- 1Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, China
- 2Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Pharmacoeconomics, School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: Cost-effectiveness of atezolizumab, as a treatment for advanced non-small-cell lung cancer (NSCLC) patients who cannot receive a platinum-containing regimen,was still unknown. Our objective was to evaluate the cost-effectiveness of atezolizumab vs. chemotherapy in this indication from the perspective of UK healthcare system.
Methods: From the global, randomised, open-label, phase III IPSOS trial, clinical inputs and patient characteristics were obtained. A partitioned survival model with three health states was built: Progression-free survival, progressed disease and death. A lifetime time horizon was applied, with an annual discount rate of 3.5%. Additionally, the willingness-to-pay threshold of £50,000/QALY was utilized. Primary outcomes were quality-adjusted life-year (QALY), costs, and incremental cost-effectiveness ratio (ICER). Sensitivity, scenario, and subgroup analyses were used to assess the reliability of base-case results. Price simulations were carried out in order to provide information for the pricing strategy at specific willingness-to-pay threshold.
Results: In the base-case analysis, atezolizumab resulted in a gain of 0.28 QALYs and an ICER of £94,873/QALY compared to chemotherapy, demonstrating no cost-effectiveness. Price simulation results revealed that atezolizumab would be preferred at a price lower than £2,215 (a reduction of 41.8%) at the willingness-to-pay threshold of £50,000. Sensitivity, scenario and subgroup analyses revealed these conclusions were generally robust, the model was most sensitive to the price of atezolizumab and subsequent medication. Furthermore, atezolizumab was found to be more cost-effective for patients displaying a positive PD-L1 expression, with an ICER of £72,098/QALY as compared to chemotherapy.
Conclusion: Atezolizumab is not cost-effective for patients with advanced NSCLC ineligible for platinum-containing regimen, potential price reduction is necessary.
1. Introduction
Globally, lung cancer is the second most frequently diagnosed cancer and is responsible for the most of cancer-related deaths. In the UK, lung cancer accounts for 13% of newly diagnosed cancer cases and is associated with 21% of cancer-related deaths (1, 2). With the process of aging, the prevalence of lung cancer has been on the rise. Non-small-cell lung cancer (NSCLC) accounts for the largest proportion among all types of lung cancer, with a staggering 88% prevalence. Additionally, over half of the patients are already in advanced stages at the time of diagnosis (3). Consequently, there is a substantial burden associated with lung cancer. In 2010, the overall expenses of lung cancer over a span of 5 years amounted to around £267 million in the UK. When considering value-based oncology, it becomes crucial to assess the relative cost-effectiveness of various treatment options (4).
For NSCLC patients with negative driver genes, the current standard treatment is platinum-based doublet chemotherapy, combined with immunotherapy and/or anti-angiogenic therapy (5). However, in the real clinical setting, a significant portion of patients cannot tolerate platinum-based chemotherapy. Initially, the majority of NSCLC patients in the real world are diagnosed with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of ≥2. In the UK, it is estimated that 53% of lung cancer patients have an ECOG PS score of ≥2 (6). In most of clinical studies focused on immunotherapy, ECOG PS-high-score and older adult patients were excluded (7, 8). Regardless of the type of treatment received, patients with an ECOG PS score ≥ 2 had a worse prognosis compared to patients with a PS score of 0–1 (9–11). Secondly, statistics showed that the average age of onset for NSCLC patients is >70 years old (1). Overall, these patients usually have some comorbidities or contraindications that make them unsuitable for platinum-based chemotherapy. For NSCLC patients who cannot tolerate platinum-based chemotherapy, recommended treatments included combination therapy, monotherapy, or palliative care (12). With such treatments, the median survival time for patients was only 9.2–9.5 months (13, 14). Therefore, it is necessary to investigate strategies that offer enhanced effectiveness and safety for individuals within these patients.
The IPSOS trial is the first and only global phase III randomized controlled validation study conducted in a population not suitable for receiving platinum-based doublet chemotherapy (14). In 23 countries across Europe, Asia, and America, the research was carried out in 91 regions. Patients who met the criteria were randomly divided into two groups, with a ratio of 2:1. One group received atezolizumab (n = 302), while the other group received chemotherapy (n = 151). The objective of this study was to assess the efficacy and safety of atezolizumab as the initial treatment among these patients (more details are provided in Supplementary Table S1). The results showed that atezolizumab significantly reduced the death risk by 22% and also decreased the risk of disease progression by 13%, which suggested that atezolizumab was a potential first-line choice for advanced NSCLC patients who cannot undergo platinum-based chemotherapy.
Atezolizumab has been approved in the UK for the treatment of five indications of NSCLC (15). (1) Adjuvant treatment for patients with stage II to IIIA NSCLC who have a high PD-L1 expression level. (2) It is recommended to be added to bevacizumab, paclitaxel, and carboplatin or nab-paclitaxel and carboplatin, as the first-line treatment for patients with advanced non-squamous NSCLC. (3) The initial therapy for adult patients with metastatic NSCLC having PD-L1 expression of 10% or more is recommended. (4) Second-line treatment for patients diagnosed with locally advanced or metastatic NSCLC, who have undergone chemotherapy previously. The disease burden for the patients of interest is significant (6), considering the IPSOS trial results were published in July 2023 in the Lancet, it is expected that the indication of atelizumab for platinum-based chemotherapy intolerant population would be expedited for approval in the UK (14). In order to offer new, effective, and safe treatment options for advanced NSCLC patients who are ineligible for treatment with a platinum-containing regimen, as well as provide a more economical solution to alleviate their disease-related financial burden and ensure the optimal allocation of limited health resources, clinicians and decision-makers need information on cost-effectiveness to make informed healthcare decisions. Therefore, we aimed to inform decision-makers about the cost-effectiveness of atezolizumab in the UK healthcare system. The analysis was conducted from the UK healthcare system perspective to provide evidence for health technology assessment submissions and establish drug pricing strategies.
2. Materials and methods
2.1. Model overview
The purpose of this cost-effectiveness analysis was to compare the clinical and economic outcomes of atezolizumab with chemotherapy in patients with advanced NSCLC who cannot receive a platinum-containing regimen.
A partitioned survival model was created, comprising three health states: progression-free survival (PFS), progressed disease (PD), and death (Supplementary Figure S1). A 10-year lifetime horizon was applied. The percentage of patients who were alive or free from progression at each cycle (cycle length: 21 days) were estimated using the areas under the OS or PFS curves. By calculating the difference between the OS and PFS curves, the PD rate was determined. In the PFS state, patients were administered treatment using either atezolizumab or single-agent chemotherapy (vinorelbine or gemcitabine). Once the disease progressed or drug-discontinuation, patients would receive subsequent treatments consistent with the IPSOS trial (14). For patients receiving no subsequent anti-cancer treatments, best supportive care (BSC) was performed, more details are available in the Supplementary Table S2.
According to the National Institute for Health and Care Excellence (NICE) reference case, the model was developed from the aspect of the UK National Health Service (NHS) and personal social services (16). Both costs and utilities were discounted annually at a rate of 3.5% (17). The threshold for willingness-to-pay was established at £50,000 for each quality-adjusted life-year (QALY), taking into consideration the population of interest receiving “end-of-life” treatment, as recommended by the NICE (18). Additional analyses were conducted using thresholds of £30,000 per QALY and £90,000 per QALY. The reported outcomes included costs, life-years, QALY, and incremental cost-effectiveness ratio (ICER).
