- 1Department of Health Policy and Management, School of Public Health, Peking University, Beijing, China
- 2Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 3Vanke School of Public Health, Tsinghua University, Beijing, China
- 4Institute for Healthy China, Tsinghua University, Beijing, China
Introduction: In 2018, the Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS recommended the adoption of the efavirenz 400 mg-based TLE (tenofovir disoproxil fumarate (TDF) + lamivudine (3TC) + efavirenz (EFV)) regimen as the primary first-line treatment for ART-naive HIV-1 infected adults in China. However, the cost-effectiveness of different TLE treatment strategies remains uncertain. This study aimed to evaluate the cost-effectiveness of various TLE treatment strategies for ART-naive HIV-1 infected adults in China.
Methods: A decision-tree Markov state transition model was employed to assess the cost-effectiveness of various TLE treatment strategies over a 10-year timeframe, from a societal perspective. Input parameters were obtained from published literature and publicly accessible information. Local data from the latest sources were used as input parameters whenever possible. The main outcome measure was the incremental costs per quality-adjusted life years (QALYs) gained. Sensitivity analyses were performed to investigate model uncertainties and determine break-even prices.
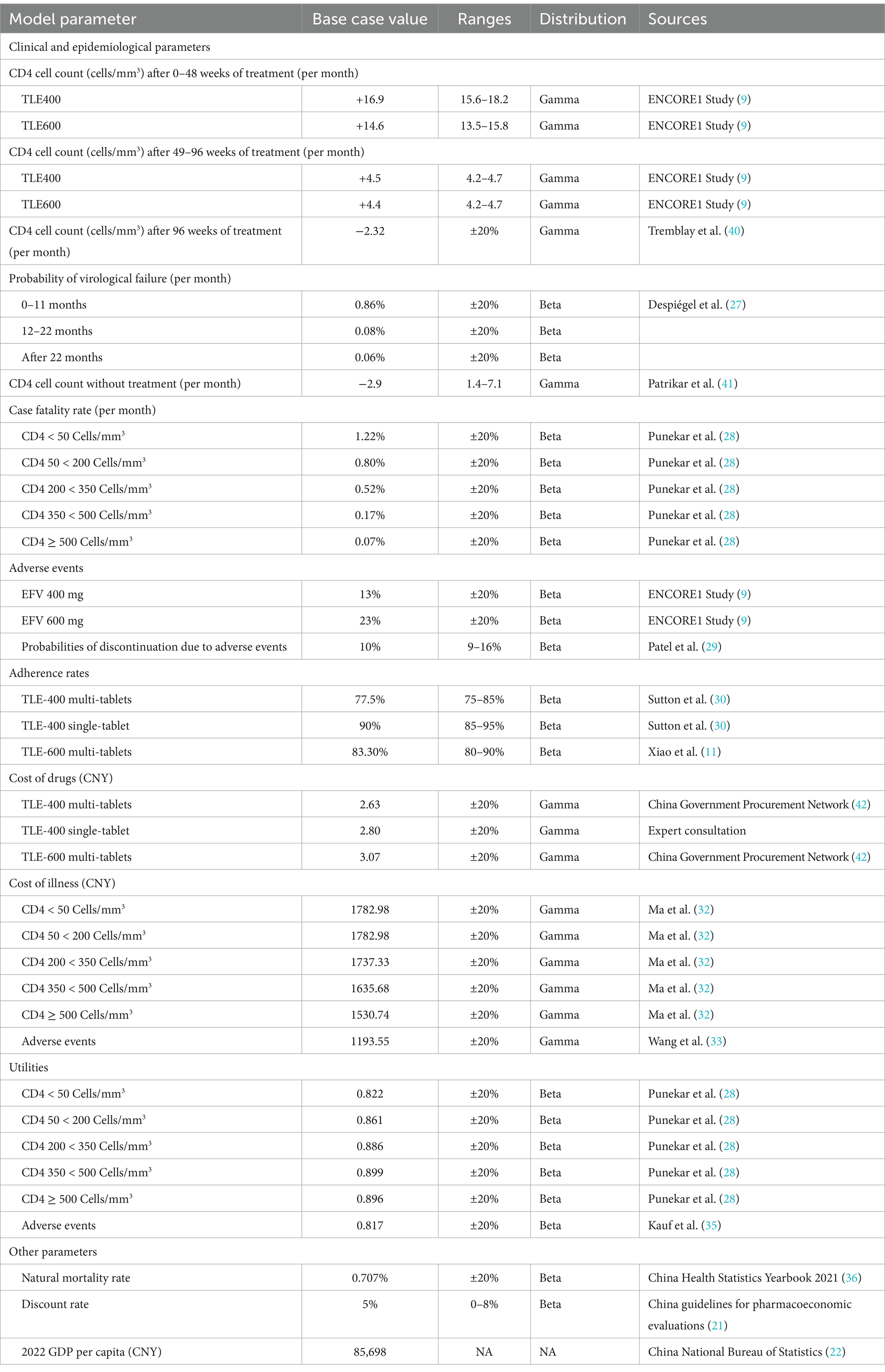
Results: Compared to the multiple-tablet regimen (MTR) consisting of efavirenz 400 mg-based TLE (TLE400) and efavirenz 600 mg-based TLE (TLE600), the single-tablet regimen (STR) of TLE400 exhibited a 10-year cost of 130733.8 CNY (compared to 122939.7 CNY and 126184.3 CNY, respectively) and an expected QALYs of 6.45 (compared to 6.27 QALYs and 6.32 QALYs, respectively) per HIV-1 patient in China. Consequently, the incremental cost-effectiveness ratios (ICERs) were 41021.6 CNY/QALY gained (equivalent to US$ 6071.2 per QALY gained) and 34996.2 CNY/QALY gained (equivalent to US$ 5179.4 per QALY gained) for TLE400 STR compared to TLE400 MTR and TLE600 MTR, respectively. The ICER for TLE400 MTR compared to TLE600 MTR was 54076.7 CNY/QALY gained (equivalent to US$ 8003.4 per QALY gained). Deterministic sensitivity analysis indicated that adherence rates to ART had the most significant influence on all three strategies. In probabilistic sensitivity analysis, TLE400 STR demonstrated a 71.4% probability of being highly cost-effective nationwide, based on the one-time national-level GDP per capita.
