Abstract
Introduction:
Newborn screening for hearing impairment (NHS) is a crucial public health issue worldwide. Often, a two-stage screening with two different testing approaches is used. We aimed to investigate the optimal screening algorithm, based on data from the literature published in the last 30 years. A particular focus of the study was to synthesize the existing evidence on two-stage newborn hearing screening regarding the refer rate (RFR), the percentage of children that did not pass the second test or were lost after the first test.
Methods:
We searched MEDLINE and Scopus for studies on two-stage NHS using transient evoked otoacoustic emissions (TEOAE) or automated auditory brainstem response (AABR). All studies on newborns who received their first test as an inpatient and a second test up to 1 month later were eligible. Random effects meta-analysis was performed to estimate RFR. Risk of bias was assessed using QUADAS-II. The unfunded study was registered in PROSPERO (CRD42023403091, available at https://www.crd.york.ac.uk/PROSPERO/view/CRD42023403091).
Results:
Eighty-five study protocols, including over 1,120,000 newborns, met the inclusion criteria. Certainty in the evidence was rated as moderate.
Discussion:
Strategies that did not involve changes to the screening method had a lower RFR. AABR-AABR: RFR = 1.3% [95% confidence interval (CI): 0.9, 1.8%], TEOAE-TEOAE: RFR = 2.7% (CI: 2.2, 3.2%), TEOAE-AABR: RFR = 3.9% (CI: 2.9, 5.1%), AABR-TEOAE: 5.9% (CI: 5.0, 6.9%). Consequently, where feasible, changing the screening method at the second screening should be avoided in order to minimize the number of follow-up examinations.
1 Introduction
A properly functioning auditory system is essential for a child’s acquisition of spoken language. Early intervention is critical for age-appropriate spoken language development in children with hearing impairment (1–5). Therefore, the Joint Committee on Infant Hearing strives for a start of interventions no later than at 3 months of age (6). Since the late 1990s, two screening methods have been available which allow a very early diagnosis: the measurement of transient evoked otoacoustic emissions (TEOAE) and automated auditory brainstem response (AABR) (7). The availability of these two methods, together with the high prevalence of the condition (1.3 per 1,000 newborns (8)), the existence of effective treatment options (e.g., hearing aids and cochlear implants), and the fact that early intervention has been shown to have positive health effects and a positive cost-benefit ratio (9–11), make congenital hearing impairment an appropriate target for inclusion in a screening program (12, 13). Universal newborn hearing screening (UNHS) was included in standard care in Germany in 2009, as specified by the Federal Joint Committee (G-BA) in §§ 47 to 57 of the Pediatrics Directive. The Pediatrics Directive outlines a two-step screening algorithm in well babies, beginning with initial TEOAE measurement in both ears, followed by bilateral AABR measurement if the initial test is not passed, and defines quality criteria. The utilization of distortion product otoacoustic emission (DPOAE) is not allowed as a screening method in this algorithm. DPOAE thresholds are limited to 50 dB HL, and the goal of newborn hearing screening (NHS) is to detect bilateral hearing loss with a threshold of ≥35 dB HL (14).
The effectiveness of any screening program depends on a number of factors, one of which is its specificity. With regard to the NHS program, the refer rate (RFR) is a relevant factor in this context, including newborns who are lost to follow-up after not passing the initial test, as well as those who receive a “fail” result on the second test. In practice, this equates to the percentage of screened infants who require referral to a pediatric audiologist for further diagnostic examinations after not passing the screening tests (positive screening).
It is crucial to strive for a minimal RFR, as it also encompasses false-positive findings as well as babies who are lost to follow-up. False-positive findings are of particular concern as they can cause anxiety for affected families and necessitate a costly and time-consuming intensive assessment, thereby further straining scarce resources in pediatric audiology practices and outpatient clinics.
The number of newborns lost to follow-up can be reduced by improving the practicality of the process, thereby increasing staff compliance. The occurrence of false-positive screening results, for example those resulting from amniotic fluid or debris in the auditory canal or a noisy environment, can be reduced by employing multi-step testing, with reasonable possible algorithms being TEOAE-TEOAE, AABR-AABR, or TEOAE-AABR.
The advantage of TEOAE is that it is a cost-effective and easily applicable method, whereas AABR is more accurate in terms of identifying false-positive results and is also capable of detecting brainstem hearing loss (6). The German Federal Joint Committee (G-BA) has established that a “fail” result of the initial TEOAE examination should be validated by an AABR in order to keep the RFR as low as possible. The quality target is an RFR that does not exceed 4% (14). The UK even requested lower RFRs (acceptable: 3%, achievable: 2%) (15).
Nevertheless, evaluations of the German NHS for the years 2011/12 (8) and 2017/18 (16, 17) have shown that the recommended screening-algorithm (TEOAE-AABR) is often not followed. In more than 50% of cases where infants do not pass the first test, a second TEOAE measurement is performed instead of the required AABR. Additionally, this second TEOAE test yielded a “fail” result in only about 10% of cases, compared to 20% for infants who underwent a second test using AABR. The failure rate of the second test was particularly high when the screening method was altered. Analysis of these data demonstrated that the second test showed the lowest failure rate with the TEOAE-TEOAE algorithm at 9.62%, while the highest rate was observed with TEOAE-AABR at 26.59%.
Accordingly, international recommendations suggest that a second TEOAE test should be performed after an initial TEOAE result of “fail” in newborns without risk factors for hearing impairment (“well-babies”) (6, 15). In light of these recommendations and the results of the NHS evaluation in Germany, it was necessary to evaluate whether the established algorithm in Germany (TEOAE-AABR) is a viable and optimal option, potentially applicable to other countries as well. Therefore, this study reviewed the current literature to investigate the quality of available screening tests and to find the optimal screening algorithm based on data published in the last 30 years. The screening algorithm should be cost-effective and easy to apply, with high sensitivity to detect all hearing losses, and high specificity to minimize the occurrence of false-positive results. The study focused specifically on synthesizing the existing evidence related to RFR in the two-stage NHS to determine the best two-step screening algorithm based on one of the two tests (TEOAE and AABR).
2 Methods
2.1 Model
The study population consists of newborns with relevant hearing impairment (“diseased,” D+) and those without (“healthy,” D−). The prevalence of hearing impairment in the population is unknown. The first stage of screening is performed using a method with an unknown sensitivity of SE1 and an unknown specificity of SP1. The observed test positivity rate of the first stage (PR1) defines the percentage of newborns who should receive a second test and are considered “failed.” Thus, the rate of positive results from the first stage is also referred to as the “failure rate.” However, not all newborns with a positive first test proceed to the second stage. The observed proportion ρ of positively tested newborns is lost (“loss rate”). This loss is assumed to be independent of hearing status (newborns drop out for reasons unrelated to the first test result). Thus, only a proportion of (1 − ρ) newborns undergo the second stage screening test.
The second stage of screening is performed using a method with an unknown sensitivity of SE2 and an unknown specificity of SP2. Again, the proportion of test-positives among the children in the second stage is of interest. The proportion of newborns who “fail” both tests, together with those who are lost after a first positive test, form the refer rate RFR. If the prevalence of hearing impairment is low, we obtain the following relationship connecting observed variables with an unknown parameter (SP2): RFR = PR1 * {ρ + (1 − ρ)*(1 − SP2)}. For small prevalence estimates, the RFR is linearly related to the failure (positive) rate of the first stage PR1. This linear relationship is determined by the loss rate ρ and the specificity of the second stage test SP2.
In the Supplementary Data 1 and Supplementary Figure 1, we provide a detailed mathematical description of how unobserved quantities of the second stage screening process relate to the observed quantities (unobserved: SE1, SP1, SE2, SP2, prevalence; observed PR1, PR2, ρ).
2.2 Study protocol
The meta-analysis was prospectively registered in PROSPERO (CRD42023403091). The amended review protocol can be found at and downloaded from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023403091.
2.3 Eligibility criteria
For this review, the following PICO criteria were applied:
(P) Population: (Well) babies undergoing a two-stage hearing screening: (1) Initial screening as an inpatient in the maternity clinic; (2) Second test up to a maximum of 1 month later; (3) No use of the test method distortion product otoacoustic emissions (DPOAE); (4) Not exclusively newborns from NICU (neonatal intensive care unit).
(I) Intervention: Two-stage hearing screening using TEOAE, AABR, or a combination of both.
(C) Comparator: not applicable.
(O) Outcome: RFR after two screening steps.
The population should preferably consist of well babies. Studies that included newborns with risk factors for hearing impairment or babies from the NICU were included if the study data did not clearly distinguish between well babies and these newborns. However, studies that included only newborns with risk factors or from the NICU were excluded from this review.
The review considered studies in which both the first and the second tests were completed within 1 month after birth. The rationale behind this strict inclusion criteria was to ensure maximum homogeneity among the studies to ascertain that differences were attributable to the selected screening algorithm rather than to differences in study setting or patient age.
2.4 Search method
We searched MEDLINE using PubMed and Scopus for relevant articles without language or geographic restrictions from the time of their inception through February 9th, 2024. The following search strategy was used for both databases:
(“newborn hearing screening”) OR (“neonatal hearing screening”) OR (“infant hearing screening”) OR (((“newborn screening”) OR (“neonatal screening”) OR (“infant screening”)) AND (“hearing”)) OR ((“hearing screening”) AND (“newborn” OR “neonatal” OR “infant”))
A broader search strategy without specification of test method (TEOAE or AABR) was chosen to avoid missing relevant publications that may not specify the test method in the title or abstract. This decision was based on a small pilot search conducted to test the search strategy, which showed that including the type of screening test in the search resulted in missing some articles that were already known to be relevant to the review.
All articles had an abstract in English. If the full texts of the selected articles were not written in a language that the authors speak, online translation tools (DeepL, Google Translator) were used to translate them into English where possible. Only peer-reviewed publications were included.
2.5 Study records
The search strategy was saved in Citavi version 6.11. The data from the selected publications were extracted into Excel spreadsheets.
One reviewer searched the information sources and screened the titles and abstracts of the identified studies for inclusion and classified each study as eligible or ineligible. The study was classified as potentially eligible if it could not be clearly excluded based on its title and abstract. The full texts of all (potentially) eligible studies were then retrieved and reviewed by two additional reviewers. Again, studies were marked as eligible or ineligible for inclusion and the selection was discussed with the first reviewer until all three reviewers agreed. The first reviewer extracted the required data and made the preliminary decision on study inclusion based on the availability of the data for extraction. The second reviewer double-checked the extracted data and made corrections, which were discussed with all three reviewers and led to the final inclusion of all studies with available data.
2.6 Data extraction and items
Data extracted for the study description included the following: first author and year of publication, screening test combination (i.e., TEOAE-TEOAE, TEOAE-AABR, AABR-TEOAE, AABR-AABR), years of screening, country, number of newborns screened, name of screening device(s), time of first test and second test, and whether the study was conducted only in well babies or also in newborns with risk factors/from the NICU.
Data extracted for quantitative analysis included the following: number of newborns screened with the first test and type of first test (TEOAE/AABR), number of newborns who passed the first test, number of newborns who did not pass the first test, number of newborns who did not pass the first test and did not return for the second test (lost-to-follow-up), number of newborns screened for the second time and type of second test (TEOAE/AABR), number of newborns passing the second test, number of newborns who did not pass the second test, and number of newborns referred after not passing both screening tests.
