- 1Department of Pharmacy, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Preventive Medicine, School of Public Health, Zunyi Medical University, Zunyi, China
- 3Department of Chronic Diseases, Center for Diseases Control and Prevention of Zhijin County, Zhijin, China
- 4Department of Epidemiology, School of Medicine, Jinan University, Guangzhou, China
Background: Oxidative stress plays an important role in the pathogenesis of endemic fluorosis. We analyzed associations between oxidative stress-related gene polymorphisms (PON1 rs662, CAT rs769217, rs2300182, and SOD2 rs11968525) and skeletal fluorosis, and examined potential gene–environment interactions with dietary vitamin C, vitamin E, zinc, and selenium intake.
Methods: A cross-sectional study was conducted in the Zhijin County, Guizhou Province of China. Skeletal fluorosis was identified according to the Chinese Diagnostic Criteria of Endemic Skeletal Fluorosis. Dietary information was assessed through face-to-face interviews by trained interviewers using a 75-item food frequency questionnaire. The genotype was detected by high throughput TaqMan-MGB RT-PCR technology. Odds ratios (ORs) and 95% CIs were calculated using an unconditional logistic regression model.
Results: Intake of vitamin E, zinc, and selenium was found to be inversely associated with the risk of skeletal fluorosis. The multivariable-adjusted ORs were 0.438 (95% CI: 0.268 to 0.715, P-trend < 0.001) for vitamin E, 0.490 (95% CI: 0.298 to 0.805, P-trend = 0.001) for zinc, and 0.532 (95% CI: 0.324 to 0.873, P-trend = 0.010) for selenium when comparing the highest with the lowest quartile. The relationship for vitamin C was not observed after adjustment for risk factors. Furthermore, participants with PON1 rs662 AA genotype had a significantly decreased risk of skeletal fluorosis compared with those with the GG genotype (OR = 0.438, 95% CI: 0.231 to 0.830). GG + AG genotype carriers were 2.212 times more likely to have skeletal fluorosis than AA carriers (OR = 2.212, 95% CI: 1.197 to 4.090). Compared with AA carriers, AG carriers had a 2.182 times higher risk of skeletal fluorosis (OR = 2.182, 95% CI: 1.143 to 4.163). Although we observed the risk of skeletal fluorosis was higher with a lower intake of antioxidant nutrients, the potential interactions between nutrient intake and genetic polymorphisms were not observed.
Conclusion: Participants with a higher intake of vitamin E, zinc, and selenium have a lower likelihood of skeletal fluorosis. In addition, the PON1 rs662 polymorphism is related to skeletal fluorosis.
Introduction
Fluorosis has become a public health problem worldwide. Excessive fluoride intake can disrupt the processes of bone formation and resorption, which may lead to bone turnover disorders and further result in skeletal fluorosis (1). More than 16.1 million dental fluorosis patients and 1.8 million patients with skeletal fluorosis have been reported in the Chinese province of Guizhou. Patients with skeletal fluorosis experience persistent pain and bone and joint damage. They are physically limited and cannot perform labor-intensive work and may even become permanently disabled. Skeletal fluorosis decreases the quality of life and the patients become a social and economic burden on society (2, 3). Therefore, it is imperative to prevent and control skeletal fluorosis.
Oxidative stress has been reported to participate in the pathogenesis of endemic fluorosis (4, 5). Oxidative stress was defined as a disturbance in the balance between the production of reactive oxygen species (ROSs) and antioxidant defenses, including enzymatic and non-enzymatic systems. Non-enzymatic systems are composed of dietary vitamin C, vitamin E, zinc, and selenium (6). Some studies have shown that antioxidant nutrients, such as vitamin C and vitamin E, had a protective effect against fluorosis (7–9), while other studies have not found this effect (10). Conflicting results may be due to states of oxidative stress being elevated in part of the participants. It was thought that susceptibility to fluorosis was related to gene polymorphism (11–13). However, these studies did not consider modification by genetics.
Antioxidant enzymes, such as glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), and paraoxonase (PON), are an important barrier to oxidative damage (4, 14, 15). It was reported that single nucleotide polymorphisms (SNPs) of genes encoding the antioxidant enzymes contribute to genetic changes that affect the activity and function of the enzymes (16, 17). PON is a family (PON-1, PON-2, PON-3) of antioxidant enzymes with anti-inflammatory functions. PON-1 RR Q192R (rs662) is a common functional SNP in PON1. The PON-1 rs662 polymorphism was associated with higher enzymatic activity, which may lead to different disease susceptibility (18). Studies reported that CAT and SOD enzyme activities decreased in a fluorosis patient group compared with a control group (19, 20). Studies reported evidence that coal-burning fluorosis was related to decreased SOD activity and gene expression (17, 21). The above studies indicate that oxidative stress is not only affected by exogenous antioxidants, but that gene polymorphism also plays a role. In addition, some studies reported oxidative stress-related gene polymorphisms in PON 1, SOD, and CAT were associated with bone health (14, 22–24). Deng et al. found that the SOD2 gene played a significant role in BMD variation and pathogenesis of osteoporosis and observed the strongest association signals at SNP rs11968525 (25). Thus, oxidative stress-related gene polymorphisms might be associated with skeletal fluorosis.
Although dietary antioxidants were important to assist with decreasing oxidative stress, the circle level of antioxidants and oxidative stress were affected by SNP of PON1, SOD, GPX, etc. (15, 26, 27). Some studies have shown that dietary intake of vitamin C, vitamin E, and selenium were interacted with SNP on disease (27–30). A previous study reported PON1 polymorphisms to modify the association between lycopene and oxidative stress parameters and bone turnover markers, and thus moderated the risk of osteoporosis (22). Therefore, oxidative stress-related gene polymorphisms may modify the association between antioxidant nutrients and skeletal fluorosis. Therefore, we investigated associations between the SNPs PON1 rs662, CAT rs769217, rs2300182, and SOD2 rs11968525 and skeletal fluorosis. We further examined potential gene interactions with dietary vitamin C, vitamin E, zinc, and selenium intake.
Subjects and Methods
Study Subjects
A population-based cross-sectional study was conducted between July and August 2015 in a coal-burning area of Zhijin County in the Guizhou Province, China. A two-stage, clustered random sampling method was used in this study. The three towns of Chadian, Chengguan, and Puweng were randomly selected from 10 towns in Zhijin County. Then, we randomly further selected 4 villages from each selected town. The 12 villages selected for the study were Dazai, Ganhe, Gaofeng, Guihua, Guohua, Hehua, Hualuo, Jiangyan, Moda, Shangzai, Yutang, and Xianfeng. Participants who have lived in Zhijin County for at least 10 years and aged 18–75 years were recruited through village doctors and the Center for Disease Control and Prevention (CDC) from the randomly selected villages (31). Participants were excluded if they had a prior history of cancer, coronary heart disease, stroke, gout, or kidney disease. They were also excluded if their dietary habits had manifestly changed during the previous 5 years, or if they had chronic diseases that might affect their dietary habits, such as gastritis, diabetes, and hypertension. In addition, the participants with incomplete questionnaire information were also excluded. A total of 894 participants were successfully interviewed and 165 subjects with no blood samples were excluded from the study. Finally, only 729 participants were included in this analysis. In addition to the questionnaire, each participant also received a clinical examination. Skeletal fluorosis was identified according to the Chinese Diagnostic Criteria of Endemic Skeletal Fluorosis (WS192-2008, China) (32).