Conclusion: In the context of treating HIV-1 infected adults in China, the STR of TLE400 demonstrated cost-effectiveness when compared to both the MTR of TLE400 and the MTR of TLE600.
Introduction
Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) is a chronic condition characterized by compromised immune function, resulting in a progressive decline in Cluster of Differentiation 4 (CD4) cell count and an increase in viral load. This leads to a gradual deterioration of the body’s ability to defend against infections (1). HIV/AIDS remains a significant global public health concern, attracting substantial attention from governments worldwide, including China (2). Since the identification of the first HIV case in China in 1985, the prevalence of HIV infection has steadily risen (3). According to the Chinese Center for Disease Control and Prevention (China CDC), as of the end of 2020, the number of individuals living with HIV in China reached 1.053 million, with a cumulative reported death toll of 351,000 (4). In 2021, HIV/AIDS-related deaths accounted for 19,623 cases, representing 88% of all infectious disease-related deaths (5).
In response to the escalating HIV burden, China has implemented various proactive strategies and interventions. One notable policy milestone is the National Free Antiretroviral Therapy Program (NFATRP) that commenced in 2003. This program offers cost-free diagnosis, counseling, treatment, and healthcare services to all individuals living with HIV. The NFATRP recommends the utilization of TLE therapy as the primary first-line antiretroviral therapy (ART) regimen, comprising tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and efavirenz (EFV). However, prior studies have emphasized that the standard dosage of 600 mg EFV in the TLE regimen (TLE600) is associated with an increased occurrence of adverse effects, including abnormal liver function, dyslipidemia, depression, suicidal ideation, and reduced adherence rates (6–8).
Nonetheless, a pivotal study known as ENCORE1 has provided compelling evidence that an alternative regimen utilizing a lower dose of 400 mg EFV is non-inferior to the standard dose regimen of 600 mg EFV in treatment-naive patients, as demonstrated at both 48 and 96 weeks (9). Similarly, several noteworthy studies conducted in China have indicated that the 400 mg EFV regimen is associated with a reduced incidence of drug-related adverse events, decreased treatment discontinuation rates, and favorable safety profiles, including optimal virological and immunological efficacy, among adult patients (10–13). Based on these findings, the updated Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS in 2021 have recommended the adoption of the 400 mg EFV-based TLE regimen (TLE400) as the primary first-line treatment for HIV-infected adults in China (14).
Based on evidence derived from previous trials, the TLE400 STR has been widely adopted in numerous developed countries (15–17). Comparative studies between the STR and conventional MTR have demonstrated superior adherence rates, reduced hospitalization, and increased patient satisfaction scores (18, 19). However, in the context of China, the TLE400 STR is not currently available, thereby creating a knowledge gap concerning its efficacy and safety within the Chinese population. Consequently, there is a need to assess whether this alternative regimen should be introduced to the Chinese population and to comprehensively understand its potential health economic implications. To address this gap and provide valuable insights into the potential trade-offs associated with the TLE400 STR, our study aimed to prospectively evaluate its cost-effectiveness for ART-naive HIV-1 infected adults in China.
Methods
Model design
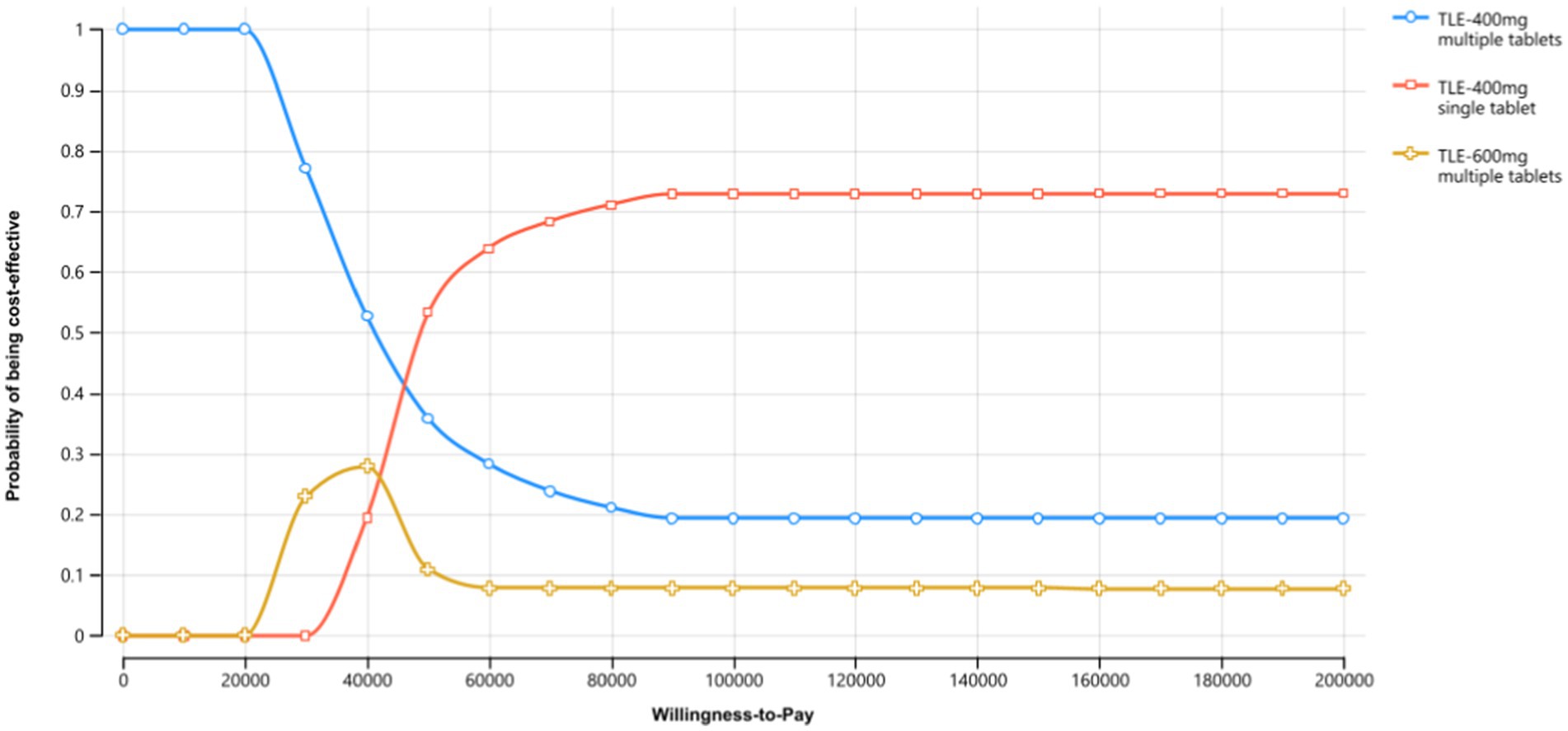
To evaluate and compare the clinical and economic performance of different treatment regimens, we utilized a decision-tree Markov state transition model in our study. The model focused on assessing the TLE400 STR, TLE400 MTR, and TLE600 MTR as first-line treatments for ART-naive HIV-1 infected adults (aged ≥18 years) in China. The decision tree component of the model comprised three branches representing the three treatment regimens: TLE400 single-tablet, TLE400 multiple-tablet, and TLE600 multiple-tablet (Figure 1A). Each branch was further divided into two strategies: adherence to ART and no adherence to ART. These strategies led to specific nodes in the Markov model. Based on existing research, we developed a Markov model that incorporated CD4 cell levels and clinical events, encompassing five HIV-infected states categorized by CD4 cell counts and three clinical events (Figure 1B). We selected CD4 cell counts as the outcome measure primarily based on data availability and comparability. The model operated in monthly cycles over a 10-year period, simulating disease-related costs and health outcomes for HIV-1 patients aged 18 and above in China.