2.2. Population and health state transitions
Clinical inputs and patient characteristics were all extracted from the IPSOS trial (14). The population of interest were individuals with stage IIIB or stage IV NSCLC, who were ineligible for platinum-based therapy, had an ECOG PS of 2–3, mean age of 75 years, and possessed wild-type EGFR or ALK gene mutations. Besides, males comprised 73% of the population. In addition, according to a NICE technology appraisal (17), the parameters assumed for the population in this model were a mean weight of 74.1 kg, an average height of 170 cm, and a body surface area of 1.85 m2.
The OS and PFS probabilities were derived from the Kaplan–Meier curves documented in the IPSOS trial (14, 19). The required data was extracted using the GetData Graph Digitizer software. Guyot’s method was utilized to reconstruct the estimates of individual patient data (IPD) (20). The accuracy of the IPD reconstruction was assessed using the root mean square error (RMSE). The RMSEs of reconstructed IPDs were ranging from 0.002 to 0.006, which indicated a high accuracy. Subsequently, the reconstructed IPD was utilized to fit various parametric functions, including exponential, gamma, Gompertz, Weibull, generalized gamma, log-normal, log-logistic, fractional polynomial (FP), restricted cubic spline (RCS), and Royston-Parmar spline (RP) models. The goodness-of-fit criteria for model selection were evaluated based on the Akaike information criterion (AIC) and visual inspection (21).
Furthermore, age-based adjustments were made to the mortality rate to ensure it would not fall below that of the general population in the UK (22).
We opted for the Gompertz model for OS and RP-hazard models for PFS of atezolizumab. As for the PFS and OS of chemotherapy, we selected the RP-odds and Log-normal models. Detailed parameters are presented in Supplementary Table S3. Further information of goodness-of-ft results can be found in Supplementary Table S4 and Supplementary Figure S2.
2.3. Adverse events
Adverse events (AEs) rates were extracted from the IPSOS trial (14), considering only AEs of grade 3 or higher with an incidence exceeding 1% in either group. These events encompassed dyspnoea, anemia, neutropenia, leukopenia, nausea, vomiting, rash, decreased white blood cell count, and decreased neutrophil count. For additional details regarding incidences, durations and costs, refer to Supplementary Tables S5, S7.
2.4. Treatment duration
As per the clinical trial design, we assumed that treatment would continue until disease progression or unacceptable toxicity occurred. Median treatment duration for patients receiving atezolizumab and chemotherapy were 6 and 4 cycles, respectively. Observations revealed discontinuation rates of 13% for atezolizumab and 14% for chemotherapy (14). Since specific discontinuation times were not available for each patient, we employed the DEALE method to estimate the cyclical rate (23). The cyclical discontinuation rates for atezolizumab and chemotherapy were 2.3 and 3.7%, respectively.
2.5. Health state utilities
A disutility approach was used, which took into account the decrease in utility as age and gender increase, based on the population norms of the UK EQ-5D-3L (24). The decrement can be summarized as follows:
The reported utility values in each study were used to calculate the disutility associated with each health state by subtracting it from the general population utility. Then, these disutility values were deducted from the population norms. The base-case analysis utilized the disutilities reported from IMpover150 (25), a study that examined the treatment of metastatic non-squamous NSCLC with atezolizumab. Using EQ-5D questionnaire, The utility values for PFS and PD when undergoing treatment were calculated as 0.71 and 0.69, respectively. The disutilities of PFS and PD when not receiving treatment were obtained from van den Hout’s research (26). Likewise, it was observed that the patients in van den Hout’s study adequately represented the population of interest. Disutilities of AEs were included, and values were all from NICE committee papers, the duration-adjusted negative effects caused by AEs were assumed to occur during the initial cycle (27–29). More details can be found in Supplementary Tables S6, S7.
2.6. Treatment unit costs and health state costs
Only direct medical costs were considered in our study, encompassed the costs of acquiring active-treatment drugs and follow-up items, along with the expenses of AE management, BSC, and end-of-life care. The prices of generic drugs were obtained from the electronic market information tool (eMIT) for the year 2022 (30). Prices of medications were obtained from the listed price outlined in the 2022 British National Formulary (BNF) (31). We assumed complete vial sharing for all weight-based medications, as a conservative estimate (4). In this study, we utilized the NHS 2021–2022 reference cost (code SB12Z) (32) to calculate administration costs (£287/cycle) for all intravenous drugs (See more in Supplementary Tables S8, S9). Due to the lack of reported by the IPSOS trial regarding the usages of vinorelbine and gemcitabine, we assumed that in our base-case analysis, 50% of the patients received intravenous vinorelbine, while the remaining patients received gemcitabine. Besides, dosing intensity was assumed to be 100% for both groups due to lack of report. Healthcare resource utilized during the follow-up period included CT chest scan, chest radiography, electrocardiogram, outpatient visit, community nurse, clinical nurse specialist, general practitioner surgery, general practitioner home visit, and therapist visit. Prices of follow-up items were obtained from 2021–2022 NHS reference costs and the 2022 Personal Social Services Research Unit (PSSRU) costs (33). Follow-up care costs were £326 and £506 per cycle for PFS and PD in this model, respectively, more information is available in Supplementary Table S10. AE management costs were obtained from NHS or NICE committee papers targeting on advanced NSCLC (Supplementary Table S5) (17, 34). BSC was consisted by radiotherapy, morphine, bisphosphonate, steroids, nonsteroidal anti-inflammatory drugs, denosumab and dietitian, doses and prices of above items were taken from NHS, BNF or eMIT. In our base-case analysis, cost for BSC was £379 per cycle. The costs of end-of-life care was considered a one-time expense. Based on a NICE committee paper (17), the average cost per episode of end-of-life care in our model was £4,773, details for costs of BSC and end-of-life care are available in Supplementary Table S11. The prices of all mentioned items were adjusted to 2022 using the PSSRU annual inflation hospital and community health services index.
2.7. Sensitivity analyses
We conducted deterministic sensitivity analysis (DSA) to examine the impact of crucial parameters on the ICER, and the findings were presented as tornado diagrams. All parameters were modified either within the designated 95% confidence intervals (CI) or by ranging the base-case values (±20%). Detailed sources of uncertainty are provided in Supplementary Table 12.
A Monte Carlo simulation was performed with 10,000 iterations to conduct probabilistic sensitivity analysis (PSA) on the base-case. Additionally, we conducted 1,000 iterations for the PSA of scenario and subgroup analyses. For cost, we opted for the gamma distribution, while for probability, proportion, and utility, we chose the beta distribution. The scatter plots were utilized to visually present the outcomes of the base-case PSA. Afterwards, probability of being cost-effectiveness at the willingness-to-pay threshold ranged from £0 to £150,000 was tested by utilizing cost-effectiveness acceptability curves (CEAC).
2.8. Scenario analysis
In this study, we conducted scenario analyses considering uncertainties in model structure and parameters, such as uncertainty in survival data extrapolation, patient medication adherence, medication patterns, and in medication duration, and heterogeneity of utility values.
