- 1Department of Medicine III, Gastroenterology, Metabolic Diseases and Intensive Care, University Hospital RWTH Aachen, Aachen, Germany
- 2Department of Biobehavioral Health Sciences, School of Nursing, University of Pennsylvania, Philadelphia, PA, United States
- 3Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 4The Institute for Translational Medicine and Therapeutics, The Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Background: Non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease are among the most common liver diseases worldwide, and there are currently no Food and Drug Administration (FDA)-approved treatments. Recent studies have focused on lifestyle changes to prevent and treat NAFLD. Omega-3 supplementation is associated with improved outcomes in patients with chronic liver disease. However, it is unclear whether Omega-3 supplementation can prevent the development of liver disease, particularly in individuals at an increased (genetic) risk.
Methods: In this UK Biobank cohort study, we established a multivariate cox proportional hazards model for the risk of incident liver disease during an 11 year follow up time. We adjusted the model for diabetes, prevalent cardiovascular disorders, socioeconomic status, diet, alcohol consumption, physical activity, medication intake (insulin, biguanides, statins and aspirin), and baseline characteristics.
Results: Omega-3 supplementation reduced the risk of incident liver disease (HR = 0.716; 95% CI: 0.639, 0.802; p = 7.6 × 10−9). This protective association was particularly evident for alcoholic liver disease (HR = 0.559; 95% CI: 0.347, 0.833; p = 4.3 × 10−3), liver failure (HR = 0.548; 95% CI: 0.343, 0.875; p = 1.2 × 10−2), and non-alcoholic liver disease (HR = 0.784; 95% CI: 0.650, 0.944; p = 1.0 × 10−2). Interestingly, we were able to replicate the association with reduced risk of NAFLD in a subset with liver MRIs (HR = 0.846; 95% CI: 0.777, 0.921; p = 1.1 × 10−4). In particular, women benefited from Omega-3 supplementation as well as heterozygous allele carriers of the liver-damaging variant PNPLA3 rs738409.
Conclusions: Omega-3 supplementation may reduce the incidence of liver disease. Our study highlights the potential of personalized treatment strategies for individuals at risk of metabolic liver disease. Further evaluation in clinical trials is warranted before Omega-3 can be recommended for the prevention of liver disease.
Introduction
Non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease are two of the most prevalent liver diseases worldwide, affecting millions of people across the globe (1–3). Despite their high prevalence and potential for serious complications, there are currently no Food and Drug Administration (FDA)-approved treatments for either condition (4). Recent research has focused on the potential benefits of lifestyle modifications, including targeted dietary interventions and supplements, for their prevention and treatment (5–8). Among the lifestyle modifications studied for their potential benefits in treating NAFLD and alcoholic liver disease are Omega-3 fatty acids, including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) (9). Omega-3 fatty acids have been shown to have a variety of health benefits, including reduced inflammation and improved cardiovascular health. Recent studies have shown that supplementation with DHA can improve liver and visceral fat in children with NAFLD, and supplementation with DHA and EPA can reduce liver fat (5, 6). However, it is unknown if Omega-3 supplementation has a preventive effect against the development of liver disease in the general population. Given the increasing prevalence of liver disease and the lack of FDA-approved treatments, it is compelling to investigate the potential benefits of Omega-3 fatty acid supplementation for the prevention of liver disease. Therefore, we used the UK Biobank to investigate the effect of Omega-3 fatty acid supplementation on the incidence of liver disease in a large population-based cohort.
Methods
UK biobank
For our study, we used the UK Biobank Resource with application number 71300. The study was approved by the Northwest Multicenter Research Ethics Committee and conducted in accordance with the Declaration of Helsinki and Istanbul after written informed consent was obtained. The UK Biobank (UKB) is a multicenter research study with 22 participating centers. Enrolment studies were conducted between 2006 and 2010. The respective date was set as baseline. Death or end of data collection in May 2021 was defined as the end of follow-up. After informed consent was obtained for clinical data collection and genotyping, a total of 502,511 individuals aged 37 to 73 years were enrolled in the study. The diagnoses included in our study were coded using the ‘International Classification of Diseases and Related Health Problem' (ICD-10). The latter were assigned to the patients and continuously updated via the hospital admission code. Death data were collected in the UKB via the National Death Register, including the leading diagnosis of death and the date of death.
Briefly, Omega-3 supplementation was tracked in a questionnaire and validated by lipidomic data, which were measured in 96,701 individuals by nuclear magnetic resonance. We included participants without any baseline diagnosis of liver disease (K70-K77, C22.0) and performed an analysis of reported Omega-3 intake with new diagnosis of liver disease over the 11-year study period. Detailed information can be found in Supplementary material (Supplementary Table S1).
Exclusion criteria and missing data
We excluded individuals with any diagnosis of pre-existing liver diseases (K70-K77, C22.0), HIV infection (B20-B24), chronic hepatitis (B18) or missing body mass index (BMI) data (Figure 1). We also excluded individuals with pathological alcohol consumption (alcohol consumption >60 g/day for men and >40 g/d for women) (10). Individuals who consumed alcohol below the pathological cut-off were not excluded. In addition, we excluded one patient due to missing survival data (Figure 1).
