- 1Department of Nursing, College of Health Science, Oda Bultum University, Chiro, Ethiopia
- 2Faculty of Health Science, Rift Valley University, Adama, Ethiopia
Background: Virological failure on first-line antiretroviral therapy (ART) remains a major challenge in the management of HIV/AIDS in resource-limited settings, including Ethiopia. However, the prevalence of virological failure and its associated factors among adult patients on first-line ART in West Hararghe, Ethiopia, are not well documented. Therefore, this study aimed to assess virological treatment failure and its determinants among people living with HIV (PWH) in West Hararghe, Eastern Ethiopia.
Methods: A retrospective cohort study was conducted using routine HIV-related data from a health facility providing services in West Hararghe between 01 January 2017 and 31 December 2020. Sociodemographic, behavioral, clinical, and HIV-related data were collected through medical chart reviews. Virological treatment failure was defined as a plasma viral load above 1,000 copies/mL based on two consecutive viral load measurements. A logistic regression model was used to identify factors associated with virological treatment failure.
Results: A total of 257 records of PWH were reviewed and included in this analysis. Of these, 11.67% experienced virological failure while on first-line ART. Baseline undernutrition (AOR = 3.717: 1.051, 13.139), non-disclosure of serostatus (AOR = 4.453: 1.340, 14.793), early (≤ 30 days) ART initiation (AOR = 0.235: 0.064, 0.859), a history of missed daily ART doses (AOR = 3.156: 1.007, 9.891), and the use of a dolutegravir (DTG)-based regimen (AOR = 0.275: 0. 085, 0.895) were statistically associated with virological failure on first-line ART.
Conclusion: Virological failure on first-line ART was found to be significantly high in West Hararghe. Factors such as undernutrition, non-disclosure of serostatus, interruption of ART doses, and the use of DTG-based regimens were identified as significant predictors of virological treatment failure. Healthcare providers should focus on the accelerated initiation of ART (preferably with a DTG-based regimen) and supplemental nutritional therapy for patients with undernutrition.
1 Introduction
The primary goal of antiretroviral therapy (ART) is to suppress viral replication, increase patients’ survival rate through the reduction of HIV transmission, prevent HIV-related illness, avert AIDS-related deaths, help patients live a normal lifespan, and enhance both health and economic outcomes (1–3). Patients on ART are recommended to undergo periodic viral load monitoring to ensure effective and durable treatment outcomes (4, 5). Viral load monitoring has become the standard of care for detecting ART failure and has been explicitly recommended in international guidelines (2, 6).
The World Health Organization (WHO) recommends isoniazid (INH) and cotrimoxazole preventive therapy (CPT) to reduce morbidity and mortality associated with opportunistic infections in individuals living with HIV. INH is recommended for individuals with a positive tuberculin skin Test (TST) and a negative chest X-ray to prevent the risk of developing active TB. CPT is recommended for individuals with a CD4 count below 200 cells/mm3 to protect against opportunistic infections such as Pneumocystis pneumonia and bacterial infections (7). Research indicates that CPT can reduce mortality by up to 60% when initiated alongside antiretroviral therapy (ART), particularly in patients with a low CD4 count (8).
According to Ethiopia’s national guidelines, a viral load of more than 1,000 copies/mL in a patient who has been on ART for at least 6 months indicates either therapeutic failure due to antiretroviral resistance or poor adherence to treatment (9). Patients whose viral loads are not suppressed at retesting after adherence support for 3–6 months are classified as having virological failure due to probable drug resistance and are switched to second-line therapy (3, 9).
Before 2016, virological monitoring was rarely conducted in Ethiopia because of limited access to viral load testing facilities. As a result, a great deal of research conducted in Ethiopia focused on the survival outcome before the scale-up of regular viral load services, when clinical and immunological assessments were the primary methods for diagnosing treatment failure (10–13).
Studies conducted in Ethiopia have reported varying rates of virological failure, ranging from 1 to 9 per 100 person-years, with proportions ranging from 11 to 28% of patients failing treatment (14–18). However, the prevalence of first-line ART failure and its associated factors among people living with HIV (PWH) in West Hararghe, Ethiopia, are not well understood. This region experiences distinct socioeconomic practices and a relatively high HIV prevalence following the implementation of routine viral load monitoring.
Previous Ethiopian studies used clinical and immunologic criteria to assess factors influencing treatment failure, whereas we propose the identification of patient characteristics to determine risk factors associated with treatment failure (19, 20).
A clear distinction between participants with and without ART failure is important for identifying predictors of treatment failure using virological (plasma viral load) criteria (6). Understanding the prevalence of virological failure and its determinants is crucial for early prevention and improving the quality of life for HIV patients on first-line ART. However, virological failure and its associated factors among adult patients on first-line ART in West Hararghe are not well documented. Therefore, this study aimed to determine the prevalence of virological failure and its determinants among PWH in West Hararghe, Eastern Ethiopia. Identifying these determinants will allow us to develop or improve interventions that will ultimately improve HIV treatment outcomes among adults in this region (Figure 1).
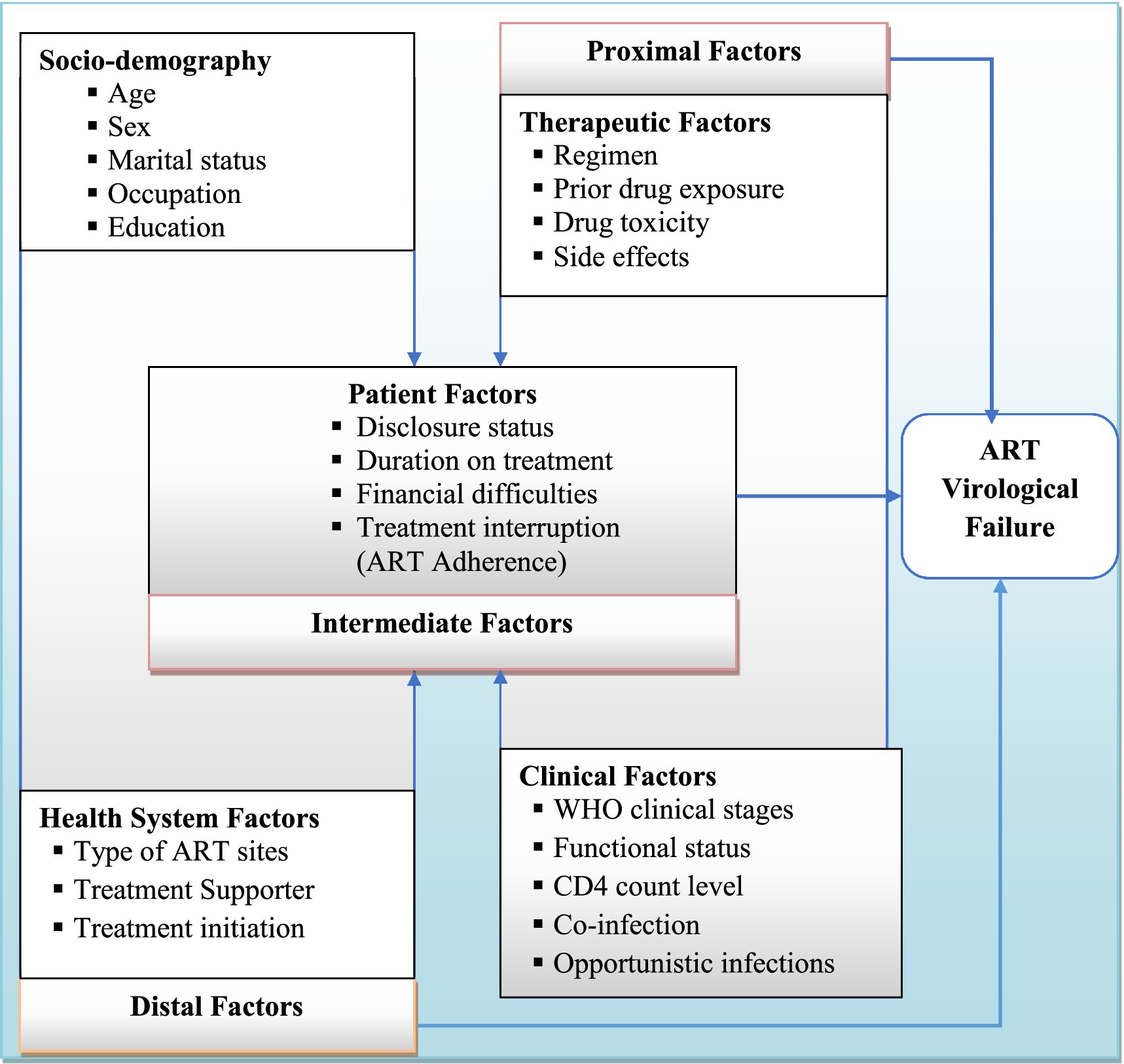
Figure 1. Conceptual framework describing predictors of ART virological treatment failure [Source: developed by the investigators, combining variables from the literature review (10–13, 19)].
