- 1Faculty of Health Sciences, Department of Medicine, University of Cape Town, Cape Town, South Africa
- 2Department of Clinical Sciences, Nigerian Institute of Medical Research, Lagos, Nigeria
- 3Non-Communicable Diseases Research Unit, South African Medical Research Council, Cape Town, South Africa
- 4Department of Medicine, University College Hospital, Ibadan, Nigeria
Introduction: The advent of antiretroviral therapy (ART) has converted HIV from a death sentence to a chronic disease. Subsequently, weight changes, including the development of overweight/obesity have been observed following ART initiation. Our study aimed to assess weight changes and the associated factors among ART-naïve people living with HIV (PLWH) following enrollment in an ART clinic in Lagos, Nigeria.
Methodology: Data were collected among adult ART-naïve PLWH enrolled at a large ART clinic over 10 consecutive years. Weight changes within the first 6 months of enrolment were determined by actual and relative weight differences expressed in kilogram (kg) and percentages (%) respectively. Weight changes were classified as neutral weight change, weight gain and weight loss. Logistic regressions were applied to identify variables associated with weight changes with statistical significance set at p < 0.05.
Results: A total of 6,737 study participants had their weights available at both visits. Most study participants were females (67.2%), employed (83.3%), married (57.1%), and had normal range body mass index (53.5%). Almost half (49.5%) of the study participants gained weight, while 25.5% recorded weight loss. Baseline variables, including viral load ≥ 100,000 copies/ml, CD4 counts ≤ 200 cells/μL, WHO clinical stages 3 and 4, male gender, presence of anaemia and tuberculosis were associated with weight gain after ART initiation.
Conclusion: Considering the high proportion of participants that gained weight, this study highlights the importance of monitoring weight changes following ART initiation. This will facilitate the identification of PLWH at greater risk for cardiometabolic diseases and other weight-related health outcomes.
Introduction
Globally, approximately 39.9 million people were living with HIV (PLWH) in 2023, with a significant proportion on effective antiretroviral therapy (ART) (1). The advent of ART has transformed HIV/AIDS from a fatal illness to a chronic condition with PLWH having a similar lifespan to the HIV-uninfected population (2). Nevertheless, the survival of PLWH is being undermined by the rising incidence of non-communicable diseases (NCDs). This could reverse some of the anticipated gains of HIV care if not curtailed. The prevalent NCDs in this population include cardiometabolic disorders such as obesity, mental health disorders, chronic pulmonary diseases and chronic kidney diseases among others (3, 4). The drivers of NCDs have been attributed to the effect of antiretrovirals (ARV), lifestyle and behavioral changes (5).
Weight gain, an indicator of successful ARV treatment, has now come under scrutiny. The drawbacks of weight gain include increased risks of insulin resistance and dyslipidaemia (6). Weight gain could also impact mental health, arising from body image issues, anxiety, and low self-esteem (7). Excessive weight gain results in obesity, an independent risk factor for morbidity and mortality related to cardiovascular and other diseases (6, 8). Newer ARV regimens, especially integrase strand inhibitors (INSTIs), have been associated with significant weight gains among PLWH (9). Furthermore, the adoption of unwholesome lifestyles by PLWH, such as the consumption of unhealthy diets, decreased physical activity, and sedentary work routines is likely to lead to weight gain (10).
In sub-Saharan Africa (SSA), where the HIV burden is highest globally, studies have evaluated weight changes among PLWH following enrollment into care (9, 11). Most studies have aligned with weight gain following ART initiation globally and SSA, with the highest weight gain observed in the first year of ART initiation (6, 12). Attributable factors for the observed weight gain include female gender, black race, clinical status at ART initiation (low CD4 counts, high viral loads, advanced clinical staging of HIV disease), diets, and older age (13–15). However, there is limited evidence on weight changes among PLWH in Nigeria, one of the countries with a high HIV burden on the continent (16, 17). Lagos State, the economic capital of Nigeria, is home to over 18 million residents and an HIV prevalence of 1.3% (18). Our study aimed to assess weight changes and their associated factors following ART initiation among ART-naïve PLWH at an ART clinic in Lagos, Nigeria.
Materials and methods
Study setting
The Clinical Sciences Department, within the Nigerian Institute of Medical Research (NIMR) operates an ART clinic. The ART clinic has been in existence since 2002 and cumulatively enrolled more than 25,000 PLWH. The clinic provides care and management to adults, pregnant women, adolescents, and infants exposed or infected with HIV. The ART clinic is located in the country’s economic capital, Lagos State. Services received in the facility are free of charge and adhere to the country’s national guidelines for HIV prevention, treatment, and care.
Data of PLWH accessing care and treatment at the facility were captured in an electronic database and maintained on a server onsite. Each person is assigned a unique identification number, which is used to collate data relating to clinical, therapeutic, and laboratory parameters. At enrolment, patients are offered counseling services (pre- and post-confirmation of HIV diagnosis) and an informed consent process. A consent form is provided to document their choice of the use of their data and biological samples for research purposes. Their responses do not influence the care and treatment received in the facility. The clinic offers only outpatient services while patients requiring admission are referred to facilities closest to their residence or choice.
Study design, population, and data collection
This is a longitudinal review of data collected among adult ART-naïve PLWH enrolled in the clinic over 10 years (January 1, 2010 – December 31, 2019). Participants were included in the study if they had their weights recorded at baseline and scheduled their first follow-up clinic visit (6 (± 2) months post-enrolment) irrespective of their ART status. Those who were younger than 18 years of age, ART-experienced, or receiving care for post-exposure prophylaxis (PEP) were excluded from the study. All eligible patients attending the clinic were included in the study.
At enrolment and follow-up visits, the nurse took the patients’ vital signs (temperature, weight, height, blood pressure). Weights to the nearest 0.1 kg were measured with patients standing on the weighing scale, barefooted in an erect posture. Heights were recorded with the marker placed at the crown of the head and measurements were taken to the nearest 0.1 cm. Blood pressure (BP) measurements were obtained with the patient in a sitting position (at least 5 min before the assessment) using a digital BP monitor with an appropriate cuff size. The BP readings were taken three times with the average recorded as the patient’s readings while the weights and heights of study participants were observed once.
At baseline and subsequent clinic visits, the clinician conducts a detailed clinical history and physical examination for the study participants. At the baseline visit, the patient’s WHO clinical staging was determined and baseline investigations were conducted (full blood count, cluster of differentiation 4 (CD4) count, viral load, serum creatinine, random blood glucose, Hepatitis B and C screening). Chest X-ray and sputum evaluation were done based on the national guidelines for possible tuberculosis co-infection (19).
Data collected for the study at the baseline clinic visit included sociodemographic characteristics (age, gender, education and marital status, occupation, and year of enrolment), anthropometry (weight, height), blood pressure, comorbidities at presentation (hypertension, diabetes mellitus, tuberculosis, anaemia, Hepatitis B and C), HIV specific characteristics (viral load, CD4 counts, and WHO clinical staging), and laboratory investigations (haemoglobin, Hepatitis B and C) at enrolment in the clinic. Data relating to anthropometric measures (weight and height), HIV-specific characteristics (viral load, CD4 counts and ART status), and anaemia were collected for review at the scheduled follow-up visit.
