- 1Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Teachers College, Columbia University, New York, NY, United States
Objective: To explore the correlated clinical and psychological factors of stigmatization and investigate the relationship between stigma and white matter abnormalities in epilepsy patients.
Methods: Stigmatization was obtained by a three-item stigma scale in 256 epilepsy patients with genetic or unknown etiology. Personality and quality of life (QOL) were assessed by Eysenck Personality Questionnaire (EPQ) and QOL-31 questionnaire respectively. One hundred and fourteen of them were performed Hamilton Depression Scale-17 (HAMD) and scanned with diffusion tensor imaging in 3T MRI. Fractional anisotropy (FA) values of frontotemporal contact fibers were calculated.
Results: There were about 39.8% patients felt stigma, with the highest score (Score 3) in 8.2% (21/256). Stigma scores were significantly negatively correlated with education (P < 0.01), age of onset (P < 0.05), extraversion score of EPQ (P < 0.01), total and all the subscale QOL scores (P < 0.001), and positively correlated with duration (P < 0.01), HAMD score (P < 0.001), neuroticism score of EPQ (P < 0.001). We found negative correlation between stigma scores and FA values of right superior longitudinal fasciculus and left cingulum (P < 0.05). Logistic regression results showed that FA value of left cingulum (P = 0.011; OR = 0.000), social function (P = 0.000; OR = 0.935) of QOL, and neuroticism score of EPQ (P = 0.033; OR = 1.123) independently correlated to felt stigma.
Conclusion: Felt stigma in epilepsy patients was found to be correlated with neuroticism, depression, and deficient social function of QOL, which might be predisposed by the impairment of the left cingulum. Our results provide preliminary evidence for the underlying neural circuits in stigmatization.
Introduction
Epilepsy is a common neurological disease characterized by epileptic seizures. About 1% of people worldwide (50 million) have epilepsy, and nearly 80% of cases occur in developing countries (1). Epilepsy can have adverse effects on social and psychological wellbeing. These effects may include social isolation, stigmatization, or disability. Stigmatization is a major contributor to the burden associated with the condition. It can also affect the families of those with the disorder (1, 2).
In many parts of the world, people with epilepsy and their families suffer from stigma. Goffman first explored the notion of epilepsy as a stigmatized illness in the early 1960's (3). It was defined as “a distinguishing mark (especially) of disgrace” or as “an attribute that is deeply discrediting.” Jacoby further explored the concept and distinguished “enacted stigma” from “felt stigma” (4). “Enacted stigma” refers to episodes of discrimination against people with epilepsy, solely on the grounds of their social unacceptability; whereas “felt stigma” refers to the shame associated with being epileptic and the fear of enacted stigma (4). In the studies of prevalent epilepsy, up to 50% of individuals reported experiencing epilepsy-related stigma (5, 6). In China, about 9 million people with epilepsy, and that 63% are untreated or not properly treated (7). More than 40% of people with epilepsy did not tell others about their disease, and 36% thought that people treated them differently because of their epilepsy (8). Concealment of epilepsy, concerns related to social life, and concerns related to future occupation were found as the predictors of felt stigma (6), which refers to the expected discrimination and shameful feelings that stop people from seeking help or sharing their experiences with others (9).
Stigma can affect people economically, socially, and culturally, including the education for children and adolescents, and work opportunities for adults (1, 10, 11). Felt stigma can reduce the quality of life even when seizures are well controlled (12, 13). In a study of 250 patients with epilepsy, 20% were unemployed (14). In Asia, epilepsy is a common negative reason for marriages and pregnancy (1, 10). Reducing stigma—helping patients “out of the shadows” is currently a major focus of activity of epilepsy support groups globally (15, 16).
Studies reported the public or patients' attitudes toward epilepsy (17). Only certain aspects of knowledge have an impact on the underlying cause of stigma in epilepsy. Previous studies demonstrated that feelings of stigma were associated with seizure severity (18), patient's age and seizure classification (mesial temporal lobe epilepsy) (19, 20). Felt stigma was also found to correlate with psychosocial aspects of patients' lives, including culture (15), education gap (21), lack knowledge of their diseases (22), lower household wealth (18), married status (23), depressive and social anxiety (24–27), etc. The social discrimination and felt stigma of epilepsy patients may be improved to a certain extent with the improvement of society's attitude toward epilepsy, despite far more struggle to fill the gap of culture, income, etc. It remains considerable efforts on the understanding of the underlying neurobiology of inner perception in epilepsy patients, which may provide new insights on the treatment of stigmatization in epilepsy.
With the development of neuroimaging technology, the underlying organic impairment of psychiatric symptoms in epilepsy can be detected. Kavanaugh et al. (28) suggested white matter integrity within frontotemporolimbic (FTL) and non-FTL regions correlates with depressive symptomatology in temporal lobe epilepsy. In our previous study, we found that the functional metabolism of the hippocampus was a risk factor relating to depressive symptoms in patients with epilepsy (29). Furthermore, abnormalities in the integrity of the white matter can be detected by diffusion tensor imaging (DTI). Mao et al. (30) found significantly lower mean FA values compared with healthy controls in the bilateral frontotemporal contact fibers, such as uncinate fasciculus, cingulum bundle, arcuate fasciculus, and the right arcuate fasciculus were independent risk factors of psychoticism in epilepsy patients. These all provided important insights into the pathophysiological mechanisms in epilepsy.