Studies providing data on simultaneous TEOAE and AABR testing were added to both screening test combinations: TEOAE followed by AABR and AABR followed by TEOAE. Otherwise, such studies would have had to be excluded as the order of the tests was not sequential.
The following data were derived from the variables collected: the failure rate after the first test step was calculated as the quotient of the number of newborns who did not pass the first test and all newborns screened in the first step. Similarly, the failure rate after the second test step was calculated as the quotient of the number of newborns who did not pass the second test and all newborns screened in the second step. The RFR was calculated as the sum of the number of newborns who did not pass the second test and the number of newborns who did not pass the first test and did not return for the second test, divided by the total number of newborns screened.
2.7 Outcomes and prioritization
Data were sought to calculate the RFR after the two-step NHS, with the failure rate after the first test included in the analysis. For the analysis, it was essential to obtain the number of infants who passed and did not pass each screening step. Therefore, studies that only reported the results after both screening steps could not be included in the review. This review focused mainly on well babies, but studies conducted in both well babies and newborns with risk factors or from the NICU were also included.
2.8 Risk of bias in individual studies and publication bias
The quality of the included studies was assessed using a modified version of the QUADAS-II tool developed for diagnostic accuracy studies (18). Of the original four domains, the “reference test” domain was not applicable to our study because we focused on the two-stage screening process without knowing the true hearing loss status. Therefore, we replaced the domain “index test” by “first test” and “reference test” by “second test.” The risk of bias for each included study was assessed independently by two reviewers. Disagreements were resolved by discussion with a mediator. The risk of bias was assessed at the study level.
Publication bias was not expected to have a significant impact on the literature found. It was assumed that all studies on two-stage NHS would be worthy of publication, as they describe not only the quality of the NHS, but also its implementation and problems. Selective reporting within studies, e.g., favoring one of the two screening tests, is also unlikely to be a problem. Therefore, methods to assess the risk of publication bias were not used in this meta-analysis.
2.9 Confidence in cumulative evidence
The strength of the overall body of evidence was assessed using Grading of Recommendations, Assessment, Development and Evaluation (19).
2.10 Subgroup analyses
Subgroup analyses were not specified in the study protocol. However, based on the characteristics of the included studies, we decided to analyze only well-baby studies as a subgroup. An additional post hoc sensitivity analysis was performed to assess the effect of outliers in the TEOAE-TEOAE group.
2.11 Statistical analysis
Continuous variables were summarized by median (minimum–maximum) or presented as box plots, and categorical variables were presented by frequency (%). Due to the exploratory nature of our study, adjustment for multiple testing was not considered. Statistical significance was claimed at 5% level (p < 0.05) or for non-overlapping 95% confidence intervals. Calculations were performed using R Version 4.3.2 (20). Random effects meta-analysis of the RFR was performed using the R package rmeta and the DerSimonian–Laird approach. Heterogeneity indices Q and I2 were calculated using the random effect estimates and random effect weights. Meta-regression was performed using the rma function of the package metafor. For the visualization of the risk of bias, we used the source code of the rob_summary function of the robvis package to generate a similar graph adapted to our needs. All data and analysis scripts are available in the Open Science Framework repository at https://osf.io/nuk4p/.
3 Results
The PRISMA flow diagram for the search and study selection process is shown in Figure 1. Out of the 5,886 records identified (PubMed: n = 3,356, Scopus: n = 2,530), 2,000 duplicates were removed prior to screening. From a total of 3,886 records screened, a total of 3,563 records were excluded because the titles and abstracts of these articles were not relevant to our research question. A full-text search was conducted on the remaining 323 records. Seven records could not be retrieved. Out of the 316 reports that were screened, 239 were excluded. In many cases this was due to a failure to provide the required data at each screening step (n = 77), an unsuitable study design (n = 50), or not performing the first or second test within the specified time frame of 1 month (n = 44). Additional reasons for exclusion are listed in Figure 1. N = 7 reports comprised more than one screening protocol [n = 6 two protocols (21–26) and n = 1 three protocols (27)]. Of the reports with more than one protocol, three (24, 26, 27) provided data on simultaneous TEOAE and AABR testing and were included in both the TEOAE-AABR and AABR-TEOAE groups. A total of 77 reports with 85 study protocols were included in the meta-analysis.
Figure 1
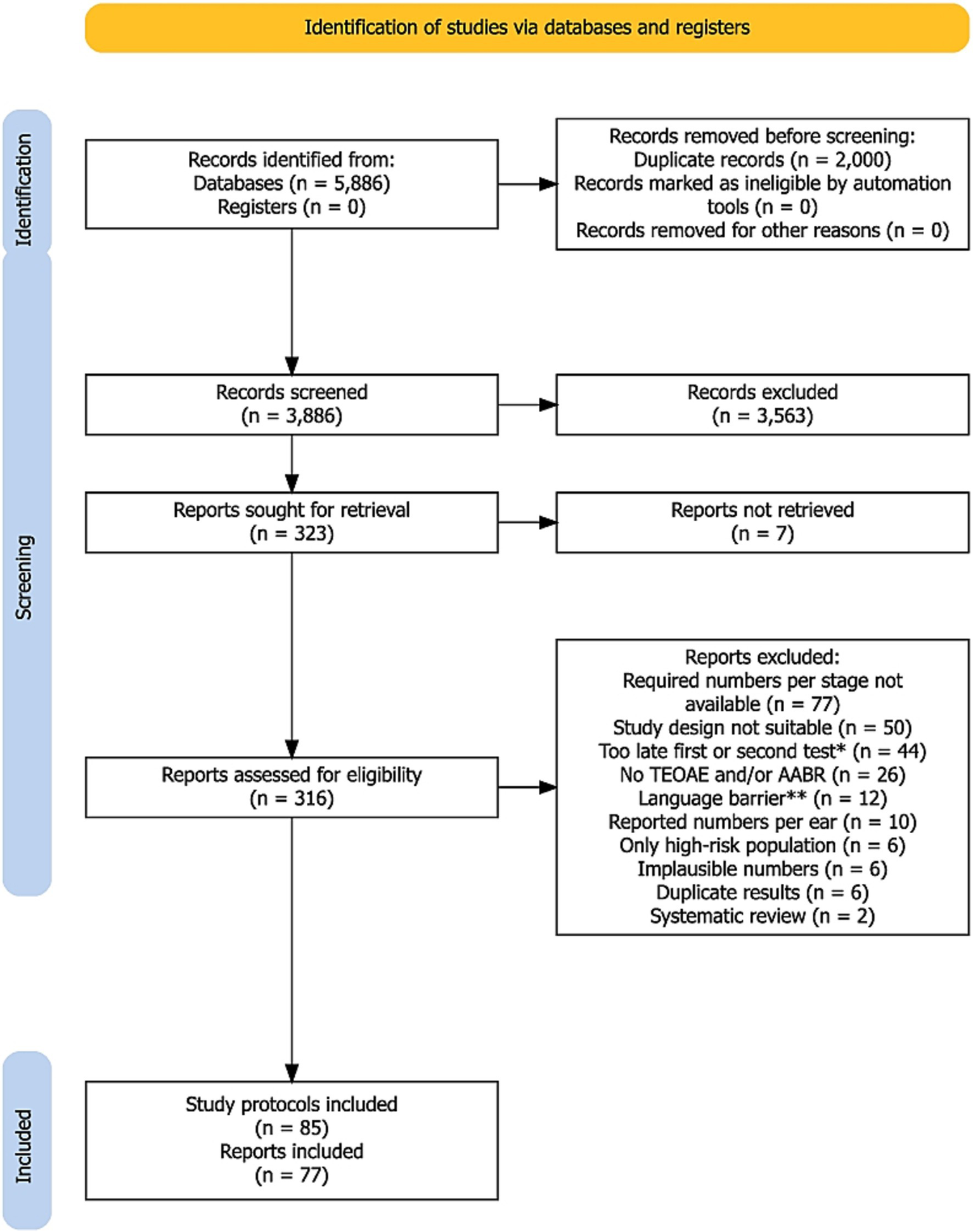
PRISMA flow diagram of the search and study selection process. *Includes also studies where the time point of the first or second test was not specified. **Language barrier refers to reports that could not be automatically translated into English online. A detailed list of the 239 excluded reports is available from the authors upon request.
The analysis included 85 study protocols from 77 reports (7 of those with more than one study protocol) with 1,125,617 newborns. Table 1 provides an overview of the study characteristics.
