multifidus lead to adherence of the fascia (10) disproportionately increasing pain with movement (19), causing a disordered feedback loop: plastic changes of 1a afferent proprioception muscle spindles and supraspinal motor coordination reflexes (82, 83) lead to spinal instability and neuromotor reflex dysfunction (84). Central conditioning and fear reduce movements (85), compounding the effect of immobilization, but can be reduced with early movement and exercise (73). These neuromotor changes with aLBP predict cLBP (4), manifesting as poor proprioceptive control and stability (82, 86). Thus the superiority of multimodal over opioid treatments can be explained: opioid lethargy may reduce movement leading to increased pain, while early anti-inflammatory medications and cold followed by heat and exercise mitigate the physiologic progression of mechanical nociceptive processes.
Cellular discoveries: mechanical force ion channels
While pain and inflammatory processes have been extensively described, the role of mechanical force in cellular adaptation has only been recognized in the last decades. Ion channels typically depolarize with electric and chemical activity. Piezo1 channels are activated directly by pressure and held open for ion influx with proteins using this force (e.g. Yoda1, Jedi1) (87). Effects include musculoskeletal growth, vascular effects, and specific channels related to pain (87, 88). These channels are reversibly opened, so intermittent pressure, i.e. vibration can repeatedly activate cell activity (109), including specifically fatty lipid remodeling (89).
Vibration for pain, vascular and myofascial rehabilitation
M-Stim simultaneously employs vibratory effects described in different disciplines to achieve varied effects. Vibration for nociception and itch was first well described by Wall in 1960, predating his more famous 1965 gate control theory (90). In the early 1990s, Salter identified the neurotransmitters and optimal frequency to block nociception. Pacinian fast-touch mechanoreceptors transmit vibration impulses directly to the dorsal horn, rather than joining wide dynamic range neurons with less specific nociception inhibition (10, 24, 25, 27, 91–93). Pacinian ATP released optimally at 200 Hz becomes adenosine, the purine responsible for presynaptic inhibition (24, 94). Single-motor 200 Hz devices have been found effective in over 100 studies for sharp nociceptive pain, with greater efficacy when placed in multiple dermatomes (27, 95).
This short-term neuromodulatory effect alone cannot explain the chronic improvement in LBP. Lundeberg's vibrating back plates reduced pain better with 200 Hz than 100 Hz, with duration-dependent sustained relief (36, 96). Since Lundeberg's time, vibration in this range has demonstrated varied tissue effects associated with repair. For example, frequencies in the 150 Hz range increase blood flow and wound healing via nitric oxide release (97, 98). Potentially, applying either single frequency across a field over time addressed hypoperfusion in the multifidus and erector spinae. Some frequencies of vibration directly inhibit fatty muscular changes, and may reverse them over time (7, 39, 99). Multiple frequencies increase range of motion through tissue changes (100–102); specific frequencies reduce inflammatory calcitonin gene-related peptide (40). Inhibitory pain relief relies on oxytocin (103–105), which vibration increases, and a growing dataset from the field of kinesiology supports vibration to directly reconfigure neuromotor derangement and fascial and muscle dysfunction (29, 106). By using multiple motors, stochastic constructive interference arises in the areas between nodes, giving exponentially greater variety in amplitude and nodal locations to address multiple physiologic derangements in a field. Therapeutic synergies may therefore arise not just from thermomechanical combinations, but specific combinatory mechanical stimulation patterns.
Pain and physics-based energy modalities
That biology-based nutrients and chemical compounds have specialized effects on the human body is axiomatic: indications for vitamin C vs. vitamin B12, or antacids vs. antidepressants differ to the point of malpractice if misprescribed. Our findings support a similar specificity for evolving physics-based modalities. Just as biochemical therapeutics must match receptor targets and physiologic pathways, administration of energy via electricity, force, light and radiation must match the physiology, mechanism, and mechanical properties of the target tissues.
Current occurs when charged particles flow through the least electrically-resistant tissue between positive and negative electrodes. Electrical stimulation external to tissue generates electrical and magnetic fields. Periodic mechanical force from sound or vibration propagates as pressure waves, while kinetic energy causes cellular and tissue shear or deformation. Thermal energy passes via changed molecular energy, while light energy is absorbed, scattered, and refracted depending on wavelength (116, 117).
While charge is the currency of biologic function (118), not all functions generate or respond to current. Pain incorporates biologic, chemical, and electrophysiologic events. Noxious temperature, pressure, or chemical exposures trigger nociception via cation and ligand-gated protein channels. These peripheral pain signals propagate centrally via voltage-gated sodium channels, the phylogenetically simplest and fastest method to conduct depolarization. Once the voltage-mediated message reaches the central cell bodies, released pain neurotransmitters activate transduction and response through synaptic mechanisms. Activated and blocked neurotransmitter receptors change cell functions, using cations through complex protein channels as second messengers or to depolarize intracellularly (107).
