Abstract
Introduction:
Preventive measures have been implemented in hospitals during COVID-19, but how these guidelines affected mental health among healthcare workers (HCWs) remains to be determined. On another note, reliable psychological and blood-based markers are needed to promptly identify HCWs at-risk to develop distress. Extracellular vesicles (EVs) originating from brain cross the blood–brain barrier and are detectable in blood, giving them a highly valuable potential for biomarker discovery. In HCWs with or without psychological distress, we investigated how perceived stress during COVID-19 impacted mental health. We then longitudinally evaluated the inflammatory cargo from neuron-, astrocyte-, and microglial-derived EVs that may be associated with psychological distress.
Methods:
Our prospective study that included an initial visit (02/2021–08/2021), and two follow-up visits 3 and 6 months later (last visit; 03/2022). HCWs (n = 15) completed questionnaires for perception of risk, COVID-19-specific posttraumatic symptomatology, psychological distress and burnout, as well as sleep quality. Blood was collected at each visit to characterizing inflammation from brain-derived EVs. Multiple regressions were conducted for all psychological/biological parameters based on the HCWs’ final score for psychological distress.
Results:
Onset of psychological distress was associated early hyperarousal. Moreover, severe distress was associated with increased astrocyte-specific levels of anti-inflammatory interleukin-10 and pro-inflammatory interferon-ɣ.
Discussion:
Our findings—that need to be replicated in larger studies—suggest that early hyperarousal may be predictive of later onset of psychological distress in HCWs. They also unravel a novel area of biomarker discovery study in psychiatry as inflammation from brain-derived EVs could help targeting “at-risk” individuals.
1 Introduction
Professional distress and burnout cost more than $20 billion to the Canadian government, and more than 30% of physicians reporting exhaustion and reduced performance at work (1).
The recent COVID-19 pandemic constituted an unprecedented situation forcing the World Health Organization to declare an international public health emergency in March 2020. In Canada, more than 4,950,000 reported infections and 68,000 deaths have been reported as of September 2024. During prior infectious outbreaks such as the severe acute respiratory syndrome (SARS), 2009 H1N1 influenza flu, and Middle East respiratory syndrome, healthcare workers (HCWs) have faced “a high risk of infection and inadequate protection from contamination, overwork, frustration, discrimination, isolation, patients with negative emotions, a lack of contact with their families, and exhaustion” (2). Meta-analyses, published during the early stages of the COVID-19 pandemic, highlighted the deleterious mental health outcomes during the COVID-19 pandemic, with a higher prevalence in HCWs vs. the general population (3, 4). Persistent fear (i.e., fear of being infected or infecting a close relative) shaped their capacity to deliver health care and were associated with poor mental health (5). Public authorities provided hospitals with guidelines (i.e., training, protective equipment) to prevent infection among their employees, but how implemented preventive measures affect fear toward risk for infection (perception and/or protection) and mental health outcomes in HCWs remain to be determined (6). While accessibility to vaccination decreased rates for posttraumatic stress disorder (PTSD), new virus variants were associated with rising incidence of PTSD (3).
However, not only the number of cases of the recent COVID-19 pandemic is far greater than the 2002–2003 SARS epidemic, but its basic reproduction number (R0) is significantly higher (7). Chinese studies found that early on 70% of frontline HCWs showed signs of distress, and 30% suffer from insomnia due to the COVID-19 outbreak (8, 9). Altogether, these factors highlight the unprecedented pressure that healthcare professionals have experienced and the need to reduce and prevent such a burden. Importantly, there is an urgent need to find psychological and accessible (i.e., blood-based) physiological markers to identify and promptly take charge of those HCWs that are at risk for prolonged disability due to distress, burnout or insomnia resulting from their work during a global health emergency crisis.
Numerous clinical studies support a role for inflammation as a pathophysiological mechanism underlying stress-related and sleep disorders (10). Professional burnout and persistent insomnia have been associated with higher circulating levels of tumor necrosis factor-α (TNF-α) and other pro-inflammatory factors in workers (11–13). Still, few data are available on the bidirectional relationship between inflammation, professional distress, and insomnia in HCWs, especially in the context of a global health crisis. Importantly, a limitation of the focus on whole plasma levels of inflammatory markers in biomarker discovery studies is that they do not reflect neurological changes, as their tissue and cellular origins cannot be precisely determined.
Extracellular vesicles (EVs) are secreted membrane vesicles (40–100 nm in diameter) produced by most cell types, including central nervous system (CNS) cells such as neurons, astrocytes, and microglia (14). Separating these EVs based on their cellular origin may be a major challenge. As EVs can cross the blood–brain barrier, astrocyte- (ADEs), neuron- (NDEs), and microglia-derived (MDEs) EVs can be readily detected in plasma by magnetic immunocapture with antibodies for markers specific to each brain cell type. Indeed, glutamate aspartate transporter (GLAST), L1 cell adhesion molecule (L1CAM) and transmembrane protein 119 (TMEM119) markers show specificity for astrocytes (15), neuronal (16), and microglial (17) cells, respectively. Importantly, EVs are cargo that can store and release inflammatory cytokines far from their origin (18). CNS biomarkers for brain diseases usually require invasive cerebrospinal fluid sampling as plasma levels may not be related to CNS-specific origins. As they are measurable in plasma, brain-derived EVs may be an incredible tool to develop accessible blood-based biomarkers of psychiatric disorders (19–21). Emerging evidence strengthens the reactive response of brain-derived exosomes in distinct mental health context (e.g., psychosis or major depressive disorder (MDD)), and supports their contribution to psychopathologies in proof-of-concept studies, in which brain-derived exosomes from mentally ill patients induces psychiatric-like behaviors in rodents (22–24). With inflammation known as a core mechanism in mental pathophysiologies (10), enriching EVs from specific brain cell types and studying their inflammatory content would allow us to define a specific neuroimmune biosignature associated with psychological distress-related symptomatology.
During the COVID-19 global pandemic, the HCWs faced a multifactorial context that is distinct from their usual daily lives (i.e., newly triggering and traumatizing event interacting with posttraumatic symptoms, and implemented preventive measures). In a longitudinal prospective study on HCWs from Centre Hospitalier Universitaire (CHU) de Québec healthcare facility, we aimed to: (1) determine whether individual perceptions of the implemented preventive measures in hospitals affected the psychological distress among HCWs; (2) establish the predictive relationship between posttraumatic symptoms, burnout, sleep and psychological distress; and (3) identify brain-derived inflammatory markers that are measurable in blood and associated with psychological distress in a context of the global health crisis.
2 Materials and methods
2.1 Recruitment
Male or female participants (n = 18; 18–59 yrs. old) were initially recruited while working full-time as a CHU de Québec healthcare employee (doctor, nurses, physiotherapist and respiratory therapist) with direct contact with patients during the COVID-19 crisis. Exclusion criteria included: (1) past or current positive test for COVID 19, as length of the neuroinflammatory effect of past COVID-19 infection is still not known (25); (2) current nicotine or cannabis use; (3) diabetes diagnosis; or (4) depression or burnout diagnosis within the past 6 months. Each participant was met 3 times: at the initial visit (02/2021–08/2021), as well as 3 and 6 months later (last visit in 03/2022). The 3- and 6-month time points have been selected to assess the healthy, adaptive response and maladaptive, pathological consequences, respectively (26). During the first visit, all participants provided written informed consent, and demographics information were collected via a self-report survey. At each visit, all six French version questionnaires (see section 2.2.) were self-completed (27–30). Blood was drawn into EDTA-treated tubes and centrifuged to collect plasma (1,500 rpm for 10 min; storage at −80°C until use). Blood drawn have always been performed between 8h00AM and 11h30AM. Fifteen participants (n = 15; 1 man and 14 women) completed all three visits. The protocol was approved by the institutional review board of the CHU de Québec (#2021–5,394).