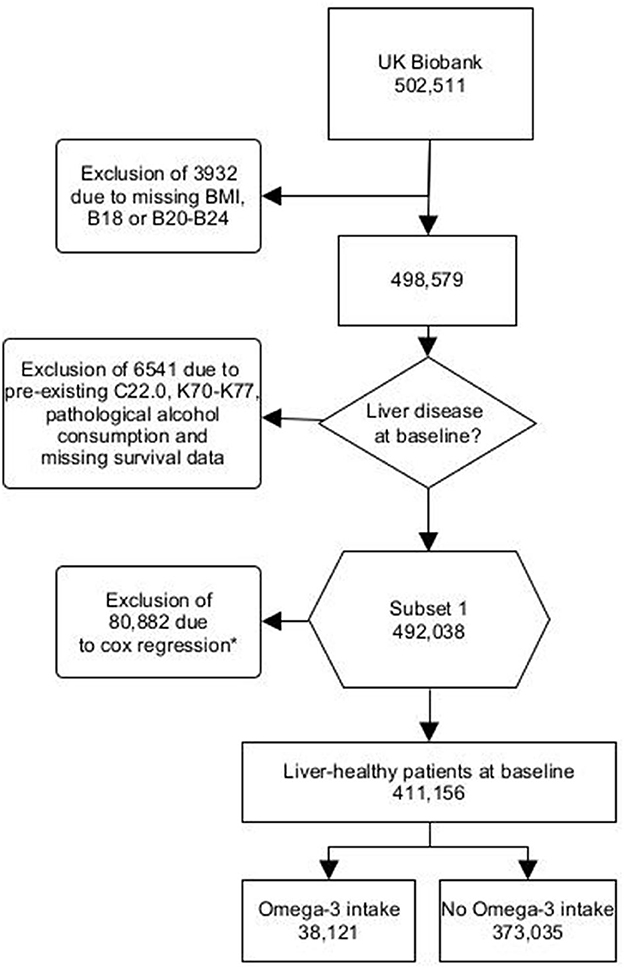
Figure 1. Flowchart UK Biobank Omega-3 users - B18, Chronic viral hepatitis; B20, Human immunodeficiency virus disease resulting in infectious and parasitic disease; B21, Human immunodeficiency virus disease resulting in malignant neoplasms; B22, Human immunodeficiency virus disease resulting in other specified diseases; B23, Human immunodeficiency virus disease resulting in other conditions; B24, Unspecified human immunodeficiency virus disease; C22.0, Hepatocellular carcinoma; K70, Alcoholic liver disease; K71, Toxic liver disease; K72, Hepatic failure, not elsewhere classified; K73, Chronic hepatitis, not elsewhere classified; K74, Fibrosis and cirrhosis of liver; K75, Other inflammatory liver diseases; K76, Other diseases of liver; K77, Liver disorders in diseases classified elsewhere. *Missing values were excluded from the cox proportional hazards model. This was the case for physical activity, diet, alcohol consumption and socioeconomic status.
Furthermore, patients were excluded from the multivariate cox proportional hazards model. Patients who reported being unable to walk (response option: “unable to walk:) were considered as “0 days/week walked”. Missing data for vegetable, fruit, fish and meat intakes were excluded. Likewise, missing data for alcohol consumption (in g/d) and socioeconomic status (Townsend Index) were excluded (Figure 1).
Omega-3 intake
Omega-3 intake was recorded using a numerical code (Supplementary Table S1). We used data from the UKB that included people with regular supplementation of Omega-3 defined as daily, weekly or monthly Omega-3 intake. Non-users did not consume any supplemental Omega-3 fatty acids. Moreover, we used the plasma metabolic profile data available in the UKB as intake control.
Metabolomics
Using nuclear magnetic resonance, the UKB determined the metabolic profiles of 105,348 participants, of whom 96,701 individuals were included in our study. 90,965 had a measurement of Omega-3 serum level. We compared beta between individuals taking Omega-3 and those without intake to compare metabolites. The Bonferroni correction was performed to avoid type I error due to multiple testing. Values -log10(p/59) >3.1 were considered significant (Supplementary Table S2, Figure 2). We further examined the distribution of Omega-3 intake in patients with lipidomic data and additionally repeated the analyses (Supplementary Table S3).
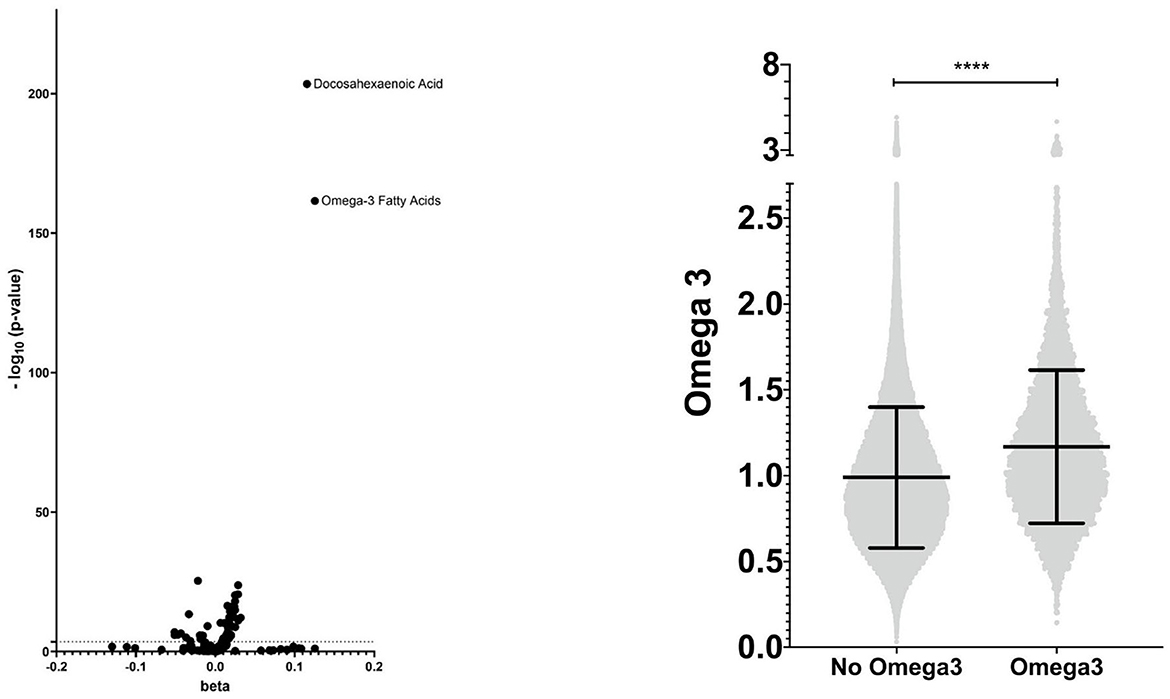
Figure 2. Metabolic profile - visualization of the lipidomic profile of Omega-3 users compared with non-users and distribution of Omega-3 serum levels. **** means <0.0001.
