- Department of Clinical Laboratory, Children's Hospital of Nanjing Medical University, Nanjing, China
Background: Studies have shown that young maternal age at childbirth can increase the risk of attention-deficit/hyperactivity disorder (ADHD) in offspring, but a study of the U.S. population has not been reported. Moreover, there is no reported research on young and advanced maternal age at childbirth and whether it can contribute to the risk of learning disability (LD) in offspring.
Methods: This study evaluated the association between young and advanced maternal age at childbirth and offspring risk of ADHD and LD in the U.S. population. Using data from 8,098 participants included in the National Health and Nutrition Examination Survey (NHANES) conducted in 1999–2004, we analyzed the association between maternal age at childbirth and ADHD and LD risk in offspring. Odds ratios (ORs) and 95% confidence intervals (CIs) for maternal age at childbirth in association with ADHD and LD risk in offspring were estimated using multivariate logistic regression models after adjustment for age, sex, race, body mass index (BMI), poverty income ratio, smoking status during pregnancy, and NHANES cycle. Restricted cubic spline (RCS) models were used to evaluate potential non-linear relationships. Sensitivity analyses were performed to ensure the reliability of the results.
Results: Among all participants, the offspring of subjects with a maternal age at childbirth of 18–24 years had an increased risk of ADHD (OR = 1.34, 95% CI: 1.01, 1.79) and LD (OR = 1.36, 95% CI: 1.06, 1.79) or either ADHD or LD (OR = 1.48, 95% CI: 1.20, 1.81). Additionally, compared with subjects with a maternal age at childbirth of 25–29 years, subjects with a maternal age at childbirth of 35–39 years had lower odds of having offspring with ADHD (OR = 0.60, 95% CI: 0.36, 1.00) and higher odds of having offspring with LD (OR = 1.34, 95% CI: 1.01, 1.78). The relationship between maternal age at childbirth and LD risk presented a U-shaped curve.
Conclusions: These results provide epidemiological evidence showing that young and advanced maternal age at childbirth are associated with ADHD and LD risk.
Introduction
Maternal age at childbirth is defined as the age of the mother at the time of delivery. Generally, a young maternal age at childbirth is defined as an age of 19 years old or younger, and an advanced maternal age is defined as an age of at least 35 years old (1). The effects of young and advanced maternal age at childbirth may affect the health outcomes of mothers and their offspring. Previous studies have suggested that a young maternal age at childbirth is associated with maternal cardiovascular disease risk (2) and low birth weight, preterm birth, and neonatal mortality (3). Several adverse health effects are associated with advanced maternal age at childbirth, including cerebral palsy, neurocognitive disorders, psychiatric disorders (4), stillbirth (5–7) and maternal cancer risk (8, 9); however, some studies have reported that advanced maternal age does not seem to be associated with the long-term morbidity of offspring (10).
Neurological disorders in children manifest as abnormalities in neurocognition, self-regulation, and adaptive functioning (11). Abnormal neurobehavioral function in children can cause health problems and other issues, such as emotional disorders (12), lower education levels (13), antisocial behavior (14), and premature mortality (15). Among neurological disorders, attention-deficit/hyperactivity disorder (ADHD) and learning disability (LD) are common in children. ADHD and LD are defined as persistent inattention and hyperactivity and a reduced ability to learn, respectively. The global prevalence of ADHD in children is ~5% (16), and the prevalence is reported to be 18.1% in Tunisian adolescents (17), 8.8% in Nigerian children (18), and 6.26% in Chinese children and teenagers (19). The prevalence rates of LD in the U.S. and in a city in India were shown to be 9.7 (20) and 3.08% (21), respectively. Previous studies on the risk factors for ADHD and LD have mainly focused on genetic factors (15, 22–24), while ~10–40% of the risk may be related to factors such as premature birth and smoking during pregnancy for ADHD (25, 26) and low birth weight (27), maternal BMI before pregnancy (28), and maternal anemia (29) for LD. Although many previous studies have suggested that ADHD is highly related to hereditary factors, no gene with a definite pathogenic effect has been identified (30). Although Thapar et al. (15) found that both prenatal and perinatal factors may be risk factors for ADHD and LD, these risk factors are not fully understood. Therefore, fully understanding these risk factors may effectively prevent the occurrence of ADHD and LD.
A recent study found that a young maternal age at childbirth was positively correlated with the risk of ADHD in offspring in Denmark (31). However, there is a lack of relevant research in the U.S. Because immigrants from multiple countries live in the U.S., the genetic background and cultural differences of the population are different from those of Denmark. Additionally, the prevalence of ADHD in the U.S. is much higher than the average level globally (32). The average maternal age at first childbirth increased from 1970 to 2006 and from 2011 to 2012 in the U.S. (33). This study was performed due to the lack of U.S. research on the association between maternal age at childbirth and ADHD and LD risk in offspring. This study is the first to explore the association between maternal age at childbirth and ADHD and LD in offspring and to evaluate the non-linear relationship in the U.S.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention every 2 years, and the main purpose is to assess the health and nutritional status of children and adults in the U.S. The user data agreement is available online (https://www.cdc.gov/nchs/data\_access/restrictions.htm). The NCHS Research Ethics Review Board approved these NHANES cycles. All subjects provided written informed consent. Data on ADHD and LD outcomes are available only for the 1999–2004 cycle; thus, we obtained publicly available NHANES data generated through surveys conducted in 1999–2004, including data from a total of 31,126 participants. By using unique survey participant identifiers, we combined information on the participants' characteristics with their questionnaire information. For maternal age at childbirth, data are available only for offspring aged 0–15 years, and the age ranges for ADHD and LD evaluation were 4–19 and 4–15 years, respectively. We therefore excluded participants under 4 and over 15 years of age. Although pregnancy may affect the outcomes of analysis, we did not exclude pregnant subjects (n = 7) in our analysis. Ultimately, 8,098 subjects were included in our study: 3,977 boys and 4,121 girls. The flow chart for inclusion and exclusion is shown in Figure 1.