2 Materials and methods
2.1 Study area and period
The study was conducted at an ART site in the West Hararghe zone from January 2017 to December 2020. The West Hararghe zone is located in the eastern part of Ethiopia, 316 km from Addis Ababa, the capital city of Ethiopia. Based on the 2007 Census conducted by the Central Statistical Agency (CSA), this zone had a total population of 1,871,706, an increase of 47.16% since 1994. According to the West Hararghe Zone Administration Health Information System 2018 annual report, approximately 1,605 adults were newly started on ART during the 4-year data retrieval period (West Hararghe Zone Health Bureau Office, HIV Prevention and Control Unit, 2020). Free access to ART began in 2005 in West Hararghe, where there are currently 14 primary care health facilities and two general hospitals public hospitals providing this service.
According to the 2018 Ethiopian ART guidelines, the preferred first-line regimen for adults is a once-daily dose of tenofovir (TDF) and lamivudine (3TC) combined with dolutegravir (DTG) or efavirenz (EFV) (TDF + 3TC + DTG or TDF + 3TC + EFV,) depending on the patient’s clinical conditions (21). Since Aug 2016, West Hararghe has implemented routine viral load monitoring with enhanced adherence counseling (EAC) for individuals with a detectable viral load, using referral testing services. The study was conducted among adults who initiated first-line ART between 01 January 2017 and 31 December 2020 at Chiro General Hospital, West Hararghe. The data for this study were collected between 15 December 2021 and 31 December 2021.
2.2 Study design
A retrospective cohort study design was used.
2.3 Population
Adults aged 15 years and above who initiated first-line ART in the West Hararghe zone were potential study participants. The actual study participants were randomly selected adults aged 15 years and above who initiated first-line ART during the data collection period and met the inclusion criteria.
2.4 Inclusion and exclusion criteria
The study included all individuals who had completed at least 6–12 months of follow-up after starting first-line ART and had available viral load evaluation results. Since virological failure is defined based on viral load measurements taken at least 6–12 months after ART initiation, the observational window was extended to capture the second viral load result for the participants who underwent extended EAC (21). To meet this definition, the participants were required to have undergone at least two viral load evaluations following 6 months on ART. The eligibility criteria for ART initiation were based on the Ethiopian national guidelines. These guidelines recommend initiating ART for all HIV-infected individuals regardless of the CD4 cell count. Only individuals with complete viral load data who met the inclusion criteria were included in the outcome analysis. Patients with incomplete viral load data and those who started ART at another site (with incomplete baseline data recording) were excluded. Incomplete viral load data were defined as having fewer than two viral load measurements during the follow-up period.
2.5 Sample size determination and sampling technique
2.5.1 Sample size determination
The sample size was determined using EpiInfo 7 version 3.5.3, based on factors associated with virological failure from a previous Ethiopian study. A double population proportion assumption was applied for a two-sided hypothesis test with a 95% significance level, 80% power, and an equal number of exposed and unexposed groups. Among the risk factors reported in a study conducted in Northeast Ethiopia, CD4 status (CD4 level < 500 cells/mL compared to > 500 cells/mL) of patients (AOR = 4.78) (22) yielded the maximum sample size. A detailed summary of the sample size calculation for the risk factors is provided in Supplementary Table 1. The final intended maximum sample size for this study was 272, with an additional 20% contingency to account for potential participant dropout or data loss.
2.5.2 Sampling procedure and sampling technique
Among the 876 PWH receiving care at Chiro General Hospital, 272 patient records were randomly selected based on the year of ART initiation.
The sample size was proportionally allocated to each year, depending on the actual number of new adults initiated on ART during that specific year. The sampling frame was created using patient registers and electronic databases, based on the years of ART initiation. Finally, a simple random sampling technique was used to select a proportionate number of patient records.
2.6 Data collection tool and procedure
Data were collected by reviewing the patients’ medical records using an extraction tool prepared in English. A structured record review checklist was developed based on the data elements from the nationally standardized HIV patient intake and follow-up formats, which were created according to the WHO patient monitoring guidelines. Peer-reviewed published literature (10, 13, 23) was used to collect data from the ART register, patient’s cards, and ART intake forms.
Health professionals who were trained in comprehensive HIV care were selected as supervisors and data collectors to ensure the quality of the data. Health Information and Communication Technology (HICT) staff, assigned to manage the data for this study, worked at different sites. The supervisors collected the checklists after ensuring their completeness and consistency; if they found any problems, they cross-checked the data against the source. All missing data from the patients’ medical records were cross-checked with the ART electronic database and laboratory registers.
2.7 Study variables
2.7.1 Dependent variables
The primary outcome of interest was virological failure, which was defined as a persistently detectable viral load of greater than or equal to 1,000 copies/mL, based on two consecutive measurements within a three-month interval, after at least 6 months of ART.
2.7.2 Independent variables
The main exposure was ART adherence, which the WHO defines as the extent to which a person’s behavior corresponds with the agreed-upon recommendations from a healthcare provider (3). Other exposure variables extracted from the patients’ baseline and follow-up records included exposure characteristics measured at ART initiation—such as age, sex, marital status, education, occupation, disclosure, availability of a caretaker, type of facility, and the duration of ART since confirmation of HIV positive. Baseline and follow-up characteristics included body mass index (BMI), functional status, WHO clinical stage, CD4 level, opportunistic infections, comorbidities, duration on ART, reported side effects, drug toxicity, first-line ART substitutions, missed ART doses, and treatment adherence.
2.8 Operational definitions
2.8.1 Virological ART treatment failure
According to the WHO (6, 9), virological ART treatment failure is defined as a second viral load measurement greater than 1,000 copies/mL, taken 3 months after an initial detectable viral load in a patient who has been on ART for at least 6 months. Therefore, the patients with a second viral load result greater than 1,000 copies/mL were categorized as having virological treatment failure, whereas those with a second viral load result less than 1,000 copies/mL were categorized as having virological treatment suppression.