Therapeutic physics interventions depend not only on the targets’ mechanism of action, but mechanical factors inherent in the tissues. Piezo1 channels are activated by mechanical shear, pressure, or stretch deformation. Typically calcium then flows intracellularly to act as a second messenger to initiate cascades of cellular activity (108). Repeated delivery of kinetic energy may be beneficial up to a cellular tolerance, accounting for specific vibration frequencies in different tissues. (109). Vectors, frequency and amplitude must match tissue tolerance and desired outcomes.
Study findings relating to energy and tissue targets
The high number of MVC participants may have contributed to M-Stim's greater impact on disability, particularly for the aLBP progression. Fascial adherence or paraspinal fatty changes from immobilization, whether through bracing or pain relief, are more common after trauma than overuse. Vibratory effects of vasodilation and inhibiting fatty changes may better address this type of derangement. The trend toward better outcomes with movement over bed-use may support the contention in the multifidus literature supporting neuromotor reflex repair (32). The kinesiology literature suggests vibration with active muscle contraction is preferred to reset dysfunctional reflexes from pain and asynchronous proprioception (29). Interestingly, the “30 minutes 3× a day for 3 days” recommended by Fattorini et al. was frequently the use pattern for strong responders. Future studies evaluating acute-to-chronic transition should evaluate the contribution of activity, stretching, or passive use in conjunction with vibration to outcomes. In addition, using big data to correlate etiology, thermal use, duration, and the most chosen therapy cycles are areas for future investigation.
A striking finding was the comparison of two non-invasive interventions against paraspinal muscle stimulator surgery. As studies with that intervention lacked a control, our data could provide some insight using the common criteria. However, while spine research supports PROMIS Pain Interference indicators of disability as superior to both ODI and Likert Scales (110, 111), the bell curve distribution makes a 1:1 crosswalk estimation more difficult. To bias toward the null hypothesis, we used the more lenient crosswalk for moderate scores (ODI 20 = PI 57.7) from the LBP literature rather than the general PROMIS PI score cut-point of 60. As the sample size for our participants was both much smaller and followed for less time, these results must be interpreted with caution.
While all participants in the DuoTherm pilot study endorsed “would recommend”, this study did not ask participants to rate satisfaction with the devices. Changes in pain catastrophizing would have been interesting to know, but this information was not collected. Future research evaluating enjoyment or greater feelings of agency with the devices would be helpful to ascertain these contributions to the effectiveness of M-Stim.
Limitations
In the context of LPB research broadly, this study has numerous strengths, including subject BMI and socioeconomic status that better represent US national averages than typical surgical or post-op studies of similar severity. The use of an active control allowed for successful blinding, which is extremely rare in device studies. Moreover, the 6-month follow-up illuminated comparisons beyond the time expected for reversion to the mean or placebo effects. Daily collection of not just prescribed but prior and external opioid sources covered a full month, while many opioid LPB studies only follow use for prescriptions given at the point of care and at a weekly or monthly cadence. With regards to limitations, the choice of a chiropractic office to avoid prescribing bias could reduce generalizability, although the percent using opioids (51%) was similar to other non-chiropractic studies (2). As both early physical care and spinal manipulation are associated with improved relief, the rapidity of pain resolution we found in both groups may differ from other outpatient environments. While MVC injuries are more likely to lead to chronic pain (112), the preponderance these participants might introduce bias in favor of M-Stim, as vibration and thermal interventions are particularly well-suited for mechanical pain.
Because cLBP research reports PROMIS and disability measures to be more relevant than NRS, we used “mild disability” as described in PROMIS guidelines as our cut-off for ongoing cLBP (110, 111). While the difference in transition from acute to chronic pain was statistically significant, the percent of TENS patients who still had mild pain at 3 months (72%) was higher than previously reported. However, any more stringent criteria would have also reduced the percentage of M-Stim participants without ongoing 3-month pain. The sample size of true acute patients should be replicated with larger numbers, and more detailed descriptions of etiology (2, 73, 113).
While the primary LMM analysis using intention to treat was significant for both acute pain and disability in both chronicity groups, analyses for smaller subsets may have been underpowered. Because the LMM analysis and subset results ubiquitously favored M-Stim, to emphasize responder signal we felt it was appropriate not to apply a Bonferroni correction. Relative risks for chronic subsets should be considered with this decision in mind. Contrariwise, the broad socioeconomic inclusion criteria may have artificially reduced statistical power: subjects with ongoing lawsuits, high pain catastrophizing and depression are typically excluded by E-Stim multifidus and other surgical intervention studies to avoid blunting the significance of physiologic interventions.
