- Department of Public Health, Medical College, Qinghai University, Xining, China
Background: Echinococcosis is a severe zoonotic disease that imposes a substantial burden on human life. This meta-analysis aimed to summarize available data on the prevalence of human echinococcosis and identify the key risk factors for echinococcosis in the Chinese general population.
Methods: Relevant studies were comprehensively searched in the PubMed, EMBASE, Web of Science, Cochrane, Chinese National Knowledge Infrastructure (CNKI), Chongqing VIP Information (VIP), Wanfang and SinoMed databases until August 22, 2020. A random-effects model was used to estimate the pooled odds ratio (OR) and 95% confidence interval (95% CI). The I2 and Q statistics were calculated to evaluate the heterogeneity, and potential sources of heterogeneity were identified using sensitivity analysis and subgroup analysis. Publication bias was estimated by funnel plots and Egger's test.
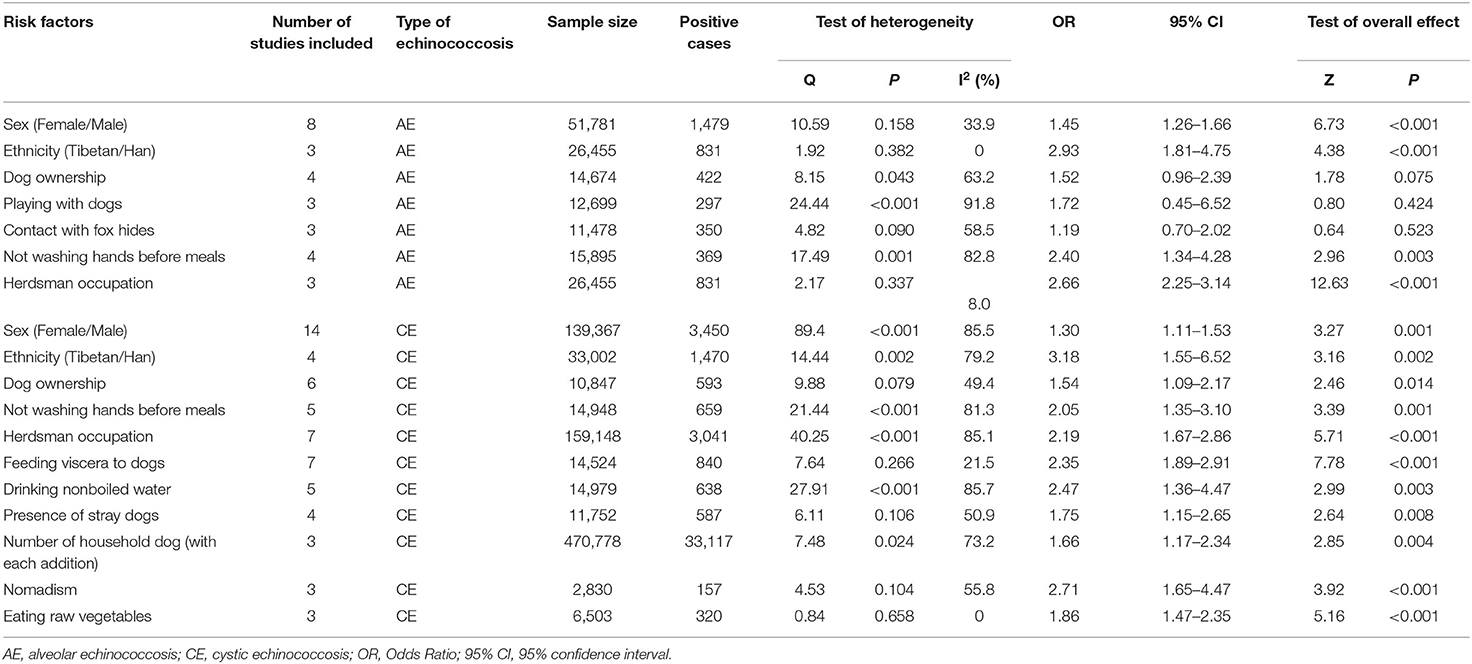
Results: A total of 1026 studies were identified through the database search, of which 26 were eligible for this meta-analysis. The pooled prevalence of AE and CE were 2.88% and 5.66%, respectively. Ethnicity (OR = 2.93, 95% CI: 1.81–4.75; I2 = 0), herdsman occupation (OR = 2.66, 95% CI: 2.25–3.14; I2 = 8.0%), not washing hands before meals (OR = 2.40, 95% CI: 1.34–4.28; I2 = 82.8%) and being female (OR = 1.45, 95% CI: 1.26–1.66; I2 = 33.9%) were risk factors for AE. The top five risk factors for CE were ethnicity (OR = 3.18, 95% CI: 1.55–6.52; I2 = 79.2%), nomadism (OR = 2.71, 95% CI: 1.65–4.47; I2 = 55.8%), drinking nonboiled water (OR = 2.47, 95% CI: 1.36–4.47; I2 = 85.7), feeding viscera to dogs (OR = 2.35, 95% CI: 1.89–2.91; I2 = 21.5%), and herdsman occupation (OR = 2.19, 95% CI: 1.67–2.86; I2 = 85.1%).
Conclusions: This study generalized articles that have contributed to our current understanding of the epidemic of human echinococcosis (AE and CE) in China over the years. The results support that the ethnicity and dog-related factors are major risk factors for both CE and AE. The identification of echinococcosis risk factors may aid researchers and policymakers in improving surveillance and preventive measures aimed at reducing Echinococcus granulosus and Echinococcus multilocularis infection in humans.
Introduction
Echinococcosis is widely known as a zoonotic and natural-focal disease in which HUMANs play the role of aberrant, dead-end intermediate hosts. Cystic echinococcosis (CE) and alveolar echinococcosis (AE) are the two most common forms of human echinococcosis and are caused by the larval stages of Echinococcus granulosus and Echinococcus multilocularis, respectively (1). Dogs are the usual definitive host of E. granulosus (2), whereas dogs and foxes are the main definitive hosts of E. multilocularis (3). Both are transmitted by the fecal-oral route through contact with infected definitive hosts or with food or water contaminated with E. granulosus or E. multilocularis eggs. The annual numbers of new cases of CE and AE are estimated at 188,000 and 18,200, respectively, leading to a corresponding total of 184,000 and 666,000 disability-adjusted life years (DALYs) (4). AE has a higher mortality rate than that of CE, which is one of the major reasons for the greater global AE burden (5); It is also called “worm cancer” (6).
Echinococcus parasites can inhabit any part of the human body, but mainly favor the liver, lungs, brain and abdomen. Once a parasite attaches to the human body, health deteriorates. CE is endemic in pastoral areas around the world, where it is often maintained by herders feeding viscera from infected ruminants to dogs. For AE, in addition to the original life cycle in wild canids, a life cycle has also been established in domestic dogs, which are the most significant transmitters of AE in China (7). AE infection is maintained through dog predation on small rodents (8). Therefore, compared to those of E. granulosus, the potential risk factors for E. multilocularis are more complex because its life cycle involves multiple wild canids as final hosts and a large number of small mammals (mostly rodents) as intermediate hosts (9).