2.8.2 Treatment adherence
This was measured as the percentage of missed doses within a month, assessed through the remaining pill count or patient self-report. Clinicians consider good, fair, and poor adherence if the percentage of missed dose is > 95%, 85–94, and <85%, respectively, (21).
2.8.3 Functional status
It was defined as working, capable of going out of home and performing routine activities; ambulatory, capable of self-care and going to the toilet without assistance; and bedridden, unable to go to the toilet without assistance (9).
2.8.4 Treatment supporters
This refers to a specific individual who provides emotional, social, or practical support related to HIV treatment.
2.9 Data quality management
To ensure the quality of the data, different mechanisms were used, including the careful design of the data extraction tool, pretesting the checklist, appropriate recruitment, orientation and assignment of data collectors, and follow-up with data collectors and supervisors. Data clerks and case managers assisted the data collectors by identifying patient records. The investigators and supervisors checked the collected data for completeness, and corrective measures were taken accordingly, including cross-checking with the data source. The collected data were entered, coded, cleaned, edited, and explored before analysis.
2.10 Data processing and analysis
A standard coding guide, data entry procedures, and detailed computer editing specifications were prepared to ensure consistency in the data management process. The data were entered into the computer using the EpiData version 3.02 software and then exported to STATA 15 for cleaning and analysis. Univariable analyses such as frequencies, medians, inter-quartile ranges, means, and proportions were used to describe sociodemographic, baseline, and follow-up characteristics.
Dividing the number of participants who experienced virological failure by the total number of participants included in the analysis (257) yielded the proportion of participants who experienced virological failure. We determined the number of times each individual’s viral load exceeded the LOD (>1,000 copies/mL) during the follow-up period. Then, we calculated the average number of these occurrences across all participants who experienced virological failure.
Factors associated with virological first-line ART failure were identified using bivariable and multivariable binary logistic regression models. Variables with a p-value ≤ 0.25 in the bivariable binary logistic regression model were included in the multivariable binary logistic regression model to identify factors associated with virological first-line treatment failure. A significance level of 0.05 was used to guide the interpretation of relationships in the final multivariable model, with odds ratios (ORs) and 95% confidence intervals (CIs) calculated.
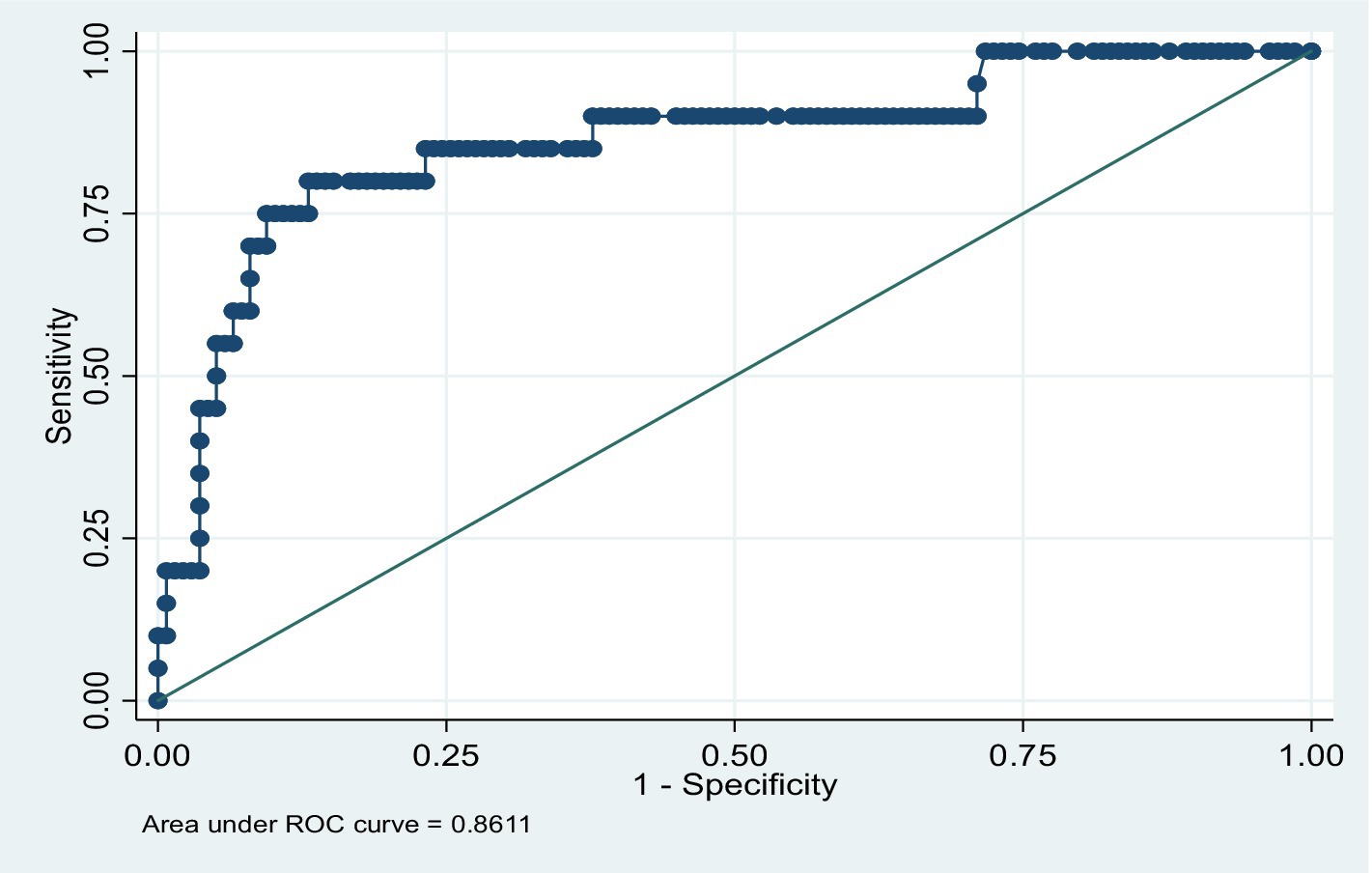
After the model was built, post-model estimation diagnostics were performed. The goodness-of-fit (GOF) of the model was assessed using the Hosmer–Lemeshow test. This was performed by dividing the predicted probabilities into declines and computing a Hosmer–Lemeshow chi-squared test that compared the predicted and observed frequencies (24). An analysis was conducted to evaluate and assess the accuracy of the model’s predictions. The area under the receiver operating characteristic (ROC) curve, which ranges from 0 to 1, provides a measure of the model’s ability to discriminate between participants who experience the outcome of interest versus those who do not. A value close to 1 indicates that the model has good predictive ability.
3 Results
3.1 Sociodemographic characteristics
A total of 257 people living with HIV (PWH) were included in the study; 94.48% of the records were complete. All included participants had been on first-line ART for at least 6 months and had undergone at least two viral load assessments within a three-month interval. Female patients comprised 162 (63.14%) of the participants, and the median age was 35 years (IQR = 28–41). The majority of the participants (95.29%) were from urban areas. Most patients had received formal education (71.55%), (see Table 1).