Table 1
| Screening protocol | Author | Year(s) | Country | No. of screened newborns | Time of first test | Time of second test | High-risk/NICU includeda |
|---|---|---|---|---|---|---|---|
| TEOAE-TEOAE | Aidan et al. (35) | 1995–1997 | France | 1,421 | 48 h | Within the first month | No |
| TEOAE-TEOAE | Alanazi (36) | 2020d,j | Saudi Arabia | 20,171 | Before hospital discharge | Within 2 weeks | Yes |
| TEOAE-TEOAE | Arjmandi et al. (37) | 2009–2010 | Iran | 1,232 | 10 dayse | After 2–3 weeks | Yes |
| TEOAE-TEOAE | Arora et al. (38) | 2017–2019 | India | 1,200 | <72 h | After 3–4 weeks | Yes |
| TEOAE-TEOAE | Arslan et al. (39) | 2007–2008 | Türkiye | 2,229 | Within 7 days, before hospital discharge | Within 15 days after 1st screening | Yes |
| TEOAE-TEOAE | Azizi et al. (40) | 2006–2007 | Iran | 3,818 | <48 h | After 2–4 weeks | Yes |
| TEOAE-TEOAE | Benito-Orejas et al. (21) | 2001–2003 | Spain | 2,454 | Within 48 h, NICU: infants tested on discharge day | Within the first month | Yes |
| TEOAE-TEOAE | Bevilacqua et al. (41) | 2010i,j | Brazil | 11,466 | 24 h | Within 20 days after birth | Yes |
| TEOAE-TEOAE | Busse et al. (22) | 2018–2019 | Albania | 778 | 24–48 h | After 14 days | No |
| TEOAE-TEOAE | Calevo et al. (42) | 2001 | Italy | 3,238 | 48–72 h | Within the third week of life | Yes |
| TEOAE-TEOAE | Calevo et al. (43) | 2002–2004 | Italy | 32,502 | 48–72 h | Within the third week of life | No |
| TEOAE-TEOAE | Cavalcanti et al. (44) | 2007–2009 | Brazil | 3,724 | 36–48 h | 1 week after first test | Nof |
| TEOAE-TEOAE | Chapchap and Segre (45) | 1996–1999 | Brazil | 4,196 | 48–72 h (NICU: prior discharge) | Within 30 days (no specification) | Yes |
| TEOAE-TEOAE | Clarke et al. (23) | 2001–2002 | United Kingdom | 81 | 21 hb | Before hospital discharge | No |
| TEOAE-TEOAE | De Capua et al. (46) | 1998–2006 | Italy | 19,700 | 96 hb, NICU: at post-menstrual age of 37–41 weeks | 10–20 days after birth | Yes |
| TEOAE-TEOAE | Diego Gimenes Lopes et al. (47) | 2016–2019 | Brazil | 1,553 | Before hospital discharge | Within 30 days | Yes |
| TEOAE-TEOAE | Eibenstein et al. (48) | 2007–2012 | Italy | 4,579 | Before hospital discharge | Within 2 weeks | No |
| TEOAE-TEOAE | Eibenstein et al. (49) | 2013–2014 | Italy | 3,120 | Before hospital discharge | Within 2 weeks | No |
| TEOAE-TEOAE | Erturk et al. (25) | 2002–2003 | Türkiye | 500 | Before hospital discharge | After 3 weeks | Yes |
| TEOAE-TEOAE | Escobar-Ipuz et al. (50) | 2007–2017 | Spain | 9,350 | <48 h | Before hospital discharge | Yes |
| TEOAE-TEOAE | Farahani et al. (51) | 2013 | Iran | 2,784 | Day 1 or day 2 | 1–2 weeks after first test | No |
| TEOAE-TEOAE | Ferlito et al. (52) | 2018 | Italy | 37,562 | 48–72 h | Within the first month | Yes |
| TEOAE-TEOAE | George et al. (53) | 2015 | Bahrain | 1,834 | Before hospital discharge | 1 week after discharge | Yes |
| TEOAE-TEOAE | Fusetti et al. (54) | 2005–2007 | Italy | 1,400 | Within 1 week of life | 2 weeks after first test | No |
| TEOAE-TEOAE | Gilbey et al. (55) | 2010–2011 | Israel | 4,958 | Before hospital discharge | Day after 1st screening | No |
| TEOAE-TEOAE | Guastini et al. (56) | 2006–2009 | Italy | 8,671 | 48–72 h, NICU: when stable general condition | 2 weeks after first test | Yes |
| TEOAE-TEOAE | Gül et al. (28) | 2010–2011 | Türkiye | 2,363 | Before hospital discharge | 2 weeks after first test | Yes |
| TEOAE-TEOAE | Habib and Abdelgaffar (57) | 1996–2004 | Saudi Arabia | 11,986 | <48 h | 5th day | No |
| TEOAE-TEOAE | Hatzopoulus et al. (58) | 2003–2004 | Albania | 450 | 24–48 h | Within 4 weeks of first test | No |
| TEOAE-TEOAE | Jakubikova et al. (59) | 2003j | Slovak Republic | 3,048 | 4th–12th day at hospital discharge | 1 month after first test | Yes |
| TEOAE-TEOAE | Kayiran et al. (60) | 2004–2009 | Türkiye | 8,052 | Before hospital discharge | 1 week later | No |
| TEOAE-TEOAE | Konukseven et al. (61) | 2007–2009 | Türkiye | 1,917 | <48 h | After 10 days | No |
| TEOAE-TEOAE | Korres et al. (62) | 2006g,j | Greece | 22,195 | 48–72 h | After 1 month | Yes |
| TEOAE-TEOAE | Korres et al. (63) | 2006h,j | Greece | 25,032 | 48–72 h | After 1 month | Yes |
| TEOAE-TEOAE | Kosmidou et al. (64) | 2018–2020 | Greece | 1,491 | First days of life | Within the first month | No |
| TEOAE-TEOAE | Lin et al. (65) | 2000–2002 | Taiwan | 5,938 | >24, before discharge | 1 month later | No |
| TEOAE-TEOAE | Lotfi and Movallali (66) | 2002–2004 | Iran | 7,718 | 3–36 h | 15–30 days old | No |
| TEOAE-TEOAE | Magnani et al. (67) | 2010–2013 | Italy | 10,359 | 24–48 h | Within 3 weeks from birth | No |
| TEOAE-TEOAE | Molini et al. (68) | 2010–2012 | Italy | 18,796 | 24–36 h | 1 month of age | No |
| TEOAE-TEOAE | Molteni (69) | 1999–2005 | Italy | 10,454 | 3rd day of life | 1 month | Yes |
| TEOAE-TEOAE | Pastorino et al. (70) | 1997–2001 | Italy | 19,290 | 36–48 h (vaginal delivery), 3–5 days (C-section) | 15–30 days after discharge | No |
| TEOAE-TEOAE | Pedersen et al. 2008 (71) | 2006 | Denmark | 1,627 | 2–30 days | 2–30 days | Yes |
| TEOAE-TEOAE | Prpic et al. (72) | 2002–2006 | Croatia | 11,746 | 2 or 3 days (NICU: when stable) | 3 weeks after first test | Yes |
| TEOAE-TEOAE | Pyarali et al. (73) | 2021 | Pakistan | 267 | Before hospital discharge | Before hospital discharge | Yes |
| TEOAE-TEOAE | Satish et al. (74) | 2015–2017 | India | 26,487 | <48 h | 1 week after first test | Yes |
| TEOAE-TEOAE | Sennaroglu and Akmese (75) | 2009–2010 | Türkiye | 1,840 | Before hospital discharge/within 10 days | After 15 days | Yes |
| TEOAE-TEOAE | Sequi Canet et al. (76) | 2002–2013 | Spain | 14,015 | As late as possible prior discharge | Age of 1 month | Yes |
| TEOAE-TEOAE | Sheng et al. (27) | 2018–2019 | China | 1,340 | <48 h | <48 h | No |
| TEOAE-TEOAE | Tasci et al. (77) | 2007–2008 | Türkiye | 15,323 | 24–48 h | Within 10 days | No |
| TEOAE-TEOAE | Tatli et al. (78) | 2002–2003 | Türkiye | 711 | Last working day prior hospital discharge | 1 week after first test | Yes |
| TEOAE-TEOAE | Unlu et al. (79) | 2009–2013 | Türkiye | 2,933 | Day 5 | Day 15 | No |
| TEOAE-TEOAE | Vaid et al. (80) | 2005–2007 | India | 1,238 | <72 h | 1 month | No |
| TEOAE-TEOAE | Vos et al. 2014 (81) | 2007–2012 | Belgium | 245,219 | 48–72 h | Day 3 or 4 (following day after first test) | No |
| TEOAE-TEOAE | Welzl-Müller et al. (82) | 1997j | Austria | 2,338 | Within the first days | Within 1–2 days after first test | No |
| TEOAE-TEOAE | Yorulmaz et al. (29) | 2011–2016 | Türkiye | 13,693 | Before hospital discharge | 14 days later | Yes |
| TEOAE-AABR | Dort et al. (24) | 2000j | Canada | 64 | Before hospital discharge | Before hospital discharge | Yes |
| TEOAE-AABR | Lin et al. (83) | 2004–2005 | Taiwan | 3,540 | >48 h | Before hospital discharge | Yes |
| TEOAE-AABR | Mazlan et al. (84) | 2010–2019 | Malaysia | 50,633 | <24 h | Within 2 weeks | Yes |
| TEOAE-AABR | Nennstiel-Ratzel et al. (85) | 2003–2005 | Germany | 16,767 | Before hospital discharge | Before hospital discharge | Yes |
| TEOAE-AABR | Olusanya et al. (86) | 2005–2006 | Nigeria | 1,150 | 24–48 h | Before hospital discharge | No |
| TEOAE-AABR | Olusanya and Bamigboye (87) | 2005–2007 | Nigeria | 4,718 | 24 h, SCBUc: shortly before discharge | Before hospital discharge | Yes |
| TEOAE-AABR | Ong et al. (26) | 2018 | Philippines | 247 | Before hospital discharge | Before hospital discharge | Yes |
| TEOAE-AABR | Pasha et al. (88) | 2006–2014 | Iran | 40,930 | Before hospital discharge | 1 month of age | No |
| TEOAE-AABR | Sheng et al. (27) | 2018–2019 | China | 2,005 | <72 h | <72 h | No |
| AABR-TEOAE | Dort et al. (24) | 2000k | Canada | 64 | Before hospital discharge | Before hospital discharge | Yes |
| AABR-TEOAE | Ong et al. (26) | 2018 | Philippines | 247 | Before hospital discharge | Before hospital discharge | Yes |
| AABR-TEOAE | Sheng et al. (27) | 2018–2019 | China | 2,005 | <72 h | <72 h | No |
| AABR-AABR | Alothman et al. (89) | 2021 | Saudi Arabia | 199,034 | 24 h/prior discharge | Prior discharge | Yes |
| AABR-AABR | Al Shamisi and Roy (90) | 2010–2019 | United Arab Emirates | 37,661 | Before hospital discharge | 1 month | Yes |
| AABR-AABR | Ayas and Yaseen (91) | 2017–2020 | United Arab Emirates | 1,821 | 24–48 h or shortly before discharge | 2 weeks after first test | No |
| AABR-AABR | Benito-Orejas et al. (21) | 2004–2006 | Spain | 3,117 | Within 48 h, NICU: tested on discharge day | Within 1 month after birth | Yes |
| AABR-AABR | Busse et al. (22) | 2018–2019 | Albania | 1,129 | 24–48 h | After 14 days | Yes |
| AABR-AABR | Clarke et al. (23) | 2001–2002 | United Kingdom | 81 | 24 hb | Before hospital discharge | No |
| AABR-AABR | Clemens and Davis (92) | 1999–2000 | United States | 3,142 | Before hospital discharge | Within 12–24 h after first test, prior discharge | No |
| AABR-AABR | Erturk et al. (25) | 2002–2003 | Türkiye | 500 | Before hospital discharge | After 3 weeks | Yes |
| AABR-AABR | Fan et al. (93) | 2005–2008 | Taiwan | 7,139 | Before hospital discharge | At 1 month of age | No |
| AABR-AABR | Gupta et al. (94) | 2011–2012 | India | 2,265 | 24–48 h, preterm babies >34 postmenstrual weeks | Within 7 days after 1st test | Yes |
| AABR-AABR | Huang et al. (95) | 2009–2010 | Taiwan | 15,790 | 24–36 h | 36–60 h of age | Yes |
| AABR-AABR | Iwasaki et al. (96) | 2000–2001 | Japan | 4,085 | 48–72 h | 5–6 days after birth, prior discharge | Yes |
| AABR-AABR | Kelly et al. (97) | 2014–2016 | United States | 31,984 | 6 h (vaginal delivery), 12 h (C-section) | Before hospital discharge | No |
| AABR-AABR | Messner et al. (98) | 1998–1999 | United States | 5,771 | <24 h | Before hospital discharge | No |
| AABR-AABR | Oruc et al. (99) | 2018 | Türkiye | 5,399 | Before hospital discharge | 15 days later | Yes |
| AABR-AABR | Shim et al. (100) | 2005–2015 | South Korea | 3,059 | 24 h | Within 1 month | Yes |
| AABR-AABR | Tanyeri Toker et al. (101) | 2020–2021 | Türkiye | 570 | <72 h | 7–15 days of age | No |
| AABR-AABR | Tsuchiya et al. (102) | 1999–2004 | Japan | 8,979 | Day 4 | 1 month of age | No |
Characteristics of the included study protocols.
All studies are considered to be level of evidence 2b, individual cohort study/low-quality randomized control study.
Studies that include only healthy newborns or exclude newborns with risk factors were scored as “no,” NICU, newborn intensive care unit.
Median age at first test.