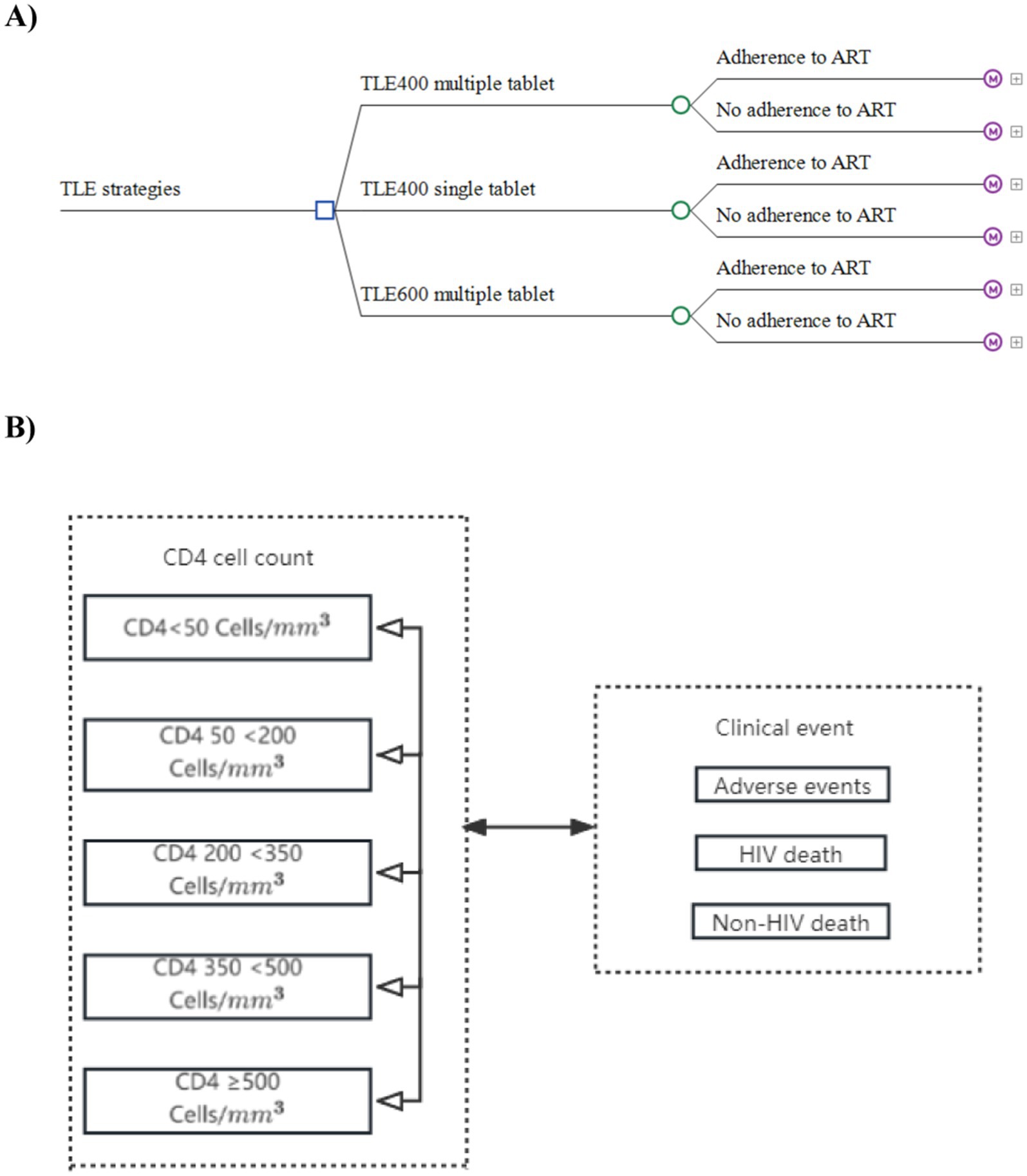
Figure 1. Markov decision tree for TLE400 single-tablet, TLE400 multiple-tablet, and TLE600 multiple-tablet regimen strategies. (A) Markov decision tree. (B) Markov diagram for HIV progression based on CD4 cell counts.
The analyses in our study were conducted from a societal perspective, which takes into account both direct and indirect costs and effects. To ensure comparability, all costs were discounted to 2022 Chinese Yuan (exchange rate: 1 CNY = US$ 0.148) and adjusted for inflation as necessary (20). Health outcomes were assessed in terms of quality-adjusted life years (QALYs) gained, with the incremental costs per QALY gained serving as the primary outcome measure. Both costs and effectiveness were discounted at a rate of 5%, following the recommendations outlined in the China guidelines for pharmacoeconomic evaluations (21). Since specific cost-effectiveness thresholds for drugs in China were unavailable, we adopted one-time the 2022 national level GDP per capita (85698.0 CNY, equivalent to US $12683.3) as the threshold to evaluate the cost-effectiveness of the three treatment strategies (22). The model utilized in our analysis was developed using TreeAge Pro 2022 (TreeAge Software, Inc., Williamstown, MA).