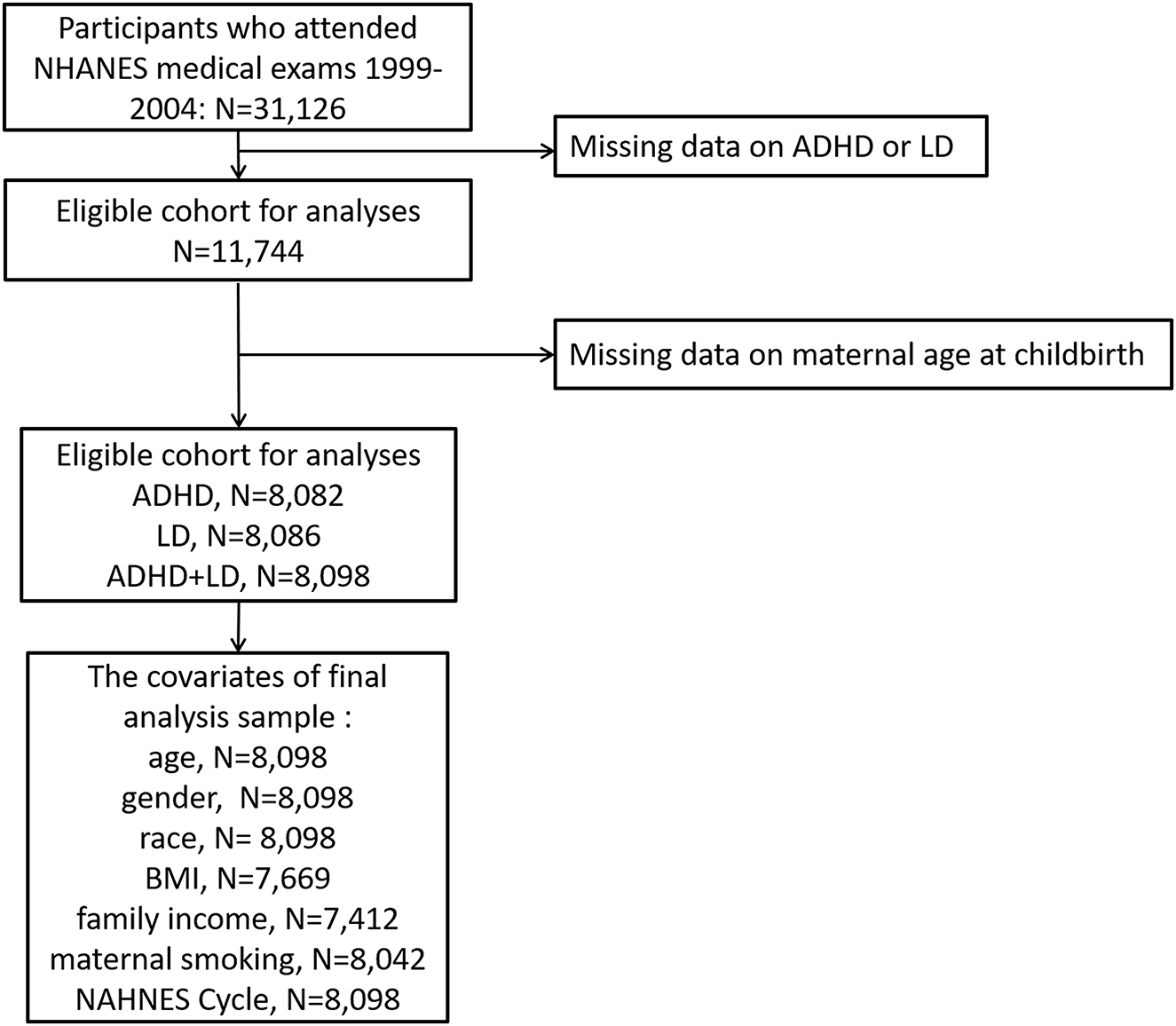
Figure 1. Eligible participants and those included in the analyses of the associations between maternal age at childbirth and the risk of ADHD and LD in offspring in the U.S. population.
Assessment of maternal age at childbirth and ADHD and LD risk
This information was based on parental/guardian responses to the following NHANES interview question, asked twice regarding both LD and ADHD (for children ≤ 15 years of age): “How old was your biological mother when you were born?” Age was recorded as a continuous variable. The outcomes of ADHD and LD were obtained from parental reports of LD and/or ADHD. Specifically, the data were based on parental/guardian responses to two NHANES interview questions: “Has a doctor or health professional ever told you that you have attention deficit disorder?” and “Has a representative from a school or a health professional ever told you that you have a learning disability?” For children ≥4 years old, the same questions were asked regarding both LD and ADHD. The answer to both of these questions was “yes” or “no.” The answer was included as a binary variable for subsequent analysis. We divided children into those with parental/guardian reports of ADHD, those with parental/guardian reports of LD, and those with parental/guardian reports of either LD or ADHD.