SCBU, special care baby unit.
Data from 3 years were analyzed.
Mean age at first test.
Originally only well babies, newborns transferred to special care baby unit were counted as losses to follow-up.
Three years after program initiation.
Additional data from two additional years were presented.
During 3 years.
Unknown dates, the publication year of the article is given.
All included studies are cohort studies and have a level of evidence 2b, individual cohort study/low-quality randomized control study, following definitions given in https://guides.library.stonybrook.edu/evidence-based-medicine/levels_of_evidence.
Of the 85 study protocols, n = 55 (64.7%) studies examined the TEOAE-TEOAE test combination, n = 9 (10.6%) examined the TEOAE-AABR test combination n = 3 (3.5%) examined the AABR-TEOAE test combination, and n = 18 (21.2%) examined the AABR-AABR test combination. The median study size across all study protocols was n = 3,238 newborns (min–max: 64–245,219). The median study size for TEOAE-TEOAE was n = 3,724 newborns (min–max: 81–245,219), for TEOAE-AABR it was n = 3,540 newborns (min–max: 64–50,633), for AABR-TEOAE it was n = 247 newborns (min–max: 64–2,005), and for AABR-AABR it was n = 3614 newborns (min–max: 81–199,034).
Figure 2A presents the loss rates of newborns who did not pass the first test and did not attend the second test for all test combinations. Depending on the study, loss rates of up to 72% were reported. Median loss rates were 14% for TEOAE-TEOAE, 5% for TEOAE-AABR, and zero for AABR-TEOAE and AABR-AABR. Figure 2B shows the RFR of all study protocols. Median RFRs of 2.5, 4.8, 6.5, and 1.1% were found for the TEOAE-TEOAE, TEOAE-AABR, AABR-TEOAE, and AABR-AABR combinations, respectively. All but three studies (23, 28, 29), reported RFR below 10%. Out of the 85 study protocols, 57 (67.1%) showed a RFR below 4%.
Figure 2
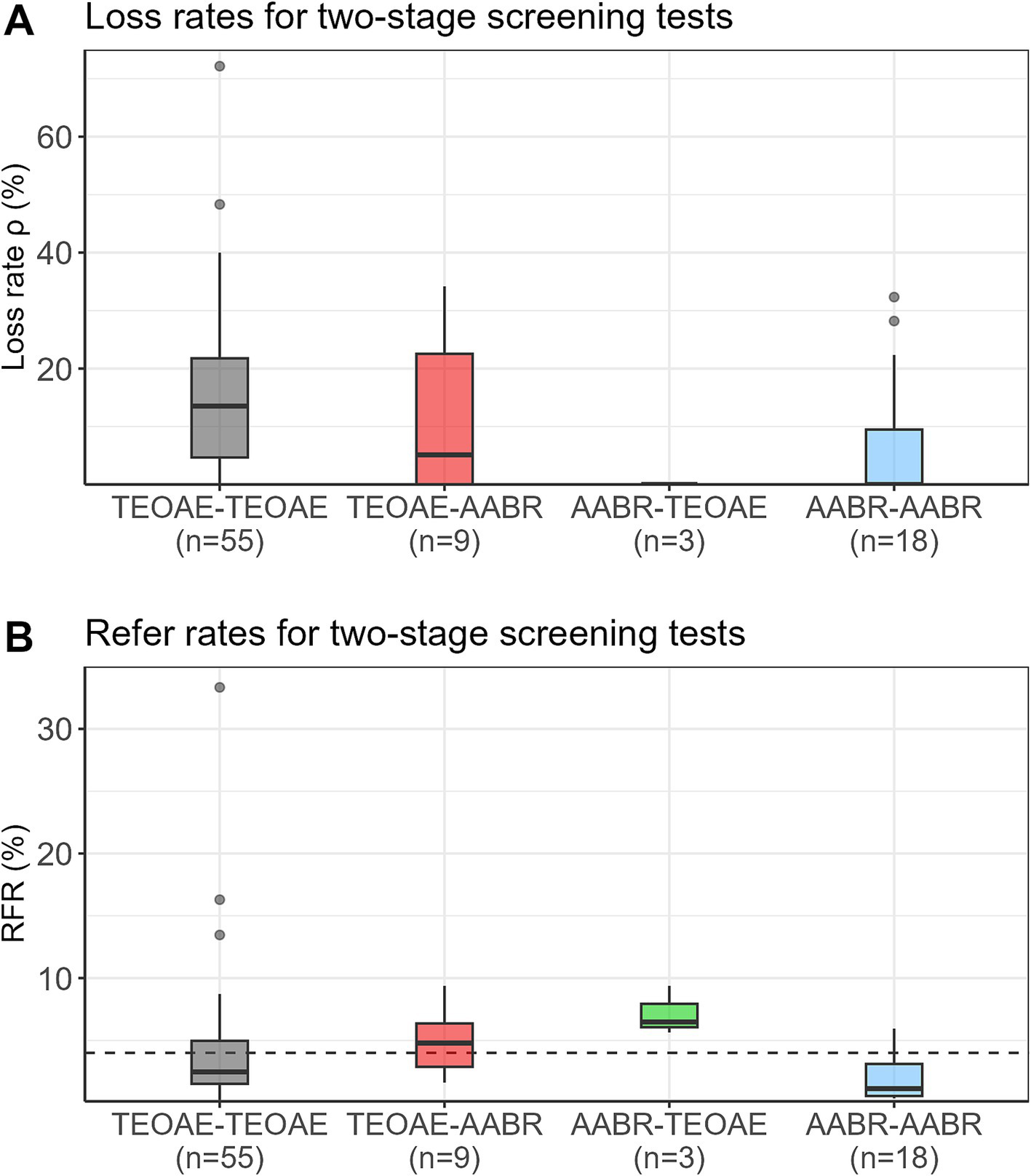
Loss rates (ρ) (A) and refer rates (RFR) (B) for the four different screening test combinations. The dashed horizontal line indicates the 4% threshold quality criteria defined in the Pediatrics Directive for the RFR. AABR, automated auditory brainstem response; TEOAE, transient evoked otoacoustic emission.
3.1 Random effects meta-analysis of RFR
Forest plots of the random effects meta-analysis of RFR are presented in Figure 3 for the TEOAE-TEOAE test combination and in Figure 4 for the other test combinations. A summary of the meta-analysis results is presented in Table 2.
Figure 3
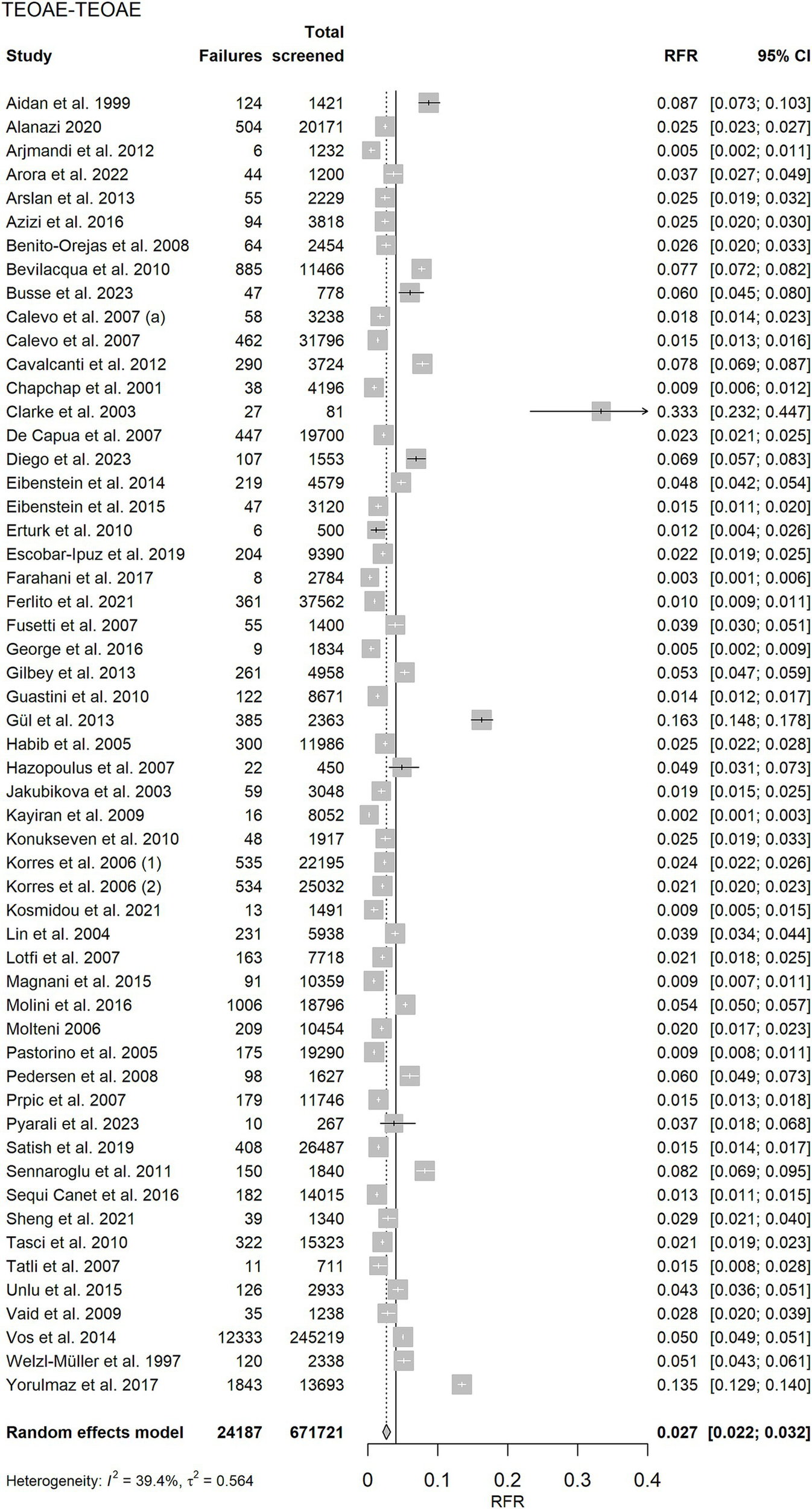
Random effects meta-analysis of refer rate (RFR) for the 55 TEOAE-TEOAE study protocols. The table shows the number of newborns who did not pass the first and second test (“Failures”), the total number of screened newborns (“Total screened”), and the RFR with 95% confidence interval (95% CI) for each study. The summary estimate, including the 95% CI is shown as a grey diamond on a scale ranging from 0 to 40%. The vertical solid line indicates the 4% threshold quality criteria defined in the Pediatrics Directive for the RFR. TEOAE, transient evoked otoacoustic emission.