Model parameters and data sources
Clinical and epidemiological parameters
The clinical and epidemiological parameters utilized in our model were primarily obtained from large-scale randomized controlled trials, such as the ENCORE1 study, and domestic high-quality literature (9, 23). To ensure consistency and relevance, the trials and literature included subjects who met the following criteria: being over 18 years old, ART-naive, and non-pregnant. The initial CD4 count for individuals in the model was randomly determined using a gamma distribution, and an equation was developed to establish the upper limit of the CD4 count in the model. The baseline CD4 cell level for patients was assumed to be 352 cells/mm3 with a standard deviation of 209 cells/mm3, based on findings from a retrospective cohort study conducted in Henan province spanning over 14 years (24). The monthly increases in CD4 cell count in the model were derived from the ENCORE1 study, which involved patients receiving TLE400 and TLE600 regimens during different time intervals (0–48 weeks and 49–96 weeks) (9, 23). Following 96 weeks of the last ART treatment, based on a US observational study, it was assumed that patients would experience a decline in CD4 cell count (25, 26).
The monthly probabilities of treatment failure after initiation and the monthly decrease in CD4 cell count without treatment were obtained from relevant literature sources (27). Mortality rates for patients in different CD4 cell states were obtained from domestically published modeling research (28). The probabilities of severe adverse reactions for TLE400 and TLE600 were sourced from the ENCORE1 study (9, 23), while the rate of treatment interruption due to adverse reactions of EFV was derived from a systematic review study (29). Specific clinical and epidemiological parameters for each disease state during the model simulation can be found in Table 1.
Adherence rates
Adherence rates are crucial for the successful implementation of antiretroviral therapy in HIV patients. In our study, the adherence rate for the TLE600 MTR was obtained from a randomized controlled trial conducted specifically with HIV patients in China (11). However, since the TLE400 STR is not currently available in the domestic market, empirical research on its adherence and direct comparative studies on adherence between the TLE400 STR and MTR are unavailable. To address this data gap, we assumed regarding adherence rates based on a study that compared adherence between single-tablet and multiple-tablet HIV regimens (30). The adherence rates utilized in our analysis were assigned to the TLE400 STR and MTR, respectively (as shown in Table 1).
Cost data
The cost data utilized in this study were obtained from multiple sources. The prices of the three TLE treatment strategies were mainly sourced from the Chinese government procurement network and expert consultations (31). Specifically, in 2022, the prices of three TLE treatment strategies were 2.63 CNY (TLE400 MTR), 2.80 CNY (TLE400 STR), and 3.07 CNY (TLE600 MTR), respectively. The drug price of the TLE400 STR was obtained through expert consultations, while the drug prices of the TLE400 MTR and TLE600 MTR were extracted from the Chinese government procurement network in 2022. The costs associated with HIV-infected states stratified by CD4 cell counts were derived from a large-scale field survey conducted by the China CDC in Henan Province in 2015 (32). The field survey encompassed various cost components, including direct medical costs and non-direct medical costs related to antiretroviral therapy. Direct medical costs included drug costs, pre-examination, CD4 cell testing, follow-up costs, outpatient costs, and hospitalization costs.
The non-direct medical costs considered in this study encompassed transportation costs, accompanying costs, nutrition costs, lost labor costs, and ART management costs. The cost of illness in 2015 was adjusted to 2022 using the Consumer Price Index (CPI) for the period from 2015 to 2022 to account for inflation. The cost of treating adverse reactions was obtained from a domestic literature focusing on the medical costs and economic evaluation of HIV patients in Shijiazhuang, Hebei province (33). This study provided detailed information on the treatment costs associated with adverse reactions in HIV patients receiving TLE strategies in China. To obtain national-level costs, all local costs were adjusted by applying the health expenditure as a weight. The total cost divided by CD4 cells is presented in Table 1.
Utilities
Due to the unavailability of utility weights specifically for the baseline population and utility scores for patients with HIV-1 infection in China, utility scores utilized in this study were obtained from published literature in other countries (34, 35). These utility scores were adapted to represent the health-related quality of life for individuals in different health states associated with HIV-1 infection. The specific parameters associated with the utility scores utilized in the model are presented in Table 1. These parameters were derived from relevant literature that assessed the health-related quality of life in HIV-1 patients. It is important to acknowledge that the utility scores may not directly apply to the Chinese population, as there may be cultural and contextual variations that can influence health-related quality of life. However, in the absence of country-specific data, these scores provide a reasonable estimation for the utility weights employed in the analysis. Table 1 shown detailed utility scores corresponding to each health state in the model.
Other parameters
Our study incorporates several additional parameters, including natural mortality rates, discount rates, and GDP per capita in 2022. These parameters are crucial for the analysis and were obtained from reliable sources. The natural mortality rates were derived from the China Health Statistics Yearbook 2021 (36). Discount rates, which are applied to both costs and effectiveness, were determined based on the China guidelines for pharmacoeconomic evaluations (21). The GDP per capita in 2022, denoted in CNY, was obtained from the China National Bureau of Statistics (22).
Sensitivity analyses
Deterministic sensitivity analyses (DSA) and probabilistic sensitivity analysis (PSA) were performed to test the robustness of the model results and to examine the uncertainty in the model. DSA was conducted to explore the sensitivity of the results to plausible variations in data inputs, where relevant parameters changed one at a time within the range of plausibility specified in Table 1. For parameters with unknown uncertainty range, the plausibility range was assumed to be 20% of the base value. The PSA was conducted using Monte Carlo simulation (N = 1,000 iterations) to assess the impact of changing multiple parameters simultaneously. The results of the DSA and PSA were summarized with tornado diagrams and cost-effectiveness acceptability curves, respectively. Scenario sensitivity analyses were conducted to estimate the break-even prices by varying the prices of TLE strategies.
