- 1Department of Child Healthcare, Shenzhen Longhua Maternity and Child Healthcare Hospital, Shenzhen, China
- 2Department of Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China
- 3Department of Neurosurgery, Wu Tsai Neuroscience Institute, Stanford University School of Medicine, Stanford, CA, United States
- 4Department of Biostatistics, School of Public Health, Sun Yat-sen University, Guangzhou, China
Background: Weak handgrip strength (HGS) has been linked to adverse health outcomes including stroke. However, the joint associations of HGS weakness and asymmetry between limbs with stroke incidence remain underexplored.
Methods: This cohort study analyzed data of participants aged ≥45 years from three waves (2011, 2013, and 2015) of the China Health and Retirement Longitudinal Study. Weak HGS was defined according to the recommendation of European Working Group on Sarcopenia in Older People. Asymmetric HGS was defined if the HGS ratio of both hands was over 1.1 or below 0.9. New-onset stroke was confirmed through self-report of physician’s diagnosis.
Results: A total of 10,966 participants without stroke at baseline were included in the analysis. During the 4 years follow-up, there were 262 (2.39%) new-onset stroke cases. Compared to individuals with non-weak and symmetric HGS, those with HGS asymmetry alone and weakness alone were associated with hazards of 1.09 (95% confidence interval [CI]: 0.80–1.48) and 1.27 (95%CI: 0.86–1.88) for new-onset stroke, respectively, while co-occurrence of both HGS asymmetry and weakness was associated with 1.80 (95%CI: 1.24–2.60) greater hazard for new-onset stroke after controlling for confounders. Such associations were consistent in older adults aged ≥60 years, but not in those aged<60 years.
Conclusion: Individuals with both weak and asymmetric HGS tended to have greater risk of new-onset stroke, compared to those with normal HGS, or with either weak or asymmetric HGS alone. Our finding suggested that examining HGS asymmetry alongside weakness may help to improve the risk-stratification and target prevention of stroke, particularly in the older population.
Background
Globally, stroke is the third leading cause of mortality and a major contributor to long-term disability (1). In 2019, it was responsible for 11.6% of global deaths and 5.7% of the total disability-adjusted life-years (DALYs) (1). With the rapidly ageing population and increases in the prevalence of hypertension and diabetes mellitus in China, the burden of stroke is on the rise, posing an enormous challenge to families and the society as a whole (2, 3). Therefore, identifying modifiable risk factors associated with stroke incidence is of great importance in formulating prevention and treatment strategies.
Handgrip strength (HGS) is a convenient, reliable, and inexpensive assessment of upper limb muscle strength, which could reflect the overall muscle capacity and physical fitness (4). Low HGS has been linked to several adverse health outcomes, including cardiovascular disease, cognitive impairment, disability, and mortality (5–7). Based on the evidence, routine implementation of HGS measurement has been recommended in health care and community settings, especially for the ageing population (6). Nevertheless, most of the current research focuses solely on HGS weakness, while its asymmetry was less discussed (8).
Asymmetric HGS has been found to be detrimental to physical health (9–14). For example, a longitudinal analysis using data from the Health and Retirement Study (HRS) has reported that both HGS weakness and asymmetry independently predicted future activity limitations in Americans aged ≥50 years (9). Likewise, HGS asymmetry has also been found to be a significant predictor of neurodegenerative disorders among Chinese adults aged 60 years and over (10). In addition, several studies have demonstrated a combined effect of HGS weakness and asymmetry on health outcomes, showing greater risks of morbidity accumulation (11), reduced cognitive functioning (12), and functional disability (13, 14) in individuals with both weak and asymmetric HGS.
As the largest organ in the body, skeletal muscle is central to whole-body energy metabolism. Declines in skeletal muscle, reflected by HGS weakness, may result in metabolic dysfunctions like impaired glucose uptake and insulin resistance (15). In addition, HGS weakness has been identified as a risk factor to cardiovascular risk factors, including increased levels of blood pressure (BP), cholesterol, and inflammation (16–18). These conditions can subsequently contribute to an elevated risk of stroke (19–21). Therefore, prospective studies have revealed that HGS weakness was significantly associated with increased risk of stroke (5, 22). However, while HGS asymmetry indicates dysfunction of overall strength capacity, its combined impacts with HGS weakness in the development of new-onset stroke is underexplored. In this study, we aimed to investigate the associations of HGS weakness and asymmetry with the risk of incident stroke among Chinese middle-aged and older adults. Our study may contribute to the establishment of a clinical approach that combines HGS weakness and asymmetry for stroke risk stratification and targeted prevention.
Methods
Study design and population
Data of Chinese adults aged 45 years and older were drawn from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative survey aiming at promoting scientific research on healthy ageing (23–26). The baseline survey was conducted from June 2011 to March 2012, with participants selected from 450 villages/resident communities across China using a multistage probability-proportional-to-size sampling method. The respondents were followed up every 2 years, with a small share of new participants recruited in each follow-up survey.
In this cohort study, we used data from baseline, 2013, and 2015 follow-up surveys. At baseline, a total of 17,708 participants were recruited (Figure 1). We excluded participants aged under 45 years or without age information (N = 488), participants lacking HGS data from both hands (N = 3,984) or having HGS measurement only from one hand (N = 387), participants with missing information on stroke status (N = 41), or body mass index (BMI) (N = 196), and participants with prevalent stroke at the baseline survey (N = 235), leaving 12,377 eligible participants free of stroke at baseline. We further excluded 741 participants who were lost to follow-up, and 670 participants who lacked information on stroke status in both 2013 and 2015 CHARLS follow-up assessments. Finally, a total of 10,966 participants were included in the current analysis.