MRI confirmed steatosis
In UKB 40,797 individuals underwent liver MRIs to determine proton density fat fractions. Steatosis was defined as a liver fat fraction of >5% (11). A total of 31,216 individuals who received liver MRIs were included in the study.
Genetic disposition
The UKB provided genetic analyses of 488,377 individuals. We investigated carriers of known liver-associated variants. Variants examined were patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409, transmembrane 6 superfamily member 2 (TM6SF2) rs58542926, hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) rs72613567, and mitochondrial amidoxime reducing component 1 (MTARC1) rs2642438. We examined wildtype, heterozygous, and homozygous minor allele carriers.
Primary and secondary outcomes
The primary outcome was the development of incident liver disease (K70-K77) or a new diagnosis of hepatocellular carcinoma (C22.0) after baseline. As a secondary outcome, we replicated the results using multivitamin supplements, vitamin C, or vitamin B12.
Multivariate cox proportional hazards model
Covariates included in the multivariate cox proportional hazards model were age, sex, BMI, ethnicity, diabetes mellitus, hypertension, ischaemic heart disease, dyslipidemia, number of medications taken, as well as intake of insulin, biguanides, statins and aspirin. In addition, we integrated physical activity with “Number of days/week walked”, “Number of days/week of moderate physical activity”, and “Number of days of vigorous physical actitivity”. We further included nutritional factors with the help of daily vegetable and fruit consumption, and weekly consumption of fish and meat. In addition, alcohol consumption (g/d) was included as a covariate to account for alcohol consumption below the pathological cut-off. We determined socioeconomic status using the Townsend Index as a covariate (12).
Statistical analysis
In the multivariate cox proportional hazards model, we considered influencing confounders as covariates. We included the covariates: Age, sex, BMI, ethnicity, diabetes mellitus, hypertension, ischemic heart disease, dyslipidemia, number of medications taken, insulin, biguanides, statins, aspirin, “number of days/week walked”, “number of days/week of moderate activity”, “number of days/week of vigorous activity”, daily fruit and vegetable consumption, weekly fish and meat consumption, daily alcohol consumption in g/d, and the Townsend index as a socioeconomic factor. Results are expressed as mean ± standard deviation (±SD). The 95% confidence intervals (CI) are given in parentheses for the hazard ratios (HR). Statistical significance was set at p-value < 0.05. Statistical analyses and graphical visualization were performed using R version 4.1.2 (R Foundation for Statistical Computing; Vienna, Austria), SPSS Statistics version 27 (IBM; Armonk, NY, USA), Prism version 8.0.1 (GraphPad, LaJolla, CA, USA), and yEd Graph Editor version 3.21.1.