Covariates
Several factors related to outcomes were adjusted in our logistic regression analysis, including age (continuous variable), sex (categorical variable), race (categorical variable), body mass index (BMI, continuous variable), poverty income ratio (PIR, categorical variable), and NHANES cycle (categorical variable). Information regarding age, sex, race, and the PIR was obtained by questionnaires conducted during face-to-face interviews. Height and body weight were recorded in a physical examination and were used to calculate BMI. Information related to smoking during pregnancy was based on parental/guardian responses to the following question: “Did your biological mother smoke at any time while she was pregnant?”
Statistical methods
Continuous variables are presented as the mean and standard deviation, and categorical variables are presented as the frequency and percentage. Because BMI and PIR values were missing for 429 and 686 participants, respectively, to ensure that the sample size was not reduced, we used the mean value imputation method. We divided participants into the following groups according to maternal age at childbirth: ≤ 17, 18–24, 25–29, 30–34, 35–39, and ≥40 years age groups. Considering that both young and advanced maternal age at childbirth may be risk factors for ADHD or LD, we regarded 25–29 years as the reference age (34). Logistic regression was used to assess the association between maternal age at childbirth and ADHD, LD and either ADHD or LD risk after adjusting for age, sex, race, BMI, PIR, smoking during pregnancy, and NHANES cycle. In addition, we used restricted cubic spline (RCS) regression to investigate the non-linear trend between maternal age at childbirth and the risk of ADHD, LD and either ADHD or LD. For the sensitivity analysis, we excluded subjects without BMI or PIR data in logistic regression analysis. P < 0.05 was considered a statistically significant difference. In multiple comparisons, to avoid false positives, the Bonferroni correction was used in our analysis, with P-values < (0.05/5 [group] = 0.01) considered statistically significant. Stata version 14.0 (StataCorp) was used for the statistical analyses in the present study.
Results
The average maternal age at childbirth in our study was 25.6 ± 6.1 years old (Table 1). Maternal age at childbirth differs among races; this age was the lowest in the non-Hispanic Black population (24.5 ± 6.1 years old) and the highest in the non-Hispanic White population (27.3 ± 5.8 years old). On average, subjects with high family income had a higher maternal age at childbirth than those with low family income (27.4 ± 5.8 vs. 24.0 ± 6.0 years old, P < 0.001). In addition, maternal age at childbirth did not differ significantly on the basis of smoking during pregnancy (25.6 ± 6.0 vs. 25.6 ± 6.1 years old, P = 0.961).
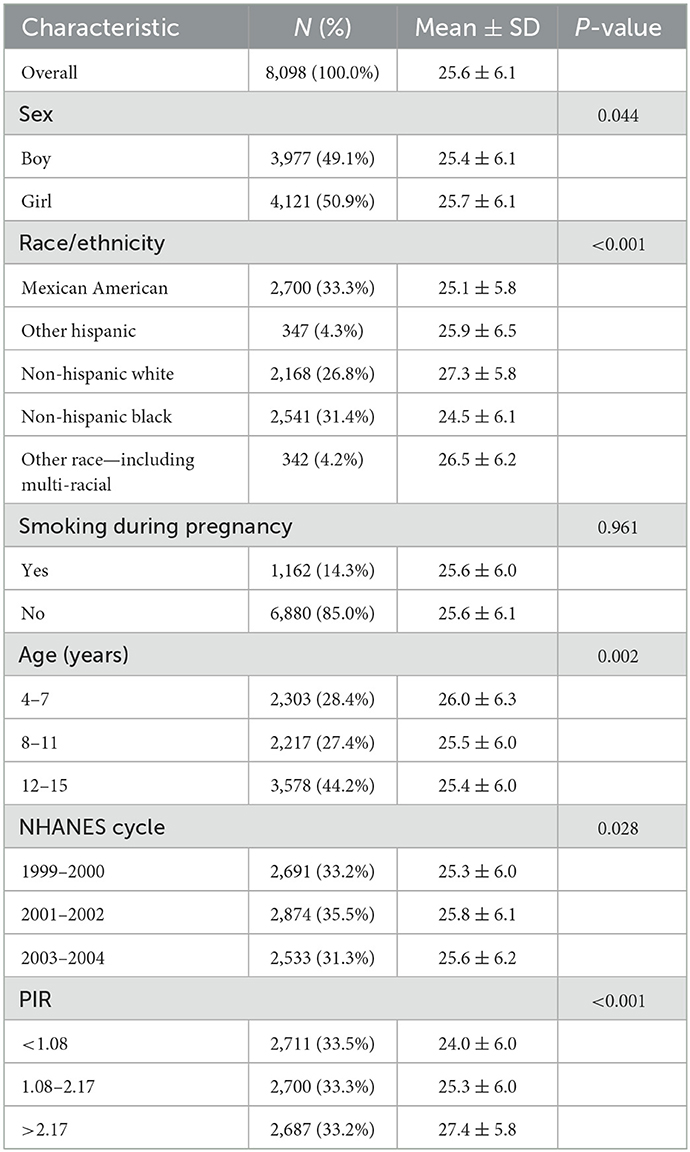
Table 1. Characteristics of maternal age of children included in the study (NHANES 1999–2004; n = 8,098).
