- 1School of Nursing, Capital Medical University, Beijing, China
- 2Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Objective: Preterm infants may face neurodevelopmental challenges linked to altered brain maturation processes. This study aimed to investigate the impact of in-hospital breast milk intake on brain resting-state functional connectivity (rs-FC) and neurological assessment at discharge in preterm infants.
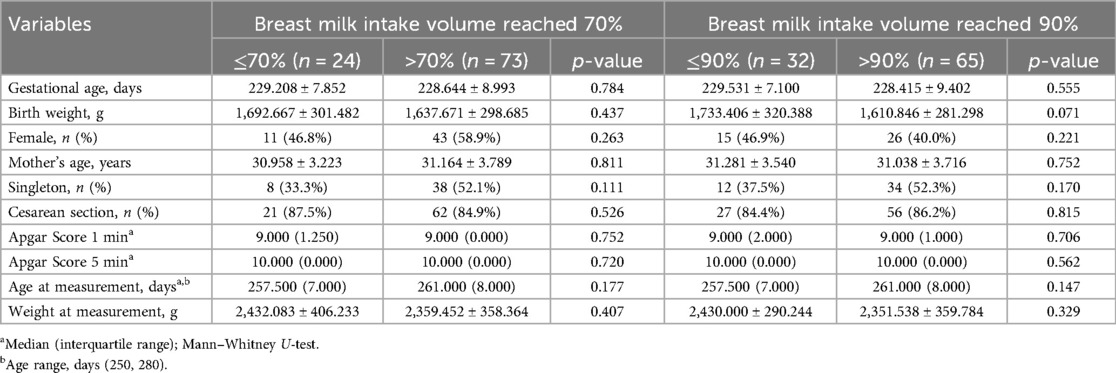
Methods: We collected data on breast milk intake from 97 preterm infants, evaluated neurological outcomes using the Amiel-Tison Neurological Assessment (ATNAT), and assessed rs-FC via functional near-infrared spectroscopy (fNIRS). Groups were stratified by breast milk intake proportion (cutoffs of >70% vs. ≤70%; cutoffs of >90% vs. ≤90%), and conducted logistic regression analysis to explore the relationship between rs-FC and neurological assessment.
Results: Preterm infants with >70% breast milk intake exhibited significantly higher ATNAT levels (p = 0.004) and stronger rs-FC (p = 0.001) between the right precentral gyrus (PCG) and inferior parietal lobe (IPL). The >90% intake group also showed higher ATNAT levels (p = 0.008) and further rs-FC enhancements (PCG-PFL: p = 0.016; PCG-IPL: p = 0.008). Logistic regression confirmed rs-FC as a predictor of optimal neurological assessment [p = 0.011, Exp (B) = 0.206, 95% CI: 0.062– 0.682].
Conclusion: Higher in-hospital breast milk intake (>70% of total enteral nutrition) improves rs-FC and neurological outcomes in preterm infants, with dose-dependent effects.
Introduction
Approximately 15 million preterm infants are born annually worldwide, accounting for more than 1 in 10 infants (1). The incidence of preterm infants in China is approximately 7.0% (2). Preterm birth affects critical steps of third-trimester and early postnatal brain development, leading to a higher incidence of neurological disorders, including issues with cognition, language, motor skills, and social emotions (3–5). These infants have greater nutritional needs, and breastfeeding plays a crucial role in their neurological development (6, 7).
The duration of breastfeeding correlates positively with microstructural changes in brain (8, 9), particularly in regions such as the frontal and temporal lobes, the internal capsule, the corticospinal tract peripheral regions, the superior longitudinal fasciculus, and the superior fronto-occipital fasciculus. These areas are associated with higher cognitive functions, including executive function, planning, socio-emotional processing, and language (10). Breast milk is associated with improved structural connectivity of developing brain networks and greater fractional anisotropy in these major white matter fasciculi (10). Such structural improvements likely underlie the observed functional connectivity enhancements. Breast milk has the potential to enhance neurodevelopmental outcomes in preterm infants by promoting the healthy development of visual, language, motor, memory, higher cognitive, and emotional functions, thus improving overall neurodevelopmental outcomes. The benefits of breast milk on neurological development may persist into old age, with a particular emphasis on enhancing verbal reasoning abilities (11).
Due to the immaturity of various developmental aspects in preterm infants, their organ function and adaptability are generally inferior compared to term infants, necessitating that preterm infants receive specialized care in the neonatal intensive care unit (NICU) (12, 13). The majority of preterm infants in the NICU receive partial breastfeeding, which means breast milk constitutes a part of the nutritional intake for preterm infants in the NICU. Despite the well-established association between breast milk and the neurological development of preterm infants (14), the effects of early in-hospital breast milk intake volume on their neurological assessment remain unclear. Therefore, this study aimed to investigate the impact of in-hospital breast milk intake on brain resting-state functional connectivity (rs-FC) and neurological assessment in preterm infants. Functional Near-Infrared Spectroscopy was chosen to measure infants' FC for its suitability in neonatal neuroimaging, and Amiel-Tison neurological assessment for its validated reliability in assessing preterm neurological development. We hypothesized that infants with higher in-hospital breast milk intake would exhibit better neurological outcomes at discharge.