2.2 Questionnaires
2.2.1 Kessler psychological distress scale (K10)
The K10 survey includes 10 questions (scored from 1-none of the time to 5-all the time) to evaluate risk for psychological distress by assessing anxiety and depression symptoms experienced during the 4 preceding weeks. The psychometric properties of the K10 have been extensively examined in several civilian and occupational populations, and show very good reliability (Cronbach’s α = 0.88–0.93) (31). At the third visit, a total score of 25 (ranging from 10 to 50) was used as a cutoff score to divide the participants into “no/low psychological distress” or “moderate/severe psychological distress” group (32). This categorization was used to retrospectively compare other psychological and biological parameters (Figure 1).
Figure 1
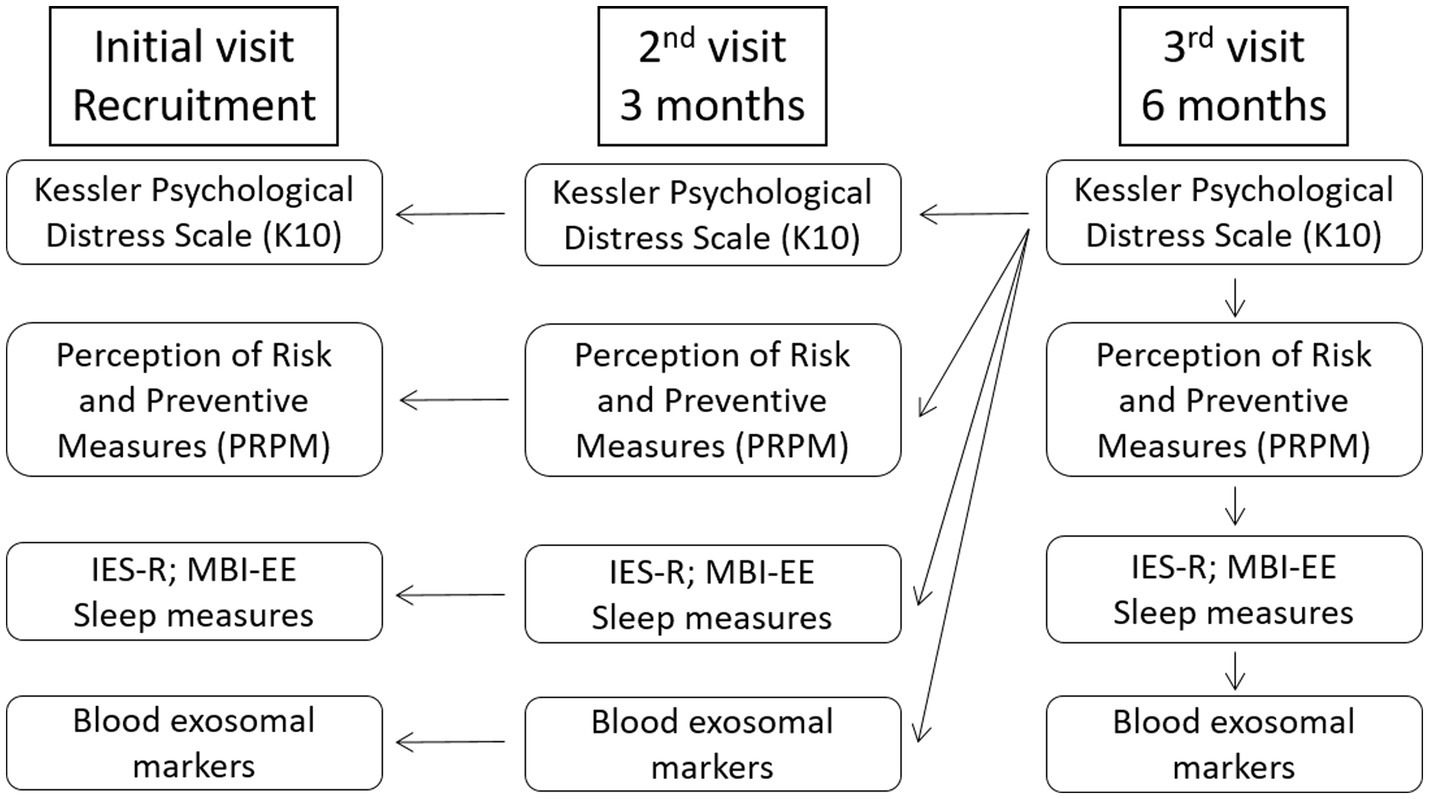
Experimental design. At the final visit, a total K10 (Kessler Psychological Distress Scale) score of 25 was used as a cutoff score to divide the participants into “no/low psychological distress” or “moderate/severe psychological distress” group. This categorization was then used as an independent variable for all other psychological and biological parameters. Insomia severity and sleep quality were assessed using the Insomnia Severity Index (ISI) and Pittsburgh Sleep Quality Index (PSQI) questionnaires, respectively. IES-R, Impact of Event Scale-Revised; MBI-EE, Maslach Burnout Inventory-Emotional Exhaustion.
2.2.2 Impact of event scale revised (IES-R)
The IES-R questionnaire includes 22 questions divided into 3 subscales of symptoms: intrusion, avoidance/numbing, and hyperarousal. The IES-R used in our study has good internal consistency (α = 0.81–0.93 for its 3 subscales and total score) and reliability (correlation coefficients = 0.71–0.76 for its 3 subscales and total score) (27). Participants scored each question from 0 (not at all) to 4 (extremely) based on their COVID-19-specific experience within the 7 preceding days. The sum of all 3 scores (0–88 range) was used for statistics (27).
2.2.3 Perception of risk and preventive measures (PRPM)
The PRPM questionnaire developed by Maunder and colleagues (2006) includes 18 items (scored from 1-strongly disagree to 5-strongly agree) assessing 3 distinct constructs that have satisfactory internal consistency (Cronbach’s α = 0.76–0.89): (1) adequacy of training, protection and support (i.e., training for control procedures, protective equipment, and emotional support); (2) job stress (i.e., conflict between colleagues, perceived stress, and increased workload/overtime); and (3) perception of stigma and interpersonal avoidance (i.e., coping strategies in regards to avoidance from close friends and family fearing to be contaminated). The average of all 3 scores was used for statistics (33).
2.2.4 Malash burnout inventory-emotional exhaustion scale (MBI-EE)
Risk for burnout was determined using the EE subscale of the MBI questionnaire shows satisfactory internal consistency (Cronbach’s α = 0.76–0.83) while used in occupational contexts (34). The survey includes 9 items for which participant responds with a score of 0 (never) to 6 (everyday). The sum (0 to 54) was used for statistics, with a total score of ≥ 18 considered as a risk of burnout (34).