Definitions
The study outcome of interest is weight change observed among ART-naïve PLWH at enrolment and 6 months post-ART initiation. Weight changes were assessed by actual and relative weight differences expressed in kilograms (kg) and percentages (%) respectively. Weight changes were classified into 3 categories using weight differences in kilograms and percentages. The categories for weight changes in kg are neutral weight change ±1 kg, weight gain ≥ 1 kg, and weight loss ≥ 1 kg. In terms of weight changes expressed in percentages, the categories were neutral weight change (<5%), weight gain ≥ 5%, and weight loss ≥ 5%. These cut-points were selected following recent studies within the HIV cohort. A study among veterans in the United States of America (USA) recorded a median weight change of 2.7 kg (5 pounds) over a one-year follow-up period post-ART initiation (20). Other studies using weight difference expressed in percentages utilized 5% weight change while another correlated 2.5% weight differentials with mortality at 1 month (21, 22). Due to the short duration of the follow-up (6 months), we aligned our definition of weight changes to prior studies with similar profiles 2.7 kg weight gain within a year of ART initiation or percentage (2.5% or 5%) change. In addition, PLWH with weight loss exceeding 5 and 10% have been classified to be either in WHO Clinical Stage 3 or 4, respectively (23).
Body mass index (BMI) was calculated as the weight (in kilogram, kg) divided by height squared (in metres) – kg/m2 (24). BMI was classified as underweight (< 18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (≥ 30.0) (24).
Hypertension was defined as systolic and/or diastolic BP (SBP and DBP) greater than or equal to 140 mmHg and 90 mmHg, respectively, in addition to prior history of hypertension diagnosis or use of anti-hypertensive medications (irrespective of current BP readings) (25). Anaemia was defined as haemoglobin level less than 10 g/dL (26). Study participants were diagnosed with diabetes mellitus in the presence of a prior diagnosis (from clinical history or medical records) and/or were currently on treatment. Tuberculosis was diagnosed following clinical history, physical examination, chest X-ray findings and sputum examination based on national guidelines (27).
Viral load was classified into two categories (≤ 100,000 and ≥ 100,001 copies per millimetre) while CD4 counts were classified according to the CDC Staging (23).
Study participants were initiated on ART based on the prevailing national treatment guidelines for the care and management of HIV at the time of enrolment into the clinic which have seen numerous revisions throughout the study period (28, 29).
Statistical analysis
Data were presented in counts and percentages for categorical variables with mean (and standard deviation) or median (and 25th – 75th percentiles) for continuous variables. The data was evaluated for normality and multicollinearity, while Chi-square test was used to determine the relationship between categorical variables and observed weight changes. Student t-test was used to compare the means of variables. We explored the predictors of weight changes among participants who initiated ART using both univariate and multivariate regression analysis (including variables such as age, sex, occupation, marital status, anaemia, ART status, ART regimen, year of enrolment, as well as HIV-specific factors and other clinical variables). The multivariable regression analysis was adjusted for age and gender for the two models (absolute and differential weight changes). Statistical significance was set at p-value < 0.05 and 95% Confidence intervals provided. Data was analyzed using Statistical Package for Social Sciences (SPSS) version 29 (IBM SPSS Inc., Chicago, IL).
Ethical approval
Ethical approval was sought and obtained from the Institutional Review Board (IRB) of the Nigerian Institute of Medical Research (NIMR) [IRB-21-066]. Additionally, ethical approval was also been obtained from the Health Research Committee of the University of Cape Town, South Africa [HREC 176/2022].
Results
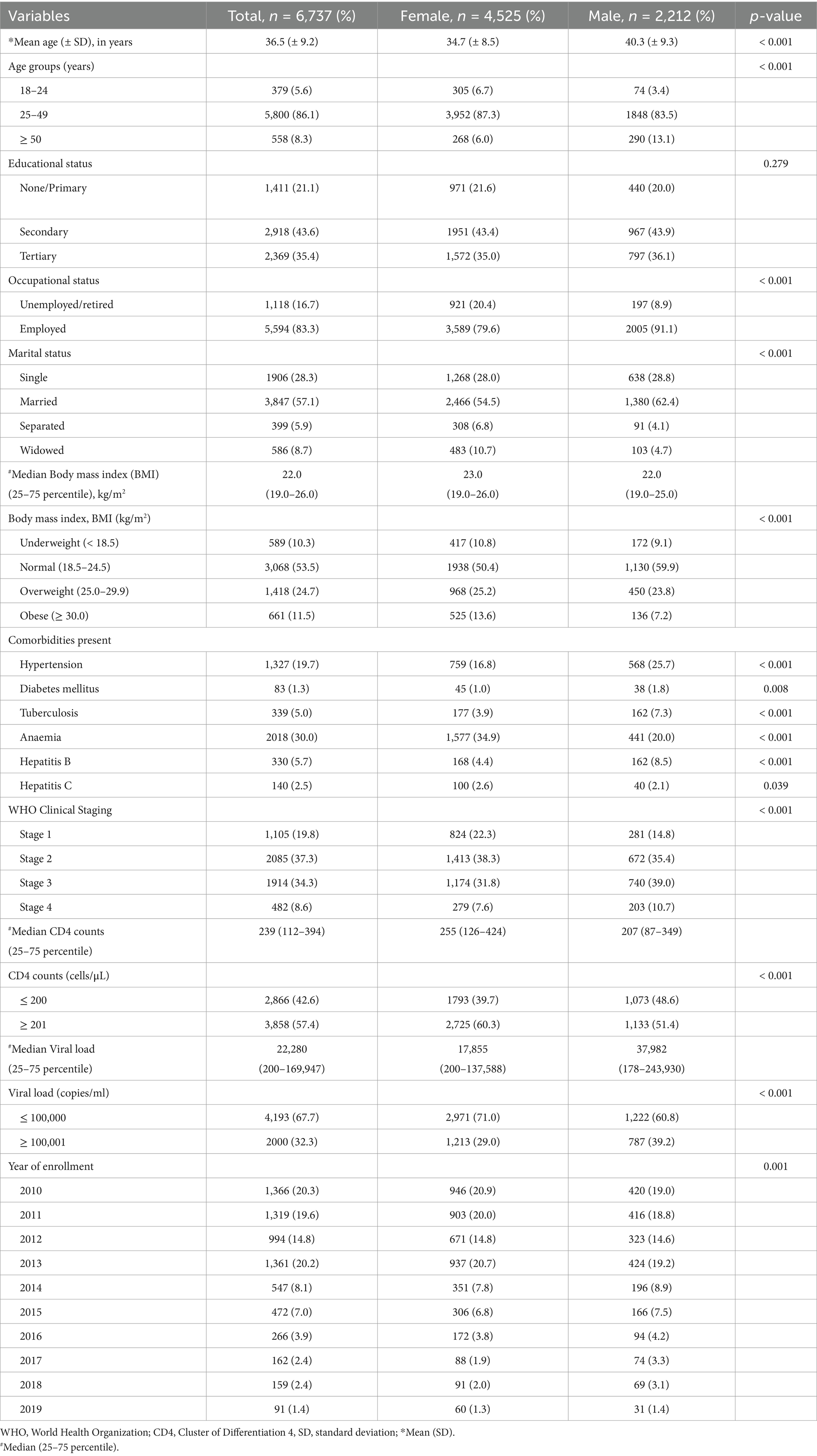
Among 10,516 ART-naïve PLWH enrolled during the study period, 6,737 study participants had their weights available at both baseline and 6-month follow-up clinic visits. The mean age (± SD) of study participants at enrollment was 36.5 (± 9.2) years, with males found to be older than females (40.3 vs. 34.7 years, respectively, p < 0.0001). At enrolment, all sociodemographic, laboratory, and HIV-specific factors were significantly different by gender except for educational status. A majority of study participants were females (67.2%), employed (83.3%), married (57.1%), and had normal BMI (53.5%). In addition, hypertension (19.7%) was the most common comorbidity recorded at the baseline visit. Concerning HIV-specific characteristics, 67.7 and 57.4% of participants had viral load ≤100,000 copies/ml and CD4 counts ≥ 201 cells/μL, respectively. A majority of the study participants were either in WHO Clinical Stages 1 or 2 (57.1%) (Table 1).