To date, many studies have examined the risk factors for echinococcosis, each study focusing on different areas. The geographic distribution and prevalence of echinococcosis vary from region to region and are mainly influenced by biological and abiotic factors. The biological factors include host species, transmission mechanism, density and prevalence among definitive hosts (5), and the abiotic factors include environmental, socioeconomic and behavioral factors. A previous study (10) on environmental and socioeconomic risk factors for CE in western China showed that the ratio of grassland positively correlated with the prevalence of human CE, whereas the gross domestic product and land surface temperature (in spring) were independently negatively correlated with disease prevalence. Wang Qian (11) reported that owning fox hides, letting flies land on food, using open streams as drinking water sources and playing with dogs were significant behavioral risk factors for AE. However, it is difficult to identify the primary high-risk factors for echinococcosis because of differences in the groups, type of echinococcosis and study region among studies. Therefore, the present meta-analysis pooled the results of previous studies and aimed to analyze the main risk factors for AE and CE.
Materials and Methods
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines when performing the literature search. Two researchers (T.Z. and B.L.) independently searched for relevant articles published in four English (PubMed, Embase, Web of Science, and the Cochrane Library) and four Chinese (China National Knowledge Infrastructure, China Science and Technology Journal Database, Wanfang Data and SinoMed) databases from their inception to August 22, 2020. The search terms were [(“echinococcosis” OR “echinococcoses” OR “echinococcus infection” OR “hydatidosis” OR “hydatidoses” OR “hydatid cyst” OR “hydatid disease” OR “echinococcus granulosus infection”) AND (“risk factor” OR “population at risk” OR “homo sapiens” OR “man” OR “human”) AND (“People's Republic of China” OR “Chinese” OR “China”)]. In addition, the references of reviews and meta-analyses were manually screened to identify additional potentially relevant studies.
Eligibility Criteria
Studies were eligible if they met the following inclusion criteria: (1) the research was conducted with Chinese residents; (2) the diagnoses of AE and CE were based on a combination of serological and ultrasonic methods; and (3) the odds ratios (ORs) and their 95% confidence intervals (95% CIs) could be obtained directly or calculated from the study.
Studies were excluded if (1) the publications were neither in Chinese nor in English; (2) the sample size was ≤ 30 (12); (3) no risk factors were reported; (4) several articles were based on data from one study sample, only the article with the most comprehensive results was included; and (5) the publication was low quality based on its overall Newcastle-Ottawa Scale (NOS) or Agency for Healthcare Research and Quality (AHRQ) score.
Quality Assessment
Two authors (TZ and BL) independently assessed the quality of the studies. We employed NOS and AHRQ scores to assess the quality of cross-sectional studies and case-control studies. NOS scores range from 0 to 8; scores of 7–8, 4–6 and 0–3 indicate a study of high, medium and low quality, respectively (13, 14). AHRQ scores are between 0 and 11, with scores of 8–11 indicating high quality and scores of 4–7 and 0–3 indicating moderate and low quality, respectively (15). Any disagreements during this process were resolved by discussion with the third author YL.
Data Extraction