Results
Base-case analysis
Our findings indicate that the TLE400 STR is associated with improved health outcomes, but higher costs compared to both the TLE600 and TLE400 MTR (see Table 2). Over a 10-year period, the average cost for a patient receiving the TLE400 STR was 130733.8 CNY, resulting in a total of 6.45 QALYs gained. In contrast, the costs and total QALYs for the TLE600 and TLE400 MTRs were lower, at 126184.3 CNY (with 6.32 QALYs gained) and 122939.7 CNY (with 6.27 QALYs gained), respectively.
Table 2. Cost-effectiveness analysis results for TLE400 single-tablet, TLE400 multiple-tablet, and TLE600 multiple-tablet regimens in China.
The incremental cost-effectiveness ratios (ICERs) for the TLE400 STR compared to the TLE600 MTR and the TLE400 MTR were 35313.6 CNY/QALY (equivalent to US$ 5226.4 per QALY gained) and 41706.1 CNY/QALY (equivalent to US$ 6172.5 per QALY gained), respectively. Both ICER values were below the threshold of one-time China’s GDP per capita (85698.0 CNY, equivalent to US$ 12683.3) in 2022, indicating that the TLE400 STR was considered cost-effective in comparison to the other two regimens. Moreover, when comparing the TLE400 MTR with the TLE600 MTR, the ICER for the TLE600 regimen versus the TLE400 regimen was 55893.5 CNY/QALY (equivalent to US$ 8272.2 per QALY gained), which was also below the one-time national-level GDP per capita threshold in 2022. Therefore, the TLE600 MTR was found to be more cost-effective when compared to the TLE400 MTR.
Sensitivity analyses
In the sensitivity analyses, we conducted various assessments to evaluate the robustness of the model results by examining key parameters. In the deterministic sensitivity analyses, the parameters that exhibited the most substantial impact on the results were adherence rates to ART, utilities, the price of TLE400 STR and costs (Figure 2). Alterations in these parameters resulted in variations in the ICERs of the treatment strategies. In the probabilistic sensitivity analysis, which involved Monte Carlo simulation with 1,000 iterations, the TLE400 STR demonstrated a 71.4% probability of being highly cost-effective at the national level when compared to the threshold of one-time GDP per capita in 2022 (Figure 3).
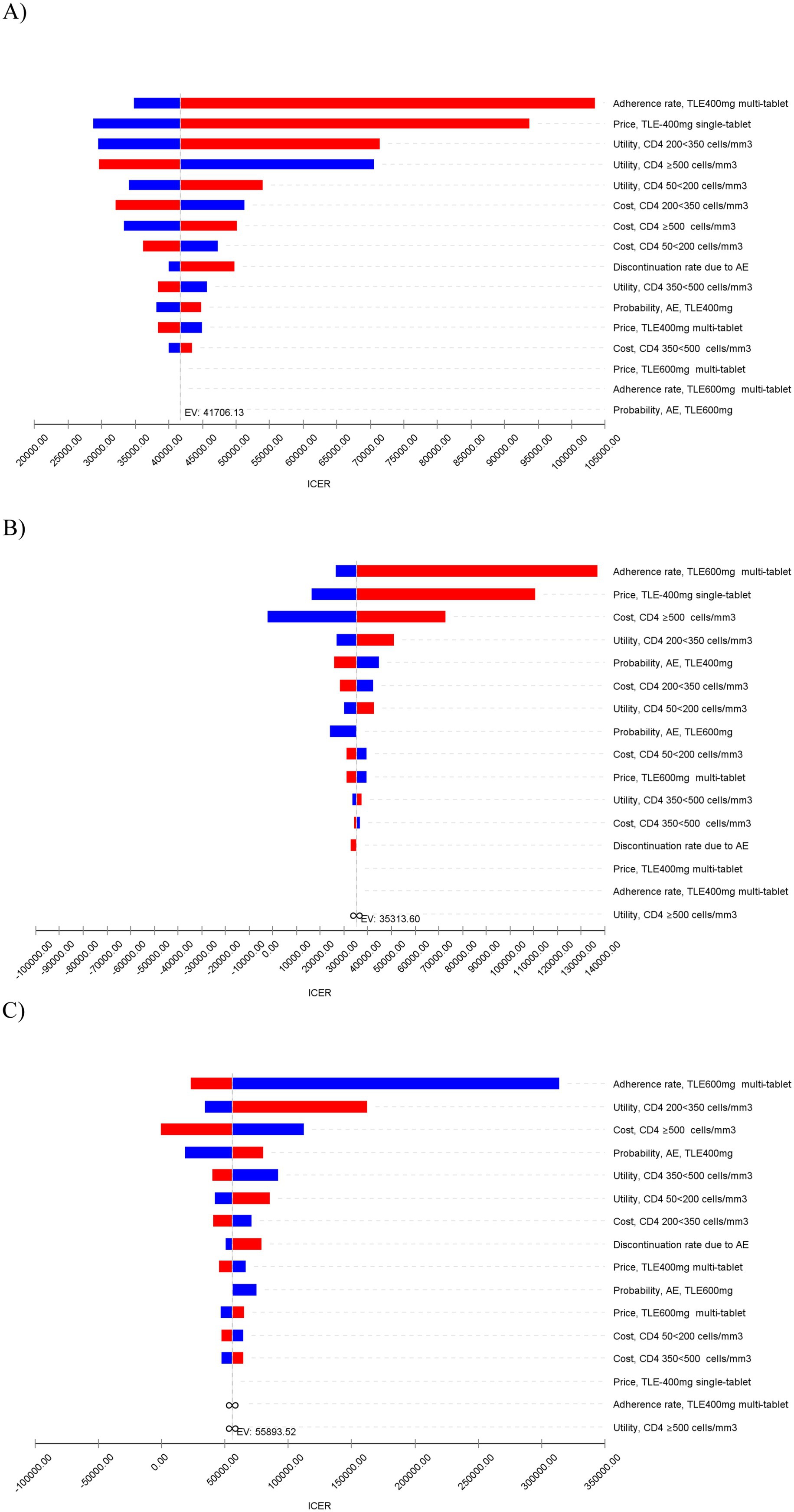
Figure 2. Tornado diagram of deterministic sensitivity analyses for the most influential model parameters on ICER (CNY/QALY gained). (A) TLE400 STR vs. TLE400 MTR. (B) TLE400 STR vs. TLE600 MTR. (C) TLE400 MTR vs. TLE600 MTR.