Figure 4
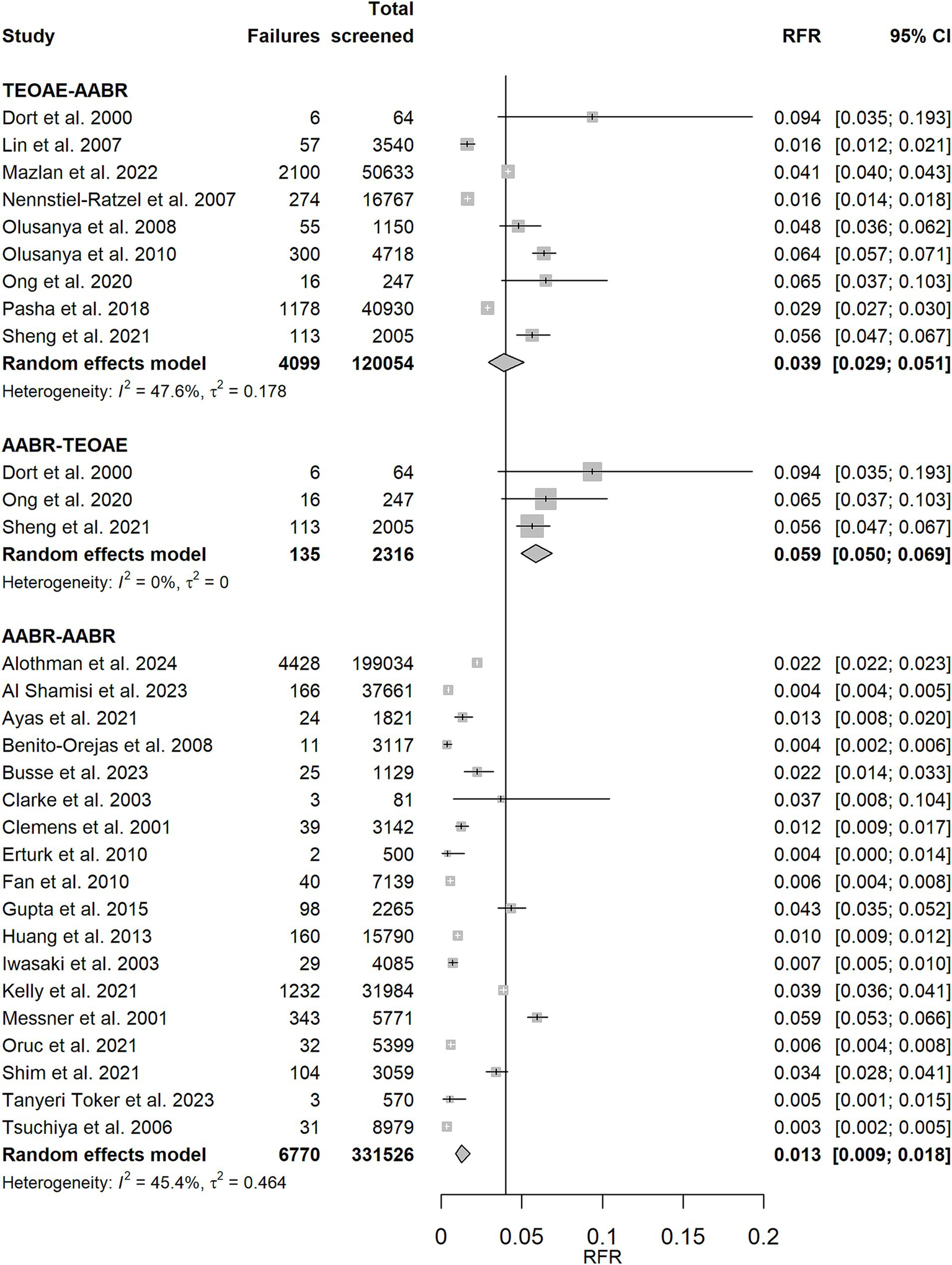
Random effects meta-analysis of refer rate (RFR) for TEOAE-AABR, AABR-TEOAE, and AABR-AABR study protocols. The table shows the number of newborns who did not pass the first and second test (“Failures”), the total number of screened newborns (“Total screened”), and the RFR with 95% confidence interval (95% CI) for each study. The summary estimate per test combination, including the 95% CI, is shown as a grey diamond on a scale ranging from 0 to 20%. The vertical solid line indicates the 4% threshold quality criteria defined in the Pediatrics Directive for the RFR. AABR, automated auditory brainstem response; TEOAE, transient evoked otoacoustic emission.
Table 2
| Test combination | RFR (95% CI) | I 2 | tau2 | Q | df | p |
|---|---|---|---|---|---|---|
| TEOAE-TEOAE | 2.7% (2.2, 3.2%) | 39.4% | 0.564 | 89.14 | 54 | 0.002 |
| TEOAE-AABR | 3.9% (2.9, 5.1%) | 47.6% | 0.178 | 15.27 | 8 | 0.054 |
| AABR-TEOAE | 5.9% (5.0, 6.9%) | 0% | 0 | 1.76 | 2 | 0.416 |
| AABR-AABR | 1.3% (0.9, 1.8%) | 45.4% | 0.464 | 31.14 | 17 | 0.019 |
Results of the random effects meta-analysis of the refer rate.
RFR, refer rate; CI, confidence interval; TEOAE, transient evoked otoacoustic emission; AABR, automated auditory brainstem response. The p-value shown in the right column relates to the Cochrane’s Q statistic (Q) for heterogeneity. Statistically significant p-values (p < 0.05) are printed in bold.
Strategies that do not involve a change in the screening test method showed the lowest RFR [AABR-AABR: RFR = 1.3% (CI: 0.9, 1.8%), TEOAE-TEOAE: RFR = 2.7% (CI: 2.2, 3.2%)]. The upper limits of their 95% confidence intervals are below the recommended quality threshold of 4%. When the screening test method is changed between stage 1 and stage 2, the corresponding 95% confidence intervals cover or exceed the 4% threshold [TEOAE-AABR: RFR = 3.9% (CI: 2.9, 5.1%), AABR-TEOAE: 5.9% (CI: 5.0, 6.9%)]. Studies of both screening combinations TEOAE-TEOAE and AABR-AABR show a moderate degree of heterogeneity as quantified by I2 (39.4 and 45.4%, respectively).
Excluding the TEOAE-TEOAE study protocol with a remarkably high RFR of 33.3% (23) and the two other studies with RFR >10% (28, 29), as shown in Supplementary Figure 2, reduces the summary estimate for RFR from 2.7% (CI: 2.2, 3.2%) to 2.4% (CI: 2.0, 2.8%). The results for RFR for well babies (Supplementary Figures 3, 4) are comparable to those of all studies: AABR-AABR and TEOAE-TEOAE show the lowest RFR (less than 4%). Changing the test method results in a higher RFR.
The results of the meta-regression of the failure rate of the first test and the RFR are presented in Supplementary Table 1 and Supplementary Figure 5. The results show that AABR-AABR is the best screening protocol.
3.2 Bias assessment
Figure 5 shows the results of the bias assessment. While there was a low risk of bias in the use of the tests (first test: 98.8%, second test: 100%), about half of the studies had a high risk of bias in patient selection and in flow and timing (49.4 and 52.9%, respectively). In terms of applicability concerns, about 3 out of 4 studies had high concerns for patient selection (72.9%). Similar to the risk of bias, both tests showed only low applicability concerns (first test: 97.6%, second test: 100%). The study-level bias assessment for all domains can be found in Supplementary Table 2.
Figure 5
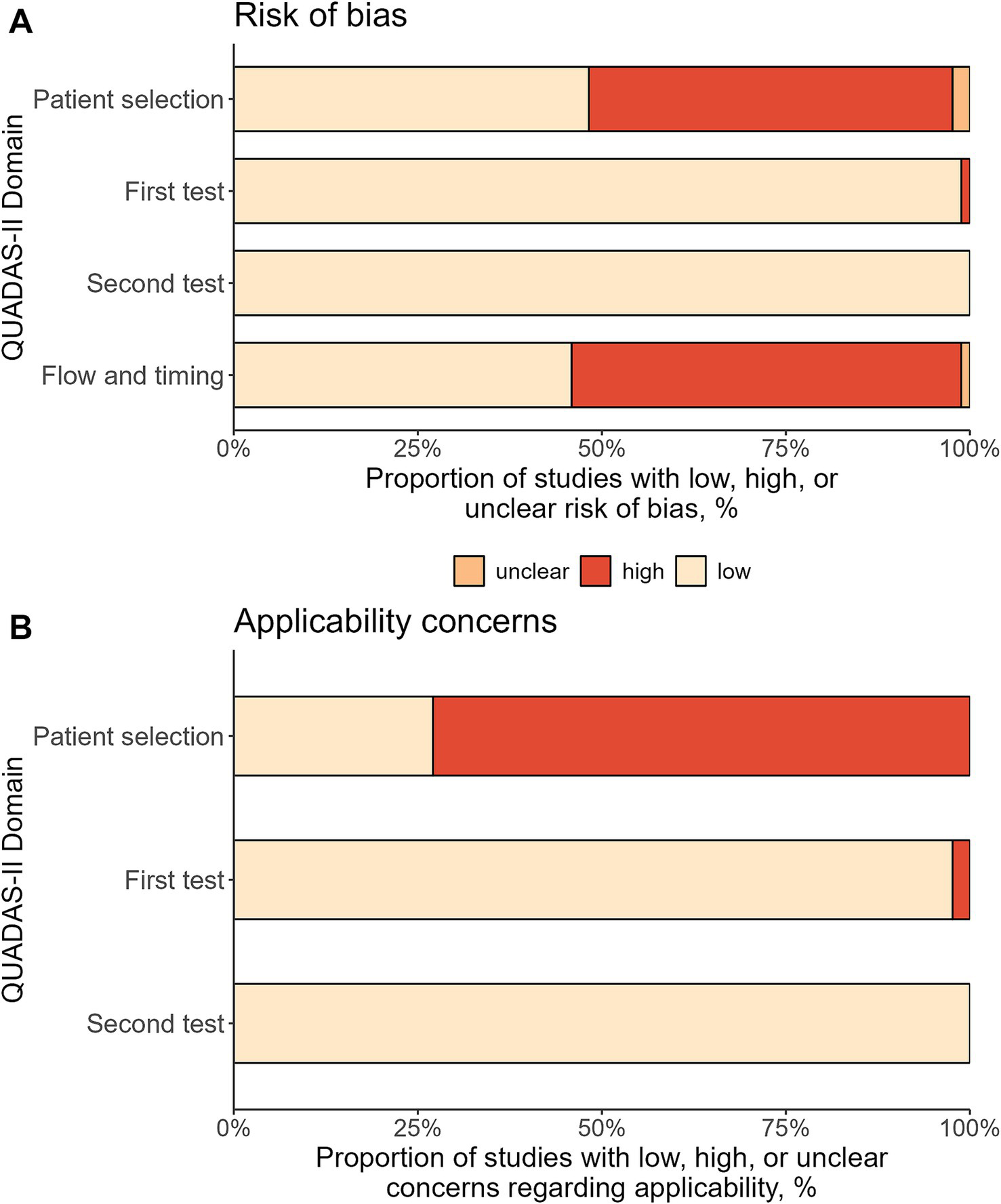
Bias assessment using QUADAS-II tool. The figure shows the percentage of the included 85 study protocols with low, high, or unclear risk of bias (A) and concerns regarding applicability (B).
The GRADE summary of findings table is included in Supplementary Table 3. We rated all evidence generated in this meta-analysis as moderate, which is the second highest GRADE evidence category. The level of evidence for the RFR from the random effects meta-analysis was lowered to moderate because of the moderate heterogeneity found, and for test combinations with test method change between the two stages because of the small number of pooled studies.