Two authors (TZ and BL) independently extracted data and information from the studies including the first author, year of publication, region, type of echinococcosis (AE or CE), study design, sample size, number of positive cases, participant age in years, sex, participant race/ethnicity, whether the participants were herdsmen, ang/or raised dogs, the kind/number of animal hosts, participant hand washing status, and the OR value and its 95% CI, or the original data from which the OR could be calculated.
Meta-Analysis
The ORs and 95% CIs of the associated factors were pooled using random-effects models if at least three studies reported data on the same factor (16). The results were represented using forest plots. Additionally, the Q test was used to test the level of heterogeneity between studies; the percentage of total variation in the results due to heterogeneity was assessed based on the I2 statistic. An I2 < 25%, 25–50%, 50–75% and 75–100% represents no, moderate, large and extreme heterogeneity, respectively (17). In this study, P < 0.05 and I2 > 50% were considered to indicate significant heterogeneity between studies (18). Sensitivity analysis using the leave-one-out method was performed to evaluate the stability and reliability of the results. In addition, Egger's test (19) and funnel plots were used to test for the presence of publication bias. The prevalence of AE and CE in endemic areas was estimated using a random-effects model that combined the prevalence reported in previous cross-sectional studies.
We employed subgroup analysis to explore the source of heterogeneity on the basis of study design (i.e., case-control study or cross-sectional study) and geographic distribution of the studies (Ningxia, Qinghai or Xinjiang). Data were analyzed using the R (4.0.0) package meta (20, 21), and p < 0.05 was considered statistically significant.
Results
Study Selection
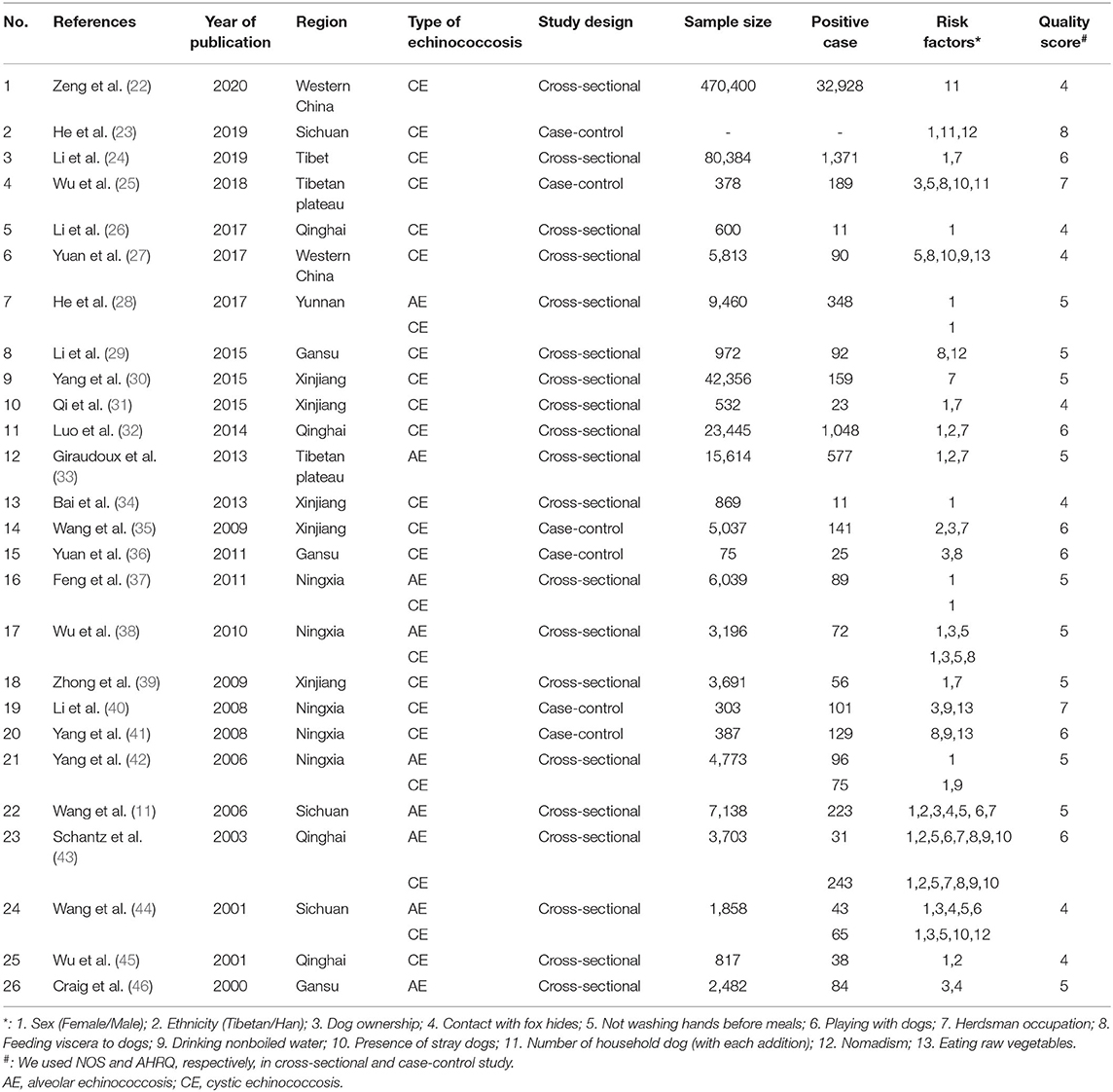
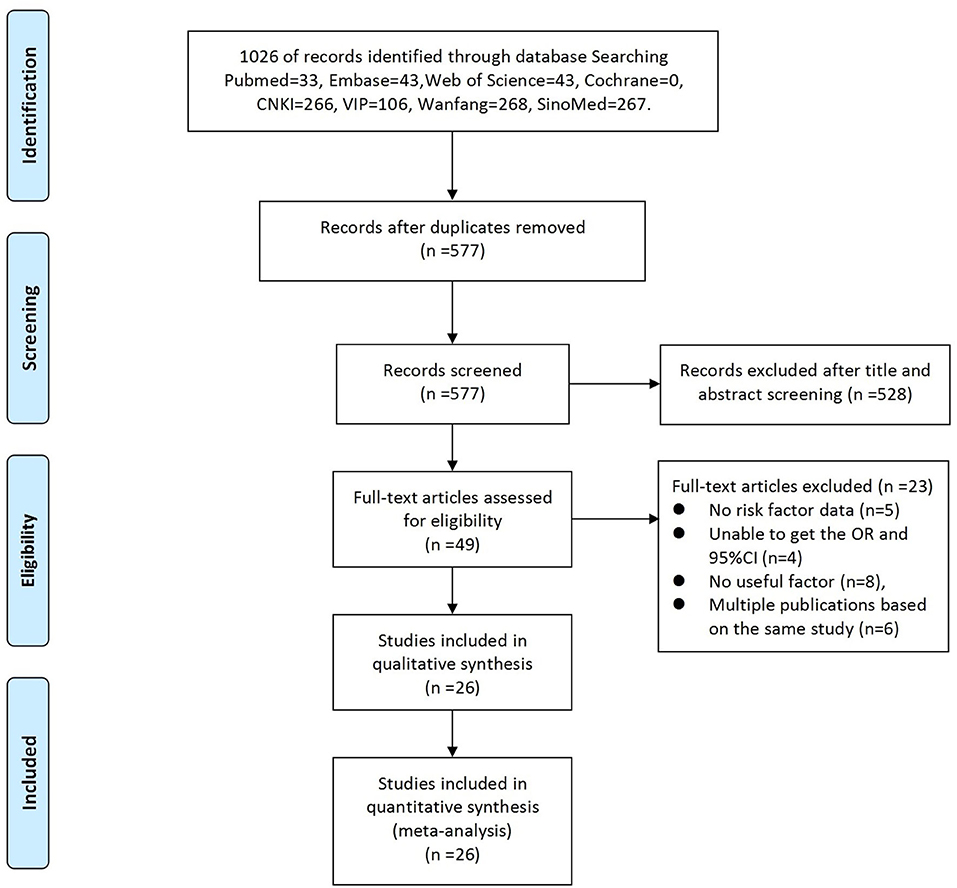
A total of 1,026 articles were originally identified; of these, 449 were excluded as duplicates. Thus, 577 studies were screened; of these, 26 (Table 1) were eligible and subsequently included in this meta-analysis. The literature selection process is detailed in Figure 1, and the basic characteristics of the included studies are shown in Table 1. All the cross-sectional studies (20/26) were of medium quality. The case-control studies (6/26) included three medium-quality and three high-quality studies (Table 1). No cohort studies were included in our analysis.
Overall, the included studies covered 690,322 individuals (AE studies = 54,338, CE studies = 635,984), of which 38,358 had echinococcosis (AE cases = 1,588, CE cases = 36,770) according to the combined diagnosis based on ultrasound and serological methods. The included studies varied in location, including the Tibetan Autonomous Region (n = 1), Qinghai Province (n = 4), Western China (n = 2), Yunnan Province (n = 1), Gansu Province (n = 3), Xinjiang Province (n = 5), Ningxia Province (n = 5), Sichuan Province (n = 3), and the Tibetan Plateau (n = 2). Three of the included studies reported only on AE-infected patients, seventeen reported only on CE-infected patients, and six studies reported on both AE-and CE-infected patients.
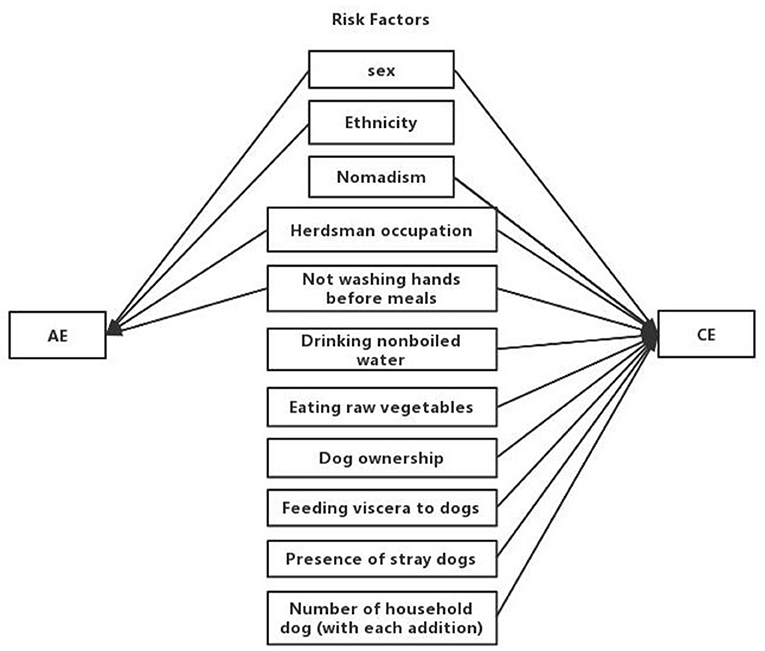
In total, thirteen potential risk factors reported were included in the meta-analysis: participant sex, ethnicity, dog ownership, contact with fox hides, not washing hands before meals, playing with dogs, herdsman occupation, feeding viscera to dogs, drinking nonboiled water, nomadism, eating raw vegetables, the presence of stray dogs, and number of household dogs. The risk factors associated with AE and CE are shown in Figure 2.