The ethics of CHARLS study was approved by the Biomedical Ethics Review Committee of Peking University (IRB approval number: IRB00001052-11015 and IRB00001052-11014) (24). Each participant has signed a written consent form to join the study.
Measurement of handgrip strength
HGS in kilograms was assessed by a standardized dynamometer (Yuejian WL-1000, China) (24, 27, 28). If participants had undergone surgery, or experienced swelling, inflammation, severe pain, or injury to one or both hands within the last 6 months, or declined to have their HGS measured, no assessment was taken. During the assessment, each participant was guided by trained interviewers to stand with their shoulder in a neutral position; if participants were unable to stand without assistance, a sitting position was allowed. They were then instructed to hold the dynamometer with their elbow flexed at a 90° angle and squeeze the handle with their maximum effort for a few seconds. The measurement was repeated twice for each hand.
To define weak HGS, the maximum value of HGS from both hands was used. According to the recommendation of European Working Group on Sarcopenia in Older People (EWGSOP), participants were first stratified into quartiles based on BMI in males and females, respectively. Then, participants with the lowest 20% of HGS in each stratum were defined as having weak HGS. In the current analysis, the cut-off values for weak HGS in females were 19.0, 21.0, 22.0, and 22.5 kg for those with a BMI ≤21.2, 21.3–23.6, 23.7–26.3, and > 26.3 kg/m2, respectively. The cut-off values for weak HGS in males were 29.0, 32.0, 34.0, and 35.0 kg for those with a BMI ≤20.4, 20.5–22.4, 22.5–25.0, and > 25.0 kg/m2, respectively.
According to the “10% rule,” HGS of the dominant hand is approximately 10% higher than that of the non-dominant hand (13). Therefore, if the HGS ratio of both hands was over 1.1 or below 0.9, the participant was defined as having asymmetric HGS; otherwise, HGS was defined as symmetric.
All participants were further categorized into four groups according to HGS weakness and asymmetry, i.e., no weakness and asymmetry, asymmetry alone, weakness alone, and both weakness and asymmetry.
Measurement of stroke
Self-reported stroke status was assessed by the question “Have you been diagnosed with stroke by a doctor?” (29). Answers to this question were “yes” or “no.” A participant was considered as having new-onset stroke if he/she has reported a negative answer at baseline and a positive answer at any of the 2013 and 2015 follow-up surveys. The onset time for stroke was identified as the age at which the stroke was first diagnosed. In cases where this information was absent, we used the midpoint between the most recent wave indicating a stroke diagnosis and the preceding wave when the participant was free of stroke to determine the stroke onset time.
Covariates
Information on age, sex, educational background, marital status, area of residence, smoking and drinking status were collected by trained investigators through face-to-face interviews. Educational background was categorized into three groups: (1) illiterate or without formal education, (2) primary school, and (3) middle school or above. Marital status was classified as married/cohabitated and unmarried groups. Area of residence was divided into rural and urban areas. Ethnicity was classified into Han ethnicity and other ethnic minorities, as the Han ethnicity is the most populous ethnic group in China. Smoking and drinking status were categorized as current users versus non-current users.
BMI was calculated as weight (kg) divided by the square of height (m2). In the Chinese population, a BMI of 28 kg/m2 or above was defined as obesity according to the recommendations of Working Group on Obesity in China (30, 31). BP was measured by a digital sphygmomanometer (Omron™ HEM-7200 Monitor, Dalian, China) (24). Glycosylated hemoglobin (HbA1c) and plasma glucose levels were assessed using the affinity high-performance liquid chromatography (HPLC) and the enzymatic colorimetric test, respectively. Physician-diagnosed diabetes mellitus, hypertension, dyslipidemia, and heart diseases were self-reported by each participant. Hypertension was ascertained if one or more of the following criteria was met: (1) with physician-diagnosed hypertension, (2) a mean systolic BP ≥ 140 mmHg, (3) a mean diastolic BP ≥ 90 mmHg, and (4) on anti-hypertensive drugs (32, 33). According to the American Diabetes Association criteria, a participant was defined as having diabetes mellitus if he/she had random plasma glucose ≥11.1 mmol/L, and/or fasting plasma glucose ≥7.0 mmol/L, and/or HbA1c ≥ 6.5%, and/or with self-reported physician-diagnosed diabetes mellitus, and/or on glucose-lowering drugs or insulin treatment (34–36).
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) for continuous data and number (percentage) for categorical data. Characteristics across different HGS weakness and asymmetry groups were compared using one-way analysis of variance (ANOVA) test for continuous data and χ2 test for categorical variables. Cox proportional hazards regression analyses were used to examine the association between different HGS weakness and asymmetry status and the risk of new-onset stroke. Participants with no weakness and asymmetry was treated as the reference group in all models. The association was first assessed in a crude model. The adjusted model was controlled for age, sex, educational background, marital status, area of residence, current smoking and drinking status, BMI (not adjusted in weak HGS-related analysis), diabetes mellitus, hypertension, dyslipidemia, and heart diseases, according to previous similar studies (5, 22, 37). The results were presented as hazard ratio (HR) with 95% confidence interval (CI).