Previous researchers studied the incidence and correlated factors of stigma in all epilepsy patients, including seizures secondary to or comorbid other diseases. This study aims to display (1) the incidence of felt stigma in epilepsy patients with genetic and unknown etiology in China; (2) clarify clinical and psychosocial factors associated with felt stigma in epilepsy; (3) further identify the neural correlates of felt stigma in patients with epilepsy by DTI.
Materials and Methods
Subjects
A total of 256 epilepsy patients who were diagnosed according to 2017 classification and Terminology of the International League against Epilepsy (ILAE) (31) were enrolled from June 2009 to October 2014 at the Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China. One hundred and fourteen of them performed Hamilton Depression Scale (HAMD) and DTI scan. A patient was included: (1) if the patient aged from 16 to 65 years, (2) if the patient appeared normal on conventional brain MRI, (3) if the patient did not have any disease other than epilepsy, and (4) if the patient did not have a family history of psychiatric illnesses.
The study protocol was approved by the local institutional review board at the author's affiliated institutions. Written informed consent was obtained from each study participant.
Clinical Characteristic
A clinical questionnaire including age, gender, and length of education was applied. Mini-mental state examination (MMSE) (32) tests were administered at the initial visit.
Socioeconomic status information was obtained from each participant including education, employment, income, and insurance. The income level was divided into two groups according to the local minimum living guarantee standard.
The duration of epilepsy, the type and frequency of seizures, and drug treatment history were elicited and recorded. Seizure severity was evaluated by the National Hospital Seizure Severity Scale (NHS3) (33).
Social Stigma, Quality of Life and Psychological Assessment
An 18-item Brief Psychiatric Rating Scale (BPRS) was performed for psychiatric evaluation. Twenty-one of them scored over 35 on BPRS and were referred to psychiatrists for consultation, and none of them were diagnosed with psychosis.
Felt Stigma was assessed by a three-item scale, developed originally for use in stroke patients (34), and subsequently adapted for use in epilepsy (35). Patients provided a simple yes/no response to indicate whether they feel other people are uncomfortable with them, whether other people treat them like an inferior person, and whether other people would prefer to avoid them because of epilepsy. Respondents whose score on the scale is zero are deemed not to feel stigmatized. The higher the positive score, the more stigmatized respondents are considered to feel. Previous studies showed that the stigma scale is a reliable and valid measure (36, 37). The stigma scale was translated to Chinese with words that people with various education levels could understand.
The patients were assessed by 17-item HAMD to recognize depressive symptoms. The range of the scale scores is from 0 to 54. The Chinese version of HAMD had been tested and proved to have good validity and reliability (38).
We adapted the QOL-31 questionnaire to evaluate the epilepsy patient's quality of life (39). This questionnaire contains 31 questions and seven subscales; each of the subscales assesses a different domain of QOL: seizure worry, overall quality of life, emotional wellbeing, energy/fatigue, cognitive function, medication effects, and social function. The Chinese version has previously been demonstrated good reliabilities (alpha = 0.94) and validity. The reliabilities for the individual subscales were observed with good test-retest (0.87–0.97) and internal consistency (0.58–0.88). Higher scores in the QOLIE-31 are indicative of a better QOL.
Personality disorder traits were assessed by the Chinese version of the Eysenck Personality Questionnaire (EPQ) (40), consisting of four categories of temperament: psychoticism (P), extraversion (E), neuroticism (N), and the lie (L) scale.
MRI Acquisition and Data Processing
MRI was performed on a General Electric 3T EXCITE HD scanner with a standard eight-channel phased array head coil. A standard protocol of conventional imaging was performed to measure conventional sagittal and axial T2-weighted fast spin-echo and coronal T1-weighted spin-echo imaging sequences. In all epilepsy patients, MRI scans were obtained in the interictal period (at least 1 week after the last seizure).
DTI Protocol and Data Analysis
Using a single-shot spin-echo echo planar image (SE-EPI) sequence, DTI data without diffusion weighting (b = 0) were acquired simultaneously in 25 non-collinear directions (b = 1,000 s/mm2). Moreover, 22 contiguous slices were acquired with a 3 mm slice thickness and with no gap. They were performed using acquisition with a 128 × 128 matrix; and a 256 x 256 mm field of view (FOV). The other acquisition parameters were: repetition time (TR) = 6,000 ms; echo-time (TE) = 76.2 ms; number of excitations (NEX) = 2.
DTI data were processed using the software FuncTool (GE Healthcare). The principal 3D orientation of the major eigenvector was color-coded per voxel. Each diffusion tensor was sampled 6 times to optimize the signal-to-noise ratio (SNR). Isotropic diffusion-weighted, ADC, and fractional anisotropy (FA) maps were generated. By positioning the region of interest (ROI) (33 ± 0.4 mm2) the targeted fibers, we got the FA values of the bilateral arcuate fasciculus (AF), uncinate fasciculus (UF), cingulum bundle (CB), fornix, superior longitudinal fasciculus (SLF) and anterior commissure (AC) as we previously described (30).
Statistical Analysis
Numerical variables are expressed as mean ± standard deviation (s.d.) or median [interquartile range (IQR)]. Statistical analysis was performed using the SPSS software version15.0 (SPSSInc., Chicago, IL). Differences were made by χ2 test for categorized data, an unpaired Student t-test for continuous data that were roughly normally distributed. Correlation between stigma scores and clinical characteristics, using Spearman correlation and logistic regression test. We used forward conditional methods to screen variables, which were included at a level of 0.05 and excluded at a level of 0.1. All tests were two-sided with a significance level of P < 0.05.