Twenty cross-sectional studies conducted before August 2020 reported the prevalence of echinococcosis; the pooled prevalence of AE and CE in endemic districts were 2.34% (95% CI: 1.74–3.13%) and 4.45% (95% CI: 2.53–7.71%), respectively.
Potential Risk Factors for AE
Seven risk factors for AE were indicated among the studies, and a meta-analysis was executed on nine cross-sectional studies (11, 28, 33, 37, 38, 42–45). The results of the meta-analysis and forest plots are summarized in Table 2, Figure 3.
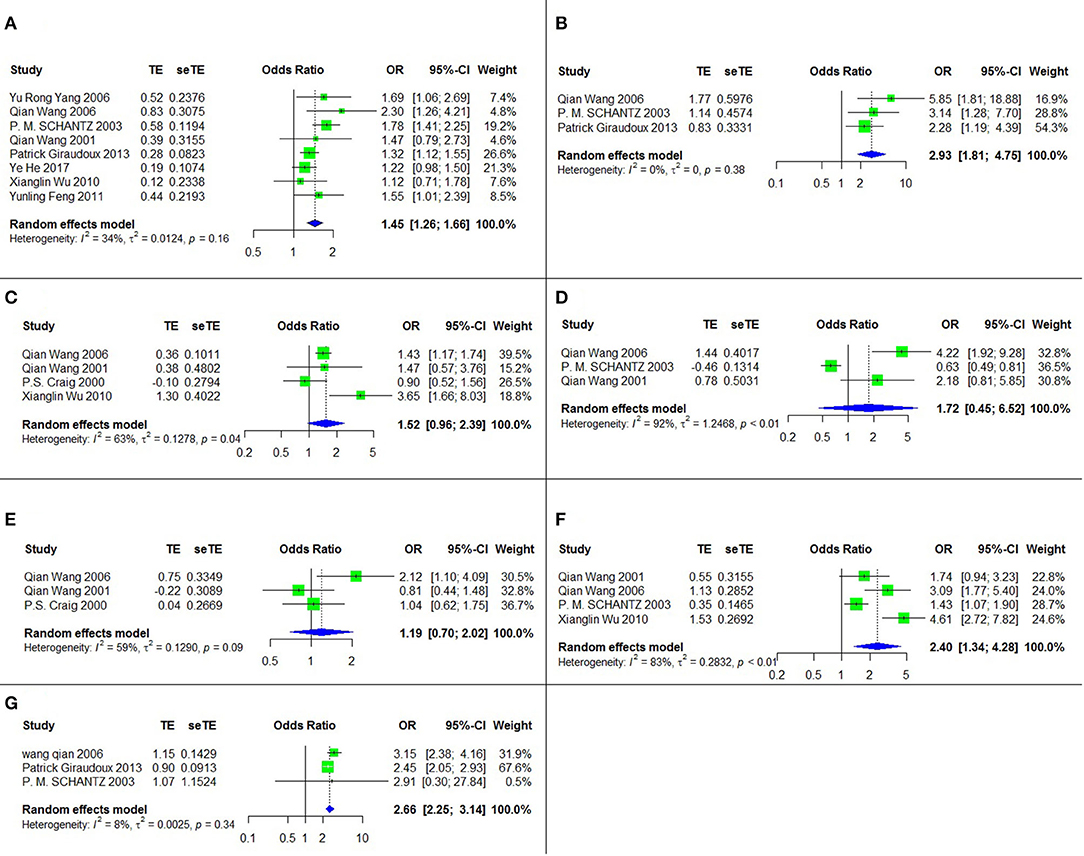
Figure 3. The forest chart of AE factors. (A) Sex (female/male); (B) Ethnicity (Tibetan/Han); (C) Dog ownership; (D) Playing with dogs; (E) Contanct with fox hides; (F) Not washing hands before meals; (G) Herdsman occupation.
Four of these seven risk factors were statistically significant. They are listed according to the strength of the correlation as follows: ethnicity (Tibetan vs. Han) (OR = 2.93, 95% CI: 1.81–4.75; p < 0.001), herdsman occupation (OR = 2.66, 95% CI: 2.25-3.14; p < 0.001), not washing hands before meals (OR = 2.40, 95% CI: 1.34–4.28; p = 0.003) and sex (female vs. male) (OR = 1.45, 95% CI: 1.26–1.66; p < 0.001).
Potential Risk Factors for CE
Eleven risk factors for CE were recognized among the relevant studies, and a meta-analysis was performed on twenty-three papers including six case-control studies and seventeen cross-sectional studies (Table 1). The results are shown in Table 2, Figure 4.