Results
Omega-3 is associated with risk reduction for incident liver disease
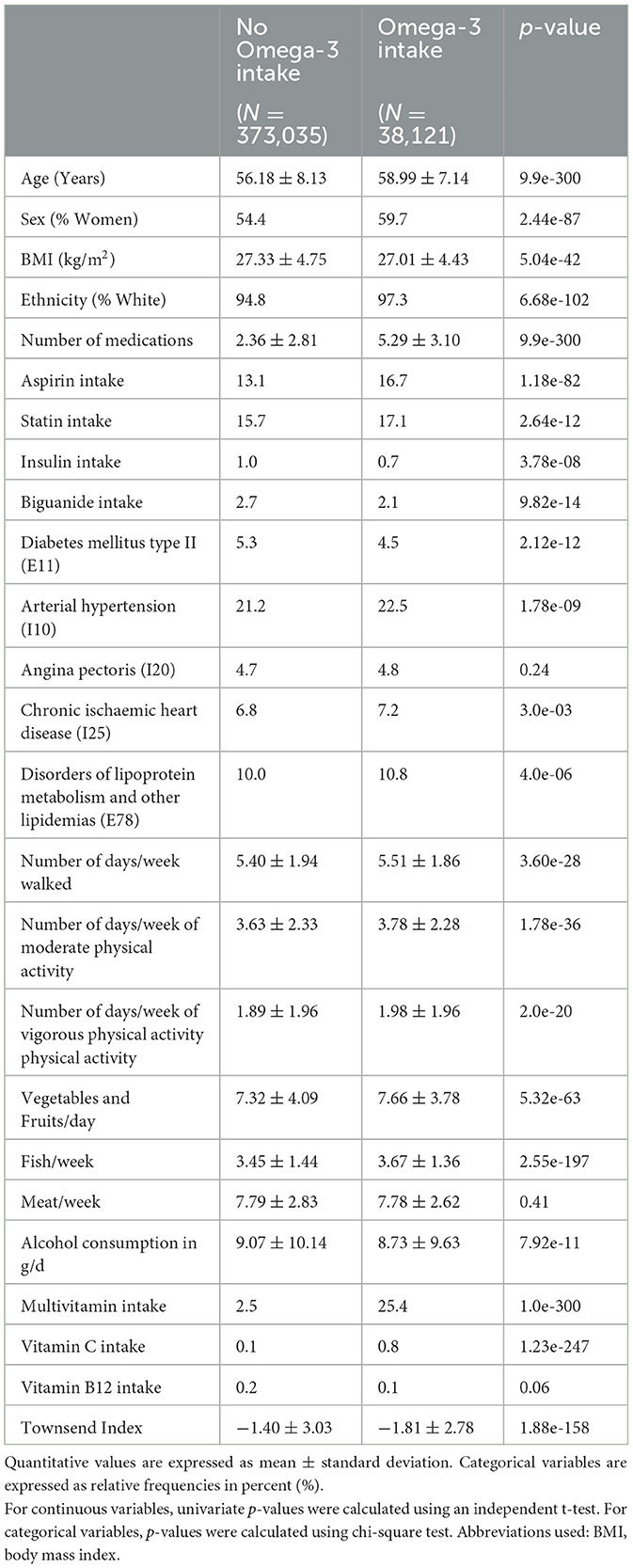
A total of 411,156 individuals were included, 373,035 participants without intake of Omega-3 (non-users) and 38,121 with regular (self-reported as daily, weekly, or monthly) intake of Omega-3 (Omega-3 users) (Figure 1, Supplementary Table S2). First, we validated the reported Omega-3 intake by using lipidomic data (Figure 2). Indeed, Omega-3 users had significantly increased levels of Omega-3 (+19.0%), compared to non-users (Figure 2, Supplementary Table S2).
Table 1 provides a comparison of the basic characteristics between two groups: those with no Omega-3 intake and those with Omega-3 intake. Interestingly, patients with Omega-3 took more medications at baseline, but were more active, ate more vegetables and fish, and came from a socioeconomically advantageous background (Table 1).
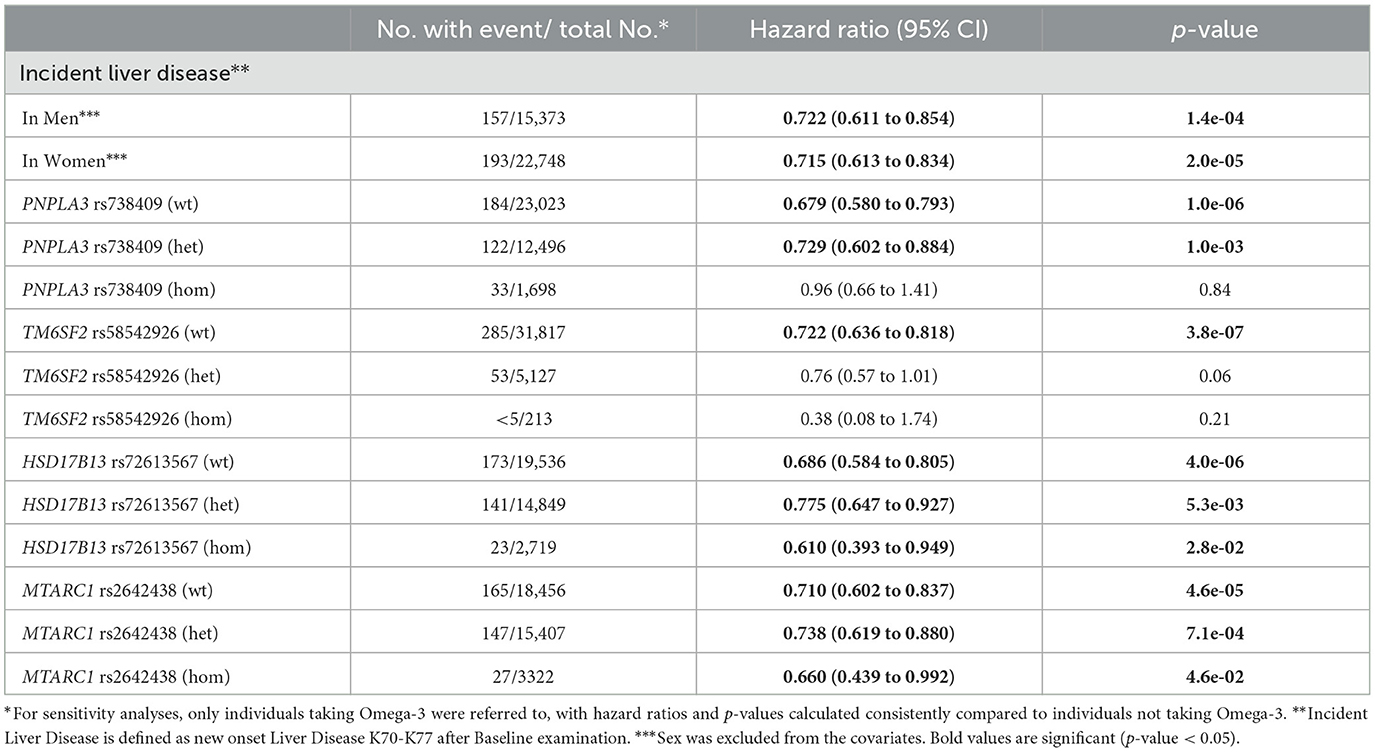
We then analyzed the incidence of liver diseases: Omega-3 users had a significantly lower incidence of overall liver disease than non-users (HR = 0.716; 95% CI: 0.639, 0.802; p = 7.6 × 10−9) (Table 2). In particular, new “alcoholic liver disease” (K70) (HR = 0.559; 95% CI: 0.347, 0.833; p = 4.3 × 10−3) as well as hepatic failure (HR = 0.548; 95% CI: 0.343, 0.875; p = 1.2 × 10−2) were significantly reduced in Omega-3 users. In addition, we found a risk reduction for “other diseases of liver” (K76), including NAFLD (HR = 0.784; 95%CI: 0.650, 0.944; p = 1.0 × 10−2). We replicated this association in a subgroup of UKB patients who underwent MRI. Here, Omega-3 intake was associated with less MRI-confirmed steatosis (HR = 0.846; 95% CI: 0.777, 0.921; p = 1.1 × 10−4) (Table 2).