The dataset was assessed for normality using the Kolmogorov–Smirnov test, and all variables were normally distributed (p < 0.001), the study variables were also evaluated for interactions/collinearity with the collinearity tolerance and statistics variance inflation factor (VIF) values showing low levels for multicollinearity (Supplementary Table 1).
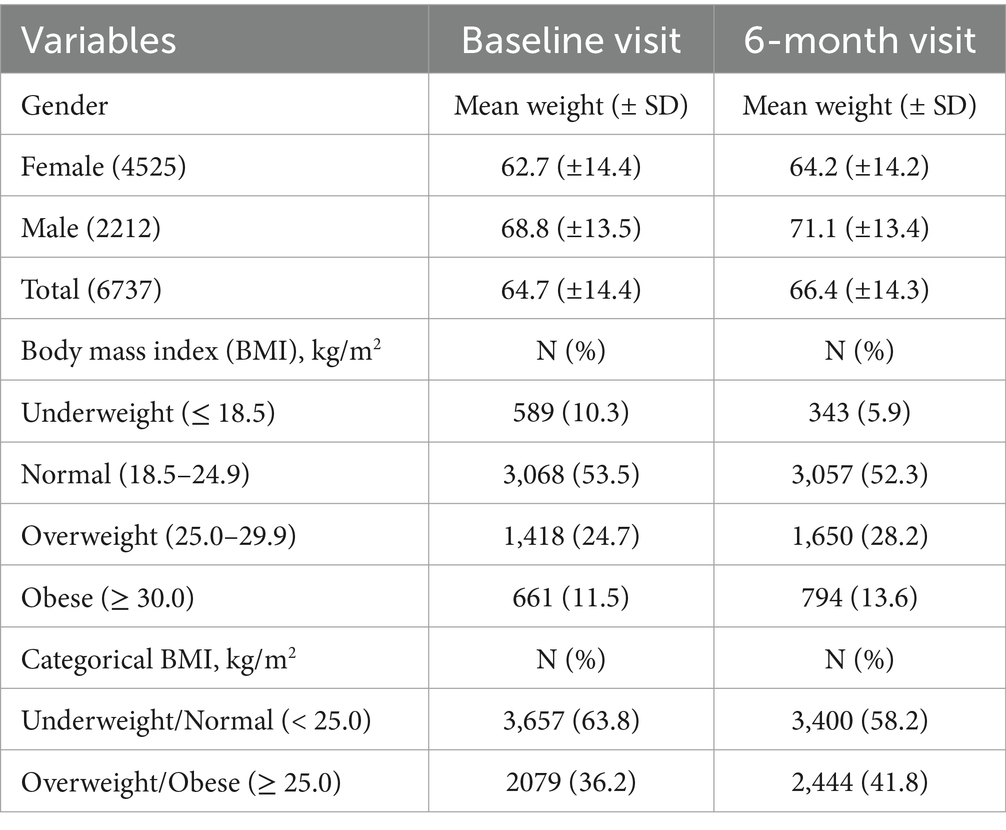
The mean weights (± SD) of study participants at baseline and 6-month clinic visits were 64.7 (±14.4)kg and 66.4 (±14.3) kg, respectively, with the difference found to be statistically significant, p < 0.001 (Table 2). The mean BMI (± SD) at baseline and 6 months were 23.6 kg/m2 (± 5.8) and 24.9 kg/m2 (± 5.1), respectively. The paired sample difference (± SD) of the mean BMIs was −1.2 kg/m2 (± 5.1) with the difference found to be significant (p < 0.001). In addition, the proportion of study participants with underweight and normal BMI (BMI < 25.0 kg/m2) decreased from 63.8 to 58.2% with about 50% reduction in the prevalence of underweight PLWH at 6 months when compared to the baseline, Table 2. The median weight change among study participants with recorded weight gain was 2.0 (25 – 75th percentile, −1.0 – 5.0) kg. Furthermore, the use of antiretroviral agents was shown to be associated with weight gain and loss irrespective of the regimens initiated (p < 0.05), Supplementary Table 2.
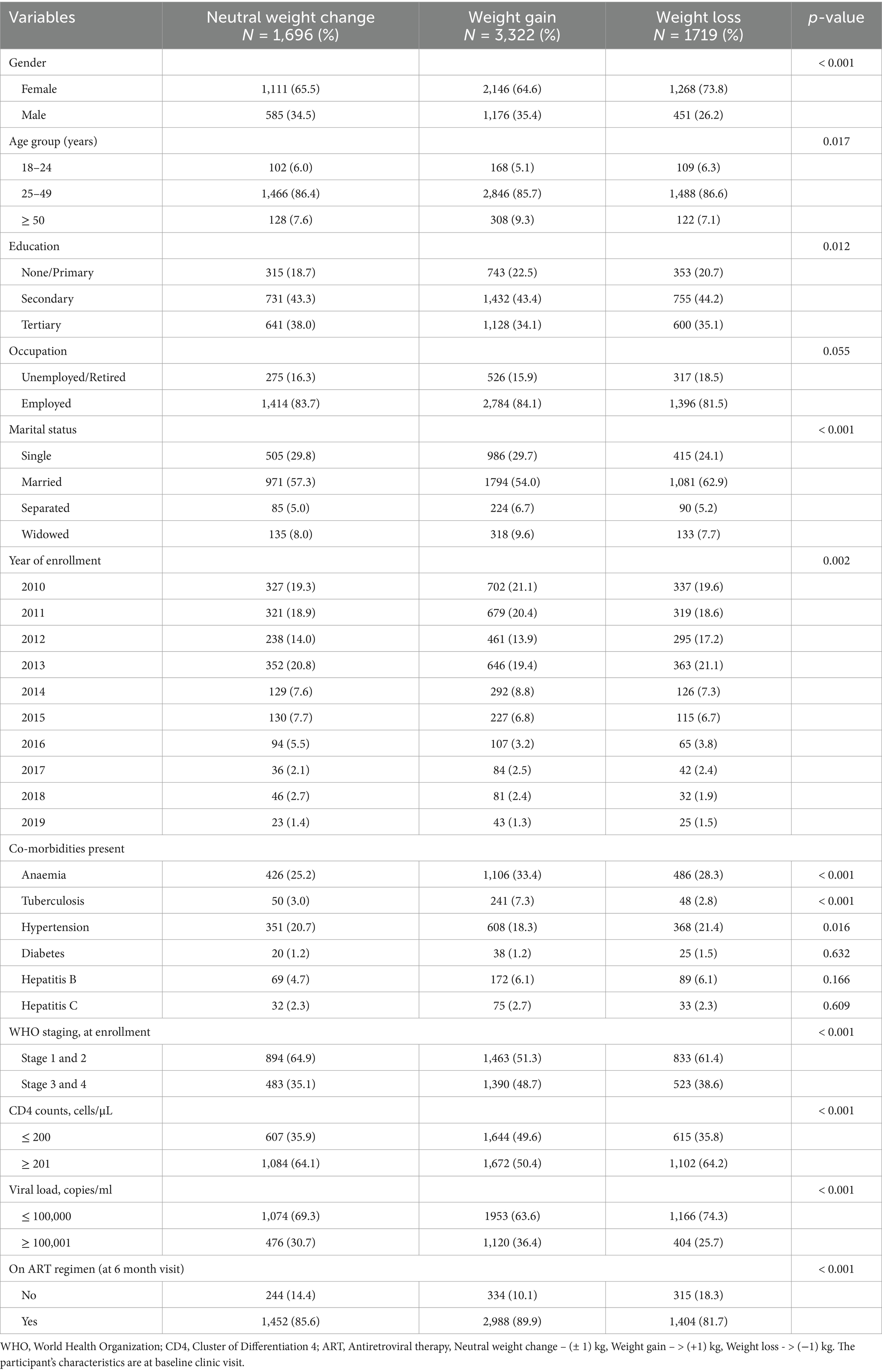
Male study participants had relatively higher weights at both baseline and 6-month visits when compared to their female counterparts (p < 0.001). Overall, a significant proportion of participants gained weight (49.5%), while 25.5% lost weight and 25.0% had neutral weight change. This trend was similar for females (weight gain, 47.4%; weight loss, 28.0%; and no change, 24.6%) and males (weight gain, 53.2%; weight loss, 20.4%; and no change, 26.4%). Eight hundred and ninety-three (893) PLWH were yet to commence ART at their 6-month clinic visit, with 38.5% (n = 344) and 35.3% (n = 315) of them recording actual weight gain and loss, respectively (Table 3). Using weight changes by percentage cutoffs, the majority of PLWH had neutral weight change (3 316, 49.2%), followed by those with weight gain (2 390, 35.5%) and weight loss (1 031, 15.3%) (Table 4).