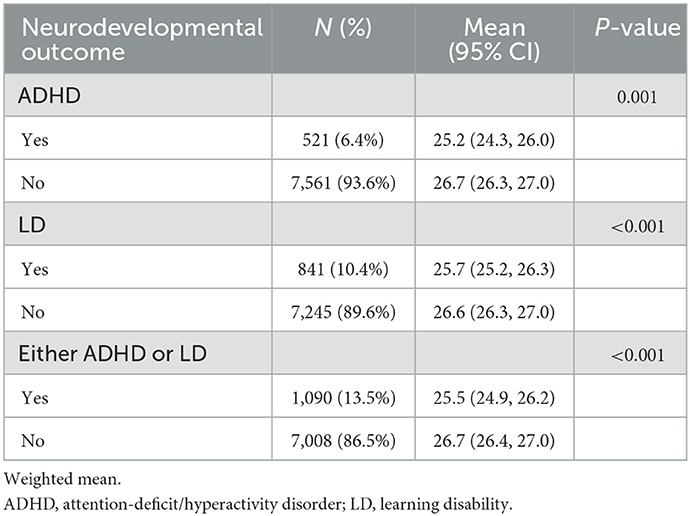
Table 2 shows that the percentages of offspring with ADHD, LD and either ADHD or LD in our study were 6.4, 10.4, and 13.5%, respectively. The maternal age at childbirth for the group with ADHD, LD, or either ADHD or LD was younger than that for the group without ADHD, LD, or either ADHD or LD (25.2 vs. 26.7 years old, P = 0.001 for ADHD; 25.7 vs. 26.6 years old, P < 0.001 for LD; 25.5 vs. 26.7 years old, P < 0.001 for either ADHD or LD).
The logistic regression results showed that compared to subjects aged 25–29 years, in subjects younger than 25 years old and older than 39 years old at childbirth, the risk of ADHD, LD, and either ADHD or LD in offspring was increased (Table 3) after adjusting for age, sex, race, BMI, PIR, smoking during pregnancy, and NHANES cycle. Among these groups, the adjusted OR for subjects aged 18–24 years was 1.48 (95% CI: 1.20, 1.81) for the risk of either ADHD or LD in offspring, with a P-value < 0.01 (Bonferroni adjustment of the P-value). In addition, we also observed that compared to subjects aged 25–29 years at childbirth, in subjects aged 30–39 years, the risk of ADHD was reduced, while the risk of LD and the risk of either ADHD or LD in offspring was increased. However, these results were not statistically significant, which may be related to our small sample size. The sensitivity analysis showed that the results were robust (Supplementary Table S1).
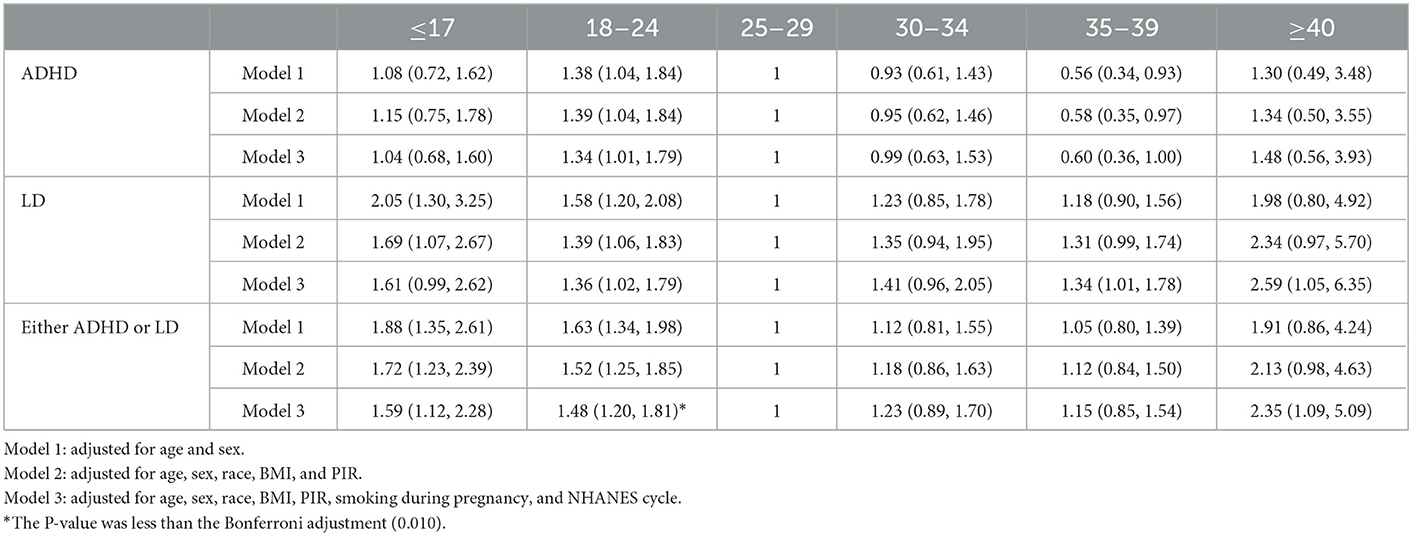
Table 3. Association between maternal age at childbirth and parent-reported LD, ADHD, and either LD or ADHD.
