- 1Ministry of Education Key Laboratory of Biomedical Engineering, College of Biomedical Engineering and Instrument Science, Hangzhou, China
- 2Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, NSW, Australia
- 3Department of Nutrition and Food Hygiene, School of Public Health, Chronic Disease Research Institute, Zhejiang University School of Medicine, Hangzhou, China
Background: Implementation intention formed by making a specific action plan has been proved effective in improving physical activity (PA) and dietary behavior (DB) for the general, healthy population, but there has been no meta-analysis of their effectiveness for patients with chronic conditions. This research aims to analyze several explanatory factors and overall effect of implementation intention on behavioral and health-related outcomes among community-dwelling patients.
Methods: We searched CIHNAL (EBSCO), PUBMED, Web of Science, Science Direct, SAGE Online, Springer Link, Taylor & Francis, Scopus, Wiley Online Library, CNKI, and five other databases for eligible studies. Random-effects meta-analysis was conducted to estimate effect sizes of implementation intention on outcomes, including PA, DB, weight, and body mass index. And the eligible studies were assessed by the Cochrane Collaboration's tool for risk of bias assessment. Sensitivity analysis adopted sequential algorithm and the p-curve analysis method.
Results: A total of 54 studies were identified. Significant small effect sizes of the intervention were found for PA [standard mean difference (SMD) 0.24, 95% confidence interval (CI) (0.10, 0.39)] and for the DB outcome [SMD −0.25, 95% CI (−0.34, −0.15)]. In moderation analysis, the intervention was more effective in improving PA for men (p < 0.001), older adults (p = 0.006), and obese/overweight patients with complications (p = 0.048) and when the intervention was delivered by a healthcare provider (p = 0.01).
Conclusion: Implementation intentions are effective in improving PA and DB for community dwelling patients with chronic conditions. The review provides evidence to support the future application of implementation intention intervention. Besides, the findings from this review offer different directions to enhance the effectiveness of this brief and potential intervention in improving patients' PA and DB.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=160491.
Introduction
Non-communicable chronic diseases (NCDs) are the leading cause of death, killing 40 million (70%) people globally in 2016. They are the major public health challenge and have caused high economic burden around the world, with an estimated accumulative loss of 30 trillion between 2011 and 2013 according to the World Economic Forum (1). Obesity/overweight [body mass index over 25 kg/m2 is considered overweight, and over 30 kg/m2 is considered obese (2)] is a well-recognized risk factor that directly impacts mortality and quality of life for people with the above chronic conditions (3).
Two primary risk factors in obesity/overweight are physical inactivity and unhealthy diet, which can cause a range of complications, i.e., heart attack and diabetes (4, 5). The World Health Organization (WHO) defines regular physical activity as moderate intensity physical activity at least 150 min per week for adults (2). Healthy diet involves less sodium intake (2), less fat intake (6, 7), and more intake of fruits and vegetables (8, 9). Regular physical activity and healthy diet can improve blood lipid markers (10, 11), lessen blood pressure (11), and improve psychological wellbeing (12). Thus, they are important facilitators for health and wellbeing of patients with chronic conditions. Improving physical activity (PA) and dietary behavior (DB) is commonly key objectives of behavioral interventions in chronic disease management (13, 14).
Understanding the determinants of behavioral performance is the prerequisite for implementing theory-based behavioral interventions, which subtly influence these determinants and ultimately help people to achieve behavioral goals. “Intention” has long been used by behavioral scientists as a proxy predictor of behavior (15, 16). For example, the theory of planned behavior (TPB) links one's behavioral intention with behavior. It considers behavioral intention as an individual's willingness to act, thus is an immediate predictor of behavior (15). Here, the notion of intention refers to “goal intention” because it connects people's motivations to their behaviors. However, the “intention-action” gap recognizes that strong intentions do not always translate into the corresponding behaviors (17–19). Gollwitzer attributed this gap to ambiguity of goal intention, causing by distraction before initiating the goal behavior and individual forgetfulness. Since goal intention only refers to one's goal behavior and motivations (e.g., “I intend to do more exercise”), its content is often ambiguous and does not attach any situational elements (e.g., when, where, how) to behavior. Every time, individuals who intend to perform the goal behavior have to firstly think when, where or how to act. If the situational content could not be addressed immediately and appropriately, individuals are likely to fall into attention fatigue, and be distracted by another immediately foreseeable rewarding action and give up the original goal (20). This has led to the creation of the concept of implementation intention, which is designed to facilitate the translation of intention into action.
Implementation intention is an explicit form of planning that acts upon elaboration of goal intention via specifying the situational content that triggers the goal behavior (21). The mechanism is that, if individuals plan the goal behavior connected with specific situation, then, as long as the situation matches, a person could automatically recollect the planned schema and activate the corresponding behavior. The more concrete the plan is, the less effort is required to activate the needed behavior, which renders the individuals less likely to be distracted (20, 21). The implementation intention intervention is realized by requesting individuals to make concrete behavioral plans by specifying situational elements of “when,” “where,” “how,” e.g., “I plan to do the brisk walking at 3 p.m. at the park near my house 3 times per week,” or making “if-then” statements (22), e.g., “If it is rainy outside, then I will do the brisk walking on the treadmill in the gym nearby.”
Previous meta-analyses studies (19, 23–25), including a large one that analyzed 94 independent studies (25), found that implementation intention has either small to medium or medium to large effects on goal attainment related to healthy eating and exercising among general population. Their pieces of research revealed several factors in intervention design, which could make a difference on the planning effect on PA and DB improvement. For PA, the intervention was favored by combining with barrier management (24) and reinforcement (26), and was more effective in clinical and student samples. While, for DB, the intervention effect was stronger for men than women (19), and in condition when promoting healthy behaviors than diminishing unhealthy ones (23). Other stimulus included was when there was less controlled (23) and no monitoring (19). However, none of the past pieces of research studied the effect on the weight-related outcome and specifically targeted people with chronic conditions. Moreover, there are still underlying moderators to be studied in order to give full play to this intervention.
Due to an acknowledged capacity to fill the “intention-action” gap, and the high demand for effective chronic disease management, there is a high demand for the application of implementation intention intervention to chronic disease management. Compared to the general population, the frequent medical tests and doctor visits, as well as disease symptoms, cause people to have a higher perceived risk (27) and protective motivation (28, 29), and thus a higher intention of health behavior. To date, the published meta-analyses have all been conducted in the general population; further research is required to understand the relevant issues that may impact the effectiveness of implementation intention for patients with chronic disease. For example, what is the influence of the factors, e.g., gender, age, education level, and disease, on the effectiveness of this intervention? What is the best plan pattern, single plan focused on the single behavioral goal, e.g., PA or DB, or multiple plans focused on more than one goal, e.g., both PA and DB? Will the other bundled interventions, i.e., reminders, or different intervention delivery, impact the intervention outcome of patient groups? Will the intervention effect be varied from different follow-up periods? Answers to these questions are important for effective implementation of planning intervention. Therefore, we conducted a systematic review and meta-analysis with a smaller but more focused topic to generate evidence for patients with community-dwelling chronic disease about the effect and potential moderators of implementation intention on improving PA and DB.
Materials and Methods
The review was conducted according to Cochrane Handbook for Systematic Reviews of Interventions (30) and PRISMA guidelines (31). The checklist was available in Supplementary Table 1. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020160491) prior to undertaking the research. Prior to the registration, we had carried out a feasibility analysis in order to better organize and allocate the research assignment through screening search results against two original eligibility criteria—implementation intention intervention and patients with chronic condition. After consultation and discussion with medical statisticians, we completed the registration, and then the research entered the implementation stage.
Search Strategies
We searched CIHNAL (EBSCO), PsycInfo (EBSCO), Psychology and Behavioral Sciences Collection (EBSCO), psyARTICLES (EBSCO), MEDLINE (EBSCO), PUBMED, WEB OF SCIENCE, Wiley Online Library, ScienceDirect, SAGE Journals Online, Springer, Taylor & Francis, Scopus for English literature, CNKI, and WANFANG for Chinese literature published during January 1, 1990 to January 1, 2022. The search was focused on identifying RCT that applied implementation intention intervention in chronic disease management. Keywords related to Implementation Intention included “implementation intention,” “action planning,” and “action plan,” and keywords about chronic diseases were modified to suit the different search strategies for databases mentioned above. Details of the search strategies in all databases are presented in Supplementary Table 2. We also searched and identified articles from the reference lists of the included studies and published meta-analyses. Finally, a further search on Google Scholar for the first author of all included studies was conducted to capture any articles that might be missed by the above process.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) RCT design; (2) the participants were adult outpatients diagnosed with one or more chronic diseases, including cardiovascular disease, diabetes, chronic lung disease, obese/overweight, and dyslipidemia, etc.; (3) the intervention group received implementation intention interventions aimed at improving PA and/or DB, where the participants were asked to make action plans, detailing the situation and action to achieve the goal. Whereas, there was no restriction on the form (e.g., paper or electronic) or process (with or without the assistance of a healthcare provider) of plan making; (4) outcome measurement included the patients' health behavior or weight outcomes. Studies were excluded when (1) the patients with severe mental disorder, gestation or physical disability to control for factors that are not of research interest; (2) the plan made by the patients failed to meet the principle of implementation intention, or had no specific instruction; (3) no description about the plan form in the original version of the article.