Univariable and multivariable logistic regressions were employed to determine the predictors of actual and differential weight gain observed at the 6-month clinic visit. On multivariable regression, the models were adjusted for age and gender (Tables 5 and 6). Study participants who were males (AOR: 1.35, 95% CI: 1.16–1.56), had tuberculosis (AOR: 2.17, 95% CI: 1.56–3.02), anaemia (AOR: 1.21, 95% CI: 1.04–1.40), CD4 counts ≤ 200 cells/μL (AOR: 1.34, 95% CI: 1.17–1.54), viral load ≥ 100,001 copies/ml (AOR: 1.21, 95% CI: 1.04–1.40), and WHO Clinical stages 3 and 4 (AOR: 1.33, 95% CI: 1.15–1.53) at presentation to the ART clinic were associated with actual weight gain at their 6-month follow-up clinic visit. Study participants with co-morbid hypertension (AOR: 0.81, 95% CI: 0.70–0.94) were less likely to have weight gain at their subsequent clinic visit when compared to their baseline weights (Table 5). In addition, PLWH commenced on Nevirapine-based regimen had comparatively higher weight gains than other drug regimens (Table 5).
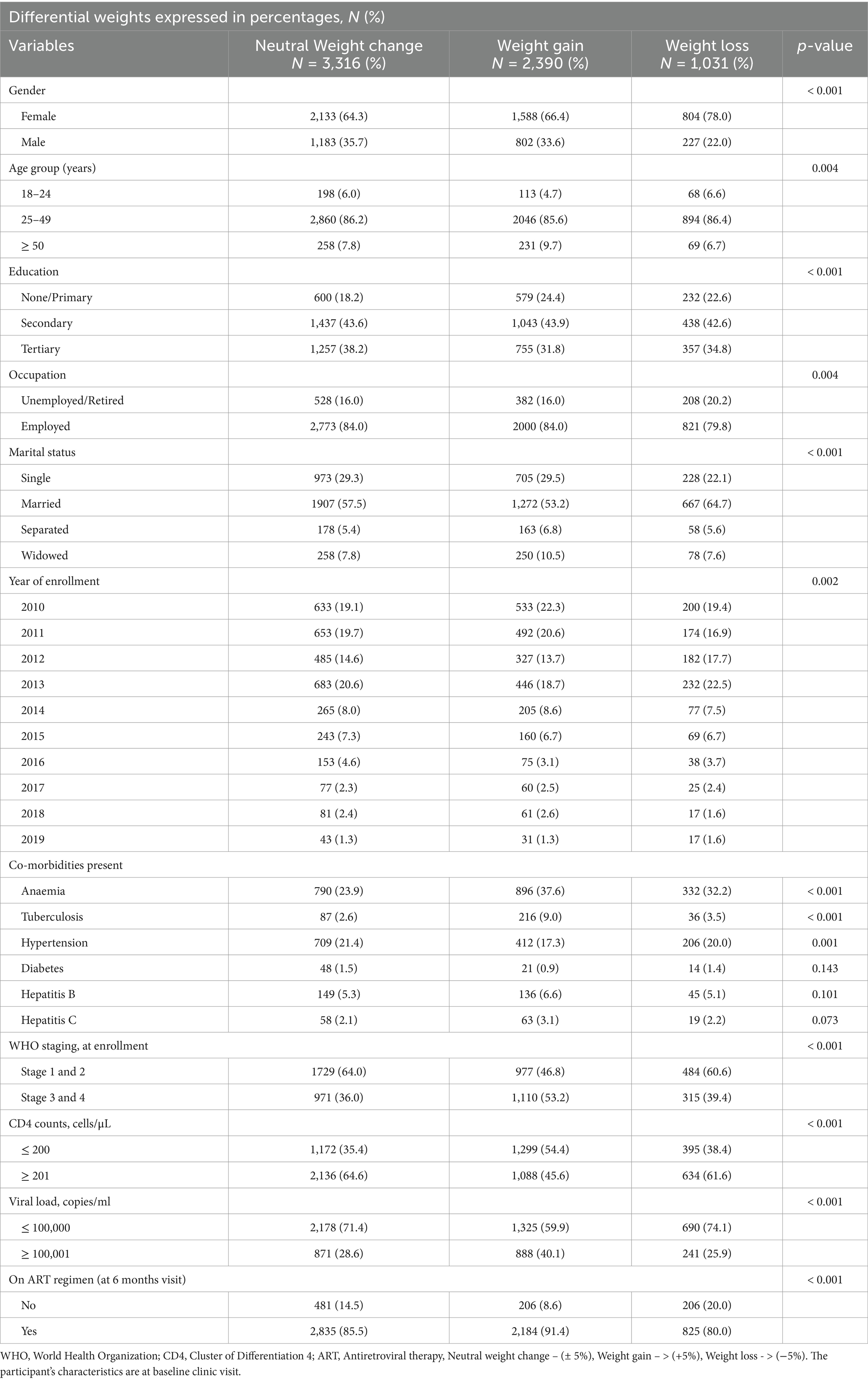
Table 4. Participants characteristics presented by cut point of 5% relative change in weight at 6 months.