When maternal age at childbirth was considered as a continuous variable using RCS regression models (Figure 2), a U-shaped association of maternal age at childbirth with the risk of either ADHD or LD in offspring was observed (P-value = 0.007). The risk of either ADHD or LD in offspring was lowest among subjects aged 25–29 years at childbirth. When performing RCS regression models of the LD risk outcome, we observed a similar but more pronounced U-shaped curve with a greater slope (P-value = 0.005). However, the non-linear association between maternal age at childbirth and ADHD risk in offspring was not significant (P-value = 0.842).
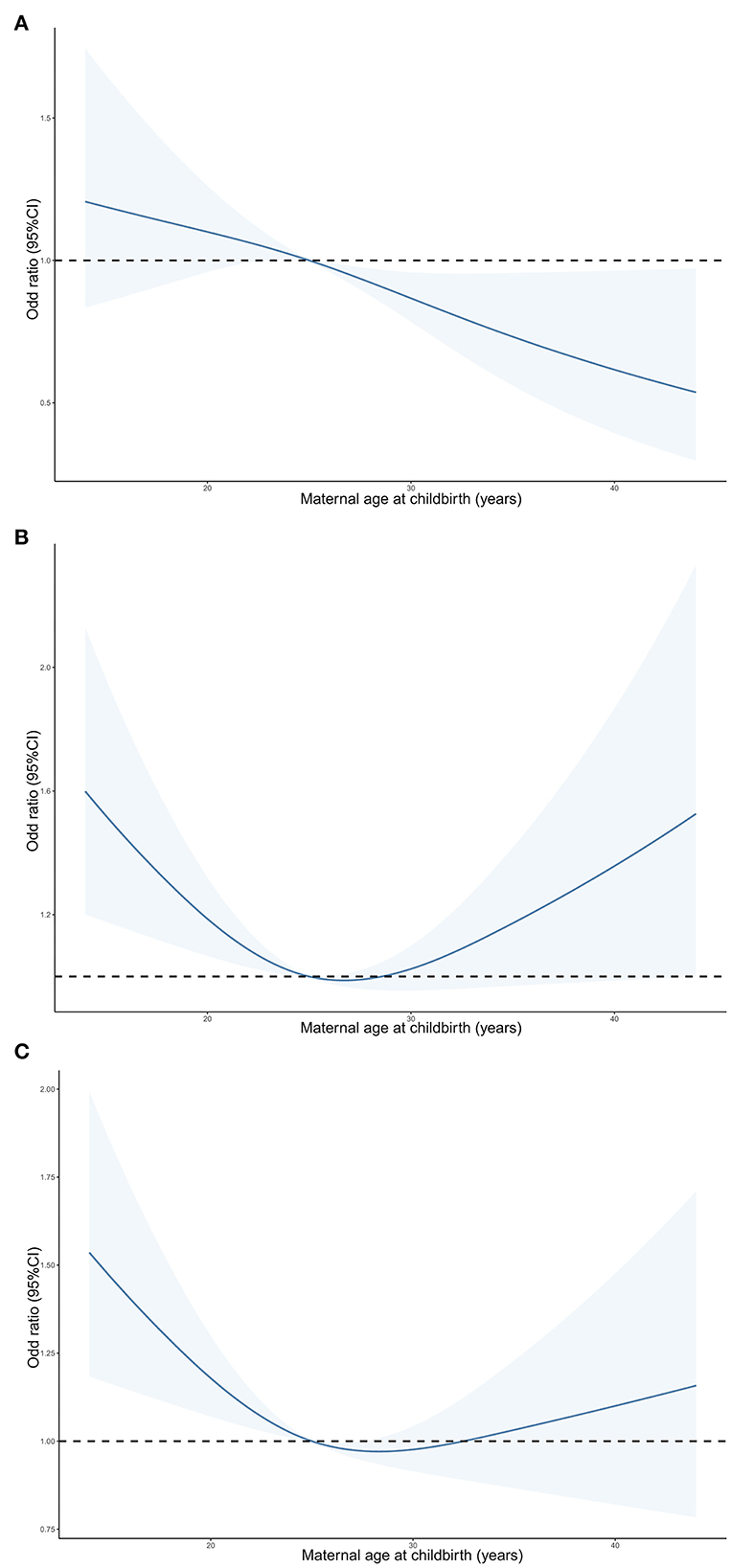
Figure 2. Predicted spline curves for the associations of maternal age at childbirth with the risk of ADHD (A), LD (B) and either ADHD or LD (C) in offspring according to RCS regression models. ADHD, attention-deficit/hyperactivity disorder; LD, learning disability.
Discussion
This is one of the first studies to report that young maternal age at childbirth may increase the risk of ADHD and LD in offspring in the U.S. population and that advanced maternal age at childbirth seems to likely increase the risk of LD in offspring. In addition, we also found that these associations differ by sex. Furthermore, the relationship between maternal age at childbirth and the risk of LD in offspring presents a U-shaped curve.