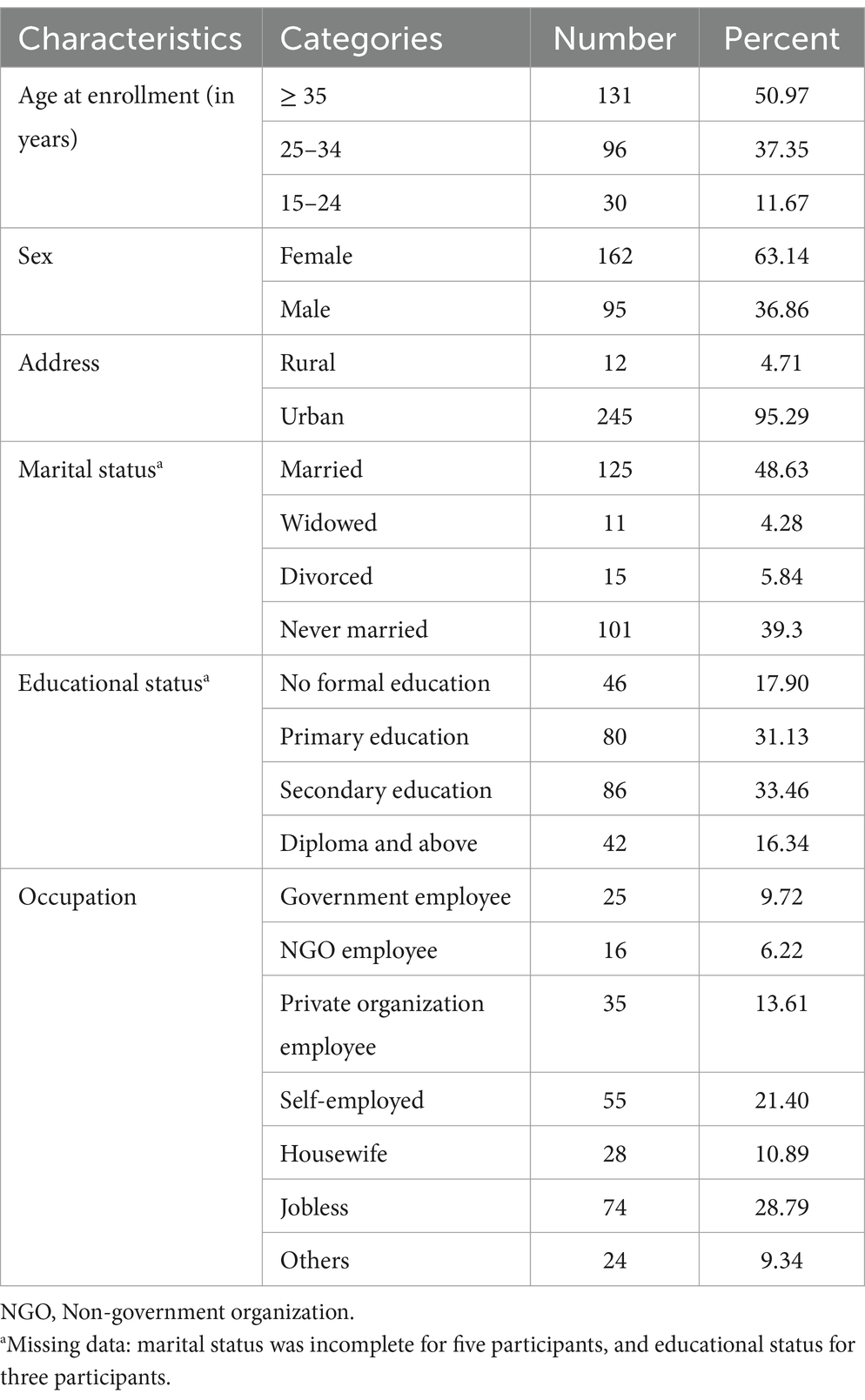
Table 1. Sociodemographic characteristics of the adults who received first-line ART from 2017 to 2020 in West Hararghe (n = 257).
3.2 Baseline, follow-up, clinical, and programmatic characteristics
BMI measurements at 6, 12, and 24 months were included to assess changes in body composition over time and to identify potential associations with virological failure. By analyzing BMI trends, we can gain insights into the nutritional status of participants and guide interventions.
The median weight of the study participants was 56 kg (IQR = 49 kg – 64.5 kg), with a median BMI of 20.57 at enrollment. The median BMI at the 6th month was 21.50 (IQR = 18.91–24.21), at the 12th month was 22.10 (IQR = 19.36–24.75), and at the 24th month was 22.23 (IQR = 19.33–25.28). Only 42 (18.34%) of the participants were unable to perform their usual work, either inside or outside their house, at the time of enrollment in this study. Of these, 13.97% were ambulatory, 4.37% were bedridden, and 81.66% were able to perform activities of daily living. Similarly, 119 (46.30%) participants were categorized as being at advanced WHO clinical stage III or V of HIV/AIDS.
A total of 166 participants (66.94%) disclosed their HIV-positive serostatus to their family members, including spouses, parents, own children, siblings, relatives, or friends. The majority of the participants were initiated on the TDF + 3TC + EFV regimen (63.43%), regardless of any criteria (46.69%), within a median duration of 5 days (IQR = 0–22) after testing HIV-positive. Only 94 (36.57%) of the patients initiated the regimen TDF + 3TC + DTG, which included the newly introduced drug DTG.
At the 6th month, 30.09% of the participants were at an advanced WHO clinical stage (stage III or IV) of HIV/AIDS, with 12.99% having a non-functional status (9.09% ambulatory and 3.90% bedridden). The mean duration on ART was approximately 30 months (29.66 ± 10.03). In addition, approximately 17.11% of the patients with recorded ART adherence assessments at the 6th month had poor adherence, and 33.19% of the patients had a recorded history of missed ART doses.
In this study, isoniazid (INH) prophylaxis was typically recommended for the individuals with a positive TST and a negative chest X-ray. Cotrimoxazole preventive therapy (CPT) was recommended for the individuals with a CD4 count below 200 cells/mm3.
A total of 166 patients (64.73%) completed isoniazid (INH) prophylaxis, while only 37.74% completed cotrimoxazole preventive therapy (CPT). In addition, 31 patients (11.95%) were diagnosed with TB at the time of follow-up (Table 2).
