- 1School of Stomatology, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2School of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Zhejiang-Hong Kong Joint Laboratory of Liver and Spleen Simultaneous Treatment in Traditional Chinese Medicine, Hangzhou, Zhejiang, China
Background: Rhizoma alismatis is a traditional Chinese medicine with a long history. It is an important part of many prescriptions and is often used to treat water metabolism-related diseases in clinical practice. At present, there are 12 species of R. alismatis, of which only Alisma plantago-aquatica L. and A. plantago-aquatica subsp. orientale (Sam.) Sam are used as traditional Chinese medicine.
Objective: Based on the scientific literature, this paper aims to provide comprehensive and up-to-date information on the botany, traditional uses, phytochemistry, pharmacology, toxicology and processing methods of R. alismatis. Furthermore, it seeks to analyze current research findings to establish a new foundation and direction for future studies.
Methods: Multidisciplinary research domains including botanical identification, ethnopharmacological applications, phytochemical constituents, pharmacological activities, toxicological profiles, and processing techniques, drawing upon extensive data retrieved from PubMed, Web of science, CNKI, and other authoritative databases.
Results: Traditional Chinese medicine believes that R. alismatis has the effects of promoting water and dampness and venting heat. Modern studies have found that its extracts and isolated compounds have diuretic, liver protection, lowering blood pressure, lowering blood glucose, anti-cancer, anti-inflammatory and antioxidant activities. The toxicity of R. alismatis has long been a controversial topic, and it is generally held that no obvious adverse reactions occur within the prescribed dosage range.
Conclusion: Modern studies partially explains the traditional concept of R. alismatis’ functions and the corresponding pharmacodynamic material basis. It is necessary to further study the network relationship between traditional usage, modern pharmacology and toxicity, and standardize the cultivation, processing and circulation system of R. alismatis.
1 Introduction
Rhizoma alismatis (known as “Zexie” in Chinese), a perennial aquatic herb of the genus Alisma (Alismataceae), predominantly inhabits temperate and subtropical marshlands across the Northern Hemisphere (Commission, 2020). Among 12 globally recognized Alisma species, six are endemic to China, with Alisma plantago-aquatica L. and A. plantago-aquatica subsp. orientale (Sam.) Sam holding particular medicinal significance. These species were first documented in the Book of Songs·Wei Feng (ca. 11th-7th century BCE) (Shu et al., 2016) and subsequently classified as a superior-grade (shang pin) botanical drug in the Shennong’s Herbal Classic (ca. 200–300 CE) for their dampness-resolving, diuretic, and kidney-tonifying properties (Anonymous, 2022). In clinical practice, it is often used as a sovereign drug, and compatible with different TCMs for the treatment of numerous diseases.
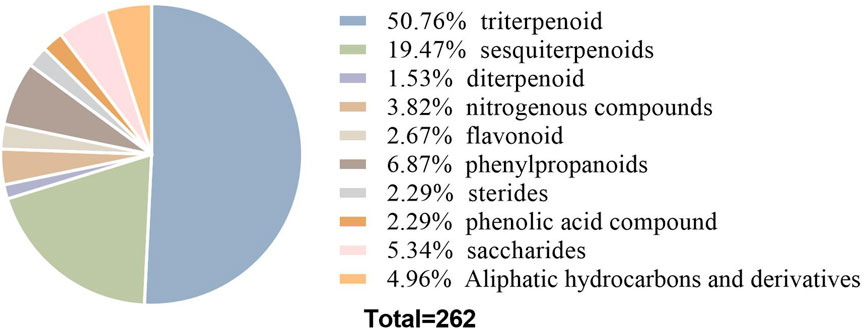
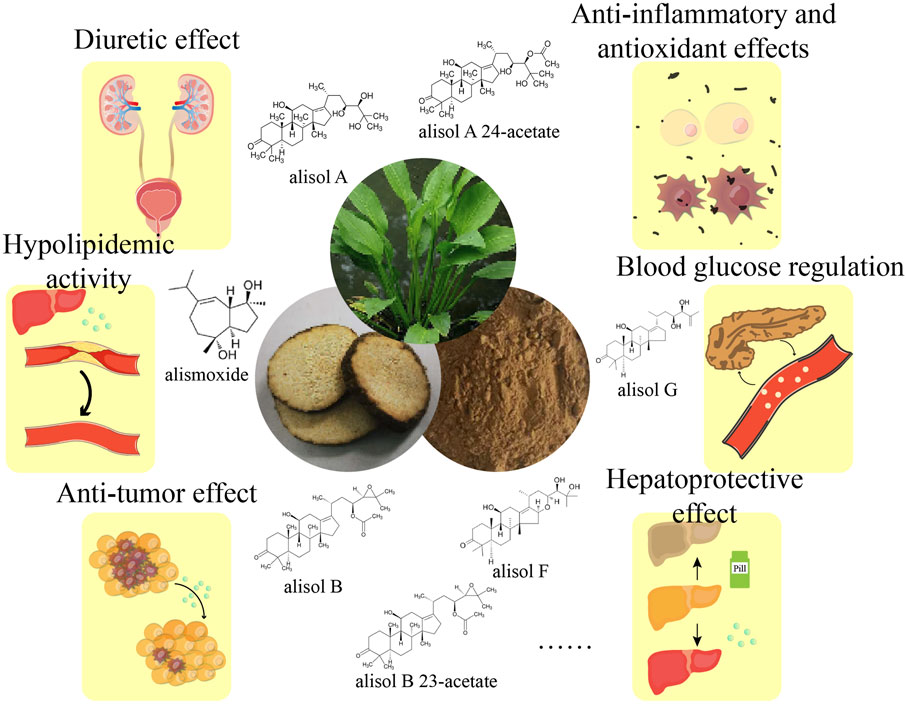
Rhizoma alismatis encompasses numerous chemical constituents, of which 262 metabolites have been identified thus far. According to the structure, these metabolites can be primarily classified into seven categories: terpenoids, sugars, nitrogen-containing compounds, phenylpropanoids, flavonoids, steroids and phenolic acids. Among them, terpenoids are particularly abundant, including triterpenoids, sesquiterpenes and diterpenes. Triterpenes are considered to be the main active ingredients of R. alismatis. The structure of triterpenoids is mostly prototerpene tetracyclic triterpenoids, including alisol A, alisol B, alisol C, and alisol B 23-acetate etc. (Xiao et al., 2024). Modern pharmacology has demonstrated its diuretic (Huang et al., 2016; Li R. et al., 2020; Wang et al., 2008), kidney stone protective, lipid-lowering (Miao et al., 2016; Yan P. et al., 2022), hypoglycemic (Lau et al., 2008), liver-protecting (Hur et al., 2007; Sun et al., 2020) and antibacterial (Jin et al., 2012) and anti-tumor (Li L. et al., 2020; Liu et al., 2019; Lou et al., 2019; Wu et al., 2023; Xu et al., 2015; Zhang et al., 2016) effects.
In recent years, the toxicity of Chinese botanical drugs has been paid more and more attention. Although TCM theory posits that R. alismatis is non-toxic and may confer tonic benefits with prolonged use (Anonymous, 2022; Jing, 1957), modern pharmacological studies have elucidated its dose-dependent toxicological characteristics, including dermal toxicity (Cash crop team of Guangdong Agriculture, 1970), gastrointestinal reactions and systemic toxicity (Chen and Zhou, 1998). However, most of the above situations occur when the dose is much higher than the conventional dose and the high dose is rich in the extract of R. alismatis terpenoids. Therefore, it is necessary to conduct in-depth and systematic research to objectively and comprehensively evaluate the clinical toxicity and adverse reactions of R. alismatis.
Pharmaceutical processing (Paozhi), a critical practice in TCM, modulates the therapeutic profile of medicinal materials through targeted physicochemical modifications (Qiu, 2003). Rhizoma alismatis undergoes multiple processing techniques, including: salt-water immersion, wine stir-frying (Zhong et al., 2005), salt stir-frying, bran stir-frying, soil stir-frying (Wang, 1993). Contemporary practice prioritizes bran-frying and salt-processing due to their distinct pharmacodynamic outcomes. Bran-fried R. alismatis demonstrates enhanced dampness-resolving properties with spleen-tonifying and turbidity-reducing capacities, whereas salt-processed variants exhibit yin-nourishing effects coupled with heat-clearing and diuretic actions (Yan et al., 2020; Zhong et al., 2005). Mechanistic studies attribute these functional shifts to processing-induced triterpenoid profile alterations, particularly the content of protostane-type triterpenes (Yan et al., 2023).
Drawing on data and feedback from clinical practice, we have identified key challenges in the application of R. alismatis. These challenges stem from issues in its cultivation, material quality, processing and preparation, contradictions between traditional uses and modern pharmacological research, as well as ongoing debates concerning its toxicity. To address these gaps, we have undertaken a comprehensive effort by compiling extensive literature, conducting a systematic analysis, and incorporating the latest pharmacological evidence. This work aims to provide a comprehensive and up-to-date resource to support the effective and safe clinical use of R. alismatis.
2 Research methodology
This study conducted a systematic review of R. alismatis research spanning 5 decades (1970–2024). A comprehensive analysis was performed on publications addressing botanical characteristics, ethnomedicinal applications, phytochemical profiles (including secondary metabolites), pharmacological properties, toxicological assessments, and processing methodologies. The search protocol utilized multidisciplinary databases (Scopus, Web of science, PubMed, ACS Publications) alongside specialized platforms (CNKI, WanFang) and broad-coverage engines.
Search strategies incorporated controlled vocabulary and natural language terms: 1. Base term: R. alismatis, 2. Thematic expansions: chemical composition, traditional medicine, toxicity, pharmacological activity. 3. Functional modifiers: diuretic, anti-inflammatory, antioxidant, hypoglycemic, hepatoprotective, antibacterial, anti-tumor. 4. Process terminology: processing. The search results yielded a substantial number of articles, which were then evaluated for relevance based on their titles and abstracts, utilizing Boolean operators (AND, OR) to optimize the search strategy. We also meticulously reviewed the reference lists of these papers to identify any additional articles pertinent to this literature review. Literature inclusion criteria: Studies on R. alismatis, relevance to the review scope, and coverage of botany, ethnomedicine, pharmacology, toxicology, chemistry, and processing methods. The exclusion criteria were articles without full texts and irrelevant articles that fell outside the scope of this review.
Unlike existing reviews, this study critically addresses research limitations and emphasizes the necessity to: analyze historical changes in the functional uses of Alisma in medicine; integrate reported high-abundance active metabolites; clarify active metabolites and their key molecular targets and mechanisms of action; summarize key aspects of traditional medicinal applications and their alignment or conflict with modern pharmacology; and consolidate toxicological data by clearly distinguishing extract types, administration doses, and treatment durations. Furthermore, this work proposes strategies for enhancing efficacy and reducing toxicity, discusses underlying mechanisms, and aims to promoting the effective clinical application of R. alismatis. Key unresolved issues include the standardized cultivation and processing of R. alismatis, in-depth investigation of specific pharmacological activities, and the interactive effects—both beneficial and toxic—of Alisma in combination with other drugs. Accordingly, this study synthesizes current knowledge on the chemical composition, pharmacological activities, and toxicological data of Alisma, identifies persistent challenges, and proposes strategic research directions to advance its therapeutic application.
3 Study selection
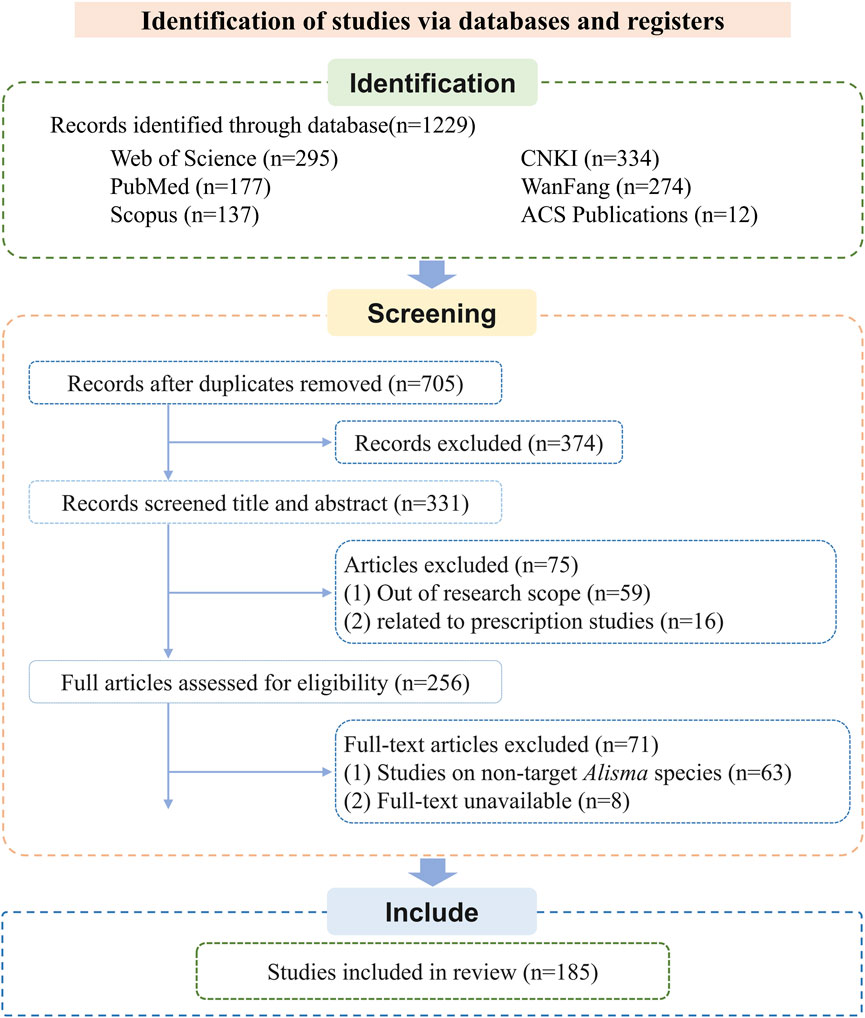
A systematic literature search was performed across six major scientific databases, resulting in the identification of 1,229 records. The screening procedure adhered to an adapted PRISMA flowchart framework (Page et al., 2021), with specific modifications as illustrated in Figure 1. The databases searched were as follows: Web of Science (n = 295), PubMed (n = 177), Scopus (n = 137), ACS Publications (n = 12), CNKI (n = 334), and WanFang Data (n = 274). Following duplicate removal using EndNote and subsequent manual verification, 705 articles remained for preliminary assessment. Through title and abstract screening, 374 records were excluded. The remaining 331 publications underwent full-text review. Of these, 59 were excluded for falling outside the research scope, 16 pertained to prescription studies, 63 involved non-target Alisma species, and 8 were excluded due to unavailability of the full text. After this rigorous selection process, 185 studies satisfied all predefined inclusion criteria and were included in the qualitative synthesis.
4 Results
4.1 The source and origin of Rhizoma alismatis
According to the Flora Reipublicae Popularis Sinicae, the Plant List and the Plant Science Data Center, there are 12 species of the genus Alisma, which are mainly distributed in temperate and subtropical regions of the northern hemisphere. They are Alisma plantago-aquatica L., A. plantago-aquatica subsp. orientale (Sam.) Sam, Alisma canaliculatum A. Braun & C.D. Bouché, Alisma lanceolatum With., Alisma gramineum Lej., Alisma subcordatum Raf., Alisma annuum Lojac., Alisma difformifolium Steud., Alisma intermedium Griff. ex Voigt, Alisma taeniifolium Steud., Alisma triviale Pursh, Alisma wahlenbergii (Holmb.) Juz. (Figure 2). Among them, only A. plantago-aquatica L. and A. plantago-aquatica subsp. orientale (Sam.) Sam have been included in the Chinese Pharmacopoeia, with a documented medicinal history of 2,000 years, and have been proven to possess significant therapeutic value (Commission, 2020).