2.2.5 Measures of sleep quality
Insomnia symptoms during the preceding month were evaluated with the 7-item Insomnia Severity Index (ISI) questionnaire, which has high internal consistency (Cronbach’s α = 0.88) (35). A total score (ranging from 0 to 28) of ≥ 15 considered as clinical insomnia (36). The 7-component Pittsburgh Sleep Quality Index (PSQI) is the gold standard self-administered measure of sleep, with high internal consistency (α > 0.80) across all types of populations (37). Score of each component (i.e., subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction) is weighted equally on a 0–3 scale. The sum of all 7 components score is used as a global PSQI score (0–21 range), for which higher score reflect poorer sleep quality (37).
2.3 Enrichment of astrocyte-(ADEs), microglia-(MDEs), and neuron-derived EVs (NDEs) and measurement of inflammatory content
Plasma was first treated with thrombin, then centrifuged for eliminating fibrin. Total EVs were precipitated from plasma samples using the ExoQuick Precipitation solution (System Biosciences, Inc., United States) and centrifugation (1,500 g; 1 h at 4°C) (38). With the Exo-Flow Capture kit (System Biosciences, Inc.), total EVs were incubated with magnetic-streptavidin beads coupled with biotinylated-specific antibodies: mouse anti-human biotinylated GLAST (for ADEs; Miltenyi Biotec, Inc., United States), and L1CAM (for NDEs; Invitrogen, CA) or purified anti-TMEM119 antibody (for MDEs; Biolegend, United States) that was previously biotinylated with FluoReporter™ MinioBiotin-XX labeling kit (Invitrogen, CA). Eluted GLAST-positive (ADEs), TMEM-119-positive (MDEs) and L1CAM-positive (NDEs) proteins were then quantified for several inflammatory markers (see section 2.4). Data were normalized with total protein concentrations in ADEs, MDEs, or NDEs samples as measured with the Bradford assay. Briefly, total protein concentrations were calculated as follows: [protein quantity in brain-derived EVs] = [protein quantity in total EVs] - [protein quantity in supernatant after antibody incubation]. Control quality of the enrichment of brain-derived EVs was validated via flow cytometry (Supplementary Figure 1). Levels of interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-6 (IL-6), TNF-α, monocyte chemoattractant protein-1 (MCP-1), interleukin-10 (IL-10), interleukin-13 (IL-13) and interleukin-1 receptor antagonist (IL-1ra) were determined in eluted ADEs, NDEs, and MDEs using multiplex V-PLEX® immunoassays (Meso Scale Discovery, United States), according to the manufacturer’s instructions.
2.4 Statistical analyses
For demographic parameters, tables of contingency, Fisher’s exact test (sex and profession) or Kolmogorov–Smirnov test (age) were used with GraphPad Software v9. ROUT outlier tests (Q = 0.5%) did not detect any outlier. Gaussian distribution was evaluated with Shapiro–Wilk test. All other statistical analyses were performed using the SPSS (IBM Corporation, United States). Multiple linear regressions assessed the association between the final K10 score (3rd visit) and all biological (i.e., inflammatory cargo from brain-derived EVs) and psychological parameters. The time point (i.e., visit) was included as a within-subject factor, and the final K10 score was between-subject factors. The K10 score at recruitment (1st visit) was included as a covariate in the regression model. A p value < 0.05 was considered as statistically significant. Considering α = 0.05 and a desired statistical power 80%, we were able to detect effect sizes (η2) of 0.29–0.79 for both psychological and biological variables, which are considered as large effect sizes. The design for our statistical analyses has been revised by the biostatistical services available at our research center.
3 Results
3.1 Demographic analyses
Fifteen (14 females, 93.3%; 1 male, 6.7%) of the 18 recruited participants completed all 3 visits: at the recruitment visit (February to August 2021), as well as 3 and 6 months later (last visit to March 2022). 86.7% of the participants were occupying a nursing position, whereas 13.3% of participants held another appointment in which they were providing healthcare services to patients (e.g., physical therapist). After classification based on the K10 score at the final visit, 10 participants (66.7%) had no or low psychological distress, while five individuals (33.3%) had moderate or severe distress. Table 1 shows demographic info for no/low and moderate/severe distress groups. HCWs with moderate/severe psychological risk tended to be older (45.0 ± 12.3 yrs) vs. those with lower levels of distress (36.1 ± 8.1 yrs; p = 0.051).
Table 1
| Demographic factors | No/low psychological distress | Moderate/severe psychological distress | p |
|---|---|---|---|
| n | 10 (66.7%) | 5 (33.3%) | |
| Sex | 0.333 | ||
| Male | 0 (0.0%) | 1 (20.0%) | |
| Female | 10 (100.0%) | 4 (80.0%) | |
| Profession | 0.524 | ||
| Nurse | 8 (80.0%) | 5 (100.0%) | |
| Other | 2 (20.0%) | 0 (0.0%) | |
| Age (mean ± SD) | 36.1 ± 8.1 | 45.0 ± 12.3 | 0.051 |
Demographic info from healthcare workers at CHU de Québec.
Fifteen of the eighteen recruited participants completed the study. Participants were assigned to the no/low or moderate/severe psychological distress group based on their K10 score during the last (3rd) visit. Fisher’s exact test (sex and profession) or Kolmogorov–Smirnov test (age) was performed for statistical analyses. Bold values represent statistically different values.
3.2 Psychological distress in HCWs was not associated with differences in individual perceptions of the implemented preventive measures in hospitals
We first evaluated the perceived stress in the work environment using the PRPM questionnaire’s constructs: (1) adequacy of training, protection, and support received from the healthcare facility; (2) job stress; and (3) perceived stigma and interpersonal avoidance. PRPM scores were compared across all 3 visits based on their psychological distress at the final (3rd) visit. All constructs did not change throughout the study (i.e., no time effect or interaction with K10 score), and were not associated with psychological distress (Supplementary Table 1).
3.3 Early hyperarousal symptoms, not burnout or sleep quality, were associated with the development of psychological distress in HCWs
3.3.1 Posttraumautic symptomatology
With the IES-R questionnaire, we assessed whether COVID-19-specific posttraumatic symptoms are associated with and/or predict psychological distress in HCWs. No association was observed between intrusion or avoidance symptoms and the category of psychological distress (Table 2). Regression analyses revealed an overall effect of final psychological distress status on hyperarousal symptoms facing the COVID-19 pandemic (F1,2 = 7.82; p < 0.05; no time × K10 interaction; Table 2). Post-hoc tests confirmed that HCWs with moderate/severe psychological distress displayed higher levels of hyperarousal symptoms at the 2nd (p < 0.01) and 3rd (p < 0.05) visits vs. their colleagues with no/low distress (Table 2).
