- 1Applied Research Division, Centre for Surveillance and Applied Research, Health Promotion and Chronic Disease Prevention Branch, Public Health Agency of Canada, Ottawa, ON, Canada
- 2Surveillance and Epidemiology Division, Centre for Immunization and Respiratory Infectious Diseases, Infectious Diseases Programs Branch, Public Health Agency of Canada, Ottawa, ON, Canada
- 3Behaviours, Environments and Lifespan Division, Centre for Surveillance and Applied Research, Health Promotions and Chronic Disease Prevention Branch, Public Health Agency of Canada, Ottawa, ON, Canada
- 4School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
- 5Centre for Indigenous Statistics and Partnerships, Statistics Canada, Ottawa, ON, Canada
- 6Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
- 7Division of Cardiac Prevention & Rehabilitation, University of Ottawa Heart Institute, Ottawa, ON, Canada
- 8Vaccine Safety, Vaccine Surveillance, Public Health Agency of Canada, Ottawa, ON, Canada
Introduction: Over the last decade, e-cigarette use has been on the rise but with growing health concerns. The objective of this systematic review was to update findings for chronic health outcomes associated with e-cigarette use from the 2018 National Academies of Sciences, Engineering, and Medicine (NASEM) report.
Methods: Three bibliographic databases were searched to identify studies comparing the chronic health effects of e-cigarette users (ECU) to non-smokers (NS), smokers, and/or dual users indexed between 31 August 2017 and 29 January 2021. Two independent reviewers screened abstracts and full texts. Data were extracted by one reviewer and verified by a second one. Outcomes were synthesized in a narrative manner using counts and based on statistical significance and direction of the association stratified by study design and exposure type. Risk of bias and certainty of evidence was assessed. The protocol was prospectively registered on Open Science Framework https://osf.io/u9btp.
Results: A total of 180 articles were eligible. This review focused on 93 studies for the 11 most frequently reported outcomes and from which 59 reported on daily e-cigarette use. The certainty of evidence for all outcomes was very low because of study design (84% cross-sectional) and exposure type (27% reported on exclusive ECU, i.e., never smoked traditional cigarettes). Overall, the summary of results for nearly all outcomes, including inflammation, immune response, periodontal and peri-implant clinical parameters, lung function, respiratory symptoms, and cardiovascular disease, suggested either non-significant or mixed results when daily ECU was compared to NS. This was also observed when comparing exclusive ECU to NS. The only notable exception was related to oral health where most (11/14) studies reported significantly higher inflammation among daily ECU vs. NS. Compared to the smokers, the exclusive-ECUs had no statistically significant differences in inflammation orperiodontal clinical parameters but had mixed findings for peri-implant clinical parameters.
Conclusions: This review provides an update to the 2018 NASEM report on chronic health effects of e-cigarette use. While the number of studies has grown, the certainty of evidence remains very low largely because of cross-sectional designs and lack of reporting on exclusive e-cigarette exposure. There remains a need for higher quality intervention and prospective studies to assess causality, with a focus on exclusive e-cigarette use.
Introduction
Electronic cigarettes (e-cigarettes) were introduced in North America in 2006 (1). Since their introduction, e-cigarette use, also known as vaping, has been on the rise in Canada, the United States (US) (2–6), and European countries (7). Globally, estimation of the number of adult e-cigarette users has risen from 58.1 million in 2018 to 68 million in 2020 (8). Vaping is defined as the inhalation of vapor produced by heating liquids typically containing nicotine and flavoring elements, including heat-not-burn (HNB) tobacco products. E-cigarette use devices are battery-operated, contain a heating element, a mouthpiece, and a chamber to hold the liquid (1, 9). Vaping liquids generally contain propylene glycol, glycerin, and flavorings, and may contain nicotine (1, 10). E-cigarette use devices are not exclusively used to vape a liquid, they may also be used to vape tetrahydrocannabinol (THC) (11), dried cannabis, tobacco products, or psychoactive substances (12). A survey conducted among high school students in seven European countries from 2016 to 2017 showed that on average, 34% of the students had tried e-cigarettes (13). Among high school students in the US, e-cigarette use increased from 1.5% (220,000 individuals) in 2011 to 20.8% (3.05 million) in 2018 (6). In Canada, e-cigarette use among youth increased from 11% in 2013 to 16% in 2019 (14, 15). Similarly in Italy, youth e-cigarette users substantially increased from 0% in 2010 to 7.4% in 2014 and to 17.5% in 2018, and exclusive e-cigarette users recorded an almost three-fold significant increase from 2.9% in 2014 to 8.2% in 2018 (16). More recent data continue to show substantial increases in the proportion of youth who vape in Canada and the US (17).
Vaping has been associated with poisonings as a result of ingesting vaping liquids and burns associated with device malfunctions. Other acute health effects such as increased heart rate shortly after vaping, endothelial dysfunction, modulation of the sympathetic nervous system, and changes in pulmonary physiology have been documented (1, 11, 18–24). More recently, vaping has emerged as a significant public health issue because of rapid rise in the number of hospitalizations for what has been termed e-cigarette- or vaping use-associated lung injury (EVALI/ VALI) (25). As of February 2020, the Centers for Disease Control and Prevention in the US had identified 2,807 known cases of EVALI hospitalizations or deaths (26). Between 1 September 2019 and 31 December 2020, 20 cases of vaping-associated lung illness were detected by active surveillance in Canada (27, 28). These emerging vaping-related health issues have contributed to the need to better understand the broader health impacts of vaping.
To date, systematic reviews of peer-reviewed literature are largely categorized into two groups: those examining the health effects of vaping (10, 29–37) and those examining the efficacy of e-cigarettes as a smoking cessation aid (38, 39). Among those that have examined the health effects associated with vaping, findings have mostly been inconclusive. A number of study limitations have likely contributed to the equivocal results including limited sample size, cross-sectional design or lack of long-term follow-up, imprecision concerning exposure definitions, overall small number of available studies, and conflicts of interest where studies were funded or associated with e-cigarette manufacturers. It must be recognized that the emergence of significant health impacts of e-cigarette use may require significant longer-term exposure and review than is currently possible given their relatively recent introduction to the marketplace.
In 2018, the National Academies of Sciences, Engineering, and Medicine (NASEM) published the most comprehensive report to date addressing the health consequences of e-cigarette use. This report concluded that vaping was associated with some physiological effects on humans (e.g., acute changes in heart rate, possible increased risk of periodontal disease, increase in incident injuries, and poisoning). However, evidence of the long-term chronic health effects was not yet available, particularly of outcomes related to cardiovascular and respiratory health, immune biomarkers (e.g., change in oxidative stress), and pregnancy (19). Despite very limited evidence, the report suggested that vaping may be less harmful than smoking conventional cigarettes (19). Since the release of the NASEM report, new vaping devices and liquids have become available, and research on chronic health effects has grown considerably. The objective of this systematic review was to examine all relevant peer-reviewed literature published since the NASEM report and synthesize findings on vaping-related chronic health effects taking into consideration the volume and quality of evidence for key health outcomes.
Methods
This review was prospectively registered on the Open Science Framework (https://osf.io/u9btp) (40) and adhered to the PRISMA statement (41). The review was initially designed to be a rapid one to provide a quick update on the NASEM report (19). However, it was converted to a full systematic review. Because of the sheer volume of research that focused on chronic health impacts of e-cigarette use, this review focuses on outcomes with the largest number of studies assessing the impact of daily e-cigarette use exposure, which was defined as daily vaping for at least 1 month.
Study inclusion criteria
Population
All human in-vivo studies were eligible.
Exposure
The primary exposure was vaping. Vaping refers to e-cigarette use, including the use of e-liquids with or without nicotine and HNB tobacco products. Those who use e-cigarettes (vape) are referred to as “e-cigarette users”. E-cigarette use was assessed by level of exposure to include the following groups: (1) “daily e-cigarette users,” defined as those who have vaped every day for at 1 month (≥4 weeks) or who reported daily use; (2) “occasional e-cigarette users,” defined as those who reported vaping for <1 month or who reported occasional use; (3) “unclear e-cigarette users,” defined as those who were “ever users” (e.g., asking participants if they had vaped in the past year or month, or simply asking them if they have ever vaped) or with no description of duration of use or definition of what constitutes a “vaper.” If a study combined both daily and occasional e-cigarette users in the same group, the users are summarized as occasional users. Additionally, to reduce potential confounding due to cigarette exposure, the daily and occasional e-cigarette users groups were further stratified by former smoking status with “exclusive e-cigarette users,” defined as those with no history of conventional tobacco cigarette smoking. Studies examining the effects of second-hand smoke exposure, withdrawal symptoms, effects of different doses of nicotine, and effects of cannabis and THC, and, smoking cessation studies that did not include the health effects of vaping as principal outcome were excluded.
Comparators
Health effects among e-cigarette users were compared to “non-smokers” (no reported e-cigarette use or conventional tobacco cigarette use), “traditional smokers” (current conventional tobacco cigarette use), and “dual-users” (defined as those who use both e-cigarettes daily occasionally or of no clear duration and conventional tobacco cigarettes).
Outcomes
Chronic physical health effects were selected based on the research available comparing daily e-cigarette users to at least one comparison group. Studies on acute, transient changes (e.g., change in blood pressure 1 h after vaping) and withdrawal symptoms were excluded. Acute effects referred to those that developed suddenly and lasted for a short period (e.g., few days). Chronic effects were those that developed more slowly and present over an extended period of time (e.g., established disease). Studies that concentrated on e-cigarette or vaping use-associated lung injury (EVALI), mental health outcomes, sleep duration, or behavioral outcomes, as well as biomarkers of exposure to chemicals/metals in body fluid, were excluded.
Study designs
Quantitative studies were eligible, including cross-sectional, cohort, case control, and randomized controlled trials (RCTs). Experimental studies that examined the effects of switching from combustible tobacco cigarettes to vaping (e.g., smoking cessation modality) were eligible provided they also reported health effects as their primary outcome after at least 1 month of use. If the duration of use was less than once a month, baseline outcomes were used, and the study was categorized as cross-sectional. Literature/systematic reviews, dissertations, case reports, case series, qualitative studies, and in vitro and animal studies were also excluded.
Publication status and language
Studies were restricted to those published and indexed in the peer review literature in English or French. Only literature indexed between 31 August 2017 and 29 January 2021 was considered for this review. Conference publications were excluded.
Search strategy
Research librarians were consulted, and the search strategy of the NASEM report was used with the addition of a new “vaping” MESH subject heading available in the MEDLINE database (i.e., vaping/). The following three databases were searched on 20 September 2019 with an update on 19 January 2021: Ovid MEDLINE, Ovid PsycINFO, and Ovid EMBASE. The search strategy used is shown in Supplementary Tables S1-1–S1-3). Gray literature was excluded from this systematic review.
Study selection and data extraction
Following the search, titles and abstracts of studies were imported into RefWorks (RefWorks, Bethesda, MD, United States), and duplicates were removed using the “dedupe” function. The titles and abstracts were then imported into Covidence (Veritas Health Innovation, Melbourne, Australia), where additional duplicates were removed. Two independent reviewers screened all the titles and abstracts to identify potentially relevant studies. Conflicts were discussed and resolved by discussion and consensus. If a consensus could not be reached, a third reviewer was consulted. The full texts of all potentially relevant studies were then independently screened by two reviewers. Again, remaining discrepancies were discussed with a third reviewer. Systematic reviews were hand-searched for additional studies. Standardized data extraction forms were developed and completed using Google forms. All extracted data were verified by a second reviewer for accuracy.
Risk of bias appraisal and conflict of interest
The included studies were assessed for risk of bias (RoB) using a modified Critical Appraisals Skills Programme (CASP) Checklist specific for each study design (42). The CASP checklist tools assess measurement bias, recruitment bias, confounding factors, precision, and external generalizability. An additional item assessed for possible conflict of interest if funding or contributions to support the study were received from the tobacco/e-cigarette industry or the pharmaceutical industry.