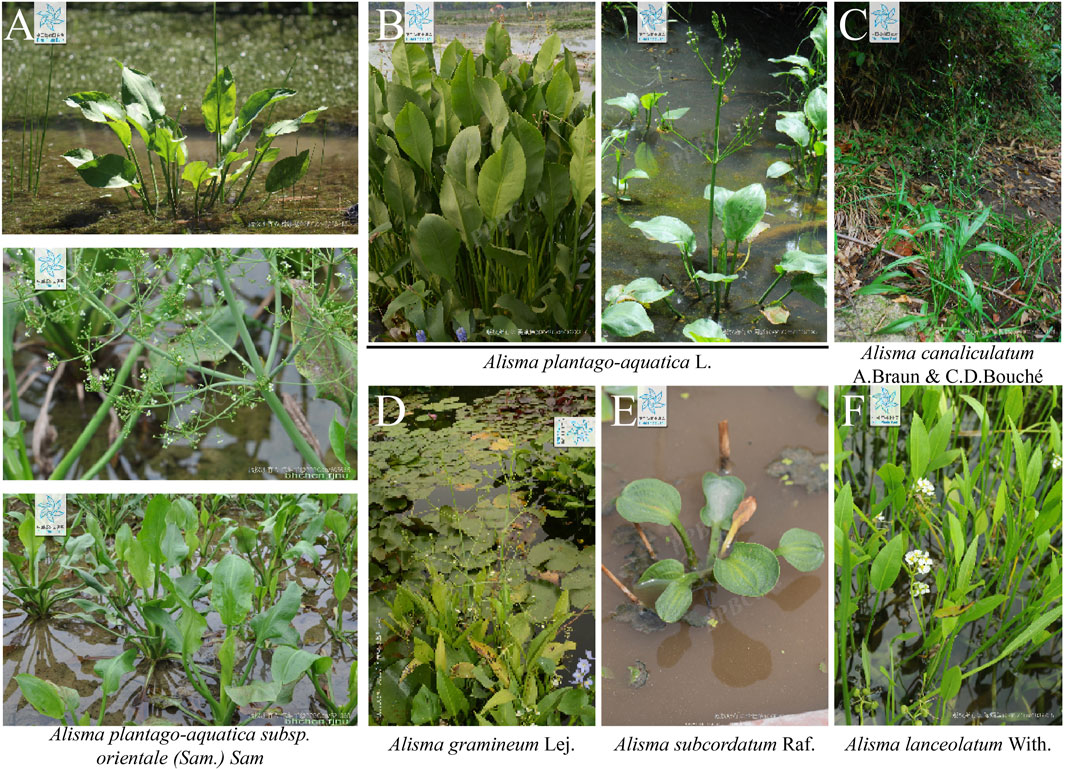
Figure 2. Morphological characteristics of several major Rhizoma alismatis species: (A) Alisma plantago-aquatica subsp. orientale (Sam.) Sam; (B) Alisma plantago-aquatica L.; (C) Alisma canaliculatum A.Braun and C.D. Bouché; (D) Alisma gramineum Lej.; (E) Alisma subcordatum Raf. (F) Alisma lanceolatum With (Cite from PPBC https://ppbc.iplant.cn/).
Rhizoma alismatis, a classical herbal medicine in TCM, maintains significant commercial importance in global herbal markets. Annual domestic consumption in China reaches approximately 8,000 metric tons, with an additional 1,000 metric tons exported to Japan, South Korea, and Southeast Asian countries (Liu et al., 2020). Morphological distinctions among these varieties are noteworthy. It can be divided into three categories in China. Jian R. alismatis (Fujian, Jiangxi) typically presents round-to-oval morphology with yellowish-white coloration, exhibiting irregular transverse annular grooves and lacking basal tubercular protrusions. Its compact texture reveals substantial yellowish-white starchy parenchyma upon cross-section. In contrast, Chuan R. alismatis (Sichuan) displays oval morphology with yellowish-brown periderm, regular concentric striations, and characteristic basal tubercles, demonstrating lighter mass with reduced starch content. Guang R. alismatis (Guangxi) shares oval morphology but features discontinuous concentric markings with multiple tubercular protrusions (Zhao et al., 2024). Quality assessment parameters prioritize large size, firm texture, yellowish-white coloration, and high starch content as superior characteristics, whereas smaller dimensions, rough epidermis, fragmentation, and scorched-yellow discoloration indicate inferior quality. Although Jian R. alismatis is traditionally regarded as the premium variety, its limited production (representing <10% of national output) contrasts sharply with Chuan R. alismatis’s market dominance (>90% total production). Guang R. alismatis’s comparatively inferior quality places it at a competitive disadvantage (Gu et al., 2019). Phylogenetic investigations using ITS2 sequencing have revealed significant taxonomic discrepancies in commercial samples. Song et al. (Ma et al., 2015) demonstrated that 93% of market specimens labeled as Alisma plantago-aquatica subsp. orientale (Sam.) Sam. actually corresponded to A. plantago-aquatica L., with authentic Alisma orientale representing only 7% of sampled materials. Chemotaxonomic analysis further indicates that Chuan R. alismatis aligns with A. plantago-aquatica L. in protostane-type tetracyclic triterpenoid profiles, while Jian R. alismatis corresponds to A. orientale characteristics (Liu, 2019). However, current cultivation patterns demonstrate interspecific hybridization across production regions, with multiple varieties cultivated within single geographic zones exhibiting phenotypic convergence (Tian et al., 2020). The above phenomenon indicates that the influence of planting environment on the quality of R. alismatis may be greater than that of the varieties of R. alismatis, which also explains to a certain extent why the TCM R. alismatis is mainly distinguished by the place of origin in China. The above situation reveals that there are serious problems in the process of cultivation, planting management and circulation of R. alismatis. It is urgent to establish a scientific and standardized management system for the cultivation and circulation.
4.2 Traditional Chinese medicine Rhizoma alismatis, classical prescription and related proprietary Chinese medicine products
4.2.1 Rhizoma alismatis Chinese herbal properties
According to the Pharmacopoeia of the People’s Republic of China (2020 edition), R. alismatis documented pharmacological actions include diuresis promotion, dampness elimination, turbidity resolution, and hypolipidemic effects (Commission, 2020). However, historical textual analysis reveals significant evolution in its perceived medicinal properties across dynastic periods (Niu et al., 2024). Early pharmacopeias like “Sheng Nong’s herbal classic” (Han Dynasty) and “Famous Doctor Bielu” (Northern and Southern Dynasties), and “Xinxiu Bencao”, “Yaoxing Lun” and “Rihuazi Bencao” (Tang Dynasty) emphasized its diuresis, viscera-nourishing properties, yin-activating and potential skin protection capacity. Clinical applications focused on managing tinnitus, dystocia, hematuria, and infertility through diarrhea inhibition and essence stabilization.
These points were inherited in “Compendium of Materia Medica” and “Huiyan of Materia Medica”, but divergent perspectives also emerged, as recorded in “Bencao Yanyi” and “Bencao Yueyan”. While maintaining consensus on diuretic-dampness removal efficacy, debates arose regarding its tonifying effects. Some texts paradoxically described nourishing of five viscera organs and yin deficiency supplementation. Then to the Qing Dynasty and modern, the effect of R. alismatis on diuresis was discussed in depth, purging fire of liver and kidney meridians, expelling the water of bladder and triple energizer, diuresis-removing dampness, reducing turbidity and lipid in “herbal reading”, “herbal justice”, “ten lectures on medication experience” and the 2020 edition of “Pharmacopoeia of the People’s Republic of China”. It is aimed at adverse urination, edema and fullness, diarrhea and less urine, phlegm and vertigo, hot and astringent pain, and hyperlipidemia in clinically, but tonic effect of long-term use are no longer mentioned. The shift in people’s understanding of the functions of R. alismatis is a thought-provoking issue. Factors such as environmental changes, artificial cultivation, and sufficient food supplies have all influenced perceptions of this botanical drug. If we can explore the underlying reasons behind this transformation, it would be a meaningful endeavor to revive its tonic properties and reemphasize its role in nourishment.
4.2.2 Commonly used classical prescriptions of Rhizoma alismatis and its related Chinese patent medicines
Rhizoma alismatis has been used in many classic prescriptions, among which Zexie Decoction and Wuling Powder are renowned. Zexie Decoction originates from the “Synopsis of the Golden Chamber”. It consists of two types of medicine: R. alismatis (Zexie) and Atractylodes macrocephala Koidz. (Baizhu) (Zhang, 2018). This prescription is a classic prescription for clinical treatment of phlegm and fluid retention syndrome. Rhizoma alismatis is a sovereign drug, diuresis and dampness, Rhizoma atractylodis macrocephalae is a minister drug, invigorating spleen and replenishing qi. Wuling Powder, composed of five medicines: Poria cocos (Schw.) Wolf (Fuling), Polyporus umbellatus (Pers.) Fries (Zhuling), bran-fried A. macrocephala Koidz. (Fu chao Baizhu), R. alismatis (Zexie), and Cinnamomum cassia (L.) J. Presl (Guizhi), can treat the dehydration and water storage of diabetes. The water metabolism of the human body involves multiple organs such as lung, spleen, stomach, kidney, bladder and triple energizer. Any dysfunction of the organs involved in the regulation of water can cause the syndrome of water-dampness stagnation.
The modernization of pharmaceutical technologies has driven the transformation of traditional Chinese medicinal materials into standardized dosage forms, including granule preparations, capsules, pills, and tablets, for commercial distribution. Rhizoma alismatis-containing patent medicines occupy a substantial market share, with over 90 formulations officially documented in the Pharmacopoeia of the People’s Republic of China (2020 edition) (Commission, 2020). Longqing Tablet possesses the functions of clearing heat and detoxifying, cooling blood, removing dampness and promoting diuresis. It is clinically used for the treatment of urinary tract infection, benign prostatic hyperplasia, acute and chronic prostatitis, and other heat stranguria resulting from dampness-heat in the lower jiao (Chen et al., 2016). Xuezhiling Tablet is composed of R. alismatis (Zexie), Cassia obtusifolia L. (Juemingzi), Crataegus pinnatifida Bunge (Shanzha) and Polygonum multiflorum Thunb. (Zhiheshouwu). Rhizoma alismatis is used as the sovereign drug for its diuresis, turbidity-dissolving and lipid-lowering effect, and is clinically used for the treatment of hyperlipidemia caused by phlegm block. Wuling Capsule is mainly employed for warming yang, transforming qi, removing dampness and promoting water circulation (Liu and Wang, 2023; Ma et al., 2024; Mou et al., 2022; Yang et al., 2015) (Supplementary Table S1).
4.3 Chemical composition of Rhizoma alismatis
Current phytochemical investigations have identified over 260 compounds in R. alismatis, encompassing carbohydrates (3 polysaccharides, 6 oligosaccharides, 5 monosaccharides), terpenoids (133 triterpenoids, 51 sesquiterpenes, 4 diterpenes), nitrogenous compounds (10), phenylpropanoids (18), flavonoids (7), steroids (6), phenolic acids (6), and 13 aliphatic hydrocarbon derivatives (Liu et al., 2020; Dai et al., 2023). Terpenoids dominate the chemical profile, representing 70.6% of total constituents (Figure 3), with triterpenoids and sesquiterpenes being particularly abundant.
4.3.1 Carbohydrate
At present, 14 kinds of carbohydrates have been reported in Rhizoma alismatis, 3 kinds of polysaccharides: alisman SI, alisman PII, alisman PIIIF; six kinds of oligosaccharides: manninotriose, verbascotetraose, verbascose, raffinose, stachyose, sucrose; five monosaccharides: β-D-fructofuranose, 5-hydroxymethylfurfuraldehyde, α-D-fructofuranose, ethylα-D- fructofuranoside, ethylβ-D-fructofuranoside, nine of which are fructose-derived carbohydrates (Zhang et al., 2009).
Alisman SI is a glucan composed only of D-glucose. Methylation analysis, nuclear magnetic resonance and enzymatic degradation studies have shown that alisman SI has a highly branched glucan type structure, composed mainly of α-1, 4-linked D-glucopyranosyl residues, and partially with α-1, 6-linked units. It has both 3,4 - and 4, 6-branch points (Shimizu et al., 1994). Alisman PII is an acidic polysaccharide composed of L-arabinose: D-galactose: D-glucuronic acid in a 4:9:2 M ratio. Its core structural features include a bony chain composed of β-1, 3-linked D-galactose units (Tomoda et al., 1994). Huang Suoyi et al. (Li et al., 2012) extracted R. alismatis polysaccharide by water extraction and alcohol precipitation method, and determined that the polysaccharide content of R. alismatis was 5.783% by phenol-sulfuric acid method.
4.3.2 Terpenoids
Terpenoids can also be divided into triterpenes, sesquiterpenes and diterpenoids (see Supplementary Table S2; Figures 4–6 for details). Among these triterpenes, alisol A, alisol B and its acetate as well as alisol C 23-acetate are initially isolated from R. alismatis in 1970 (Murata et al., 1970). These triterpenes are all derived from the alisol B 23- acetate which is highly content in the fresh plants (Peng, 2006). Most of their chemical skeletons are prototerpene tetracyclic triterpenoids represented by alisol A-X and its derivatives and alismanol A-Q. Only four diterpenoids were detected, namely oriediterpenone, oriediterpenol, oriediterpenoside and 12-deoxyphorbol-13a-pentadecanoate.
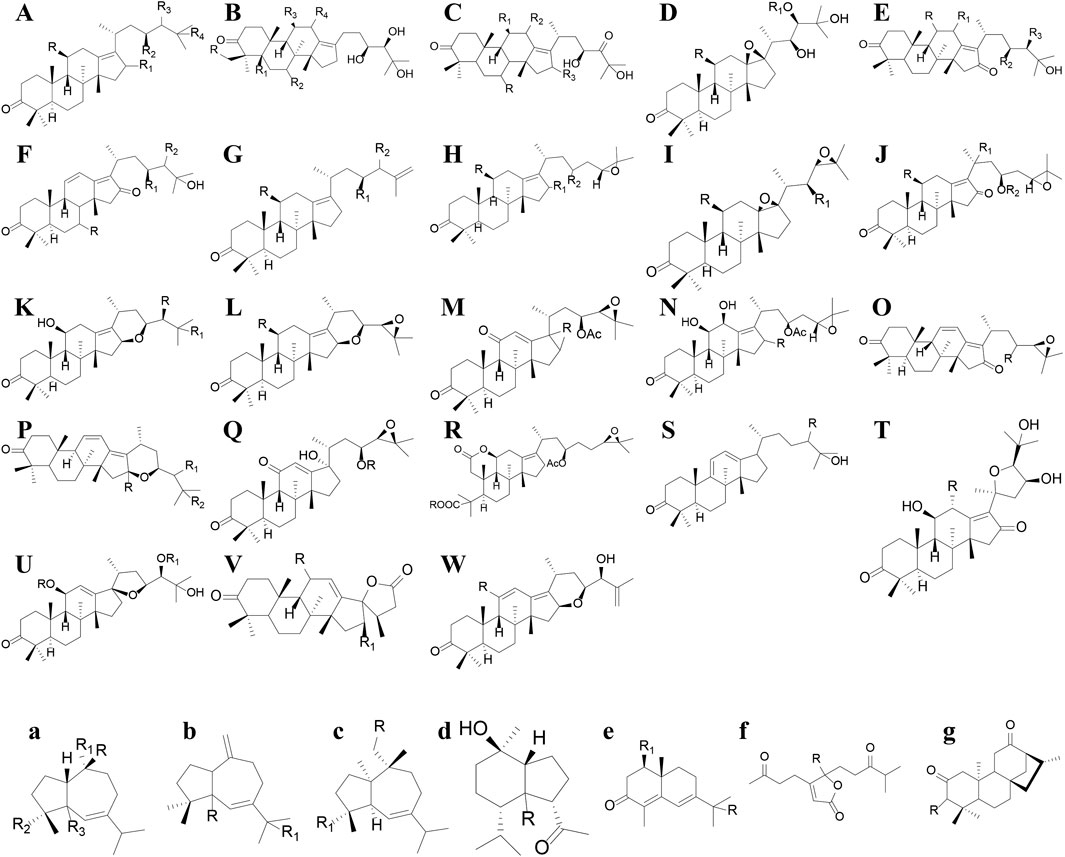
Figure 4. The nucleus of the triterpenes and Sesquiterpenes in Rhizoma alismatis. The nucleus of the triterpenes in Rhizoma alismatis (A–W). The nucleus of the sesquiterpenes in R. alismatis (a–g).