Data Extraction and Quality Assessment
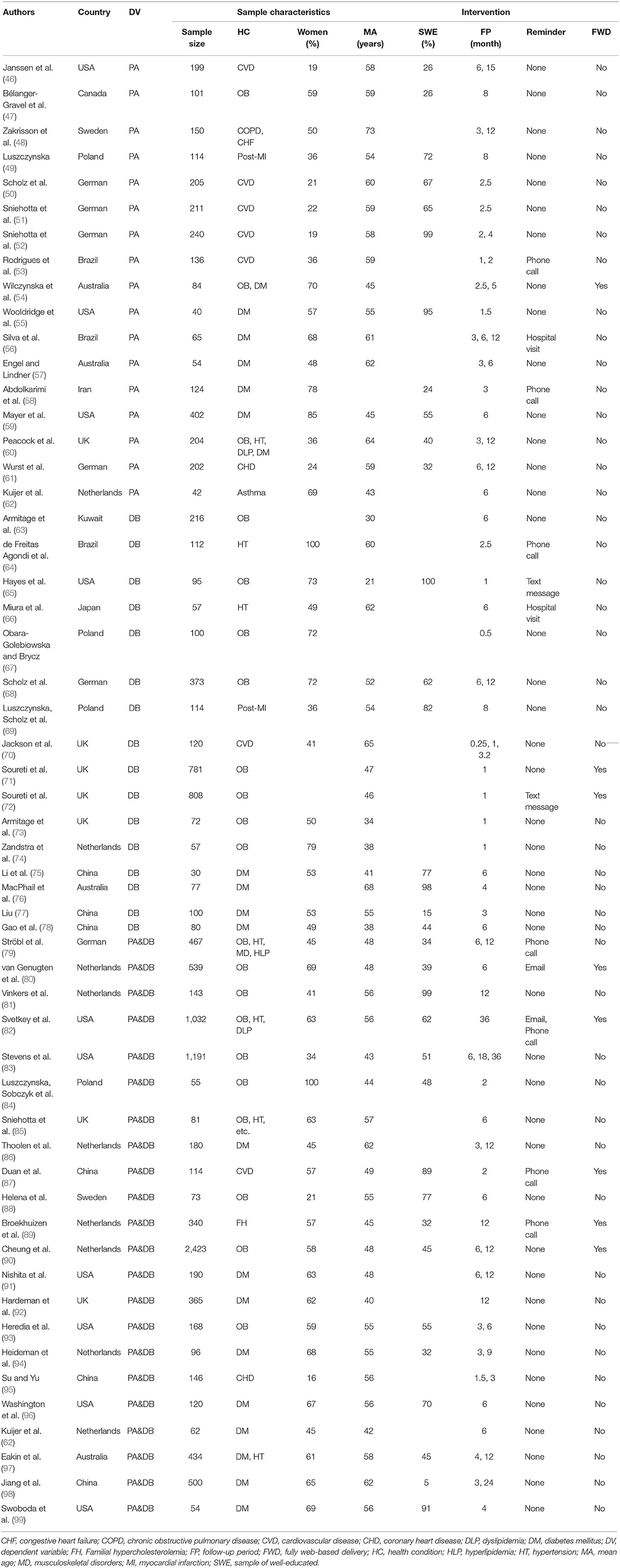
One reviewer (HL) completed the data extraction and quality assessment of the included studies, and a second reviewer (DW) verified the extracted data. Similarly, disagreements were resolved by consensus with involvement of a third reviewer (ND). Four information items were extracted whenever possible: (1) basic study information, including authors, published year, trial location, and a dependent variable; (2) sample information, i.e., sample size, gender, mean age, education level, and health condition; (3) information about implementation intention intervention, including planned intervention duration, intervention delivery (either delivered by a healthcare provider or fully web based), and a reminder. The latter two were coded as dichotomous data yes/no; and (4) outcome information, including health behavior outcomes (PA and DB), and physiological outcomes (body mass index and weight). The education level was described as the proportion of a well-educated sample (the eighth column), which is assessed in three ways: (1) the proportion of a sample with a high education proportion or education year ≥ 9, (2) education background was General Educational Development (GED) or beyond, (3) not specified but assessed as “high” by the author. The follow-up period was divided by 4 and 28, respectively, when converting the time unit from “day” and “week” into “month.” For studies with multiple measurements of PA or DB, the primary one was extracted and involved into subsequent calculation. If not specified, then the first one being reported in the result part of the original paper was chosen.
Two reviewers (HL and DW) independently assessed the risk of bias in individual studies, applying the Cochrane risk-of-bias tool (30), including: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each item was rated in three levels: “high risk,” “unclear risk,” or “low risk” in accordance with the instructions in the Cochrane handbook. For each of the six risk items, proportions of studies with low, high, and unclear risk levels were calculated. Only studies with more than three (> 3) low risk items and less than two (<2) high risk items were rated as high-quality studies. Stratified pooled effect sizes were calculated for the high-quality studies. Differences were resolved by consensus among the three reviewers.
Study Selection
Two reviewers (HL and DW) simultaneously and independently completed the review of titles, abstracts, and full texts after removing duplicates. Handing searching of a reference list and Google Scholar was conducted after first completion of full text identification by the two reviewers independently. Disagreements were resolved through discussion and consensus, together with the third reviewer (ND).
Meta-Analyses
All meta-analyses were conducted using Stata 12 (Stata Statistical Software, College Station, Texas, United States) (32). Outcome estimates calculation was using random-effect models, respectively, for PA, DB, weight, and body mass index (BMI). According to the guidance from Cochrane Handbook for Systematic Review, it is necessary to standardize the results before comparison of health behavior outcomes (PA and DB) that were measured in a variety of ways in the previous studies. So, we used the standard mean difference (SMD) to represent effect size of health behavior outcomes (i.e., PA and DB) for the included studies, and mean difference (MD) for effect size of an outcome for weight and BMI. By convention, the cutting value of 0.2, 0.5, and 0.8 of SMD suggests “small,” “medium,” or “large” effect size, respectively (33). For studies with repeated measures for each outcome, only the measure with the follow-up period close to the average value was included in the calculation. The average follow-up period was calculated by dividing the sum of the follow-up period of all measurements by the number of measurements. For instance, a total of 17 PA outcome data were extracted from the included studies where the sum of their corresponding follow-up period was 115 months; then, the average value was 6.8 (115/17) months. If a trial measured a patient's PA outcome respectively at 6, 18, and 36 months, only the 6-month outcome would be included in analysis because it was the one most close to the average value of 6.8 months, and p < 0.05 was considered statistically significant for all models. And for three-arm RCT studies, if two intervention groups implemented the same planning interventions but were different in other ways, the control group sample was split into two to make up two comparisons. While if two intervention groups implemented different planning interventions, they were combined into one according to the Section 5, Chapter 16, in Cochrane handbook (30).
Heterogeneity among studies for each outcome were assessed by I square, with p < 0.01 considered significantly different. I square is measured in percentage, where values of 25, 50, and 75% represent low, moderate, and high heterogeneity (34). Next, a set of single meta regression analyses was performed to the variables that might impact the intervention effect when the number of cases was over 10. The regression analyses were to identify the potential sources of heterogeneity (30). The other purpose was to explore to what extent those variables correlated with the outcome. Egger test was conducted to assess potential bias due to small study effects if cases for each indicator were more than 10, (35) as well as visual inspection of symmetry of funnel plots (36–38).