Material and methods
Participants
This observational study enrolled 106 preterm infants admitted to the neonatal intensive care unit (NICU) of a Grade III Level A hospital between December 2019 and February 2021. These infants had a gestational age of 30–34 weeks and Apgar scores ≥7 at 1 and 5 minutes. Exclusion criteria included congenital malformations, chromosomal abnormalities, moderate-to-severe hypoxic-ischemic encephalopathy, grade IV periventricular/intraventricular hemorrhage, cystic periventricular leukomalacia, congenital genetic metabolic diseases, and parental history of depression, mental illness, or congenital brain development disorders. Parental history of depression or mental illness was excluded to avoid confounding by heritable neurodevelopmental risks. Ethical approval was obtained from the hospital's ethics committee (approval number: 2019-058), and written informed consent was obtained from the parents. The study was registered as ChiCTR1900027648 on ClinicalTrials.gov and followed a previously published protocol (15). Due to COVID-19 pandemic-related home confinement and isolation policies, the recruitment period was extended, and the discharge follow-up section of the protocol was omitted.
In-hospital breast milk intake
Mothers were encouraged to initiate breast milk expression immediately after delivery, with colostrum recommended for preterm infants as soon as available. Breast milk was the preferred nutritional source for preterm infants, provided the mother could express and deliver it to the NICU. Standardized feeding guidelines (16) were followed, supplemented with human milk fortifier (Similac HMFortifi, Abbott), once preterm infants reached enteral feed volumes of 80 ml/kg/day. In cases where parental consent was obtained for the use of donor human milk, it was utilized when the mother's own milk was insufficient, with preterm formula utilized as a last resort. For infants without parental consent, preterm formula was provided when maternal or donor human milk was insufficient. Daily nutritional intake in the NICU was documented electronically, encompassing both maternal and donor human milk. Detailed records of nutritional intake and volume were recorded daily, with the proportion of breast milk consumption calculated as the volume of breast milk intake relative to the total enteral nutrition volume during hospitalization.
Neurological assessment at discharge
The neurological assessment of preterm infants was assessed at discharge by a trained NICU nurse using the Amiel-Tison neurological assessment (ATNAT). ATNAT is effective in detecting abnormal neurological signs, exhibiting good inter- and intra-assessor reliability and validity (17, 18). Consisting of 35 items, ATNAT utilizes a non-quantitative scoring system based on a three-point ordinal scale: “0” for typical responses, “1” for moderately abnormal responses, and “2” for definitely abnormal responses. Scores were assigned accordingly by the nurse, who remained blinded to infants' breast milk intake. The assessment typically takes 10–15 min to complete. To simplify analysis, responses categorized as “1” and “2” were combined to indicate non-optimal neurological assessment due to the infrequent occurrence of definitely abnormal responses (n = 2). Consequently, infants were classified as having either optimal or non-optimal neurological assessment.
fNIRS measurements
Functional connectivity (FC) is investigated in early brain development to understand the functional integration of different brain regions (19). FC represents the statistical relationship between brain areas, forming functional connectivity networks (FCNs) (20). Functional Near-Infrared Spectroscopy (fNIRS) is a noninvasive neuroimaging technique sensitive to the developmental integration of circulatory, neurovascular, and metabolic functions in neonatal and infant brains (21). Previous studies have validated fNIRS for assessing the cerebrum (22), and recent studies had extended its utility to studying the neonatal cerebral cortex (23, 24).
Resting-state (rs) FC was measured using fNIRS at preterm infants' discharge. Infants were placed in a quiet, dimly lit room after feeding, where they wore fNIRS headgear. Data collection began once infants acclimated to the environment and headgear, with their actions recorded during the process. Soft silicone pads within the headgear and a headrest at the occipital lobe position aided measurements. Data collection occurred with infants wearing a electrocardiogram monitor, supervised by the same nurse.
Hemodynamic changes were collected using NirSmart (NirScan Inc., HuiChuang, Beijing), measuring oxygenated and deoxygenated hemoglobin concentration changes in the cortex at 760 and 850 nm wavelengths. The device, equipped with 58 channels (22 sources and 16 detection probes), had a sampling rate of 10 Hz. Optode probes were placed at T3, T4, Fpz, and Oz points following the international 10–20 system, with a 2.0 cm distance between the source and detector defining each measurement channel. The measurement area encompassed the prefrontal lobe, occipital lobe, bilateral motor area, and bilateral temporal lobe, comprising the regions of interest (ROIs) for this study. ROIs (n = 15) were determined based on Brodmann areas (25), as presented in Supplementary Table 1. Figure 1 illustrates the brain areas covered by the fNIRS array. Data were recorded using NIRScan software (NirScan Inc., HuiChuang, Beijing).
