- 1Department of Immunology and Microbiology, Geisel School of Medicine at Dartmouth, Lebanon, NH, United States
- 2Bodymind Science, LLC, Arlington, VT, United States
- 3Department of Medicine, Geisel School of Medicine at Dartmouth, Lebanon, NH, United States
Vitamin D deficiency and insufficiency (VDD) are widely recognized as risk factors for respiratory tract infections. Vitamin D influences expression of many genes with well-established relevance to airway infections and relevant to immune system function. Recently, VDD has been shown to be a risk factor for acquisition and severity of COVID-19. Thus, treating VDD presents a safe and inexpensive opportunity for modulating the severity of the disease. VDD is common in those over 60 years of age, many with co-morbid conditions and in people with skin pigmentation sufficient to reduce synthesis of vitamin D. Exposure to fine particulate air pollution is also associated with worse outcomes from COVID19. Vitamin D stimulates transcription of cathelicidin which is cleaved to generate LL37. LL37 is an innate antimicrobial with demonstrated activity against a wide range of microbes including envelope viruses. LL37 also modulates cytokine signaling at the site of infections. Fine particles in air pollution can interfere with LL37 destruction of viruses and may reduce effective immune signaling modulation by LL37. While vitamin D influences transcription of many immune related genes, the weakened antimicrobial response of those with VDD against SARS-CoV-2 may be in part due to reduced LL37.
Conclusion: Vitamin D plays an important role reducing the impact of viral lung disease processes. VDD is an acknowledged public health threat that warrants population-wide action to reduce COVID-19 morbidity and mortality. While vitamin D influences transcription of many immune related genes, the weakened antimicrobial response of those with VDD against SARS-CoV-2 may be in part due to reduced LL37. Action is needed to address COVID-19 associated risks of air pollution from industry, transportation, domestic sources and from primary and second hand tobacco smoke.
Introduction
Innate Immune Responses in the Context of COVID-19
Mammals have complex immune systems that integrate and coordinate adaptive and innate responses to microbial threats. Innate immune protection is the first line of defense, and is the entire defense against a novel pathogen before the slower adaptive immune system has an opportunity to respond. Humans have multiple layers of innate protection including barrier protection, cellular surveillance and communications between cells found at mucosal surfaces with other parts of the immune system. As part of this system of defense, virtually all metazoan animals, including humans, release antimicrobial peptides (AMPs) that both kill invading microbes and act as immune signaling mediators. AMPs are key element in successfully maintaining boundaries between the mammalian host and the ubiquitous microbial flora to which all life forms are exposed. An example of antimicrobial innate protection is cathelicidin (hCAP18), a broad-spectrum antimicrobial AMP known for its role in protecting against Mycobacterium tuberculosis, the organism that causes tuberculosis.
A cationic peptide LL-37, derived from cleavage of the cathelicidin peptide, binds to target microbes, creating a pore in vulnerable bacteria or destroying the envelope of envelope viruses such as those of the Corona virus family (1). Vitamin D (VD) activates the vitamin D receptor which is a transcription factor that influences transcription of hundreds of genes including promoting transcription of the hCAP18 gene that encodes cathelicidin. Some VD regulated genes are key to balanced responses of the immune system against many bacterial and viral infections. Recent publications (2, 3) link Vitamin D deficiency to severity of COVID-19. We postulate that with sufficient VD, that LL37 helps to clear the SARS-CoV-2 virus and helps to regulate the immune system responses. Other reports show that carbon and other nanoparticles (4) in air pollution cause citrullination of LL37 (5), which blocks its ability to destroy or disable viruses such as SARS-CoV-2.
COVID-19 Susceptibility
A key question about COVID-19 illness is what differentiates those individuals who became seriously ill with long term health impact or death, from those who also test positive for carrying SARS-CoV-2 or having been exposed, remain symptom free or with relatively mild disease. While there are a vast array of correlations including, age, sex, ethnicity, and health status at the time of infection, most of these variables cannot be therapeutically manipulated. It makes clinical sense to identify and remediate issues that can be therapeutically adjusted, such as vitamin D sufficiency.