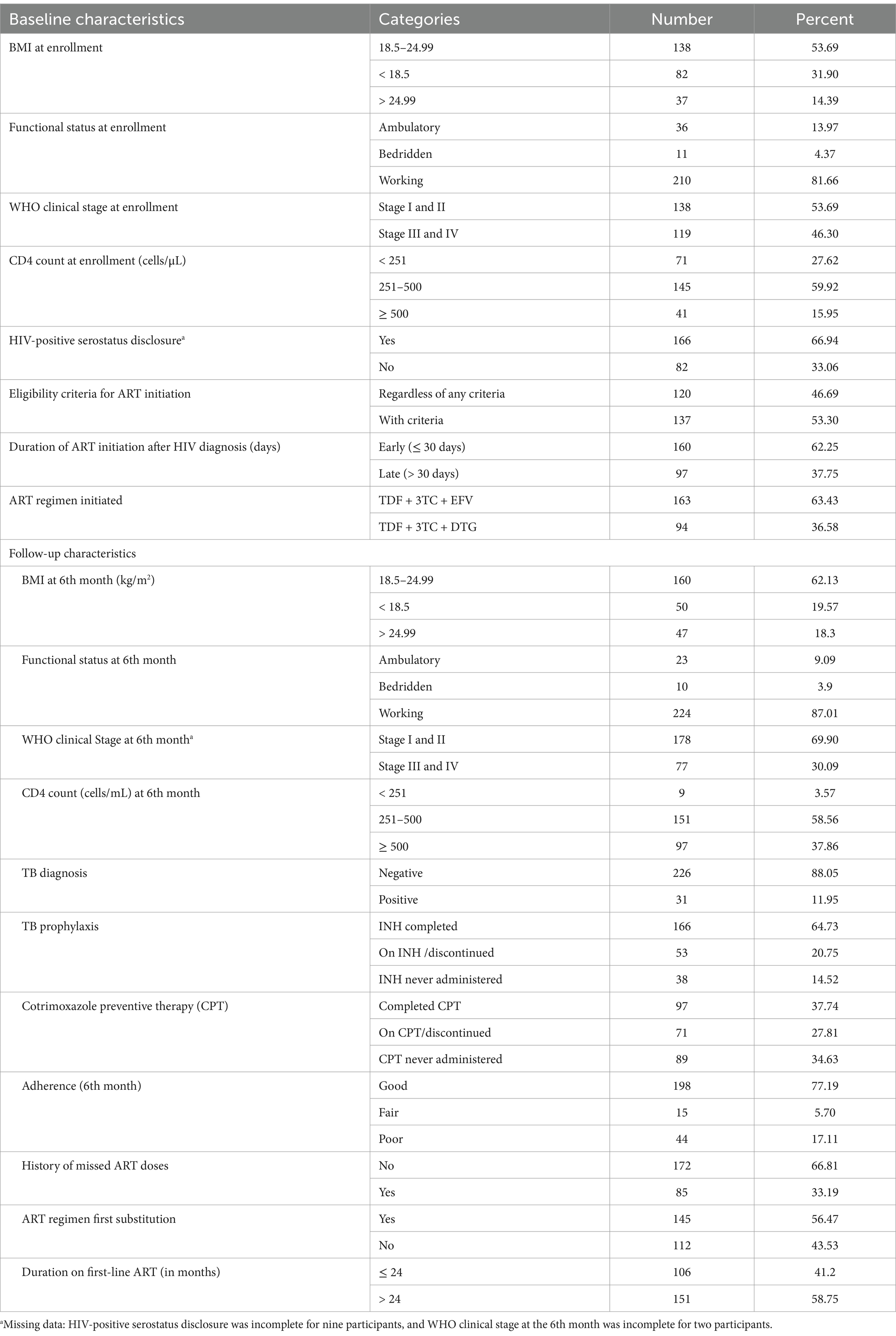
Table 2. Baseline and follow-up clinical and programmatic characteristics of the adults who received first-line ART in West Hararghe from 2017 to 2020 (n = 257).
3.3 Virological first-line ART failure
More than 1 in 10 patients experienced virological failure on first-line ART (11.67, 95%CI = 7.69, 15.80) (Table 3).

Table 3. Sociodemographic and clinical factors significantly associated with virological failure on first-line ART among the adults enrolled from 2017 to 2020 in West Hararghe (n = 257).
3.4 Factors associated with Virological ART failure
Potential factors associated with virological failure on first-line ART were identified using bivariable logistic regression (p ≤ 0.25) and were further analyzed using multivariable logistic regression. In the bivariable logistic regression analysis, sex, age at enrollment, baseline BMI, HIV-positive serostatus disclosure, baseline WHO clinical stage, duration before ART initiation after HIV diagnosis, a history of missed ART doses, and substitution of the DTG-based ART regimen were selected for multivariable logistic regression. Finally, five predictors—baseline BMI, HIV-positive serostatus disclosure, duration before ART initiation after HIV diagnosis, a history of missed ART doses, and substitution of the DTG-based ART regimen—were found to have a statistically significant association with virological failure on first-line ART during the multivariable logistic regression analysis.
The multivariable logistic regression analysis revealed that the patients who did not disclose their HIV-positive serostatus showed higher odds (AOR = 4.453: 1.340, 14.793) of virological ART failure as compared to the patients who disclosed their status. In addition, the undernourished patients (baseline BMI ≤ 18.5) had higher odds (AOR = 3.717: 1.051, 13.139) of virological ART failure than the patients with a considerably normal nutritional status (BMI = 18.5–24.99).
The PWH who initiated ART > 30 days after HIV diagnosis showed higher odds of virological ART failure than those who initiated ART ≤ 30 days after the diagnosis. In the same way, the participants who initiated the newly recommended DTG-based regimen or whose ART regimen was substituted with the new first-line ART regimen (TDF + 3TC + DTG) showed lower odds (AOR = 0.275: 0. 085, 0.895) of virological ART failure compared to the patients on other regimens. After ART initiation, the patients with a recorded history of missed ART doses had higher odds (AOR = 3.156: 1.007, 9.891) of virological ART failure compared to their counterparts (Table 3).
Finally, after the model was built, post-model estimation diagnostics were performed. The GOF test for the virological outcome logistic model showed acceptable results (Pearson chi-squared (10) = 10.68, p = 0.3828). The ROC also showed good predictive ability for the model (86.11% area under the curve), with an acceptable classification rate (correctly classified = 88.61%) (Figure 2).
4 Discussion
Despite the successful implementation of HIV treatment programs for PWH, we reported relatively high rates of treatment failure among the PWH who received first-line ART regimens in West Hararghe. This incidence of treatment failure was significantly associated with HIV-positive serostatus non-disclosure, undernutrition (baseline BMI < 18.5), a history of missed ART doses (any missed daily dose), late (> 30 days) initiation of ART after HIV diagnosis, and the use of non-DTG-based ART regimens. Nutritional status (underweight) and HIV serostatus non-disclosure were baseline patient characteristics that negatively affected virological failure. Interruptions in ART doses (inadequate adherence) were another identified risk factor that might result in drug resistance, thereby increasing the odds of virological failure. The odds of virological failure were lower for the patients who initiated ART within 30 days of HIV diagnosis and for those on the DTG-based regimen, compared to their counterparts.
The present findings revealed that more than one-tenth of the enrolled adults experienced virological failure on first-line ART—an important clinical concern in West Hararghe—despite the 95–95–95 national action plan aimed at achieving over 95% viral suppression, as recommended by UNAIDS (25). This finding is more or less similar to previous study reports from Ethiopia (11.5%) and Uganda (11%) (16, 26, 27). Other similar studies reported virological failure rates of 10.24% (28), 13% (29) from Ethiopia, and 9% across four African countries (30). Previous studies conducted in Ethiopia (14, 22, 31), India (32), China (33), and Swaziland (34) also reported higher rates than those observed in the present study. These slight variations might be due to differences in socioeconomic status (35, 36), study populations (36), sample sizes, availability of medical services, and the methods used to investigate virological failure, all of which may influence the detection of treatment failure (37).