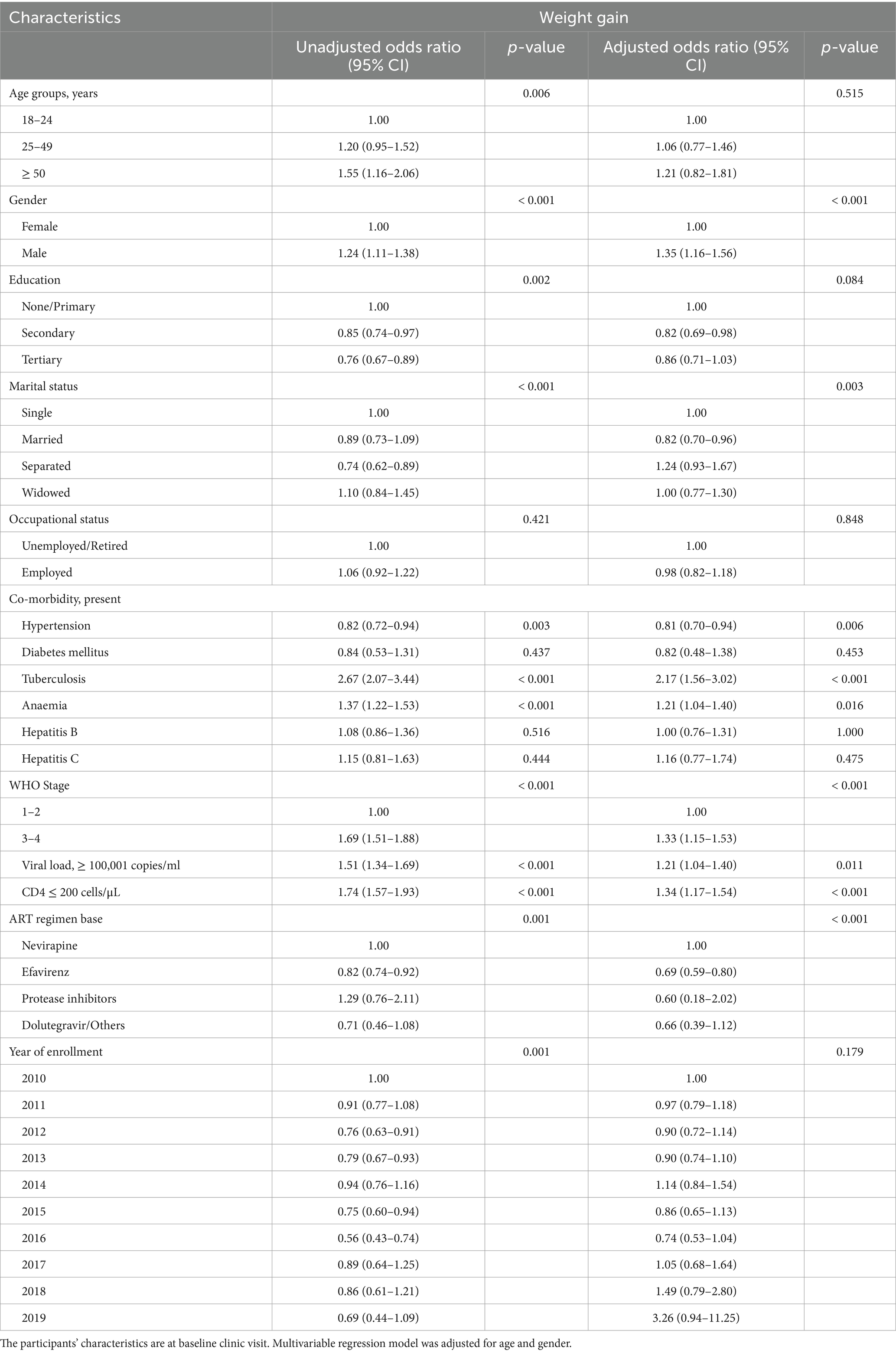
Table 5. Predictors of absolute weight gain (in kg) using univariable and multivariable logistic regression.
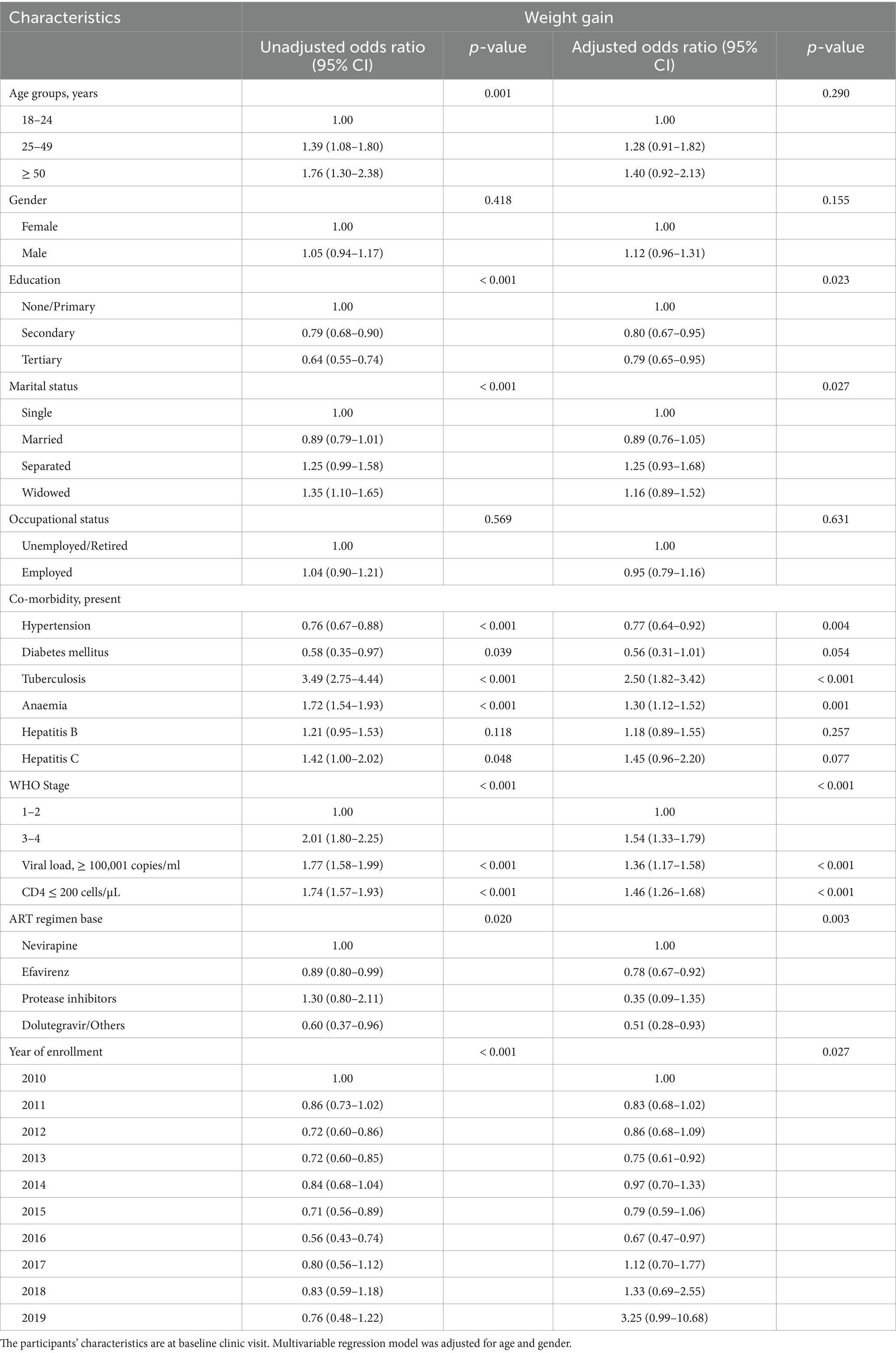
Table 6. Predictors of differential weight gain (in percentages) using univariable and multivariable logistic regression.
Furthermore, exploring the predictors of weight gain using weight differentials expressed in percentages revealed similar findings. Study participants with comorbid tuberculosis (AOR: 2.50, 95% CI: 1.82–3.42), anaemia (AOR: 1.30, 95% CI: 1.12–1.52), CD4 counts ≤ 200 cells/μL (AOR: 1.46, 95% CI: 1.26–1.68), viral load ≥ 100,001 copies/ml (AOR: 1.36, 95% CI: 1.17–1.58), and WHO Clinical stages 3 and 4 (AOR: 1.54, 95% CI: 1.33–1.79) at presentation to the ART clinic were more likely to have gained weight at their 6-month follow-up clinic visit. Conversely, study participants with comorbid hypertension (AOR: 0.77, 95% CI: 0.64–0.92), secondary (AOR: 0.80, 95% CI: 0.67–0.95) and tertiary (AOR: 0.79, 95% CI: 0.65–0.95) educational levels, and year of enrollment (2013, AOR: 0.75, 95% CI: 0.61–0.92; 2016, AOR: 0.67, 95% CI: 0.47–0.97) had less likelihood of weight gain (Table 6).
Discussion
Our study highlights weight changes observed within six months of care offered to.
PLWH at a large ART clinic in Lagos, Nigeria. Our findings reaffirm the impact of gender, anaemia, hypertension, tuberculosis, advanced HIV disease (WHO clinical stages 3 and 4, low CD4 counts) and high viral load on weight changes in the study population.