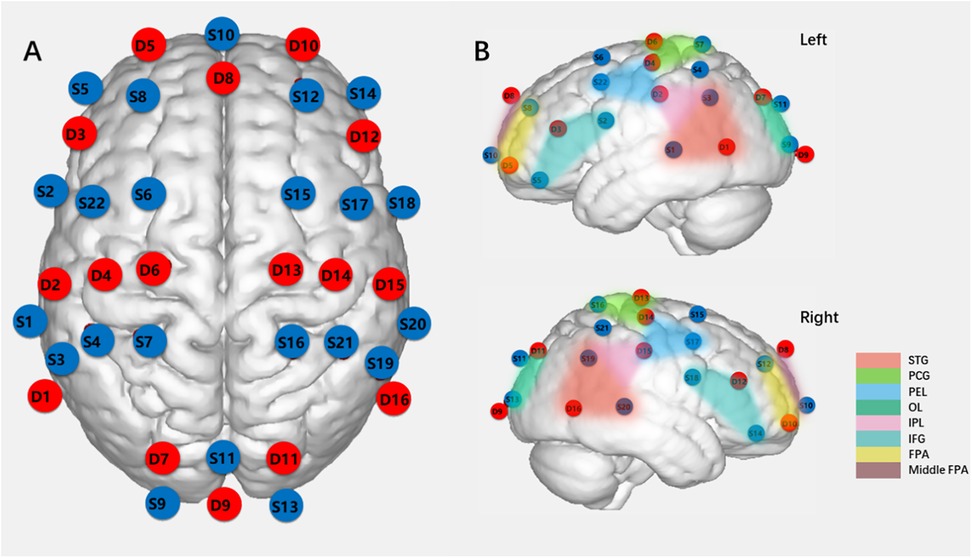
Figure 1. Schematic diagram of light sources and detection probes locations and ROIs (ROIs = 15). (A) Locations of 22 light sources and 16 dtection probes on fNIRs headcap (S:light source, D: dtection probe). (B) ROIs (Regions of interest), (STG:, superior temporal gyrus; PCG, precentral gyrus; PEL, posterior frontal lobe; OL, occipital lobe; IPL, inferior parietal lobe; IFG, inferior frontal gyrus; FPA, frontopolar area.).
fNIRS data processing
fNIRS data preprocessing was conducted using HomER2 (26), a MATLAB-based graphical user interface program. Channels with poor light intensity (signal-to-noise ratio <1.5), indicative of suboptimal optode-scalp contact, were excluded. Raw optical intensity was normalized to optical density (OD) by dividing by the mean intensity, yielding a relative (percent) concentration change. OD data were further converted to oxy-hemoglobin (HbO) and deoxy-hemoglobin (HbR) using the modified Beer–Lambert law (27).
During measurements, intervals corresponding to preterm infants' movements, crying, or other actions were manually marked as invalid by the same NICU nurse. Considering the minimum duration of −2 to 8 s required for infants' hemodynamic response function to return to baseline levels (28), 10 s of data were excluded after each invalid section across all channels to ensure the inclusion of only resting-state periods. Valid data sections were included only if they were consecutively at least 5 s long without interruption. Participants with ≥300 s of total valid data (29) and >70% reserved channels were included for further analysis. Artifact correction involved wavelet filtering (30) and principal component analysis (31), with a band-pass filter (0.01–0.1 Hz) applied to remove low-frequency noise and physiological interference.
Statistical analysis
Demographic and clinical characteristics between groups were compared using the Mann–Whitney U test or two-sample t-test in SPSS 26.0. After obtaining FC values (Correlation, COR; Coherence, COH; Phase Locking Value, PLV) derived from hemodynamic signals (oxy-hemoglobin, HbO; deoxy-hemoglobin, HbR; total hemoglobin), between-group t-tests were conducted, with significant results corrected for multiple comparisons using the false discovery rate (FDR) method (32). COR reflects linear temporal relationships between hemodynamic signals (HbO, HbR and total hemoglobin) in distinct brain regions. COH measures frequency-dependent synchronization of signals, capturing shared oscillatory activity. PLV quantifies phase consistency between signals across trials, indicating stable inter-regional coupling. These metrics are widely validated in neonatal fNIRS studies (30–32). A 15 × 15 p-value matrix (COR of HbR) illustrating the results of between-group comparisons was generated.