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was proved by the Medical Ethics Committee of Zunyi Medical University (No. 2014-1-003). Written informed consent was obtained from all the participants.
Data Collection Pertaining to Diet and Lifestyle
Interviews were conducted by trained interviewers who administered a structured questionnaire in a personal interview. The content of the questionnaire included: (1) socio-demographic characteristics (age, gender, ethnicity, marital status, and education level); (2) lifestyle habits (smoking, drinking alcohol, drinking tea, sedentary frequency, vitamin supplement consumption, use of improved stoves, domestic fuel type, use of coal to roast grains and chilies, and washing dry grains and chilies before use); (3) dietary habits in the year before the interview; (4) relevant disease history (hypertension, diabetes, gout, kidney disease, cancer, heart-related diseases, and stroke). Smokers were defined as individuals who smoked at least five packs of cigarettes a year. Alcohol drinkers were defined as participants who drank at least once a week for at least 6 months. Those who drank tea at least twice weekly were defined as tea drinkers. Bodyweight and height were measured with the participant in light clothing, without shoes by previously trained field researchers. The BMI (kg/m2) was calculated using weight and height measures.
A 75-item food frequency questionnaire (FFQ) was used to investigate dietary intake in the year before the subjects by trained interviewers during a face-to-face interview. The FFQ included commonly consumed food groups (cereal products, vegetables, fruits, red and processed meat, poultry, fish, shrimp, egg, dairy products, legumes, fungus, algae, nuts, beverages, and soups), and the intake frequency (never, per year, per month, per week, or per day), and the weight of each ingested food. Energy and nutrient intakes were calculated using the Chinese Food Composition Database (33). The validity and reproducibility of the FFQ have been assessed (34).
Real-Time Polymerase Chain Reaction Genotyping
Venous blood samples of 5 ml were collected in an EDTA anticoagulant tube from each participant on the day of investigation, and stored at −70°C until use. Genomic DNA was extracted using a whole blood DNA extraction kit (Tiangen Biotech, Beijing, China) according to the manufacturer's instructions. Genotyping was performed with high throughput TaqMan-MGB RT-PCR technology. The genotyping was performed on a Roche Lightcycler® 480 platform Software Real-time Fluorescence Quantitative PCR Instrument (Roche, Applied Biosystems). The PCR reaction system was a volume of 6 μl: 0.25 μl TaqMan universal PCR mixture, 0.25 μl of SNP genotyping mixture, 4.5 μl of dd H2O, and 1 μl of DNA. PCR amplification conditions were as follows: initial heating at 95°C for 4 min, followed by 40 cycles of 95°C for 7 s and 60°C for 40 s. Two blank controls were set for every 96 well plates. We repeated genotyping at random for 10% of the sample.