Three sensitivity analyses were further conducted to test the robustness of the findings: (1) we examined the associations in different age groups, i.e., <60 years and ≥ 60 years, (2) we excluded participants who developed stroke within the first 2 years of follow-up to avoid reverse causality, and (3) we defined asymmetric HGS if the HGS ratio of both hands was over 1.2 or below 0.8 to test the robustness of the findings.
Data analyses were performed with Stata/SE 15.1 (Stata-Corp, College Station, TX, United States). All tests were two-sided and a value of p <0.05 was considered to be statistically significant.
Results
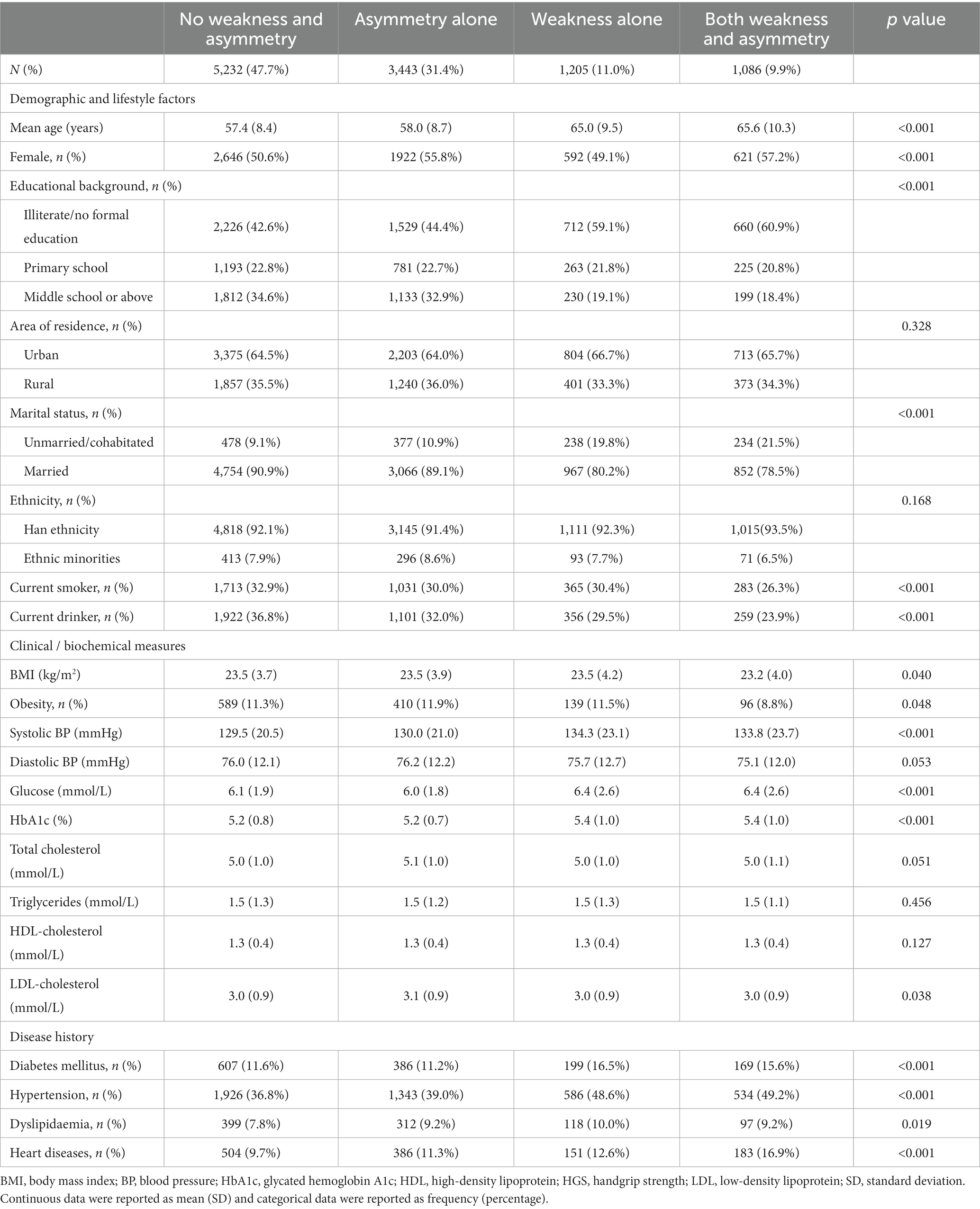
Of the 10,966 participants aged 45 years or older, 5,781 (52.7%) were females and the mean age was 59.2 ± 9.4 years at baseline. Figure 2 depicts a histogram of the HGS ratio among participants, showing that the majority (58.7%) had symmetric HGS, i.e., HGS ratio between 0.9 and 1.1. The number of participants with non-weak and symmetric HGS, asymmetric HGS alone, weak HGS alone, and both weak and asymmetric HGS were 5,232 (47.7%), 3,443 (31.4%), 1,205 (11.0%), and 1,086 (9.9%), respectively (Table 1). Compared to participants without HGS weakness or asymmetry, those with both weak and asymmetric HGS were older, more likely to be females, unmarried, less educated, less obese; and they were also more likely to have higher levels of systolic BP and glucose profiles, and with higher prevalence of self-reported chronic diseases, including diabetes mellitus, hypertension, dyslipidemia, and heart diseases.