Furthermore, multinomial logistic regression was performed in SPSS 26.0 to explore the relationships between FC and ATNAT scores, with confounding factors such as sex, mother's age, singleton status, delivery mode, gestational age, birth weight, and breast milk intake included as covariates. Covariates (sex, gestational age, etc.) were selected a priori based on their established impact on preterm outcomes (12, 15). A p-value of <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
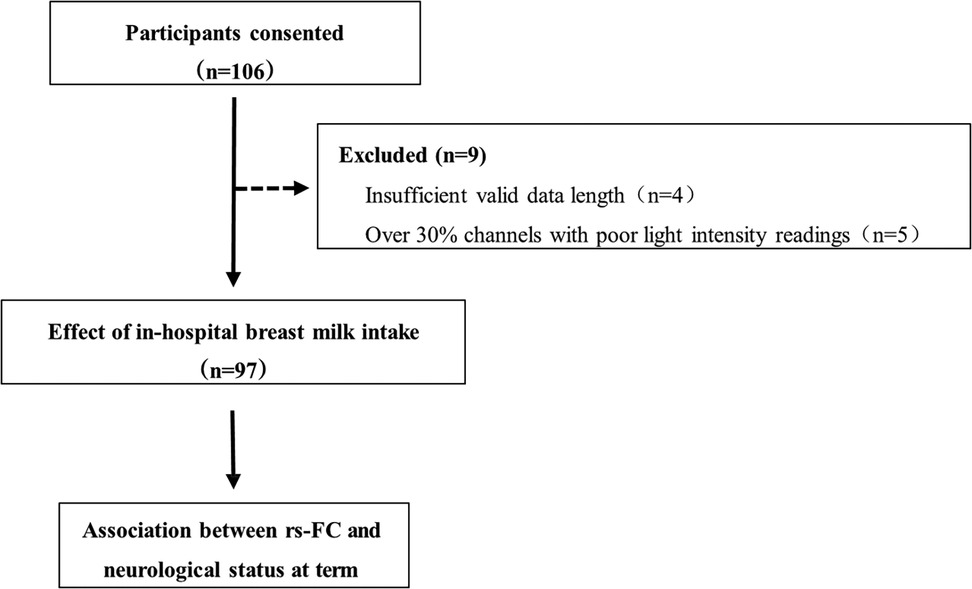
A total of 106 participants were recruited for the study. Nine preterm infants were initially measured but subsequently excluded from the analyses due to insufficient valid data (<300 s), and five due to poor channel light intensity readings (>30%), as shown in Figure 2. Consequently, the study included 97 preterm infants, among whom 73 had breast milk intake exceeding 70% of in-hospital total enteral nutrition, while 24 had intake equal to or less than 70% (Table 1). Furthermore, subgroup analyses were conducted to assess the impact of breast milk intake, comparing infants with over 90% intake to those with 90% or less. Table 1 presents the demographic and clinical characteristics stratified by breast milk intake thresholds. The proportion of breast milk intake during hospitalization ranged from 5.73% to 100% for preterm infants.
Functional brain connectivity
FC between the right frontal lobe and right occipital lobe was significantly increased in preterm infants breastfed with >70% in the hospital (p = 0.001, FDR, Figure 3A1) compared to those breastfed with ≤70%. This connectivity involved the right precentral gyrus (PCG) and the right inferior parietal lobe (IPL), as illustrated in Figure 3B1. Furthermore, in preterm infants receiving >90% of in-hospital breast milk, FC in these neural systems was further enhanced, particularly in the right frontal lobe. Specifically, significant differences were observed in FC between the right PCG and right posterior frontal lobe (PFL) (p = 0.016, FDR, Figure 3A2), as well as between the right PCG and right IPL (p = 0.008, FDR). The anatomical labels depicting altered FC are presented in Figure 3B2.
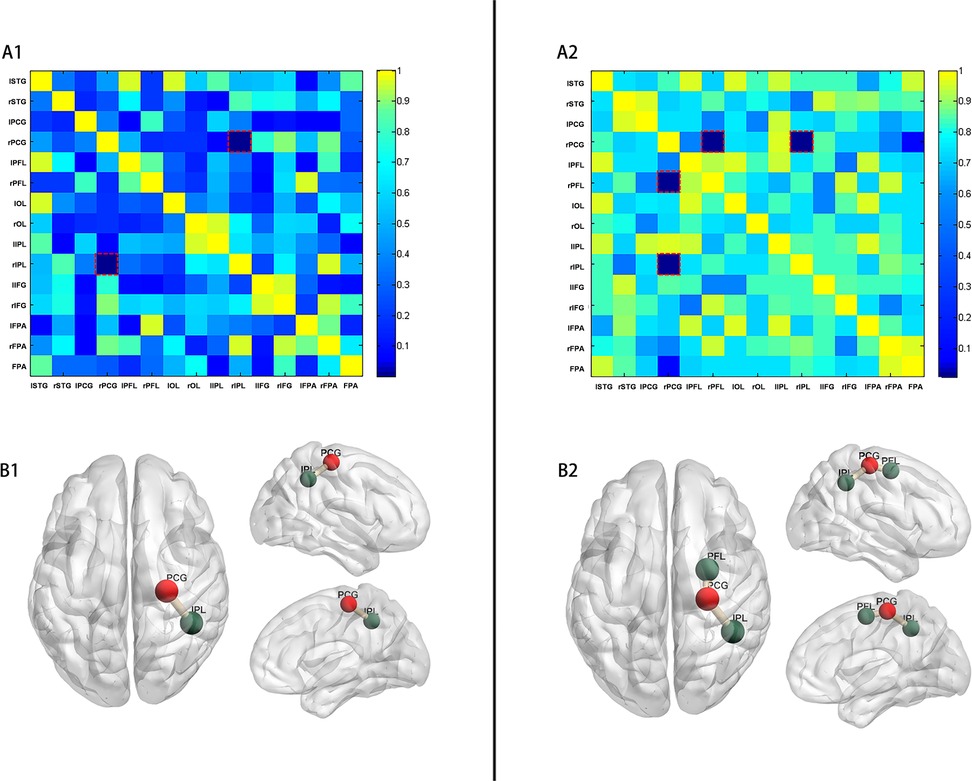
Figure 3. p-values of comparison of FC (COR of HbR). (A) p-values (adjusted p-values after FDR) matrix of t-test for FC (COR of HbR) between two groups of preterm infants (A1: preterm infants with in-hospital breast milk intake of >70% and ≤70%; A2: preterm infants with in-hospital breast milk intake of >90% and ≤90%). The horizontal and vertical coordinates represent the ROI number. (B) Resting-state FC was increased between the right PCG and the right IPL (p = 0.001) in preterm infants with in-hospital breast milk intake of >70% (B1). Resting-state FC was increased between the right PCG and the right IPL (p = 0.008) and between the right PCG and the right PFL (p = 0.016) in preterm infants with in-hospital breast milk intake of >90% (B2). (PCG, precentral gyrus; IPL, inferior parietal lobe; PFL, posterior frontal lobe).
Neurological assessment at term
Significant differences were observed in the neurological assessment at discharge (p = 0.004) between preterm infants with >70% and those with ≤70% of in-hospital breast milk intake. A higher proportion of breast milk intake among preterm infants was associated with optimal neurological assessment at term age. Similarly, significant differences were noted in the neurological assessment at discharge (p = 0.008) between preterm infants with >90% and those with ≤90% of in-hospital breast milk intake volume.
Logistic regression analysis was conducted with demographic and clinical characteristics as covariates (sex, mother's age, singleton status, delivery mode, gestational age, birth weight, and breast milk intake), FC as a predictor, and ATNAT results as the outcome variable. The results are presented in Table 2. The regression model between FC (specifically, between the right PCG and right IPL) and ATNAT was statistically significant (p = 0.011, B = −1.578, Exp[B] = 0.206, 95% confidence interval [CI]: 0.062– 0.682; Hosmer and Lemeshow Test: Chi-square = 5.637, p = 0.688).