Although male gender was associated with weight gain, similar to findings in Ethiopia and other low-resource settings (30, 31), some studies reported greater weight gain following ART initiation in females (12, 32) while others found no difference by gender (33, 34). The weight difference observed could be attributed to different ART regimen compositions, prevailing socioeconomic conditions (such as poverty level, employment status, food security), dietary patterns, and body composition of PLWH before ART initiation (30, 31, 33). The patriarchal system, economic privileges, and access to nutritious food disproportionately favor males, potentially contributing to the disparity in weight gain observed. While gender was associated with weight gain, other health conditions like hypertension had the opposite effect.
Hypertension was associated with lower odds of weight gain in this study, which may be attributed to PLWH with co-morbid hypertension adhering to lifestyle advice to eat a healthy diet and exercise regularly (35, 36). Although the association of overweight/obesity with prevalent or incident hypertension among PLWH has been reported in several studies, there is a dearth of evidence on weight changes following hypertension diagnosis in this population (37, 38). However, our finding would require validation among the HIV cohort.
Study participants with Advanced HIV disease (CD4 counts ≤ 200 cells/μL, and WHO Clinical Stage 3 and 4) and high viral load (≥ 100,000 copies/ml) at baseline clinic visit had comparatively higher weight gain in concordance with other studies (15, 39, 40). Following ART initiation, the interruption of HIV adverse effects (sustained inflammation and accelerated catabolism) and resolution of opportunistic infections, especially in the gastrointestinal tract (GIT) is thought to be responsible for the return to health and resultant weight gain (41, 42). However, other studies have shown that PLWH without advanced HIV disease at ART initiation were also associated with weight gain (43, 44). This disparity may be due to the study population composition (age, gender, and BMI distribution) as well as burden of opportunistic infections and AIDS-defining conditions. Furthermore, other studies showed no association between viral load counts at baseline and weight changes following ART initiation (45, 46).
Anaemia at baseline clinic visit was associated with weight gain at the 6th-month clinic visit, similar to findings in the USA (20). However, this contrasts with other studies that have shown impaired weight gain among PLWH with anaemia at ART initiation (47, 48). Anaemia among PLWH is mainly caused by persistent chronic inflammation, opportunistic infections as well as HIV infection (26, 49). Furthermore, anaemia has been associated with advanced HIV disease, HIV disease progression, and mortality (23, 50).
Wasting syndrome, with weight loss being a key clinical feature, is associated with both HIV and tuberculosis infections (51, 52). In addition, plasma leptin levels responsible for appetite and food intake are reduced in the presence of tuberculosis infection (53). Following initiation of appropriate therapy (HIV and tuberculosis treatment), comparatively higher weight gains have been recorded among PLWH with co-morbid tuberculosis when compared to those without tuberculosis, similar to a study in Kenya (54).
The majority of our study participants received a non-nucleoside reverse transcriptase inhibitor (NNRTI) (either nevirapine or efavirenz) at ART initiation as the preferred first-line regimen. Weight gain observed amongst PLWH on efavirenz-based regimen lagged behind those initiated on nevirapine-based regimen. Weight gain attributed to dolutegravir-based regimen could not be fully demonstrated as its use was introduced only in 2019 at our ART clinic (i.e., several years after the commencement of this study). Due to the late introduction of dolutegravir based regimen, the effects of dolutegravir did not align with findings from other settings. Furthermore, Protease inhibitors (PI) based regimens are used as second-line treatment options in the clinic. Thus, a smaller proportion of the study population were on dolutegravir and PI based regimens. The use of dolutegravir based regimen had been reported to increase weight among ART-naïve PLWH significantly (55). Although dolutegravir use was limited in this study, future studies should elucidate its association with weight gain among PLWH in Nigeria. Nevertheless, this study aligns with weight gain among PLWH irrespective of the regimen initiated (20).
Weight gain following ART initiation has been shown to be a harbinger of good treatment outcome. However, there are potentials for the development of cardiometabolic disorders such as diabetes, obesity, hypertension and dyslipidaemia (13, 56). Thus, healthcare providers should monitor the long-term impact of ARVs and perceived increased cardiovascular disease risks. Appropriate management of diet, lifestyle modifications, and regular assessment of weight (and BMI) could mitigate non-AIDS adverse events following ART initiation.
Strengths and limitations
This study explored weight changes among ART-naïve PLWH initiating therapy over a ten-year period in Lagos, Nigeria. We believe our findings are generalisable with other HIV care settings despite being a single site study due to the large sample size and ease of access to the ART clinic. Study limitations include absence of data on dietary intake, physical activity, cotrimoxazole prophylaxis (duration and strength), and adherence to ARVs. In addition, the study did not account for co-morbid HIV/AIDS-related (infectious) opportunistic infections (aside from tuberculosis) and adverse events following ART initiation. Finally, the 6-month follow-up period may also limit the visualization of the long-term effects of ARVs on weight gain in PLWH.
Conclusion
This study explored weight changes and associated predictors among ART-naïve PLWH initiating therapy in Lagos, Nigeria. Weight gain was associated with male gender, advanced HIV disease (low CD4 counts and WHO Clinical stages 3 and 4), high viral load, anaemia, and comorbid tuberculosis at presentation to the ART clinic. Excess weight gain following ART initiation has emerged as a public health concern with particular emphasis on the associated clinical sequelae (nutritional and metabolic disorders) in the face of scarce resources in the health ecosphere within the country. Beyond favorable treatment outcomes (virological suppression, weight gain, absence of AIDS-defining illness), the monitoring of increased cardiometabolic disease risk should be incorporated into the HIV care continuum to abate the growing threat of NCDs in this cohort.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB), Nigerian Institute of Medical Research (IRB-21-066) and Health Research Committee (HREC), University of Cape Town, South Africa (HREC 176/2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
OO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NP: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. NO: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing, Visualization. BS: Conceptualization, Formal analysis, Project administration, Supervision, Validation, Visualization, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is funded by GSK under the EDCTP2 programme (TMA2017GSF-1962), supported by the European Union. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC, NIMR, or the funders.
Acknowledgments
The data unit has been invaluable in the retrieval of data. We also acknowledge the contributions of current and past staff of the department, whose efforts have contributed to the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1545676/full#supplementary-material
References
1. UNAIDS. Global HIV and AIDS statistics - fact sheet. (2024). Available online at: www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (Accessed March 3, 2025).