Statistical Analysis
The data were coded and doubly entered by two data clerks into Epi-Data version 3.1 to avoid clerical errors using side-by-side comparison, and the data were then exported to SPSS for windows version 18 statistical software. The chi-squared (χ2) test was used to detect whether the genotype distribution satisfied the Hardy–Weinberg equilibrium among the two groups.
Dietary intake of vitamin C, vitamin E, zinc, and selenium showed skewed distribution, which was classified into quartiles based on the total distribution among the two groups, and the lowest quartile was used as the reference category. The association between the risk of skeletal fluorosis and nutrients or genetic polymorphism was examined using odds ratios (ORs) and 95% CI, which were calculated using the unconditional logistic regression model. The potential interactions between nutrient intake and genetic polymorphism in a dominant genetic model were examined in the multivariate unconditional logistic regression model by adding interactive terms. Age, gender, ethnicity, marital status, education level, smoking, alcohol drinking, tea-drinking, improved stove use, fuel type and using coal to roast grains and chilies, washing dry grains and chilies before use, total energy intake, BMI, sedentary frequency, and vitamin supplement consumption were all added to the model. All the tests of significance were two-sided. Statistical significance was determined at the p < 0.05 level.
Results
General Characteristics
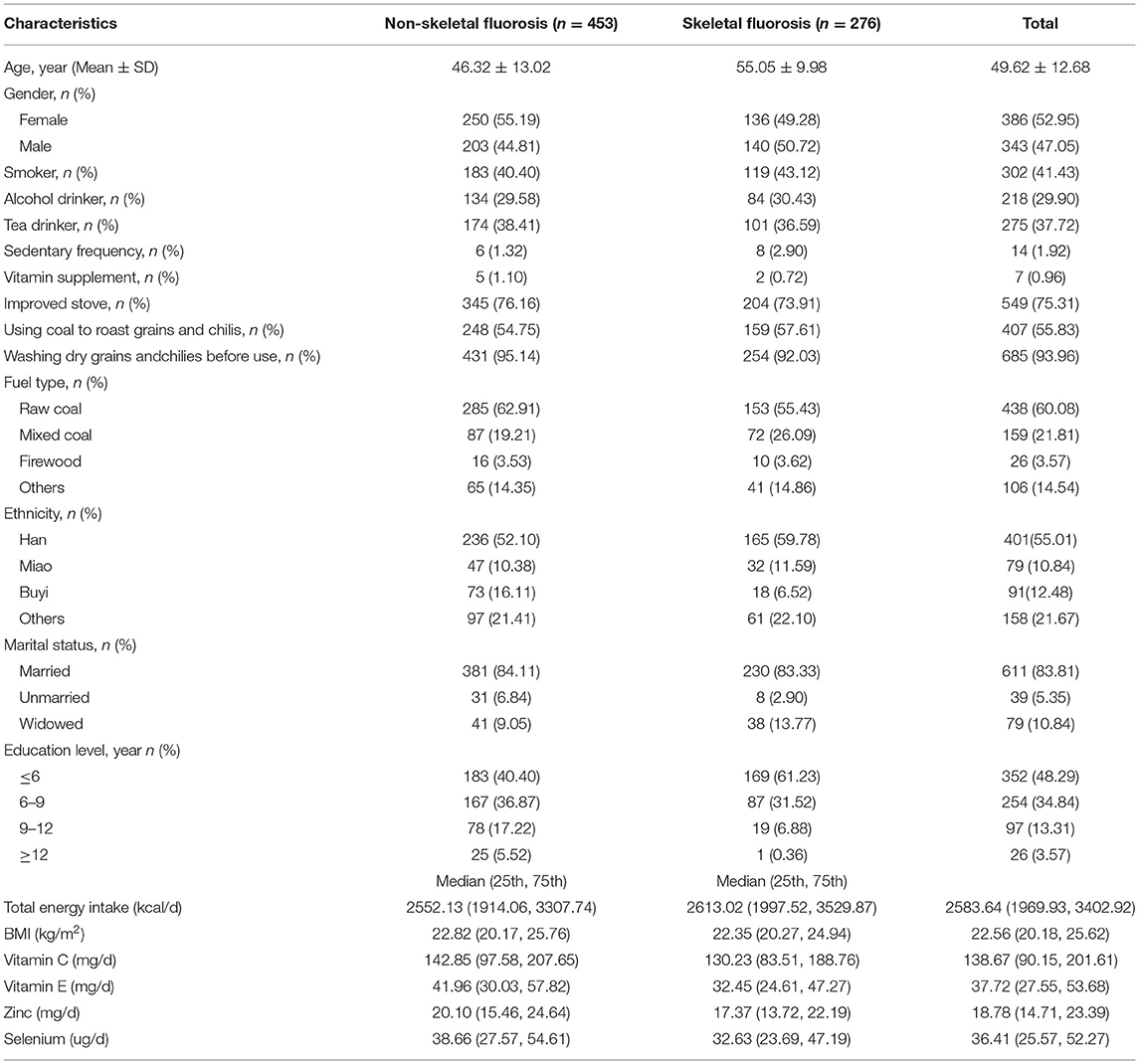
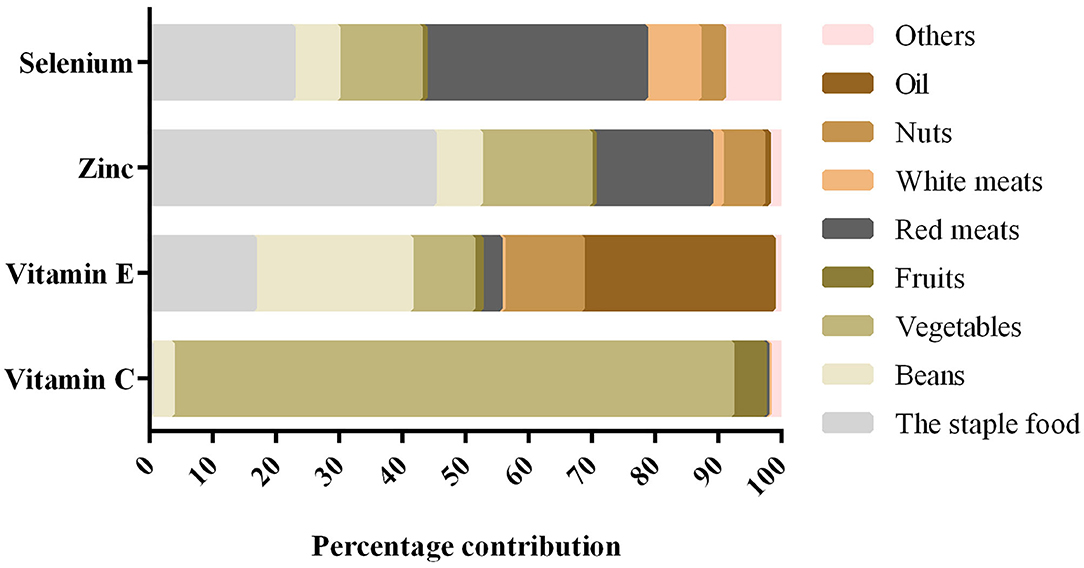
Of the 729 subjects who participated in this study, 37.9% were diagnosed with skeletal fluorosis. Among the 276 patients with skeletal fluorosis included 136 women (49.28%) and 140 men (50.72%), with a mean age of 55.05 ± 9.98 years. Among the 453 patients without skeletal fluorosis included 250 women (55.19%) and 203 men (44.81%), with a mean age of 46.32 ± 13.02 years. The socio-demographic characteristics of the participants are presented in Table 1. The main food sources of antioxidant nutrients are shown in Figure 1. Vegetables and oil were the main sources of vitamin C and vitamin E, respectively. Zinc and selenium were predominantly consumed from the staple food and red meats, respectively.
Association Between Dietary Nutrient Intake and Skeletal Fluorosis
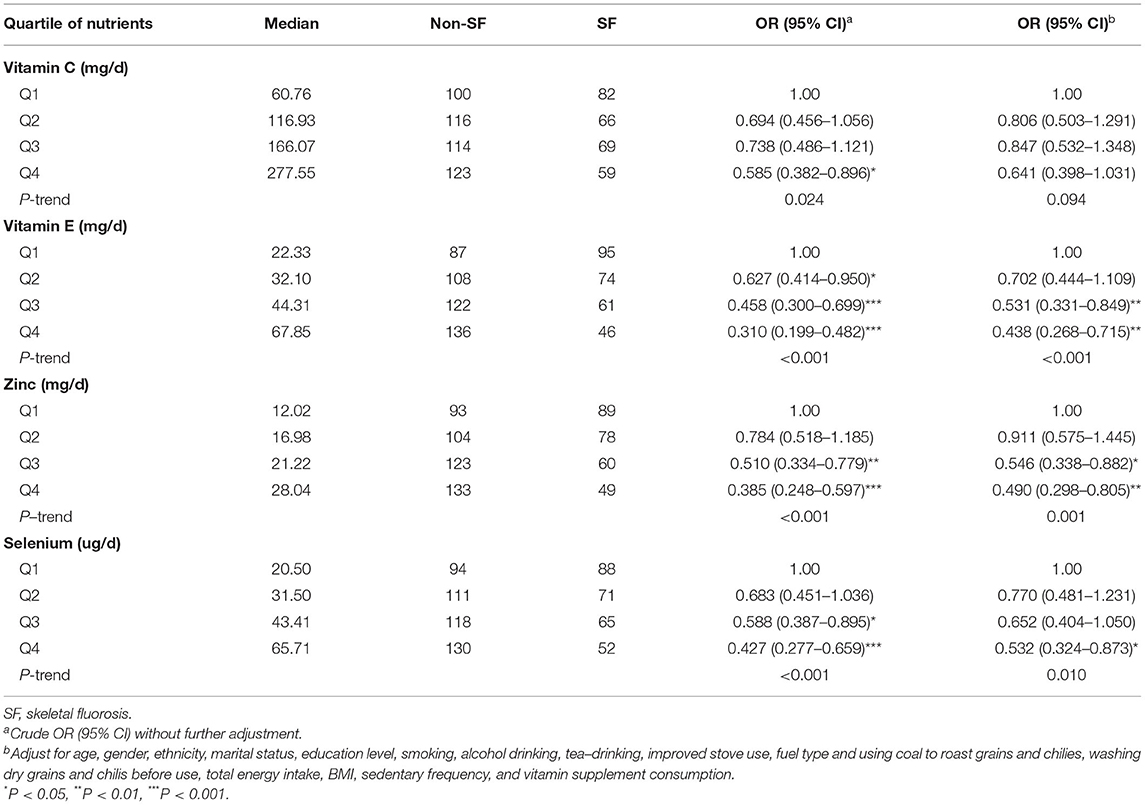
As shown in Table 2, intake of vitamin E, zinc, and selenium was inversely associated with the risk, of skeletal fluorosis. The multivariable-adjusted ORs were 0.438 (95% CI: 0.268 to 0.715, P-trend < 0.001) for vitamin E, 0.490 (95% CI: 0.298 to 0.805, P-trend = 0.001) for zinc, and 0.532 (95% CI: 0.324 to 0.873, P-trend = 0.010) for selenium when comparing the highest with the lowest quartile. Intake of vitamin C was not associated with the risk of skeletal fluorosis after adjusting for potential confounding factors.