In the price analysis scenario, we explored the impact of varying the prices of the TLE strategies on the ICER values. As the price of the TLE400 STR increased, the ICER values for both comparisons (TLE400 STR vs. TLE400 MTR and TLE400 STR vs. TLE600 MTR) also increased. The break-even prices, at which the ICER values were equal to the one-time national-level GDP per capita in 2022, were determined to be 9.4 CNY per pill for the comparison of TLE400 STR and TLE400 MTR, and 7.8 CNY per pill for the comparison of TLE400 STR and TLE600 MTR. The detailed results and visual representations of the scenario analysis were shown in Appendix Figures 1, 2.
Discussion
Our study provides valuable insights into the cost-effectiveness of the TLE400 STR compared to the TLE400 MTR and TLE600 MTR for the treatment of HIV-1 infected adults in China. This study is the first to comprehensively analyze all three types of TLE regimens and specifically focuses on the TLE400 STR in the Chinese context. Our findings indicate that the TLE400 STR is a cost-effective option compared with the two MTR strategies in China. The results of our sensitivity analysis also underscore the importance of adherence rates to ART in the economic evaluation model. PSA indicates that the TLE400 STR is likely to be a cost-effective option when considering the uncertainties associated with multiple parameters simultaneously. The TLE400 STR demonstrated higher adherence rates compared to the TLE400 MTR and TLE600 MTR. This can be attributed to the individual benefits of dose reduction, particularly in terms of reducing dose-related toxic effects associated with efavirenz (9, 23). Lower EFV doses have been shown to result in fewer central nervous system adverse events and treatment-related discontinuations, leading to improved self-reported adherence (13, 37). Moreover, the high daily pill burden of multiple-tablet regimens is known to decrease adherence to ART and increase the likelihood of discontinuation (18, 30, 38). In contrast, single-tablet regimens are associated with better adherence rates and non-inferior clinical outcomes. These factors contribute to the higher adherence rates and larger QALYs gained with the TLE400 STR in our study.
Considering the cost-effectiveness analysis, the TLE400 STR is deemed cost-effective despite its slightly higher costs compared to the other two regimens. The ICERs of the TLE400 STR remain below the recommended threshold of one-time China’s GDP per capita, indicating that the regimen offers good value for its cost. Therefore, the TLE400 STR is considered a more valuable treatment option for HIV-1 infected patients in China. Additionally, deterministic sensitivity analysis indicated that adherence rates to ART had the most significant influence on all three strategies. According to existing literature, the number of tablets in a regimen is an important factor affecting adherence rates to ART (18, 30).
Furthermore, our scenario analysis provides insights into the break-even prices for the TLE400 STR based on the recommended threshold of one-time GDP per capita (39). The analysis demonstrates that lowering the prices of TLE strategies, specifically through the reduction of EFV dose, can enhance the cost-effectiveness of the regimens. This has significant implications as it not only benefits patients in terms of improved health outcomes but also alleviates the financial burden on the government and society.
It is important to acknowledge that our analysis has a limited time horizon of 10 years, focusing on short-term outcomes, as long-term data specific to China are not readily available. Future research could address this limitation by investigating the long-term implications and cost-effectiveness of the TLE400 STR over an extended period. Such research would provide a more comprehensive understanding of the economic value of the TLE400 STR and its sustained impact on healthcare outcomes and costs.
Limitations
While our study provides valuable insights, it is important to acknowledge several limitations. Firstly, the lack of specific data in China required us to reference parameters from studies conducted in other countries and regions. This introduces potential differences in clinical parameters, case fatality rates, and utilities. For example, we acknowledged that using generic utility value sets from China might hold more weight than specific value sets from another country. However, sensitivity analyses conducted in our study indicated no significant impact on our results. Nevertheless, obtaining more representative data from Chinese HIV-infected patients would enhance the robustness of future studies. Secondly, our model did not consider stratification by biological factors, such as body weight. It has been observed that Chinese HIV-infected patients with lower body weight may experience higher efavirenz plasma concentration compared to those with higher body weight (12). Incorporating such stratification into the model would provide a more accurate assessment of the cost-effectiveness of the TLE400 STR for different patient populations. Future studies should aim to collect data specifically for patients with low body weight in China. Thirdly, our model did not account for the impact of treatments associated with first-line treatment failures. This simplification was made to mitigate excessive uncertainties and data limitations. However, it is important to acknowledge that the inclusion of such treatments may provide additional benefits and cost savings associated with the TLE400 STR. Future studies that incorporate comprehensive data on treatment failures would yield a more comprehensive evaluation of the regimen’s cost-effectiveness. Fourthly, we acknowledged that the use of CD4 count as the outcome measure had certain limitations, as many global bodies had since adopted viral load as the measure of clinical outcomes. Overall, while our study provides valuable insights into the cost-effectiveness of the TLE400 STR, it is essential to consider these limitations when interpreting the results. Future research should aim to address these limitations and provide more accurate estimations of the economic value of the TLE400 STR in the context of HIV-1 treatment in China.
Conclusion
In conclusion, our study demonstrates that the TLE400 STR is a cost-effective treatment strategy for HIV-1 infected adults in China. The TLE400 STR, compared to the TLE400 MTR and TLE600 MTR, offers improved health outcomes despite higher costs, making it economically favorable. The lower efavirenz dose (400 mg) in the STR shows potential advantages in terms of higher adherence rates and better health outcomes. Incorporating the TLE400 STR as the preferred first-line treatment in the National Free AIDS Antiretroviral Drug List, at an optimal price, would provide healthcare professionals and policymakers with an additional option to address the growing burden of HIV in China. However, it is crucial to acknowledge the limitations of our study, including the reliance on data from other countries and the need for more specific data on the Chinese population, particularly for patients with low body weight. Further research, including clinical trials and studies incorporating local data, is warranted to validate our findings and provide more precise estimations of the cost-effectiveness of the TLE400 STR. Overall, the inclusion of this regimen in the national HIV treatment guidelines has the potential to bring significant benefits to individuals living with HIV and the healthcare system in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HZ: Conceptualization, Formal analysis, Methodology, Software, Writing – review & editing. YK: Methodology, Validation, Writing – original draft, Writing – review & editing. HC: Data curation, Validation, Writing – review & editing. SL: Investigation, Project administration, Supervision, Writing – review & editing. PF: Investigation, Resources, Supervision, Validation, Writing – review & editing. ZL: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can befound online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1429461/full#supplementary-material
Abbreviations
ART, Antiretroviral therapy; CPI, Consumer Price Index; EFV, Efavirenz; ICERs, Incremental cost-effectiveness ratios; MTR, Multi-tablet regimen; NFATRP, National Free Antiretroviral Therapy Program; QALYs, Quality-adjusted life years; STR, Single-tablet regimen; TDF, Tenofovir disoproxil fumarate; TLE, Combination of tenofovir disoproxil fumarate, lamivudine, and efavirenz; TLE400, 400 mg EFV-based TLE regimen; TLE600, 600 mg EFV-based TLE regimen; 3TC, Lamivudine.