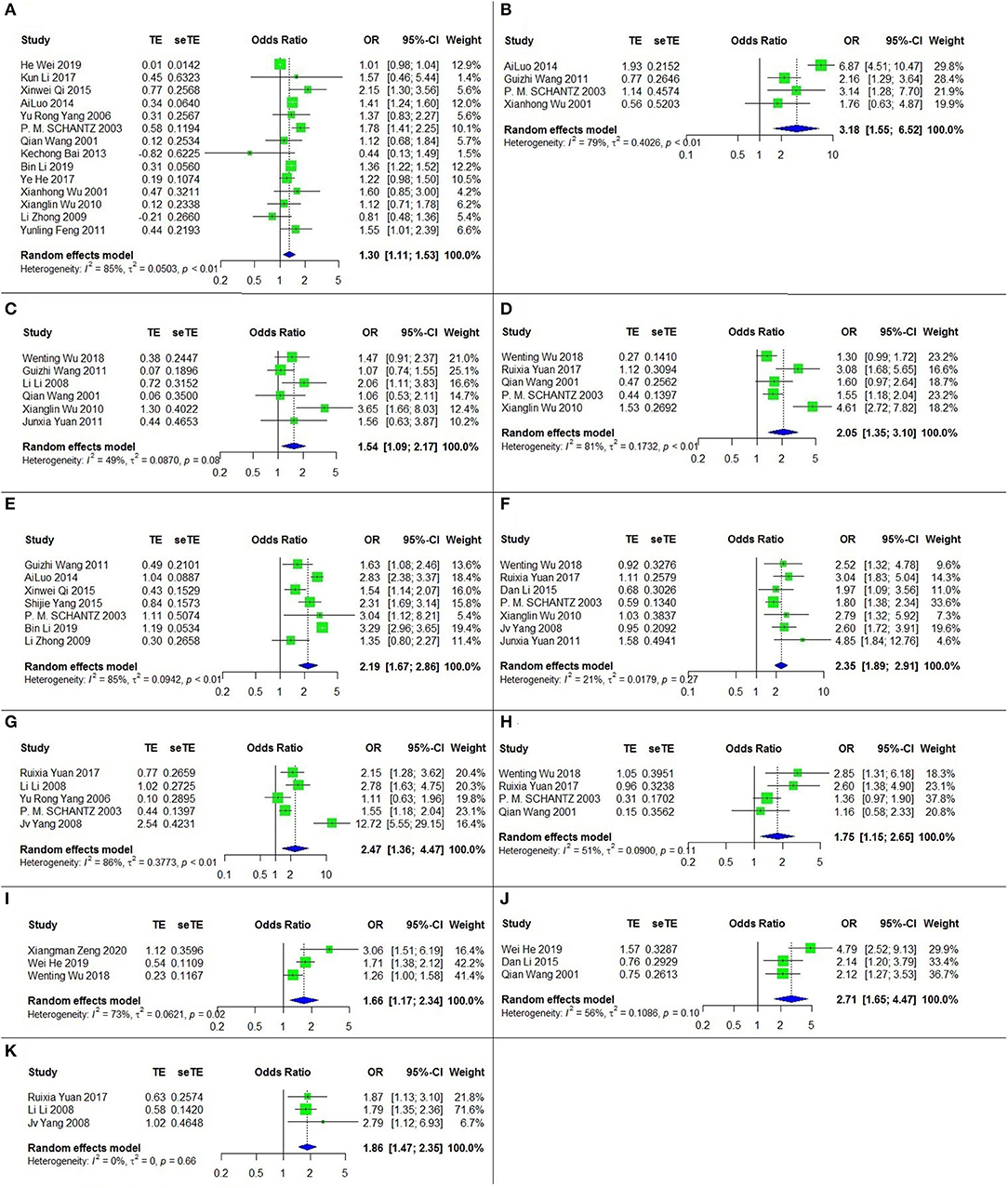
Figure 4. The forest chart of CE factors. (A) Sex (female/male); (B) Ethnicity (Tibetan/Han); (C) Dog ownership; (D) Not washing hands before meals; (E) Herdsman occupation; (F) Feeding viscera to dogs; (G) Drinking nonboiled water; (H) Presence of stray dogs; (I) Number of household dog (with each addition); (J) Nomadism; (K) Eating raw vegetables.
All of these eleven risk factors were statistically significant. The top three, according to the strength of the correlation, were ethnicity (Tibetan vs. Han) (OR = 3.18, 95% CI: 1.55–6.52; p = 0.002), nomadism (OR = 2.71, 95% CI: 1.65-4.47; p < 0.001) and drinking nonboiled water (OR = 2.47, 95% CI: = 1.36–4.47; p = 0.003).
Sensitivity Analysis
The sensitivity analysis revealed that the results were stable for most of the risk factors. However, when we removed two of the studies [(27, 38)] on AE, the heterogeneity of playing with dogs and dog ownership declined markedly, and their corresponding results became statistically significant. Similarly, when we removed three of the studies related to CE (23, 25, 32), the heterogeneity of sex, ethnicity and the presence of stray dogs dropped below 50%. More details are shown in Supplementary Material.
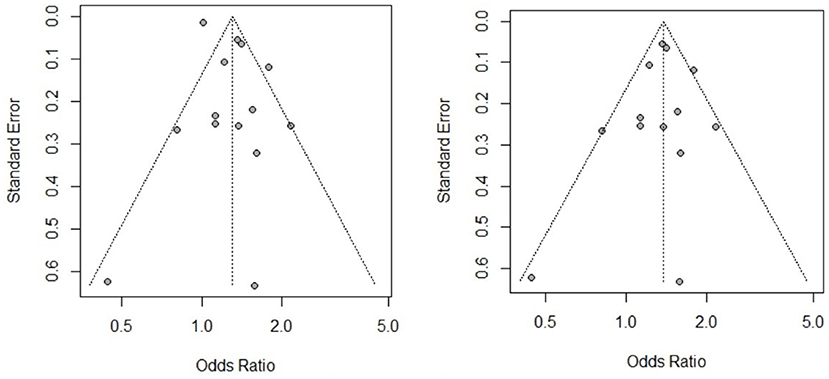
Publication Bias
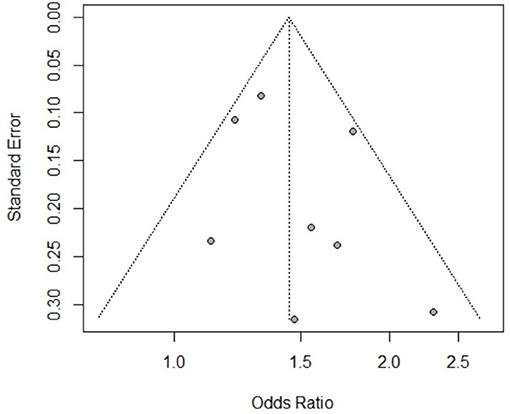
The publication bias was assessed for all the risk factors included in this study (see Supplementary Material). Based on the results of Egger's test and the funnel charts, three of the risk factors for CE (participant sex, herdsman occupation and feeding viscera to dogs) exhibited publication bias. Other risk factors did not exhibit bias; for instance, the p value of Egger's test for the participant sex, as a risk factor of AE, was >0.05, and the funnel chart was largely symmetric (Figure 5).
Subgroup Analysis
The study design-specific subgroup analysis examined only risk factors for CE because all of the AE studies had a cross-sectional design. The results are shown in Supplementary Material. In the case-control studies, two risk factors were significant: dog ownership (OR = 1.35, 95% CI: 1.03-1.83; p = 0.029) and feeding viscera to dogs (OR = 2.76, 95% CI: 2.00–3.83; p < 0.001). In the cross-sectional studies, seven risk factors were identified. The top three, according to the strength of the correlation, were ethnicity (OR = 3.71, 95% CI: 1.60–8.59; p = 0.002), not washing hands before meals (OR = 2.37, 95% CI: 1.40–4.00; p = 0.003) and herdsman occupation (OR = 2.30, 95% CI: 1.74–3.04; p < 0.001). The heterogeneity of all the CE risk factors decreased to varying degrees in the study design-specific subgroup analysis.
In general, the studies were widely geographically distributed, including seven Chinese provinces. The provinces of Ningxia, Qinghai, and Xinjiang were included in the study region-specific subgroup analysis, and had three risk factors, two risk factors, and one risk factor, respectively. Only one risk factor for AE was identified (participant sex in Ningxia, OR = 1.44, 95% CI: 1.11–1.86; P = 0.006). The significant risk factors for CE were participant sex in Ningxia (OR = 1.34, 95% CI: 1.03–1.75; P = 0.029) and Qinghai (OR = 1.49, 95% CI: 1.33–1.66; P < 0.001) and herdsman occupation in Xinjiang (OR = 1.73, 95% CI: 1.37–2.19; P < 0.001). The Supplementary Material shows this in more detail.