Table 2
| Posttraumatic symptoms | Visits | No/low distress | Moderate or severe distress | K10 3rd visit | Visit | Visit * K10 3rd visit |
|---|---|---|---|---|---|---|
| Intrusion | 1 | 8.480 ± 1.229 | 11.640 ± 1.768 | F1,2 = 3.086 p = 0.104 η2 = 0.205 |
F1,2 = 0.375 p = 0.691 η2 = 0.030 |
F1,2 = 1.049 p = 0.366 η2 = 0.080 |
| 2 | 5.989 ± 1.553 | 11.023 ± 2.235 | ||||
| 3 | 5.715 ± 0.977 | 7.770 ± 1.406 | ||||
| Avoidance | 1 | 6.744 ± 1.512 | 9.312 ± 2.176 | F1,2 = 2.295 p = 0.156 η2 = 0.161 |
F1,2 = 0.116 p = 0.891 η2 = 0.01 |
F1,2 = 0.310 p = 0.736 η2 = 0.025 |
| 2 | 3.360 ± 1.364 | 7.280 ± 1.963 | ||||
| 3 | 4.245 ± 1.004 | 6.310 ± 1.445 | ||||
| Hyperarousal | 1 | 4.064 ± 1.057 | 6.671 ± 1.521 |
F
1,2
= 7.815
p = 0.016* η 2 = 0.394 |
F1,2 = 0.040 p = 0.961 η2 = 0.003 |
F1,2 = 0.606 p = 0.554 η2 = 0.048 |
| 2 | 1.938 ± 0.743 | 6.125 ± 1.070 ‡‡ | ||||
| 3 | 2.569 ± 0.628 | 5.063 ± 0.904 ‡ | ||||
| Total score | 1 | 19.288 ± 3.062 | 27.624 ± 4.407 | F1,2 = 3.807 p = 0.075 η2 = 0.241 |
F1,2 = 0.088 p = 0.916 η2 = 0.007 |
F1,2 = 0.211 p = 0.811 η2 = 0.017 |
| 2 | 10.999 ± 3.382 | 21.002 ± 4.867 | ||||
| 3 | 12.529 ± 2.152 | 19.143 ± 3.097 |
Evaluation of COVID pandemic-specific posttraumatic symptoms in healthcare workers with no/low or moderate/severe psychological distress.
Intrusion, avoidance, hyperarousal, and overall symptoms were evaluated using the Impact of Event Scale-Revised (IES-R) questionnaire. Participants were divided into no/low vs. moderate/severe psychological group based on the K10 score at the final (K10 3rd visit). Data are presented as the marginalized mean ± SD. *p ≤ 0.05 for main regression effect; ‡p ≤ 0.05 and ‡‡p ≤ 0.01 following post-hoc comparisons with the corresponding (i.e., same visit) no/low group. Bold values represent statistically different values.
3.3.2 Risk of burnout and insomnia
The final K10 score did not affect the risk for burnout (MBI-EE) or ISI-reported insomnia severity in HCWs (Supplementary Table 2), as the score remained below the cutoff score for clinical insomnia. In regard to the self-reported PSQI questionnaire, no significant difference was found on sleep quality, latency, duration and efficiency, as well as on use of sleep medication and daytime dysfunction across all three visits between both distress groups (Supplementary Table 2). An interaction between time and the final K10 score was also observed (F1,2 = 4.956, p < 0.05) on sleep disturbances. HCWs with no/low distress showing lower sleep disturbance at the 2nd and final visit (p < 0.05 vs. the average levels at the first visit; Supplementary Table 2).
3.4 High levels of IFN-γ and IL-10 from astrocyte-derived EVs were predictive of late moderate to severe psychological distress
Astrocyte-, microglia- and neuron-derived EVs (Table 3; Supplementary Table 3) were isolated from plasma to characterize the inflammatory cargo from distinct CNS-specific sources. In astrocyte-derived EVs, main effects of the final K10 category were found on IFN-γ (F1,2 = 5.61, p < 0.05), IL-10 (F1,2 = 48.90, p < 0.001), and IL-6 (F1,2 = 5.91, p < 0.05). Astrocyte-derived EVs from HCWs with moderate/severe psychological distress at the final visit showed high levels of IFN-γ at the 2nd visit (p < 0.01) and IL-10 at each visit (p < 0.01 or p < 0.05) as compared to HCWs with no/low distress. A tendency for higher astrocyte-levels of IL-6 was also observed in the moderate/severe group vs. the no/low group at the 2nd visit (p = 0.066; Table 3). A time × K10 interaction was also observed (F1,2 = 5.15, p < 0.05) on TNF-α levels from astrocyte-derived exosomes, with HCWs with moderate/severe distress showing higher levels of TNF-α from astrocyte-derived exosomes at the 2nd visit (p < 0.05 vs. the average levels at the final visit; Table 3).
Table 3
| Immune cargo | Visits | No/low distress | Moderate or Severe distress | K10 3rd visit | Visit | Visit * K10 3rd visit |
|---|---|---|---|---|---|---|
| Astrocyte-derived exosomes | ||||||
| IFN-γ | 1 | 0.051 ± 0.026 | 0.052 ± 0.037 |
F
1,2
= 5.612
p = 0.035* η 2 = 0.319 |
F1,2 = 0.443 p = 0.647 η2 = 0.036 |
F1,2 = 2.684 p = 0.089 η2 = 0.183 |
| 2 | 0.035 ± 0.025 | 0.188 ± 0.037 ‡‡ | ||||
| 3 | 0.027 ± 0.029 | 0.038 ± 0.042 | ||||
| IL-10 | 1 | 0.002 ± 0.002 | 0.016 ± 0.003 ‡‡ |
F
1,2
= 44.899
p = 0.001*** η 2 = 0.789 |
F1,2 = 1.648 p = 0.214 η2 = 0.121 |
F1,2 = 0.307 p = 0.738 η2 = 0.025 |
| 2 | 0.009 ± 0.003 | 0.029 ± 0.004 ‡‡ | ||||
| 3 | 0.002 ± 0.003 | 0.016 ± 0.004 ‡ | ||||
| IL-6 | 1 | 0.022 ± 0.009 | 0.030 ± 0.013 |
F
1,2
= 5.912
p = 0.032* η 2 = 0.330 |
F1,2 = 0.212 p = 0.810 η2 = 0.017 |
F1,2 = 0.808 p = 0.457 η2 = 0.063 |
| 2 | 0.011 ± 0.011 | 0.051 ± 0.016 | ||||
| 3 | 0.010 ± 0.009 | 0.024 ± 0.013 | ||||
| TNF-α | 1 | 0.016 ± 0.009 | 0.020 ± 0.013 | F1,2 = 2.408 p = 0.147 η2 = 0.167 |
F1,2 = 0.673 p = 0.519 η2 = 0.053 |
F
1,2
= 5.155
p = 0.014* η 2 = 0.300 |
| 2 | 0.015 ± 0.007 | 0.069 ± 0.010 ω | ||||
| 3 | 0.027 ± 0.011 | 0.006 ± 0.015 | ||||
| Neuron-derived exosomes | ||||||
| MCP-1 | 1 | 0.016 ± 0.011 | 0.051 ± 0.016 |
F
1,2
= 8.150
p = 0.014* η 2 = 0.404 |
F1,2 = 0.794 p = 0.464 η2 = 0.062 |
F1,2 = 0.877 p = 0.429 η2 = 0.068 |
| 2 | 0.013 ± 0.006 | 0.019 ± 0.008 | ||||
| 3 | 0.011 ± 0.007 | 0.030 ± 0.010 | ||||
| Microglia-derived exosomes | ||||||
| MCP-1 | 1 | 0.033 ± 0.013 ω | 0.019 ± 0.019 | F1,2 = 1.705 p = 0.216 η2 = 0.124 |
F1,12 = 0.003 p = 0.997 η2 = 0 |
F1,2 = 2.709 p = 0.087 η2 = 0.184 |
| 2 | 0.030 ± 0.036 | 0.132 ± 0.052 | ||||
| 3 | 0.004 ± 0.005 | 0.017 ± 0.007 | ||||
Immune cargo from brain-derived extracellular vesicles in healthcare workers with no/low or moderate/severe psychological distress.