Distribution Characteristics of Genotypes
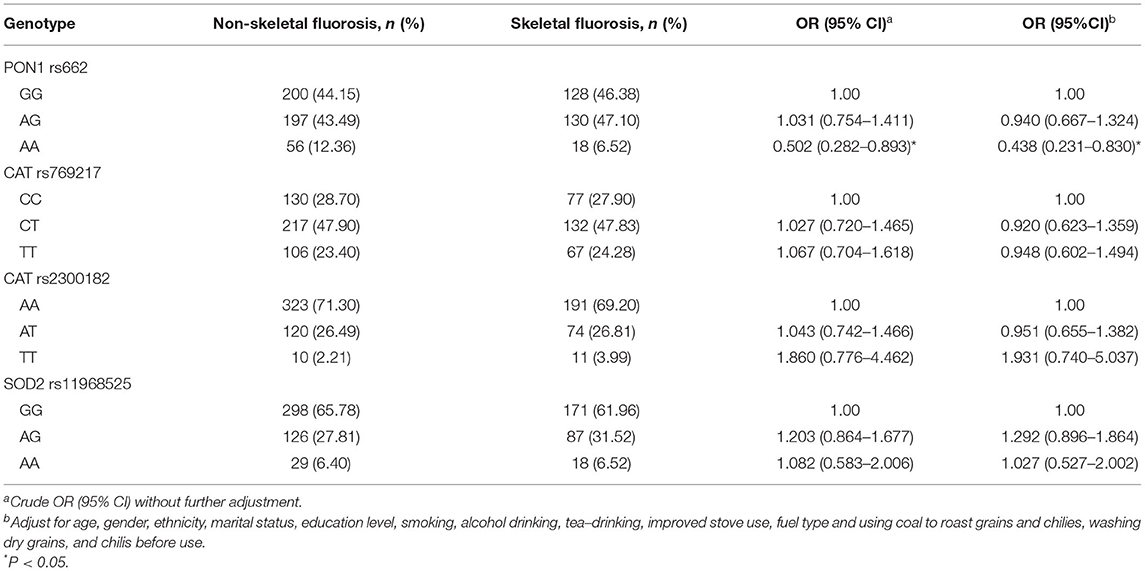
Testing for deviation from Hardy–Weinberg equilibrium was performed using the χ2 test. The genotype distributions of PON1 rs662, CAT rs769217, rs2300182, and SOD2 rs11968525 polymorphisms of both patient groups were found to be in Hardy–Weinberg equilibrium (P>0.05) (Supplementary Table 1). The PON1 rs662 polymorphism was related to the risk of skeletal fluorosis. After adjusting for confounding factors, the relationship remained the same. Participants with the AA genotype had a significantly decreased risk of skeletal fluorosis than those with the GG genotype (OR = 0.438, 95% CI: 0.231 to 0.830). Moreover, CAT rs769217, rs2300182, and SOD2 rs11968525 polymorphisms were not associated with skeletal fluorosis (Table 3).
Association Between Different Genetic Models of Oxidative Stress-Related Genes and Skeletal Fluorosis
The PON1 rs662, CAT rs2300182, rs769217, and SOD2 rs11968525 SNPs were divided into four different genetic models listed in Table 4. Two univariate models (dominant and accumulative) showed that the SNP PON1 rs662 was associated with the risk of skeletal fluorosis, after adjusting for confounding factors. GG+AG genotype carriers were 2.212 times more likely to have skeletal fluorosis than those with AA (OR = 2.212, 95% CI: 1.197 to 4.090). Compared with AA genotype carriers, AG genotype carriers had 2.182 times higher risk of skeletal fluorosis (OR = 2.182, 95% CI: 1.143 to 4.163). However, the four different genetic models did not show a relationship to skeletal fluorosis for SNPs CAT rs2300182, rs769217, and SOD2 rs11968525.
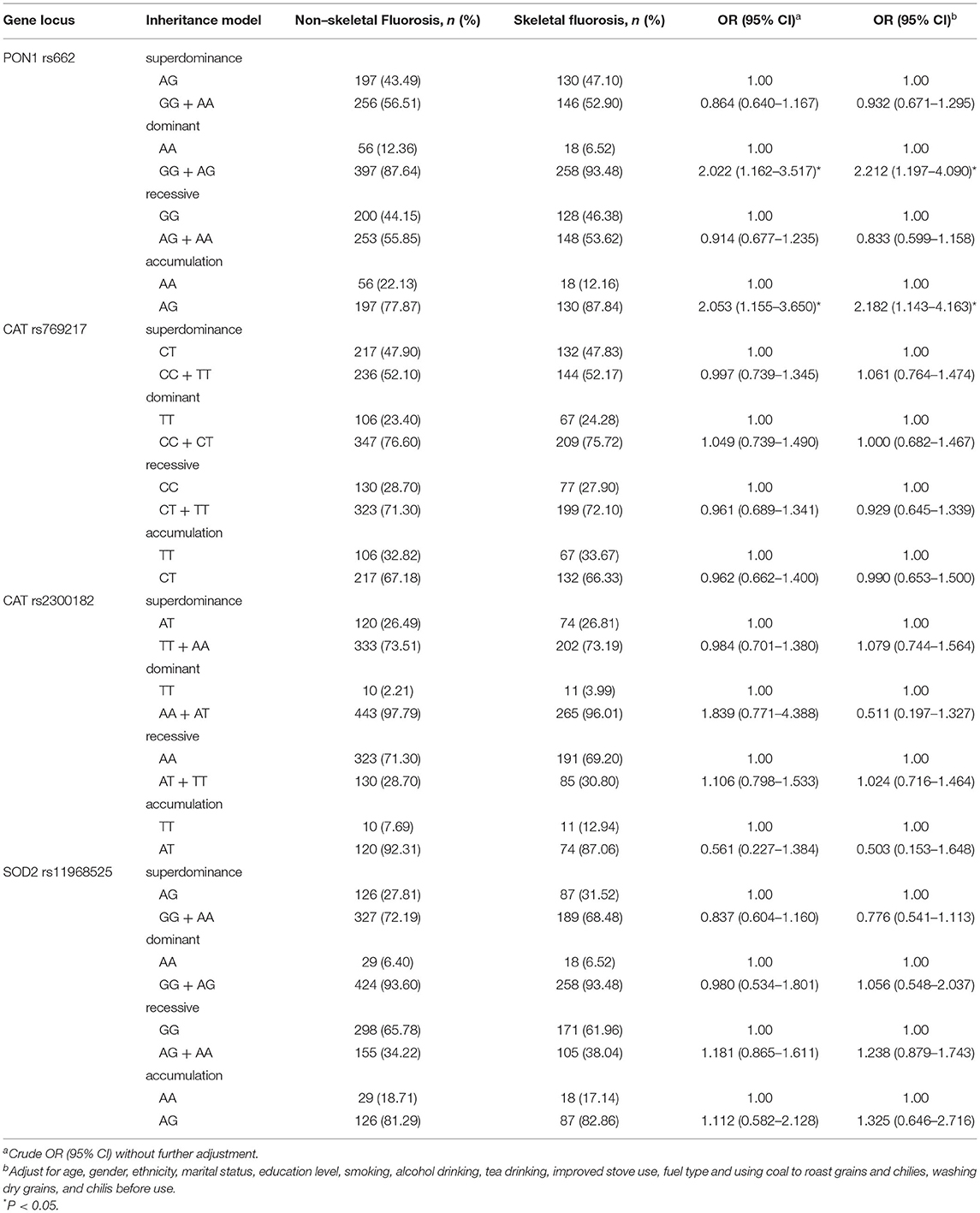
Table 4. Distribution and risk estimation of SNP polymorphisms in non-skeletal fluorosis and skeletal fluorosis.