1. In scenario 1, we only considered standard parametric survival models.
2. In scenario 2, the utility values of PFS and PD states reported in the IMpover110 trial (PFS: 0.76; PD, 0.69) were utilized.
3. In scenario 3, dosing intensity for both atezolizumab and chemotherapy was assumed to be the same as the IMpover110 trial (35).
4. In scenario 4, it is assumed that patients take vinorelbine orally.
5. In scenario 5, active treatment during the PD state persisted until 3 months prior to death.
6. In scenario 6, we adjusted the utilization ratio of vinorelbine or gemcitabine within the range of 0–100%.
2.9. Subgroup analysis
The ICER, probability of being cost-effective at the selected willingness-to-pay threshold, and cost of being cost-effective at the chosen willingness-to-pay threshold for atezolizumab in each subgroup were calculated using subgroup-specific hazard ratios (HRs) of PFS and OS based on Cox proportional hazards models. We considered the subgroup factors of age (≥80, 70–79, or < 70), sex (male or female), race (white or Asian), ECOG PS score (0–1, 2, or 3), tobacco use history (previous, current, or never), histology (non-squamous or squamous), stage (IIIB or IV), brain or liver metastases (yes or no), number of metastatic sites (≥3 or < 3) and PD-L1 expression level (<1, 1–49%, or > 50%).
2.10. Price simulation
The price simulation analysis incorporated fluctuating prices ranging from £1,000 to £3,800, with increments of £10, as per the results from our base-case analysis. Furthermore, Monte Carlo simulation of 1,000 iterations were performed to conduct PSAs for each respective price.
The values, ranges, and sources for all parameters utilized in this model are summarized in Supplementary Table S12.
3. Results
3.1. Model validation
The model’s face validity, encompassing its structure, assumptions, data sources, and results, underwent evaluation by clinical experts. The validation results demonstrated a strong fit of our model, as the survival rates for both PFS and OS were consistent with the original data obtained from the IPSOS trial (Supplementary Figure S2). 4-year OS or PFS rates of both atezolizumab and chemotherapy were less than 10%, indicated little uncertainty regards extrapolation (14).
3.2. Base-case analysis results
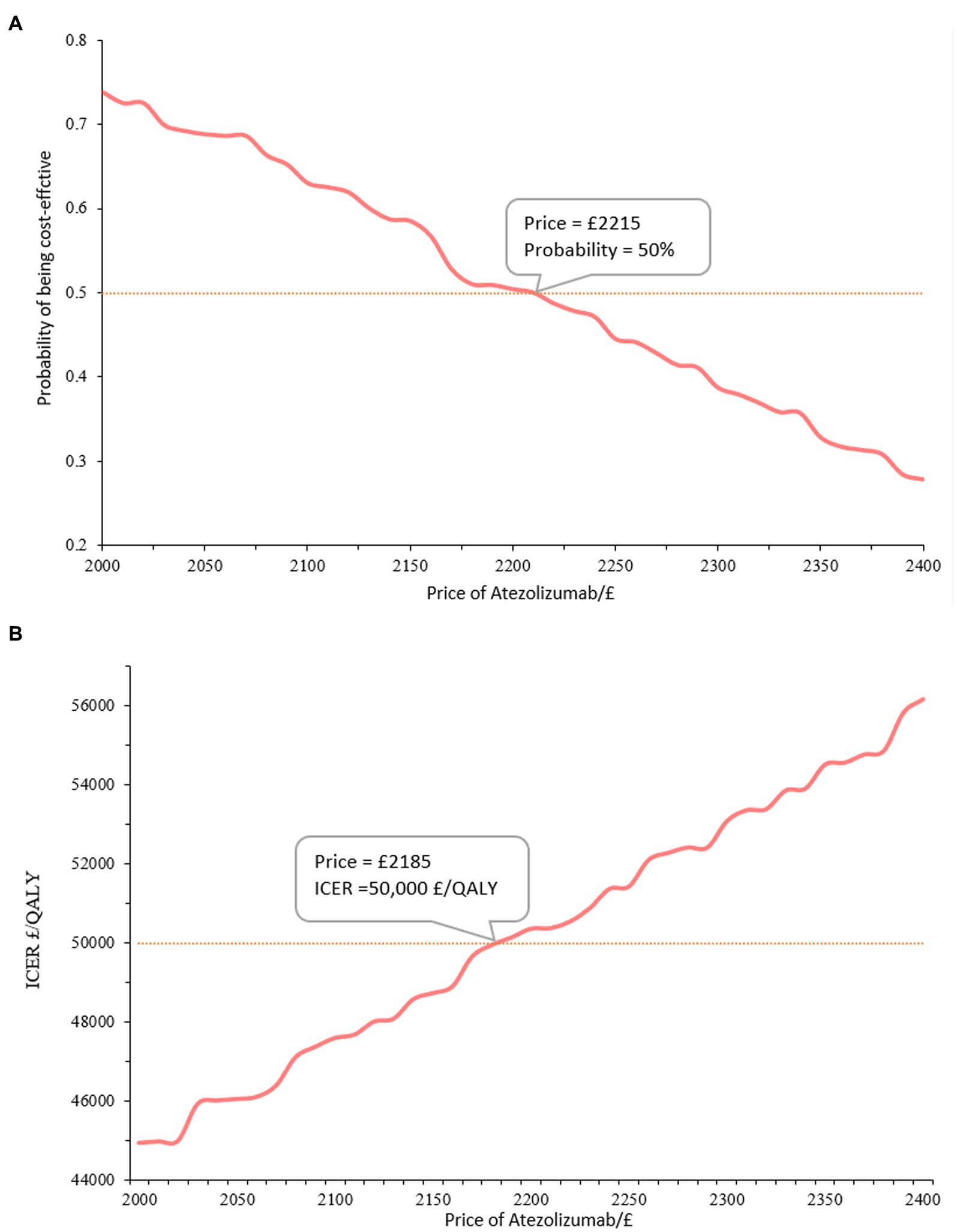
To conclude, Atezolizumab is not an economical option for patients with advanced NSCLC ineligible for treatment with a platinum-containing regimen as compared to chemotherapy at the current price of £3807.69/1,200 mg. Atezolizumab can be deemed cost-effective only when priced below £2215/1,200 mg at the willingness-to-pay threshold of £50,000/QALY.
The findings of the base-case analysis are outlined in Table 1. The lifetime costs for atezolizumab and chemotherapy amounted to £56,950 and £30,744, respectively. Atezolizumab exhibited a gain of 0.46 life-years and 0.28 QALYs in contrast to chemotherapy. The ICER of atezolizumab compared to chemotherapy were £94,873/QALY, which was higher than the recommended willingness-to-pay threshold of £50,000/QALY, indicating that atezolizumab was not cost-effective when compared to chemotherapy at the current price of £3807.69/1,200 mg. We also conducted an alternative analysis focusing solely on PFS. The ICER for atezolizumab compared to chemotherapy was £213,196/QALY. Breakdown results of costs are provided in Supplementary Table S13.