Previous epidemiological studies have shown an increased risk of ADHD in the offspring of mothers with young maternal age at childbirth in the Swedish population (35); similarly, the risk of ADHD in offspring is also increased for mothers with young maternal age at childbirth in the Danish population (31). The results of the above two studies are similar to ours and may be explained because the development of ADHD is related to neurotransmission pathways. Therefore, we speculated that the education levels of the subjects with advanced maternal age at childbirth were higher, and these individuals may have greater health knowledge during pregnancy. Therefore, compared to participants in the 25–29-year-old group, participants in advanced maternal age groups may pay more attention to nutritional supplements and additives during pregnancy, such as folic acid, which are beneficial for neural development, thus reducing the risk of ADHD in their offspring. Other potential reasons are that individuals in the U.S. population have complex ethnic backgrounds. The genetic backgrounds of different races are quite different, and genetic factors are considered to be related to the development of ADHD (36, 37). In contrast to the results of the present study, a study of the Danish population revealed that advanced maternal age at childbirth (over 35 years old) is a protective factor against ADHD in offspring. While our results showed that the risk of ADHD in offspring of individuals older than 39 years is likely to be increased, the difference was not significant, which may be related to the small sample size of our study. Another reason for the difference in study results is that the Danish study did not group and analyze the data of people over 40 years old.
At present, few studies have been conducted on maternal age at childbirth and LD risk in offspring, which may be related to the diagnosis of LD without International Classification of Disease (ICD) standards. Although LD evaluation is subjective, it is easy to perform and can reflect certain neural functions (38, 39). At present, the etiology of LD is still unclear. Some scholars have suggested that genetic factors may play a role, and the symptoms of LD are also broader. Adolescents who exhibit abnormalities in reading, writing, listening, speaking, and performing math are suggested to have LD. However, the cause of LD in offspring is complex and may not be related to a neural pathway. In this study, we observed a U-shaped curve of maternal age at childbirth and offspring risk of LD. It is suggested that the offspring of individuals with younger and more advanced maternal ages at childbirth have a greater risk of LD. At present, most of the existing studies suggest that young maternal age at childbirth increases the risk of health effects on offspring. However, controversy exists for subjects with advanced maternal age at childbirth. An appropriate older maternal age is beneficial for offspring with regard to financial status and education level (1). The social economic position and resources of older mothers are greater than those of younger mothers, and over time, the offspring of older mothers experience the effects of improvements in public health conditions and educational expansion. Although negative effects occur during the perinatal period, there is no difference between the offspring of older mothers and those of younger mothers during adulthood (40). However, it should be noted that the oocytes of older mothers are aged, and accumulated DNA damage (41) and the imbalance in mitochondrial homeostasis (42) in aged oocytes have a negative impact on the development of fertilized eggs (43). DNA damage and mitochondrial function are closely related to nerve development (44, 45). These characteristics of aged oocytes may explain the decreased learning ability of offspring. However, some researchers suggest that some patients are classified as having an LD because of their parents' misunderstanding of the education process and higher parental expectations for offspring (46). The intelligence or neurodevelopment of offspring may not be abnormal, but the specific molecular mechanism of the relationship between age and LD risk still requires further research.
Our results indicate that maternal age at childbirth is related to the risk of LD in offspring. Due to the recall bias present in retrospective cohorts, animal studies may clarify the cause and effect relationship. Some existing mechanistic studies have confirmed that the spatial learning capacity of mice at 32–35 weeks is lower than that of mice at 9–12 weeks, which may be related to the decrease in the expression of vitamin D receptor (VDR) in the early embryogenesis process (47). In addition, compared with 3-month-old mice, 15–18-month-old mice showed anxiety-like behavior, and the gene expression pattern of the hippocampus was also changed (48). We also speculated that late production age may be related to egg quality (49). These possible mechanisms may explain the link between maternal age at childbirth and ADHD and LD risk in offspring. However, there are few studies on the mechanism of the relationship between young maternal age at childbirth and ADHD and LD risk in offspring, possibly because few animal models exist. We speculated that the link between young maternal age at childbirth and ADHD and LD risk in offspring may arise because the family income and education levels of young mothers are lower than those of older mothers (50). Additionally, Fall et al. found that young maternal age at childbirth may be associated with poor birth outcomes and nutrition for offspring in low- and middle-income countries (1).
As an advantage, this study is the first to report the association between maternal age at childbirth and offspring ADHD and LD risk in the U.S. population. We found that young or advanced maternal age at childbirth may lead to increased ADHD and LD risk in offspring. Through the results of subgroup analysis, we identified the susceptible population, which may provide a basis for research on the underlying mechanism or prevention. Applying the RCS model is helpful to determine the association between maternal age at childbirth and the risk of ADHD and LD in offspring.
Although the present study results have some scientific significance, there are still several limitations of our study as a whole. First, genetic factors have a great influence on ADHD and LD risk. Even if we consider race as a covariate for adjustment and further analysis, we still cannot exclude the bias of genetic factors. Second, some maternal lifestyles during pregnancy also affect offspring ADHD and LD risk, although we adjusted for the variable of smoking during pregnancy. In fact, there are additional factors that may affect ADHD and LD risk in offspring, such as gestational hypertension and gestational diabetes, but we could not adjust for them because no such data are contained in the NHANES database. Third, in this study, some covariates were missing. To avoid reducing the sample size, we imputed data for the missing variables, but strictly speaking, this approach may have biased the actual results. Fourth, the outcome variables in this study were based on self-reported data, leading to potential subjective bias. It is thus necessary for future research to use objective indicators.
In conclusion, the present study results indicated a U-shaped association between maternal age at childbirth and the risk of ADHD and LD in offspring in a nationally representative U.S. survey. Future studies with large sample sizes and mechanistic studies are needed to confirm the risk of ADHD and LD in offspring.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NHANES.