Sensitivity analyses were undertaken using sequential algorithm and p-curve analysis. The former was done by performing a series of meta-analyses with one study removed each time to assess the reliability of the estimates (39). Besides, we were advised to conduct p-curve analysis, where p-curve refers to the distribution of significant p-values (p ≤ 0.05) obtained from statistical tests across a group of studies. For the past few years, p-curve analysis has been recommended to test for publication bias (40–43). The bias is closely correlated with the existence of p-hacking, which means that researchers may keep performing statistical analyses on datasets with overlapping observations until they obtain a significant p-value from a sequence of p-values, and then “selectively” report it (44). This will lead to the p-curve's shape of p-hacked study left skewed, where p-curve's shape of no-effect study is uniform, and of truly effect is right skewed (41, 42, 44). In brief, p-curve provides an intuitive way to estimate the potential risk for publication bias and average power of evidential value of a set of studies. And Simonsohn's team have developed an online app 4.06 and provided a user guide for conveniently conducting the p-curve analysis (www.p-curve.com). However, later researchers found that this method is only robust to the condition where heterogeneity is low, thus is not recommended for the estimates with high heterogeneity (40, 43, 45). So, we made use of the online tool and the guide to perform the analysis merely on the estimates, which was low in heterogeneity [I2 <50% accordingly (40)].
Results
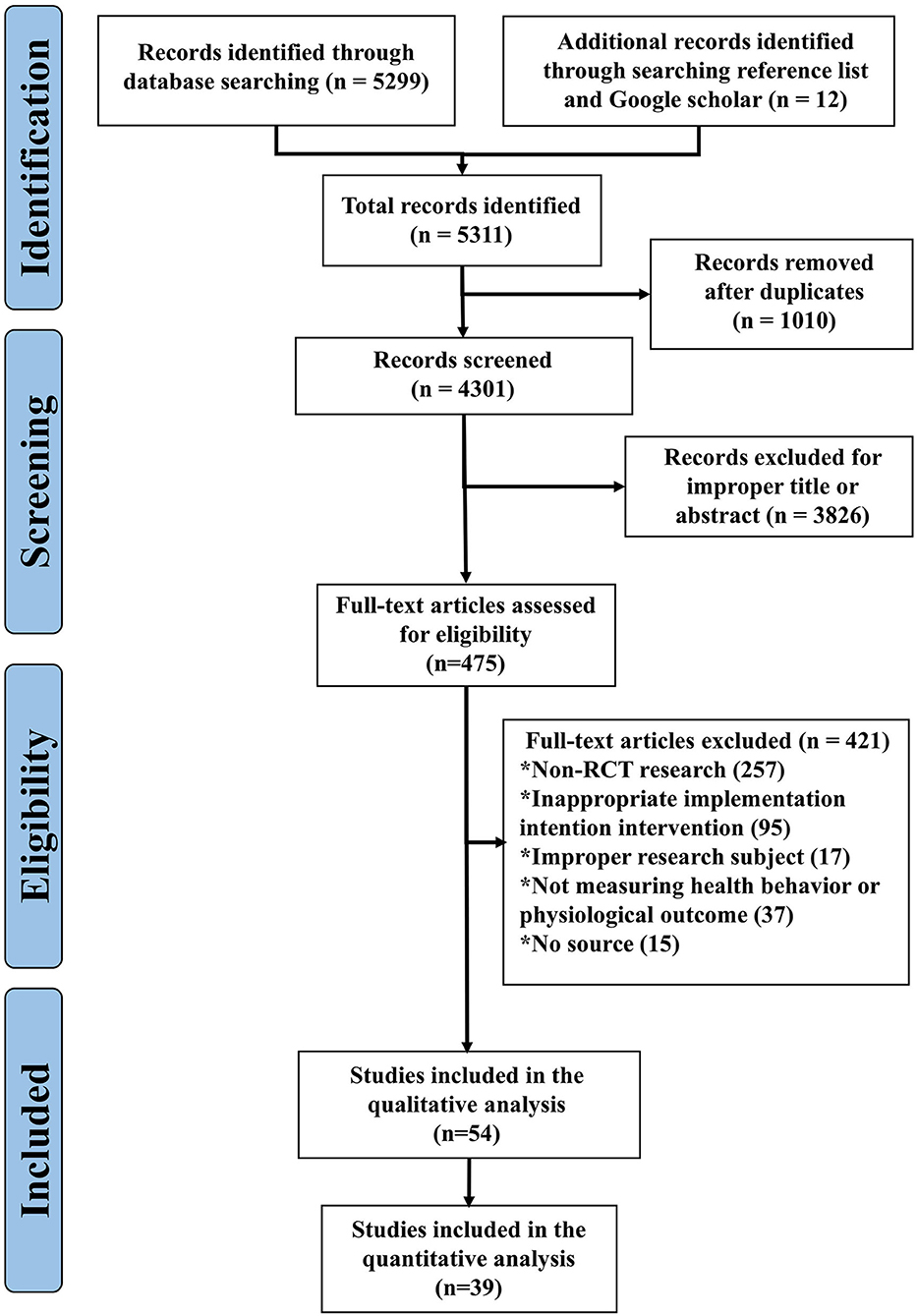
A total of 5,299 records published from January 1, 1990 to January 1, 2022 were identified. After removing duplicates, 475 were eligible for full-text review. Additionally, 12 studies were found through further searching the reference lists of the identified articles during data extraction (Figure 1). The full-text screening identified 54 studies that met the inclusion criteria, of which 39 were available for quantitative analysis (Figure 1).
Study Characteristics
Characteristics of the included studies are summarized in three aspects: basic information, sample characteristics, and interventions (Table 1). Basic information included the first author, country, published year, and dependent variable of the intervention. The majority of studies were conducted in developed countries (73%) and European countries (51%). The country included USA (18%), Netherlands (15%), UK (13%), German (11%), China (11%), Poland (7%), Australia (7%), Brazil (5%), Sweden (5%), Canada (2%), Japan (2%), Kuwait (2%), and Iraq (2%).
Sample characteristics were represented by sample size, health condition, gender, mean age, and the educational level. An obese/overweight sample accounted for 30%, obesity with complications for 39%, cardiovascular disease/coronary heart disease/congestive heart failure /hypertension for 33%, diabetes mellitus for 37%, dyslipidemia for the same 9%, chronic lung disease for 4%, and musculoskeletal disorders for 3%. In 29 studies, the female participants outnumbered the male participants. The majority of the subjects in the included studies were middle-aged or older (mean: 52 years; median: 55 years). Of 34 studies which provided education information, 56% had over more than a half well-educated sample.
Information about implementation intention intervention included the follow-up period, reminder form, and delivery form. The follow-up period ranged from 7 days to 36 months. Mean values of the follow-up period for the PA outcome (whose data were accessible) were 6.7 months per measurement. It was 4.7 months per measurement for DB, for BMI was 7.1 months per measurement, and for weight was 11.5 months per measurement. Only 14 studies arranged reminders after planning. Reminder forms included the hospital visit (4%), phone call (13%), text message (4%), and email (4%). Notably, the form of the “hospital visit” refers that the participants received on-site reinforcement of their action plans during the face-to-face follow-up (56, 66). Only 15% of the planning interventions were fully web based; the rest were all delivered by a healthcare provider. Thirty-three studies involved single-plan interventions (17 aimed at improving PA and 16 at improving DB), while the remaining 22 involved multi-plan interventions.
Quality Assessment
Studies reporting either the PA or DB outcome suffered from a majority of high and unclear risk in blinding of the participants and personnel (84 and 55%, respectively) and selective reporting (58 and 61%, respectively). Half of the studies reporting DB outcome were also at high and unclear risk in blinding of outcome assessment and allocation concealment. A total of nine studies with the PA outcome were assessed as high quality, and the pooled effect size was statistically significant with similarly high heterogeneity [SMD, 0.32; 95% CI (0.09, 0.55), p = 0.007, I2 = 74%]. Estimated effect size for 10 high-quality studies with the DB outcome was also significant but smaller [SMD −0.18, 95% CI (−0.28, −0.07), p < 0.001, I2 = 42%]. Pooled effect size for high-quality studies was not statistically significant for either weight or the BMI outcome (both ps > 0.05). Quality assessment for the health behavior outcome and risk of bias assessments within individual study is presented in Supplementary Figure 1 and Supplementary Table 3.