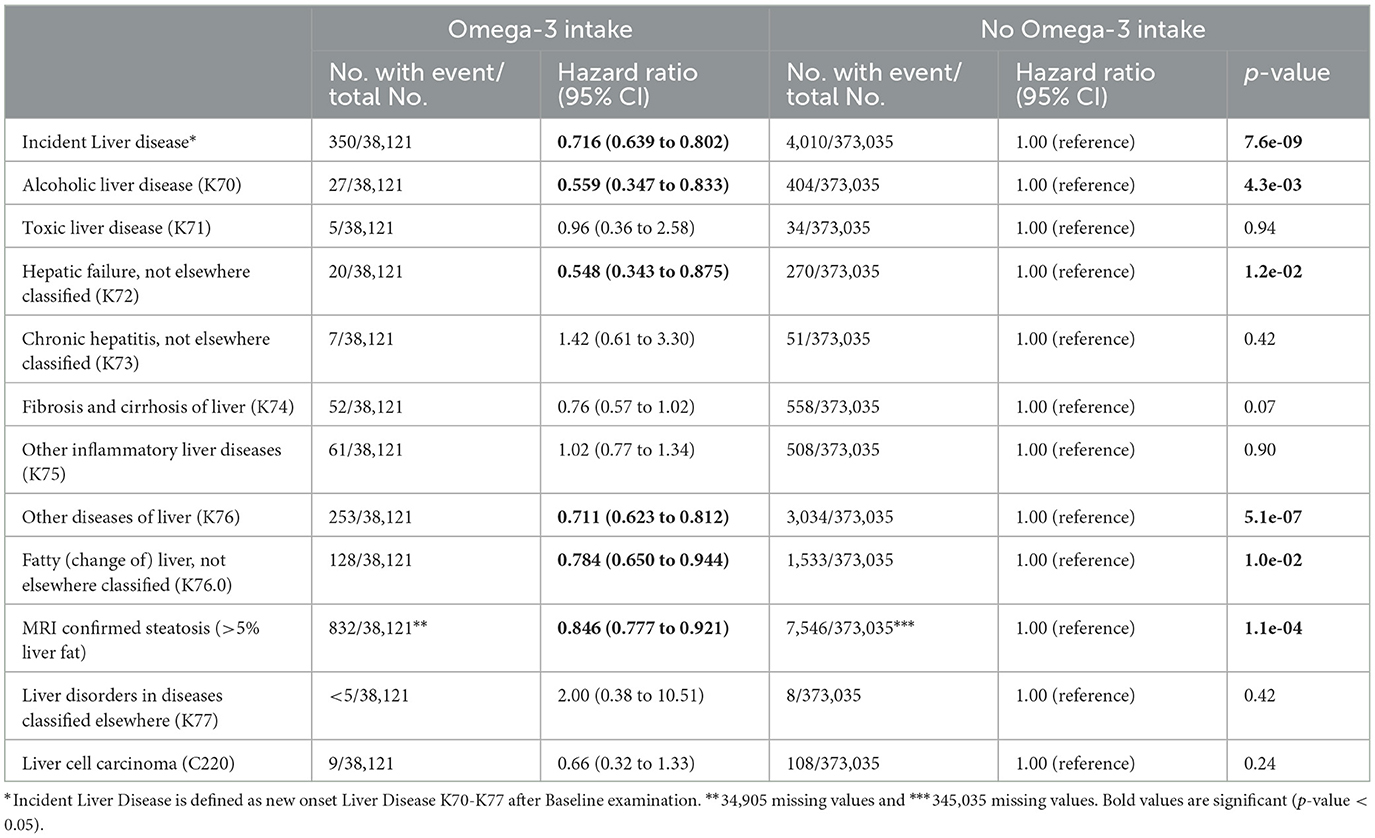
Table 2. Omega-3 intake and the development of incident liver disease and hepatocellular carcinoma in individuals without prior liver disease in UKB.
Men with regular Omega-3 intake showed a 27.8% reduction in the risk of a new diagnosis of liver disease (HR = 0.722; 95% CI: 0.611, 0.854; p = 1.4 × 10−4), whereas women showed a 28.5% reduction (HR = 0.715, 95% CI: 0.613, 0.834; p = 2.0 × 10−5) (Table 3).