Appreciation of the importance of Vitamin D in the COVID-19 pandemic, requires an understanding of its role as a transcription factor for hundreds of genes, many of which are associated with immune protection (6, 7). Additionally it requires recognition that life style, geography, economics and social customs have influenced the risk of vitamin D insufficiency and deficiency (VDD) that exists in much of the world's population. We note here that vitamin D deficiency, and reduced AMPs associated with it, can be further impacted by exposure to carbon and other forms of nanoparticle-associated air pollution. Air pollution exposure is another risk factor for severe illness from COVID-19 (8).
Vitamin D
Vitamin D is normally made by humans through exposure to adequate levels of sunlight. Broadly this means daily sun exposure to the skin for approximately 10 min. For the sun to provide adequate UVB to activate vitamin D production, the sun must be more than 45 degrees above the horizon. While the conditions for adequate UVB availability occur daily in equatorial regions of the Earth, they are only seasonally available at mid and high latitude locations. The process of acquiring Vitamin D from sunlight involves UVB converting 7-dehydrocholesterol in the skin to previtamin D3, and subsequently to vitamin D3. Vitamin D can also be obtained through some foods, generally from those that are fortified, and through supplementation (9).
Vitamin D insufficiency and deficiency are defined as follows: Vitamin D deficiency exists when 25-hydroxyvitamin D (25(OH)D) is measured at below 20 ng/ml (50 nmol/liter). Vitamin D insufficiency is defined as 25(OH)D being measured at between 21–29 ng/ml (52.5–72.5 nmol/liter) (10). VDD is found widely in industrialized societies world wide, but more so in mid and higher latitude locations as well as in older adults and in populations of color (9, 11). Relevant to the COVID-19 pandemic, extrapolating from data found at the Johns Hopkins University Corona Virus Resource Center maps showing locations and size of COVID-19 cases worldwide, to date, the greatest density of disease is occurring above 30 degrees latitude (12). Most of Europe, Asia and North America lie within this zone.
Recent news and academic reports chronicle a disproportionate percentage of people of color in the US who are hospitalized and die of COVID-19 (13–16). Extensive evidence exists that African Americans as a group, historically have significantly lower serum Vitamin D levels than Americans of European descent. This risk factor is shared, to a lesser extent by others with greater skin pigmentation and who lack adequate daily UVB sunlight exposure (17–19). VDD is shared by those whose lifestyle choices, occupation or geographic location, limit their regular exposure to sun. VDD is also widely seen in populations where religion or social custom involves wearing clothing that fully covers the body.
Cathelicidin and LL37
Human innate immune molecule LL37, the cationic active fragment of cathelicidin (hCAP18) displays antimicrobial activity against a wide range of microbes including viral, bacterial, parasitic, and fungal microorganisms (20). The hCAP18 gene, encoding cathelicidin the precursor to LL37, is transcriptionally regulated in part by vitamin D steroid hormone metabolite, 1α,25-dihydroxyvitamin D (1,25(OH)2D) (21). Following cleavage of the cathelicidin peptide, LL37 is active against bacteria and viruses. Additionally, LL37 acts to modulate immune responses and functions in concert with toll-like receptors and other signaling mechanisms to communicate the nature of threat to the immune system (22–25). This nuanced modulation of the immune system serves to limit over and under responses to microbial challenges.
LL37 is reported to have attenuated the replication of a number of viruses including several classified as Class IV single stranded (SS) enveloped RNA viruses similar to the Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) that causes COVID-19 illness. LL37 has demonstrated anti-viral activity against diverse viruses include Respiratory Syncytial Virus (RSV) (24, 26) Influenza A (27), hepatitis C (HCV) (28), Dengue virus (DENV) (29), HIV-1 (30) Vaccinia Virus (31), and others.
In vitro studies of cyclic mechanical stretch of human bronchial epithelial cells, show a down regulation of hCAP18 and the induction of a proinflammatory response (32). Reduction of hCAP18 means reduction of LL37. This report could have implications in terms of the decision to mechanically ventilate patients with disease symptoms similar to those found in COVID-19. Additional related studies are warranted.