Discussion
The study results indicate that preterm infants with >70% in-hospital breast milk intake exhibit better neurological assessment and enhanced development of rs-FC in the brain compared to those with ≤70% intake. Furthermore, our findings suggest that variations in rs-FC might correspond to differences in the neurological assessment of preterm infants. This study offers new insights into the neural basis of divergent neurological outcomes among preterm infants.
The present study unveiled an increase in rs-FC between the right PCG and the right supramarginal gyrus in preterm infants with an in-hospital breast milk intake volume exceeding 70%, with this positive effect demonstrating a dose-dependent pattern: infants with >90% breast milk intake exhibited stronger rs-FC than the >70% group, implying that higher proportions may yield incremental benefits. However, a definitive threshold requires validation in larger cohorts with granular intake data. Our findings support mother-infant bonding practices and DHM supply in NICU. These interventions may inhance infants' breast milk intake in NICU.
The PCG, serving as the primary motor cortex, and the supramarginal gyrus, situated in the IPL, jointly contribute to motor complexity processing, crucial for action perception and execution (33). Notably, previous research has highlighted a positive correlation between enhanced fine motor skills and enlarged PCG size in children (34). Furthermore, stroke patients exhibited improved upper limb movement following training, associated with increased FC of both the primary motor cortex and supramarginal gyrus (35).
The PFL encompasses the lateral and medial divisions of the pre-motor cortex and supplementary motor cortex. Alterations in rs-FC of the motor area have been observed in preterm infants, with a prevalent decrease in FC of the pre-motor cortex noted in very preterm infants compared to full-term counterparts (36). The development of executive functions, indicative of meaningful outcomes, has garnered attention (37). The observed increase in FC implies an enhancement in executive function related to volitional movement, suggesting that a higher proportion of early breast milk intake might contribute to improved executive function in preterm infants.
Blesa et al. (8) demonstrated an augmented fractional anisotropy-weighted connectivity, as observed through MRI, in infants who received ≥75% exclusive breast milk feeds compared to those who did not. Moreover, the degree of anatomical connectivity was further enhanced in these neural systems among infants who received ≥90% exclusive breast milk. Our results also indicate that increased functional connectivity (FC) serves as a predictor of the neurological assessment of preterm infants, independent of factors such as gestational age, birth weight, sex, singleton status, delivery mode, weight at assessment, and breast milk intake. This suggests that a higher proportion of breast milk intake might influence the neurological assessment of preterm infants by enhancing rs-FC. Preterm infants exhibiting increased FC were more likely to be assessed with optimal neurological assessment at their corrected gestational age at term. Evidence has suggested that early breast milk exposure may exert lasting effects on functional connectivity (38). For instance, longitudinal MRI studies report sustained improvements in white matter microstructure and cognitive outcomes in preterm infants receiving high breast milk volumes during hospitalization (10, 39). While our data are limited to the neonatal period, future studies should explore whether rs-FC enhancements observed here correlate with long-term neurodevelopmental trajectories.