For cross-sectional studies, questions assessing the completeness and length of cohort follow-ups were removed. Furthermore, CASP items assessing whether the studies fit with the existing literature were removed, as the intention of this review was to examine and synthesize the evidence. A study was considered with high RoB if one or more of the items that assessed the “validity of the results” on the CASP checklist was not met (Supplementary Tables S4-1–S4-4).
Data synthesis
Because of heterogeneity in the measures used to assess specific outcomes, findings were summarized using a narrative synthesis approach. Narrative syntheses describe the certainty of evidence and overall direction of associations between e-cigarette users and health outcomes relative to study comparison groups (non-smokers, cigarette smokers, and dual-users). The direction of association was determined by “vote counting,” where it summarized the number of studies per health outcome that reported “better,” “poorer,” or “null” associations with e-cigarette users compared to non-smokers, dual-users, and cigarette smokers. In the vote counting table, the studies were stratified by e-cigarette use exposure (daily, occasional, or unclear duration) and study design (RCT, cohort, pre-post, case-control, and cross-sectional). Results for the sub-analysis of studies that included “exclusive e-cigarette users” (i.e., no history of cigarette smoking) are also provided.
The narrative synthesis summarizes the findings only for daily e-cigarette users. It does not summarize the findings for occasional users or if the duration of e-cigarette use was unclear because of increased level of exposure uncertainty within the groups. The results for exclusive e-cigarette users or daily users were highlighted, when available. If a study reported on both daily and occasional e-cigarette use, only findings for daily users were included in the vote counting. If two studies analyzed the same outcome from the same survey (cohort), the one with most recent publication date was included unless the older one used a larger sample size from the cohort (e.g., pooling two waves of data collection). In order to minimize over-counting of studies reporting on multiple related indicators or biomarkers, the overall direction of association was based on the direction of at least 60% of the findings. Two vote counts were given in cases when there was a 50% split in the direction of associations, or three vote counts for a 33% split. Footnotes in the vote-counting tables identify study results contributing to more than one vote count.
The overall certainty of evidence by outcome was assessed using a modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, adapted for narrative syntheses (43, 44), and was summarized based on the highest level of study design (i.e., RCTs before cross-sectional studies). Supplementary Table S5 provides a summary of judgment decision rules applied in GRADE. The certainty of evidence was assessed for all studies that reported on findings for daily e-cigarette users and exclusive e-cigarette users separately, and was further stratified by study design (i.e., RCTs, pre-post and cohort studies were considered of higher quality than cross-sectional and case-control studies).
Results
Description of studies
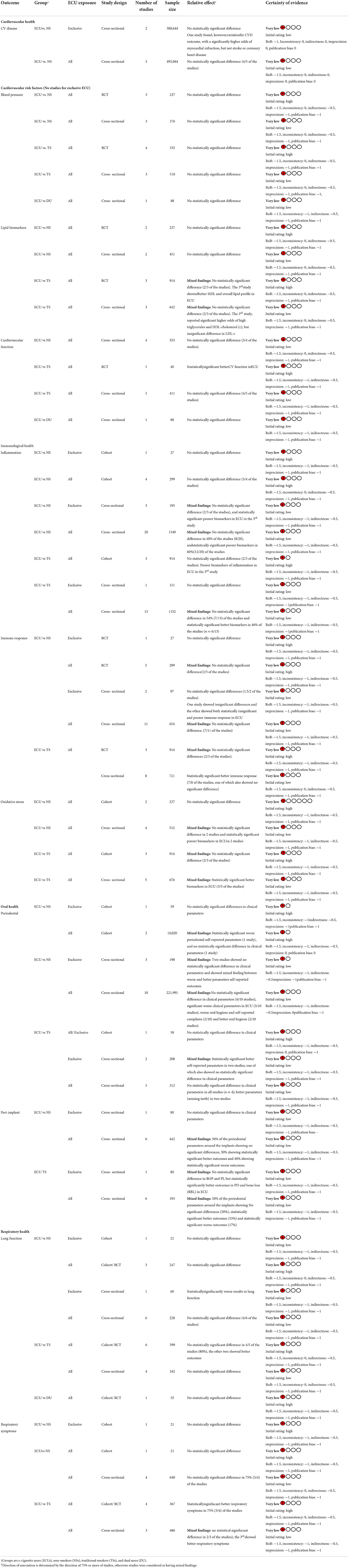
Figure 1 displays the screening process and reasons for exclusion in each stage. The database search identified 14,443 potentially relevant studies; of these, 180 met the inclusion criteria. Supplementary Tables S2-1–S2-4 provide the study characteristics. Supplementary Tables S3-1–S3-4 provide the findings from each study. Supplementary Tables S4-1–S4-4 summarize the RoB assessments for daily e-cigarette users. Outcome measures in Supplementary materials presents a list of combined outcomes included in the systematic reviews, and Supplementary material [Full-texts reviewed studies and reasons for studies exclusion (excel file)] provides the reason for exclusion. Findings are summarized for 93 studies that reported on one or more of the 11 most common outcomes (Table 1) and Supplementary Tables in Supplements S2, S3. Fifty-nine studies assessed daily e-cigarette users, but only 11 reported on exclusive e-cigarette users. Thirty-four percent (n = 32) of the studies assessed outcomes from more than one health domain (e.g., respiratory and cardiovascular (CV) or respiratory and oral). Moreover, many studies assessed several outcomes within a health domain (e.g., cardiovascular disease (CVD), blood pressure, and lipids profiles; Table 1).
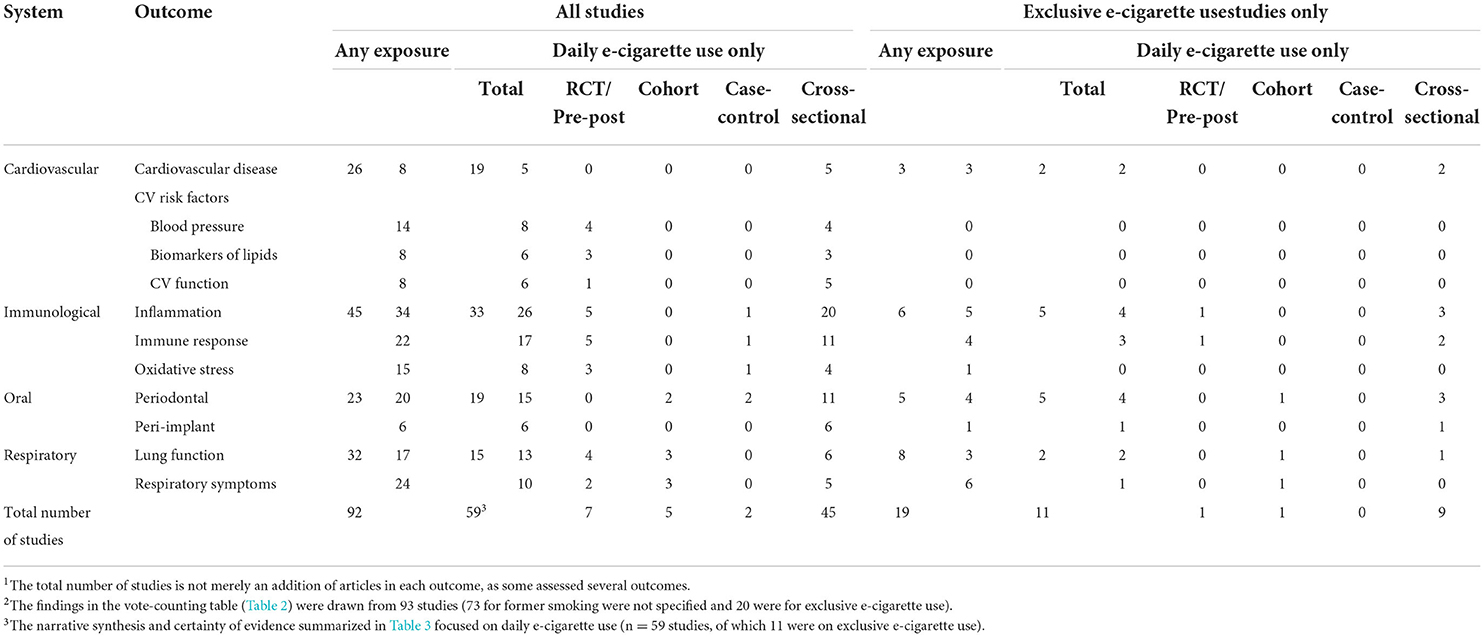
Table 1. Number of studies1 by physiological system and health outcome of daily users, and sub-set summary for exclusive e-cigarette users stratified by study design.
From the 93 included studies, the majority (78%, n = 71) used a cross-sectional design with very few cohort (9%, n = 9), RCTs (8.4%, n = 8), quasi-experimental (2%, n = 2), and case-control studies (2%, n = 2). For the majority (79%, n = 72) of the studies, the former smoking status of e-cigarette users was not reported. Table 1 presents the number of studies for each health outcome stratified by study design, daily exposure, and whether e-cigarette use was exclusive (i.e., daily e-cigarette users that never smoked conventional tobacco cigarettes). Half of the studies were conducted in the US (53.8%, n =4 9), with fewer from Middle Eastern/Arab countries (19.7%, n = 18), European countries (14.2%, n = 13), and eastern Asia (4.3%, n = 4). Most of the study populations included adults. Only three (69–71) included samples from youth. Fifty-nine studies (65%) reported on daily e-cigarette users, but only 11 of (19%) were on exclusive e-cigarette users (i.e., had never smoked cigarettes). These studies are the focus of health outcomes in the narrative synthesis section below.
Risk of bias and conflicts of interest
The RoB among the studies reviewed is summarized in Supplementary Tables S4-1–S4-4. For all the 11 outcomes assessed, studies examining daily e-cigarette users had a high RoB, which contributed to reduction in the certainty of evidence. All the RCTs (n = 7) were considered to have a high RoB largely because of recruitment bias and confounding factors. Most of the RCTs (71%), had no (n = 2) or unclear (n = 3) randomization in the assignment of the e-cigarette users to the control groups. In all the RCTs (n=7), patients, health workers, or study personnel were not blind to treatment (i.e., e-cigarette use) (n = 5) or it was unclear (n = 2) whether there was blinding. For most of the RCTs (86%), the groups were not (n = 4) or it was unclear (n = 3) if they were similar at the start of the trial.
In the cohort studies (n = 5), 80% (n = 3) had a high risk of selection bias (i.e., due to convenience samples), and all of them had a high RoB due to confounding factors. For the majority (83%), attrition was not a problem, and all the studies had a reasonable follow-up period (min = 6 months, max = 60 months, and average = 33.6 months). In the cross-sectional studies (n = 45), the majority (77%) had a high risk of selection bias and bias in 80% of the studies due to lack of controlling for confounding factors (e.g., duration of e-cigarette use, and smoking).
Twenty percent of the studies examining daily e-cigarette use (n = 12/59) had a potential conflict of interest (COI) related to direct or indirect involvement of the tobacco or e-cigarette or pharmaceutical industry. A large proportion of the more rigorously designed studies had a potential COI; 62% (5/8) of the RCTs and 33% (3/9) the cohort studies compared to the 18% (13/71) of the cross-sectional studies (Supplementary Tables S2.1–S2.4).