Fine Particles in Air Pollution May Interfere With Vitamin D Protection
Correlation has also been observed between exposure to higher levels of air pollution and increased levels of COVID-19 illness and deaths (8, 13).
While exposure to air pollution certainly reduces lung function in multiple ways, one possibility is the impact of carbon and other types of nanoparticles (NP) found in air pollution to inactivate LL-37. NP have been shown to interfere with Vitamin D-associated innate immune protection by at least three known mechanisms, interference with antiviral activities and signaling and changes in lung tissue remodeling. See Figure 1.
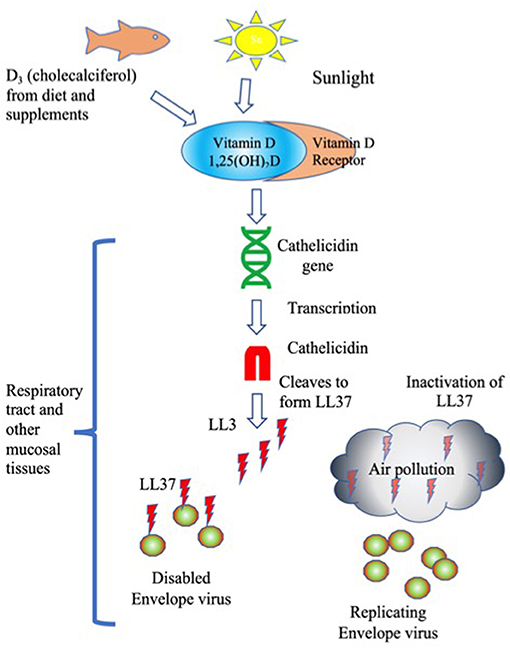
Figure 1. LL37 Inactivation of envelope viruses is stimulated by Vitamin D and blocked by air pollution. Humans obtain Vitamin D from sunlight, and from supplements and food. The active form of Vitamin D, 1,25(OH)2D binds to the Vitamin D receptor, which stimulates transcription of Cathelicidin. Cathelicidin is cleaved to generate the cationic antimicrobial peptide LL37. LL37 binds to and disables envelope viruses. Air pollution inactivates LL37 by removing the charge, leaving viruses to replicate unimpeded.
Carbon NP are reported to interfere with the anti-viral actions of LL37 (33). Studies simulating cell culture exposure to industrial and transportation-associated air pollution showed that when LL37 binds to carbon NP, it is structurally altered leading to reduction of antibacterial and antiviral activities (33). Additionally, LL37 normally modulates the immune response to lipopolysaccharide (LPS) that is part of the surface of gram-negative bacteria. LL37 neutralization of the effects of LPS, as measured by decreases in TNF-alpha concentrations, is impacted by carbon NP.
The effects of fine particles in air pollution have more far reaching effects. Recent research demonstrates that LL37 can be altered by enzymatic activity of peptidyl arginine deiminases (PAD) (5). The process, called citrullination, involves changing the positively charged arginine in LL37 to citrulline and thus changing its charge from positive to neutral. This effectively removes the mechanism by which LL37 is able to destroy viruses and bacteria (5, 33). Additionally, neutralization of charge by citrullination is responsible for disabling its ability to dampen inflammatory responses to viral infections.
Air pollution from transportation and industry are high in many of the most significant COVID-19 hot spots globally (8, 13). Fine particles in air pollution that have been linked to citrullination of proteins include a variety of materials used in industry such as nickel nanoparticles (4) and carbon nanotubules (34). Exposure to primary and second hand tobacco smoke is also associated with protein citrullination (35). In addition to industrial and transportation associated air pollution, carbon nanoparticles are also generated by wood or other domestic types of fires. This may be of particular importance in areas where fires are used for cooking or heating homes.