2. Harris, TG, Rabkin, M, and El-Sadr, WM. Achieving the fourth 90: Healthy aging for people living with HIV. AIDS (2018). 32:1563–1569. doi: 10.1097/QAD.0000000000001870
3. Patel, P, Rose, CE, Collins, PY, Nuche-Berenguer, B, Sahasrabuddhe, VV, Peprah, E, et al. Noncommunicable diseases among HIV-infected persons in low- income and middle-income countries: a systematic review and meta-analysis. AIDS. (2018) 32:S5–S20. doi: 10.1097/QAD.0000000000001888
4. Achwoka, D, Mutave, R, Oyugi, JO, and Achia, T. Tackling an emerging epidemic: the burden of non-communicable diseases among people living with hiv/aids in sub-Saharan Africa. Pan African Med J. (2020) 36:1–9. doi: 10.11604/pamj.2020.36.271.22810
5. d’Arminio Monforte, A, Bonnet, F, Bucher, HC, Pourcher, V, Pantazis, N, Pelchen-Matthews, A, et al. What do the changing patterns of comorbidity burden in people living with HIV mean for long-term management? Perspective from Eurpean HIV cohorts. HIV Med. (2020). 21:3–16. doi: 10.1111/hiv.12935
6. Chandiwana, NC, Siedner, MJ, Marconi, VC, Hill, A, Ali, MK, Batterham, RL, et al. Weight gain after HIV therapy initiation: pathophysiology and implications. J Clin Endocrinol Metab. (2024) 109:e478–87. doi: 10.1210/clinem/dgad411
7. Raggio, GA, Looby, SE, Robbins, GK, Park, ER, Sweek, EW, Safren, SA, et al. Psychosocial correlates of body image and lipodystrophy in women aging with HIV. J Assoc Nurses AIDS Care. (2020) 31:157–66. doi: 10.1097/JNC.0000000000000139
8. Olawepo, JO, Pharr, JR, Kabir, R, and Olutola, A. Health care providers’ perceptions about overweight and obesity among people living with human immunodeficiency virus in Nigeria. Qual Health Res. (2021) 31:2147–57. doi: 10.1177/10497323211023164
9. Romo, ML, Esber, AL, Owuoth, J, Maswai, J, Singoei, V, Iroezindu, M, et al. Impact of weight gain with dolutegravir on antiretroviral adherence and viral suppression in four African countries. HIV Med. (2023) 24:1066–74. doi: 10.1111/hiv.13501
10. Moyo-Chilufya, M, Maluleke, K, Kgarosi, K, Muyoyeta, M, Hongoro, C, and Musekiwa, A. The burden of non-communicable diseases among people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. EClinicalMedicine. (2023) 65:102255. doi: 10.1016/j.eclinm.2023.102255
11. Mavarani, L, Albayrak-Rena, S, Potthoff, A, Hower, M, Dolff, S, Sammet, S, et al. Changes in body mass index, weight, and waist-to-hip ratio over five years in HIV-positive individuals in the HIV Heart aging study compared to the general population. Infection. (2023) 51:1081–91. doi: 10.1007/s15010-023-02009-8
12. Sax, PE, Erlandson, KM, Lake, JE, McComsey, GA, Orkin, C, Esser, S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. (2020) 71:1379. doi: 10.1093/cid/ciz999
13. Patel, YS, and Malvestutto, CD. Beyond the numbers: weight gain risk factors, implications, and interventions among individuals with HIV. J AIDS HIV Treat. (2024) 6:1–10. doi: 10.33696/AIDS.6.047
14. Stires, H, Lamori, J, Chow, W, Zalewski, Z, Vidulich, A, Avina, M, et al. Weight gain and related comorbidities following antiretroviral initiation in the 2000s: a systematic literature review. AIDS Res Hum Retrovir. (2021) 37:834–41. doi: 10.1089/aid.2020.0216
15. Kanters, S, Renaud, F, Rangaraj, A, Zhang, K, Limbrick-Oldfield, E, Hughes, M, et al. Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy - a systematic literature review and network meta-analysis. EClinicalMedicine. (2022) 48:101412. doi: 10.1016/j.eclinm.2022.101412
16. Olawepo, JO, Pharr, JR, Cross, CL, Kachen, A, Olakunde, BO, and Sy, FS. Changes in body mass index among people living with HIV who are new on highly active antiretroviral therapy: a systematic review and meta-analysis. AIDS Care Psychol Socio-Medical Aspects AIDS/HIV. (2021) 33:326–36. doi: 10.1080/09540121.2020.1770181
17. Nduka, CU, Uthman, OA, Kimani, PK, and Stranges, S. Body fat changes in people living with HIV on antiretroviral therapy. AIDS Rev. (2016) 18:198–211.
18. Federal Ministry of Health Nigeria. Nigeria HIV/AIDS Indicator and impact survey 2018 Technical Report. (2019). Available online at: www.health.gov.ng (Accessed April 20, 2023).
19. Federal Ministry of Health (FMoH) Nigeria, National Tuberculosis L& BUCP, National AIDS & STIs control Programme. National Guidelines for the management of TB/HIV co-infection in Nigeria. Abuja; (2021). Available online at: www.ntblcp.org.ng/resources/the-2021-national-guidelines-for-the-management-of-tb-hiv/ (Accessed March 28, 2024).
20. Yuh, B, Tate, J, Butt, AA, Crothers, K, Freiberg, M, Leaf, D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. (2015) 60:1852–9. doi: 10.1093/cid/civ192
21. Sudfeld, CR, Isanaka, S, Mugusi, FM, Aboud, S, Wang, M, Chalamilla, GE, et al. Weight change at 1 mo of antiretroviral therapy and its association with subsequent mortality, morbidity, and CD4 T cell reconstitution in a Tanzanian HIV-infected adult cohort. Am J Clin Nutr. (2013) 97:1278–87. doi: 10.3945/ajcn.112.053728
22. Liu, E, Spiegelman, D, Semu, H, Hawkins, C, Chalamilla, G, Aveika, A, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. (2011) 204:282–90. doi: 10.1093/infdis/jir246
23. Weinberg, JL, and Kovarik, CL. The WHO clinical staging system for HIV/AIDS. Amer Med Assoc J Ethics. (2010) 12:202–6. doi: 10.1001/virtualmentor.2010.12.3.cprl1-1003
24. WHO. Obesity: preventing and managing the global epidemic. 894, WHO Technical Report Series. (2000). Available online at: https://apps.who.int/iris/handle/10665/42330 (Accessed March 20, 2023).
25. Unger, T, Borghi, C, Charchar, F, Khan, NA, Poulter, NR, Prabhakaran, D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
26. Brokering, KL, and Qaqish, RB. Management of Anemia of chronic disease in patients with the human immunodeficiency virus. Pharmacotherapy. (2003) 23:1475–85. doi: 10.1592/phco.23.14.1475.31949
27. Kwaghe, AV, Umeokonkwo, CD, and Aworh, MK. Evaluation of the national tuberculosis surveillance and response systems, 2018 to 2019: national tuberculosis, leprosy and buruli ulcer control programme, Abuja, Nigeria. Pan African Med J. 35:54. doi: 10.11604/pamj.2020.35.54.21493
28. Federal Ministry of Health Nigeria, National AIDS and STI control Programme. National Guidelines for HIV Prevention Treatment and Care (2016). Available at: www.prepwatch.org/wp-content/uploads/2017/08/nigeria_national_guidelines_2016.pdf (Accessed March 21, 2023).
29. National AIDS and STIs Control Programme, Federal Ministry of Health Nigeria. National Guidelines for HIV prevention, treatment and care. Abuja. (2020). Available online at: https://www.prepwatch.org/wp-content/uploads/2022/06/Nigeria-Guidelines-for-HIV-Prevention-Treatment-and-Care.pdf (Accessed September 21, 2023).