Discussion
The pooled prevalence of AE and CE were 2.34% (95% CI: 1.74–3.13%) and 4.45% (95% CI: 2.53–7.71%), respectively, in the included individuals. However, the prevalence of AE was significantly higher than that previously calculated in China (0.96%) (3). Since we aim at understanding the risk factors for AE and CE, several studies on prevalence were not included due to restrictions in the search terms (i.e., “risk factor”), which could partially explain the discrepancy. Notably, a recent nationwide study revealed that the prevalence of CE in China has decreased to 0.07% (47), demonstrating that the current prevention and control measures have had a significant impact.
Sex, ethnicity, not washing hands before meals and herdsman occupation were found to be common risk factors for AE and CE in this meta-analysis. Similar to previous studies (48, 49), we found that women were more likely to develop echinococcosis than men, because they are more involved in housework, such as food preparation and pet care, and therefore have more opportunities to contact infected dogs, soil and vegetables (50). Furthermore, as a result of their increased number of regular abdominal ultrasound examinations to monitor reproductive health, women of childbearing age infected with echinococcosis have a greater chance of early detection (51), which will lead to detection bias. In a case-control study, Alaouadi (52) found that women are more susceptible to echinococcosis than men because women‘s higher estrogen levels might promote echinococcosis growth. Ethnicity could be a confounding factor because most Tibetans living in western China are herders (32), who thus regularly come into contact with infected canid definitive hosts.
The main route of human infection is through fecal-oral transmission because echinococcosis can spread via the ingestion of food, soil, or water contaminated with the feces of infected mammals (53). In line with previous study showing that no washing hand before meals was one of the risk factors for echinococcosis (27), and one meta-analysis on risk factors for global echinococcosis indicating that eating raw unwashed vegetables and drinking piped water were associated with higher odds of infecting through the accidental ingestion of worm eggs (54), the present analysis confirms the causal effects of poor hygienic habits on the higher risk of disease. Our study also revealed that nomadism is a risk factor for AE and CE, which is similar to the results of a previous meta-analysis conducted in Iran (19). Nomads live in areas with poor sanitation and economic disadvantages, where they are exposed to infected animals and have a higher risk of becoming infected.
The contribution of dogs to the spread of echinococcosis cannot be ignored. Dog ownership, feeding viscera to dogs, the presence of stray dogs and number of household dogs were all risk factors for CE, but there was insufficient evidence to conclude that these were also risk factors for AE. This finding contradicts previous studies, which reported that dog-related factors were linked to both AE and CE (55, 56). The high heterogeneity of dog-related factors for AE could partially help to explain the inconsistency; the potential mechanism however should be further studied. In China, there are a large number of pet dogs and stray dogs, and a previous meta-analysis found that the combined prevalence of E. multilocularis and E. granulosus in dogs was 7.3% (57). Furthermore, because dogs belonging to rural families are less likely to obtain nutritious food, their diets are supplemented by hunting small mammals, which are intermediate hosts of E. multilocularis (58), or by being fed livestock viscera, which supports the lifecycle of E. granulosus (59). The large number of infected dogs and close contact with dogs are the causes of the high rates of E. multilocularis and E. granulosus in humans.
Coming into contact with fox hides was not a significant risk factor in this meta-analysis. After sensitivity analysis, the I2 for this factor changed to 0.00, but overall effect was still not significant. However, previous studies have reported that exposure to foxes increases the risk of AE infection (50, 60). Thus, Schweiger (61) found an increase in the fox population starting in 1985; after 10–15 years, the number of human AE cases significantly increased. A plausible explanation could be urbanization (62), which has resulted in an increased number of foxes appearing in people‘s living quarters. Increased opportunities for people to come into contact with foxes has increased the infection risk in the human population.
The analysis of the overall effect of the CE-related risk factors sex, herdsman occupation and feeding viscera to dogs revealed publication bias. In our subgroup analysis based on study design, the publication bias disappeared, and the heterogeneity decreased. For example, as shown in Figure 6, when we excluded the case-control study, the CE risk factor sex became significant, and the funnel chart became symmetrical. Therefore, differences in study design may be the source of heterogeneity. We also conducted a subgroup meta-analysis based on study region. Sex was identified as a risk factor in several regions, but heterogeneity was not completely eliminated. As a result, it remains unclear whether the study region was the source of heterogeneity.
The results suggested that women and Tibetans should receive increased attention for echinococcosis prevention and control. Government-based interventions should be considered and implemented in these groups to raise awareness about the disease and take preventive measures. For instance, regular disease education and community screening should both be provided. Establishing good hygiene practices in the general population also prevents the long-term implications of echinococcosis. Dogs, the main vectors of transmission, play a key role in the prevention and control of echinococcosis. Thus, to improve public health, echinococcus infection in dogs must be properly managed and monitored, such as implementing monthly deworming and an effective registration of all dogs. In addition to the above suggestions, other echinococcosis control measures in China have been conducted, such as using EG95 antigen-based subunit vaccine to induce a robust immune response to infection in goats and sheep (63) as well as establishing the Belt and Road Network for the Elimination and Control of Echinococcosis and Cysticercosis (B&R-NEC), which provided the research and development capacity required to meet echinococcosis control targets (64). The establishment of an online scientific research platform and the use of animal vaccines can enable people to better understand and control the spread of echinococcosis.
Our study had several limitations, most relating to the lack of data available in the literature. Although we identified some risk factors for echinococcosis, more factors need to be analyzed, such as environmental and economic factors. Other limitations are related to the design of the included studies. All were observational studies (case-control and cross-sectional studies) that have inherent limits; for instance, observational studies are prone to selection bias and space bias (65). In addition, the timing of exposure and outcome could not be determined in these studies. Moreover, an insufficient number of regions were studies; specifically, only three areas were analyzed in the subgroup meta-analysis based on study region, and the details of echinococcosis risk factors in each region could not be identified.
Conclusion
In summary, understanding the risk factors for echinococcosis provides a scientific basis to guide the formulation of prevention and control measures. Of the risk factors examined, for both AE and CE, the most important was ethnicity. Tibetans are at the highest risk of echinococcosis and thus must be closely monitored. The evidence for dog-related risk factors is also convincing, albeit at a lower level than that of ethnicity. Preventative measures of echinococcosis in humans should aim at raising the awareness of the disease in target groups and dog management. A series of national control measures, including regular dog deworming, public health education and community screening, should be implemented.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
TZ and SL: study design and drafting of the manuscript. TZ, BL, and YL: data collection, analysis, and interpretation. All authors approval of the final version for publication.