Figure 2. Histogram of HGS ratio. Participants with a HGS ratio over 1.1 or below 0.9 were defined as having asymmetric HGS; otherwise, symmetric HGS was defined. HGS, handgrip strength.
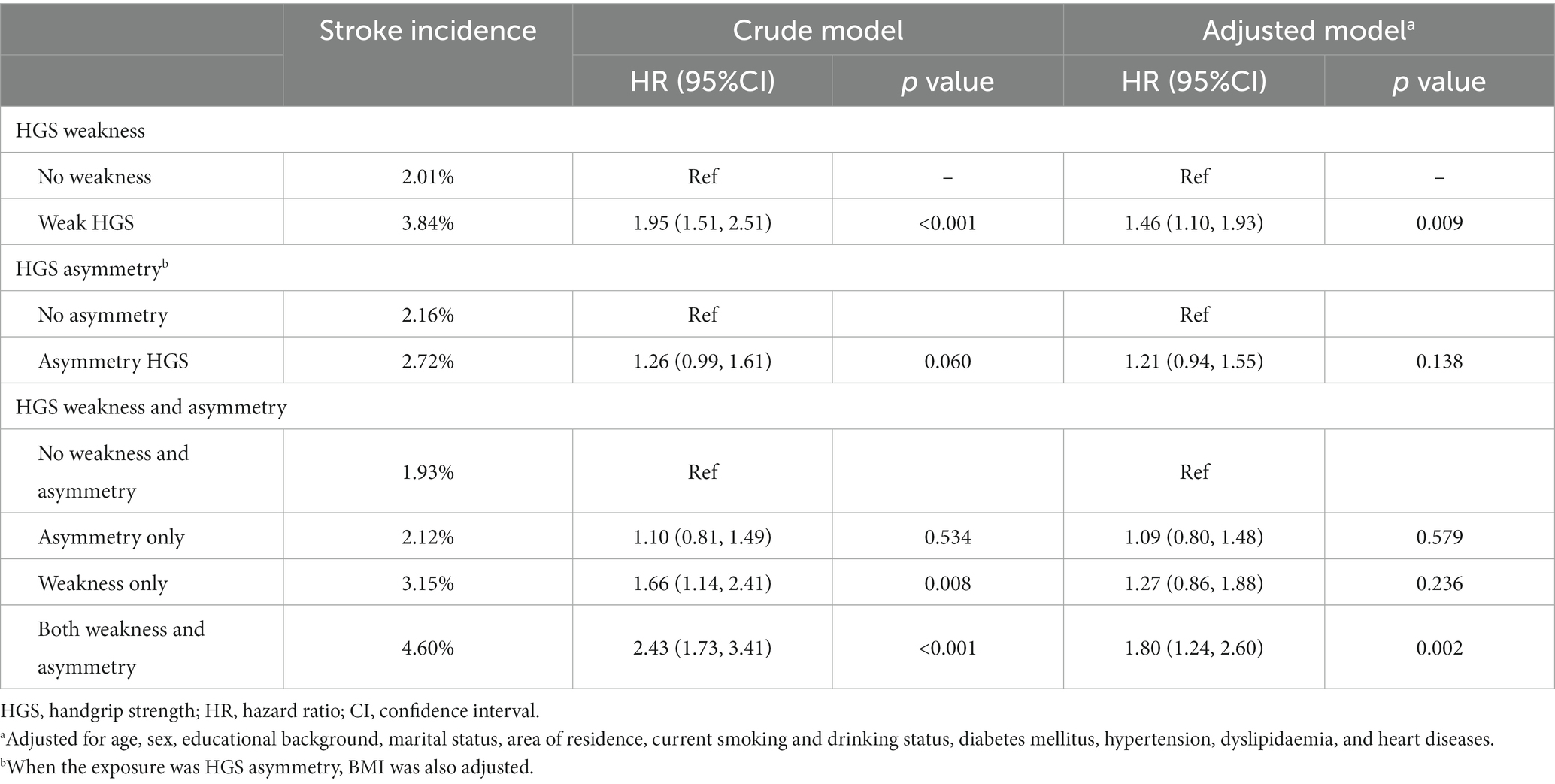
During 4 years of follow-up, there were 262 (2.39%) new-onset stroke cases. Compared to non-weak HGS group, those with HGS weakness were more likely to develop stroke (2.01% versus 3.84%, p < 0.001) (Table 2). In contrast, the incidence of stroke was comparable between participants with or without asymmetric HGS (2.72% versus 2.16%, p = 0.060). When weakness and asymmetry of HGS were taken into consideration at the same time, we found that participants with both conditions had the highest incidence rate of stroke compared to the other three groups (p < 0.001).