Finally, the superiority of M-Stim in those with higher BMI may reflect a reduced penetration of TENS rather than superior M-Stim efficacy.
Conclusion
In comparison to prescription TENS, adding M-Stim devices significantly reduced the transition of acute to chronic LBP, and restored function significantly more in subjects with higher severity. The combination of multiple therapies addressing the physiology of acute, intermediate, and chronic injury, particularly with stochastic harmonic interaction of specific focal vibration frequencies in a novel array, may be potentially useful to address the growing epidemic of low back pain (63, 114, 115).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kaizo Clinical Research Institute, Kaizo Health. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization. JE-S: Writing – review & editing, Conceptualization, Data curation, Investigation, Project administration, Supervision. OT: Writing – review & editing, Data curation, Formal analysis, Investigation, Visualization. JW: Writing – review & editing, Data curation, Formal analysis, Visualization. KS: Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Project administration. LC: Writing – review & editing, Conceptualization, Methodology. ML: Conceptualization, Funding acquisition, Methodology, Resources, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by National Institutes of Health grants R44DA049631 (AB), R44DA058952 (AB) as part of the Help End Addiction Longterm (HEAL) Program.
Conflict of interest
AB was employed by Harmonic Scientific LLC (parent company MMJ Labs LLC).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. No generative AI was used in the preparation of this manuscript, however generative AI was used to fact-check summaries of physics descriptions, and to create the visual heat map graphics from the use data and the LMM graphs from the outcome over time data.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1625420/full#supplementary-material
References
1. Clark S, Horton R. Low back pain: a major global challenge. Lancet. (2018) 391(10137):2302. doi: 10.1016/S0140-6736(18)30725-6
2. Delitto A, Patterson CG, Stevans JM, Brennan GP, Wegener ST, Morrisette DC, et al. PCORI Final Research Reports. Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—the TARGET Trial. Washington, DC: Patient-Centered Outcomes Research Institute (PCORI), University of Pittsburgh (2021). Copyright © 2021. All Rights Reserved.
3. James G, Millecamps M, Stone LS, Hodges PW. Dysregulation of the inflammatory mediators in the Multifidus muscle after spontaneous intervertebral disc degeneration SPARC-null mice is ameliorated by physical activity. Spine. (2018) 43(20):E1184–e94. doi: 10.1097/BRS.0000000000002656
4. Alshehri MA, van den Hoorn W, Klyne DM, van Dieën JH, Cholewicki J, Hodges PW. Poor lumbar spine coordination in acute low back pain predicts persistent long-term pain and disability. Eur Spine J. (2024) 33(6):2380–94. doi: 10.1007/s00586-024-08205-w
5. Langevin HM. Fascia mobility, proprioception, and myofascial pain. Life (Basel). (2021) 11(7):668.34357040
6. Ekşi M, Özcan-Ekşi EE. Fatty infiltration of the erector spinae at the upper lumbar spine could be a landmark for low back pain. Pain Pract. (2024) 24(2):278–87. doi: 10.1111/papr.13302
7. Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). (2016) 7:69. doi: 10.3389/fendo.2016.00069
8. James G, Chen X, Diwan A, Hodges PW. Fat infiltration in the multifidus muscle is related to inflammatory cytokine expression in the muscle and epidural adipose tissue in individuals undergoing surgery for intervertebral disc herniation. Eur Spine J. (2021) 30(4):837–45. doi: 10.1007/s00586-020-06514-4
9. Stecco A, Giordani F, Fede C, Pirri C, De Caro R, Stecco C. From muscle to the myofascial unit: current evidence and future perspectives. Int J Mol Sci. (2023) 24(5):4527. doi: 10.3390/ijms24054527
10. Wilke J, Schleip R, Klingler W, Stecco C. The lumbodorsal fascia as a potential source of low back pain: a narrative review. BioMed Res Int. (2017) 2017:5349620. doi: 10.1155/2017/5349620
11. Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. (1994) 19(2):165–72. doi: 10.1097/00007632-199401001-00009
12. Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. (2003) 13(4):371–9. doi: 10.1016/S1050-6411(03)00044-0
13. Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. (1992) 5(4):383–9. discussion 97. doi: 10.1097/00002517-199212000-00001
14. Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am. (2012) 50(4):569–85. doi: 10.1016/j.rcl.2012.04.005
15. James G, Ahern BJ, Goodwin W, Goss B, Hodges PW. Targeted multifidus muscle activation reduces fibrosis of multifidus muscle following intervertebral disc injury. Eur Spine J. (2024) 33(6):2166–78. doi: 10.1007/s00586-024-08234-5
16. Ballantyne JC. Spinal interventions for chronic back pain. BMJ. (2025) 388:r179. doi: 10.1136/bmj.r179
17. Sharif S, Jazaib Ali MY, Kirazlı Y, Vlok I, Zygourakis C, Zileli M. Acute back pain: the role of medication, physical medicine and rehabilitation: WFNS spine committee recommendations. World Neurosurg X. (2024) 23:100273. doi: 10.1016/j.wnsx.2024.100273
18. Werthman AM, Jolley BD, Rivera A, Rusli MA. Emergency department management of low back pain: a comparative review of guidelines and practices. Cureus. (2024) 16(2):e53712. doi: 10.7759/cureus.53712
19. Langevin HM, Fox JR, Koptiuch C, Badger GJ, Greenan-Naumann AC, Bouffard NA, et al. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord. (2011) 12:203. doi: 10.1186/1471-2474-12-203
20. Tankha H, Gaskins D, Shallcross A, Rothberg M, Hu B, Guo N, et al. Effectiveness of virtual yoga for chronic low back pain: a randomized clinical trial. JAMA Netw Open. (2024) 7(11):e2442339. doi: 10.1001/jamanetworkopen.2024.42339
21. Jauregui JJ, Cherian JJ, Gwam CU, Chughtai M, Mistry JB, Elmallah RK, et al. A meta-analysis of transcutaneous electrical nerve stimulation for chronic low back pain. Surg Technol Int. (2016) 28:296–302.27042787
22. Vance CGT, Dailey DL, Chimenti RL, Van Gorp BJ, Crofford LJ, Sluka KA. Using TENS for pain control: update on the state of the evidence. Medicina (Kaunas). (2022) 58(10):1332. doi: 10.3390/medicina58101332
23. Qaseem A, Wilt TJ, McLean RM, Forciea MA, Denberg TD, Barry MJ, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med. (2017) 166(7):514–30. doi: 10.7326/M16-2367
24. Salter MW, Henry JL. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. (1987) 22(2):631–50. doi: 10.1016/0306-4522(87)90359-9
25. Li Q, Liu P, Wang Z, Li X. Vibration therapy to improve pain and function in patients with chronic low back pain: a systematic review and meta-analysis. J Orthop Surg Res. (2023) 18(1):727. doi: 10.1186/s13018-023-04217-2
26. Lurie RC, Cimino SR, Gregory DE, Brown SHM. The effect of short duration low back vibration on pain developed during prolonged standing. Appl Ergon. (2018) 67:246–51. doi: 10.1016/j.apergo.2017.10.007
27. Casale R, Hansson P. The analgesic effect of localized vibration: a systematic review. Part 1: the neurophysiological basis. Eur J Phys Rehabil Med. (2022) 58(2):306–15. doi: 10.23736/S1973-9087.22.07415-9
28. Sakai Y, Morita Y, Kawai K, Fukuhara J, Ito T, Yamazaki K, et al. Targeted vibratory therapy as a treatment for proprioceptive dysfunction: clinical trial in older patients with chronic low back pain. PLoS One. (2024) 19(7):e0306898. doi: 10.1371/journal.pone.0306898
29. Fattorini L, Rodio A, Filippi GM, Pettorossi VE. Effectiveness of focal muscle vibration in the recovery of neuromotor hypofunction: a systematic review. J Funct Morphol Kinesiol. (2023) 8(3):103. doi: 10.3390/jfmk8030103
30. Mirzoev TM. Mechanotransduction for muscle protein synthesis via mechanically activated Ion channels. Life (Basel). (2023) 13(2):341. doi: 10.3390/life13020341
31. Deckers K, De Smedt K, Mitchell B, Vivian D, Russo M, Georgius P, et al. New therapy for refractory chronic mechanical low back pain-restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodulation. (2018) 21(1):48–55. doi: 10.1111/ner.12741
32. Gilligan C, Volschenk W, Russo M, Green M, Gilmore C, Mehta V, et al. Long-term outcomes of restorative neurostimulation in patients with refractory chronic low back pain secondary to multifidus dysfunction: two-year results of the ReActiv8-B pivotal trial. Neuromodulation. (2023) 26(1):87–97. doi: 10.1016/j.neurom.2021.10.011
33. Gilligan C, Volschenk W, Russo M, Green M, Gilmore C, Mehta V, et al. Five-year longitudinal follow-up of restorative neurostimulation shows durability of effectiveness in patients with refractory chronic low back pain associated with multifidus muscle dysfunction. Neuromodulation. (2024) 27(5):930–43. doi: 10.1016/j.neurom.2024.01.006
34. Baxter AL, Thrasher A, Etnoyer-Slaski JL, Cohen LL. Multimodal mechanical stimulation reduces acute and chronic low back pain: pilot data from a HEAL phase 1 study. Front Pain Res (Lausanne). (2023) 4:1114633. doi: 10.3389/fpain.2023.1114633