This study also revealed that HIV serostatus disclosure decreases the odds of virological failure. It is well established that non-disclosure of HIV serostatus negatively impacts ART adherence [(38, 39), which can subsequently lead to virological failure (38, 40). Unlike the findings of this study, a previous Ethiopian study reported a higher incidence of virological failure among adults who had disclosed their HIV-positive status, possibly due to stigma and discrimination (41). HIV-positive serostatus non-disclosure and ART interruption might result in the loss of opportunities to suppress viral replication, ultimately leading to virological failure (42, 43). If not handled properly, disclosure of HIV-positive status to friends and family may lead to a loss of support (44) and HIV serostatus-related discrimination (45). Similarly, missing ART doses can increase the odds of virological failure, as shown in this study and studies conducted elsewhere (30, 46). Missed ART doses (inadequate ART adherence) may lead to treatment failure, possibly due to the development of acquired drug resistance (47, 48).
In this study, the undernourished patients (baseline BMI < 18.5 kg/m2) showed higher odds of virological failure than the patients with normal baseline nutritional status (baseline BMI = 18.5–24.99 kg/m2) (AOR = 3.717: 1.051, 13.139). This is comparable to a previous study conducted in Ethiopia (10, 49). Other studies from Ethiopia (10, 19, 50, 51) showed similar findings. A possible reason for this could be that patients with low BMI have poor nutritional status, which leads to weakened immunity, a blunted immune response, and poor virological outcomes (52–54).
Late ART initiation (initiation within 30 days of HIV diagnosis) was significantly associated with increased odds of virological failure. This finding is consistent with reports from other studies, which suggest that early ART initiation may benefit HIV patients by enhancing viral suppression and survival (55–57). This study suggests that accelerated ART initiation generally leads to improved virological outcomes, thus supporting the recommendation in the Ethiopian ART guidelines for accelerated ART initiation.
Factors associated with virological failure were explored, including the type of first-line ART regimen initiated. DTG-based regimes were statistically associated with lower rates of virological failure compared to other first-line ART regimens. This finding is in line with reports from studies that found evidence of virological suppression being associated with regimens containing DTG (58, 59). Based on the WHO recommendation (3), Ethiopia’s ART guidelines were revised in August 2018 to prioritize the first-line use of integrase inhibitors (DTG) over EFV (21). Non-nucleoside reverse-transcriptase inhibitor drug resistance, particularly to EFV, is an important key factor driving the switch to DTG (60), while DTG-based regimens are potent treatment options (59, 61). Poor adherence is one of the factors that contribute to drug adaptability and resistance development (62).
4.1 Strengths and limitations of the study
Since the implementation of the test-and-treat approach, along with the recent use of DTG for first-line ART and routine viral monitoring, variables not reported in other similar studies, to the best of the author’s knowledge, were considered for verification. This study has some limitations. It was conducted at a single site, which might have limited the generalizability of the findings to other settings. In addition, due to the small sample size, certain variables might have been either over- or under-estimated in their impact on viral suppression. Finally, the design of the study did not include viral genotyping to confirm the presence of resistance or determine the incidence rate and hazard ratio for direct measurement of risk. Although these are important programmatic interests, they would require a cohort study, which is beyond the scope of this research.
5 Conclusion
Virological first-line treatment failure was significantly higher in West Hararghe. Hindering factors identified in this study, such as undernutrition, non-disclosure serostatus, and interruptions in ART doses, may contribute to the higher burden of HIV treatment failure. The study findings also emphasize the early initiation of ART (preferably DTG-based regimens) with frequent virological monitoring to sustain the ART treatment program. All stakeholders in the West Hararghe HIV control program need to review the recommended standards of care and interventions to address the identified contributing factors. Healthcare providers should take into account the patient’s nutritional status and serostatus disclosure when enrolling patients. In conjunction with the early initiation of ART, a comprehensive nutritional assessment and supplemental nutritional therapy for undernutrition could be important. Future studies should be conducted using prospective and qualitative designs to identify factors influencing virological treatment outcomes among adults on ART.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Haramaya University Institutional Health Research Ethics Review Committee (IHRERC). The studies were conducted per the local legislation and institutional requirements. Informed, voluntary, written, and signed consent were obtained from heads of the health care facilities not required from the participants we ensured the confidentiality and anonymity of the secondary data used. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the heads and staff of the health facilities for their continuous support during the data collection period. They would also like to thank the data collectors for their willingness to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1440504/full#supplementary-material
References
1. Brogan, A, Talbird, SE, Davis, AE, Wild, L, and Flanagan, D. Is increased screening and early antiretroviral treatment for HIV-1 worth the investment? An analysis of the public health and economic impact of improvement in the UK. HIV Med. (2019) 20:668–80. doi: 10.1111/hiv.12788
2. Marcus, JL, Leyden, WA, Alexeeff, SE, Anderson, AN, Hechter, RC, Hu, H, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000-2016. JAMA Netw Open. (2020) 3:e207954–4. doi: 10.1001/jamanetworkopen.2020.7954
3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach World Health Organization (2016).
5. WHO. Data quality assessment of national and partner HIV treatment and patient monitoring data and systems: implementation tool. Geneva, Switzerland: World Health Organization (2018).
6. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Summary of key features and recommendations, June 2013. Geneva, Switzerland: World Health Organization (2013).
7. Assefa, DG, Zeleke, ED, Bekele, D, Ejigu, DA, Molla, W, Woldesenbet, TT, et al. Isoniazid preventive therapy for prevention of tuberculosis among people living with HIV in Ethiopia: a systematic review of implementation and impacts. Int J Environ Res Public Health. (2022) 20:621. doi: 10.3390/ijerph20010621
8. Hoffmann,, et al. Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS (London, England). (2010) 24:1709–16. doi: 10.1097/QAD.0b013e32833ac6bc
9. FMOH. National Guidelines for comprehensive HIV prevention. Addis Ababa: Care and Treatment; Ethiopian Ministry of Health (2016).
10. Agezew, T, Tadesse, A, Derseh, L, and Yimer, M. Incidence and predictors of first line anti-retroviral therapy failure among adults receiving HIV Care in North West Ethiopia: a hospital-based follow-up study. J Infect Dis Epidemiol. (2019) 5:345. doi: 10.23937/2474-3658/1510072
11. Assefa, A, Gelaw, B, Getnet, G, and Yitayew, G. The effect of incident tuberculosis on immunological response of HIV patients on highly active anti-retroviral therapy at the university of Gondar hospital, Northwest Ethiopia: a retrospective follow-up study. BMC Infect Dis. (2014) 14:468. doi: 10.1186/1471-2334-14-468
12. Haile, D, Takele, A, Gashaw, K, Demelash, H, and Nigatu, D. Predictors of treatment failure among adult antiretroviral treatment (ART) clients in bale zone hospitals, south eastern Ethiopia. PLoS One. (2016) 11:e0164299. doi: 10.1371/journal.pone.0164299
13. Sisay, C, Bekele, A, Sisay, A, Mekonen, H, Terfa, K, Melese, D, et al. Incidence and predictors of anti-retroviral treatment (ART) failure among adults receiving HIV Care at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. J AIDS Clin Res. (2017) 8:2. doi: 10.4172/2155-6113.1000749
14. Desta, AA, Woldearegay, TW, Futwi, N, Gebrehiwot, GT, Gebru, GG, Berhe, AA, et al. HIV virological non-suppression and factors associated with non-suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: a retrospective study. BMC Infect Dis. (2020) 20:1–10. doi: 10.1186/s12879-019-4732-6
15. Gesesew, HA, Ward, P, Woldemichael, K, and Mwanri, L. Immunological failure in people with HIV (PWH) adults from 2003 to 2015 in Southwest Ethiopia: a retrospective cohort study. BMJ Open. (2018) 8:e017413. doi: 10.1136/bmjopen-2017-017413
16. Getaneh, Y, Egziabhier, AG, Zealiyas, K, Tilahun, R, Girma, M, Michael, GG, et al. Treatment failure among people living with HIV taking antiretroviral therapy in Ethiopia. BioRxiv. (2019):577049. doi: 10.1101/577049
17. Lenjiso, GA, Endale, BS, and Bacha, YD. Clinical and immunological failure among HIV-positive adults taking first-line antiretroviral therapy in Dire Dawa, eastern Ethiopia. BMC Public Health. (2019) 19:771. doi: 10.1186/s12889-019-7078-5
18. Tadesse, BT, Chala, A, Mukonzo, J, Chaka, TE, Tadesse, S, Makonnen, E, et al. Rates and correlates of short term Virologic response among treatment-Naïve people with HIV (PWH) children initiating antiretroviral therapy in Ethiopia: a multi-center prospective cohort study. Pathogens. (2019) 8:161. doi: 10.3390/pathogens8040161
19. Ahmed, M, Merga, H, and Jarso, H. Predictors of virological treatment failure among adult HIV patients on first-line antiretroviral therapy in Woldia and Dessie hospitals, Northeast Ethiopia: a case-control study. BMC Infect Dis. (2019) 19:305. doi: 10.1186/s12879-019-3924-4
20. Rutherford, GW, Anglemyer, A, Easterbrook, PJ, Horvath, T, Vitoria, M, Penazzato, M, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. (2014) 28:S161–9. doi: 10.1097/QAD.0000000000000236
21. FMOH. National Guidelines for comprehensive HIV prevention. Addis Ababa: Care and Treatment; Ethiopian Ministry of Health (2018).