Ethics statement
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board approved these NHANES cycles. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Material preparation, data processing, analysis were carried out, and the first draft of the manuscript was written by GL. All authors commented on the previous versions of the manuscript, contributed to the conception and design of the study, read, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.923133/full#supplementary-material
References
1. Fall CH, Sachdev HS, Osmond C, Restrepo-Mendez MC, Victora C, Martorell R, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). Lancet Global Health. (2015) 3:e366–77. doi: 10.1016/S2214-109X(15)00038-8
2. Rosendaal NTA, Pirkle CM. Age at first birth and risk of later-life cardiovascular disease: a systematic review of the literature, its limitation, and recommendations for future research. BMC Public Health. (2017) 17:627. doi: 10.1186/s12889-017-4519-x
3. Gibbs CM, Wendt A, Peters S, Hogue CJ. The impact of early age at first childbirth on maternal and infant health. Paediat Perinat Epidemiol. (2012) 26:259–84. doi: 10.1111/j.1365-3016.2012.01290.x
4. Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol. (2012) 24:187–93. doi: 10.1097/GCO.0b013e3283517908
5. Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS ONE. (2017) 12:e0186287. doi: 10.1371/journal.pone.0186287
6. Carolan M, Frankowska D. Advanced maternal age and adverse perinatal outcome: a review of the evidence. Midwifery. (2011) 27:793–801. doi: 10.1016/j.midw.2010.07.006
7. Carolan M. Maternal age ≥45 years and maternal and perinatal outcomes: a review of the evidence. Midwifery. (2013) 29:479–89. doi: 10.1016/j.midw.2012.04.001
8. Merrill RM, Fugal S, Novilla LB, Raphael MC. Cancer risk associated with early and late maternal age at first birth. Gynecol Oncol. (2005) 96:583–93. doi: 10.1016/j.ygyno.2004.11.038
9. Nassar AH, Usta IM. Advanced maternal age. Part II: long-term consequences. Am J Perinatol. (2009) 26:107–12. doi: 10.1055/s-0028-1090593
10. Pariente G, Wainstock T, Walfisch A, Sheiner E, Harlev A. Advanced maternal age and the future health of the offspring. Fetal Diagn Therapy. (2019) 46:139–46. doi: 10.1159/000493191
11. Hagan JF, Balachova T, Bertrand J, Chasnoff I, Dang E, Fernandez-Baca D, et al. Neurobehavioral disorder associated with prenatal alcohol exposure. Pediatrics. (2016) 138:1553. doi: 10.1542/peds.2015-1553
12. Lecendreux M, Konofal E, Faraone SV. Prevalence of attention deficit hyperactivity disorder and associated features among children in France. J Attent Disord. (2011) 15:516–24. doi: 10.1177/1087054710372491
13. Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M. Functional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry. (2006) 67:524–40. doi: 10.4088/JCP.v67n0403
14. Marshal MP, Molina BS. Antisocial behaviors moderate the deviant peer pathway to substance use in children with ADHD. J Clin Child Adolesc Psychol. (2006) 35:216–26. doi: 10.1207/s15374424jccp3502_5
15. Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. (2016) 387:1240–50. doi: 10.1016/S0140-6736(15)00238-X
16. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. (2018) 5:175–86. doi: 10.1016/S2215-0366(17)30167-0
17. Mhalla A, Guedria A, Brahem T, Amamou B, Sboui W, Gaddour N, et al. ADHD in tunisian adolescents: prevalence and associated factors. J Attent Disord. (2018) 22:154–62. doi: 10.1177/1087054717702217
18. Umar MU, Obindo JT, Omigbodun OO. Prevalence and correlates of ADHD among adolescent students in Nigeria. J Attent Disord. (2018) 22:116–26. doi: 10.1177/1087054715594456
19. Wang T, Liu K, Li Z, Xu Y, Liu Y, Shi W, et al. Prevalence of attention deficit/hyperactivity disorder among children and adolescents in China: a systematic review and meta-analysis. BMC Psychiatry. (2017) 17:32. doi: 10.1186/s12888-016-1187-9
20. Altarac M, Saroha E. Lifetime prevalence of learning disability among US children. Pediatrics. (2007) 119:S77–83. doi: 10.1542/peds.2006-2089L
21. Padhy SK, Goel S, Das SS, Sarkar S, Sharma V, Panigrahi M. Prevalence and patterns of learning disabilities in school children. Indian J Pediat. (2016) 83:300–6. doi: 10.1007/s12098-015-1862-8
22. Banaschewski T, Becker K, Dopfner M, Holtmann M, Rosler M, Romanos M. Attention-deficit/hyperactivity disorder. Deutsches Arzteblatt Int. (2017) 114:149–59. doi: 10.3238/arztebl.2017.0149
23. Roberts JL, Hovanes K, Dasouki M, Manzardo AM, Butler MG. Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders or learning disability presenting for genetic services. Gene. (2014) 535:70–8. doi: 10.1016/j.gene.2013.10.020
24. Becker N, Vasconcelos M, Oliveira V, Santos FCD, Bizarro L, Almeida RMM, et al. Genetic and environmental risk factors for developmental dyslexia in children: systematic review of the last decade. Dev Neuropsychol. (2017) 42:423–45. doi: 10.1080/87565641.2017.1374960
25. Sciberras E, Mulraney M, Silva D, Coghill D. Prenatal risk factors and the etiology of ADHD-review of existing evidence. Curr Psychiatry Rep. (2017) 19:1. doi: 10.1007/s11920-017-0753-2
26. Huang L, Wang Y, Zhang L, Zheng Z, Zhu T, Qu Y, et al. Maternal smoking and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Pediatrics. (2018) 141:2465. doi: 10.1542/peds.2017-2465
27. Johnson EO, Breslau N. Increased risk of learning disabilities in low birth weight boys at age 11 years. Biol Psychiatry. (2000) 47:490–500. doi: 10.1016/S0006-3223(99)00223-1
28. Andersen CH, Thomsen PH, Nohr EA, Lemcke S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur Child Adolesc nt Psychiatry. (2018) 27:139–48. doi: 10.1007/s00787-017-1027-6
29. Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. (2019) 18:1–12. doi: 10.1001/jamapsychiatry.2019.2309
30. Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry. (2016) 3:555–67. doi: 10.1016/S2215-0366(16)00096-1
31. Hvolgaard Mikkelsen S, Olsen J, Bech BH, Obel C. Parental age and attention-deficit/hyperactivity disorder (ADHD). Int J Epidemiol. (2017) 46:409–20. doi: 10.1093/ije/dyw073
32. Mahone EM, Denckla MB. Attention-deficit/hyperactivity disorder: a historical neuropsychological perspective. J Int Neuropsychol Soc JINS. (2017) 23:916–29. doi: 10.1017/S1355617717000807