35. Lundeberg T. Naloxone does not reverse the pain-reducing effect of vibratory stimulation. Acta Anaesthesiol Scand. (1985) 29(2):212–6. doi: 10.1111/j.1399-6576.1985.tb02188.x
36. Lundeberg T. The pain suppressive effect of vibratory stimulation and transcutaneous electrical nerve stimulation (TENS) as compared to aspirin. Brain Res. (1984) 294(2):201–9. doi: 10.1016/0006-8993(84)91031-X
37. Zhao L, Qi P, Wang X, Su X, Liao L. Local analgesia for the relief of pain in children undergoing venipuncture and intravenous cannulation: a systematic review and network meta-analysis. BMC Anesthesiol. (2025) 25(1):115. doi: 10.1186/s12871-025-02991-6
38. Su HC, Hsieh CW, Lai NM, Chou PY, Lin PH, Chen KH. Using vibrating and cold device for pain relieves in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr Nurs. (2021) 61:23–33. doi: 10.1016/j.pedn.2021.02.027
39. Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. (2007) 104(45):17879–84. doi: 10.1073/pnas.0708467104
40. Thammanichanon P, Kaewpitak A, Binlateh T, Leethanakul C. Interval vibration reduces orthodontic pain via a mechanism involving down-regulation of TRPV1 and CGRP. In Vivo. (2020) 34(5):2389–99. doi: 10.21873/invivo.12052
41. Syabariyah S, Nurachmah E, Widjojo BD, Prasetyo S, Sanada H, Irianto , et al. The effect of vibration on the acceleration of wound healing of diabetic neuropathic foot ulcer: a prospective experimental study on human patients. Healthcare (Basel). (2023) 11(2):191.36673559
42. Johnson JM, Kellogg DL Jr. Thermoregulatory and thermal control in the human cutaneous circulation. Front Biosci (Schol Ed). (2010) 2:825–53. doi: 10.2741/s105
43. Gulick DT, Campbell S, Palombaro K. Effectiveness of vibration on myofascial trigger points. W J Yoga Phys Ther Rehabil. (2023) 4(2):1–6.
44. Darnall BD, Colloca L. Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: a review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol. (2018) 139:129–57. doi: 10.1016/bs.irn.2018.07.022
45. Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH task force on research standards for chronic low back pain. Phys Ther. (2015) 95(2):e1–e18. doi: 10.2522/ptj.2015.95.2.e1
46. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524–32. doi: 10.1037/1040-3590.7.4.524
47. Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. (2007) 45(5 Suppl 1):S3–s11. doi: 10.1097/01.mlr.0000258615.42478.55
48. PROMIS. Pain Interference Scoring Manual 2022 (2025). p. 7. Available online at: https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Pain_Interference_Scoring_Manual_03June2022.pdf (Accessed July 22, 2025).
49. Hung M, Saltzman CL, Kendall R, Bounsanga J, Voss MW, Lawrence B, et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. (2018) 476(10):2027–36. doi: 10.1097/CORR.0000000000000419
50. Tang X, Schalet BD, Hung M, Brodke DS, Saltzman CL, Cella D. Linking oswestry disability index to the PROMIS pain interference CAT with equipercentile methods. Spine J. (2021) 21(7):1185–92. doi: 10.1016/j.spinee.2021.02.012
51. Gilligan C, Volschenk W, Russo M, Green M, Gilmore C, Mehta V, et al. An implantable restorative-neurostimulator for refractory mechanical chronic low back pain: a randomized sham-controlled clinical trial. Pain. (2021) 162(10):2486–98. doi: 10.1097/j.pain.0000000000002258
52. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 khz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res. (2018) 11:2929–41. doi: 10.2147/JPR.S188795
53. Johnson MI, Paley CA, Jones G, Mulvey MR, Wittkopf PG. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. (2022) 12(2):e051073. doi: 10.1136/bmjopen-2021-051073
54. Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39(2):175–91. doi: 10.3758/BF03193146
55. Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. (2016) 19(7):E985–e1000.27676689
56. Seyedhoseinpoor T, Taghipour M, Dadgoo M, Sanjari MA, Takamjani IE, Kazemnejad A, et al. Alteration of lumbar muscle morphology and composition in relation to low back pain: a systematic review and meta-analysis. Spine J. (2022) 22(4):660–76. doi: 10.1016/j.spinee.2021.10.018
57. GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. (2023) 5(6):e316–e29. doi: 10.1016/S2665-9913(23)00098-X
58. Casiano VE, Sarwan G, Dydyk AM, Varacallo M. Back Pain. StatPearls. Treasure Island, FL: StatPearls Publishing LLC. (2024). StatPearls Publishing Copyright © 2024.
59. Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. US Health care spending by payer and health condition, 1996–2016. JAMA. (2020) 323(9):863–84. doi: 10.1001/jama.2020.0734
60. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. An updated assessment of utilization of interventional pain management techniques in the medicare population: 2000–2013. Pain Physician. (2015) 18(2):E115–E27. doi: 10.36076/ppj/2015.18.E115
61. Gaskin D, Richard P. The Economic Costs of Pain in the United States. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press (2011). p. 1–16.
62. Leventhal EL, Nathanson LA, Landry AM. Variations in opioid prescribing behavior by physician training. West J Emerg Med. (2019) 20(3):428–32. doi: 10.5811/westjem.2019.3.39311
63. Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. (2017) 32(1):21–7. doi: 10.1007/s11606-016-3810-3
64. Murphy SM. The cost of opioid use disorder and the value of aversion. Drug Alcohol Depend. (2020) 217:108382. doi: 10.1016/j.drugalcdep.2020.108382
65. Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. AHRQ Comparative Effectiveness Reviews. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality (US) (2018).
66. Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. (2018) 391(10137):2368–83. doi: 10.1016/S0140-6736(18)30489-6
67. Mezei L, Murinson BB. Pain education in north American medical schools. J Pain. (2011) 12(12):1199–208. doi: 10.1016/j.jpain.2011.06.006
68. Severijns P, Goossens N, Dankaerts W, Pitance L, Roussel N, Denis C, et al. Physiotherapy-led care versus physician-led care for persons with low back pain: a systematic review. Clin Rehabil. (2024) 38(12):1571–89. doi: 10.1177/02692155241282987
69. Borsbo B, Gerdle B, Peolsson M. Impact of the interaction between self-efficacy, symptoms and catastrophising on disability, quality of life and health in with chronic pain patients. Disabil Rehabil. (2010) 32(17):1387–96. doi: 10.3109/09638280903419269
70. Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low back pain. Pain. (2018) 21(2):487–501. doi: 10.1016/j.jpain.2018.07.001
71. Dehghan M, Farahbod F. The efficacy of thermotherapy and cryotherapy on pain relief in patients with acute low back pain, a clinical trial study. J Clin Diagn Res. (2014) 8(9):LC01–4.25386469
72. National Safety Council. Workers’ compensation costs. National Safety Council (2024). p. 1387–96. Available online at: https://injuryfacts.nsc.org/work/costs/workers-compensation-costs/ (Accessed July 22, 2025).
73. Chang D, Lui A, Matsoyan A, Safaee MM, Aryan H, Ames C. Comparative review of the socioeconomic burden of lower back pain in the United States and globally. Neurospine. (2024) 21(2):487–501. doi: 10.14245/ns.2448372.186
74. Rogers AH, Heggeness LF, Smit T, Zvolensky MJ. Opioid coping motives and pain intensity among adults with chronic low back pain: associations with mood, pain reactivity, and opioid misuse. J Behav Med. (2023) 46(5):860–70. doi: 10.1007/s10865-023-00416-8
75. Wang X, Martin G, Sadeghirad B, Chang Y, Florez ID, Couban RJ, et al. Common interventional procedures for chronic non-cancer spine pain: a systematic review and network meta-analysis of randomised trials. BMJ. (2025) 388:e079971.39971346
76. Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses. (2007) 68(1):74–80. doi: 10.1016/j.mehy.2006.06.033
77. Smith C, Kruger MJ, Smith RM, Myburgh KH. The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med. (2008) 38(11):947–69. doi: 10.2165/00007256-200838110-00005
78. Kunkle BF, Kothandaraman V, Goodloe JB, Curry EJ, Friedman RJ, Li X, et al. Orthopaedic application of cryotherapy: a comprehensive review of the history, basic science, methods, and clinical effectiveness. JBJS Rev. (2021) 9(1):e20.00016. doi: 10.2106/JBJS.RVW.20.00016
79. Coletti RH. The ischemic model of chronic muscle spasm and pain. Eur J Transl Myol. (2022) 32(1):10323. doi: 10.4081/ejtm.2022.10323
80. O’Sullivan P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man Ther. (2005) 10(4):242–55. doi: 10.1016/j.math.2005.07.001
81. Tsao H, Tucker KJ, Hodges PW. Changes in excitability of corticomotor inputs to the trunk muscles during experimentally-induced acute low back pain. Neuroscience. (2011) 181:127–33. doi: 10.1016/j.neuroscience.2011.02.033