4.3.3 Other types of compounds
The remaining compounds in R. alismatis include nitrogen compounds, phenylpropanoid compounds, steroids, flavonoids, phenolic acids, aliphatic hydrocarbons and derivatives (see Supplementary Table S3; Supplementary Figure S1), accounting for about 30% of the identified compounds. Among them, the nitrogen compounds were mainly bases, nucleosides and indole components, and the steroids were mainly β-sitosterol, carotene and its derivatives.
4.4 Pharmacological activities of Rhizoma alismatis
Rhizoma alismatis is rich in chemical substances. Modern pharmacological studies have shown that it also has a variety of biological activities, such as diuretic inhibition of kidney stone formation, anti-inflammatory and antioxidant effects, blood lipid regulation, hypoglycemic, liver protection and antibacterial, anti-tumor effects (Figure 7; Table 1).
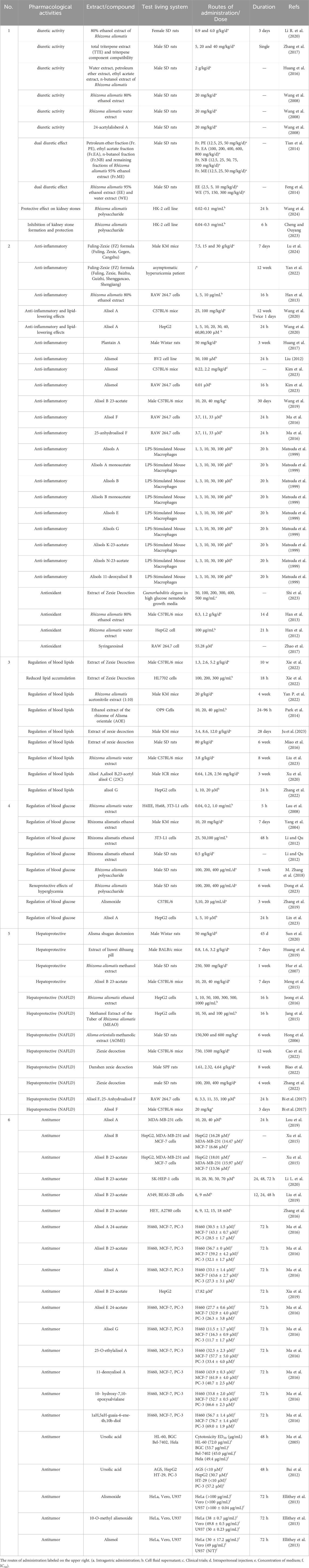
4.4.1 Diuretic and inhibition of renal stone formation
Rhizoma alismatis is an important diuretic. Animal experiments showed that the diuretic effect of 80% ethanol extract of R. alismatis was stronger than that of water extract (Wang et al., 2008). Water, n-butanol, ethyl acetate and petroleum ether were used to extract R. alismatis directly. The results showed that the ethyl acetate extract of R. alismatis had the best diuretic effect (Huang et al., 2016). The results of HPLC analysis showed that the content of triterpenoids in the 80% ethanolic extract (Wang et al., 2008) and ethyl acetate extract (Huang et al., 2016) of R. alismatis were high, suggesting its diuretic effect may be related to the terpenoids. Zhang X. et al. (2017) extracted R. alismatis with 80% ethanol, conducted concentration under reduced pressure, successively adsorbed with AB-8 macroporous resin and polyamide resin, eluted with gradient ethanol, concentrated, and freeze-dried to obtain the total triterpene extract (TTE) of R. alismatis. Then they determined the content of triterpenes in TTE by high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF-MS) and found that alisol A, alisol A 24-acetate, alisol B, alisol B 23-acetate, and alisol C 23-acetate were the main triterpenes in TTE, accounting for 72.2% (722.38 mg/g) of the total triterpene metabolites. And the results of diuretic experiment showed that TTE increased the urine volume of rats by 15.4%, 69.23% and 48.08% at the doses of 5, 20 and 40 mg/kg, respectively. Further study on the diuretic effect of different combinations of five triterpenoids in TTE found that when the ratio of alisol B 23-acetate: alisol B: alisol A 24-acetate: alisol A: alisol C 23-acetate was 7.2 : 0.6: 2.8 : 3.0: 6.4, the diuretic effect was the best (Zhang et al., 2017). Therefore, it can be ascertained that triterpenoids are the key functional substances of R. alismatis diuretic effect. In addition, studies have shown that the diuretic mechanism of R. alismatis is related to the reabsorption of kidney. The diuretic effect of R. alismatis can significantly increase the concentration of Na+, K+ and Cl− in urine, and reduce the level of aquaporin-2 (AQP-2) mRNA in rat renal medullary cells and HK-2 cells (Li R. et al., 2020). As a response protein of antidiuretic hormone and a key protein of urine reabsorption, the reduction of AQP-2 explains the reason of R. alismatis diuretic (Kharin and Klussmann, 2023).
However, further studies have found that diuretic effect has duality (Chen et al., 2014; Feng et al., 2014). Feng et al. (2014) extracted R. alismatis with 95% ethanol, and obtained petroleum ether, ethyl acetate and n-butanol extracts by systematic solvent extraction after decompression and concentration. The diuretic experiment in rats showed that 100 and 400 mg/kg doses of ethyl acetate extract and 12.5, 25 and 50 mg/kg doses of n-butanol extract could significantly increase urine volume and urinary electrolyte excretion in rats, but 800 mg/kg dose of ethyl acetate fraction and 75 mg/kg and 100 mg/kg doses of n-butanol fraction significantly reduced. In the subsequent experiments, the 95% ethanol extract of R. alismatis was subjected to diuretic experiments. It was found that the urine volume of rats was increased at the doses of 2.5, 5 and 10 mg/kg, but decreased at the doses of 20, 40 and 80 mg/kg, while the water extract of R. alismatis was not significantly different. At present, it is believed that the dual diuretic effect of R. alismatis may be related to the sodium chloride cotransporter in the distal renal tubule. Lin Ruichao believes that the ethanol extract of R. alismatis may inhibit the activity of sodium-chloride cotransporter in the distal renal tubule at the doses of 2.5, 5 and 10 mg/kg, and increase the transport of Na+ to the distal segment of the distal renal tubule and the upper collecting duct, and the increase of Na+ concentration leads to the increase of K+ loss. Stimulation of the aldosterone-sensitive sodium pump increases Na+ reabsorption to exchange K+ and H+ for urine excretion. At the doses of 20, 40 and 80 mg/kg, the inhibitory effect of the ethanol extract of R. alismatis on the sodium-chloride cotransporter in the distal renal tubules would fail due to the huge permeability difference, and the urine would be reduced due to the passive absorption of water and the effect of antidiuretic hormones. This view does not explain the different effects of different concentrations of R. alismatis ethanol extract on sodium-chloride cotransporters in distal renal tubules. The specific mechanism of action is not clear and needs further study. It is worth noting that the diuretic dose of 95% ethanol extract prepared by Lin Rui chao et al. was significantly lower than that of the enriched n-butanol fraction (which is rich in diuretic active triterpenes). Except for the batch differences of medicinal materials and the sensitivity of experimental animals, the diuretic effect of 95% ethanol extract was significantly better than that of four different extraction fractions (Water, n-butanol, ethyl acetate and petroleum ether extract), suggesting that there may be a synergistic interaction between diuretic and non-diuretic components in R. alismatis extract to enhance diuretic effect. However, this very aspect remains unexplored in current research.
Because of its excellent diuretic effect, R. alismatis is often used for the treatment of kidney stones. CaOx stones are the most common in kidney stones, which mainly exist in the form of CaOx monohydrate (COM) and CaOx dihydrate (COD). Compared with COD crystals, COM crystals show stronger cell affinity and significant cytotoxicity. Therefore, Wang et al. (2024) established a cell injury model by stimulating HK-2 cells with 100 nM calcium oxalate monohydrate, and found that R. alismatis polysaccharide could significantly improve the survival rate of HK-2 cells by reducing the levels of inflammatory factors such as NLRP3, TNF-α, IL-6 and NO, inhibiting apoptosis, and reducing the expression of adhesion molecule CD44 on the surface of HK-2 cells. Cheng and Ouyang (2023) believed that the carboxymethyl derivative of R. alismatis polysaccharide could alleviate the damage of HK-2 cells and reduce the formation and damage of kidney stones by affecting the crystal structure of calcium oxalate. In addition, some studies have pointed out that there is a certain correlation between obesity and kidney stones (Kim et al., 2022), and the water and dampness effects of R. alismatis may also alleviate the occurrence of kidney stones by regulating lipids. It can be seen that there may be a complex mechanism affecting the metabolism of water, sugar and lipid in the whole body to treat kidney stones.
4.4.2 Anti-inflammatory and antioxidant effects
Although R. alismatis has not been used as an anti-inflammatory drug, recent studies have found that it shows good anti-inflammatory effects in different disease models. The 80% ethanol extract of Alisma orientalis can alleviate LPS-induced acute lung injury by inhibiting the expression of inflammatory genes such as NF-κB, COX-2, IL-1β and iNOS (Han et al., 2013). Monomer components within R. alismatis, such as Alisol A (Wang et al., 2020), plantain A (Huang et al., 2017), Alismol (Liu, 2012), alisol A 24-acetate, alisol B 23-acetate (Wang et al., 2019), alisols A monoacetate (Matsuda et al., 1999), alisols B (Matsuda et al., 1999), alisols B monoacetate (Matsuda et al., 1999), alisols E (Matsuda et al., 1999), Alisol F, alisols G (Matsuda et al., 1999), alisols K-23-acetate (Matsuda et al., 1999), alisols N-23-acetate (Matsuda et al., 1999), alisols 11-deoxyalisol B (Matsuda et al., 1999) and 25-anhydro-Ali-F (Ma et al., 2016), all of which have been documented to have potent anti-inflammatory activity.
In addition to anti-inflammatory, R. alismatis extract also showed good antioxidant activity. The 80% ethanol extract of R. alismatis can alleviate LPS-induced acute lung injury by enhancing the activity of Nrf2 and promoting the expression of its downstream regulatory genes NQO-1, HO-1 and GCLC (Han et al., 2013). The water extract of R. alismatis can significantly reduce the levels of reactive oxygen species (ROS) and active aldehydes in HepG2 cells induced by palmitic acid, and alleviate steatosis and cell damage in HepG2 cells (Han et al., 2012). Syringaresinol, a phenylpropanoid compound in R. alismatis, showed good antioxidant properties in DPPH scavenging assay (Zhao et al., 2017). Based on the aforementioned findings, the anti-inflammatory and antioxidant activities of individual active components have been primarily demonstrated in in vitro cell-based assays, whereas the corresponding efficacy in vivo has been observed mainly with R. alismatis extracts and their formulated preparations. This suggests that synergistic interactions among the active components of R. alismatis may contribute to its anti-inflammatory and antioxidant effects in vivo.
4.4.3 Blood-lipid regulation
Clinical experiments have found that R. alismatis can significantly reduce blood lipids and have been verified in several animal models of hyperlipidemia. Zexie Decoction, as a classical prescription, is widely used in clinical treatment of hyperlipidemia. Cell and mouse models have confirmed that Zexie Decoction can significantly improve symptoms such as lipid accumulation and insulin resistance by inhibiting the activity of SREBPs and the expression of its target gene FKBP38 (Xie et al., 2022). In the hyperlipidemia model, CYP450 enzyme subtype enzyme activity decreased, which will affect the liver’s ability to metabolize therapeutic drugs. Ju et al. (2023) conducted a study on the disformulation of Zexie Decoction (water extract) and compared the effects of Zexie Decoction group, single medicine Zexie group and Baizhu group on CYP450 enzyme activity in hyperlipidemia mice. It was found that Zexie Decoction can restore the expression of CYP3A4 and enhance its activity, in which Zexie played a leading role in Zexie Decoction.
A large number of studies have shown that R. alismatis has good lipid-lowering activity when use alone. The 95% ethanol extract of R. alismatis can effectively reduce the levels of serum total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) in hyperlipidemia rats. Nineteen biomarkers were obtained by urinary metabolomics, among which ascorbalamic acid, indolelactic acid, 1-hydroxypyrene, 3-methyluridine and 4-heptanone. 4-aminohippuric acid, hypoxanthine, creatinine and indole-3-carboxylic acid were positively correlated with hyperlipidemia. Whereas metabolites such as xanthosine, urocanic acid, propionylcarnitine, xanthine, cytidine, N-acetylneuraminic acid, phenylacetylglycine, methylhippuric acid, D-arginine and hippuric acid were negatively correlated with it. After taking R. alismatis, these markers tended to normal levels and alleviated the dysfunction caused by changes in metabolites (Miao et al., 2016). Hongliang Jiang (Yan et al., 2022) et al. combined metabolomics, network pharmacology, and lipidomics to identify 18 potential active compounds and 83 potential therapeutic targets in the plasma of hyperlipidemic mice based on metabolomics. They focused on the PPAR signaling pathway and validated by qPCR that ALB, TNF, IL1B, MMP9, PPARA, and PPARG may be the 6 upstream key targets regulated by R. alismatis. Kang-Beom Kwon et al. (Park et al., 2014) found that 70% ethanol extract of R. alismatis could inhibit the differentiation of OP9 adipocytes in bone marrow stromal cells by reducing the expression of C/EBP β in mice. Haoxin Wu et al. (Liu et al., 2023) found that the area of plaques, blood HDL-C and TG, and serum CHO and LDL-C levels were improved in APOE−/− hyperlipidemic mouse models after taking R. alismatis extract. Combining network pharmacology analysis and validation, it was suggested that R. alismatis may improve its atherosclerotic progression by inhibiting the phosphorylation of PI3K/AKT and the expression of SREBP-1.
The lipid-regulating effects of R. alismatis decoctions and single extracts have closed related with its unique triterpenoids. Such as alisol G, alisol A 23-acetate, 16-oxo-11-anhydroalisol A, alisol B, alisol B 23-acetate, and alismanol J may improve HepG2 cell uptake of LDL by regulating PCSK9 (Zhang et al., 2022). Acyl coenzyme a-cholesterol acyltransferase (ACAT), one of the lipid-regulating targets of R. alismatis, plays an important role in the balance of cholesterol metabolism in cells and organisms. Its activity is influenced by the ratio of alisol A, alisol B, and 23-acetyl alisol C, with the n-terminal lipid-regulating activity of ACAT being stronger than the transmembrane domain when the ratio is 3:1:1 (Xu et al., 2020). In summary, the lipid-lowering effect of R. alismatis is related to triterpenoids, which may affect the key indicators of lipid metabolism diseases such as TC, TG, LDL-C and HDL-C by affecting the intake of low-density lipoprotein in hepatocytes and inhibiting the differentiation and autophagy of adipocytes.
4.4.4 Regulating blood glucose
In TCM, Rhizoma alismatis is frequently utilized as one of the prescriptions for the management of diabetes. Studies have manifested that R. alismatis can effectively reduce blood glucose levels both in vivo and in vitro (Lau et al., 2008; Yang et al., 2004). Polysaccharide is considered to be one of the effective metabolites of hypoglycemic. For example, the T2DM model rats established by high-fat diet combined with intraperitoneal injection of streptozotocin (30 mg/kg) were fed with R. alismatis polysaccharide for 6 weeks, the levels of SOD and GSH-Px in liver tissue of rats were decreased, and insulin resistance and lipid metabolism were improved (Zhang et al., 2018). Studies have also shown that R. alismatis polysaccharide may improve the symptoms of diabetic rats such as dry and yellow hair, significant weight loss and renal injury by regulating PPAR-γ/LXR-α/ABCG1 signaling pathway (Dong et al., 2023). Besides polysaccharide, the ethanol extract of R. alismatis can slow down the release of glucose by inhibiting the activity of α-glucosidase and increase the glucose uptake of 3T3-L1 adipocytes to inhibit adipogenesis (Li and Qu, 2012). In vivo and in vitro experiments confirmed that alisol A (Lin et al., 2023) and epoxy alisolene (Zhang et al., 2019) could affect cell glucose absorption capacity and reduce fasting blood glucose in type 2 diabetic mice, respectively. The development of its hypoglycemic effect requires further validation of its efficacy and safety in humans, as well as clarification of the specific mechanisms of action of its various active constituents. Concurrently, attention must be paid to the rational use of R. alismatis, including investigations into its potential for concomitant use with existing medications. Careful monitoring is essential to ensure medication safety.