4 Discussion
A meta-analysis was conducted to identify the optimal screening algorithm for a two-stage NHS using combinations of TEOAE or AABR tests. The study analyzed 85 study protocols with over 1,120,000 newborns who completed both the first and second hearing tests within 1 month after birth. The results showed that the refer rate (RFR) was lower when there was no change in the screening method used. The aggregated RFR was 1.3% for the AABR-AABR test combination and 2.7% for TEOAE-TEOAE.
The following discussion focuses on newborn hearing screening in the German setting. However, we believe that the discussion will be of broader interest to other countries, as a two-stage algorithm is typically used in NHS programs. In all population-based health programs globally, regular monitoring, evaluation and quality management are essential to ensure the effectiveness, efficiency and sustainability of the program, thereby enabling continuous improvement. In the case of the NHS, this includes the validation of screening algorithms that should meet the required quality standards, particularly with respect to false-positive findings, while simultaneously ensuring cost-effectiveness and applicability in the clinical setting.
The RFR is an important quality parameter in the NHS, as screenings with a refer result must be followed up by a pediatric audiologist. These specialists are scarce in Germany (as well as in other countries), which can lead to long waiting times for families with children who have not passed the screening tests. In addition, false-positive results can cause unnecessary anxiety for parents (30). A low RFR in the NHS can be achieved primarily through a multi-step screening algorithm. Therefore, when the NHS was introduced in 2009, the German Pediatrics Directive required an AABR control if the first hearing test was not passed and an RFR of less than 4% at discharge, in line with national and international quality targets at the time (14, 31).
The German NHS evaluation data for the years 2011/2012 and the follow-up evaluation data for 2017/2018 both indicate that a second hearing test following an initial screening test with a “fail” result considerably reduces the RFR, as this second test was passed in over 80% (8, 16). However, it is worth noting, that this second measurement was performed with a TEOAE in more than half of the tests, which is contrary to the German Pediatrics Directive. In follow-up evaluation interviews this was explained by the longer measurement duration and the increased susceptibility to interference of the AABR. The follow-up evaluation analysis revealed a considerably higher rate of refer results in the second test when a different test method was used than in the first test (8, 16). This is in line with the finding of this meta-analysis that the highest RFR were observed when the test method was changed (TEOAE-AABR or AABR-TEOAE). The rational behind this observation remains unclear, but it may be attributable to the examiner’s minor familiarity with the less frequently used second stage screening method.
In the German follow-up evaluation, the TEOAE-TEOAE algorithm had a lower RFR (9.62%) than AABR-AABR (13.98%) (16). In contrast, in this meta-analysis the AABR-AABR test combination had the lowest RFR, even lower than that of the TEOAE-TEOAE algorithm. This was also observed in the subgroup analysis on “well babies” and may be attributed to the higher lost-to-follow-up rate in the studies reporting TEOAE-TEOAE results (see Figure 2). Likewise, the questionnaire-based EUSCREEN study demonstrated a lower RFR for programs utilizing aABR in comparison to those employing OAE exclusively (32). In addition, studies utilizing this test combination exhibited a significantly higher heterogeneity (see Tables 2, Q statistic) and were often based on routine clinical data, whereas data for the AABR-AABR test sequence were mainly derived from clinical studies. The AABR diagnostic is the “gold standard” for detecting most hearing disorders. However, since TEOAE offers the most practical screening setting and TEOAE-TEOAE has the second best refer rate, the authors recommend this combination for well-babies. This recommendation is also applicable on a global scale, particularly in developing countries, as TEOAE is a cost-effective and easily applicable method that does not necessitate the use of costly consumable materials. One limitation of TEOAE is its inability to detect retrocochlear causes of hearing loss, such as auditory neuropathy (AN). However, this condition is rare in well babies as, i.e., Boudewyns et al. (33) estimated the incidence of AN in a population of newborns at the well-baby clinic with 0.09/1000 live births. Retrocochlear hearing loss is most common in children with risk factors for hearing disorders such as hyperbilirubinemia. Therefore, in contrast to the recommendation of using TEOAE-TEOAE for well-babies, newborns with known risk factors should always be screened using AABR-AABR.
To our knowledge, this is the first systematic review of quality measures using a two-stage screening design and quantitative data from original studies to assess the optimal screening algorithm. Strengths of the study include the large number of newborns included and the high number of study reports.
Because of the strict inclusion criteria (first inpatient screening, second screening within the first month), we did not include studies with outpatient screening only or screening within 6 weeks after birth. We chose these criteria in order to achieve the greatest possible homogeneity among the studies and to be more confident that the differences we found were due to the chosen screening algorithm rather than to differences in study setting or patient age. However, even with these strict inclusion criteria, the results showed a moderate amount of heterogeneity, so we lowered the overall level of evidence for this study.
One limitation of our study is the assumption of homogeneity of sensitivities and specificities across all studies, which ignores the heterogeneity, caused by differently qualified staff and in different settings (i.e., quiet vs. noisy). As these factors influencing the screening result are only described in detail in a few reports, they could not be considered in the meta-analysis. Similarly, the reports often lack information on whether data from children with risk factors for hearing impairment were included. However, they usually provide information on whether children from the NICU were included. In the subgroup analysis including only well babies, the AABR-AABR and TEOAE-TEOAE algorithms showed the lowest RFR (below 4%), and higher RFR were found after changing the test method. These findings confirm the overall results as the sampled population is less heterogeneous (only well babies).
A further limitation of our study was the inability to investigate the sensitivity of the different algorithms, as data on the outcome of babies with positive screening results were not available in the vast majority of studies. The implementation of standardized recording of children with hearing disorders, including the etiology (congenital or acquired), is necessary to enable the investigation of false-negative screening results and thus the sensitivity of the different algorithms.
In its most recent 2019 position paper, the Joint Committee on Infant Hearing recommends performing at least two screening attempts with the same method or an AABR after TEOAE before discharge of a well baby. TEOAE testing after an initial AABR with a refer result is also acceptable for well babies, as the lost-to-follow-up rate for outpatient follow-up is very high (6, 34). The UK screening program guidelines recommend performing a further TEOAE test with an interval of at least 5 h if the first TEOAE test is not passed for well babies (15).
The results of the meta-analysis and the data analysis of the German follow-up evaluation should provide evidence for adjusting the German Pediatrics Directive regarding the method of the second test to improve the RFR and align with international recommendations. Staff compliance with performing a second test before discharge is expected to improve if TEOAE tests are allowed, as TEOAE tests are faster and easier to perform than an AABR measurement. In contrast, changing the method of the second hearing test after failing the initial test results in a higher RFR without evident advantages. Therefore, the TEOAE-TEOAE screening algorithm for well babies could also be recommended for other countries, given that TEOAE represents a screening method that is reliable, cost-effective, and easy to apply in the clinical setting. However, in children with risk factors for perinatal hearing impairment, both hearing tests should always be performed with an AABR test, as specified in the German Pediatrics Directive (14) and international guidelines.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: Open Science Framework repository at https://osf.io/nuk4p/.
Author contributions
KM: Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. UN: Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing. CM: Data curation, Validation, Writing – original draft, Writing – review & editing. UM: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. IB: Conceptualization, Data curation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Anja Friedrichs for the full-text retrieval of non-open access studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1566478/full#supplementary-material
References
1.
Korver AMH Konings S Dekker FW Beers M Wever CC Frijns JHM et al . Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. (2010) 304:1701–8. doi: 10.1001/jama.2010.1501
2.
McCann DC Worsfold S Law CM Mullee M Petrou S Stevenson J et al . Reading and communication skills after universal newborn screening for permanent childhood hearing impairment. Arch Dis Child. (2009) 94:293–7. doi: 10.1136/adc.2008.151217
3.
Pimperton H Blythe H Kreppner J Mahon M Peacock JL Stevenson J et al . The impact of universal newborn hearing screening on long-term literacy outcomes: a prospective cohort study. Arch Dis Child. (2016) 101:9–15. doi: 10.1136/archdischild-2014-307516
4.
Wolff R Hommerich J Riemsma R Antes G Lange S Kleijnen J . Hearing screening in newborns: systematic review of accuracy, effectiveness, and effects of interventions after screening. Arch Dis Child. (2010) 95:130–5. doi: 10.1136/adc.2008.151092
5.
Yoshinaga-Itano C Manchaiah V Hunnicutt C . Outcomes of universal newborn screening programs: systematic review. J Clin Med. (2021) 10:2784. doi: 10.3390/jcm10132784
6.
Joint Committee on Infant Hearing . Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv. (2019) 4:1–44. doi: 10.15142/fptk-b748
7.
Grandori F . European consensus statement on neonatal hearing screening. J Laryngol Otol. (1998) 112:1219. doi: 10.1017/s002221510014294x
8.
Nennstiel U Brockow I Söhl K Zirngibl A , am Zehnhoff-DinnesenAMatulatPet al. Endbericht zur Evaluation des Neugeborenen-Hörscreenings 2011/2012. Available online at: https://www.g-ba.de/downloads/40-268-4395/2017-05-18_Kinder-RL_Annahme_Endbericht_NHS-Bericht.pdf. (Accessed September 8, 2024)
9.
Grosse SD Mason CA Gaffney M Thomson V White KR . What contribution did economic evidence make to the adoption of universal newborn hearing screening policies in the United States?Int J Neonatal Screen. (2018) 4:25. doi: 10.3390/ijns4030025
10.
Rohlfs A Wiesner T Drews H Muller F Breitfuss A Schiller R et al . Interdisciplinary approach to design, performance, and quality management in a multicenter newborn hearing screening project. Discussion of the results of newborn hearing screening in Hamburg (part II). Eur J Pediatr. (2010) 169:1453–63. doi: 10.1007/s00431-010-1229-0
11.
Sharma R Gu Y Ching TYC Marnane V Parkinson B . Economic evaluations of childhood hearing loss screening programmes: a systematic review and critique. Appl Health Econ Health Policy. (2019) 17:331–57. doi: 10.1007/s40258-018-00456-1
12.
Wilson JMG Jungner G . Principles and practice of screening for disease. Geneva: World Health Organization (1968).
13.
Andermann A Blancquaert I Beauchamp S Dery V . Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. (2008) 86:317–9. doi: 10.2471/blt.07.050112
14.
Gemeinsamer Bundesausschuss . Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krankheiten bei Kindern (Kinder-Richtlinie). Available online at: https://www.g-ba.de/downloads/62-492-3190/Kinder-RL_2023-05-12_iK-2023-07-13.pdf. (Accessed September 8, 2024)
15.
Government Digital Service UK . Newborn hearing screening programme (NHSP) operational guidance. Guidance 6: patient journey from screen to referral. Available online at: https://www.gov.uk/government/publications/newborn-hearing-screening-programme-nhsp-operational-guidance/6-patient-journey-from-screen-to-referral. (Accessed September 8, 2024)
16.
Nennstiel U Brockow I Hanauer M Heißenhuber A Marzi C Söhl K et al . Endbericht zur Folge-Evaluation des Neugeborenen-Hörscreenings 2017/2018. Available online at: https://www.g-ba.de/downloads/40-268-9045/2022-11-17_Kinder-RL_Abnahme-Endbericht-Folge-Evaluation-NHS_Bericht.pdf. (Accessed September 8, 2024)
17.