Interaction Between Oxidative Stress-Related Gene Polymorphisms and Nutrient Intake
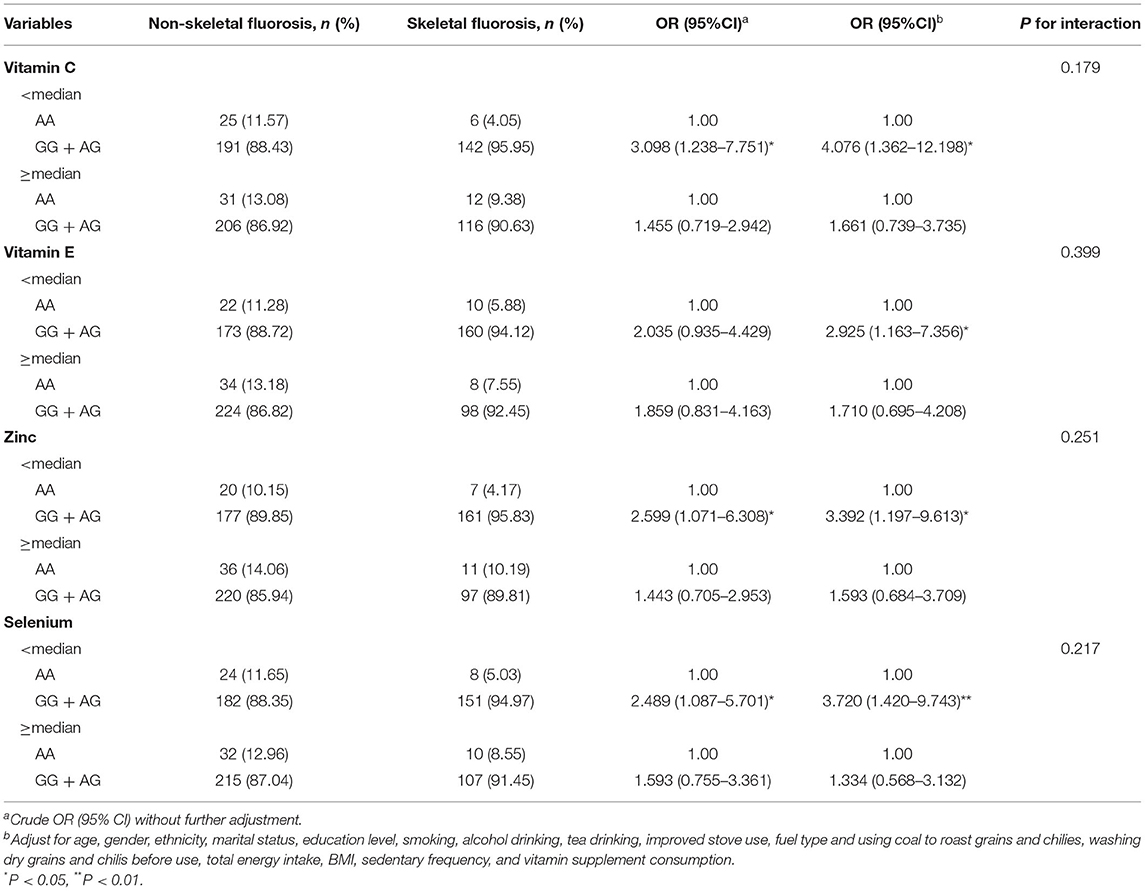
As presented in Table 5, patients with diets below the median nutrient intake level with the SNP PON1 rs662 and GG + AG genotype had a higher risk of skeletal fluorosis, compared with AA genotype carriers. The multivariable-adjusted ORs were 4.076 (95% CI: 1.362 to 12.198) for vitamin C, 2.925 (95% CI: 1.163 to 7.356) for vitamin E, 3.392 (95% CI: 1.197 to 9.613) for zinc, and 3.720 (95% CI: 1.420 to 9.743) for selenium. However, the interaction between nutrients and the PON1 polymorphism has not been associated with the risk of skeletal fluorosis. Moreover, there was no significant interaction effect between nutrient intake and the CAT rs2300182 and SOD2 rs11968525 polymorphisms, related to the risk of skeletal fluorosis (Supplementary Tables 2–4).
Discussion
This study showed that the intake of vitamin E, zinc, and selenium in the patient diet was inversely associated with the occurrence of skeletal fluorosis. It also showed that the PON1 rs662 SNP was related to the occurrence of skeletal fluorosis.
Oxidative stress is the main contributing mechanism to coal-burning fluorosis, and antioxidant nutrients play an important role in coal-burning fluorosis (35). In this study, we found that a higher intake of antioxidant nutrients is associated with lower occurrence of skeletal fluorosis. A previous study found that vitamin E had a positive protective effect on experimental chronic fluorosis in rats (7). Another animal study also reported that vitamin E could significantly reduce the damage to reproductive function in male rabbits exposed to fluoride (36). Liu et al. (37) reported that vitamin C, vitamin E, folic acid, and other antioxidants can enhance the body's antioxidant capacity, and thus, prevent the occurrence of skeletal fluorosis. An epidemiological study showed that a diet rich in antioxidant nutrients could reduce the risk of fluorosis (38). Vitamin E is an important antioxidant in the body and is well-known for its antioxidant activity and free radical scavenging function, which works by scavenging free radicals, active oxygen, and inhibiting lipid peroxidation (39). Thus, vitamin E is believed to exert its protective effect mainly by destroying the oxygen-free radicals produced by fluoride that destroy cells (36). It has also been found to protect hard tissue from fluoride damage by preventing excessive accumulation of fluoride in bones and teeth (40). Vitamin E has been shown to prevent fluorosis-induced spermatogenic cell apoptosis by inhibiting oxidative stress-mediated JNK and ERK signaling pathways (39). The aforementioned studies may partially explain the protective effects of vitamin E against skeletal fluorosis.
We also found that the dietary intake of zinc and selenium was inversely associated with skeletal fluorosis. Zinc is an essential trace element for the human body and a component of certain enzymes and proteins. In agreement with our findings, an Indian study showed that compared with the control group, the serum zinc level in the fluorosis group was significantly lower (41). Moreover, Ersoy et al. (42) conducted a case-control study of 30 patients with fluorosis, and 30 healthy controls showed that chronic fluorosis was related to lowered serum zinc levels. They considered that the significant decrease of serum zinc in the fluorosis patient group may be related to the increased excretion of zinc in urine, the decrease of plasma proteins, such as albumin, and the inability of albumin to transport zinc in its binding form. Similarly, selenium is a typical trace element that is beneficial to human and animal health. An animal experiment showed that a certain concentration of selenium reduced toxicity caused by fluorine (43). Epidemiological studies also showed that, compared with healthy children in low-risk fluorosis areas, the levels of calcium and zinc in the blood of children with chronic fluorosis were 5 times lower and the levels of selenium were 2 times lower (9). Selenium could have an antioxidant function by scavenging free radicals and repairing membrane damage, and it reduced apoptosis induced by fluoride (44, 45). In addition, selenium alleviated NaF-induced apoptosis by increasing the expression of HSP70 and alleviated oxidative stress by regulating the levels of SIRT1 and antioxidant enzymes (46). Miao et al. (47) observed that a certain concentration of selenium could enhance the activity of SOD and GPX antioxidant enzymes, reduce the toxic effect of fluoride, improve liver function, and inhibit apoptosis to a certain extent. These results suggested that fluorosis was not only related to antioxidant nutrients but could also involve other causes.