3.3. Sensitivity analyses
The results of the DSA are shown in Figure 1. The variables that had the greatest impact on the ICER were the price of atezolizumab, percents of patients received subsequent paclitaxel and atezolizumab, discontinuation rate of atezolizumab, and the utility of PD. The price of atezolizumab had the greatest impact on ICER, but in this part, we only considered a limited range of fluctuations, which meant that even at the lowest price, atezolizumab was still not cost-effective. The proportion of patients receiving immune checkpoint inhibitors and other drugs after progression also had a significant impact on ICER, mainly due to the high price of these drugs. Similarly, discontinuation rates related to patient compliance and medication safety were significant factors affecting ICER. Additionally, the impact of utility value on ICER could not be ignored, as it was clearly related to the patient’s effectiveness. Lastly, the influence of discount rates was unquestionable, as they were closely related to the results of output and input indicators. Overall, after allowing parameter fluctuated within the specified upper and lower limits, it was established that atezolizumab was unlikely to exhibit cost-effectiveness at the threshold of £50,000/QALY. Nevertheless, this conclusion may be reevaluated if the threshold was set at £90,000/QALY.
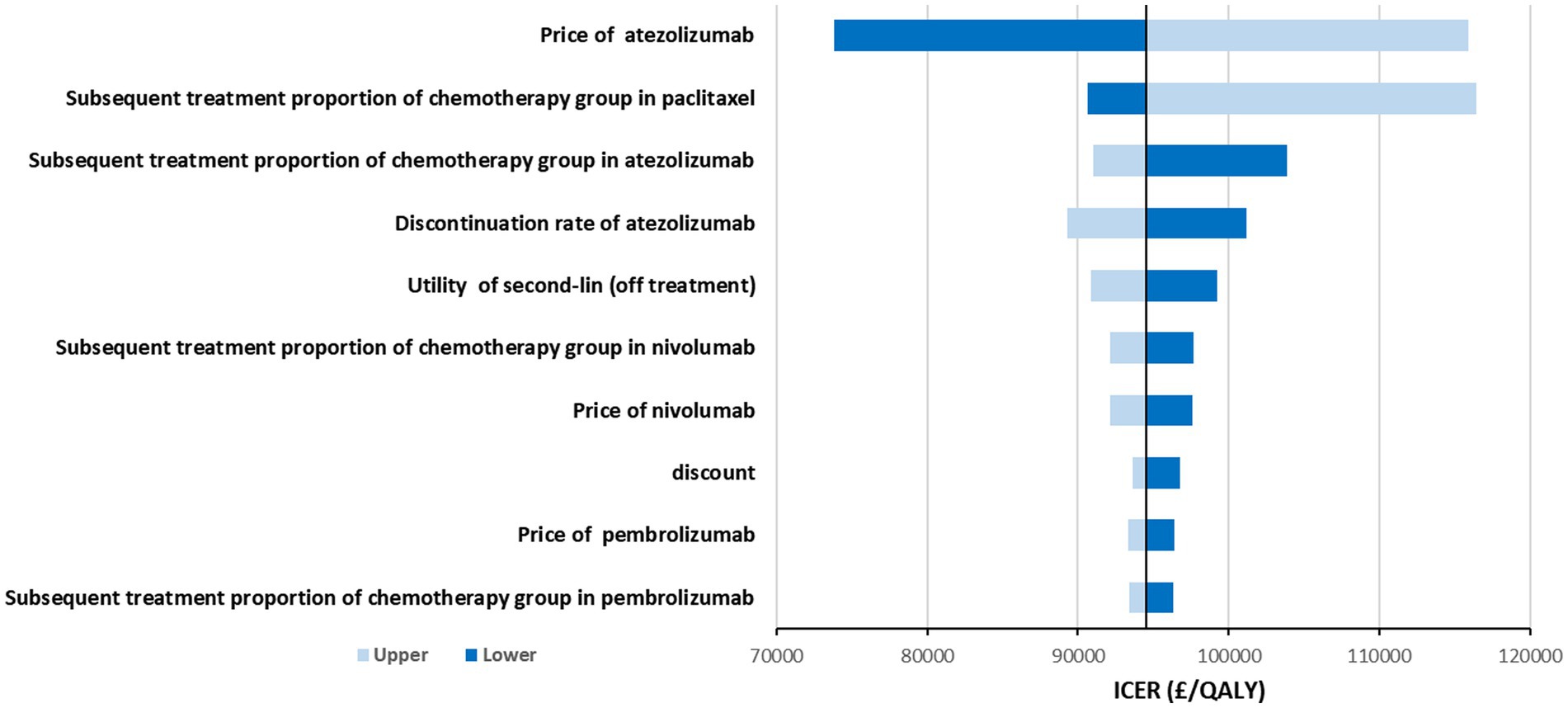
Figure 1. Tornado diagram shows the association of variables with the ICER of atezolizumab vs. chemotherapy.
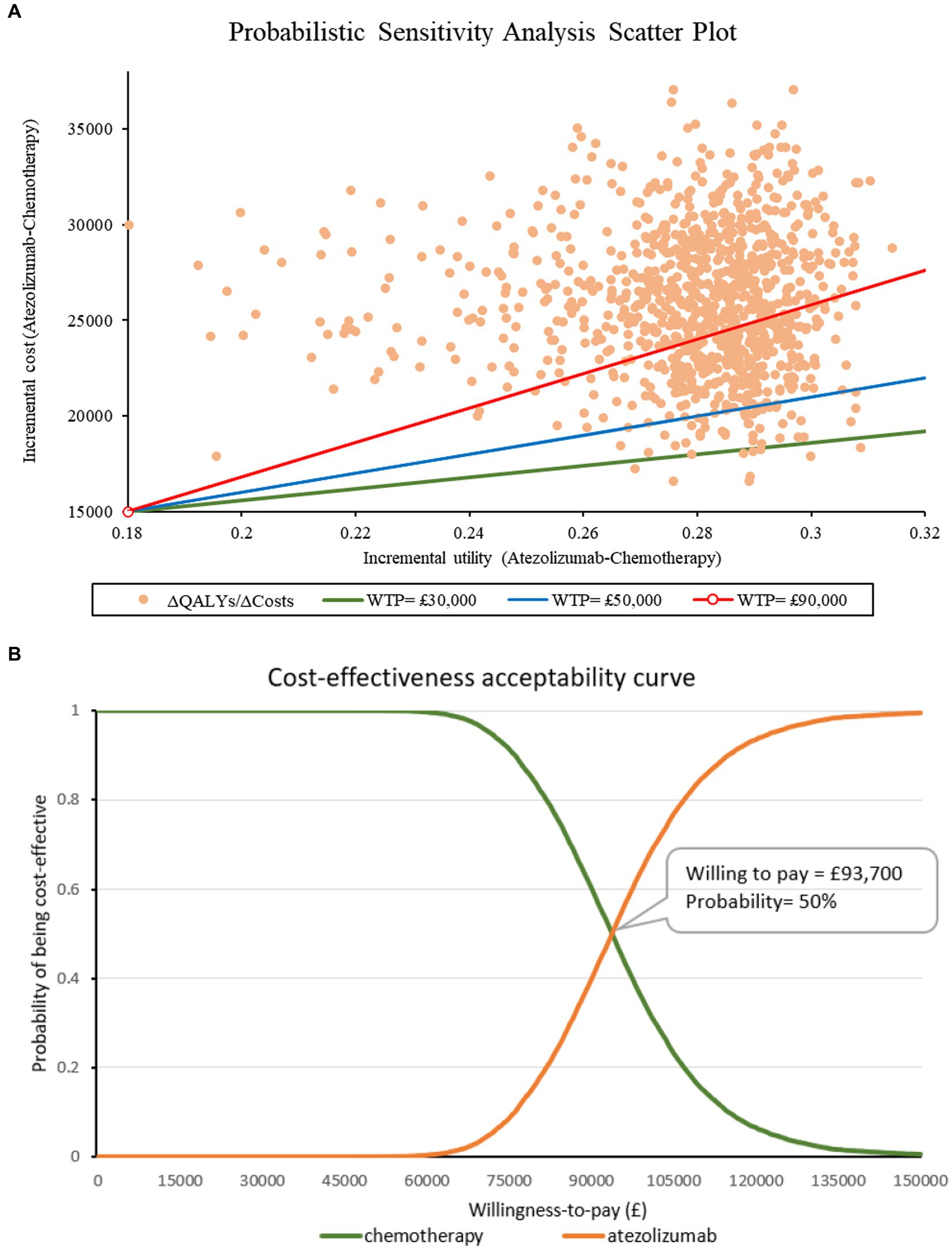
The scatter plot and CEAC curve can be found in Figure 2. The results from the PSA showed that the average cost of atezolizumab was £56,943 and the average cost of chemotherapy was £30,754. Moreover, the average effects for these two drugs are 0.86 and 0.58 QALYs, respectively. Following 10,000 iterations, the average ICER is calculated to be £94,384. Atezolizumab was considered to be cost-ineffective at the threshold of £50,000/QALY. Even with a higher threshold of £90,000/QALY, the probability of atezolizumab being cost-effective remained at 40%. The CEAC curve suggested that atezolizumab would be cost-effective if the threshold surpasses £93,700/QALY. Nevertheless, attaining this threshold within the present healthcare landscape in the UK poses significant challenges.