Meta-Analyses
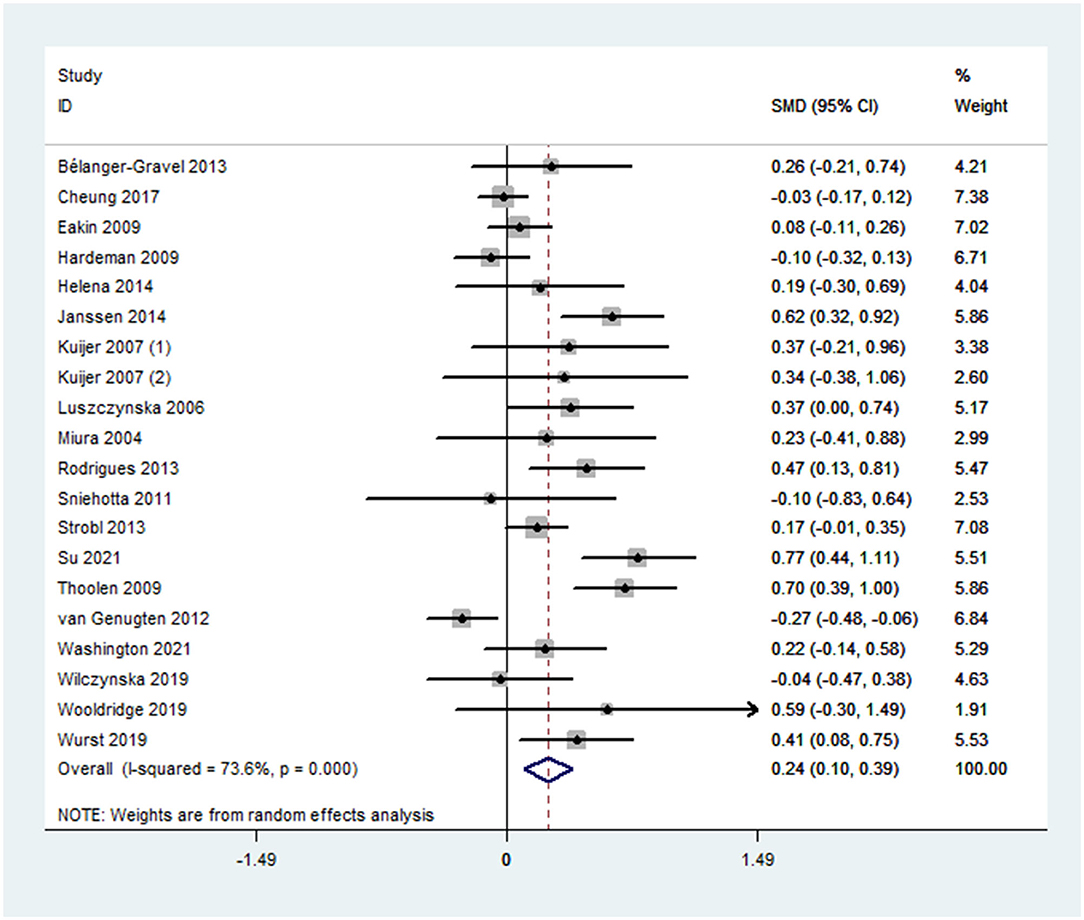
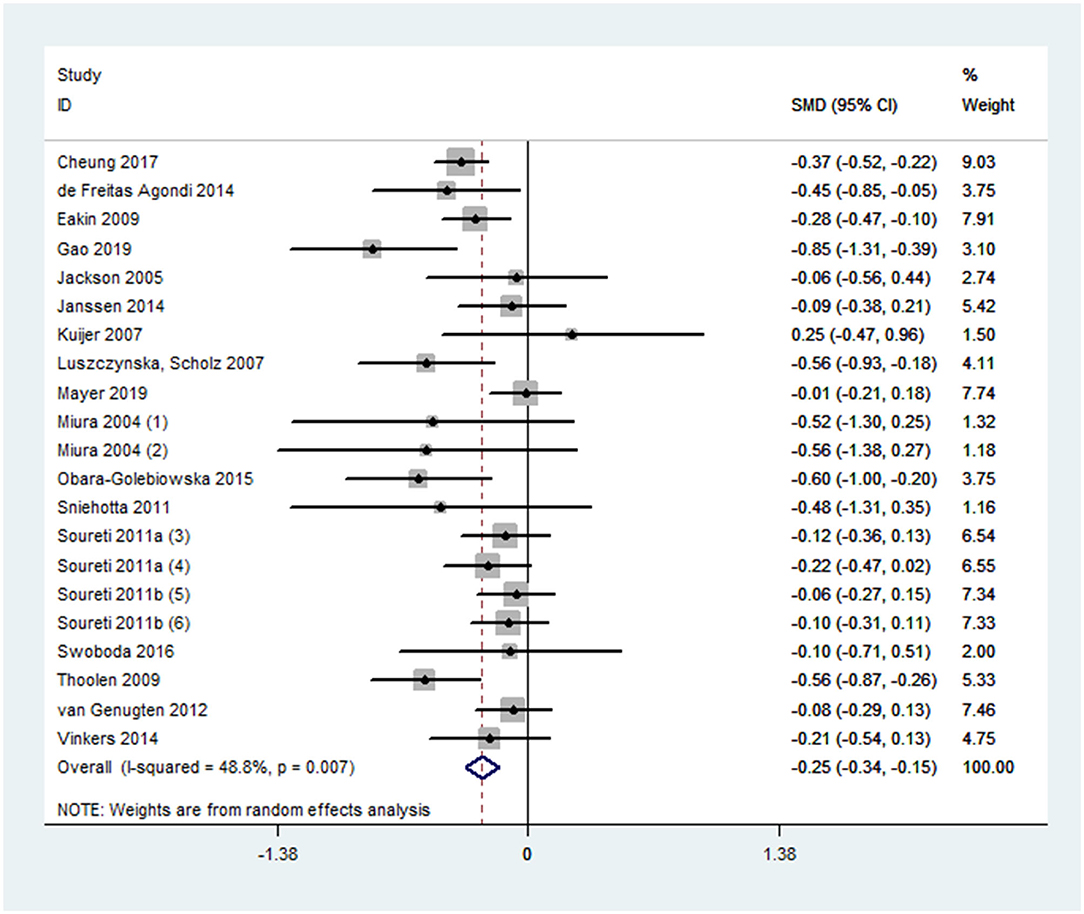
Overall effect size for PA outcomes calculated from 20 cases was significant yet small [SMD 0.24, 95% CI (0.10, 0.39), p < 0.001] (Figure 2). The severity of heterogeneity (p < 0.001, I2 = 74%) suggested the potential presence of moderators. Overall effect for the DB outcome was significant yet small [SMD, −0.25, 95% CI (−0.31, −0.15], p < 0.001]. Random-effect meta-analysis of 21 data sets from 18 studies resulted with a low level of heterogeneity (p = 0.007, I2 = 49%) (Figure 3). Neither estimates for effect size on BMI (p = 0.28) nor weight (p = 0.24) were significant. Data information for meta-analyses is available in Supplementary Table 4.
Moderation Analyses
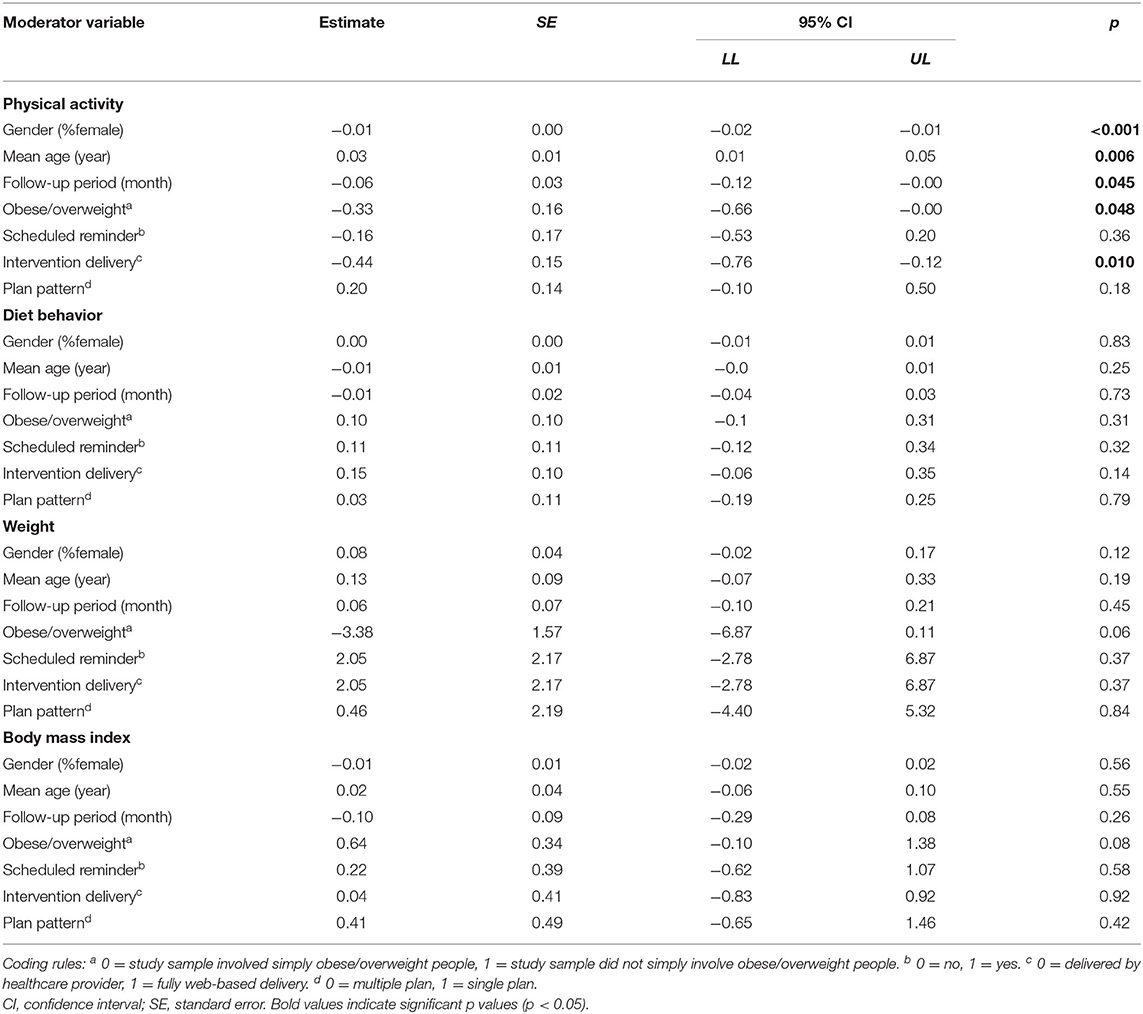
For the PA outcome, separated meta-regression analysis indicated that the effect of implementation intention was significantly influenced by age, gender, health condition, and intervention delivery (Table 2). Older age [β = 0.03, 95% CI (0.01, 0.05), p = 0.006, adjusted R2 = 50.48%] was significantly associated with larger effect size. While women [β = −0.01, 95% CI (−0.02, −0.01), p = 0.004, Adjusted R2 = 83.40%], simple obese or overweight condition [β = −0.33, 95% CI (−0.66, −0.00), p = 0.048, Adjusted R2 = 26.71%] and fully web-based delivery [β = −0.44, 95% CI (0.76, −0.12), p = 0.010, Adjusted R2 = 40.13%] had significantly negative correlation with the effect size. No significant moderator was identified either for DB, BMI or weight.