4.4.5 Hepatoprotective effect
The hepatoprotective effect of R. alismatis is primarily manifested in the alleviation of drug-induced liver injury and the treatment of fatty liver, hepatitis, and liver fibrosis (Choi et al., 2019). For drug-induced liver injury, Zexie prescription, extract and monomer components have a certain protective effect. Zexie Shugan Decoction can reverse thioacetamide (TAA)-induced liver injury and fibrosis by inhibiting α-SMA protein, reducing collagen area and fibrosis score (Sun et al., 2020). The methanol extract of R. alismatis was continuously fed to rats injected with bromobenzene for 1 week, which increased the content of epoxide hydrolase and glutathione s-transferase, decreased the content of aminopyridine n-demethylase and aniline hydroxylase, and alleviated the liver injury caused by bromobenzene (Hur et al., 2007). In addition, the methanol extract of R. alismatis inhibited tunicamycin-induced triglyceride accumulation by inhibiting endoplasmic reticulum stress (Jang et al., 2015), alleviating its lipid toxicity. Alisol B 23-acetate can reduce the intake of ANIT and increase the excretion of bile acid by down-regulating NTCP protein and up-regulating efflux transporters (Bsep, Mrp2 and Mdr2). Alisol B 23-acetate can also reduce bile acid synthesis by inhibiting CYP7A1 and CYP8B1, and increase Bal and Baat expression by up-regulating Sult2a1 gene to increase bile acid metabolism. From the perspectives of enhancing the ability of liver to metabolize drugs and maintaining the bile acid homeostasis of the liver, the liver injury of ANIT-mediated cholestasis hepatotoxicity mice was effectively alleviated (Meng et al., 2015). Alisol F (20 mg/kg/d, i. p.) can effectively reduce the levels of AST, ALT and inflammatory factors induced by LPS/d-gal by affecting the MAPKs/NF-kB/STAT3 pathway, and improve liver pathological damage (Bi et al., 2017).
At the same time, R. alismatis is considered to be an effective candidate drug for NAFLD (Choi et al., 2019). Zexie Decoction is commonly used in clinical practice. Comparing the differentially expressed genes, differentially expressed lipid molecules and differentially expressed intestinal microflora between the Zexie Decoction group and the control group (Zhang et al., 2022), it is speculated that Zexie Decoction may play a therapeutic role by regulating fatty acid synthesis, correcting lipid metabolism disorders and reducing inflammatory response. Sirt1 and AMPK are the key to Zexie Decoction promoting fatty acid oxidation (FAO) (Cao et al., 2022). Biao et al. (2022) found that Danshen Zexie Decoction could inhibit ROS/NLRP3/IL-1β signaling pathway and improve many liver function indexes by activating Nrf2 in NAFLD rat model fed with high fat diet. Continuous feeding of obese mice induced by high-fat diet at doses of 100, 300 mg/kg/d can effectively inhibit liver ER stress and hepatic steatosis. In addition to the decoction, R. alismatis extraction can also inhibit the steatosis caused by oleic acid, palmitic acid (Jeong et al., 2016), and high-fat diet (Jang et al., 2015); reduces oxidative stress and inflammation levels in the liver (Hong et al., 2006).
4.4.6 Anti-tumor effect
A considerable number of studies have demonstrated that extracts and monomer metabolites of R. alismatis significantly inhibit the proliferation of various tumor cells, including breast cancer (Lou et al., 2019), colorectal cancer (Xu et al., 2015), liver cancer (Li L. et al., 2020), lung cancer (Liu et al., 2019) and ovarian cancer (Zhang et al., 2016). Wu et al. (2023) proposed that R. alismatis is a potential low-toxicity anticancer agent. Its active ingredient, alisol (a tetracyclic triterpenoid alcohol), can be metabolized into various derivatives and is suggested to inhibit cancer cell proliferation and migration by modulating key signaling pathways such as mTOR, Bax/Bcl-2, CHOP, caspase, NF-κB, and IRE1. However, the clinical application of R. alismatis as an anti-tumor drug remains limited, as the existing evidence primarily derives from in vitro studies (Table 1; Figure 7). Therefore, it is essential to further validate its anti-tumor efficacy and elucidate the underlying mechanisms, particularly through in vivo experiments, to account for critical factors like bioavailability and metabolic processing.
4.4.7 Alignment and discrepancies: modern pharmacology and traditional medicine
The investigation into R. alismatis presents a compelling narrative of convergence and expansion between its millennia-old applications in TCM and contemporary pharmacological inquiry. At its core, a remarkable consistency exists, where modern science provides a mechanistic foundation for ancient wisdom. TCM attributes to R. alismatis the properties of diuresis promotion, dampness elimination, turbidity resolution. Modern pharmacology robustly validates this. However, this consistency is elegantly complemented by a critical divergence and deepening of understanding. The discovery of its effects beyond the traditional scope, such as significant anti-inflammatory, anti-diabetic nephropathy, and potential anti-cancer properties, exemplifies this divergence. These are not contradictions but rather expansions, revealing new therapeutic dimensions unexplored by classical texts. However, the tonifying and nourishing functions and skin-protective effects recorded in ancient texts have not yet been confirmed by modern pharmacology. Are they undiscovered secrets or previously misunderstood concepts? This undoubtedly warrants in-depth investigation.
According to the holistic framework of TCM, R. alismatis possesses sweet and cold properties. Upon ingestion, its therapeutic actions are directed toward, or it “guides” its effects to, the Kidney and Bladder meridians. Coincidentally, toxicology has found that long-term excessive use of R. alismatis can cause kidney damage (see Section 4.5 for details). Thus, the dialogue between tradition and modernity regarding R. alismatis is not one of conflict but of synergistic validation and discovery. Modern pharmacology confirms the profound intuition of traditional practice by elucidating the scientific “how,” while simultaneously transcending it by uncovering novel biological activities, thereby enriching the potential applications of this ancient remedy in modern evidence-based therapy.
4.5 The toxicity of Rhizoma alismatis
The toxicity of R. alismatis is controversial in clinical application and basic research, which also affects people’s understanding of its efficacy. Some ancient TCM books stated that R. alismatis is cold, sweet and non-toxic. For instance, “Shennong’s Herbal Classic” described that long-term use of R. alismatis can enhance the acuity of ears and eyes, suppress hunger, prolong lifespan, lighten the body and enhance complexion. “Leigong processing medicinal solution” recorded that R. alismatis is sweet and salty, cold and non-toxic. “Bencao Jingjie” also held the opinion that the taste of R. alismatis is sweet and non-toxic. On the contrary, there are also records suggesting that R. alismatis has a certain degree of toxicity. The toxicity of the whole plant of R. alismatis is mentioned in “The main toxic plants in the south and their treatment of poisoning”, with the underground root being more toxic. The symptoms of poisoning include itching, redness, blistering on the skin, abdominal pain, diarrhea and other gastrointestinal symptoms after consumption (Cash crop team of Guangdong Agriculture, 1970). In the “commonly used traditional Chinese medicine and adverse reactions” record: “A large dose or long-term application of R. alismatis can lead to water electrolyte imbalance and hematuria, and even acidosis, and can cause nausea, vomiting, abdominal pain and liver function impairment”, clearly proposing its liver and kidney toxicity (Chen and Zhou, 1998). The 2020 edition of the “Pharmacopoeia of the People’s Republic of China” divides toxic Chinese medicines into three categories: 1. high toxicity, 2. toxic, 3. small toxicity; however, R. alismatis is not included. Currently, there is a big controversy regarding whether R. alismatis is toxic. According to the existing experimental evidence, the toxicity of R. alismatis is conditional and acceptable. The following will discuss the research reports on the toxicity of botanical drug extracts and monomer metabolites of R. alismatis (Figure 8).
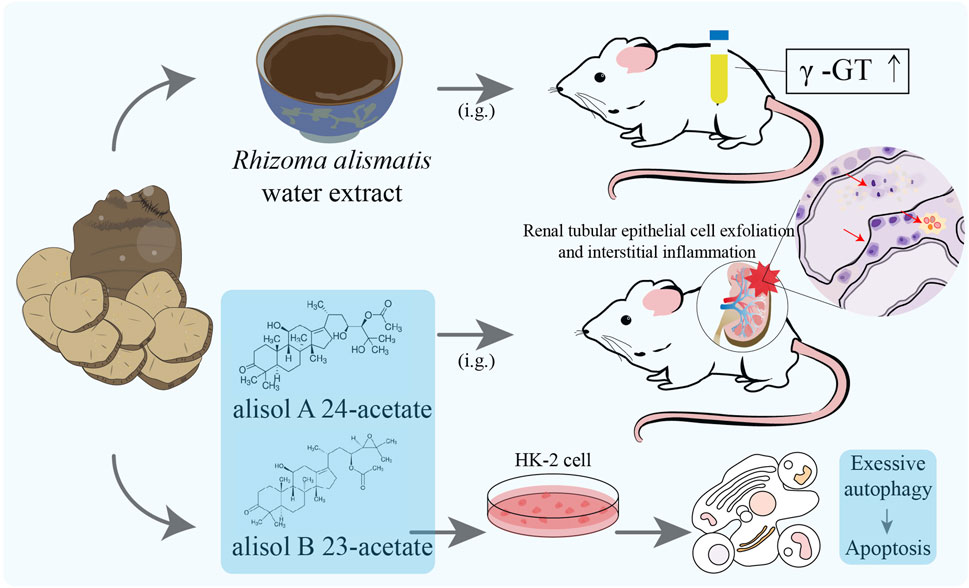
Figure 8. Toxicity study of Rhizoma alismatis. Urinary γ-glutamyltransferase (γ-GT) activity was significantly increased in 60 days after continuous administration of the normal dose of 20 and 40 times of Rhizoma alismatis water extract; Inflammation of the renal interstitium, exfoliation of renal tubular epithelial cells and morphological changes were observed in Alisol B 23-acetate (0.4 g/kg/day) and Alisol A 24-acetate (0.5 g/kg/day) group rats (6 months) and marked with red arrows; After Alisol B 23-acetate (15 μM) and alisol A 24-acetate (6 μM) were added to the culture medium, HK-2 cells showed significant apoptosis and autophagy.
4.5.1 Toxicity study of Rhizoma alismatis extracts
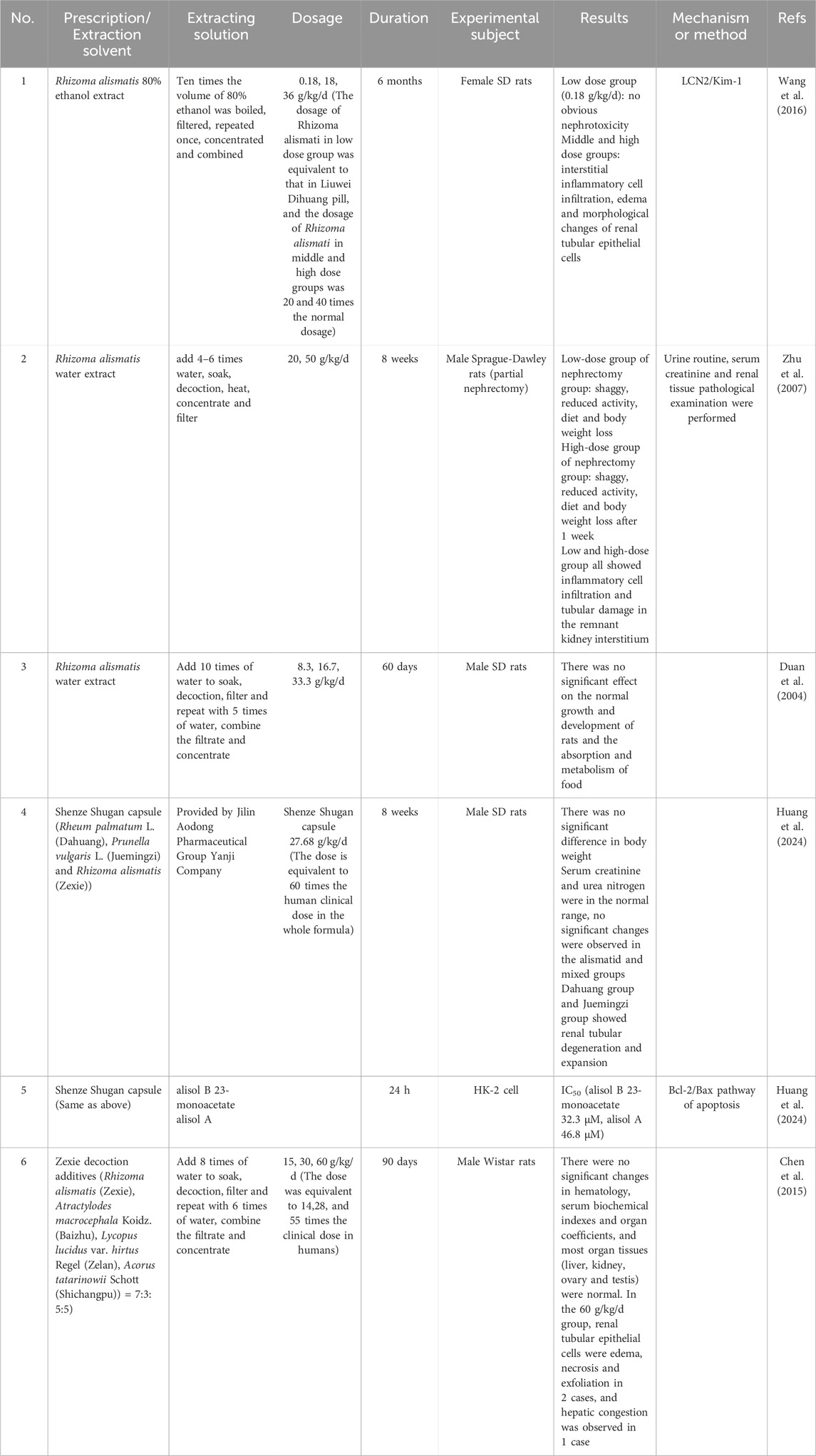
The metabolites of Chinese herbal medicine are complex and diverse, and the effective metabolites of Chinese herbal medicine are often extracted and concentrated by water and alcohol extraction. Here, the toxicity will be discussed based on the water extract and alcohol extract (Table 2).
The water extract of Rhizoma alismatis contains more polysaccharides, while its alcohol extract is mainly composed of nitrogen-containing compounds, phenylpropanamide compounds, and terpenoids. The difference in material composition often affects its efficacy and toxicity. Wang et al. (2016) boiled R. alismatis with 10 times the volume of 80% ethanol, filtered, and concentrated to obtain the ethanol extract of R. alismatis, and carried out a long-term nephrotoxicity experiment on the ethanol extract of R. alismatis. After 6 months of continuous administration, renal interstitial inflammatory cell infiltration and renal tubular epithelial cell edema were found in rats in the middle and high dose groups (20 and 40 times of the equivalent dose of R. alismatis in Liuwei Dihuang Pills). However, the low dose group (the equivalent dose of R. alismatis in Liuwei Dihuang Pills) did not show significant nephrotoxicity.
Although the water extract of R. alismatis has weaker diuretic and relieving drug-induced liver injury than the alcohol extract (Feng et al., 2014; Jang et al., 2021), its water extract may also be toxic. Zhu et al. (2007) fed normal rats and right nephrectomy rats with 20 and 50 g/kg water extract of R. alismatis for 8 weeks respectively. It was found that the right nephrectomy rats fed with water extract of R. alismatis showed adverse reactions such as loose hair, reduced activity, reduced diet and weight loss. The renal sections showed renal interstitial inflammatory cell infiltration and tubular damage.
Duan et al. (2004) studied its subchronic toxicity (60 days) on normal rats with 50, 100 and 200 times of safe dosage of the water extract of R. alismatis. The blood urea nitrogen (BUN) in the high dose group showed an increasing trend, but did not exceed the normal range; the activity of urinary γ-glutamyltransferase (γ-GT) in the middle and high dose groups was significantly higher than control group. However, histopathological examination showed that there was no obvious pathological damage in the liver, kidney, spleen and testis of the rats in each experimental group. Based on these studies of R. alismatis extract, the metabolism of R. alismatis requires the participation of the kidney, and its toxicity depends more on the function of the kidney. Therefore, R. alismatis needs special attention for patients with renal dysfunction or injury, and cannot be taken for a long time and overdose.