Breast milk contains neuroprotective agents such as lactoferrin, insulin-like growth factor-1 (IGF-1), and long-chain polyunsaturated fatty acids (LC-PUFAs), which modulate neuroinflammation, synaptic plasticity, and myelination (14). Human milk oligosaccharides (HMOs) may further promote gut-brain axis signaling, indirectly supporting connectivity. Fortification with LC-PUFAs or HMOs could theoretically amplify these benefits, though evidence remains limited (40). A recent study found that breast milk was protective for preterm infants' emotionally reactiveand sleep problems (41), enhancing the positive effect of breast milk to brain development.
Limitations
The present study has several limitations. The relatively small sample size may limit the generalizability of the findings. Additionally, the absence of normality tests for rs-FC connectivity values before conducting t-tests may have influenced the results. In future research, we intend to address these limitations by expanding the sample size and conducting comprehensive statistical tests to enhance the reliability and validity of our findings. This study focused on outcomes at discharge; however, whether rs-FC improvements persist beyond NICU care or correlate with long-term outcomes remains unknown. Future longitudinal studies should address this critical gap. While this study considered some confounding factors, completely eliminating them is challenging due to potential unobserved confounders or measurement errors. Therefore, while the use of covariates in this study may mitigate their impact, it cannot completely eliminate their influence.
Conclusion
The study findings suggested that preterm infants with a higher in-hospital breast milk intake were more likely to demonstrate optimal neurological assessment compared to those with a lower intake. Moreover, this effect exhibits a volume-dependent relationship. Additionally, our results indicated that variations in rs-FC may correspond to differences in the neurological assessment of preterm infants.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Women's Hospital, School of Medicine, Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
RY: Conceptualization, Funding acquisition, Investigation, Writing – review & editing, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft. HW: Data curation, Resources, Supervision, Writing – review & editing. QC: Investigation, Methodology, Writing – review & editing. DC: Data curation, Investigation, Writing – review & editing. JZ: Resources, Supervision, Writing – review & editing. SY: Investigation, Writing – review & editing. FW: Supervision, Writing – review & editing. XX: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the Scientific Research Fund of the National Health and Health Commission-Zhejiang Major Medical Science and Technology Plan (WKJ-ZJ-2008) and Beijing Social Science Fund Planning Project (23XCC021).
Acknowledgments
The authors are grateful to the participants in the study and nurses and physicians of NICU of Women's Hospital School of Medicine Zhejiang University for facilitating the collection of samples. The authors also acknowledge Fan Qu, who assisted in the writing and editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1508840/full#supplementary-material
Abbreviations
ATNAT, Amiel-Tison neurological assessment; COH, coherence; COR, correlation; FC, functional connectivity; FCNs, functional connectivity networks; FDR, false discovery rate; fNIRS, functional near-infrared spectroscopy; HbO, oxy-hemoglobin; HbR, deoxy-hemoglobin; IPL, inferior parietal lobe; NICU, neonatal intensive care unit; OD, optical density; PCG, precentral gyrus; PFL, posterior frontal lobe; PLV, phase locking value; ROIs, regions of interest; rs-FC, resting-state functional connectivity.
References
1. WHO. Preterm birth [Internet]. (2018). Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed October 10, 2024).
2. National Health Commission of People’s Republic of China. Interpretation of preterm infants health care work norms and policies. (2017). Available at: http://www.nhc.gov.cn/fys/mrgzdt/201703/0816ecd330c04c338c3fd468f1baf29f.shtml (Accessed October 10, 2024).