Sensitivity analyses results validated the base-case conclusion. At the threshold of £50,000/QALY, atezolizumab was not cost-effective at the current public price.
3.4. Scenario analysis
In general, the uncertainty related to the structural assumptions and parameter estimates examined had a negligible impact on the base-case conclusion. In scenario 1, utilizing the approaches commonly used that solely incorporate standard parametric models, the findings revealed that the ICER of atezolizumab in comparison to chemotherapy amounted to £99,040/QALY; In scenario 2, employing the utility values documented by the IMpover110 trial, the ICER was £90,974/QALY; In scenario 3, by modifying the dosing intensity for both drugs, the ICER amounted to £88,219/QALY; The ICER of atezolizumab against chemotherapy was £97,354/QALY in scenario 4, where the duration of second-line treatment was altered; In scenario 5, vinorelbine was administered orally, the ICER was £93,335/QALY; In scenario 6, when the usage ratio of gemcitabine ranged from 0 to 100%, the ICER ranged from £92,000 to £98,000/QALY. All scenarios resulted in comparable ICERs and reached the same conclusion. More details, refer to Supplementary Table S14 and Supplementary Figure S3.
3.5. Subgroup analysis
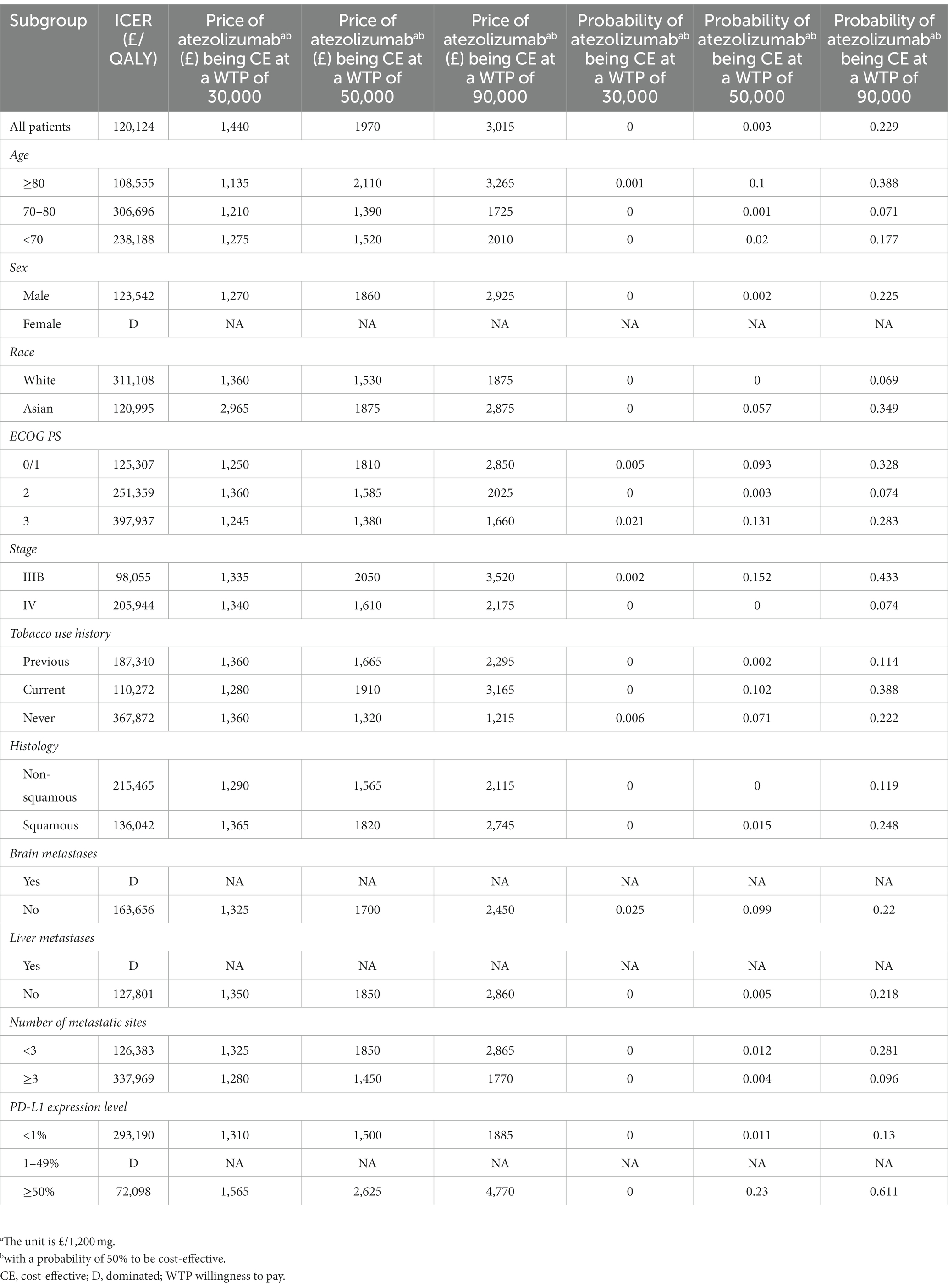
The subgroup analysis results are summarized in Table 2. Overall, the ICERs showed a significant association with HRs, indicating improved outcomes with lower risks of disease progression and death. The ICER for atezolizumab compared to chemotherapy in the entire patient cohort was £120,124/QALY. To meet the cost-effectiveness threshold of £50,000/QALY, the price would need to be £1,970. Atezolizumab did not show favorable results in any of the subgroups at the £50,000/QALY threshold. Atezolizumab was found to be more cost-effective for patients with positive PD-L1 expression (ICER: £72,098/QALY). However, atezolizumab performed worse in patients with PD-L1 expression levels ranging from 1 to 49%, as well as in female patients and those with liver or brain metastases. For other factors, such as age, sex, race, ECOG PS, tobacco use history, histology, number of metastatic sites, and disease stage, did not affect the conclusion, and ICER for atezolizumab compared to chemotherapy in all of these subgroups were over £100,000/QALY. Further details are in Table 2.