30. Huisin’t Veld, J, Balestre, E, Buyze, J, Menten, J, Jaquet, A, Cooper, DA, et al. Determinants of weight evolution among HIV-positive patients initiating antiretroviral treatment in low-resource settings. J Acquir Immune Defic Syndr. (2015) 70:146–54. doi: 10.1097/QAI.0000000000000691
31. Bantie, B, Gebeyehu, NA, Adella, GA, Kassie, GA, Mengstie, MA, Abebe, EC, et al. Trends of body mass index changes among adults on antiretroviral therapy in Northwest Ethiopia: a longitudinal data analysis. Sci Rep. (2024) 14:5265. doi: 10.1038/s41598-024-53701-0
32. Bares, SH, Smeaton, LM, Scott, SE, Smith, BA, Godfrey, C, and McComsey, GA. The association between weight gain, sex, and immune activation following the initiation of antiretroviral therapy. J Infect Dis. (2021) 224:1765–74. doi: 10.1093/infdis/jiab210
33. Pantazis, N, Papastamopoulos, V, Antoniadou, A, Adamis, G, Paparizos, V, Metallidis, S, et al. Changes in body mass index after initiation of antiretroviral treatment: differences by class of Core drug. Viruses. (2022) 14. doi: 10.3390/v14081677
34. Bansi-Matharu, L, Phillips, A, Oprea Phd, C, Grabmeier-Pfistershammer, K, Günthard, HF, De Wit, S, et al. Association between contemporary antiretrovirals and increase in body mass index: results from the prospective RESPOND cohort study. Lancet HIV. (2021) 8:e711–22. doi: 10.1016/S2352-3018(21)00163-6
35. Verma, N, Rastogi, S, Chia, YC, Siddique, S, Turana, Y, Cheng, H, et al. Non-pharmacological management of hypertension. J Clin Hypertens. (2021) 23:1275–83. doi: 10.1111/jch.14236
36. Blumenthal, JA, Hinderliter, AL, Smith, PJ, Mabe, S, Watkins, LL, Craighead, L, et al. Effects of lifestyle modification on patients with resistant hypertension: results of the TRIUMPH randomized clinical trial. Circulation. (2021) 144:1212–26. doi: 10.1161/CIRCULATIONAHA.121.055329
37. Aridegbe, M, Adeoye, I, and Oguntade, A. Obesity, hypertension, and dyslipidemia among human immunodeficiency virus patients in Abeokuta Ogun state, Nigeria. Nigerian J Cardiol. (2019) 16:83. doi: 10.4103/njc.njc_10_18
38. Commodore-Mensah, Y, Agyemang, C, Aboagye, JA, Echouffo-Tcheugui, JB, Beune, E, Smeeth, L, et al. Obesity and cardiovascular disease risk among Africans residing in Europe and Africa: the RODAM study. Obes Res Clin Pract. (2020) 14:151–7. doi: 10.1016/j.orcp.2020.01.007
39. Bares, SH, Smeaton, LM, Xu, A, Godfrey, C, and McComsey, GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Women’s Health. (2018) 27:1162–9. doi: 10.1089/jwh.2017.6717
40. Grabar, S, Potard, V, Piroth, L, Abgrall, S, Bernard, L, Allavena, C, et al. Striking differences in weight gain after cART initiation depending on early or advanced presentation: results from the ANRS CO4 FHDH cohort. J Antimicrob Chemother. (2023) 78:757–68. doi: 10.1093/jac/dkad007
41. Bailin, SS, Gabriel, CL, Wanjalla, CN, and Koethe, JR. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep. (2020) 17:138–50. doi: 10.1007/s11904-020-00483-5
42. Buzón-Martín, L. Weight gain in HIV-infected individuals using distinct antiretroviral drugs. AIDS Rev. (2020) 22:158–67. doi: 10.24875/AIDSRev.M20000036
43. Weldesenbet, AB, Ayele, TA, Sisay, MM, Tusa, BS, and Kebede, SA. Predictors of change in weight among people living with hiv on antiretroviral treatment in west hararghe zone, Ethiopia: a retrospective longitudinal study. HIV/AIDS Research Palliative Care. (2020) 12:373–80. doi: 10.2147/HIV.S262663
44. Herrin, M, Tate, JP, Akgün, KM, Butt, AA, Crothers, K, Freiberg, MS, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquired Immune Deficiency Syndromes Lippincott Williams and Wilkins. (2016) 73:228–36. doi: 10.1097/QAI.0000000000001071
45. Lakey, W, Yang, LY, Yancy, W, Chow, SC, and Hicks, C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retrovir. (2013) 29:435–40. doi: 10.1089/aid.2012.0234
46. Tang, AM, Sheehan, HB, Jordan, MR, Van, DD, Terrin, N, Dong, K, et al. Predictors of weight change in male HIV-positive injection drug users initiating antiretroviral therapy in Hanoi. Vietnam AIDS Res Treat. (2011) 2011:1–8. doi: 10.1155/2011/890308
47. Ezeamama, AE, Guwatudde, D, Sikorskii, A, Kabagambe, EK, Spelts, R, Vahey, G, et al. Impaired hematologic status in relation to clinical outcomes among HIV-infected adults from Uganda: a prospective cohort study. Nutrients. (2018) 10:475. doi: 10.3390/nu10040475
48. Brentlinger, PE, Silva, WP, Vermund, SH, Valverde, E, Buene, M, and Moon, TD. Practical management of HIV-associated Anemia in resource-limited settings: prospective observational evaluation of a new Mozambican guideline. AIDS Res Hum Retrovir. (2016) 32:12–25. doi: 10.1089/aid.2015.0030
49. Curkendall, SM, Richardson, JT, Emons, MF, Fisher, AE, and Everhard, F. Incidence of anaemia among HIV - infected patients treated with highly active antiretroviral therapy. HIV Med. (2007) 8:483–90. doi: 10.1111/j.1468-1293.2007.00500.x
50. Jin, M, Yang, Z, Li, J, Liu, X, and Wu, Z. Factors influencing survival status of HIV/AIDS after HAART in Huzhou City, eastern China. Can J Infect Dis Med Microbiol. (2022) 2022:1–7. doi: 10.1155/2022/2787731
51. Federal Ministry of Health Nigeria. The National Strategic Plan for tuberculosis and leprosy control (2010-2015). (2011). Available online at: https://www.hfgproject.org/wp-content/uploads/2015/02/Nigeria-National-Strategic-Plan-for-Tuberculosis-and-Leprosy-Control_2010-2015.pdf (Accessed March 20, 2023).
52. Mhiri, C, Bklec, L, Di Costanzo, B, Georges, A, and Gherardil, R. The slim disease in African patients with AIDS. Transact. Royal Society Tropical Med Hygiene. (1992) 86:303–6. doi: 10.1016/0035-9203(92)90323-5
53. Ye, M, and Bian, LF. Association of serum leptin levels and pulmonary tuberculosis: a meta-analysis. J Thorac Dis. (2018) 10:1027–36. doi: 10.21037/jtd.2018.01.70
54. Bourgi, K, Ofner, S, Musick, B, Griffith, B, Diero, L, Wools-Kaloustian, K, et al. Weight gain among treatment-naïve persons with HIV receiving Dolutegravir in Kenya. J Acquir Immune Defic Syndr. (1988) 91:490–6. doi: 10.1097/QAI.0000000000003087
55. Esber, AL, Chang, D, Iroezindu, M, Bahemana, E, Kibuuka, H, Owuoth, J, et al. Weight gain during the dolutegravir transition in the African cohort study. J Int AIDS Soc. (2022) 25:e25899. doi: 10.1002/jia2.25899
56. McComsey, GA, Emond, B, Shah, A, Bookhart, BK, Rossi, C, Milbers, K, et al. Association between weight gain and the incidence of Cardiometabolic conditions among people living with HIV-1 at high risk of weight gain initiated on antiretroviral therapy. Infect Dis Ther. (2022) 11:1883–99. doi: 10.1007/s40121-022-00673-1
Keywords: weight changes, people living with HIV, antiretroviral therapy, naïve, Lagos, Nigeria
Citation: Odubela OO, Peer N, Odunukwe NN, Musa AZ, Salako BL and Kengne AP (2025) Weight changes among antiretroviral therapy-naïve people living with human immunodeficiency virus in Lagos, Nigeria. Front. Public Health. 13:1545676. doi: 10.3389/fpubh.2025.1545676
Edited by:
Satish Rojekar, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Ibe Michael Usman, Kampala International University Western Campus, UgandaDereje Tsegaye, Mattu University, Ethiopia
Deepika Godugu, St John’s University, United States
Kinjal Jayeshkumar Parikh, Cipla, India
Copyright © 2025 Odubela, Peer, Odunukwe, Musa, Salako and Kengne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oluwatosin Olaseni Odubela, b2R1YnN0b3NpbjA4QGdtYWlsLmNvbQ==
 Oluwatosin Olaseni Odubela
Oluwatosin Olaseni Odubela Nasheeta Peer
Nasheeta Peer Nkiruka Nnonyelum Odunukwe2
Nkiruka Nnonyelum Odunukwe2 Andre Pascal Kengne
Andre Pascal Kengne