Cell type-specific exosomal levels of interferon-γ (IFN-γ), interleukin-10 (IL-10), IL-6, IL-13, IL-1β, IL-6, tumor necrosis-factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) were measured with Meso Scale Discovery technology in the astrocyte-, neuron-, and microglia-derived exosomes from plasma samples during each visit. The full dataset is described in Supplementary Table 2. Data are presented as the marginalized mean ± SD (pg/mg proteins). *p ≤ 0.05; ***p ≤ 0.001; ‡p ≤ 0.05 and ‡‡p ≤ 0.01 following post-hoc comparisons with the corresponding (i.e., same visit) no/low group; ω p ≤ 0.05 following post-hoc comparisons with the 3rd dataset for the same group. Bold values represent statistically different values.
In regards to neuron-specific levels, a main effect of final K10 score was revealed on neuron-specific levels of MCP-1 (F1,2 = 8.15, p < 0.05), with highly distressed HCWs tending to display higher levels of neuron-specific MCP-1 at the initial visit (p = 0.098 vs. the no/low group at the same visit; Table 3). Finally, a tendency for a time × final K10 interaction was observed for microglia-derived exosomal MCP-1 (F1,2 = 2.71, p = 0.087), with levels lowering across time in HCWs with no/low distress group (p < 0.05 at the 3rd vs. 1st visit; Table 3). Astrocyte-, neuron-, or microglia-derived exosomal expression of immune markers that did not vary across time in both no/low vs. moderate/severe distress groups are presented in Supplementary Table 3.
4 Discussion
We longitudinally assessed the perception of HCWs toward the implemented preventive measures to limit COVID-19 infection within the healthcare facility, as well as their posttraumatic symptomatology, psychological distress, burnout and insomnia symptoms. Blood-based inflammatory cargo specific to brain-derived EVs was also characterized at several time points across a 6-month period. Participants were assigned to the no/low or moderate/severe distress group based on their final K10 psychological score, and their responses to the questionnaires were retrospectively analyzed to identify biopsychosocial markers that are associated with and/or are predictive of psychological distress.
We found that HWCs with moderate/severe distress tended to be older than their colleagues with no/low psychological distress. In line with our observation, age and years of experience of HCWs were positively associated with mean scores for perceived stress and depression during the COVID-19 pandemic (39). As older workers with extensive experience have increased workload, responsibilities and overtime, they may endorse higher levels of stress and be more at-risk for psychological distress (40). It cannot be excluded that older HCWs may also have additional responsibilities outside of work than their younger colleagues. On another note, HCWs older than 50 years were more at risk to develop severe outcomes and recovered more slowly following COVID-19 infection (41). These acknowledged risks may have increased the HCWs’ perceived stress and mental health issues. We also observed an unbalanced distribution of biological sex and occupation in the cohort, with only one male participant and 86% of participants being nurses, which is a female-predominant occupation. Biological sex has been consistently reported as a predictor of different mental health conditions, with women more at risk for major depressive disorder, anxiety, and PTSD (42, 43). In the COVID-19 context, a recent umbrella review of 87 meta-analyses examining HCWs’ mental health did not revealed sex-specific adverse mental outcomes and perceived stress (44). In line with our study, most of the meta-analyses were exclusively recruiting nurses or had higher proportions of nurses within their sample (45–48). Though Maunder and colleagues found increased risk for emotional exhaustion in nurses vs. other healthcare professionals during the COVID-19 pandemic (49), the umbrella review reported no specific effect of the job category on mental health symptoms (44).
We found that psychological distress was not associated with perceived adequacy of training, job stress, or stigma/interpersonal avoidance. In a large cohort of 1875 HCWs across 12 Ontario hospitals, increased risk due to personal protective equipment predicted adverse psychological outcomes (30). Discrepancies between this investigation and our study may be explained by our limited sample size, as well as by the highest proportion of nurses in our study. Despite the reported high efficacy of personal protective equipment and hygiene measures applied during the pandemic, nurses were at higher risk for infection and psychological impact (50). Another investigation involving 539 HCWs from 2 Ontario hospitals in Fall 2020 and Winter 2021 revealed that high self-efficacy for COVID-19 prevention and control correlated with decreased psychological distress (49). Altogether with our findings, this study suggests that risk for psychological distress may be modulated mostly by coping strategies, not perceived stress. Indeed, maladaptive coping strategies (e.g., behavioral disengagement, self-blame, and venting) were predictors of psychological distress, while humor and positive reframing were negatively associated with (51). Psychosocial factors (e.g., marriage, education, work department) and lifestyle habits (e.g., practice of mindfulness, exercise), which were not assessed in our study, may affect the perception of job stress and its management (52, 53). Still, the implementation of preventive measures and training remains critical for minimizing the harmful effects on mental health and quality of health services available to the population (54).
High psychological distress was not associated with COVID-19-specific avoidance or intrusion symptoms. Importantly, early hyperarousal symptoms were predictive of later onset psychological distress. In a longitudinal study colliding psychological data during four COVID-19 waves from 2019 to 2023 in China, exaggerated startle response and hyperarousal were the central symptoms across all four waves (55). Altogether with large meta-analyses reporting posttraumatic symptoms in 32% of HCWs during the COVID-19 pandemic (3, 56), our data suggest that hyperarousal symptoms while facing the health crisis may be a specific psychological factor that may be monitored to promote mental health surveillance among HCWs. Interestingly, fear for personal health has been shown as the strongest predictor of PTSD symptoms in HCWs during the COVID-19, underscoring the urgent need for targeted mental health interventions (57).
Recent meta-analyses reported that burnout and insomnia affected 37–42% of HCWs during the COVID-19 pandemic (3, 4). Sleep disturbances have also been associated with posttraumatic stress disorder, depression or anxiety in front-line HCWs during the COVID-19 outbreak (40, 56). Here, HCWs with high psychological distress did not report changes in sleep parameters, whereas reduced sleep disturbances were observed across time in HCWs with no/low distress. This finding may suggest that HCWs not developing high levels of distress were using resources to promote sleep quality, and facilitate resilience during the pandemic. A strength of our investigation was that both objective and subjective questionnaires were used, adding robustness to our observed associations between psychological distress and sleep quality.