22. Nega, J, Taye, S, Million, Y, Rodrigo, C, and Eshetie, S. Antiretroviral treatment failure and associated factors among HIV patients on first-line antiretroviral treatment in Sekota, Northeast Ethiopia. AIDS Res Ther. (2020) 17:1–9. doi: 10.1186/s12981-020-00294-z
23. Zhao, Y, Wu, Z, McGoogan, JM, Sha, Y, Zhao, D, Ma, Y, et al. Nationwide cohort study of antiretroviral therapy timing: treatment dropout and Virological failure in China, 2011–2015. Clin Infect Dis. (2019) 68:43–50. doi: 10.1093/cid/ciy400
24. Hosmer, DW, Lemeshow, S, and Sturdivant, RX. Applied logistic regression. Hoboken, New Jersey, USA: John Wiley & Sons (2013).
26. Bulage, L, Ssewanyana, I, Nankabirwa, V, Nsubuga, F, Kihembo, C, Pande, G, et al. Factors associated with Virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, august 2014–July 2015. BMC Infect Dis. (2017) 17:326. doi: 10.1186/s12879-017-2428-3
27. Hailu, GG, Hagos, DG, Hagos, AK, Wasihun, AG, and Dejene, TA. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of northern Ethiopia. PLoS One. (2018) 13:e0196259. doi: 10.1371/journal.pone.0196259
28. Derseh, BT, Shewayerga, B, Dagnew Mekuria, A, and Admasu Basha, E. Virological treatment failure among adult HIV/AIDS patients from selected hospitals of north Shoa zone, Amhara region, Ethiopia. Infect Drug Resist. (2020) 13:4417–25. doi: 10.2147/IDR.S280966
29. Haile, T, Hawulte, B, and Alemayehu, S. A retrospective cross-sectional study on the prevalence and factors associated with virological non-suppression among HIV-positive adult patients on antiretroviral therapy in Woliso town, Oromia, Ethiopia. Int J Med Health Sci. (2021) 15:158–64.
30. Kiweewa, F, Esber, A, Musingye, E, Reed, D, Crowell, TA, Cham, F, et al. HIV virologic failure and its predictors among people with HIV (PWH) adults on antiretroviral therapy in the African cohort study. PLoS One. (2019) 14:e0211344. doi: 10.1371/journal.pone.0211344
31. Wendie, TF, and Workneh, BD. Prevalence and predictors of Virological failure among adults living with HIV in south Wollo zone, Northeast Ethiopia: a retrospective cohort study. HIV/AIDS. (2020) 12:393. doi: 10.2147/HIV.S266460
32. Shet, A, Neogi, U, Kumarasamy, N, DeCosta, A, Shastri, S, and Rewari, BB. Virological efficacy with first-line antiretroviral treatment in India: predictors of viral failure and evidence of viral resuppression. Trop Med Int Health. (2015) 20:1462–72. doi: 10.1111/tmi.12563
33. Yuan, D, Liu, M, Jia, P, Li, Y, Huang, Y, Ye, L, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. (2020) 20:1–10. doi: 10.1186/s12879-020-05124-1
34. Jobanputra, K, Parker, LA, Azih, C, Okello, V, Maphalala, G, Kershberger, B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. (2015) 10:e0116144. doi: 10.1371/journal.pone.0116144
35. Plymoth, M, Sanders, EJ, van der Elst, EM, Medstrand, P, Tesfaye, F, Winqvist, N, et al. Socio-economic condition and lack of virological suppression among adults and adolescents receiving antiretroviral therapy in Ethiopia. PLoS One. (2020) 15:e0244066. doi: 10.1371/journal.pone.0244066
36. Burch, LS, Smith, CJ, Anderson, J, Sherr, L, Rodger, AJ, O’Connell, R, et al. Socioeconomic status and treatment outcomes for individuals with HIV on antiretroviral treatment in the UK: cross-sectional and longitudinal analyses. Lancet Public Health. (2016) 1:e26–36. doi: 10.1016/S2468-2667(16)30002-0
37. Dombrowski, JC, Kent, JB, Buskin, SE, Stekler, JD, and Golden, MR. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS. (2012) 26:77–86. doi: 10.1097/QAD.0b013e32834dcee9
38. Abdullahi, IJ, Deybasso, HA, and Adlo, AM. Determinants of virological failure among patients on first-line antiretroviral therapy in Central Oromia, Ethiopia: a case–control study. HIV/AIDS. (2020) 12:931. doi: 10.2147/HIV.S281672
39. Dessie, G, Wagnew, F, Mulugeta, H, Amare, D, Jara, D, Leshargie, CT, et al. The effect of disclosure on adherence to antiretroviral therapy among adults living with HIV in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. (2019) 19:1–8. doi: 10.1186/s12879-019-4148-3
40. Morkphrom, E, Ratanasuwan, W, Sittironnarit, G, and Rattanaumpawan, P. Non-disclosure of HIV serostatus to sexual partners: prevalence, risk factors and clinical impact in patients with HIV. HIV Med. (2021) 22:194–200. doi: 10.1111/hiv.13005
41. Teshome Yimer, Y, and Yalew, AW. Magnitude and predictors of anti-retroviral treatment (ART) failure in private health facilities in Addis Ababa, Ethiopia. PLoS One. (2015) 10:e0126026. doi: 10.1371/journal.pone.0126026
42. Bayu, B, Tariku, A, Bulti, AB, Habitu, YA, Derso, T, and Teshome, DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case–control study. HIV/AIDS-Research and Palliative Care. (2017) 9:153. doi: 10.2147/HIV.S139516
43. Mesic, A, Spina, A, Mar, HT, Thit, P, Decroo, T, Lenglet, A, et al. Predictors of virological failure among people living with HIV receiving first line antiretroviral treatment in Myanmar: retrospective cohort analysis. AIDS Res Ther. (2021) 18:1–12. doi: 10.1186/s12981-021-00336-0
44. Mi, T, Li, X, Zhou, G, Qiao, S, Shen, Z, and Zhou, Y. Hiv disclosure to family members and medication adherence: role of social support and self-efficacy. AIDS Behav. (2020) 24:45–54. doi: 10.1007/s10461-019-02456-1
45. Yonah, G, Fredrick, F, and Leyna, G. HIV serostatus disclosure among people living with HIV/AIDS in Mwanza, Tanzania. AIDS Res Ther. (2014) 11:1–5. doi: 10.1186/1742-6405-11-5