82. Meier ML, Vrana A, Schweinhardt P. Low back pain: the potential contribution of supraspinal motor control and proprioception. Neuroscientist. (2019) 25(6):583–96. doi: 10.1177/1073858418809074
83. Tsao H, Galea MP, Hodges PW. Driving plasticity in the motor cortex in recurrent low back pain. European journal of pain (London. England. (2010) 14(8):832–9. doi: 10.1016/j.ejpain.2010.01.001
84. Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. (2003) 151(2):262–71. doi: 10.1007/s00221-003-1457-x
85. Klyne DM, Moseley GL, Sterling M, Barbe MF, Hodges PW. Individual variation in pain sensitivity and conditioned pain modulation in acute low back pain: effect of stimulus type, sleep, and psychological and lifestyle factors. J Pain. (2018) 19(8):942.e1–.e18. doi: 10.1016/j.jpain.2018.02.017
86. O’Sullivan PB. Lumbar segmental ‘instability': clinical presentation and specific stabilizing exercise management. Man Ther. (2000) 5(1):2–12. doi: 10.1054/math.1999.0213
87. Kono Y, Kajiya H, Nagano R, Tominaga C, Maeda H, Fujita T, et al. Piezo1 promotes double-directional differentiation from human periodontal ligament progenitor cells. J Oral Biosci. (2025) 67(2):100651. doi: 10.1016/j.job.2025.100651
88. Lei L, Wen Z, Cao M, Zhang H, Ling SK, Fu BS, et al. The emerging role of Piezo1 in the musculoskeletal system and disease. Theranostics. (2024) 14(10):3963–83. doi: 10.7150/thno.96959
89. Ambattu LA, Del Rosal B, Conn CE, Yeo LY. High-frequency MHz-order vibration enables cell membrane remodeling and lipid microdomain manipulation. Biophys J. (2025) 124(1):25–39. doi: 10.1016/j.bpj.2024.10.007
90. Wall PD, Cronly-Dillon JR. Pain, itch, and vibration. Arch Neurol. (1960) 2:365–75. doi: 10.1001/archneur.1960.03840100003002
91. Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. (1993) 41(2):125–56. doi: 10.1016/0301-0082(93)90006-E
92. Boucher JA, Abboud J, Nougarou F, Normand MC, Descarreaux M. The effects of vibration and muscle fatigue on trunk sensorimotor control in low back pain patients. PLoS One. (2015) 10(8):e0135838. doi: 10.1371/journal.pone.0135838
93. De Koninck Y, Henry JL. Peripheral vibration causes an adenosine-mediated postsynaptic inhibitory potential in dorsal horn neurons of the cat spinal cord. Neuroscience. (1992) 50(2):435–43. doi: 10.1016/0306-4522(92)90435-5
94. Salter MW, De Koninck Y, Henry JL. ATP-sensitive K+ channels mediate an IPSP in dorsal horn neurones elicited by sensory stimulation. Synapse. (1992) 11(3):214–20. doi: 10.1002/syn.890110306
95. Ballard A, Khadra C, Adler S, Trottier ED, Le May S. Efficacy of the buzzy device for pain management during needle-related procedures: a systematic review and meta-analysis. Clin J Pain. (2019) 11(3):214–20. doi: 10.1097/AJP.0000000000000690
96. Lundeberg TC. Vibratory stimulation for the alleviation of chronic pain. Acta Physiol Scand Suppl. (1983) 523:1–51.6609524
97. Skoglund CR. Vasodilatation in human skin induced by low-amplitude high-frequency vibration. Clin Physiol. (1989) 9(4):361–72. doi: 10.1111/j.1475-097X.1989.tb00990.x
98. Yu CO, Leung KS, Jiang JL, Wang TB, Chow SK, Cheung WH. Low-Magnitude high-frequency vibration accelerated the foot wound healing of n5-streptozotocin-induced diabetic rats by enhancing glucose transporter 4 and blood microcirculation. Sci Rep. (2017) 7(1):11631. doi: 10.1038/s41598-017-11934-2
99. Benedetti MG, Boccia G, Cavazzuti L, Magnani E, Mariani E, Rainoldi A, et al. Localized muscle vibration reverses quadriceps muscle hypotrophy and improves physical function: a clinical and electrophysiological study. Int J Rehabil Res. (2017) 40(4):339–46. doi: 10.1097/MRR.0000000000000242
100. Pamukoff DN, Pietrosimone B, Lewek MD, Ryan ED, Weinhold PS, Lee DR, et al. Whole-body and local muscle vibration immediately improve quadriceps function in individuals with anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. (2016) 97(7):1121–9. doi: 10.1016/j.apmr.2016.01.021
101. Kneis S, Wehrle A, Ilaender A, Volegova-Neher N, Gollhofer A, Bertz H. Results from a pilot study of handheld vibration: exercise intervention reduces upper-limb dysfunction and fatigue in breast cancer patients undergoing radiotherapy: vibBRa study. Integr Cancer Ther. (2018) 17(3):717–27. doi: 10.1177/1534735418766615