Results
Demographic and Disease Characteristics of Study Participants
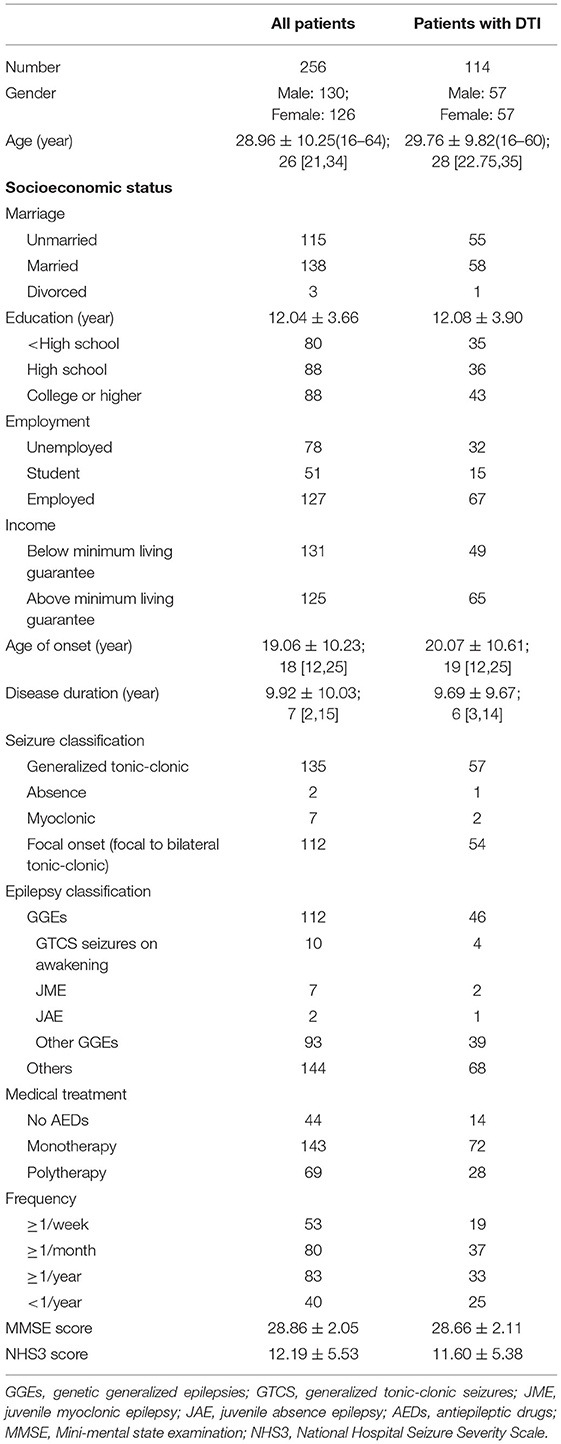
We include 256 epilepsy patients with a normal MMSE score and without any other disease, among whom 114 patients were performed HAMD and DTI assessments. All patients were Han Chinese. Demographic and clinical characteristics of all epilepsy patients were shown in Table 1.
Felt Stigma, Quality of Life, Depression, Personality, and Frontotemporal Contact Fibers Assessment in Epilepsy Patients
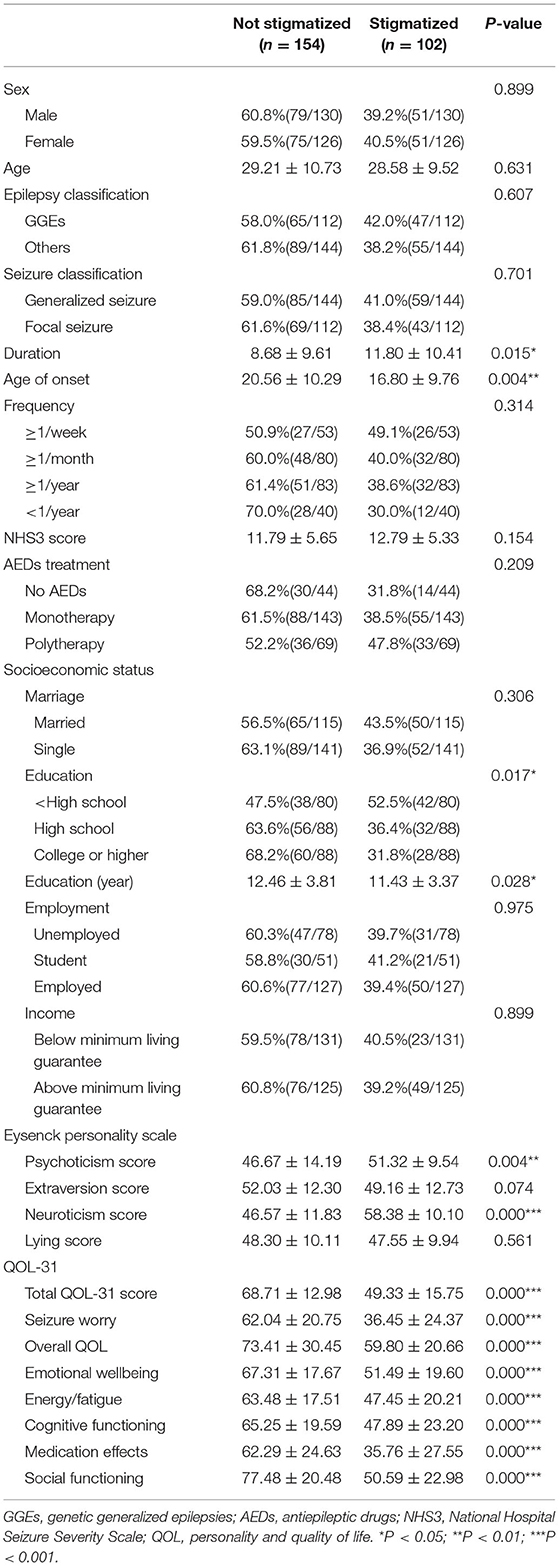
We evaluated the stigma, personality, depression, and quality of life traits by corresponding scales. Notably, we found a high incidence of felt stigma in epilepsy patients. There were about 39.8% (102 / 256) patient's felt stigma, with Score 1 in 17.2% (44 / 256), Score 2 in 14.5% (37 / 256) and the highest score (Score 3) present in 8.2% (21 / 256) patients. The other 60.2% (154 / 256) answered “NO” to all three questions. The distribution of answers to each question was shown in Table 2.