3.6. Price simulation
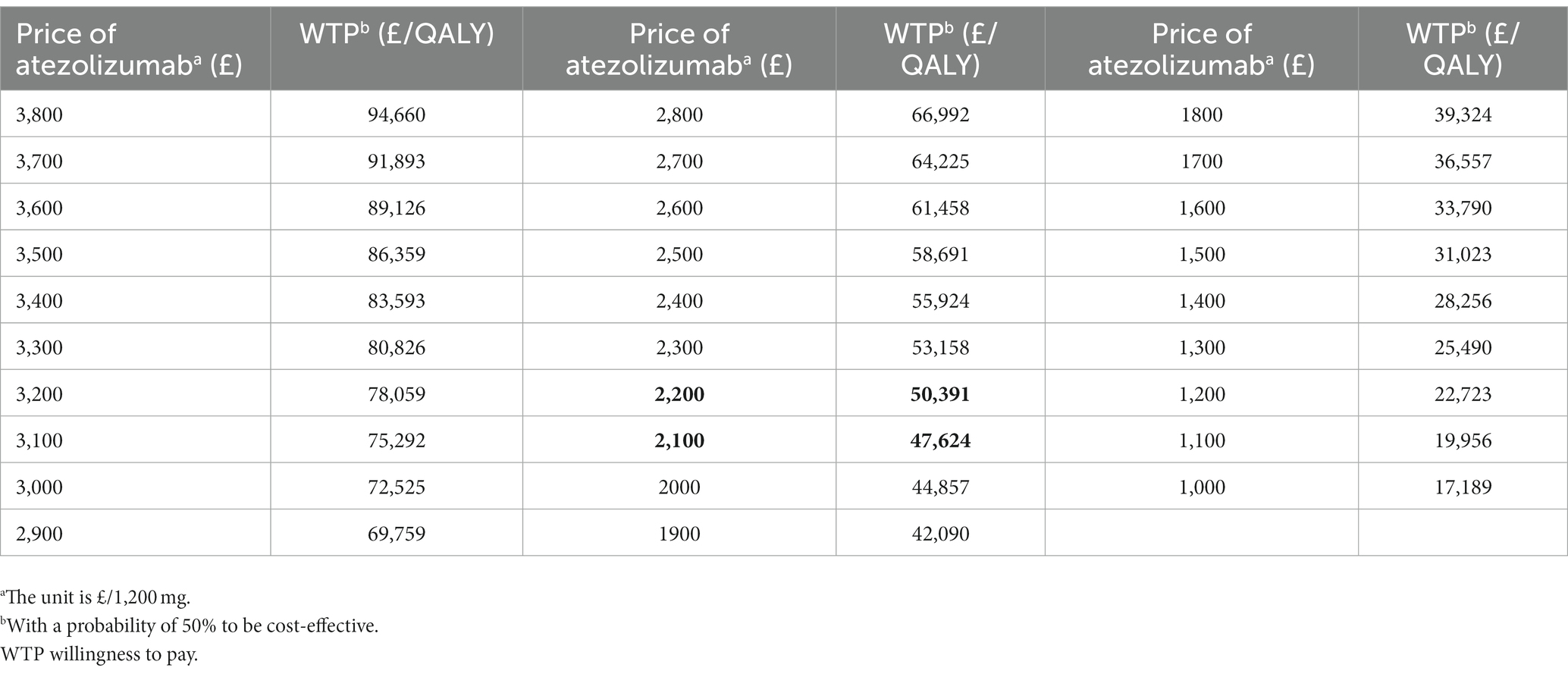
Overall, there is a positive correlation between the cost of atezolizumab and the ICER when compared to chemotherapy. It was concluded that atezolizumab was considered cost-effective if priced below £2,215/1,200 mg, given the willingness-to-pay threshold of £50,000/QALY. Atezolizumab was deemed cost-effective when the price was below £1,465 at a £30,000/QALY threshold. Additionally, for a price of £3,640, atezolizumab was considered cost-effective when the threshold was £90,000/QALY. More findings are depicted in Figure 3 and Table 3. There has been approved Patient Access Scheme for atezolizumab currently in the UK for several indications including advanced NSCLC (17), we believe that implementing the recommended price reductions for atezolizumab for NSCLC patients who are ineligible for treatment with a platinum-containing regimen would be practicality and feasible.
4. Discussion
Atezolizumab was the first to demonstrate a significant enhancement in the survival of patients with advanced lung cancer who have an intolerance to platinum-based chemotherapy (14), irrespective of the type of pathology, PD-L1 expression, or PS score. The real-world NEJ057 study (13) also corroborated this finding. Immunotherapy regimens had significantly higher median OS compared to chemotherapy (19.8 months vs. 9.5 months). Regarding toxicity, findings from both the IPSOS and NEJ057 studies suggested that using immune-mono therapy could significantly decrease the occurrence of severe AEs. Based on the IPSOS trial, it was found that 16% of patients receiving atezolizumab experienced treatment-related severe AEs, while the figure for patients receiving chemotherapy is 33%. Clinical treatment guidelines and strategies is expected to enter a new era of immune-mono therapy. Considering the exorbitant price of atezolizumab compared to the standard treatments, physicians and patients confront the challenge of evaluating its cost-effectiveness. The escalating healthcare costs justify concerns regarding value-based oncology.
This study aimed to fulfill the unmet need for an economic assessment of this novel indication. According to our analysis, atezolizumab was found to be less favorable compared to chemotherapy. When considering a WTP threshold of £50,000/QALY over a lifetime time horizon, atezolizumab incurred an extra cost of £26,206 and had an additional effect of 0.28 QALYs. This led to an ICER of £94,873/QALY in comparison to chemotherapy. Considering parameters’ uncertainty, we used the 95% CI as their range of variation. For parameters with only standard deviation or errors, we assume to calculate a 95% CI based on their distribution. For parameters with standard deviation or confidence interval, we assume they vary within a range of ±20%. The findings from the sensitivity analyses provided evidence that the results of the base-case analysis were generally stable and reliable. The factors that had the greatest impacts on economic outcomes were the price of atezolizumab, second-line medication, discontinuation rate of atezolizumab, and utility for PD. To address the uncertainties around the structural assumptions and parameter estimates, multiple scenario analyses were conducted. The selection of survival models, dosing patterns, utility values, and other factors minimally influenced the base-case results and yielded consistent conclusions. Nevertheless, for patients with positive PD-L1 expression, atezolizumab performed better, with an ICER of £72,079/QALY compared to chemotherapy. While for patients with a PD-L1 expression level of 1–49%, female patients, and those with liver or brain metastases, atezolizumab could be cost-effective only when its unit cost lower than chemotherapy. The high diversity of subgroup results reminds us that patient characteristics during the administration of drugs in clinical practice is crucial for utilizing healthcare resources in a rational manner.