PON1 is located on chromosome 7q21.3-q22.1 and consists of 354 amino acids. PON1 can hydrolyze a variety of substrates, such as organophosphorus, aromatic esters, lactones, low-density lipoprotein (LDL), and cholesterol, to prevent oxidative damage and lipid peroxidation (48). PON1 is considered to be involved in the development of oxidative stress-related diseases, such as osteoporosis, atherosclerosis, coronary heart disease, diabetes, and metabolic syndrome (49–52). In this study, we first reported that PON1 rs662 polymorphism was associated with the risk of skeletal fluorosis. The carriers of the AA genotype had a lower risk of skeletal fluorosis than carriers of GG, GG + AG, and AG genotypes. Some studies explored the relationship between the PON1 rs662 polymorphism and bone health. In agreement with our findings, a case-control study from China reported that the GG genotype and the G allele of the rs662 polymorphism were closely related to the increased risk of ankylosing spondylitis (53). However, another case-control study found that the carriers of the AA genotype of rs662 in PON1 were more susceptible to osteonecrosis of the femoral head than GG genotype carriers (OR = 2.53, 95% CI: 1.05 to 6.07) (48). In addition, there were studies which had confirmed that the PON1 gene polymorphism was associated with osteoporosis (23, 54). A reasonable explanation for the inconsistency of these studies may be because of the different distribution of PON1 polymorphisms in different diseases, which leads to different disease susceptibility. More and more evidence indicated that there was a biochemical relationship between lipid oxidation and skeletal biology. Increased lipid oxidation causes oxidative stress and reduces Wnt signaling, thereby reducing osteoblast differentiation and survival, and inducing osteoclast differentiation through the cAMP-mediated pathway (55). Mackinnon et al. (22) found that the PON1 gene polymorphism was associated with multiple markers of bone conversion, and PON1 could catalyze the decomposition of peroxides, reduce the accumulation of lipid peroxidation products, and reduce the effect of oxidative stress on bone formation. Although the PON1 polymorphism can affect bone metabolism by participating in lipid oxidation and reducing oxidative stress, the exact mechanism needs to be confirmed by further studies.
In this study, we also analyzed the interactions between nutrient intake and gene polymorphisms. A previous study showed that PON1 polymorphisms modified the association between serum concentrations of lycopene and oxidative stress parameters and bone turnover markers and therefore, decreased the risk of osteoporosis (22). A population-based study showed that high-selenium levels interacted with potential genes associated with oxidative stress (56). Moreover, two studies showed a statistically significant interaction between SOD2 with dietary intake of vitamin E and selenium affecting the risk of cancer (30, 57). Although PON1 rs662 GG + AG carriers had a higher risk of skeletal fluorosis than the AA genotype at low-antioxidant nutrient levels, none of PON1 rs662, CAT rs2300182, and SOD2 rs11968525 interacted with antioxidant nutrients on skeletal fluorosis. No interaction was observed which may be attributed to the small sample.
This study has some limitations. At first, the study was a cross-sectional study design, the causal relationship could not be determined, although we minimized possible reverse causation by excluding participants with essential changes in dietary habits over the past 5 years. Second, the oxidative stress induced by fluoride was affected by the activity of antioxidant enzymes, and polymorphisms of antioxidant enzyme genes affected enzyme activities. Although we collected blood for genotype identification, we did not measure the activity of antioxidant enzymes in the blood. Third, skeletal fluorosis has a close relationship with lifestyle (37). We only collected sedentary frequency without detailed physical activity level, so only sedentary frequency was adjusted to control for the effect of activity level. Finally, for interaction analysis, the sample size of this study was relatively small and antioxidant nutrients were stratified by median rather than quartiles, which may have obscured statistical differences. Larger sample studies are needed to demonstrate the interactions.
Conclusion
In summary, this study showed that the participants with a higher intake of vitamin E, zinc, and selenium had a lower likelihood of skeletal fluorosis. In addition, the PON1 rs662 polymorphism is related to the occurrence of skeletal fluorosis. Larger studies are needed to determine whether there is an interaction between PON1 rs662 polymorphism and antioxidant nutrients.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Zunyi Medical University. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JL and FZ designed the study and conducted the study. NT was responsible for data management, analysis, and revised critically for important intellectual content, and completed supplement analysis of data. LL was responsible for writing the first draft of the manuscript and revised it. JL, FZ, and QC critically revised the manuscript. XZ, QY, DC, and ZS carried out the study. All authors participated in data interpretation, review, and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81460497 and 82060595) and Guizhou Province Foundation for postgraduate Scientific Research Fund Project YJSKYJJ [2021]191. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors want to thank the study subjects for their willingness to participate and the students who participated in the recruitment of subjects and the interviews in this study. We also thank the Center of Disease Control and Prevention of the Zhijin County for providing administrative support to our study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.849173/full#supplementary-material
References
1. Yang C, Wang Y, Xu H. Treatment and Prevention of Skeletal Fluorosis. Biomed Environ Sci. (2017) 30:147–9. doi: 10.3967/bes2017.020
2. Liu J, Yang S, Luo MJ, Zhao X, Zhang YM, Luo Y. Association of dietary carotenoids intake with skeletal fluorosis in the coal-burning fluorosis area of guizhou province. Biomed Environ Sci. (2018) 31:438–47. doi: 10.3967/bes2018.057
3. Ma Y, Yao Y, Zhong N, Angwa LM, Pei J. The dose-time effects of fluoride on the expression and DNA methylation level of the promoter region of bMP-2 and bMP-7 in rats. Environ Toxicol Pharmacol. (2020) 75:103331. doi: 10.1016/j.etap.2020.103331
4. Srivastava S, Flora SJS. Fluoride in drinking water and skeletal fluorosis: a review of the global impact. Curr Environ Health Rep. (2020) 7:140–6. doi: 10.1007/s40572-020-00270-9
5. Li W, Jiang B, Cao X, Xie Y, Huang T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated caspase pathways. Chem Biol Interact. (2017) 261:27–34. doi: 10.1016/j.cbi.2016.11.021
6. Geybels MS, van den Brandt PA, van Schooten FJ, Verhage BA. Oxidative stress-related genetic variants, pro- and antioxidant intake and status, and advanced prostate cancer risk. Cancer Epidemiol Biomarkers Prev. (2015) 24:178–86. doi: 10.1158/1055-9965.EPI-14-0968
7. Oner AC Dede S, Yur F, Oner A. The effect of vitamin C and vitamin E on DNA damage, oxidative status, and some biochemical parameters in rats with experimental fluorosis. Fluoride. (2020) 53:154–63.
8. Wang YX, Xiao X, Zhan XA. Antagonistic effects of different selenium sources on growth inhibition, oxidative damage, and apoptosis induced by fluorine in broilers. Poult Sci. (2018) 97:3207–17. doi: 10.3382/ps/pey192
9. Tkachenko H, Kurhaluk N, Skaletska N, Maksin V, Osadowski Z. Elemental status and lipid peroxidation in the blood of children with endemic fluorosis. Biol Trace Elem Res. (2021) 199:1237–45. doi: 10.1007/s12011-020-02243-3
10. Aydin N, Dede S, Tanritanir P. The distribution of minerals in some tissues of sheep with fluorosis. Fluoride. (2014) 47:43−8.