Sensitivity Analyses
Sequential algorithm analyses showed overall modest variations in effect size for PA (between 0.21 and 0.27) and DB (between −0.27 and −0.23), suggesting that the estimates were relatively stable (Supplementary Table 5). The funnel plots were symmetrical for both PA and DB outcomes (Supplementary Figure 2). Results of egger's test were not statistically significant (PA: p = 0.12, DB: p = 0.26). Since the estimate for PA was accompanied with severe heterogeneity, we had not conducted the p-curve analysis on it. For the DB outcome, descriptively, 88% of all p-values were lower than 0.01, and 12% lied in (0.01, 0.02), and the same proportion lied in (0.02, 0.03). None was larger than 0.03. The p-curve was significantly right skewed, indicating evidential value (Supplementary Figure 3). Furthermore, both of the binomial test (p = 0.004) and continuous test (full p-curve and half p-curve: ps < 0.001) for evidential value [98%, 90% CI (94%, 99%)] were significant. The above findings suggested no significant sign of publication bias.
Discussion
Summary of Main Results
To our knowledge, this is the first systematic review and meta-analysis of effects of implementation intention intervention on both PA and DB for community dwelling people with NCD. This review identified 54 studies that applied implementation intention to chronic disease management for community-dwelling outpatients over the world. We studied multiple moderators for the effects of implementation intention intervention for specific groups as recommended (22). Pooled effect sizes for PA and DB were 0.24 [95% CI (0.10, 0.39)] and −0.25 [95% CI (−0.31, −0.15)], respectively, demonstrating significant, small effect of making a specific plan on PA and DB improvement for community-dwelling patients. No significant effect was detected for BMI or weight. Men, older people, and people without obesity/overweight achieved a better PA outcome. Intervention delivered by the healthcare provider has better planning effect than those of fully web based. Delivery by people can enhance the planning effect to improve a DB outcome, whereas a reminder seems to produce negative effect on planning.
Comparison With Previous Findings
Our result of significant, yet small effect of implementation intention on PA is supported by a previous meta-analysis on both chronic disease and healthy population (24), while in disagreement with another meta-analysis that found no significant results for PA under implementation intention intervention (26). Unlike the moderate effect found among general population (19, 23), there is only small effect on DB improvement among patients with chronic disease. The estimate for a PA outcome was accompanied with high heterogeneity (I2 = 76% in this paper), with the previous meta-analyses identifying the existence of a reminder (26), and different planning forms (24), etc., as possible sources of heterogeneity. There can be many factors causing the difference between the population with chronic conditions and normal, healthy population, e.g., gender, age, different initial health conditions and lifestyles. We will further discuss the influences of several factors on intervention effect.
Interpretation of Findings
It is found that men perform better than women in PA improvement. Different from the finding by Vilà et al. that planning for fat intake reduction is more powerful for men than for women (19), we did not find any gender difference in the DB outcome. Perhaps, it is because our analysis included a broadened scope of DB indicators, which include fat intake (46, 72, 86), fat score, food frequency (66, 85), etc., whereas Vilà et al. only investigated the effect of implementation intention of fat intake behavior (19).
For age, some studies found that weight loss intervention is particularly difficult in young adult population (65, 100). And a high drop-out rate was reported in the younger patient sample in several included studies (74, 79, 80). On the other hand, the reduced effect of implementation intention on PA improvement in the obese/overweight population than the opposite may be explained by a lack of self-awareness of own obese conditions and the associated harm to health (85), the behavioral goal that is not attainable, i.e., desired rapid and remarkable weight loss (81), and a lack of knowledge about strategies to lose weight (65).
As expected, healthcare provider-guided delivery has achieved a better outcome than fully web-based delivery in PA improvement. This finding differed from a previous meta-analysis study that found fully web-based intervention achieved better behavior improvement, such as increased exercise time, increased engagement (101), etc. It is reported that fully web-based intervention tended to have a high rate of “loss to follow-up” or low exposure (72, 81). For example, one study reported that only 15% of the patients finished all of the four intervention modules online (80). Another study found over a 50% missing rate of study population (90). Furthermore, unguided interventions could lead to a lack of goals and poor focused plans (102, 103). Thus, face-to-face support from a healthcare provider for patients has been inferred as a facilitator for effective planning intervention so far.
We did not find any significant effect of a reminder on the implementation intention for PA, opposite to what found in the general population (26). Similarly, there was no effect of a reminder on the DB outcome. Scheduling a plan reminder acts as a prompt for patients to adhere to their plans. There are a variety of forms [e.g., phone calls (64), text messages (71), emails (80)], frequencies, and contents of a reminder. Thus, the homogeneity of the reminder among included studies was far from satisfactory. Furthermore, neither the PA nor the DB outcome is significantly influenced by the plan pattern. Swoboda et al. conducted a study with a single-plan intervention, a multiple-plan intervention, and a blank control group. The descriptive data presented in their study appeared to imply that the single planned intervention model resulted in greater improvement. They did not, however, compare the differences between two plan patterns. Although both exercise and diet improvement are important, it is unclear whether changing multiple behaviors at once or changing different behaviors sequentially is better for patients with chronic disease. Future research should be conducted to compare the outcomes of various plan patterns.
Limitations
This study has several limitations. We considered only populations with multiple chronic conditions, which limited the generalizability of the findings but allowed us to narrow our focus, whereas, overweight, which has common health consequences to obesity, was also included in this review despite the fact that it is not an illness and does not match the inclusion criteria for patients with chronic disease. Besides, the exclusion of non-RCT studies could have resulted in data loss. However, during the protocol-drafting phase, we believed that including RCTs would provide more unbiased estimates if we could obtain an adequate number of articles. Additionally, because only English or Chinese language studies were included, it was possible for publications in other languages to be overlooked. Furthermore, none of the studies met all Cochrane risk bias quality criteria; quality of evidence was not optimal. Another limitation is that high heterogeneity for physical activity was identified, reflecting the integrity of chronic disease management and high inconsistency in outcome appraisal of implementation intention intervention. For regression analysis, we acknowledged that the regression of gender and age of a sample might have introduced aggregation bias since we did not collect individual patient data. In addition, the study used a novel method, p-curve analysis, as supplementary of sensitivity analysis. We noted that exclusion of non-significant study (p > 0.05) was recognized as an inherent limitation of p-curve analysis (39). However, “although excluding non-significant results makes p-curve noisier (that is, less efficient in estimating the real effect size), it does not make p-curve biased” (p. 675). So, in this paper, we only used it as a sensitivity analysis method rather than the method to estimate the overall effect size (37, 40).