The association of baseline HGS weakness and asymmetry with the risk of new-onset stroke was presented in Table 2. In the crude model, weakness was associated with 1.95 times (95%CI: 1.51–2.51) greater risk of future stroke attack, whereas asymmetry was not significantly associated with the risk of stroke (HR: 1.26, 95%CI: 0.99–1.61). The findings were consistent in fully adjusted models. When HGS weakness and asymmetry were considered at the same time, using non-weak and symmetric HGS as reference, individuals with both weak and asymmetric HGS tended to have elevated risk of stroke (adjusted HR: 1.80, 95%CI: 1.24–2.60) compared to those with weakness alone (adjusted HR: 1.09, 95%CI: 0.80–1.48) or asymmetry alone (adjusted HR: 1.27, 95%CI: 0.86–1.88), albeit the confidence intervals were generally overlapped. Subgroup analysis by age showed similar associations in participants aged ≥60 years, but not in their counterparts who were younger than 60 years (Supplementary Table S1). Consistent results were also found after excluding incident stroke in 2013 CHARLS survey (Supplementary Table S2) or using a different criterion for asymmetric HGS (Supplementary Figure S1).
Discussion
The present study reported the individual and joint associations of HGS weakness and asymmetry with new-onset stroke among middle-aged and older Chinese in a cohort setting. We demonstrated that individuals with both weak and asymmetric HGS had a tendency of increased risk of new-onset stroke, compared to those with normal HGS, or with either weak or asymmetric HGS alone. Our findings highlighted the importance of examining HGS asymmetry alongside its weakness to improve the risk-stratification and target prevention of stroke.
The significant association between HGS weakness and incident stroke has been demonstrated by several previous studies (5, 22, 38). For example, the Prospective Urban Rural Epidemiology (PURE) study with data from 17 countries has reported a 9% increased risk of incident stroke associated with every 5 kg reduction in HGS (5). A cohort study of over 280,000 participants from UK biobank has also revealed that weak HGS was linked to a higher hazard for both ischemic and hemorrhagic stroke (38). Moreover, a prospective cohort study using data from CHARLS has reported that weakness and declines in HGS were associated with stroke incidence in middle-aged and older Chinese (22). In accord with above-mentioned studies, we also demonstrated that HGS weakness was associated with increased risk of stroke.
The exact mechanisms underlying the association between HGS weakness and risk of stroke are not fully understood. Skeletal muscle is a major organ of energy metabolism, and when muscle mass is reduced, the uptake of glucose is decreased accordingly. This reduction can subsequently lead to insulin resistance (15), a well-established risk factor of stroke (19). Furthermore, previous studies have demonstrated that HGS weakness was associated with increased level of inflammatory factors (18), such as C-reactive protein and interleukin-6, which in turn could contribute to a heightened vulnerability to stroke incidence (20). In addition, weak HGS might be a product of long-term unhealthy lifestyles and imbalanced nutrition (39, 40). These factors are associated with an increased risk of stroke, irrespective of age (41, 42). Therefore, the significant association between HGS weakness and new-onset stroke appears to be physiologically plausible.
Compared to HGS weakness, the association between HGS asymmetry and health outcomes has been underexplored. A cohort analysis using data from CHARLS has demonstrated that individuals with asymmetric HGS had increased hazard of neurodegenerative disorders during the 4 years follow-up, whereas HGS weakness was not an independent contributor to the outcome (10). Another longitudinal study with over 18,000 American adults aged 50 years or above has shown that HGS asymmetry and weakness were independently associated with increased risk of morbidity accumulation, while the odds were even greater in individuals with co-occurrence of the two conditions (11). Similar findings regarding the combined effect of HGS asymmetry and weakness have also been reported on incident functional disabilities (13, 14), low cognitive function (12), and depression (43). Our study further extends the knowledge of previous studies by demonstrating an augmented risk of stroke in individuals with both weak and asymmetric HGS, albeit no significant association was observed when examining the individual association of HGS asymmetry with new-onset stroke. The exact mechanism underlying the increased risk estimates of stroke in participants with both weak and asymmetric HGS remains unclear. Strength asymmetry between limbs might be a precursor of declines in overall strength capacity, and the co-occurrence of HGS weakness and asymmetry might represent a more severe muscle dysfunction than either condition alone (11). This may help explain the exaggerated risk of stroke in participants with both weak and asymmetric HGS. As such, our study further supports the combined assessments of HGS weakness and asymmetry in health screening to identify high-risk individuals for target prevention of stroke.
Subgroup analysis by age further showed that the associations remained consistent in adults aged ≥60 years, while such associations became statistically non-significant in individuals below 60 years. The mechanisms driving this variation are yet to be fully elucidated. In our study sample, younger adults aged <60 years generally achieved higher educational levels. This could potentially render them more perceptive and responsive to weak HGS, subsequently reducing their stroke risk. In addition, middle-aged individuals usually maintain a more active lifestyle compared to their older counterparts. This heightened physical engagement might mitigate the associations between HGS and stroke susceptibility in the younger population. Our research underscores the importance of HGS monitoring and intervention, particularly in the older population.