Health outcomes: Narrative synthesis
Table 1 summarizes the total number of studies for each outcome and those that reported on daily or exclusive e-cigarette use stratified by study design. Many of the studies assessed more than one outcome. Supplementary Table S2 provides all the outcomes assessed in each study and provides details on population characteristics, and Outcome measures in Supplementary materials provides all lists of combined outcomes. Table 2 provides the vote-count by outcome based on the statistical significance and directionality of effect for comparisons (i.e., e-cigarette users compared to non-smokers, cigarette smokers, or dual-users). The vote counting reflects the number of comparisons in each group. A sub-summary of the results for exclusive e-cigarette users is provided, although very few studies examined the health outcomes of exclusive e-cigarette use. In total, 59 studies assessed daily e-cigarette users related to CV (n = 19), immunological (n = 33), oral (n = 19), and respiratory (n = 15) health outcomes. Hereafter we focus our narrative synthesis on these 59 studies.
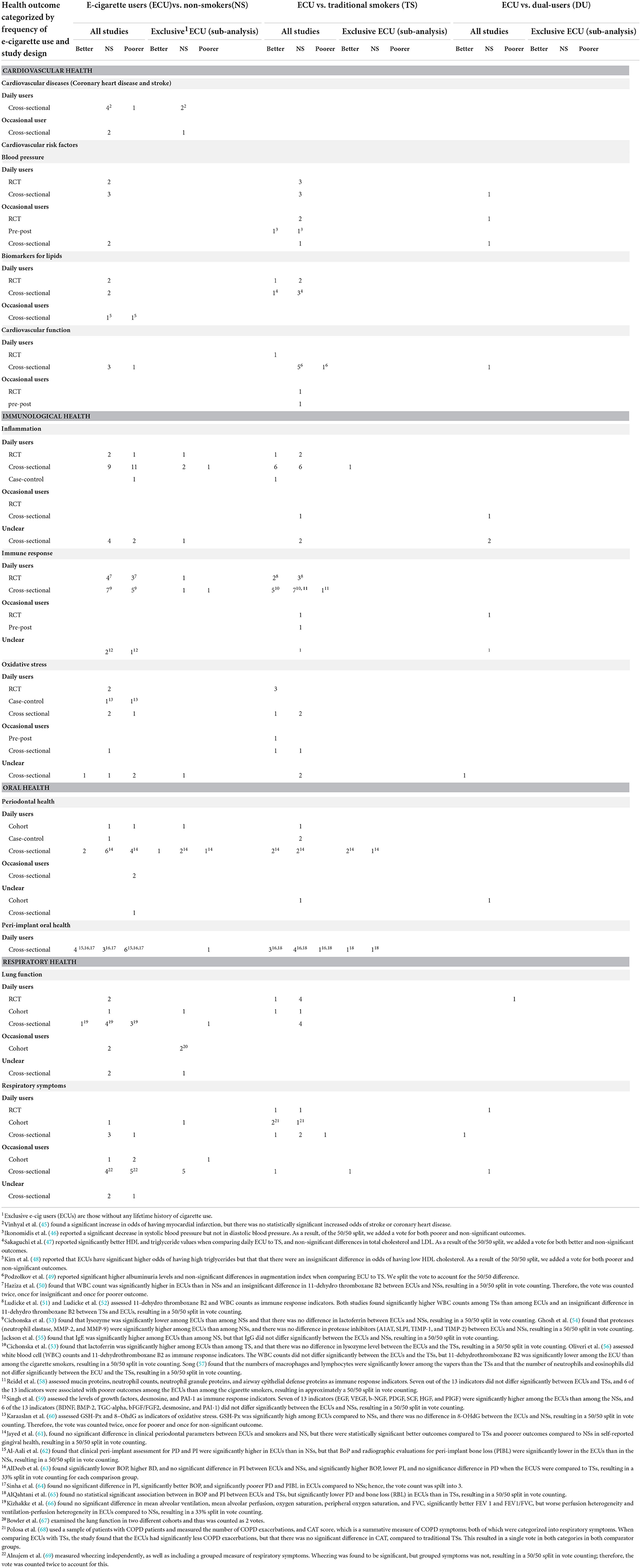
Table 2. Vote counting for all the studies (including those on exclusive e-cigarette use) and a sub-analysis for exclusive e-cigarette use only.
Certainty of evidence
The certainty of the evidence for all the health outcomes was very low regardless of daily or exclusive e-cigarette use. High RoB and study design were key contributor to reducing the certainty of evidence. Table 3 provides the certainty of evidence for each outcome separately for high and low quality study designs. Results are reported separately for daily and exclusive e-cigarette users. The Table summarizes the relative direction of association and certainty of the evidence. The outcome descriptions summarize the highest quality of evidence available with an emphasis on daily and exclusive e-cigarette use and study design.
Cardiovascular health
Twenty-five studies (45–52, 56, 72–88) explored cardiovascular (CV) health including cardiovascular disease (CVD) (n = 8), blood pressure (n = 14), biomarkers of lipid metabolism (n = 8), and CV function (n = 8). Nineteen studies assessed the association of daily e-cigarette use with CV health, five RCT (50–52, 73, 87), and 14 cross-sectional studies (45, 47, 49, 56, 74–83).
Cardiovascular disease
Eight studies (45, 48, 76, 78, 82, 83, 85, 86) assessed the odds of having CVD in e-cigarette users compared to non-smokers; five (45, 76, 78, 82, 83) concentrated on daily e-cigarette users, two of which assessed (45, 82) exclusive e-cigarette users. None compared daily e-cigarette users to cigarette smokers or dual-users. Indicators of CVD included self-reported premature CVD (i.e., disease age <65 years), myocardial infarction, ischemic attack (stroke), congestive heart failure, peripheral artery disease, and coronary heart disease. All evidence was cross-sectional and found no significant difference in the odds of CVD between daily e-cigarette users or exclusive e-cigarette users and non-smokers (45, 76, 78, 82, 83). One study looking at multiple CVD outcomes did find significantly higher odds of myocardial infarction for exclusive e-cigarette users compared to non-smokers, but there was no difference for stroke or coronary heart disease (76).
Blood pressure
Fourteen studies (46, 48, 50, 51, 72–75, 79, 80, 84, 86–88) assessed blood pressure, but only eight (50, 51, 73–75, 79, 80, 87) compared blood pressure results of daily e-cigarette users to those of non-smokers, smokers and/or dual users (four cross-sectional and four RCTs). None of the eight studies reported on exclusive e-cigarette use. Blood pressure indicators included measured effects on systolic, diastolic, and mean blood pressure or hypertension status. No significant difference was observed for daily e-cigarette users compared to non-smokers, cigarette smokers, or dual users.
Biomarkers of lipid metabolism
Eight studies (47, 50–52, 56, 77, 86, 88) assessed lipid profiles, six (47, 50–52, 56, 77) reported results for daily e-cigarette user, and none assessed exclusive e-cigarette use. Lipid biomarkers included total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and/or triglycerides. The findings suggested no significant difference for any biomarker between daily e-cigarette users and non-smokers, regardless of study design. One of the three RCT studies (52), however, suggested better HDL at 6 months follow-up in daily e-cigarette users than in cigarette smokers. In contrast, two cross-sectional studies found no significant difference in lipids between daily e-cigarette users and cigarette smokers (56, 77), and one reported mixed findings suggesting better HDL and triglycerides in e-cigarette users but no significant differences in total cholesterol and LDL (47).
Cardiovascular function
Eight studies (46, 49, 72, 75, 79–81, 87) assessed CV function comparing e-cigarette users to non-smokers, smokers, or dual users. Six (49, 75, 79–81, 87) included daily e-cigarette users. None was conducted on exclusive e-cigarette users. A wide range of markers was explored, with the most common being differences in heart rate, pulse wave velocity, and arterial stiffness. Most (75%) of the studies found no statistically significant differences between daily e-cigarette users and non-smokers (79–81) or dual users (79). A single RCT (87) found that daily e-cigarette users had better CV function indicators than smokers. No differences were observed among the cross-sectional studies (49, 75, 79–81).
Immunological health
Forty-five studies (46, 47, 50–58, 60, 62–65, 69, 72, 77, 80, 87, 89–112) examined inflammation, immune response, and oxidative stress, from which 33 (six RCTS (50–52, 87, 99, 100), two case controls (60, 111), and 25 cross-sectional studies (47, 53–58, 62–65, 77, 89–98, 101–103) included daily e-cigarette use. Only six studies (55, 64, 65, 98, 99, 103) included exclusive e-cigarette use.
Inflammation
Twenty-six studies compared biomarkers of inflammation between daily e-cigarette users and non-smokers and/or smokers (47, 50–53, 56–58, 60, 62–65, 89–100, 103). Most of these studies were related to oral health (n = 14) (53, 62, 63, 65, 89–94, 97, 103), followed by respiratory health (n = 7) (57, 58, 95, 96, 98–100), CV health (n = 4) (47, 50–52), and a single study with biomarkers of inflammation non-specific to a physiological system (56). Only four studies reported on exclusive e-cigarette use (65, 98, 99, 103). The biomarkers of inflammation included cytokine and protein biomarkers of inflammation, inflammatory mediators, anti-inflammatory lipid mediators, factors inhibiting inflammation, and other biomarkers of inflammation (e.g., soluble intercellular adhesion molecule-1 and gingival crevicular fluid volume. For a complete list of biomarkers, refer to Supplementary Table S2 and Outcome measures in Supplementary materials). The results differed based on the physiological system. Table 2 provides the direction of association for the three physiological systems combined (CV, respiratory, and oral health), and system-specific findings are outlined below.
For inflammation related to oral health, the majority of cross-sectional studies (79%, 11/14), one of which included exclusive e-cigarette use (65), found that inflammation was significantly higher in daily e-cigarette users than in non-smokers. Comparing daily e-cigarette users to smokers, only one study included exclusive e-cigarette users and found no statistically significant differences in inflammation (103). For the remaining studies, half reported significantly lower levels of inflammation among daily e-cigarette users than among cigarette smokers (60, 63, 92), and the other half reported no significant (53, 97, 103) associations. The inflammation biomarkers included gingival crevicular fluid volume and various cytokines.
For inflammation related to respiratory health, two RCTs (99, 100) reported on daily e-cigarette users, one of which reported on exclusive e-cigarette users (99), and neither found significant differences in inflammation compared to non-smokers. The other RCT (100) found significantly higher inflammation in daily e-cigarette users than in non-smokers. Four of the five cross-sectional studies comparing daily e-cigarette users to non-smokers found no significant differences in inflammation (57, 95, 96, 98), including one that looked at exclusive e-cigarette users (98). One study found significantly higher inflammation in daily e-cigarette users than in non-smokers (58). Respiratory health biomarkers were measured via sputum and serum for pulmonary health, other inflammatory biomarkers included nasal lavage fluid, nasal epithelial lining and bronchoalveolar. For a complete list of biomarkers, refer to Supplementary Table S2 and Outcome measures in Supplementary materials.
For inflammation related to CV health, both RCTs (50, 51) and a cross-sectional study (47) found no significant difference in inflammation between daily e-cigarette users and non-smokers; none were exclusive e-cigarette users. Comparing daily e-cigarette users to smokers, two RCTs (50, 51) reported no significant differences, while one RCT (51) and a cross-sectional study (47) found lower levels of inflammation among daily e-cigarette users. The biomarkers of inflammation included HS-C reactive protein, sICAM-1, fibrinogen, and biomarkers of platelet activation. For a complete list of biomarkers, refer to Supplementary Table S2 and Outcome measures in Supplementary materials.