Conclusion
To the best of our knowledge, this is the first meta-analysis on the effect of implementation intention intervention on community dwelling patients with chronic conditions. At a time of growing concern about chronic disease management, our findings support implementation intention as a promising behavior change intervention for both physical activity and dietary behavior improvement, especially for men, older people, and people with chronic disease but without obese/overweight condition in improving physical activity. Support from a healthcare provider was identified as a facilitator for the intervention effect. And no significant influence was found for the follow-up period, plan pattern, or reminder.
However, with the development of internet and communication technology, it is remained to explore finer and more humanized design to realize effective online planning intervention and plan reinforcement, e.g., a human-computer interaction technique, healthcare system involved both people with specific chronic conditions, and a healthcare provider. It is advisable to analyze the influence of different reminders with different forms in terms of frequency, form, and delivery. Furthermore, the quality and the consistency of study design also need to be improved.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Database searching was performed by HL and DW. HL, DW, and ND collectively conducted the data analysis and visualization. HL drafted the work, which had been critically revised for important content by PY. All authors contributed to selecting search terms and defining inclusion/exclusion criteria, contributed to the article, and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2020YFC2006405), the Key Research and Development Program of Guangxi Zhuang Autonomous of China (No. 2020AB33002), the Key Research and Development Program of Zhejiang, China (No. 2021C03111), and the Alibaba Cloud.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Ting Song is kindly acknowledged for valuable discussion and helpful suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.721223/full#supplementary-material
References
1. Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. (2013) 369:1336–43. doi: 10.1056/NEJMra1109345
2. WHO. Global Status Report on Noncommunicable Diseases 2014 Geneva: World Health Organization Press (2014).
3. Fitzpatrick SL, Stevens VJ. Adult obesity management in primary care, 2008–2013. Prev Med. (2017) 99:128–33. doi: 10.1016/j.ypmed.2017.02.020
4. Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. (2013) 369:954–64. doi: 10.1056/NEJMra1203528
5. TLGH. Getting to the heart of non-communicable diseases. Lancet Glob Health. (2018) 6:e933. doi: 10.1016/S2214-109X(18)30362-0
6. Allott EH, Arab L, Su LJ, Farnan L, Fontham ET, Mohler JL, et al. Saturated fat intake and prostate cancer aggressiveness: results from the population-based North Carolina-Louisiana prostate cancer project. Prostate Cancer Prostatic Dis. (2017) 20:48–54. doi: 10.1038/pcan.2016.39
7. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. (2019) 322:1178–87. doi: 10.1001/jama.2019.13771
8. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. doi: 10.1093/ije/dyw319
9. Volpe SL. Fruit and vegetable intake and prevention of chronic disease. ACSMs Health Fit J. (2019) 23:30–1. doi: 10.1249/FIT.0000000000000474
10. Alhassan A, Young J, Lean ME, Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. (2017) 266:87–94. doi: 10.1016/j.atherosclerosis.2017.09.028
11. Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A. The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. (2019) 58:2159–74. doi: 10.1007/s00394-018-1804-0
12. Huang X, Yang H, Wang HH, Qiu Y, Lai X, Zhou Z, et al. The association between physical activity, mental status, and social and family support with five major non-communicable chronic diseases among elderly people: a cross-sectional study of a rural population in Southern China. Int J Environ Res Public. (2015) 12:13209–23. doi: 10.3390/ijerph121013209
13. Hernandez TL, Brand-Miller JC. Nutrition therapy in gestational diabetes mellitus: time to move forward. Diabetes Care. (2018) 41:1343–5. doi: 10.2337/dci18-0014
14. Thompson PD, Eijsvogels TM. New physical activity guidelines: a call to activity for clinicians and patients. JAMA. (2018) 320:1983–4. doi: 10.1001/jama.2018.16070
15. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. (1991) 50:179–211. doi: 10.1016/0749-5978(91)90020-T
16. Sheppard BH, Hartwick J, Warshaw PR. The theory of reasoned action: a meta-analysis of past research with recommendations for modifications and future research. J Consum Res. (1988) 15:325–43. doi: 10.1086/209170
17. Rhodes RE, de Bruijn GJ. How big is the physical activity intention–behaviour gap? a meta-analysis using the action control framework. Br J Health Psychol. (2013) 18:296–309. doi: 10.1111/bjhp.12032
18. Sheeran P. Intention—behavior relations: a conceptual and empirical review. Eur Rev Soc Psychol. (2002) 12:1–36. doi: 10.1080/14792772143000003
19. Vilà I, Carrero I, Redondo R. Reducing fat intake using implementation intentions: a meta-analytic review. Br J Health Psychol. (2017) 22:281–94. doi: 10.1111/bjhp.12230
20. Gollwitzer PM. Goal achievement: the role of intentions. Eur Rev Soc Psychol. (1993) 4:141–85. doi: 10.1080/14792779343000059
21. Gollwitzer PM. The volitional benefits of planning. In: Bargh PMGJA, editor. The Psychology of Action: Linking Cognition and Motivation to Behavior. New York, NY: The Guilford Press (1996). p. 287–312.
22. Hagger MS, Luszczynska A, De Wit J, Benyamini Y, Burkert S, Chamberland P-E, et al. Implementation intention and planning interventions in health psychology: recommendations from the synergy expert group for research and practice. Psychol Health. (2016) 31:814–39. doi: 10.1080/08870446.2016.1146719
23. Adriaanse MA, Vinkers CD, De Ridder DT, Hox JJ, De Wit JB. Do implementation intentions help to eat a healthy diet? a systematic review and meta-analysis of the empirical evidence. Appetite. (2011) 56:183–93. doi: 10.1016/j.appet.2010.10.012
24. Bélanger-Gravel A, Godin G, Amireault S. A meta-analytic review of the effect of implementation intentions on physical activity. Health Psychol Rev. (2013) 7:23–54. doi: 10.1080/17437199.2011.560095
25. Gollwitzer PM, Sheeran P. Implementation intentions and goal achievement: a meta-analysis of effects and processes. Adv Exp Soc Psychol. (2006) 38:69–119. doi: 10.1016/S0065-2601(06)38002-1
26. Silva MAVd, Sao-Joao TM, Brizon VC, Franco DH, Mialhe FL. Impact of implementation intentions on physical activity practice in adults: a systematic review and meta-analysis of randomized clinical trials. PLoS ONE. (2018) 13:e0206294. doi: 10.1371/journal.pone.0206294
27. Ferrer R, Klein WM. Risk perceptions and health behavior. Curr Opin Psychol. (2015) 5:85–9. doi: 10.1016/j.copsyc.2015.03.012
28. Xiao H, Li S, Chen X, Yu B, Gao M, Yan H, et al. Protection motivation theory in predicting intention to engage in protective behaviors against schistosomiasis among middle school students in rural China. PLoS Negl Trop Dis. (2014) 8:e3246. doi: 10.1371/journal.pntd.0003246
29. Ansari-Moghaddam A, Seraji M, Sharafi Z, Mohammadi M, Okati-Aliabad H. The protection motivation theory for predict intention of Covid-19 vaccination in iran: a structural equation modeling approach. BMC Public Health. (2021) 21:1165. doi: 10.1186/s12889-021-11134-8
30. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: John Wiley & Sons (2019). Available online at: www.trainingcochraneorg/handbook (accessed March 18, 2021).
31. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
32. Egger M, Davey-Smith G, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context: John Wiley & Sons Hoboken, NJ: John Wiley & Sons, Inc (2008).
33. Lipsey MW, Wilson DB. Applied Social Research Methods Series. Ventura County, CA: Practical meta-analysis: SAGE publications, Inc (2001).