46. Kyaw, NTT, Harries, AD, Kumar, AMV, Oo, MM, Kyaw, KWY, Win, T, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005-2015. PLoS One. (2017) 12:e0171780. doi: 10.1371/journal.pone.0171780
47. Hawkins, C, Ulenga, N, Liu, E, Aboud, S, Mugusi, F, Chalamilla, G, et al. HIV virological failure and drug resistance in a cohort of Tanzanian people with HIV (PWH) adults. J Antimicrob Chemother. (2016) 71:1966–74. doi: 10.1093/jac/dkw051
48. Rupérez, M, Pou, C, Maculuve, S, Cedeño, S, Luis, L, Rodríguez, J, et al. Determinants of virological failure and antiretroviral drug resistance in Mozambique. J Antimicrob Chemother. (2015) 70:2639–47. doi: 10.1093/jac/dkv143
49. Bayou, B, Sisay, A, and Kumie, A. Assessment of the magnitude and associated factors of immunological failure among adult and adolescent people with HIV (PWH) patients in St. Luke and Tulubolo hospital, Oromia region, Ethiopia. Pan Afr Med J. (2015) 21:291. doi: 10.11604/pamj.2015.21.291.6831
50. Sunkanmi, F, Paul, Y, Peter, D, Nsikan, A, Joseph, J, Opada, E, et al. Factors influencing viral load non-suppression among people living with HIV (PLHIV) in Borno state, Nigeria: a case of Umaru Shehu ultra-modern hospital. J Adv Med Res. (2020) 32:98–105. doi: 10.9734/jammr/2020/v32i330388
51. Zenu, S, Tesema, T, Reshad, M, and Abebe, E. Determinants of first-line antiretroviral treatment failure among adult patients on treatment in Mettu Karl specialized hospital, south West Ethiopia; a case control study. PLoS One. (2021) 16:e0258930. doi: 10.1371/journal.pone.0258930
52. Abba, A, Fokam, J, Kamgaing, RS, Yimga, JF, Ka’e, AC, Nka, AD, et al. Correlation between the immuno-virological response and the nutritional profile of treatment-experienced people with HIV (PWH) patients in the east region of Cameroon. PLoS One. (2021) 16:e0229550. doi: 10.1371/journal.pone.0229550
53. Han, W, Jiamsakul, A, Jantarapakde, J, Yunihastuti, E, Choi, JY, Ditangco, R, et al. Association of body mass index with immune recovery, virological failure and cardiovascular disease risk among people living with HIV. HIV Med. (2021) 22:294–306. doi: 10.1111/hiv.13017
54. Izudi, J, Alioni, S, Kerukadho, E, and Ndungutse, D. Virological failure reduced with HIV-serostatus disclosure, extra baseline weight and rising CD4 cells among HIV-positive adults in northwestern Uganda. BMC Infect Dis. (2016) 16:1–8. doi: 10.1186/s12879-016-1952-x
55. Boyd, M, Boffito, M, Castagna, A, and Estrada, V. Rapid initiation of antiretroviral therapy at HIV diagnosis: definition, process, knowledge gaps. HIV Med. (2019) 20:3–11. doi: 10.1111/hiv.12708
56. Zhou, C, Zhang, W, Lu, RR, Ouyang, L, Xing, H, Shao, YM, et al. Benefits of early and immediate initiation of antiretroviral therapy among HIV patients in Chongqing, China. Biomed Environ Sci. (2020) 33:282–5. doi: 10.3967/bes2020.039
57. Coffey, S, Bacchetti, P, Sachdev, D, Bacon, O, Jones, D, Ospina-Norvell, C, et al. RAPID antiretroviral therapy: high virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS (London, England). (2019) 33:825–32. doi: 10.1097/QAD.0000000000002124
58. NAMSAL ANRS 12313 Study Group Kouanfack, C, Mpoudi-Etame, M, Omgba Bassega, P, Eymard-Duvernay, S, Leroy, S, et al. Dolutegravir-based or low-dose Efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. (2019) 381:816–26. doi: 10.1056/NEJMoa1904340
59. Venter, WD, Moorhouse, M, Sokhela, S, Fairlie, L, Mashabane, N, Masenya, M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. (2019) 381:803–15. doi: 10.1056/NEJMoa1902824
60. Günthard, HF, Saag, MS, Benson, CA, del Rio, C, Eron, JJ, Gallant, JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society–USA panel. JAMA. (2016) 316:191–210. doi: 10.1001/jama.2016.8900
61. Phillips, AN, Bansi-Matharu, L, Venter, F, Havlir, D, Pozniak, A, Kuritzkes, DR, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. (2020) 7:e193–200. doi: 10.1016/S2352-3018(19)30400-X
62. Ramadhani, HO, Thielman, NM, Landman, KZ, Ndosi, EM, Gao, F, Kirchherr, JL, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among people with HIV (PWH) adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. (2007) 45:1492–8. doi: 10.1086/522991
Glossary
ABC - Abacavir
AOR - Adjusted Odd Ratio
ART - Anti-Retroviral Therapy
ARV - Antiretroviral drugs
BMI - Body Mass Index
AZT - Azidothymidine (also known as Zidovudine, abbreviated as ZDV)
CD4 cells - Cluster for Differentiation 4 cells, type of T-lymphocyte bearing CD4
CPT - Cotrimoxazole preventive therapy
DTG - Dolutegravir
EAC - Enhanced Adherence Counseling
EFV - Efavirenz
EPHI - Ethiopian Public Health Institute
MUAC - Middle Upper Arm Circumference
NNRTI - Nonnucleoside Reverse Transcriptase Inhibitor
NVP - Nevirapine
PWH - People with HIV
3TC - Lamivudine
TDF - Tenofovir
TB - Tuberculosis
WHO - World Health Organization
Keywords: HIV/AIDS, virological failure, first-line ART, early ART initiation, West Hararghe
Citation: Zerihun E, Tesema K and Abera F (2025) Virological treatment failure and associated factors among adults on first-line antiretroviral therapy in West Hararghe, Ethiopia. Front. Public Health. 13:1440504. doi: 10.3389/fpubh.2025.1440504
Edited by:
Peter Kojo Quashie, University of Ghana, GhanaReviewed by:
Wei Li Adeline Koay, Medical University of South Carolina, United StatesJames Aboagye, University of Ghana, Ghana
Copyright © 2025 Zerihun, Tesema and Abera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ebisa Zerihun, ZWJpc2F6MDc3QGdtYWlsLmNvbQ==
 Ebisa Zerihun
Ebisa Zerihun Kenesa Tesema
Kenesa Tesema Fekadu Abera
Fekadu Abera