Immune response
Seventeen studies (47, 50–58, 60, 77, 98–102) assessed immune response between daily e-cigarette users and non-smokers and/or smokers, of which three included exclusive e-cigarette users (55, 98, 99). The biomarkers of immune response included immune infiltration measures, white blood cell counts, platelet activity, and levels of growth factors, proteins, and enzymes associated with immune response. For a complete list of the biomarkers, refer to Supplementary Table S2 and Outcome measures in Supplementary materials. Seven studies explored indicators of immune response linked to respiratory health (54, 57, 58, 98–100, 102), four were related to CV health (47, 50–52), two were related to oral health (53, 60), and four were unrelated to a specific physiological system (55, 56, 77, 101). There were no clear patterns by immune response system, and the results were mixed for both RCT and cross-sectional study designs. Comparing exclusive e-cigarette users to non-smokers, one RCT (99) and a cross-sectional study (98) suggested no statistically significant difference in biomarkers of immune response related to respiratory health. This finding contradicted the RCT (100) results for daily e-cigarette users compared to non-smokers that found statistically significant lower levels for immune response biomarkers following inoculation with live attenuated influenza virus (100). Two out of the three RCTs (51, 52) and a cross-sectional study (47) comparing immune response related to CV health among daily e-cigarette users compared to non-smokers showed no significant differences, while the third RCT (50) showed non-significant differences in fibrinogen levels but higher white blood cells among daily e-cigarette users. A cross-sectional (53) and a case-control study (60) related to oral health (53, 60) and cross-sectional studies related to non-specific physiological system (55, 56, 77, 101) showed mixed findings with non-significant (53, 55, 56, 101), poorer (53, 60), and better (56, 77) immune response for daily e-cigarette users than for non-smokers.
No studies compared exclusive e-cigarette users to smokers. Two of the three RCTs (50, 51) reported no statistically significant differences in immune response biomarkers related to CV health between daily e-cigarette users and smokers, and the third (52) reported statistically significant lower white blood cells in daily e-cigarette users. Cross-sectional associations supported the mixed findings, reporting no statistical significance (53, 56–58, 77, 101), lower (53, 56, 102), and higher immune response (58) for daily e-cigarette users than for smokers.
Oxidative stress
Eight studies assessed differences in biomarkers of oxidative stress between daily e-cigarette users and non-smokers or smokers; three RCTs assessed CV health (50–52), one case-control (60) and one cross-sectional study (58) assessed respiratory health, and three cross-sectional studies were not linked to a specific physiological system (47, 56, 77). None of the studies examined exclusive e-cigarette users. The biomarkers of oxidative stress included salivary malondialdehyde, salivary mucins, and urinary 8-isoprostane. For a complete list of biomarkers, refer to Supplementary Table S2 and Outcome measures in Supplementary materials. The findings from all the RCTs suggested no significant differences in biomarkers of oxidative stress related to CV health between daily e-cigarette users and non-smokers (50, 51). Overall, the cross-sectional studies suggested mixed findings between daily e-cigarette users and non-smokers, with two (47, 77) finding no significant differences (one included markers linked to CV health) and two (58, 60) finding greater oxidative stress among daily e-cigarette users than among non-smokers. Comparing daily e-cigarette users to smokers, two of the three RCTs (50, 51) reported no significant difference, while the third showed statistically significant lower levels of biomarkers of oxidative stress for daily e-cigarette users (50). Cross-sectional associations comparing daily e-cigarette users to smokers suggested no significant differences in 40% (2/5) of the studies (58, 77), while 60% (3/5) showed significantly lower levels of biomarkers for e-cigarette users (47, 56, 60), one of which was related to CV health (47).
Oral health
Twenty-three studies (60–65, 71, 74, 89, 90, 92, 93, 103, 111, 113–121) assessed periodontal (n = 20) and peri-implant (n = 6) oral health, among which 19 included daily e-cigarette use (60–65, 74, 89, 90, 92, 93, 103, 111, 113–118) (15 periodontal and 6 peri-implants) and five (61, 65, 103, 113, 115) included exclusive e-cigarette use (4 periodontal and one peri-implants).
Periodontal health
The periodontal health measures included clinical assessments [e.g., plaque index (PI), bleeding on probing (BOP), probing depth (PD), marginal bone loss (MBL), clinical attachment loss (CAL)], oral hygiene (number of missing teeth), self-reported measures of hard (teeth and bones) and soft (gums) tissues, and combined measures (self-reported dental health complaints and periodontal index).
Fifteen studies (60–65, 71, 74, 89, 90, 92, 93, 103, 111, 113–118) reported on periodontal health comparisons for daily e-cigarette use, four (61, 103, 113, 115) of which reported on exclusive e-cigarette use. From the 15 studies, two were cohort studies (115, 116), one of which assessed exclusive e-cigarette use. The cohort study (115) that compared exclusive e-cigarette users to non-smokers found no significant difference in clinical periodontal parameters at 3- and 6-month follow-ups (i.e., PI, BOP, PD, CAL, and number of missing teeth), while the other cohort showed significantly worse self-reported periodontal parameters (116) in daily e-cigarette users than in non-smokers. Cross-sectional studies on daily e-cigarette use reported no differences in 5/6 (83.3%) of the studies that assessed clinical parameters (61, 89, 93, 103, 118), two of which were on exclusive e-cigarette use (61, 103), while the sixth study showed poorer clinical outcomes in daily e-cigarette users than in non-smokers (114). Similarly, a case control (60) showed worse clinical periodontal outcomes (significantly higher PI, PD, CAL and MBL) for daily e-cigarette users than for non-smokers.
Two of the cross-sectional studies, however, showed poorer oral hygiene (number of missing teeth) for daily e-cigarette users than for non-smokers (103, 117), one of them was on exclusive e-cigarette use (103). One study comparing exclusive e-cigarette users to non-smokers showed a poorer self-reported clinical diagnosis of gum disease, bone loss, or periodontal disease (61). Finally, two studies (113, 118) reported significantly better outcomes for daily e-cigarette users (fewer missing teeth and marginal bone loss) than for non-smokers, and neither of these were on exclusive e-cigarette use.
The studies showed no significant difference in periodontal clinical outcomes but significantly better self-reported outcomes for daily e-cigarette users and exclusive e-cigarette users when compared to smokers. One cohort study showed no significant difference in periodontal clinical parameters between exclusive e-cigarette users and smokers (115). Three cross-sectional studies (60, 61, 90, 118), one of which looked at exclusive e-cigarette use (61) and a case-control supported the non-significant differences in clinical parameters, while two exclusive e-cigarette use studies reported better self-reported gingival health (61) and missing teeth (118) compared to smokers.
Peri-implant
Six studies (62–65, 90, 92) assessed differences in peri-implant parameters between daily e-cigarette users and non-smokers (62–65, 90, 92) and smokers (63, 65, 92), one of which assessed exclusive e-cigarette use (90). The outcomes included clinical periodontal assessments (i.e., PI, BOP, PD) around implants and radiographic parameters including peri-implant bone loss, clinical attachment loss, and radiographic bone loss (RBL). All the studies were cross-sectional, and most reported on more than one clinical outcome. For studies comparing daily e-cigarette users to non-smokers (62–65, 90, 92), most of the results were inconsistent within studies and led to “vote-splitting” (refer to Table 3). Four studies (62–64, 92) showed a mix of results, non-significant differences in PI (62–64, 92) and PD (62), poorer PD (63), BOP, PIBL (62) and MBL (92), and/or better clinical parameter BOP (63, 64, 92) for daily e-cigarette users than for non-smokers. Only one study showed significantly better clinical parameters (90), and only one study included exclusive users (65) showing poorer clinical parameters for the majority of clinical markers (PI, PD, and RBL) than non-smokers. Variable results for outcome measures were also seen within the studies comparing daily e-cigarette users with smokers, also leading to vote-splitting for these studies (63, 65, 92), including the one that assessed exclusive e-cigarette use (65). Clinical parameters of e-cigarette users either failed to differ from those of smokers (63, 90, 92), showed better (62, 63, 92), or worse clinical parameters (63).
Respiratory health
Thirty-two studies (47, 50–52, 54, 59, 66–70, 73, 74, 86, 96, 112, 122–137) reported on respiratory symptoms and lung function, of which 15 (47, 50–52, 54, 66, 68, 73, 74, 96, 122, 126, 131–133) included daily e-cigarette use, two of which were on exclusive e-cigarette use (131, 132). From the 15 studies, 13 (47, 50–52, 54, 66, 68, 73, 96, 122, 131–133) reported on lung function or spirometry measures including forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, peak expiratory force (PEF), forced expiratory time (FET), and maximal forced expiratory flow at 25 and 75% of the pulmonary volume (MFEF25–75) and 10 (47, 50, 68, 73, 74, 96, 122, 126, 132, 133) reported on respiratory symptoms including self-reported coughing, wheezing, chest tightness, difficulties breathing, and phlegm/sputum production.
Lung function
Thirteen studies reported on lung function comparing daily e-cigarette users to non-smokers or smokers, of which nine [two RCTs (50, 51), one cohort (132), and six cross-sectional studies] (47, 54, 66, 96, 122, 131) compared the lung function of daily e-cigarette users to that of non-smokers, and only two of them were on exclusive e-cigarette use (131, 132). The two RCTs (50, 51) and half of the cross-sectional studies (47, 54, 96) comparing daily e-cigarette users to non-smokers and the cohort study (132) comparing exclusive e-cigarette users to non-smokers found no significant differences. Two cross-sectional studies (122, 131), one of which examined exclusive e-cigarette use (66), found statistically significant worse lung function in daily e-cigarette users than in non-smokers (FVC in one study (122) and in most of the measures [FVC, FEV1, FEV1/FVC ratio, and MFEF 25%–75% in the other (131)]). One additional cross-sectional study (66) reported mixed findings (no significant difference in mean alveolar ventilation, mean alveolar perfusion, oxygen saturation, peripheral oxygen saturation, and FVC in daily e-cigarette users but significantly better FEV1 and FEV1/FVC and worse perfusion heterogeneity and ventilation perfusion heterogeneity than in non-smokers). All the studies reporting poorer lung function between daily e-cigarette users and non-smokers were cross-sectional.
Ten studies compared the respiratory function of daily e-cigarette users to that of smokers, and none of them looked at exclusive e-cigarette use. Almost all of the studies reported no differences between these groups, including three out of the four RCTs (50, 51, 73), one cohort (133), and all the cross-sectional studies (47, 54, 96, 122). The other two studies (one RCT and one cohort) reported better lung function in daily e-cigarette users (52, 68) than in smokers.
Respiratory symptoms
Ten studies (two RCTs (52, 73), three cohorts (68, 132, 133), and five cross-sectional studies (47, 74, 96, 122, 126) compared respiratory symptoms between daily e-cigarette users and non-smokers or smokers, of which only one examined exclusive e-cigarette users (132). Among the cross-sectional studies comparing daily e-cigarette users to non-smokers (47, 74, 96, 122, 132), all but one reported no significant difference in respiratory symptoms. The one cohort study that examined exclusive e-cigarette use (132) also found no difference compared to non-smokers. One cross-sectional study (122) did report a significant difference, with daily e-cigarette users demonstrating increased respiratory symptoms when compared to non-smokers.
The results were mixed across the studies comparing daily e-cigarette users to smokers (47, 50, 68, 73, 96, 122, 133). None assessed exclusive e-cigarette use. Three of the four cohort and RCT studies found fewer respiratory symptoms (52, 68, 133); two cohorts (68, 133) found fewer exacerbations of chronic obstructive pulmonary disease (COPD) symptoms among COPD patients using e-cigarettes, one also reported lower (better) COPD assessment test scores (133), and an RCT found significantly less coughing (52) in e-cigarette users than in smokers. However, one RCT (73) reported no significant difference in combined respiratory symptoms in daily e-cigarette users compared to smokers. For the three cross-sectional studies, one showed better respiratory symptoms (47), and two reported non-significant differences (96, 122) between daily e-cigarette users and smokers. Respiratory symptoms were one of the few outcomes where e-cigarette users were compared to dual users. An RCT reported no difference (73) and a cross-sectional study (126) reported better lung function for e-cigarette users than for dual users. None compared exclusive e-cigarette users to smokers or dual users.