Circulating (i.e., total blood levels regardless of their cellular origin) immune factors as biomarkers of psychiatric disorders face limitations with the limited specificity to neurological changes and to the heterogenous symptomatology of mental disorders (20–22). The emergence of brain-derived EVs as a novel approach to study neurological changes has led to the identification of biomarkers of neurodegeneration in rodents (58, 59), non-human primates (60), and humans (38). Here we studied temporal changes in levels of pro- and anti-inflammatory regulators released by astrocyte-, microglia-, and neuron-derived EVs from the HCWs’ blood samples. Symptoms of distress in HCWs were associated with increased astrocyte-specific levels of TNF-α as early as the second visit. As TNF-α is a known key player in several autoimmune diseases and regulator of CNS functional homeostasis in healthy state (61), altered levels of TNF-α from astroglial EVs may have a harmful effect in HCWs with severe psychological distress. Moreover, the onset of psychological distress in HCWs was related to early increases in astrocyte-specific levels of pro-inflammatory IL-6 and IFN-γ. Combat veterans with PTSD exhibit higher circulating blood levels of IFN-γ, in parallel with elevated levels of T helper lymphocytes and lower levels of regulatory T cells (62). IL-6 and IFN-γ have been associated with in depressive-like behavior, fatigue, and sleep alterations in rodents (63). We also found an early increase in levels of anti-inflammatory IL-10 in astrocyte-derived EVs from HCWs with moderate/severe psychological distress. In line with our findings, increased circulating (i.e., non-CNS-specific) levels of IL-10 have been reported in individuals with MDD or suicidal ideation (10). In addition to astrocyte-specific immune changes, HCWs reporting moderate/severe distress displayed non-detectable levels of microglia or neuron-specific mediators early on during the pandemic. In the CNS, astrocytes represent the most abundant cell type in the brain, which might explain our brain cell-specific detectable variabilities in inflammatory cargo. Overall, we demonstrated that the neuroimmune mechanisms underlying psychological distress in HWCs are specific to the astroglial cell type, and that early immune alterations from brain origins may be associated with late psychological distress. The contribution of immune content from EVs to a psychological condition is complex and a lot of work remains to be done to enhance our understanding of their roles. To our knowledge, our study is the first one evaluating the inflammatory cargo released by brain-derived EVs in association with the severity of self-reported psychological symptoms and demonstrating its feasibility. It is to be noted that the term “EVs” instead of “exosomes” were used following a general consensus from experts for “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate” (64). Further investigations in the field of psychiatry may use these brain-derived markers to distinguishing symptoms of the large psychiatric spectrum and to creating specific biotypes.
Of note, our longitudinal investigation included limitations. First, only 15 participants (out of 90 participants as a primary objective) completed the study, limiting our statistical power and our capacity to exclude potential Type 1 or 2 statistical errors. Our statistical plan was revised accordingly with the help of the biostatistics platform from the CHU de Québec-Université Laval research center, and reported significantly moderate to large effect sizes on hyperarousal symptoms and immune regulators from astrocyte-derived EVs. Given the increased risk for false-positive or false-negative interpretation, caution is warranted in regards to the generalization of the findings (65), and further studies including larger sample sizes are needed.
Our limited sample size may be explained by several factors. First, the overtime work schedules that HCWs were encountering during the COVID-19 outbreak was a challenge for our recruitment, as they were required to provide our team with a blood sample in addition to completing all the questionnaires. Second, past positive test for COVID-19 was considered as an exclusion criterion to avoid confounding bias on neuroinflammation (i.e., brain-derived EVs), excluding a substantial portion of HCWs who have been exposed to the virus, and narrowing our targeted population. The selective inclusion of HCWs without any past COVID-19 positive test throughout the study may generate a selection bias, as the study cohort may not me representative of the sample population. As all HCWs had to pass three mandatory screening tests per week, latent COVID-19 infection in our cohort is unlikely. Moreover, comparing our psychological and biological parameters between non-infected and infected HCWs would have needed a much larger sample size. As history of past COVID-19 infection has been associated with increased risk for psychological symptoms (e.g., anxiety, depression, and PTSD) (66), further investigation should verify whether past COVID-19 infection interacts with the association between psychological distress and blood-based EV markers. We also observed an unbalanced distribution of biological sex and occupation in the cohort, with only one male participant and 86% of participants being nurses. Though this limited the statistical power to identify sex- and occupation-dependent outcomes, a recent umbrella review reported no specific effect of sex or job category on mental health outcomes (44). Finally, the collected data on symptomatology were self-reported, thus not associated with confirmed clinical diagnosis in order to identify blood-based biomarkers of a specific psychiatric diagnosis. Further studies should include a larger sample of participants with better distribution of sex and occupation, include non-HCWs as controls, and incorporate medical and medication (e.g., immunosuppressive) information. Importantly, studies of brain-derived EVs as blood-based biomarkers of diagnosis and risk need to be conducted in other psychiatric contexts to develop of novel avenues in the diagnosis and treatment of several mental illnesses.
5 Conclusion
In sum, the implementation of preventive measures in health facilities remains essential to minimize adverse effects on health and on the quality of health services available to the population, but the perceived adequacy of training, stigma and interpersonal avoidance did not impact the severity of psychological distress in our sample of HCWs. Though replication studies with larger sample sizes are needed, our study highlighted that early reported hyperarousal symptoms were associated with late psychological distress symptoms in HCWs. Lastly, late psychological distress tended to be associated with early neuroinflammatory EVs changes specific to astrocytes. All in all, our findings suggest that psychological markers (i.e., hyperarousal) and blood-based biomarkers (i.e., immune cargo from brain-derived EVs) should be investigated further for early identification of HCWs “at-risk” for psychological distress to provide them with timely and adequate support. Our study also unravels the valuable potential of brain-derived EVs for early biomarkers of risk in psychiatry.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review board of the CHU de Québec (#2021–5394). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CC: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. TH: Data curation, Formal analysis, Writing – review & editing. EB: Funding acquisition, Methodology, Resources, Writing – review & editing. CM: Funding acquisition, Methodology, Writing – review & editing. JD: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by funding the Centre Thématique de Recherche en Neurosciences to Jessica Deslauriers (PI), as well as Eric Boilard, Chantal Mérette and Charles M Morin as co-Is. Jessica Deslauriers is also recipient of a Fonds de Recherche du Québec – Santé J1 research scholarship.
Acknowledgments
We would like to warmly thank Chantal Mérette for her assistance in experimental design, as well as the participants to the study and the members of the clinical and evaluative research platform of the CHU de Québec-Université Laval research center for their assistance with blood collection and revised biostatistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1560129/full#supplementary-material
References
1.
National Physician Health Survey . Canadian Medical Association (CMA). CMA national physician health survey: a national snapshot. Ottawa: The Association. (2017). Available at: https://digitallibrary.cma.ca/link/digitallibrary18
2.
Preti E Di Mattei V Perego G Ferrari F Mazzetti M Taranto P et al . The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. (2020) 22:43. doi: 10.1007/s11920-020-01166-z
3.
Andhavarapu S Yardi I Bzhilyanskaya V Lurie T Bhinder M Patel P et al . Post-traumatic stress in healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. (2022) 317:114890. doi: 10.1016/j.psychres.2022.114890
4.
de Sousa GM de O TVD MLP d MG MLG C de Lima-Araújo GL Schuch FB et al . Mental health in COVID-19 pandemic: a Meta-review of prevalence Meta-analyses. Front Psychol. (2021) 12:703838. doi: 10.3389/fpsyg.2021.703838
5.
Majid U Hussain SAS Zahid A Haider MH Arora R . Mental health outcomes in health care providers during the COVID-19 pandemic: an umbrella review. Health Promot Int. (2023) 38:1–11. doi: 10.1093/heapro/daad025
6.
Radha K George G Varghese A Joseph J Vijayanarayanan N . Prevalence of physical and psychological impacts of wearing personal protective equipment on health care workers during COVID-19: a systematic review and Meta-analysis. Indian J Occup Environ Med. (2022) 26:140–50. doi: 10.4103/ijoem.ijoem_32_22
7.