Vitamin D in Tissue Remodeling
Another mechanism of vitamin D protection against lung disease involves its role in balanced breakdown and repair of lung and other mucosal tissues. Primary mediators of breakdown of extracellular matrix are the matrix metalloproteinase (MMP) family of proteases, some of whose members are secreted from cells and support tissue repair and remodeling. The actions of MMPs are balanced by a family of inhibitors, tissue inhibitors of metalloproteinases (TIMP). Vitamin D has demonstrated regulatory effects on MMPs and their TIMP inhibitors (36). Possibly relevant to fine particles in air pollution, in studies of VDD mice exposed to second hand tobacco smoke, the balance between breakdown and repair is lost. This does not occur with tobacco smoke exposure or VDD alone. Under conditions of both smoke and VDD, the process is dominated by increased MMP-9 relative to its specific inhibitor, TIMP1, contributing to the breakdown of lung tissues (37).
Bioavailability of Vitamin D
Studies involving vitamin D intervention or passive monitoring of VDD associate diseases, reportedly a positive correlation exists between either circulating levels of 25-hydroxyvitamin D (38) or a dose dependent effect of Vitamin D administration and beneficial outcomes. However, vitamin D metabolism has a variety of complex steps that modulate generation of the active 1,25-dihydroxyvitamin D (1,25(OH)2D) (6, 39). Complicating bioavailability, and potentially relevant to the COVID-19 pandemic, is that there are differences in Vitamin-D-binding protein in humans that are specific to populations of European vs. African ancestry (39). This is a complex topic that warrants additional attention, to understand its implications, especially with the racial differential in morbidity and mortality from COVID-19 illness.
Another issue that impacts bioavailability is dosing, specifically the benefits of daily intake of Vitamin D vs. bolus dosing. While bolus dosing studies show rapid correction of VDD, the increase in 25(OH) Vitamin D was of short duration (40–42). In contrast, daily dosing has been shown to produce sustained serum levels of 25(OH)Vitamin D (43). Measurement of Vitamin D is readily available and supplementation is inexpensive and safe if done properly. As to the role of daily vs. bolus dosing strategies, how, one, the other or a combination of approaches would impact VDD in COVID19 is unclear.
Discussion
COVID-19 is an immediate society-altering public health crisis and understanding why severity varies from life threatening to asymptomatic is crucial to resolve this pandemic. We have postulated that vitamin D plays a pivotal role in modulating severity of COVID-19 illness; that LL37 plays a role in the clearance of the SARS-CoV-2 virus and in modulating the immune system responses; and that fine particles in air pollution may interfere with protections afforded by vitamin D and LL37. Minimally discussed to date, is strong established evidence of the importance of Vitamin D sufficiency in reducing the impact of viral lung disease processes that have implications for mitigating COVID-19. Given the relative benefits of protection afforded by attaining and maintaining Vitamin D sufficiency, it raises the potential beneficial impact of immediate attention by public health and medical providers to perform in depth studies of the relationship of Vitamin D to COVID-19 illness. Additionally, protocols for prevention, treatment and reduction of symptoms warrant immediate attention. Further, attention must be focused on the risks and long-term mitigation of exposure to fine particles from industrial and transportation associated air pollution as well as from primary and second hand tobacco smoke and from other domestic sources.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
MC-G: conception of hypothesis, manuscript, and project management. SF: manuscript and editing. PP: manuscript and editing. KC: medical expertise, manuscript, editing.
Funding
This work was supported by the Flight Attendant Medical Research Institute (FAMRI), grants 130051 and 130052.
Conflict of Interest
MC-G and PP were employed by the company Bodymind Science, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. David Lieb, for consultation on relevant virology. We thank Norris Cotton Cancer Center and the Flight Attendant Medial Research Institute for their support.
References
1. Steinstraesser L, Tippler B, Mertens J, Lamme E, Homann HH, Lehnhardt M, et al. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. (2005) 2:2. doi: 10.1186/1742-4690-2-2
2. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. (2020). doi: 10.1007/s40520-020-01570-8. [Epub ahead of print].