34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
35. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36. Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc Ser A Stat Soc. (1988) 151:419–45. doi: 10.2307/2982993
37. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/S0895-4356(01)00377-8
38. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. (2011) 343:d4002. doi: 10.1136/bmj.d4002
39. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
40. McShane BB, Böckenholt U, Hansen KT. Adjusting for publication bias in meta-analysis: an evaluation of selection methods and some cautionary notes. Perspect Psychol Sci. (2016) 11:730–49. doi: 10.1177/1745691616662243
41. Simonsohn U, Simmons JP, Nelson LD. Better P-curves: making P-curve analysis more robust to errors, fraud, and ambitious P-hacking, a reply to Ulrich and Miller (2015). J Exp Psychol Gen. (2015) 144:1146–52. doi: 10.1037/xge0000104
42. Simonsohn U, Nelson LD., Simmons JP. p-Curve and effect size: Correcting for publication bias using only significant results. Perspect Psychol Sci. (2014) 9:666–81. doi: 10.1177/1745691614553988
43. van Aert RC, Wicherts JM, van Assen MA. Conducting meta-analyses based on p values: Reservations and recommendations for applying p-uniform and p-curve. Perspect Psychol Sci. (2016) 11:713–29. doi: 10.1177/1745691616650874
44. Simonsohn U, Nelson LD, Simmons JP. P-curve: a key to the file-drawer. J Exp Psychol Gen. (2014) 143:534. doi: 10.1037/a0033242
45. Hill CR, Samendinger S, Rymal AM. P-curve analysis of the köhler motivation gain effect in exercise settings: a demonstration of a novel technique to estimate evidential value across multiple studies. Ann Behav Med. (2020) 55:543–56. doi: 10.1093/abm/kaaa080
46. Janssen V, De Gucht V, van Exel H, Maes S. A self-regulation lifestyle program for post-cardiac rehabilitation patients has long-term effects on exercise adherence. J Behav Med. (2014) 37:308–21. doi: 10.1007/s10865-012-9489-y
47. Bélanger-Gravel A, Godin G, Bilodeau A, Poirier P. The effect of implementation intentions on physical activity among obese older adults: a randomised control study. Psychol Health. (2013) 28:217–33. doi: 10.1080/08870446.2012.723711
48. Zakrisson AB, Arne M, Hasselgren M, Lisspers K, Ställberg B, Theander K, et al. complex intervention of self-management for patients with COPD or CHF in primary care improved performance and satisfaction with regard to own selected activities; a longitudinal follow-up. J Adv Nurs. (2019) 75:175–86. doi: 10.1111/jan.13899
49. Luszczynska A. An implementation intentions intervention, the use of a planning strategy, and physical activity after myocardial infarction. Soc Sci Med. (2006) 62:900–8. doi: 10.1016/j.socscimed.2005.06.043
50. Scholz U, Sniehotta FF, Burkert S, Schwarzer R. Increasing physical exercise levels: Age-specific benefits of planning. J Aging Health. (2007) 19:851–66. doi: 10.1177/0898264307305207
51. Sniehotta FF, Scholz U, Schwarzer R. Action plans and coping plans for physical exercise: a longitudinal intervention study in cardiac rehabilitation. Br J Health Psychol. (2006) 11:23–37. doi: 10.1348/135910705X43804
52. Sniehotta FF, Scholz U, Schwarzer R, Fuhrmann B, Kiwus U, Völler H. Long-term effects of two psychological interventions on physical exercise and self-regulation following coronary rehabilitation. Int J Behav Med. (2005) 12:244–55. doi: 10.1207/s15327558ijbm1204_5
53. Rodrigues RCM, João TMS, Gallani MCBJ, Cornélio ME, Alexandre NMC. The“ Moving Heart Program”: an intervention to improve physical activity among patients with coronary heart disease. Rev Lat Am Enfermagem. (2013) 21:180–9. doi: 10.1590/S0104-11692013000700023
54. Wilczynska M, Lubans DR, Paolini S, Plotnikoff RC. Mediating effects of the ‘eCoFit’ physical activity intervention for adults at risk of, or diagnosed with, type 2 diabetes. Int J Behav Med. (2019) 26:512–21. doi: 10.1007/s12529-019-09800-8
55. Wooldridge JS, Ranby KW, Roberts S, Huebschmann AG. A couples-based approach for increasing physical activity among adults with type 2 diabetes: a pilot feasibility randomized controlled trial. Diabetes Educ. (2019) 45:629–41. doi: 10.1177/0145721719881722
56. Silva M, São-João TM, Cornelio ME, Mialhe FL. Effect of implementation intention on walking in people with diabetes: an experimental approach. Rev Saude Publica. (2020) 54:103. doi: 10.11606/s1518-8787.2020054002024
57. Engel L, Lindner H. Impact of using a pedometer on time spent walking in older adults with type 2 diabetes. Diabetes Educ. (2006) 32:98–107. doi: 10.1177/0145721705284373
58. Abdolkarimi M, Morowatisharifabad M, Asadpour M. The Effect of implementation intention on improving physical activity level and cardiovascular fitness in patients with type 2 diabetes: a randomized control study. IJDO. (2021) 13:26–32. doi: 10.18502/ijdo.v13i1.5747
59. Mayer VL, Vangeepuram N, Fei K, Hanlen-Rosado EA, Arniella G, Negron R, et al. Outcomes of a weight loss intervention to prevent diabetes among low-income residents of East Harlem, New York. Health Educ Behav. (2019) 46:1073–82. doi: 10.1177/1090198119868232
60. Peacock OJ, Western MJ, Batterham AM, Chowdhury EA, Stathi A, et al. Effect of novel technology-enabled multidimensional physical activity feedback in primary care patients at risk of chronic disease – the MIPACT study: a randomised controlled trial. Int J Behav Nutr Phys Act. (2020) 17:99. doi: 10.1186/s12966-020-00998-5
61. Wurst R, Kinkel S, Lin J, Goehner W, Fuchs R. Promoting physical activity through a psychological group intervention in cardiac rehabilitation: a randomized controlled trial. J Behav Med. (2019) 42:1104–16. doi: 10.1007/s10865-019-00047-y
62. Kuijer RG, De Ridder DTD, Colland VT, Schreurs KMG, Sprangers MAG. Effects of a short self-management intervention for patients with asthma and diabetes: Evaluating health-related quality of life using then-test methodology. Psychol Health. (2007) 22:387–411. doi: 10.1080/14768320600843226
63. Armitage CJ, Alganem S, Norman P. Randomized controlled trial of a volitional help sheet to encourage weight loss in the Middle East. Prev Sci. (2017) 18:976–83. doi: 10.1007/s11121-017-0807-z
64. de Freitas Agondi R, Cornélio ME, Rodrigues RCM, Gallani M-C. Implementation intentions on the effect of salt intake among hypertensive women: a pilot study. Nurs. Res Pract. (2014) 2014:196410. doi: 10.1155/2014/196410
65. Hayes JF, Balantekin KN, Graham AK, Strube MJ, Bickel WK, Wilfley DE. Implementation intentions for weight loss in college students with overweight and obesity: a proof-of-concept randomized controlled trial. Transl Behav Med. (2021) 11:359–68. doi: 10.1093/tbm/ibaa038
66. Miura S-i, Yamaguchi Y, Urata H, Himeshima Y, Otsuka N, Tomita S, et al. Efficacy of a multicomponent program (patient-centered assessment and counseling for exercise plus nutrition [PACE+ Japan]) for lifestyle modification in patients with essential hypertension. Hypertension Res. (2004) 27:859–64. doi: 10.1291/hypres.27.859
67. Obara-Golebiowska M, Brycz H. Strategies of return to self-regulation among obese people: Implementation of goal's intention and motivation to weight reduction. Baltic J Health Phys Act. (2015) 7:59–65. doi: 10.29359/BJHPA.07.2.05
68. Scholz U, Ochsner S, Luszczynska A. Comparing different boosters of planning interventions on changes in fat consumption in overweight and obese individuals: a randomized controlled trial. Int J Psychol. (2013) 48:604–15. doi: 10.1080/00207594.2012.661061
69. Luszczynska A, Scholz U, Sutton S. Planning to change diet: a controlled trial of an implementation intentions training intervention to reduce saturated fat intake among patients after myocardial infarction. J Psychosom Res. (2007) 63:491–7. doi: 10.1016/j.jpsychores.2007.06.014
70. Jackson C, Lawton R, Knapp P, Raynor DK, Conner M, Lowe C, et al. Beyond intention: Do specific plans increase health behaviours in patients in primary care? a study of fruit and vegetable consumption. Soc Sci Med. (2005) 60:2383–91. doi: 10.1016/j.socscimed.2004.10.014
71. Soureti A, Murray P, Cobain M, van Mechelen W, Hurling R. Web-based risk communication and planning in an obese population: exploratory study. J Med Internet Res. (2011) 13:e100. doi: 10.2196/jmir.1579
72. Soureti A, Murray P, Cobain M, Chinapaw M, van Mechelen W, Hurling R. Exploratory study of web-based planning and mobile text reminders in an overweight population. J Med Internet Res. (2011) 13:e118. doi: 10.2196/jmir.1773