Based on the results presented above, price simulations were conducted to explore suitable pricing for atezolizumab for studied patients. It was found that atezolizumab would be cost-efective at a price of £2,215/1,200 mg (a reduction of 41.8%) at the threshold of £50,000/QALY. For patients with positive PD-L1 expression, atezolizumab would be cost-efective at a price of £2,625/1,200 mg (a reduction of 31.1%). At the threshold of £90,000/QALY, atezolizumab would be cost-effective after a 4.4% price reduction for overall patients, and atezolizumab was economical at the current price for PD-L1 positive patients. The benefit of atezolizumab in PD-L1 positive patients was also observed in those with stage II-IIIA NSCLC in the adjuvant setting (4) and those with metastatic NSCLC receiving first- or second-line treatment (36, 37). Nevertheless, atezolizumab was deemed less cost-effective for patients with liver or brain metastases.
The direct and indirect costs associated with advanced NSCLC, particularly as the disease progresses, place a significant financial burden on healthcare systems, society, patients, and caregivers. Therefore, it is of utmost importance to develop newer, more economical, and safer treatments for NSCLC that can effectively slow down or halt the progression of the disease. The well-known fact is that chemotherapy has limited practicality in advanced NSCLC patients, especially in those who are ineligible for treatment with a platinum-containing regimen. Our findings suggest that atezolizumab seems to be a potential choice. However, the current price is a deterrent to it becoming the standard of care (SOC). Therefore, price concession is necessary, and our research provide reference for decision-makers. Overall, this research contributes to the development of a new SOC for patients with NSCLC who are unable to tolerate the AEs of the most powerful treatments, and to expedite the approval of this SOC in the UK, thereby providing new treatment options for patients and helping to alleviate the economic burden associated with the disease. Through this study, we aim to inform the UK decision-makers that atezolizumab has the potential to be a new SOC for NSCLC patients who are ineligible for treatment with a platinum-containing regimen. However, at current pricing, it is not yet a cost-effective choice. Furthermore, atezolizumab can be deemed cost-effective only when priced below £2215/1,200 mg at the willingness-to-pay threshold of £50,000/QALY.
The model structure and approach employed in this study are in line with the NICE appraisal of atezolizumab monotherapy for untreated PD-L1 positive metastatic NSCLC (36). For accuracy, reliable sources of information such as NHS reference costs, the PSSRU, and eMIT were utilized. The strengths of this study lie primarily in the innovative research topic, high-quality clinical data, the consideration of various scenarios, and the extensive sensitivity analysis. As far as we know, no other analysis has evaluated the cost-effectivenes of atezolizumab for patients with advanced NSCLC who cannot receive a platinum-containing regimen. Additionally, we performed price simulations to offer decision-makers a more comprehensive comprehension of the economic value attached to atezolizumab. Further discussion is necessary due to the implications of this study. Decision-makers in the UK should be informed about the price at which atezolizumab would be deemed cost-effective for patients with advanced NSCLC who cannot receive treatment containing platinum. Furthermore, our evidence may support the UK health technology assessment submissions for this indication. Finally, our analysis explored the cost-effectiveness outcomes of the 28 prespecified subgroups in the IPSOS trial. NSCLC is in the era of precision treatment (38), economic information for the subgroups may assist in tailoring treatment choices.
Our study has several limitations. First, lack of individual data compelling us to make assumptions about proportional hazards in subgroup analyses. This may cause bias in the calculation of survival rate, thereby leading to errors in the results of subgroup analyses; Second, omitting grade 1 or 2 AEs may have introduced biases, causing the actual cost of treatments to be underestimated. Nevertheless, the sensitivity analysis results indicated that this limitation had minimal impact. Third, at this stage, we did not study the availability and affordability, implying that further research is required. Fourth, as the lack of report in the IPSOS trial (14), the incidences of all grade 3–5 AEs were unavailable. Instead, we could solely consider AEs of any grade with an incidence difference exceeding 5% between groups. This theoretically might result in the underestimation of costs. We contend that its impact was restricted given that the grade 3–5 AEs integrated into our model closely resembled those in the IPSOS trial (14). Our model included 83 events, whereas the IPSOS trial documented 84 events. Fifth, EQ-5D based utility was not collected in the IPSOS trial, and the varying information used for the utility values from different trials might have an influence on the outcomes. Despite conducting a scenario analysis, the impact is still uncertain.
5. Conclusion
From the perspective of the UK healthcare system, atezolizumab is not an economical option for patients with advanced NSCLC ineligible for treatment with a platinum-containing regimen. Moreover, atezolizumab can be deemed cost-effective only when priced below £2215/1,200 mg at the willingness-to-pay threshold of £50,000 per QALY. Our study may offer evidence to guide the assessment of therapeutic alternatives and pricing setting for advanced NSCLC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JX: Data curation, Supervision, Writing – review & editing. JL: Investigation, Methodology, Writing – review & editing. WT: Supervision, Writing – review & editing, Funding acquisition, Resources, Validation, Visualization. XZ: Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Program of National Natural Science Foundation of China (grant no. 72174207), provided by WT. No potential biases to declare in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1282374/full#supplementary-material
References
1. Cancer Research UK . Lung cancer statistics (2022). Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lungcancer#heading-Zero (Accessed April 14, 2022).
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Lu, T, Yang, X, Huang, Y, Zhao, M, Li, M, Ma, K, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. (2019) 11:943–53. doi: 10.2147/CMAR.S187317
4. Yip, CY, Greystoke, A, Abogunrin, S, Belleli, R, di Maio, D, Rouse, P, et al. Cost-effectiveness analysis of adjuvant atezolizumab in stage II-IIIA non-small cell lung cancer expressing ≥50% PD-L1: a United Kingdom health care perspective. Lung Cancer. (2023) 179:107171. doi: 10.1016/j.lungcan.2023.03.007
5. National Institute for Health and Care Excellence . Lung cancer: diagnosis and management (NICE guideline NG122). Available at: https://www.nice.org.uk/guidance/ng122, (2019) (Accessed July 29, 2023).
6. Royal College of Physicians . National Lung Cancer Audit annual report 2022. (2022). Available at: https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2022 (Accessed November 29, 2022).
7. National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. (2022). Available at: https://seer.cancer.gov/statfacts/html/lungb.html (Accessed October 13, 2022).