3. Alipio M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with Coronavirus-2019 (COVID-2019). SSRN Electronic J. (2020) doi: 10.2139/ssrn.3571484
4. Mohamed BM, Boyle NT, Schinwald A, Murer B, Ward R, Mahfoud OK, et al. Induction of protein citrullination and auto-antibodies production in murine exposed to nickel nanomaterials. Sci Rep. (2018) 8:679. doi: 10.1038/s41598-017-19068-1
5. Casanova V, Sousa FH, Shakamuri P, Svoboda P, Buch C, D'Acremont M, et al. Citrullination alters the antiviral and immunomodulatory activities of the human cathelicidin LL-37 during rhinovirus infection. Front Immunol. (2020) 11:85. doi: 10.3389/fimmu.2020.00085
6. Hewison M. Vitamin D and immune function: an overview. Proc Nutrition Soc. (2012) 71:50–61. doi: 10.1017/S0029665111001650
7. de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. (2014) 18:660. doi: 10.1186/s13054-014-0660-4
8. Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. medRxiv. (2020) doi: 10.1101/2020.04.05.20054502
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
11. Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. (2000) 85:4125–30. doi: 10.1210/jcem.85.11.6962
13. Gupta S. Why African-Americans may be especially vulnerable to COVID-19. Science News. 2020. Available online at: https://www.sciencenews.org/article/coronavirus-why-african-americans-vulnerable-covid-19-health-race
14. Pettus EW. Mississippi shows wide racial gap in impact of coronavirus. AP news. April 8, 2020. Available online at: https://apnews.com/50932ad39cca0513c450219e4a4587a7
15. Reyes C, Husain N, Gutowski C, St Clair S, Pratt G. Chicago's coronavirus disparity: black chicagoans are dying at nearly six times the rate of white residents, data show. Chicago Tribune. (2020). Available online at: https://www.chicagotribune.com/coronavirus/ct-coronavirus-chicago-coronavirus-deaths-demographics-lightfoot-20200406-77nlylhiavgjzb2wa4ckivh7mu-story.html
16. Stafford K, Hoyer M, Morrison A. Outcry over racial data grows as virus slams black Americans. AP News. (2020). Available online at: https://apnews.com/71d952faad4a2a5d14441534f7230c7c
17. Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. (2008) 57:183–91. doi: 10.1016/j.metabol.2007.08.023
18. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. (2016). 104:454–61. doi: 10.3945/ajcn.115.127985
19. Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. (2000) 72:1529–34. doi: 10.1093/ajcn/72.6.1529
20. Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Current Eye Res. (2005) 30:385–94. doi: 10.1080/02713680590934111
21. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. (2007) 179:2060–3. doi: 10.4049/jimmunol.179.4.2060
22. Ahmed A, Siman-Tov G, Keck F, Kortchak S, Bakovic A, Risner K, et al. Human cathelicidin peptide LL-37 as a therapeutic antiviral targeting Venezuelan equine encephalitis virus infections. Antiviral Res. (2019) 164:61–9. doi: 10.1016/j.antiviral.2019.02.002
23. Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. (2011) 6:25333. doi: 10.1371/journal.pone.0025333
24. Currie SM, Findlay EG, McHugh BJ, Mackellar A, Man T, Macmillan D, et al. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS ONE. (2013) 8:73659. doi: 10.1371/journal.pone.0073659
25. Jadhav NJ, Gokhale S, Seervi M, Patil PS, Alagarasu K. Immunomodulatory effect of 1, 25 dihydroxy vitamin D3 on the expression of RNA sensing pattern recognition receptor genes and cytokine response in dengue virus infected U937-DC-SIGN cells and THP-1 macrophages. Int Immunopharmacol. (2018) 62:237–43. doi: 10.1016/j.intimp.2018.07.019
26. Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM. Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Research Notes. (2016) 9:11. doi: 10.1186/s13104-015-1836-y
27. Tripathi S, Wang G, White M, Qi L, Taubenberger J, Hartshorn KL. Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic influenza A viruses. PLoS ONE. (2015) 10:124709. doi: 10.1371/journal.pone.0124706