Multivitamins, vitamin C and vitamin B12 are not associated with risk reduction for incident liver disease
It is important to consider potential confounding factors, such as other supplements or medications, that may have an impact on the outcome being studied, to improve the accuracy of the results and draw more valid causal inferences. Therefore, we studied the intake of multivitamins, vitamin C, or vitamin B12 nutritional supplements in the UKB. Vitamin C and vitamin B12 have shown associations with liver health in previous studies (13–15). Nevertheless, our results showed no significant benefit for incident liver disease (Table 4).
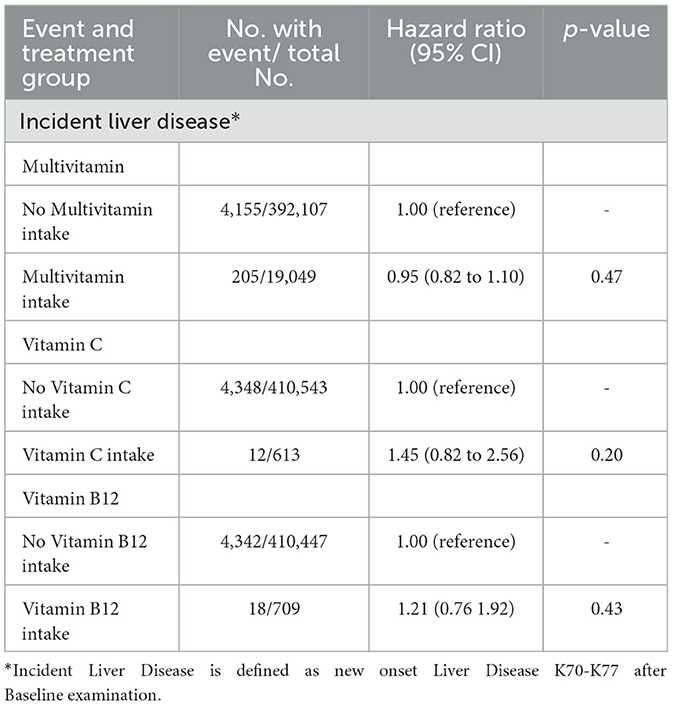
Table 4. Intake of different nutritional supplements and the development of incident liver disease in individuals without prior liver disease in UKB.
Omega-3 intake is protective regardless of genetic risk
Common genetic variants in PNPLA3 and TM6SF2 have been shown to increase the risk and severity of NAFLD, whereas single nucleotide polymorphisms (SNPs) in MTARC1 and HSD17B13 have protective effects (16–19). Similar to the results in the general population, Omega-3 intake resulted in a 27.1% risk reduction of incident liver disease in heterozygous carriers of the minor allele of PNPLA3 rs738409 (HR = 0.729; 95% CI: 0.602, 0.884; p = 1.0 × 10−3) (Table 3). Importantly, this association was not observed in homozygous carriers of the minor allele of PNPLA3 rs738409. In contrast, minor allele carriers of TM6SF2 rs58542926 showed no significant risk reduction. We next analyzed whether Omega-3 supplementation has an additive protective effect on common protective genetic variants. Interestingly, in homozygous carriers of the minor allele of HSD17B13 rs72613567 the protective effect of Omega-3 supplementation was strikingly higher than in the general population (HR = 0.610; 95% CI: 0.393, 0.949; p = 2.8 × 10−2). Similar results were obtained for MTARC1 rs2642438, suggesting additive hepatoprotective effects or potential synergistic effects (Table 3). Additionally, we repeated the analyses in the smaller subset of patients with measured Omega-3 levels and found comparable results (Supplementary Table S3).
Omega-3 intake in individuals with lipidomic data
Finally, we examined the individuals who received an analysis of the lipidomic data and evaluated the differences between Omega-3 users and non-users in more detail (Supplementary Table S3). The incidence of liver disease was significantly lower among Omega-3 supplement users (81 cases/8,747 individuals) compared to non-users (847 cases/82,218 individuals), indicating a potential protective effect of Omega-3 supplementation against liver disease. Overall, Omega-3 intake was significantly associated with a lower risk of liver disease (HR = 0.726; 95% CI: 0.573, 0.921; p = 8.0 × 10−3). Stratified analysis by gender revealed a significant association in women (HR = 0.653; 95% CI: 0.467, 0.912; p = 1.2 × 10−2), but not in men.
Discussion
Dietary modification is a cornerstone in the treatment of NAFLD. However, research on how targeted dietary interventions can be used for primary prevention is limited. This study aimed to investigate the relationship between Omega-3 fatty acid consumption and the development of liver disease in a large and diverse population-based cohort, including both non-alcoholic and alcoholic liver disease. We found that regular Omega-3 fatty acid consumption was associated with a significant risk reduction in liver disease development, particularly for (non-)alcoholic liver disease.
Notably, our investigation included a comprehensive analysis of both general and genetic risk factors for liver disease, and we verified the participants' self-reported Omega-3 intake using lipidomic data.
Our findings indicate that regular Omega-3 fatty acid consumption is associated with a significant reduction in the risk of liver disease, particularly NAFLD. This is consistent with previous studies demonstrating the potential benefits of Omega-3 supplementation in treating NAFLD (5, 8). We further showed that ICD-10 coded NAFLD was significantly reduced in Omega-3 users. Moreover, a significant reduction in the risk of MRI-confirmed steatosis has been observed.