Personality traits were evaluated by EPQ scale, and the average scores were 48.55 ± 12.70, 50.87 ± 12.53, 51.35 ± 12.57, 48.00 ± 10.03 for psychoticism, extraversion, neuroticism and lying subscales, respectively. The average scores of HAMD scale in epilepsy patients was 5.04 ± 5.63 [3(1,7)]. We measured quality of life in epilepsy patients using QOL-31. The total QOL-31 score was 60.90 ± 20.00, with seizure worry for 51.72 ± 25.54, overall QOL for 67.92 ± 27.70, emotion wellbeing for 60.93 ± 20.00, energy-fatigue for 57.02 ± 20.20, cognitive function for 58.25 ± 22.74, medical effects for 51.59 ± 28.90 and social function for 66.64 ± 25.22.
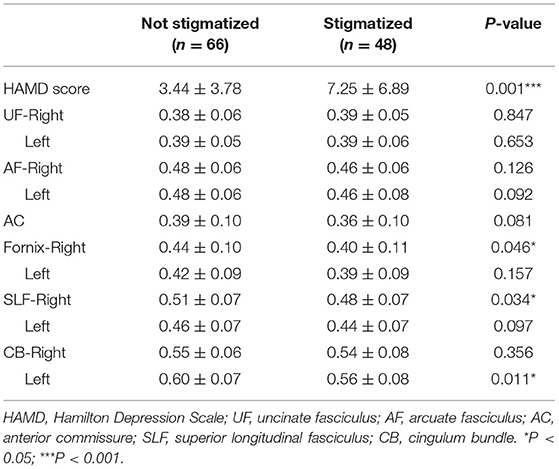
Using DTI, we estimated frontotemporal contact fibers by detecting FA values of UF (Right 0.38 ± 0.06; Left 0.39 ± 0.05), AF (Right 0.47 ± 0.06; Left 0.47 ± 0.07), AC 0.38 ± 0.10, Fornix (Right 0.42 ± 0.10; Left 0.41 ± 0.09), SLF (Right 0.49 ± 0.07; Left 0.46 ± 0.07) and CB (Right 0.54 ± 0.07; Left 0.58 ± 0.08).
Factors Correlated to Felt Stigma in Epilepsy Patients
Patient's felt stigma were found to have shorter education years (P < 0.05), longer duration (P < 0.05), and younger age of onset (P < 0.01). Moreover, stigmatized epilepsy patients manifested higher HAMD score (P = 0.001), higher psychoticism (P < 0.01) and neuroticism (P < 0.001) score of EPQ scale, and lower QOL-31 score (P < 0.001). No differences were found between epilepsy patients with and without stigma in age, gender, marriage, income, employment, seizure frequency, numbers of AEDs application, MMSE, and NHS3 scores (P > 0.05) (Tables 3, 4, Supplementary Tables 1, 2).
We further explored the correlation between stigma score and clinical variables, personality, emotion and quality of life traits by Spearman correlation analysis. We performed analysis between stigma scores and age of the patients, disease duration, age of onset, numbers of AEDs, frequency of epilepsy, MMSE score, NHS3 score, education, HAMD score, EPQ score and QOL-31 score. Stigma scores were significantly negatively correlated with education (r = −0.167, P = 0.008), age of onset (r = −0.134, P = 0.032), extraversion score of EPQ (r = −0.182, P = 0.004), total and all the subscale QOL scores (Total QOL: r = −0.606, P = 0.000; Seizure worry: r = −0.520, P = 0.000; Overall QOL: r = −0.303, P = 0.000; Emotional wellbeing: r = −0.470, P = 0.000; Energy-fatigue: r = −0.390, P = 0.000; Cognitive functioning: r = −0.389, P = 0.000; Medication effects: r = −0.479, P = 0.000; Social functioning: r = −0.596, P = 0.000), and positively correlated with duration (r = 0.170, P = 0.006), HAMD score (r = 0.361, P = 0.000), neuroticism score of EPQ (r = 0.501, P = 0.000). Other variables did not display significant correlation.
FA values of frontotemporal contact fibers were summarized in Table 4. We found decreased FA values of the right fornix, right SLF, and left CB in stigmatized epilepsy patients. Spearman correlated analysis showed negative correlations between stigma scores and FA values of right SLF (r = −0.187, P = 0.046) and left CB (r = −0.240, P = 0.010).