28. Matsumura T, Sugiyama N, Murayama A, Yamada N, Shiina M, Asabe S, et al. Antimicrobial peptide LL-37 attenuates infection of hepatitis C virus. Hepatol. Res. (2016) 46:924–32. doi: 10.1111/hepr.12627
29. Jadhav NJ, Patil PS, Alagarasu K. Effect of full-length and truncated variants of LL-37 on dengue virus infection and immunomodulatory effects of LL-37 in dengue virus infected U937-DC-SIGN cells. Int J Peptide Res Therapeutics. (2020) 26:547–55. doi: 10.1007/s10989-019-09861-z
30. Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. (2007) 5:410–5. doi: 10.2174/157016207781023947
31. Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. (2004) 172:1763–7. doi: 10.4049/jimmunol.172.3.1763
32. Karadottir H, Kulkarni NN, Gudjonsson T, Karason S, Gudmundsson GH. Cyclic mechanical stretch down-regulates cathelicidin antimicrobial peptide expression and activates a pro-inflammatory response in human bronchial epithelial cells. PeerJ. (2015) 3:e1483. doi: 10.7717/peerj.1483
33. Findlay F, Pohl J, Svoboda P, Shakamuri P, McLean K, Inglis NF, et al. Carbon nanoparticles inhibit the antimicrobial activities of the human cathelicidin LL-37 through structural alteration. J Immunol. (2017) 199:2483–90. doi: 10.4049/jimmunol.1700706
34. Mohamed BM, Verma NK, Davies AM, McGowan A, Crosbie-Staunton K, Prina-Mello A, et al. Citrullination of proteins: a common post-translational modification pathway induced by different nanoparticles in vitro and in vivo. Nanomedicine. (2012) 7:1181–95. doi: 10.2217/nnm.11.177
35. Alsalahy MM, Nasser HS, Hashem MM, Elsayed SM. Effect of tobacco smoking on tissue protein citrullination and disease progression in patients with rheumatoid arthritis. Saudi Pharmaceutical J. (2010) 18:75–80. doi: 10.1016/j.jsps.2010.02.002
36. Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. (2009) 133:126–31. doi: 10.1016/j.clim.2009.06.009
37. Crane-Godreau MA, Black CC, Giustini AJ, Dechen T, Ryu J, Jukosky JA, et al. Modeling the influence of vitamin D deficiency on cigarette smoke-induced emphysema. Front Physiol. (2013) 4:132. doi: 10.3389/fphys.2013.00132
38. Han JE, Alvarez JA, Jones JL, Tangpricha V, Brown MA, Hao L, et al. Impact of high-dose vitamin D3 on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition. (2017) 38:102–8. doi: 10.1016/j.nut.2017.02.002
39. Holick MF. Bioavailability of vitamin D and its metabolites in black and white adults. N Engl J Med. (2013) 369:2047–8. doi: 10.1056/NEJMe1312291
40. Kearns M, Binongo J, Watson D, Alvarez J, Lodin D, Ziegler T, et al. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutrition. (2015) 69:193–7. doi: 10.1038/ejcn.2014.209
41. Ketha H, Thacher TD, Oberhelman SS, Fischer PR, Singh RJ, Kumar R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24, 25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio. Bone. (2018) 110:321–5. doi: 10.1016/j.bone.2018.02.024
42. Martineau AR, Nanzer AM, Satkunam KR, Packe GE, Rainbow SJ, Maunsell ZJ, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. (2009) 13:119–25.
Keywords: COVID-19, Vitamin D deficiency, cathelicidin/LL37, air pollution, citrullination of peptide, carbon nanoparticles, African American, tobacco smoke
Citation: Crane-Godreau MA, Clem KJ, Payne P and Fiering S (2020) Vitamin D Deficiency and Air Pollution Exacerbate COVID-19 Through Suppression of Antiviral Peptide LL37. Front. Public Health 8:232. doi: 10.3389/fpubh.2020.00232
Received: 23 April 2020; Accepted: 18 May 2020;
Published: 28 May 2020.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Patrick Geraghty, SUNY Downstate Medical Center, United StatesErnest J. Plata, Wiley College, United States
Copyright © 2020 Crane-Godreau, Clem, Payne and Fiering. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mardi A. Crane-Godreau, bWFyZGkuZGFydG1vdXRoLnJlbGF0ZWRAZ21haWwuY29t
 Mardi A. Crane-Godreau
Mardi A. Crane-Godreau Kathleen J. Clem
Kathleen J. Clem Peter Payne
Peter Payne Steven Fiering
Steven Fiering