4.5.2 Toxicity study of monomer compounds of Rhizoma alismatis
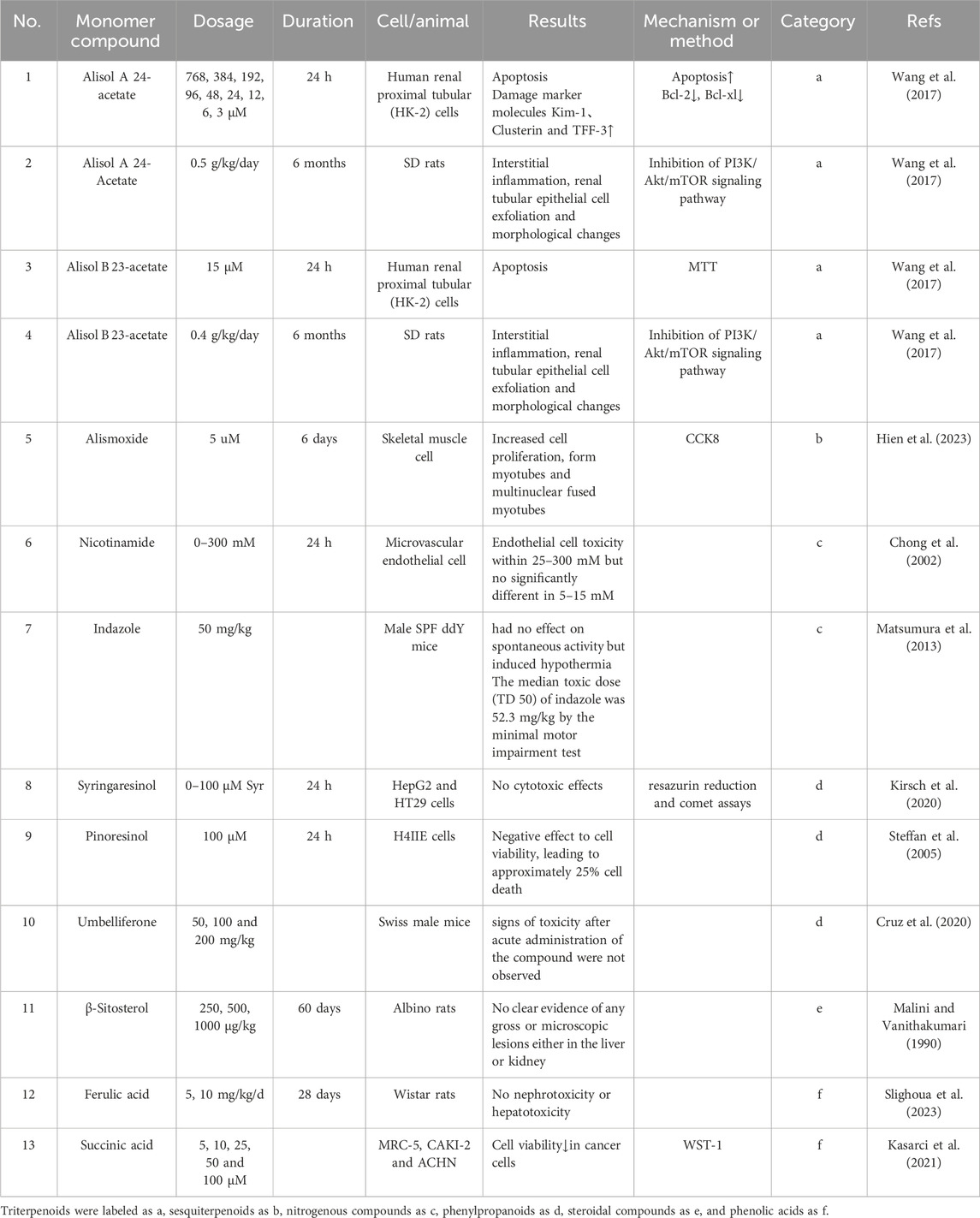
In this section, the literature reports of effective monomers of Rhizoma alismatis were collected, and the literature was sorted out according to monomer composition, dose, model, toxicity results, mechanism of action, and monomer category (Table 3). The results showed that the toxicity study of the main active ingredient terpenoids in R. alismatis mainly stayed in cell experiments, while animal experiments and human experiments were less reported. In addition, R. alismatis contains the same metabolites of other plants, such as nicotinamide, indazole, β-sitosterol and so on. These compounds have been proved to have potential toxicity and adverse reactions (Chong et al., 2002; Matsumura et al., 2013), but the content of R. alismatis in normal use is much lower than its adverse reaction dose. Subsequently, some in-depth toxicity studies on the terpenoids of R. alismatis are needed.
4.5.2.1 Toxicity of terpenoids
The terpenoids in R. alismatis include triterpenoids, sesquiterpenes and diterpenes, among which the triterpenoids (133) in R. alismatis have been studied most deeply. Triterpenoids’ chemical skeletons are prototerpane-type tetracyclic triterpenes represented by alisol A-X and its derivatives and alismanol A-Q, which are also characteristic metabolites of R. alismatis. Alisol A 24-acetate, alisol B 23-acetate, Alisol A and Alisol B are the main chemical components in the water extract of R. alismatis (Luo et al., 2021; Wang et al., 2017).
Alisol B 23-acetate and alisol A 24-acetate were continuously fed to rats at 0.4 g/kg/day and 0.5 g/kg/day for 6 months, respectively. Inflammation of renal interstitium, exfoliation of renal tubular epithelial cells and morphological changes were observed in two group rats, suggesting that these two compounds have potential renal toxicity and could potentially be the primary cause of toxicity elicited by R. alismatis. In vitro experiments demonstrated that alisol B 23-acetate and alisol A 24-acetate (treated with 6 μM and 15 μM, respectively) could significantly induce HK-2 cell apoptosis and autophagy by down-regulating the levels of Bcl-2 and Bcl-xl and inhibiting the PI3K/AKT/mTOR pathway. Simultaneously, the expression of Kim-1, Clusterin, and TFF-3, which are renal drug toxicity biomarkers, was enhanced. Evidently, the compounds alisol B 23-acetate and alisol A 24-acetate were verified as nephrotoxic compounds. Additionally, research indicated that the inhibition of autophagy could effectively reverse the apoptosis induced by alisol B 23-acetate and alisol A 24-acetate, suggesting that the nephrotoxicity of alisol B 23-acetate and alisol A 24-acetate was associated with their excessive induction of autophagy through inhibiting PI3K/Akt/mTOR signaling pathway (Table 3).
Although compounds, alisol B 23-acetate and alisol A 24-acetate showed good growth inhibitory effects on cancer cells such as H460, MCF-7, PC-3 and HepG2 (Table 2), their IC50 values were all greater than 17.82 μM. Obviously, renal epithelial cell HK-2 is more sensitive to it, that is, it may have caused adverse reactions of renal injury while exerting anti-cancer effects. Therefore, the potential anti-cancer activity of these compounds remains to be discussed. Other triterpenoids (Table 3, column a) have been reported in addition to the toxicity of tumor cells and macrophages (Table 3), there are almost no reports on toxicity and adverse reactions such as liver and kidney.
At present, 58 sesquiterpenes have been isolated from R. alismatis. According to their structures, they can be divided into guaiacol type, apostichopus type, germacane type, xanthene type and phyllane type (Mengxiang et al., 2023). The guaiacol type represented by alismoxide orientalol A ∼ G and its derivatives is the main type. At present, the research on its toxicity mainly stays in cell experiments (Table 3). Four diterpenes were isolated from R. alismatis, including oriediterpenol, oriediterpenol, oriediterpenoside, 12-deoxyphorbol-13α-pentadecanoate. At present, there is no report on the above diterpene monomer components.
4.5.2.2 The toxicity of other metabolites
Other metabolites of R. alismatis include nitrogen-containing compounds, phenylpropanoids, steroids, flavonoids, phenolic acids, and aliphatic hydrocarbons and derivatives. The toxicity studies of some compounds are listed in Table 3 (category: c-f).
Nicotinamide and indazole are nitrogen-containing compounds that have been studied for toxicity in R. alismatis. Nicotinamide is a form of vitamin B3, which has a dual role in cell growth. It enhances cell viability and replication at a dose close to 5 mM (Chong et al., 2002), and when the dose is higher than 20 mM, it can lead to apoptosis. The results of human intervention test showed that children continued to take 25–50 mg/kg per day for 5 years, and the elderly took 1.5 g NAM twice a day for 6 months without adverse reactions, indicating its safety. At present, the reported adverse reactions of nicotinamide are mainly headache, dizziness and vomiting caused by large-scale use on an empty stomach. Such adverse reactions can gradually recover after stopping taking (Hwang and Song, 2020), and the concentration contained in R. alismatis is far less than the concentration of side effects. Indazole has anti-inflammatory, antibacterial, anti-HIV, anti-arrhythmia, anti-fungal and anti-tumor effects. At present, there are about 40 kinds of therapeutic agents based on indazole for clinical application or clinical trials. At a dose of 50 mg/kg, there is no significant effect on mice, but it will lead to a decrease in body temperature in mice. The minimum motor dysfunction test shows that the median toxic dose of indazole is 52.3 mg/kg (Matsumura et al., 2013). Umbelliferone, a phenylpropanoid compound, has been shown to have anti-diabetes, anti-cancer, anti-infection, anti-rheumatoid arthritis, neuroprotection, and improvement of liver, kidney, and myocardial tissue damage. However, it has a dose-dependent cytotoxicity (Lin et al., 2023). At a concentration of 200 μg/mL, it inhibits the activity of human bone marrow stem cells. When the concentration exceeds 500 μM, it exhibits cytotoxic effects on human, rat, mouse, and rabbit hepatocytes; however, no obvious acute toxicity symptoms were observed in the acute toxicity test of umbelliferone in mice at 200 mg/kg (Cruz et al., 2020). The steroidal compound β-sitosterol from R. alismatis has been proven 30 years ago that 60 days of continuous administration at a dose of 1 mg/day does not cause liver and kidney toxicity, and has developed a variety of health products that are considered to be non-toxic compounds. Phenolic acids are a class of organic acids containing phenolic rings, which are present in a variety of plants and have anti-oxidation, anti-inflammatory, and hypotensive effects. Ferulic acid in R. alismatis was continuously fed at a dose of 5–10 mg/kg for 28 days without hepatorenal toxicity. No significant cytotoxicity was observed in MRC-5 cell lines treated with 25 μM and 50 μM in vitro (Kasarci et al., 2021). In addition to the mentioned metabolites, there are more than 100 kinds of non-terpenoids in R. alismatis, and its toxicity research has not been reported in the relevant literature.
4.6 Processing of Rhizoma alismatis
The processing methods of R. alismatis are various. Lei Gong’s theory of processing was first recorded in the Southern and Northern Dynasties: fine file, wine soaking for one night, wet out, dry out. In the Song Dynasty, the processing methods of wine soaking, stir-frying, steaming after wine soaking were added. To Ming Dynasty, the processing methods of water soaking, steaming, simmering and steaming after rice swill soaking appeared. In the Qing Dynasty, in addition to the main processing methods of the previous generation, the processing methods of salt water mixing, salt water stir-frying, wine stir-frying, wine mixing and baking were also put forward (Zhong et al., 2005).
The processing of Rhizoma alismatis has been continuously developed and adjusted, and the following mainstream processing approaches have emerged, including stir-frying, salt stir-frying, bran stir-frying, and soil stir-frying. Currently, the most commonly used processing varieties are bran stir-frying and salt stir-frying (Figure 9A) (Wang, 1993). The processing method is as follows: bran stir-frying: take bran, sprinkle it into a hot pot, wait until smoke emerges, add R. alismatis slices (RA), stir-fry until they turn brown, remove it, sieve the bran, and allow it to cool (RA: bran = 10:1, m/m). Wine stir-fried: add RA in a 100 °C hot pot, stir-fry several times, spray well with wine, stir-fry dry, take out and cool (RA: wine = 20: 1, m/v). Salt stir-fry: take RA, add salt water to mix well, stuffy, place in the pot, heat with fire, fry dry, remove, cool (RA: salt = 50: 1, m/m). Soil stir-frying: heat the pot, take RA, place it in the pot, immediately scatter the soil powder into it, turn it evenly with an iron rake, stir until the soil powder is evenly adhered to the tablets, remove the sieve to remove the soil powder, and stay cool.
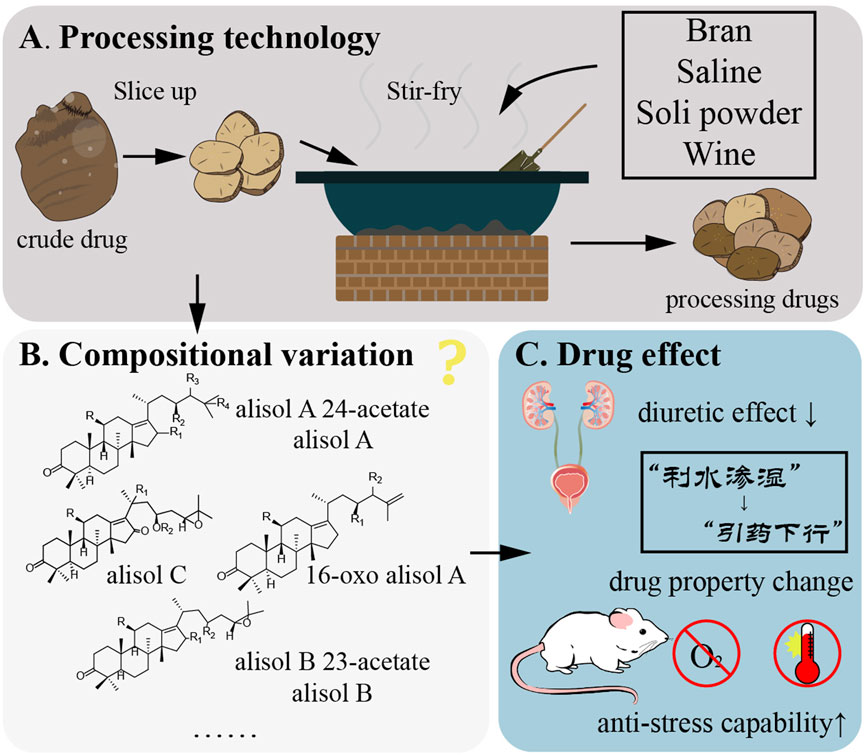
Figure 9. Processing methods of Rhizoma alismatis. (A) Rhizoma alismatis were sliced up and stir-fry with bran, saline, soli powder and wine. (B) Rhizoma alismatis compounds change after different processing methods (C). Processing affect the Rhizoma alismatis functions including diuretic effect and anti-stress capability.
The view of TCM believes that different processing methods can change the medicinal properties of R. alismatis. It can promote water and dampness without damaging yin. Bran stir-fried R. alismatis has the characteristics of dampness, spleen, turbidity and clearing (Yan et al., 2020). Salt stir-fried R. alismatis has the effect of guiding medicine downward, enhancing yin-nourishing, heat-clearing and diuresis (Zhong et al., 2005). At present, the study on the functional differences of R. alismatis with different processing methods is still in the preliminary stage, and the effect of different processing methods on diuretic effect is still controversial (Figures 9B,C) (Wei et al., 2013; Yan et al., 2020; Zeng et al., 2011). In addition, the water extract of R. alismatis raw products and salt stir-fry products can alleviate pulmonary edema in edema mice (Zeng et al., 2011) and affect the anti-stress effect of mice. Taking water extract concentrate of raw products for 8–15 days will weaken the anti-fatigue, hypoxia tolerance and high temperature resistance of mice, while salt stir-fry R. alismatis can increase the anti-stress ability of mice (Wei et al., 2013). This may be related to the toxicity of R. alismatis, and salt disposing can neutralize some of the toxic metabolites. Xu et al. (Zhang et al., 2012) confirmed the stronger spleen-strengthening effect of bran stir-fry R. alismatis by detecting the content of serum gastrin h, the activity of Na+-K+-ATPase in rat duodenum and the contraction amplitude of isolated duodenal smooth muscle in rats. This finding aligns with the tonifying and beneficial effects of R. alismatis recorded in classical texts, thereby laying the groundwork for the development of its new applications.