33. Matthews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief. (2014) 152:1–8.
34. Sheen JJ, Wright JD, Goffman D, Kern-Goldberger AR, Booker W, Siddiq Z, et al. Maternal age and risk for adverse outcomes. Am J Obstet Gynecol. (2018) 219:390.e1–e15. doi: 10.1016/j.ajog.2018.08.034
35. Chang Z, Lichtenstein P, D'Onofrio BM, Almqvist C, Kuja-Halkola R, Sjolander A, et al. Maternal age at childbirth and risk for ADHD in offspring: a population-based cohort study. Int J Epidemiol. (2014) 43:1815–24. doi: 10.1093/ije/dyu204
36. Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Disease Prim. (2015) 1:15020. doi: 10.1038/nrdp.2015.20
37. Tarver J, Daley D, Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. (2014) 40:762–74. doi: 10.1111/cch.12139
38. Whitsell LJ. Learning disorders as a school health problem. Neurological and psychiatric aspects. Calif Med. (1969) 111:433–45.
39. Erenberg G. Learning disabilities: an overview. Semin Neurol. (1991) 11:1–6. doi: 10.1055/s-2008-1041198
40. Barclay KaM, M. Advanced maternal age and offspring outcomes: reproductive aging and counterbalancing period trends. Popul Dev Rev. (2016) 42:69–94. doi: 10.1111/j.1728-4457.2016.00105.x
41. Goldmann JM, Seplyarskiy VB, Wong WSW, Vilboux T, Neerincx PB, Bodian DL, et al. Germline de novo mutation clusters arise during oocyte aging in genomic regions with high double-strand-break incidence. Nat Genet. (2018) 50:487–92. doi: 10.1038/s41588-018-0071-6
42. May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferre-L'Hotellier V, Moriniere C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. (2016) 22:725–43. doi: 10.1093/humupd/dmw028
43. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. (2009) 15:573–85. doi: 10.1093/humupd/dmp014
44. McKinnon PJ. Genome integrity and disease prevention in the nervous system. Genes Dev. (2017) 31:1180–94. doi: 10.1101/gad.301325.117
45. Khacho M, Harris R, Slack RS. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat Rev Neurosci. (2019) 20:34–48. doi: 10.1038/s41583-018-0091-3
46. Graham HR, Minhas RS, Paxton G. Learning problems in children of refugee background: a systematic review. Pediatrics. (2016) 137:3994. doi: 10.1542/peds.2015-3994
47. Mao WJ, Wu ZY, Yang ZH, Xu YW, Wang SQ. Advanced maternal age impairs spatial learning capacity in young adult mouse offspring. Am J Transl Res. (2018) 10:975–88.
48. Sampino S, Stankiewicz AM, Zacchini F, Goscik J, Szostak A, Swiergiel AH, et al. Pregnancy at advanced maternal age affects behavior and hippocampal gene expression in mouse offspring. J Gerontol Ser A Biol Sci Med Sci. (2017) 72:1465–73. doi: 10.1093/gerona/glx016
49. Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol. (2018) 9:327. doi: 10.3389/fendo.2018.00327
Keywords: maternal age, attention-deficit/hyperactivity disorder, learning disability, NHANES, epidemiology
Citation: Gao L, Li S, Yue Y and Long G (2023) Maternal age at childbirth and the risk of attention-deficit/hyperactivity disorder and learning disability in offspring. Front. Public Health 11:923133. doi: 10.3389/fpubh.2023.923133
Received: 19 April 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Jay Pan, Sichuan University, ChinaReviewed by:
Jitendra Kumar Sinha, GloNeuro Academy, IndiaFei Jiang, Soochow University Medical College, China
Copyright © 2023 Gao, Li, Yue and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulin Yue,  eXVlbGlsbGluQDE2My5jb20=; Guangfeng Long,
eXVlbGlsbGluQDE2My5jb20=; Guangfeng Long,  c3p3bGdmemF5c0AxNjMuY29t
c3p3bGdmemF5c0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ling Gao†
Ling Gao† Yulin Yue
Yulin Yue Guangfeng Long
Guangfeng Long