3. Tokariev A, Stjerna S, Lano A, Metsäranta M, Palva JM, Vanhatalo S. Preterm birth changes networks of newborn cortical activity. Cereb Cortex. (2019) 29(2):814–26. doi: 10.1093/cercor/bhy012
4. Tibrewal M, Cheng B, Dohare P, Hu F, Mehdizadeh R, Wang P, et al. Disruption of interneuron neurogenesis in premature newborns and reversal with estrogen treatment. J Neurosci. (2018) 38(5):1100–13. doi: 10.1523/JNEUROSCI.1875-17.2017
5. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. (2018) 125(1):16–25. doi: 10.1111/1471-0528.14832
6. Burnett AC, Anderson PJ, Lee KJ, Roberts G, Doyle LW, Cheong JLY, et al.. Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics. (2018) 141(1):e20171958. doi: 10.1542/peds.2017-1958
7. Boutwell BB, Young JTN, Meldrum RC. On the positive relationship between breastfeeding & intelligence. Dev Psychol. (2018) 54(8):1426–33. doi: 10.1037/dev0000537
8. Grevet LT, Teixeira DS, Pan PM, Jackowski AP, Zugman A, Miguel EC, et al. The association between duration of breastfeeding and the trajectory of brain development from childhood to young adulthood: an 8-year longitudinal study. Eur Child Adolesc Psychiatry. (2024) 33(6):1863–73. doi: 10.1007/s00787-023-02283-9
9. Núñez C, García-Alix A, Arca G, Agut T, Carreras N, Portella MJ, et al. Breastfeeding duration is associated with larger cortical gray matter volumes in children from the ABCD study. J Child Psychol Psychiatry. (2023) 64(7):1067–79. doi: 10.1111/jcpp.13790
10. Blesa M, Sullivan G, Anblagan D, Telford EJ, Quigley AJ, Sparrow SA, et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. (2019) 184:431–9. doi: 10.1016/j.neuroimage.2018.09.045
11. Rantalainen V, Lahti J, Henriksson M, Kajantie E, Mikkonen M, Eriksson JG, et al. Association between breastfeeding and better preserved cognitive ability in an elderly cohort of Finnish men. Psychol Med. (2018) 48(6):939–51. doi: 10.1017/S0033291717002331
12. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Netw Open. (2021) 4(5):e219382. doi: 10.1001/jamanetworkopen.2021.9382
13. Yang Y, Lu H. Breastfeeding in hospitalised preterm infants: a survey from 18 tertiary neonatal intensive care units across mainland China. J Paediatr Child Health. (2020) 56(9):1432–7. doi: 10.1111/jpc.14967
14. Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7
15. Yang R, Zhang Y, Wang H, Xu X. Effects of in-hospital breast feeding on brain function development in preterm infants in China: study protocol for a prospective longitudinal cohort study. BMJ Open. (2020) 10:e038879. doi: 10.1136/bmjopen-2020-038879
16. General Office of China National Health. Commission. Preterm infant health care work norms. (2017). Available at: http://www.nhc.gov.cn/fys/s3585/201703/d5656db4e43a49ff9d14470864b0fb16.shtml (Accessed October 10, 2024).
17. Deschênes G, Gosselin J, Couture M, Lachance C. Interobserver reliability of the Amiel-Tison neurological assessment at term. Pediatr Neurol. (2004) 30(3):190–4. doi: 10.1016/j.pediatrneurol.2003.09.005
18. Simard MN, Lambert J, Lachance C, Audibert F, Gosselin J. Interexaminer reliability of Amiel-Tison neurological assessments. Pediatr Neurol. (2009) 41(5):347–52. doi: 10.1016/j.pediatrneurol.2009.05.010
19. Mohammadi-Nejad AR, Mahmoudzadeh M, Hassanpour MS, Wallois F, Muzik O, Papadelis C, et al. Neonatal brain resting-state functional connectivity imaging modalities. Photoacoustics. (2018) 10:1–19. doi: 10.1016/j.pacs.2018.01.003
20. Tokariev A, Roberts JA, Zalesky A, Zhao X, Vanhatalo S, Breakspear M, et al. Large-scale brain modes reorganize between infant sleep states and carry prognostic information for preterms. Nat Commun. (2019) 10(1):2619. doi: 10.1038/s41467-019-10467-8
21. Lee CW, Blanco B, Dempsey L, Chalia M, Hebden JC, Caballero-Gaudes C, et al. Sleep state modulates resting-state functional connectivity in neonates. Front Neurosci. (2020) 14:347. doi: 10.3389/fnins.2020.00347
22. Sortica da Costa C, Cardim D, Molnar Z, Kelsall W, Ng I, Czosnyka M, et al. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr Res. (2019) 86(2):247–53. doi: 10.1038/s41390-019-0410-z
23. Frie J, Bartocci M, Lagercrantz H, Kuhn P. Cortical responses to alien odors in newborns: an fNIRS study. Cereb Cortex. (2018) 28(9):3229–40. doi: 10.1093/cercor/bhx194
24. Arimitsu T, Minagawa Y, Yagihashi T, Uchida MO, Matsuzaki A, Ikeda K, et al. The cerebral hemodynamic response to phonetic changes of speech in preterm and term infants: the impact of postmenstrual age. NeuroImage Clin. (2018) 19:599–606. doi: 10.1016/j.nicl.2018.05.005
25. Jamie W. The Student’s Guide to Cognitive Neuroscience. 3rd ed. 27 Church Road, Hove, East Sussex, BN3 2FA: Psychology Press (2019).