Liu Y Gayle AA Wilder-Smith A Rocklöv J . The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. (2020) 27:27. doi: 10.1093/jtm/taaa021
8.
Lai J Ma S Wang Y Cai Z Hu J Wei N et al . Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. (2020) 3:e203976–6. doi: 10.1001/jamanetworkopen.2020.3976
9.
Zhang C Yang L Liu S Ma S Wang Y Cai Z et al . Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psych. (2020) 11:306. doi: 10.3389/fpsyt.2020.00306
10.
Yuan N Chen Y Xia Y Dai J Liu C . Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. (2019) 9:233–13. doi: 10.1038/s41398-019-0570-y
11.
Toker S Shirom A Shapira I Berliner S Melamed S . The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J Occup Health Psychol. (2005) 10:344–62. doi: 10.1037/1076-8998.10.4.344
12.
Känel von R Bellingrath S Kudielka BM . Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J Psychosom Res. (2008) 65:51–9. doi: 10.1016/j.jpsychores.2008.02.007
13.
Bargellini A Barbieri A Rovesti S Vivoli R Roncaglia R Borella P . Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. (2000) 57:453–7. doi: 10.1136/oem.57.7.453
14.
Raposo G Stoorvogel W . Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. (2013) 200:373–83. doi: 10.1083/jcb.201211138
15.
Jungblut M Tiveron MC Barral S Abrahamsen B Knöbel S Pennartz S et al . Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia. (2012) 60:894–907. doi: 10.1002/glia.22322
16.
Hlavin ML Lemmon V . Molecular structure and functional testing of human L1CAM: an interspecies comparison. Genomics. (1991) 11:416–23.
17.
Bennett ML Bennett FC Liddelow SA Ajami B Zamanian JL Fernhoff NB et al . New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci. (2016) 113:E1738–46. doi: 10.1073/pnas.1525528113
18.
Fitzgerald W Freeman ML Lederman MM Vasilieva E Romero R Margolis L . A system of cytokines encapsulated in ExtraCellular vesicles. Sci Rep. (2018) 8:8973–11. doi: 10.1038/s41598-018-27190-x
19.
Kawikova I Askenase PW . Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res. (2015) 1617:63–71. doi: 10.1016/j.brainres.2014.09.070
20.
Saeedi S Israel S Nagy C Turecki G . The emerging role of exosomes in mental disorders. Transl Psychiatry. (2019) 9:122–11. doi: 10.1038/s41398-019-0459-9
21.
Vaughn MN Winston CN Levin N Rissman RA Risbrough VB . Developing biomarkers of mild traumatic brain injury: promise and Progress of CNS-derived exosomes. Front Neurol. (2021) 12:698206. doi: 10.3389/fneur.2021.698206
22.
Desmeules C Corbeil O Huot-Lavoie M Béchard L Brodeur S Demers M-F et al . Psychotic disorders and exosomes: an overview of current evidence and future directions. Psychiatry Res. (2024) 339:116066. doi: 10.1016/j.psychres.2024.116066
23.
Dai J Zhang M-Z He Q-Q Chen R . The emerging role of exosomes in schizophrenia. Psychiatry Res. (2023) 327:115394. doi: 10.1016/j.psychres.2023.115394
24.
Wei Z-X Xie G-J Mao X Zou X-P Liao Y-J Liu Q-S et al . Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. (2020) 45:1050–8. doi: 10.1038/s41386-020-0622-2
25.
Vanderheiden A Klein RS . Neuroinflammation and COVID-19. Curr Opin Neurobiol. (2022) 76:102608. doi: 10.1016/j.conb.2022.102608
26.
Minihan S Songco A Fox E Ladouceur CD Mewton L Moulds M et al . Affect and mental health across the lifespan during a year of the COVID-19 pandemic: the role of emotion regulation strategies and mental flexibility. Emotion. (2024) 24:67–80. doi: 10.1037/emo0001238
27.
Brunet A St-Hilaire A Jehel L King S . Validation of a French version of the impact of event scale-revised. Can J Psychiatr. (2003) 48:56–61. doi: 10.1177/070674370304800111
28.
Lange M Licaj I Boulouard M Garon D Richard E Le Bas J et al . Psychological impact of the COVID-19 outbreak in community pharmacists: a longitudinal study. J Am Pharm Assoc (2003). (2022) 62:1359–63. doi: 10.1016/j.japh.2021.12.004
29.
Morin CM Bélanger L LeBlanc M Ivers H Savard J Espie CA et al . The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. (2009) 169:447–53. doi: 10.1001/archinternmed.2008.610
30.
Honarmand K Yarnell CJ Young-Ritchie C Maunder R Priestap F Abdalla M et al . Personal, professional, and psychological impact of the COVID-19 pandemic on hospital workers: a cross-sectional survey. PLoS One. (2022) 17:e0263438. doi: 10.1371/journal.pone.0263438
31.
Kessler RC Andrews G Colpe LJ Hiripi E Mroczek DK Normand SLT et al . Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. (2002) 32:959–76. doi: 10.1017/s0033291702006074
32.
Serraglio A Carson N Ansari Z . Comparison of health estimates between Victorian population health surveys and National Health Surveys. Aust N Z J Public Health. (2003) 27:645–8. doi: 10.1111/j.1467-842x.2003.tb00614.x
33.
Maunder RG Lancee WJ Balderson KE Bennett JP Borgundvaag B Evans S et al . Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerging Infect Dis. (2006) 12:1924–32. doi: 10.3201/eid1212.060584
34.
West CP Dyrbye LN Sloan JA Shanafelt TD . Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. (2009) 24:1318–21. doi: 10.1007/s11606-009-1129-z
35.
Manzar MD Jahrami HA Bahammam AS . Structural validity of the insomnia severity index: a systematic review and meta-analysis. Sleep Med Rev. (2021) 60:101531. doi: 10.1016/j.smrv.2021.101531
36.
Bastien CH Vallières A Morin CM . Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
37.
Buysse DJ Reynolds CF Monk TH Berman SR Kupfer DJ . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213.
38.
Winston CN Romero HK Ellisman M Nauss S Julovich DA Conger T et al . Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci. (2019) 13:1005. doi: 10.3389/fnins.2019.01005
39.
Schroeder S Kelly D Leighton K . Influence of years of experience and age on hospital workforce compassion satisfaction, anxiety, depression, stress, and burnout during pandemic: implications for retention. Psychol Health Med. (2023) 28:1741–54. doi: 10.1080/13548506.2022.2159988
40.
Vanhaecht K Seys D Bruyneel L Cox B Kaesemans G Cloet M et al . COVID-19 is having a destructive impact on health-care workers’ mental well-being. Int J Qual Health Care. (2021) 33. doi: 10.1093/intqhc/mzaa158
41.
Li Q Xiong L Cao X Xiong H Zhang Y Fan Y et al . Age at SARS-CoV-2 infection and psychological and physical recovery among Chinese health care workers with severe COVID-19 at 28 months after discharge: a cohort study. Front Public Health. (2023) 11:1–6. doi: 10.3389/fpubh.2023.1086830
42.
Liu Y Zhang N Bao G Huang Y Ji B Wu Y et al . Predictors of depressive symptoms in college students: a systematic review and meta-analysis of cohort studies. J Affect Disord. (2019) 244:196–208. doi: 10.1016/j.jad.2018.10.084
43.