The effective metabolites of R. alismatis are mainly prototerpenoid triterpenes, including Alisol A, Alisol B, Alisol C, etc. From the biological secondary metabolic pathway, they are derived from the high content of alisol B 23-acetate in fresh medicinal materials, and alisol A 24-acetate, alisol B, alisol A accounted for the vast majority. After processing, its chemical composition remains nearly unchanged, but the proportion of each substance varies. For instance, alisol B, alisolenol and epoxide alisolene in wine stir-fry R. alismatis and salt stir-fry R. alismatis did not change significantly compared with raw products; however, the contents of alisol B 23-acetate and 23-acetyl alisol C were significantly reduced, and the decrease rate was salt stir-fry > wine stir-fry > bran stir-fry > raw products. Additionally, the contents of alisol A and alisol A 24-acetate in wine stir-fry R. alismatis and salt stir-fry R. alismatis increased (Cao et al., 2016; Wei et al., 2021). However, the change of alisol B 23-acetate in bran stir-fry R. alismatis was controversial (Dai and Jia, 2008). Based on the existing literature, the change of diuretic effect caused by different processing methods is mainly attributed to the change of the proportion of triterpenoids. Tong et al. (Yan et al., 2023) compared and analyzed the blood-entering components in the serum of rats after taking row R. alismatis and salt stir-R. alismatis by ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF- MS). It was found that the number of metabolites (5 prototype components and 9 metabolites) in the serum of rats given the water extract of salt stir-R. alismatis was lower than that of the raw water extract (5 prototype components and 15 metabolites), and the response intensity of the prototype metabolites 16-oxo alisol A, alisol B and alisol C in the serum of rats in the salt stir-fry R. alismatis group increased, while the types and response intensity of metabolic components generally decreased. It shows that the salt stir-R. alismatis may promote the absorption of prototype components by slowing down the metabolic rate of terpenoids.
5 Conclusion and discussion
With the innovation of detection technology, more and more chemical constituents have been found from R. alismatis, but most of them are extremely trace components. The detection of these new metabolites will not change our overall understanding of the function and toxicity. The content and ratio of bioactive components in R. alismatis, critical to its pharmacological profile, thus warrant focused attention. According to the development history of TCM, R. alismatis, the functions of diuresis-removing dampness, clearing heat and detoxifying, dissolving turbidity and lipid-lowing, which are recognized by traditional Chinese medicine. However, the tonic effect has been rarely mentioned, which may be related to the changes of material deficiency and nutrient deficiency in ancient times and the current material abundance and nutrient abundance. Modern pharmacological studies have shown that R. alismatis has diuretic, anti-kidney stone, anti-inflammation, hypoglycemic and lipid-lowering effects, which to a certain extent explains the effects of its TCM such as removing water and dampness, dissipating heat and removing fluids, and reducing turbidity and lipid-lowering. In addition, it also has liver protection and anti-tumor effects, but the latter is not verified in vivo. The Shennong Bencao Jing (Classic of Herbal Medicine) documents dermatological properties of R. alismatis—a pharmacological dimension rarely addressed in historical discourse and insufficiently investigated in contemporary research. This underexplored therapeutic potential carries significant implications for advancing phytotherapeutic innovations, particularly in dermatological applications.
One of the most controversial points of R. alismatis is the presence and size of its toxicity. Traditional Chinese medicine tends to regard Rhizoma alismati as a non-toxic medicinal material. However, modern pharmacological studies have found that it may cause some adverse reactions and poisoning events under certain circumstances. Triterpenoids are the main active ingredients in R. alismatis, for example, alisol B 23-acetate and alisol A 24-acetate have diuretic, lipid-lowering and liver-protecting effects (Meng et al., 2015; Wang et al., 2019; Zhang X. et al., 2017), but they can cause interstitial nephritis, apoptosis and exfoliation of renal tubular epithelial cells in rats at much higher doses and prolonged administration. These results suggest that triterpenoids are not only the effective components of R. alismatis, but also potential nephrotoxic substances. Alisol B 23-acetate and alisol A 24-acetate serve dual roles as both primary active constituents and principal toxic agents in R. alismatis. Intriguingly, some studies have linked their content to plucking time, processing and species (Chuan, Jian, and Guang R. alismatis). The alisol B 23-acetate content in R. alismatis harvested in April is significantly higher than in those harvested from January to March (Wen et al., 1998), which may be attributed to seasonal variations causing fluctuations in plant hormones. Studies have found that external application of salicylic acid, gibberellins, or abscisic acid can influence the alisol B 23-acetate content in R. alismatis (Li et al., 2015). Similarly, endogenous levels of plant hormones are affected by cultivation environments and climatic conditions, leading to regional variations. This partially explains the influence of species-specific differences on its chemical composition. The Chinese Pharmacopoeia mandates a minimum total content of 0.10% for alisol B 23-acetate and alisol C 23-acetate (two critical triterpenoid markers) in authenticated R. alismatis dry materials. However, the separate comparison of 24-acetylalismatiol A and 23-acetylalismatiol B could not distinguish R. alismatis from different origins. However, the inclusion of multiple variables (including alismoxide, alisol C 23-acetate, alisol A, alismol, alisol B, alisol B 23-acetate, 11-deoxyalisol B, 11-deoxyalisol C, alisol O, 24-acetylalisol A, and 11-deoxy-23-acetylalisol B) (Jin et al., 2020; Luo et al., 2010; Weng et al., 2022) enabled effective geographical differentiation through comprehensive analytical approaches. Specifically, hierarchical cluster analysis (HCA), principal component analysis (PCA), and Pearson correlation analysis collectively demonstrated significant discriminative power in characterizing regional variations.
To mitigate adverse effects of R. alismatis, diverse processing methods have been implemented. Salt-processing and bran-frying significantly alter the morphological characteristics of the botanical drug, while processing parameters (adjuvant concentration, temperature, duration) critically influence its bioactive constituent profiles (Yan et al., 2024). Processing of R. alismatis significantly reduces its adverse reactions compared to the crude drug. We hypothesize that the concentrations of its primary toxic components, alisol B 23-acetate and alisol A 24-acetate (as mentioned in Section 4.5), should be markedly decreased. However, existing literature presents conflicting results regarding this matter (Cai and Lin, 2021; Cao et al., 2016; Lu, 2013; Wei et al., 2021). This phenomenon suggests that: 1. Beyond the potential for undiscovered toxins in R. alismatis, our attention should shift to the combined levels of alisol B 23-acetate and alisol A 24-acetate; 2. The synergistic or antagonistic effects during processing may mitigate the adverse reactions. During this process, we also identified issues and challenges in the processing procedures. we observed inconsistent trends in the changes of bioactive components before and after processing, which could be attributed to variations in raw materials or a lack of standardization in the processing techniques. Numerous factors influence the content of these components, including the plant variety, cultivation region, climate, harvesting time, and processing methods. These complexities pose significant challenges to the standardized production of Rhizome alismatis. Herbal compatibility in traditional formulations may offer solutions. Preliminary evidence demonstrates reduced toxicity in compound preparations like Shenze Shugan Capsule (Dahuang, Juemingzi, Zexie) (Table 2), where R. alismatis appears to attenuate toxicity of co-administered botanical drugs (Dahuang and Juemingzi have always been considered to be highly toxic Chinese herbal medicines (Yang et al., 2024; Zhang et al., 2023)). In addition, toxicity experiments showed that even if 50–100 times the normal dose of long-term administration of R. alismatis water extract, there was no significant difference in behavioral activity, coat luster, mental state, drinking water and feces of rats. Liver, spleen, stomach, testis and other parts did not observed abnormalities expect for kidney. In the medium and high dose groups, the kidneys showed mild adverse effects, that is, the renal tubular epithelial cells showed swelling and degeneration and inflammatory cell infiltration in the renal interstitium (Duan et al., 2004). But severe adverse reactions were observed in the residual kidneys of partial nephrectomy mice (Zhu et al., 2007). In conclusion, R. alismatis is generally safe to take under the conventional dose. However, its toxicity depends on the metabolic capacity of the kidney, and patients with impaired renal function should be careful to take long-term high-doses. In addition, the production of safe and high-quality traditional Chinese medicine is inseparable from the source, that is, the high-quality provenances. There are 12 species of R. alismatis worldwide. At present, only two species, Alisma plantago-aquatica L. and Alisma orientale (Sam.) Juz., are included in the Chinese Pharmacopoeia, and the whole traceability system has not been established for planting and circulation. With the accelerating internationalization of TCM, it is suggested to carry out systematic evaluation of “Chinese medicinal properties” and quality of various varieties of R. alismatis, screen high-quality varieties, and establish a standardized cultivation, processing and circulation system, so as to provide a guarantee for the production of high-quality Chinese medicine R. alismatis and ensure the clinical efficacy of TCM.
Author contributions
TP: Validation, Conceptualization, Data curation, Methodology, Investigation, Writing – original draft, Writing – review and editing, Formal Analysis, Software. RT: Writing – review and editing, Formal Analysis, Data curation. JW: Investigation, Writing – review and editing. JG: Visualization, Methodology, Writing – review and editing. FJ: Visualization, Writing – review and editing, Supervision. BC: Resources, Project administration, Funding acquisition, Writing – review and editing, Validation, Supervision, Writing – original draft.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82374102) and Scientific research item, School of Life Sciences, Zhejiang Chinese Medical University (2022FH001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1722483/full#supplementary-material
References
Bai, K.-K., Yu, Z., Chen, F.-L., Li, F., Li, W.-Y., and Guo, Y.-H. (2012). Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. & Med. Chem. Lett. 22 (7), 2488–2493. doi:10.1016/j.bmcl.2012.02.009
Bi, X., Wang, P., Ma, Q., Han, L., Wang, X., Mu, Y., et al. (2017). Anti-inflammatory activities and liver protection of alisol F and 25-Anhydroalisol F through the inhibition of MAPK, STAT3, and NF-κB activation in vitro and in vivo. Molecules 22 (6), 951. doi:10.3390/molecules22060951
Biao, Y., Chen, J., Liu, C., Wang, R., Han, X., Li, L., et al. (2022). Protective effect of danshen zexie decoction against non-alcoholic fatty liver disease through inhibition of ROS/NLRP3/IL-1β pathway by Nrf2 signaling activation. Front. Pharmacol. 13, 877924. doi:10.3389/fphar.2022.877924
Cai, D., and Lin, Y. (2021). Effects of different processing methods on 23-O-Acetylalisol B in rhizoma alismatis from zichuan. Chin. J. Ethnomedicine Ethnopharmacy 30 (21), 45–48.
Cao, L., Li, Q., Fang, Q., Wang, X., Zhou, X., and Yuxia, Y. (2016). Effects of three processing methods on four triterpenoids in alismatis Rhizoma. Chin. Tradit. Pat. Med. 38 (09), 1994–1998. doi:10.3969/j.issn.1001-1528.2016.09.025
Cao, Y., Shi, J., Song, L., Xu, J., Lu, H., Sun, J., et al. (2022). Multi-omics integration analysis identifies lipid disorder of a non-alcoholic fatty liver disease (NAFLD) mouse model improved by zexie–baizhu decoction. Front. Pharmacol. 13, 858795. doi:10.3389/fphar.2022.858795
Cash crop team of Guangdong Agriculture (1970). Mainly poisonous plants in the south. Beijing: Science Press.
Chen, D., and Zhou, L. (1998). Common Chinese medicine and adverse reactions. Beijing: Military Medical Science Press.
Chen, D.-Q., Feng, Y.-L., Tian, T., Chen, H., Yin, L., Zhao, Y.-Y., et al. (2014). Diuretic and anti-diuretic activities of fractions of alismatis rhizoma. J. Ethnopharmacol. 157, 114–118. doi:10.1016/j.jep.2014.09.022
Chen, G., Liu, Y., Li, P., Wang, Y., Zhao, S., and Zhang, S. (2015). Long-term toxicity experiment on modified zexie tang. Chin. J. Exp. Traditional Med. Formulae 21 (20), 145–149. doi:10.13422/j.cnki.syfjx.2015200145
Chen, X., Qu, Y., Wang, S., and Wang, G. (2016). Clinical study on the upper and lower urinary tract infections treated withDifferent dosage of longqing tablets. Acta Chin. Med. 31 (06), 899–901. doi:10.16368/j.issn.1674-8999.2016.06.251
Cheng, X.-Y., and Ouyang, J.-M. (2023). Carboxymethylated rhizoma alismatis polysaccharides regulate calcium oxalate crystals growth and reduce the regulated crystals’ cytotoxicity. Biomolecules 13 (7), 1044. doi:10.3390/biom13071044
Choi, E., Jang, E., and Lee, J.-H. (2019). Pharmacological activities of Alisma orientale against nonalcoholic fatty liver disease and metabolic syndrome: literature review. Evidence-Based Complementary Altern. Med. 2019, 2943162–15. doi:10.1155/2019/2943162
Chong, Z. Z., Lin, S.-H., and Maiese, K. (2002). Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J. Vasc. Res. 39 (2), 131–147. doi:10.1159/000057762
Commission, C. P. (2020). Pharmacopoeia of the people's republic of China. Beijing: China Medical Science Press.
Cruz, L. F., Figueiredo, G. F. d., Pedro, L. P., Amorin, Y. M., Andrade, J. T., Passos, T. F., et al. (2020). Umbelliferone (7-hydroxycoumarin): a non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. & Pharmacother. 129, 110432. doi:10.1016/j.biopha.2020.110432
Dai, X., and Jia, T. (2008). Effect of different processing methods on the content of main components in rhizoma alismatis. 2008 Academic Seminar Chin. Med. Process. Branch China Assoc. Traditional Chin. Med. Camphor Trees Jiangxi, China.