26. Huppert TJ, Diamond SG, Franceschini MA, Boas DA. Homer: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. (2009) 48(10):D280–98. doi: 10.1364/ao.48.00d280
27. Kocsis L, Herman P, Eke A. The modified beer-Lambert law revisited. Phys Med Biol. (2006) 51(5):N91–8. doi: 10.1088/0031-9155/51/5/N02
28. Taga G, Watanabe H, Homae F. Developmental changes in cortical sensory processing during wakefulness and sleep. Neuroimage. (2018) 178:519–30. doi: 10.1016/j.neuroimage.2018.05.075
29. Bulgarelli C, de Klerk CCJM, Richards JE, Southgate V, Hamilton A, Blasi A. The developmental trajectory of fronto-temporoparietal connectivity as a proxy of the default mode network: a longitudinal fNIRS investigation. Hum Brain Mapp. (2020) 41(10):2717–40. doi: 10.1002/hbm.24974
30. Di Lorenzo R, Pirazzoli L, Blasi A, Bulgarelli C, Hakuno Y, Minagawa Y, et al. Recommendations for motion correction of infant fNIRS data applicable to multiple data sets and acquisition systems. Neuroimage. (2019) 200:511–27. doi: 10.1016/j.neuroimage.2019.06.056
31. Delgado Reyes LM, Bohache K, Wijeakumar S, Spencer JP. Evaluating motion processing algorithms for use with functional near-infrared spectroscopy data from young children. Neurophotonics. (2018) 5(2):025008. doi: 10.1117/1.NPh.5.2.025008
32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodol). (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
33. Li X, Krol MA, Jahani S, Boas DA, Tager-Flusberg H, Yücel MA. Brain correlates of motor complexity during observed and executed actions. Sci Rep. (2020) 10(1):10965. doi: 10.1038/s41598-020-67327-5
34. Bolk J, Padilla N, Forsman L, Broström L, Hellgren K, Åden U. Visual-motor integration and fine motor skills at 6 (1/2) years of age and associations with neonatal brain volumes in children born extremely preterm in Sweden: a population-based cohort study. BMJ Open. (2018) 8(2):e020478. doi: 10.1136/bmjopen-2017-020478
35. Wang H, Xu G, Wang X, Sun C, Zhu B, Fan M, et al. The reorganization of resting-state brain networks associated with motor imagery training in chronic stroke patients. IEEE Trans Neural Syst Rehabil Eng. (2019) 27(10):2237–45. doi: 10.1109/TNSRE.2019.2940980
36. Gozdas E, Parikh NA, Merhar SL, Tkach JA, He L, Holland SK. Altered functional network connectivity in preterm infants: antecedents of cognitive and motor impairments? Brain Struct Funct. (2018) 223(8):3665–80. doi: 10.1007/s00429-018-1707-0
37. Corradi-Dell’Acqua C, Ronchi R, Thomasson M, Bernati T, Saj A, Vuilleumier P. Deficits in cognitive and affective theory of mind relate to dissociated lesion patterns in prefrontal and insular cortex. Cortex. (2020) 128:218–33. doi: 10.1016/j.cortex.2020.03.019
38. Diéguez E, Nieto-Ruiz A, Martín-Pérez C, Sepúlveda-Valbuena N, Herrmann F, Jiménez J, et al. Association study between hypothalamic functional connectivity, early nutrition, and glucose levels in healthy children aged 6 years: the COGNIS study follow-up. Front Nutr. (2022) 9:935740. doi: 10.3389/fnut.2022.935740
39. Poppe T, Tottman AC, Gamble GD, Jiang Y, Silva AE, Nguyen L, et al. Neonatal nutrition and brain structure at 7 years in children born very preterm. JAMA Netw Open. (2025) 8(1):e2456080. doi: 10.1001/jamanetworkopen.2024.56080
40. Wejryd E, Freiholtz Jern E, Marchini G, Åden U, Landberg E, Abrahamsson T. Human milk oligosaccharides in breast milk at two weeks of age in relation to neurodevelopment in 2-year-old children born extremely preterm. An Explorative Trial. Nutrients. (2025) 17(5):832. doi: 10.3390/nu17050832
Keywords: preterm infants, NICU, breast milk, neurological assessment, breastfeeding
Citation: Yang R, Wang H, Cai Q, Chen D, Zhu J, Yuan S, Wang F and Xu X (2025) Higher in-hospital proportion of breast milk intake improves brain functional connectivity and neurological assessment in preterm infants. Front. Pediatr. 13:1508840. doi: 10.3389/fped.2025.1508840
Received: 10 October 2024; Accepted: 31 March 2025;
Published: 16 April 2025.
Edited by:
Xinran Liu, Peking University People’s Hospital, ChinaReviewed by:
Marcel Henrique Marcondes Sari, Federal University of Paraná, BrazilKatherine M. Ottolini, Children's National Hospital, United States
Copyright: © 2025 Yang, Wang, Cai, Chen, Zhu, Yuan, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, ZmFuZ3dAemp1LmVkdS5jbg==; Xinfen Xu, eHV4aW5mQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Rui Yang1,†
Rui Yang1,† Qian Cai
Qian Cai Jiajun Zhu
Jiajun Zhu Xinfen Xu
Xinfen Xu