Brockow I Sohl K Hanauer M Heissenhuber A Marzi C Am Zehnhoff-Dinnesen A et al . Newborn hearing screening in Germany-results of the 2011/2012 and 2017/2018 evaluations. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2023) 66:1259–67. doi: 10.1007/s00103-023-03779-0
18.
Whiting PF Rutjes AWS Westwood ME Mallett S Deeks JJ Reitsma JB et al . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19.
Guyatt GH Oxman AD Vist GE Kunz R Falck-Ytter Y Alonso-Coello P et al . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
20.
R Core Team . R: a language and environment for statistical computing. Available online at: https://www.R-project.org/. (Accessed September 8, 2024)
21.
Benito-Orejas JI Ramirez B Morais D Almaraz A Fernandez-Calvo JL . Comparison of two-step transient evoked otoacoustic emissions (TEOAE) and automated auditory brainstem response (AABR) for universal newborn hearing screening programs. Int J Pediatr Otorhinolaryngol. (2008) 72:1193–201. doi: 10.1016/j.ijporl.2008.04.011
22.
Busse AML Qirjazi B Mackey AR Kik J Goedegebure A Hoeve HLJ et al . Implementation of newborn hearing screening in Albania. Int J Neonatal Screen. (2023) 9:28. doi: 10.3390/ijns9020028
23.
Clarke P Iqbal M Mitchell S . A comparison of transient-evoked otoacoustic emissions and automated auditory brainstem responses for pre-discharge neonatal hearing screening. Int J Audiol. (2003) 42:443–7. doi: 10.3109/14992020309081514
24.
Dort JC Tobolski C Brown D . Screening strategies for neonatal hearing loss: which test is best?J Otolaryngol. (2000) 29:206–10. PMID:
25.
Erturk BB Genc GA Ozkan S . Comparison of hearing screening protocols for universal newborn hearing screening in Turkey. J Int Adv Otol. (2010) 6:223–30.
26.
Ong KMC Rivera AS Chan AL Chiong CM . Determining concordance and cost impact of otoacoustic emission and automated auditory brainstem response in newborn hearing screening in a tertiary hospital. Int J Pediatr Otorhinolaryngol. (2020) 128:109704. doi: 10.1016/j.ijporl.2019.109704
27.
Sheng H Zhou Q Wang Q Yu Y Liu L Liang M et al . Comparison of two-step transient evoked otoacoustic emissions and one-step automated auditory brainstem response for universal newborn hearing screening programs in remote areas of China. Front Pediatr. (2021) 9:655625. doi: 10.3389/fped.2021.655625
28.
Gül A Aliosmanoglu C Cengul E . The results of newborn hearing screening in Diyarbakir Children’s Hospital. Duzce Med J. (2013) 15:4–6.
29.
Yorulmaz A Genç U Yılmaz FH Küçüksümbül S . Evaluation and importance of our newborn hearing screening results. Med Bull Haseki. (2017) 55:111–8. doi: 10.4274/haseki.3469
30.
Hewlett J Waisbren SE . A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J Inherit Metab Dis. (2006) 29:677–82. doi: 10.1007/s10545-006-0381-1
31.
Gemeinsamer Bundesausschuss . Tragende Gründe zum Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Kinder-Richtlinien: Einführung eines Neugeborenen-Hörscreenings. Available online at: https://www.g-ba.de/downloads/40-268-641/2008-06-19-Kinder-H%C3%B6rscreening_TrG.pdf. (Accessed September 8, 2024)
32.
Mackey AR Bussé AML Hoeve HLJ Goedegebure A Carr G Simonsz HJ et al . Assessment of hearing screening programmes across 47 countries or regions II: coverage, referral, follow-up and detection rates from newborn hearing screening. Int J Audiol. (2021) 60:831–40. doi: 10.1080/14992027.2021.1886351
33.
Boudewyns A Declau F van den Ende J Hofkens A Dirckx S Van de Heyning P . Auditory neuropathy spectrum disorder (ANSD) in referrals from neonatal hearing screening at a well-baby clinic. Eur J Pediatr. (2016) 175:993–1000. doi: 10.1007/s00431-016-2735-5
34.
Hunter LL Meinzen-Derr J Wiley S Horvath CL Kothari R Wexelblatt S . Influence of the WIC program on loss to follow-up for newborn hearing screening. Pediatrics. (2016) 138:e20154301. doi: 10.1542/peds.2015-4301
35.
Aidan D Avan P Bonfils P . Auditory screening in neonates by means of transient evoked otoacoustic emissions: a report of 2,842 recordings. Ann Otol Rhinol Laryngol. (1999) 108:525–31. doi: 10.1177/000348949910800601
36.
Alanazi AA . Referral and lost to system rates of two newborn hearing screening programs in Saudi Arabia. Int J Neonatal Screen. (2020) 6:50. doi: 10.3390/ijns6030050
37.
Arjmandi F Fahangfar B Mehrabi S Toghiani A Sohrabi H . Prevalence of deafness and hearing screening in newborns in Isfahan. J Res Med Sci. (2012) 17:S233–6.
38.
Arora RD Jati M Nagarkar NM Galhotra A Agrawal S Mehta R et al . Experience, challenges and outcome of implementing universal new born hearing screening in a medical college hospital set up. Indian J Otolaryngol Head Neck Surg. (2022) 74:3841–6. doi: 10.1007/s12070-021-02633-6
39.
Arslan S Isik AU Imamoglu M Topbas M Aslan Y Ural A . Universal newborn hearing screening; automated transient evoked otoacoustic emissions. B-ENT. (2013) 9:122–31. PMID:
40.
Azizi A Amirian F Dargahi A Beidaghi S Mohammadi M . Evaluation of universal newborn hearing screening with TEOAE and ABR: a cross-sectional study with the literature review. Int J Trop Med. (2016) 11:84–9.
41.
Bevilacqua MC de Freitas Alvarenga K Costa OA Moret ALM . The universal newborn hearing screening in Brazil: from identification to intervention. Int J Pediatr Otorhinolaryngol. (2010) 74:510–5. doi: 10.1016/j.ijporl.2010.02.009
42.
Calevo MG Mezzano P Zullino E Padovani P Scopesi F Serra G et al . Neonatal hearing screening model: an Italian regional experience. J Matern Fetal Neonatal Med. (2007) 20:441–8. doi: 10.1080/14767050701398090
43.
Calevo MG Mezzano P Zullino E Padovani P Serra G STERN Group . Ligurian experience on neonatal hearing screening: clinical and epidemiological aspects. Acta Paediatr. (2007) 96:1592–9. doi: 10.1111/j.1651-2227.2007.00475.x
44.
Cavalcanti HG Guerra RO . The role of maternal socioeconomic factors in the commitment to universal newborn hearing screening in the northeastern region of Brazil. Int J Pediatr Otorhinolaryngol. (2012) 76:1661–7. doi: 10.1016/j.ijporl.2012.07.041
45.
Chapchap MJ Segre CM . Universal newborn hearing screening and transient evoked otoacoustic emission: new concepts in Brazil. Scand Audiol Suppl. (2001) 30:33–6. doi: 10.1080/010503901750166600
46.
De Capua B Costantini D Martufi C Latini G Gentile M De Felice C . Universal neonatal hearing screening: the Siena (Italy) experience on 19,700 newborns. Early Hum Dev. (2007) 83:601–6. doi: 10.1016/j.earlhumdev.2007.01.001
47.
Diego Gimenes Lopes J Disconzi Dallegrave C Hellmann Delfino N Lauxen R Marcelino T Eduardo Monteiro Zappelini C . Epidemiological profile of neonates in hearing screening at a maternity of a tertiary hospital in the state of Santa Catarina, Brazil. Int Arch Otorhinolaryngol. (2023) 27:e412–22. doi: 10.1055/s-0043-1770918
48.
Eibenstein A Varakliotis T Saccoccio A Cisternino S Gamma R Mattei A et al . Newborn hearing screening with TEOAE: our experience on 4759 infants. Otorinolaringologia. (2014) 64:27–32.
49.
Eibenstein A Saccoccio A Di Rubbo V Rosati N Varakliotis T Tizio A et al . Newborn hearing screening program: our experience. Otorinolaringologia. (2015) 65:97–101.
50.
Escobar-Ipuz FA Soria-Bretones C Garcia-Jimenez MA Cueto EM Torres Aranda AM Sotos JM . Early detection of neonatal hearing loss by otoacoustic emissions and auditory brainstem response over 10 years of experience. Int J Pediatr Otorhinolaryngol. (2019) 127:109647. doi: 10.1016/j.ijporl.2019.109647
51.
Farahani F Hamidi Nahrani M Seifrabiei MA Emadi M . The effect of mode of delivery and hospital type on newborn hearing screening results using otoacoustic emissions: based on screening age. Indian J Otolaryngol Head Neck Surg. (2017) 69:1–5. doi: 10.1007/s12070-016-0967-3
52.
Ferlito S Maniaci A Cocuzza S La Mantia I Di Mauro P Poli G et al . Universal newborn hearing screening in the Italian Region of Sicily in 2018. Acta Otorhinolaryngol Ital. (2021) 41:356–63. doi: 10.14639/0392-100X-N1162
53.
George SA Alawadhi A Al Reefy H Riskalla A . Newborn hearing screening. Bahrain Med Bull. (2016) 38:148–50.
54.
Fusetti M Fioretti A Ottaviano I Simaskou M Eibenstein A Melpignano G . Newborn hearing screening with otoacoustic emissions: our experience. Otorinolaringologia. (2007) 57:99–104.
55.
Gilbey P Kraus C Ghanayim R Sharabi-Nov A Bretler S . Universal newborn hearing screening in Zefat, Israel: the first two years. Int J Pediatr Otorhinolaryngol. (2013) 77:97–100. doi: 10.1016/j.ijporl.2012.10.004
56.
Guastini L Mora R Dellepiane M Santomauro V Mora M Rocca A et al . Evaluation of an automated auditory brainstem response in a multi-stage infant hearing screening. Eur Arch Otorrinolaringol. (2010) 267:1199–205. doi: 10.1007/s00405-010-1209-z
57.
Habib HS Abdelgaffar H . Neonatal hearing screening with transient evoked otoacoustic emissions in Western Saudi Arabia. Int J Pediatr Otorhinolaryngol. (2005) 69:839–42. doi: 10.1016/j.ijporl.2005.01.018
58.
Hatzopoulos S Qirjazi B Martini A . Neonatal hearing screening in Albania: results from an ongoing universal screening program. Int J Audiol. (2007) 46:176–82. doi: 10.1080/14992020601145310
59.
Jakubikova J Kabatova Z Zavodna M . Identification of hearing loss in newborns by transient otoacoustic emissions. Int J Pediatr Otorhinolaryngol. (2003) 67:15–8. doi: 10.1016/s0165-5876(02)00285-9
60.
Kayiran SM Genc E Erdil A Gurakan B . Results of American hospital newborn hearing screening program. Turk Arch Ped. (2009) 44:135–7.
61.