11. Yang D, Liu Y, Chu Y, Yang Q, Jiang W, Chen F, et al. Association between vitamin d receptor gene fokI polymorphism and skeletal fluorosis of the brick-tea type fluorosis: a cross sectional, case control study. BMJ Open. (2016) 6:e011980. doi: 10.1136/bmjopen-2016-011980
12. Li BY, Yang YM, Liu Y, Sun J, Ye Y, Liu XN, et al. Prolactin rs1341239 t allele may have protective role against the brick tea type skeletal fluorosis. PLoS ONE. (2017) 12:e0171011. doi: 10.1371/journal.pone.0171011
13. Rahila C, Narayanan MBA, Kumar SGR. Association of cOL1A2 (PvuII) gene polymorphism with risk and severity of dental fluorosis – a case control study. Saudi Dental Journal. (2019) 31:463–8. doi: 10.1016/j.sdentj.2019.05.004
14. Oh B, Kim SY, Kim DJ, Lee JY, Lee JK, Kimm K, et al. Associations of catalase gene polymorphisms with bone mineral density and bone turnover markers in postmenopausal women. J Med Genet. (2007) 44:e62. doi: 10.1136/jmg.2006.042259
15. Min J, Park H, Park B, Kim YJ, Park J, Lee H, et al. Paraoxonase gene polymorphism and vitamin levels during pregnancy: relationship with maternal oxidative stress and neonatal birthweights. Reprod Toxicol. (2006) 22:418–24. doi: 10.1016/j.reprotox.2006.01.003
16. Abd El Azeem RA, Zedan MM, Saad EA, Mutawi TM, Attia ZR. Single-nucleotide polymorphisms (SNPs) of antioxidant enzymes sOD2 and gSTP1 genes and sLE risk and severity in an egyptian pediatric population. Clin Biochem. (2021) 88:37–42. doi: 10.1016/j.clinbiochem.2020.11.010
17. Wang Q, Cui KP, Xu YY, Gao YL, Zhao J, Li DS, et al. Coal-burning endemic fluorosis is associated with reduced activity in antioxidative enzymes and cu/Zn-SOD gene expression. Environ Geochem Health. (2014) 36:107–15. doi: 10.1007/s10653-013-9522-2
18. Reichert CO, de Macedo CG, Levy D, Sini BC, Monteiro AM, Gidlund M, et al. Paraoxonases (PON) 1, 2, and 3 polymorphisms and pON-1 activities in patients with sickle cell disease. Antioxidants (Basel). (2019) 8:252. doi: 10.3390/antiox8080252
19. Zheng B, Shi C, Muhammed FK, He J, Abdullah AO, Liu Y. Gastrodin alleviates bone damage by modulating protein expression and tissue redox state. FEBS Open Bio. (2020) 10:2404–16. doi: 10.1002/2211-5463.12991
20. Ozbey U, Deger Y, Yur F, Cambay Z, Ozbey G. Investigation of blood antioxidant enzyme levels and glutathione peroxidase, catalase, and superoxide dismutase gene polymorphism in sheep with fluorosis. Fluoride. (2017) 50:374–82.
21. Li HL Yu YN, Chen Y, Huang L. Effect of fluoride on oxidative stress and mn-SOD expression in rats with endemic fluorosis of coal burning. Zhonghua Bing Li Xue Za Zhi. (2012) 41:627–30. doi: 10.3760/cma.j.issn.0529-5807.2012.09.012
22. Mackinnon ES, El-Sohemy A, Rao AV, Rao LG. Paraoxonase 1 polymorphisms 172T → A and 584A → G modify the association between serum concentrations of the antioxidant lycopene and bone turnover markers and oxidative stress parameters in women 25-70 years of age. J Nutrigenet Nutrigenomics. (2010) 3:1–8. doi: 10.1159/000316636
23. Toptaş B, Kurt Ö, Aydogan HY, Yaylim I, Zeybek Ü, Can A, et al. Investigation of the common paraoxonase 1 variants with paraoxonase activity on bone fragility in turkish patients. Mol Biol Rep. (2013) 40:6519–24. doi: 10.1007/s11033-013-2770-5
24. Mlakar SJ, Osredkar J, Prezelj J, Marc J. Antioxidant enzymes gSR, sOD1, sOD2, and cAT gene variants and bone mineral density values in postmenopausal women: a genetic association analysis. Menopause. (2012) 19:368–76. doi: 10.1097/gme.0b013e31822d5b10
25. Deng FY, Lei SF, Chen XD, Tan LJ, Zhu XZ, Deng HW. An integrative study ascertained sOD2 as a susceptibility gene for osteoporosis in chinese. J Bone Miner Res. (2011) 26:2695–701. doi: 10.1002/jbmr.471
26. Hernandez-Guerrero C, Parra-Carriedo A. Ruiz-de-Santiago D, Galicia-Castillo O, Buenrostro-Jauregui M, Diaz-Gutierrez C. Genetic polymorphisms of antioxidant enzymes cAT and sOD affect the outcome of clinical, biochemical, and anthropometric variables in people with obesity under a dietary intervention. Genes Nutr. (2018) 13:1. doi: 10.1186/s12263-017-0590-2
27. Zanon-Moreno V, Asensio-Marquez EM, Ciancotti-Oliver L, Garcia-Medina JJ, Sanz P, Ortega-Azorin C, et al. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol Vis. (2013) 19:231–42.
28. Park CY, Jung S, Kim MK, Choi BY, Shin MH, Shin DH, et al. Habitual dietary intake of beta-carotene, vitamin c, folate, or vitamin e may interact with single nucleotide polymorphisms on brachial-ankle pulse wave velocity in healthy adults. Eur J Nutr. (2016) 55:855–66. doi: 10.1007/s00394-015-0896-z
29. Sandsveden M, Bengtsson Y, Melander O, Rosendahl AH, Manjer J. Genetic variation interacts with selenium exposure regarding breast cancer risk: assessing dietary intake, serum levels and genetically elevated selenium levels. Nutrients. (2022) 14:826. doi: 10.3390/nu14040826
30. Tang H, Dong X, Day RS, Hassan MM Li D. Antioxidant genes, diabetes and dietary antioxidants in association with risk of pancreatic cancer. Carcinogenesis. (2010) 31:607–13. doi: 10.1093/carcin/bgp310
31. Chen T, Tao N, Yang S, Cao D, Zhao X, Wang D, et al. Association between dietary intake of one-Carbon metabolism-Related nutrients and fluorosis in guizhou, china. Front Nutr. (2021) 8:700726. doi: 10.3389/fnut.2021.700726
32. Ministry of Health of the People's Republic of China. Industry Standard of the People's Republic of China Diagnosis for Endemic Skeletal Fluorosis (WS/T 192-2008). Beijin: People's Medical Publishing House (2008).