Felt Stigma Correlated to Left Cingulum, Social Function, and Neuroticism Independently
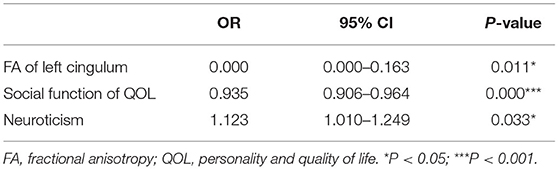
We carried out logistic regression to identify the most notable impact factor of stigma. We set stigma score as a dependent variable, and age, gender, marriage, income, employment, education, disease duration, age of onset, numbers of AEDs, seizure frequency, seizure type and MMSE, NHS3, HAMD, EPQ, QOL-31 scores and FA values of frontotemporal fibers as independent variables. The results showed that FA value of left CB [P = 0.011; OR = 0.000 (95% CI = 0.000–0.163)], social function [P = 0.000; OR = 0.935 (95% CI = 0.906–0.964)] of QOL, and neuroticism score of EPQ [P = 0.033; OR = 1.123 (95% CI = 1.010–1.249)] were significant factors included in the regression model of stigma scores (Table 5).
Discussion
Stigma is one of the common features of epilepsy in both developed and developing countries. It can cause serious harm to the physical, mental, and social wellbeing of a person with epilepsy.
In this study, we found that about 39.8% of epilepsy patients showed felt stigma. Similarly, previous epidemiological surveys showed the incidence of stigmatized persons was 30~62.5% in European (35, 41). In China, about 40% of people with epilepsy did not tell others about their disease (which could be a symbol of stigma), and 36% thought that people treated them differently because of their epilepsy (8).
Previous studies adopted a variety of scales to evaluate the degree of social stigma in epilepsy patients. In the 1980's, a 21-item stigma scale was used (42), and in these years, scales such as internalized stigma of mental illness (ISMI) scale (43), the original or modified version of the Parent/Child Stigma Scale developed by Austin et al. (44, 45), the impact of epilepsy scale by Jacoby et al. (46), were designed, modified and adapted. In this study, we use a three-item felt stigma scale, which was one of the most widely used stigma scales and adapted in large-scale epidemiological investigations (35). It is a brief and fast questionnaire in that patients were asked three questions for “yes” or “no” answers, and could be applied to a wide population.
Various factors might be contributing to the stigma of epilepsy. Felt stigma was linked to higher seizure frequency, recency of seizures, younger age at epilepsy onset or longer duration, lower educational level, poorer knowledge about epilepsy, and younger age (47). Consistently, we found shorter education years, longer duration, and younger age of onset might have impacts on felt stigma. Previous studies demonstrated that perceived stigma was shown to vary inversely with age, with younger groups tending to feel more stigmatized compared to the older population (47). And younger age was significantly associated with greater perceptions of stigma in patients with 9 and 14 years of age (19). No relationship was found between felt stigma and age, sex, wealth, seizure type/frequency in this study. We failed to detect differences in stigma scores among three groups (16–20y, 21–44y, and 45–65y) either. The variability of age enrollment might be the explanation for the uncorrelated with age in this study. The presence of seizures emerged as the most common factor associated with higher degrees of perceived stigma, with stigma increasing with seizure severity (19, 47, 48). Our results indicated a similar tendency in patients with seizure frequency less than once per year, compared to those with more than once per week. As previously mentioned, felt stigma in epilepsy is associated with depression (24, 49) and negatively impacts on quality of life (23, 24, 50). We reported the degree of depression and all aspects of quality of life, especially social function had significant correlations to felt stigma as well. In a study of public attitudes toward epilepsy in the United Kingdom, more than a fifth of people questioned agreed with a statement that “people with epilepsy have more personality problems than others” (51). Several studies have demonstrated that perceived stigma is a critical factor for interictal aggression and neurotic personality in people with epilepsy (12, 52–54). Other researchers reported that introverted personality was independently associated with the felt stigma of epilepsy (55). In our results, although both the neuroticism and extraversion of personality were associated with stigma scores, we found that neurotic personality was a more notable aspect of felt stigma through logistic regression analysis.
The underlying neurobiology of stigmatization in epilepsy is still unclear. Some preliminary data illuminated the neural underpinnings of stigma. Krendl et al. (56) investigated the functional anatomic using fMRI and reported the subcortical (e.g., amygdala) and cortical responses (e.g., lateral PFC and anterior cingulate) were correlated to stigma perception. Raij et al. (57) showed stigma resistance in psychiatric patients was associated with rostro-ventral medial prefrontal cortex. Nakamura et al. (58) indicated superficial-intracalcarine cortex connectivity was associated with a better response to the anti-stigma interventions. In epilepsy patients, white matter abnormalities have been identified in several DTI studies (59–61). In our previous study, we also found decreased FA value in the bilateral UF, CB, AF (30). The integrity of white matter, especially frontotemporal contact fibers, was associated with psychiatric symptoms, such as depression (62) and personality disorders (30). In this study, we found a possible relationship between felt stigma and FA values of the right fornix, right SLF, and left CB. CB is a bundle of axons projecting from the posterior cingulate gyrus to the entorhinal cortex and the hippocampus is a terminal node of the inferior CB pathway. The impairment of CB was reported to be contributed to neuroticism (63, 64), depression (65), and schizophrenia (66). In this study, we enrolled the patients with epilepsy of genetic or unknown etiology to avoid confounding impacts on DTI and psychosocial factors from the lesions themselves. Our logistic regression results revealed that left CB might be an independent risk predictor of stigmatization in epilepsy patients.