Dai, M., Jin, S., Song, C., and Li, S. (2023). Research progress on chemical constituents and pharmacological effects of Alisma rhizoma and its processed products. Chin. Traditional Herb. 54 (05), 1620–1635. doi:10.7501/j.issn.0253-2670.2023.05.029
Dong, Y., Zhang, X., and Wang, J. (2023). Protective effect of Alisma orientalis polysaccharides on diabetes nephropathy rats by activating PPAR-γ/LXR-α/ABCG1 signaling pathway. Drug Eval. Res. 46 (07), 1480–1487. doi:10.7501/j.issn.1674-6376.2023.07.011
Duan, X., Wang, J., Yin, X., Yan, S., and Xiao, Y. (2004). A 60-day feeding study of Rhizoma alismatis orientalis in SD rats. Chin. J. Food Hygiene (02), 108–111. doi:10.13590/j.cjfh.2004.02.003
Ellithey, M., Lall, N., Hussein, A., and Meyer, D. (2013). Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboreum. Mar. Drugs 11 (12), 4917–4936. doi:10.3390/md11124917
Feng, Y., Chen, H., Tian, T., Chen, D., Zhao, Y., and Lin, R. (2014). Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of alismatis rhizoma. J. Ethnopharmacol. 154 (2), 386–390. doi:10.1016/j.jep.2014.04.017
Gu, L., Huang, L., Xe, P., and Yuan, J. (2019). The research status of Chinese medicinal herbs zexie (rhizoma alismatis)and lts enlightenment to Guangxi. 25 (06), 60–62+66. doi:10.13862/j.cnki.cn43-1446/r.2019.06.017
Han, C. W., Kang, E. S., Ham, S. A., Woo, H. J., Lee, J. H., and Seo, H. G. (2012). Antioxidative effects ofAlisma orientaleextract in palmitate-induced cellular injury. Pharm. Biol. 50 (10), 1281–1288. doi:10.3109/13880209.2012.673629
Han, C. W., Kwun, M. J., Kim, K. H., Choi, J.-Y., Oh, S.-R., Ahn, K.-S., et al. (2013). Ethanol extract of alismatis rhizoma reduces acute lung inflammation by suppressing NF-κB and activating Nrf2. J. Ethnopharmacol. 146 (1), 402–410. doi:10.1016/j.jep.2013.01.010
Hien, T. T. T., Thang, H. D., Tuan, H. A., Tai, P. T., Tung, N. M., Van Khoi, N., et al. (2023). Four new lignans obtained from the leaves of Schisandra cauliflora and their effect on skeletal muscle cell proliferation. J. Nat. Med. 77 (4), 928–938. doi:10.1007/s11418-023-01712-y
Hong, X., Tang, H., Wu, L., and Li, L. (2006). Protective effects of the Alisma orientalis extract on the experimental nonalcoholic fatty liver disease. J. Pharm. Pharmacol. 58 (10), 1391–1398. doi:10.1211/jpp.57.10.0013
Huang, X., Zhang, X., Li, X., Zhao, W., Xu, W., and Wu, S. (2016). Diuretic effect of different extract parts of rhizoma alismatis on rats. Fujian J. Traditional Chin. Med. 47 (05), 21–23. doi:10.13260/j.cnki.jfjtcm.011216
Huang, Y., Yu, Q., Chen, Y., Cheng, M., and Xie, L. (2017). Phenolic constituents from Alisma plantago-aquatica linnaeus and their anti-chronic prostatitis activity. Chem. Central J. 11 (1), 120. doi:10.1186/s13065-017-0350-9
Huang, Y., Li, D., Cheng, B., Liu, G., Zhang, Y.-X., and Zhou, W.-X. (2019). Active fraction combination from liuwei dihuang decoction (LW-AFC) ameliorates corticosterone-induced long-term potentiation (LTP) impairment in mice in vivo. J. Ethnopharmacol. 236, 147–154. doi:10.1016/j.jep.2019.03.002
Huang, Q., Fan, M., Ji, F., Wang, Y., Ding, H., Xu, J., et al. (2024). The safety evaluation of Shenze Shugan capsule and mechanism of apoptosis induced by five potentially nephrotoxic components. J. Ethnopharmacol. 324, 117777. doi:10.1016/j.jep.2024.117777
Hur, J. M., Choi, J. W., and Park, J. C. (2007). Effects of methanol extract ofalisma orientale rhizome and its major component, alisol B 23-acetate, on hepatic drug metabolizing enzymes in rats treated with bromobenzene. Archives Pharmacal Res. 30 (12), 1543–1549. doi:10.1007/bf02977323
Hwang, E., and Song, S. (2020). Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules 10 (5), 687. doi:10.3390/biom10050687
Jang, M.-K., Han, Y.-R., Nam, J., Han, C., Kim, B., Jeong, H.-S., et al. (2015). Protective effects of Alisma orientale extract against hepatic steatosis via inhibition of endoplasmic reticulum stress. Int. J. Mol. Sci. 16 (11), 26151–26165. doi:10.3390/ijms161125944
Jang, K., Ye, X., Xiong, F., Zhang, Y., Yang, L., Xiong, A., et al. (2021). The protective effects and mechanism of alismatis rhizoma extracts against senecionine-induced acute liver injury in mice. Acta Pharm. Sin. 56 (03), 823–830. doi:10.16438/j.0513-4870.2020-1659
Jeong, H.-S., Cho, Y.-H., Kim, K.-H., Kim, Y., Kim, K.-S., Na, Y.-C., et al. (2016). Anti-lipoapoptotic effects of Alisma orientalis extract on non-esterified fatty acid-induced HepG2 cells. BMC Complementary Altern. Med. 16 (1), 239. doi:10.1186/s12906-016-1181-2
Jin, H.-G., Jin, Q., Ryun Kim, A., Choi, H., Lee, J. H., Kim, Y. S., et al. (2012). A new triterpenoid from Alisma orientale and their antibacterial effect. Archives Pharmacal Res. 35 (11), 1919–1926. doi:10.1007/s12272-012-1108-5
Jin, L., Wang, Y., Ye, S., Li, C., Peng, G., and Zheng, Y. (2020). Establishment of HPLC fingerprints of Alisma orientale from different growing areas and chemical pattern recognition. Chin. Tradit. Pat. Med. 42 (01), 139–144. doi:10.3969/j.issn.1001-1528.2020.01.030
Ju, A., Zhou, Y., Shan, W., Liu, l., and Li, Q. (2023). Effects of zexie decoction on CYP450 enzyme subtype in hyperlipidemia model mice. J. Pharm. Res. 42 (12), 959–963. doi:10.13506/j.cnki.jpr.2023.12.002
Kasarci, G., Ertugrul, B., Iplik, E. S., and Cakmakoglu, B. (2021). The apoptotic efficacy of succinic acid on renal cancer cell lines. Med. Oncol. 38 (12), 144. doi:10.1007/s12032-021-01577-9
Kharin, A., and Klussmann, E. (2023). Many kinases for controlling the water channel aquaporin-2. J. Physiology 602 (13), 3025–3039. doi:10.1113/jp284100
Kim, S. Y., Yoo, D. M., Bang, W. J., and Choi, H. G. (2022). Obesity is positively associated and alcohol intake is negatively associated with nephrolithiasis. Nutrients 14 (19), 4122. doi:10.3390/nu14194122
Kim, K. H., Kim, S., Kwun, M. J., Lee, J. Y., Oh, S.-R., Choi, J.-Y., et al. (2023). Alismol purified from the tuber of Alisma orientale relieves acute lung injury in mice via Nrf2 activation. Int. J. Mol. Sci. 24 (21), 15573. doi:10.3390/ijms242115573
Kirsch, V., Bakuradze, T., and Richling, E. (2020). Toxicological testing of syringaresinol and enterolignans. Curr. Res. Toxicol. 1, 104–110. doi:10.1016/j.crtox.2020.09.002
Lau, C. H., Chan, C. M., Chan, Y. W., Lau, K. M., Lau, T. W., Lam, F. C., et al. (2008). In vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytotherapy Res. 22 (10), 1384–1388. doi:10.1002/ptr.2513
Li, Q., and Qu, H. (2012). Study on the hypoglycemic activities and metabolism of alcohol extract of alismatis rhizoma. Fitoterapia 83 (6), 1046–1053. doi:10.1016/j.fitote.2012.05.009
Li, X., Wei, Q., Shi, L., and Huang, S. (2012). The extraction of polysaccharides from alisman orientali and determination of lts content. Shandong Chem. Ind. 41 (07), 26–28. doi:10.19319/j.cnki.issn.1008-021x.2012.07.009
Li, Q., Zhou, Y.-f., Liu, Y.-y., Ke, L.-l., Huo, Y.-j., and Pan, D.-r. (2015). Effects of Different Elicitors on Accumulation of Alisol B 23-acetate in [Alisma orientale(Samuels) Juzep.]. Hubei Agric. Sci. 54 (05), 1125–1130. doi:10.14088/j.cnki.issn0439-8114.2015.05.025
Li, L., Cheng, J., Zhu, D., Shi, X., Wei, Y., Chen, S., et al. (2020). The effects of alisol B 23-acetate in hepatocellular carcinoma via inducing cell apoptosis and inhibiting cell migration and invasion. General Physiology Biophysics 39 (03), 219–228. doi:10.4149/gpb_2020005
Li, R., Li, Z., Chen, Y., Bu, W., Ding, W., Yang, B., et al. (2020). The structural composition of components contributes to the superiority of the geoherb Alisma orientale for “diuresis and diffusing dampness”. RSC Adv. 10 (65), 39385–39395. doi:10.1039/c9ra08469j
Lin, Y., Hong, L., Qiu, Z., Wang, C., Cai, Z., Yang, X., et al. (2023). Effect of alisol A on glucose absorption in vitro. Chin. J. Ethnomedicine Ethnopharmacy 32 (20), 20–23. doi:10.3969/j.issn.1007-8517.2023.20.zgmzmjyyzz202320006
Lin, Z., Cheng, X., and Zheng, H. (2023). Umbelliferon: a review of its pharmacology, toxicity and pharmacokinetics. Inflammopharmacology 31 (4), 1731–1750. doi:10.1007/s10787-023-01256-3
Liu, Y. (2012). Inhibitory effect of alismol to MMP3 expression and nitric oxide produced in microglial cells. Exp. Technol. Manag. 29 (10), 47–50. doi:10.16791/j.cnki.sjg.2012.10.014
Liu, S. (2019). Studies on the Constituents from Water Extract of Alismatis Rhizoma and their Differences of Origins [Doctor, China Academy of Chinese Medical Sciences]. Available online at: https://next.cnki.net/middle/abstract?v=J38Mj5ylEu918OmqXcLQ2CFW4JlESufSY-1ebXdBHNVSGpy0h3th-nifKNZRWH7PCpq7NZIV6hm7uo9MCzqnp4Vh7LZplCEAnTvpgepHPA314L5Q-XLm7KVFpWfz_DIArlBwVPDNzP8JNz6HwPW4HP4RSpb_NOE86gdLgz5hF2S_Rk5Dkj9rTSuj3ccbWIvqI8BE9qfVDpk=&uniplatform=NZKPT&language=CHS&scence=null
Liu, M., and Wang, L. (2023). Effects and safety of supplementary wuling san in the treatment of Obese type 2 diabetes. Pract. Clin. J. Integr. Traditional Chin. West. Med. 23 (09), 93–96. doi:10.13638/j.issn.1671-4040.2023.09.026
Liu, S., Sheng, W., Li, Y., Zhang, S., Zhu, J., Gao, H., et al. (2019). Chemical constituents from alismatis rhizoma and their anti-inflammatory activities in vitro and in vivo. Bioorg. Chem. 92, 103226. doi:10.1016/j.bioorg.2019.103226
Liu, Y., Xia, X. C., Meng, L. Y., Wang, Y., and Li, Y. M. (2019). Alisol B 23-acetate inhibits the viability and induces apoptosis of non-small cell lung cancer cells via PI3K/AKT/mTOR signal pathway. Mol. Med. Rep. 20 (2), 1187–1195. doi:10.3892/mmr.2019.10355
Liu, S., Liu, X., Tian, S., Zhu, J., Gao, H., Yan, L., et al. (2020). Qualitative and quantitative research on nucleosides of Alismatis Rhizoma from different regions. China J. Chinese Materia Medica 45 (07), 1558–1565. doi:10.19540/j.cnki.cjcmm.20190621.201
Liu, R., Sun, Y., Di, D., Zhang, X., Zhu, B., and Wu, H. (2023). PI3K/AKT/SERBP-1 pathway regulates Alisma orientalis beverage treatment of atherosclerosis in APOE −/− high-fat diet mice. Pharm. Biol. 61 (1), 473–487. doi:10.1080/13880209.2023.2168020
Lou, C., Xu, X., Chen, Y., and Zhao, H. (2019). Alisol A suppresses proliferation, migration, and invasion in human breast cancer MDA-MB-231 cells. Molecules 24 (20), 3651. doi:10.3390/molecules24203651
Lu, X. h. (2013). Determination by HPLC of the content of 24-Acetyl alisol A in crude and salted rhizoma alismatis. Inn. Mong. J. Traditional Chin. Med. 32 (20), 43. doi:10.16040/j.cnki.cn15-1101.2013.20.055
Lu, M., Yin, J., Xu, T., Dai, X., Liu, T., Zhang, Y., et al. (2024). Fuling-zexie formula attenuates hyperuricemia-induced nephropathy and inhibits JAK2/STAT3 signaling and NLRP3 inflammasome activation in mice. J. Ethnopharmacol. 319, 117262. doi:10.1016/j.jep.2023.117262
Luo, Y., Qiu, L., Xu, W., Li, X., and Wu, S. (2010). Comparison of the contents of 4 triterpenoids in Alisma japonica from different producing areas. J. Chin. Med. Mater. 33 (12), 1849–1851. doi:10.13863/j.issn1001-4454.2010.12.013
Luo, S., Huang, W., Liu, Y., Lu, Y., Li, G., and Tang, W. (2021). Establishment of fingerprinting of water extracts of alismatis rhizoma based on ultra-high performance chromatography. Shaanxi J. Agric. Sci. 67 (01), 49–52. doi:10.3969/j.issn.0488-5368.2021.01.013
Ma, C.-M., Cai, S.-Q., Cui, J.-R., Wang, R.-Q., Tu, P.-F., Hattori, M., et al. (2005). The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 40 (6), 582–589. doi:10.1016/j.ejmech.2005.01.001
Ma, X., Yao, H., Xin, T., Chen, X., and Song, J. (2015). SNP ldentification of alisma orientale,alisma plantago-aquatica andAlismatis rhizoma in the market based on DNA barcoding. Chin. Pharm. J. 50 (17), 1474–1478. doi:10.11669/cpj.2015.17.003
Ma, Q., Han, L., Bi, X., Wang, X., Mu, Y., Guan, P., et al. (2016). Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale. Phytochemistry 131, 150–157. doi:10.1016/j.phytochem.2016.08.015
Ma, J., Yuan, H., Shang, C., and Liu, C. (2024). Mechanism and research progress of wuling powder on treating edema. J. Liaoning Univ. Traditional Chin. Med. 26 (09), 163–167. doi:10.13194/j.issn.1673-842x.2024.09.031
Malini, T., and Vanithakumari, G. (1990). Rat toxicity studies with β-sitosterol. J. Ethnopharmacol. 28 (2), 221–234. doi:10.1016/0378-8741(90)90032-o
Matsuda, H., Kageura, T., Toguchida, I., Murakami, T., Kishi, A., and Yoshikawa, M. (1999). Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide-activated macrophages: absolute stereostructures of alismaketones-B 23-acetate and -C 23-acetate. Bioorg. & Med. Chem. Lett. 9 (21), 3081–3086. doi:10.1016/s0960-894x(99)00536-3
Matsumura, N., Kikuchi-Utsumi, K., Sakamaki, K., Watabe, M., Aoyama, K., and Nakaki, T. (2013). Anticonvulsant action of indazole. Epilepsy Res. 104 (3), 203–216. doi:10.1016/j.eplepsyres.2012.11.001
Meng, Q., Chen, X., Wang, C., Liu, Q., Sun, H., Sun, P., et al. (2015). Alisol B 23-acetate protects against ANIT-induced hepatotoxity and cholestasis, due to FXR-mediated regulation of transporters and enzymes involved in bile acid homeostasis. Toxicol. Appl. Pharmacol. 283 (3), 178–186. doi:10.1016/j.taap.2015.01.020
Mengxiang, D., Shuna, J., Chengwu, S., and Sen, L. (2023). Research progress on chemical constituents and pharmacological effects ofAlisma rhizoma and its processed products. Chin. Traditional Herb. Drugs 54 (05), 1620–1635. doi:10.7501/j.issn.0253-2670.2023.05.029
Miao, H., Zhang, L., Chen, D. Q., Chen, H., Zhao, Y. Y., and Ma, S. C. (2016). Urinary biomarker and treatment mechanism of rhizoma alismatis on hyperlipidemia. Biomed. Chromatogr. 31 (4), e3829. doi:10.1002/bmc.3829
Mou, Y., Wang, X., Wang, T., Wang, Y., Wang, H., Zhao, H., et al. (2022). Clinical application and pharmacological mechanism of wuling powder in the treatment of ascites: a systematic review and network pharmacological analysis. Biomed. & Pharmacother. 146, 112506. doi:10.1016/j.biopha.2021.112506
Murata, T., Imai, Y., Hirata, T., and Miyamoto, M. (1970). Biological-active trieterpenes of alismatis rhizoma. I. Isolation of the alisols. Chem. Pharm. Bull. 18 (7), 1347–1353. doi:10.1248/cpb.18.1347
Niu, L., Wu, L., and Yuan, Z. (2024). Evolvement of efficacy of alismatis rhizoma. Chin. J. Mod. Appl. Pharm. 41 (12), 1682–1685. doi:10.13748/j.cnki.issn1007-7693.20223844
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.)372 , n71. doi:10.1136/bmj.n71
Park, Y.-J., Kim, M.-S., Kim, H.-R., Kim, J.-M., Hwang, J.-K., Yang, S.-H., et al. (2014). Ethanol extract ofAlismatis rhizomeInhibits adipocyte differentiation of OP9 cells. Evidence-Based Complementary Altern. Med. 2014, 415097. doi:10.1155/2014/415097
Peng, G. (2006). Progress in the study on chemical constituents of Alisma orientalis. Nat. Prod. Res. Dev. doi:10.16333/j.1001-6880.2006.02.041
Shi, H., Zheng, Y., Zhao, J., Li, Y., Jia, H., Hou, X., et al. (2023). Zexie decoction reduce glucose-dependent lipid accumulation and oxidative stress in Caenorhabditis elegans. Phytomedicine 120, 155036. doi:10.1016/j.phymed.2023.155036
Shimizu, N., Ohtsu, S., Tomoda, M., Gonda, R., and Ohara, N. (1994). A glucan with immunological activities from the tuber of Alisma orientale. Biol. Pharm. Bull. 17 (12), 1666–1668. doi:10.1248/bpb.17.1666
Shu, Z., Pu, J., Chen, L., Zhang, Y., Rahman, K., Qin, L., et al. (2016). Alisma orientale: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Am. J. Chin. Med. 44 (02), 227–251. doi:10.1142/s0192415x16500142
Slighoua, M., Amrati, F. E.-Z., Chebaibi, M., Mahdi, I., Al Kamaly, O., El Ouahdani, K., et al. (2023). Quercetin and ferulic acid elicit estrogenic activities in vivo and in silico. Molecules 28 (13), 5112. doi:10.3390/molecules28135112
Steffan, B., Wätjen, W., Michels, G., Niering, P., Wray, V., Ebel, R., et al. (2005). Polyphenols from plants used in traditional Indonesian medicine (Jamu): uptake and antioxidative effects in rat H4IIE hepatoma cells. J. Pharm. Pharmacol. 57 (2), 233–240. doi:10.1211/0022357055317
Sun, Y., Pan, H., Shen, S., Xia, Z., Yu, Z., Li, C., et al. (2020). Alisma shugan decoction (ASD) ameliorates hepatotoxicity and associated liver dysfunction by inhibiting oxidative stress and p65/Nrf2/JunD signaling dysregulation in vivo. Med. Sci. Monit. 26, e921738. doi:10.12659/msm.921738
Tian, T., Chen, H., and Zhao, Y. Y. (2014). Traditional uses, phytochemistry, pharmacology, toxicology and quality control of (Sam.) Juzep: a review. J. Ethnopharmacol. 158, 373–387. doi:10.1016/j.jep.2014.10.061
Tian, S., Zhao, X., Liu, S., Feng, W., Chen, L., Yan, L., et al. (2020). Regional differences analysis of alismatis rhizoma based on UPLC characteristic chromatogram and determination of eight terpenoids. China J. Chin. Materia Medica 45 (07), 1545–1557. doi:10.19540/j.cnki.cjcmm.20200324.201
Tomoda, M., Gonda, R., Shimizu, N., and Ohara, N. (1994). Characterization of an acidic polysaccharide having immunological activities from the tuber of Alisma orientale. Biol. Pharm. Bull. 17 (5), 572–576. doi:10.1248/bpb.17.572
Wang, S. (1993). Effect of different processing methods on dissolution of total components of rhizoma alismatis. Lishizhen Med. Materia Medica Res. (01), 29.