References
1. Arts, EJ, and Hazuda, DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. (2012) 2:a007161. doi: 10.1101/cshperspect.a007161
2. World Health Organization. HIV-AIDS. (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (Accessed 18 December, 2024).
3. Xiwen, Z. The AIDS epidemic situation and the achievement of prevention and control in China. J Med Res. (1999) 11:1–5.
4. He, N. Research Progress in the epidemiology of HIV/AIDS in China. China CDC Wkly. (2021) 3:1022–30. doi: 10.46234/ccdcw2021.249
5. NHC Government. Overview of notifiable infectious diseases in China. (2021) Available online at: http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml (Accessed 31 March, 2023).
6. Mollan, KR, Smurzynski, M, Eron, JJ, Daar, ES, Campbell, TB, Sax, PE, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med. (2014) 161:1–10. doi: 10.7326/M14-0293
7. Rajesh, R, Sudha, V, Varma, D, and Sonika, S. Association between medication adherence outcomes and adverse drug reactions to highly active antiretroviral therapy in Indian human immunodeficiency virus-positive patients. J Young Pharm. (2012) 4:250–60. doi: 10.4103/0975-1483.104369
8. EACS. HIV Guidelines. (2015). Available online at: https://www.eacsociety.org/media/guidelines_8.0-english-revised_20160610.pdf (Accessed 18 December, 2024).
9. Encore Study Group 1. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. (2014) 383:1474–82. doi: 10.1016/S0140-6736(13)62187-X
10. Xu, L, Peng, W, Song, X, Li, Y, Han, Y, Zhu, T, et al. Pharmacodynamics of efavirenz 400 mg in treatment-naïve Chinese HIV-infected patients in a prospective cohort study. BMC Infect Dis. (2021) 21:112. doi: 10.1186/s12879-021-05802-8
11. Xiao, J, Xiao, J, Liu, Y, Li, B, Zhang, L, Han, J, et al. Efficacy and safety of Efavirenz 400 mg-based regimens switching from 600 mg-based regimens in people living with HIV with virological suppression in China: a randomized, open-label, non-inferiority study. Int J Infect Dis. (2022) 117:48–55. doi: 10.1016/j.ijid.2022.01.051
12. Guo, F, Cheng, X, Hsieh, E, Du, X, Fu, Q, Peng, W, et al. Prospective plasma efavirenz concentration assessment in Chinese HIV-infected adults enrolled in a large multicentre study. HIV Med. (2018) 19:440–51. doi: 10.1111/hiv.12607
13. Chen, J, Chen, R, Shen, Y, Wei, H, Wang, X, Zhang, R, et al. Efficacy and safety of lower dose tenofovir disoproxil fumarate and efavirenz versus standard dose in HIV-infected, antiretroviral-naive adults: a multicentre, randomized, noninferiority trial. Emerg Microbes Infect. (2020) 9:843–50. doi: 10.1080/22221751.2020.1752609
14. HIV/AIDS Hepatitis C Research Group. Chinese Guidelines for HIV/AIDS Diagnosis and Treatment. Chin J Clin Infect Dis. (2021) 2021:321–43. doi: 10.3760/cma.j.cn112138-20211006-00676
15. Dravid, A, Betha, TP, Sharma, AK, Gawali, R, Mahajan, U, Kulkarni, M, et al. Efficacy and safety of a single-tablet regimen containing tenofovir disoproxil fumarate 300 mg, lamivudine 300 mg and efavirenz 400 mg as a switch strategy in virologically suppressed HIV-1-infected subjects on nonnucleoside reverse transcriptase inhibitor-containing first-line antiretroviral therapy in Pune, India. HIV Med. (2020) 21:578–87. doi: 10.1111/hiv.12912
16. Truong, WR, Schafer, JJ, and Short, WR. Once-daily, single-tablet regimens for the treatment of HIV-1 infection. P T. (2015) 40:44–55. doi: 10.1177/1060028016682531
17. Dubrocq, G, and Rakhmanina, N. The pharmacokinetics, pharmacodynamics, and clinical role of fixed dose combination of tenofovir disoproxil fumarate, lamivudine and reduced dose efavirenz (TLE-400) in treating HIV-1 infection. Expert Opin Drug Metab Toxicol. (2018) 14:773–9. doi: 10.1080/17425255.2018.1498840
18. Altice, F, Evuarherhe, O, Shina, S, Carter, G, and Beaubrun, AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. (2019) 13:475–90. doi: 10.2147/PPA.S192735
19. Arribas, JR, Pialoux, G, Gathe, J, Di Perri, G, Reynes, J, Tebas, P, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. (2014) 14:581–9. doi: 10.1016/S1473-3099(14)70782-0
20. World Bank Group. Official exchange rate (LCU per US$, period average)—China. (n.d.). Available online at: https://data.worldbank.org/indicator/PA.NUS.FCRF?locations=CN (Accessed 29 May, 2024).
22. National Bureau of Statistics. China's per capita GDP. (2022). Available online at: https://data.stats.gov.cn/search.htm?s=10/5000 (Accessed 29 May, 2024).