73. Armitage CJ, Norman P, Noor M, Alganem S, Arden MA. Evidence that a very brief psychological intervention boosts weight loss in a weight loss program. Behav Ther. (2014) 45:700–7. doi: 10.1016/j.beth.2014.04.001
74. Zandstra EH, den Hoed W, van der Meer N, van der Maas A. Improving compliance to meal-replacement food regimens. Forming implementation intentions (conscious IF-THEN plans) increases compliance. Appetite. (2010) 55:666–70. doi: 10.1016/j.appet.2010.09.021
75. Li L, Han LQ, Wang J, Dang YQ. Observation on the application of diet and exercise card in patients with type 2 diabetes. Chinese Baby. (2019) 10:13. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwMzAyEg1t teXNqMjAxOTE1MDA4GggyNGs2dWliZg%3D%3D
76. MacPhail M, Mullan B, Sharpe L, MacCann C, Todd J. Using the health action process approach to predict and improve health outcomes in individuals with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2014) 7:469–79. doi: 10.2147/DMSO.S68428
77. Liu F. Effects of individualized dietary intervention on type 2 diabetes. Med Inform. (2015) 18: 8439. doi: 10.3969/j.issn.1006-1959.2015.25.526
78. Gao L, Tao J, Liu Q. Application of diet goal based health education in early type 2 diabetes patients. J Nurs. (2019) 26:75–8. doi: 10.16460/j.issn1008-9969.2019.23.075
79. Ströbl V, Knisel W, Landgraf U, Faller H. A combined planning and telephone aftercare intervention for obese patients: effects on physical activity and body weight after one year. J Rehabil Med. (2013) 45:198–205. doi: 10.2340/16501977-1095
80. van Genugten L, van Empelen P, Oenema A. Intervention use and action planning in a web-based computer-tailored weight management program for overweight adults: randomized controlled trial. JMIR Res Protoc. (2014) 3:e31. doi: 10.2196/resprot.2599
81. Vinkers CD, Adriaanse MA, Kroese FM, De Ridder DT. Efficacy of a self-management intervention for weight control in overweight and obese adults: a randomized controlled trial. J Behav Med. (2014) 37:781–92. doi: 10.1007/s10865-013-9530-9
82. Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. (2008) 299:1139–48. doi: 10.1001/jama.299.10.1139
83. Stevens VJ, Obarzanek E, Cook NR, Lee I-M, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med. (2001) 134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007
84. Luszczynska A, Sobczyk A, Abraham C. Planning to lose weight: randomized controlled trial of an implementation intention prompt to enhance weight reduction among overweight and obese women. Health Psychol. (2007) 26:507. doi: 10.1037/0278-6133.26.4.507
85. Sniehotta FF, Dombrowski SU, Avenell A, Johnston M, McDonald S, Murchie P, et al. Randomised controlled feasibility trial of an evidence-informed behavioural intervention for obese adults with additional risk factors. PLoS ONE. (2011) 6:e23040. doi: 10.1371/journal.pone.0023040
86. Thoolen BJ, Ridder Dd, Bensing J, Gorter K, Rutten G. Beyond good intentions: the role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychol Health. (2009) 24:237–54. doi: 10.1080/08870440701864504
87. Duan YP, Liang W, Guo L, Wienert J, Si GY, Lippke S. Evaluation of a web-based intervention for multiple health behavior changes in patients with coronary heart disease in home-based rehabilitation: pilot randomized controlled trial. J Med Internet Res. (2018) 20:e12052. doi: 10.2196/12052
88. Helena I, Margareta E, Eva L, Pernilla Å. Tailored behavioral medicine intervention for enhanced physical activity and healthy eating in patients with obstructive sleep apnea syndrome and overweight. Sleep Breath. (2014) 18:655–68. doi: 10.1007/s11325-013-0929-x
89. Broekhuizen K, van Poppel MN, Koppes LL, Kindt I, Brug J, van Mechelen W. Can multiple lifestyle behaviours be improved in people with familial hypercholesterolemia? results of a parallel randomised controlled trial. PLoS ONE. (2012) 7:e50032. doi: 10.1371/journal.pone.0050032
90. Cheung KL, Schwabe I, Walthouwer MJ, Oenema A, Lechner L, De Vries H, et al. Effectiveness of a video-versus text-based computer-tailored intervention for obesity prevention after one year: a randomized controlled trial. Int J Environ Res. (2017) 14:1275. doi: 10.3390/ijerph14101275
91. Nishita C, Cardazone G, Uehara DL, Tom T. Empowered diabetes management:life coaching and pharmacist counseling for employed adults with diabetes. Health Educ Behav. (2013) 40:581–91. doi: 10.1177/1090198112465088
92. Hardeman W, Kinmonth AL, Michie S, Sutton S. the ProActive Project T. Impact of a physical activity intervention program on cognitive predictors of behaviour among adults at risk of Type 2 diabetes (ProActive randomised controlled trial). Int J Behav Nutr Phys Act. (2009) 6:16. doi: 10.1186/1479-5868-6-16
93. Heredia NI, Lee M, Hwang KO, Reininger BM, Fernandez ME, McNeill LH. Health coaching to encourage obese adults to enroll in commercially-available weight management programs: the path to health study. Contemp Clin Trials. (2019) 83:1–9. doi: 10.1016/j.cct.2019.06.006
94. Heideman WH, de Wit M, Middelkoop BJC, Nierkens V, Stronks K, et al. Diabetes risk reduction in overweight first degree relatives of type 2 diabetes patients: effects of a low-intensive lifestyle education program (DiAlert) a randomized controlled trial. Patient Educ Couns. (2015) 98:476–83. doi: 10.1016/j.pec.2014.12.008
95. Su JJ, Yu DS-f. Effects of a nurse-led eHealth cardiac rehabilitation programme on health outcomes of patients with coronary heart disease: A randomised controlled trial. Int J Nurs Stud. (2021) 122:104040. doi: 10.1016/j.ijnurstu.2021.104040
96. Washington-Plaskett T, Idris MY, Mubasher M, Ko Y-A, Islam SJ, Dunbar S, et al. Impact of technology-based intervention for improving self-management behaviors in black adults with poor cardiovascular health: a randomized control trial. Int J Environ Res Public Health. (2021) 18:3660. doi: 10.3390/ijerph18073660
97. Eakin E, Reeves M, Lawler S, Graves N, Oldenburg B, et al. Telephone counseling for physical activity and diet in primary care patients. Am J Prev Med. (2009) 36:142–9. doi: 10.1016/j.amepre.2008.09.042
98. Jiang Y, Mao F, Dong W, Zhang X, Dong J. Lasting effects of a community-based self-management intervention for patients with type 2 diabetes in china: outcomes at 2-year follow-up of a randomized trial. Asia Pac J Public Health. (2021) 33:30–8. doi: 10.1177/1010539520975266
99. Swoboda CM, Miller CK, Wills CE. Setting single or multiple goals for diet and physical activity behaviors improves cardiovascular disease risk factors in adults with type 2 diabetes:a pragmatic pilot randomized trial. Diabetes Educ. (2016) 42:429–43. doi: 10.1177/0145721716650043
100. Lanoye A, Brown KL, LaRose JG. The transition into young adulthood: a critical period for weight control. Curr Diab Rep. (2017) 17:114. doi: 10.1007/s11892-017-0938-4
101. Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med. Internet Res. (2004) 6:e40. doi: 10.2196/jmir.6.4.e40
102. De Vet E, Gebhardt WA, Sinnige J, Van Puffelen A, Van Lettow B, De Wit JB. Implementation intentions for buying, carrying, discussing and using condoms: the role of the quality of plans. Health Educ Res. (2011) 26:443–55. doi: 10.1093/her/cyr006
Keywords: physical activity, diet behavior, chronic disease management, implementation intention planning, behavioral interventions
Citation: Lin H, Yu P, Yang M, Wu D, Wang Z, An J, Duan H and Deng N (2022) Making Specific Plan Improves Physical Activity and Healthy Eating for Community-Dwelling Patients With Chronic Conditions: A Systematic Review and Meta-Analysis. Front. Public Health 10:721223. doi: 10.3389/fpubh.2022.721223
Received: 06 June 2021; Accepted: 19 April 2022;
Published: 19 May 2022.
Edited by:
Dan J. Graham, Colorado State University, United StatesReviewed by:
Basil H. Aboul-Enein, University of London, United KingdomLingtong Min, Northwestern Polytechnical University, China
Shan Nan, Hainan University, China
Jianqun Dong, Chinese Center for Disease Control and Prevention, China
Copyright © 2022 Lin, Yu, Yang, Wu, Wang, An, Duan and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Deng, emp1LmRlbmduaW5nQGdtYWlsLmNvbQ==
†These authors share first authorship
 Hui Lin
Hui Lin Ping Yu
Ping Yu Min Yang
Min Yang Dan Wu
Dan Wu Zhen Wang
Zhen Wang Jiye An
Jiye An Huilong Duan
Huilong Duan Ning Deng
Ning Deng