34. Zhang CX, Ho S. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac J Clin Nutr. (2009) 18:240–50. doi: 10.6133/apjcn.2009.18.2.13
35. Liu J, Yang S, Luo MJ, Chen T, Ma XJ, Tao N, et al. Association between dietary patterns and fluorosis in guizhou, china. Front Nutr. (2019) 6:189. doi: 10.3389/fnut.2019.00189
36. Kumar N, Sood S, Arora B, Singh M, Beena Roy PS. To study the effect of vitamin d and e on sodium-Fluoride-induced toxicity in reproductive functions of male rabbits. Toxicol Int. (2012) 19:182–7. doi: 10.4103/0971-6580.97220
37. Liu G, Ye Q, Chen W, Zhao Z, Li L, Lin P. Study of the relationship between the lifestyle of residents residing in fluorosis endemic areas and adult skeletal fluorosis. Environ Toxicol Pharmacol. (2015) 40:326–32. doi: 10.1016/j.etap.2015.06.022
38. Keshavarz S, Ebrahimi A, Nikaeen M. Fluoride exposure and its health risk assessment in drinking water and staple food in the population of dayyer, iran, in 2013. J Educ Health Promot. (2015) 4:72. doi: 10.4103/2277-9531.171785
39. Tian Y, Xiao Y, Wang B, Sun C, Tang K, Sun F. Vitamin e and lycopene reduce coal burning fluorosis-induced spermatogenic cell apoptosis via oxidative stress-mediated jNK and eRK signaling pathways. Biosci Rep. (2018) 38:BSR20171003. doi: 10.1042/BSR20171003
40. Blaszczyk I, Birkner E, Gutowska I, Romuk E, Chlubek D. Influence of methionine and vitamin e on fluoride concentration in bones and teeth of rats exposed to sodium fluoride in drinking water. Biol Trace Elem Res. (2012) 146:335–9. doi: 10.1007/s12011-011-9251-2
41. Khandare AL, Validandi V, Boiroju N. Fluoride alters serum elemental (Calcium, magnesium, copper, and zinc) homeostasis along with erythrocyte carbonic anhydrase activity in fluorosis endemic villages and restores on supply of safe drinking water in school-going children of nalgonda district, india. Biol Trace Elem Res. (2018) 185:289–94. doi: 10.1007/s12011-018-1271-8
42. Ersoy IH, Koroglu BK, Varol S, Ersoy S, Varol E, Aylak F, et al. Serum copper, zinc, and magnesium levels in patients with chronic fluorosis. Biol Trace Elem Res. (2011) 143:619–24. doi: 10.1007/s12011-010-8892-x
43. Feng P, Wei JR, Zhang ZG. Influence of selenium and fluoride on blood antioxidant capacity of rats. Exp Toxicol Pathol. (2012) 64:565–8. doi: 10.1016/j.etp.2010.11.014
44. Feng P, Wei J, Zhang Z. Intervention of selenium on chronic fluorosis-induced injury of blood antioxidant capacity in rats. Biol Trace Elem Res. (2011) 144:1024–31. doi: 10.1007/s12011-011-9087-9
45. Gao JP, Tian XL, Yan XR, Wang Y, Wei JN, Wang XT, et al. Selenium exerts protective effects against fluoride-Induced apoptosis and oxidative stress and altered the expression of bcl-2/caspase family. Biol Trace Elem Res. (2021) 199:682–92. doi: 10.1007/s12011-020-02185-w
46. Li W, Dong S, Chen Q, Chen C, Dong Z. Selenium may suppress peripheral blood mononuclear cell apoptosis by modulating hSP70 and regulate levels of sIRT1 through reproductive hormone secretion and oxidant stress in women suffering fluorosis. Eur J Pharmacol. (2020) 878:173098. doi: 10.1016/j.ejphar.2020.173098
47. Miao K, Zhang L, Yang S, Qian W, Zhang Z. Intervention of selenium on apoptosis and fas/FasL expressions in the liver of fluoride-exposed rats. Environ Toxicol Pharmacol. (2013) 36:913–20. doi: 10.1016/j.etap.2013.08.003
48. Li JM Li Y, Wang L. The genetic association between pON1 polymorphisms and osteonecrosis of femoral head: a case-control study. Medicine (Baltimore). (2017) 96:e8198. doi: 10.1097/MD.0000000000008198
49. Zhao FL, Guo LJ, Wang XF, Zhang YK. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. (2021) 16:4. doi: 10.1007/s11657-020-00854-w
50. Racis M, Stanisławska-Sachadyn A, Sobiczewski W, Wirtwein M, Krzemiński M, Krawczyńska N, et al. Association of genes related to oxidative stress with the extent of coronary atherosclerosis. Life (Basel). (2020) 10:210. doi: 10.3390/life10090210
51. Iwanicka J, Iwanicki T, Niemiec P, Nowak T, Krauze J, Grzeszczak W, et al. Relationship between rs854560 pON1 gene polymorphism and tobacco smoking with coronary artery disease. Dis Markers. (2017) 2017:1540949. doi: 10.1155/2017/1540949
52. Luo JQ, Ren H, Liu MZ, Fang PF, Xiang DX. European versus asian differences for the associations between paraoxonase-1 genetic polymorphisms and susceptibility to type 2 diabetes mellitus. J Cell Mol Med. (2018) 22:1720–32. doi: 10.1111/jcmm.13453
53. Xu H, Qu Y. Correlation of pON1 polymorphisms with ankylosing spondylitis susceptibility: a case-control study in chinese han population. Medicine (Baltimore). (2017) 96:e7416. doi: 10.1097/MD.0000000000007416
54. Kim BJ, Kim SY, Cho YS, Kim BJ, Han BG, Park EK, et al. Association of paraoxonase 1 (PON1) polymorphisms with osteoporotic fracture risk in postmenopausal korean women. Exp Mol Med. (2011) 43:71–81. doi: 10.3858/emm.2011.43.2.009
55. Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic wnt signaling in the skeleton. J Biol Chem. (2009) 284:27438–48. doi: 10.1074/jbc.M109.023572
56. Galan-Chilet I, Tellez-Plaza M, Guallar E, De Marco G, Lopez-Izquierdo R, Gonzalez-Manzano I, et al. Plasma selenium levels and oxidative stress biomarkers: a gene-environment interaction population-based study. Free Radic Biol Med. (2014) 74:229–36. doi: 10.1016/j.freeradbiomed.2014.07.005
Keywords: skeletal fluorosis, antioxidant nutrients, oxidative stress, gene polymorphism, cross-sectional study
Citation: Tao N, Li L, Chen Q, Sun Z, Yang Q, Cao D, Zhao X, Zeng F and Liu J (2022) Association Between Antioxidant Nutrients, Oxidative Stress-Related Gene Polymorphism and Skeletal Fluorosis in Guizhou, China. Front. Public Health 10:849173. doi: 10.3389/fpubh.2022.849173
Received: 05 January 2022; Accepted: 13 April 2022;
Published: 13 May 2022.
Edited by:
Cain Craig Truman Clark, Coventry University, United KingdomReviewed by:
Conor MacDonald, INSERM U1018 Centre de Recherche en Épidémiologie et Santé des Populations (CESP), FranceRoch A. Nianogo, University of California, Los Angeles, United States
Copyright © 2022 Tao, Li, Chen, Sun, Yang, Cao, Zhao, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, bGl1anVuX3ptY0BzaW5hLmNvbQ==; Fangfang Zeng, emVuZ2Zmam51QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Na Tao1†
Na Tao1† Fangfang Zeng
Fangfang Zeng Jun Liu
Jun Liu