102. Khalifeloo M, Naghdi S, Ansari NN, Akbari M, Jalaie S, Jannat D, et al. A study on the immediate effects of plantar vibration on balance dysfunction in patients with stroke. J Exerc Rehabil. (2018) 14(2):259–66. doi: 10.12965/jer.1836044.022
103. Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, et al. Is oxytocin “nature’s medicine"? Pharmacol Rev. (2020) 72(4):829–61. doi: 10.1124/pr.120.019398
104. Poisbeau P, Grinevich V, Charlet A. Oxytocin signaling in pain: cellular, circuit, system, and behavioral levels. Curr Top Behav Neurosci. (2018) 35:193–211. doi: 10.1007/7854_2017_14
105. Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. (2007) 1098:312–22. doi: 10.1196/annals.1384.006
106. Casale R, Damiani C, Maestri R, Fundarò C, Chimento P, Foti C. Localized 100 Hz vibration improves function and reduces upper limb spasticity: a double-blind controlled study. Eur J Phys Rehabil Med. (2014) 50(5):495–504.24651209
107. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. (2009) 139(2):267–284. doi: 10.1016/j.cell.2009.09.028
108. Lacroix JJ, Wijerathne TD. PIEZO channels as multimodal mechanotransducers. Biochem Soc Trans. (2025) 53(1):293–302. doi: 10.1042/bst20240419
109. Lewis AH, Cui AF, McDonald MF, Grandl J. Transduction of Repetitive Mechanical Stimuli by Piezo1 and Piezo2 Ion Channels. Cell Rep. (2017) 19(12):2572–2585. doi: 10.1016/j.celrep.2017.05.079
110. Brodke DS, Goz V, Voss MW, Lawrence BD, Spiker WR, Hung M. PROMIS PF CAT outperforms the ODI and SF-36 physical function domain in spine patients. Spine. (2017) 42(12):921–9. doi: 10.1097/BRS.0000000000001965
111. Bernstein DN, St John M, Rubery PT, Mesfin A. PROMIS Pain interference is superior to the likert pain scale for pain assessment in spine patients. Spine. (2019) 44(14):E852–e6. doi: 10.1097/BRS.0000000000002979
112. Nolet PS, Emary PC, Kristman VL, Murnaghan K, Zeegers MP, Freeman MD. Exposure to a motor vehicle collision and the risk of future back pain: a systematic review and meta-analysis. Accid Anal Prev. (2020) 142:105546. doi: 10.1016/j.aap.2020.105546
113. Costa Menezes L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. (2012) 184(11):E613–24. doi: 10.1503/cmaj.111271
114. Jones CMP, Day RO, Koes BW, Latimer J, Maher CG, McLachlan AJ, et al. Opioid analgesia for acute low back pain and neck pain (the OPAL trial): a randomised placebo-controlled trial. Lancet. (2023) 402(10398):304–12. doi: 10.1016/S0140-6736(23)00404-X
115. Dobson KG, Mustard C, Carnide N, Furlan A, Smith PM. Impact of persistent pain symptoms on work absence, health status and employment 18 months following disabling work-related injury or illness. Occup Environ Med. (2022) 79(10):697–705. doi: 10.1136/oemed-2022-108383
116. Ansari MA, Erfanzadeh M, Mohajerani E. Mechanisms of laser-tissue interaction: II. Tissue thermal properties. J Lasers Med Sci. (2013) 4(3):99–106.25606316
117. Ebadi S, Henschke N, Forogh B, Nakhostin Ansari N, van Tulder MW, Babaei-Ghazani A, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. (2020) 7(7):Cd009169. doi: 10.1002/14651858.CD009169.pub3
Keywords: mechanical low back pain, prevention, focal mechanical vibration, PROMIS (patient-reported outcomes measurement information system), spine biomechanics, vibration, acute low back pain (aLBP), chronic low back pain (cLBP)
Citation: Baxter AL, Etnoyer-Slaski JL, Tucker O, Williams JAR, Swartout K, Cohen LL and Lawson ML (2025) Novel multimodal mechanical stimulation is superior to TENS to treat and prevent chronic low back pain: a randomized controlled trial. Front. Pain Res. 6:1625420. doi: 10.3389/fpain.2025.1625420
Received: 8 May 2025; Accepted: 8 July 2025;
Published: 18 August 2025.
Edited by:
Laura Case, University of California, San Diego, United StatesReviewed by:
Michael W. Salter, University of Toronto, CanadaErkan Özduran, Sivas Numune Hospital, Türkiye
Copyright: © 2025 Baxter, Etnoyer-Slaski, Tucker, Williams, Swartout, Cohen and Lawson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Lynn Baxter, YWJheHRlckBhdWd1c3RhLmVkdQ==
†Deceased
 Amy Lynn Baxter
Amy Lynn Baxter Jena L. Etnoyer-Slaski3
Jena L. Etnoyer-Slaski3 Lindsey L. Cohen
Lindsey L. Cohen