23. Carey, D, Puls, R, Amin, J, Losso, M, Phanupak, P, Foulkes, S, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis. (2015) 15:793–802. doi: 10.1016/S1473-3099(15)70060-5
24. Wang, D, Ma, S, Ma, Y, Guo, H, Li, P, Yang, C, et al. Effect of traditional Chinese medicine therapy on the trend in CD4(+) T-cell counts among patients with HIV/AIDS treated with antiretroviral therapy: a retrospective cohort study. Evid Based Complement Alternat Med. (2021) 2021:5576612. doi: 10.1155/2021/5576612
25. Lifson, AR, Krantz, EM, Eberly, LE, Dolan, MJ, Marconi, VC, Weintrob, AC, et al. Long-term CD4+ lymphocyte response following HAART initiation in a US military prospective cohort. AIDS Res Ther. (2011) 8:1–11. doi: 10.1186/1742-6405-8-2
26. Mauskopf, J, Brogan, AJ, Talbird, SE, and Martin, S. Cost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infection. AIDS. (2012) 26:355–64. doi: 10.1097/QAD.0b013e32834e87e6
27. Despiégel, N, Anger, D, Martin, M, Monga, N, Cui, Q, Rocchi, A, et al. Cost-effectiveness of dolutegravir in HIV-1 treatment-naive and treatment-experienced patients in Canada. Infect Dis Ther. (2015) 4:337–53. doi: 10.1007/s40121-015-0071-0
28. Punekar, YS, Guo, N, Tremblay, G, Piercy, J, Holbrook, T, and Young, B. Improving access to antiretrovirals in China: economic analyses of dolutegravir in HIV-1 patients. Cost Eff Resour Alloc. (2019) 17:26. doi: 10.1186/s12962-019-0195-2
29. Patel, DA, Snedecor, SJ, Tang, WY, Sudharshan, L, Lim, JW, Cuffe, R, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1–infected patients: a systematic review and network meta-analysis. PLoS One. (2014) 9:e105653. doi: 10.1371/journal.pone.0105653
30. Sutton, SS, Hardin, JW, Bramley, TJ, D'Souza, AO, and Bennett, CL. Single-versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care. (2016) 22:242–8. doi: 10.1371/journal.pone.0170661. eCollection 2017
31. CCGP. Chinese government procurement network. (2022). Available online at: http://www.ccgp.gov.cn/ (Accessed 31 March, 2023).
32. Ma, L. Single AIDS family different antiviral treatment strategy, cost-effectiveness and utility research. Beijing, China: Chinese Center for Disease Control and Prevention (2016).
33. Chao, W, Guo, L, Ling, D, Haicong, Z, Yujun, Z, and Caixia, F. Cost-effectiveness analysis of tenofovir dipivoxil in adults with initially treated acquired immune deficiency syndrome. Clin Misdiagn Misther. (2020) 33:29–34. doi: 10.3969/j.issn.1002-3429.2020.11.007
34. Young, J, Smith, C, Teira, R, Reiss, P, Jarrín Vera, I, Crane, H, et al. Antiretroviral pill count and clinical outcomes in treatment-naïve patients with HIV infection. HIV Med. (2018) 19:132–42. doi: 10.1111/hiv.12562
35. Kauf, TL, Roskell, N, Shearer, A, Gazzard, B, Mauskopf, J, Davis, EA, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. (2008) 11:1144–53. doi: 10.1111/j.1524-4733.2008.00326.x
36. Commission NH. China health statistical yearbook 2021. Beijing: China Union Medical College Press (2021).
37. Li Hui, CY-Q, Ya-guang, C, and Shi-man, R. Progress in antiviral treatment of HIV with efavirenz 400 mg dose. Chin J AIDS STD. (2021) 27:113–6. doi: 10.13419/j.cnki.aids.2021.08.29
38. Griffith, DC, Farmer, C, Gebo, KA, Berry, SA, Aberg, J, Moore, RD, et al. Uptake and virological outcomes of single- versus multi-tablet antiretroviral regimens among treatment-naïve youth in the HIV research network. HIV Med. (2019) 20:169–74. doi: 10.1111/hiv.12695
39. World Health Organization. (2003). Making choices in health: WHO guide to cost-effectiveness analysis. Available online at: http://apps.who.int/iris/bitstream/handle/10665/42699/9241546018.pdf?sequence=1 (Accessed 31 March, 2023).
40. Tremblay, G, Chounta, V, Piercy, J, Holbrook, T, Garib, SA, Bukin, EK, et al. Cost-effectiveness of Dolutegravir as a first-line treatment option in the HIV-1-infected treatment-naive patients in Russia. Value Health Reg Iss. (2018) 16:74–80. doi: 10.1016/j.vhri.2018.08.001
41. Patrikar, S, Basannar, DR, Bhatti, VK, Kotwal, A, Gupta, RM, and Grewal, RS. Rate of decline in CD4 count in HIV patients not on antiretroviral therapy. Med J Armed Forces India. (2014) 70:134–8. doi: 10.1016/j.mjafi.2013.08.005
42. CCGP. Funding for prevention and control of major infectious diseases, AIDS prevention and control project, antiviral treatment drugs/prevention of mother-to-child transmission drugs procurement project. (2022). Available online at: http://www.ccgp.gov.cn/cggg/zygg/cjgg/202211/t20221113_19000182.htm; http://www.ccgp.gov.cn/cggg/dfgg/zbgg/202209/t20220905_18591594.htm; http://www.ccgp.gov.cn/cggg/dfgg/zbgg/202208/t20220829_18553355.htm (Accessed 31 March, 2023).
Keywords: HIV, TLE regimen, cost-effectiveness, efavirenz, antiretroviral therapy
Citation: Zhang H, Kong Y, Chen H, Luo S, Fan P and Li Z (2025) The cost-effectiveness analysis of single-tablet efavirenz-based regimen among HIV-1 infected adults in China. Front. Public Health. 13:1429461. doi: 10.3389/fpubh.2025.1429461
Edited by:
Sam Agatre Okuonzi, Ministry of Health, UgandaReviewed by:
Abdulmuminu Isah, University of Nigeria, Nsukka, NigeriaJolem Mwanje, African Centre for Health Social and Economic Research, South Sudan
Copyright © 2025 Zhang, Kong, Chen, Luo, Fan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengyang Fan, cGVuZ3lhbmcuZmFuQHZpYXRyaXMuY29t; Zhihui Li, emhpaHVpbGlAbWFpbC50c2luZ2h1YS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Haijun Zhang1,2†
Haijun Zhang1,2† Yuhao Kong
Yuhao Kong Sitong Luo
Sitong Luo