Funding
The study was supported by the National Natural Science Foundation of China (81860606) and the Natural Science Foundation of Qinghai Province (2019-ZJ-906).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the researchers who participated in this survey and the research investigators for their great help on the data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.821265/full#supplementary-material
References
1. Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. (2010) 114:1-16. doi: 10.1016/j.actatropica.2009.11.001
2. Khan A, Ahmed H, Simsek S, Afzal MS, Cao J. Spread of cystic echinococcosis in pakistan due to stray dogs and livestock slaughtering habits: research priorities and public health importance. Front Public Health. (2019) 7:e412. doi: 10.3389/fpubh.2019.00412
3. Wang X, Dai G, Li M, Jia W, Guo Z, Lu J. Prevalence of human alveolar echinococcosis in China: a systematic review and meta-analysis. BMC Public Health. (2020) 20:1105. doi: 10.1186/s12889-020-08989-8
4. Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. (2015) 12:e1001920. doi: 10.1371/journal.pmed.1001920
5. Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol. (2017) 95:315-493. doi: 10.1016/bs.apar.2016.11.001
7. Baumann S, Shi R, Liu WY, Bao HH, Schmidberger J, Kratzer W, et al. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. (2019) 47:703-27. doi: 10.1007/s15010-019-01325-2
8. Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang WB, et al. Echinococcosis: advances in the 21st Century. Clin Microbiol Rev. (2019) 32:e00075-18. doi: 10.1128/CMR.00075-18
9. Eckert J, Thompson RC. Historical aspects of echinococcosis. Adv Parasitol. (2017) 95:1-64. doi: 10.1016/bs.apar.2016.07.003
10. Huang D, Li RD, Qiu J, Sun XD, Yuan RX, Shi YY, et al. Geographical environment factors and risk mapping of human cystic echinococcosis in Western China. Int J Environ Res Public Health. (2018) 15:1729. doi: 10.3390/ijerph15081729
11. Wang Q, Qiu J, Yang W, Schantz PM, Raoul F, Craig PS, et al. Socioeconomic and behavior risk factors of human alveolar echinococcosis in Tibetan communities in Sichuan, People's Republic of China. Am J Trop Med Hyg. (2006) 74:856-62. doi: 10.4269/ajtmh.2006.74.856
12. Weber EJ, Hoo ZH. Why sample size estimates? Emerg Med J. (2018) 35:755-6. doi: 10.1136/emermed-2018-207763
13. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:e45. doi: 10.1186/1471-2288-14-45
14. Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: a systematic review and meta-analysis. Br J Sports Med. (2019) 53:270-81. doi: 10.1136/bjsports-2017-098890
15. Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. (2015) 61:78-89. doi: 10.1016/j.comppsych.2015.05.001
16. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. (2009) 9:e80. doi: 10.1186/1471-2288-9-80
17. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193-206. doi: 10.1037/1082-989X.11.2.193
18. Hoaglin DC. Misunderstandings about Q and 'Cochran's Q test' in meta-analysis. Stat Med. (2016) 35:485-95. doi: 10.1002/sim.6632
19. Mahmoudi S, Mamishi S, Banar M, Pourakbari B, Keshavarz H. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infect Dis. (2019) 19:e929. doi: 10.1186/s12879-019-4458-5
21. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153-60. doi: 10.1136/ebmental-2019-300117
22. Zeng XM, Guan YY, Wu WP, Wang LY, Cai HX, Fang Q, et al. Analysis of factors influencing cystic echinococcosis in northwest non-qinghai tibetan plateau regions of China. Am J Trop Med Hyg. (2020) 102:567-73. doi: 10.4269/ajtmh.18-0703
23. He W, Wang Q, Huang Y, Yu WJ, Zhang GJ, Liao S, et al. Risk factors of human cystic echinococcosis in Shiqu County Sichuan Province: a case-control study. Chinese J Schistosomiasis Control. (2019) 31:486-90. doi: 10.16250/j.32.1374.2019003
24. Li B, Quzhen GS, Xue CZ, Han S, Chen WQ, Yan XL, et al. Epidemiological survey of echinococcosis in Tibet Autonomous Region of China. Infect Dis Poverty. (2019) 8:29. doi: 10.1186/s40249-019-0537-5
25. Wen-Ting W, Wei-Ping W, Ya-Yi G, Shuai H, Chui-Zhao X, Xu W, et al. A case-control study on risk factors of cystic echinococcosis in humans in Tibetan areas. Chinese J Schistosomiasis Control. (2018) 30:161-4. doi: 10.16250/j.32.1374.2018059
26. Li K, Zhang L, Zhang H, Lei Z, Luo H, Mehmood K, et al. Epidemiological investigation and risk factors of Echinococcus granulosus in yaks (Bos grunniens), Tibetan pigs and Tibetans on Qinghai Tibetan plateau. Acta Trop. (2017) 173:147-52. doi: 10.1016/j.actatropica.2017.06.019
27. Yuan R, Wu H, Zeng H, Liu P, Xu Q, Gao L, et al. Prevalence of and risk factors for cystic echinococcosis among herding families in five provinces in western China: a cross-sectional study. Oncotarget. (2017) 8:91568-76. doi: 10.18632/oncotarget.21229
28. Ye H, Jiaxiang Y. Analysis for epidemiological factors of echinococcosis. China Trop Med. (2017) 17:418-20. doi: 10.13604/j.cnki.46-1064/r.2017.04.25
29. Li D, Gao Q, Liu J, Feng Y, Ning W, Dong Y, et al. Knowledge, attitude, and practices (KAP) and risk factors analysis related to cystic echinococcosis among residents in Tibetan communities, Xiahe County, Gansu Province, China. Acta Trop. (2015) 147:17-22. doi: 10.1016/j.actatropica.2015.02.018
30. Shi-jie Y, Min L, Tian T. Epidemiological characteristics of human hydatidosis and risk factors in Habahe County, Xinjiang. Dis Surveill. (2015) 30:485-8.
31. Xinwei Q, Xiaohui F, Kesteren FV, Haitao L, Tao S, Xinyu D, et al. Epidemic status of Echinococcus granulosus and risk factors of human cycstic echinococcosis in Hoboksar Mongolian Autonomous County of Xinjiang. Chinese J Endemiol. (2015) 34:56-60.
32. Luo A, Wang H, Li JQ, Wu HS, Yang F, Fang PQ. Epidemic factors and control of hepatic echinococcosis in Qinghai province. J Huazhong Univ Sci Technol Med Sci. (2014) 34:142-5. doi: 10.1007/s11596-014-1246-8
33. Giraudoux P, Raoul F, Pleydell D, Li TY, Han XM, Qiu JM, et al. Drivers of echinococcus multilocularis transmission in China: small mammal diversity, landscape or climate? PLoS Negl TropDis. (2013) 7:e2045. doi: 10.1371/journal.pntd.0002045
34. Bai K, Wang X. Epidemic characteristics and related influencing factors of hydatid disease. China J Pharm Econ. (2013):414-5.
35. Guizhi W. Risk factors analysis of some endemic areas of cystic echinococcosis in northern Xinjiang. [master‘s thesis]. Xinjiang: Xinjiang Medical University (2009).
36. Junxia Y, Xiaoqin R. Epidemic status and influencing factors of hydatidosis in Pingchuan district in 2009. Chin Community Doctors. (2011) 13:224
37. Yun-Ling F, Xang-lin W, Li L. Investigation on echinococcosis epidemiological of female population in the rural areas of ningxia. Mod Prev Med. (2011) 38:3214-5,8.
38. Xiang-lin W, Min Z, Li L. Hydatid disease risk factor analysis of the human the rural area of Ningxia. China Med Herald. (2010) 84:1763-4.