Strengths and limitations
The major strength of our study is the cohort design with a nationwide sample using standardized protocols. We not only investigated the individual impact of HGS asymmetry and weakness on stroke incidence, but also the combined impact. However, there are still some limitations that deserve further discussion. First, stroke was defined based on self-report of a physician’s diagnosis, which might cause potential recall bias and misclassification of stroke. Nevertheless, chronic diseases reported by participants has been demonstrated to be reliable compared with information extracted from medical record (44, 45). In addition, in longitudinal studies, the bias from such misclassification is often non-differential with respect to stroke outcome events, thus biasing the measure of association towards the null. Consequently, our results were likely more conservative than the true association. Second, since brain images were not applied to determine history of stroke at baseline, we could not rule out the possibility of minor stroke or transient ischemic attack (TIA) without typical symptoms or medical diagnosis. Nevertheless, it is usually not feasible to do so for every participant in large epidemiological study. The sensitivity analysis excluding incident stroke cases in the first 2 years of follow-up also revealed augmented risk of stroke in participants with both weak and asymmetric HGS, suggesting the robustness of our findings. Third, there was no information regarding the types of stroke attack. Therefore, we were unable to differentiate whether HGS weakness and asymmetry had different associations with ischemic and hemorrhagic stroke. Fourth, although many confounders have been controlled in the statistical models, we could not rule out potential residual confounding effects of other factors that were not captured in the present study, such as family history of stroke or dietary pattern and quality (46–49). In addition, although physical activity is recognized as an important risk factor for stroke (50), it was not included in the analysis due to the lack of data for 58.3% of the included participants. Furthermore, the modified International Physical Activity Questionnaire (IPAQ)-short form used in CHARLS adopted categorical choices to collect participant’s time spent on different intensities of physical activity, which might lead to imprecise estimates of energy expenditure and physical activity levels. Therefore, we did not consider this factor in the current study. Future research should further investigate whether physical activity could alter the associations observed in the present study.
Conclusion
Our study demonstrated that HGS weakness in combination with its asymmetry was associated with an increased risk of new-onset stroke in Chinese middle-aged and older adults. The risk estimates tended to be larger than that observed in individuals with normal HGS, or those only with weak HGS or asymmetric HGS. Our findings could provide valuable insights into early identification and intervention for stroke development. Targeting individuals with both weak and asymmetric HGS might have substantial benefits in lowering the risk of stroke. However, future randomized controlled trials are needed to confirm the conclusion.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found in a public, open access repository, and can be accessed at China Health and Retirement Longitudinal Study (CHARLS) http://charls.pku.edu.cn/index/en.html. Requests to access the datasets should be directed to https://charls.pku.edu.cn/en/. The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The ethics of CHARLS was approved by the Biomedical Ethics Review Committee of Peking University (IRB approval number: IRB00001052-11015 and IRB00001052-11014) in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in CHARLS. The current study is a secondary data analysis based on the public data of CHARLS, which is exempt from further ethical approval according to relevant regulations.
Author contributions
BC and VG: conceptualization and supervision. VG: data curation. YZ and VG: formal analysis, funding acquisition, and writing – original draft. YZ, WC, BC, JL, and VG: methodology. WC, BC, LL, and VG: validation. YZ, WC, BC, LL, JL, and VG: writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Shenzhen Science and Technology Innovation grant (No. JCYJ20220531091200001) and the Natural Science Foundation of Guangdong Province (2023A1515010425). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Acknowledgments
We thank the Peking University National Center for Economic Research for providing the data of the China Health and Retirement Longitudinal Study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1251262/full#supplementary-material
References
1. Diseases, GBD, and Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Li, Z, Jiang, Y, Li, H, Xian, Y, and Wang, Y. China's response to the rising stroke burden. BMJ. (2019) 364:l879. doi: 10.1136/bmj.l879
3. Zhao, D, Liu, J, Wang, M, Zhang, X, and Zhou, M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
4. McGrath, RP, Kraemer, WJ, Snih, SA, and Peterson, MD. Handgrip strength and health in aging adults. Sports Med. (2018) 48:1993–2000. doi: 10.1007/s40279-018-0952-y
5. Leong, DP, Teo, KK, Rangarajan, S, Lopez-Jaramillo, P, Avezum, A Jr, Orlandini, A, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. (2015) 386:266–73. doi: 10.1016/S0140-6736(14)62000-6
6. Bohannon, RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging. (2019) 14:1681–91. doi: 10.2147/CIA.S194543
7. Soysal, P, Hurst, C, Demurtas, J, Firth, J, Howden, R, Yang, L, et al. Handgrip strength and health outcomes: umbrella review of systematic reviews with meta-analyses of observational studies. J Sport Health Sci. (2021) 10:290–5. doi: 10.1016/j.jshs.2020.06.009