Discussion
This systematic review provides a narrative synthesis of the most reported chronic health effects of e-cigarette use since the release of the NASEM review published in 2018 (19). When the NASEM report was drafted, human studies were not available for several plausible e-cigarette use-linked health outcomes (e.g., clinical CV outcomes, changes in respiratory function and respiratory diseases, and pregnancy outcomes), and conclusions were frequently based on evidence from studies using in vitro or animal models. Despite the recent surge in e-cigarette use-related studies (n = 180 studies involving 18 physiological health systems and presenting more than 40 outcomes), gaps in the evidence remain, and this review recognizes the need for stronger study designs and methods. It must also be recognized that the latency for the appearance of symptoms or signs of many health conditions is long; the recency of the introduction and use of e-devices may therefore preclude such findings during the timeframe of their use.
Brief summary
To our knowledge, this is the first systematic review that stratifies outcomes by study design, exclusive e-cigarette use, and duration of e-cigarette exposure comparing e-cigarette users to non-smokers, traditional smokers, or dual-users, and assesses the certainty of evidence. The certainty of the evidence for all 11 outcomes was very low, owing to limitations in reporting and accounting for e-cigarette use exposure (exclusive, daily, and occasional), large cross-sectional designs, heterogeneity in findings, and validity of outcome measures. Only 51% of all the studies [62% of the 11 outcomes (n = 59)] reported on health effects associated with daily e-cigarette users. Moreover, only 20% of all the studies (21% of the 11 outcomes, n = 20), of which 11 were on daily users, reported on exclusive e-cigarette use. The lack of data on exclusive e-cigarette users and on the smoking history of daily e-cigarette users is a problem that makes it difficult to disentangle the long-term effects of e-cigarette use from the confounding effects of past cigarette smoking exposure. Establishing dose-response exposure gradients, both for e-cigarette users (i.e., frequency and duration for exclusive e-cigarette use) and in relation to comparison groups (i.e., compared to non-smokers and frequency and duration for former and current smokers and dual-users), would greatly improve the strength of the evidence by increasing confidence in comparison results. While new evidence has emerged, it is largely based on cross-sectional studies (81.7% of all the studies and 78% among the 11 selected outcomes) where causation is inferred rather than confirmed. Although the review identified some cohort and experimental studies, they were limited because of small samples and the potential for selection bias. There remains a need for ongoing, large, experimental and cohort studies among exclusive e-cigarette users that consider important individual factors such as health status, socioeconomic status, smoking duration, and history using valid and consistent health outcome measures.
In summary, among the experimental and cohort studies that compared exclusive e-cigarette users to non-smokers (n=6), no statistically significant differences was found for CVD (45, 82), respiratory inflammation (99), immune response (99), periodontal clinical parameters (115), peri-implant parameters (65), lung function (132), and respiratory symptoms (132). The relatively short duration of exposure to e-cigarette use may, at this point, limit the likelihood of identifying differences that may well emerge with longer exposure periods.
Comparing exclusive e-cigarette users to smokers, there were no statistically significant differences in biomarkers of oral inflammation (103), periodontal clinical parameters (115), and peri-implant parameters for BOP and PI, and there was better PD and bone loss (65). No studies compared exclusive e-cigarette users to non-smokers or smokers for either CV risk factors or oxidative stress, and none compared the lung function or respiratory symptoms of exclusive e-cigarette users to those of smokers. Moreover, no studies compared exclusive e-cigarette users to dual users.
Comparisons to the literature
This review intended to update and fill the evidence gaps identified in the NASEM report (19) and more recent systematic reviews (32, 33, 35, 36) on chronic CV health effects of e-cigarette use. Previous reviews have found insufficient evidence on CV health outcomes, specifically changes in heart rate (32, 36), blood pressure (32, 35, 36) and cardiac geometry and function (19). Additionally, although based on few studies and limited duration of exposure, previous reviews have found no significant difference in the odds of CVD among e-cigarette users compared to non-smokers (33), but there were significant negative impacts on endothelial function, thrombogenesis, arterial stiffness, and long-term risk for coronary events (36). It is worth noting that the previous review evidence has been drawn from studies with varied exposure assessment methods such as including occasional e-cigarette users and not controlling for former cigarette use (33, 36). Despite the greater number of studies identified by the present review, the overall certainty of the evidence is very low and largely suggests no significant differences between e-cigarette users and non-smokers or smokers in terms of CVD, blood pressure, lipid profiles, and CV function. However, among the few examples where differences were observed, most were in the direction that would be hypothesized by exposure status, that is, e-cigarette users had poorer outcomes than non-smokers but better outcomes than smokers.
The NASEM report (19) also elaborated that although substantive evidence exists that e-cigarette components can induce oxidative stress, there was and remains limited evidence on long term exposure of humans resulting from e-cigarette use. The present review found no significant differences between daily e-cigarette users and non-smokers in biomarkers of oxidative stress related to CV health (50, 51), although the certainty of evidence from the RCT studies was very low. Additionally, no differences were identified in inflammatory biomarkers and immune response between exclusive e-cigarette users and non-smokers (99), but a reduction in oral inflammation in exclusive e-cigarette users compared to smokers (103) was observed. However, the certainty of evidence in all the cases was also very low.
Despite the growing number of studies that examine oral health, the findings from this review are mixed. Consistent results were observed for oral health inflammation, where 11 out of 15 cross-sectional studies reported significantly higher inflammation among daily e-cigarette users than among non-smokers. For most of the other oral health outcomes, however, more recent evidence cannot confirm the previous limited evidence of deterioration in periodontal health of e-cigarette users compared to that of non-smokers or the hypothesis that switching from cigarettes to e-cigarettes (19) reduces the risk of periodontal disease.
Implications for research and practice
Despite the recent surge in e-cigarette use-related studies, gaps in the evidence remain, and this review calls for more research studies with stronger study designs and methods that focus on comparing exclusive e-cigarette users to non-smokers. The majority of studies in this review assessed health outcomes in healthy adults without consideration of within-population differences. As such, the findings of this review may not be generalizable to younger populations and specific subgroups (e.g., females vs. males, or ethnic groups). This is a key concern, as the number of youth who have never smoked previously but have used e-cigarettes is growing (3, 6, 17). More studies examining the health effects and risks in specific subpopulations (e.g., age group, sex, and ethnicity) are needed.
We found COI to be a concern; 20% (12/59) of the studies assessing the chronic health effects of daily e-cigarette use, included in this systematic review had a potential COI resulting from direct or indirect funding or affiliation with the pharmaceutical and tobacco industries Caution should be exercised when interpreting the evidence from these studies. Most studies with a COI reported either favorable or non-significant findings related to e-cigarette use and health outcomes.
We identified a substantial number of studies that reported on both injuries and poisonings (n = 19); asthma (n = 18, daily e-cigarette use = 2); COPD (n = 13, daily e-cigarette use = 3); genetic expressions (n = 12, daily e-cigarette use = 9); metabolic health (n = 9, daily e-cigarette use=3); self-reported health (n = 8, daily e-cigarette use = 2); pregnancy outcomes (n = 7, daily e-cigarette use = 0); cancer (n = 5, daily e-cigarette use = 1); sleep (n = 5, daily e-cigarette use = 0); cognitive health (n = 3, daily e-cigarette use = 1); reproductive health (n = 2, daily e-cigarette use = 1); sensory outcomes (n = 3, daily e-cigarette use = 2); neurological health (n = 1, daily e-cigarette use = 0); COVID-19 outcomes (n = 3, daily e-cigarette use = 1); voice quality (n = 1, daily e-cigarette use = 1), and multiple physiological systems (n = 1, daily e-cigarette use = 1). These studies were not narratively summarized in this review due to lower volume of evidence compared to the other health outcomes we reported on. The evidence is summarized, however, in appendices (available upon request) and recognizes the growing interest in studying the chronic health effects of e-cigarette use.
Strengths and limitations
The strengths of this review include an a priori established study protocol with clear inclusion and exclusion criteria, development of search strategy by consultation with an information specialist, consideration of study design, specific/precise definition of e-cigarette use exposures (i.e., exclusive, daily, and occasional e-cigarette users) to further clarify the associations with various comparison groups (i.e., non-smokers, smokers, and dual users), and use of GRADE to assess the certainty of evidence associated with e-cigarette use.
The review included only in vivo studies on humans and did not take into consideration in vitro/mechanistic studies involving e-cigarettes.
Because of the heterogeneity of outcome assessment methods and effect statistics, the findings were summarized in a narrative manner based on the direction of association and statistical significance; the summary measures of effect and dose-response were not interpreted. Most of the studies did not report on e-cigarette or e-liquid brands or products used. This limited the analysis concerning the health effects of specific e-cigarette products and might have contributed to the mixed findings. Finally, much of the evidence was limited by the lack of report on e-cigarette use characteristics of duration and previous smoking exposure.
Conclusions
This review provides an update on the human in vivo evidence on chronic health effects of e-cigarette use presented in the 2018 NASEM report. While there have been a large number of studies examining the chronic health effects of e-cigarette use, the certainty of the evidence remains very low largely because of cross-sectional study design, few studies with daily or exclusive e-cigarette use exposure, and potential COI. There remains a need for higher-quality interventional and prospective studies to assess causality, with a focus on exclusive e-cigarette users, to improve the certainty in longer-term health outcomes of e-cigarette users when compared to non-smokers, smokers, and dual-users. Additionally, future studies would benefit from exploring effects on different population groups (e.g., youth, young adults, ethnic groups) and effects of different e-cigarette compositions. The variability in the composition of e-cigarette liquids in different e-cigarette brands, with potential varying amounts of nicotine, heavy metals, flavorings, etc., may have contributed to the heterogeneity across the results, and warrants better reporting and investigation in future studies. The recency of the introduction and use of e-cigarette devices may also preclude the identification, at this point, of significant findings reflecting disordered function and enhanced risk of disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RW, FB, MG, SP, SM, and WT conceptualized and designed the study. RW, FB, SP, MG, and WT performed data screening, extraction and analyses, and drafted the initial manuscript. AM, SS, AC, EW, AH, and JL assisted with the data screening and extraction. EW and AH assisted in data analysis and drafting the appendices tables. AP provided expertise for ECU and the physiological systems and assisted in interpreting the findings. All authors reviewed and revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Katherine Merucci from the Public Health Agency of Canada (PHAC)/Health Canada Health Library for her assistance in developing and conducting an updated search strategy. The authors would also like to thank the Substance Related Harms Division at the PHAC for lending their expertise in the development of this review, Alejandra Jaramillo Garcia, MSc from PHAC for her feedback on the GRADE assessment methodology used, Howard Morrison, PhD from PHAC (retired) for his feedback on the interpretation of different physiological systems, Amr Elgamal, MD, FCCP, and director of the intensive care unit at Martinsburg VAMC, USA, Ihab Abdel Fatah, MBBCh, PhD, Faculty of Medicine, Menoufia University, Ahmed Abdel Hamid, DDS, PhD and Nahed Ateya, DDM, PhD, Faculty of Dentistry Alexandria University, and Fathia Awny, MBBCh, MSc, Ministry and Health and Population, Egypt (retired), for their feedback on the initial interpretation and grouping of the respiratory, CV, oral health, and immunological outcomes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the government of Canada.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.959622/full#supplementary-material
Abbreviations
BOP, bleeding on probing; CAL, clinical attachment loss; CASP; Critical Appraisals Skills Programme; COI, conflict of interest; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; CVD, cardiovascular disease; DUs, dual users; EVALI, e-cigarette- or vaping-associated lung illness; FEV1, first second of forced expiration; FVC, forced vital capacity; FET, forced expiratory time; GCF, gingival crevicular fluid; GRADE, grading of recommendation assessment, development, and evaluation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HNB, heat-not-burn; MBL, marginal bone loss; MFEF, maximal forced expiratory flow; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; NASEM, National Academies of Sciences, Engineering, and Medicine; PEF, peak expiratory force; PD, probing depth; PI, plaque index; PHAC, Public Health Agency of Canada; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RBL, radiographic bone loss; RoB, risk of bias; THC, tetrahydrocannabinol; TSs, traditional smokers; USA, United States of America.