However, the current study has some limitations. Only epilepsy patients with genetic and unknown etiology were included in this study. We only focused on the impairment of frontotemporal contact fibers and its correlation to stigmatization. The changes of other whiter matter required further study. In this study, we only investigate the “felt stigma,” where the other aspect- “enacted stigma” was not studied. In this study, only 114 cases were evaluated HAMD and DTI, which were performed as the subjects in the last regression analysis. This study preliminarily exhibited the neural correlates of felt stigma in epilepsy patients. Further studies are required to understand underlying neurobiology in stigmatization of epilepsy.
Conclusion
The current study illuminated a high prevalence of felt stigma in epilepsy patients in China. Felt stigma was found to be significantly associated with neurotic personality and social function of the quality of life. Felt stigma was also crucially related to the integrity of the left cingulum. The results pointed out the demanded social supporting caregivers for people with epilepsy and the necessity of screening for psychiatric symptoms in chronic epilepsy courses. The relationship between white matter and felt stigma suggested a new direction for future studies to free epilepsy patients from stigma.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee, Zhongshan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LM: study concept and design, enrolment of the study participants, and study coordination. KW: data analysis, interpretation, and revising the manuscript. QZ: acquisition of MRI data. JW: acquisition of clinical data. YZ: acquisition of data. WP: assessment of clinical scales. JD: study concept and design, interpretation of data, and revising the manuscript for content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.879895/full#supplementary-material
References
2. UK NCGC. The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care: Pharmacological Update of Clinical Guideline 20. London: Royal College of Physicians (UK) (2012).
3. Goffman E. Stigma: notes on the management of spoiled identity. Am Sociol Rev. Upper Saddle River, NJ: Prentice Hall (1986).
4. Jacoby A. Felt versus enacted stigma: a concept revisited. evidence from a study of people with epilepsy in remission. Soc Sci Med. (1994) 38:269–74. doi: 10.1016/0277-9536(94)90396-4
5. Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia. (1997) 38:353–62. doi: 10.1111/j.1528-1157.1997.tb01128.x
6. Aydemir N, Kaya B, Yildiz G, Oztura I, Baklan B. Determinants of felt stigma in epilepsy. Epilepsy Behav. (2016) 58:76–80. doi: 10.1016/j.yebeh.2016.03.008
7. Wang WZ, Wu JZ, Wang DS, Dai XY, Yang B, Wang TP, et al. The prevalence and treatment gap in epilepsy in China: an ILAE/IBE/WHO study. Neurology. (2003) 60:1544–5. doi: 10.1212/01.WNL.0000059867.35547.DE
8. Li S, Wu J, Wang W, Jacoby A, de Boer H, Sander JW. Stigma and epilepsy: the Chinese perspective. Epilepsy Behav. (2010) 17:242–5. doi: 10.1016/j.yebeh.2009.12.015
10. Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia. (2019) 60 Suppl 1:7–21. doi: 10.1111/epi.14458
11. Kariuki SM, Thomas PT, Newton CR. Epilepsy stigma in children in low-income and middle-income countries. Lancet Child Adolesc Health. (2021) 5:314–6. doi: 10.1016/S2352-4642(21)00090-0
12. Lee SA. Felt stigma in seizure-free persons with epilepsy: associated factors and its impact on health-related quality of life. Epilepsy Behav. (2021) 122:108186. doi: 10.1016/j.yebeh.2021.108186
13. Tombini M, Assenza G, Quintiliani L, Ricci L, Lanzone J, Di Lazzaro V. Epilepsy and quality of life: what does really matter? Neurol Sci. (2021) 42:3757–65. doi: 10.1007/s10072-020-04990-6
14. Lim KS, Wo SW, Wong MH, Tan CT. Impact of epilepsy on employment in Malaysia. Epilepsy Behav. (2013) 27:130–4. doi: 10.1016/j.yebeh.2012.12.034
16. Price P, Kobau R, Buelow J, Austin J, Lowenberg K. Improving understanding, promoting social inclusion, and fostering empowerment related to epilepsy: epilepsy Foundation public awareness campaigns−2001 through 2013. Epilepsy Behav. (2015) 44:239–44. doi: 10.1016/j.yebeh.2014.12.044
17. Novotna I, Rektor I. The long-term development of public attitudes towards people with epilepsy in the Czech Republic: 1981, 1984, 1998 and 2009 studies. Acta Neurol Scand. (2017) 135:454–8. doi: 10.1111/ane.12619
18. Rice DR, Cisse FA, Djibo HA, Tassiou NR, Sakadi F, Bah AK, et al. Epilepsy stigma in the Republic of Guinea and its socioeconomic and clinical associations: a cross-sectional analysis. Epilepsy Res. (2021) 177:106770. doi: 10.1016/j.eplepsyres.2021.106770
19. Austin JK, Perkins SM, Dunn DW. A model for internalized stigma in children and adolescents with epilepsy. Epilepsy Behav. (2014) 36:74–9. doi: 10.1016/j.yebeh.2014.04.020