In the UKB, we developed a unique approach to study the interaction between genetics and Omega-3 intake. Especially for heterozygous PNPLA3 rs738409 minor allele carriers, regular Omega-3 intake showed a benefit. Minor allele carriers of the rare variant TM6SF2 rs58542926, which is harmful to the liver (17), did not show statistically significant associations, which may have been due to a lack of power. The HSD17B13 and MTARC1 variants, which have been linked to lower rates of NAFLD (20, 21), showed opposite associations. Both heterozygous and homozygous minor allele carriers showed a significant risk reduction for MTARC1 rs2642438 and HSD17B13 rs72613567. Interestingly, our data indicate an additive synergistic effect of Omega-3 supplementation and protective variants of HSD17B13 and MTARC1. Replication of the results in the subgroup of individuals with lipidomic data confirmed this to a large extent, however, for some genetic variants the number of cases was too small to draw a conclusion. To prevent the lack of significance due to a small sample size, analyzing larger cohorts is recommended, particularly for homozygous minor allele carriers.
One explanation for the hepatoprotective association between Omega-3 intake and liver disease may be that Omega-3 fatty acids improve insulin sensitivity, which is strongly associated with protection against NAFLD (22). In addition, Omega-3 fatty acids may directly affect liver fat metabolism (23). Studies have suggested that Omega-3 fatty acids may increase the breakdown of fat in the liver, leading to a reduction in the accumulation of liver fat, which is consistent with the results of our study (24). Omega-3 fatty acids may also have anti-inflammatory properties, and liver inflammation is a key component in the development and progression of liver diseases (22). The anti-inflammatory, insulin-sensitizing, and lipid-metabolizing effects of Omega-3 fatty acids may contribute to their potential benefits in the prevention of liver diseases, although the exact mechanisms are not fully understood.
This is the largest study to date to demonstrate a primary preventive effect in a prospective and well-characterized cohort. Moreover, gene-dietary interactions have not been studied at a population-based level before and this research may uncover personalized treatment strategies for individuals at risk for metabolic liver diseases. Deciphering gene-environment interactions in metabolic liver disease holds promise for the development of patient-tailored dietary strategies. Nevertheless, our study has limitations. First, we used ICD-10 diagnoses as outcomes in our study, which were continuously updated. In spite of this, we confirmed the negative association between Omega-3 supplementation and hepatic steatosis using MRI data. However, we recommend the replication of genetic findings in a larger cohort. Second, the frequency of Omega-3 intake was not available for analysis except for the statement that it was taken regularly. We attempted to mitigate these limitations by assessing the blood metabolites indicative of Omega-3 use which strongly supports the reported intake (Figure 2, Supplementary Table S2). Furthermore, Omega-3 supplementation may indicate an overall healthier lifestyle, which could not be entirely excluded. To correct for factors associated with a healthier lifestyle, we included pre-existing conditions, physical activity, dietary factors, other vitamin supplements, and socioeconomic status (Table 1). Moreover, we cannot completely exclude the influence of different cooking oils on Omega-3 serum levels by correcting for dietary factors. In addition, the lack of analysis on specific Omega-3 associated fatty acids such as eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) is a limitation that should be addressed in cohorts with corresponding data to provide a nuanced understanding of the relationship between Omega-3 fatty acids and liver health. Bias due to misclassification was minimized by sensitivity analyses with different endpoints (Tables 2–4). To further mitigate the risk of residual confounding, we performed sensitivity analyses in subgroups in which the association persisted robustly (Tables 3, 4).
Our study raises the question of whether Omega-3 supplementation should be recommended to people at a high risk of liver disease. Supplementation with Omega-3 fatty acids was particularly beneficial for women. However, these associations need to be confirmed in randomized trials before recommending Omega-3 for protection against liver disease.
In conclusion, this study demonstrated the primary preventive associations of Omega-3 supplementation with the development of incident liver disease, which warrants further evaluation in clinical trials.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: approved registration at UK Biobank is required. Requests to access these datasets should be directed to https://www.ukbiobank.ac.uk.
Ethics statement
The studies involving human participants were reviewed and approved by the Northwest Multicenter Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MV: conceptualization, methodology, software, formal analysis, writing—original draft, and validation. KC and ES: resources, writing—review and editing, and validation. KSS, LH, and MR: writing—review and editing. KMS: conceptualization, writing—review and editing, and funding acquisition. CS: conceptualization, software, writing—review and editing, supervision, funding acquisition, and validation. All authors contributed to the article and approved the submitted version.
Funding
CS is supported by a grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University (PTD 1-13/IA 532313) and the NRW Rueckkehr Programme of the Ministry of Culture and Science of the German State of North Rhine-Westphalia (MKW). KMS is supported by the Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine-Westphalia (MKW) under the Excellence strategy of the federal government and the Laender.
Acknowledgments
This research was conducted using the UK Biobank Resource under application number 71300. Access to the UK Biobank data was provided by MV and CS. Copyright © 2023, NHS England. Reused with permission of NHS England and/or UK Biobank. All rights reserved. This work uses data provided by patients and collected by the NHS as part of their care and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1192099/full#supplementary-material
Abbreviations
NAFLD, Non-alcoholic fatty liver disease; FDA, Food and Drug Administration; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; UKB, UK Biobank; ICD-10, International Classification of Diseases and Related Health Problem; BMI, body mass index; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2; HSD17B13, hydroxysteroid 17-beta dehydrogenase; MTARC1, mitochondrial amidoxime reducing component 1; SD, standard deviation; CI, confidence interval; HR, hazard ratio; SNP, single nucleotide polymorphism.