39. Li Z. An Epidemiology Study on the Cystic Echinococcosis in Xinyuan County. [master's thesis]. [Xinjiang]: Xinjiang Medical University. (2009).
41. Jv Y, Li L, Tianxi L. Case control study of cystic hydatid disease in highrisk areas of Ningxia. In: Proceedings of the Second National Symposium on Zoonoses. Jiangsu Taizhou. (2008). p. 349–53.
42. Yang YR, Sun T, Li Z, Zhang J, Teng J, Liu X, et al. Community surveys and risk factor analysis of human alveolar and cystic echinococcosis in Ningxia Hui Autonomous Region, China. Bull World Health Organ. (2006) 84:714-21. doi: 10.2471/BLT.05.025718
43. Schantz PM, Wang H, Qiu J, Liu FJ, Saito E, Emshoff A, et al. Echinococcosis on the Tibetan Plateau: prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology. (2003) 127:S109-20. doi: 10.1017/S0031182003004165
44. Wang Q, Qiu JM, Schantz P, He JG, Ito A, Liu FJ. Investigation of risk factors for development of human hydatidosis among households raising livestock in tibetan areas of Western Sichuan Province. Chin J Parasitol Parasit Dis. (2001) 19:93-6.
45. Xian-hong W, Duo-long H. An epidemiological investigation on hydatid disease in Gonghe County, Qinghai Province. Endem Dis Bull. (2001) 16:29-31.
46. Craig PS, Giraudoux P, Shi D, Bartholomot B, Barnish G, Delattre P, et al. An epidemiological and ecological study of human alveolar echinococcosis transmission in south Gansu, China. Acta tropica. (2000) 77:167–77. doi: 10.1016/S0001-706X(00)00134-0
47. Wang LY, Qin M, Liu ZH, Wu WP, Xiao N, Zhou XN, et al. Prevalence and spatial distribution characteristics of human echinococcosis in China. PLoS Negl Trop Dis. (2021) 15:e0009996. doi: 10.1371/journal.pntd.0009996
48. ZarrabI AhrabI S, MadanI R, Montazer BavILI M, Babazadeh BedoustanI A. Incidence of cystic echinococcosis in the East Azerbaijan, Iran, during 2011-2017: a retrospective epidemiological study. Clin Exp Health Sci. (2020) 11:158–162. doi: 10.33808/clinexphealthsci.708408
49. Khatonaki H, Mazaherifar S, Shokoohi G, Hatami N, Vafai Z, Javdani F, et al. The epidemiology and medical care costs of Echinococcus granulosusis in Jahrom, southern Iran from 2007 to 2017. Infect Ecol Epidemiol. (2020) 10:1821503. doi: 10.1080/20008686.2020.1821503
50. Ping L, Jinhua L, Yin L, Jingli K, Qi D, Na Z, et al. The epidemic situation and causative analysis of echinococcosis. China Anim Health Inspection. (2016) 33:48-51.
51. Paternoster G, Boo G, Wang C, Minbaeva G, Usubalieva J, Raimkulov KM, et al. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance data. Lancet Glob Health. (2020) 8:e603-11. doi: 10.1016/S2214-109X(20)30038-3
52. Rfjjouob A. Effect of age and sex on immunological activation in relation to infection with Echinococcus granulosus. J University Babylon. (2016)24:1935-46.
53. Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, et al. The echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. (2017) 96:259-369. doi: 10.1016/bs.apar.2016.09.006
54. Torgerson PR, Robertson LJ, Enemark HL, Foehr J, van der Giessen JWB, Kapel CMO, et al. Source attribution of human echinococcosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2020) 14:e0008382. doi: 10.1371/journal.pntd.0008382
55. Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, et al. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. (2017) 11:e0005801. doi: 10.1371/journal.pntd.0005801
56. Possenti A, Manzano-Roman R, Sanchez-Ovejero C, Boufana B, La Torre G, Siles-Lucas M, et al. Potential risk factors associated with human cystic echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. (2016) 10:e0005114. doi: 10.1371/journal.pntd.0005114
57. Gong QL, Ge GY, Wang Q, Tian T, Liu F, Diao NC, et al. Meta-analysis of the prevalence of Echinococcus in dogs in China from 2010 to 2019. PLoS Negl Trop Dis. (2021) 15:e0009268. doi: 10.1371/journal.pntd.0009268
58. Liu CN, Xu YY, Cadavid-Restrepo AM, Lou ZZ, Yan HB, Li L, et al. Estimating the prevalence of Echinococcus in domestic dogs in highly endemic for echinococcosis. Infect Dis Poverty. (2018) 7:77. doi: 10.1186/s40249-018-0458-8
59. Laivacuma S, Deksne G, Jokelainen P, Ivanovs A, Zaharova L, Zeltina I, et al. Risk Factors for human cystic Echinococcosis in Latvia. Vector Borne Zoonotic Dis. (2019) 19:430-3. doi: 10.1089/vbz.2018.2354
60. Bastien M, Vaniscotte A, Combes B, Umhang G, Germain E, Gouley V, et al. High density of fox and cat faeces in kitchen gardens and resulting rodent exposure to Echinococcus multilocularis and Toxoplasma gondii. Folia Parasitol. (2018) 65:2018.002. doi: 10.14411/fp.2018.002
61. Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis. (2007) 13:878-82. doi: 10.3201/eid1306.061074
62. Wu T, Perrings C, Kinzig A, Collins JP, Minteer BA, Daszak P. Economic growth, urbanization, globalization, and the risks of emerging infectious diseases in China: a review. Ambio. (2017) 46:18-29. doi: 10.1007/s13280-016-0809-2
63. Liu F, Li L, Liu Y, Sun C, Liu C, Wu X, et al. Development of reverse genetics system for small ruminant morbillivirus: rescuing recombinant virus to express Echinococcus granulosus EG95 antigen. Virus Res. (2019) 261:50-5. doi: 10.1016/j.virusres.2018.12.008
64. Qian M-B, Zhou XN. Walk together to combat echinococcosis. Lancet Infect Dis. (2018) 18:946. doi: 10.1016/S1473-3099(18)30466-3
Keywords: risk factor, human, meta-analysis, echinococcosis, China
Citation: Zhang T, Li B, Liu Y and Liu S (2022) Risk Factors Associated With Echinococcosis in the General Chinese Population: A Meta-Analysis and Systematic Review. Front. Public Health 10:821265. doi: 10.3389/fpubh.2022.821265
Received: 24 November 2021; Accepted: 19 April 2022;
Published: 17 May 2022.
Edited by:
Francisco Westermeier, FH Joanneum, AustriaReviewed by:
Helga Waap, Instituto Nacional Investigaciao Agraria e Veterinaria (INIAV), PortugalMajid Fasihi Harandi, Kerman University of Medical Sciences, Iran
Copyright © 2022 Zhang, Li, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shou Liu, bGl1c2hvdTIwMDRAYWxpeXVuLmNvbQ==
 Tiantian Zhang
Tiantian Zhang Bin Li
Bin Li Shou Liu
Shou Liu