20. Gabriel D, Ventura M, Samoes R, Freitas J, Lopes J, Ramalheira J, et al. Social impairment and stigma in genetic generalized epilepsies. Epilepsy Behav. (2020) 104:106886. doi: 10.1016/j.yebeh.2019.106886
21. Moshe SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. (2015) 385:884–98. doi: 10.1016/S0140-6736(14)60456-6
22. Mameniskiene R, Sakalauskaite-Juodeikiene E, Budrys V. People with epilepsy lack knowledge about their disease. Epilepsy Behav. (2015) 46:192–7. doi: 10.1016/j.yebeh.2015.03.002
23. Bautista RE, Shapovalov D, Shoraka AR. Factors associated with increased felt stigma among individuals with epilepsy. Seizure. (2015) 30:106–12. doi: 10.1016/j.seizure.2015.06.006
24. Leaffer EB, Hesdorffer DC, Begley C. Psychosocial and sociodemographic associates of felt stigma in epilepsy. Epilepsy Behav. (2014) 37:104–9. doi: 10.1016/j.yebeh.2014.06.006
25. Wei Z, Ren L, Yang L, Liu C, Cao M, Yang Q, et al. The relationship between social anxiety and felt stigma in patients with epilepsy: a network analysis. Seizure. (2021) 92:76–81. doi: 10.1016/j.seizure.2021.08.014
26. Margolis SA, Gonzalez JS, Faria C, Kenney L, Grant AC, Nakhutina L. Anxiety disorders in predominantly African American and Caribbean American adults with intractable epilepsy: the role of perceived epilepsy stigma. Epilepsy Behav. (2019) 99:106450. doi: 10.1016/j.yebeh.2019.106450
27. Tombini M, Assenza G, Quintiliani L, Ricci L, Lanzone J, Ulivi M, et al. Depressive symptoms and difficulties in emotion regulation in adult patients with epilepsy: Association with quality of life and stigma. Epilepsy Behav. (2020) 107:107073. doi: 10.1016/j.yebeh.2020.107073
28. Kavanaugh B, Correia S, Jones J, Blum A, LaFrance WJ, Davis JD. White matter integrity correlates with depressive symptomatology in temporal lobe epilepsy. Epilepsy Behav. (2017) 77:99–105. doi: 10.1016/j.yebeh.2017.07.035
29. Peng WF, Ding J, Mao LY, Li X, Liang L, Chen CZ, et al. Increased ratio of glutamate/glutamine to creatine in the right hippocampus contributes to depressive symptoms in patients with epilepsy. Epilepsy Behav. (2013) 29:144–9. doi: 10.1016/j.yebeh.2013.07.004
30. Mao LY, Ding J, Peng WF, Ma Y, Zhang YH, Chen CZ, et al. Disease duration and arcuate fasciculus abnormalities correlate with psychoticism in patients with epilepsy. Seizure. (2011) 20:741–7. doi: 10.1016/j.seizure.2011.07.002
31. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position Paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
33. O'Donoghue MF, Duncan JS, Sander JW. The national hospital seizure severity scale: a further development of the chalfont seizure severity scale. Epilepsia. (1996) 37:563–71. doi: 10.1111/j.1528-1157.1996.tb00610.x
34. Hyman MD. The stigma of stroke. its effects on performance during and after rehabilitation. Geriatrics. (1971) 26:132–41.
35. Baker GA, Brooks J, Buck D, Jacoby A. The stigma of epilepsy: a European perspective. Epilepsia. (2000) 41:98–104. doi: 10.1111/j.1528-1157.2000.tb01512.x
36. Jacoby A, Baker GA, Steen N, Potts P. Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a UK Community study. Epilepsia. (1996) 37:148–61. doi: 10.1111/j.1528-1157.1996.tb00006.x
37. Abetz L, Jacoby A, Baker GA, McNulty P. Patient-based assessments of quality of life in newly diagnosed epilepsy patients: validation of the NEWQOL. Epilepsia. (2000) 41:1119–28. doi: 10.1111/j.1528-1157.2000.tb00317.x
38. Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, et al. Validity and reliability of the Chinese hamilton depression rating scale. Br J Psychiatry. (1988) 152:660–4. doi: 10.1192/bjp.152.5.660
39. Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. (1998) 39:81–8. doi: 10.1111/j.1528-1157.1998.tb01278.x
40. Gong XY. The revision of Eysenck personality questionnaire in China. Psychol Sci Newsletter. (1984) 4:11–8. doi: 10.1016/0191-8869(84)90008-4
41. Brigo F, Igwe SC, Ausserer H, Tezzon F, Nardone R, Otte WM. Epilepsy-related stigma in European people with epilepsy: correlations with health system performance and overall quality of life. Epilepsy Behav. (2015) 42:18–21. doi: 10.1016/j.yebeh.2014.11.015
42. Ryan R, Kempner K, Emlen AC. The stigma of epilepsy as a self-concept. Epilepsia. (1980) 21:433–44. doi: 10.1111/j.1528-1157.1980.tb04091.x
43. Ritsher JB, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res. (2003) 121:31–49. doi: 10.1016/j.psychres.2003.08.008
44. Austin JK, Shafer PO, Deering JB. Epilepsy familiarity, knowledge, and perceptions of stigma: report from a survey of adolescents in the general population. Epilepsy Behav. (2002) 3:368–75. doi: 10.1016/S1525-5050(02)00042-2
45. Austin JK, MacLeod J, Dunn DW, Shen J, Perkins SM. Measuring stigma in children with epilepsy and their parents: instrument development and testing. Epilepsy Behav. (2004) 5:472–82. doi: 10.1016/j.yebeh.2004.04.008
46. Jacoby A, Baker G, Smith D, Dewey M, Chadwick D. Measuring the impact of epilepsy: the development of a novel scale. Epilepsy Res. (1993) 16:83–8. doi: 10.1016/0920-1211(93)90042-6