References
1. Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
2. Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. (2010) 53:372–84. doi: 10.1016/j.jhep.2010.04.008
3. Sahasrabuddhe V, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. (2012) 104:1808–14. doi: 10.1093/jnci/djs452
4. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. (2018) 113.:6 doi: 10.1038/s41395-018-0088-6
5. Pacifico L, Bonci E, di Martino M, Versacci P, Andreoli G, Silvestri LM, et al. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovas Dis. (2015) 25:734–41. doi: 10.1016/j.numecd.2015.04.003
6. Scorletti E, Bhatia L, Mccormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the WELCOME* study. Hepatology. (2014) 60: 1211–21. doi: 10.1002/hep.27289
7. Parker HM, Cohn JS, O'connor HT, Garg ML, Caterson ID, George J, et al. Effect of fish oil supplementation on hepatic and visceral fat in overweight men: a randomized controlled trial. Nutrients. (2019) 11:475. doi: 10.3390/nu11020475
8. Lee CH, Fu Y, Yang SJ, Chi CC. Effects of Omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: a systematic review and meta-analysis. Nutrients. (2020) 12:2769. doi: 10.3390/nu12092769
9. Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. (2018) 9:345–81. doi: 10.1146/annurev-food-111317-095850
10. Stockwell T, Chikritzhs T, Holder H, Single E, Elena M, Jernigan D, et al. International Guide for Monitoring Alcohol Consumption and Harm. Geneva: World Health Organization. (2000).
11. Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, et al. Characterisation of liver fat in the UK Biobank cohort. PLoS ONE. (2017) 12: e0172921. doi: 10.1371/journal.pone.0172921
12. Townsend P. Townsend Deprivation Index. National Database for Primary Care Groups and Trusts. Colchester, UK: UK Data Service. (1998).
13. Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: results from the NHANES 1999–2004. Nutrients. (2022) 14:1224. doi: 10.3390/nu14061224
14. Talari HR, Molaqanbari MR, Mokfi M, Taghizadeh M, Bahmani F, Tabatabaei SMH, et al. The effects of vitamin B12 supplementation on metabolic profile of patients with non-alcoholic fatty liver disease: a randomized controlled trial. Sci. Rep. (2022) 12:14047. doi: 10.1038/s41598-022-18195-8
15. He Z, Li X, Yang H, Wu P, Wang S, Cao D, et al. Effects of oral vitamin C supplementation on liver health and associated parameters in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Front Nutr. (2021) 8:745609. doi: 10.3389/fnut.2021.745609
16. Liu YL, Patman GL, Leathart JBS, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >g polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. (2014) 61:75–81. doi: 10.1016/j.jhep.2014.02.030
17. Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. (2014) 46:2901. doi: 10.1038/ng.2901
18. Wang P, Wu CX Li Y, Shen N. HSD17B13 rs72613567 protects against liver diseases and histological progression of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2020) 24:352–56. doi: 10.26355/eurrev_202009_22842
19. Schneider C v., Schneider KM, Conlon DM, Park J, Vujkovic M, Zandvakili I, et al. A genome-first approach to mortality and metabolic phenotypes in MTARC1 p.Ala165Thr (rs2642438) heterozygotes and homozygotes. Med. (2021) 2:851–863. doi: 10.1016/j.medj.2021.04.011
20. Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-Truncating HSD17B13 variant and protection from chronic liver disease. New England J Med. (2018) 378:1096–106. doi: 10.1056/NEJMoa1712191
21. Emdin CA, Haas ME, Khera A, Aragam K, Chaffin M, Klarin D, et al. A missense variant in mitochondrial amidoxime reducing component 1 gene and protection against liver disease. PLoS Genet. (2020) 16:1096–106. doi: 10.1371/journal.pgen.1008629
22. Lepretti M, Martucciello S, Aceves MAB, Putti R, Lionetti L. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. (2018) 10:350. doi: 10.3390/nu10030350
23. Leslie MA, Cohen DJA, Liddle DM, Robinson LE, Ma DWL. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. (2015) 14:1–18. doi: 10.1186/s12944-015-0049-7
Keywords: Omega-3 fatty acids, NAFLD, liver disease, primary prevention, alcoholic liver disease (ALD)
Citation: Vell MS, Creasy KT, Scorletti E, Seeling KS, Hehl L, Rendel MD, Schneider KM and Schneider CV (2023) Omega-3 intake is associated with liver disease protection. Front. Public Health 11:1192099. doi: 10.3389/fpubh.2023.1192099
Received: 22 March 2023; Accepted: 04 July 2023;
Published: 19 July 2023.
Edited by:
Jennifer K. Frediani, Emory University, United StatesReviewed by:
Xiaobin Zao, Beijing University of Chinese Medicine, ChinaKarsten H. Weylandt, Brandenburg Medical School Theodor Fontane, Germany
Copyright © 2023 Vell, Creasy, Scorletti, Seeling, Hehl, Rendel, Schneider and Schneider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolin Victoria Schneider, Y3NjaG5laWRlckB1a2FhY2hlbi5kZQ==
†These authors share last authorship
 Mara Sophie Vell
Mara Sophie Vell Kate Townsend Creasy
Kate Townsend Creasy Eleonora Scorletti3
Eleonora Scorletti3 Miriam Daphne Rendel
Miriam Daphne Rendel Kai Markus Schneider
Kai Markus Schneider Carolin Victoria Schneider
Carolin Victoria Schneider