8. Roberts, HC, Denison, HJ, Martin, HJ, Patel, HP, Syddall, H, Cooper, C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. (2011) 40:423–9. doi: 10.1093/ageing/afr051
9. Parker, K, Rhee, Y, Tomkinson, GR, Vincent, BM, O'Connor, ML, and McGrath, R. Handgrip weakness and asymmetry independently predict the development of new activity limitations: results from analyses of longitudinal data from the US health and retirement study. J Am Med Dir Assoc. (2021) 22:821–826 e1. doi: 10.1016/j.jamda.2020.11.006
10. Chen, Z, Ho, M, and Chau, PH. Handgrip strength asymmetry is associated with the risk of neurodegenerative disorders among Chinese older adults. J Cachexia Sarcopenia Muscle. (2022) 13:1013–23. doi: 10.1002/jcsm.12933
11. Klawitter, L, Vincent, BM, Choi, BJ, Smith, J, Hammer, KD, Jurivich, DA, et al. Handgrip strength asymmetry and weakness are associated with future morbidity accumulation in Americans. J Strength Cond Res. (2022) 36:106–12. doi: 10.1519/JSC.0000000000004166
12. McGrath, R, Cawthon, PM, Cesari, M, Al Snih, S, and Clark, BC. Handgrip strength asymmetry and weakness are associated with lower cognitive function: a panel study. J Am Geriatr Soc. (2020) 68:2051–8. doi: 10.1111/jgs.16556
13. Collins, K, Johnson, N, Klawitter, L, Waldera, R, Stastny, S, Kraemer, WJ, et al. Handgrip strength asymmetry and weakness are differentially associated with functional limitations in older Americans. Int J Environ Res Public Health. (2020) 17:3231. doi: 10.3390/ijerph17093231
14. McGrath, R, Vincent, BM, Jurivich, DA, Hackney, KJ, Tomkinson, GR, Dahl, LJ, et al. Handgrip strength asymmetry and weakness together are associated with functional disability in aging Americans. J Gerontol A Biol Sci Med Sci. (2021) 76:291–6. doi: 10.1093/gerona/glaa100
15. Merz, KE, and Thurmond, DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. (2020) 10:785–809. doi: 10.1002/cphy.c190029
16. Ji, C, Zheng, L, Zhang, R, Wu, Q, and Zhao, Y. Handgrip strength is positively related to blood pressure and hypertension risk: results from the National Health and nutrition examination survey. Lipids Health Dis. (2018) 17:86. doi: 10.1186/s12944-018-0734-4
17. Li, D, Guo, G, Xia, L, Yang, X, Zhang, B, Liu, F, et al. Relative handgrip strength is inversely associated with metabolic profile and metabolic disease in the general population in China. Front Physiol. (2018) 9:59. doi: 10.3389/fphys.2018.00059
18. Tuttle, CSL, Thang, LAN, and Maier, AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. (2020) 64:101185. doi: 10.1016/j.arr.2020.101185
19. Wieberdink, RG, Koudstaal, PJ, Hofman, A, Witteman, JC, Breteler, MM, and Ikram, MA. Insulin resistance and the risk of stroke and stroke subtypes in the nondiabetic elderly. Am J Epidemiol. (2012) 176:699–707. doi: 10.1093/aje/kws149
20. Jenny, NS, Callas, PW, Judd, SE, McClure, LA, Kissela, B, Zakai, NA, et al. Inflammatory cytokines and ischemic stroke risk: the REGARDS cohort. Neurology. (2019) 92:e2375–84. doi: 10.1212/WNL.0000000000007416
21. Nwabuo, CC, Appiah, D, Moreira, HT, Vasconcellos, HD, Yano, Y, Reis, JP, et al. Long-term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: the CARDIA study. Eur J Prev Cardiol. (2021) 28:1445–51. doi: 10.1177/2047487320915342
22. Liu, G, Xue, Y, Wang, S, Zhang, Y, and Geng, Q. Association between hand grip strength and stroke in China: a prospective cohort study. Aging (Albany NY). (2021) 13:8204–13. doi: 10.18632/aging.202630
23. Lin, L, Wang, HH, Lu, C, Chen, W, and Guo, VY. Adverse childhood experiences and subsequent chronic diseases among middle-aged or older adults in China and associations with demographic and socioeconomic characteristics. JAMA Netw Open. (2021) 4:e2130143. doi: 10.1001/jamanetworkopen.2021.30143
24. Zhao, Y, Hu, Y, Smith, JP, Strauss, J, and Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
25. Lin, L, Bai, S, Qin, K, Wong, CKH, Wu, T, Chen, D, et al. Comorbid depression and obesity, and its transition on the risk of functional disability among middle-aged and older Chinese: a cohort study. BMC Geriatr. (2022) 22:275. doi: 10.1186/s12877-022-02972-1
26. Lin, L, Cao, B, Chen, W, Li, J, Zhang, Y, and Guo, VY. Association of adverse childhood experiences and social isolation with later-life cognitive function among adults in China. JAMA Netw Open. (2022) 5:e2241714. doi: 10.1001/jamanetworkopen.2022.41714
27. Lin, L, Sun, W, Lu, C, Chen, W, and Guo, VY. Adverse childhood experiences and handgrip strength among middle-aged and older adults: a cross-sectional study in China. BMC Geriatr. (2022) 22:118. doi: 10.1186/s12877-022-02796-z
28. Qin, K, Lin, L, Lu, C, Chen, W, and Guo, VY. Association between systemic inflammation and activities of daily living disability among Chinese elderly individuals: the mediating role of handgrip strength. Aging Clin Exp Res. (2021) 34:767–74. doi: 10.1007/s40520-021-02003-w
29. Qin, K, Bai, S, Chen, W, Li, J, and Guo, VY. Association of comorbid depression and obesity with cardiometabolic multimorbidity among middle-aged and older Chinese adults: a cohort study. Arch Gerontol Geriatr. (2023) 107:104912. doi: 10.1016/j.archger.2022.104912
30. Zhou, BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.
31. Lin, L, Chen, W, Sun, W, Chen, M, Li, J, Shen, J, et al. Associations between adverse childhood experiences and obesity in a developing country: a cross-sectional study among middle-aged and older Chinese adults. Int J Environ Res Public Health. (2022) 19:6796. doi: 10.3390/ijerph19116796
32. Program, NHBPE. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Bethesda (MD): National Heart, Lung, and Blood Institute (US) (2004).