47. Kwon CS, Jacoby A, Ali A, Austin J, Birbeck GL, Braga P, et al. Systematic review of frequency of felt and enacted stigma in epilepsy and determining factors and attitudes toward persons living with epilepsy-report from the international league against epilepsy task force on stigma in epilepsy. Epilepsia. (2022) 63:573–97. doi: 10.1111/epi.17135
48. Bielen I, Friedrich L, Sruk A, Prvan MP, Hajnsek S, Petelin Z, et al. Factors associated with perceived stigma of epilepsy in Croatia: a study using the revised epilepsy stigma scale. Seizure. (2014) 23:117–21. doi: 10.1016/j.seizure.2013.10.008
49. Rafael F, Houinato D, Nubukpo P, Dubreuil CM, Tran DS, Odermatt P, et al. Sociocultural and psychological features of perceived stigma reported by people with epilepsy in Benin. Epilepsia. (2010) 51:1061–8. doi: 10.1111/j.1528-1167.2009.02511.x
50. Viteva E. Impact of stigma on the quality of life of patients with refractory epilepsy. Seizure. (2013) 22:64–9. doi: 10.1016/j.seizure.2012.10.010
51. Jacoby A, Gorry J, Gamble C, Baker GA. Public knowledge, private grief: a study of public attitudes to epilepsy in the United Kingdom and implications for stigma. Epilepsia. (2004) 45:1405–15. doi: 10.1111/j.0013-9580.2004.02904.x
52. Seo JG, Kim JM, Park SP. Perceived stigma is a critical factor for interictal aggression in people with epilepsy. Seizure. (2015) 26:26–31. doi: 10.1016/j.seizure.2015.01.011
54. Margolis SA, Nakhutina L, Schaffer SG, Grant AC, Gonzalez JS. Perceived epilepsy stigma mediates relationships between personality and social well-being in a diverse epilepsy population. Epilepsy Behav. (2018) 78:7–13. doi: 10.1016/j.yebeh.2017.10.023
55. Lee SA, Yoo HJ, Lee BI. Factors contributing to the stigma of epilepsy. Seizure. (2005) 14:157–63. doi: 10.1016/j.seizure.2005.01.001
56. Krendl AC, Macrae CN, Kelley WM, Fugelsang JA, Heatherton TF. The good, the bad, and the ugly: an fMRI investigation of the functional anatomic correlates of stigma. Soc Neurosci. (2006) 1:5–15. doi: 10.1080/17470910600670579
57. Raij TT, Korkeila J, Joutsenniemi K, Saarni SI, Riekki TJ. Association of stigma resistance with emotion regulation - functional magnetic resonance imaging and neuropsychological findings. Compr Psychiatry. (2014) 55:727–35. doi: 10.1016/j.comppsych.2013.10.010
58. Nakamura Y, Okada N, Ando S, Ohta K, Ojio Y, Abe O, et al. The association between amygdala subfield-related functional connectivity and stigma reduction 12 months after social contacts: a functional neuroimaging study in a subgroup of a randomized controlled trial. Front Hum Neurosci. (2020) 14:356. doi: 10.3389/fnhum.2020.00356
59. Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. (2009) 46:373–81. doi: 10.1016/j.neuroimage.2009.01.055
60. Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O'Neill TJ, et al. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. Neuroimage. (2010) 49:2033–44. doi: 10.1016/j.neuroimage.2009.10.055
61. Deppe M, Kellinghaus C, Duning T, Moddel G, Mohammadi S, Deppe K, et al. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology. (2008) 71:1981–5. doi: 10.1212/01.wnl.0000336969.98241.17
62. Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. (2013) 38:49–56. doi: 10.1503/jpn.110180
63. Madsen K, Jernigan T, Skimminge A, Mortensen E, Knudsen G, Baaré W. Cingulum Bundle Asymmetry Predicts Trait Neuroticism: A DTI Study. (2009). doi: 10.1016/S1053-8119(09)70905-X
64. Madsen KS, Jernigan TL, Vestergaard M, Mortensen EL, Baare W. Neuroticism is linked to microstructural left-right asymmetry of fronto-limbic fibre tracts in adolescents with opposite effects in boys and girls. Neuropsychologia. (2018) 114:1–10. doi: 10.1016/j.neuropsychologia.2018.04.010
65. Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry. (2012) 72:296–302. doi: 10.1016/j.biopsych.2012.01.022
Keywords: epilepsy, stigma, QOL, diffuse tensor imaging, neuroticism
Citation: Mao L, Wang K, Zhang Q, Wang J, Zhao Y, Peng W and Ding J (2022) Felt Stigma and Its Underlying Contributors in Epilepsy Patients. Front. Public Health 10:879895. doi: 10.3389/fpubh.2022.879895
Received: 20 February 2022; Accepted: 28 March 2022;
Published: 26 April 2022.
Edited by:
Wulf Rössler, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Jacopo Lanzone, Sant'Isidoro Hospital Ferb Onlus Trescore Balneario, ItalyEkaterina Viteva, Plovdiv Medical University, Bulgaria
Gloria Tedrus, Pontifical Catholic University of Campinas, Brazil
Copyright © 2022 Mao, Wang, Zhang, Wang, Zhao, Peng and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Ding, ZGluZy5qaW5nQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work
 Lingyan Mao
Lingyan Mao Keying Wang
Keying Wang Qianqian Zhang
Qianqian Zhang Jing Wang
Jing Wang Yanan Zhao
Yanan Zhao Weifeng Peng
Weifeng Peng Jing Ding
Jing Ding