Konukseven O Genc A Muderris T Kayikci MK Turkyilmaz D Ozturk B et al . Can automated auditory brainstem response be used as an initial stage screening test in newborn hearing screening programs?J Int Adv Otol. (2010) 6:231–8.
62.
Korres SG Balatsouras DG Nikolopoulos T Korres GS Ferekidis E . Making universal newborn hearing screening a success. Int J Pediatr Otorhinolaryngol. (2006) 70:241–6. doi: 10.1016/j.ijporl.2005.06.010
63.
Korres SG Balatsouras DG Gkoritsa E Eliopoulos P Rallis E Ferekidis E . Success rate of newborn and follow-up screening of hearing using otoacoustic emissions. Int J Pediatr Otorhinolaryngol. (2006) 70:1039–43. doi: 10.1016/j.ijporl.2005.10.018
64.
Kosmidou P Tzifas S Lygeros S Danielides G Nikolopoulos T Dimitriou G et al . Newborn hearing screening: analysing the effectiveness of early detection of neonatal hearing loss in a Hospital in Greece. Cureus. (2021) 13:e19807. doi: 10.7759/cureus.19807
65.
Lin C Huang C Lin C Lin Y Wu J . Community-based newborn hearing screening program in Taiwan. Int J Pediatr Otorhinolaryngol. (2004) 68:185–9. doi: 10.1016/j.ijporl.2003.10.007
66.
Lotfi Y Movallali G . A universal newborn hearing screening in Iran. Iran Rehabil J. (2007) 5:8–11.
67.
Magnani C Bacchi G Borghini AM Delmonte D Fava G Occasio AM et al . Universal newborn hearing screening: the experience of the University Hospital of Parma. Acta Biomed. (2015) 86:273–7. PMID:
68.
Molini E Calzolaro L Lapenna R Ricci G . Universal newborn hearing screening in Umbria region, Italy. Int J Pediatr Otorhinolaryngol. (2016) 82:92–7. doi: 10.1016/j.ijporl.2016.01.007
69.
Molteni G . Early detection of newborn hearing impairment by transiently evoked otoacoustic emissions and auditory evoked potentials. Personal experience in 10,454 children. Otorinolaringologia. (2006) 56:93–6.
70.
Pastorino G Sergi P Mastrangelo M Ravazzani P Tognola G Parazzini M et al . The Milan project: a newborn hearing screening programme. Acta Paediatr. (2005) 94:458–63. doi: 10.1111/j.1651-2227.2005.tb01918.x
71.
Pedersen L Møller TR Wetke R Ovesen T . Neonatal hearing screening. A comparison of automatic auditory brainstem audiometry and otoacoustic emissions. Ugeskr Laeger. (2008) 170:642–6. PMID:
72.
Prpic I Mahulja-Stamenkovic V Bilic I Haller H . Hearing loss assessed by universal newborn hearing screening—the new approach. Int J Pediatr Otorhinolaryngol. (2007) 71:1757–61. doi: 10.1016/j.ijporl.2007.07.015
73.
Pyarali M Akhtar S Adeel M Mallick SA Uneeb SN Aslam A . Neonatal hearing screening programme and challenges faced by the developing country: a tertiary care hospital experience. J Pak Med Assoc. (2023) 73:1788–93. doi: 10.47391/JPMA.6264
74.
Satish HS Anil Kumar R Viswanatha B . Screening of newborn hearing at a tertiary care hospital in South India. Indian J Otolaryngol Head Neck Surg. (2019) 71:1383–90. doi: 10.1007/s12070-018-1454-9
75.
Sennaroglu G Akmese PP . Risk factors for hearing loss and results of newborn hearing screening in rural area. J Int Adv Otol. (2011) 7:343–50.
76.
Sequi Canet JM Sala Langa MJ Collar Del Castillo JI . Results from ten years newborn hearing screening in a secondary hospital. An Pediatr. (2016) 85:189–96. doi: 10.1016/j.anpedi.2015.11.003
77.
Tasci Y Muderris II Erkaya S Altinbas S Yucel H Haberal A . Newborn hearing screening programme outcomes in a research hospital from Turkey. Child Care Health Dev. (2010) 36:317–22. doi: 10.1111/j.1365-2214.2009.01029.x
78.
Tatli MM Bulent Serbetcioglu M Duman N Kumral A Kirkim G Ogun B et al . Feasibility of neonatal hearing screening program with two-stage transient otoacoustic emissions in Turkey. Pediatr Int. (2007) 49:161–6. doi: 10.1111/j.1442-200X.2007.02344.x
79.
Unlu I Guclu E Yaman H . When should automatic auditory brainstem response test be used for newborn hearing screening?Auris Nasus Larynx. (2015) 42:199–202. doi: 10.1016/j.anl.2014.10.005
80.
Vaid N Shanbhag J Nikam R Biswas A Ears B . Neonatal hearing screening–the Indian experience. Cochlear Implants Int. (2009) 10:111–4. doi: 10.1179/cim.2009.10.Supplement-1.111
81.
Vos B Lagasse R Leveque A . Main outcomes of a newborn hearing screening program in Belgium over six years. Int J Pediatr Otorhinolaryngol. (2014) 78:1496–502. doi: 10.1016/j.ijporl.2014.06.019
82.
Welzl-Muller K Boheim K Stephan K Schlogel H Stadlmann A Nekahm D . Optimizing hearing screening by transient evoked otoacoustic emissions in newborn infants. HNO. (1997) 45:227–32. doi: 10.1007/s001060050109
83.
Lin H Shu M Lee K Lin H Lin G . Reducing false positives in newborn hearing screening program: how and why. Otol Neurotol. (2007) 28:788–92. doi: 10.1097/mao.0b013e3180cab754
84.
Mazlan R Raman K Abdullah A . A 10-year retrospective analysis of newborn hearing screening in a tertiary hospital in Malaysia. Egypt J Otolaryngol. (2022) 38:135. doi: 10.1186/s43163-022-00331-w
85.
Nennstiel-Ratzel U Arenz SV Kries R Wildner M Strutz J . Modellprojekt Neugeborenen-Hörscreening in der Oberpfalz. HNO. (2007) 55:128–34. doi: 10.1007/s00106-006-1383-x
86.
Olusanya BO Wirz SL Luxon LM . Hospital-based universal newborn hearing screening for early detection of permanent congenital hearing loss in Lagos, Nigeria. Int J Pediatr Otorhinolaryngol. (2008) 72:991–1001. doi: 10.1016/j.ijporl.2008.03.004
87.
Olusanya BO Bamigboye BA . Is discordance in TEOAE and AABR outcomes predictable in newborns?Int J Pediatr Otorhinolaryngol. (2010) 74:1303–9. doi: 10.1016/j.ijporl.2010.08.010
88.
Pasha YZ Zamani M Fard AH Pasha EZ . Screening of hearing in newborn infants: follow-up and outcome after 40930 births in Babol, Northern Iran. Arch Iran Med. (2018) 21:382–6.
89.
Alothman N Elbeltagy R Mulla R . Universal newborn hearing screening program in Saudi Arabia: current insight. J Otol. (2024) 19:35–9. doi: 10.1016/j.joto.2024.01.002
90.
Al Shamisi AM Roy ME . Newborn hearing screening at a single tertiary care hospital in the United Arab Emirates. Aud Vestib Res. (2023) 32:54–63. doi: 10.18502/avr.v32i1.11322
91.
Ayas M Yaseen H . Emerging data from a newborn hearing screening program in Sharjah, United Arab Emirates. Int J Pediatr. (2021) 2021:2616890. doi: 10.1155/2021/2616890
92.
Clemens CJ Davis SA . Minimizing false-positives in universal newborn hearing screening: a simple solution. Pediatrics. (2001) 107:E29. doi: 10.1542/peds.107.3.e29
93.
Fan JY Chen LS Lai JC Chen MK Chen HC . A pre-paid newborn hearing screening programme: a community-based study. B-ENT. (2010) 6:265–9. PMID:
94.
Gupta S Sah S Som T Saksena M Yadav CP Sankar MJ et al . Challenges of implementing universal newborn hearing screening at a tertiary care centre from India. Indian J Pediatr. (2015) 82:688–93. doi: 10.1007/s12098-015-1688-4
95.
Huang H Chiang S Shiau Y Yeh W Ho HC Wang L et al . The universal newborn hearing screening program of Taipei City. Int J Pediatr Otorhinolaryngol. (2013) 77:1734–7. doi: 10.1016/j.ijporl.2013.08.004
96.
Iwasaki S Hayashi Y Seki A Nagura M Hashimoto Y Oshima G et al . A model of two-stage newborn hearing screening with automated auditory brainstem response. Int J Pediatr Otorhinolaryngol. (2003) 67:1099–104. doi: 10.1016/s0165-5876(03)00199-x
97.
Kelly AF Kelly PK Shah M . Auditory brainstem response pass rates correlate with newborn hour of life and delivery mode. J Pediatr. (2021) 230:100–5. doi: 10.1016/j.jpeds.2020.10.036
98.
Messner AH Price M Kwast K Gallagher K Forte J . Volunteer-based universal newborn hearing screening program. Int J Pediatr Otorhinolaryngol. (2001) 60:123–30. doi: 10.1016/s0165-5876(01)00507-9
99.
Oruc MA Alan Y Mercan GC Taner CE Oncel MY Alan M et al . Results of newborn hearing screening in tepecik education and research hospital. Anatol J Family Med. (2021) 4:68–73. doi: 10.5505/anatoljfm.2021.83997
100.
Shim J Kim H Kwon Y Chang J Park E Im GJ . Results of a 10-year hearing screening using automated auditory brainstem response in newborns: the two-step AABR method. Int J Pediatr Otorhinolaryngol. (2021) 151:110947. doi: 10.1016/j.ijporl.2021.110947
101.
Tanyeri Toker G Kumbul YC Cetinkol AE Aslan H Baba P Oncel MY . Is gestational COVID-19 a risk factor for congenital hearing loss?Otol Neurotol. (2023) 44:115–20. doi: 10.1097/MAO.0000000000003761
102.
Tsuchiya H Goto K Yunohara N Matsuoka M Nishioka M Nakamura Y et al . Newborn hearing screening in a single private Japanese obstetric hospital. Pediatr Int. (2006) 48:604–7. doi: 10.1111/j.1442-200X.2006.02274.x
Summary
Keywords
hearing screening, newborn, meta-analysis, referral rate, transient evoked otoacoustic emissions, automated auditory brainstem response
Citation
Manz K, Nennstiel U, Marzi C, Mansmann U and Brockow I (2025) Quality measures of two-stage newborn hearing screening: systematic review and meta-analysis. Front. Public Health 13:1566478. doi: 10.3389/fpubh.2025.1566478
Received
24 January 2025
Accepted
31 March 2025
Published
16 April 2025
Volume
13 - 2025
Edited by
Silvia Palma, AUSL Modena, Italy
Reviewed by
Christian Desloovere, University Hospitals Leuven, Belgium
Donata Gellrich, LMU Munich University Hospital, Germany
Updates
Copyright
© 2025 Manz, Nennstiel, Marzi, Mansmann and Brockow.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsi Manz, manz@ibe.med.uni-muenchen.de
†These authors have contributed equally to this work and share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.