8. Lilenbaum, RC, Cashy, J, Hensing, TA, Young, S, and Cella, D. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol. (2008) 3:125–9. doi: 10.1097/JTO.0b013e3181622c17
9. Reck, M, Rodríguez-Abreu, D, Robinson, AG, Hui, R, Csőszi, T, Fülöp, A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
10. West, H, McCleod, M, Hussein, M, Morabito, A, Rittmeyer, A, Conter, HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
11. Reck, M, Mok, TSK, Nishio, M, Jotte, RM, Cappuzzo, F, Orlandi, F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
12. Hanna, NH, Schneider, BJ, Temin, S, Baker, S Jr, Brahmer, J, Ellis, PM, et al. Therapy for stage IV non-small-cell lung Cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. (2020) 38:1608–32. doi: 10.1200/JCO.19.03022
13. Uematsu, M., Tsukita, Y., Tozuka, T., Kushiro, K., Hosokawa, S., Sumi, T., et al. First-line immune checkpoint inhibitors alone or in combination with chemotherapy in real-life elderly patients with advanced non-small cell lung cancer (NEJ057). (2023) ASCO.
14. Lee, SM, Schulz, C, Prabhash, K, Kowalski, D, Szczesna, A, Han, B, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. (2023) 402:451–63. doi: 10.1016/S0140-6736(23)00774-2
15. Project Orbis . Medicines and Healthcare products Regulatory Agency and their membership in Project Orbis. (n.d.) Available at: https://www.medicines.org.uk/emc/product/8442/smpc. (Accessed July 25, 2023).
16. National Institute for Health and Care Excellence . Guide to the methods of technology appraisal 2013. Available at: http://www.nice.org.uk/article/PMG9/chapter/Foreword, (2013) (Accessed April 17, 2018).
17. National Institute for Health and Care Excellence . Atezolizumab for adjuvant treatment of resected non-small-cell lung cancer [TA823]. (2022). Available at: https://www.nice.org.uk/guidance/ta823 (Accessed July 25, 2023).
18. Shah, KK, Cookson, R, Culyer, AJ, and Littlejohns, P. NICE's social value judgements about equity in health and health care. Health Econ Policy Law. (2013) 8:145–65. doi: 10.1017/S1744133112000096
19. Lee, SM, Schulz, C, Prabhash, K, Han, B, Szczesna, A, Cortinovis, DL, et al. Results from a Phase 3 study of first-line (1L) atezolizumab (atezo) vs single-agent chemotherapy (chemo) in patients (pts) with NSCLC not eligible for a platinum-containing regimen. Ann Oncol. (2022) 33:S1418–9. doi: 10.1016/j.annonc.2022.08.052
20. Guyot, P, Ades, AE, Ouwens, MJNM, and Welton, NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
21. Kearns, B, Stevenson, MD, Triantafyllopoulos, K, and Manca, A. Generalized linear models for flexible parametric Modeling of the Hazard function. Med Decis Mak. (2019) 39:867–78. doi: 10.1177/0272989X19873661
22. Janssen-Heijnen, ML, van Steenbergen, LN, Steyerberg, E, Visser, O, de Ruysscher, DK, and Groen, HJ. Long-term excess mortality for survivors of non-small cell lung cancer in the Netherlands. J Thorac Oncol. (2012) 7:496–502. doi: 10.1097/JTO.0b013e318241f80b
23. Beck, JR, Pauker, SG, Gottlieb, JE, Klein, K, and Kassirer, JP. A convenient approximation of life expectancy (the "DEALE"). II. Use in medical decision-making. Am J Med. (1982) 73:889–97. doi: 10.1016/0002-9343(82)90787-2
24. Ara, R, and Brazier, JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. (2010) 13:509–18. doi: 10.1111/j.1524-4733.2010.00700.x
25. Socinski, MA, Jotte, RM, Cappuzzo, F, Orlandi, F, Stroyakovskiy, D, Nogami, N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
26. van den Hout, WB, Kramer, GWPM, Noordijk, EM, and Leer, JWH. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst. (2006) 98:1786–94. doi: 10.1093/jnci/djj496
27. National Institute for Health and Care Excellence . Nivolumab with chemotherapy for neoadjuvant treatment of resectable non-small-cell lung cancer [TA876]. (2023). https://www.nice.org.uk/guidance/ta876 (Accessed July 25, 2023).
28. Zhao, M, Shao, T, Chi, Z, and Tang, W. Effectiveness and cost-effectiveness analysis of 11 treatment paths, seven first-line and three second-line treatments for Chinese patients with advanced wild-type squamous non-small cell lung cancer: a sequential model. Front Public Health. (2023) 11:1051484. doi: 10.3389/fpubh.2023.1051484
29. Shao, T, Zhao, M, Liang, L, and Tang, W. Serplulimab plus chemotherapy vs chemotherapy for treatment of US and Chinese patients with extensive-stage small-cell lung Cancer: a cost-effectiveness analysis to inform drug pricing. BioDrugs. (2023) 37:421–32. doi: 10.1007/s40259-023-00586-6
30. National Health Service . Drugs and pharmaceutical electronic market information tool (eMIT). https://www.gov.uk/government/publications/drugs-andpharmaceutical-electronic-market-information-emit, (2021) (Accessed 25 July, 2023).
31. National Institute for Health and Care Excellence . British National Formulary. https://www.nice.org.uk/bnf-uk-only, (2021) (Accessed 25 July, 2023).
32. National health service . National Cost Collection for the NHS 2019/20. https://www.england.nhs.uk/national-cost-collection/, (2021) (Accessed July 25, 20232).
33. PSSRU . Personal social service research unit in the UK. (2023). https://www.pssru.ac.uk/ (Accessed July 25, 2023).
34. National Institute for Health and Care Excellence . Lorlatinib for untreated ALK-positive advanced non-small-cell lung cancer [TA909]. (2023). https://www.nice.org.uk/guidance/ta909 (Accessed July 25, 2023).
35. Jassem, J, de Marinis, F, Giaccone, G, Vergnenegre, A, Barrios, CH, Morise, M, et al. Updated overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. (2021) 16:1872–82. doi: 10.1016/j.jtho.2021.06.019
36. National Institute for Health and Care Excellence . Atezolizumab monotherapy for untreated advanced non-small-cell lung cancer [TA705]. Available at: https://www.nice.orguk/guidance/ta705, (2021) (Accessed October 29, 2022).
37. National Institute for Health and Care Excellence . Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy [TA520]. Available at: https://www.nice.org.uk/guidance/ta520, (2018) (Accessed March 15, 2021).
Keywords: atezolizumab, non-small-cell lung cancer, UK, platinum-ineligible, cost-effectiveness, price simulation
Citation: Jiang Y, Zhao M, Xi J, Li J, Tang W and Zheng X (2023) Cost-effectiveness analysis of atezolizumab in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen: a United Kingdom health care perspective. Front. Public Health. 11:1282374. doi: 10.3389/fpubh.2023.1282374
Edited by:
Thomas T. H. Wan, University of Central Florida, United StatesReviewed by:
Walid Shalata, Soroka Medical Center, IsraelMehmet Yesilbas, Best Edit & Proof, United States
Bing-Long Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Jiang, Zhao, Xi, Li, Tang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zheng, emhlbmd4cEBuanVjbS5lZHUuY24=; Wenxi Tang, dG9rYW1teUBjcHUuZWR1LmNu
†These authors have contributed equally to this work
 Yunlin Jiang
Yunlin Jiang Mingye Zhao
Mingye Zhao Jiayi Xi3
Jiayi Xi3 Wenxi Tang
Wenxi Tang Xueping Zheng
Xueping Zheng