33. Lin, L, Wang, HH, Liu, Y, Lu, C, Chen, W, and Guo, VY. Indoor solid fuel use for heating and cooking with blood pressure and hypertension: a cross-sectional study among middle-aged and older adults in China. Indoor Air. (2021) 31:2158–66. doi: 10.1111/ina.12872
34. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
35. Chen, D, Liang, Z, Sun, H, Lu, C, Chen, W, Wang, HHX, et al. Association between hypertriglyceridemic-waist phenotype and risk of type 2 diabetes mellitus in middle-aged and older Chinese population: a longitudinal cohort study. Int J Environ Res Public Health. (2021) 18:9618. doi: 10.3390/ijerph18189618
36. Lin, L, Lu, C, Chen, W, and Guo, VY. Daytime napping and nighttime sleep duration with incident diabetes mellitus: a cohort study in Chinese older adults. Int J Environ Res Public Health. (2021) 18:5012. doi: 10.3390/ijerph18095012
37. Wu, Y, Wang, W, Liu, T, and Zhang, D. Association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: a meta-analysis of prospective cohort studies. J Am Med Dir Assoc. (2017) 18:551.e17:551.e35. doi: 10.1016/j.jamda.2017.03.011
38. Kim, Y, Hwang, S, Sharp, SJ, Luo, S, Au Yeung, SL, and Teerlink, CC. Genetic risk, muscle strength, and incident stroke: findings from the UK biobank study. Mayo Clin Proc. (2021) 96:1746–57. doi: 10.1016/j.mayocp.2021.01.034
39. Robinson, SM, Jameson, KA, Batelaan, SF, Martin, HJ, Syddall, HE, Dennison, EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. (2008) 56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x
40. Hamer, M, and Stamatakis, E. Screen-based sedentary behavior, physical activity, and muscle strength in the English longitudinal study of ageing. PLoS One. (2013) 8:e66222. doi: 10.1371/journal.pone.0066222
41. Lee, CD, Folsom, AR, and Blair, SN. Physical activity and stroke risk: a meta-analysis. Stroke. (2003) 34:2475–81. doi: 10.1161/01.STR.0000091843.02517.9D
42. Chowdhury, R, Stevens, S, Gorman, D, Pan, A, Warnakula, S, Chowdhury, S, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. (2012) 345:e6698. doi: 10.1136/bmj.e6698
43. Hurh, K, Park, Y, Kim, GR, Jang, SI, and Park, EC. Associations of handgrip strength and handgrip strength asymmetry with depression in the elderly in Korea: a cross-sectional study. J Prev Med Public Health. (2021) 54:63–72. doi: 10.3961/jpmph.20.315
44. Muggah, E, Graves, E, Bennett, C, and Manuel, DG. Ascertainment of chronic diseases using population health data: a comparison of health administrative data and patient self-report. BMC Public Health. (2013) 13:16. doi: 10.1186/1471-2458-13-16
45. Payette, Y, de Moura, CS, Boileau, C, Bernatsky, S, and Noisel, N. Is there an agreement between self-reported medical diagnosis in the CARTaGENE cohort and the Québec administrative health databases? Int J Popul Data Sci. (2020) 5:1155. doi: 10.23889/ijpds.v5i1.1155
46. Baden, MY, Shan, Z, Wang, F, Li, Y, Manson, JE, Rimm, EB, et al. Quality of plant-based diet and risk of total, ischemic, and hemorrhagic stroke. Neurology. (2021) 96:e1940–53. doi: 10.1212/WNL.0000000000011713
48. Guo, VY, Cao, B, Wu, X, Lee, JJW, and Zee, BC. Prospective association between diabetic retinopathy and cardiovascular disease-a systematic review and Meta-analysis of cohort studies. J Stroke Cerebrovasc Dis. (2016) 25:1688–95. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.009
49. Yu, S, Su, Z, Miao, J, Yu, Y, Zhang, S, Wu, J, et al. Different types of family history of stroke and stroke risk: results based on 655,552 individuals. J Stroke Cerebrovasc Dis. (2019) 28:587–94. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.038
50. Kyu, HH, Bachman, VF, Alexander, LT, Mumford, JE, Afshin, A, Estep, K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. (2016) 354:i3857. doi: 10.1136/bmj.i3857
Keywords: handgrip strength, weakness, asymmetry, stroke, cohort study
Citation: Zhang Y, Chen W, Cao B, Lin L, Li J and Guo VY (2023) Associations of handgrip weakness and asymmetry with new-onset stroke in Chinese middle-aged and older adults: a cohort study. Front. Public Health. 11:1251262. doi: 10.3389/fpubh.2023.1251262
Edited by:
Hiroyuki Sasai, Tokyo Metropolitan Institute of Gerontology, JapanReviewed by:
Hiro Kishimoto, Kyushu University, JapanNatsuki Shimizu, Saitama Medical University, Japan
Cândida Alves, Federal University of Maranhão, Brazil
Copyright © 2023 Zhang, Chen, Cao, Lin, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Cao, YmluZ2Nhb0BzdGFuZm9yZC5lZHU=; Vivian Yawei Guo, Z3VveXcyM0BtYWlsLnN5c3UuZWR1LmNu
 Yuying Zhang
Yuying Zhang Weiqing Chen
Weiqing Chen Bing Cao3*
Bing Cao3* Li Lin
Li Lin Jinghua Li
Jinghua Li Vivian Yawei Guo
Vivian Yawei Guo