Wang, L., Wu, Q., Zhang, Q., Peng, G., and Ding, A. (2008). Basic study of diuretic active compounds in rhizoma alismatis. West China J. Pharm. Sci. 23 (06), 670–672. doi:10.13375/j.cnki.wcjps.2008.06.014
Wang, C., Ma, L., Hou, X., Chen, H., Feng, L., and Jia, X. (2016). Study on nephrotoxicity of ethanol extract from alismatis rhizoma in rats and its molecular mechanism. China J. Chin. Materia Medica 41 (18), 3432–3438. doi:10.4268/cjcmm20161819
Wang, C., Feng, L., Ma, L., Chen, H., Tan, X., Hou, X., et al. (2017). Alisol A 24-Acetate and alisol B 23-Acetate induced autophagy mediates apoptosis and nephrotoxicity in human renal proximal tubular cells. Front. Pharmacol. 8, 172. doi:10.3389/fphar.2017.00172
Wang, Y., Zhao, J., Liang, J., Tian, X., Huo, X., Feng, L., et al. (2017). A bioactive new protostane-type triterpenoid from Alisma plantago-aquatica subsp. orientale (sam.) sam. Nat. Prod. Res. 33 (6), 776–781. doi:10.1080/14786419.2017.1408106
Wang, B., Chen, L., Dai, L., Fang, W., and Wang, H. (2019). Alisol B 23-Acetate ameliorates lipopolysaccharide-induced cardiac dysfunction by suppressing toll-like receptor 4 (TLR4)/NADPH oxidase 2 (NOX2) signaling pathway. Med. Sci. Monit. 25, 8472–8481. doi:10.12659/msm.918252
Wang, K., Zhang, B., Song, D., Xi, J., Hao, W., Yuan, J., et al. (2020). Alisol A alleviates arterial plaque by activating AMPK/SIRT1 signaling pathway in apoE-Deficient mice. Front. Pharmacol. 11, 580073. doi:10.3389/fphar.2020.580073
Wang, Z., Liu, L., Li, C., Zhao, Y., Tong, X., Cheng, X., et al. (2024). Carboxymethylated rhizoma alismatis polysaccharides reduces the risk of calcium oxalate stone formation by reducing cellular inflammation and oxidative stress. Urolithiasis 52 (1), 63. doi:10.1007/s00240-024-01565-4
Wei, H., Zeng, C., Li, X., Huang, Z., Chen, Y., Yan, X., et al. (2013). Experimental study on anti-stress effect of rhizoma alismatis before and after processing in Fujian province. J. Guangxi Univ. Chin. Med. 16 (01), 3–5. doi:10.3969/j.issn.1008-7486.2013.01.002
Wei, W., Xiang, F., Xu, N., and Zhou, X. (2021). Determination of seven major components in different processed products of alismatis rhizoma. China Pharm. 24 (12), 2284–2288. doi:10.19962/j.cnki.issn1008-049X.2021.12.031
Wen, H., Li, W., Peng, G., and Chi, Y. (1998). Content variety of alisol B 23-Acetate in rhiozma alismatis reaped at different time. J. Chin. Med. Mater. 21 (12), 595–596. doi:10.13863/j.issn1001-4454.1998.12.001
Weng, Y., li, L., Ni, L., You, P., Xu, W., Huang, H., et al. (2022). Determination and chemomterics analysis of contents in alismatis rhizoma decocted powder from different areas. Chin. J. Hosp. Pharm. 42 (01), 1–6. doi:10.13286/j.1001-5213.2022.01.01
Wu, Y., Wang, X., Yang, L., Kang, S., Yan, G., Han, Y., et al. (2023). Potential of alisols as cancer therapeutic agents: investigating molecular mechanisms, pharmacokinetics and metabolism. Biomed. & Pharmacother. 168, 115722. doi:10.1016/j.biopha.2023.115722
Xia, J., Luo, Q., Huang, S., Jiang, F., Wang, L., Wang, G., et al. (2019). Alisol B 23-acetate-induced HepG2 hepatoma cell death through mTOR signaling-initiated G1 cell cycle arrest and apoptosis: a quantitative proteomic study. Chin. J. Cancer Res. 31 (2), 375–388. doi:10.21147/j.issn.1000-9604.2019.02.12
Xiao, X., Jing, Y., Li, C., Zhang, N., and Guo, C. (2024). Research progress of main chemical constituents and pharmacological effects of alismatis rhizoma. J. Xinxiang Med. Univ. 41 (04), 378–382. doi:10.7683/xxyxyxb.2024.04.015
Xie, Z., Li, E.-w., Gao, G., Du, Y., Wang, M., Wang, H., et al. (2022). Zexie tang targeting FKBP38/mTOR/SREBPs pathway improves hyperlipidemia. J. Ethnopharmacol. 290, 115101. doi:10.1016/j.jep.2022.115101
Xu, W., Li, T., Qiu, J., Wu, S., Huang, M., Lin, L., et al. (2015). Anti-proliferative activities of terpenoids isolated from Alisma orientalis and their structure-activity relationships. Anti-Cancer Agents Med. Chem. 15 (2), 228–235. doi:10.2174/1871520614666140601213514
Xu, F., Chen, J., Zhang, Y., Wu, Q., Shen, Y., Gu, W., et al. (2020). Molecular insight into the mechanism of lipid regulating effect of Alisma orientalis based on ACAT. Int. J. Biol. Macromol. 158, 1141–1162. doi:10.1016/j.ijbiomac.2020.04.260
Yan, G., Lan, M., Qiu, J., Li, X., Xu, W., and Wu, S. (2020). Study on changes of chemical composition and diuretic effect in alismatis rhizoma after processing. Chin. J. Inf. Traditional Chin. Med. 27 (04), 59–65. doi:10.3969/j.issn.1005-5304.201906015
Yan, J., Zhou, Y., Yang, Q., Wu, J., and He, X. (2022). Evaluation of the safety and efficacy of a fuling-zexie decoction for people with asymptomatic hyperuricemia: protocol for a prospective, double-blinded, randomized, placebo-controlled clinical trial. Trials 23 (1), 517. doi:10.1186/s13063-022-06479-3
Yan, P., Wei, Y., Wang, M., Tao, J., Ouyang, H., Du, Z., et al. (2022). Network pharmacology combined with metabolomics and lipidomics to reveal the hypolipidemic mechanism ofAlismatis rhizomain hyperlipidemic mice. Food & Funct. 13 (8), 4714–4733. doi:10.1039/d1fo04386b
Yan, L., Ou, Z., Wang, Y., Zhang, Y., Cheng, Y., Wang, Z., et al. (2023). Comparative analysis of serum pharmacochemistry of alismatis RhizomaBefore and after salt processing based on UPLC-Q-TOF-MS. Chin. J. Exp. Traditional Med. Formulae 29 (23), 122–130. doi:10.13422/j.cnki.syfjx.20230866
Yan, B., Xie, L., Xie, Y., Wang, A., and Tan, W. (2024). Multi-index comprehensive evaluation of alismatis rhizoma processing technology. Asia-Pacific Tradit. Med. 20 (01), 25–29. doi:10.11954/ytctyy.202401006
Yang, X., Huang, Z., Cao, W., Chen, w., Tao, J., Chen, H., et al. (2004). Effects of rhizome alismatis extract on blood biochemicalindices and insulin in hyperglycemic mice. Chin. J. of Clin. Rehabilitation(06), 1196–1197. doi:10.3321/j.issn:1673-8225.2004.06.064
Yang, Y., Zhang, D., Liu, J., Hu, L., Xue, Q., Ding, X., et al. (2015). Wuling san protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J. Ethnopharmacol. 169, 49–59. doi:10.1016/j.jep.2015.04.011
Yang, J., Xiao, S., Li, L., Zhu, A., Xiao, W., and Wang, Q. (2024). Actin dysregulation mediates nephrotoxicity of cassiae semen aqueous extracts. Toxics 12 (8), 556. doi:10.3390/toxics12080556
Zeng, C., Yang, K., Lu, G., Zeng, Z., Zheng, Y., and Huang, K. (2011). Experimental study on diuretic effect of alismatis rhizoma before and after salt processing in Guangxi. Guangxi J. Traditional Chin. Med. 34 (01), 55–56. doi:10.3969/j.issn.1003-0719.2011.01.028
Zhang, C., Zhou, A., and Zhang, m. (2009). Chemical constituents of Alisma orientalis and their immunosuppressive function. China J. Chin. Materia Medica 34 (08), 994–998. doi:10.3321/j.issn:1001-5302.2009.08.016
Zhang, H., Xie, X., Chen, Y., Ren, G., Wang, X., and Xu, Z. (2012). The invigorating spleen function by Alisma orientalis before and after processing. Chin. J. Exp. Traditional Med. Formulae 18 (10), 187–190. doi:10.13422/j.cnki.syfjx.2012.10.059
Zhang, L., Xu, Y., Tang, Z., Xu, X., Chen, X., Li, T., et al. (2016). Effects of alisol B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest, apoptosis, migration and invasion inhibition. Phytomedicine 23 (8), 800–809. doi:10.1016/j.phymed.2016.04.003
Zhang, X., Li, X., Lin, N., Zhao, W., Huang, X., Chen, Y., et al. (2017). Diuretic activity of compatible triterpene components of alismatis rhizoma. Molecules 22 (9), 1459. doi:10.3390/molecules22091459
Zhang, Z., Huo, X., Tian, X., Feng, L., Ning, J., Zhao, X., et al. (2017). Novel protostane-type triterpenoids with inhibitory human carboxylesterase 2 activities. RSC Adv. 7 (46), 28702–28710. doi:10.1039/c7ra04841f
Zhang, J., Jin, Q., Li, S., Wu, J., Wang, Z., Hou, J., et al. (2018). Orientalol L–P, novel sesquiterpenes from the rhizome of Alisma orientale (Sam.) Juzep and their nephrotoxicity on HK2 cells. New J. Chem. 42 (16), 13414–13420. doi:10.1039/c8nj02027b
Zhang, M., Chen, J., and Zhou, X. (2018). Improvement effects of Alisma orientalis polysaccharide on insulin resistance and lipid metabolism disorder in type 2 diabetes mellitus rats and its mechanism study. China Pharm. 29 (01), 42–45. doi:10.6039/j.issn.1001-0408.2018.01.11
Zhang, W., Wang, M., Liu, H., Wang, Q., and Chen, Q. (2019). Hypoglycemic effect of alismoxide in type 2 diabetic mice. Chin. Pharmacol. Bull. 35 (09), 1240–1244. doi:10.3969/j.issn.1001-1978.2019.09.011
Zhang, F., Wu, J., Ruan, H., Xia, J., Xue, H., Wang, J., et al. (2022). ZeXie decoction alleviates non-alcoholic fatty liver disease in rats: the study of genes, lipids, and gut microbiotas. Biochem. Biophysical Res. Commun. 632, 129–138. doi:10.1016/j.bbrc.2022.09.097
Zhang, J., Yan, X., Jin, Q., Chen, J., Yang, L., Wei, W., et al. (2022). Novel triterpenoids from Alisma plantago-aquatica with influence on LDL uptake in HepG2 cells by inhibiting PCSK9. Phytomedicine 105, 154342. doi:10.1016/j.phymed.2022.154342
Zhang, F., Wu, R., Liu, Y., Dai, S., Xue, X., Li, Y., et al. (2023). Nephroprotective and nephrotoxic effects of rhubarb and their molecular mechanisms. Biomed. & Pharmacother. 160, 114297. doi:10.1016/j.biopha.2023.114297
Zhao, X., Wang, G., Wang, Y., Tian, X., Zhao, J., Huo, X., et al. (2017). Chemical constituents from Alisma plantago-aquatica subsp. orientale (Sam.) Sam and their anti-inflammatory and antioxidant activities. Nat. Prod. Res. 32 (23), 2749–2755. doi:10.1080/14786419.2017.1380024
Zhao, L., Lin, L., Xian, R., Wang, Y., Lan, Z., Pan, X., et al. (2024). Medicinal alisma rhizoma status about commodity specifications and grade division of Chinese. J. Chengdu Univ. Traditional Chin. Med. 47 (02), 75–80. doi:10.13593/j.cnki.51-1501/r.2024.02.075
Zhong, L., Dou, Z., Gong, Q., Zhang, D., and Duan, Q. (2005). Overview of research on the source, harvesting and processing of rhizoma alismatis. J. Jiangxi Univ. Chin. Med. (03), 54–55. doi:10.3969/j.issn.1005-9431.2005.03.025
Keywords: Rhizoma alismatis, phytochemistry, pharmacology, toxicology, processing
Citation: Pan T, Tang R, Wang J, Gao J, Jiang F and Chen B (2025) Botany, traditional uses, phytochemistry, pharmacology, toxicology and processing of Rhizoma alismatis: a review. Front. Pharmacol. 16:1722483. doi: 10.3389/fphar.2025.1722483
Received: 10 October 2025; Accepted: 12 November 2025;
Published: 04 December 2025.
Edited by:
Yulia Smyatskaya, Peter the Great St.Petersburg Polytechnic University, RussiaReviewed by:
Majid Sharifi-Rad, Zabol University, IranChuanzhi Kang, China Academy of Chinese Medical Sciences, China
Copyright © 2025 Pan, Tang, Wang, Gao, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Buyang Chen, MTc4NTU4Mjg2ODNAMTYzLmNvbQ==; Fusheng Jiang, amZzMTAyMEAxNjMuY29t; Jia Gao, Z2FvamlhamlhMTAwQDEyNi5jb20=
†These authors have contributed equally to this work
 Tianhan Pan
Tianhan Pan Ruonan Tang2†
Ruonan Tang2† Fusheng Jiang
Fusheng Jiang Buyang Chen
Buyang Chen