References
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. (2019) 143:e20182741. doi: 10.1542/peds.2018-2741
2. Shiplo S, Czoli CD, Hammond D. E-cigarette use in Canada: prevalence and patterns of use in a regulated market. BMJ Open. (2015) 5:e007971. doi: 10.1136/bmjopen-2015-007971
3. Hammond D, Reid JL, Rynard VL, Fong GT, Cummings KM, McNeill A, et al. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: repeat national cross sectional surveys. BMJ. (2019) 365:l2219. doi: 10.1136/bmj.l2219
4. Reid JL, Rynard VL, Czoli CD, Hammond D. Who is using e-cigarettes in Canada? Nationally representative data on the prevalence of e-cigarette use among Canadians. Prev Med. (2015) 81:180–3. doi: 10.1016/j.ypmed.2015.08.019
5. Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB. Changes in electronic cigarette use among adults in the United States, 2014-2016. JAMA. (2018) 319:2039–41. doi: 10.1001/jama.2018.4658
6. Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students - United States, 2011-2018. MMWRMorbidity Mortal Wkly Rep. (2018) 67:1276–7. doi: 10.15585/mmwr.mm6745a5
7. Laverty AA, Filippidis FT, Vardavas CI. Patterns, trends and determinants of e-cigarette use in 28 European Union Member States 2014–2017. Prev Med. (2018) 116:13–8. doi: 10.1016/j.ypmed.2018.08.028
8. Jerzyński T, Stimson G V, Shapiro H, Król G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct J. (2021) 18:1–10. doi: 10.1186/s12954-021-00556-7
9. Government of C. About vaping, Vol. 2020. (2019). Available online at: https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping.html (accessed April 15, 2022).
10. Hua M, Talbot P. Potential health effects of electronic cigarettes: a systematic review of case reports. Prev Med Rep. (2016) 4:169–78. doi: 10.1016/j.pmedr.2016.06.002
11. Government of C. Injuries and poisonings from vaping products including e-cigarettes, Vol. 2019. (2018). Available online at: https://health-infobase.canada.ca/datalab/ecigarette-blog.html (accessed March 21, 2022).
12. Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B. E-Cigarettes: A review of new trends in cannabis use. Int J Environ Res Public Health. (2015) 12:9988–10008. doi: 10.3390/ijerph120809988
13. Kinnunen JM, Rimpelä AH, Lindfors PL, Clancy L, Alves J, Hoffmann L, et al. Electronic cigarette use among 14-to 17-year-olds in Europe. Eur J Public Health. (2021) 31:402–8. doi: 10.1093/eurpub/ckaa145
14. Government of Canada. Canadian Tobacco and Nicotine Survey (CTNS): 2013 summary. Government of Canada. (2015). Available online at: https://www.canada.ca/en/health-canada/services/canadian-alcohol-drugs-survey/2013-summary.html (accessed February 8, 2022).
15. Government of Canada. Canadian Tobacco and Nicotine Survey (CTNS): summary of results for 2019. Government of Canada. (2020). Available online at: https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary.html#n4 (accessed February 8, 2022).
16. Gorini G, Gallus S, Carreras G, De Mei B, Masocco M, Faggiano F, et al. Prevalence of tobacco smoking and electronic cigarette use among adolescents in Italy: Global Youth Tobacco Surveys (GYTS), 2010, 2014, 2018. Prev Med. (2020) 131:105903. doi: 10.1016/j.ypmed.2019.105903
17. Hammond D, Rynard VL, Reid JL. Changes in prevalence of vaping among youths in the United States, Canada, and England from 2017 to 2019. JAMA Pediatr. (2020) 174:797–800. doi: 10.1001/jamapediatrics.2020.0901
18. Corey CG, Chang JT, Rostron BL. Electronic nicotine delivery system (ENDS) battery-related burns presenting to US emergency departments, 2016. Inj Epidemiol. (2018) 5:4. doi: 10.1186/s40621-018-0135-1
19. National Academies of Sciences E, Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press (2018).
20. Ang E, Tuthill D, Thompson J. E-cigarette liquid ingestion: a fast growing accidental issue in children. Arch Dis Child. (2018) 103:1091. doi: 10.1136/archdischild-2018-314886
21. Wylie C, Heffernan A, Brown JA, Cairns R, Lynch AM, Robinson J. Exposures to e-cigarettes and their refills: calls to Australian Poisons Information Centres, 2009-2016. Med J Aust. (2019) 210:126. doi: 10.5694/mja2.12032
22. Rossheim ME, McDonald KK, Soule EK, Gimm GW, Livingston MD, Barnett TE, et al. Electronic cigarette explosion/burn and poisoning related emergency department visits, 2018-2019. Am J Emerg Med. (2020) 38:2637–40. doi: 10.1016/j.ajem.2020.08.017
23. Tsai MC, Byun MK, Shin J, Crotty Alexander LE. Effects of e-cigarettes and vaping devices on cardiac and pulmonary physiology. J Physiol. (2020) 598:5039–62. doi: 10.1113/JP279754
24. Chatterjee S, Caporale A, Tao JQ, Guo W, Johncola A, Strasser AA, et al. Acute e-cig inhalation impacts vascular health: a study in smoking naïve subjects. Am J Physiol - Hear Circ Physiol. (2021) 320:H144–58. doi: 10.1152/ajpheart.00628.2020
25. Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, DeSisto CL, et al. Characteristics of a multistate outbreak of lung injury associated with e-cigarette use, or vaping - United States, 2019. MMWRMorbidity Mortal Wkly Rep. (2019) 68:860–4. doi: 10.15585/mmwr.mm6839e1
26. Centers for Disease C Prevention. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products, Vol. 2020 (2020). Available online at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed September 14, 2022).
27. Government of C. Vaping-associated lung illness, Vol. 2020. (2020). Available online at: https://www.canada.ca/en/public-health/services/diseases/vaping-pulmonary-illness.html (accessed March 21, 2022).
28. Baker MM, Procter TD, Belzak L, Ogunnaike-Cooke S. Vaping-associated lung illness (VALI) in Canada: a descriptive analysis of VALI cases reported from September 2019 to December 2020. Health Promot Chronic Dis Prev Canada. (2022) 42:37–44. doi: 10.24095/hpcdp.42.1.06
29. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes, Vol. 69. (2014), p. 248–60. doi: 10.1016/j.ypmed.2014.10.009
30. Yang I, Sandeep S, Rodriguez J. The oral health impact of electronic cigarette use: a systematic review. Crit Rev Toxicol. (2020) 50:97–127. doi: 10.1080/10408444.2020.1713726
31. Kennedy CD. van SM, McKee M, Pisinger C. The cardiovascular effects of electronic cigarettes: a systematic review of experimental studies. Prev Med. (2019) 127:105770. doi: 10.1016/j.ypmed.2019.105770
32. Garcia PD, Gornbein JA, Middlekauff HR. Cardiovascular autonomic effects of electronic cigarette use: a systematic review. Clin Auton Res. (2020) 30:507–19. doi: 10.1007/s10286-020-00683-4
33. Goniewicz ML, Miller CR, Sutanto E, Li D. How effective are electronic cigarettes for reducing respiratory and cardiovascular risk in smokers? A systematic review. Harm Reduct J. (2020) 17:1–9. doi: 10.1186/s12954-020-00440-w
34. Cardenas VM, Fischbach LA, Chowdhury P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: a systematic review of the literature. Tob Induc Dis. (2019) 17:52. doi: 10.18332/tid/104724
35. Martinez-Morata I, Sanchez TR, Shimbo D, Navas-Acien A. Electronic cigarette use and blood pressure endpoints: a systematic review. Curr Hypertens Rep. (2021) 23:1–10. doi: 10.1007/s11906-020-01119-0
36. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, et al. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol. (2019) 26:1219–28. doi: 10.1177/2047487319832975
37. Wills TA, Soneji SS, Choi K, Jaspers I, Tam EK. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur Respir J. (2021) 57:1901815. doi: 10.1183/13993003.01815-2019
38. Malas M, van der Tempel J, Schwartz R, Minichiello A, Lightfoot C, Noormohamed A, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. (2016) 18:1926–36. doi: 10.1093/ntr/ntw119
39. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. (2016) 4:116–28. doi: 10.1016/S2213-2600(15)00521-4
40. Bang F, Wasfi R, DeGroh M, Prince Ware S. Chronic health effects associated with vaping: a rapid review. OSF. (2021). Available online at: https://osf.io/u9btp/
41. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
42. Critical Appraisal Skills Programme. CASP Checklists. UK. (2018). Available online at: https://casp-uk.net/casp-tools-checklists/ (accessed September 15, 2020).
43. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
44. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. (2017) 22:85–7. doi: 10.1136/ebmed-2017-110668
45. Vindhyal MR, Okut H, Ablah E, Ndunda PM, Kallail KJ, Choi WS. Cardiovascular outcomes associated with adult electronic cigarette use. Cureus. (2020) 12:e9618. doi: 10.7759/cureus.9618
46. Ikonomidis I, Vlastos D, Kourea K, Kostelli G, Varoudi M, Pavlidis G, et al. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette. Circulation. (2018) 137:303–6. doi: 10.1161/CIRCULATIONAHA.117.029153
47. Sakaguchi C, Nagata Y, Kikuchi A, Takeshige Y, Minami N. Differences in levels of biomarkers of potential harm among users of a heat-not-burn tobacco product, cigarette smokers, and never-smokers in Japan: a post-marketing observational study. Nicotine Tob Res. (2021) 23:1143–52. doi: 10.1093/ntr/ntab014
48. Kim CY, Paek YJ, Seo HG, Cheong YS, Lee CM, Park SM, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep. (2020) 10:5612. doi: 10.1038/s41598-020-62545-3
49. Podzolkov VI, Bragina AE, Druzhinina NA, Vasil'eva LV, Osadchiy KK, Dubchak AE, et al. Relation between tobacco smoking/electronic smoking and albuminuria/vascular stiffness in young people without cardiovascular diseases. Kidney Blood Press Res. (2020) 45:467–76. doi: 10.1159/000507510
50. Haziza C, Bourdonnaye de La, Donelli A, Skiada D, Poux V, Weitkunat R, et al. Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the menthol tobacco heating system 2.2 for three months (Part 2). Nicotine Tob Res. (2020) 22:549–59. doi: 10.1093/ntr/ntz084
51. Ludicke F, Picavet P, Baker G, Haziza C, Poux V, Lama N, et al. Effects of switching to the menthol tobacco heating system 22, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settin. Nicotine Tob Res. (2018) 20:173–82. doi: 10.1093/ntr/ntx028
52. Ludicke F, Ansari SM, Lama N, Blanc N, Bosilkovska M, Donelli A, et al. Effects of switching to a heat-not-burn tobacco product on biologically-relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol Biomarkers Prev. (2019) 28:1934–43. doi: 10.1158/1055-9965.EPI-18-0915
53. Cichonska D, Kusiak A, Kochanska B, Ochocinska J, Swietlik D. Influence of electronic cigarettes on selected antibacterial properties of saliva. Int J Environ Res Public Health. (2019) 16:4433. doi: 10.3390/ijerph16224433
54. Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR, Alexis NE, et al. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. (2019) 200:1392–401. doi: 10.1164/rccm.201903-0615OC
55. Jackson M, Singh KP, Lamb T, McIntosh S, Rahman I. Flavor preference and systemic immunoglobulin responses in e-cigarette users and waterpipe and tobacco smokers: a pilot study. Int J Environ Res Public Health. (2020) 17:640. doi: 10.3390/ijerph17020640
56. Oliveri D, Liang Q, Sarkar M. Real-world evidence of differences in biomarkers of exposure to select harmful and potentially harmful constituents and biomarkers of potential harm between adult e-vapor users and adult cigarette smokers. Nicotine Tob Res. (2020) 22:1114–22. doi: 10.1093/ntr/ntz185
57. Song MA, Freudenheim JL, Brasky TM, Mathe EA, McElroy JP, Nickerson QA, et al. Biomarkers of exposure and effect in the lungs of smokers, nonsmokers, and electronic cigarette users. Cancer Epidemiol Biomarkers Prev. (2020) 29:443–51. doi: 10.1158/1055-9965.EPI-19-1245
58. Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. (2018) 197:492–501. doi: 10.1164/rccm.201708-1590OC
59. Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR, et al. Systemic biomarkers in electronic cigarette users: implications for non-invasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. (2019) 5:182–2019. doi: 10.1183/23120541.00182-2019
60. Karaaslan F, Dikilitas A, Yigit U. The effects of vaping electronic cigarettes on periodontitis. Aust Dent J. (2020) 65:143–9. doi: 10.1111/adj.12747
61. Javed F, Abduljabbar T, Vohra F, Malmstrom H, Rahman I, Romanos GE. Comparison of periodontal parameters and self-perceived oral symptoms among cigarette smokers, individuals vaping electronic cigarettes, and never-smokers. J Periodontol. (2017) 88:1059–65. doi: 10.1902/jop.2017.170197
62. Al-Aali K, ArRejaie A, Abduljabbar T, Vohra F, Akram Z. Peri-implant parameters, tumor necrosis factor-alpha, and interleukin-1 beta levels in vaping individuals. Clin Implant Dent Relat Res. (2018) 20:410–5. doi: 10.1111/cid.12597
63. Al Deeb M, Alresayes S, Mokeem SA, Alhenaki A, AlHelal A, Shafqat S, et al. Clinical and immunological peri-implant parameters among cigarette and electronic smoking patients treated with photochemotherapy: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2020) 31:101800. doi: 10.1016/j.pdpdt.2020.101800
64. Sinha DK, Vishal Kumar A, Khan M, Kumari R, Kesari M. Evaluation of tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-1beta levels among subjects vaping e-cigarettes and nonsmokers. J Fam Med Prim care. (2021) 9:1072–5. doi: 10.4103/jfmpc.jfmpc_902_19
65. AlQahtani MA, Alayad AS, Alshihri A, Correa FOB, Akram Z. Clinical peri-implant parameters and inflammatory cytokine profile among smokers of cigarette, e-cigarette, and waterpipe. Clin Implant Dent Relat Res. (2018) 20:1016–21. doi: 10.1111/cid.12664
66. Kizhakke Puliyakote AS, Elliott AR, Sa RC, Anderson KM, Crotty Alexander LE, Hopkins SR. Vaping disrupts ventilation-perfusion matching in asymptomatic users. J Appl Physiol. (2021) 130):308–17. doi: 10.1152/japplphysiol.00709.2020
67. Bowler RP, Hansel NN, Jacobson S, Barr RG, Make BJ, Han MK, et al. Electronic cigarette use in US adults at risk for or with COPD: Analysis from two observational cohorts. J Gen Intern Med. (2017) 32:1315–22. doi: 10.1007/s11606-017-4150-7
68. Polosa R, Morjaria JB, Prosperini U, Busa B, Pennisi A, Malerba M, et al. COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up. Ther Adv Chronic Dis. (2020) 11:2040622320961617. doi: 10.1177/2040622320961617
69. Alnajem A, Redha A, Alroumi D, Alshammasi A, Ali M, Alhussaini M, et al. Use of electronic cigarettes and secondhand exposure to their aerosols are associated with asthma symptoms among adolescents: a cross-sectional study. Respir Res. (2020) 21:300. doi: 10.1186/s12931-020-01569-9
70. Tackett AP, Keller-Hamilton B, Smith CE, Hebert ET, Metcalf JP, Queimado L, et al. Evaluation of respiratory symptoms among youth e-cigarette users. JAMA Netw Open. (2020) 3:e2020671. doi: 10.1001/jamanetworkopen.2020.20671
71. Akinkugbe AA. Cigarettes, e-cigarettes, and adolescents' oral health: findings from the population assessment of tobacco and Health (PATH) Study. JDR Clin Transl Res. (2019) 4:276–83. doi: 10.1177/2380084418806870
72. George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, et al. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol. (2019) 74:3112–20. doi: 10.1016/j.jacc.2019.09.067
73. Pulvers K, Nollen NL, Rice M, Schmid CH, Qu K, Benowitz NL, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among african american and latinx smokers: a randomized clinical trial. JAMA Netw Open. (2020) 3:26324. doi: 10.1001/jamanetworkopen.2020.26324
74. Aherrera A, Aravindakshan A, Jarmul S, Olmedo P, Chen R, Cohen JE, et al. E-cigarette use behaviors and device characteristics of daily exclusive e-cigarette users in Maryland: implications for product toxicity. Tob Induc Dis. (2020) 18:93. doi: 10.18332/tid/128319
75. Arastoo S, Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, et al. Acute and chronic sympathomimetic effects of e-cigarette and tobacco cigarette smoking: role of nicotine and non-nicotine constituents. Am J Physiol - Hear Circ Physiol. (2020) 319:H262–70. doi: 10.1152/ajpheart.00192.2020
76. Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med. (2018) 55:455–61. doi: 10.1016/j.amepre.2018.05.004
77. Badea M, Gaman L, Delia C, Ilea A, Leasu F, Henriquez-Hernandez LA, et al. Trends of lipophilic, antioxidant and hematological parameters associated with conventional and electronic smoking habits in middle-age Romanians. J Clin Med. (2019) 8:665. doi: 10.3390/jcm8050665
78. Farsalinos KE, Polosa R, Cibella F, Niaura R. Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Ther Adv Chronic Dis. (2019) 10:1–10. doi: 10.1177/2040622319877741
79. Fetterman JL, Keith RJ, Palmisano JN, McGlasson KL, Weisbrod RM, Majid S, et al. Alterations in vascular function associated with the use of combustible and electronic cigarettes. J Am Heart Assoc. (2020) 9:e014570. doi: 10.1161/JAHA.119.014570
80. Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Lakhani K, et al. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am J Physiol Heart Circ Physiol. (2020) 319:H547–56. doi: 10.1152/ajpheart.00307.2020
81. Ip M, Diamantakos E, Haptonstall K, Choroomi Y, Moheimani RS, Nguyen KH, et al. Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: implication for sudden death risk. Am J Physiol Circ Physiol. (2020) 318:H1176–84. doi: 10.1152/ajpheart.00738.2019
82. Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, et al. Association between e-cigarette use and cardiovascular disease among never and current combustible-cigarette smokers. Am J Med. (2019) 132:949. doi: 10.1016/j.amjmed.2019.02.016
83. Rodu B, Plurphanswat N. A re-analysis of e-cigarette use and heart attacks in PATH wave 1 data. Addiction. (2020) 115:2176–9. doi: 10.1111/add.15067
84. Leavens ELS, Ford BR, Ojo-Fati O, Winkelman TNA, Vickery KD, Japuntich SJ, et al. Electronic cigarette use patterns and chronic health conditions among people experiencing homelessness in MN: a statewide survey. BMC Public Health. (2020) 1:20. doi: 10.1186/s12889-020-09919-4
85. Parekh T, Pemmasani S, Desai R. Risk of stroke with e-cigarette and combustible cigarette use in young adults. Am J Prev Med. (2020) 58:446–52. doi: 10.1016/j.amepre.2019.10.008
86. Wang JB, Olgin JE, Nah G, Vittinghoff E, Cataldo JK, Pletcher MJ, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS ONE. (2018) 13:e0198681. doi: 10.1371/journal.pone.0198681
87. Ikonomidis I, Katogiannis K, Kostelli G, Kourea K, Kyriakou E, Kypraiou A, et al. Effects of electronic cigarette on platelet and vascular function after four months of use. Food Chem Toxicol. (2020) 141:111389. doi: 10.1016/j.fct.2020.111389
88. Kim T, Choi H, Kang J, Kim J. Association between electronic cigarette use and metabolic syndrome in the Korean general population: a nationwide population-based study. PLoS ONE. (2020) 15:e0237983. doi: 10.1371/journal.pone.0237983
89. Al-Hamoudi N, Alsahhaf A, Al Deeb M, Alrabiah M, Vohra F, Abduljabbar T. Effect of scaling and root planing on the expression of anti-inflammatory cytokines (IL-4, IL-9, IL-10, and IL-13) in the gingival crevicular fluid of electronic cigarette users and non-smokers with moderate chronic periodontitis. J Periodontal Implant Sci. (2020) 50:74–82. doi: 10.5051/jpis.2020.50.2.74
90. Alqahtani F, Alqahtani M, Albaqawi AH, Al-Kheraif A, Javed F. Comparison of cotinine levels in the peri-implant sulcular fluid among cigarette and waterpipe smokers, electronic-cigarette users, and nonsmokers. Clin Implant Dent Relat Res. (2019) 21:702–7. doi: 10.1111/cid.12813
91. Alqahtani S, Cooper B, Spears CA, Wright C, Shannahan J. Electronic nicotine delivery system-induced alterations in oral health via saliva assessment. Exp Biol Med. (2020) 245:1319–25. doi: 10.1177/1535370220941258
92. ArRejaie AS. Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers. J Periodontol. (2019) 90:367–74. doi: 10.1002/JPER.18-0045
93. BinShabaib M, ALHarthi SS, Akram Z, Khan J, Rahman I, Romanos GE, et al. Clinical periodontal status and gingival crevicular fluid cytokine profile among cigarette-smokers, electronic-cigarette users and never-smokers. Arch Oral Biol. (2019) 102:212–7. doi: 10.1016/j.archoralbio.2019.05.001