Semmlinger V Leithner C Klöck LM Ranftl L Ehring T Schreckenbach M . Prevalence and predictors of nonresponse to psychological treatment for PTSD: a Meta-analysis. Depress Anxiety. (2024) 2024:9899034. doi: 10.1155/2024/9899034
44.
Boucher VG Dahl M Lee J Faulkner G Beauchamp MR Puterman E . An umbrella review and meta-analysis of 87 meta-analyses examining healthcare workers’ mental health during the COVID-19 pandemic. J Affect Disord. (2025) 375:423–36. doi: 10.1016/j.jad.2025.01.109
45.
Annaloro C Serpenti F Saporiti G Galassi G Cavallaro F Grifoni F et al . Viral infections in HSCT: detection, monitoring, clinical management, and immunologic implications. Front Immunol. (2020) 11:569381. doi: 10.3389/fimmu.2020.569381
46.
Maqbali Al M Badi Al K Sinani Al M Madkhali N Dickens GL . Clinical features of COVID-19 patients in the first year of pandemic: a systematic review and Meta-analysis. Biol Res Nurs. (2022) 24:172–85. doi: 10.1177/10998004211055866
47.
Galanis P Vraka I Fragkou D Bilali A Kaitelidou D . Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. (2021) 77:3286–302. doi: 10.1111/jan.14839
48.
Varghese A George G Kondaguli SV Naser AY Khakha DC Chatterji R . Decline in the mental health of nurses across the globe during COVID-19: a systematic review and meta-analysis. J Glob Health. (2021) 11:05009. doi: 10.7189/jogh.11.05009
49.
Maunder RG Heeney ND Kiss A Hunter JJ Jeffs LP Ginty L et al . Psychological impact of the COVID-19 pandemic on hospital workers over time: relationship to occupational role, living with children and elders, and modifiable factors. Gen Hosp Psychiatry. (2021) 71:88–94. doi: 10.1016/j.genhosppsych.2021.04.012
50.
Rodríguez-Rey R Guerra Corral M Collazo-Castiñeira P Collado S Caro-Carretero R Cantizano A et al . Predictors of mental health in healthcare workers during the COVID-19 pandemic: the role of experiential avoidance, emotion regulation and resilience. J Adv Nurs. (2024) 80:4089–102. doi: 10.1111/jan.16122
51.
Dehon E Zachrison KS Peltzer-Jones J Tabatabai RR Clair E Puskarich MA et al . Sources of distress and coping strategies among emergency physicians during COVID-19. West J Emerg Med. (2021) 22:1240–52. doi: 10.5811/westjem.2021.9.53406
52.
Amiri S Alajlouni O Al-Rawi SO Samra A Jamil G Kieu A et al . Effect of Mediterranean diet and physical activity on healthcare professional depression, burnout and professional fulfillment during COVID-19. Int J Occup Saf Ergon. (2025) 31:240–7. doi: 10.1080/10803548.2024.2424098
53.
Khazaee-Pool M Pashaei T Yazdani F Ponnet K . Exploring healthcare workers’ perceptions and experiences regarding post-traumatic stress disorder after 2 years of the last global pandemic. BMC Health Serv Res. (2025) 25:861. doi: 10.1186/s12913-025-13004-0
54.
Gross JV Mohren J Erren TC . COVID-19 and healthcare workers: a rapid systematic review into risks and preventive measures. BMJ Open. (2021) 11:e042270. doi: 10.1136/bmjopen-2020-042270
55.
Dong Q Yang Y Ma M Ou W Lv G Huang M et al . Posttraumatic stress symptoms in healthcare workers during the COVID-19 pandemic: a four-wave longitudinal study. Psychiatry Res. (2023) 327:115406. doi: 10.1016/j.psychres.2023.115406
56.
Scherer IJ Holmes PV Harris RBS . The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiol Behav. (2011) 102:225–33. doi: 10.1016/j.physbeh.2010.11.014
57.
Włoszczak-Szubzda A Goniewicz M Gómez-Salgado J Al-Wathinani AM Goniewicz K . Predictors of post-traumatic stress disorder among healthcare workers during the COVID-19 pandemic in Poland. Medicine (Baltimore). (2025) 104:e41821. doi: 10.1097/MD.0000000000041821
58.
Silverman JM Christy D Shyu CC Moon K-M Fernando S Gidden Z et al . CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)G93A ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem. (2019) 294:3744–59. doi: 10.1074/jbc.RA118.004825
59.
Spinelli M Natale F Rinaudo M Leone L Mezzogori D Fusco S et al . Neural stem cell-derived exosomes revert HFD-dependent memory impairment via CREB-BDNF Signalling. IJMS. (2020) 21:11–5 doi: 10.3390/ijms21238994
60.
Kumar A Kim S Su Y Sharma M Kumar P Singh S et al . Brain cell-derived exosomes in plasma serve as neurodegeneration biomarkers in male cynomolgus monkeys self-administrating oxycodone. EBioMedicine. (2021) 63:103192. doi: 10.1016/j.ebiom.2020.103192
61.
Gonzalez CN . Role of tumor necrosis factor-alpha in the central nervous system: a focus on autoimmune disorders. Front Immunol. (2023) 14:1213448. doi: 10.3389/fimmu.2023.1213448
62.
Zhou J Nagarkatti P Zhong Y Ginsberg JP Singh NP Zhang J et al . Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. (2014) 9:e94075. doi: 10.1371/journal.pone.0094075
63.
Dantzer R O’Connor JC Freund GG Johnson RW Kelley KW . From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
64.
Witwer KW Théry C . Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. (2019) 8:1648167. doi: 10.1080/20013078.2019.1648167
65.
Tipton E Hallberg K Hedges LV Chan W . Implications of small samples for generalization: adjustments and rules of thumb. Eval Rev. (2017) 41:472–505. doi: 10.1177/0193841X16655665
66.
Mohammadian Khonsari N Shafiee G Zandifar A Mohammad Poornami S Ejtahed H-S Asayesh H et al . Comparison of psychological symptoms between infected and non-infected COVID-19 health care workers. BMC Psychiatry. (2021) 21:170. doi: 10.1186/s12888-021-03173-7
Summary
Keywords
healthcare workers, preventive measures, psychological distress, insomnia, job stress, extracellular vesicles, inflammation
Citation
Canivet C, Hébert T, Boilard E, Morin CM and Deslauriers J (2025) Mental health among healthcare workers during COVID-19: a study to oversee the impact of the risk perception and relationship with inflammation from blood-based extracellular vesicles. Front. Public Health 13:1560129. doi: 10.3389/fpubh.2025.1560129
Received
22 January 2025
Accepted
30 July 2025
Published
21 August 2025
Volume
13 - 2025
Edited by
Feten Fekih-Romdhane, Tunis El Manar University, Tunisia
Reviewed by
Aquartuti Tri Darmayanti, School of Health Sciences Mamba’ul Ulum, Indonesia
Raphaela Schneider, University Hospital Bochum, Ruhr-Universität Bochum, Germany
Abzal Zhumagaliuly, Kazakh National Medical University, Kazakhstan
Updates
Copyright
© 2025 Canivet, Hébert, Boilard, Morin and Deslauriers.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica Deslauriers, jessica.deslauriers@pha.ulaval.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.