94. Ganesan SM, Dabdoub SM, Nagaraja HN, Scott ML, Pamulapati S, Berman ML, et al. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci Adv. (2020) 6:aaz0108. doi: 10.1126/sciadv.aaz0108
95. Gavrilin MA, McAndrew CC, Prather ER, Tsai M, Spitzer CR, Song MA, et al. Inflammasome adaptor ASC is highly elevated in lung over plasma and relates to inflammation and lung diffusion in the absence of speck formation. Front Immunol. (2020) 11:461. doi: 10.3389/fimmu.2020.00461
96. Perez MF, Atuegwu NC, Mortensen EM, Oncken C. The inflammatory biomarker YKL-40 is elevated in the serum, but not the sputum, of e-cigarette users. Exp Lung Res. (2021) 47:55–66. doi: 10.1080/01902148.2020.1847216
97. Pushalkar S, Paul B, Li Q, Yang J, Vasconcelos R, Makwana S, et al. Electronic cigarette aerosol modulates the oral microbiome and increases risk of infection. iScience. (2020) 23:100884. doi: 10.1016/j.isci.2020.100884
98. Lee AC, Chakladar J, Li WT, Chen C, Chang EY, Wang-Rodriguez J, et al. (2020). Tobacco, but not nicotine and flavor-less electronic cigarettes, induces ace2 and immune dysregulation. Int J Mol Sci, 21, 1–16. doi: 10.3390/ijms21155513
99. Song MA, Reisinger SA, Freudenheim JL, Brasky TM, Mathe EA, McElroy JP, et al. Effects of electronic cigarette constituents on the human lung: a pilot clinical trial. Cancer Prev Res. (2020) 13:145–51. doi: 10.1158/1940-6207.CAPR-19-0400
100. Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, et al. Electronic-cigarette use alters nasal mucosal immune response to live-attenuated influenza virus: a clinical trial. Am J Respir Cell Mol Biol. (2021) 64:126–37. doi: 10.1165/rcmb.2020-0164OC
101. Kelesidis T, Tran E, Arastoo S, Lakhani K, Heymans R, Gornbein J, et al. Elevated cellular oxidative stress in circulating immune cells in otherwise healthy young people who use electronic cigarettes in a cross-sectional single-center study: implications for future cardiovascular risk. J Am Heart Assoc. (2020) 9:e016983. doi: 10.1161/JAHA.120.016983
102. Shields PG, Song MA, Freudenheim JL, Brasky TM, McElroy JP, Reisinger SA, et al. Lipid laden macrophages and electronic cigarettes in healthy adults. EBioMedicine. (2020) 60:102982. doi: 10.1016/j.ebiom.2020.102982
103. Mokeem SA, Alasqah MN, Michelogiannakis D, Al-Kheraif AA, Romanos GE, Javed F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1beta and IL-6 levels in cigarette- and waterpipe-smokers and E-cig users. Environ Toxicol Pharmacol. (2018) 61:38–43. doi: 10.1016/j.etap.2018.05.016
104. Faridoun A, Sultan AS, Jabra-Rizk MA, Weikel D, Varlotta S, Meiller TF. Salivary biomarker profiles in E-cigarette users and conventional smokers: A cross-sectional study. Oral Dis. (2021) 27:277279. doi: 10.1111/odi.13533
105. Mainous AG, Yadav S, Hong YR, Huo J. e-Cigarette and conventional tobacco cigarette use, dual use, and C-reactive protein. J Am Coll Cardiol. (2020) 75:22712273. doi: 10.1016/j.jacc.2020.02.061
106. Menicagli R, Marotta O, Serra R. Free radical production in the smoking of e-cigarettes and their possible effects in human health. Int J Prev Med. (2020) 11:53. doi: 10.4103/ijpvm.IJPVM_424_19
107. Moon J, Lee H, Kong M, Kim H, Oh Y. Association between electronic cigarette use and levels of high-sensitivity C-reactive protein and uric acid. Asia Pac J Public Health. (2020) 32:3541. doi: 10.1177/1010539519899777
108. Sakamaki-Ching S, Williams M, Hua M, Li J, Bates SM, Robinson AN, et al. Correlation between biomarkers of exposure, effect and potential harm in the urine of electronic cigarette users. BMJ Open Respir Res. (2020) 7:e000452. doi: 10.1136/bmjresp-2019-000452
109. Stokes AC, Xie W, Wilson AE, Yang H, Orimoloye OA, Harlow AF, et al. Association of cigarette and electronic cigarette use patterns with levels of inflammatory and oxidative stress biomarkers among US adults: Population assessment of tobacco and health study. Circulation. (2021) 143:869–71. doi: 10.1161/CIRCULATIONAHA.120.051551
110. Ye D, Gajendra S, Lawyer G, Jadeja N, Pishey D, Pathagunti S, et al. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: A pilot study. J Periodontol. (2020) 91:12741283. doi: 10.1002/JPER.19-0457
111. Ibraheem WI, Fageeh HI, Preethanath RS, Alzahrani FA, Al-Zawawi AS, Divakar DD, et al. Comparison of RANKL and osteoprotegerin levels in the gingival crevicular fluid of young cigarette- and waterpipe-smokers and individuals using electronic nicotine delivery systems. Arch Oral Biol. (2020) 115:104714. doi: 10.1016/j.archoralbio.2020.104714
112. Ashford K, McCubbin A, Rayens MK, Wiggins A, Dougherty K, Sturgill J, et al. ENDS use among college students: salivary biomarkers and persistent cough. Addict Behav. (2020) 108:106462. doi: 10.1016/j.addbeh.2020.106462
113. Mokeem SA, Abduljabbar T, Al-Kheraif AA, Alasqah MN, Michelogiannakis D, Samaranayake LP, et al. Oral Candida carriage among cigarette- and waterpipe-smokers, and electronic cigarette users. Oral Dis. (2019) 25:319–26. doi: 10.1111/odi.12902
114. Aldakheel FM, Alduraywish SA, Jhugroo P, Jhugroo C, Divakar DD. Quantification of pathogenic bacteria in the subgingival oral biofilm samples collected from cigarette-smokers, individuals using electronic nicotine delivery systems and non-smokers with and without periodontitis. Arch Oral Biol. (2020) 117:104793. doi: 10.1016/j.archoralbio.2020.104793
115. ALHarthi SS, BinShabaib M, Akram Z, Rahman I, Romanos GE, Javed F. Impact of cigarette smoking and vaping on the outcome of full-mouth ultrasonic scaling among patients with gingival inflammation: a prospective study. Clin Oral Investig. (2019) 23:2751–8. doi: 10.1007/s00784-018-2725-2
116. Atuegwu NC, Perez MF, Oncken C, Thacker S, Mead EL, Mortensen EM. Association between regular electronic nicotine product use and self-reported periodontal disease status: population assessment of tobacco and health survey. Int J Environ Res Public Health. (2019) 16:1263. doi: 10.3390/ijerph16071263
117. Huilgol P, Bhatt SP, Biligowda N, Wright NC, Wells JM. Association of e-cigarette use with oral health: a population-based cross-sectional questionnaire study. J Public Health. (2019) 41:354–61. doi: 10.1093/pubmed/fdy082
118. Vohra F, Bukhari IA, Sheikh SA, Albaijan R, Naseem M. Comparison of self-rated oral symptoms and periodontal status among cigarette smokers and individuals using electronic nicotine delivery systems. J Am Coll Health. (2020) 68:788–93. doi: 10.1080/07448481.2019.1709476
119. Vora MV, Chaffee BW. Tobacco-use patterns and self-reported oral health outcomes: a cross-sectional assessment of the Population Assessment of Tobacco and Health study, 2013-2014. J Am Dent Assoc. (2019) 150:332–44. doi: 10.1016/j.adaj.2018.12.004
120. Jeong W, Choi DW, Kim YK, Lee HJ, Lee SA, Park EC, et al. Associations of electronic and conventional cigarette use with periodontal disease in South Korean adults. J Periodontol. (2020) 91:55–64. doi: 10.1002/JPER.19-0060
121. Ghazali AF, Ismail AF, Daud A. Caries experience among cigarette and E-cigarette users: a 6-month prospective study. J Pharm Sci Res. (2019) 11:2566–9.
122. AboElNaga HH. Electronic cigarettes: not an advantageous alternative to conventional smoking in asthma. Egypt J Chest Dis Tuberc. (2018) 67:427–32. doi: 10.4103/ejcdt.ejcdt_83_18
123. Boddu SA, Bojanowski CM, Lam MT, Advani IN, Scholten EL, Sun X, et al. Use of e-cigarettes with conventional tobacco is associated with decreased sleep quality in women. Am J Respir Crit Care Med. (2019) 200:1431–4. doi: 10.1164/rccm.201904-0890LE
124. Braymiller JL, Barrington-Trimis JL, Leventhal AM, Islam T, Kechter A, Krueger EA, et al. Assessment of nicotine and cannabis vaping and respiratory symptoms in young adults. JAMA Netw Open. (2020). doi: 10.1001/jamanetworkopen.2020.30189
125. Brozek GM, Jankowski M, Zejda JE. Acute respiratory responses to the use of e-cigarette: an intervention study. Sci Rep. (2019) 9:6844. doi: 10.1038/s41598-019-43324-1
126. Cassidy RN, Tidey JW, Colby SM. Exclusive e-cigarette users report lower levels of respiratory symptoms relative to dual e-cigarette and cigarette users. Nicotine Tob Res. (2020) 22:S54–60. doi: 10.1093/ntr/ntaa150
127. Diamantopoulou E, Barbouni A, Merakou K, Lagiou A, Farsalinos K. Patterns of e-cigarette use, biochemically verified smoking status and self-reported changes in health status of a random sample of vapeshops customers in Greece. Intern Emerg Med. (2019) 14:843–51. doi: 10.1007/s11739-018-02011-1
128. Giovanni SP, Keller TL, Bryant AD, Weiss NS. Littman AJ. Electronic cigarette use and chronic respiratory symptoms among US adults. Am J Respir Crit Care Med. (2020) 201:1157–60. doi: 10.1164/rccm.201907-1460LE
129. Hedman L, Backman H, Stridsman C, Bosson JA, Lundback M, Lindberg A, et al. Association of electronic cigarette use with smoking habits, demographic factors, and respiratory symptoms. JAMA Netw Open. (2018) 1:e180789. doi: 10.1001/jamanetworkopen.2018.0789
130. Li D, Sundar IK, McIntosh S, Ossip DJ, Goniewicz ML, O'Connor RJ, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. (2019) 29:140–7. doi: 10.1136/tobaccocontrol-2018-054694
131. Meo SA, Ansary MA, Barayan FR, Almusallam AS, Almehaid AM, Alarifi NS, et al. Electronic cigarettes: impact on lung function and fractional exhaled nitric oxide among healthy adults. Am J Mens Health. (2019) 13:1557988318806073. doi: 10.1177/1557988318806073
132. Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, et al. Health impact of E-cigarettes: a prospective 35-year study of regular daily users who have never smoked. Sci Rep. (2017) 7:13825. doi: 10.1038/s41598-017-14043-2
133. Polosa R, Morjaria JB, Prosperini U, Russo C, Pennisi A, Puleo R, et al. Health effects in COPD smokers who switch to electronic cigarettes: a retrospective-prospective 3-year follow-up. Int J COPD. (2018) 13:2533–42. doi: 10.2147/COPD.S161138
134. Schneller LM, Quinones Tavarez Z, Goniewicz ML, Xie Z, McIntosh S, Rahman I, et al. Cross-sectional association between exclusive and concurrent use of cigarettes, ENDS, and cigars, the three most popular tobacco products, and wheezing symptoms among US adults. Nicotine Tob Res. (2020) 22:S76–84. doi: 10.1093/ntr/ntaa199
135. Dai H, Khan AS. A longitudinal study of exposure to tobacco-related toxicants and subsequent respiratory symptoms among U.S. adults with varying e-cigarette use status. Nicotine Tobacco Res. (2020) 22:S61–9. doi: 10.1093/ntr/ntaa180
136. Xie W, Kathuria H, Galiatsatos P, Blaha MJ, Hamburg NM, Robertson RM, et al. Association of electronic cigarette use with incident respiratory conditions among US adults from 2013 to 2018. JAMA Netw Open. (2020) 3:e2020816. doi: 10.1001/jamanetworkopen.2020.20816
Keywords: vaping, E-cigarette (e-cig), electronic cigarette, health, chronic health effects
Citation: Wasfi RA, Bang F, de Groh M, Champagne A, Han A, Lang JJ, McFaull SR, Melvin A, Pipe AL, Saxena S, Thompson W, Warner E and Prince SA (2022) Chronic health effects associated with electronic cigarette use: A systematic review. Front. Public Health 10:959622. doi: 10.3389/fpubh.2022.959622
Received: 01 June 2022; Accepted: 29 August 2022;
Published: 06 October 2022.
Edited by:
Tilman Steinert, ZfP Südwürttemberg, GermanyReviewed by:
Cassio Almeida-da-Silva, University of the Pacific, United StatesLuke Clancy, Dublin Institute of Technology, Ireland
Copyright © 2022 Wasfi, Bang, de Groh, Champagne, Han, Lang, McFaull, Melvin, Pipe, Saxena, Thompson, Warner and Prince. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rania A. Wasfi, cmFuaWEud2FzZmlAcGhhYy1hc3BjLmdjLmNh; Stephanie A. Prince, c3RlcGhhbmllLnByaW5jZS53YXJlQHBoYWMtYXNwYy5nYy5jYQ==
 Rania A. Wasfi
Rania A. Wasfi Felix Bang2
Felix Bang2 Andrew Lawrence Pipe
Andrew Lawrence Pipe Stephanie A. Prince
Stephanie A. Prince